GST-FF层析填料说明书
- 格式:pdf
- 大小:409.64 KB
- 文档页数:2
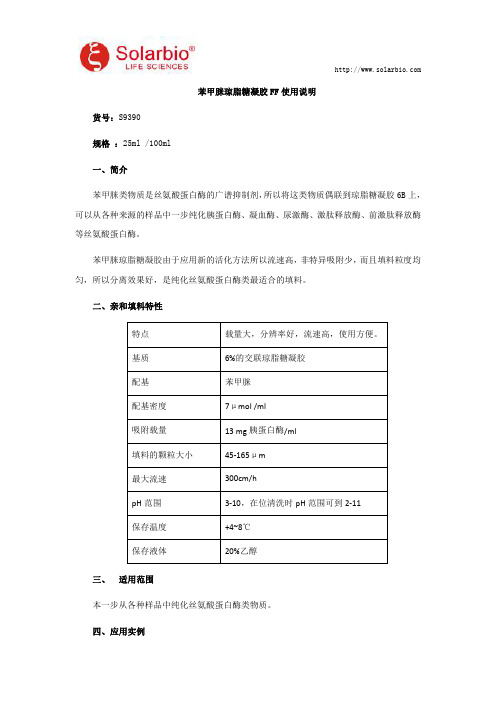
苯甲脒琼脂糖凝胶FF使用说明货号:S9390规格:25ml/100ml一、简介苯甲脒类物质是丝氨酸蛋白酶的广谱抑制剂,所以将这类物质偶联到琼脂糖凝胶6B上,可以从各种来源的样品中一步纯化胰蛋白酶、凝血酶、尿激酶、激肽释放酶、前激肽释放酶等丝氨酸蛋白酶。
苯甲脒琼脂糖凝胶由于应用新的活化方法所以流速高,非特异吸附少,而且填料粒度均匀,所以分离效果好,是纯化丝氨酸蛋白酶类最适合的填料。
二、亲和填料特性特点载量大,分辨率好,流速高,使用方便。
基质6%的交联琼脂糖凝胶配基苯甲脒配基密度7μmol/ml吸附载量13mg胰蛋白酶/ml填料的颗粒大小45-165μm最大流速300cm/hpH范围3-10,在位清洗时pH范围可到2-11保存温度+4~8℃保存液体20%乙醇三、适用范围本一步从各种样品中纯化丝氨酸蛋白酶类物质。
四、应用实例实验名称:苯甲脒琼脂糖凝胶FF分离尿激酶。
实验步骤:(1)苯甲脒琼脂糖凝胶FF装柱,1.6×20m,柱床体积为10ml(2)用缓冲液1平衡2-5个床体积,流速为2ml/min(3)取尿激酶粗品200mg溶解在20ml的缓冲液1中,用0.45μm的滤膜过滤,上样,流速为1ml/min(4)用缓冲液1再洗2-5个床体积,流速为2ml/min(5)最后缓冲液2进行洗脱,流速为2ml/min,收集洗脱峰,用SDS-PAGE纯化后的效果。
(6)用纯水洗5个柱床体积,然后分别用100mM,pH8.0Tris-HCl缓冲液含1M NaCl 和100mM,pH4.0的醋酸缓冲液含1M NaCl交替洗涤3次,再用纯水洗3个柱床体积,然后再用20%的乙醇流洗3个柱床体积,流速为2ml/min,柱子置于+4~8℃环境中保存。
(7)色谱图见图1,SDS-PAGE结果见图2。
缓冲液组成:缓冲液1:100mM pH7.4的PBS缓冲液;缓冲液2:100mM醋酸缓冲液,pH4.0。
也可以用这个填料特异去除各种融合蛋白酶切后的丝氨酸蛋白酶,例如凝血酶以及胰蛋白酶类蛋白酶。

离子交换柱说明书翻译目录1. 简介2. 所需材料3. 处理填料4. 组装柱子5. 填料装柱6. 平衡7. 样品处理8. 操作流速9. 吸附10. 洗脱11. 再生12. 在位清洗(CIP)13. 清洁14. 保存附录A附录B附录C15. 订货须知1. 简介CM Sepharose Fast Flow, DEAE Sepharose Fast Flow, Q Sepharose Fast Flow和SP Sepharose Fast FloW这些都是离子交换色谱的填料,高效且具有优良的流动性能。
以下说明以在推荐柱子XK 16/20 中填充填料为例,不同尺寸的柱子请参考“附录A”修改填柱方案。
Sepharose FF填料也可以装在Tricorn 高性能层析柱中。
请阅读并遵循装柱指示手册。
技巧详情请翻阅"Ion Exchange Chromatography and Chromatofocusing: Principles and Methods"2. 所需材料填料、柱子、泵、梯度混合器、进样阀、量筒、烧杯、真空瓶、注射器、玻璃棒、缓冲液填料缓冲液应与初始缓冲液相同,缓冲液推荐见附录 C 中的表 2 和表3。
装柱时应避免使用高粘度的缓冲液,若使用,则需降低流速。
3. 处理填料1) 所有材料至室温2) 倾倒填料,洗掉存储液,替换为缓冲液,配成的匀浆总体积为32.5ml(75%的填料,25%的缓冲液)注意:Sepharose Fast FloW离交柱CM, DEAE 和Q存储在20%的酒精中,SP Sepharose Fast Flow存储在20%的乙醇和0.2M的醋酸钠中3) 给匀浆脱气4. 组装柱子1) 装柱前确保每个组件完好无损,特别是网2) 柱底连接注射器或者泵3) 柱底浸没在缓冲液,用泵或注射器将缓冲液润洗整柱体,确保装柱无气泡4) 塞上出口,装上柱底5) 用缓冲液冲洗柱子,使柱底保持一小段液位,并保持柱垂直5. 填料装柱以下说明以XK 16/20 柱为例,不同尺寸的柱子请参考“附录A”。

CM Sepharose Fast Flow 使用说明CM Sepharose Fast Flow是一种以琼脂糖凝胶为基质的弱阳离子交换剂,具有流速快、载量高等特征,适用于各种等电点的蛋白质分离。
该离子交换剂以羧甲基作为带电基团,在pH 6 ~ 13的范围内均处于带电状态,并在整个层析过程始终保持高容量。
A 理化性质:离子交换剂类型:弱阳离子离子载量:0.09 ~ 0.13 m mol / ml 填料颗粒结构:6%高度交联琼脂糖粒度(直径)范围:45 - 165 μm平均粒度:90 μm最大线性流速:750 cm / h ( 25℃,100 kPa,K50/30 FF柱,14-16 cm柱床高,流动相0.1 M NaCl )耐压上限:0.3 Mpa(3 bar,42 psi)工作pH范围: 6 - 13pH稳定性:长期:4 - 13在柱清洗:2 - 14对化学试剂的稳定性:对所有常用的水相缓冲液、1.0 M醋酸、1.0 M NaOH、8 M 尿素、8 M盐酸胍、乙醇、甲醇等稳定物理稳定性:因pH或离子强度的改变而引起的体积变化可忽略耐压热性:在0.1 M醋酸钠中,可承受121℃、30 min 的高压灭菌B 填料预处理:购买的CM Sepharose Fast Flow是预先溶胀好的,保存于20%乙醇中;使用前倾出20%乙醇溶液→加入初始buffer(buffer:沉淀填料= 1:3体积比)→得填料悬液。
注:初始buffer中不能含有使粘度显著增加的试剂。
C 装柱:将所有需要使用的仪器和试剂平衡至实验进行的环境温度;对填料悬液抽气;以buffer冲洗柱子两端的部件,排除气泡死角,尤其要确保滤网上无气泡;安装下端柱部件,关闭出水阀,使柱中存留几厘米的buffer;以连续的液流沿玻璃棒倾加填料悬液至柱中,玻璃棒下端抵靠柱内壁,尽量避免气泡的引入;立即以buffer充满剩余的柱体积,安装上端柱部件,并将其连接到泵;打开下端的出水阀,调节泵使流速合适,即装柱流速:建议≥6.7 cm/min(15 cm柱床高,25℃,低粘度buffer);注:如果无法达到建议的装柱流速,可将泵调至最大流速;但在随后的层析洗柱的过程中,流速不能超过装柱流速的75%.保持装柱流速一段时间,至柱床高度恒定(大约需要流过3倍柱床体积的buffer)。

SP Seplife FF层析介质说明书1.产品介绍SP Seplife FF层析介质是蓝晓科技自主研发的一种新型高度交联的琼脂糖层析介质,是将磺酸基丙基键合在琼脂糖凝胶过滤层析介质上形成的一种强阳离子交换层析介质,具有高流速、低反压、高动态载量、良好的化学稳定性和机械性能,非特异性吸附低,回收率高,方便进行规模放大,可缩短生产时间,提高生产效率。
广泛用于生物制药和生物工程下游蛋白质、核酸及多肽的离子交换分离。
2.性能介绍产品牌号SP Seplife FF外观白色球状凝胶,无臭无味种类强阳离子交换填料基质Seplife 6FF配基磺酸基丙基粒径(μm)50~160pH稳定性4~13(长期),3~14(短期,在位清洗[CIP])在以下液体中稳定:所有常用的水相缓冲液;1mol/L 氢氧化钠;化学稳定性8mol/L 尿素;8mol/L 盐酸胍;70% 乙醇吸附量(mg /ml)>55mg/ml (溶菌酶)离子交换量(mmol /ml)0.18~0.25工作温度4~40℃耐热性121℃,水中30min最高流速*(cm/h)750耐压(MPa)0.3应用用于生物制药和生物工程下游蛋白质、核酸及多肽的离子交换层析纯化*检测条件:层析柱16mm×300mm;*柱床高15cm;温度25℃;流动相为0.1mol/L NaCl。
