有机硅树脂教材
- 格式:ppt
- 大小:570.50 KB
- 文档页数:43
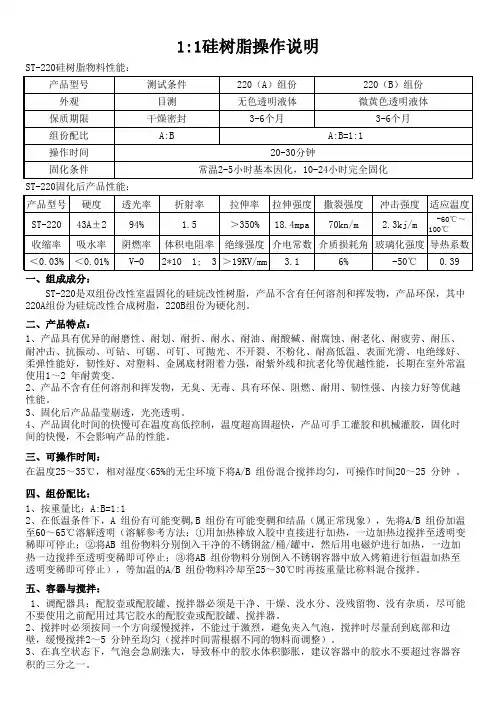
1:1硅树脂操作说明ST-220硅树脂物料性能:ST-220固化后产品性能:一、组成成分: ST-220是双组份改性室温固化的硅烷改性树脂,产品不含有任何溶剂和挥发物,产品环保,其中220A组份为硅烷改性合成树脂,220B组份为硬化剂。
二、产品特点:1、产品具有优异的耐磨性、耐划、耐折、耐水、耐油、耐酸碱、耐腐蚀、耐老化、耐疲劳、耐压、耐冲击、抗振动、可钻、可锯、可钉、可抛光、不开裂、不粉化、耐高低温、表面光滑、电绝缘好、柔弹性能好,韧性好、对塑料、金属底材附着力强,耐紫外线和抗老化等优越性能,长期在室外常温使用1~2 年耐黄变。
2、产品不含有任何溶剂和挥发物,无臭、无毒、具有环保、阻燃、耐用、韧性强、内接力好等优越性能。
3、固化后产品晶莹剔透,光亮透明。
4、产品固化时间的快慢可在温度高低控制,温度超高固超快,产品可手工灌胶和机械灌胶,固化时间的快慢,不会影响产品的性能。
三、可操作时间:在温度25~35℃,相对湿度<65%的无尘环境下将A/B 组份混合搅拌均匀,可操作时间20~25 分钟 。
四、组份配比:1、按重量比:A:B=1:12、在低温条件下,A 组份有可能变稠,B 组份有可能变稠和结晶(属正常现象),先将A/B 组份加温至60~65℃溶解透明(溶解参考方法:①用加热棒放入胶中直接进行加热,一边加热边搅拌至透明变稀即可停止;②将AB 组份物料分别倒入干净的不锈钢盆/桶/罐中,然后用电磁炉进行加热,一边加热一边搅拌至透明变稀即可停止;③将AB 组份物料分别倒入不锈钢容器中放入烤箱进行恒温加热至透明变稀即可停止),等加温的A/B 组份物料冷却至25~30℃时再按重量比称料混合搅拌。
五、容器与搅拌:1、调配器具:配胶壶或配胶罐、搅拌器必须是干净、干燥、没水分、没残留物、没有杂质,尽可能不要使用之前配用过其它胶水的配胶壶或配胶罐、搅拌器。
2、搅拌时必须按同一个方向缓慢搅拌,不能过于激烈,避免夹入气泡,搅拌时尽量刮到底部和边壁,缓慢搅拌2~5 分钟至均匀(搅拌时间需根据不同的物料而调整)。

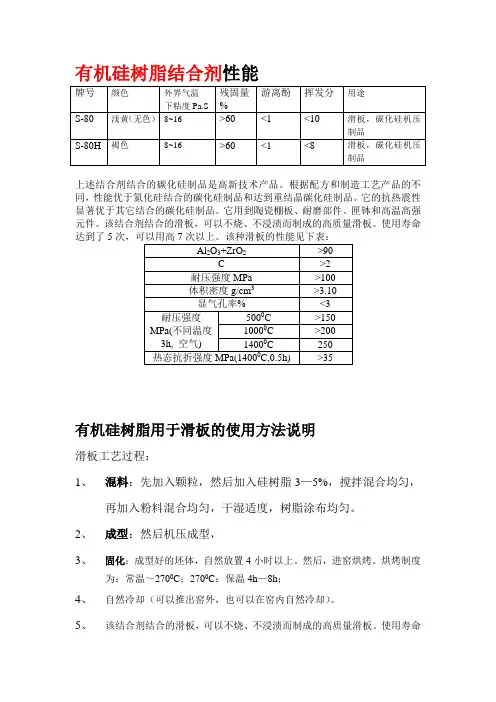
有机硅树脂结合剂性能
上述结合剂结合的碳化硅制品是高新技术产品。
根据配方和制造工艺产品的不同,性能优于氮化硅结合的碳化硅制品和达到重结晶碳化硅制品。
它的抗热震性显著优于其它结合的碳化硅制品。
它用到陶瓷棚板、耐磨部件、匣钵和高温高强元件。
该结合剂结合的滑板,可以不烧、不浸渍而制成的高质量滑板。
使用寿命达到了5
有机硅树脂用于滑板的使用方法说明
滑板工艺过程:
1、混料:先加入颗粒,然后加入硅树脂3—5%,搅拌混合均匀,
再加入粉料混合均匀,干湿适度,树脂涂布均匀。
2、成型:然后机压成型,
3、固化:成型好的坯体,自然放置4小时以上。
然后,进窑烘烤。
烘烤制度
为:常温~2700C;2700C:保温4h—8h;
4、自然冷却(可以推出窑外,也可以在窑内自然冷却)。
5、该结合剂结合的滑板,可以不烧、不浸渍而制成的高质量滑板。
使用寿命
达到了5次,可以用高7次以上。
注意事项:。



8SiliconesIn contrast to most organic polymers,in silicones the backbone is made of silicon and oxygen.Silicon is together with carbon in the fourth group of the periodic system,therefore a similar behavior of these elements can be expected.8.1HISTORYKipping∗started with the synthesis of organic silicon compounds by treat-ing SiCl4with magnesium-based organometallic compounds.These com-pounds are now called Grignard reagents,invented by Victor Grignard in 1900.Hyde†,at Corning,developed aflexible,high temperature binder for glassfibers and synthesized thefirst silicone polymer.The potential appli-cations in otherfields,such as electric industries soon became apparent.Eugene George Rochow‡at General Electric developed synthesis of silicones that is now used.1,2Hisfirst patent dates at1941.3,4In1949,the silly putty was invented by James Wright when mixing silicone oil with boric acid.Silly putty acts like both a rubber and a putty.∗Frederic Stanley Kipping,born in Upper Broughton(UK)1863,died in1949†James Franklin Hyde,born in Solvay,New York1903,died in1999‡Eugene George Rochow,born in Newark,New Jersey1909,died in2002321322Reactive Polymers Fundamentals and Applications8.2MONOMERS8.2.1ChlorosilanesThe synthesis of silanes and siloxanes starts from chlorosilanes such as di-methyldichlorosilane.Other products are derived from this compound that also serve as monomers.Thus,in silicone chemistry,the term monomer is not as clearly defined as in otherfields of polymer chemistry.8.2.2SilsesquioxanesSilsesquioxane resins are used in industrial applications in the automotive, aerospace,naval,and other manufacturing industries.Silsequioxane resins exhibit excellent heat andfire resistant properties that are desirable for such applications.These properties make the silsesquioxane resins attractive for use infiber-reinforced composites for electrical laminates,and structural use in automotive components,aircraft,and naval vessels.There is a need for rigid silsesquioxane resins that has increased flexural strength,flexural strain,fracture toughness,and fracture energy, without significant loss of modulus or loss of thermal stability.In addi-tion,rigid silsesquioxane resins have low dielectric constants and are use-ful as interlayer dielectric materials.Rigid silsesquioxane resins are also useful as abrasion resistant coatings.These applications require that the silsesquioxane resins exhibit high strength and toughness.5The formation of silsesquioxanes is shown in Figure8.1.Silsesqui-oxanes are organosilicon compounds with the formula[RSiO3/2]n.