Chapter 8 ATOMIC ELECTRON CONFIGURATIONS AND
- 格式:ppt
- 大小:1.81 MB
- 文档页数:42

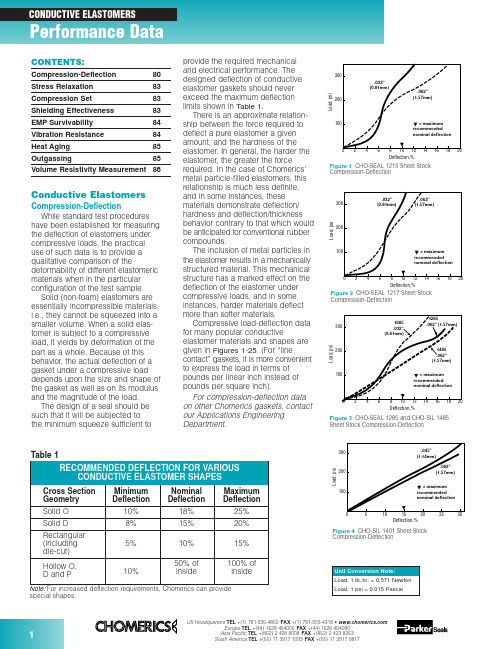
Figure 3CHO-SEAL 1285 and CHO-SIL 1485Sheet Stock Compression-DeflectionCompression-Deflectionspecial shapes.Conductive ElastomersCompression-DeflectionWhile standard test procedures have been established for measuring the deflection of elastomers under compressive loads, the practical use of such data is to provide a qualitative comparison of thedeformability of different elastomeric materials when in the particular configuration of the test sample.Solid (non-foam) elastomers are essentially incompressible materials;i.e., they cannot be squeezed into a smaller volume. When a solid elas-tomer is subject to a compressive load, it yields by deformation of the part as a whole. Because of this behavior, the actual deflection of a gasket under a compressive load depends upon the size and shape of the gasket as well as on its modulus and the magnitude of the load.The design of a seal should be such that it will be subjected to the minimum squeeze sufficient toprovide the required mechanical and electrical performance. The designed deflection of conductive elastomer gaskets should never exceed the maximum deflection limits shown in Table 1.There is an approximate relation-ship between the force required to deflect a pure elastomer a given amount, and the hardness of the elastomer. In general, the harder the elastomer, the greater the force required. In the case of Chomerics’metal particle-filled elastomers, this relationship is much less definite,and in some instances, these materials demonstrate deflection/hardness and deflection/thickness behavior contrary to that which would be anticipated for conventional rubber compounds.The inclusion of metal particles in the elastomer results in a mechanically structured material. This mechanical structure has a marked effect on the deflection of the elastomer under compressive loads, and in some instances, harder materials deflect more than softer materials.Compressive load-deflection data for many popular conductiveelastomer materials and shapes are given in Figures 1-25. (For “linecontact” gaskets, it is more convenient to express the load in terms of pounds per linear inch instead of pounds per square inch).For compression-deflection data on other Chomerics gaskets, contact our Applications Engineering Department.Compression-DeflectionCompression-DeflectionCONTENTS:Compression-Deflection 80Stress Relaxation 83Compression Set 83Shielding Effectiveness 83EMP Survivability 84Vibration Resistance 84Heat Aging 85Outgassing85Volume Resistivity Measurement86Figure 80.125 in. (3.18 mm) Dia. O-Strip Compression-DeflectionDeflection,%Compression-DeflectionCompression-DeflectionL o a d , l b ./i n c hDeflection, %Figure 210.156 in. (3.96 mm) High Hollow D-Strip Compression-DeflectionL o a d , l b ./i n c hDeflection, %Figure 220.312 in. (7.92 mm) High Hollow D-Strip Compression-DeflectionFigure 200.250 in. (6.35 mm) Dia. Hollow O-Strip Compression-DeflectionL o a d , l b ./i n c hDeflection, %L o a d , l b ./i n c hDeflection, %Figure 230.250 in. (6.35 mm) Dia. Hollow P-Strip Compression-DeflectionFigure 240.360 in. (9.14 mm) Dia. Hollow P-Strip Compression-DeflectionL o a d , l b ./i n c hDeflection, %L o a d , l b ./i n c hDeflection,%Figure 190.156 in. (3.96 mm) Dia. Hollow O-Strip Compression-DeflectionL o a d , l b ./i n c hDeflection, %Figure 170.250 in. (6.35 mm) Wide Rectangular Strip Compression-Deflection0.40.81.20.20.61.0C o m p r e s s i o n F o r c e (l b /i n )00.5 1.50.10.2Deflection (inch)1356P/N 10-09-W864-XXXXFigure 250.410 in. (10.41 mm) High V-Strip Compression-DeflectionStress RelaxationAs important as Compression Set and Compression-Deflection, is the Stress Relaxation characteristic of a gasket.If a rubber is subject to a com-pressive load, it will deflect. There is a stress/strain relationship, which for rubbers is generally non-linear except for very small deflections.After the load is applied, a stress decay occurs within the polymer resulting from the internal rearrange-ment of the molecular structure. An approximate rule is that the relaxed stress for cured silicone will finally settle at 70 to 75 percent of the initial stress.There are two ways in which a rubber gasket can be loaded to a desired value. One way is to load it to a point, let it relax, and reapply the load to restore the original stress. The next time it will relax, but not so much.If this is repeated a sufficient number of times, the correct static load on the gasket will reach equilibrium.A more practical way to reach the design value of stress is to load the gasket to 125 percent of its final design value, so that after the relax-ation process is completed the gasket will settle to 100 percent of the design load. This is very