Benzoylmesaconine_63238-67-5_DataSheet_MedChemExpress
- 格式:pdf
- 大小:81.86 KB
- 文档页数:1

1、盐酸醋丁洛尔(Acebutolol Hydrochloride)化学名:N-[3-乙酰基-4-[2羟基-3-[(1-甲基乙基)氨基]丙氧基]苯基]丁酰胺盐酸盐;英文名:N-[3-Acetyl-4-[2-hydroxy-3-[(l-methylethyl)amino]Propoxy ]phenyl ]butanamide hydrochloride分子式:C18H28N2O4·HCl分子量:371.891\对丁酰胺基苯酚3\对丁酰胺基苯乙酮5\2-羟基-5-丁酰胺基苯乙酮8\2-环氧乙基甲氧基-5-丁酰胺基-苯乙酮10\醋丁洛尔2、醋谷胺铝(Aceglutamide Aluminum)化学名:N2-乙酰-L-谷氨酰胺四羟基三铝英文名:N2-Acetyl-L-glutamine aluminium complcx分子式:C35H59Al3N10O24分子量:1084.861\N-乙酰谷酰胺3、阿克他利(Actarit)化学名:4-乙酰胺基苯乙酸英文名:4-(Acetylamino)benzeneacetic acid 分子式:C10H11NO3分子量:193.211\4-氨基苯乙酸2\4-氨基苯乙酸乙酯3\4-乙酰胺基苯乙酸乙酯4\4-乙酰胺基苯乙酸,阿克他利4、阿昔洛韦(Acyclovir)化学名:2-氨基1,9-二氢-9-[(2-羟基乙氧基)甲基]-6H-嘌呤-6-酮英文名:2-amino-l,9-dihydro-9-[(2-hydroxyethoxy)methyl]-6H-purin-6-one 分子式:C8H11N5O3分子量:225.21\鸟嘌呤3\9-(2-苯甲酰氧基乙氧基甲基) 鸟嘌呤4\阿昔洛韦阿苯达唑(Albendazole)化学名:[5-(丙硫基)-l H-2-苯并咪唑基]氨基甲酸甲酯英文名:[5-(propylthio)-l H-benzimidazol-2-yl]carbamic acid methyl ester 分子式:C12H15O2S分子量:265.341\3-氯-6-硝基乙酰苯胺3\2-硝基-5-丙硫基苯胺4\4-丙硫基-邻苯二胺5\2-氨基-5-丙硫基苯并咪唑===================================6、阿拉普拉(Alacepril)化学名:(S)-N-[l-[3-(乙酰硫基)-2-甲基丙酰]-L-脯氨酰]-L-苯丙氨酸英文名:(S)N-[l-[3-(Acetylthio)-2-methyl-l-oxopropyl]-L-prolyl]-L-phenylalanine 分子式:C20H26N2O5S分子量:406.501\l-(D-3-乙酰硫基-2-甲基丙酰)-L-脯氨酸2\L-苯丙氨酸-叔丁酯盐酸盐4\l-(D-3-乙酰硫基-2-甲基丙酰)-L-脯氨酰-L-苯丙氨酸-叔丁酯6\阿拉普拉7、阿氯芬酸(Alclofenac)化学名:3-氯-4-(2-丙烯氧)苯乙酸英文名:3-Chloro-4-(2-propenyloxy)benzeneacetic acid分子式:C11H11O3Cl分子量:2261\邻氯苯酚3\邻氯烯丙氧苯5\2-氯-4-氯甲基-烯丙氧苯6\3-氯-4-烯丙氧苯乙腈7\3-氯-4-(2-丙烯氧)苯乙酸,阿氯芬酸8、阿法骨化醇(Alfacalcidol)化学名:(1α,3β,5Z,7E)-9,10-并环胆甾-5,7,10(19)三烯-1,3-二醇英文名:(1α,3β,5Z,7E)-9,10-Secocholesta-5,7,10(19)-triene-1,3-diol分子式:C27H44O2分子量:400641\胆固醇2\ 3β-羟基-5α-胆甾烷-6-酮3\ 6-亚乙二氧基-5α-胆甾烷-3β-醇4\ 6-亚乙二氧基-胆甾烷-3 –酮4\\ 2α-溴-6-亚乙二氧基-胆甾烷-3-酮5\ 6-亚乙二氧基-l-胆甾烯-3-酮6\6-亚乙二氧基-lα ,2α–环氧胆甾烷-3-酮7\6,6-亚乙二氧基-胆甾烷-lα ,3(α,β)-双醇8\5α-胆甾烷-6-酮-lα,3(α,β)-双醇8\5α-胆甾烷-lα,3(α,β)-二乙酰氧-6-酮9\5α-胆甾烷-lα,3β-二乙酰氧-6-醇10\ lα,3β-二乙酰氧胆固醇10\\lα,3β-二乙酰氧-7-溴-胆固醇11\ lα,3β-二乙酰氧-胆甾-5,7-二烯12\ lα,3β-二乙酰氧-前维生素D313 lα,3β-二乙酰氧-维生素D314\ lα-羟维生素D39、阿明洛芬(Alminoprofen)化学名:2-[对-(2-甲基烯丙基)-氨基苯基]丙酸;α-甲基-4-[(2-甲基-2-丙烯基)氨基]苯乙酸英文名:α-Methyl-4-[(2-methyl-2-propenyl)amino]benzeneacetic acid分子式:C13H17NO2分子量:219.291\2-(对硝基苯基)丙酸甲酯2\2-(对氨基苯基)丙酸甲酯4\2-[对(2-甲基烯丙基)氨基苯基]丙酸甲酯盐酸盐5\阿明洛芬10阿普唑仑(Alprazolam)化学名:8-氯-l-甲基-6-苯基-4H-[1,2,4]三唑并[4,3-α][1,4]苯并二氮杂草英文名:8-Chloro-l-methyl-6-phenyl-4H-[1,2,4]triazolo[4,3-α][1,4]benzodiazepine 分子式:C17H13ClN4分子量:308.771\ (2-氨基础理论-氯苯基)-苯甲酮2\ 甘氨酸乙酯盐酸盐3\7-氯-5-苯基-2-氧代-2,3-二氢-l H-1,4苯并二氨杂卓5\7-氯-5-苯基-2-硫代-2,3-二氢-l H-1,4苯并二氮杂卓7\ 2-(2-乙酰肼基)-7-氯-5-苯基-3 H-1,4苯并二氮杂卓8\阿普唑仑11盐酸阿普洛尔(Alprenolo Hydrochloride)化学名:1-[(1-甲基乙基)氨基]-3-[2-(2-丙烯基)苯氧基]-2-丙醇盐酸盐英文名:1-[(1-Methylethyl)amino]-3-[2-(2-propenyl)phenoxy]-2-propanol hydrochloride 分子式:C15H23O2N·HCl分子量:285.821\ 2-丙烯苯酚2\ 表氯醇3\ 1-(邻丙烯苯氧)-2,3-环氧丙烷5\1-(邻丙烯苯氧)-2-羟-3-异丙氨-丙烷,阿普洛尔12盐酸氨溴索(Ambroxol Hydrochloride)化学名:4-[[(2-氨基-3,5-二溴苯基)甲基]氨基]环已醇盐酸盐英文名:4-[[(2-Amino-3,5-dibromophenyl)methyl]amino]cyclohexanol hydrochloride 分子式:C13H18Br2N2O·HCl分子量:414.571\ 2-氨基-3,5-二溴-N-(羟基环已基)苯甲酰胺2\ 4-(2-氨基-3,5-二溴苄胺基)-环已醇3\ 盐酸氨溴索13氨芬酸钠(Amfenac Sodium)化学名:2-氨基-3-苯甲酰苯乙酸钠二水合物英文名:2-Amino-3-benzoylbenzeneacetic acid sadinmsalt dihydrate 分子式:C15H2NO3Na·2H2O分子量:277.181\ 1-氨基-1,3-二氢吲哚-2-酮2\苯基丙酮3\1-(2-甲基苯亚乙基亚氨基)1,3-二氢吲哚-2-酮4\ 2-(2-甲基-3-苯基吲哚-7-基)乙酸乙酯5\ 2-乙酰胺基-3-苯甲酰苯乙酸乙酯6\ 7-苯甲酰1,3-二氢吲哚-2-酮7\ 2-氨基-3-苯酰苯乙酸8\氨芬酸钠14苯磺酸氨氨地平(Amlodipine Besilate)化学名:(±)-2-(2-氨基乙氧甲基)-4-(2-氯苯基)-1,4-二氢-6-甲基-3,5-吡啶二羧酸-3-乙酯-5-甲酯苯磺酸盐英文名:(±)-2-[(2-Aminoethoxy)methyl]-4-(2-chlorophenyl)-1,4-dihydro-6-methyl-3,5-pyridinedicarboxy lic acid-3-ethyl-5-methylester Benzenesulfonate分子式:C20H25ClN2O5·C6H5SO3H分子量:567.061、2-氯苯甲醛2、乙酰乙酸甲酯3、2-(2-氯苄叉基)乙酰乙酸甲酯4、4-氯-3-氧代丁酸乙酯,氯乙酰乙酸乙酯5、2-叠氮乙醇6、2-叠氮乙氧基乙酰乙酸乙酯7、2-[(2-叠氮乙氧基)甲基]-4-(2-氯苯基)-3-乙氧羰基-5-甲氧羰基-6-甲基-1,4二氢吡啶氨氯地平15盐酸氨磺洛尔(Amosulalol Hydrochloride)化学名:5-(±)-5-[1-羟基-2-[[2-(2-甲氧基苯氧基)乙基]氨基]乙基]-2-甲基苯磺酰胺盐酸盐英文名:5-(±)-5-[1-Hydroxy-2-[[2-(2-methoxyphenoxy)ethyl]amino]ethyl]-2-methylbenzenesalfonamide hydrochloride分子式:C18H24N2O5S·HCl分子量:416.931\ 5-溴乙酰-2-甲基苯磺酰胺2\ N-[2(2-甲氧基)苯氧基乙基]苄胺3\ 5-[N-苯甲基-N-[[2-(2-甲氧基)苯氧基]乙基]氨基]乙酰-2-甲基苯磺酰胺4\ 5-[1-羟基-2-[[N-苯甲基-N-2-(2-甲氧基)苯氧基乙基]氨基]乙基]-2-甲基苯磺酰胺5\5-[1-羟基-2-[[2-(2-甲氧基苯氧基)乙基]氨基]乙基]-2-甲基苯磺酰胺盐酸盐,盐酸氨磺洛尔16阿莫西林(Amoxicillin)化学名:[2S-[2α,5α,6β(S﹡)]]-6-[[氨基(4-羟苯基)乙酰基]氨基]-3,3-二甲基-7-氧代-4-硫-1-氮杂二环[3,2,0]庚烷-2-羧酸英文名:[2S-[2α,5α,6β(S ﹡)]]-6-[[Amino(4-hydroxyphenyl)acetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3,2,0]h eptane-2-carboxylic acid分子式:C16H19N3O5·3H2O分子量:419.411\ ( ±)苄氧甲酰氨-4-羟苯乙酸2\ (-)苄氧甲酰氨-对羟苯乙酸3\ 6-氨基-青霉烷酸4\6-[(-)-苄氧甲酰氨-对羟基苯乙酰胺]青霉烷酸5\ 阿莫西林17氨苄西林(Ampicillin)化学名:[2S-[2α,5α,6β(S﹡)]]-6-[(氨基苯乙酰)氨基]-3,3-二甲基7-氧代-4-硫杂-1-氮杂二环[3,2,0]庚烷-2-羧酸英文名:[2S-[2α,5α,6β(S ﹡)]]-6-[(Aminophenylacetyl)amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3,2,0]heptane-2-car boxylic acid分子式:C16H19N3O4S分子量:.349.421\D(-)-α-氨基-α-苯乙酸2\ 氯甲酸苄酯3\ D(-)-苄氧基甲酰氨基-α-苯乙酸4\ 氯甲酸乙酯5\[3]和[4]的混合酸无水物6\ 6-氨基青霉烷酸7\ 6-[D(-)-α-(苄氧基甲酰氨基)苯乙酰氨]青霉烷酸8\氨苄西林18、氨力农(Amrinone)化学名:5-氨基-(3,4′-双吡啶)-6(1H)-酮英文名:5-Amino-(3,4′-bipyridin)-6(1H)-one 分子式:C10H9N3O分子量:187.201\ 4-甲基吡啶2\ 2-(4-吡啶基)-3-二甲胺基丙烯醛3\ 氰基乙酰胺4\ 3-氰基-5-(-4-吡啶基)-2(1H)-吡啶酮5\ 3-氨甲酰基-5-(-4-吡啶基)-2(1H)-吡啶酮19\阿加曲班(Argatroban)化学名:1-[5-[(氨基亚氨基甲基)氨基]-1-氧代-2[[(1,2,3,4四氢-3-甲基-8-喹啉基)磺酰]氨基]戊基]-4-甲基-2-哌啶羧酸英文名:1-[5-[(Aminoiminomethyl)amino]-1-oxo-2-[[(1,2,3,4-tetrahydro-3-methyl-8-quinolinyl)sulfonyl]a mino]pentyl]-4-methyl-2-piperidinecarboxylic acid分子式:C23H36N6O5S分子量:508.6420盐酸阿罗洛尔(Arotinolol Hydrochloride)化学名:(±)-5-[2-[[3-[(1,1-二甲基乙基)氨基]-2-羟基丙基]硫]-4-噻唑基]-2-噻吩酰胺盐酸盐英文名:(±)-5-[2-[[3-[(1,1-Dimethylethyl)amino]-2-hydroxypropyl]thio]-4-thiazolyl]-2-thiophenecarboxa mide hydrochloride分子式:C15H21N3O2S3·HCl分子量:.408.11\ 5-乙酰基噻吩-2-羧酸2\ 5-乙酰噻吩-2-甲酰氨3\ 5-溴乙酰噻吩-2-甲酰氨4\ 二硫代氨基甲酸铵5\ 5-(2-巯基4-噻唑基)-2-噻吩甲酰氨7\ 5-[2-(3’-叔丁基氨基-2’-羟基丙基硫)-4-噻唑基]-2-噻吩甲酰氨8\ 盐酸阿罗洛尔21阿司咪唑(Astemizole)化学名:1-[(4-氟苯基)甲基]-N-[1-[2-(4-甲氧苯基)乙基]-4-哌啶基]-1H-2-苯并唑咪基胺英文名:1-[(4-Fluorophenyl)methyl] -N-[1-[2-(4-methoxyphenyl)ethyl]-4-piperidinyl] -1H-benzimidazol-2-amine分子式:C28H31FN4O分子量:.458.251\ 1-异硫氰基-2-硝基苯酯2\ 4-氨基-1-哌啶甲酸乙酯3\ N-(2-硝基苯基)-N’-(1-乙氧甲酰-4-哌啶基)硫脲4\ N-(2-氨基苯基)-N’-(1-乙氧甲酰-4-哌啶基)硫脲5\ 2-(1-乙氧甲酰-4-哌啶基氨基)苯并咪唑6\ 4-氟苄基氯7\1-(4-氟苄基)-2-(1-乙氧甲酰-4-哌啶基氨基)苯并咪唑8\1-(4-氟苄基)-2-(4-哌啶基氨基)苯并咪唑9\甲磺酸对甲氧苯基乙酯10 阿司咪唑22阿替洛尔(Atenolol)化学名:4[2-羟基-3-[(1-甲基乙基)氨基]丙氧基]苯乙酰胺英文名:4-[2-Hydroxy-3-[(1-methylethyl)amino]propoxy]benzeneacetamide 