2007年广东省高中生化学奥林匹克竞赛复赛试题(含答案)
- 格式:doc
- 大小:198.50 KB
- 文档页数:5
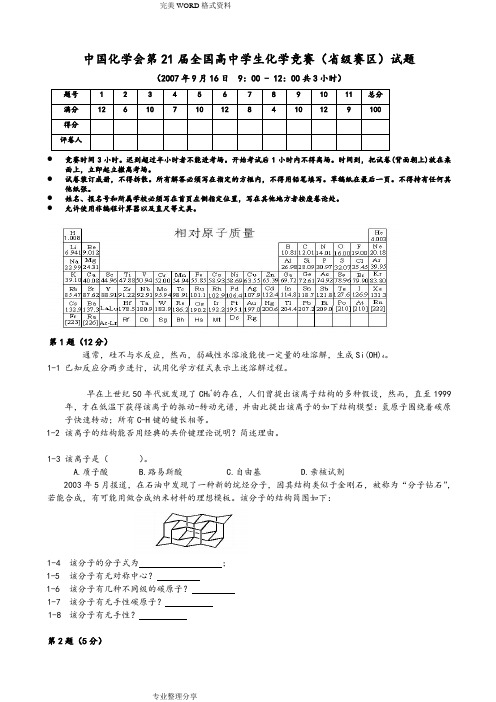
中国化学会第21届全国高中学生化学竞赛(省级赛区)试题(2007年9月16日 9:00 - 12:00共3小时)题号 1 2 3 4 5 6 7 8 9 10 11 总分满分12 6 10 7 10 12 8 4 10 12 9 100得分评卷人●竞赛时间3小时。
迟到超过半小时者不能进考场。
开始考试后1小时内不得离场。
时间到,把试卷(背面朝上)放在桌面上,立即起立撤离考场。
●试卷装订成册,不得拆散。
所有解答必须写在指定的方框内,不得用铅笔填写。
草稿纸在最后一页。
不得持有任何其他纸张。
●姓名、报名号和所属学校必须写在首页左侧指定位置,写在其他地方者按废卷论处。
●允许使用非编程计算器以及直尺等文具。
第1题(12分)通常,硅不与水反应,然而,弱碱性水溶液能使一定量的硅溶解,生成Si(OH)4。
1-1已知反应分两步进行,试用化学方程式表示上述溶解过程。
早在上世纪50年代就发现了CH5+的存在,人们曾提出该离子结构的多种假设,然而,直至1999年,才在低温下获得该离子的振动-转动光谱,并由此提出该离子的如下结构模型:氢原子围绕着碳原子快速转动;所有C-H键的键长相等。
1-2该离子的结构能否用经典的共价键理论说明?简述理由。
1-3该离子是()。
A.质子酸B.路易斯酸C.自由基D.亲核试剂2003年5月报道,在石油中发现了一种新的烷烃分子,因其结构类似于金刚石,被称为“分子钻石”,若能合成,有可能用做合成纳米材料的理想模板。
该分子的结构简图如下:1-4该分子的分子式为;1-5该分子有无对称中心?1-6该分子有几种不同级的碳原子?1-7该分子有无手性碳原子?1-8该分子有无手性?第2题(5分)羟胺和用同位素标记氮原子(N﹡)的亚硝酸在不同介质中发生反应,方程式如下:NH2OH+HN﹡O2→ A+H2ONH2OH+HN﹡O2→ B+H2OA、B脱水都能形成N2O,由A得到N﹡NO和NN﹡O,而由B只得到NN﹡O。

2007年广东省联赛答案解释1、C;渗透压只跟血浆中的分子、离子浓度有关,与蛋白质的分子质量无关。
(1)概念:渗透压指的是溶质分子通过半透膜的一种吸水力量,其大小取决于溶质颗粒数目的多少,而与溶质的分子量、半径等特性无关。
由于血浆中晶体溶质数目远远大于胶体数目,所以血浆渗透压主要由晶体渗透压构成。
血浆胶体渗透压主要由蛋白质分子构成,其中,血浆白蛋白分子量较小,数目较多(白蛋白>球蛋白>纤维蛋白原),决定血浆胶体渗透压的大小。
(2)渗透压的作用晶体渗透压——维持细胞内外水平衡胶体渗透压——维持血管内外水平衡2、B;A:红细胞本身没有线粒体,不能进行有氧呼吸;B:无氧呼吸停止后,Na-K泵停止工作,K+外流导致血浆中的浓度升高;C:跟外排作用无关;D:物质代谢减慢才对。
3、C;A:最多通气为零,不可能为负值;B:8.5KPa以后基本趋于平衡;D:反射弧的效应器不应该是肺泡,而是呼吸肌。
4、C;AD:都是内环境里的缓冲物质,可以跟酸中和;B:因为蛋白质是弱酸性的,所以和钠构成强碱弱酸盐,也有缓冲特性,可以与酸反应;C:这反应应该也可以发生,但题目说的是内环境中,而血红蛋白只在红细胞内,所以不属于内环境。
5、C;昆虫除原尾目无触角、高等双翅目和膜翅目幼虫的触角退化外,其它种类都有1对触角。
触角长在昆虫两只复眼的中上方,昆虫活动的时候,这两根触角总是不停地摆动着,东察西探,象是寻找猎物的雷达。
触角是主要的感觉器官,有嗅觉、触觉和听觉的功能。
触角能够帮助昆虫寻找食物和配偶,并探明身体前方有无障碍物。
在有些昆虫中,触角还有其它用处,例如雄性芫菁在交配时用来握住雌虫,魔蚊幼虫用来捕捉食物,仰泳蝽的触角在水中能平衡身体,水龟虫则用来帮助呼吸。
触角都长在头前面的两个叫做触角窝的小坑里。
触角通常有许多小节组成,基本上可以分为三大节。
靠近触角窝的一节通常比较短粗,是支撑上面各节的,相当于树叶的柄,叫做柄节。

3 3 中国化学会第 21 届(2007 年)全国高中学生化学竞赛(省级赛区)试题答案及评分标准H1.008相对原子质量He 4.003Li BeB C N O F Ne 6.941 9.01210.81 12.01 14.01 16.00 19.00 20.18 Na MgAl Si P S Cl Ar 22.99 24.3126.98 28.09 30.97 32.07 35.45 39.95 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 39.10 40.08 44.96 47.88 50.94 52.00 54.94 55.85 58.93 58.69 63.55 65.39 69.72 72.61 74.92 78.96 79.90 83.80Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb TeI Xe 85.47 87.62 88.91 91.22 92.91 95.94 98.91 101.1 102.9 106.4 107.9 112.4 114.8 118.7 121.8 127.6 126.9 131.3 Cs Ba Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn 132.9 137.3 La -Lu 178.5 180.9 183.9 186.2 190.2 192.2 195.1 197.0 200.6 204.4 207.2 209.0 [210] [210] [222] Fr Ra[223] [226] A c-Lr Rf Db Sg Bh Hs Mt Ds Rg第 1 题(12 分)通常,硅不与水反应,然而,弱碱性水溶液能使一定量的硅溶解,生成 Si(OH)4。
1-1 已知反应分两步进行,试用化学方程式表示上述溶解过程。
(2 分)Si + 4OH = SiO 44+ 2 H 2 (1 分) SiO 44+ 4H 2O = Si(OH)4 + 4OH (1 分)若写成 Si + 2OH+ H 2O = SiO 2+ 2 H 2SiO 2+ 3H 2O = Si(OH)4 + 2OH,也得同样的分。

中国化学会第21届全国高中学生化学竞赛(省级赛区)试题(2007年9月16日 9:00 - 12:00共3小时)题号 1 2 3 4 5 6 7 8 9 10 11 总分满分 12 6 10 7 10 12 8 4 10 12 9100 得分 评卷人● 竞赛时间3小时。
迟到超过半小时者不能进考场。
开始考试后1小时内不得离场。
时间到,把试卷(背面朝上)放在桌面上,立即起立撤离考场。
● 试卷装订成册,不得拆散。
所有解答必须写在指定的方框内,不得用铅笔填写。
草稿纸在最后一页。
不得持有任何其他纸张。
● 姓名、报名号和所属学校必须写在首页左侧指定位置,写在其他地方者按废卷论处。
●允许使用非编程计算器以及直尺等文具。
第1题(12分)通常,硅不与水反应,然而,弱碱性水溶液能使一定量的硅溶解,生成Si(OH)4。