reproducible.Figure 26shows a typical stress relaxation curve for Chomerics’conductive elastomers.Compression SetWhen any rubber is deformedfor a period of time, some of the defor-mation is retained permanently even after the load is removed. The amount of permanent deformation, asmeasured by ASTM D395, is termed “Compression Set.” Compression set is measured under conditions of constant deflection (ASTM D395Method B) and is normally expressed as a percentage of the initialdeflection, not as a percentage of the initial height.For gaskets that are used once, or where the gasket/flange periphery relationship is constant (such as a door gasket), compression set is of minor significance if the original load condition and the service temperature are within the design limitations of the gasket material.For gaskets that are randomlyreseated one or more times in normal service life, it is important that the maximum change in gasket thickness does not exceed twice the maximum mismatch between the opposing mating surfaces.Shielding EffectivenessMost shielding effectiveness data given in Table 3 of the Conductive Elastomer section (pages 32-34) is based on a MIL-G-83528B testmethod, with a 24 in. x 24 in. aperture in a rigid enclosure wall and about 100 psi on the gasket. It is a valid and useful way of comparing variousgasket materials, but does not reflect the shielding effectiveness one can expect at seams of typical enclosures.CHO-TM-TP08 is a modified version of the MIL test that provides typical values achieved in actual applications.Since many factors will affect the actual shielding effectiveness of anenclosure seam (flange design,stiffness, flatness, surface resistivity,fastener spacing, enclosuredimensions, closure force, etc.), the only way to determine shielding effectiveness for real enclosures is to test them.Figures 28and 29provide dataon shielding effectiveness for actualFigure 27Formula for Calculation of Compression Setenclosures. The data in Figure 28shows the difference in attenuation between a shelter door closed with no gasket and the same door closed against a CHO-SEAL 1215 hollow D-strip gasket. Instead of single data points at each frequency tested, a range of data is shown for eachfrequency, representing the worst and best readings measured at many points around the door. Figure 29 shows the effects of closure force on shielding effectiveness of an enclosure tested at high frequencies (1-40 GHz) using CHO-SEAL 1215 solid D-strip gaskets.In order to establish reasonable upper limits on gasket resistivity, it is necessary to understand the rela-tionship between flange interface resistance and EMI leakage through the flange. Figure 30presents this relationship for an aluminum enclosure 3 in. x 3 in. x 4 in. deep, measured at 700 MHz. Die-cut gaskets 0.144 in.wide by 0.062 in. thick, in a wide range of resistivities, were clamped between the gold-plated flanges of thisenclosure. Simultaneous measure-ments of flange interface resistance (all attributable to the gaskets) versus RF leakage through the seamproduced a classic S-shaped curve.For the gasket configuration used in this test, the dramatic change in shielding effectiveness occursbetween gasket volume resistivities of 0.01 and 0.4 ohm-cm. Since real enclosures do not have gold-plated flanges, but rather have surfacefinishes (such as MIL-C-5541 Class 3chromate conversion coatings) which also increase in resistance over time, it is recommended that gasket volume resistivity be specified at 0.01 ohm-cm max. for the life of the equipment.Frequency, HzA t t e n u a t i o n (dB )Figure 28Shielding Effectiveness of a Shelter Door Gasket (14 kHz to 10 GHz)kA/inch of gasket (peak-to-peak).Pure silver (1224) and silver-plated-aluminum filled (1285) gaskets have less current carrying capability than silver-plated-copper materials, but are generally acceptable for EMP hardened systems (depending on specific EMP threat levels, gasket cross section dimensions, etc.).Vibration ResistanceCertain conductive elastomers are electrically stable during aircraft-level vibration environments, while others are not. The key factor which deter-mines vibration resistance is theshape and surface texture of the filler particles. Smooth, spherical fillers (such as those used in silver-plated-Figure 32Scanning Electron Microscopy Illustrates EMP Damage Mechanism for Silver/Glass ElastomersL e a k a g e (d B )Vibration (g)Figure 33Effects of Vibration on Shielding Effectiveness of Conductive Elastomer GasketsEMP SurvivabilityIn order for an enclosure to continue providing EMI isolationduring and after an EMP environment,the conductive gaskets at joints and seams must be capable of carrying EMP-induced current pulses without losing their conductivity. Figure 31shows the EMP current response of various types of conductive elastomer