[R7Si7O9(OH)3],as shown in Figure8.1,can be synthesized in one step via the hydrolytic condensation of RSiCl3or RSi(OMe)3.A single Si-O-Si linkage in a fully condensed R8Si8O12framework can be cleaved selectively by strong acids(e.g.,HBF4/BF3or triflic acid.68.2.3Hydrogen SilsesquioxanesHydrogen-silsesquioxane resins are useful precursor substances for silica-containing ceramic coatings.Hydrogen silsesquioxane resins are ladder or cage polymers.7The general structure is shown in Figure8.2.When tri-chlorosilane is subjected to hydrolytic condensation caused by direct con-tact with water,the reaction occurs abruptly,and gels are formed.Accord-ingly,various methods for manufacturing hydrogen-silsesquioxane resinsSilicones 323Si Cl RH 2O Figure 8.1:Formation of Silsesquioxanes:[R 7Si 7O 9(OH )3]Si O Si O Si O SiOO O O O O Si O Si O Si O SiO Si OHSiHO HO H H H H H H H H n H HHSiSi O SiOSi O Si O Si H O Figure 8.2:Hydrogen silsesquioxane resins.7Top:Ladder Form,Bottom:Cage Form324Reactive Polymers Fundamentals and Applicationsthat do not form gels have been proposed.The hydrogen-silsesquioxane resin can be manufactured in an aromatic hydrocarbon solution of trichlo-rosilane.The hydrolytic condensation is then performed as a two-phase reaction with concentrated sulfuric acid.Concentrated sulfuric acid and aromatic hydrocarbon react to pro-duce an arylsulfonic acid hydrate,and the water in this hydrate contributes to the hydrolytic condensation of trichlorosilane.Therefore,the hydrogen-silsesquioxane resin produced by this hydrolytic condensation is obtained from the organic phase.When water is added to the concentrated sulfuric acid phase in or-der to recover and reuse the arylsulfonic acid,precipitation occurs,thus rendering the arylsulfonic acid unsuitable for reuse.For this reason,large quantities of organic solvent and sulfuric acid are lost using this method.A method for complete reuse of the solvent,the sulfuric acid and surfac-tants,essentially without loss of these compounds,has been described.The method utilizes a two-phase system consisting of an aqueous phase:1.An aqueous solution consisting of sulfuric acid and an organicsulfonic acid,e.g.,p-toluenesulfonic acid monohydrate,and2.The organic phase consisting of a diluted solution of organic sulf-onic acid in a halogenated hydrocarbon solvent.The trichlorosil-ane must be soluble in this solvent,and the solvent should not reactwith sulfuric acid.Examples are isopropyl chloride,chlorobenz-ene,and others.This method results in hydrogen-silsesquioxane resins at a high yield. The loss of the organic solvent used in the organic phase is small,and the precipitation of benzenesulfonic acid,etc.,in the aqueous phase due to su-persaturation can also be eliminated.The organic solvent,the sulfuric acid and the organic sulfonic acid used in the aqueous phase can be effectively reused.88.2.4Alkoxy SiloxanesExamples of alkoxy siloxanes are listed in Table8.1.Trifunctional silox-ane units and tetrafunctional siloxane units are used to improve the physical properties of curable epoxy resins.Branched silicone resins with trifunc-tional siloxane units are highly heat-resistant and have an excellent capac-ity forfilm-formation,which is why they are used as electrical insulatingSilicones325Table8.1:Epoxy-containing Siloxanes9SiloxaneMethyltrimethoxysilaneMethyltriethoxysilaneEthyltrimethoxysilaneEthyltriethoxysilaneVinyltrimethoxysilanePhenyltrimethoxysilane3,3,3-TrifluoropropyltrimethoxysilaneDimethyldimethoxysilaneMethylphenyldimethoxysilaneMethylvinyldimethoxysilaneDiphenyldimethoxysilaneDimethyldiethoxysilaneMethylphenyldiethoxysilaneTetramethoxysilaneTetraethoxysilane(TEOS)TetrapropoxysilaneDimethoxydiethoxysilanematerials,and