管理体系手册-英文版.doc
- 格式:doc
- 大小:459.00 KB
- 文档页数:45

医疗器械质量管理体系核查资料英文版Title: Documents for Inspection of Medical Device Quality Management SystemIntroduction:A robust and effective quality management system is crucial for the manufacturing and distribution of medical devices. To ensure compliance with regulatory standards and maintain the highest quality standards, regular inspections and audits of the quality management system are essential. This article presents an overview of the documents required for the inspection of a medical device quality management system.1. Quality Manual:The quality manual serves as the foundation of the quality management system. It outlines the organization's quality policy, objectives, and responsibilities. During the inspection, the conformity of the quality manual with relevant regulations and standards will be evaluated.2. Standard Operating Procedures (SOPs):SOPs provide detailed instructions on processes and activities within the quality management system. These procedures cover various aspects, including design control, purchasing, manufacturing, packaging, labeling, storage, anddistribution. Inspectors will review the SOPs to ensure their compliance with applicable regulations and their effectiveness in achieving quality objectives.3. Risk Management Documents:Medical device manufacturers must have a robust risk management process in place. Documentation related to risk management, such as risk management plans, risk assessments, and risk control measures, should be available for inspection. The inspector will evaluate the adequacy of the risk management process and its integration throughout the quality management system.4. Corrective and Preventive Actions (CAPA):CAPA procedures are essential for identifying, investigating, and resolving problems that may arise during the manufacturing or distribution of medical devices. The inspection will involve reviewing the CAPA documentation, including records of non-conformities, root cause analyses, corrective actions, and preventive measures. The effectiveness of the CAPA system will be assessed to ensure continuous improvement.5. Training Records:Proper training ensures that personnel involved in thequality management system are competent and capable of performing their assigned tasks. Inspection of training records will determine if employees have received sufficient training on quality procedures, regulations, and any specific device-related requirements.6. Internal and External Audit Reports:Internal and external audits play a critical role in identifying potential issues and areas for improvement within the quality management system. The inspection will involve reviewing audit reports to assess the thoroughness of the audits, the identification of non-conformities, and the implementation and effectiveness of corrective actions.Conclusion:The inspection of a medical device quality management system requires a comprehensive review of various documents. These documents provide evidence of compliance with regulatory standards and the effectiveness of the system in ensuring the production and distribution of safe and effective medical devices. By meticulously assessing these documents, regulatory bodies can ensure that manufacturers maintain the highest quality standards and protect the well-being of patients.。

SA8000-2023修订版(中文版和英文版)
概述
SA8000是一种社会责任标准,旨在确保企业在管理、劳动条
件和对员工的权益方面达到一定的最低标准。
本文档介绍了
SA8000-2023修订版的内容和变化。
主要变化
1. 管理体系要求
- 强调企业管理层的责任,包括领导力和承诺。
- 要求企业建立有效的沟通渠道,使员工能够表达意见和关切。
- 强调在供应链管理中的交流和合作。
2. 劳动条件要求
- 强化对雇佣合同的监督和执行,确保员工享有合理的工作条
件和权益。
- 要求提供合理的工作时间和休息时间。
- 鼓励培训和职业发展机会,提高员工的技能和素质。
3. 工资和福利要求
- 规定必须支付合理的工资,以满足员工的基本需求。
- 要求提供福利措施,如医疗保险和退休金计划。
4. 儿童劳动和强制劳动禁止
- 禁止雇用未满足法定工作年龄要求的儿童。
- 禁止强制劳动和任何形式的人身剥削。
5. 员工权益和福利
- 强调员工享有自由结社和集体谈判的权利。
- 要求提供安全和健康的工作环境,预防事故和职业病发生。
- 保护员工的隐私和个人信息。
结论
SA8000-2023修订版的目标是促进企业社会责任的履行,保护员工权益,改善劳动条件。
通过遵守这一标准,企业可以建立良好的声誉,提高业务的可持续性和竞争力。

世界500强名企的KPI绩效管理操作手册——精华版KPI(Key Performance Indication)即关键业绩指标,是通过对组织内部某一流程的输入端、输出端的关键参数进行设置、取样、计算、分析,衡量流程绩效的一种目标式量化管理指标,是把企业的战略目标分解为可运作的远景目标的工具,是企业绩效管理系统的基础。
KPI是现代企业中受到普遍重视的业绩考评方法。
KPI可以使部门主管明确部门的主要责任,并以此为基础,明确部门人员的业绩衡量指标,使业绩考评建立在量化的基础之上。
建立明确的切实可行的KPI 指标体系是做好绩效管理的关键。
KPI法符合一个重要的管理原理--“二八原理”。
在一个企业的价值创造过程中,在在着“20/80”的规律,即20%的骨干人员创造企业80%的价值;而且在每一位员工身上“二八原理”同样适用,即80%的工作任务是由20%的关键行为完成的。
因此,必须抓住20%的关键行为,对之进行分析和衡量,这样就能抓住业绩评价的重心。
一、建立关键业绩指标体系遵循的原则1、目标导向。
即KPI必须依据企业目标、部门目标、职务目标等来进行确定。
2、注重工作质量。
因工作质量是企业竞争力的核心,但又难以衡量,因此,对工作质量建立指标进行控制特别重要。
3、可操作性。
关键业绩指标必须从技术上保证指标的可操作性,对每一指标都必须给予明确的定义,建立完善的信息收集渠道。
4、强调输入和输出过程的控制。
设立KPI指标,要优先考虑流程的输入和输出状况,将两者之间的过程视为一个整体,进行端点控制。
二、确立KPI指标应把握的要点1、把个人和部门的目标与公司的整体战略目标联系起来。
以全局的观念来思考问题。
2、指标一般应当比较稳定,即如果业务流程基本未变,则关键指标的项目也不应有较大的变动。
3、指标应该可控制,可以达到。
4、关键指标应当简单明了,容易被执行这所接受和理解。
5、对关键业绩指标要进行规范定义,可以对每一KPI指标建立“KPI定义指标表”。

ISO9001程序文件-中英文1.0目的:为确保质量管理体系持续有效运行,使其充分符合ISO9001:2000标准的要求,特制定本程序,以规定开展相应的审核活动,来评价本厂质量管理体系是否有效,是否需要采取纠正及预防措施。
Purpose: In order to ensure the effective and continuous implementation of the quality management, fully meet the requirements of ISO9001:2000 standard, specially make the procedure so as to implement audit action and verify the effectiveness of the quality management system and to take corrective or preventive action if needed.2.0 范围:本厂所开展的内部质量审核的全部活动均适用本程序。
Scope: applies to all the actions relating to internal quality audit.3.0 职责 Responsibility3.1 管理者代表:负责年度内审计划的拟定并计划组织实施。
Management Representative: responsible for the annual internal audit plan and its implementation.3.2 内审组长:负责制定审核日程并具体组织、指导内审作业。
