益生菌肠道微生物的基因组学英文论文及翻译
- 格式:doc
- 大小:79.50 KB
- 文档页数:6

益生菌的介绍英文作文### Probiotics: Your Gut's Best Friend。
Probiotics are live microorganisms that, when consumed in adequate amounts, provide health benefits to the host. They are often referred to as "good" or "helpful" bacteria because they help keep your gut healthy.Probiotics are found in a variety of fermented foods, such as yogurt, kefir, sauerkraut, kimchi, and miso. They can also be taken as supplements in capsule or powder form.How do probiotics work?Probiotics work by colonizing the gut and competing with harmful bacteria for food and space. They also produce antimicrobial substances that can kill or inhibit the growth of harmful bacteria.In addition, probiotics help to:Improve digestion and absorption of nutrients。
Boost the immune system。
Reduce inflammation。
Lower cholesterol levels。

probiotics翻译probiotics(益生菌)是一类有益于人体健康的微生物,主要包括某些菌种,如乳酸菌和双歧杆菌等。
这些微生物在人体内起到平衡肠道菌群、增强免疫力、改善消化系统功能等作用。
Probiotics可以通过食物或补充剂的形式摄入。
以下是一些常见的probiotics使用方法和中英文对照例句:1. Yogurt is a popular food source of probiotics.酸奶是常见的益生菌食物来源。
2. Some people take probiotic supplements to support their gut health.一些人服用益生菌补充剂来维护肠道健康。
3. Probiotics can help restore the natural balance of bacteria in the gut.益生菌有助于恢复肠道内细菌的自然平衡。
4. These probiotic strains have been shown to improve digestion and reduce bloating.这些益生菌菌株已被证明可以改善消化功能并减少腹胀。
5. Probiotics are believed to strengthen the immune system and prevent certain infections.人们认为益生菌可以增强免疫系统并预防某些感染。
6. It is important to choose a probiotic product with a sufficient number of live bacteria.选择含有足够数量活菌的益生菌产品非常重要。
7. Probiotics should be stored in a cool and dry place to maintain their viability.益生菌应存放在阴凉干燥的地方以保持其活性。

肠道微生物组与健康:机制见解摘要:肠道微生物群现在被认为是有助于调节宿主健康的关键元素之一。
几乎所有的身体部位都被微生物定植,这表明与我们的器官存在不同类型的串扰。
由于分子工具和技术(即宏基因组学、代谢组学、脂质组学、宏转录组学)的发展,宿主和不同微生物之间发生的复杂相互作用正在逐步被破译。
如今,肠道微生物群偏差与许多疾病有关,包括肥胖、2 型糖尿病、肝脂肪变性、肠病(IBD)和几种类型的癌症。
因此,表明涉及免疫、能量、脂质和葡萄糖代谢的各种途径受到影响。
在这篇综述中,特别关注对该领域当前理解的批判性评估。
讨论了许多解释肠道细菌如何与保护或疾病发作有因果关系的分子机制。
我们检查了公认的代谢物(即短链脂肪酸、胆汁酸、三甲胺 N-氧化物),并将其扩展到最近确定的分子作用物(即内源性大麻素、生物活性脂质、酚衍生化合物、晚期糖基化终产物和肠联基因)及其特异性受体,如过氧化物酶体增殖物激活受体α (PPARα)和γ (PPARγ)、芳烃受体(AhR)和 G 蛋白偶联受体(即 GPR41、GPR43、GPR119、武田 G 蛋白偶联受体 5)。
总而言之,了解将肠道微生物与健康联系起来的复杂性和分子方面将有助于为已经开发的新疗法奠定基础。
人类肠道微生物组人类微生物组在这里被认为是微生物、它们的基因和产物的集合,它们从出生起就在我们体内定植并垂直转移。
虽然所有身体部位都被定植(图 1),但在肠道中发现的微生物数量最高,这已经得到了广泛的研究。
在这里,我们回顾了解决肠道微生物、其活性和介质分子如何促进我们健康的主要和最新发现。
图 1 根据不同身体部位的细菌总丰度。
不同器官中细菌数的边界,由细菌浓度和体积得出。
在健康受试者中,口腔和唾液微生物组包含数百万种微生物,这些微生物每天与我们的食物一起吞咽,但它们在肠道中的持久性受到许多因素的阻碍,包括胃的酸度、十二指肠内外胆汁酸(BA)的产生、消化酶和抗菌蛋白许多其他主要变量会影响进一步的下游微生物定植,例如 pH 值、氧浓度和氧化还原电位等化学参数、粘液、胆汁和抗体的生物产生,以及物理方面,包括肠道结构、蠕动和转运时间(图 1)。

微生物与细菌的英文作文英文回答:Microorganisms and bacteria are both essential components of the natural world. They play significant roles in various aspects of our lives, including health, environment, and industry. However, there are some differences between the two.Microorganisms, also known as microbes, are microscopic organisms that can only be seen under a microscope. They include various types such as bacteria, fungi, viruses, and protozoa. Microbes are found everywhere, from the soil to the air, and even inside our bodies. They have a wide range of functions and can be both beneficial and harmful.Bacteria, on the other hand, are a specific type of microorganism. They are single-celled organisms that lack a nucleus and other membrane-bound organelles. Bacteria can be found in various environments, including soil, water,and the human body. They are known for their ability to reproduce rapidly, which is why they can cause infections and diseases.One major difference between microorganisms and bacteria is their size. Microorganisms can be seen only under a microscope, while bacteria are large enough to be seen with the naked eye. Another difference is their classification. Microorganisms are a broader category that includes bacteria, fungi, viruses, and protozoa, while bacteria are a specific type of microorganism.In terms of their roles, both microorganisms and bacteria have significant impacts on our lives. They are involved in food production, such as the fermentation of yogurt and cheese. They also play a crucial role in the environment by decomposing organic matter and recycling nutrients. Additionally, bacteria are used in various industrial processes, such as the production of antibiotics and biofuels.In terms of their effects on human health,microorganisms and bacteria can be both beneficial and harmful. Beneficial microorganisms, like probiotics, help maintain a healthy digestive system and boost the immune system. However, harmful bacteria can cause infections and diseases, such as pneumonia and urinary tract infections.In conclusion, microorganisms and bacteria are both important components of the natural world. They have various functions and impacts on our lives. While microorganisms are a broader category that includes bacteria, fungi, viruses, and protozoa, bacteria are a specific type of microorganism. Both have significant roles in health, environment, and industry.中文回答:微生物和细菌都是自然界中重要的组成部分。

益生菌、胃肠道微生物和宿主之间相互作用的研究进展王丽凤张和平*(内蒙古农业大学乳品生物技术与工程教育部重点实验室国家奶牛产业技术研发中心乳制品加工研究室呼和浩特010018)摘要目前国内外的研究工作集中于了解肠道共生菌和益生菌以及人类宿主之间的相互作用。
利用组学技术,以便于了解益生菌和共生菌之间以及细菌环境和宿主胃肠道组织之间的相互作用。
利用测序技术对栖居在胃肠道内细菌的研究显示人体的复杂性随不同人群和个体的变化而变化。
此外,转录推动了我们对细菌(包括共生菌和益生菌)与胃肠道间复杂相互作用的洞悉。
本综述从胃肠道内微生物的作用等方面概括这一领域的最新研究进展,并在此基础上提出对未来的展望。
关键词益生菌;胃肠道;微生物;相互作用文章编号1009-7848(2011)04-0147-07益生菌是乳制品和功能性食品工业的重要组成部分,带来了数十亿美元的市场。
益生菌的多方面作用包括预防感染,降低腹泻发病率,抗微生物活性,病原菌的竞争性排斥,免疫耐受,减少大肠癌生物标志物,上皮屏障功能,增加细胞免疫力,增加体液反应,降低血胆固醇水平,减少过敏肠道疾病症状等。
目前研究的大多数益生菌来自乳杆菌属和双歧杆菌属。
乳酸杆菌与发酵产品相关,尤其在奶制品中应用最多。
最近向食品中添加双歧杆菌的研究不断增多,大多作为有益添加剂。
多数菌种天然存在于胃肠道,这些微生物通常能够抗酸、耐受胆汁。
某些菌株还具有发酵果糖诸如人类不能消化的低聚果糖(FOS )和半乳甘露寡糖(GOS )的能力,这些果糖能为胃肠道内的一些共生菌和益生菌提供一定生长优势[1]。
胃肠道是一个约有500种、100万亿微生物的复杂器官,大概是人类体内细胞总数的10多倍[2]。
对于这些细菌的遗传组成成分,可以在胃肠道中翻译编码成具有大量生理功能的基因储存器,对人体宿主的胃肠道产生有益作用。
胃肠道已经演变成为一个营养和微生物丰富的生存部位,细菌在其中不断地旺盛生长。
组学技术诸如转录组技术、宏基因组学和代谢组学的使用,促进人们了解胃肠道中益生菌如何生长以及共生菌如何发挥作用。

2.4. Chemical and microbial analyses Analysis of DM and CP concentration in the experimental diets, excreta and probiotic products was done according to AOAC (1990 methods (930.05 and 976.05, respectively. The GE was measured by using the bomb calorimeter (model 1261, Parr Instrument Co., Moline, IL, and chromium concentration was determined with an automated spectrophotometer (Jasco V-650, Jasco Corp., Tokyo, Japan according to the procedure of Fenton and Fenton (1979. The microbiological assay of faecal samples (d 14 and 28 and intestinal digesta (d 28 was conducted by culturing in different media for the determination of total anaerobic bacteria (Tryptic soy agar, Bifidobacterium spp. (MRS agar, Lactobacillus spp. (MRS agar+0.02% NaN3+0.05% L-cystine hydrochloride monohydrate, Clostridium spp. (TSC agar and coliforms (violet red bile agar. The microbiological assay of probiotic products was also carried out by culturing technique. The L. acidophilus was enumerated using MRS agar+0.02%NaN3+0.05% L-cystine hydrochloride monohydrate, B. Subtilis by using plate count agar, S. cerevisiae and A. oryzae by potato dextrose agar. The anaerobic conditions during the assay of anaerobic were created by using gas pack anaerobic system (BBL, No. 260678; Difco, Detroit, MI. The tryptic soy agar (No. 236950, MRS agar (No. 288130, violet red bile agar (No. 216695, plate count agar (No. 247940, and potato dextrose agar (No. 213400 used were purchased from Difco Laboratories (Detroit,MI, and TSC agar(CM0589 was purchased from Oxoid (Hampshire, UK. The pH of probiotic products was determined by pH meter (Basic pH Meter PB-11, Sartorius, Germany.2.5. Small intestine morphology Three cross-sections for each intestinal sample were prepared after staining with azure A and eosin using standard paraffin embedding procedures. A total of 10 intact, welloriented crypt-villus units were selected in triplicate for each intestinal cross-section as described previously (Jin et al., 2008. Villus height was measured from the tip of the villi to the villus crypt junction, and crypt depth was defined as the depth of the invagination between adjacent villi. All morphological measurements (villus height and crypt depth were made in 10-μm increments by using animage proce ssing and analysis system (Optimus software version 6.5, Media Cybergenetics, North Reading, MA.2.6. Statistical analysesAll the data obtained in the current study were analyzed in accordance with a rand omized complete block design using the GLM procedure of SAS (SAS Inst. Inc., C ary, NC. In Exp. 1, one-way analysis of variance test was used and when signific ant differences (Pb0.05 were determined among treatment means, they were separ ated by using Duncan's multiple range tests. In Exp. 2, the data were analyzed as a 2×2 factorial arrangement of treatments in randomized complete block design. T he main effects of probiotic products (LF or SF, antibiotic (colistin or lincomycin, a nd their interaction were determined by the Mixed procedures of SAS. However, as the interaction (probiotic x antibiotic was not statistically significant (Pb0.05, it wa s removed from the final model. The pen was the experimental unit for all analysis in both experiments. The bacterial concentrations were transformed (log before st atistical analysis.3.1. Experiment 13.1.1. Growth performance and apparent total tract digestibilityDietary treatments had no effect on the performance of pigs during phase I (Table 3. However, during phase II and the overall experimental period, improved (Pb0.05 ADG, ADFI and G:F were observed in pigs fed PC, LF and SF dietswhen compared with pigs fed NC diet. Moreover, pigs fed PC and SF diets had hi gher (Pb0.05 ADG and better G:F than pigs fed LF diet during phase II and the o verall experimentalperiod. The dietary treatments had no influence on the ATTDof DM and GE; however, pigs fed PC and SF diets had greater ATTD of CP whe n compared with pigs fed NC and LF diets (Table 4.3.1.2. Bacterial population in faecesDietary treatments had no effect on the faecal total anaerobes and Bifidobacterium spp. population at d 14 and 28, and Lactobacillus spp. at d 14 (Table 5. However, pigs fed PC (d 14 and 28 and SF (d 28 diets had less (Pb0.05 faecal Clostridium spp. and coliforms than pigs fed NC diet.Moreover, pigs fed SF diet had greater (Pb0.05 faecal Lactobacillus spp. populatio n (d 28 than pigs fed NC, PC and LF diets.3.2. Experiment 23.2.1. Growth performance and apparent total tract digestibilityDuring phase I, pigs fed SF diet consumed more feed than pigs fed LF diet, wher eas the ADG and ADFI were similar between pigs fed LF and SF diets (Table 6. During phase II and the overall experimental period, pigs fed SF diet showed better ADG(Pb0.01, ADFI (Pb0.01 and G:F (Pb0.05 thanpigs fed LF diet. Howev er, different antibiotics had no effect on the performance of pigs. Pigs fed SF diet had greater ATTD of DM and CP during phases I and II (Pb0.01 and 0.001, respe ctively when compared with pigs fed LF diet (Table 7.However, different antibiotics had no effect on the ATTD of DM, CP and GE.3.2.2. Bacterial population in intestinePigs fed SF diet had greater (Pb0.05 Lactobacillus spp. And less Clostridium spp. (Pb0.01 and coliform (Pb0.05 population in the ileum than pigs fed LF diet (Table 8. Additionally,higher (Pb0.05 caecal Bifidobacterium spp. Population was observed in pigs fed SF diet. Antibiotics had no effect on the ileal microbial population; however, pigs fed colistin diet had less number of Bifidobacterium spp. (Pb0.05 and coliforms (Pb0.01 inthe cecum, whereas, feeding of lincomycin diet resulted in reduced (Pb0.05 caecal Clostridium spp.population.3.2.3. Small intestinal morphologyThe different probiotic products and antibiotics had no influence on the morphology of different segments of the small intestine, except for the greater (Pb0.05 villus height:crypt depth at the jejunum and ileum noticed in pigs fed lincomycin diet (Table 9.4.DiscussionPrevious studies on probiotics lack information on the method of production used, however, the preparation of probiotics by LF method is fairly common (Patel et al., 2004. The probiotic products used in the present study differedfrom the previous reports in that harvested probiotic microbes were added directly to the diets. In this study, the microbial biomass grown on the CB was directly sprayed onthe carrier (corn and soybean meal to obtain LF probiotic product. In case of the SF probiotic product, corn and soybean meal was used as a substrate during fermentation and as a carrier of probiotic microbes. We have reported previously that multi-microbe probiotic product prepared by SF method was better than the probiotic product prepared by submerged liquid fermentation in improving performance, nutrient retention and reducing harmful intestinal bacteria in broilers (Shim et al., 2010. In the current study, LF and SF method was used and corn–soybean meal was used as a substrate forthe growth of potential probiotic microbes under optimum conditions.2.4 化学和微生物分析在试验日粮干物质和粗蛋白含量的分析中,排泄物和益生菌产品是根据AOAC(1990方法(分别为930.05和976.05 分析。