heat-resistant paints and coatings.98.2.5Epoxy-modified SiloxanesSiloxanes with pendent epoxy groups are listed in Table8.2.Epoxy-con-taining silicone resins are prepared either by the co-hydrolysis and conden-sation of epoxy-containing trialkoxysilane and diorganodialkoxysilane or by the base-catalyzed equilibration polymerization of cyclic diorganosil-oxane and epoxy-containing trialkoxysilane.9Epoxy-containing silicone resins have broad molecular weight distributions and do not exhibit a soft-ening point or a distinct glass transition temperature.8.2.6SilaferrocenophanesSilaferrocenophanes are of considerable interest because they may serve as precursors to unusual ceramic materials.Polymers can be made by ring opening polymerization as shown in Figure8.3.Other ferroceno-phanes bridged by heteroatoms such as germanium and phosphorus have been synthesized.In the presence of methylphenylchlorosilane or diphen-ylchlorosilane,i.e.,silanes with pendent hydrogen,telechelic polymers can326Reactive Polymers Fundamentals and ApplicationsTable8.2:Epoxy-containing Siloxanes9Siloxane3-Glycidoxypropyl(methyl)dimethoxysilane3-Glycidoxypropyl(methyl)diethoxysilane3-Glycidoxypropyl(methyl)dibutoxysilane2-(3,4-Epoxycyclohexyl)ethyl(methyl)dimethoxysilane2-(3,4-Epoxycyclohexyl)ethyl(phenyl)diethoxysilane2,3-Epoxypropyl(methyl)dimethoxysilane2,3-Epoxypropyl(phenyl)dimethoxysilane3-Glycidoxypropyltrimethoxysilane(GLYMO)3-Glycidoxypropyltriethoxysilane3-Glycidoxypropyltributoxysilane2-(3,4-Epoxycyclohexyl)ethyltrimethoxysilane2-(3,4-Epoxycyclohexyl)ethyltriethoxysilane2,3-Epoxypropyltrimethoxysilane2,3-EpoxypropyltriethoxysilaneSi 33SiCH3CH3Figure8.3:Ring Opening Polymerization of SilaferrocenophanesSilicones327Table8.3:Products Obtained by the Rochow Synthesis10Silane Yields[%]Boiling Points[°C]Methyldichlorosilane0.541Methyltrichlorosilane8–1866Dimethyldichlorosilane80–8570Trimethylchlorosilane2–457be produced with the hydrogen bearing silanes as end group11,12Apart from silaferrocenophanes,ferrocenophanes with conjugated double bonds instead of silicon are of interest because of their electrical properties.13 8.2.7Synthesis8.2.7.1Direct SynthesisSilicones are synthesized via methylchlorosilanes by the Müller-Rochow process.The reaction is carried out at temperatures of250to300°C and 2to5bars.A copper catalyst used with antimony,cadmium,aluminum, zinc,and tin is effective for improving the activity.However,lead would act as an inhibitor.Afinely homogenized mixture of silicon and copper is introduced into afluidized bed reactor.The reactor isfluidized by gaseous methyl-chloride.The reactants are separated from the solid components and on cooling a crude liquid silane mixture is obtained.Silicon conversions of 90to98%and methylchloride conversions of30to90%can be achieved. The reaction is strongly exothermic and requires a precise control.Dimeth-yldichlorosilane is the main product.Other major products obtained are shown in Table8.3.The selectivity for producing dimethyldichlorosilane is highly sensitive to trace amounts of other metals present.The selectivity for dimethyldichlorosilane is reduced if the Cu,Zn,or Sn concentrations exceed the generally used concentrations or if the reaction temperature exceeds300°C.A silver promoter increases the selectivity to dimethyl-dichlorosilane.14,15The crude silane mixture is then separated in distilla-tion columns.A high separating capacity is needed,because the boiling points of CH3SiCl3and(CH3)2SiCl2differ by only4°C.A high purity is required,because