Internal audit leader: responsible for making audi agenda and instructing audit.3.3 内审员:负责按审核计划要求对相关单位实施审核。

**********有限公司企业标准QM/YS-2016质量手册按ISO9001:2015要求编制版本号: A/0受控号:2016-5-1发布 2016-5-1实施**********有限公司发布修订记录目录批准令本《质量手册》是依据ISO9001:2015质量管理体系标准要求,结合本公司产品生产特点、生产规模和体制实际情况,为确保和提高产品质量,健全质量管理体系而编制。
本手册规定了本公司的质量方针和目标,对产品实现过程的持续改进、质量管理体系的有效运行规定了准则和方法。
本手册是本公司质量管理体系运行开展各项质量活动的指导性文件、法规性文件,现予以发布。
本公司全体员工务必认真学习,严格遵照执行,确保本手册得以认真有效的实施。
本手册于二○一六年五月一日起正式实施。
凡于本手册不一致的质量文件一律以本手册为准。
总经理: ***二○一六年五月一日管理者代表任命书为了便于公司ISO9001质量管理体系的有效推行,由总经理任命***先生为本公司管理者代表,其职责和权限为:1、负责按ISO9001标准建立保持并经济有效地实施文件化质量体系,领导各职能部门开展质量活动;2、负责方针目标管理,及时向总经理汇报质量管理体系运行情况,负责质量管理体系内部审核的组织领导工作,并提供质量体系改进的依据和建议;3、负责组织贯彻实施企业经营管理决策、目标方针,完善各项管理制度,不断提高公司管理水平;4、领导内部质量审核活动,协调解决质量管理体系运行中的不一致等问题;5、负责做好对过程的监视和测量及数据分析的领导控制工作;6、负责质量管理体系有关事宜的外部联络工作;7、负责提高公司员工文化、生活水平,营造良好的作业环境和安全舒适的生活环境;8、负责公司重大纠正/预防措施的审批和组织实施。
总经理:***本公司宗旨:品质稳定----我们成功的基石公司的质量方针:开拓进取,群策群力;持续精进,客户满意。
释义:1、在当前的市场竞争中,保持质量管理体系运行的持续有效性是企业承诺的主题,其根本目的在于为社会和顾客提供满足要求的产品。
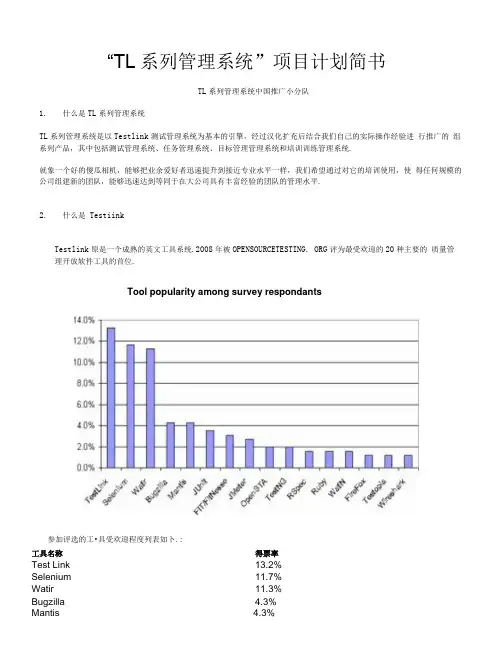
“TL系列管理系统”项目计划简书TL系列管理系统中国推广小分队1.什么是TL系列管理系统TL系列管理系统是以Testlink测试管理系统为基本的引擎,经过汉化扩充后结合我们自己的实际操作经验进行推广的组系列产品,其中包括测试管理系统、任务管理系统、目标管理管理系统和培训训练管理系统.就象一个好的傻瓜相机,能够把业余爱好者迅速提升到接近专业水平一样,我们希望通过对它的培训使用,使得任何规模的公司组建新的团队,能够迅速达到等同于在大公司具有丰富经验的团队的管理水平.2.什么是TestiinkTestlink原是一个成熟的英文工具系统.2008年被OPENSOURCETESTING. ORG评为最受欢迎的20种主要的质量管理开放软件工具的首位.Tool popularity among survey respondants参加评选的工•具受欢迎程度列表如卜.:工具名称得票率Test Link13.2%Selenium11.7%Watir11.3%Bugzilla 4.3%Mantis 4.3%JUnit 3.5% FIT/FitNesse 3.1% JMeter 2.7% OpenSTA 1.9% Test NG 1.9% RSpec 1.6% Ruby 1.6% WatiN 1.6% Fire Fox 1.2% Testopia 1.2% Wireshark 1.2% Autolt 0.8% Canoo Web Test 0.8% easyb 0.8% Emma 0.8% Findbug 0.8% jsUnit 0.8% Marathon 0.8% PHPUnit 0.8% RTH 0.8% Web Inject 0.8% Web LOAD 0.8% WET 0.8% All other xUnits - too many to list 0.4% allpairs 0.4% Android 0.4% bugzillametrics 0.4% buildbot 0.4% checkstyle 0.4% Cobertura 0.4% Concordion 0.4% cppunit 0.4% cruise control 0.4% CubicTest 0.4% cunit 0.4% Dogtail 0.4% dotproject 0.4% Easy Mock 0.4% Eclipse 0.4% Eventum 0.4% executequery 0.4% figleaf 0.4% FireBug 0.4% FireWatir 0.4% Grinder 0.4% HL7Test Harness 0.4% htmlunit 0.4% Hudson 0.4% hyperic 0.4%jenny 0.4%JRuby 0.4%jwebunit 0.4%Lou Wilson's VBA/Excel Web Test Automation Tool 0.4% nHapi 0.4%nose 0.4%NotePad+ + 0.4%NUnit 0.4%Paros 0.4%Perl 0.4%Perl WWW, Mechanize 0.4%perlclip 0.4%perl-Win32GuiTest 0.4%PHP 0.4%pmd 0.4%Pylot 0.4%Python 0.4%QaTraq 0.4%Ruby WebBench 0.4%Sahi 0.4%SalomeTMF 0.4%SAMIE 0.4%SandCastle 0.4%Seagul 0.4%simpletest 0.4%SoapUl 0.4%STAF 0.4%STAF & STAX 0.4%SymbianOS Unit test 0.4%TestComplete 0.4%The Grinder 0.4%twill 0.4%Unitils 0.4%utPLSQL 0.4%Watij 0.4%xUnit 0.4%Testlink已经作为开发和测试技术团队的管理工具在国外数I•家大公司使用,其中世界知名品牌的公司有:INTELYAHOOCOMCASTQUANTUM McAfeeExpediaTestlink是一种专门用来为产品测试管理的团队协作工具.团队成员对一个产品或者系统进行质量测试管理的时候,对于所有做出来的产品需求功能检验、测试案例设计、测试任务分配和管理质量跟踪等全部能够用这个系统来记录管理.由于Testlink具有基于网络的优势,成员能够在不同地点实现共同协作.它的使用,强制性地把产品质量测试这样一个经验性很强的活动,变成了一项有明确要求指导的,有清晰任务分工的,有便捷报表管理的,可以组织管理很多团队成员参加的,能够追踪历史性和机械性的操作.把Testlink和一个具有丰富经验的测试设计师的具体产品知识相结合,能够把一个经验不是很丰富的新团队,迅速提高到等同于大公司里具有丰富经验团队的水平.对于需要团队协作开发和测试的产品制造公司,培训队伍熟练使用这个工具,是一种最具高回报的管理投资。

最新版本可持续发展- HSF-环境管理体系手册简介本手册汇集了最新版本的可持续发展的环境管理体系,通常简称为HSF(HuiShengFeng)-环境管理体系手册。
该手册旨在帮助组织实施和维护可持续发展的环境管理体系,以促进环境保护和资源可持续利用。
目标本手册的目标是实现以下几点:- 确保组织遵守相关环境法规和标准;- 审视组织的环境问题,并采取必要的措施予以改进;- 减少环境污染和资源浪费;- 提高员工和利益相关方对可持续发展的认识和参与度;- 促进组织在市场中的可持续发展竞争力。
内容概要本手册的内容包括以下几个方面:1. 环境管理体系要求介绍了建立和维护HSF环境管理体系的要求,包括确定环境政策、制定目标和计划、执行措施和监控等。
2. 环境法规和标准列举了与环境保护相关的法律法规和标准,包括国家和地方政府制定的环境保护相关法律法规和国际标准。
3. 环境评估和管理介绍了环境评估和管理的基本原理和方法,包括环境影响评估、环境风险评估和环境管理计划的制定等。
4. 环境监测与报告说明了环境监测的目的和方法,包括环境指标的监测和数据采集、环境报告的编制和提交等。
5. 环境培训和宣传介绍了组织内部环境培训的重要性和方法,包括员工培训计划的制定和宣传策略的设计等。
6. 持续改进强调了持续改进的重要性,并提供了改进措施的选定和实施方法。
7. 文档控制和审核说明了文档控制和审核的要求,包括文档的编制、审核和审查流程等。
结论HSF-环境管理体系手册是一个有效的工具,可以帮助组织建立和维护可持续发展的环境管理体系。
通过遵守相关法规和标准,实施环境管理措施,持续改进和加强环境培训,组织可以实现环境保护和资源可持续利用的目标,提高自身在市场中的竞争力。

文件管理与控制程序Documents management and control procedure1.目的Purpose确保环境管理体系文件、适用的外来文件(有关的法律、法规、标准、相关方提供的文件或规范)使用的有效性。
To assure the2.适用范围 scope适用于对环境管理体系相关文件及适用的外来文件的控制。
It is apply to theenvironment management system relation documents and usable external documents’ control.3.职责Responsibility3.1 环工组:负责环境管理体系文件及适用的外来文件的归口管理;负责监控文件的执行。
The environment team: to manage and be in charge of environment management documents and external documents.3.2 各部门individual department:确保各相关场所均使用现行文件的有效版本。
To ensureThe relative workplace is using the current effective documents.4.工作程序Work procedure4.1文件控制范围包括:documents control’s scopea)环境管理手册;environment management manual;b)环境管理体系程序文件;environment management system procedure documents;c)环境管理体系作业指导文件;the work instruction documents for environment management system;d)环境记录表格;environment record form;e)外来文件。

ISO9001:2000中英文对照Quality management systems — Requirements质量管理体系——要求1 Scope1 范围1.1 General1.1 总则This International Standard specifies requirements for a quality management system where an organization本标准为有下列需求的组织规定了质量管理体系要求:a) needs to demonstrate its ability to consistently provide product that meets customer and applicable regulatory requirements, and需要证实其有能力稳定地提供满足顾客和适用的法律法规要求的产品;b) aims to enhance customer satisfaction through the effective application of the system, including processes for continual improvement of the system and the assurance of conformity to customer and applicable regulatory requirements.通过体系的有效应用,包括体系持续改进的过程以及保证符合顾客与适用的法律法规要求,旨在增进顾客满意。
NOTE In this International Standard, the term “product”applies only to the product intended for, or required by, a customer.注:在本标准中,术语“产品”仅适用于预期提供给顾客或顾客所要求的产品。