关于益生菌食品的研究与发展的论文在欧美等国家,以乳酸菌发酵的乳制品发展已有上百年的历史,其在乳制品市场占有相当大的比例。
据英国某调研公司调查,欧共体国家中对乳酸菌乳制品的消费,每年都以17%左右的比例增长。
在日本、欧洲,活性乳酸菌发酵酸奶在乳制品中的比例高达80%,在北美也有30%。
而在我国台湾地区,活性乳酸菌发酵酸奶的消费量也已超过70%。
1962年,Bogdanov从保加利亚乳杆菌中分离出了3种具有抗癌活性的糖肽,首次报道了乳酸菌的抗肿瘤作用。
1965年, Lilly D. M.和Stillwell R. H.在《科学》杂志上发表的论文“益生菌—由微生物产生的生长促进因素”中最先使用益生菌Probiotic这个定义来描述一种微生物对其他微生物促进生长的作用。
20世纪70年代初由沃斯(Woese)、奥森(Olsen)等提出16s rRNA寡核苷酸序列分析法来对菌进行鉴定。
构建了现已被确认的全生命系统进化树,越来越多的细菌依据16SrDNA被正确分类或重分类,给乳酸菌的鉴定和肠内菌群分析带来极大方便。
1971年,Sperti用益生菌(Probiotic)描述刺激微生物生长的组织提取物。
1974年,Paker将益生菌定义为对肠道微生物平衡有利的菌物。
1977年,微生态学(Microecology)由德国人Volker Rush首先提出。
他在赫尔本建立了微生态学研究所,并从事对双歧杆菌、乳杆菌、大肠杆菌等活菌作生态疗法的研究与应用。
Gilliland对肠道乳杆菌的降低胆固醇作用进行了研究,提出了乳酸菌在生长过程中通过降解胆盐促进胆固醇的分解代谢,从而降低胆固醇含量的观点。
1979年中国的微生态学研究开始。
自中国微生物学会人畜共患病病原学专业委员会下属的正常菌群学组的成立.1988年2月15日中华预防医学会微生态学分会的成立有了学术组织。
1988年《中国微生态学杂志》创刊。
80年代初大连医科大学康白教授首先研制成功促菌生(蜡杆芽胞杆菌)。

doi:10.3971/j.issn.1000-8578.2023.22.1087益生菌Akkermansia muciniphila在肿瘤发生发展及治疗中的作用赵宏慧,王玉栋Research Progress on Role of Probiotic Akkermansia Muciniphila in Oncogenesis, Development and Treatment of TumorZHAO Honghui, WANG YudongDepartment of Medical Oncology, The Fourth Hospital of Hebei Medical University, Shijiazhuang 050011, ChinaCorrespondingAuthor:WANGYudong,E-mail:*****************.cnAbstract: Although tumor treatment models have been continuously improved in clinical practice, cancer remains a serious threat to human health. The effect of probiotics on tumor therapy has received extensive attention. As a common colonizer of the intestinal mucosa, Akkermansia muciniphila(AKK) has a well-defined role in metabolic diseases, but its complex role in tumor development and therapeutic efficacy has not been fully elucidated. The unique properties and physiological roles of AKK play an important role in different solid tumors and it may be a potential biomarker. This article provides a review of previous studies and proposes clinical strategies to influence the abundance of AKK to provide a theoretical reference for the development of next-generation probiotics and the reshaping of the tumor treatment landscape.Key words: Akkermansia muciniphila; Probiotics; Cancer; Tumor therapyFunding: Natural Science Foundation of Hebei Province (No. H2020206551); Beijing Xisike Clinical Oncology Research Foundation (No. Y-MSDPU2021-0202)Competing interests: The authors declare that they have no competing interests.摘 要:尽管肿瘤治疗模式在临床实践中已不断完善,但肿瘤仍严重威胁着人类健康。
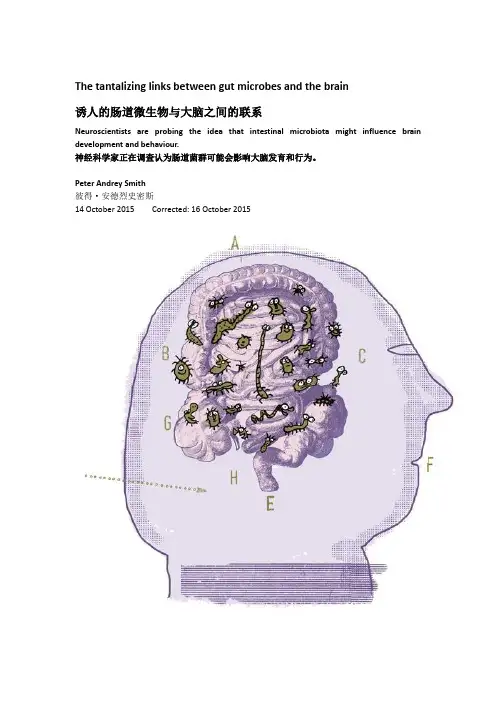
The tantalizing links between gut microbes and the brain诱人的肠道微生物与大脑之间的联系Neuroscientists are probing the idea that intestinal microbiota might influence brain development and behaviour.神经科学家正在调查认为肠道菌群可能会影响大脑发育和行为。
Peter Andrey Smith彼得·安德烈史密斯14 October 2015 Corrected: 16 October 2015Illustration by Serge BlochNearly a year has passed since Rebecca Knickmeyer first met the participants in her latest study on brain development. Knickmeyer, a neuroscientist at the University of North Carolina School of Medicine in Chapel Hill, expects to see how 30 newborns have grown into crawling, inquisitive one-year-olds, using a battery of behavioural and temperament tests. In one test, a child's mother might disappear from the testing suite and then reappear with a stranger. Another ratchets up the weirdness with some Halloween masks. Then, if all goes well, the kids should nap peacefully as a noisy magnetic resonance imaging machine scans their brains.“We try to be prepared for everything,” Knickmeyer says. “We know exactly what to do if kids make a break for the door.”Knickmeyer is excited to see something else from the children — their faecal microbiota, the array of bacteria, viruses and other microbes that inhabit their guts. Her project (affectionately known as 'the poop study') is part of a small but growing effort by neuroscientists to see whether the microbes that colonize the gut in infancy can alter brain development.The project comes at a crucial juncture. A growing body of data, mostly from animals raised in sterile, germ-free conditions, shows that microbes in the gut influence behaviour and can alter brain physiology and neurochemistry.In humans, the data are more limited. Researchers have drawn links between gastrointestinal pathology and psychiatric neurological conditions such as anxiety, depression, autism, schizophrenia and neurodegenerative disorders — but they are just links.“In general, the problem of causality in microbiome studies is substantial,” says Rob Knight, a microbiologist at the University of California, San Diego. “It's very difficult to tell if microbial differences you see associated with diseases are causes or consequences.” There are many outstanding questions. Clues about the mechanisms by which gut bacteria might interact with the brain are starting to emerge, but no one knows how important these processes are in human development and health.That has not prevented some companies in the supplements industry from claiming that probiotics — bacteria that purportedly aid with digestive issues — can support emotional well-being.Pharmaceutical firms, hungry for new leads in treating neurological disorders, are beginning to invest in research related to gut microbes and the molecules that they produce.Scientists and funders are looking for clarity. Over the past two years, the US National Institute of Mental Health (NIMH) in Bethesda, Maryland, has funded seven pilot studies with up to US$1 million each to examine what it calls the 'microbiome–gut–brain axis' (Knickmeyer's research is one of these studies). This year, the US Office of Naval Research in Arlington, Virginia, agreed topump around US$14.5 million over the next 6–7 years into work examining the gut's role in cognitive function and stress responses. And the European Union has put €9 million (US$10.1 million) towards a five-year project called MyNewGut, two main objectives of which target brain development and disorders.Nature special:Human microbiotaThe latest efforts aim to move beyond basic observations and correlations — but preliminary results hint at complex answers. Researchers are starting to uncover a vast, varied system in which gut microbes influence the brain through hormones, immune molecules and the specialized metabolites that they produce.“There's probably more speculation than hard data now,” Knickmeyer says. “So there's a lot of open questions about the gold standard for methods you should be applying. It's very exploratory.”Gut reactionsMicrobes and the brain have rarely been thought to interact except in instances when pathogens penetrate the blood–brain barrier — the cellular fortress protecting the brain against infection and inflammation. When they do, they can have strong effects: the virus that causes rabies elicits aggression, agitation and even a fear of water. But for decades, the vast majority of the body's natural array of microbes was largely uncharacterized, and the idea that it could influence neurobiology was hardly considered mainstream. That is slowly changing.Studies on community outbreaks were one key to illuminating the possible connections. In 2000, a flood in the Canadian town of Walkerton contaminated the town's drinking water with pathogens such as Escherichia coli and Campylobacter jejuni. About 2,300 people suffered from severe gastrointestinal infection, and many of them developed chronic irritable bowel syndrome (IBS) as a direct result.During an eight-year study1 of Walkerton residents, led by gastroenterologist Stephen Collins at McMaster University in Hamilton, Canada, researchers noticed that psychological issues such as depression and anxiety seemed to be a risk factor for persistent IBS. Premysl Bercik, another McMaster gastroenterologist, says that this interplay triggered intriguing questions. Couldpsychiatric symptoms be driven by lingering inflammation, or perhaps by a microbiome thrown out of whack by infection?The McMaster group began to look for answers in mice. In a 2011 study2, the team transplanted gut microbiota between different strains of mice and showed that behavioural traits specific to one strain transmitted along with the microbiota. Bercik says, for example, that “relatively shy” mice would exhibit more exploratory behaviour when carrying the microbiota of more-adventurous mice. “I think it is surprising. The microbiota is really driving the behavioural phenotype of host. There's a marked difference,” Bercik says. Unpublished research suggests that taking faecal bacteria from humans with both IBS and anxiety and transplanting it into mice induces anxiety-like behaviour, whereas transplanting bacteria from healthy control humans does not.Such results can be met with scepticism. As the field has developed, Knight says, microbiologists have had to learn from behavioural scientists that how animals are handled and caged can affect things such as social hierarchy, stress and even the microbiome.And these experiments and others like them start with a fairly unnatural model: germ-free — or 'gnotobiotic' — mice. These animals are delivered by Caesarean section to prevent them from picking up microbes that reside in their mothers' birth canals. They are then raised inside sterile isolators, on autoclaved food and filtered air. The animals are thus detached from many of the communal microbes that their species has evolved with for aeons.In 2011, immunologist Sven Pettersson and neuroscientist Rochellys Diaz Heijtz, both at the Karolinska Institute in Stockholm, showed that in lab tests, germ-free mice demonstratedless-anxious behaviour than mice colonized with natural indigenous microbes3. (Less anxiety is not always a good thing, evolutionarily speaking, for a small mammal with many predators.) When the Karolinska team examined the animals' brains, they found that one region in germ-free mice, the striatum, had higher turnover of key neurochemicals that are associated with anxious behaviour, including the neurotransmitter serotonin. The study also showed that introducing adult germ-free mice to conventional, non-sterile environments failed to normalize their behaviour, but the offspring of such 'conventionalized' mice showed some return to normal behaviour, suggesting that there is a critical window during which microbes have their strongest effects.By this time, many researchers were intrigued by the mounting evidence, but results stemmed mostly from fields other than neuroscience. “The groups working on this are primarily gut folks, with a few psychology-focused