MG subgroup classification and therapeutic strategies
- 格式:pdf
- 大小:1.82 MB
- 文档页数:14

MGRS化疗方案 化疗方案是针对恶性肿瘤患者的一种常见治疗方式。MGRS化疗方案是一种针对某些特定类型的白血病的治疗方案。本文将详细介绍MGRS化疗方案的原理、疗效以及应用范围。
一、MGRS化疗方案的原理 MGRS(Multidrug Resistance Gene)化疗方案是基于对白血病细胞的多药耐药机制进行干预的治疗方案。该方案主要通过抑制细胞内的多药耐药基因的表达,增加化疗药物对白血病细胞的杀伤效应。
二、MGRS化疗方案的疗效 MGRS化疗方案在一定程度上能够提高白血病患者的治疗效果。通过抑制多药耐药基因的表达,降低白血病细胞对化疗药物的耐药性,使治疗更为有效。
三、MGRS化疗方案的应用范围 MGRS化疗方案主要适用于那些被诊断为具有多药耐药特点的白血病患者。这些患者往往对传统的化疗方案疗效不佳,容易出现复发。采用MGRS化疗方案可以提高治疗效果,降低复发率。
四、MGRS化疗方案的具体操作步骤 1. 评估患者的药物耐药情况。通过检测多药耐药基因的表达水平,确定患者是否适合接受MGRS化疗方案。 2. 设计个体化的治疗方案。根据患者的具体情况,结合MGRS化疗方案的原理,制定针对性的治疗方案。
3. 实施化疗治疗。按照制定的方案进行化疗治疗,包括用药剂量和治疗周期等方面的具体安排。
4. 监测治疗效果。针对化疗治疗过程中的不适症状,随时进行监测和调整治疗方案。
五、MGRS化疗方案的优势和注意事项 1. 优势: a. 提高治疗效果。MGRS化疗方案可以增加化疗药物对白血病细胞的杀伤效应,提高治疗效果。
b. 个体化治疗。MGRS化疗方案根据患者的个体情况进行调整,提高治疗效果。
2. 注意事项: a. 治疗过程中需密切监测患者的病情和治疗反应,及时调整方案。 b. 注意化疗带来的不良反应,采取相应的支持治疗措施。 六、MGRS化疗方案的临床应用案例 以某患者的治疗为例,患者被诊断为特定类型的白血病,且表达多药耐药基因。经过连续3个疗程的MGRS化疗方案治疗后,患者的白血病细胞数量明显减少,病情得到有效控制,且无明显不良反应。 结语: MGRS化疗方案是一种针对特定类型白血病的治疗方案,通过抑制多药耐药基因的表达,提高化疗效果,减少复发率。然而,每个患者的情况各不相同,治疗方案需个体化调整,并需密切监测治疗效果和不良反应。MGRS化疗方案在未来可能得到更广泛的应用。
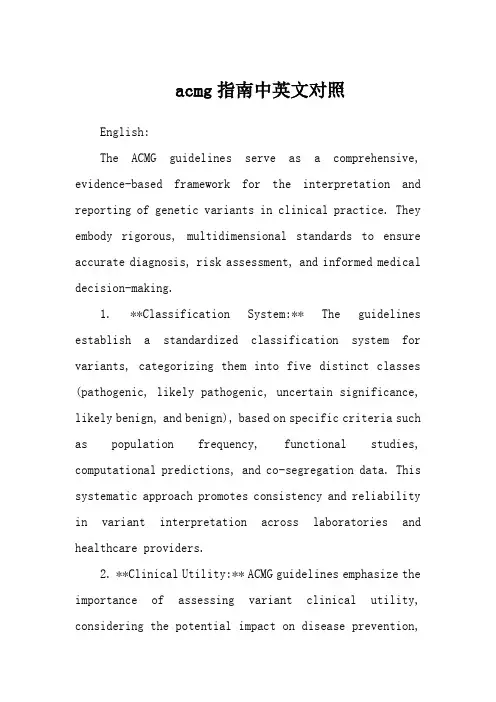
acmg指南中英文对照English:The ACMG guidelines serve as a comprehensive, evidence-based framework for the interpretation and reporting of genetic variants in clinical practice. They embody rigorous, multidimensional standards to ensure accurate diagnosis, risk assessment, and informed medical decision-making.1. **Classification System:** The guidelines establish a standardized classification system for variants, categorizing them into five distinct classes (pathogenic, likely pathogenic, uncertain significance, likely benign, and benign), based on specific criteria such as population frequency, functional studies, computational predictions, and co-segregation data. This systematic approach promotes consistency and reliability in variant interpretation across laboratories and healthcare providers.2. **Clinical Utility:** ACMG guidelines emphasize the importance of assessing variant clinical utility, considering the potential impact on disease prevention,diagnosis, treatment, or prognosis. This focus ensures that reported findings have clear relevance to patient care, guiding appropriate interventions and management strategies.3. **Ethical Considerations:** Recognizing the ethical complexities surrounding genetic testing, the guidelines address issues such as informed consent, privacy, and return of results. They advocate for transparent communication with patients regarding the implications, limitations, and potential emotional consequences of genetic information, fostering autonomy and trust in the healthcare relationship.4. **Interdisciplinary Collaboration:** The guidelines stress the necessity of interdisciplinary collaboration involving clinicians, genetic counselors, laboratory scientists, and bioinformaticians. Such teamwork ensures comprehensive evaluation of genetic data, effective translation of complex findings into