分子式:C14H22N2O3分子量:266.341\ 2-(4-环氧乙烷基甲氧苯基)-乙酰胺2\ 异丙胺3\1-对氨基甲酰甲基苯氧基-3-异丙胺基-2-丙醇,阿替洛尔23盐酸阿扎司琼(Azasetron Hydrochloride)化学名:(±)-N-1-氮杂二环[2,2,2]-3-辛基-6-氯-3,4-二氢-4-甲基-3-氧代-2H-1,4-苯并噁嗪-8-甲酰胺盐酸盐英文名:(±)-N-1-Azabicyclo[2,2,2]oct-3-yl-6-chloro-3,4-dihydro-4-methyl-3-oxo-2H-1,4-benzoxazine-8-carb oxamide hydrochloride分子式:C17H20ClN3O3·HCl分子量:368.281\ 5-氯-2-羟基苯甲酸甲酯2\ 5-氯-2-羟基-3-硝基苯甲酸甲酯3\ 5-氯-2-羟基-3-氨基苯甲酸甲酯5\氯乙酰氯6\ 6-氯-4-甲基-3-氧代-3,4-二氢-2H-1,4-苯并噁嗪-8-甲酸甲酯7\ 6-氯-4-甲基-3-氧代-3,4-二氢-2H-1,4-苯并噁嗪-8-甲酸8\ 6-氯-4-甲基-3-氧代-3,4-二氢-2H-1,4-苯并噁嗪-8-甲酰氯9\ 1-氮杂-3-二环[2,2,2]辛基胺10\ 盐酸阿扎司琼24盐酸氮卓斯丁(Azelastine Hydrochloride)化学名:4-[(4-氯苯基)甲基]-2-(六氢-1-甲基-1H-4-氮杂卓基)-1(2H)-酞嗪酮盐酸盐英文名:4-[(4-Chlorophenyl)methyl]-2-(hexahydro-1-methyl-1H-azepin-4-yl)-1(2H)-phthalazinonehydroc hloride分子式:C22H25N3Cl2O分子量:418.371、2-4氯苯乙酰酰基苯甲酸2、硫酸肼3、4-(4-氯苄基)-1(2H)-酞嗪酮2-(2-氯乙基)-N-甲基吡咯烷盐酸盐5、4-(4-氯苄基)-2[N-甲基全氢化氮杂卓基-(4)-]-1(2H)-酞嗪酮6、4-(4-氯苄基)-2-[N-甲基全氢化氮杂卓基-(4)-]-1-(2H)酞嗪酮盐酸盐,盐酸氮卓斯丁25奥(Azulene)化学名:环戊二烯并环庚三烯英文名:Cyclopentacycloheptene 分子式:C10H8分子量:128.161\ 1,6-己二酸2\ 6-羰基-壬二酸3\ 5-(2-乙酰基-1-环戊烯基)-戊酸4\ 2,3,5,6,7,8-六氢-1H–奥-4-酮5\ 八氢,奥-4-酮6\ 十氢奥-4-醇7\奥26盐酸巴氨西林(Bacampicillin Hydrochloride)化学名:[2S-[2α,5α,6β(S﹡)]]-6-[(氨基苯基乙酰)氨基]-3,3-二甲基-7-氧代4-硫杂-1-氮杂二环[3,2,0]庚烷-2-羧酸-1-[(乙氧羰基)氧基]乙酯盐酸盐英文名:[2S-[2α,5α,6β(S﹡)]]-6-[(Aminophenylacetyl)amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3,2,0]heptane-2-car boxylic acid 1-[(ethoxycarbonyl)oxy]ethyl ester hydrochloride分子式:C21H27N3O7S·HCl分子量:502.001\ 碳酸-1-氯乙酯乙酯3\碳酸-1-溴乙酯乙酯4\ 6-[(氨基苯基乙酰)氨基]-3,3-二甲基7-氧代号-硫杂志-氮杂二环[3,2,0]庚烷-2-羧酸5\ 乙酰乙酸甲酯6\ 6-[2-(甲氧羰基)-1-甲基乙烯基氨基苯基(乙酰)氨基]-3,3-二甲基-7-氧代-4-硫杂-1-氮杂二环[3,2,0]庚烷-2-羧酸7\ 盐酸巴氨西林27巴氯芬(Baclofen)化学名:β-(氨甲基)-4-氯苯丙酸英文名:β-(Aminomethyl)-4-chlorobenzenepropanoic acid分子式:C10H12ClNO2分子量:213.661\ 对氯肉桂酸乙酯2\ 硝基甲烷3\ β-硝甲基4-氯苯丙酸乙酯4\ 4-(对氯苯)-2-吡咯烷酮28苄达酸(Bendazac)化学名:[(1-苄基-1H-3-吲唑基)氧基]乙酸英文名:[(1-Phenylmethyl) -1H-indazol-3-yl]-oxy]-acetic acid 分子式:C16H14H2O3分子量:282.301\ 1-苄基-1H-3-吲唑醇钠2\ 氯乙晴3\ 1-苄-3-吲唑-氧乙腈4\ 苄达酸29盐酸苄丝肼(Benserazide Hydrochloride)化学名:DL-丝氨酸-2-[(2,3,4-三羟苯基)甲基]酰肼盐酸盐英文名:DL-Serine-2-[(2,3,4-trihydroxyphenyl)methyl]-hydrazidc hydrochloride 分子式:C10H15N3O5·HCL分子量:293.711\ DL-丝氨酰肼盐酸盐2\ 2,3,4-三羟苯甲醛3\ DL-丝氨酰-2-(2,3,4-三羟基亚苄基)酰肼盐酸盐4\ 盐酸苄丝肼30苯溴马隆(Benzbromarone)化学名:(3,5-二溴-4-羟基苯基)-(2-乙基-3-苯并呋喃)-甲酮英文名:(3,5-Dibromo-4-hydroxyphenyl)(2-ethyl-3-benzofuranyl)methanone 分子式:C17H12Br2O3分子量:424.091\ 水杨醛2\ 一氯丙酮3\2-乙酰苯并呋喃4\ 水合肼5\ 2-乙基苯并呋喃6\ 对甲氧基苯甲酰氯7\ 2-乙基3-茴香酰-苯井呋喃8\ 吡啶盐酸盐9\2-乙基地-3-(对-羟-苯甲酰)-苯并呋喃10\ 苯溴马隆31二丙酸倍他米松(Betamethasone Dipropionate)化学名:(11β,16β)-9-氟-11,17,21-三羟-16-甲基-孕甾-1,4-二烯-3,20-双酮-17,21-二丙酸酯英文名:(11β,16β)-9-Fluoro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione-17,21-dipropionate分子式:C28H37FO7分子量:504.591\ 9α-氟-11β,17α,21-三羟基-16β-甲基-5α-孕甾烷-3,20-二酮-21-乙酸酯3\ 11β三甲基硅烷氧基-9α-氟-17α,21-二羟基-16β-甲基-5α-孕甾烷-3,20-二酮-21-乙酸酯4\ 11β三甲基硅烷氧基-9α-氟-17α,21-二羟基-16β-甲基-5α-孕甾烷-3,20-二酮5\ 丙酸酐6\对甲苯磺酸7\11β三甲基硅烷氧基-9α-氟-17α,21-二羟基-16β-甲基-5α-孕甾烷-3,20-二酮-17,21-二丙酸酯8\ 2,3-二氯-5,6-二腈-苯醌(DDQ)9\ 11β三甲基硅烷氧-17α,21-二羟基-基-9α-氟-16β-甲基孕甾-1,4-二烯-3,20-二酮-17,21-二丙酸酯10\ 9α-氟-11β,17α,21-三羟基-16β-甲基孕甾-1,4-二烯-3,20-二酮-17,21-二丙酸酯, 二丙酸倍他米松32倍他米松磷酸钠(Betamethasone Sodium phosphate)化学名:9α-氟-11β,17α,21-三羟基-16β-甲基孕甾-1,4-二烯3,20-二酮-21-(二氢磷酸酯)二钠盐英文名:(11β,16β)-9-Fluoro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione-21-(dihydrogenph osphate)disodium salt分子式:C22H29FNaO8P分子量:494.431\ 3α-乙酰氧基-16-孕甾烯-11,20-二酮3\ 3α-乙酰氧基-16α,17α-亚甲偶氮孕甾-11,20-二酮4\ 3α-乙酰氧基-16-甲基-16-孕甾烯- 11,20-二酮6\ 16α,17α-环氧-3α-羟基-16β-甲基孕甾-11,20-二酮7\ 3α,17α-二羟基-16β-甲基孕甾- 11,20-二酮8\ 21-溴-3α,17α-二羟基-16β-甲基孕甾- 11,20-二酮10\ 3α,17α-21-三羟基-16β-甲基孕甾- 11,20-二酮-21-乙酸酯11\N-溴琥珀酰亚胺12\ 17α,21-二羟基-16β-甲基孕甾- 3,11,20-三酮-21-乙酸酯13\ 4-溴-17α,21-二羟基-16β-甲基孕甾-3, 11,20-三酮-21乙酸酯14\ 盐酸氨基脲15\ 17α,21-二羟基-16β-甲基-4-孕甾烯- 3,11,20-三酮-21-乙酸酯的-3-缩氨基脲16\ 17α,21-二羟基-16β-甲基-4-孕甾烯- 3,11,20-三酮-21-乙酸酯17\ 17α,21-二羟基-16β-甲基-4-孕甾烯- 3,11,20-三酮-21-乙酸酯的-3,20-二缩氨基脲19\11β,17α,21-三羟基-16β-甲基-4-孕甾烯- 3,,20-二酮的-3,20-二缩氨基脲20\ 11β,17α,21-三羟基-16β-甲基-4-孕甾烯- 3,20-二酮21\ 乙酐22\ 11β,17α,21-三羟基-16β-甲基-4-孕甾烯- 3,,20-二酮-21-乙酸酯23\ 甲磺酰氯24\ 17α,21-三羟基-16β-甲基-4,9(11)-孕甾二烯- 3,,20-二酮-21-乙酸酯25\ 9α-溴-11β,17α,21-三羟基-16β-甲基-4-孕甾烯- 3, 20-二酮-21-乙酸酯27\ 9β,11β-环氧- 17α,21-二羟基-16β-甲基-4-孕甾烯- 3, 20-二酮-21-乙酸酯28\ 9α-氟-11β,17α,21-三羟基-16β-甲基-4-孕甾烯- 3, 20-二酮-21-乙酸酯30\9α-氟-11β,17α,21-三羟基-16β-甲基-1,4-孕甾二烯- 3, 20-二酮-21-乙酸酯31\倍他米松32\ 9α-氟-11β,17α,21-三羟基-16β-甲基-4-孕甾烯- 3, 20-二酮-21-甲磺酸酯33\ 9α-氟-16β-甲基-11β,17α–二羟基- 3, 20-二氧代-21-碘-1,4-孕甾二烯34\ 9α-氟-16β-甲基11β,17α,21-三羟基- 3, 20-二氧代1,4-孕甾二烯-21-磷酸二氢酯35\倍他米松磷酸钠33\盐酸贝凡洛尔(Bevantolol Hydrochloride)化学名:1-[[2-(3,4-二甲氧基苯基)乙基]胺基]-3-(3-甲苯氧基)-2-丙醇盐酸盐英文名:1-[[2-(3,4-Dimethoxyphenyl)ethyl]amino]-3-(3-methylphenoxy)-2-propanol hydrochloride分子式:C20H27NO4·HCl分子量:381.901\ 3-甲基苯酚2\ 氯甲基环氧乙烷3\ 3-甲苯氧基环氧乙烷4\2-(3,4-二甲氧苯基)乙胺5\盐酸贝凡洛尔34\盐酸溴己新(Bromhexine Hydrochloride)化学名:2-氨基-3,5二溴-N-环已基- N-甲基苯甲铵盐酸盐英文名:2-Amino-3,5-dibromo-N-cyclohexyl-N-methylbenzenemethanamine hydrochloride 分子式:C14H21N2Br2Cl分子量:412.631\ 2-硝基溴苄2\ N-甲基环已胺3\ N-(2-硝基苄)- N-甲基环已胺5\ N-(2-氨基苄)- N-甲基环已胺6\盐酸溴已新35\溴哌利多(Bromperidol)化学名:4[4-(4-溴苯基)-4羟基-1-哌啶基]-1-(4-氟苯基)-1-丁酮英文名:4[4-(4-Bromophenyl)-4-hydroxy-1-piperidinyl]-1-(-4-fluorophenyl)-1-butanone 分子式:C21H23BrFNO2分子量:420.331\4-溴苯基溴化镁2\ 1-(乙氧羰基)-4-氧代哌啶3\ 1-(乙氧羰基)-4-羟基-4-(4-溴苯基)哌啶4\ 4-羟基-4-(4-溴苯基)哌啶5\ 1-(4-氯丁酰)-4氟苯6\ 溴哌利多36\溴替唑仑(Brotizolam)化学名:2-溴-4-(2-氯苯基)-9-甲基-6H-噻嗯并-[3,2-f ]-[1,2,4]-三唑并-[4,3-α]-[1,4]-二氮杂卓英文名:2-Bromo-4-(2-chlorophenyl)-9-methyl-6H-thieno-[3,2-f ]-[1,2,4]-triazolo-[4,3-α]-[1,4]-diazepine 分子式:C22H19Br分子量:363.311\ 7-溴-5-(2-氯苯基)-1,3-二氢噻嗯-[2,3-e]-1,4-二氮杂卓-2-酮2\ 7-溴-5-(2-氯苯基)-1,3-二氢-[2,3-e]-噻嗯-1,4-二氮杂卓-2-硫酮3\ 7-溴-5-(2-氯苯基)-2-肼基-1,3-二氢-[2,3-e]- 噻嗯-1,4-二氮杂卓4\ 正乙酸二乙酯5\溴替唑仑37富马酸溴长春胺(Brovincamine Fumarate)化学名:(3α,14β,16α)-11-溴-14,15-二氢-14-羟基象牙烯宁-14-羧酸甲酯富马酸盐英文名:(3α,14β,16α)-11-Bromo-14-15-dihydro-14-hydroxyeburnamenine-14-carboxylic acid methylester fumarate分子式:C21H25BrN2O3·C4H4O4分子量:549.351\ (3α,14β,16α)-长春胺2\溴长春胺38布地奈德(Budesonide)化学名:(11β,16α)-16,17-[亚丁基双(氧)]-11,21-二羟基孕甾-1,4-二烯-3-20-二酮英文名:(11β,16α)-16,17-[Butylidenebis(oxy)]-11,21-dihydroxypregna-1,4-diene-3,20-dione分子式:C25H34O6分子量:430.551\ 16α-羟基泼尼松龙2\布地奈德39\盐酸布那唑嗪(Bunazosin Hydrochloride)化学名:1-(4-氨基-6,7-二甲氧基-2-喹唑啉基)-6-氢-4-(1-氧代丁基)-1H-1,4-二氮卓盐酸盐英文名:1-(4-Amino-6,7-dimethoxy-2-quinazolinyl)hexahydro-4-(1-oxobutyl)-1H-1,4-diazepinehydrochlo ride分子式:C19H27N5O3·HCl分子量:409.931\ 2-氯-4-氨基-6,7-二甲氧基喹唑啉2\ N-甲酰高哌嗪3\ 2-(N-甲酰高哌嗪基)-4-氨基-6,7二甲氧基喹唑啉4\ 2-高哌嗪基-4-氨基-6,7-二甲氧基喹唑啉盐酸盐5\ n-丁酰氯6\ 盐酸布那唑嗪40盐酸布尼洛尔(Bunitrolol Hydrochloride)化学名:2-[3-[(1,1-二甲基乙基)氨基]-2羟基丙氧基]苄腈盐酸盐英文名:2-[3-[(1,1-dimethylethyl)amino]-2-hydroxypropoxy]benzonitrile hydrochloride 分子式:C14H20ON2O2·HCl分子量:248.81\ 2-环氧乙烷甲氧基-苄腈3\盐酸布尼洛尔。


