上市新药达洛鲁胺(Darolutamide)合成检索总结报告
- 格式:pdf
- 大小:496.22 KB
- 文档页数:10

上市新药Tenapanor(坦帕诺)合成检索总结报告一、Tenapanor(坦帕诺)简介Tenapanor(坦帕诺)是由Ardelyx公司研发,于2019年9月在美国上市,主要用于成人便秘性肠易激综合征的治疗。
Tenapanor(坦帕诺)是一种钠/氢交换蛋白-3(NHE3)抑制剂。
NHE3在小肠和结肠表面表达,主要负责吸收食物中的钠离子。
本品抑制NHE3,能够减少钠离子在小肠和结肠的吸收,导致水向肠道内分泌,从而加快肠道蠕动并导致粪便疏松。
Tenapanor(坦帕诺)不良反应:严重腹泻。
Tenapanor(坦帕诺)分子结构式如下:CAS:1234423-95-0英文名称:Tenapanor中文名称:坦帕诺二、Tenapanor(坦帕诺)合成路线三、Tenapanor (坦帕诺)合成检索总结报告(一)Tenapanor (坦帕诺)中间体3的合成合成方法实验步骤参考文献合成方法一Into aIL 3-necked round-bottom flask purged and maintained with an inert atmosphere of nitrogen,was placed a solution of 2-bromo l-(3-bromophenyl)ethanone 1(55g,199.28mmol,1.00equiv),1,4-dioxane (300mL),TEA (40g,396.04mmol,1.99eqmv),and (2,4-dichlorophenyl)-N-methylmethanamme 2(38g,201.06mmol,1.01equiv).The resulting solution was stirred for 2h at 25o C.The solids 3were filtered out and the filtrate was used without any further purification.WO2010/78449;(2010);(A2)English (二)Tenapanor (坦帕诺)中间体4的合成合成方法实验步骤参考文献合成方法一Into a IL 3-necked round-bottom flaskpurged and maintained with an inert atmosphere of nitrogen,was placed a solution of 2-((2,4-dichlorobenzyl)(methyl)ammo)-l-(3-bromophenyl)ethanone 3(77g,198.97mmol,1.00equiv,the oretical yield)in methanol (300mL)This was followed by the addition of NaBH 4(15g,394.74mmol,1.98equiv)in several batches at 0o C.The resulting solution was stirred for 30min at 0o C m a water/ice bath.The reaction was then quenched by the addition of 100mL of acetone The resulting mixture was concentrated under vacuum.The resulting solution was extracted with 3x100mL of ethyl acetate and the organic layers combined and dϖed over anhydrous sodium sulfate.The residue was applied onto a silica gel column with ethyl acetate/petroleum ether (1:100).This resulted in 50g (65%)of 2-((2,4-dichlorobenzyi)(methyl)amino)-l-(3-bromophenyl)ethanol 4as a yellow oil.WO2010/78449;(2010);(A2)English (三)Tenapanor (坦帕诺)中间体5的合成合成方法实验步骤参考文献合成方法一Into a 500-mL 3-necked round-bottom flask,was placed a solution of 2-((2,4dichIorobenzyl)(methyl)ammo)-l-(3-bromophenyl)ethanol 4(25g,64.27mmol,1.00equiv)in dichloromethane (100mL).This was followed by the addition of sulfuric acid (100mL)dropwise with stirring at 0-5o C.The resulting solution was stirred for 4h at room temperature.The resulting solution was diluted with of ice water.The pH value of the solution was adjusted to 8with sodium hydroxide.The resulting solution was extracted with 3x300mL of dichloromethane and the organic layers combined and dried over anhydrous sodium sulfate and concentrated under vacuum.The crude product was re-crystallized from petroleum ether ethyl acetate in the ratio of 8:1.This resulted in 15g (63%)of 4-(3-bromophenyl)-6,8-di ‐chloro-2-methyl-l,2,3,4-tetrahydroisoquinoline 5as a white solid.WO2010/78449;(2010);(A2)English (四)Tenapanor (坦帕诺)中间体6的合成。

药物Dasabuvir(达萨布韦)合成检索总结报告
一、Dasabuvir(达萨布韦)简介
Dasabuvir(达萨布韦)于2014年12月19日在美国上市。
Dasabuvir (达萨布韦)是HCV NS5B RNA-依赖RNA聚合酶抑制剂,适用于基因1型慢性丙肝感染。
Dasabuvir(达萨布韦)不良反应有:疲劳、瘙痒、感觉虚弱或缺乏能量、恶心及失眠。
Dasabuvir(达萨布韦)分子结构式如下:
英文名称:Dasabuvir 英文名称:Dasabuvir sodium salt
中文名称:达萨布韦中文名称:达萨布韦钠盐本文主要对Dasabuvir(达萨布韦)的合成路线、关键中间体的合成方法及实验操作方法进行了文献检索并作出了总结。
二、Dasabuvir(达萨布韦)合成路线
三、Dasabuvir(达萨布韦)合成检索总结报告(一) Dasabuvir(达萨布韦)中间体2的合成
(二) Dasabuvir(达萨布韦)中间体3的合成
(三) Dasabuvir(达萨布韦)中间体5的合成方法一。

上市新药Tafamidis(他法米迪)合成检索总结报告一、Tafamidis(他法米迪)简介Tafamidis(他法米迪)是由Foldrx Pharms公司研发,2019年5月3日在美国上市,tafamidis(他法米迪)于2011年11月16日己在欧洲上市,tafamidis meglumine(他法米迪司甲葡胺)于2013年9月20日已在日本上市。
Tafamidis(他法米迪)主要用于治疗由甲状腺素介导的淀粉样变性(ATTR-CM)引起的心脏病。
Tafamidis(他法米迪)是一种转甲状腺素蛋白(TTR)稳定剂,可与TTR特异性结合,稳定TTR的四聚体形态,从而延缓导致ATTR-CM的淀粉样蛋白沉积的产生。