质量和食品安全管理体系方针颁布令
***食品有限公司各部门、全体员工应按以下方针要求开展质量和食品安全管理体系方面的工作:
科学管理、安全卫生,顾客至上、持续改进
本方针与公司总体经营宗旨相适应、协调,它是公司经营方针的重要组成部分。
体现了公司对质量的核心追求,体现了公司满足要求和持续改进的承诺,同时为制定质量和食品安全目标提供了框架。
全体员工应深入理解质量和食品安全方针的内涵并落实到工作实践中去,为提高公司质量和食品安全水平而努力。
为确保质量方针的贯彻和实施,公司制定的质量和食品安全目标:
○1合同履行率达到100%;
○2顾客满意度98%以上;
○3出厂产品卫生指标合格率达到98%;
○4生产、环境卫生指标合格率达到99%以上。
总经理(签名):
日期:
任命书
公司各部门、全体员工:
为实现公司质量和食品安全方针、目标,确保公司质量和食品安全管理体系的过程得到建立、实施、保持和持续改进,任命***为本公司质量和食品安全管理小组组长(管理者代表)。
除其原职位的职责外,作为管理者代表赋予其主要职责是:
●负责按ISO9001:2017和ISO22000:2005标准的要求建
立、实施、保持,改进质量和食品安全管理体系;
●向总经理报告质量和食品安全管理体系的运行情况和改
进的需求;
●在整个公司内促进质量意识的形成;
●领导食品安全小组开展工作,并为其成员安排相关的培
训和教育;
●做好内部审核的组织工作,协助总经理做好管理评审;
●就质量和食品安全管理体系的有关事宜与外部联络。
总经理(签名):
日期:。
※※目錄※※※※0.0修订履历※※1.0颁布令本《EHS管理手册》由公司总经理组织相关部门,依据GB/ T24001:2004(idt ISO14001:2004)及GB/T18001:2011 (idt OHSAS18001:2007)标准要求,结合本公司实际情况编制而成,经认真审核,现予颁布实施。
《EHS管理手册》,内容包括:a)EHS方针;b)公司EHS管理体系所形成的程序或对其引用;c)EHS管理体系过程之间的顺序和相互作用的表述;本《EHS管理手册》从颁布之日起,要求公司各部门、全体员工严格贯彻执行!总经理:日期:年月日2.0 EHS管理者代表任命书为贯彻执行ISO14001《环境管理体系一规范及使用指南》和OHSAS18001《职业安全卫生管理体系—规范》,为了在本公司有效建立实施和保持一个完善的EHS管理体系,特任命薛春天先生为公司EHS管理者代表,并担当如下职责权限:a).确保按照ISO14001《环境管理体系一规范使用指南》和OHSAS18001《职业安全卫生管理体系—规范》的要求建立、实施与保持EHS管理体系;b).向最高管理者汇报EHS管理体系的运行情况;c).为EHS管理体系的改进提供依据;d).负责EHS管理体系有关事宜外部联络。
总经理:日期:年月日3.0 EHS管理方针1.环境体系方针:遵守环境法服务于社会改善持续上节约降消耗全员共参与齐建绿色厂2.职业健康与安全管理方针强化安全管理,提高安全意识;逐级落实责任,做好事前预防;遵守安全法规,齐建安全企业总经理:日期:年月日4.0 EHS管理体系4.1 总要求4.1.1 建立并保持EHS管理体系,确定文件化的EHS方针、目标和指标,通过EHS因素的识别与评价的结果,制订出EHS管理方案和运行控制程序并有效实施,以达到不断提高EHS绩效的目的。
4.1.2本公司EHS管理体系包含公司生产及服务全过程。
4.1.3本手册是公司EHS管理体系运行的依据,是EHS管理体系评审以及第三方对EHS管理体系认证的体系文件。
ISO22000-2018食品安全管理体系管理手册及程序文件全套资料ISO22000-2018食品安全管理体系管理手册及程序文件全套资料22000认证文件清单22000体系文件框架:1. 食品安全管理手册2. 程序文件3. 质量计划3.1. 前提方案(GMP、SSOP)3.2 HACCP计划4. 作业指导书4.1 操作规程、规章制度4.2 各种记录表格5. 支持性材料6. 其他材料(资质性证明材料等)一、食品安全管理(质量)手册总体来说,手册按照ISO22000-2018标准框架进行编写正文前(手册目录前)需要有:1. 手册批准令(手册使用的说明)2. 手册的修改记录3. 企业概况4. 食品安全小组组长任命书手册正文内应含有:1. 食品安全方针目标2. 组织机构框图及各部门的职责3. 其他,如适用范围、引用标准、术语及定义二、程序文件ISO22000-2018标准中要求形成文件的程序有:1. 文件控制2. 记录控制3. 操作性前提方案(也可以是知道书或几计划的形式)4. 处置受不合格影响的产品5. 纠正措施6. 纠正7. 潜在不安全产品的处置8. 召回9. 内部审核10.风险与机遇的识别应对11.组织环境此外,操作性前提方案需要形成程序文件的地方很多,我们可以对照GMP和SSOP中的相关条款,结合本企业的实际情况来确定需要形成文件的相关程序。
三、前提方案(PRPS方案、SSM方案)按照ISO22000标准中规定的11项或提供以下文件:1. GMP2. SSOP此外,还要提供:建立并有效实施产品的标识、追溯和回收计划:建立并有效实施加工设备与设施的预防性维护保养程序建立并有效实施教育与培训计划文件资料的控制实验室管理手册或实验室管理制度汇编加工工艺控制等除后两项外,前四项可以列入程序文件之中。
四、HACCP计划1. 产品描述2. 原料、辅料以及内包装材料的描述3. (生产加工)工艺流程图4. (生产加工)工艺过程说明5. 危害分析表6. HACCP计划表7. CCP点的监控程序也可以怎加CCP点的纠偏控制程序、验证程序五、HACCP计划支持性材料1. 危害分析的技术资料2. CL值得确定依据3. 生产加工工艺流程图的确认4. 控制措施组合的确认5. 各种图纸6. 与企业产品有关的法律法规、文件清单7. 与企业产品有关的标准六、三级文件1. 作业指导书、操作规程、规章制度等文件编号及清单2. 记录表格编号及清单七、资质性证明材料八、食品安全体系运行记录1. CCP点监控记录2. CCP点的纠偏记录3. 不合格控制记录4. 产品的可追溯性记录5. 内部审核记录6. 管理评审记录7. 产品召回演练X X X食品有限公司QM/ABC2018-01ISO22000:2018食品安全管理手册A 版编制:年月日审核:年月日批准:年月日受控状态:分发号:2018-08-24发布 2018-09-01 实施XXX食品有限公司0.1目录0封面0.1目录0.2颁布令0.3质量方针和质量目标的声明0.4公司简介0.5任命书1范围2规范性引用文件3术语和定义4 组织的环境4.1理解组织及其环境4.2理解相关方的需求和期望4.3确定食品安全管理体系的范围4.4食品安全管理体系5 领导作用5.1领导作用和承诺5.2食品安全方针5.3 组织的岗位、职责和权限6 策划6.1应对风险和机遇的措施6.2食品安全目标及其实现的策划6.3变更的策划7 支持7.1资源7.1.1总则7.1.2人员7.1.3基础设施7.1.4工作环境7.1.5外部开发食品安全管理体系要素的控制7.1.6 外部提供过程、产品和服务的控制7.2能力7.3意识7.4沟通7.4.1总则7.4.2外部沟通7.4.3内部沟通7.5成文信息8运行8.1运行策划和控制8.2前提方案8.3可追溯性8.4应急准备和响应8.4.1总则8.4.2紧急情况和事故的处理8.5危害控制8.5.1危害分析预备步骤8.5.2危害分析8.5.3控制措施和控制措施组合的确认8.5.4危害控制计划(HACCP计划/OPRP计划)8.6前提方案PRPs和危害控制计划信息更新8.7监视和测量的控制8.8前提方案PRPs和危害控制计划的验证8.8.1验证8.8.2验证活动结果的分析8.9不符合产品和过程的控制8.9.1总则8.9.2纠正措施8.9.3纠正8.9.4潜在不安全产品的处理8.9.5撤回/召回9食品安全管理体系绩效评价9.1监视、测量、分析和评价9.1.1总则9.1.2分析和评价9.2内部审核9.3管理评审10改进10.1不符合和纠正措施10.2食品安全管理体系更新10.3持续改进11附件11.1 CCP判断树11.2程序文件清单11.3 职能分配表11.4组织机构图0.2颁令布本公司按照ISO/DIS 22000:2018《食品安全管理体系食品链中各类组织的要求》标准编制成食品安全管理手册。
质量/环境/职业健康安全QESH综合管理手册依据GB/T 19001-2016 / ISO 9001:2015GB/T 24001-2016 / ISO14001:2015OHSAS18001:2007编制文件编号:YD-A-001版次:01编制:日期:审核:日期:批准:日期:01 目录01 目录01 目录0.2 修订履历0.3 颁布令为了适应公司的发展需要,满足社会及法律法规要求,在总结改进质量、环境、职业健康安全管理体系的基础上,公司依据《GB/T 19001-2016 / ISO 9001:2015质量管理体系要求》、《GB/T 24001-2016 / ISO14001:2015环境管理体系要求》、《OHSAS18001:2007职业健康安全管理体系要求》的要求,重新编制了公司第二版《QESH综合管理手册》(以下简称:手册)现予以批准颁布实施。
手册阐明了公司的质量、环境、职业健康安全方针和目标,对公司的管理体系做了具体描述,是指导公司实施管理体系的纲领和行为准则。
质量、环境、职业健康安全是企业的生命,其管理关系企业的效益和环境的可持续发展,质量、环境、职业健康安全管理体系是确保产品质量和环境保护的基础。
本手册既是向顾客和认证机构提供质量、环境、职业健康安全保证能力的证据,也是本公司管理者向顾客和相关方做出的质量、环境、职业健康安全的承诺。
在公司内部,它是本公司的质量、环境、职业健康安全管理法规,全体员工必须按手册的要求开展质量、环境、职业健康安全活动,向顾客提供满意的产品和服务;保护环境,减少污染。
公司要求全体员工认真学习手册文件,并严格按手册规定的要求从事各项质量、环境、职业健康安全管理活动;确保本公司的质量、环境、职业健康安全有效运行,并得到持续改进。
本手册版权属于XXXXXXXXXXXX有限公司所有,其它单位不许复制。
本手册自2017年9月6日起正式实施,自实施之日起公司全体员工必须遵照执行。
中华人民共和国国家标准质量管理体系——要求Quality management systems —Requirements1范围1.1总则本标准为同时有下列需求的组织规定了质量管理体系要求:a) 需要证实其有能力稳定地提供满足顾客和适用的法律法规要求的产品;b) 通过体系的有效应用,包括持续改进体系的过程以及保证符合顾客与适用的法律法规要求,旨在增强顾客满意。
注:在本标准中,术语”产品”仅适用于提供的预期产品,不适用于非预期的副产品。
应用本标准规定的所有要求是通用的,意在适用于各种类型、不同规模和提供不同产品的组织。
当本标准的任何要求由于组织及其产品的特点而不适用时,可以考虑进行删减。
除非删减仅限于第7章中那些不影响组织提供满足顾客和适用法律法规要求的产品的能力或责任的要求, 否则不能声称符合本标准。
2 引用标准通过在本标准中的引用,下列标准包含了构成本标准规定的内容。
对版本明确的引用标准,该标准的增补或修订不适用。
但是,鼓励使用本标准的各方探讨使用下列标准最新版本的可能性。
GB/T 19000-2000 质量管理体系—基础和术语(idt ISO9000:2000)3术语和定义本标准采用GB/T19000-2000给出的术语和定义。
本标准描述供应链所使用的以下术语经过了更改,以反映当前的使用情况:供方组织顾客本标准中的术语“组织”用以取代GB/T19001-1994所使用的术语“供方”,术语“供方”用以取代术语“分承包方”。
本标准中所出现的术语“产品”,也可指“服务”。
4质量管理体系4.1 总要求组织应按本标准的要求建立质量管理体系,形成文件,加以实施和保持,并持续改进。
组织应:a) 识别质量管理体系所需的过程及其在组织中的应用(见1.2);b) 确定这些过程的顺序和相互作用;c) 确定为确保这些过程的有效运作和控制所需的准则和方法;d) 确保可以获得必要的资源和信息,以支持这些过程的运作和监视;e) 测量、监视和分析这些过程;f) 实施必要的措施,以实现对这些过程所策划的结果和对这些过程的持续改进。