1-1 已知反应分两步进行,试用化学方程式表示上述溶解过程。
早在上世纪50年代就发现了CH 5+的存在,人们曾提出该离子结构的多种假设,然而,直至1999年,才在低温下获得该离子的振动-转动光谱,并由此提出该离子的如下结构模型:氢原子围绕着碳原子快速转动;所有C-H 键的键长相等。
1-2 该离子的结构能否用经典的共价键理论说明?简述理由。
1-3 该离子是( )。
A.质子酸B.路易斯酸C.自由基D.亲核试剂2003年5月报道,在石油中发现了一种新的烷烃分子,因其结构类似于金刚石,被称为“分子钻石”,若能合成,有可能用做合成纳米材料的理想模板。
该分子的结构简图如下:1-4 该分子的分子式为 ;1-5 该分子有无对称中心?1-6 该分子有几种不同级的碳原子? 1-7 该分子有无手性碳原子? 1-8 该分子有无手性?第2题(5分)羟胺和用同位素标记氮原子(N ﹡)的亚硝酸在不同介质中发生反应,方程式如下:NH 2OH+HN ﹡O 2→ A +H 2ONH 2OH+HN ﹡O 2→ B +H 2OA 、B 脱水都能形成N 2O ,由A 得到N ﹡NO 和NN ﹡O ,而由B 只得到NN ﹡O 。
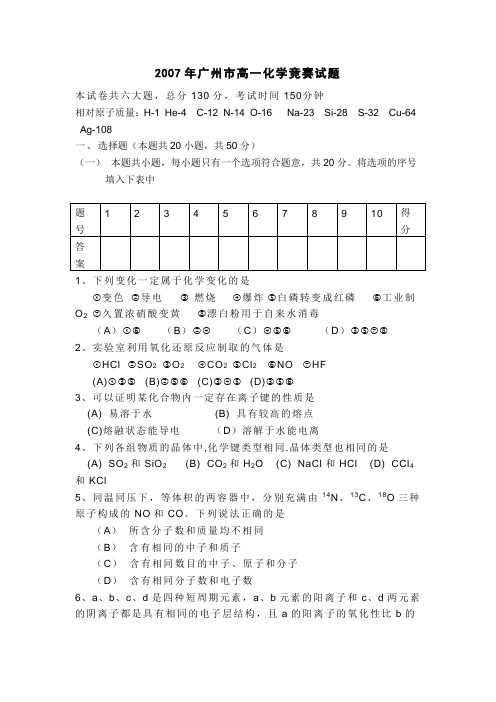
2007年广州市高一化学竞赛试题本试卷共六大题,总分130分,考试时间150 分钟相对原子质量:H-1 He-4 C-12 N-14 O-16 Na-23 Si-28 S-32 Cu-64 Ag-108一、选择题(本题共20小题,共50分)(一)本题共小题,每小题只有一个选项符合题意,共20分。
将选项的序号填入下表中1、下列变化一定属于化学变化的是①变色②导电③燃烧④爆炸⑤白磷转变成红磷⑥工业制O2⑦久置浓硝酸变黄⑧漂白粉用于自来水消毒(A)①⑥(B)②④(C)④⑤⑥(D)③⑤⑦⑧2、实验室利用氧化还原反应制取的气体是①HCl ②SO2③O2④CO2⑤Cl2⑥NO ⑦HF(A)①③⑤(B)②⑤⑥(C)③④⑤(D)③⑤⑥3、可以证明某化合物内一定存在离子键的性质是(A) 易溶于水(B) 具有较高的熔点(C)熔融状态能导电(D)溶解于水能电离4、下列各组物质的晶体中,化学键类型相同.晶体类型也相同的是(A) SO2和SiO2(B) CO2和H2O (C) NaCl和HCl (D) CCl4和KCl5、同温同压下,等体积的两容器中,分别充满由14N、13C、18O三种原子构成的NO和CO。
下列说法正确的是(A)所含分子数和质量均不相同(B)含有相同的中子和质子(C)含有相同数目的中子、原子和分子(D)含有相同分子数和电子数6、a、b、c、d是四种短周期元素,a、b元素的阳离子和c、d两元素的阴离子都是具有相同的电子层结构,且a的阳离子的氧化性比b的阳离子氧化性弱,c的阴离子所带的电荷比d的阴离子所带的电荷多,则它们的原子序数大小关系为(A)b>a>d>c (B)c>b>a>d (C)a>b>c>d (D)b>a>c>d7、下列关于“摩尔”概念的说法正确的选项是①在标准状况下,1mol任何气体都是22.4L②1mol物质,只有在标准状况下,才约是22.4L③1 molCuSO4是160g④含有6.02×1023个微粒的物质叫1 mol(A)①正确(B)②正确(C)③④正确(D)都不正确8、在pH=3的溶液中,可以大量共存的是(A)Al3+、Ca2+、NO3-、Cl-(B) Na+、K+、S2-、Br-(C)K+、Na+、AlO2-、NO3-(D) K+、Na+、SO42-、S2O32-9、在NaOH溶液中溶解度最小的或跟NaOH最少反应的是(A)Sb2O3(B)Bi2O3(C)As2O5(D)As2O310、对于反应H-+ NH3=H2+ NH2-的不正确的说法是(A)属于置换反应(B)H-是还原剂(C)H2既是氧化产物又是还原产物(D)氧化产物与还原产物的化学计量数之比为1:1(二)本题共10小题,每小题3分,共30分。

中国化学会第21届全国高中学生化学竞赛(省级赛区)试题(2007年9月16日 9:00 - 12:00共3小时)题号 1 2 3 4 5 6 7 8 9 10 11 总分满分12 6 10 7 10 12 8 4 10 12 9 100得分评卷人●竞赛时间3小时。
迟到超过半小时者不能进考场。
开始考试后1小时内不得离场。
时间到,把试卷(背面朝上)放在桌面上,立即起立撤离考场。
●试卷装订成册,不得拆散。
所有解答必须写在指定的方框内,不得用铅笔填写。
草稿纸在最后一页。
不得持有任何其他纸张。
●姓名、报名号和所属学校必须写在首页左侧指定位置,写在其他地方者按废卷论处。
●允许使用非编程计算器以及直尺等文具。
第1题(12分)通常,硅不与水反应,然而,弱碱性水溶液能使一定量的硅溶解,生成Si(OH)4。
1-1已知反应分两步进行,试用化学方程式表示上述溶解过程。
早在上世纪50年代就发现了CH5+的存在,人们曾提出该离子结构的多种假设,然而,直至1999年,才在低温下获得该离子的振动-转动光谱,并由此提出该离子的如下结构模型:氢原子围绕着碳原子快速转动;所有C-H键的键长相等。
1-2该离子的结构能否用经典的共价键理论说明?简述理由。
1-3该离子是()。
A.质子酸B.路易斯酸C.自由基D.亲核试剂2003年5月报道,在石油中发现了一种新的烷烃分子,因其结构类似于金刚石,被称为“分子钻石”,若能合成,有可能用做合成纳米材料的理想模板。
该分子的结构简图如下:1-4该分子的分子式为;1-5该分子有无对称中心?1-6该分子有几种不同级的碳原子?1-7该分子有无手性碳原子?1-8该分子有无手性?。
2007广东高考化学试题详解2007广东高考化学试题详解化学组郑云可能用到的原子量:H 1 C 12 N 14 O 16 Na 23 Mg 24 Al 27 S 32Cl 35.5 K 39 Ca 40 Mn 55 Fe 56 Pt 195第Ⅰ卷选择题(共70分)一.选择题(本题包括10小题,每小题3分,共30分。
每小题只有1个选项符合题意)1.铋(Bi)在医药方面有重要应用。
下列关于20983Bi和21083Bi的说法正确的是A 20983Bi和21083Bi都含有83个中子B 20983Bi和21083Bi互为同位素C 20983Bi和21083Bi的核外电子数不同D 20983Bi和21083Bi分别含有126和127个质子[命题意图]:考查同位素概念和构成原子的各粒子之间的关系,难度,易。
[解题思路]:20983Bi和21083Bi互为同位素,质子数相同,电子数同,中子数不同。
答案:BC 0.5mol·L-1CuCl2溶液中含有3.01×1023个Cu2+D 标准状况下,33.6LH2O含有9.03×1023个H2O分子[命题意图]:考查物质的量基本计划,阿伏加德罗常数,高考热点之一,难度,中。
[解题思路]:解题时要注意回忆概念、分析原理注意运算公式的适用范围。
B选项4.6gNO2气体中理论上含有1mol NO2分子,约6.02×1023个NO2分子,由于2NO2 N 2O4,故应小于6.02×1023个,具体多少无法计算。
C选项中要考虑Cu2+的水解,也无法确定其数目,D选项标准状况下H2O冰水混合物,不能用标准状况下气体摩尔体积22.4L/ mol来计算。
答案:A4.许多国家十分重视海水资源的综合利用。