gaskets. Note that gaskets based on silver-plated-glass fillers (1350)become nonconductive at low levels of EMP current, and should therefore not be used when EMP is a design consideration. Figure 32is an electron microscope photo which clearly shows the damage mechanism.Silver-plated-copper filled (1215)gaskets have the highest resistance to EMP type currents, showing no loss of conductivity even at 2.50102030405060Shielding Degradation, dBIn t e r f a c e R e s i s t a n c e , m i l l i o h m sFigure 30Interface Resistance vs. Shielding Degradation at a Flange Jointglass materials) tend to move apart during vibration, leading to dramatic increases in resistance and loss of shielding effectiveness (although they normally recover their initial properties after the vibration has ended). Rough, less spherical particles resist vibration with very little electrical degradation. Figure 33shows the effects of vibration on three types of conductive gaskets.Although Chomerics’ silver-plated-copper filled 1215 gasket, with rough,irregular particle agglomerations,exhibits excellent stability during vibration, users of conductive elastomers should be aware that smooth, spherical silver-plated-copper fillers can be almost asunstable as silver-plated-glass fillers.Frequency, GHzA t t e n u a t i o n (dB )Figure 29Effect of Closure Force on Shielding Effectiveness (1 GHz to 40 GHz)Heat AgingThe primary aging mechanism which affects electrical stability of conductive elastomers is the oxidation of filler particles. Formaterials based on pure silver fillers,particle oxidation is not generally a problem because the oxide of silver is relatively soft and reasonably conductive. If the filler particles are non-noble (such as copper, nickel,aluminum, etc.) they will oxidize readily over time and become nonconductive. Even silver-plated base metal powders, such as silver-V o l u m e R e s i s t i v i t y (o h m -c m )Hours at 150°C (Solid Line)Hours at 125°C (Dotted Line)Figure 34Typical heat aging characteristics of Chomerics’ plated-powder-filled conductiveelastomers. Flanged 1000-hr test recommended for qualification. Unflanged 48-hr. test recommended for QC acceptance.plated-copper or silver-plated-aluminum will become non-conductive over time if the plating is not done properly (or if other processingvariables are not properly controlled).These are generally batch control problems, with each batch being potentially good or bad.The most reliable method of predicting whether a batch will be electrically stable is to promote the rate at which poorly plated or processed particles will oxidize, by heat aging in an air circulating oven.For qualification, 1000 hours (42 days)at maximum rated use temperature (with the gasket sample deflected 7-10% between flanges) is the recommended heat aging test for accelerating the effects of long-term aging at normal ambient tempera-tures. A quicker heat aging test,which correlates well with the 1000hour test and is useful for QC acceptance testing, involves a 48hour/150°C oven bake with thegasket sample on an open wire-grid tray (rather than being clamped between flanges). Figure 34shows typical data for volume resistivity versus time for each of these tests.Note:It is essential that no source of free sulfur be placed in the aging oven, as it will cause the material to degrade electrically and mask any oxidation aging tendencies. Common sources of sulfur are neoprenes,most cardboards and other paper products.OutgassingMany spacecraft specifications require that nonmetallic components be virtually free of volatile residues which might outgas in the hard vacuum environment of space. The standard test method for determining outgassing behavior is ASTM E595-93, which provides for measurement of total mass loss (TML) and collected volatile condensable materials (CVCM) in a vacuum environment. Data for a number of Chomerics conductive elastomers,based on ASTM E595-93 testing done by NASA Goddard SpaceflightCenter, is presented in Table 2. The normal specification limits or guide-lines on outgassing for NASA applications are 1% TML max.,and 0.1% CVCM max.。