14260Technical Data SheetRevision Date:25.07.14Rev 5Bay 150,Shannon Industrial Estate,Shannon County Clare,Ireland 2Ton EpoxyORDERING INFORMATIONStock No.1426020222Package Size 50ml Cartridge 200ml CartridgeDescriptionA high strength,non-shrinking,adhesive/potting compound specially formulated for high clarity,good impact strength,and water resistance.The adhesive bond is resistant to weathering,solvents,and wide variations in temperature.Features∙100%reactive,no solvents∙Good water and chemical resistance ∙Fills gaps and voids∙Room temperature curingRecommended applications∙Bonding or potting electronic components and assemblies ∙Creating moisture-resistant seals∙Suitable for bonding ceramics,ferrous and non-ferrous,ferrites,wood,glass and concretePRODUCT DATA Physical Properties (Uncured)ColourClear Mixed Viscosity8,000cps Mixed Ratio by Volume 1:1Mixed Ratio by weight 1.2:1Mixed Density1.10gm/cc Working time 28 grams @ 23˚C8–12minutes Fixture time @23oC 30–35minutes Functional cure @ 23˚C 2 hours Full Cure12hoursPhysical Properties (Cured)7days cured @24˚CAdhesive tensile shear,ASTM D100215.5MPa (0.25mm bondline)T peel4-5N/10mm Tensile Elongation1%Service temperature,dry-40–93oC Cured hardness,ASTM D224083DDielectric strength,ASTM D14924KV/mm Compression strength,ASTM D69575.86MPaSpecific Volume909cm 3/kg7days room temperature cure (30days immersion)Chemical ResistanceAcetic (dilute)10%Poor Acetone FairAmmonia Very Good Corn Oil Excellent Cutting oil Excellent Ethanol Poor Petrol (unleaded)Excellent Glycols/Antifreeze Excellent Hydrochloric 10%Poor Isopropanol Poor Kerosene Excellent MEK Poor Mineral Spirits Excellent Motor Oil Excellent Sodium Hydroxide 10%Very Good Sulphuric 10%PoorPlease consult ITW Devcon for other chemicalsTechnical Data SheetRevision Date:25.07.14Rev 5Bay 150,Shannon Industrial Estate,Shannon County Clare,Ireland 2Ton EpoxyAPPLICATION INFORMATION General Surface Preparation2Ton Epoxy works best on clean surfaces.Surfaces should be free of heavy deposits of grease,oil,dirt or other contaminants or cleaned with industrial cleaning equipment such as vapor degreasers or hot aqueous baths.Abrading or roughing the surfaces of metals will increase the microscopic bond area significantly and optimize the bond strength.MixingThis product is available in two cartridge sizes.The cartridge should be used with the appropriate manual Applicator Gun and Static Mixer Nozzle.The Static Mixer Nozzle enables the material to be thoroughly mixed when dispensed,and thus can be applied directly to the surfaces to be bonded.Please note:Once the product goes beyond its working time the nozzle has to be thrown away and a new nozzle used for further dispensing.ApplicationApply mixed epoxy directly to one surface in an even film or as a bead.Assemble with the mating part within the recommended working time.Obtain firm contact between the parts to minimize any gap and ensure good contact of the epoxy between the mating parts,clamping may optimize this part of the process.A small volume of epoxy should flow out the edges to show there is adequate gap filling.For very large gaps,apply epoxy to both surfaces and spread to cover the entire area,or make a bead pattern,which will allow flow throughout the joint.Let bonded assemblies stand for the recommended functional cure time before handling.They are capable of withstanding processing forces at this point,but should not be dropped,shocked or heavily loaded.Storage Shelf LifeDevcon Epoxy Adhesives should be stored in a cool dry place when not used for a longperiod of time.A shelf life of 3years from date of manufacture can be expected when stored at room temperature (22ºC)in their original containers.The expiry will be displayed on the product packaging.Precaution For complete safety and handling information,please refer to the appropriate Material Safety Data Sheets prior to using this product.WarrantyDevcon will replace any material found to be defective.Because the storage,handling and application of this material is beyond our control we can accept no liability for the results obtained.DisclaimerAll information on this data sheet is based on laboratory testing and is not intended for design purposes.ITW Devcon makes no representations or warranties of any kind concerning this data.For product information visit alternatively for technical assistance please call +35361771501(Ireland)or +49(0)431717910(Germany)14260。
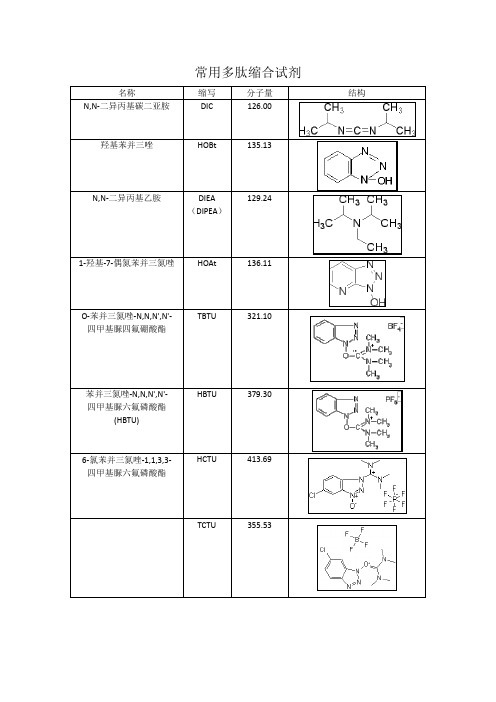
常用多肽缩合试剂名称缩写分子量结构N,N-二异丙基碳二亚胺DIC 126.00羟基苯并三唑HOBt 135.13129.24N,N-二异丙基乙胺DIEA(DIPEA)1-羟基-7-偶氮苯并三氮唑HOAt 136.11TBTU 321.10O-苯并三氮唑-N,N,N',N'-四甲基脲四氟硼酸酯HBTU 379.30苯并三氮唑-N,N,N',N'-四甲基脲六氟磷酸酯(HBTU)HCTU 413.696-氯苯并三氮唑-1,1,3,3-四甲基脲六氟磷酸酯TCTU 355.53PyBOP 520.40六氟磷酸苯并三唑-1-基-氧基三吡咯烷基磷常用多肽缩合试剂名称缩写分子量结构PyAOP 521.38(3H-1,2,3-三唑并[4,5-b]吡啶-3-氧基)三-1-吡咯烷基鏻六氟磷酸盐DCC 206.33N,N'-二环己基碳二亚胺4-二甲氨基吡啶DMAP 122.17DBU 152.241,8-二氮杂双环[5.4.0]十一碳-7-烯1,1’-羰基二咪唑CDI 162.15HATU 380.232-(7-偶氮苯并三氮唑)-N,N,N',N'-四甲基脲六氟磷酸酯HOOBt 163.103-羟基-1,2,3-苯并三嗪-4(3H)-酮Cl-HOBt 169.576-氯-1-羟基苯并三氮唑EDC.HCl 191.71-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐TATU 322.1O-(7-氮杂苯并三氮唑)-N,N,N',N'-四甲基脲四氟硼酸盐常用多肽缩合试剂名称缩写分子量结构TPTU 297.10O-(1,2-二氢-2-氧-吡啶基)--1,1,3,3-四甲基脲四氟硼酸盐TSTU 301.10O-(N-琥珀酰亚胺基)-N NN'N'-四甲基四氟硼酸脲PyBrOP 466.20三吡咯烷基溴化鏻六氟磷酸盐TCFH 280.58N,N,N',N'-四甲基氯甲脒六氟磷酸盐DEPBT 299.233 -二乙氧基磷酰基-1,2,3-苯唑4(3H)-酮TOTU 328.1O-[(乙氧基羰基)氰基甲胺]-N,N,N',N'-四甲基硫尿四氟硼酸苯并三氮唑-1-基氧基三(二甲基氨基)磷鎓六氟磷酸盐BOP(卡特缩合剂)442.50N,N,N',N'-四甲基-O-(3,4-二氢-4-氧代-1,2,3-苯并三嗪-3-基)脲四氟硼酸盐TDBTU 349.09。