3.使用方法3.1 装柱装柱按照标准操作规程操作。
必须保证每种材料都处于工作温度,凝胶装柱前需要脱气。
3.2平衡使用2~5倍柱床体积的上样平衡液平衡柱子,务必使流出液的电导和pH同上样缓冲液的电导和pH完全一致。
平衡液是低浓度的缓冲溶液,如NaOAC、PBS等。
常用的平衡液是0.1mol/L 醋酸缓冲液,pH5.0。
3.3上样(1)样品用平衡液配制,浑浊的样品要离心和过滤后上样。
盐浓度太大的样品处理后再配。
(2)一般情况是让目标产品结合在柱子上,用平衡液洗去杂质,再选择一种洗脱液洗下目标产品。

蓝胶亲和介质(Blue QZT 6FF)产品说明书1. 产品简介蓝胶亲和介质广泛用于白蛋白、干扰素、脂蛋白、凝血因子和激酶、脱氢酶等需要NAD+和NADH+辅助因子的酶的分离纯化。
Blue QZT 6FF亲和介质以自制的琼脂糖凝胶为基质,以Cibacron Blue 3G为配基,具有很高的物理化学稳定性,配基不易脱落,寿命长,应用广泛。
2. 产品特性3. 应用Blue QZT 6FF亲和介质广泛用于白蛋白、干扰素、脂蛋白、凝血因子和激酶、脱氢酶等需要NAD+和NADH+辅助因子的酶的分离纯化。
层析操作通常包括装柱、平衡、进料、淋洗、洗脱、再生等步骤。
平衡:用5~10CV的平衡缓冲液(20 mM PB,pH 7.0)平衡层析柱,至流出液电导和pH不变(与平衡液一致)。
进料:样品缓冲液应尽可能与平衡液一致。
固体样品可用平衡液溶解配制;低浓度样品溶液可用平衡液透析;高浓度样品溶液可用平衡液稀释。
为了避免堵塞层析柱,样品应经离心或微滤(0.45μm)处理。
进料量根据介质的载量和料液中目标蛋白的含量计算。
淋洗:上样完毕后继续用平衡缓冲液淋洗至基线。
洗脱:用洗脱缓冲液(20 mM PB+1M MgCl2(或KCl),pH 7.0)洗脱,收集流出液。
再生、原位清洗:介质使用数次(5-10次,具体次数与原料的种类和来源及实验要求有关)后,需要对介质进行再生、在位清洗。
(1)用0.1M的NaOH以40cm/h的速度清洗柱子3-5CV,然后用20%的乙醇清洗3CV,缓冲液洗到中性即可重复使用;(2)对疏水性结合的蛋白、脂蛋白和脂类物质,可用3~4 CV的70%乙醇或30%异丙醇清洗,并用3~10 CV的纯水冲洗。
其它注意事项:在装柱、使用和保存柱子的时候,要避免柱子流干,气泡进入;第1次使用时先用2M NaCl冲洗5CV,洗去残留的染料,平衡后即可使用。
具体的应用实例如下:蛋白:;层析介质:Blue QZT 6FF介质(CV=1.0 ml);对照介质:CV=1.0 ml, 国际领先品牌;样品:;平衡缓冲液:20 mM PB,pH 7.0;洗脱缓冲液:20 mM PB+1M MgCl2,pH 7.0;流速:0.5 ml/min。

ProteinAt Beads 4FF 的预装柱说明书货号:YA2471规格:1*1mL /1*5mL /5*1mL /3*1mL+1*5mL /5*5mL保存:2-8℃产品说明:Protein At Beads 4FF 是用于分离和纯化单克隆抗体、多克隆抗体或Fc-融合蛋白的亲和层析介质。
Protein A 是一种分离自金黄色葡萄球菌的细胞壁蛋白,主要通过Fc 片段结合哺乳动物IgG 。
Protein At Beads 4FF 的配体蛋白Protein At 是在天然蛋白A 的基础上进行生物工程突变得到的一种耐碱蛋白A(Alkaline tolerate ,故缩写为At)。
Protein At Beads 4FF 是以高度交联的4%琼脂糖凝胶为基质,可以在相对较高的流速下进行单克隆抗体和多克隆抗体的纯化。
表1:介质性能参数介质高度交联的4%琼脂糖平均粒径~90μm配体耐碱性Protein A结合载量>40mg 人lgG/ml 介质化学稳定性可耐受抗体纯化过程中的所有试剂工作pH 3-12在线清洗0.1-0.5M NaOH 线性流速50-300cm/h 保存20%乙醇纯化流程:1、Buffer 的准备所用水和缓冲液在使用之前建议用0.22μm 或0.45μm 滤膜过滤。
平衡/洗杂液:0.15M NaCl 、20mM Na 2HPO 4,pH7.0洗脱液:0.1M 甘氨酸,pH 3.0中和液:1M Tris-HCl ,pH 8.5。
2、样品准备上柱之前要确保样品溶液有合适的离子强度和pH 值,可以用平衡/洗杂液对血清样品、腹水或细胞培养液稀释,或者样品用平衡/洗杂液透析。
样品在上样前建议离心或用0.22μm 或0.45μm 滤膜过滤,减少杂质,提高蛋白纯化效率和防止堵塞柱子。
3、样品纯化1.上柱:将泵管道中注满去离子水。
去掉上塞子,将层析柱连接至色谱系统中。
再折断下口,将预装柱接到色谱系统中,并旋紧。
GE Healthcare操作指南11-0012-38 AD HiTrap亲和柱———————————————————————————————————————HisTrap FF crude 1 ml and 5 mlHisTrap TM FF crude是一种预带电荷的Ni Sephrose TM 6 Fast Flow即用的预装柱。
该预装柱的目的是采用固定化金属配体亲和层析 (IMAC) 来纯化带有组氨酸标记的重组蛋白质。
在细胞完全破碎后,样品无需预离心及过滤就可将未澄清的细胞裂解液上到柱上。
为确保裂解完全,我们建议延长样品的机械处理时间。
Ni Sephrose 6 Fast Flow具有低 (Ni2+) 离子脱落并与用于蛋白质纯化的许多添加剂相兼容。
柱与介质的相结合的特殊设计提供了快速、简单、及方便的纯化。
纯化时间的缩短通常使有害影响减到最少,例如,敏感的目标蛋白的降解和氧化,因此, 纯化时间的缩短极其重要。
HisTrap FF crude可用注射器、恒流泵,或液相层析系统,如 ÄKTAdesign TM 层析系统或FPLC TM系统的操作。
小心:含鎳。
可能引起过敏反应。
货号名称供货数量*可提供指定的包装大小。
接头配件盒提供的接头用途供货数量1/16”阳/路厄氏阴将注射器连接到HiTrap柱的顶端 1无法兰/M6阴管道接头将管道(如,恒流泵P1)连接到HiTrap柱的底部* 1无法兰/M6阳管道接头将管道(如,恒流泵P1)连接到HiTrap柱的顶部** 1活接头1/16” 阴/M6阳通过HiTrap柱的底部连接到固有的FPLC系统 1 活接头M6阴/ 1/16” 阳通过HiTrap柱的顶部连接到固有的FPLC系统 1 堵头阴,1/16” 封住HiTrap柱的底部2、5或7* 也需要活接头1/16” 阴/M6阳** 也需要活接头M6阴/ 1/16” 阳目录1.说明 42.一般事项73.样品制备114.纯化操作125.纯化效率的优化146.按比例放大157.柱的清洗及保存158.保存169.故障排除1610.定购信息20 附录A 221说明介质的性质HisTRap FF crude 1-ml和 5-ml柱是用亲和介质Ni Sepharose 6 Fast Flow的预装柱,由具有固定鳌合基团的高度交联的球状琼脂糖组成。
Ni-琼脂糖凝胶6FF(His标签纯化树脂)说明书货号:P2010规格:5mL/ 10mL/25mL(凝胶体积)保存:4°C保存,有效期至少一年。
产品说明:Ni NTA Beads 6FF是以高度交联的6%琼脂糖凝胶为基质,配体与Ni NTA Beads 相同,蛋白的载量可以大于40mg 6×His-taggedprotein/ml 介质;微球粒径为45–165μm。
