Role of Mitochondria in Parvovirus Pathology
- 格式:pdf
- 大小:2.92 MB
- 文档页数:10

靶向哺乳动物细胞线粒体的核酸转运付爱玲【摘要】Mitochondrial DNA (mtDNA)genome mutations and defects are the essential mechanism of a various of mitochondrial dysfunction associated with diseases. The studies of targeting de-livery nucleic acid into mammalian mitochondria can thoroughly correct mtDNA mutation, rescue mtDNA impairment and then reverse the progress of diseases. There’s obvious differences be-tween nucleic acid import pathway of mammalian mitochondria and gene transfection of nuclei. In this paper, the effective strat-egies of delivering DNA and RNA(tRNA,rRNA,mRNA and an-tisense RNA)into mitochondria have been reviewed, as well as the challenges and development.%线粒体 DNA(mitochondrial DNA, mtDNA)的遗传性突变和缺陷是多种线粒体功能失调相关疾病的根本原因。
靶向线粒体递送核酸,可从根本上纠正 mtDNA 突变、挽救 mtD-NA 损伤、阻断疾病进程。
哺乳细胞内线粒体的核酸转运途径与细胞核的基因转染大不相同。
该文综述了向哺乳动物细胞线粒体递送 DNA 和 RNA(tRNA、rRNA、mRNA 和反义RNA)的有效策略,并对其存在问题和发展趋势做一阐述。


MIT大牛卢冠达:巧借CRISPR,首次发明人体细胞DNA“录音机”生物探索编者按提起MIT的年轻华裔科学家,大家可能对CRISPR领域的张锋比较熟悉。
今天,小编要介绍的却是另外一位“80后”大牛,学术背景横跨电气工程、计算机科学、合成生物学以及医学等多个领域的卢冠达博士。
近日,他带领的研究小组在Science发表了最新研究成果:利用CRISPR技术,首次开发出了人体细胞DNA“录音机”。
Timothy K. Lu博士(图片来源MIT)8月18日,卢冠达博士发表的这项新成果声称,其研究小组开发出一种记录人类细胞DNA复杂历史的方法。
据悉,这是首个可以记录人类细胞中事件持续时间和/或强度的模拟记忆存储系统。
而这一研究中的新方法借助了目前生命科学领域非常热门的基因编辑系统CRISPR/Cas9。
近几年,除卢冠达博士外,一些其他研究人员也曾设计出一些方法用于记录细菌细胞中的这种模拟信息。
但值得注意的是,此前,一直没有人在人类细胞中做到这一点。
就在上周,这一领域迎来了新的进展。
事实上,早在2014年11月14日,卢冠达(Timothy K. Lu)博士就曾在Science上发表过一篇题为“Genomically encoded analog memory with precise in vivo DNA writing in living cell populations”的论文。
在这一研究中,他带领的研究小组发明了一种DNA“录音机”,可将DNA转化为可读的形式,记录细菌细胞几个星期的生活史,描述活细胞各种各样的记忆。
1特殊设计据介绍,以往利用CRISPR技术编辑基因时,研究人员需要创建一个与靶序列相匹配的RNA向导链。
为了编码记忆,卢冠达博士的研究小组采用了不同的方法:他们设计的向导链能够识别编码相同向导链的DNA,即自我靶向向导RNA(self-targeting guide RNA)。
由于大部分的突变会导致DNA序列的部分缺失,研究人员设计的RNA向导链长于通常的20个核苷酸。

mTORC1/2双重抑制剂OSI -027抑制高氧诱导的肺成纤维细胞增殖和分化*吴黎虹, 唐坤, 党红星△, 符跃强, 刘成军, 李静, 许峰(重庆医科大学附属儿童医院重症医学科,国家儿童健康与疾病临床医学研究中心,儿童发育疾病研究教育部重点实验室,儿科学重庆市重点实验室,重庆 400014)[摘要] 目的:分析哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin , mTOR )复合物1/2(mTORcomplex 1/2, mTORC1/2)双重抑制剂OSI -027对高体积分数氧(高氧)所致人胚肺成纤维细胞增殖和分化的抑制作用。
方法:高氧(95% O 2)处理人胚肺成纤维细胞MRC -5建立增殖分化模型,分为对照组、高氧组、高氧+OSI -027组和高氧+雷帕霉素组。
Western blot 检测α-平滑肌肌动蛋白(α-smooth muscle actin , α-SMA )、I 型胶原蛋白(collagen type I , Col I )、增殖细胞核抗原(proliferating cell nuclear antigen , PCNA )、细胞周期蛋白D1(cyclin D1)、RhoA 、Rho 相关含卷曲螺旋蛋白激酶1(Rho -associated coiled -coil -containing protein kinase 1, ROCK1)、蛋白激酶B (protein kinase B , PKB/AKT )、p -AKT 和mTOR 的表达; CCK -8实验检测细胞活力;流式细胞术检测细胞周期。
结果:与对照组相比,PCNA 、cyclin D1、Col I 和α-SMA 表达随高氧处理时间增加而增加(P <0.05)。
与高氧组相比,OSI -027及雷帕霉素干预后,细胞活力下降,细胞周期被抑制在G 1期(P <0.05)。

Science细述基因组卫士piRNA的故事与绝大多数动物一样,人类基因组不少都来自于一种自私的DNA链——转座子。
这类遗传物质能够在染色体不同位点间跳跃,导致基因失活甚至引发癌症。
在生殖细胞系中,转座子的跳跃还可能导致不孕。
“对于绝大多数动物来说,无法控制转座子都会最终导致物种灭绝,”麻省大学医学院的生化遗传学家Phillip Zamore说。
正因如此,一类特殊的RNA分子(piRNA)就成了动物基因组的大英雄。
piRNA发现于2006年,在动物生殖细胞系中它与特定蛋白一同束缚转座子。
这种蛋白- RNA的组合形成了一个分子防御系统,科学家们将其比作基因组的免疫系统。
与免疫系统相似,piRNA系统能够区分敌我,启动应答,并且去适应新出现的入侵者。
这些基因组卫士们还能够记住曾经入侵的转座子。
在进化过程中piRNA的复杂性曾经历了爆炸式增长,科学家们认为人体内的piRNA 总共可能有数百万种。
近年来,研究人员开始慢慢理解piRNA约束转座子的机制,但人们依然不了解细胞制造piRNA的过程,也不清楚这些RNA在生殖细胞系以外还有何功能。
Zamore指出,在哺乳动物中piRNA沉默转座子只是其功能的一小部分,尽管这也是人们目前唯一了解的部分。
2012年前后piRNA研究领域开启了令人兴奋的新时代,该领域的重要成果纷纷登上Science、Nature、Cell等顶尖杂志。
本期Science杂志就对近来的piRNA研究进行了系统性总结和展望。
piRNA的发现piRNA的发现始于Piwi蛋白研究,该蛋白是一些动物繁衍后代所必需的。
2006年Alexei Aravin和Gregory Hannon两个研究小组几乎同时在Nature和Science杂志发表文章,他们在小鼠体内发现了数千种与Piwi蛋白合作的小RNA。
人们将这类RNA称为Piwi-互作RNA或piRNA。
piRNA与microRNAs、siRNA有许多不同之处,Dicer酶是microRNAs 和siRNA成熟所必需的酶,但piRNA生成并不需要这种酶。
2008最佳生物显微照片:柄翅卵蜂居首中国网时间:2008-12-04柄翅卵蜂新浪科技讯北京时间12月4日消息,据国外媒体报道,2008年奥林巴斯生物数字(Olympus BioScapes)显微摄影大赛于日前揭晓。
来自英国的作品“柄翅卵蜂”夺冠,清晰的呈现了这种为世界上最小的昆虫之一的黄蜂种类,长度仅为0.21毫米。
:以下为本次大赛的前十名获奖作品:第一名:柄翅卵蜂作者:斯派克·沃尔克(Spike Walker)城市:英国斯塔福德郡彭克里奇技术:莱因伯格照明法玛瑙石化木第二名:玛瑙石化木作者:托马斯·谢尔勒(Thomas Shearer)城市:美国明尼苏达州德卢斯技术:偏振光第三名:第三纪层贵藻类化石作者:斯蒂芬·纳吉(Stephen Nagy)城市:美国明尼苏达州赫勒拿技术:Jamin-Lebedeff干涉相衬第四名:彩虹小斑马鱼作者:艾伯特·潘(Albert Pan)单位:美国哈佛大学技术:荧光第五名:串叶松香草作者:雪莉·欧文斯(Shirley Owens)城市:美国法拉盛技术:激光扫描共聚焦第六名:蜗牛齿舌作者:戴维·沃尔克(David Walker)城市:英国西约克郡哈德斯菲尔德技术:偏振光第七名:成年老鼠的海马体作者:尼尔·梅尔文(Neal Melvin)城市:美国得克萨斯州达拉斯技术:荧光第八名:虱性车轮虫作者:戈德·古恩斯(Gerd Günther)城市:德国杜塞尔多夫技术:微分干涉相衬第九名:玉虫作者:查尔斯·克莱布斯(Charles Krebs)城市:美国伊萨夸技术:立体显微镜第十名:星状硅藻类作者:皮特·兹纳克尔(Petr Znachor)单位:捷克巴德杰维契水生生物研究所技术:荧光(孝文)。
Journal of Kunming Medical UniversityCN 53-1221R昆明医科大学学报2019,40(1):118耀122线粒体和胚胎发育关系的研究进展张尊月1,2,3),李坪4),唐莉1),王昆华2,3),龙艳喜1),王华伟1,2,3)(1)昆明医科大学第一附属医院生殖遗传科,云南昆明650032;2)云南省消化病研究所,云南昆明650032;3)云南省消化疾病工程技术中心,云南昆明650032;4)昆明医科大学人体解剖学与组织胚胎学系,云南昆明650500)[摘要]线粒体是卵母细胞或胚胎细胞中数量最多的细胞器之一,提供细胞90%以上所需的能量,同时参与细胞周期的全部过程。
在卵子、精子或胚胎发育过程中,线粒体的数量、分布及活性均受到严密有序的调控。
卵母细胞老化、人类辅助生殖技术对卵子、精子老化、胚胎的处理手段、细胞内氧化应激反应或线粒体基因突变等均会影响线粒体功能,进而导致卵子或精子质量差,进而影响胚胎发育潜能。