XXXXXX有限公司XXXXXX科技有限公司XXXXXX技术有限公司QEO Management Manual (Basis of ISO9001:2008/ISO14001:2004/OHSAS18001:2007)Document No.: HM-QEOM-2015Version: D/0Compile : Distribute No :Review : Control state :Approve : Effect date : 2015 .01. 10Each department: After receiving the modification and revision of the document, the holdingThis <QEO Manual> is worked out based on ISO9001:2008, ISO14001:2004, OHSAS18001:2007, also is the programmatic document of QEO system, and is the public commitment for Customer & Social & Staff, and is the basic standard of all the employees’ behavior.This manual is a controlled document and approved & issued & implemented by General Manager. And Administrative is responsible for all the management about this manual. Nobody can show this manual to any other person without Management representative’s authorization. This manual should be returned to Administrative if the manual holder leaves company.The holder should keep the manual carefully, and shall not be damaged, lost, scribbled.The Dept. manager should summarize all advice and feedback to Administrative timely if any changes; Administrative should review the applicability and validity periodically, if necessary, should revise the manual based on relevant prevision of <Document control procedure>.Through audit, <QEO Manual > is up to the standard of ISO9001:2008(Quality management system), ISO14001:2004(Environment management system), OHSAS18001:2007(Occupational Health and Safety management system), and meets the actual situation of our company, also meets the requirement of our QEO system.This manual is issued now, hope all employees comply with it.General Manager:2015.1.10To implement the standard of ISO9001:2008, ISO14001:2004, OHSAS18001:2007, and enhance the leadership of the operation of QEO system, so company decides to appoint XXX to be the Management representative.Responsibility of Management representative:1. ensure that processes needed for the quality management system are established, implemented.2. organize & coordinate & judge all the important items on behalf of top management.3. report to top management on the performance of the quality management system and any need for improvement and solve any other problem.4. publicize and carry out relevant laws,rules,regulations,standards.Ensure the promotion of awareness of meeting customer requirements and social commitment5.liaise with external parties on matters relating to the quality management systemAppoint XXX to be employee representative.Responsibility of employee representative1.be responsible to all employees, and reflect the views and status of OHSAS’s operation to management representative or general manager.2. acquire the OHSAS’s satisfaction of employee and relevant party, communicate and advise timely.General Manager:2015.1.101.1 Policy:Quality first and treat the customer as supremacy; Full participation and continuous improvement.Safety first and precaution crucial; Law-abiding and anti-consumption.What’s said above is that providing the best quality to customers efficiently/fast/timely, though continuous improvement&anti-consumption&law-abiding.1.2 Objective:A:Customer satisfaction ≥85%B:Scarp rate ≤1%C:Reduce the consumption of resourceD:The noise and waste water will meet the standardE:Prevent and reduce the impact of environment by civilized services and beautify environment, so that meet the requirement of standardF:Safety first and precaution crucial, improve OHS’s conditions and eliminate or reduce the OHS’s risk of staff & relevant party. Completely eradicate any fire-accident or major accident, the rate should be ≤0.2% G:Observe the laws/regulations/other requirements of nation or localAlso, we are looking forward to strengthening cooperation about environment&safety with our suppliers and customers.This policy is applicable to entire company and other designated agents.Promise:We should satisfy customers’requirement by high-quality goods and best service. All employees should understand the policy and objective and requirement, and strengthen the consciousness of quality and environment safety, should strictly implement relevant regulations so that ensure the quality of goods and service.General Manager2015.1.10XXXXXX有限公司、XXXXXX科技有限公司、XXXXXX技术有限公司are located in the center of JIANGSU,ZHEJIANG,SHANGHAI, the north shore of Hangzhou Bay sea-crossing Bridge, relying on the 1-hour economic circle, and nearby SHANGHAI,HANGZHOU,SUZHOU,NINGBO and other cities. The transportation is very convenient.Haiyan Hama Hardware is created in 2002, it covers 5000 M2 and adds 33333 M2 in 2009.The company engages in the production/sale/trade of stamp parts,C type steel,spring nut, C type fastening parts,molds.The address is below:XXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXX mainly engages in engineer consulting, developing and other services, and the production/sale/trade of industrial bracket, solar energy bracket, steel structure etc.We have advanced stamp forging equipments, best management system and exquisite process to ensure the stability of product quality. We have professional design team to design new products to meet customers’needs. We are at the leading position in the peer and our products are praised by all customers which exported to Europe, Mid East, Southeast Asia and other countries.Quality, integrity, pioneering and innovative are the purposes of our company, and we adhere the policy of Quality first and treat the customer as supremacy; Full participation and continuous improvement to provide customers with the newer, more accurate