Ni NTA Beads 6FF除了可以耐受苛刻的试剂条件(多种还原剂、去污剂、高浓度变性剂等)外,因其耐压的基质,可以耐受最高0.3 MPa 的压力,更稳定,因此该产品更适合用于工业大规模蛋白的纯化,可以在相对较高的流速下,实现对目的蛋白的纯化。
本产品悬浮液为含20%乙醇的1×PBS,已螯合Ni2+。
操作方法:1、样品制备(1)细菌或酵母表达的蛋白1)挑取单菌落到LB 培养基中,根据载体使用说明加入相应浓度的诱导剂诱导相应的时间。
2)表达结束后,将培养液转移到离心杯中,7,000rpm,离心15min收集菌体,然后加入1/10 体积的Lysis Buffer 和PMSF,PMSF在破碎前加入,最终浓度为1mM。
加入溶菌酶(工作浓度为0.2-0.4mg/ml,如果表达的宿主细胞内含pLysS 或pLysE,可以不加溶菌酶),(同时也可加入其他蛋白酶抑制剂,但不能影响目的蛋白与树脂的结合)。
3)将菌体沉淀悬浮起来,(如果菌液浓度高,也可考虑加入10μg/ml RNase A 和5μg/ml DNase I),混匀,放置于冰上,然后冰上超声破碎细胞,至菌液基本保持澄清。
4)将澄清的破碎液转移至离心管中,10,000rpm下,4 度离心20-30 分钟。
取上清,置于冰上备用或-20℃保存。
(2)酵母、昆虫和哺乳细胞分泌表达可溶性蛋白1)将细胞培养液转移至离心杯,5000rpm 下,离心10min,收集菌体得上清,如上清中不含EDTA、组氨酸和还原剂等物质,即可直接加入柱子使用;如含有EDTA、组氨酸和还原剂等物质,需用1×PBS 4℃下透析才能加入柱子。
层析柱(色谱柱)填料介质的装载及洗脱操作规程工业制备层析柱(色谱柱)的填料装载充填和洗脱操作的规范直接影响色谱分离纯化工艺过程的效率,迈思通公司经过多年的实践经验和该行业专家的指导,编写了关于《层析柱(色谱柱)吸附介质的充填及洗脱规程》的方法,供广大用户和科研者参考。
一、柱头安装是否合适及是否严密的检查1、检查下柱头是否按图纸要求安装?(要求按图纸要求安装)2、检查下柱头内,是否安装了防止吸附剂(例如硅胶)漏出的滤布(或滤网)。
3、所用滤布(滤网)的材质要求能耐所用溶剂的腐蚀,而且孔径很小,一般要求使用500目以上孔径大小的滤布(网)。
4、下柱头安好,紧固后,从柱上端(开口)加入所用洗脱剂中极性较小的一种溶剂(例如已烷、石油醚、甲苯等),使溶剂液面比下柱头上部分离出20-30厘米,仔细观察下柱头是否漏溶剂。
如果漏,则必须坚固(或重装),直至不漏为止。
5、检查确实不漏后,放出柱内溶剂,准备装吸附剂(例如硅胶等)。
二、充填吸附剂以充填(或称装填)硅胶为例,作如下说明:1、干法装柱①、取吸附剂(例如硅胶)记下硅胶的量,(等装完柱后,检查剩余硅胶量,就可以知道装入柱中的硅胶量了)。
②、工作人员戴上口罩,准备好木槌,有人从柱上端倒入一定时的硅胶,柱下边有人用木槌敲击柱外壁,振实硅胶;一边加硅胶,一边敲击柱;最好硅胶加到什么高度,就在此高度敲击;且向上下移动敲击。
如此,直至硅胶装满时为止;再上下移动敲击一阵子;至硅胶上平面不再下沉,而且硅胶面很实时为止。
③、如果体系中没有设预柱,层析柱中上端硅胶不把装满,留出空间待“上样”用。
④、如果有预柱的体系,层析柱应装满硅胶。
⑤、干法装柱,粉尘大,且敲击声大,柱外表易变成不光亮,但不耗溶剂。
2、湿法装柱①、同上,准备一定时的硅胶,且记录下硅胶量。
②、准备一只30-50升的耐溶剂腐蚀的塑料桶,例入极性小的且配洗脱剂时需用的溶剂(例如已烷,石油醚、甲苯等),再例入硅胶,搅拌混合。
Amicon® Pro AffinityConcentration Kit - GSTPurification of GST-tagged recombinant proteins.Catalog Nos. ACR5000GS, ACK5003GS, ACK5010GS, ACK5030GS,ACK5050GS, ACK5100GSFOR RESEARCH USE ONLYNot for use in diagnostic procedures.USA & Canada Phone: +1(800) 437-7500 Fax: +1 (951) 676-9209Australia +61 3 9839 2000IntroductionThe expression and purification of recombinant proteins is central to protein regulation, structure and function studies. Purification has been greatly facilitated by the addition of small affinity tags to protein sequences such as the glutathione S-transferase domain (GST). GST agarose resin is an affinity chromatography matrix, when used in concert with the Amicon®Pro Affinity Concentration device, offers rapid purification of GST fusion proteins, native GST, or glutathione-binding proteins. GST-tagged proteins bind specifically to reduced glutathione in near-neutral, non-denaturing conditions (e.g., phosphate buffer). Following resin capture of the target protein, unbound lysate components are removed by spin-based clearing and washing steps. Bound protein is recovered by centrifugation following competitive displacement with buffer containing free, reduced glutathione.By condensing the entire purification workflow into one device, the Amicon® Pro device eliminates the need for multiple sample transfers thereby minimizing protein loss. The large exchange device reservoir (up to 9ml) accommodates a range of sample capacities as well as reduces the need for multiple spin steps during the wash and elution phases. Direct coupling to an Amicon® Ultra-0.5 device further provides simultaneous concentration during the elution phase. Lastly, the Amicon® Pro