even a small amount of CH3SiCl3leads to branched and eventually gelled products.328Reactive Polymers Fundamentals and Applications8.2.7.2HydrosilylationThe hydrosilylation reaction consists of the addition of hydrogen-contain-ing silanes to products with double or triple bonds.This reaction is suitable for introducing organo functions into silicone compounds.Therefore,hy-drosilylation is extensively used to synthesize organofunctional silicones with pendant vinyl groups,amino groups,etc.16In a further step,chlor-ine atoms,hydrogen atoms,and alkoxy groups can undergo a nucleophilic substitution.The hydrosilylation reaction requires often high temperatures. Vinyl Groups.The hydrosilylation of aromatic compounds containing vinyl unsaturation can lead to radical polymerization of the monovinylaro-matic compounds,especially at elevated temperature.The use of radical polymerization inhibitors,such as phenols or quinones,is often necessary, however,most of these inhibitors are not sufficiently active at elevated temperatures and require the presence of oxygen to improve their activ-ity.However,special conditions and precautions make the use of a radical polymerization inhibitor unnecessary.Styrene andα-methylstyrene can be hydrosilylated with heptamethyltrisiloxane with a Karstedt platinum cata-lyst at90°C.17When4-vinyl-1-cyclohexene is reacted with a hydrogenchlorosil-ane,both the vinylic double bond and the double bond in the cyclohexene ring react.Thereby an organic silicon compound of the formula given in Figure8.4is obtained in which the hydrogenchlorosilane is added to each of the two double bonds in4-vinyl-1-cyclohexene.The cyclohexane ring within the molecule imparts a high hardness and scratch resistance and is useful as a coupling agent to be added to paints for use in automobiles, buildings and adhesives.The compound is also useful as an intermediate to an alkoxysilane coupling agent.188.2.7.3Grignard SynthesisThe Grignard synthesis is suitable to introduce organic groups to silicon. The Grignard synthesis is used on a laboratory scale.An example for a Grignard synthesis is shown in Figure8.5.With water,methylphenyldi-chlorosilane condenses to a linear polymer.Silicones329Si H CH 3Cl Cl Si H CH 3Cl Cl SiSi CH 3Cl ClCl Cl CH 3SiCH 3Cl Si CH 3ClCl +++Figure 8.4:Hydrosilylation of 4-Vinyl-1-cyclohexeneMg Br 2CH 3SiClCl Cl +SiClCl CH 2CH 3MgClBr+Mg Br Si ClCl +Si Cl Cl Cl MgClBr+Figure 8.5:Grignard Synthesis330Reactive Polymers Fundamentals and Applications8.2.7.4CondensationHydrolysis of chlorosilanes results in silanols.These silanols are not stable and undergo a polycondensation.Intramolecular and intermolecular con-densation takes place.Intermolecular condensation yields linear siloxanes, and intramolecular condensation yields cyclic products.When trichloro-silanes undergo hydrolysis,highly crosslinked silicone resins are obtained. The reaction can be catalyzed by acids.An equilibrium between the linear siloxanes and cyclic siloxanes can be established.If the catalyst is deactivated,the condensation stops and the cyclic products that consist mostly of a tetramer can be removed by distillation. On the other hand,cyclic siloxanes can be transformed to polymers in the presence of alkali.If the catalyst is not deactivated then cyclic siloxane forms until the equilibrium is established.In equilibrium ca.20%of cyclic products are present,which is relevant to the recycling of polysiloxanes.Chain Stoppers.To obtain stable or functional terminal groups,chain stoppers are added.The reaction proceeds under continuous cleavage and recombination of siloxane bonds.The reaction is catalyzed by acids.Bodying.Bodying is a technology that consists of the base-catalyzed depletion of the