and better quality products.We would like to make friends with people all over the world, let’s create a better future together.Spring NutMterial( Wire PackageChannelSaw WeldMaterial Shaping Burring Punch Galvanize Package Ship FittingMaterial Cut Saw Stamp Galvanize Package ShipWeld BurringShip1.Scope1.1Generala) This manual demonstrates the ability to consistently provide product that meets customer and applicable statutory and regulatory requirementsb)Enhance customer satisfaction and achieve environment and occupational health&safety through the effective application of the system, including processes for continual improvement of the system and the assurance of conformity to customerc)Accord with the policy which is declared through the effective implementation and improve the system, and show the conformity to outside.1.2 QEO Manual is the programmatic document of the management system, it clarifies as follows:a) the scope of system, including any excisions and the rationalityb)including all the processes and cites that work out the documents required by management systemc)the interaction description among the processes of the QEO management system1.3Applicable scopea)Quality system: All the management behavior and process in productionb)Environment system: All the management behavior and process in production located No.388 North Zhenzhong Road,Shendang Haiyanc) All the management behavior and process in production located No.388 North Zhenzhong Road,Shendang Haiyan1.4ExcisionThe products are based on the enterprise standard: Q/YHM01-2013(Q330424J13.1188-2013)and Q/YHM02-2013(Q330424J13.1406-2013), and it doesn’t involve the design and development of new products. But it doesn’t affect the company to provide products to customer, so slash the article of 7.3ISO9000:2005 《Quality management systems Fundamentals and vocabulary》ISO9001:2008 《Quality management systems Requirement》ISO14001:2004 《Environmental management systems Requirements with guidance for use》ISO14004:2004 《Environmental management systems General guidelines on principles, systems and supporting techniques》OHSAS18001:2007 《Occupational health and safety management systems Requirements》ISO19011:2011 《And (or) guidelines for quality and environmental management system auditing》All the terms and definitions are based on the standard of ISO9001:2008, ISO14001:2004, OHSAS18001:2007.3.1 QEO management system: Short for Quality, Environment, Occupational health and safety.3.2 Continual improvement: The organization shall continually improve the suitability, and effectiveness of the environmental management system based on the policy to enhance environmental performance3.3 Environment: Surroundings in which an organization operates including air, water, land, natural resources, flora, fauna, humans and their interrelations3.4 Environment aspect:Element of an organization's activities or products or services that interacts or can interact with the environment3.5 Environment impact: Change to the environment, whether adverse or beneficial, wholly or partially resulting from an organization's environmental aspects3.6 Significant environment aspect: The aspect can or maybe result from an important environmental impact3.7 Objective: Objective set by the organization consistent with the environmental policy/in terms of OH&S performance, that an organization sets itself to achieve, Objective should be quantified wherever practicable.3.8 Environment performance: performance related to the management of environmental aspects3.9 Environment policy: intentions and direction of an organization related to environmental performance, it provides the scheme for the establishment of behavior and objective and target.3.10 Environment target: Come from environment objective, or the detail requirement to achieve the environment obj ective, it’s applicable to whole organization or partly,target should be quantified wherever practicable.3.11 Interested party: person or organization that can affect, be affected by, or perceive itself to be affected by a decision or activity3.12 Prevention of pollution:reduce or control any type of pollutant, use of processes , practices, materials, products,can include process changes, efficient use of resources, material and energy substitution, recycling.3.13 Incident: events in which in injury or ill health or fatality occurred, or could have occurred.3.14 Hazard: source, situation, or act with potential for harm in terms of human injury or ill health, or a combination of these3.15 Major hazard: the hazard cant be accept or is prohibited after identification3.16 Nonconformity: can be any deviation from relevant work standards, practices, procedures, legal requirements etc. Directly or indirectly result from in injury, property loss etc.3.17 Risk: combination of the likelihood of an occurrence of a hazardous event3.18 Risk assessment: process of evaluating the risk and deciding whether or not the risk is acceptable. 