SAT II 最新化学词汇表Part 1Chapter 1acid 酸apparatus 仪器, 装置aqueous solution 水溶液arrangement of electrons 电子排列assumption 假设atom 原子(化学变化中的最小粒子)atomic mass 原子量atomic number 原子序数atomic radius 原子半径atomic structure 原子结构be composed of 由……组成bombardment 撞击boundary 界限cathode rays 阴极射线cathode-ray oscilloscope (C.R.O) 阴极电子示波器ceramic 陶器制品charge-clouds 电子云charge-to-mass ratio (e/m) 质荷比(质谱分析时样品质量的测量以质量与其离子电荷之比表示)chemical behavior 化学行为chemical property 化学性质(物质在化学变化中表现出来的性质)clockwise 顺时针方向的(counter clockwise)compound 化合物(由不同元素组成的纯净物)configuration 构型copper 铜correspond to 相似corrosive 腐蚀d-block elements d 区元素deflect 使偏向, 使转向derive from 源于deuterium 氘diffuse mixture 扩散混合物distance effect 距离效应distil 蒸馏distinguish 区别distribution 分布doubly charged (2+) ion 正二价离子dye 染料effect of electric current in solutions 电流在溶液里的影响electrical charge 电荷electrical field 电场electrically neutral atom 电中性原子electricity 电electrolysis 电解electron 电子(负电荷粒子, 电量等于4.77×10-10绝对静电单位)electron shielding 电子屏蔽element 元素(具有相同核电荷数即荷内质子数的一类原子的总称)emission spectrum 发射光谱(根据发射光源和激发能量方式所产生的特征电磁波谱) energy level 能态, 能级(稳态能量, 有相同主量数的电子壳层)fertilizer 肥料first ionization energy 一级电离能fluorescent screen 荧光屏fluoride 氟化物fuel 燃料fundamental substance 基础物质fuzzy 模糊的galaxy 星系, 银河gas 气体gaseous state 气态gravity 重力Group I 第一族Heisenberg’s uncertainty principle 海森堡测不准原理hydrofluoric acid 氢氟酸identical 同一的, 相等的in terms of 根据, 在……方面innermost 最内的, 最深的interaction 相互作用internal structure 内部结构interpret 解释investigate 研究, 调查ionization energy 电离能(从原子或分子中移走一个电子至无穷远处所需的能量, 以电子伏特eV表示)ionize 电离isotope 同位素(原子里具有相同的质子数和不同的中子数的同一元素的原子互称同位素)J.J. Thomson’s e/m experiment 汤姆森质何比实验Latin 拉丁lepton 轻粒子liquid 液体magnet 磁铁magnetic field 磁场Maltese Cross 马耳他十字marble 大理石mass number 质量数matter 物质metal foil 金箔meteorite 陨星microbe 微生物, 细菌Millikan’s ‘oil-drop’experiment 密立根油滴实验model-building 模型建筑mole 摩尔(表示一个系统的物质的量的单位, 该系统中所包含的基本单元数与12g碳12即12C的原子数目相等, 每摩尔物质含有阿佛加德罗常数个微粒)molecule 分子(保持物质的化学性质的最小粒子)narrow beam 狭窄的光线negative electrode (cathode) 阴极negligible 可以忽略的neutron 中子nitrate 硝酸盐noble gas 稀有气体normal pressures 常压nuclear charge (原子) 核电荷nuclear model for atoms 原子核模型nuclear reaction 核反应nucleus (pl. nuclei) 核Orbital 轨道paraffin wax 石蜡particle 微粒, 粒子Pauli exclusion principle 保里不相容原理(每个原子轨道至多只能容纳两个电子;而且, 这两个电子自旋方向必须相反)Periodic Table 周期表physical property 物理性质(物质不需要发生化学变化就表现出来的性质, 如颜色、状态、气味、熔沸点、密度等)plastics 塑料plum-pudding 李子布丁positive charge 正电荷(带有质子的物质, 用丝绸摩擦玻璃棒, 在棒上会产生正电荷) positive electrode (anode) 阳极positively charged particle (ion) 离子potential difference 电位prediction 预言principal quantum number 主量子数(标示轨道电子的波函数, 包括轨道角动量和自旋量子数, 电子的能级和距原子核的平均距离主要取决于主量子数)probe 探测, 探究protium 氕proton 质子quantum (pl. quanta) 量子(一个电子转移到原子的下一层轨道时发出的有限辐射能单位)quantum mechanics 量子力学Quantum Theory 量子理论quark 夸克(组成基本粒子的更小的粒子)radioactive source 放射源repel 排斥repulsion 斥力respectively 分别地rung 梯级scattering effect 散射作用Schrödinger equation 薛定谔(波动) 方程(一偏微分方程, 描述基本粒子波动性) scintillation 火花shell 电子壳层shielding effect 屏蔽效应simpler substance 单质(指由同种元素组成的纯净物)solid 固体sphere 球spin 自旋stable state 稳态sub-atomic particle 原子内的粒子subset 子集, 小团体successive ionization energy 逐级电离能symbol 符号symmetry 对称the lowest-energy orbital 最低能量轨道transition elements 过渡元素tritium 氚X-ray X 射线α-particles α粒子, 即alpha-particle (带有两个质子和中子的粒子, 即氦原子核, 对物质的穿透力较强, 流速约为光速的1/10)α-ray α射线β-particles β粒子β-ray β射线γ-particles γ粒子γ-ray γ射线Chapter 2abbreviation 缩写absorption 吸收abundance 丰度accelerate 加速alloy 合金alter 改变atmospheric pressure 大气压Avogadro’s constant 阿佛加德罗常数(12g12C含有的原子数, 约为6.02×1023) azide 叠氮化物balance chemical equation 配平化学方程式balance ionic equation 配平离子方程式benzene 苯blast furnace 高炉bromide 溴化物bulk 体积burette 滴定管butane 丁烷carbon dioxide 二氧化碳carbon monoxide 一氧化碳carbonate 碳酸盐collide with 冲突combustion analysis 燃烧分析concentration 浓度conical flask 锥形瓶convert 转化covalent bonds 共价键(原子间通过共用电子对形成的化学键)decimal place 小数位deposit 沉淀物detonator 炸药dioxide 二氧化物dissolve 溶解drop wise 逐滴地electric current 电流empirical formulae 实验式, 经验式(只表示化合物中原子间最简单比例关系, 非分子式, 而为成分式)end-point 终点enthalpy 焓(热力学状态函数, 单位质量的热含量, 恒压下系统改变状态时增加的热含量等于内能与体系体积与压力乘积之和)equation 方程式acetic acid 乙酸filament 灯丝formula (pl. formulae) 化学式(用元素符号来表示物质组成的式子)granule 颗粒Group I the alkali metal 第一族, 碱金属Group II the alkaline earth metal 第二族, 碱土金属Group III- 第三族Group IV Carbonic Group 碳族Group V Nitric Group 氮族Group VI Oxygenic Group 氧族Group VII the halogens 第七主族, 卤族hexane 己烷horizontal axis 横坐标hydrocarbon 碳氢化合物, 烃hydrochloric acid 盐酸hydrogen peroxide 过氧化氢hydroxide 氢氧化物hypothesis 假设indicator 指示剂inspect 检查, 查看iodide 碘化物ionic compound 离子型化合物(电负性相差大的两种元素相互作用, 发生电子转移, 变为正、负离子, 正、负离子结合形成离子型化合物)iron oxide 氧化铁low pressure 低压mass spectrometer 质谱仪methane 甲烷mixture 混合物(由两种或多种物质混合而成的, 这些物质相互间没有发生反应, 混合物里各物质都保持原来的性质)molar mass 摩尔质量(1摩尔物质的质量)molarity 摩尔浓度, 也叫物质的量浓度(以1升即1立方分米溶液里含有多少摩溶质来表示溶液组成的物理量)molecular formulae 分子式(根据元素分析和分子量表示的化学式)monoxide 一氧化物negative ion (=anion) 阴离子neutralize 中和nitric acid 硝酸non-metal 非金属octane 辛烷organic compound 有机化合物oxidation state 氧化态oxide 氧化物peroxide 过氧化物phosphate 磷酸盐pipette 移液管positive ion (=cation) 阳离子precipitation reaction 沉淀反应reactant 反应物reaction 反应reagent 试剂, 反应物red-ox reaction 氧化还原反应relative atomic mass 相对原子质量(以碳12原子的质量的1/12约1.66×10-27kg作为标准, 其他原子的质量跟它比较所得的值)relative formula mass 相对式量relative isotopic mass 相对同位素质量relative molecular mass 相对分子质量(化学式中各原子的相对原子质量的总和) room temperature 室温singly charged 单核stoichiometric ratio 化学计量比stoichiometry 化学计量法sulphate 硫酸盐sulphide 硫化物sulphite 亚硫酸盐sulphuric acid 硫酸temperature 温度thermite 铝热剂, 灼热剂titration 滴定法(将已知浓度的标准溶液加到被测溶液中, 直到反应完成, 借以测定其浓度)vaporize 汽化vertical axis 纵坐标vice versa 反之亦然volume 体积weld 焊接Chapter 3adjacent molecule 相邻的分子amide 酰胺(含-CONH2基)ammonia 氨atmosphere 大气层atomic orbital 原子轨道attractive force 吸引力biochemical compound 