silanol groups in a silicone resin prepared by the hydro-lysis and condensation of organoalkoxysilane.In this process the molec-ular weight of the silicone resin is simply increased,while control of the molecular weight,softening point,and glass transition temperature is not possible.9Crosslinking.The degree of crosslinking depends on the presence of either tetrachlorosilane SiCl4for the production of very rigid resins,or (CH3)2SiCl2for softer grades.8.2.8ManufactureCommercially produced silicone resins comprise:•Non-meltable solids•Soluble reactive resins•Silsesquioxanes•High reactive alkoxysiloxanes with molecular weight.8.3MODIFIED TYPES8.3.1Chemical ModificationsReactive alkoxysiloxanes can undergo a reaction with functional organic resins.The modification of methylpolysiloxanes is achieved by substitut-ing the methyl groups with other organic groups,e.g.,lower alkyl chains or functional groups like vinyl groups,or by copolymerization with organic polymers,e.g.,poly(ethylene oxide)or poly(propylene oxide).8.3.1.1Amine FunctionsAminofunctional silicones impart extreme softness.Such materials are ap-preciated in textiles because of the improved wear comfort.In textile dye-ing uniformity of colorfixation is achieved by efficient control of foaming in the dyeing bath.8.3.1.2Functionalized SilanesReactive silanes or siloxanes can also include functionalities such as:vin-yl,hydride,allyl,or other unsaturated groups.For surface coating,hexa-methyldisiloxane and tetramethyldivinyldisiloxane are used.5Mixtures of siloxanes with trimethyl silyl groups and dimethylvinyl silyl groups are also common.8.3.1.3Crosslinking AgentsCrosslinking agents include alkoxysilanes such as methyltrimethoxysilane, dimethyldimethoxysilane,etc.,or oxime silanes,for example,methyltris-(methylethylketoxime)silane.19Crosslinking accelerators include amines, tin compounds such as dibutyltin diacetate,or dibutyltin dilaurate.198.3.2FillersThe silicone network does not exhibit much mechanical strength.Mechan-ical strength is imparted by the interaction of afiller with the polymer. Fumed silica shows the strongest reinforcing effect.Otherfillers include quartzflour,iron oxide,and carbon black.8.3.3Reinforcing MaterialsFiber reinforced,silicone matrix resin compositesfind many applications in structural components.Fiber reinforcement often takes the form of wo-ven glassfiber mats.Woven carbonfiber mats offer a higher modulus reinforcing media,but they are more expensive than glassfibers.Other fiber compositions such as aramid,nylon,polyester,and quartzfibers may be used for reinforcement.Otherfibrous forms,such as non-woven mats and layers of loosefibers,may also be used in silicone-based composite applications.20Fiber reinforced,silicone matrix resin composites in multilayer lam-inated form are strong andfire resistant.Theyfind applications in interiors of airplanes and ships.They are also used in electrical applications,such as wiring boards and printed circuit boards,requiringflexural strength and low weight.Suitable resin types are typically highly branched and crosslinked polymer molecules,when cured.To facilitate the impregnation process, silicone precursor formulations may be diluted with toluene.The toluene is then evaporated from the composite.8.4CURING8.4.1Curing by CondensationCuring by condensation releases alcohol,amines,acetic acid,or other volatile reaction products.The polymerization reaction does proceed in the absence of wa-ter.This fact is utilized in one component systems that form polymers by means of atmospheric humidity.To avoid premature curing,the com-ponents are packed in compartments that are free of moisture and tight to permeation of moisture.Methoxysilanes can condense with chlorosilanes