Tafamidis(他法米迪)不良反应:由于临床试验患者数较少,目前未观察到不良反应。
Tafamidis(他法米迪)分子结构式如下:CAS:594839-88-0英文名称:Tafamidis中文名称:他法米迪化学名称:2-(3,5.二氯苯基)-1,3-苯并噁唑-6-羧酸二、Tafamidis(他法米迪)合成路线三、Tafamidis (他法米迪)合成检索总结报告(一)Tafamidis (他法米迪)中间体3的合成序号实验步骤参考文献1In a 2000ml round bottom flask 6.67g 4-amino-3-hydroxy-benzoic acid 1was dissolved in a mixture of THF (126ml)and water (12.6ml)at room temperature (24°C).The 3,5-dichlorobenzoyl chloride 2(1.2eq.)was added within 20-30minutes to the reaction mixture at 20°C and stirred for an hour.1.2eq.of trimethylamine was added to the reaction mixture and stirred for further 30min.The reaction mixture was charged with 1000ml 0.1M HCl aq.solution.A precipitate formed and was filtered by vacuum filtration.The obtained filter cake was washed with water.The wet filter cake was dissolved in 300ml of 0.5M NaOH aq.solution and was filtered in order to separate insoluble particles.The mother liquor was washed with DCM and neutralized with 1M HC1aq.solution until pH =7-8.The formed slurry was charged with acetonitrile and filtered byWO2019/175263;(2019);(A1)English。
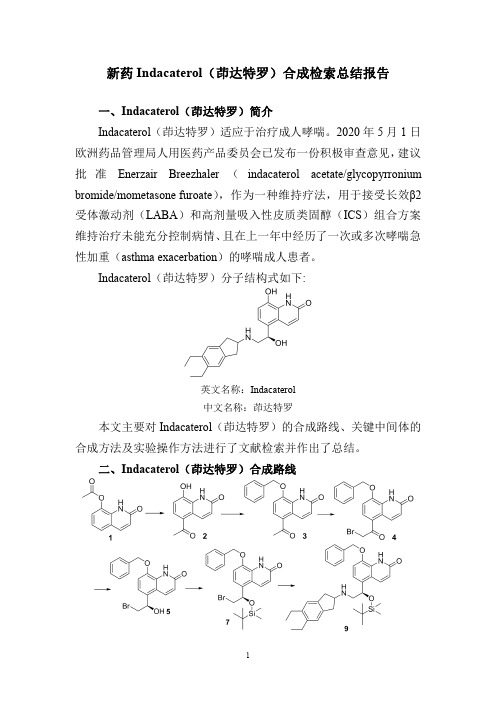
新药Indacaterol(茚达特罗)合成检索总结报告一、Indacaterol(茚达特罗)简介Indacaterol(茚达特罗)适应于治疗成人哮喘。
2020年5月1日欧洲药品管理局人用医药产品委员会已发布一份积极审查意见,建议批准Enerzair Breezhaler(indacaterol acetate/glycopyrronium bromide/mometasone furoate),作为一种维持疗法,用于接受长效β2受体激动剂(LABA)和高剂量吸入性皮质类固醇(ICS)组合方案维持治疗未能充分控制病情、且在上一年中经历了一次或多次哮喘急性加重(asthma exacerbation)的哮喘成人患者。
Indacaterol(茚达特罗)分子结构式如下:英文名称:Indacaterol中文名称:茚达特罗本文主要对Indacaterol(茚达特罗)的合成路线、关键中间体的合成方法及实验操作方法进行了文献检索并作出了总结。
二、Indacaterol(茚达特罗)合成路线三、Indacaterol (茚达特罗)合成检索总结报告(一)Indacaterol (茚达特罗)中间体2的合成合成方法实验步骤参考文献操作方法一A slurry ofaluminum chloride (85.7g,640mmol)in 1,2-dichloroethane (280ML)was cooled in ice,and the product 1(56.8g,280mmol)was added.The mixture was warmed to room temperature and then heated at 85°C.After 30min,acetyl chloride (1.5ML,21mmol)was added and the mixture was heated an additional 60min.The reaction mixture was then cooled and added to 1N hydrochloric acid (3L)at 0°C.with good stirring.After stirring for 2h,the solids were collected on a Buchner funnel,washed with water (3×250ML)and dried under reduced pressure.The crude product isolated from several batches (135g)was combined and triturated with dichloromethane (4L)for 6h.The product was collected on a Buchner funnel and dried under reduced pressure to give 5-acetyl-8-hydroxy-2(1H)-quinolinone 2(121g).US2004/167167;(2004);(A1)English;US2003/153597;(2003);(A1)English;US2003/229058;(2003);(A1)English;WO2004/89892;(2004);(A2)English;US2005/182092;(2005);(A1)English(二)Indacaterol (茚达特罗)中间体3的合成合成方法实验步骤参考文献Crude 5-acetyl-8-hydroxy-(1H)-quinolin-2-one 2(8.13g,40mmol,1.0eq.)is added to N,N,diisopropylethylamine (6.46g,50mmol,1.25eq.)and acetone (64mL).The suspension is heated to reflux temperature and water is added (8.2mL).Benzylbromide (7.52g,44mmol,1.10eq.)is addedWO2004/87668;(2004);(A1)操作方法一drop-wise and the reaction is maintained for6-7hours atreflux temperature until all starting material has reacted.Water(20mL)is added at T=58°C and the mixture iscooled down to20-25°C.The product is filtered,washedwith acetone/water(1/1,2×8.5mL)and then with water(4×8mL).The crude product3is dried overnight undervacuum(60°C).Yield:10.77g(91.7%).Purity of the crudeproduct:99.5%.The product may be recrystallized fromacetone/water.English;WO2005/123684;(2005);(A2)English;操作方法二32.52g(0.16mol)of5-acetyl-8-hydroxy-(1H)-quinolin-2-one2was added to a500mL reaction flask,200mL ofN,N-dimethylformamide was added for dissolution,27.2g(0.20mol)of anhydrous potassium carbonate