people collaborating,” says Melanie Gareau, a physiologist at the University of California, Davis. “So the findings tended to describe peripheral and behavioural changes rather than changes to the central nervous system.”Innovations in the microbiomeBut Pettersson and Diaz Heijtz's research galvanized the field, suggesting that researchers could get past observational phenomenology and into the mechanisms affecting the brain. Nancy Desmond, a programme officer involved in grant review at the NIMH, says that the paper sparked interest at the funding agency soon after its publication and, in 2013, the NIMH formed a study section devoted to neuroscience research that aims to unravel functional mechanisms and develop drugs or non-invasive treatments for psychological disorders.Judith Eisen, a neuroscientist at the University of Oregon in Eugene, earned a grant to study germ-free zebrafish, whose transparent embryos allow researchers to easily visualize developing brains. “Of course, 'germ-free' is a completely unnatural situation,” Eisen says. “But it provides the opportunity to learn which microbial functions are important for development of any specific organ or cell type.”Chemical explorationMeanwhile, researchers were starting to uncover ways that bacteria in the gut might be able to get signals through to the brain. Pettersson and others revealed that in adult mice, microbial metabolites influence the basic physiology of the blood–brain barrier4. Gut microbes break down complex carbohydrates into short-chain fatty acids with an array of effects: the fatty acid butyrate, for example, fortifies the blood–brain barrier by tightening connections between cells (see 'The gut–brain axis').Recent studies also demonstrate that gut microbes directly alter neurotransmitter levels, which may enable them to communicate with neurons. For example, Elaine Hsiao, a biologist now at the University of California, Los Angeles, published research5 this year examining how certain metabolites from gut microbes promote serotonin production in the cells lining the colon — an intriguing finding given that some antidepressant drugs work by promoting serotonin at the junctions between neurons. These cells account for 60% of peripheral serotonin in mice and more than 90% in humans.Read next: Microbiomes raise privacy concernsLike the Karolinska group, Hsiao found that germ-free mice have significantly less serotonin floating around in their blood, and she also showed that levels could be restored by introducing to their guts spore-forming bacteria (dominated by Clostridium, which break down short-chain fatty acids). Conversely, mice with natural microbiota, when given antibiotics, had reduced serotonin production. “At least with those manipulations, it's quite clear there's a cause–effect relationship,” Hsiao says.But it remains unclear whether these altered serotonin levels in the gut trigger a cascade of molecular events, which in turn affect brain activity — and whether similar events take place in humans, too. “It will be important to replicate previous findings, and translate these findings into human conditions to really make it to the textbooks,” Hsiao says.For John Cryan, a neuroscientist at University College Cork in Ireland, there is little question that they will. His lab has demonstrated6 that germ-free mice grow more neurons in a specific brain region as adults than do conventional mice. He has been promoting the gut–brain axis to neuroscientists, psychiatric-drug researchers and the public. “If you look at the hard neuroscience that has emerged in the last year alone, all the fundamental processes that neuroscientists spend their lives working on are now all shown to be regulated by microbes,” he says, pointing to research on the regulation of the blood–brain barrier, neurogenesis in mice and the activation of microglia, the immune-like cells that reside in the brain and spinal cord.At the 2015 Society for Neuroscience meeting in Chicago, Illinois, this month, Cryan and his colleagues plan to present research showing that myelination — the formation of fatty sheathing that insulates nerve fibres — can also be influenced by gut microbes, at least in a specific part of the brain. Unrelated work7 has shown that germ-free mice are protected from an experimentally induced condition similar to multiple sclerosis, which is characterized by demyelination of nerve fibres. At least one company, Symbiotix Biotherapies in Boston, Massachusetts, is alreadyinvestigating whether a metabolite produced by certain types of gut bacterium might one day be used to stem the damage in humans with multiple sclerosis.A move to therapyTracy Bale, a neuroscientist at the University of Pennsylvania in Philadelphia, suspects that simple human interventions may already be warranted. Bale heard about Cryan's work on the radio programme Radiolab three years ago. At the time, she was researching the placenta, but wondered how microbes might fit into a model of how maternal stress affects offspring.In research published this year8, Bale subjected pregnant mice to stressful stimuli. She found that it noticeably reduced the levels of Lactobacilli present in the mice's vaginas, which are the main source of the microbes that colonize the guts of offspring. These microbial shifts carried over to pups born vaginally, and Bale detected signs that microbiota might affect neurodevelopment, especially in males.Read next:Gut–brain link grabs neuroscientistsIn work that her group plans to present at the Society for Neuroscience meeting, Bale has shown that by feeding vaginal microbiota from stressed mice to Caesarean-born infant mice, they can recapitulate the neurodevelopmental effects of having a stressed mother. Bale and her colleagues are now wrapping up research investigating whether they can treat mice from stressed mums with the vaginal microbiota of non-stressed mice.The work, Bale says, has “immediate translational effects”. She points to a project headed by Maria Dominguez-Bello, a microbiologist at the New York University School of Medicine, in which babies born by means of Caesarean section are swabbed on the mouth and skin with gauze taken from their mothers' vaginas. Her team wants to see whether these offspring end up with microbiota similar to babies born vaginally. “It's not standard of care,” Bale says, “but I will bet you, one day, it will be.”Many are still sceptical about the link between microbes and behaviour and whether it will prove important in human health — but scientists seem more inclined to entertain the idea now than they have in past. In 2007, for example, Francis Collins, now director of the US National Institutes of Health, suggested that the Human Microbiome Project, a large-scale study of the microbes that colonize humans, might help to unravel mental-health disorders. “It did surprise a few people who assumed we were talking about things that are more intestinal than cerebral,” Collins says. “It was a little bit of leap, but it's been tentatively backed up.”Funding agencies are supporting the emerging field, which spans immunology, microbiology and neuroscience, among other disciplines. The NIMH has offered seed funding for work on model systems and in humans to probe whether the area is worth more-substantial investment, a move that has already brought more researchers into the fold. The MyNewGut project in Europe has an even more optimistic view of the value of such research, specifically seeking concrete dietary recommendations that might alleviate brain-related disorders.Today, Knickmeyer's project on infants represents what she calls “a messy take-all-comers kind of sample”. Among the brain regions that Knickmeyer is scanning, the amygdala and prefrontal cortex hold her highest interest; both have been affected by microbiota manipulations in rodent models. But putting these data together with the dozens of other infant measures that she is taking will be a challenge. “The big question is how you deal with all the confounding factors.” The children's diets, home lives and other environmental exposures can all affect their microbiota and their neurological development, and must be teased apart.Knickmeyer speculates that tinkering with microbes in the human gut to treat mental-health disorders could fail for other reasons. Take, for instance, how microbes might interact with the human genome. Even if scientists were to find the therapeutic version of a “gold Cadillac of microbiota”, she points out, “maybe your body rejects that and goes back to baseline because your own genes promote certain types of bacteria.” There is much more to unravel, she says. “I'm always surprised. It's very open. It's a little like a Wild West out there.”Nature 526,312–314 (15 October 2015) doi:10.1038/526312aTweet Follow @NatureNewsCorrectionsCorrected:An earlier version of this story incorrectly stated that the US Office ofNaval Research agreed to commit US$52 million into gut–brain research. Infact, the figure is closer to $14.5 million over the next 6–7 years. Thetext has now been corrected.References:1.Marshall, J. K. et al. Gut 59, 605–611 (2010).Show context Article PubMed2.Bercik, P. et al. Gastroenterology 141, 599–609 (2011).Show context Article PubMed ChemPort3.Diaz Heijtz, R. et al. Proc. Natl Acad. Sci. USA 108, 3047–3052 (2011).Show context Article PubMed4.Braniste, V. et al. Sci. Transl. Med. 6, 263ra158 (2014).Show context Article PubMed ChemPort5.Yano, J. M. et al. Cell 161, 264–276 (2015).Show context Article PubMed ChemPort6.Ogbonnaya, E. S. et al. Biol. Psychiatry 78, e7–e9 (2015).Show context Article PubMed7.Lee, Y.-K., Menezes, J. S., Umesaki, Y. & Mazmanian, S. K. Proc. Natl Acad. Sci. USA 108, 4615–4622 (2010).Show context Article PubMed8.Jašarević, E., Howerton, C. L., Howard, C. D. & Bale, T. L. Endocrinology 156, 3265–3276 (2015). Show context Article PubMed ChemPortNature:肠道微生物与大脑之间的诱人关系很多发现常常让我感到吃惊。

f-erysipelotrichaceae作用-概述说明以及解释1.引言1.1 概述F-Erysipelotrichaceae是一种常见的肠道菌群,属于革兰氏阳性菌。
近年来,随着研究的深入,人们对F-Erysipelotrichaceae在人体中的作用逐渐引起了关注。
它们不仅参与调节肠道微生态平衡,还可能影响宿主的健康状态。
本文将着重探讨F-Erysipelotrichaceae的定义、特征,以及其在人体中的作用,旨在加深对该菌群的了解,并为进一步研究其与健康之间的关联提供一定的参考。
1.2 文章结构文章结构部分主要介绍了本文的整体框架和各个部分的内容安排。
本文分为引言、正文和结论三个部分。
在引言部分,我们首先概述了文章要探讨的主题,介绍了F-Erysipelotrichaceae这一微生物群的概念以及其在人体内的作用。
随后,对整篇文章的结构进行了简要介绍,明确了各个章节的主要内容和逻辑顺序。
在正文部分,我们将详细解释F-Erysipelotrichaceae的定义与特征,包括其分类、生理特性等方面的内容。
接着,我们将着重探讨F-Erysipelotrichaceae在人体中的作用,包括其与免疫系统、代谢功能等方面的关系。
最后,我们会分析F-Erysipelotrichaceae与健康的关联,探讨其在疾病发生和预防中的重要作用。
在结论部分,我们将对F-Erysipelotrichaceae的重要性进行总结,并探讨未来研究方向。
最后,我们将用简短的结语结束全文,强调F-Erysipelotrichaceae在人体健康中的重要性,并鼓励进一步深入研究。
1.3 目的2.3 目的本文旨在探讨F-Erysipelotrichaceae在人体中的作用,并深入分析其与健康的关联。
通过对该菌群的定义与特征进行介绍,我们希望能够更全面地了解其在人体内的功能和意义。
同时,通过对已有研究成果的总结和分析,我们试图揭示F-Erysipelotrichaceae在调节人体健康方面的重要性,为未来的相关研究提供参考和启示。