CD-ROM 8325 - 1Revision 0December 1996METHOD 8325SOLVENT EXTRACTABLE NONVOLATILE COMPOUNDS BY HIGH PERFORMANCE LIQUID CHROMATOGRAPHY/PARTICLE BEAM/MASS SPECTROMETRY (HPLC/PB/MS)1.0SCOPE AND APPLICATION1.1This method describes the use of high performance liquid chromatography (HPLC),coupled with particle beam (PB) mass spectrometry (MS), for the determination of benzidines and nitrogen-containing pesticides in water and wastewater. The following compounds can be determined by this method:Compound CAS No.aBenzidine 92-87-5Benzoylprop ethyl 33878-50-1Carbaryl 63-25-2o-Chlorophenyl thiourea 5344-82-13,3'-Dichlorobenzidine 91-94-13,3'-Dimethoxybenzidine 119-90-43,3'-Dimethylbenzidine 612-82-8Diuron 330-54-1Linuron (Lorox)330-55-2Monuron 150-68-5Rotenone 83-79-4Siduron1982-49-6Chemical Abstract Service Registry Numbera 1.2The method also may be appropriate for the analysis of benzidines and nitrogen-containing pesticides in non-aqueous matrices. The method may be applicable to other compounds that can be extracted from a sample with methylene chloride and are amenable to separation on a reverse phase liquid chromatography column and transferable to the mass spectrometer with a particle beam interface.1.3Preliminary investigation indicates that the following compounds also may be amenable to this method: Aldicarb sulfone, Carbofuran, Methiocarb, Methomyl (Lannate), Mexacarbate (Zectran), and N-(1-Naphthyl)thiourea. Ethylene thiourea and o-Chlorophenyl thiourea have been successfully analyzed by HPLC/PB/MS, but have not been successfully extracted from a water matrix.1.4Tables 4 - 6 present method detection limits (MDLs) for the target compounds, ranging from 2 to 25 µg/L. The MDLs are compound- and matrix-dependent.1.5This method is restricted to use by, or under the supervision of, analysts experienced in the use of HPLC and skilled in the interpretation of particle beam mass spectrometry. Each analyst must demonstrate the ability to generate acceptable results with this method.2.0SUMMARY OF METHOD2.1The target compounds for this method must be extracted from the sample matrix prior to analysis.2.1.1Benzidines and nitrogen-containing pesticides are extracted from aqueousmatrices at a neutral pH with methylene chloride, using a separatory funnel (Method 3510), a continuous liquid-liquid extractor (Method 3520), or other suitable technique.2.1.2Solid samples are extracted using Methods 3540 (Soxhlet), 3541 (AutomatedSoxhlet), 3550 (Ultrasonic extraction), or other suitable technique.2.2An aliquot of the sample extract is introduced into the HPLC instrument and a gradient elution program is used to chromatographically separate the target analytes, using reverse-phase liquid chromatography.2.3Once separated, the analytes are transferred to the mass spectrometer via a particle beam HPLC/MS interface. Quantitation is performed using an external standard approach.2.4An optional internal standard quantitation procedure is included for samples which contain coeluting compounds or where matrix interferences preclude the use of the external standard procedure.2.5The use of ultraviolet/visible (UV/VIS) detection is an appropriate option for the analysis of routine samples, whose general composition has been previously determined.3.0INTERFERENCES3.1Refer to Methods 3500 and 8000 for general discussions of interferences with the sample extraction and chromatographic separation procedures.3.2Although this method relies on mass spectrometric detection, which can distinguish between chromatographically co-eluting compounds on the basis of their masses, co-elution of two or more compounds will adversely affect method performance. When two compounds coelute, the transport efficiency of both compounds through the particle beam interface generally improves, and the ion abundances observed in the mass spectrometer increase. The degree of signal enhancement by coelution is compound-dependent.3.2.1This coelution effect invalidates the calibration curve and, if not recognized, willresult in incorrect quantitative measurements. Procedures are given in this method to check for co-eluting compounds, and must be followed to preclude inaccurate measurements.3.2.2An optional internal standard calibration procedure has been included for use ininstances of severe co-elution or matrix interferences.3.3 A major source of potential contamination is HPLC columns which may contain silicon compounds and other contaminants that could prevent the determination of method analytes. Generally, contaminants will be leached from the columns into mobile phase and produce a variable background. Figure 1 shows unacceptable background contamination from a column with stationary phase bleed.CD-ROM8325 - 2Revision 0December 1996CD-ROM 8325 - 3Revision 0December 19963.4Contamination may occur when a sample containing low analyte concentrations is analyzed immediately after a sample containing relatively high analyte concentrations. After analysis of a sample containing high analyte concentrations, one or more method blanks should be analyzed.Normally, with HPLC, this is not a problem unless the sample concentrations are at the percent level.4.0APPARATUS AND MATERIALS4.1High performance liquid chromatograph (HPLC) - An analytical system with programmable solvent delivery system and all necessary accessories including 5 µL injection loop,analytical columns, purging gases, etc. The solvent delivery system must be capable, at a minimum,of handling a binary solvent system, and must be able to accurately deliver flow rates between 0.20- 0.40 mL/min. Pulse dampening is recommended, but not required. The chromatographic system must be able to be interfaced with a mass spectrometer (MS). An autoinjector is recommended and should be capable of accurately delivering 1 - 10 µL injections without affecting the chromatography.4.1.1HPLC Columns - An analytical column is needed, and a guard column is highlyrecommended.4.1.1.1Analytical Column - Reverse phase column, C chemically bonded to184-10 µm silica particles, 150 - 200 mm x 2 mm, (Waters C-18 Novapak or equivalent).Residual acidic sites should be blocked (endcapped) with methyl or other non-polargroups and the stationary phase must be bonded to the solid support to minimize columnbleed. Select a column that exhibits minimal bleeding. New columns must beconditioned overnight before use by pumping a 75 - 100% v/v acetonitrile:water solutionthrough the column at a rate of about 0.05 mL/min. Other