不需要化学变化就能够从海水中获得的物质是A 氯、溴、碘B 钠、镁、铝C 烧碱、氢气D 食盐、淡水[命题意图]:考查海水成分,化学与生活、化学与技术内容。
中国化学会第21届全国高中学生化学竞赛(省级赛区)试题(2007年9月16日 9:00 - 12:00共3小时)题号 1 2 3 4 5 6 7 8 9 10 11 总分满分 12 6 10 7 10 12 8 4 10 12 9100 得分 评卷人● 竞赛时间3小时。
迟到超过半小时者不能进考场。
开始考试后1小时内不得离场。
时间到,把试卷(背面朝上)放在桌面上,立即起立撤离考场。
● 试卷装订成册,不得拆散。
所有解答必须写在指定的方框内,不得用铅笔填写。
草稿纸在最后一页。
不得持有任何其他纸张。
● 姓名、报名号和所属学校必须写在首页左侧指定位置,写在其他地方者按废卷论处。
●允许使用非编程计算器以及直尺等文具。
第1题(12分)通常,硅不与水反应,然而,弱碱性水溶液能使一定量的硅溶解,生成Si(OH)4。
1-1 已知反应分两步进行,试用化学方程式表示上述溶解过程。
早在上世纪50年代就发现了CH 5+的存在,人们曾提出该离子结构的多种假设,然而,直至1999年,才在低温下获得该离子的振动-转动光谱,并由此提出该离子的如下结构模型:氢原子围绕着碳原子快速转动;所有C-H 键的键长相等。
1-2 该离子的结构能否用经典的共价键理论说明?简述理由。
1-3 该离子是( )。
A.质子酸B.路易斯酸C.自由基D.亲核试剂2003年5月报道,在石油中发现了一种新的烷烃分子,因其结构类似于金刚石,被称为“分子钻石”,若能合成,有可能用做合成纳米材料的理想模板。
该分子的结构简图如下:1-4 该分子的分子式为 ;1-5 该分子有无对称中心?1-6 该分子有几种不同级的碳原子? 1-7 该分子有无手性碳原子? 1-8 该分子有无手性?第2题(5分)羟胺和用同位素标记氮原子(N ﹡)的亚硝酸在不同介质中发生反应,方程式如下:NH 2OH+HN ﹡O 2→ A +H 2ONH 2OH+HN ﹡O 2→ B +H 2OA 、B 脱水都能形成N 2O ,由A 得到N ﹡NO 和NN ﹡O ,而由B 只得到NN ﹡O 。
Model Test 8Part I Listening Comprehension (15 minutes) Section A1. A. 3 hours B. 4 hours C. 5 years old. D. 2 minutes ago2. A. That’s all right. B. No, of course not. C. Yes, I’d love to. D. Yes, I would.3. A. Is seven thirty OK? B. At the same place. C. 4 hours. D. Sure.4. A. Don’t mention it.B. I’ve been there.C. It’s not far from here.D. It is very small, but beautiful.5. A. Here you are. B. It is here. C. Yes, I would. D. I’ve got two. Section B6. A. On the street. B. In a hotel. C. At home. D. In a car.7. A. Making the room colorful.B. Painting the room green.C. Choosing what the man likes.D. Keeping the room as quiet as possible.8. A. He was a quiet person in the past.B. He plays football quite well.C. He seldom goes to the football match now.D. He likes the football match very much.9. A. Jane does not like dancing.B. Jane is not available.C. Jane’s leaving the day after tomorrow.D. Jane will go to the dance on her own.10. A. At a post office. B. At a supermarket. C. At a bank. D. In a restaurant. Section CHow often one hears children wishing they were grown up, and old people wishing they were young again. Each age has its_11______ and its pains, and the happiest person is the one who enjoys what each age gives him without wasting his time in_12________ .Childhood is a time when there are few_13______ to make life difficult. If a child has good parents, he is fed, looked after and loved, whatever he may do. It is_14______ that he will ever again in his life be given so much without having to do anything_15______ _. In addition, life is always presenting new things to the child—things that have lost their interest for older people because they are too well-known. But a child has his pains: he is not so free to do what he wishes to do; he is continually being told not to do things, or being punished for what he has done wrong.Part II Vocabulary & Structure (15 minutes) Section A16. This pocket book gives information about how to make a bookshelf on your own.A. spaciousB. criticalC. specificD. crucial17.Susan wants to know whether the committee members the suggested measures.A. have agreed to C. have agreed withB. have