[关键词]线粒体;卵母细胞;胚胎发育[中图分类号]R715.5[文献标志码]A [文章编号]2095-610X (2019)01-01118-05Research Progress on the Relationship between Mitochondriaand Embryonic DevelopmentZHANG Zun-yue 1,2,3),LI Ping 4),TANG Li 1),WANG Kun-hua 2,3),LONG Yan-xi 1),WANG Hua-wei 1,2,3)(1)Department of reproduction and genetics ,The First Affiliated Hospital of Kunming Medical University ,Kunming Yunnan 650032;2)Yunnan Institute of Digestive Disease ,the First Affiliated Hospital of Kunming Medical University ,Kunming Y unnan 650032;3)Y unnan Engineering Technology Research Center of Digestive Disease ,The First Affiliated Hospital of Kunming Medical University ,Kunming 650032;4)Department of Human Anatomy and Histology/Embryology ,Kunming Medical University ,Yunnan Kunming650500,China )[Abstract ]Mitochondria is one of the most abundant organelles in oocytes or embryos ,providing almost allthe energy required by cells and participating in the necessary process of cell survival and development .During the development of oocytes ,sperm or embryos ,the number ,distribution and activity of mitochondria are closely and orderly regulated.Oocytes aging and the treatment of oocytes or embryos by human assisted reproduction technology ,the oxidative stress response in cells or the mutation of mitochondrial gene can all affect the mitochondrial function ,thus lead to poor quality of oocytes or sperm and further affect the potential of embryo development.[Key words ]Mitochondria ;Oocyte ;Embryonic development [收稿日期]2018-10-19收稿[基金项目]国家自然科学基金资助项目(31100769,3171101074);云南省科技厅-昆明医科大学项目(2017FE467-130);云南省卫生厅项目(2017NS002,2018NS084);昆明医科大学生殖与遗传科技创新团队项目(CXTD201708)[作者简介]张尊月(1989~),女,黑龙江鸡西人,博士,助理研究员,主要从事生殖遗传学研究工作。
生物的英语试题及答案一、选择题(每题1分,共10分)1. What is the basic unit of life?A. CellB. OrganC. TissueD. System2. Which of the following is not a characteristic of living organisms?A. GrowthB. ReproductionC. RespirationD. Inertia3. What is the process by which plants convert sunlight into energy?A. RespirationB. PhotosynthesisC. FermentationD. Digestion4. Which of the following is a type of genetic mutation?A. Gene duplicationB. Chromosomal deletionC. Both A and BD. None of the above5. What is the term for the study of the relationships among species?A. TaxonomyB. PhylogeneticsC. EcologyD. Ethology6. What is the primary function of the mitochondria in a cell?A. DNA replicationB. Protein synthesisC. Energy productionD. Waste disposal7. Which of the following is a hormone?A. InsulinB. GlucoseC. OxygenD. Carbon dioxide8. What is the correct sequence of the biologicalclassification hierarchy?A. Kingdom, Phylum, Class, Order, Family, Genus, SpeciesB. Species, Genus, Family, Order, Class, Phylum, KingdomC. Kingdom, Species, Genus, Family, Order, Class, PhylumD. Phylum, Class, Order, Family, Species, Genus, Kingdom9. What is the process by which new species arise?A. EvolutionB. Natural selectionC. SpeciationD. All of the above10. What is the role of chlorophyll in photosynthesis?A. To absorb light energyB. To produce waterC. To release oxygenD. To store energy二、填空题(每题1分,共5分)11. The process by which an organism develops from a single cell to a mature individual is called ________.12. The study of the structure of organisms is known as________.13. In genetics, the basic unit of heredity is the ________.14. The largest organ in the human body is the ________.15. The scientific method of classifying organisms based on evolutionary relationships is called ________.三、简答题(每题5分,共10分)16. Explain the role of DNA in the cell.17. Describe the process of cellular respiration.四、论述题(每题15分,共15分)18. Discuss the importance of biodiversity and the threats it faces.答案:一、选择题1-5: A, D, B, C, B6-10: C, A, A, A, A二、填空题11. Development12. Anatomy13. Gene14. Skin15. Phylogenetics三、简答题16. DNA is the molecule that carries the genetic instructions used in the growth, development, functioning, andreproduction of all known living organisms and many viruses.It is the blueprint for the organism's traits and functions. 17. Cellular respiration is the process by which cellsconvert nutrients into energy in the form of ATP (adenosine triphosphate). It involves the breakdown of glucose in the presence of oxygen to produce carbon dioxide, water, and energy.四、论述题18. Biodiversity is crucial for the health of ecosystems, asit ensures the stability and resilience of these systems. It provides a variety of ecosystem services such as pollination, pest control, and nutrient cycling. Threats to biodiversity include habitat destruction, climate change, overexploitation, pollution, and the introduction of invasive species.结束语:通过这份生物英语试题及答案,我们不仅复习了生物学的基本概念和过程,还加深了对生物多样性重要性及其面临的挑战的理解。