clinically meaningful information, and optimal patient care.5. **Continuous Updating:** Reflecting the rapidly evolving field of genetics, the ACMG guidelines undergo regular review and updating to incorporate new scientificdiscoveries, technological advancements, and emerging best practices. This commitment to staying current ensures that the guidelines remain at the forefront of precision medicine, providing clinicians with the most up-to-date guidance for genetic testing and interpretation.In summary, the ACMG guidelines represent a multifaceted, high-quality standard in genetic testing and interpretation. They encompass a rigorous classification system, prioritize clinical utility, address ethical considerations, promote interdisciplinary collaboration, and embrace continuous updating – all crucial elements that collectively contribute to accurate diagnoses, informed decision-making, and improved patient outcomes in the realm of medical genetics.Chinese:ACMG 指南作为临床实践中遗传变异解读和报告的综合性、循证框架,体现了确保精准诊断、风险评估及明智医疗决策的严格、多维度标准。

MGMT启动子甲基化在预测联合治疗后高风险低级别胶质瘤患者预后的重要性根据一项新的研究,MGMT启动子甲基化可作为高风险,低级别胶质瘤患者接受联合治疗的生存预后生物标志物。
之前来自 NRG Oncology/RTOG 0424 的一个研究小组发现,大多数接受替莫唑胺联合放射治疗的高风险,低级别胶质瘤患者与标准治疗相比总生存期增加了3年。
低级别胶质瘤具有较高的肿瘤异质性,中位生存期可能从几个月到十多年不等。
该研究中的高危患者有多种复发危险因素。
但该研究小组没有检测患者的MGMT启动子甲基化状态。
该基因位点的甲基化使MGMT蛋白表达沉默,并增加替莫唑胺的敏感性。
6月28号发表在“JAMA Oncology”上的另一项由俄亥俄州立大学Arnab Chakravarti 领导的研究,再次对临床试验所收集的样本MGMT启动子甲基化状态和IDH1/2突变状态进行检测。
结果表明MGMT启动子甲基化患者在联合治疗后生存率是MGMT启动子-未甲基化患者的2倍。
最初的研究共包括129名患者,其中75名患者的组织样本可以使用Illumina HumanMethylation 450 甲基化分析平台确定MGMT启动子甲基化状态。
而那些未能确定的患者也表现出了与确定者类似的治疗前特征。
在这75名患者中,有57名患者有MGMT启动子甲基化;其中47名有IDH1/2突变;7名无突变。
18名为未甲基化;其中3名有IDH1/2突变;10名无突变。
大多数MGMT启动子-未甲基化为星形细胞瘤。
MGMT启动子-未甲基化与联合治疗后较差的总体生存率和无进展生存期显著相关。
风险值分为3.52和3.06。
此外,考虑到IDH1/2突变状态与胶质瘤中的超突变表型和MGMT启动子甲基化相关。
然而,即使在多变量分析中排除IDH1/2的突变状态,MGMT启动子状态仍然与总体生存率和无进展生存期显著相关。
但作者也指出由于样本量小以及缺乏历史对照的分子数据,研究存在一定局限性。


单克隆球蛋⽩病的诊断及治疗MGRS肾脏意义的单克隆球蛋⽩病(MGRS)是由国际肾脏病和单克隆球蛋⽩病研究组提出,以区别良恶性⾎液疾病导致的单克隆球蛋⽩病。
MGRS 是指 B 淋巴细胞和浆细胞增殖性疾病导致副蛋⽩⾎症肾损害,但其不能达到多发性⾻髓瘤(MM)、华⽒巨球蛋⽩⾎症(WM)、慢性淋巴细胞⽩⾎病(CLL)或者恶性淋巴瘤的诊断标准。
轻链管型肾病(LCCN)常由 MM 引起,其不属于 MGRS 导致的肾脏损害。
MGRS 相关肾损害单克隆免疫球蛋⽩(Ig)沉积可以发⽣在肾⼩球、肾⼩管间质和肾⾎管。
肾⼩球⽑细⾎管和系膜区是单克隆 Ig 最常见沉积部位。
MGRS 肾损害类型常根据 Ig 沉积部位区分。
MGRS 相关⼩肾⼩球球病变分为有序沉积和⽆序沉积。
有序沉积病变包括轻链淀粉样变肾病、1型和 2 型冷球蛋⽩⾎症肾病和免疫结晶样肾⼩球病。
⽆序沉积病变包括 MIDD、增⽣性肾⼩球病伴单克隆 Ig 沉积(PGNMID)和 C3 肾⼩球病伴单克隆免疫球蛋⽩病。
MGRS 相关肾⼩管间质病变包括轻链近端⼩管病伴或不伴 Fanconi 综合征。
MGRS 相关肾病的临床特点患者年龄为中⽼年,⼤部分患者年龄>50 岁。
MIDD 和淀粉样变多见于男性,PGNMID 多见于⼥性。
肾⼩球病的患者常表现为蛋⽩尿、肾功能损害、镜下⾎尿及⾼⾎压。
淀粉样变性肾损害的患者常⽆明显⾎尿及⾼⾎压,常出现肾病⽔平蛋⽩尿。
肾外表现常见于 AL 淀粉样变性、1 型冷球蛋⽩⾎症、MIDD。
其主要肾外受累器官为肝脏、肺、⽪肤、关节和周围神经。
肾⼩管疾病的主要表现为慢性肾功能异常、管性蛋⽩尿和近端⼩管功能异常(糖尿、1 型肾⼩管酸中毒及氨基酸尿等),⾻软化等。
肾外受累器官如肝脏、脾脏、淋巴结、肺脏及⾓膜等。
MGRS 相关肾病在移植肾有⾼复发倾向。
复发时间各种肾脏病变各异。
化疗或者⾃体⼲细胞移植(ASCT)可以明显预防或延缓复发。
MGRS 相关肾病的诊断由于其肾脏受累部位的多样性和临床表现的多样性,并且并⾮所有 MG 患者都可以诊断为MGRS 相关肾病,所以其诊断较难。