The genomics of probiotic intestinal microorganismsSeppo Salminen1 , Jussi Nurmi2 and Miguel Gueimonde1(1) Functional Foods Forum, University of Turku, FIN-20014 Turku, Finland(2) Department of Biotechnology, University of Turku, FIN-20014 Turku, FinlandSeppo SalminenEmail: *********************Published online: 29 June 2005AbstractAn intestinal population of beneficial commensal microorganisms helps maintain human health, and some of these bacteria have been found to significantly reduce the risk of gut-associated disease and to alleviate disease symptoms. The genomic characterization of probiotic bacteria and other commensal intestinal bacteria that is now under way will help to deepen our understanding of their beneficial effects.While the sequencing of the human genome [1, 2] has increased ourunderstanding of the role of genetic factors in health and disease, each human being harbors many more genes than those in their own genome. These belong to our commensal and symbiotic intestinal microorganisms - our intestinal 'microbiome' - which play an important role in maintaining human health and well-being. A more appropriate image of ourselves would be drawn if the genomes of our intestinal microbiota were taken into account. The microbiome may contain more than 100 times the number of genes in the human genome [3] and provides many functions that humans have thus not needed to develop themselves. The indigenous intestinal microbiota provides a barrier against pathogenic bacteria and other harmful food components [4–6]. It has also been shown to have a direct impact on the morphology of the gut [7], and many intestinal diseases can be linked to disturbances in the intestinal microbial population [8].The indigenous microbiota of an infant's gastrointestinal tract is originally created through contact with the diverse microbiota of the parents and the immediate environment. During breast feeding, initial microbial colonization is enhanced by galacto-oligosaccharides in breast milk and contact with the skin microbiota of the mother. This early colonization process directs the microbial succession until weaning and forms the basis for a healthy microbiota. The viable microbes in the adultintestine outnumber the cells in the human body tenfold, and the composition of this microbial population throughout life is unique to each human being. During adulthood and aging the composition and diversity of the microbiota can vary as a result of disease and the genetic background of the individual.Current research into the intestinal microbiome is focused on obtaining genomic data from important intestinal commensals and from probiotics, microorganisms that appear to actively promote health. This genomic information indicates that gut commensals not only derive food and other growth factors from the intestinal contents but also influence their human hosts by providing maturational signals for the developing infant and child, as well as providing signals that can lead to an alteration in the barrier mechanisms of the gut. It has been reported that colonization by particular bacteria has a major role in rapidly providing humans with energy from their food [9]. For example, the intestinal commensal Bacteroides thetaiotaomicron has been shown to have a major role in this process, and whole-genome transcriptional profiling of the bacterium has shown that specific diets can be associated with selective upregulation of bacterial genes that facilitate delivery of products of carbohydrate breakdown to the host's energy metabolism [10, 11]. Key microbial groups in the intestinal microbiota are highly flexible in adapting to changes in diet, and thus detailed prediction of their actions and effects may be difficult. Although genomic studies have revealed important details about the impact of the intestinal microbiota on specific processes [3, 11–14], the effects of species composition and microbial diversity and their potential compensatory functions are still not understood.Probiotics and healthA probiotic has been defined by a working group of the International Life Sciences Institute Europe (ILSI Europe) as "a viable microbial food supplement which beneficially influences the health of the host" [15]. Probiotics are usually members of the healthy gut microbiota and their addition can assist in returning a disturbed microbiota to its normal beneficial composition. The ILSI definition implies that safety and efficacy must be scientifically demonstrated for each new probiotic strain and product. Criteria for selecting probiotics that are specific for a desired target have been developed, but general criteria that must be satisfied include the ability to adhere to intestinal mucosa and tolerance of acid and bile. Such criteria have proved useful but cumbersome in current selection processes, as there are several adherence mechanisms and they influence gene upregulation differently in the host. Therefore, two different adhesion studies need to be conducted on each strain and theirpredictive value for specific functions is not always good or optimal. Demonstration of the effects of probiotics on health includes research on mechanisms and clinical intervention studies with human subjects belonging to target groups.The revelation of the human genome sequence has increased our understanding of the genetic deviations that lead to or predispose to gastrointestinal disease as well as to diseases associated with the gut, such as food allergies. In 1995, the first genome of a free-living organism, the bacterium Haemophilus influenzae, was sequenced [16]. Since then, over 200 bacterial genome sequences, mainly of pathogenic microorganisms, have been completed. The first genome of a mammalian lactic-acid bacterium, that of Lactococcus lactis, a microorganism of great industrial interest, was completed in 2001 [17]. More recently, the genomes of numerous other lactic-acid bacteria [18], bifidobacteria [12] and other intestinal microorganisms [13, 19, 20] have been sequenced, and others are under way [21]. Table 1lists the probiotic bacteria that have been sequenced. These great breakthroughs have demonstrated that evolution has adapted both microbes and humans to their current state of cohabitation, or even symbiosis, which is beneficial to both parties and facilitates a healthy and relatively stable but adaptable gut environment.Table 1Lessons from genomesLactic-acid bacteria and bifidobacteria can act as biomarkers of gut health by giving early warning of aberrations that represent a risk of specific gut diseases. Only a few members of the genera Lactobacillus and Bifidobacterium, two genera that provide many probiotics, have been completely sequenced. The key issue for the microbiota, for probiotics, and for their human hosts is the flexibility of the microorganisms in coping with a changeable local environment and microenvironments.This flexibility is emphasized in the completed genomes of intestinal and probiotic microorganisms. The complete genome sequence of the probiotic Lactobacillus acidophilus NCFM has recently been published by Altermann et al. [22]. The genome is relatively small and the bacterium appears to be unable to synthesize several amino acids, vitamins and cofactors. Italso encodes a number of permeases, glycolases and peptidases for rapid uptake and utilization of sugars and amino acids from the human intestine, especially the upper gastrointestinal tract. The authors also report a number of cell-surface proteins, such as mucus- and fibronectin-binding proteins, that enable this strain to adhere to the intestinal epithelium and to exchange signals with the intestinal immune system. Flexibility is guaranteed by a number of regulatory systems, including several transcriptional regulators, six PurR-type repressors and ninetwo-component systems, and by a variety of sugar transporters. The genome of another probiotic, Lactobacillus johnsonii [23], also lacks some genes involved in the synthesis of amino acids, purine nucleotides and numerous cofactors, but contains numerous peptidases, amino-acid permeases and other transporters, indicating a strong dependence on the host.The presence of bile-salt hydrolases and transporters in these bacteria indicates an adaptation to the upper gastrointestinal tract [23], enabling the bacteria to survive the acidic and bile-rich environments of the stomach and small intestine. In this regard, bile-salt hydrolases have been found in most of the sequenced genomes of bifidobacteria and lactic-acid bacteria [24], and these enzymes can have a significant impact on bacterial survival. Another lactic-acid bacterium, Lactobacillus plantarum WCFS1, also contains a large number of genes related to carbohydrate transport and utilization, and has genes for the production of exopolysaccharides and antimicrobial agents [18], indicating a good adaptation to a variety of environments, including the human small intestine [14]. In general, flexibility and adaptability are reflected by a large number of regulatory and transport functions.Microorganisms that inhabit the human colon, such as B. thetaiotaomicron and Bifidobacterium longum [12], have a great number of genes devoted to oligosaccharide transport and metabolism, indicating adaptation to life in the large intestine and differentiating them from, for example, L. johnsonii [23]. Genomic research has also provided initial information on the relationship between components of the diet and intestinal microorganisms. The genome of B. longum [12] suggests the ability to scan for nutrient availability in the lower gastrointestinal tract in human infants. This strain is adapted to utilizing the oligosaccharides in human milk along with intestinal mucins that are available in the colon of breast-fed infants. On the other hand, the genome of L. acidophilus has a gene cluster related to the metabolism of fructo-oligosaccharides, carbohydrates that are commonly used as prebiotics, or substrates to肠道微生物益生菌的基因组学塞波萨米宁,尤西鲁米和米格尔哥尔摩得(1)功能性食品论坛,图尔库大学,FIN-20014芬兰图尔库(2)土尔库大学生物技术系,FIN-20014芬兰图尔库塞波萨米宁电子邮件:seppo.salminen utu.fi线上发表于2005年6月29日摘要肠道有益的共生微生物有助于维护人体健康,一些这些细菌被发现显着降低肠道疾病的风险和减轻疾病的症状。
Animal(2012),6:10,pp1620–1626&The Animal Consortium2012doi:10.1017/S1751731112000481The effect of chitooligosaccharide supplementation on intestinal morphology,selected microbial populations,volatile fatty acid concentrations and immune gene expression in the weaned pig A.M.Walsh,T.Sweeney,B.Bahar,B.Flynn and J.V.O’Doherty-School of Agriculture,Food Science and Veterinary Medicine,University College Dublin,Lyons Research Farm,Newcastle,Co.Dublin,Ireland(Received24March2011;Accepted29January2012;First published online2March2012)An experiment(complete randomised design)was conducted to investigate the effects of supplementing different molecular weights (MW)of chitooligosaccharide(COS)on intestinal morphology,selected microbial populations,volatile fatty acid(VFA)concentrations and the immune status of the weaned pig.A total of28piglets(24days of age,9.1kg(6s.d.0.80)live weight)were assignedto one of four dietary treatments for8days and then sacrificed.The treatments were(1)control diet(0ppm COS),(2)control diet plus5to10kDa COS,(3)control diet plus10to50kDa COS and(4)control diet plus50to100kDa COS.The COS was included in dietary treatments at a rate of250mg/kg.Tissue samples were taken from the duodenum,jejunum and ileum for morphological measurements.Digesta samples were taken from the proximal colon to measure lactobacilli and Escherichia coli populations and digesta samples were taken from the caecum and proximal colon for VFA analysis.Gene expression levels for specific cytokines were investigated in colonic tissue of the pig.Supplementation of different MW of COS had no significant effect on pig performance during the post-weaning period(days0to8;P.0.05).The inclusion of COS at all MW in the diet significantly reduced faecal scores compared with the control treatment(P,0.01).Pigs fed the10to50kDa COS had a higher villous height(P,0.05)and villous height:crypt depth ratio(P,0.05)in the duodenum and the jejunum compared with the control treatment.Pigs fed the5to10kDa COS had a lower lactobacilli population(P,0.05)and E.coli population(P,0.05)in the colon compared with the control group.Pigs offered the5to10kDa COS had significantly lower levels of acetic acid and valeric acid compared with the control group(P,0.05). The inclusion of different MW of COS had no significant effect on the expression of the cytokines tumour necrosis factor-a,Interleukin (IL)-6,IL-8and IL-10in the gastro-intestinal tract of the weaned pig.The current results indicate that a lower MW of5to10kDa COS possessed an antibacterial activity,while the higher MW of10to50kDa was optimum for enhancing the intestinal structure. Keywords:chitooligosaccharide,pig,microbiology,intestinal morphologyImplicationOur results indicate that the inclusion of chitooligosaccharides (COSs)in piglet diets may moderate several gut health para-meters that contribute to some of the common problems that occur after weaning in the absence of in-feed antibiotics.It was observed that COSs with a molecular weight(MW)of5to 10kDa were more effective in reducing Escherichia coli populations while a MW of10to50kDa enhanced the intestinal structure.IntroductionThe weaning period imposes profound social and environ-mental stresses on the piglet such as removal from the sow,change in diet and mixing of piglets from different litters. Numerous studies have reported that there is a reduction in villous height(villous atrophy)and an increase in crypt depth (crypt hyperplasia)after weaning,which leads to increased susceptibility to intestinal gut dysfunction(Spreeuwenberg et al.,2001;Pierce et al.,2006).The post-weaning period is characterised by a reduction in feed intake,poor growth rates,diarrhoea and an increased risk of disease(Lalles et al., 2007).These negative effects on piglet growth during the weaning period were managed by growth-promoting anti-biotics.However,the European Union placed a total ban on the use of in-feed antibiotic growth promoters on the1st January2006due to public concerns regarding bacterial resistant and human health issues. Chitooligosaccharides(COS)may be a potential viable alternative to traditional antimicrobials in animal production.