device offers highly efficient diafiltration in a single 15 minute spin.The kit contains sufficient GST resin, optimized buffers, and Amicon® Pro devices for 12 standard reactions.Sample Preparation GuidelinesOptimizing lysis parameters is critical for protein extraction and downstream purification. The Amicon®Pro Affinity Concentration GST kit is compatible with a range of conditions, including reducing agents (β-mercaptoethanol, DTT), chelating agents (EDTA), non-ionic detergents, and BugBuster®Protein Extraction Reagent (Cat. No. 70584), a proprietary mixture of non-ionic detergents offering a rapid cost-effective alternative to mechanical cell lysis methods such as sonication.Irrespective of extraction method, we recommend inclusion of rLysozyme™ Solution (Cat. No.71110) and Benzonase®Nuclease (Cat. No. 70746) and during protein extraction for increased cell lysis (protein yield) and a reduction in sample viscosity (improved sample handling), respectively.Protease inhibitors may also be added to the lysis buffers to protect against degradative enzymes.A listing of individual protease inhibitors and cocktails is available at /search on key words “Protease Inhibitor.” Note: Serine proteases should be used with caution if the GST fusion tag is to be cleaved via Thrombin or Factor Xa digestion. If used during protein extraction, we strongly recommend the eluted fraction be buffer exchanged prior to performing the cleavage reaction.In certain systems, high protein expression can lead to aggregation in the form of inclusion bodies.Strong denaturants such as 6 M guanidine or 8 M urea can be used to solubilize aggregates greatly enhancing yield. However, only properly folded, functional GST is capable of binding GST resin. GST fractions recovered from denaturation of inclusion bodies must be refolded to reconstitute active GST fusion constructs. To a degree, this can be accomplished through use of the Protein Refolding Kit (Cat. No. 70123).Cat. No. BWEGSFor more information, consult the GST•Bind™ Resin User Guide available at /chemicals. Search using the Cat. No. 70541.Kit ComponentsCS211421-GST Resin (3 mL) – Crosslinked agarose with immobilized reduced glutathione supplied as 50% slurry in 50 mM Phosphate Buffer pH 7.5, 0.15 M NaCl, 0.1% NaN3. The resin utilizes an 11-atom spacer arm (~16 Å) for covalent attachment of reduced glutathione via a sulfide linkage. The binding capacity is typically 5-8 mg/mL of settled resin.Buffers:o CS211416-10X GST Bind/Wash Buffer (25 mL) – 43 mM Na2HPO4, 14.7 mM KH2PO4,1.37 M NaCl, 27 mM KCl pH7.2o CS211354-10X Glutathione Reconstitution buffer (5 mL) – 500 mM Tris, pH 8.0o CS211418-Glutathione, reduced, free acid (125 mg)Amicon®Pro Devices – The kit includes 12 complete assemblies. Each device consists of an exchange device, holder tube, 50 mL collection tube, and Amicon®Ultra-0.5 centrifugal filter device. A 2 mL collection tube is included for sample recovery from the AU-0.5 device by reverse spin. The kit is available in five formats based on the nominal molecular weight limit (NMWL) of the AU-0.5: 3 (ACK5003GS), 10 (ACK5010GS), 30 (ACK5030GS), 50 (ACK5050GS), and 100 kDa (ACK5100GS). Consult the Amicon®Pro (/psp, search keywords “Amicon Pro”) and Amicon®Ultra-0.5 