生化化合物boiling point 沸点bond angle 键角(与同一原子连接的两个键之间的角度)bond enthalpybond length 键长(分子中两个原子核间的平衡距离)bonding pair 成键电子对brine 盐水brittle 脆的building-block (=monomer unit) 单体(聚合物中最简单的重复结构单元)catalyst 催化剂(能改变反应速度而它本身的组成和质量在反应前后保持不变的物质) chemical bonding 化学键(分子或晶体中, 原子或离子之间直接的、主要的和强烈的相互作用称为化学键)chemical bonding and structure 化学键及结构chloride 氯化物cleavage 裂开condense 浓缩conduct electricity 导电covalent compound 共价化合物crystal 晶体crystal lattice 晶格crystal plane 晶体平面crystalline solid 晶状固体cyclohexane 环己烷dative covalent/coordinate bond 配位键decomposition 离解density 密度dipole-dipole force 取向力dot-and-cross diagram 电子式, 点叉式double bond 双键double helix 双螺旋ductile 可塑性, 易变形的, 可延展的electric dipole 电偶极子(一对相距极近, 符号相反、数值相等的电荷所形成的体系) electrical insulator 电绝缘体electrical transformer 变压器electro-negativity 电负性(原子或基团吸引并持留价电子的能力)electron-pair 电子对electron-pair repulsion theory 电子对互斥理论(是利用中心原子周圍電子的排斥理論來預測的分子及離子(去除金屬部分) 的形狀)electrostatic attraction 静电吸引(引力)emerald 翡翠enthalpy change of vaporization 蒸发焓ethane 乙烷ethanol 乙醇, 又叫酒精evaporation 蒸发fabric 布,fiber/fibre 纤维fibrous 纤维状的formation of ions 离子的形成gaseous state 气态gemstone 宝石graphite 石墨hemoglobin 血红蛋白hard 硬的high-density poly (ethene) 高密度聚乙烯hydrated ion 水合离子(与水结合而成, 如H3O+)hydrogen bond 氢键(氢键是由于与电负性极强的元素如氟、氧等相结合的氢原子和另一分子中电负性极强的原子间所产生的引力而形成)insoluble 不溶instantaneous dipole-induced dipole forces 诱导力intermediate character 两性intermolecular force 分子间作用力(又称van der Waals’force 范德华力)interval 间隙ionic bonding 离子键(由原子得失电子后, 生成的正负离子之间, 靠静电作用而形成的化学键)ionic crystal 离子晶体(离子间通过离子键结合而成的晶体)ionic lattice 离子晶格jeweler 珠宝kinetic theory of matter 物质运动论(所有物质的分子处于恒动状态)liquid state 液态lone-pairs 孤对电子low-density poly (ethene) 低密度聚乙烯LP-LP repulsion> LP-BP repulsion> BP-BP repulsion 孤电子对—故电子对斥力>孤电子对—成键电子对斥力>成键电子对—成键电子对斥力lubricant 润滑剂magnetize 磁化malleable 有延展性的melting point 熔点metal complex 金属络合物(由金属离子与电子给予体结合而成)metallic bonding 金属键(通过自由运动的价电子将金属原子连结起来的键)metallic element 金属元素mineral 矿物质mobile electron 流动电子molecular orbital 分子轨道molten 熔化non-conductor 非导体non-linear molecule 非直线分子non-metallic element 非金属元素non-polar molecule 非极性分子non-stick properties 不黏性nylon 尼龙, 聚酰胺纤维octahedron 八面体oppositely charged electron 电性相反的电极oppositely charged ion 电性相反的离子outer-shell electron 外层电子oxonium ion 氧鎓离子hydronium ion 水合氢离子polar molecule 极性分子polarization of ions 离子极化polarized 极化poly 聚乙烯poly (ester) chain 聚酯链polychlorinated biphenyls (PCBs) 多氯联(二) 苯polymer 聚合物, 高分子polymer chain 聚合物链protein 蛋白质quartz 石英relative bond strength 相对键能repulsion 斥力ruby 红宝石sapphire 蓝宝石semi-precious stone 亚宝石single bond 单键slippery 光滑sodium chloride 氯化钠solid state 固态solubility 溶解度(物质在溶剂中达到饱和时的溶解程度) soluble 可溶sparingly soluble 难溶sublimation 升华(固体不经液态直接转变为气态) sublime 升华(固体不经液态直接转变为气态)sucrose 蔗糖surface tension 表面张力(由于表面层下面的分子与表面层下面的分子间的分子吸引, 液体表面收缩成最小表面的趋向)symmetrical distribution 对称分布tensile strength 抗拉强度tetrahedral molecule 四面体分子tetrahedron 四面体the δ+ and δ-charges δ+ 和δ-电荷three-dimensional arrangement 三维排列triangular pyramidal molecule 三角锥形分子trichloromethane 三氯甲烷trigonal planar molecule 三角锥形分子triple bond 三键unit cell 晶胞vapor pressure (蒸汽压)viscosity 黏度(流体流动阻力的表示, 为液体中黏合力和内聚力的综合效果) volatility 挥发性washing-up liquidwater is peculiar 水是特殊的weapon 武器δbond δ键δorbital δ轨道πbond π键πorbital π轨道Chapter 4 and 5antacid tablet 解酸的药片atomic radii (=atomic radius) 原子半径barium meal 钡餐Blocks of elements in the Periodic Table 周期表中元素的分区brick red 砖红色bricklaying 砌砖, 泥水业brilliant whitish flame 明亮的白色火焰bubble 泡camera lenses 照相机镜头cement 水泥chalk 白垩chemical species 化学物种clay 黏土, 泥土cliff 悬崖cloudy white precipitate 浑浊的白色沉淀covalent radius 共价半径covered with a layer of its oxide 覆盖一层氧化物薄膜crucible 坩埚crumble 粉碎d-block d区diatomic molecule 双原子分子dilute 稀释disulphur dichloride 二氯化二硫dolomite 白云石electronegative 带负电的, 负电性的electropositive 带正电的, 正电性的evolution (气体) 散出exothermic reaction 放热反应f-block f区filtration 过滤firework 焰火flare 照明弹good conductivity of heat and electricity 良好的导电导热性gypsum 石膏hydrogencarbonate 碳酸氢盐incendiary bomb 燃烧弹indigestion remedy 消化不良的治疗lanthanide and actinide elements 镧系和锕系元素Law of Octaves 八行周期律Law of Triads 三素组lime 石灰lime water 石灰水溶液limelight 灰光灯limestone 石灰石liquid phase 液相magnesium ribbon 镁条marine invertebrate 海里的无脊椎动物Mendeleev’s periodic tablemetal hydride 金属氢化物metallic radius 金属半径molten slag 熔渣monatomic ion 一价离子mortar 灰浆negative oxidation state 负化合价opaque 不透, 不传导oxidation 氧化oxidation number (abbreviated ox.