-E-mail:john.vodoherty@ucd.ie 1620Chitosan is a natural biopolymer derived by alkaline deacety-lation of chitin,which is the principal component of protective cuticles of crustaceans such as crabs,shrimps,prawns,lobsters and cell walls of some fungi such as aspergillus(Qin et al., 2006).Both chitin and chitosan are biopolymers composed of glucosamine and N-acetylated glucosamine(2-acetylamino-2-deoxy-D-glucopyranose)units linked by b(1to4)glycosidic bonds(Koide,1998).Low molecular weight(MW)COS is a water-soluble derivative of chitosan due to shorter chain lengths(Kim and Rajapakse,2005).Recently,both chitosan and its derivatives have generated considerable interest due to their biological activities,including antimicrobial,antitumour, immunoenhancing effects and the acceleration of wound healing(No et al.,2002;Liu et al.,2006)There is considerable variation in the literature on the biological properties of COS (Jeon et al.,2001;Liu et al.,2006).Most of this variation is partly due to the widely different MW used across studies.It is hypothesised that the biological properties of COS may be influenced by its MW and COS will enhance selected indices of health in weaned piglets.Material and methodsAll procedures described in this experiment were conducted under an experimental licence from the Irish Department of Health in accordance with the cruelty to Animals Act1876 and the European Communities(Amendments of the Cruelty to Animals Act1976)Regulations.Experimental dietsThe experiment was designed as a complete randomised block design and comprised four dietary treatments.Thedietary treatments were as follows:(1)control diet(0ppm COS),(2)control diet plus5to10kDa COS,(3)control diet plus10to50kDa COS and(4)control diet plus50to100kDa COS.The COS was sourced from Kitto Life Co.Ltd(Kyungki-do,Seoul,Korea)and was supplemented in the experimental diets at a concentration of250ppm.The diets were fed for 8days ad libitium,after which time the pigs were humanely sacrificed.The diets were formulated to have similar diges-tible energy(16MJ/kg)and standardised ileal digestible (SID)lysine(14g/kg)contents.All amino acids requirements were met relative to SID lysine(National Research Council, 1998).The ingredient composition and chemical analysis of the dietary treatments are presented in Table1.Animals and managementA total of28piglets(progeny of large white3(large white3landrace sows))were selected from a commercial pig unit at24days of age.The piglets had a weaning weight of9.1kg(s.d.50.80)and were blocked on the basis of litter of origin and live weight(n57).The piglets were individu-ally housed in fully slated pens(1.7m31.2m).They were individually fed and had ad libitum access to feed and water. The house temperature was thermostatically controlled at 308C throughout the experiment.This study was not a growth performance study but some performance data were recorded.The piglets were weighed at the beginning of the experiment(day0)and at the end of the experiment(day8). Food was available up to thefinal weighing and all remaining food was weighed back for the purpose of cal-culating feed efficiency.Pigs were observed for clinical signs of diarrhoea and a scoring system was applied to indicate the presence and severity of this as described by Pierce et al. (2006).Faeces scores were assigned daily for individual pigs from day0and continued until day8.The following faeces scoring system was used:15hard faeces,25slightly soft faeces in the pen,35soft,partially formed faeces,45loose, semi-liquid faeces and55watery,mucous-like faeces.Gut morphological analysisThe piglets were humanely sacrificed on day8by a lethal injection of Euthatal(pentobarbitone sodium BP–Merial Animal Ltd,Sandringham House,Essex,UK)at a rate of1ml/ 1.4kg BW.On removal of the digestive tract,sections of the duodenum(10cm from the stomach),the jejunum(60cm from stomach)and the ileum(15cm from caecum)were excised andfixed in10%phosphate-buffered formalin.The preserved segments were prepared using standard paraffin-embedding techniques.The samples were sectioned at5m m Table1Composition and chemical analysis of experimental diets (as-fed basis)Items Starter diet* Ingredient(g/kg)Whey permeate125.0 Wheat444.2 Soya bean meal142.5 Whey protein isolate130.0 Full-fat soybean80.0 Soya oil65.0 Vitamins and minerals 5.0 Lysine HCL 4.5 DL-methionine 1.6L-threonine 2.2 Analysis(g/kg,unless otherwise stated)DM892.5 CP(N36.25)224.2 GE(MJ/kg)18.2 Ash43.7 NDF110.3 Lysine-16.5 Methionine and cysteine-9.9 Threonine-10.7 Tryptophan- 2.5 Calcium-8.0 Phosphorous- 6.0 DM5dry matter;GE5gross energy.Starter diet provided(mg/kg completed diet):Cu,175;Fe,140;Mn,47;Zn, 120;I,0.6;Se,0.3;retinol,1.8;cholecalciferol,0.025;alpha-tocopherol,67; phytylmenaquinone,4;cyanocobalamin,0.01;riboflavin,2;nicotinic acid,12; pantothenic acid,10;choline chloride,250;thiamine,2;pyridoxine,0.015.*COS was included in dietary treatments T2–T4at a rate of250mg/kg.-Calculated for tabulated nutritional composition(Sauvant et al.,2004).Chitooligosaccharide in piglet diets1621thickness and stained with haemotoxylin and eosin(Pierce et al.,2006).Villous height and crypt depth were measured on the stained sections(43objective)using a light micro-scopefitted with an image analyser(Image Pro Plus,Media Cybernetics,Buckinghamshire,UK).Measurements of15well oriented and intact villi and crypts were taken for each seg-ment.Villous height was measured from the crypt–villous junction to the tip.Crypt depth was measured from the crypt–villous junction to the base.Results were expressed as the mean villous height or crypt depth in micrometres. Intestinal microfloraFor microbial analysis,digesta samples(,1061g)were aseptically recovered from the proximal colon of each pig immediately post slaughter.Digesta samples were stored in sterile containers(Sarstedt,Wexford,Ireland),placed on ice and transported to the laboratory within2h.A1.0g sample was removed from the digesta sample,serially diluted (1:10)in9.0ml aliquots of maximum recovery diluents (Oxoid,Basingstoke,UK)and spread plated(0.1ml aliquots) onto selective agars,as follows:Lactobacillus spp.were isolated on de Man,Rogosa and Sharp(MRS)agar(Oxoid) with an overnight(18to24h)incubation at378C in an atmosphere enriched with5%CO2,as recommended by the manufacturers(Oxoid).The Escherichia coli species were isolated on MacConkey agar(Oxoid)following aerobic incubation at378C for18to24h(O’Doherty et al.,2010). Target colonies of Lactobacilli and E.coli were identified by Gram stains and colony morphology(Salanitro et al.,1977). The API50CHL(BioMerieux,Biomerieux,Craponne,France) kit was used to confirm suspect Lactobacilli spp.Suspect E. coli colonies were confirmed with API20E(BioMerieux, France).This API system identifies the suspect colonies by measuring their ability to produce cytochrome oxidase. Typical colonies of each bacteria on each agar were counted, log transformed and the numbers of bacteria were expressed per gram of digesta after being serially diluted.Volatile fatty acid(VFA)analysisSamples of digesta from individual pigs were taken from the caecum and the proximal colon to measure the VFA concentration and molar proportions of VFAs.The VFA con-centrations in the digesta were determined using gas liquid chromatography according to the method described by Pierce et al.(2007).A1-g sample was diluted with distilled water (2.53weight of sample)and centrifuged at14003g for4min(Sorvall GLC–2B laboratory centrifuge,Dupont, Wilmington,DE,USA).Then,1ml of the subsequent super-natant and1m l of internal standard(0.5g3-methyl-n-valeric acid in1l of0.15mol/l oxalic acid)were mixed with3ml of distilled water.Following centrifugation to remove the precipitate,the sample wasfiltered through Whatman 0.45m m polyethersulphone membranefilters into a chromato-graphic sample vial.A1-m l sample was injected into a model 3800Varian gas chromatograph with a25m30.53mm i.d. megabore column(coating CP-Wax58(FFAP)–CB(no. CP7614))(Varian,Middelburg,the Netherlands).RNA extraction and complementary DNA(cDNA)synthesis Tissue samples were collected from the mesenteric side of the colon,rinsed with ice-cold sterile phosphate-buffered saline(Oxoid)and stripped of overlying smooth muscle cells. Approximately1to2g of the porcine colon tissue was cut into small pieces and placed in tubes containing15ml of RNAlater(Applied Biosystems,Foster City,CA,USA)and immediately stored at2208C pending RNA extraction.Total RNA was extracted from colon tissue samples(25mg)using a GenElute Mammalian Total RNA Miniprep Kit(RTN70, Sigma-Aldrich,St Louis,MO,USA)according to the manu-facturer’s instructions.To eliminate possible genomic DNA contamination,total RNA samples were subjected to DNAse I(AMPD1,Sigma-Aldrich)treatment according to the man-ufacturer’s protocol.Then RNA purification was performed using a phenol–chloroform extraction method(Chomczynski and Sacchi,2006).The total RNA was quantified using a NanoDrop-ND1000Spectrophotometer(Thermo Fisher Scien-tific,Wilmington,DE,USA)and the purity was assessed by determining the ratio of the absorbance at260and280nm. All total RNA samples had260/280nm ratios above1.8.In addition,RNA integrity was verified by visualisation of the18 and28S ribosomal RNA bands stained with ethidium bromide after gel electrophoresis on1.2%agarose gels(Egel,Invitro-gen Inc.,Carlsbad,CA,USA).Total RNA(1m g)was reverse transcribed(RT)using the RevertAid H minusfirst strand cDNA synthesis kit(Fermentas GmbH,St Leon-Rot,Germany)with oligo dT primers.Thefinal RT product was adjusted to a volume of120m l using nuclease-free water.Real-time quantitative PCRAll primers for the selected cytokines,genes such as Inter-leukin-1a(IL-1a),IL-6,IL-10,tumour necrosis factor(TNF-a) and the reference genes b-actin(ACTB),b2-microglobin (B2M),glyceraldehyde-3-phosphate dehydrogenase(GAPDH) and peptidylprolyl isomerise A(PPIA)are presented in Table2. Amplification was carried out in a reaction volume of20m l containing10m l SYBR Green Fast PCR Mastermix(Applied Biosystem),forward and reverse primer mix(1m