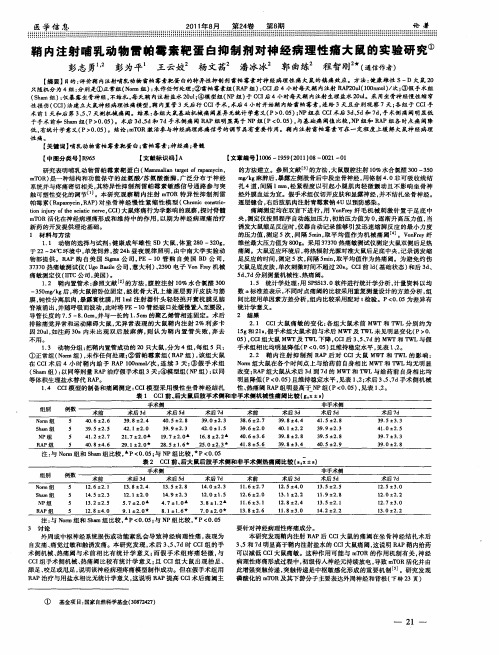
Role of Mitochondria in Parvovirus PathologyJonna Nykky,Matti Vuento,Leona Gilbert*Department of Biological and Environmental Science,and Nanoscience Center,University of Jyva¨skyla¨,Jyva¨skyla¨,FinlandAbstractProper functioning of the mitochondria is crucial for the survival of the cell.Viruses are able to interfere with mitochondrial functions as they infect the host cell.Parvoviruses are known to induce apoptosis in infected cells,but the role of the mitochondria in parvovirus induced cytopathy is only partially known.Here we demonstrate with confocal and electron microscopy that canine parvovirus(CPV)associated with the mitochondrial outer membrane from the onset of infection.During viral entry a transient depolarization of the mitochondrial transmembrane potential and increase in ROS level was detected.Subsequently,mitochondrial homeostasis was normalized shortly,as detected by repolarization of the mitochondrial membrane and decrease of ROS.Indeed,activation of cell survival signalling through ERK1/2cascade was observed early in CPV infected cells.At12hours post infection,concurrent with the expression of viral non-structural protein1,damage to the mitochondrial structure and depolarization of its membrane were apparent.Results of this study provide additional insight of parvovirus pathology and also more general information of virus-mitochondria association.Citation:Nykky J,Vuento M,Gilbert L(2014)Role of Mitochondria in Parvovirus Pathology.PLoS ONE9(1):e86124.doi:10.1371/journal.pone.0086124 Editor:Ferenc Gallyas Jr,University of Pecs Medical School,HungaryReceived September12,2013;Accepted December9,2013;Published January21,2014Copyright:ß2014Nykky et al.This is an open-access article distributed under the terms of the Creative Commons Attribution License,which permits unrestricted use,distribution,and reproduction in any medium,provided the original author and source are credited.Funding:The authors have no support or funding to report.Competing Interests:The authors have declared that no competing interests exist.*E-mail:leona.k.gilbert@jyu.fiIntroductionMitochondria are important organelles for the cell as they produce energy,regulate redox balance and maintain Ca2+ homeostasis.In cell signalling the mitochondria regulate cell responses to different cellular situations determining the fate of cell from survival to death[1–3].In viral infections,mitochondria have a role in innate immunity by activating interferon production [4].Mitochondrial dysfunction is associated with numerous diseases such as neurodegenerative diseases,diabetes and cancer [5–11].Among the factors leading to mitochondrial dysfunction are depolarization of the mitochondrial transmembrane potential (DY m),changes in expression of mitochondrial proteins and lipids, mutations in mtDNA,oxidative stress,and alterations in mitochondrial number[5–11].Many viral proteins target the mitochondria and interfere with its functions contributing to pathology of viral diseases[12,13].For example,association of hepatitis C virus(HCV)proteins with the mitochondria play an important role in pathogenesis of HCV induced chronic liver diseases and liver cancer.HCV proteins enter the mitochondria causing an increase in mitochondrial Ca2+ uptake,reactive oxygen species(ROS)production and mitochon-drial permeability transition.As a result,intrinsic cell death and changes in the liver microenvironment lead to cell transformation [14,15].One major factor in HIV pathogenesis is viral protein R (Vpr).Vpr is integrated in the mitochondrial outer membrane and it also reduces the expression of mitofusin2,which leads to mitochondrial fragmentation and depolarization of DY m inducing death of infected CD4+T lymphocytes[16].On the other hand, respiratory syncytial virus(RSV)can cause severe infections as viral non-structural protein1(NS1)interferes with mitochondrial antiviral signalling protein inhibiting the interferon production [17].Immune response is therefore delayed early in an RSV infection giving more time for viral replication.Viruses can modulate mitochondrial functions for their benefit and they can interfere with signalling networks activating growth pathways to increase metabolic activity[18,19].One example is the activation of phosphatidylinositol-3kinases/AKT(PI3K/ AKT)survival pathway by rotaviral non-structural protein1 (NSP1)in the beginning of infection[20].Another rotaviral protein,NSP4,is integrated into the mitochondrial membranes causing apoptosis through depolarization of mitochondria and release of cytochrome c[20].NSP1counteracts the