centrifugal filter device User Guides (/catalogue/module/c82301) for proper assembly/disassembly and additional product information.All reagents should be stored at 2° to 8°C (do not freeze). The Amicon Pro devices can be stored separately at room temperature.Procedures for using the Amicon® Pro Affinity Concentration Kit - GSTThe protocol is based on purification of GST-tagged protein from 1 mL of E. coli lysate using 200 µl of GST resin slurry (100 µL packed resin). The protocol is linearly scalable for 50-1000 µL of resin slurry. Due to large variability among sample preps, parameters which may require optimization include bead input, binding time, wash, and elution parameters. This protocol includes steps for simultaneous concentration during the elution step as well as buffer exchange using the Amicon®Ultra-0.5 centrifugal filter device.Note: Given the collection tube’s capacity, it is not necessary to remove filtrate between the various centrifugation steps. However, if process samples need be retained for analytical purposes, the collection tube should be cleared.Buffer Preparation10X Bind/Wash Buffer should be diluted to 1X with sterile deionized H2O; 1X Bind/Wash solution may be stored at 4°C for 1 week.Prepare 10X GST Elution Buffer containing 100 mM reduced glutathione as follows: add 4ml 10X Reconstitution buffer directly to the glutathione powder vial. Pipette repeatedly then incubate for30 minutes at room temperature. Divide buffer into working volumes and store at -20o C (400Cat. No. BWEGSµl/standard reaction); 10X GST Elution Buffer is stable for 6 months at -20o C. We do not recommend repeated freeze/thaw cycles. To minimize glutathione oxidation, dilute 10X buffer to 1X using sterile deionized water and pH to 8.0 immediately before use. Discard any unused 1X Elution Buffer.Bead Preparation1. To ensure uniform suspension, vortex the GST resin thoroughly before adding it to the device.2. Remove the collection tube cap and open the exchange device cap.3. Add 200 µL of resin slurry to the base of the exchange device. Close the exchange cap.Up to 500 µl packed resin (1000 µl slurry volume) may be added per device We recommend using wide-bore tips (Cat. No. 02-707-134, Fisher Scientific) for resin transfer.4. To remove storage buffer, centrifuge in a swinging bucket rotor at 1000 g X 1 min.5. Add 500 µL of 1X Bind Buffer. Centrifuge at 1000 g X 1 min.Protein Binding1. Add 500 µL of sample to the exchange device.Up to 9 mL of sample can be added. The volume loaded is determined by the target protein’s expression level and resin’s binding capacity.2. Incubate for 60 min at room temp with gentle agitation.We recommend upright agitation on a plate shaker at low setting.End-over-end mixing, particularly with small volumes or for extended time, may result in substantial bead loss to the sides of the feeder tube.The duration of binding time may vary with application.3. Centrifuge the device at 1000 g X 1 min in a swinging bucket rotor. Recover the sample flow-through from the 50 mL collection tube (optional).To ensure maximal protein capture, collect all resin into solution prior to centrifugation.4. Add1.5 mL of Wash Buffer. Centrifuge at 1000 g X 1 min. Recover the wash fraction from the 