l),8m l DEPC treated water and1m l of template cDNA.Quantitative real-time PCR was carried out using an ABI PRISM7500Fast sequence detection system for96-well plates(Applied Biosys-tem).The thermal cycling conditions were as follows:an initial denaturation step at958C for10min,40cycles of958C for15s, followed by608C for1min.Dissociation analyses of the PCR product were performed to confirm the specificity of the resulting PCR products.All samples were run in triplicate.The cycle threshold value(C t)is defined as the fractional cycle number at whichfluorescence passes thefixed threshold.The mean C t values of triplicates of each sample were used for calculations.Normalisation of quantitative PCR dataNormalisation of the C t values obtained from real-time PCR was performed by(i)transforming the raw C t values into relative quantities using the formula,relative quantities5 (PCR efficiency)D C t,where D C t is the change in the C t valuesWalsh,Sweeney,Bahar,Flynn and O’Doherty 1622of the sample relative to the highest expression (minimum C t value),(ii)using geNorm,a normalisation factor was obtained from the relative quantities of four most stable housekeeping genes (GAPDH,B2M,ACTB and PPIA)and (iii)the normalised fold change or the relative abundance of each of the target genes was calculated by dividing their relative quantities by the normalisation factor.Statistical analysisThe experimental data were analysed as a randomised block design using the GLM procedure of SAS (2004).The individualpig served as the experimental unit.Food intake was inclu-ded as a covariate in the model for villous height,crypt depth and villous height to crypt depth ratio in the digestive tract.The microbial counts were log transformed.The data were checked for normality using the Proc Univariate function of SAS.The means were separated using the Tukey–Kramer Test.Probability values of ,0.05were used as the criterion of statistical significance.All results are presented in the tables as least square means 6standard error of the means (s.e.).ResultsPerformance and faecal scoringThe average faecal scores of the pigs are presented in Table 3.The supplementation of different MW of COS had no significant effect on the growth performance of the pig during the 8-day experimental period (P .0.05).However,the inclusion of COS at all MW in the diet significantly reduced faecal scores com-pared with the control treatment (P ,0.01).MicrobiologyThe effect of COS supplementation at different MW on selected microbial populations in the colon of the pig is shown in Table 3.Pigs offered diets containing 5to 10kDa COS had a lower E.coli number compared with the control (P ,0.05)and the 50to 100kDa COS (P ,0.05)treatments.The 10to 50kDa treatment had a neumerically lower E.coli number compared with the control group (P 50.09).Pigs offered diets containing 5to 10kDa COS had a significantly lower population of lacto-bacilli in the colon compared with the control group (P ,0.05)and the 50to 100kDa COS diet (P ,0.01).Pigs offered 50to 100kDa COS had a higher lactobacilli number than pigs offered 10to 50kDa COS (P ,0.05).The supplementation of different MW of COS had no significant dietary effect on the lacto-bacilli :E.coli ratio in the colon of the pig.Table 2Porcine-specific primers used for real-time PCR 1.Forward primer sequence (50-30)Gene 2.Reverse primer sequence (50-30)T m (8C)IL-6 1.AGACAAAGCCACCACCCCTAA59.82.CTCGTTCTGTGACTGCAGCAGCTTATC 62.7IL-8 1.TGCACTTACTCTTGCCAGAGAACTG 61.92.CAAACTGGCTGTTGCCTTCTT 61.7IL-10 1.GCCTTCGGCCCAGTGAA 57.62.AGAGACCCGGTCAGCAACAA 59.4TNF-a 1.TGGCCCCTTGAGCATCA55.22.CGGGCTTATCTGAGGTTTGAGA 60.3GAPDH 1.CAGCAATGCCTCCTGTACCA 62.22.ACGATGCCGAAGTTGTCATG 62.1B2M 1.CGGAAAGCCAAATTACCTGAAC 59.02.TCTCCCCGTTTTTCAGCAAAT 60.0ACTB 1.CAAATGCTTCTAGGCGGACTGT 59.02.TCTCATTTTCTGCGCAAGTTAGG 60.0PPIA1.CGGGTCCTGGCATCTTGT58.02.TGGCAGTGCAAATGAAAAACTG56.5IL 5interleukin;TNF 5tumour necrosis factor;GAPDH 5glyceraldehyde-3-phosphate dehydrogenase;B2M 5b 2-microglobin;ACTB 5genes b -actin;PPIA 5peptidylprolyl isomerise A.Primers were designed using Primer Express TM software and were synthesisedby MWG Biotech (Milton Keynes,UK).Table 3Effect of COS supplementation at different MW on faecal scoring,selected microbial populations in the proximal colon and the total VFA concentration and the proportions of VFAs in the caecum of the weaned pig (least square means and s.e.;n 57)Dietary treatmentsControl 5to 10kDa 10to 50kDa50to 100kDas.e.SignificanceFaeces scoring Days 0to 84.06b 3.31a 3.44a 3.38a 0.124**Proximal colonic bacterial population (log cfu/g of digesta)Escherichia coli5.94b 4.34a 4.71a 5.81b 0.477*Lactobacilli spp.7.39bc 6.24a 6.56ab 7.56c 0.347*VFA concentrations in the caecum Total VFA (mmol/g of digesta)95.8770.25103.00116.7913.006ns Acetic acid 67.36b 45.77a 70.30b 79.20b 8.543*Propionic acid 19.8416.5123.3927.05 3.616ns Isobutyric acid 0.770.490.810.790.157ns Butyric acid 6.45 6.14 6.817.51 1.575ns Isovaleric acid 0.67b 0.45a 0.68b 0.83b 0.092*Valeric acid0.790.881.011.410.282nsCOS 5chitooligosaccharide;MW 5molecular weight;VFA 5volatile fatty acid.Probability of significance:*P ,0.05;**P ,0.01;ns,P ,0.05.Means with the same superscript alphabets within rows are not significantly different (P .0.05).Chitooligosaccharide in piglet diets1623Volatile fatty acidsThe effects of COS supplementation at different MW on the VFA concentrations in the caecum are shown in Table3.The supplementation of different MW of COS had a significant effect on the concentrations of acetic acid(P,0.05)and isovaleric acid(P,0.05)in the caecum.Pigs fed5to10kDa COS had lower levels of acetic acid and isovaleric acid compared with the control(P,0.05),10to50kDa COS (P,0.05)and50to100kDa COS(P,005).There was no significant effect of MW on VFA concentrations(P.0.05)in the proximal colon(data not shown).Gut morphologyThe effects of varying COS MW on villous height,crypt depth and the villous height:crypt depth ratios in the gastro-intestinal tract are shown in Table4.Pigs fed the10to 50kDa COS had a higher villous height in the duodenum and the jejunum compared with the control group(P,0.05), 5to10kDa COS(P,0.01)and50to100kDa COS diets (P,0.05).There was no effect of dietary treatment on crypt depth in the duodenum(P.0.05).Pigs offered the10to 50kDa COS had a higher villous height:crypt ratio in the duodenum and the jejunum compared with the control group(P,0.05)and the5to10kDa COS diet(P,0.01).Cytokine gene expression analysisThe effects of COS supplementation on the immune response in colon tissues of the pig are shown in Table5.The supplementation of different MW of COS had no significant effect on the expression of the cytokines TNF-a,IL-6,IL-8 and IL-10(P.0.05)in the gastro-intestinal tract of the pig. DiscussionThe hypothesis of the current experiment is that the biolo-gical properties of COS may be influenced by its MW and COS will enhance selected indices of health in weaned pig-lets.It was demonstrated in the current study that the lower MW of5to10kDa possessed antibacterial activity while the higher MW of10to50kDa was optimum for enhancing intestinal structure.Dietary supplementation of COS at the low MW of5to 10kDa decreased both lactobacilli and E.coli counts,while the10to50kDa COS numerically decreased E.coli popula-tions in the colon of the pig.In a study by Liu et al.(2008), COS supplementation at different concentrations reduced E. coli concentrations in the caecum of the weanling pig.E.coli is considered to be one of the most important causes of post-weaning diarrhoea in weaned pigs;therefore,a reduction inTable4Effect of COS supplementation at different MW on villous height,crypt depth and the villous height:crypt depth ratio in the gastro-intestinal tract of the weaned pig(least square means and s.e.)Dietary treatments Control5to10kDa10to50kDa50to100kDa s.e.Significance Covariate(intake) Villous height(m m)Duodenum284.0a256.0a326.3b266.2a17.38*ns Jejunum271.6a270.7a316.5b260.8a16.15*ns Ileum239.8268.3251.5242.915.07ns ns Crypt depth(m m)Duodenum305.7330.2280.1311.718.98ns ns Jejunum294.1298.4281.6268.420.93ns ns Ileum207.5242.8228.2239.811.29ns ns Villous:crypt depth ratioDuodenum 1.0a0.8a 1.2b0.9a0.08*ns Jejunum0.9a0.9a 1.2b 1.0ab0.06*ns Ileum 1.2 1.1 1.1 1.00.06ns nsCOS5chitooligosaccharide;MW5molecular weight.Probability of significance:*P,0.05;**P,0.01;ns,P,0.05.Means with the same superscript alphabets within rows are not significantly different(P.0.05).Table5Effect of COS supplementation at different MW on the immune response in unchallenged proximal colon tissues(leastsquare means of fold change in normalised relative gene expression with their s.e.;n57animals)Dietary treatments Control5to10kDa10to50kDa50to100kDa s.e.SignificanceColonTNF-a0.3660.3530.3760.3620.0568nsIL-60.2480.3590.3180.3220.0645nsIL-80.3850.5440.3700.3580.0797nsIL-100.3640.3420.3110.3070.0614ns COS5chitooligosaccharide;MW5molecular weight;TNF5tumour necrosis factor;IL5interleukin.Probability of significance:*P,0.05;**P,0.01;ns,P,0.05.Walsh,Sweeney,Bahar,Flynn and O’Doherty1624E.coli populations may reduce the incidence of diarrhoea in post-weaned pigs(Fairbrother et al.,2005).Although many species of E.coli are commensal,high levels of specific E.coli (like ETEC)will increase the risk of disease.Unfortunately, ETEC numbers were not measured in the current study.In the current study,the faecal score was decreased in pigs fed the COS diets compared with the control.These results suggest that the supplementation of the5to10kDa and10 to50kDa COS reduces E.coli populations in the colon, resulting in a lower faecal score in the post-weaning period. The50to100kDa COS led to a reduced diarrhoea score but no reduction in E.coli populations;therefore,this MW of COS may be working as a bulking agent to affect the faecal score.The50to100kDa COS may retard the rate of passage through the intestine and may have the ability to absorb water.In the current study,it was demonstrated that supple-mentation of5to10kDa COS had the strongest antimicrobial effect against both lactobacilli and E.coli.This is in agreement with other studies in which low MW COS(5to10kDa)were shown to possess strong antibacterial properties compared with higher MW COS and the antibacterial properties of COS increased at a low MW of,5kDa against Gram-negative such as E.coli(Zheng and Zhu,2003;Kittur et al.,2005).In a study by Liu et al.(2010),COS supplementation decreased E.coli populations compared with the control in the caecum of weaned pigs,while Jeon et al.