packings and column sizesmay be used if appropriate performance can be achieved.4.1.1.2Guard Column - Packing similar to that used in analytical column.4.1.2HPLC/MS interface - The particle beam HPLC/MS interface must reduce the ionsource pressure to a level compatible with the generation of classical electron ionization (EI)mass spectra, i.e., about 1 x 10 - 1 x 10Torr, while delivering sufficient quantities of analytes -4 -6to the conventional EI source to meet sensitivity, accuracy, and precision requirements. The concentrations of background components with masses greater than 62 Daltons should be reduced to levels that do not produce ions greater than a relative abundance of 10% in the mass spectra of the analytes.4.2Mass spectrometer system - The mass spectrometer must be capable of electron ionization at a nominal electron energy of 70 eV. The spectrometer should be capable of scanning from 45 to 500 amu in 1.5 seconds or less (including scan overhead). The spectrometer should produce a mass spectrum that meets the criteria in Table 1 when 500 ng or less of DFTPPO are introduced into the HPLC.4.3Data system - A computer system must be interfaced to the mass spectrometer, and must be capable of the continuous acquisition and storage on machine-readable media of all mass spectra obtained throughout the duration of the chromatographic program. The computer software must be capable of searching any HPLC/MS data file for ions of a specified mass and plotting such abundance data versus time or scan number.4.4Volumetric flasks - Class A, in various sizes, for preparation of standards.CD-ROM 8325 - 4Revision 0December 19964.5Vials - 10-mL amber glass vials with polytetrafluororethylene (PTFE)-lined screw caps or crimp tops.4.6 Analytical balance - capable of weighing 0.0001 g.4.7Extract filtration apparatus4.7.1Syringe - 10-mL, with Luer-Lok fitting.4.7.2Syringe filter assembly, disposable - 0.45 µm pore size PTFE filter in filterassembly with Luer-Lok fitting (Gelman Acrodisc, or equivalent).5.0REAGENTS5.1Reagent grade chemicals shall be used in all tests. Unless otherwise indicated, it is intended that all reagents shall conform to the specifications of the Committee on Analytical Reagents of the American Chemical Society, where such specifications are available. Other grades may be used, provided it is first ascertained that the reagent is of sufficiently high purity to permit its use without lessening the accuracy of the determination.5.2Organic-free reagent water - All references to water in this method refer to organic-free reagent water, as defined in Chapter One.5.3Solvents - All solvents must be HPLC-grade or equivalent.5.3.1Acetonitrile, CH CN 35.3.2Methanol, CH OH 35.3.3Ammonium acetate, NH OOCCH , (0.01M in water).435.4Mobile phase - Two mobile phase solutions are needed, and are designated Solvent A and Solvent B. Degas both solvents in an ultrasonic bath under reduced pressure and maintain by purging with a low flow of helium.5.4.1Solvent A is a water:acetonitrile solution (75/25, v/v) containing ammoniumacetate at a concentration of 0.01M.5.4.2Solvent B is 100 % acetonitrile.5.5Stock standard solutions - Stock solutions may be prepared from pure standard materials or purchased as certified solutions. Commercially-prepared stock standards may be used at any concentration if they are certified by the manufacturer.5.5.1Prepare stock standard solutions by accurately weighing 0.0100 g of pure materialin a volumetric flask. Dilute to known volume in a volumetric flask. If compound purity is certified at 96% or greater, the weight may be used without correction to calculate the concentration of the stock standard. Commercially-prepared stock standards may be used at any concentration if they are certified by the manufacturer or by an independent source.CD-ROM 8325 - 5Revision 0December 19965.5.1.1Dissolve benzidines and nitrogen-containing pesticides in methanol,acetonitrile, or organic-free reagent water.5.5.1.2Certain analytes, such as 3,3'-dimethoxybenzidine, may require dilutionin 50% (v/v) acetonitrile:water or methanol:water solution.5.5.1.3Benzidines may be used for calibration purposes in the free base or acidchlorides forms. However, the concentration of the standard should be calculated as thefree base.5.5.2Transfer the stock standard solutions into amber bottles with PTFE-linedscrew-caps or crimp tops. Store at -10E C or less and protect from light. Stock standard solutions should be checked frequently for signs of degradation or evaporation, especially just prior to preparing calibration standards from them.5.6Surrogate spiking solution - The recommended surrogates are benzidine-D ,8caffeine-N , 3,3'-dichlorobenzidine-D , and bis(perfluorophenyl)-phenylphosphine oxide. Prepare 152 6a solution of the surrogates in methanol or acetonitrile at a concentration of 5 mg/mL of each. Other surrogates may be included in this solution as needed. (A 10-µL aliquot of this solution added to 1L of water gives a concentration of 50 µg/L of each surrogate). Store the surrogate spiking solution in an amber vial in a freezer at -10E C or less.5.7MS performance check solution - Prepare a 100 ng/µL solution of DFTPPO in acetonitrile.Store this solution in an amber vial in a freezer at -10E C or less.5.8Calibration solutionsThis method describes two types of calibration procedures that may be applied to the target compounds: external standard calibration, and internal standard calibration. Each procedure requires separate calibration standards. In addition, the performance characteristics of the HPLC/PB/MS system indicate that it may be necessary to employ a second order regression for calibration purposes, unless a very narrow calibration range is chosen. See Method 8000 for additional information on non-linear calibration techniques.5.8.1For external standard calibration, prepare calibration standards for all targetcompounds and surrogates in acetonitrile. DFTPPO may be added to one or more calibration solutions to verify MS tune (see Sec. 7.3). Store these solutions in amber vials at -10E C or less. Check these solutions at least quarterly for