50mL collection tube (optional).Due to the large capacity of the exchange device, the volume of the wash can be increased for greater sample purity. There is no need for multiple wash steps.Sample ElutionSamples can be eluted without concentration by adding elution buffer and centrifuging (1000g X 2 minute) directly into a clean 50 ml collection tube. Given the limited volume processing capacity of the AU-0.5 device, we recommend this protocol if elution volumes > 1.5 ml are required.For simultaneous elution with concentration, attach the Amicon® Ultra-0.5 device and follow the steps outlined below.1. Remove the exchange device and insert it into the AU0.5 device.2. Place the exchange device/AU-0.5 assembly back in the holder and return the device to thecollection tube.3. Add up to 1.5 mL of Elution Buffer, gently resuspend the resin, and incubate for 5 min.Under standard conditions, one elution is sufficient for recovery of 90-95% of captured protein.4. Close the exchange device cap and screw on the collection tube cap to ensure a proper seal.5. Centrifuge at 4000 g X 15 min in a swinging bucket rotor. Concentrated samples can be bufferexchanged or recovered from the AU-0.5 device by reverse spin (see below).Cat. No. BWEGSDepending on the starting elution volume, NMWL of AU0.5 device employed, and the degree of concentration desired, the length of the spin time can range for 10-30 minutes. Please consult the Performance Characteristics section in the Amicon®Pro Affinity Concentration System User Guide (/psp, and search keywords "Amicon Pro”) for recommended guidelines., consult.6. Recover the concentrated fraction by reverse spin or proceed to Buffer Exchange (see below).Buffer Exchange (Optional if samples have been collected in the Amicon® Ultra-0.5 device)1. After sample concentration, add 1.5ml desired buffer to the exchange device/AU-0.5 assembly.2. Centrifuge device at 4000g X 15 minutes in a swinging bucket rotor. Concentrated samples canbe recovered from the AU-0.5 device by reverse spin (see below).Collect sample from the AU0.5 device by Reverse Spin(following Concentration or Buffer Exchange)1. Disassemble the exchange device/AU-0.5 assembly from the holder tube.2. Using a gentle twisting motion, detach the AU-0.5 from the exchange device.3. If there is residual sample in the exchange device tip, depress the exchange device cap to expelthe remaining sample volume into the AU-0.5.4. Hold AU-0.5 upright and slide the 2 ml collection tube on top of it.5. Invert the assembly and centrifuge (in a microcentrifuge) with a fixed angle rotor 1000g X 2min.Sample protein yield can be determined by Mid IR-based spectrometry using the DirectDetect™ biomolecular quantitation system and DirectDetect™ Assay Free Sample Cards.TroubleshootingCat. No. BWEGSIssue: Recombinant Protein is present in low amount in eluatePossible Cause SolutionProtein is insoluble and may have formed inclusion bodies. After lysate clearance, check both the supernatant and pellet for protein. Perform lysis and binding procedures under denaturing conditions.The GST fusion protein is not in the proper three dimensional conformation. Attempt to reconstitute native protein structure using Protein Refolding Kit (Cat. No. 70532).The