(2001)observed a anti-microbial effect of COS against Gram-positive bacteria such as Lactobacilli under in-vitro conditions.To explain COS antibacterial activity,two mechanisms have been proposed.Thefirst mechanism is that the posi-tively charged COS reacts with negatively charged molecules at the microbial cell surface,thereby altering cell perme-ability(Chung and Chen,2008).Therefore,COS may interact with the membrane of the cell to alter cell permeability. However,as evident from the current study,this activity may differ with varying MW as the50to100kDa group had no inhibitive effect on the selected microbial populations,while the MWs of5to10kDa and10to50kDa COS had the strongest inhibitive effect.The other antibacterial mechan-ism is the binding of COS with DNA to inhibit RNA synthesis (Liu et al.,2004).It has been proposed that COS penetrates the nuclei of the bacteria and interferes with RNA and pro-tein synthesis.It is noteworthy that all the COS samples used in the current study were soluble in aqueous solutions.Kim and Rajapakse(2005)found that COS with a MW of .30kDa were not effective as antibacterial agents due to their poor solubility in aqueous solutions at a neutral pH. Volatile fatty acids are the major end products of bacterial metabolism in the large intestine(Macfarlane and Macfarlane, 2003).Both protein and carbohydrate fermentation contribute to the production of acetic acid;however,branched-chain fatty acids such as isovaleric acid are produced from protein fermentation(Mackie et al.,1998).In the current study,the5 to10kDa group had the lowest selected microbial populations while also reducing isovaleric acid and acetic acid concen-trations in the caecum.The shift in the production of the fermentation end products is reflected in the reduction of the selected microbial populations.The quantity of VFA produced depends on the amount and composition of the substrate and on the type of microbes present in the large intestine (van Beers-Schreurs et al.,1998).Reduced VFA concentrations indicate that lower amounts of substrate were fermented as a result of a lower microbial activity in the caecum(Htoo et al.,2007).Villous height is generally reduced and crypt depth is increased,which may explain the increased occurrence of diarrhoea and reduced growth after weaning(Pluske et al., 1996).The inclusion of10to50kDa COS in the present study was found to increase the villous height and villous:crypt depth ratio in the duodenum and also in the jejunum com-pared with the control group.Very little data have been published on the effects of COS MW on gut morphology in weaned piglets;thus,the exact mechanism for the increase in villous height and villous:crypt depth ratio is unclear.It may be hypothesised that low MW COS has the potential to promote intestinal morphology through cell proliferation. The COS has been shown to influence colonic cell prolifera-tion,crypt depth and crypt circumference in mice(Torzsas et al.,1996).A study carried out by Liu et al.(2008),on different con-centrations of COS,demonstrated that200mg/kg of COS increased villous height and villous:crypt ratio in the jeju-num and ileum(Liu et al.,2008).The possible explanation for this improved intestinal structure was that COS is com-posed of N-acetyl glucosamine(Kim and Rajapakse,2005), which may bind to certain types of bacteria and possibly interfere with their adhesion to the gut tissue of host animals (Ofek et al.,2003;Liu et al.,2008).This result is in agree-ment with Moura˜o et al.(2006),who reported that an increase in villi length in the ileum of weaned rabbits was correlated to a lower intestinal microflora.A decrease in bacteria load has been shown to increase the proliferation of epithelial cells,which leads to an improved intestinal mor-phology and increased villous height(Moura˜o et al.,2006). In the present study,in pigs fed the lower MW of5to10kDa COS,a strong antimicrobial effect on both Lactobacilli and E.coli populations was observed,with no effect on villous structure,while the higher MW of10to50kDa resulted in a reduction in E.coli numbers in comparison with the control and was optimum for improving villous integrity.There were no effects of COS supplementation in colon tissue on any of the cytokines analysed.This overall lack of an effect on these inflammatory cytokines implies that COS inclusion in the diet had no effects on immune gene expression of the pigs.Mori et al.(1997)also demonstrated that chitin and its derivatives do not stimulate the production of IL-6,IL-1and TNF-a byfibroblasts.In our study,no dif-ferences were observed on growth performance between days0and8post-weaning.In conclusion,MW is an important factor to consider when investigating the biological properties of COS.On the basis of the current study,the lower MW of5to10kDa possessed antibacterial activity while the higher MW of10to50kDaChitooligosaccharide in piglet diets1625。
megamonas funiformis iolg1 基因-回复"megamonas funiformis iolg1 基因"是一种与人体肠道微生物相关的基因。
在本文中,我将逐步回答您有关该基因的问题,并进一步了解其在人类健康中的作用。
首先,让我们来了解一下megamonas funiformis这个物种。
megamonas funiformis是一种肠道潜在致病菌,属于肠道微生物的一部分。
它的存在对于人类肠道菌群的平衡和功能至关重要。
肠道微生物群是人体内最常见的微生物生态系统之一,它们在维持人体健康、免疫功能、能量代谢和疾病预防中起着重要作用。
然而,megamonas funiformis iolg1基因的具体功能尚不清楚。
在对该基因的研究中,关注的重点是确定它是否与人类健康相关。
为了更好地理解其功能,科学家们对megamonas funiformis iolg1基因进行了进一步的研究。
在研究中,科学家们使用了多种研究技术,包括基因测序和功能分析。
他们发现,在megamonas funiformis中,iolg1基因编码一种特定的蛋白质。
这种蛋白质可能扮演着特定的生物学功能,并参与人体的免疫响应、消化过程或其他与健康相关的生物过程中。
进一步的研究表明,megamonas funiformis iolg1基因的表达水平可能受到多种因素的影响,包括饮食习惯、年龄、性别和个体的遗传差异。
这些因素可能会对肠道微生物的群落结构和功能产生影响,进而影响人体的健康状况。
然而,目前对于megamonas funiformis iolg1基因的研究仍然处于初步阶段。
虽然我们可以猜测它在人体健康中的潜在作用,但我们仍需要未来更多的科学研究来确认这些假设。
更多的研究将需要考虑到其他肠道微生物的相互作用,以及环境和遗传变异对megamonas funiformis iolg1基因表达的影响。
总结来说,megamonas funiformis iolg1基因是一种与人体肠道微生物相关的基因。
hpa轴对肠道菌群的影响的英语The Impact of the HPA Axis on Gut Microbiota.The hypothalamic-pituitary-adrenal (HPA) axis is a crucial component of the neuroendocrine system, regulating the body's response to stress. The gut microbiota, on the other hand, refers to the diverse collection of microorganisms that reside in the intestines. Both the HPA axis and the gut microbiota play significant roles in maintaining overall health and well-being. Recently, there has been increasing interest in the interaction between these two systems and how they may influence each other. This article will explore the impact of the HPA axis on gut microbiota.First, it is important to understand the basic functions of the HPA axis. The HPA axis is responsible for regulating the body's stress response, which involves the release of hormones such as cortisol. During acute stress, the release of cortisol is a natural biological response tothe presence of a stressor. However, chronic stress canlead to dysfunction of the HPA axis, resulting in elevated cortisol levels.The gut microbiota, on the other hand, plays a crucial role in maintaining the normal function of the intestines.It consists of a diverse collection of microorganisms that help in digesting food, absorbing nutrients, and protecting against pathogenic bacteria. The gut microbiota also playsa significant role in immune function and brain development.Now, let's delve into the impact of the HPA axis on gut microbiota. Chronic stress, which leads to dysfunction ofthe HPA axis, can have profound effects on the gut microbiota. Elevated cortisol levels, which arecharacteristic of chronic stress, can increase intestinal permeability, also known as "leaky gut." This increase in permeability allows bacteria and other microbial productsto leak into the bloodstream, triggering a systemic inflammatory response.This inflammatory response can have far-reachingconsequences for gut health. For example, it can lead to a decrease in the diversity and abundance of beneficial bacteria, such as Lactobacillus and Bifidobacterium, which are crucial for maintaining intestinal homeostasis. At the same time, it can promote the growth of harmful bacteria, such as Escherichia coli and Clostridium difficile, which can cause gut inflammation and other digestive issues.In addition to its direct effects on gut microbiota composition, chronic stress can also alter the function of the gut microbiota. For instance, stress can impact the production of short-chain fatty acids (SCFAs), which are metabolites produced by beneficial bacteria and play a crucial role in maintaining intestinal health. SCFAs are involved in regulating inflammation, promoting intestinal barrier function, and providing energy to intestinal cells. Chronic stress can reduce the production of SCFAs, thereby compromising intestinal health and function.The impact of the HPA axis on gut microbiota is not limited to direct effects on microbiota composition and function. There is also evidence that the HPA axis caninfluence gut microbiota through its effects on the immune system. For instance, chronic stress can lead to an overactive immune response, which can trigger inflammation and alter the balance of gut microbiota. Additionally, stress can affect the communication between the gut and the brain, known as the gut-brain axis, further influencing gut microbiota composition and function.In summary, the HPA axis plays a significant role in regulating the body's response to stress. However, chronic stress can lead to dysfunction of the HPA axis, resultingin elevated cortisol levels that have profound effects on gut microbiota composition and function. These effects can lead to gut inflammation, altered immune response, and other digestive issues. Understanding the interaction between the HPA axis and gut microbiota is crucial for developing effective strategies to maintain intestinal health and prevent chronic diseases associated with stress and gut microbiota dysregulation.。
肠道微生物的英语单词The Complex World of Gut Microbiota.The gut microbiota, often referred to as the "microbiome" or the "intestinal flora," refers to the vast community of microorganisms that reside within the human gastrointestinal tract. This intricate ecosystem plays a crucial role in maintaining our overall health and well-being. The gut microbiota is composed of a diverse range of bacteria, fungi, viruses, and other microorganisms that coexist in a delicate balance.The human body is estimated to contain trillions of microbial cells, outnumbering the human cells by a ratio of 10 to 1. The majority of these microbial cells reside in the gastrointestinal tract, particularly in the colon. The gut microbiota performs various vital functions, including digesting food, synthesizing vitamins, and regulating the immune system.Functions of the Gut Microbiota.Digestion and Nutrition: The gut microbiota aids in the breakdown of dietary fiber and other complex carbohydrates, releasing short-chain fatty acids (SCFAs) such as acetate, propionate, and butyrate. These SCFAs serve as a source of energy for the host and have been linked to various health benefits, including improved insulin sensitivity and reduced inflammation.Immune System Regulation: The gut microbiota plays a crucial role in shaping and regulating the immune system. It stimulates the development of immune cells and helps maintain a balanced immune response, protecting against both infectious diseases and autoimmune conditions.Barrier Function: The gut microbiota contributes to maintaining the integrity of the gut barrier, which prevents harmful bacteria and toxins from leaking into the bloodstream. A healthy gut microbiota supports tight junctions between gut cells, ensuring a strong barrier against pathogens.Brain-Gut Axis: The gut microbiota also interacts with the brain through the gut-brain axis, influencing mood, cognition, and behavior. This axis involves a complex communication network between the gastrointestinal tract and the central nervous system, which is believed to play a role in conditions like depression, anxiety, and autism.Importance of Gut Microbiota Balance.Disruptions to the gut microbiota, known as "dysbiosis," can lead to various health issues. Changes in the composition of the microbiota can be triggered by various factors, including diet, antibiotics, stress, and chronic illnesses.Diet: The composition of the gut microbiota is significantly influenced by the diet. A diet rich in fiber and diverse in plant-based foods promotes the growth of beneficial bacteria, while a diet high in processed foods and low in fiber can lead to a decrease in microbial diversity and an increase in harmful bacteria.Antibiotics: The use of antibiotics can have a profound impact on the gut microbiota, killing off both harmful and beneficial bacteria. This can lead to a temporary imbalance in the microbiota, allowing opportunistic pathogens to proliferate.Stress: Chronic stress has been shown to alter the gut microbiota composition, leading to an increase in inflammatory markers and a decrease in beneficial bacteria.Chronic Illnesses: Conditions like inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), and obesity have been linked to alterations in the gut microbiota. These changes can contribute to the development and progression of these diseases.Modulating the Gut Microbiota.Given the crucial role of the gut microbiota in maintaining health, there has been increasing interest in modulating its composition through various strategies.Probiotics: Probiotics are live microorganisms that, when administered in adequate amounts, confer a health benefit on the host. They are commonly found in yogurt, fermented foods, and dietary supplements. Probiotics can help restore balance to the gut microbiota, improving digestive health and immune function.Prebiotics: Prebiotics are dietary fibers that promote the growth and activity of beneficial bacteria in the gut. By providing food for the probiotic bacteria, prebiotics can help support a healthy gut microbiota.Dietary Changes: Incorporating a diet rich in fiber, fruits, vegetables, and whole grains can promote the growth of beneficial bacteria and maintain gut microbiota diversity.Conclusion.The gut microbiota plays a pivotal role in maintaining human health and well-being. Its intricate balance ofmicroorganisms is essential for digestion, immune system regulation, and overall physiological functions. Disruptions to this balance can lead to various health issues, emphasizing the importance of maintaining a healthy gut microbiota through diet, lifestyle choices, and probiotic supplementation. As research in this field continues to evolve, so does our understanding of the crucial role the gut microbiota plays in our lives.。