NSP4induced apoptosis early in the infection giving time for viral replication. Another survival signalling pathway is mediated through the extracellular regulated kinases1and2(ERK1/2).ERK1/2signal cascade activates cytoplasmic and nuclear substrates that promote cell survival,cell division,differentiation and cell motility[21]. Overexpression of ERK1/2has been reported to inhibit the intrinsic mitochondria dependent apoptotic pathway[22].As a results of its functions,activation of ERK1/2signalling has been reported to be important mediator in pathogenesis of number of viruses including echovirus1[23],coxsackievirus B3[24], entrovirus71[25],vaccinia virus[26],human cytomegalovirus [27],influenza virus[28]and HIV-1[29].During virus infection the significance of ERK1/2activation is mainly to prevent apoptosis and ensure production of viral progeny. Parvoviruses are small non-enveloped viruses with linear ssDNA genome[30].Pathology of parvoviral infection is often directly connected to the cytotoxic nature of infection.Enteritis,myocar-ditis,hepatitis and reticulocytopenia are consequences of parvo-virus induced cell death[31–35].The mechanisms of cell death have been reported to be apoptosis,necrosis and death by cytoskeletal rearrangements[34,36–40].We have used canine parvovirus(CPV)as a model virus in our studies concerning parvovirus pathology and we have earlier reported that a CPV infection initiates apoptosis[41].CPV induced apoptosis involves activation of caspases(9and3)and dissipation of DY m.Caspasesare activated early in the infection,but damage to nuclear DNA and depolarization of DY m appear after beginning of viral replication[41].Others have reported that expression of CPV NS1protein induces apoptosis in a p53and Bcl-2independent fashion[42].Studies up to this point have reported the events of viral induced apoptosis from late stages of infections.Our aim here is to examine the role of the mitochondria in parvovirus pathology at very early stages of infection.The direct association of CPV with the mitochondria and the consequence of this immediate interaction could potentially be the initial triggers for intrinsic cell death. Alternatively,the NS1expression and its subsequent cytopathic effects on the cell could also be the original trigger.With both possibilities,a comparison of viral-mitochondrial involvement of early events with later events is reported.Our results demonstrate a close mitochondrial association of CPV,and damage to the morphology of the mitochondria as well as induction of oxidative stress.Interestingly a biphasic compromise of DY m was observed. More significantly,we report the activation of ERK1/2signalling in parvovirus infection.Knowledge of exact triggers of intrinsic cell death from viral infections will allow better therapeutic interven-tions and possible alleviation of pathological consequences. Materials and MethodsCells and virusNorden laboratory feline kidney(NLFK)cells[43](gift from Colin Parrish,Cornell University,Ithaca,N.Y.),permissive to CPV,were grown in Dulbecco’s modified Eagle’s medium (DMEM;Invitrogen Life Technologies,CA,USA)supplemented with10%fetal calf serum(PAA Laboratories,Pasching,Austria) and1%PenStrep(Invitrogen Life Technologies,CA,USA). Canine parvovirus type2(gift from Colin Parrish,Cornell University,Ithaca,N.Y.,USA)derived from an infectious clone, as previously described[44],was propagated in NLFK cells in 500cm2cell culture flasks(Nunc,Roskilde,Denmark)for7days and then stored at220u C.Cell debris was removed from300ml of virus culture medium by centrifugation and the supernatants were concentrated by ultrafiltration(30kDa filter,Merck Milli-pore,Darmstadt,Germany).The virus was pelleted by ultracen-trifugation at1730006g for1h and resuspended in1ml of PBS pH7.4.The suspension was sonicated with low power and extracted with chloroform.Full and empty capsids were separated from the CPV containing aqueous layer by isopycnic centrifuga-tion in45%cesium chloride gradient.Opalescent bands were collected with a syringe and capsids were pelleted by ultracentri-fugation at2450006g for4h.The pellets were resuspended in 100m l of PBS.Full CPV capsids were used to infect NLFK cells at m.o.i.10.Immunofluorescence microscopyTo study colocalization of CPV with mitochondria,fluorescence confocal microscopy was used.NLFK cells were grown on coverslips and synchronized with2mM Thymidine(Sigma-Aldrich,St.Louis,MO,USA)for18h.Cells were rinsed with DMEM and inoculated with CPV(m.o.i.10)in a small volume for 15min.Then DMEM was added and cells were incubated for2–24h.Mock and CPV infected cells were stained with300nM