was added,27.4g(0.16mol)of benzyl bromide was added dropwisewith stirring,and the reaction was stirred at roomtemperature for4hours.Potassium carbonate was removedby filtration.The residue was dissolved in1500mL of dichloromethane,washed with water3times(3300mL)anddried over anhydrous magnesium sulfate.Dichloromethanewas removed by filtration and concentrating,the residue wasground with acetone,and the solid was filtered and washedwith acetone/water(1/1,235mL)to obtain42.8g of5-acetyl-8-benzyloxy-(1H)-quinolin-2-one3with a yield of91.5%.EP2019/3556435;(2019);(A1)English操作方法三To5-acetyl-8-hydroxy-2-quinolone2(37.7g,186mmol)was added dimethylformamide(200ml)and potassiumcarbonate(34.5g,250mmol)followed by benzyl bromide(31.8g,186mmol).The mixture was stirred at roomtemperature for2.25hour and then poured into saturatedsodium chloride(3.5l)at0°C.and stirred well for1hour.The product was collected and dried on a Buchner funnel for1hour,and the resulting solids were dissolved indichloromethane and dried over sodium sulfate.The solutionwas filtered through a pad of Celite and washed withdichloromethane(5×200ml).The combined filtrate was then concentrated to dryness and the resulting solids weretriturated with ether(500ml)for2hours.The product wascollected on a Buchner funnel,washed with ether(2×250ml)and dried under reduced pressure to give5-acetyl-8-benzyloxy-2(1H)-quinolinone3(44g)as an off whitepowder.US2004/224982;(2004);(A1)English;US2004/242622;(2004);(A1)English;US2004/167167;(2004);(A1)English;US2006/35933;(2006);(A1)English;US2006/35931;(2006);(A1)English5-Acetyl-8-hydroxy-1H-quinolin-2-one2(430g,2.12mol)was suspended in N,N-dimethylformamide(3.3L),potassium carbonate(298g,2.16mol)and subsequently操作方法四benzyl bromide (298g,2.11mol)were added thereto,and the mixture was stirred at room temperature for 2hours.The insoluble matter was removed by filtration and washed with N,N-dimethylformamide.The filtrate and the washing were mixed and concentrated under reduced pressure.To the residue was added purified water (3.3L)and the mixture was stirred at room temperature overnight.The precipitate was collected by filtration,washed with purified water,and dried under vacuum to obtain 5-acetyl-8-benzyloxy-1H-quinolin -2-one 3(541g).US2012/46467;(2012);(A1)English 操作方法五15.23g of 5-acetyl-8-hydroxycarbostyril 2and 15.7g of benzyl chloride are added to a mixture of 200ml of acetone and 90ml of water,and 10.35g of potassium carbonate are added thereto.The mixture is refluxed for 12hours under stirring.Then,the mixture is evaporated under reduced pressure to remove acetone,and the residue is extracted with chloroform.The extract is washed with water,dried and evaporated under reduced pressure to remove chloroform.The residue is recrystallized from a mixture of ethyl acetate and n-hexane.10.14g of 5-acetyl-8-benzyloxycarbostyril 3are obtained as prisms.1.58g of said product are further recovered from the mother liquor.Total yield:11.72g (53.3%)US1986/4579854;(1986);(A)English(三)Indacaterol (茚达特罗)中间体4的合成合成方法实验步骤参考文献操作方法一40.0g (0.137mol)of 5-acetyl-8-benzyloxy-(1H)-quinolin-2-one 3was added to a 1liter three-necked flask,and 500mL of dichloromethane was added and stirred for dissolution.The reaction solution was cooled to about 0°C in an ice bath,and 0.267g (0.002mol)of anhydrous aluminum trichloride was added.Then 20g (0.15mol)of bromine was added dropwise,the addition was completed within about 30minutes,and the reaction mixture was increased to room temperature.At this temperature,the reaction was continued with stirring for 4hours,and the thin layer chromatography detection showed that the reaction was complete.The reaction mixture was washed with saturated sodium bicarbonate solution three times (3100mL),and the organicEP2019/3556435;(2019);(A1)English。