№) 氧化数oxidation state 氧化态oxidizing agent 氧化剂(得到电子的物质)p-block p区periodic patterns 周期律periodicity 周期性photographic flash bulb 感光photosynthesis 光合作用pitchblende 沥青铀矿plaster 石膏plaster of Pairs 熟石膏positive oxidation state 正化合价quicklime 生石灰reactivity 活动性reciprocal 倒数redox system 氧化还原体系reducing agent 还原剂(逝去电子的物质)reduction 还原refractory material 难熔物质rotary kiln 回转窑(炉)saturated solution 饱和溶液s-block s区scum 浮垢sedimentary rock 沉积岩Siemens per meter (S m-1) 西门子/米(西门子是电导实用单位, 亦称姆欧, 欧姆的倒数)single atom 单原子slaked lime 石灰(固)solid phase 固相suspension 悬浮液the outmost electrons 最外层电子the rising parts of the curve 曲线的上升部分the trend is uneven 趋势是不规则的thermal decomposition 热(分) 解toxic 有毒的tracer bullet 示踪子弹trough 曲线上的最小值valence 化合价vapor phase 气相vigorous 剧烈的Chapter 6a cream precipitate 米黄色沉淀aerosol propellant 气溶胶喷射剂ammonia solution 氨水anomalous properties 异常的性质antiseptic抗菌剂, 防腐剂apparent 透明的bacteria 细菌bleach 漂白bromine is a dark red liquid giving off a dense red vapor 溴是深红色液体, 会挥发浓的红色溴蒸气capture an electron 捕获一个电子CFCS (chlorofluorocarbons) 含氯氟烃chlorine is greenish yellow gas 氯是黄绿色气体contamination 污染covalent diatomic molecule 共价双原子分子cyclohexane 环己烷dichloromethane 二氯甲烷displacement reaction 置换反应disproportionation reaction 歧化反应electron affinity 电子亲合势(原子保持其离子电荷的亲合势)fire extinguisher 灭火器flammable 易燃的fluoride controversyfluorine is pale yellow gas 氟是淡黄绿色气体foaming agent 起泡剂germicide 杀菌剂halate 卤酸盐halide 卤化物halogen 卤族元素, 简称卤素hydrated halide ion 水合卤素离子inert 惰性的iodine in alcohol 碘酒iodine is a shiny, grey-black crystalline solid which sublimes to a purple vapor 碘是有光泽的灰黑色晶体, 会升华变成紫色碘蒸气liver damage 肝脏损伤Lubricant 滑润剂non-flammable 不易燃的organic solvent 有机溶剂organochlorine 有机氯ozone layer 臭氧层poisonous 有毒的PTFE (polytetrafluoroethene) 聚四氟乙烯PVC 聚氯乙烯refrigerant 制冷剂solvent 溶剂thyroid problem 甲状腺问题volatility 挥发性water purification 水质净化waterproof clothing 防水布Part 2Chapter 71, 2-dichloroethene 1, 2-二氯乙烯2, 2, 3-trimethylbutane 2, 2, 3-三甲基丁烷2, 2, 4-trimethypentane (iso-octane) 2, 2, 4-三甲基戊烷2-bromobutane 2-溴丁烷2-hydroxybenzoic acid 2-对羟基苯甲酸2-methylpentan-3-one 2-甲基3-戊酮3-ethylpent-2-ene 3-乙基烯acid-base reaction 酸碱反应activation energy 活化能(分子开始反应所需最低能量, 为活化分子能量与所有分子平均能量差)addition 加成alanine 丙氨酸alcohol 醇aldehyde 醛aliphatic alcohol 脂肪醇aliphatic aldehyde 脂肪醛aliphatic compounds 脂肪族化合物alkene 烯烃alkyl 烷基allotrope 同素异形体amine 胺amino acid 氨基酸ammonium cyanate 氰化铵anhydrous salt 无水盐anti-bumping stone 沸石aqueous layer 水层arene 芳烃aromatic compounds 芳香族化合物(分子里含有一个或多个苯环的化合物)aspirin 阿司匹林atoms can rotate freely about a carbon-carbon single bond 原子可绕碳-碳单键自由旋转ball-and-stick model 球棍模型benzene ring 苯环branched-chain 支链Buchner flask 布氏烧瓶, 抽滤瓶Buchner funnel 布氏漏斗(常用于真空抽滤疏松沉淀)buckminsterfullerenebut-2-ene 2-丙稀butanl-ol (=CH3CH2CH2CH2OH) 1-丁醇butanoic acid 丁酸cage 壳体, 支架calculation of percentage yields 回收率的计算capillary electrophoresis apparatus 毛细管电泳仪carbanion 负(阴) 碳离子carbocation 正(阳) 碳离子carboxylic acid 羧酸Compact 致密的condenser 冷凝器convection currents 对流气流(由温差推动)criteria for checking purity 检测纯度的标准cyclic hydrocarbon 环烃(碳原子间相互连接成环状)cyclobutane 环丁烷decane 癸烷displayed formula (=full structural formula)distillation 蒸馏法eicosane 二十烷electric heating mantle 电热炉electrophile 亲电子试剂electrophilic addition 亲电子加成electrophilic substitution 亲电子取代elimination 消去equilibrium (pl. equilibria) 平衡ester 酯anhydride 酐ethanol (=CH3CH2OH) 乙醇ethyl- 乙基ethylamine 乙胺Ethylbenzene 乙基苯free radical 自由基free-radical substitution 自由基取代functional group 官能团gas-liquid chromatography 气液色谱法general formulageodesic domes 地圆学说geometric (or cis-trans) isomer 几何异构体(顺式-反式) grooved cork 具孔塞ground glass cone-and-socket joint 磨口玻璃锥管接合处ground glass socket 磨口玻璃管halogenoalkane 卤代烃heptane 庚烷heterolytic fission 异裂(共价键断裂产生两个相反电荷的离子) homolysis/hemolytic fission 均列high-performance liquid chromatography 高效液相色谱法homologous series 同系物homolytic fission 均裂(共价键断裂产生两个自由基) hydrolysis 水解hydroxy- (-OH) 羟基hyphen 连字符immiscible liquid 不溶混液体impurity 杂质intermolecular hydrogen bond 分子间氢键Isomerism 同分异构现象ketone 酮kinetic energy 动能Kjeldahl 凯氏定氮法的凯氏liquid circulates 液体循环melting point tube containing sample 装有样品的熔点测定管methanol 甲醇methoxymethane 甲氧基甲烷methyl甲基Methylpropane 甲基丙烷molecular formulanomenclature 系统命名法nonane 壬烷nucleophile 亲核试剂nucleophilic addition 亲核加成nucleophilic substitution 亲核取代organic chemistry 有机化学paper chromatography 纸层析法paraffin oil 石蜡油pentan-3-one 3-戊酮pentane 戊烷phenyl 苯基phenylalanine 苯基丙氨酸phosphoric acid 磷酸preliminary calculation 预算propan-1-ol (=CH3CH2CH2OH) 1-丙醇propan-2-ol 2-丙醇propanal 丙醛propane 丙烷propylamine 丙胺pumice 浮石reaction mechanism 反应机制reaction pathway 反应途径recrystallization 重结晶redistilling 重蒸馏Reflux 回流rubber ring 橡胶圈rubber seal 橡胶塞separating funnel 分液漏斗side-chain 侧链skeletal formula 骨架skeletonspectroscopic technique 光谱技术stereoisomerism 立体异构体stoppered flask 已塞紧的烧瓶straight-chain 直链structural formulastructural isomer 同分异构体(化合物具有相同的分子式, 但具有不同结构) substitution 取代synthesis 合成the maximum mass of product 最大产量the neck of the reaction flask 烧瓶瓶颈thermometer 温度计thermostatically controlled heating mantle 恒温控制加热炉Thiele tube 蒂埃尔均热管thin-layer chromatography 薄层层析法three-dimensional formulaultraviolet (UV) 紫外线vacuum filtration 真空抽滤, 真空过滤vinegar 醋visible spectroscopy 可见光voltage 电压water bath 水浴wavelength 波长Chapter 82, 2, 4-trimethyolpentane 2, 2, 4-三甲基戊烷2-methylpentane 戊烷adhesive 粘合剂alkane 烃alternatives to fossil fuels 化石燃料的代替品anaerobic decay 厌氧分解bimetallic catalyst 双金属催化剂biofuels 生物燃料bitumen 沥青burn off 燃尽carbon coke 焦炭carcinogenic aldehyde methanal 致癌的醛甲烷化catalytic cracking 催化裂化(由重质组分催化裂解为轻质组分) cellulose 纤维素chemical cell 化学电池CO2 emissions CO2 的排放coal 