MitoTrackerRed(MTR,Invitrogen Life Technologies,CA,USA) for30min at37u C.Cells were fixed with4%paraformaldehyde, treated with0.1%Triton in PBS and CPV capsid proteins were labelled with monoclonal anti-CPV antibody(gift from Colin Parrish,[45])diluted in Triton solution at concentration of10m g/ ml.Anti-CPV antibody was visualised with Alexa Fluor488conjugated anti-mouse antibody(Invitrogen Life Technologies, CA,USA)at a dilution of1:200.To label mitochondria with polyclonal anti-COX IV antibody(Abcam,Cambridge,UK) mock and CPV infected NLFK cells grown on coverslips were fixed with ice cold methanol and double labelled with monoclonal mouse anti-CPV antibody and with anti-COX IV antibody at a concentration of7m g/ml.Alexa Fluor488conjugated anti-mouse antibody and Alexa594conjugated anti-rabbit antibody(dilution 1:200)were used to detect primary antibodies.The samples were examined with a laser scanning fluorescence microscope(LSM 510,Axiovert100M;Zeiss,Jena Germany)by using the excitation and emission settings appropriate for the dye used. Single confocal sections were taken from the middle of the cell. Colocalization analysisTo quantify the level of colocalization,10cells per time point from three independent experiments,30cells per time point all together,were randomly selected and imaged using confocal microscope.Levels for the laser power and detector amplification were optimized for each channel in the confocal microscope before starting the quantification.The nucleus was excluded from the image for colocalization analysis.Quantification of colocaliza-tion was determined with BioImageXD software[46].Overlap between channels was expressed as percentage Ch2voxels colocalizing with Ch1voxels.The colocalization thresholds were set manually to eliminate background fluorescence and all connected regions with fewer than three pixels were removed to eliminate photon shot noise.Immunoelectron microscopyCells were grown on8.8cm2plastic culture dishes(Nunc, Roskilde,Denmark)to80%confluency.The cells were synchro-nized and infected as stated above.After infection dishes were washed with0.1M phosphate buffer,pH7.4and the cells were fixed with PLP-fixative(0.01M sodium periodate,0.075M lysine, 2%PFA)for2h at RT and left in2%PFA overnight.After rinsing with sodium phosphate buffer,cells were permeabilized for 8min at RT with phosphate buffer containing0.01%saponin and 0.1%BSA.Primary antibody(anti-CPV antibody,used at concentration10m g/ml)and gold-conjugated secondary antibody (dilution1:50;British Biocell International,Cardiff,UK)were diluted in permeabilization buffer and incubated with cells for1h at RT.Between and after labelings,cells were washed with permeabilization buffer.Cells were postfixed with1%glutaralde-hyde in0.1M phosphate buffer for10min at RT,quenched with 50mM NH4Cl in phosphate buffer,and washed with both phosphate buffer and water.Cells were treated in the dark with HQ-silver(British Biocell International,Cardiff,UK)for2min, followed by washes with water and gold toning solutions[2% sodium acetate365min,0.05%gold chloride(Sigma-Aldrich,St. Louis,MO,USA)10min on ice,0.3%sodium thiosulphate 2610min on ice].After washes with water,the cells were postfixed with1%osmium tetroxide in0.1M phosphate buffer for 1h at4u C,dehydrated with a descending concentration series of ethanol,and stained with2%uranyl acetate.Plastic capsules filled with Epon LX-112(Ladd Research Industries,Williston,VT, USA)were placed upside-down on top of the cells.Epon was polymerized for24h at45u C and24h at60u C.After polymerization,the capsules were warmed up to100u C and removed carefully.Horizontal sections were cut,picked up on a grid,and viewed with an electron microscope JEM-1400(Jeol, Tokyo,Japan).Images were taken with iTEM software(Olympus, Mu¨nster,Germany).To analyze the impact of CPV infection on mitochondria,all mitochondria from one cell were classified asintact or damaged and counted.All together mitochondria from 30cells per time point from three independent experiments were counted.Flow cytometric analyses of membrane integrityTo analyse if a CPV infection has an effect on DY m at early time points p.i.,JC-1(Invitrogen Life Technologies,CA,USA)was used.JC-1is a fluorescent dye that is used to detect the loss of DY m.In healthy cells the dye monomers aggregate and mitochondria can be seen as red,but when mitochondria are depolarized the dye is in a monomeric form and emits green fluorescence.NLFK cells grown in8.8cm2dishes(Nunc, Roskilde,Denmark)were synchronized infected(or mock infected) with CPV and incubated for2–18h.JC-1was added to trypsinized cells at a final concentration