新药Aprocitentan(阿普昔腾坦)合成检索总结报告一、Aprocitentan(阿普昔腾坦)简介Aprocitentan(阿普昔腾坦)适应于治疗高血压。
2017年12月Janssen宣布与Idorsia达成合作协议,共同开发和推广在研高血压药物Aprocitentan及其衍生化合物。
2020年1月,Idorsia Pharmaceuticals 和Janssen开启高血压肾功能不全,慢性三期临床研究。
Aprocitentan(阿普昔腾坦)分子结构式如下:英文名称:Aprocitentan中文名称:阿普昔腾坦本文主要对Aprocitentan(阿普昔腾坦)的合成路线、关键中间体的合成方法及实验操作方法进行了文献检索并作出了总结。
二、Aprocitentan(阿普昔腾坦)合成路线路线一三、Aprocitentan(阿普昔腾坦)合成路线路线二四、Aprocitentan (阿普昔腾坦)合成路线一检索总结报告(一)Aprocitentan (阿普昔腾坦)中间体2的合成(路线一)合成方法实验步骤参考文献操作方法一A mixtureof 5-bromo-2-chloro-pyrimidine 1(100g,0.51mol),THF (1.5L),and K 2CO 3(286g,24mol)was heated to 45o C,then ethylene glycol (43ml,0.7mol)was added to thereaction mixture and maintained at 45o C for 8h.The reaction mixture was cooled to room temperature,filtered,and the residue was washed with THF (400ml).The solvent of The filtratewas replaced with ethyl acetate using Dean-Stark apparatus,and the solution was refluxed for 1h,then cooled to 15o C,filtered and washed with ethyl acetate (200ml)and dried in theoven at 60o C for 6h to obtain 110g (98%)of 2as a white solid,mp 68o C-70o anic Preparations and Procedures International ;vol.49;nb.3;(2017);p.258-264.(二)Aprocitentan (阿普昔腾坦)中间体4的合成(路线一)合成方法实验步骤参考文献操作方法To a stirred solution of 100g dimethyl(4-bromophenyl)-malonate 3(10,0.348mol)in 400cm 3methanol,30g formamide (0.66mol)and 30g sodium methoxide (0.555mol)were added at 20-25o C.The reaction mass was heated to 70o C and maintained until completion of reaction (monitored by HPLC).After completion of the reaction,methanol was distilled off from the reaction mass under reduced pressure at 70o C to obtain the syrup.The syrup was cooled to 25-30o C and diluted with 2dm 3water.The pH of the solution was adjusted to 2-2.5using conc.hydrochloricMonatshefte fur Chemie ;vol.149;nb.3;(2018);p.一acid and maintained for 45min.The obtained solid was filtered and washed with water until the pH of the filtrate became 7-7.5.The product was suck dried and dried under reduced pressure at 100C to obtain crude 4.The crude solid was dissolved in 500cm3methanol at 60-65o C and maintained for 1h.The reaction mass was cooled to 25-30o C and maintained for 30min.The obtained solid was filtered,washed with methanol,anddried at 50-55o C under reduced pressure to offer 4.Yield:70g (75.25%);purity by HPLC:99.5%;m.p.:176-180C 653-661.操作方法二To the mixture containing the intermediate 3,100g of formazan hydrochloride was added,and the mixture was stirred and heated to 25°C for 16h.Adding water to the reaction solution,stir at 25°C until clarified,stand still,take the water phase,adjust the pH of the aqueous phase to 5with hydrochloric acid solution and stir for 1h.After suction filtration,the obtained filter cake was washed with a methanol aqueous solution having a mass fraction of 80%.Drying gave 270.2g of intermediate 4,The yield was 92.6%.CN108997223;(2018);(A)Chinese 操作方法三A solution of intermediate 3(11.73g)in MeOH (100mL)was added at 0o C to a solution of sodium (2.83g)in MeOH (100mL).The mixture was stirred for 18h at rt before formamidine hydrochloride (4.10g)was added.The suspension was stirred at rt for 4h.The solvent was removed and the residue was suspended in 10%aq.citric acid (100mL)and stirred for 10min.The white precipitate was collected,washed with 10%aq.citric acid,water,evaporated three times from cyclohexane and dried under HV at 40o C to give 