releasing methyl-chloride,21as shown in Figure8.6.The reaction is catalyzed by ferric chloride.8.4.1.1Platinum Complexes for HydrosylilationAdditional crosslinking occurs by reaction of compounds with pendant vinyl groups.Certain platinum complexes catalyze the hydrosylilation re-Si CH 33O O CH 3CH 3Si CH 3Cl+Si CH 3CH 3O CH 3Si CH 3CH 3O ClFigure 8.6:Condensation of Methoxysilanes with Chlorosilanesaction.Suitable platinum catalysts are chloroplatinic acid,dichlorobis(tri-phenylphosphine)platinum(II),platinum chloride,platinum oxide,and also complexes of platinum compounds.For example,a Karstedt catalyst is a complex of chloroplatinic acid with 1,3-divinyl-1,1,3,3-tetramethyldisilox-ane and 1,3-diethenyl-1,1,3,3-tetramethyldisiloxane.5Synergistic catalyst systems are mixtures of the compounds H 2PtCl 6and RuCl 3×nH 2O.22The hydrosilylation reaction proceeds at room tem-perature.However,using inhibitors the temperature can be increased.8.4.1.2Hydrosilylation InhibitorsHydrosilylation inhibitors fall into two general classes.23One class is com-posed of materials that effectively inhibit hydrosilylation over a wide range of temperatures and can be volatilized out of the organosilicon composition to allow hydrosilylation to proceed.Examples of this class are pyridine,acrylonitrile,2-ethenylisopropanol,and perchloroethylene.The other class of inhibitors is materials that are non-volatile.The inhibitory effect of these materials is overcome by heating,whereupon hydrosilylation takes place.Examples of this latter class are the reac-tion product of a siloxane having silicon-bonded hydrogen atoms,a plat-inum catalyst,and an acetylenic alcohol,organic phosphines and phos-phites,benzotriazole,organic sulfoxides,metallic salts,aminofunctional siloxanes,ethylenically unsaturated isocyanurates olefinic siloxanes,di-alkyl carboxylic esters,and unsaturated amides.Examples of inhibitors are shown in Table 8.4.In polyethers,oxidation impurities inhibit the hydrosilylation of the polyethers,however,the exact identities of these inhibitors are unknown.They are believed to include acetal hydroperoxides,allyl hydroperoxides and free radicals localized at the tertiary carbon atoms in the hydrophobicTable8.4:Inhibitors for Platinum CatalystsInhibitor RemarksMethylbutynolEthynyl cyclohexanol Most preferred5Diphenylphosphine3-Methyl-1-dodecyn-3-ol Release coatings243,7,11-Trimethyl-1-dodecyn-3-olsegments(e.g.,propylene oxide)of unsaturated polyethers.Oxidation im-purities are most likely to occur in polyethers which have been stored for a long period with no or insufficient quantities of antioxidant.However,they may also be present in freshly prepared polyethers which may have gotten too hot in the presence of air or oxygen.Polyethers can be stabilized with mixtures of ascorbic acid and sodium ascorbate and allyl polyethers.25 8.4.1.3SaltsA commercially available curing catalyst material comprises zinc octoate and choline octoate.208.4.1.4PolymethylsilazanesPolymethylsilazanes are synthesized by the reaction of ammonia with di-methyldichlorosilane and methyltrichlorosilane.They are effective room temperature curing agents for silicone resins.However,ammonia is re-leased in the course of curing.268.5CROSSLINKINGCrosslinking can be achieved by different reactions at high temperatures for HTV-rubber and at room temperature for RTV-rubber.The liquid RTV-silicone rubber can crosslink both by condensation and by addition mech-anisms.8.5.1Condensation CrosslinkingCondensation crosslinking occurs betweenα,ω-dihydroxypoly(dimethyl-siloxane)s and silicates in the presence of inorganic compounds.The cross-linking density depends on the functionality and concentration of the cross-linking agent and the nature