of15m M and incubated for30min at37u C.As a control cells were also labelled in the presence of100nM K+-selective ionophore valinomycin(Sigma-Aldrich,St.Louis,MO,USA)that collapsed DY m.Stained cells were detected with flow cytometry using a FACSCalibur with a 488nm laser line and Cell Quest software(Becton Dickinson, Franklin Lakes,NJ,USA)and results were further analysed with FlowJo software(Tree Star,Ashland,OR,USA).A total of104 cells were considered in each assay to create the cytograms.Cells were gated to two populations according to valinomycin treated cells(depolarized mitochondria)and mock infected cells(polarized mitochondria).Amount of cells showing depolarization of DY m were normalized in comparison with the mock infected control, which was given the value1.These experiments were repeated three times.Detection of ROSThe level of intracellular reactive oxygen species(ROS)was determined with DCFDA cellular ROS detection assay kit (Abcam,Cambridge,MA,USA).Synchronized and infected cells were trypsinized and stained with20m M DCFDA for45min at 37u C.To induce formation of ROS in positive control,cells were incubated for2h with50m M tert-butyl hydroperoxide(TBHP)or with0.5m M staurosporine(STS)for16h.Samples were analysed with flow cytometry using a FACSCalibur with a488nm laser line and Cell Quest software(Becton Dickinson,Franklin Lakes,NJ, USA).Results were further analysed with FlowJo software(Tree Star,Ashland,OR,USA).A total of104cells were considered in each assay to create the cytograms.Cells were gated to two populations:first population corresponded to the production of ROS as compared to TBHP and STS treated cells;and the second population according to mock infected cells.The amount of cells positive for ROS were normalized in comparison with the mock infected control,which was given the value1.These experiments were repeated three times.Release of calciumTo study the release of calcium from intracellular stores during CPV infection,Fluo-4Direct calcium assay kit was used (Invitrogen Life Technologies,CA,USA).Measurements were done according to manufacture’s instructions.Shortly,cells grown on96-well plates were synchronized and loaded with Fluo-4for 60min at37u C.Loading solution was changed to DMEM and CPV or100nM thapsigargin(Invitrogen Life Technologies,CA, USA),an intracellular calcium releaser,were added to appropriate wells.Fluorescence intensity was measured with Victor X42030 Multilabel Reader(PerkinElmer,Waltham,MA,USA)at30min –6h after treatments using excitation wavelength485nm and emission was measured at535nm.Fluorescence intensity measurements were normalized in comparison with the mock infected control which was given the value1.Experiment was repeated three times.Activation of cell survival signallingIn order to examine the activation of cell survival signalling in CPV infected cells,phosphorylation of ERK1/2was studied. NLFK cells were grown and infected with CPV as described above.As a positive control cells were incubated in the presence of 2m M phorbol12-myristate13-acetate(PMA;Sigma-Aldrich,St. Louis,MO,USA)for1h(repeated twice).At indicated time points post infection cells were lysed in a50mM Tris-HCl (pH7.4)buffer containing150mM NaCl,1mM EDTA,1%NP-40,0.25%sodium deoxycholate and1:100diluted protease inhibitor cocktail(Sigma-Aldrich,St.Louis,MO,USA).After centrifugation at140006g and4u C,protein concentration was determined with Bradford method(Bio Rad,Hercules,CA,USA). An amount of50m g of proteins were separated on SDS-PAGE gel and transferred to nitrocellulose membrane(Schleicher&Schuell BioScience,Keene,NH).Blots were incubated with polyclonal antibody towards ERK1/2or phosphorylated ERK1/2(Cell Signaling Technology,Danvers,MA,USA)at a dilution1:1000. Detection was performed with HRP-conjugated goat anti-rabbit antibody(Dako,Glostrup,Denmark)and SuperSignal West Pico Chemiluminescent Substrate(Pierce,Rockford,IL,USA).Densi-tometry(ImageJ software,NIH,Bethesda,MD,USA)was utilized to measure the intensity of bands.Results were normalized to give a value of1for mock infected cells and CPV infected samples were compared to that.Experiments were repeated three times and results are shown as ratio of p-ERK to ERK.Inhibition of ERK1/2activationTo explore the importance of ERK1/2activation in CPV infection,percentage of infected cells and depolarization of mitochondria were determined in the presence of the MEK1/2 specific inhibitor U0126(Promega,Madison,WI,USA).U0126 was freshly dissolved at10mM in DMSO and added to the culture medium at final concentration of20m M for30min prior to virus infection and kept in the medium throughout