GST-tag is not exposed for binding to the affinityresin.The protein may require denaturing conditions for binding.The GST-tag is not present. Sequence the ligation junctions to ensure that the readingframe is correct. Check for possible internal translation starts(N-terminal tag) or premature termination sites (C-terminal tag). Recombinant protein is degraded during cell lysis. Add protease inhibitors during cell lysis.Protein forms aggregates. Add solubilizing agents such as detergents (0.1% Triton® X-100, TWEEN®-20) or increase salt concentration.pH of the Lysis or Binding buffers is incorrect. Check buffer pH; the acceptable range is 7-8. Acidic bufferswill prevent binding.Protein expression is insufficient. Optimize the growth/induction conditions.Cell Lysis is incomplete. Optimize the Cell Lysis Protocol.Cell Lysate is too viscous. If possible, dilute the lysate in Bind Buffer. Alternatively,include Benzonase® Nuclease during lysis to remove freeRNA/DNA.Protein precipitates during sample concentration while using the AU0.5 device due to over-concentration. Reduce the duration of the centrifugation time during the elution/concentration step.Protein is lost during sample concentration while using AU0.5 device. Check the protein's expected size and MWCO of AU0.5 device used. AU0.5 device is offered in 5 different MWCO formats - 3, 10, 30, 50, and 100 kDa.Issue: High Non-specific bindingPossible Cause SolutionInsufficient washing. Increase the volume of the Wash Buffer used or the number ofwash steps. Alternatively, supplement the Bind/Wash Buffers.Contaminants interact directly with the GST fusion protein. Add reducing agents such as DTT or β-mercaptoethanol to reduce disulfide bonds. Add detergents to disrupt non-specific interactions.GST fusion protein is degraded. Degraded/truncated forms of the recombinant protein will stillbind to the GST resin and appear as contaminating bands inSDS-PAGE. Perform a lysis procedure on ice and includeprotease inhibitors.Cell lysate is too concentrated. Dilute the lysate in Bind Buffer before purification.Cat. No. BWEGSCat. No. BWEGSPerformanceProduct Ordering InformationNo Devices Amicon ® Pro + AU 0.5 ml with MWCO: 3k 10k 30k 50k 100k Amicon ® Pro Affinity Concentration Kit - GSTACR5000GS ACK5003GS ACK5010GS ACK5030GS ACK5050GS ACK5100GS Amicon ® Pro AffinityConcentration System 12PKACS500312 ACS501012 ACS503012 ACS505012 ACS510012 Amicon ® Pro AffinityConcentration System 24PK ACS500324 ACS501024 ACS503024 ACS505024 ACS510024The Amicon ® Pro Affinity Concentration Kit contains reagents and devices sufficient for 12 standard reactions. Amicon ® Pro devices are also sold separately in 12 and 24 packs.Description Catalogue Number Qty/Pack GST•Bind™ Resin 70541-3/4/5 10/50/25 mL GST•Bind™ Buffer Kit 70534 1 KitPurification using Amicon Pro Affinity Concentration Kit – GST purifications of a 50 kDa GST-tagged protein the Amicon ® Pro Affinity Concentration Kit numbered lanes are: 2 – flowthrough fraction 3 – wash fraction 4 – eluted fractionNoticeThe information in this document is subject to change without notice and should not be construed as a commitment by EMD Millipore Corporation (“Millipore”) or an affiliate. Neither EMD Millipore nor any of its affiliates