agreed D. have been in agreement with18.The result of my experiment has me in my belief that his theory is right.A. confirmedB. constructedC. conductedD. concluded19.The class over, the students the dining hall.A. moved forB. made toC. made forD. entered for20.What’s the of talking with him over his present performance? He always turns adeaf ear to us.A. viewB. matterC. meansD. point21.It was almost 10 years they met again.A. beforeB. sinceC. laterD. that22.Is Beijing the place ?A. you’d like to visit most C. in which you most want to visitB. where you’d like to visit D. that you want to visit it most23.Although we in this neighborhood for 5 years so far, we haven’t got to know manypeople yet.A. wereB. have beenC. had beenD. are24.I’m for the suggestion that a special board to examine the problem.A. be set upB. will be set upC. had beenD. has to be set up25.I really appreciate my daughter with her maths.A. seeing you to help C. you to helpB. to see you helping D. your helpingSection B26.His sudden (decide) left me quite at a loss.27.It’s 6 o’clock already. We have (bare) enough time to catch the train.28.His self-confidence (able)_______ him to become a competent surgeon.29.Nowadays many people are able to receive (far) education by the distanteducation system.30.By the time you sit for the examination next month, you (complete) the course.31.Because of too much typing on computer keyboards, some young teachers have trouble(write) clearly on blackboards.32.The detective concluded that the murder (commit) in this very room.33.If he had remembered (close) the windows, the thief would not have got in.34.Though (fail) many times, Tim didn’t lose heart.35.If such a tragedy(悲剧) (occur),damage would have been incalculable. Part III Reading comprehension (40 minutes) Task 1Self-employed private physicians who charge a fee for each patient visit are the foundation of medical practice in the United States. Most physicians have a contract relationship with one or more hospitals in the community. They send their patients to these hospitals, which usually charge patients that they care for. Some hospitals belong to a city, a state or, in the case of veterans’ hospitals, a federal government agency. Others are operated by religious orders(教堂) or other non-profit groups.Some medical doctors are on salary. Salaried physicians may work as hospital staff members, or residents, who are often still in training. They may teach in medical schools, be hired by corporations to care for their workers or work for the federal government’s Public Health Service.Physicians are among the best paid professionals in the United States. In the 1980s, it was not uncommon for medical doctors to earn incomes of more than $100 000 a year. Specialists, particularly surgeons, might earn several times that amount. Physicians list many reasons why they deserve to be so well rewarded for their work. One reason is the long and expensive preparation required to become a physician in the United States. Most would be physicians first attend college for four years, which can attend medical