the experiment.Samples were prepared for immunofluorescence microscopy to determine the infection percentage,for immunoblot analysis of ERK1/2activation and for DY m analysis.Methods for immunofluorescence microscopy,immunoblot and DY m analysis were as previously described.Infection percentage was calculated at24h p.i..Experiments with inhibitor were repeated three times. Statistical testingStudent’s t-test was used to identify statistically significant differences between mock and CPV infected samples.p#0.05was considered significant.ResultsCPV colocalized with mitochondriaTo study the association between CPV and mitochondria, colocalization analysis based on confocal microscopy images was conducted.Results confirmed that cytoplasmic CPV colocalized with mitochondria from2h p.i.to22h p.i.(Fig.1A).Colocaliza-tion of CPV and mitochondria,as manifested by white merge spots distributed in the cytoplasm,was observed at all time points used(Fig.1A).Spots had an apparent perinuclear location at10–14h p.i..A quantitative estimation of colocalization was obtained with BioImage XD software and is shown as a mean of30individual cells per time point(Fig.1B).Percentage of cytoplasmic CPVcolocalizing (as defined by BioImage XD software)with mito-chondria fluctuated during used time points.In MTR labelled cells the colocalization percentage was already 13%at 2h p.i..Then colocalization decreased to 9%at 4–8h p.i..At 10h p.i.colocalization increased again to 15%and reached the highest level at 22–24h p.i.with 19%.With anti-COX IV labelledcellsFigure 1.CPV colocalizes with mitochondria.(A)Confocal immunofluorescence images of CPV infected cells.Mitochondria were labelled with anti-COX IV antibody (red)and CPV with anti-capsid antibody (green).Nucleus has been cut out from the images due to really intense fluorescence from CPV at longer time points.Colocalization of CPV with mitochondria is shown in white in lower images.Bars 5m m.(B)Percentage of colocalization of CPV with mitochondria.Colocalization analysis of confocal microscopy images was done with BioimageXD software.Mitochondria were labelled either with anti-COX IV antibody or with MitoTrackerRed (MTR).CPV was labelled with antibody recognizing intact capsids.Mock 24h p.i.Results are shown as means from 30cells from 3independent experiments 6S.D.doi:10.1371/journal.pone.0086124.g001colocalization was low during entry(3–6%at2–4h p.i.),but percentage rose after16h p.i.and reached a highest level at 24h p.i.(16%).Infection with CPV caused mitochondrial damage Immunoelectron microscopy was used to detect direct associ-ation of CPV with mitochondria and to study the ultrastructure of mitochondria in mock and CPV infected cells(Fig.2).The preparation of samples within this technique is harsh.Unfortu-nately this can lead to some loss of the integrity of mitochondria as seen in the percentage of damaged mitochondria in the mock infected sample(6%).CPV located close to mitochondria at all of the studied time points(Fig.2A).At22h p.i.virus label was seen in large plaques,not as rounded gold particles.These plaques were not located on the mitochondrial membrane as some label with the earlier time points.Some gold label was also seen occasionally inside the mitochondria at all time points.Damage to the mitochondrial morphology was seen in CPV infected cells. Infection seemed to cause disintegration of the membrane(as in Fig.2A6,10,18,22h p.i.,2B ii;marked with*).Additionally, mitochondrial membrane blebbing(Fig.2B i,arrowhead)and disappearance of cristae(Fig.2B ii,marked with C)was seen.At later time points starting14h p.i.damaged mitochondria were seen in autophagosome-like structures(Fig.2B iii,marked with,). Percentage of damaged mitochondria was counted from30cells at each time point used and the mean is shown in Figure2C.At 2h p.i.the percentage of damaged mitochondria was24%,but it decreased to17%by6h p.i..After this,the percentage rose and was the highest at18h p.i.when the percentage of damaged mitochondria reached46%.These results demonstrated that CPV infection induced damage to the mitochondria. Depolarization of mitochondrial membranes was associated with CPV infectionJC-1dye was used to study the potential of mitochondrial membrane.CPV infected cells were labelled with this dye at different times post infection.Results demonstrated that mito-chondria began to be depolarized at2h p.i.