assumes responsibility for any errors that may appear in this document. Technical AssistanceFor more information, contact the office nearest you. Up-to-date world-wide contact information is available on our web site at /offices. You can also visit the tech service page on our web site at /techserves.WarrantyEMD Millipore Corporation(“EMD Millipore”) warrants its products will meet their applicable published specifications when used in accordance with their applicable instructions for a period of one year from shipment of the products. EMD MILLIPORE MAKES NO OTHER WARRANTY, EXPRESSED OR IMPLIED. THERE IS NO WARRANTY OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE. The warranty provided herein and the data, specifications and descriptions of EMD Millipore products appearing in EMD Millipore’s published catalogues and product literature may not be altered except by express written agreement signed by an officer of EMD Millipore. Representations, oral or written, which are inconsistent with this warranty or such publications are not authorized and if given, should not be relied upon.In the event of a breach of the foregoing warranty, EMD Millipore Corporation’s sole obligation shall be to repair or replace, at its option, the applicable product or part thereof, provided the customer notifies EMD Millipore Corporation promptly of any such breach. If after exercising reasonable efforts, EMD Millipore Corporation is unable to repair or replace the product or part, then EDM Millipore shall refund to the Company all monies paid for such applicable Product. EMD MILLIPORE CORPORATION SHALL NOT BE LIABLE FOR CONSEQUENTIAL, INCIDENTAL, SPECIAL OR ANY OTHER DAMAGES RESULTING FROM ECONOMIC LOSS OR PROPERTY DAMAGE SUSTAINED BY ANY COMPANY CUSTOMER FROM THE USE OF ITS PRODUCTS.Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.(c) 2009 - 2012: Merck KGaA, Darmstadt. All rights reserved. No part of these works may be reproduced in any form without permission in writing.Benzonase®, BugBuster®, and Amicon® are registered trademarks of Merck KGaA, Darmstadt, Germany. DirectDetect™ and GST•Bind™ is a trademark of Merck KGaA, Darmstadt, Germany. Triton® is a registered trademark of Dow Chemical Company. Tween® is a registered trademark of ICI Americas, Inc.Cat. No. BWEGS。
Glutathione Sepharose 4 Fast Flow
原理
谷胱甘肽S-转移酶是一种经常被使用的Tag,可以使含有谷胱甘肽S-转移酶的重组蛋白的纯化和检测更加容易,为了使产物的纯化更简单,GST融合蛋白的一步纯化是可用的。
纯化简要概述
结合缓冲液:140mM NaCl, 2.7mM KCl, 10mM Na2HPO4 , 1.8mM KH2PO4, pH 7.3
洗脱缓冲液:50mM Tris-HCl, 10mM 还原型谷胱甘肽, pH 8.0
1,用5倍柱体积的结合缓冲液平衡柱子。
2,上样。
3,用5-10倍柱体积的结合缓冲液平衡分离柱,直到基线,即所有未结合物质都被冲洗出柱子。
4,用5倍柱体积的洗脱缓冲液进行洗脱。
5,用5-10倍体积的结合缓冲液冲洗柱子。
使用注意
1,样品上样期间和洗脱期间,保持较低的流速是非常重要的,因为GST和谷胱甘肽互相间的结合动力学作用是相对较低的。
其结合容量是依赖于蛋白质的特性,因此其产量是依赖于蛋白质的类型而变化的。
使用较低的流速或者将样品反复流过柱子几次,或许可以提高产量。
2,柱子的重复使用取决于样品的特性。
对同一样品重复使用时,需考虑避免交叉污染的问题。
在位清洗
1,用2-3倍柱体积的,高端pH值为(0.1M Tris-HCl,0.5M NaCl,pH8.0)和低端pH值为(0.1M 醋酸钠,0.5M NaCl,pH4.5)的缓冲液交替冲洗柱子。
2,重复循环3次。
3,立即用3-5个柱体积的结合缓冲液再平衡柱子。
沉淀蛋白质的去除
1,用2倍柱体积的6M的盐酸胍冲洗柱子。
2,立即用5倍柱体积的结合缓冲液冲洗柱子。
通过疏水性互相作用结合到柱子上的物质的去除
1,用3-4倍柱体积的70%的乙醇冲洗柱子(或者2倍柱体积的非离子型去污剂如1%的曲拉通-100冲洗柱子)。
2,立即用5倍柱体积的结合缓冲液冲洗柱子。
当填料在室温状态下暴露在0.1M柠檬酸盐(pH4.0),0.1M NaOH,70%的乙醇或者6M盐酸胍2小时后,其结合能力没有显著地丢失。
当填料暴露在1%的SDS 14天后,其结合能力没有明显的丢失。
储存
用5个柱体积的20%的乙醇在中性pH值的条件下冲洗介质和柱子,在4°-8°条件下储存。