school for four years. Tuition alone can exceed $10 000 a year. By the time they have obtained their medical degrees, many young physicians are deeply in debt. They still face three to five years of residency(实习期)in a hospital, the first year as an apprentice physician. The hours are long and their pay is relatively low.Setting up a medical practice is expensive, too. Sometimes several physicians will decide to establish a group practice, so they can share the expense of maintaining an office and buying equipment. These physicians also of each other’s patients in emergencies.Physicians word long hours and must accept a great deal of responsibility. Many medicalprocedures, even quite routine ones, involve risk. It is understandable that physicians want to be well rewarded for making decisions which can mean the difference between life and death.36.According to the passage, it is very unlikely that an American hospital is owned by_____.A. a churchB. a corporation.C. a cityD. a state37.The expenses for becoming a doctor are spent on _______.A. schooling and training C. facilities he or she usesB. practice in a hospital D. education he or she provides38.According to the passage, how long does it take for a would-be physician to become anindependent physician in the USA?A. About seven yearsB. Eight yearsC. The yearsD. About twelve years39.Sometimes several physicians set up a group medical practice mainly because .A.they may have more patientsB.they can take turns to work ling hoursC.facilities may be a big burden to an individualD.no one wants to assume too much responsibility40.Which of the following statements could fully express the author’s view towardsphysicians’ payment in the USA?A.Physicians’ expensive education and the characteristics of their work make them welldeserve the handsome pay.B.It is reasonable for physicians to have a large income because their work is verydangerous.C.Physicians should be better paid because they work long hours under bad conditions.D.Physicians shoulder great responsibilities, so it is understandable that they should bewell rewarded.Task 2Don’t Waste Advertising DollarsWalking down any of Shanghai’s main shopping streets this week, newcomers might think the locals have been celebrating Christmas for centuries. Christmas may not be a customary holiday in China, but businessmen in Shanghai know it will bring something more valuable than tradition: people willing to spend money. Most Chinese may feel little connection with the Christmas celebration, but with most shops offering discounts(折扣), the message couldn’t be clearer—it is the season to part with one’s hard-earned cash.Much of that marketing drive is directed towards the thousand of foreigners and forting companies seems no avoiding the season’s commercial(商业的) greetings. Along some major roads, nearly every shop window displays some symbols to the holiday: a man-made fir tree(杉树) with lights, or a snowman.With an increasing number of Westerners arriving in the city for work, young Shanghainese, eager to keep pace with fashions, have begun to show their interest