signs of deterioration.5.8.2Internal standard calibration requires the use of suitable internal standards (seeMethod 8000). Ideally, stable, isotopically-labeled, analogs of the target compounds should be used. These labeled compounds are included in the calibration standards and must also be added to each sample extract immediately prior to analysis. Prepare the calibration standards in a fashion similar to that for external standard calibration, but include each internal standard in each of the calibration standards.The concentration of the internal standards should be 50 - 100 times the lowestconcentration of the unlabeled target compounds. In addition, the concentration of the internal standards does not vary with the concentrations of the target compounds, but is held constant.Store these solutions in amber vials at -10E C or less. Check these solutions at least quarterly for signs of deterioration.5.9Internal standard spiking solution - This solution is required when internal standard quantitation is used. Prepare a solution containing each of the internal standards that will be used for quantitation of target compounds (see Sec. 5.8.2) in methanol. The concentration of this solution must be such that a 1-µL volume of the spiking solution added to a 1-mL final extract will result in a concentration of each internal standard that is equal to the concentration of the internal standard in the calibration standards in Sec. 5.8.2. Store this solution in an amber vial at -10E C or less. Check this solution at least quarterly for signs of deterioration. This solution is not necessary if only external standard calibration will be used.5.10Sodium chloride, NaCl - granular, used during sample extraction.6.0SAMPLE COLLECTION, PRESERVATION, AND HANDLING6.1See the introductory material to this chapter, Organic Analytes, Sec. 4.1.6.2Samples should be extracted within 7 days and analyzed within 30 days of extraction. Extracts should be stored in amber vials at -10E C or less.7.0PROCEDURE7.1Samples may be extracted by Method 3510 (separatory funnel), Method 3520 (continuous extractor), Method 3535 (solid-phrase extraction), or other appropriate technique. Prior to extraction, add a 10-µL aliquot of the surrogate spiking solution and 100 g of sodium chloride to the sample, and adjust the pH of the sample to 7.0. Samples of other matrices should be extracted by an appropriate sample preparation technique. The concentration of surrogates in the sample should be 20-50 times the method detection limit. Concentrate the extract to 1 mL, and exchange the solvent to methanol, following the procedures in the extraction method.7.2Establish chromatographic, particle beam interface, and mass spectrometer conditions, using the following conditions as guidance.Mobile phase purge:Helium at 30 mL/min, continuousMobile phase flow rate:0.25 - 0.3 mL/min through the columnGradient elution:Hold for 1 min at 25% acetonitrile (Solvent A), thenprogram linearly to about 70% acetonitrile (60%Solvent B) in 29 min. Start data acquisitionimmediately.Desolvation chamber temperature:45 - 80E CIon source temperature:250 - 290E CElectron energy:70 eVScan range:62 to 465 amu, at #1.5 sec/scan NOTE:Post-column addition is an option that improves system precision and, thereby, may improve sensitivity. Post-column flow rates depend on the requirements ofthe interface and may range from 0.1 to 0.7 mL/min of acetonitrile. Maintain aminimum of 30% acetonitrile in the interface.Analyte-specific chromatographic conditions are also shown in Table 2. (The particle beam interface conditions will depend on the type of nebulizer).CD-ROM8325 - 6Revision 0December 19967.2.1The analyst should follow the manufacturer's recommended conditions for theirinterface's optimum performance. The interface is usually optimized during initial installation by flow injection with caffeine or benzidine, and should utilize a mobile phase of acetonitrile/water (50/50, v/v). Major maintenance may require re-optimization.7.2.2Fine tune the interface by making a series of injections into the HPLC column ofa medium concentration calibration standard and adjusting the operating conditions (Sec. 7.2)until optimum sensitivity and precision are obtained for the maximum number of target compounds.7.3Initial calibration7.3.1Once the operating conditions have been established, calibrate the MS mass andabundance scales using DFTPPO to meet the recommended criteria in Table 1.7.3.2Inject a medium concentration standard containing DFTPPO, or separately injectinto the HPLC a 5-µL aliquot of the 100 ng/µL DFTPPO solution and acquire a mass spectrum.Use HPLC conditions that produce a narrow (at least ten scans per peak) symmetrical peak.If the spectrum does not meet the criteria (Table 1), the MS ion source must be retuned and adjusted to meet all criteria before proceeding with calibration. An average spectrum across the HPLC peak may be used to evaluate the performance of the system.Manual (not automated) ion source tuning procedures specified by the manufacturer should be employed during tuning. Mass calibration should be accomplished while an acetonitrile/water (50/50, v/v) mixture is pumped through the HPLC column and the optimized particle beam interface. For optimum long-term stability and precision, interface and ion source parameters should be set near the center of a broad signal plateau rather than at the peak of a sharp maximum (sharp maxima exhibit short-term variations with particle beam interfaces and gradient elution conditions).7.3.3System performance criteria for the medium concentration standard - Evaluatethe stored HPLC/MS data with the data system software and verify that the HPLC/PB/MS system meets the following performance criteria.7.3.3.1HPLC performance - 3,3'-dimethylbenzidine and3,3'-dimethoxybenzidine should be separated by a valley whose height is less than 25%of the average peak height of these two compounds. If the valley between them exceeds25%, modify the gradient. If this fails, the HPLC column requires maintenance. SeeSec. 