煤condensation 冷凝corrode 腐蚀cracking 裂化crude oil 原油cycloalkane 环烃cylinder 汽缸, 圆筒diesel 柴油drastic action 剧烈反应efficient combustion 有效燃烧feedstock 给料ferment 发酵fission 裂变flow rate 流速Fluid 流体fluidised bed 流化床fossil 化石fraction 分馏物fractional distillation 分馏fractional distillation column 分馏塔fusion (核) 聚变gasoline 汽油generate electricity 发电geothermal: hot rocks 地热:热岩greenhouse effect 温室效应hydrocarbons: fuels 碳氢化合物:燃料hydroelectricity 水电, 水力发电inhalation 吸入isomerization 异构化kerosene 煤油lead-acid battery 铅酸蓄电池lubricating oil 润滑油megawatt 兆瓦, 即106瓦naphtha 粗汽油natural gas 天然气non-renewable resource 不可再生资源nuclear fuels 核燃料oil refinery 炼油厂oscillating motion 振动overflow pipe 溢流管oxidation product 氧化产物oxidizer 氧化剂petrol 汽油photovoltaic cell 阻挡层光电池plant 植物rapeseed 油菜籽raw material 原料recycle 重复利用reforming 重整regeneration chamber 燃烧室residue 废料, 残渣seething mixture 沸腾的混合物separate into layers 分层sieve 滤网solar panels 太阳能(电池) 板spherical tank 球形罐spillage 溢出steady state 稳态sunflower oil 葵花油sunlight: solar heating and photovoltaics 阳光:太阳热和太阳电池tarmac 停机坪thermal energy 热能transfer of energy to the surroundings 把能量转移到四周tray (分馏塔的) 板turbine 涡轮waste products 废品weir 坝, 堰zeolite 沸石Chapter 92, 2-dimethylpropane 2, 2-二甲基丙烷CH3·(methyl) free radical 甲基自由基chain reaction 链锁反应combustion in air 在空气中燃烧complete combustion in an excess of air 在过量空气中完全燃烧concentrated sulphuric acid 浓硫酸dodecane 十二烷hydrocarbons: alkenes 碳氢化合物:烃in poorly ventilated rooms 在通风不足的房间initiation step 初级过程mechanism 机理overlap 重叠photochemical reaction 光化学反应(原子、分子、自由基或离子由吸收一个具有一定频率的光子而成为激发态所引起的反应)photo dissociation 光解作用(分子通过吸收一个光子的电磁能分裂出一个或多个原子) propagation step 增殖过程saturated hydrocarbon 饱和烃termination step 终止过程tetrachloromethane 四氯甲烷undecane 十一烷unsaturated hydrocarbon 不饱和烃waxy solid 蜡状固体Chapter 102-methylbuta-1, 3-diene 2-甲基-1, 3-二丙稀addition polymerization 加聚反应(由大量小分子(单体) 相继加成为大分子量化合物或聚合物)antifreeze 防冻剂bark 树皮cis-trans isomerism 顺-反式同分异构现象decolorize 褪色dibromo- 二溴化/代diene 二烯electron-rich 富电子electrophilic addition 亲电加成ethane-1, 2-diol 乙烷-乙二醇gas scrubber 气体洗涤器gutta-percha 杜仲胶, 古塔胶hard margarine 硬植物油horny 角状的, 粗硬的hydrocarbons: alkenes 碳氢化合物:烯烃industrial methylated spirits 工业甲基化酒精(翻译错误, 指的是掺甲醇的工业酒精) inelastic 无弹力的isoprene 异戊(间) 二烯latex 橡浆, 树乳monomer 单体(见chapter 3 building-block)multiple bond 重键(不饱和化合物中双键和三键的总称)natural rubber 天然橡胶nickel catalyst 镍催化剂non-biodegradable 不可生物降解optic nerve 视觉神经percha tree 橡胶树pollutant 污染物poly (chloroethene) 聚氯乙烯poly (phenylethene) 聚苯乙烯polymerization 聚合反应polyunsaturated 多个不饱和的propene is bubbled through a solution of bromine 把丙稀通入溴水中repeat unit 重复单元retinal 视网膜steam 蒸汽styrene 苯乙烯systematic name 系统命名traditionally vinyl chloride 氯乙烯triethyl 三乙基Ziegler-Natta catalyst 齐格勒-纳塔催化剂(由两种金属化合物反应而成, 用于烯烃、双烯烃等聚合, 生成聚乙烯、聚丙烯)planar molecule 平面分子Chapter 11acidified aqueous potassium dichromate 酸化二氯溶液acyl chloride (acylation) 酰基氯, 氯化某酰(酰化作用)adulteration 搀杂alkoxide ion (=RO-) 烷氧阴离子anaerobic process 厌氧过程ceramic wool soaked in ethanol 陶瓷羊毛corresponding alcohol 相应的醇dehydration 脱水deterrent 灭菌剂enzyme 酶ethoxide ion 乙氧基离子fermentation 发酵foul taste 恶臭fruity odor 水果香味gentle heating 微热glucose 葡萄糖infrared spectrum 红外光谱(分子只能吸收与其振动、转动频率相一致的红外线而形成特征光谱)litmus paper 石蕊试纸(检查酸碱性用)metabolism 新陈代谢miscibility with water 与水的互溶性phenolphthanlein indicator 酚酞指示剂pore 孔porous ceramic surface 多孔的陶瓷表面primary alcohol 伯醇reverse reaction 逆发应secondary alcohol 仲醇simplified equation 简化方程式tertiary alcohol 叔醇wave number 波数yeast 酵母菌Chapter 12aerosol propellant 气溶胶火箭燃料anti-inflammatory medicine 消炎药aqueous ethanol-silver nitrate 乙醇硝酸银溶液blowing agent 发泡剂bromochlorodifluoromethane (BCF) 溴氯二氟甲烷(灭火剂)circuit board 电路板combustible material 可燃物degreasing agent 除油剂electrical insulation 电绝缘材料halogenoalkanes 卤代烃ibuprofen 布洛芬, 异丁苯丙酸(解热镇痛药)ozone ‘hole’臭氧层空洞primary halogenoalkane 伯卤代烃rheumatoid arthritis 类风湿关节炎second halogenoalkane 仲卤代烃silver halide precipitate 卤化银沉淀stratosphere 平流层tertiary halogenoalkane 叔卤代烃the classification of halogenoalkanes 卤代烃的分类Part 3Chapter 13beaker 烧杯bond breaking 断键bond making 成键clamp 夹copper spiral 铜圈endothermic reaction 吸热反应(体系从环境吸收热能, 化学反应的焓变为正值) energy transfer 能量转移enthalpy changes by different routes 不同途径的焓变enthalpy cycleexothermic reaction 放热反应(体系放热给环境, 化学反应的焓变为负值)first law of thermodynamics 热力学第一定律(本质是能量守恒定律)flame calorimeter 火焰量热计graph extrapolated backwards to starting time 反推到开始时间的曲线图Haber process 哈伯合成氨法heat capacity 热容(当一系统由于加给一微小的热量δQ而温度升高δT时, Δq/δT这个量即是热容)heating-insulated vessel 隔热容器Hess’law 赫斯定律(一个化学反应的热销应决定于其始终态, 与中间过程无关) joule 焦耳〔能量和功的单位〕law of conservation of energy 能量守恒定律(在任一封闭系统中总能量保持不变) metal calorimeter 金属量热计negative value (-) 负值Pascal 帕斯卡(压强单位)perpetual motion 永恒运动polystyrene cup 聚苯乙烯杯positive value 正值reaction pathway 反应途径release large quantities energy 释放大量能量screw 螺旋桨shield 护板specific heat capacity of water 水的比热容spectator ionstandard enthalpy change of combustion 标准摩尔燃烧焓standard enthalpy change of formation 标准摩尔生成焓standard enthalpy change of reaction 