in Christmas. But some people still don’t think Christmas is an important festival in China. At least it is important than the New Year and China’s Spring Festival.41. In the first sentence, the word ―newcomers‖ (Line 1, Para. 1) probably means.A. people who are very youngB. people from other countriesC. local peoplesD. Shanghainese42. To the business people in Shanghai, Christmas will bring ________.A. profitsB. greetingsC. discountsD. tradition43. Which of the following statements is true?A.Christmas is the season for common people to earn money.B.Most Chinese people think they have something to do with Christmas.C.Christmas is the season people will hold their hard-earned money tightly.D. Few Chinese people feel they are connected with the Christmas celebration.44. Why do some young Shanghainese show great interest in Christmas?A. They want to follow the up-to-date Western fashions.B. They think Christmas is more important than New Year’ Day.C.They think themselves connected with Christmas celebration.D.They want to part with their hard-earned cash during Christmas.45. What is the author’s idea towards Christmas celebration?A.Chinese young people should not celebrate Christmas seasons.B.Young people should show their interest in not Christmas celebrations.C.Christmas is a good season for business people to earn money in China.D.Different people have different views towards the western style celebration.Task 3Arora HillsA secure place, a peaceful place, a better placeCome and live next to Nature. In the community of Arora Hills you are surrounded by the beauty of life. This exciting new community is home to three winding streams, expansive recreational parks and miles of nature trails as well as peaceful rolling hills. With more than half of Arora Hills preserved as open space, you will have room to live and space to play.Arora Hills is located next to Ovid Hazen Wells park. It is the place where you wish you had grown up. Just outside your home, you’ll enjoy playing fields, two neighborhood swimming pools, the 290-acre Ovid Hazen Wells park, a planned middle school and shopping center.Building a community that will provide the enviable(令人羡慕的) lifestyle that Arora Hills will offer, takes vision and experience. The community starts to come to life this year and will be ready for home purchases early next year. To be on the priority list, just fill out the easy form and be the first to know of community updates and special pre-opening prices and neighborhood releases.Task 4A – CBO(Chief Business Officer)B – CDO(Chief Development Officer)C – CEO(Chief Executive Officer)D – CFO(Chief Finance Officer)E – CHRO(Chief Human Resource Officer)F – CIO(Chief Information Officer)G – CMO(Chief Marketing Officer)H – CNO(Chief Negotiation Officer) I – COO(Chief Operation Officer)J – CCPO(Chief Public Relation Officer) K – CCQR(Chief Quality Control Officer) L – CCRO(Chief Research Officer)M – CCSO(Chief Sales Officer)N – CCTO(Chief Technology Officer)O – CCUO(Chief User Officer)P – CCVO(Chief Valuation Officer)Example: ( C ) 首席执行官( M ) 销售总监Task 5ASK THE LAWYERQ:I