(Fig.3)when the amount of depolarized mitochondria was1.6times the amount in mock infected cells.At4h p.i.DY m was restored to the level of mock infected cells.However,at14h p.i.depolarization of the mitochondrial membranes was seen again.At18h p.i.the amount of depolarized mitochondria was tripled when compared to mock infected cells.Valinomycin was used to induce depolarization of DY m in positive controls,and with this drug there were2764.1(p#0.05)times more depolarized mitochondria than in mock infected cells(data not shown).These results indicate that mitochondria are depolarized during CPV infection.The ROS level increases during the early phases of CPV infectionProduction of ROS during CPV infection was analysed with DCFDA.The intracellular level of ROS increased in the beginning of infection(Fig.4).The amount of cells with increased ROS level at2h p.i.was1.4times the level in mock infected cells. Production of ROS continued to increase up to6h p.i.,but returns to the level of mock infected cell from8h–22hr p.i.. Thereafter the level of ROS increased again at24h p.i..In STS treated apoptotic cells the level of ROS was6.461.5(p#0.05) times the level in mock infected cells and in TBHP treated cells the factor was 6.060.4(p#0.05)(data not shown).These results demonstrate that mitochondria are affected during virus uptake, but cells are able to recover from the triggered oxidative stress.Cytoplasmic calcium concentration remained unchanged in the beginning of infectionFluorescent calcium indicator Fluo-4was used to detect changes in cytoplasmic calcium concentration.Cells were loaded with Fluo-4and treated with CPV or thapsigargin.Thapsigargin releases calcium from intracellular stores by inhibiting calcium-ATPases on endoplasmic reticulum.CPV infected and thapsigar-gin treated cells were compared to mock infected cells. Thapsigargin induced direct increase in cytoplasmic calcium when added to the cells as detected by increase in fluorescence (Fig.5).In CPV infected cells only a minor change was observed in the fluorescence level indicating small fluctuation in cytoplasmic calcium concentration at0.5–6h p.i when compared to mock infected cells.ERK1/2was activated during a CPV infectionDue to the discovered changes in DY m at2–4h p.i.,activation of cell survival signalling in the beginning of infection was studied. We used antibody towards phosphorylated ERK1/2(p-ERK1/2) to investigate activation of ERK1/2signalling.A representative result is shown in Fig.6A.Ratio of band intensities of p-ERK1/2 to ERK1/2increased already at15min p.i.(Fig.6B).Activation was further enhanced to30min p.i..Thereafter the activation started gradually to decline and by4h p.i.the ratio was below that of mock infected cells indicating a decrease in p-ERK.For cells incubated with PMA the ERK ratio was9.860.6(p#0.05) (data not shown).When the activation of ERK1/2was inhibited by U0126the percentage of infection declined20%when compared to infection without the drug(Fig.6C).The used concentration of U0126inhibited the activation of ERK1/2 efficiently as detected with immunoblotting(Fig.6B).Inhibiting the ERK1/2activation reduced the CPV induced depolarization of DY m at2h p.i.(Fig.6D).DiscussionTaking into account the important role of mitochondria in producing energy,defects in the mitochondrial functions reflect directly to the viability of the cell.Indeed,mitochondrial dysfunction is linked to pathologies of diseases among viral diseases[8,9,13,47].As viruses are able to modulate mitochondrial function for their benefit this feature has evoked interest to be targeted for antiviral drugs.New antiviral drugs could inhibit viral changes in mitochondrial function or induce proapoptotic effect in virus-infected cells to inhibit viral replication[13,18,48].To be able to design new antiviral drugs and to develop better intervention strategies,various virus-mitochondria interactions have to be carefully studied.We have analysed the role of mitochondria in parvovirus pathology.Our data demonstrates that CPV localized to the cytoplasm near mitochondria at2–24h p.i.(Fig.1).CPV associated with mitochondria through out the infection,but was only occasionally seen inside the mitochondria(Fig.1and2).The percentage of CPV colocalized with mitochondria showed that at 2–24h p.i.a fraction of CPV is associated with the mitochondria (Fig.1).The amount of viruses associated with the mitochondria increased with longer time points after16h p.i..By this time new viruses have been made[41,49]and the virus content of the cell has increased[41]probably affecting also the amount of association of CPV with the mitochondria.Colocalization percentages vary between the two used mitochondria label (Fig.1B);MTR goes in the mitochondria,and labels the whole cell organelle as COX IV localizes at inner mitochondrial membrane.Different labelling of the mitochondria with these。