The genomics of probiotic intestinal microorganismsSeppo Salminen1 , Jussi Nurmi2 and Miguel Gueimonde1(1) Functional Foods Forum, University of Turku, FIN-20014 Turku, Finland(2) Department of Biotechnology, University of Turku, FIN-20014 Turku, FinlandSeppo SalminenEmail: *********************Published online: 29 June 2005AbstractAn intestinal population of beneficial commensal microorganisms helps maintain human health, and some of these bacteria have been found to significantly reduce the risk of gut-associated disease and to alleviate disease symptoms. The genomic characterization of probiotic bacteria and other commensal intestinal bacteria that is now under way will help to deepen our understanding of their beneficial effects.While the sequencing of the human genome [1, 2] has increased ourunderstanding of the role of genetic factors in health and disease, each human being harbors many more genes than those in their own genome. These belong to our commensal and symbiotic intestinal microorganisms - our intestinal 'microbiome' - which play an important role in maintaining human health and well-being. A more appropriate image of ourselves would be drawn if the genomes of our intestinal microbiota were taken into account. The microbiome may contain more than 100 times the number of genes in the human genome [3] and provides many functions that humans have thus not needed to develop themselves. The indigenous intestinal microbiota provides a barrier against pathogenic bacteria and other harmful food components [4–6]. It has also been shown to have a direct impact on the morphology of the gut [7], and many intestinal diseases can be linked to disturbances in the intestinal microbial population [8].The indigenous microbiota of an infant's gastrointestinal tract is originally created through contact with the diverse microbiota of the parents and the immediate environment. During breast feeding, initial microbial colonization is enhanced by galacto-oligosaccharides in breast milk and contact with the skin microbiota of the mother. This early colonization process directs the microbial succession until weaning and forms the basis for a healthy microbiota. The viable microbes in the adultintestine outnumber the cells in the human body tenfold, and the composition of this microbial population throughout life is unique to each human being. During adulthood and aging the composition and diversity of the microbiota can vary as a result of disease and the genetic background of the individual.Current research into the intestinal microbiome is focused on obtaining genomic data from important intestinal commensals and from probiotics, microorganisms that appear to actively promote health. This genomic information indicates that gut commensals not only derive food and other growth factors from the intestinal contents but also influence their human hosts by providing maturational signals for the developing infant and child, as well as providing signals that can lead to an alteration in the barrier mechanisms of the gut. It has been reported that colonization by particular bacteria has a major role in rapidly providing humans with energy from their food [9]. For example, the intestinal commensal Bacteroides thetaiotaomicron has been shown to have a major role in this process, and whole-genome transcriptional profiling of the bacterium has shown that specific diets can be associated with selective upregulation of bacterial genes that facilitate delivery of products of carbohydrate breakdown to the host's energy metabolism [10, 11]. Key microbial groups in the intestinal microbiota are highly flexible in adapting to changes in diet, and thus detailed prediction of their actions and effects may be difficult. Although genomic studies have revealed important details about the impact of the intestinal microbiota on specific processes [3, 11–14], the effects of species composition and microbial diversity and their potential compensatory functions are still not understood.Probiotics and healthA probiotic has been defined by a working group of the International Life Sciences Institute Europe (ILSI Europe) as "a viable microbial food supplement which beneficially influences the health of the host" [15]. Probiotics are usually members of the healthy gut microbiota and their addition can assist in returning a disturbed microbiota to its normal beneficial composition. The ILSI definition implies that safety and efficacy must be scientifically demonstrated for each new probiotic strain and product. Criteria for selecting probiotics that are specific for a desired target have been developed, but general criteria that must be satisfied include the ability to adhere to intestinal mucosa and tolerance of acid and bile. Such criteria have proved useful but cumbersome in current selection processes, as there are several adherence mechanisms and they influence gene upregulation differently in the host. Therefore, two different adhesion studies need to be conducted on each strain and theirpredictive value for specific functions is not always good or optimal. Demonstration of the effects of probiotics on health includes research on mechanisms and clinical intervention studies with human subjects belonging to target groups.The revelation of the human genome sequence has increased our understanding of the genetic deviations that lead to or predispose to gastrointestinal disease as well as to diseases associated with the gut, such as food allergies. In 1995, the first genome of a free-living organism, the bacterium Haemophilus influenzae, was sequenced [16]. Since then, over 200 bacterial genome sequences, mainly of pathogenic microorganisms, have been completed. The first genome of a mammalian lactic-acid bacterium, that of Lactococcus lactis, a microorganism of great industrial interest, was completed in 2001 [17]. More recently, the genomes of numerous other lactic-acid bacteria [18], bifidobacteria [12] and other intestinal microorganisms [13, 19, 20] have been sequenced, and others are under way [21]. Table 1lists the probiotic bacteria that have been sequenced. These great breakthroughs have demonstrated that evolution has adapted both microbes and humans to their current state of cohabitation, or even symbiosis, which is beneficial to both parties and facilitates a healthy and relatively stable but adaptable gut environment.Table 1Lessons from genomesLactic-acid bacteria and bifidobacteria can act as biomarkers of gut health by giving early warning of aberrations that represent a risk of specific gut diseases. Only a few members of the genera Lactobacillus and Bifidobacterium, two genera that provide many probiotics, have been completely sequenced. The key issue for the microbiota, for probiotics, and for their human hosts is the flexibility of the microorganisms in coping with a changeable local environment and microenvironments.This flexibility is emphasized in the completed genomes of intestinal and probiotic microorganisms. The complete genome sequence of the probiotic Lactobacillus acidophilus NCFM has recently been published by Altermann et al. [22]. The genome is relatively small and the bacterium appears to be unable to synthesize several amino acids, vitamins and cofactors. Italso encodes a number of permeases, glycolases and peptidases for rapid uptake and utilization of sugars and amino acids from the human intestine, especially the upper gastrointestinal tract. The authors also report a number of cell-surface proteins, such as mucus- and fibronectin-binding proteins, that enable this strain to adhere to the intestinal epithelium and to exchange signals with the intestinal immune system. Flexibility is guaranteed by a number of regulatory systems, including several transcriptional regulators, six PurR-type repressors and ninetwo-component systems, and by a variety of sugar transporters. The genome of another probiotic, Lactobacillus johnsonii [23], also lacks some genes involved in the synthesis of amino acids, purine nucleotides and numerous cofactors, but contains numerous peptidases, amino-acid permeases and other transporters, indicating a strong dependence on the host.The presence of bile-salt hydrolases and transporters in these bacteria indicates an adaptation to the upper gastrointestinal tract [23], enabling the bacteria to survive the acidic and bile-rich environments of the stomach and small intestine. In this regard, bile-salt hydrolases have been found in most of the sequenced genomes of bifidobacteria and lactic-acid bacteria [24], and these enzymes can have a significant impact on bacterial survival. Another lactic-acid bacterium, Lactobacillus plantarum WCFS1, also contains a large number of genes related to carbohydrate transport and utilization, and has genes for the production of exopolysaccharides and antimicrobial agents [18], indicating a good adaptation to a variety of environments, including the human small intestine [14]. In general, flexibility and adaptability are reflected by a large number of regulatory and transport functions.Microorganisms that inhabit the human colon, such as B. thetaiotaomicron and Bifidobacterium longum [12], have a great number of genes devoted to oligosaccharide transport and metabolism, indicating adaptation to life in the large intestine and differentiating them from, for example, L. johnsonii [23]. Genomic research has also provided initial information on the relationship between components of the diet and intestinal microorganisms. The genome of B. longum [12] suggests the ability to scan for nutrient availability in the lower gastrointestinal tract in human infants. This strain is adapted to utilizing the oligosaccharides in human milk along with intestinal mucins that are available in the colon of breast-fed infants. On the other hand, the genome of L. acidophilus has a gene cluster related to the metabolism of fructo-oligosaccharides, carbohydrates that are commonly used as prebiotics, or substrates to肠道微生物益生菌的基因组学塞波萨米宁,尤西鲁米和米格尔哥尔摩得(1)功能性食品论坛,图尔库大学,FIN-20014芬兰图尔库(2)土尔库大学生物技术系,FIN-20014芬兰图尔库塞波萨米宁电子邮件:seppo.salminen utu.fi线上发表于2005年6月29日摘要肠道有益的共生微生物有助于维护人体健康,一些这些细菌被发现显着降低肠道疾病的风险和减轻疾病的症状。