5-(4-bromophenyl)-pyrimidine-4,6-diol 4.WO2009/24906;(2009);(A1)English;WO2006/51502;(2006);(A2)English;US2012/142716;(2012);(A1)English(三)Aprocitentan (阿普昔腾坦)中间体5的合成(路线一)合成方法实验步骤参考文献操作方法200g of the intermediate 4was taken in a 3L three-necked flask.Add 300g of toluene and 180g of N,N-dimethylaniline,mechanically stirred,230g of phosphorus oxychloride was added dropwise at 30°C,and the temperature was raised to 55°C after the addition.After the solid is completely dissolved,the temperature is raised to 100°C,the reaction is carried out for 4h,and then cooled to 25°C for use.450g of water was mixed with 500g of toluene,and cooled to 25°C CN108997223;(2018);(A)。

上市新药Voxelotor(沃塞洛托)合成检索总结报告上市新药Voxelotor(沃塞洛托)合成检索总结报告一、Voxelotor(沃塞洛托)简介Voxelotor(沃塞洛托)是由Global Blood Therapeutics公司研发,于2019年11月在美国上市,主要用于治疗12岁及以上的成人和儿童患者的镰状细胞病。
Voxelotor(沃塞洛托)的作用机制是一种血红蛋白S(HbS)聚合抑制剂,能阻断聚合化及由此导致的红细胞镰状化和破坏。
Voxelotor(沃塞洛托)不良反应:头痛,腹泻,腹痛,恶心,疲劳,皮疹和发热。
Voxelotor(沃塞洛托)分子结构式如下:CAS:1446321-46-5二、Voxelotor(沃塞洛托)合成路线三、Voxelotor (沃塞洛托)合成检索总结报告(一)Voxelotor (沃塞洛托)中间体2的合成合成方法实验步骤参考文献合成方法一To s solutionof 2-bromobenzene-1,3-diol 1(5g,26.45mmol)in DCM (50ml)at 0°C was added DIPEA (11.54mL,66.13mmol)and MOMCl (4.42mL.58.19mmol).The mixture was stirred at 0°C for 1.5h,and then warmed to room temperature.The solution was diluted with DCM,washed with sat.NaHCO 3,brine,dried and concentrated to give crude product,which was purified by column (hexanes/EtOAc=4:1)to give desired product 215.58g (90%).WO2015/31285;(2015);(A1)English;US2015/225366;(2015); (A1)English;ACS Medicinal Chemistry Letters ;vol.8;nb.3;(2017);p.321-326(二)Voxelotor (沃塞洛托)中间体3的合成合成方法实验步骤参考文献合成方法一To a solution of 2-bromo-1,3-bis(methoxymethoxy)benzene 2(19.9g,71.8mmol)in THF (150mL)at -78°C.was added BuLi (2.5M,31.6mL,79.0mmol)dropwise.The solution was stirred at -78°C.for 25min (resulting white c loudy mixture),then it was warmed to 0°C.and stirred for 25min.The reaction mixture slowly turns homogenous.To the solution was added DMF at 0°C.After 25min,HPLC showed reaction completed.The mixture was quenched with sat.NH 4Cl (150mL),diluted with ether (300mL).The organic layer was separated,aq layer was further extracted with ether (2×200mL),and organic layer wascombined,washed with brine,dried and concentrated to give crude product,which was triturated to give 14.6g desired product US2015/225366;(2015);(A1)English;WO2015/31285;(2015);( A1)English;ACS Medicinal Chemistry Letters ;vol.8;nb.3;(2017);p.321-326。
新药Serlopitant(司洛匹坦)合成检索总结报告
一、Serlopitant(司洛匹坦)简介
2020年2月,宣布了Serlopitant(司洛匹坦)的二期临床试验的结果,每日口服抗瘙痒药治疗不明原因慢性瘙痒(CPUO)的安全性和有效性。
Serlopitant(司洛匹坦)被评估为可能与治疗有关的治疗-紧急不良事件的频率为治疗组10.3%,安慰剂组2.6%。
副反应发生率最高的是腹泻(6.9%)、嗜睡(5.2%)、疲劳和头痛(2.6%)。
安慰剂组最常报告的不良事件是胃食管反流病和关节痛(各2.6%)。
两名接受治疗的患者报告了三起被认为与研究药物无关的严重不良事件。
迄今为止,对2 000多人进行了药物治疗,包括接受一年以上治疗的患者。
Serlopitant(司洛匹坦)分子结构式如下:
英文名称:Serlopitant
中文名称:司洛匹坦
本文主要对Serlopitant(司洛匹坦)的合成路线、关键中间体的合成方法及实验操作方法进行了文献检索并作出了总结。
二、Serlopitant(司洛匹坦)合成路线
三、Serlopitant(司洛匹坦)合成检索总结报告(一) Serlopitant(司洛匹坦)中间体3的合成
(二) Serlopitant(司洛匹坦)中间体5的合成
(三) Serlopitant(司洛匹坦)中间体6的合成
(四) Serlopitant(司洛匹坦)中间体7的合成
(五) Serlopitant(司洛匹坦)中间体8的合成
(六) Serlopitant(司洛匹坦)中间体9的合成
(七) Serlopitant(司洛匹坦)中间体10的合成方法一。
Glycopyrronium bromide(格隆溴铵)合成检索总结报告一、Glycopyrronium bromide(格隆溴铵)简介Glycopyrronium bromide(格隆溴铵)为抗胆碱药。
临床很少用药。
它能选择性作用胃肠道,有较强抑制胃液分泌作用。
用于溃疡病、胃炎、胃酸过多症等。
其周围作用类似阿托品。
但本品抑制腺体分泌作用较强,临床多用于麻醉前给药,亦可作为消化性溃疡和缓解内脏痉挛的辅助药。
2020年5月1日欧洲药品管理局人用医药产品委员会已发布一份积极审查意见,建议批准Glycopyrronium bromide(格隆溴铵)与indacaterol acetate、mometasone furoate联合使用,作为一种维持疗法,用于接受长效β2受体激动剂(LABA)和高剂量吸入性皮质类固醇(ICS)组合方案维持治疗未能充分控制病情、且在上一年中经历了一次或多次哮喘急性加重(asthma exacerbation)的哮喘成人患者。