7.4.6.7.3.3.2Peak tailing - Examine a total ion chromatogram and examine thedegree of peak tailing. Severe tailing indicates a major problem and systemmaintenance is required to correct the problem. See Sec. 7.4.67.3.3.3MS sensitivity - The signal-to-noise ratio for any compound's spectrumshould be at least 3:1.7.3.3.4Column bleed - Figure 1 shows an unacceptable chromatogram withcolumn bleed. Figure 2 shows an acceptable ion chromatogram. Figure 3 is the massspectrum of dimethyloctadecyl-silanol, a common stationary phase bleed product. Ifunacceptable column bleed is present, the column must be changed or conditioned toproduce an acceptable background.CD-ROM8325 - 7Revision 0December 19967.3.3.5Coeluting compounds - Compounds which coelute cannot be measuredaccurately because of carrier effects in the particle beam interface. Peaks must beexamined carefully for coeluting substances and if coeluting compounds are present atgreater than 10% of the concentration of the target compound, either conditions must beadjusted to resolve the components, or internal standard calibration must be used.7.3.4Once optimized, the same instrument operating conditions must be used for theanalysis of all calibration standards, samples, blanks, etc.7.3.5Once all the performance criteria are met, inject a 5-µL aliquot of each of theother calibration standards using the same HPLC/MS conditions.7.3.5.1The general method of calibration is a second order regression ofintegrated ion abundances of the quantitation ions (Table 3) as a function of amountinjected. For second order regression, a sufficient number of calibration points must beobtained to accurately determine the equation of the curve. (See Method 8000 for theappropriate number of standards to be employed for a non-linear calibration). Non-linearcalibration models can be applied to either the external standard or the internal standardcalibration approaches described here.7.3.5.2For some analytes the instrument response may be linear over a narrowconcentration range. In these instances, an average calibration factor (externalstandard) or average response factor (internal standard) may be employed for samplequantitation (see Method 8000).7.3.6If a linear calibration model is used, calculate the mean calibration factor orresponse factor for each analyte, including the surrogates, as described in Method 8000.Calculate the standard deviation (SD) and the relative standard deviation (RSD) as well. The RSD of an analyte or surrogate must be less than or equal to 20%, if the linear model is to be applied. Otherwise, proceed as described in Method 8000.7.4Calibration verificationPrior to sample analysis, verify the MS tune and initial calibration at the beginning of each 8-hour analysis shift using the following procedure:7.4.1Inject a 5-µL aliquot of the DFTPPO solution or a mid-level calibration standardcontaining 500 ng of DFTPPO, and acquire a mass spectrum that includes data for m/z 62-465. If the spectrum does not meet the criteria in Table 1, the MS must be retuned to meet the criteria before proceeding with the continuing calibration check.7.4.2Inject a 5-µL aliquot of a medium concentration calibration solution and analyzewith the same conditions used during the initial calibration.7.4.3Demonstrate acceptable performance for the criteria shown in Sec. 7.3.3.7.4.4Using the initial calibration (either linear or non-linear, external standard or internalstandard), calculate the concentrations in the medium concentration calibration solution and compare the results to the known values in the calibration solution. If calculated concentrations deviate by more than 20% from known values, adjust the instrument and inject the standard again. If the calibration cannot be verified with the second injection, then a new CD-ROM8325 - 8Revision 0December 1996initial calibration must be performed after taking corrective actions such as those described in Sec. 7.9.7.5Sample Analysis7.5.1The column should be conditioned overnight before each use by pumping aacetonitrile:water (70% v/v) solution through it at a rate of about 0.05 mL/min.7.5.2Filter the extract through a 0.45 µm filter. If internal standard calibration isemployed, add 10 µL of the internal standard spiking solution to the 1-mL final extract immediately before injection.7.5.3Analyze a 5-µL aliquot of the extract, using the operating conditions establishedin Secs. 7.2 and 7.3.7.6Qualitative identificationThe qualitative identification of compounds determined by this method is based on retention time and on comparison of the sample mass spectrum, after background correction, with characteristic ions in a reference mass spectrum. The reference mass spectrum must be generated by the laboratory using the conditions of this method. The characteristic ions from the reference mass spectrum are defined as the three ions of greatest relative intensity, or any ions over 30% relative intensity, if less than three such ions occur in the reference spectrum. Compounds are identified when the following criteria are met.7.6.1The intensities of the characteristic ions of a compound must maximize in thesame scan or within one scan of each other. Selection of a peak by a data system target compound search routine where the search is based on the presence of a target chromatographic peak containing ions specific for the target compound at a compound-specific retention time will be accepted as meeting this criterion.7.6.2The retention time of the sample component is within ± 10% of the retention timeof the standard.7.6.3The relative intensities of the characteristic ions agree within 20% of the relativeintensities of these ions in the reference spectrum. (Example: For an ion with an abundance of 50% in the reference spectrum, the corresponding abundance in a sample spectrum can range between 30% and 70%.)7.6.4Structural isomers that produce very similar mass spectra should be identified asindividual isomers if they have sufficiently different HPLC retention times. Sufficient GC resolution is achieved if the height of the valley between two isomer peaks is less than 25% of the sum of the two peak heights. Otherwise, structural isomers are identified as isomeric