标准反应焓变(标准状态下反应的焓变) standard enthalpy changes: standard conditions 标准焓变:标准状态stirrer 搅拌器suction pump 真空泵, 抽水机vacuum flask 真空烧瓶wick 灯芯Chapter 14reaction rates 反应速率acidity 酸性, 酸度adsorb 吸附aldehyde 乙醛at normal temperatures and pressures 在常温常压下basicity 碱度;碱性Bung 塞camphor 樟脑catalytic converter 催化转化器celluloid 赛璐珞(明胶)chemical analysis 化学分析chemical kinetics 化学动力学colorimeter 色度计color intensity 色度concentration of reactants 反应物浓度constant random motion 永恒的无规则运动desorbe 解吸entropy 熵(热力学状态函数, 用于量度系统无序度, 等于吸收之热与吸热时绝对温度之商)esterification 酯化exhaust gases 排放气体factors that affect the rate of a reaction 影响反应速率的因素gas syringe 气体注射器glass delivery tube 玻璃导管heterogeneous catalysis 多相催化(催化剂与反应物处于不同相如在固体和流体相界面间发生催化作用)homogeneous catalysis 均相催化(催化剂与反应物在同相中反应)intensity of the radiation 照射的强度inverted, water-filled burette 倒置的装满水的量管latitude 纬度low-energy collisions 低能量碰撞nitrocellulose 硝化纤维素nitroglycerine 硝化甘油oxyacetylene torch 氧乙炔火炬peroxyacetyl nitrate (PAN) 硝酸过氧化乙酰ppb 十亿分之一(10-10)ppm 百万分之一(10-6)pressure sensor 压力感受器rate determining step 决定反应速率的步骤removal 去除Ribenascanning probe microscopy (SPM) 扫描显微探针sealed container 密闭容器self-sustained 自持的, 自我保持的spectrophotometer 分光光度计surface area 表面积temperature sensor 温感器(能对温度变化作出反应)the asymmetric shape of the curve 曲线的不对称形状the Boltzmann distribution 玻耳兹曼分布the collision theory of reactivity 碰撞理论(化学反应速率等于反应物分子间的碰撞数乘以有效碰撞因子)Timer 计时器Chapter 15base 碱closed system 封闭系统constancy of macroscopic propertiescotton wool 脱脂棉dynamic equilibrium 动态平衡(在一定条件下德可逆反应里, 正反应和逆反应德速率相等, 反应混合物中各组成成分德含量保持不变)fertility 肥(沃) 度forwards direction 正方向irreversible one-way reaction 不可逆单向反应keep the pressure constant 保持恒压Le Chatelier’s principle 勒沙特列原理(如果改变影响平衡的一个条件如浓度、压强或温度等, 平衡就向能够减弱这种改变的方向移动)macroscopic propertiesnail varnish remover 洗甲油Ostwald process 奥斯特瓦尔德法(制硝酸, 采用高温铂网催化剂, 将氨氧化为氧化氮, 经水吸收成硝酸)porous iron 多孔的铁reaction vessel 反应容器reverse direction 反方向reversible reaction 可逆反应strong acid 强酸the equilibrium shifts to minimize this increase 平衡就向能够减弱这种改变的方向移动weak acid 弱酸。





![[转载]【原文翻译】KESHE《光的结构》](https://img.taocdn.com/s1/m/67b51951e418964bcf84b9d528ea81c758f52e22.png)
[转载]【原文翻译】KESHE《光的结构》Chapter 9第9章The Unifying Field Theory统一场论M T Keshe 2000-2009, all rights reserved.M T Keshe 2000-2009,版权所有。
Date of release: 28.10.2009, revised 2012.发布日期:2009.10.28,2012年修订Abstract摘要In this paper the origin of electromagnetic fields or electromagnetism and how they are created within the existence of other magnetic fields are simply explained.本文简要地解释了电子磁场(electromagnetic fields)或电子磁(electromagnetism)的本源以及它们如何在其它磁场的存在中产生。
Discussion论述The creation of a magnetic field and its interaction with physical matter or other fields always leads to release of electromagnetic fields or energy equal to the energy of an electron.一个磁场的产生以及它与有形物质或其它场的相互作用总是会导致电子磁场(electromagnetic fields)或者说等同于一个电子的能量的释放。
In the universe, in environments where there are no mattersfor magnetic fields to interact with for electromagnetism to be created or to be released, electromagnetic fields do still exist and function.在宇宙中,在没有物质可以与磁场相互作用从而产生和释放电子磁场(electromagnetism)的环境中,依然有电子磁场(electromagnetic fields)存在并运作。





chapter 1 atomic structureelement n.元素all know materials can be broken down into fundamental substances we call element. 我们所知道的所有物质都可以分解成原子。
atom n.原子atom is the smallest particle of matter having all that element’s characteristics.原子时具有元素性质的最小粒子。
nucleus /’nju:kli?s,’nu?kli?s/ 原子核electron n.电子proton 质子neutron 中子compound n. 化合物:When two or more elements combine and form a compound, a chemical change takes place.当两种或两种以上的元素结合形成化合物时, 发生化学变化。
化学中的物质分为单质和化合物,大部分元素是以化合物的形式存在的。
ion n. 离子:when an atom get or lost elections,it becomes ion.原子得失电子后形成离子。
cathode n. 阴极(negative electrode)Cathode rays are attracted by a positive charge.阴极射线被阳电荷所吸引。
anode n. 阳极(positive election)A red wire is often attached to the anode.红色电线通常与阳极相联。
particle n. 粒子:微小粒子包Particles include moleculars,atoms , protons, neutrons ,electrons and ions.括分子,原子,质子,中子,电子,离子等等。



第七章 原子结构和元素周期律Chapter 7 The Atomic Structure and Periodic System of Elements这一章中,我们介绍物质的微观结构──原子结构。
化学工作者总是希望通过对物质本质的认识,来阐明元素相互化合的原理,把化学事实系统化,使化学成为可以理解的、容易加以记忆的学科。
人们利用这些原理来预言具有新功能的化合物的诞生。
例如科学家利用等电子原理(the isoelectronic principle )合成新的化合物:In 1971, the following isoelectronic compounds were known :Ni(CO)4、Co(CO)3(NO)、Fe(CO)2(NO)2、and Mn(CO)(NO)3. The last member of this series, Cr(NO)4, was unknown. However, in 1972, several chemists had sufficient faith in the isoelectronic principle to photolyze a solution of Cr(CO)6 in the presence of NO ,and thus they prepared.For many years chemists were unsuccessfully tried to prepare the perbromate ion, 4BrO -. The first successful synthesis of perbromate involved an isoelectronic species as the starting material.8324SeO -834BrO β--+ 因此,学习近代化学知识,从原子内部入手是完全必要的。
我们所关心的原子内部,对于元素及化合物的性质而言,主要集中在原子的电子结构(electronic structure of atoms ),特别是它们的价电子构型(valence electronic structure of atoms )。