Glycopyrronium bromide(格隆溴铵)分子结构式如下:英文名称:Glycopyrronium bromide中文名称:格隆溴铵本文主要对Glycopyrronium bromide(格隆溴铵)的合成路线、关键中间体的合成方法及实验操作方法进行了文献检索并作出了总结。
二、Glycopyrronium bromide(格隆溴铵)合成路线三、Glycopyrronium bromide (格隆溴铵)合成检索总结报告(一)Glycopyrronium bromide (格隆溴铵)中间体2的合成合成方法实验步骤参考文献操作方法一A mixture of2.34g (8.18mmol)(2R,5R)-2-tert-butyl-5-phenyl-5-(1-cyclopentenyl)-1,3-dioxane-4-keto (1)was dissolved in a mixed solution of 20mL of methanol and 10mL of water.4.58g of KOH was added,and the reaction was refluxed for 3hours with stirringTime.The mixture was cooled to room temperature and extracted with n-heptane.The aqueous phase was acidified with IN HCl,extracted with ethyl acetate,dried and distilled to remove the solvent.(R)-a-phenyl-a-cyclopentenyl-a-hydroxyacetic acid (2),yield 1.64g,92%CN102070631;(2016);(B)Chinese 操作方法二Intermediate (1)(540mg,1.9mmol)was dissolved in MeOH (927μL,22.9mmol).Water (1.84mL,102mmol)was added,followed by the addition of KOH (1.1g,18.8mmol).The reaction was refluxed at 130°C.for 3hours.The mixture was diluted to 250mL with saturated ammonium chloride,then washed (2×100mL hexane).The remaining aqueous emulsion was washed (2×250mL EtOAc).The EtOAc layers were combined,washed with 50mL saturated aqueous NaCl,dried over Na 2SO 4,filtered and concentrated to yield intermediate (2)as a brownish-yellow solid (290mg).US2009/69335;(2009);(A1)English;US2009/170870;(2009);(A1)English.(二)Glycopyrronium bromide (格隆溴铵)中间体3的合成。
上市新药pretomanid(普托马尼)合成检索总结报告一、pretomanid(普托马尼)简介pretomanid(普托马尼)是由Tb Alliance公司研发,并于2019年8月在美国上市的新药。
pretomanid(普托马尼)可以联合苯达喹啉(bedaquiline)和利奈唑胺(1inezolid),用于治疗肺部广泛耐药或治疗不耐受或无效的多药耐药性肺结核的成年患者。
pretomanid(普托马尼)作用机制是抑制结核杆菌的分枝菌酸的生物合成,从而阻止细胞壁的生成。
pretomanid(普托马尼)不良反应:周围神经病变,痤疮,贫血,恶心,呕吐,头痛,转氨酶升高,消化不良,食欲下降,皮疹,瘙痒,腹痛,胸膜炎疼痛,谷氨酰转移酶升高,下呼吸道感染,高淀粉酶血症,咯血,背痛,咳嗽,视力障碍,低血糖,体质量异常减轻和腹泻。
pretomanid(普托马尼)分子结构式如下:CAS:187235-37-6英文名称:Pretomanid中文名称:普托马尼二、pretomanid(普托马尼)合成路线(一)pretomanid(普托马尼)合成路线一(二)pretomanid(普托马尼)合成路线二三、pretomanid(普托马尼)合成检索总结报告(一)合成路线一的分步文献检索结果1、pretomanid (普托马尼)路线一中间体2的合成方法之一序号实验步骤参考文献1Compound 1(22.5g,101.53mmol)was dissolved in 150mL of N,N-dimethylformamide (DMF),Add 15.2g (223.36mmol)of imidazole,After stirring for 40min,A solution of tert -butyldimethylchlorosilane (19.9g,131.99mmol)in DMF (50mL)was added dropwise,After dripping,react at room temperature for 7h.After the reaction was completed,150mL of water was added to quench the reaction,and the aqueous layer was extracted with ethyl acetate (3×150mL).The organic phases were combined,washed with saturated sodium chloride solution,dried over anhydrous sodium sulfate,filtered,The solvent was removed under reduced pressure to obtain a crude product,and the crude product 2was dissolved in 60mL of ethyl acetate.90mL of n -hexane was added dropwise under an ice bath.After the drop was completed,the mixture was stirred at this temperature for 30min,and filtered.The filtrate was evaporated to dryness to give 24.3g of white solid 2,The yield was 71.26%.CN110483549;(2019);(A)2、pretomanid (普托马尼)路线一中间体2的合成方法之二序号实验步骤参考文献mixture 2-chloro-4-nitroimidazole 4(20.0g,0.14mol,100mol%)is dissolved in anhydrousEtOH (200mL),anhydrous K 2CO 3(2.82g,0.020mol,15mol%)is added at room temperature,followed by the tert -butyl-dimethyl-((S)-1-oxiranylmethoxy)-silane 3WO2007/75872;(2007);(A2)English1(22.2mL,0.11mol,0.78mol%).The reaction mixture is heated to 70o C for 6-10h.The solvent is then removed in vacuo and the reaction mixture is taken up in EtOAc.The organic layer is washed several times with water,0.5N HCI,water,brine and the solvent is removed in vacuo to give the crude alcohol