of the catalyst.8.5.2PeroxidesCrosslinking at higher temperatures in the range100to160°C is achieved by the addition of peroxides.Suitable formulations contain a small amount of vinyl groups.8.5.3Hydrosilylation CrosslinkingThe hydrosilylation reaction is suitable forfinal crosslinking or curing re-actions.8.5.3.1Thermoplastic ElastomersHydrosilylation crosslinking can be used to prepare a thermoplastic elas-tomer.A thermoplastic elastomer is a polymer or polymeric blend that can be processed and recycled in the same way as a conventional thermoplas-tic material.However,it has some properties and functional performance similar to those of vulcanized rubber at the service temperature.Elastomeric rubber blends are used in the production of high perfor-mance thermoplastic elastomers,particularly for the replacement of ther-moset rubbers in various applications.High performance thermoplastic elastomers,in which a highly vulcanized rubbery polymer is intimately dispersed in a thermoplastic matrix,are generally known as thermoplastic vulcanizates.Hydrosilylation crosslinking of a rubber acts via the unsatur-ated groups present from norbornene and diene components.Even at low concentrations of hydrosilylation agent and catalyst,a rubber can be fully crosslinked in a dynamic vulcanization process and provide a thermoplas-tic elastomer product with excellent physical properties and oil resistance.Suitable hydrosilylation agents are methylhydrogen polysiloxanes, methylhydrogen dimethyl-siloxane copolymers,bis(dimethylsilyl)alkanes, and bis(dimethylsilyl)benzene.27Platinum catalyst concentrations of0.1 to4ppm are sufficient.The preparation is done by mixing the rubber and silicone hydride at180°C.Then a solution of the platinum catalyst is added.The rubber is dynamically vulcanized by mixing until maximum torque is reached.8.6PROPERTIES8.6.1Silicone RubberSilicone rubber consists essentially of silicone polymers andfillers.Silic-one rubber formulations with molecular weights of more than100kDaltonand canflow,in contrast to other polymers.8.6.1.1HTV-silicone rubberSilicone polymers for solid silicone rubber(HTV-silicone rubber)havemolecular weights of500kDalton to1000kDalton,yet exhibit a pastyconsistency.8.6.1.2RTV-silicone rubberPourable silicone rubber(RTV-silicone rubber)has a liquid consistencyand molecular weights in the range of10kDalton to20kDalton.8.6.2Thermal PropertiesThe service temperatures of silicones cover a wide range,from−60to +250°C.Silicone rubber retains its elasticity to temperatures down to −60°C.The glass transition temperature is120°C.At temperatures greater than150°C silicone rubbers are superior to other elastomers with respect totheir thermal properties.28Silicone rubber exhibits aflash point of750°Cand an excellentflame retardancy.However,on combustion,it releasestoxic or corrosive gases.8.6.2.1Boron Siloxane CopolymersPolymers containing boron within the polymer are high temperature oxida-tively stable materials.It has been known that the addition of a carboranewithin a siloxane polymer significantly increases the thermal stability ofsuch siloxane polymers.29Hybrids of organic and inorganic components,made from1,7-Bis(chlorotetramethyldisiloxy)-m-carborane,1,3-dichloro-tetramethyldisiloxane and1,4-dilithio-1,3-butadiyne are shown in Figure8.7.Oxidative crosslinking in air is found for poly(m-carborane-di-methyl-siloxane)around420°C.21Such polymers can be converted into ceramicsSi OSi C CSi OSi CH 3CH 3CH 3CH 3Z H 3C H 3C H 3C H 3C CCCCSi O Si 33H 3C H 3C Si O Cl Si C C Si O Si Cl CH 33CH 33CH 33CH 33Z Z=B 10H 10C C Li Li Si O Si Cl ClCH 3CH 333Figure 8.7:Polymers from 1,7-Bis(chlorotetramethyldisiloxy)-m -carborane,1,3-dichlorotetramethyldisiloxane and 