3.19 Safety: eliminate the situation of unacceptable risk3.20 Ill health: Diseases caused by exposure to occupational hazards by a work activity or work-related situation3.21 Environment measure: process of measuring the characteristic related to its operation that can havea significant environment impact.3.22 Environment monitor: process that qualitative check the objective, target and legal requirements when cant be measured the characteristic related to its operation that can have a significant environment impact.3.23 OHS measurement: process of measuring the characteristic related to the OHS performance that’s quanitative3.24 OHS monitoring: process of monitoring the characteristic related to the OHS performance that’s qualitative3.25 Hazardous waste: substances of highly toxic, explosive, radioactive, radiation and infectious may be harmful to the surrounding or human health.3.26 Recyclable waste: non-hazardous waste from normal producing activity and life activity, and part can be recycle, such as the packaging material, fitting, maintain material which can be fixed.The organization shall establish, document, implement and maintain this management system and continually improve its effectiveness in accordance with the requirements of the ISO9001, ISO14001 and OHSAS18001.As follows:a)determine the processes needed for the system and their application throughout the organizationb) compile procedure, WI in accordance with the processes, to determine the sequence and interaction of these processes and the method of process controlling, ensure the operation effectivec) ensure resources and information necessary to support the operation and monitoring of these processesd) measure, monitor, analysis these processes in accordance with the responsibility and proceduree) achieve planned results through control the process implementationf) implement actions necessary to continual improvement of these processesThese processes shall be managed by the organization in accordance with the requirements of this Standard. Any outsource processes for HEAT and GALV ANIZE are controlled according to requirements of Purchasing control, and implement appropriate document of level-ⅡorⅢ4.1.1 QEO are kept separate from four processes: management activities, provision of resources, product realization, measurement, analysis and improvement, and this four processes further identify subprocess, all above processes are applicable to PDCA4.1.2Description the sequence and interaction of management activities, provision of resources, product realization, measurement, analysis and improvement4.2 Documentation requirements4.2.1 General4.2.1.1 The system documentation shall includea)documented statements of a management policy and management objectivesb) management manualc) documented procedures and records required by this systemd)documents and records that determined by the organization to ensure the effective planning, operation and control of its processes4.2.1.2 Company plans all the documents according to the QEO management system to make up a complete system, and describe the system from different side and level to the effective operation and continual improvement4.2.1.3 Administration compile <QEO management manual> and procedure document, should concern any aspects of company scale, process complication and employee competence to ensure all the documents are consistent and practicability and operational. A document can include the requirements of one or more procedure. A documented requirement of procedure also could be included several documents.4.2.1.4 QEO is dynamic and should change according to the change from outside or inside, keep suitabilityand effectiveness and adequacy.4.2.2 QEO management manual4.2.2.1 <QEO management manual> is the programmatic document that planned by management representative, compiled by administration, approved and issued by general manager.4.2.2.2 QEO management manual includes:a) the scope of QEO management system, including details of any deletion of the ISO9001:2008 and justification for any exclusionsb) the documented procedures established for the management system, or reference to themc)a description of the interaction between the processes of the management system4.2.2.3Implementation of the manual should maintain continuity and stability, when the manual content is not appropriate, it should be amended timely by administration.4.2.3 Control of documentsAdministration is the department of document controlled, and control all the document required by the QEO system, set document compilation, review, approval, issuance, use, change; recognition of current revision status, re-approval, number, recycle and cancel, recognition and distribution of external document.4.2.3.1 document compilation, review, approvala) <QEO management manual> compiled by administration and issued after approval from general managerb) procedures compiled by administration and issued after approval from general managerc)support management documents, WI(including technical documents, operation, inspection standards) and other documents are compiled by the functional departments and issued after approval from general manager4.2.3.2 Review and update of documentsThe applicability of documents(<QEO management manual>&procedure&WI&other documents) are reviewed and updated when the organization, working process, applicable laws and regulations are changed, and implement after approval from general manager4.2.3.3 document changesa) identify the changes and the current revision status by using the document control list and change recordsb) to ensure the availability of current version of the document by changing, replacing, recycling, etc.4.2.3.4 document number and releaseThe document should be numbered in order to ensure that the relevant versions of the applicable documents can be obtained in use, Issued by administration, Number and register when distributing, fill in <Document Distribute&Recycle records>.The documents distributed into company or QEO management documents are controlled, the distribution number is the control number and stamped with "controlled" chapter. The copy of the document is also stamped with "controlled" by administration.4.2.3.5 document use and managementThe department or person of using the documents are responsible for the management of documents, prevent the loss or damage of the document, to ensure documents remain legible and readily identifiable.4.2.3.6 administration is responsible for identification of external documents, and according to the need to determine the release scope of management system and operation documents, and the documents are issued and distributed according to <document control procedures> approved by the general manager4.2.3.7 document recycle and obsoleteObsolete or expired documents are recycled by administration, and destruct after the approval of the management representative. As the accumulation of knowledge as data storage failure file, the retain department apply and stamp "scrap" chapter after approval of administration manager.4.2.4 record controlAdministration is responsible for the record control,to define the controls for the identification, storage, protection, retrieval, retention and disposition of records.4.2.4.1 record numberRecords are numbered by administration. Table formats are numbered according to<record control procedures>. The picture from activities of QEO management should be numbered on the package as below name, content, production time, the producer and other related information.4.2.4.2 record form releaseThe administration is responsible for the release of the record form to each departments, and fill in the < summary of records >.4.2.4.3 record filledAll departments establish and maintain records of ISO9001:2008, ISO14001:2004 and OHSAS18001:2007 standard requirements and system documentation requirements, to provide evidence of the effective operation and compliance with the requirements. All records should be clear, complete. And the data, information should be adequacy, all records should be timely and no forgery.4.2.4.4 records collection and storageEvery department is responsible for the collection and summary of records, keep the records according to the limit time, and keep away from moisture and moth, anti-deterioration, loss prevention.4.2.4.5 record lendLend records must be approved by administration, and handle relevant procedures in the <document copy& loan record>. The borrower shall take good care of the record, keep it complete and clean,and return it timely 4.2.4.6 record disposeRecords are kept three years generally, except for special records. The expired records should be destroyed by administration, in the meantime should fill in the <document destruction record>, The records which have the value of long term preservation should be sealed after the approval of administration4.3 related documents<Document control procedure><Record control procedure>5.1Management commitmentGeneral manager shall provide evidence of its commitment to the development and implementation of the quality management system and continually improving its effectiveness as follows:a) communicating to the company the importance of meeting customer as well as statutory and regulatory requirementsb) establishing the QEO policyc) establishing quality objectives, environmental objectives and targets, and occupational health and safety objectives and indicatorsd) take charge of the audit at the planned time, and evaluate the suitability, adequacy and effectiveness of the QEO management systeme) provide adequate resources, including human resources, financial resources, infrastructure and work environment to ensure the effective operation of QEO management system5.2 interested party requirements5.2.1 customer focusa) general manager must follow the principle of customer focus, then organize relevant departments to recognize and identify customer needs and expectations through market research or communicate directly to customer, and then convert them to specific requirements (including the requirements of product, process, service), and communication in the whole company.b) general manager is responsible for the approval of the major contract, and take initiative to understand any information about customer requirements through analyzing market research, understand current and future needs from customers, and meet their requirements, and strive to exceed customer expectations.c) notification of risk sources and control measures to the customers who enter company's production area, and exert the influence to reduce the safety risk.5.2.2 other interested party requirementsIdentify requirements of other interested parties, including environmental and occupational health and safety requirements of government, employees and their families, contract, visitor, surrounding to reduce environmental hazards and occupational safety and health damage. At the same time, have the interested parties understand will result in the environmental impact and occupational health and safety impact so that regulate their behavior.5.3 QEO policyThe general manager is responsible for establishing and approving QEO policy, and to ensure that it meets the following requirements:a) appropriate to the purpose of the organization, and suitable for the company's activities, product characteristic, scale