have just started to work in China. I am employed by a Chinese company and I would like to know how do you determine tax on my salary earned in China?A:A foreign resident who is temporarily working in China, but who is employed by a legal entity not established in China, will normally be subject to Chinese taxation on the wages, salaries, and other remuneration(报酬) only if the employee is present in China for an total of more than 183 days in a year. However, if the foreign resident is employed permanently by a Chinese company, regardless of whether it is a domestic or foreign invested company(foreign invested companies are Chinese companies), or a representative office, the foreign resident’s salary will be treated as income originating from China and hence be subject to Chinese tax rules. Remuneration gained while performing work outside of China, will be regarded as income originating from outside China. You must keep in mind that if you stay in China for more than five continual full years or become a Chinese resident, you will be generally subject to Chinese taxation on your worldwide income.56. What did the questioner want to know?He wanted to know something about. 57. Who is allowed to answer questions in this column?.58. How will the tax be determined if a foreigner resident who has worked in China for morethan 183 days?He should be subject to .59. How will a foreigner be treated in taxation if he gains income outside China but hasstayed in China for 5 continual full years?He will be treated a Chinese resident is treated.60. Who would be interested in the Question and Answer?Foreigners who .Part IV Translation—English to Chinese (25 minutes)61. I imagine I’ll do some work instead of going to the movies.A. 我设想我能干点活,就不去看电影了。
2007年广东省高中生化学奥林匹克竞赛复赛试题(物理化学部分)一、(8分)有科技工作者提出苯(C 6H 6)在甲烷化条件下,在地下水中能被微生物“自然清除”而产生碳酸盐和甲烷。
试写出苯转化为碳酸盐和甲烷的化学反应方程式并配平,检验在298.15 K 下这一生物转化过程热力学上的可行性。
假设:溶液pH 值为7,碳酸盐浓度为1 mmol·L -1,甲烷浓度为100 μmol·L -1,苯浓度为1 μmol·L -1。
由文献查得298.15 K 下的其他数据如下: ∆f G m (C 6H 6, l) = 123.0 kJ·mol -1 ∆f G m (H 2O, l) = -237.2 kJ·mol -1∆f G m (-3HCO , aq) = -586.9 kJ·mol -1∆f G m (CH 4, g) = -50.8 kJ·mol -1 ∆f G m (H +, aq) = 0 kJ·mol -1甲烷在水中溶解达到饱和时其Henry 常数)CH (4H K =102.82 bar·mol -1·L, 1 bar = 105 Pa苯在饱和水溶液中的活度系数W ,H C 66γ= 2505.6苯的摩尔质量和密度分别为78.11 g·mol -1、872.6 kg·m -3,水的摩尔质量和密度分别为18.02g·mol -1、997.1 kg·m -3。
解:首先需根据反应前后碳的氧化还原状态进行配平。
苯中的碳(-I )部分氧化为碳酸根(+IV ),其余的还原为甲烷(-IV )。
C 6H 6 → x -3HCO + y CH 4 (1)这里x + y = 6。
其次,由苯氧化为-3HCO 产生的电子(5x 个)必须进入还原产物甲烷中(即3y 个电子)。
由这两个方程 5x = 3y 和x + y = 6,可得 x =2.25, y = 3.75, 即在碳酸氢根产物中氧是 2.25⨯3 = 6.75 mmol 。
因此,需要在苯的一側加上6.75 mmol 的水,即有C 6H 6 + 6.75 H 2O → 2.25-3HCO + 3.75CH 4 (2)最后,平衡反应计量(和电荷),反应物中的19.5个氢中有17.25个进入产物碳酸氢根和甲烷中,还需在产物中加入2.25个质子,即C 6H 6 + 6.75 H 2O → 2.25-3HCO + 3.75CH 4 + 2.25H + (3)上述反应方程书写正确,给2分。
现需计算反应(3)的自由能变化∆r G m 。
∆r G m = 2.25∆f G m (-3HCO , aq) + 3.75∆f G m (CH 4, aq) +2.25∆f G m (H +, aq)- ∆f G m (C 6H 6, aq) – 6.75∆f G m (H 2O, l) (4)1分要得到∆f G m (C 6H 6, aq),需用∆f G m (C 6H 6, l),将其调整为苯在“1 mol·L -1”标准浓度,该化合物在溶液中的标准摩尔生成自由能为∆f G m (C 6H 6, aq) = ∆f G m (C 6H 6, l) + RT ln W ,H C 66x (1 mol·L -1 ) + RT ln W ,H C 66γ (5)1分由于W ,H C 66x (1 mol·L -1 )约为0.0194(W ,H C 66x =1/(1+50.5)=0.0194, 注意:苯的摩尔体积为89.4cm 3·mol -1,因此在1 mol·L -1苯的水溶液中有50.5 mol 的水),并且RT ln W ,H C 66γ = E H C 66G = 19.4 kJ·mol -1,其中E H C 66G 为298.15K 时苯在饱和水溶液中的过剩自由能。