as a yellowish solid.The solid is suspended in diethyl ether and filtrated to give the final compound as a colorless powder.The remaining filtrate is concentrated and the process of precipitating the product 2with diethyl ether is repeated twice.ACS Medicinal Chemistry Letters ;vol.8;nb.12;(2017);p.1275-1280Journal of Medicinal Chemistry ;vol.52;nb.5;(2009);p.1317-13283、pretomanid (普托马尼)路线一中间体5的合成序号实验步骤参考文献14(S)-1-(tert -Butyl-dimethyl-silanyloxy)-3-(2-chloro-4-nit ro-imidazol-1-yl)-propan-2-ol 2(3.0g,8.9mmol,100mol%)is dissolved in dichloromethane (100mL)and freshly distilled 3,4-dihydro-2H-pyran (1.5g,17.8mmol,200mol%)is added to the solution,followed by pyridinium-p-to.uene sulfonate (3.4g,13.4mmol,150mol%).The reaction mixture is stirred at room temperature for 24h.The reaction mixture is quenched with saturated aq NaHCO 3solution.The organic layer is separated and the aqueous part is extracted with dichloromethane.The combined organic layers are washed with water,brine,dried on MgSO 4and the solvent is removed in vacuo to give 1-[(S)-3-(tert-Bυtyl-dimethyl-silanyloxy)-2-(tetrahydro-pyran-2-yloxy)-propy l]-2-chloro-4-nitro-1H-imidazole 5as colorless oil.WO2007/75872;(2007);(A2)English 4、pretomanid (普托马尼)路线一中间体7的合成。
上市新药Siponimod(辛波莫德)合成检索总结报告一、Siponimod(辛波莫德)简介Siponimod(辛波莫德)是由诺华公司研发,2019年3月26日在美国上市,主要用于复发型多发性硬化症(MS)成人患者的治疗,包括临床孤立综合征(CIS)、复发缓解型多发性硬化症(RRMS)和活动性继发进展型多发性硬化症(SPMS)。
Siponimod(辛波莫德)是一种鞘氨醇-1-磷酸(S1P)受体调节剂。
与SlP受体1和5具有高亲和力,阻止淋巴细胞从淋巴结流出,从而减少了外周血中的淋巴细胞数量。
Siponimod(辛波莫德)不良反应:头痛,高血压和转氨酶升高。
Siponimod(辛波莫德)分子结构式如下:CAS:1230487-00-9英文名称:Siponimod中文名称:辛波莫德化学名称:3-[[4-[(1E)-1-[[[4-环己基-3-(三氟甲基)苯基]甲氧基]亚氨基]乙基]-2-乙基苯基]-甲基]-3-氮杂环丁烷羧酸富马酸盐(2:1)二、Siponimod(辛波莫德)合成路线三、Siponimod(辛波莫德)合成检索总结报告(一)Siponimod(辛波莫德)中间体3的合成序号实验步骤参考文献1To a solution of ethyl N-hydroxyacetimidate2(3.0g,29.3mmol)in dry DMF (25mL)was added KOBu-t (3.3g,29.3mmol)and the mixture was stirred at room temperature for 20min.A solution of benzyl bromide 1(8.0g,29.3mmol)in dry DMF (5mL)was then added.The resulting mixture was stirred at room temperature for 5h.The reaction mixture was partitioned in H 2O (200mL)and 20%EtOAc/hexane (100mL).After separation,the aqueous layer was further extracted with 20%EtOAc/hexane (2X50mL).The combined organic layer was washed with brine and dried over anhydrous Na 2SO 4.After concentration,the residue was passed through a silica gel pad (a depth of 3cm)in a filtration funnel and washed with 10%EtOAc/hexane.The desired product 3was obtained after concentration as a colorless liquid (8.60g,100%).ACS Medicinal Chemistry Letters ;vol.4;nb.3;(2013);p.333-337(二)Siponimod (辛波莫德)中间体4的合成序号实验步骤参考文献1Anhydrous ZnCl 2(7.96g,58.4mmol)was dissolved in dry degassed NMP (25mL)in a 2-neck flask while heated at 100o C under argon and the resulting solution was allowed to cool to room temperature.One neck of the flask was connected with a distillation set-up while the other was sealed with septa.To the above ZnCl 2solution was added cyclohexyl magnesium chloride (2M in Et 2O,26.5mL,53mmol)via syringe.The reaction was exothermic and Et 2O was evaporated.After the completion of addition,the viscous mixture was stirred at room temperature for 5min before elevating the temperature to 80o C to allow for the complete evaporation of Et2O (ca.30min)to give an unstirrable solid.After cooled to room temperature,chloride 3(7.85g,26.5mmol)and Pd(PBut 3)2(0.678g,1.33mmol)were added.The flask was flushed with argon and sealed with septa.The mixture was then heated to 140o C and stirred for 1h.After cooled to room temperature,the mixtureACS Medicinal Chemistry Letters ;vol.4;nb.3;(2013);p.333-337。