and environmental and occupational health and safety;b) commitments of meeting requirements, pollution prevention, prevention of hazard, compliance with laws and regulations and continually improving tis effectivenessc) provide a framework for planning and evaluating the QEO objective;d) document it, implement it, maintain it and communicated it to all employeese) review for continuing suitability when internal and external environment changes5.4 planning5.4.1 Quality ObjectivesGeneral manager shall ensure that quality objectives, including those needed to meet requirements for product, are established at relevant functions and levels within the organization. The quality objectives shall be measurable and consistent with the quality policyThe quality objective is in Chapter 0.3Administration is responsible for the measurement of the objective implement, and other departments are responsible for the measurement of department objectives implement at the planned time.5.4.2 objective and target5.4.2.1 general manager shall establish the objective and targets of environment and occupational health&safety at relevant functions and levels within company .5.4.2.2 environmental objectives and targets, occupational health and safety objectives and indicators are measurable and consistent with the QEO policy. The establishment of objectives and targets should consider the followings:a) applicable laws, regulations and other requirementsb) significant environmental aspects, the risk of hazard and the statusc) optional technical pland) financial, operational requirementse) opinions from interested party5.4.2.3 objectives and targets should be in compliance with QEO policy and include the commitment of pollution prevention and continuous improvement.5.4.2.4 quality objectives, environmental objectives and targets, occupational health and safety objectives and targets shall be reviewed in accordance with planned arrangements, and if necessary to be updated.5.4.3 environmental / occupational health and safety management program5.4.3.1 Administration is responsible for organizing and maintaining the environment / occupational health and safety management program to achieve the company's policy.a) responsibility and authority gave to relevant departments in order to achieve the objectives and targetsb) the schedule and method to achieve the objectives and targets5.4.3.2 The environmental / occupational health and safety management program should be reviewed at least one time a year. If necessary, should revise the environmental / occupational health and safety management program for new projects or company's activities, products, services and operating conditions, to ensure its adaptability.5.4.4 Management system planningGeneral manager ensure to plan the QEO management system, ensure below:a) QEO management system meets the requirements of quality objectives, environmental objectives and targets, occupational health and safety objectives, and identify all processes and follow the process model of PDCAb) should plan firstly, then implement when changes to the QEO management system are planned and implemented to ensure the integrity of the quality management system is maintained.5.4.4.1 Plan timeIn the following situations, general manager shall plan the management system:a) when establish and improve the management systemb)when changes to company policy, objectives, organizational structurec)when company's resource allocation, the market are changed significantlyd) when product type, enterprise scale are changede) when relevant laws and regulations are changedf) when received major complaints from interested partyg) when internal audit or management review require;h) when some special items are not included into the existing system5.4.4.2 Plan contentThe general manager shall ensure that the resources and processes needed to achieve the objectives are identified and planned:a) develop corresponding target according to QEO policyb) recognize the process and relationship of achieving quality objectives, environmental objectives and targets and occupational health and safety objectives, and make clear provisions of these processes and control them, including permitted exclusions and rationalityc) required resources;d) responsibilitye) continuous improvement of QEO management system5.4.4.3 plan outputThe output of plan is in accordance with the management system contents, to direct the establishment and change of entire management system. And lead to changes of other levels including the management manual.5.4.5 Identification, evaluation and updating of environmental aspects5.4.5.1 Administration organizes every departments to collect information of production process, infrastructure, monitoring and measurement, raw materials, equipment update and the whole process of maintenance, packaging, product and service in accordance with the <environmental aspects identification, evaluation and update procedure> , determined the aspects that can interact and exert the effect.5.4.5.2 identification of environmental aspects should mainly consider three Tenses (past, present and future), and three states (normal, abnormal and emergency), seven types (the atmospheric emissions, wastewater emissions, soil waste, soil pollution, resources and energy utilization, noise, other problems to local area and social), two sides (environmental pollution, ecological destruction and reasonable use of resources).。