1,4-dilithio-1,3-butadiyneby pyrolysis.Carbonfibers coated with poly(carborane-siloxane-acetyl-ene)can be protected against oxidation at elevated temperatures.308.6.2.2Microcellular CeramicsMicrocellular foams were produced by means of poly(methyl methacryl-ate)as a sacrificial templating agent.Poly(methyl methacrylate)micro-beads,were mixed in with a methylsilicone resin powder.The samples were heated up to300°C and after one hour pyrolyzed at1200°C.A sili-con oxycarbide(SiOC)ceramic microcellular foam was obtained.318.6.3Electrical PropertiesSilicone rubbers and resins are very efficient in insulating.The dielectric strength,the resistivity,and the dielectric constant do not change signifi-cantly with temperature.8.6.4Surface Tension PropertiesUnmodified silicones exhibit hydrophobic properties.When spread out asfilms they impart water repellency to the carrier material.The surface tension is only around30mN/m.Silazanes significantly improve water-repellent properties of silic-one resins.19Examples of hexaorganodisilazanes include hexamethyldis-ilazane,1,3-dihexyltetramethyldisilazane,1,3-di-tert-butyltetramethyldis-ilazane,1,3-di-n-butyltetramethyldisilazane,and1,3-diphenyltetramethyl-disilazane.8.6.5AntioxidantsIron-containing polysilazanes exhibit an antioxidation effect on silicone oil and rubber.32This type of polymer was synthesized by the polycondensa-tion of silazane lithium salts with iron trichloride.The synthesis is shown in Figure8.8.The gelling time of a silicone oil increased from3to1000 hours at300°C in air by an addition of5%of polysilazane.8.6.6Gas PermeabilitySilicones have an extraordinarily high gas permeability.Theyfind use in medical applications,e.g.,as contact lenses,so that the oxygen in air canSi N Si N Si N CH 3H 3C H 3C H 3CH H CH 3BuLi SiN Si N Si N CH 33H 3C H 3C H 3C H H CH 3Li SiN Si N Si N CH 33H 3C H 3C H 3CH Li CH 3Li BuLi Si N Si N Si N CH 33H 3C H 3C H 3C Li Li CH 3Li Si Si N CH 3H 3C CH 3CH 3Si Si N 3H 3H 3C Li H 3C Si Si NCH 3H 3CH 3CH 3Li FeCl 3Si Si N CH 3H 3CH 33Li Fe Li CH 3H 3C CH 3N Si Si Li H 3C H 3CH 3NSi SiCH 3H 3C Figure 8.8:Synthesis of Iron-containing Polysilazanes。



有机硅是一种重要的高分子材料,广泛应用于建筑、电子、纺织、医疗等领域。
以下是一些关于有机硅的专业书籍推荐:1. 《有机硅化学》:这本书详细介绍了有机硅的化学性质、合成方法和应用技术,是学习有机硅化学的基础教材。
2. 《有机硅材料科学》:这本书从材料科学的角度出发,系统地介绍了有机硅的基本性质、结构与性能关系、加工工艺和应用技术。
3. 《有机硅聚合物》:这本书主要介绍了有机硅聚合物的合成、结构与性能、加工和应用,对于研究有机硅聚合物的学者和工程师具有很高的参考价值。
4. 《有机硅材料及其应用》:这本书详细介绍了有机硅材料的分类、性质、制备方法和应用领域,对于从事有机硅材料研究和开发的人员具有很大的帮助。
5. 《有机硅橡胶》:这本书主要介绍了有机硅橡胶的合成、结构与性能、加工工艺和应用,对于研究有机硅橡胶的学者和工程师具有很高的参考价值。
6. 《有机硅涂料》:这本书详细介绍了有机硅涂料的组成、性能、制备方法和应用领域,对于从事有机硅涂料研究和开发的人员具有很大的帮助。
7. 《有机硅电子材料》:这本书主要介绍了有机硅电子材料的合成、结构与性能、加工工艺和应用,对于研究有机硅电子材料的学者和工程师具有很高的参考价值。
8. 《有机硅光学材料》:这本书详细介绍了有机硅光学材料的合成、结构与性能、加工工艺和应用,对于从事有机硅光学材料研究和开发的人员具有很大的帮助。
9. 《有机硅生物医学材料》:这本书主要介绍了有机硅生物医学材料的合成、结构与性能、加工工艺和应用,对于研究有机硅生物医学材料的学者和工程师具有很高的参考价值。
10. 《有机硅纳米材料》:这本书详细介绍了有机硅纳米材料的合成、结构与性能、加工工艺和应用,对于从事有机硅纳米材料研究和开发的人员具有很大的帮助。

有机硅树脂相关参考书有机硅树脂,这个名字听起来像是个高大上的化学术语,其实没那么复杂。
想象一下,你手里拿着一瓶透明的液体,像是魔法药水一样。
哇,这可不是普通的胶水哦,这可是有机硅树脂!它在我们生活中可谓是无处不在,给我们的生活带来了不少便利。
咱们得先聊聊它的特性,像防水、防腐蚀,这些可都是它的强项。
你说这东西有多牛?嘿,居然能把水珠打得飞起,真是名副其实的“水性涂鸦”!再来聊聊它的用途。
有没有想过,平常我们用的洗碗液、沐浴露,里面可能就有有机硅的身影。
想象一下,洗完碗,手上还留着一丝丝滑滑的感觉,那是因为有机硅在里面“加分”呢。
还有那些高档化妆品,光是闻上去就像春天的花朵,实际上可能也是靠它来增加光泽感和滑顺度。
是不是觉得有机硅树脂真的是个万金油?再说说这玩意儿的制作过程。
听说这些小分子在工厂里可是大显身手,经过一系列的化学反应,变成我们所需的树脂。
简直就像变魔术一样,最初的液体经过搅拌、加热,再加上一些神秘的成分,最后呈现出光彩照人的产品。
是不是觉得化学也可以这么有趣?有机硅树脂的好处可不仅限于此。
比如它的耐热性,嘿,咱们做菜的时候,有时锅里的油可热得很呢。
这时候,如果用上有机硅树脂的锅具,那就放心大胆了,绝对不会被烫坏。
它还有个超厉害的功能,就是不易燃!想想看,万一厨房里出现了小火苗,有机硅可会帮你一把,让火焰变得不再那么可怕。
再说到环保,这可是现代人的热门话题。
有机硅树脂一般比较稳定,不容易分解,少了有害物质释放,能算得上是环保材料的一员。
是不是觉得这小东西竟然还有这样的责任感?真是不得不让人赞叹。
许多厂家也在不断研发新技术,力求让有机硅树脂在使用后能更好地回收利用,真的是在为地球出力。
再提到一些更高端的应用,像航空航天、电子设备,这些领域可是对材料的要求特别高。
有机硅树脂在这些地方表现得就像个超级英雄,能够承受极端环境,保证设备的正常运作。
咱们用的手机、电脑,里面可能就藏着有机硅的身影,真是让人心里默默点赞。