上市新药达洛鲁胺(Darolutamide)合成检索总结报
告
一、达洛鲁胺(Darolutamide)简介
达洛鲁胺(Darolutamide)是由拜耳公司研发,于2019年7月在美国上市,主要用于治疗非转移性去势抵抗性前列腺癌。
达洛鲁胺(darolutamide)是雄激素受体(AR)抑制剂。
达洛鲁胺(darolutamide)竞争性抑制雄激素与受体结合,AR核转位和AR介导的转录。
主要代谢产物酮基-达洛鲁胺(keto-darolutamide)也表现出相似的体外活性。
此外,达洛鲁胺(darolutamide)也是孕激素受体(PR)拮抗剂,体外活性约为AR的1%。
在前列腺癌的小鼠异种移植模型中,达洛鲁胺(darolutamide)可以降低体外前列腺癌细胞增殖和肿瘤体积。
达洛鲁胺(Darolutamide)不良反应:疲劳,四肢疼痛和皮疹。
达洛鲁胺(Darolutamide)分子结构式如下:
CAS:1297538-32-9
英文名称:Darolutamide
二、达洛鲁胺(Darolutamide
)合成路线
三、达洛鲁胺(Darolutamide )合成检索总结报告(一)达洛鲁胺(Darolutamide )中间体2
的合成序号
实验步骤参考文献1Acetonitrile (50ml),water (50ml),potassium carbonate (7g,2.07eqv.)and 4-bromo-2-chlorobenzonitrile 1(14.0g,1.00eqv.)were charged.The mixture was refluxed under nitrogen atmosphere for about 0.5h.The mixture was cooled to 60-70°C under nitrogen protection.Palladium(II)acetate Pd(OAc)2(0.10g,0.007eqv.)and triphenylphosphine (0.40g,0024eqv.)were added under nitrogen protection.1-(Tetrahydro-2H-pyran-2-yl)-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole (21.0g,1.17eqv.)was dissolved in acetonitrile (30ml).Air was removed by vacuum and replaced by nitrogen.This solution was added to the reaction mixture in about 0.5h at 70±3°C.The reaction
WO2016/162604;(2016);(A1)English
mixture was stirred for2hat70±3°C.The water phase was separated off and removed from the reaction mixture at65-70°C.2nil of ammonia water(25%)was added to the reaction mixture which was then cooled to20±5°C. Water(80nil)was added gradually at20±5°C.The mixture was stirred overnight at20±5°C.The crystalline product2was filtered and washed twice with acetonitrile:water1:1(20mL).The product was dried under reduced pressure at50-60°C.Yield17.18g (92.3%).HPLC-purity99.8%.
21-(tetrahydro-2H-pyran-2-yl)-5-(4,4,5,5-tetramethyl-1,3,
2-dioxaborolan-2-yl)-1H-pyrazole(1.6g,5.8mmol)and
commercially available4-bromo-2-chlorobenzonitrile1
(1.0g,4.6mmol)were dissolved in THF(16.0ml).To
this mixture bis(triphenylphosphine)palladium
(II)chloride(162.5mg,0.23mmol),sodium carbonate
(1.2g,11.1mmol)and4.5ml of water were added and
the reactionmixture was stirred at40°C overnight.The
solvent was distilledunder vacuum.The resulting mixture
was diluted with ethylacetate and washed twice with
water.The organic layer was dried over anhydrous
Na2SO4,filtered and concentrated undervacuum.The
crude product was purified by column chromatographyon
silica gel using a solvent of10%ethyl acetate inhexanes
to give intermediate2as a yellow solid(1.1g,yield
83.3%).
Yu,Jiang;Zhou,Peiting;
Hu,Mingxing;Yang,
Liuqing;Yan,Guoyi;Xu,
Ruixue;Deng,Yufang;
Li,Xinghai;
Chen,Yuanwei;
European Journal of
Medicinal Chemistry;
vol.182;(2019);Art.No:
111608
31-(tetrahydro-2H-pyran-2-yl)-5-(4,4,5,5-tetramethyl-
1,3,2-dioxaborolan-2-yl)-lH-pyrazole(6.5g;23.28mmol)
and4-bromo-2-chlorobenzonitrile1(4g;18.48mmol)
were dissolved in THF(65ml).To this mixture
bis(triphenylphosphine)-palladium(II)chloride(0.65g;
0.92mmol),sodium carbonate(4.7g;44.3mmol)and18
ml of water were added and the reaction mixture was
stirred at35°C for2.5h.The solvents were distilled to
almost dryness and water(48ml)was added.After30
min of stirring the precipitated product was filtered and
32ml of ethanol was added to the precipitation.The
suspension was stirred for15min at RT and30min at
-10°C before filtering to give3.7g of the product2.
WO2011/51540;(2011);
(A1)English
2-Chloro-4-(l-(tetrahydro-2H-pyran-2-yl)-lH-pyrazol-5-y
l)benzonitrile,4-Bromo-2-chlorobenzonitrile1(30g,139
mmol),90ml of THF and360ml of toluene were placed
in reaction vessel under nitrogen athmosphere.。