pairs.7.6.5Identification is hampered when sample components are not resolvedchromatographically and produce mass spectra containing ions contributed by more than one analyte. When HPLC peaks obviously represent more than one sample component (i.e., a broadened peak with shoulder(s) or a valley between two or more maxima), appropriate selection of analyte spectra and background spectra is important.CD-ROM8325 - 9Revision 0December 19967.6.6Examination of extracted ion current profiles of appropriate ions can aid in theselection of spectra, and in qualitative identification of compounds. When analytes coelute(i.e., only one chromatographic peak is apparent), the identification criteria may be met, buteach analyte spectrum will contain extraneous ions contributed by the coeluting compound.7.7Quantitative Analysis7.7.1Complete chromatographic resolution is necessary for accurate and precisemeasurements of analyte concentrations. Compounds which coelute cannot be measured accurately because of carrier effects in the particle beam interface. Peaks must be examined carefully for coeluting substances and if coeluting compounds are present at greater than 10% of the concentration of the target compound, either conditions must be adjusted to resolve the components, or the results for the target compound must be flagged as potentially positively biased.7.7.2Calculate the concentration of each analyte, using either the external standardor internal standard calibration. See Method 8000 for the specific equations to be employed for either the non-linear or linear calibration models.7.7.3If the response for any quantitation ion exceeds the initial calibration range of theHPLC/PB/MS system, the sample extract must be diluted and reanalyzed. When internal standard calibration is employed, additional internal standard must be added to the diluted extract to maintain the same concentration as in the calibration standards.7.8HPLC-UV/VIS Detection (optional)7.8.1Prepare calibration solutions as outlined in Sec. 5.8.7.8.2Inject 5 µL of each calibration solution onto the HPLC, using the chromatographicconditions outlined in Secs. 7.2.1 and 7.2.2. Integrate the area under the full chromatographic peak at the optimum wavelength (or at 230 nm if that option is not available) for each target compound at each concentration.7.8.3The retention time of the chromatographic peak is an important criterion foranalyte identification. Therefore, the ratio of the retention time of the sample analyte to the standard analyte should be 1.0 ± 0.1.7.8.4Calculate calibration factors or response factors as described in Method 8000,for either external standard or internal standard calibration, and evaluate the calibration linearity as described in Method 8000.7.8.5Verify the calibration at the beginning of each 8-hour analytical shift, as describedabove.7.8.6Once the calibration has been verified, inject a 5-µL aliquot of the sample extract,start the HPLC gradient elution, load and inject the sample aliquot, and begin data acquisition.Refer to Method 8000 for guidance on calculation of concentration.7.9Corrective ActionsWhen the initial calibration cannot be verified, one or more of the following corrective actions may be necessary.CD-ROM8325 - 10Revision 0December 1996。

01%甲胺基阿维菌素苯甲酸盐微乳剂0001 范围00本标准规定了1%甲胺基阿维菌素苯甲酸盐微乳剂的要求、试验方法、检验规则以及标志、标签、包装、贮运。
0000本标准适用于由符合标准的甲胺基阿维菌素苯甲酸盐原药、助溶剂、表面活性剂和水配制成的1%甲胺基阿维菌素苯甲酸盐微乳剂。
0 0该产品有效成份的其他名称、结构式和基本物化参数如下: 0 00ISO 通用名称:Emamectin Benzoate00化学名称:4’’-表-甲胺基-4”-脱氧阿维菌素苯甲酸盐000结构式:0000OOCH 3H 3CHO OH 3COCH 3HO HH H 3COO OCH 3HOHHOCH 3CH 3RO HCH 3H HOHH 3CH 2NB 1a: R =C 2H 5B 1b: R = CH 3COO - 实验式及相对分子质量(按1997国际相对原子质量计): 00Emamectin Benzoate B 1a :C 49H 75NO 13.C 7H 6O 2:1008.2600Emamectin Benzoate B 1b :C 48H 73NO 13.C 7H 6O 2:994.23 00生物活性:杀虫杀螨;0000熔点:141℃-146℃;00溶解度:溶于丙酮、甲醇、微溶于水,不溶于已烷;000稳定性:在通常贮存条件下本品稳定,对紫外光不稳定。
0 002规范性引用文件00下列文件中的条款通过本标准的引用而成为本标准的条款。
凡是注日期的引用文件,其随后所有的修改单(不包括勘误的内容)或修订版均不适用于本标准,然而,鼓励根据本标准达成协议的各方研究是否可使用这些文件的最新版本。
凡是不注日期的引用文件,其最新版本适用于本标准。
000GB/T 1250 极限数值的表示方法和判定方法000GB/T 1601 农药PH值的测定方法00GB/T 1603 农药乳液稳定性测定方法00GB/T 1604 商品农药验收规则00GB/T 1605 商品农药采样方法00GB 3796 农药包装通则00GB 4838 农药乳油包装00GB/T 19136-2003 农药热贮稳定性测定方法000GB/T 19137-2003 农药低温稳定性测定方法000HG/T 2467.5-2003 农药悬浮剂产品标准编写规范000HG/T 2467.10-2003 农药微乳剂产品标准编写规范000GB/T 6682 分析实验室用水规格和试验方法000JJF 1070 定量包装商品净含量计量检验规则000国家质量监督检验检疫总局令第75号定量包装商品计量监督管理办法0003要求003.1 外观:浅黄色均相液体,无可见的悬浮物和沉淀。
·药物研发·高效液相色谱-串联质谱法检测泮托拉唑钠原料药中的水合肼赵会明 张振洋 樊华军[英格尔检测技术服务(上海)有限公司 上海 201100]摘要建立了泮托拉唑钠原料药中的基因毒性杂质水合肼的高效液相色谱-串联质谱(LC-MSMS)检测方法。
采用反相色谱,以水-乙腈(含0.1%甲酸)为流动相,梯度洗脱,流速0.5 mL/min,以ESI正离子多反应监测(MRM)模式进行质谱检测。
结果显示,水合肼的检测限和定量限可达到0.23、0.47 ng/mL,其在0.47~9.37 ng/mL浓度范围内线性关系良好(r=0.999 9),准确度试验中低、中、高浓度回收率均在81.6%~90.9%之间。
在3批次泮托拉唑钠原料药中均未检出水合肼。
关键词高效液相色谱-串联质谱法基因毒性杂质泮托拉唑钠水合肼痕量检测中图分类号:R917; O657 文献标志码:A 文章编号:1006-1533(2022)11-0072-04引用本文 赵会明, 张振洋, 樊华军. 高效液相色谱-串联质谱法检测泮托拉唑钠原料药中的水合肼[J]. 上海医药, 2022, 43(11): 72-75.Determination of hydrazine hydrate in pantoprazole sodium by high performance liquid chromatography-tandem mass spectrometryZHAO Huiming, ZHANG Zhenyang, FAN Huajun[ICAS Testing Technology Service (Shanghai) CO., LTD., Shanghai 201100, China]ABSTRACT To establish a high-performance liquid chromatography-tandem mass spectrometry (LC-MSMS) method for the determination of hydrazine hydrate in active pharmaceutical ingredient (API) pantoprazole sodium. HPLC was carried out by reverse chromatography using water-acetonitrile containing 0.1% formic acid as flow phase and gradient elution at a flow rate of 0.5 mL/min. Mass spectrometry was performed with multi-reaction monitoring (MRM) in positive ESI mode. The detection and quantitative limits of hydrazine hydrate reached 0.23, 0.47 ng/mL and hydrazine hydrate showed good linear relationship in the range of 0.47-9.37 ng/mL (r=0.999 9). The recoveries of samples at low, medium and high-level concentrations reached81.6% to 90.9% in the accuracy experiment. No hydrazine hydrate was detected in 3 batches of pantoprazole sodium.KEY WORDS HPLC-tandem mass spectrometry; genotoxic impurities; pantoprazole sodium; hydrazine hydrate; trace determination上消化道出血是近年的临床疾病中常见且多发的一种疾病,其临床表现为呕血、黑便等,如得不到及时有效治疗,可能引发失血性休克。