于是,得 ∆f G m (C 6H 6, aq) = (123.0 – 9.8 + 19.4) kJ·mol -1= 132.6 kJ·mol -1 1分要得到∆f G m (CH 4, aq),需用∆f G m (CH 4, g),有∆f G m (CH 4, aq) = ∆f G m (CH 4, g) + RTln[)CH (4H K /bar·mol -1·L]式中,)CH (4H K 为甲烷在水中的Henry 常数,)CH (4H K =102.82 bar·mol -1·L, 1 bar = 105 Pa 。
于是,得 ∆f G m (CH 4, aq) = (-50.8 + 16.1)kJ·mol -1= -34.7 kJ·mol -1 1分将所得∆f G m (C 6H 6, aq)、∆f G m (CH 4, aq)值代入式(4),得∆r G m = [2.25⨯(-586.9) + 3.75⨯(-34.7) + 0 – (132.6) – 6.75⨯ (-237.2)] kJ·mol -1= 17.9 kJ·mol -1 1分以上计算结果表明,在标准条件(即所有反应物和产物的浓度均为1 mol·L -1,水为纯液体)下,该反应是不能进行的。
在常见条件下,有∆r G m = ∆r Gm + RT ln Q = ∆r Gm + RT ln 75.66625.275.3425.23]1][H C []H []CH []HCO [+-假设-3HCO 的活度近似为1,将相应物质的浓度代入上式,得∆r G m = (17.9 + 2.48ln 75.6625.2775.3425.23]1][01[]10[]10[]10[----) kJ·mol -1= -162 kJ·mol -1因此,在含水层的一般条件下,该反应是热力学上可行的。
1分 二、(8分)背景知识对于有机化合物L R R R 321-(其中L 为卤族元素),饱和碳原子上卤族元素的亲核取代反应有两种极端情况,一是S N 2机理,此时遵循二级反应速率定律,即]L R R R ][Nu [d ]L R R R [d 321321--=--k t式中,k 为二级反应速率常数。
-Nu 为亲核基团,L 为离去基团。
二是S N 1机理,此时反应的表观速率符合一级反应速率定律,即]L R R R [d ]L R R R [d 321321--=-k t科学家就亲核物质对离去基团的影响作了较系统研究,Swain 和Scott 发现下述规律:Br CH Nu,O H Nu32lg n s k k ⋅=⎪⎪⎭⎫⎝⎛ (1)式中,Nu k 是由亲核物质引起的亲核取代的二级反应速率常数;O H 2k 是由水(标准亲核物质)引起的亲核攻击的二级反应速率常数;n 是攻击倾向或亲核物质的亲核性的量度;s 反映有机分子对亲核物质攻击的敏感程度。
表1给出了一些物质的Br CH Nu,3n 。
表1 一些重要环境亲核物质的Br CH Nu,3n 值aa 由在水中与甲基溴或溴己烷中发生的反应测定[式(1),s = 1];b 数据取自Hine J. PhysicalOrganic Chemistry, McGraw-Hill, New York, 1962;c 数据取自Haag W. R., and Mill T. Some reactions of naturally occurring nucleophiles with haloalkanes in water. Environ. Toxicol. Chem., 1988, 7, 917-924.作为对敏感程度量度的标准,对于甲基溴的S N 2反应,定义式(1)中的s = 1.0。
假定式(1)的s 和Br CH Nu,3n 与温度无关。
估计在中性水中与简单卤代烷烃(CH 3L ,L = Cl, Br, I )发生S N 2反应时,某一给定亲核物质为了跟H 2O 竞争必须具有的近似浓度。
设s = 1.0,对于表1列出的亲核物质,两种反应的重要性相等时,即]O H []Nu [2O H %50N u 2⋅=⋅k k ,得到的浓度%50]Nu [为%50]Nu [=55.3⨯Br3CH Nu,10n - (2)%50]Nu [的大小与相对亲核性有关,其浓度从微摩尔每升级到摩尔每升级范围内变化,见表2。
表2 与卤代烷烃发生S aa 使用表1中的Br CH Nu,3n 值,设s =1,根据式(2)计算得到的%50]Nu [值。
脂卤代烃进行中性水解,其一级反应速率常数记为]O H [2O H N 2k k =。
问题:(1)从表2数据,您认为脂卤代烃与-OH 的S N 2反应受pH 值的影响如何?(2)在含有100 mmol·L -1 Cl -,2 mmol·L -1 -3NO , 1 mmol·L -1 -3HCO , 0.1 mmol·L -1 -CN 的水溶液(pH=7.0, T =298.15 K )中,试估计以低浓度(<1 mmol·L -1)存在的甲基溴的半衰期。
已知在pH=7.0、T =298.15 K 的纯水中,CH 3Br 的半衰期约为20天。
解:(1)从表2数据可知,当pH 值小于10时,脂卤代烃与-OH 的S N 2反应是不重要的,因为10 = -lg[H +],可得[OH -] = K w (H 2O)/ 10-10 mol·L -1 = 1⨯10-14/10-10 mol·L -1= 10-4 mol·L -1,此数值小于表2中的%50]Nu [值(= 4⨯10-3);而当pH 值大于10时,必须考虑脂卤代烃与-OH 的S N 2反应。
2分(2)由于所有的亲核物质的浓度都过量(远大于[CH 3Br]0),CH 3Br 发生的反应可描述为假一级反应,其一级反应速率常数为∑+=ii ikk k ]Nu []O H [Nu 2O H obs 2 (1)1分由表2数据可看出,CH 3Br 与-3NO 和-OH 的反应可忽略。
为了估计与其他亲核物质反应的速率常数,将s =1代入重排过的题给式(1),得Br3CH Nu,210O H Nu n k k ⨯= (2)1分 将表1中的Br CH Nu,3n 值代入式(2),各亲核物质i 的Nu k 代入式(1),得])CN [10]HCO [10]Cl [10]O H ([1.538.332O H obs 2---+++⨯=k k (3) 1分 将不同亲核物质的浓度代入式(3),得 O H O H obs 222.174)6.123.61003.55(k k k =+++⨯= 1分这一计算说明CH 3Br 与氯的反应的重要性是其中性水解的2倍,而与另两个亲核物质的反应仅占CH 3Br 总变化速率的10.8%[(6.3+12.6)/174.2 = 10.8%]。