1250J.Med.Chem.2010,53,1250–1260DOI:10.1021/jm901530bSynthesis and Structure-Activity Relationships of Azamacrocyclic C-X-C Chemokine Receptor4 Antagonists:Analogues Containing a Single Azamacrocyclic Ring are Potent Inhibitors of T-Cell Tropic(X4)HIV-1ReplicationGary J.Bridger,*,†Renato T.Skerlj,†,)Pedro E.Hernandez-Abad,‡David E.Bogucki,†Zhongren Wang,†Yuanxi Zhou,†Susan Nan,†Eva M.Boehringer,†Trevor Wilson,†Jason Crawford,†Markus Metz,†,)Sigrid Hatse,§Katrien Princen,§Erik De Clercq,§and Dominique Schols§†AnorMED Inc.now Genzyme Corporation,500Kendall Street,Cambridge,Massachusetts02142,‡Johnson Matthey Pharmaceutical Research,1401King Road,West Chester,Pennsylvania19380,and§Rega Institute for Medical Research,Katholieke Universiteit Leuven, Minderbroedersstraat10,B-3000Leuven,Belgium.)Genzyme Corp.,153Second Avenue,Waltham,Massachusetts02451.Received October15,2009Bis-tetraazamacrocycles such as the bicyclam AMD3100(1)are a class of potent and selective anti-HIV-1agents that inhibit virus replication by binding to the chemokine receptor CXCR4,the coreceptor for entryof X4viruses.By sequential replacement and/or deletion of the amino groups within the azamacrocyclic ringsystems,we have determined the minimum structural features required for potent antiviral activity in thisclass of compounds.All eight amino groups are not required for activity,the critical amino groups on a perring basis are nonidentical,and the overall charge at physiological pH can be reduced without compromisingpotency.This approach led to the identification of several single ring azamacrocyclic analogues such asAMD3465(3d),36,and40,which exhibit EC50’s against the cytopathic effects of HIV-1of9.0,1.0,and4.0nM,respectively,antiviral potencies that are comparable to1(EC50against HIV-1of4.0nM).Moreimportantly,however,the key structural elements of1required for antiviral activity may facilitate the designof nonmacrocyclic CXCR4antagonists suitable for HIV treatment via oral administration.IntroductionThe development of antiviral agents that inhibit alternative targets in the HIV a-replicative cycle remains an important goal in order to alleviate the side effects of currently approved agents or to overcome the problem of drug resistance.In this regard,we have focused on the development of compounds that inhibit CXCR4,the coreceptor used by T-tropic(T-cell tropic)viruses for fusion and entry of HIV into target cells of the immune system.The corresponding chemokine receptor CCR5is used by M-tropic(macrophage tropic)viruses and has been associated with the early stages of infection and replication in HIV-positive patients.1,2The transition from M-tropic to T-tropic(or dual/mixed-tropic)virus during the course of HIV infection in approximately50%of patients is associated with a faster CD4þT-cell decline and a more rapid disease progression.3-5Recently,we reported the results of clinical trials with our prototype CXCR4antagonist AMD31006-8(1)and an orally bioavailable CXCR4antagonist,(S)-N0-(1H-benzimidazol-2-ylmethyl)-N0-(5,6,7,8-tetrahydroquinolin-8-yl)butane-1,4-dia-mine(AMD070).9-11When administered to HIV positive patients whose virus was confirmed to use CXCR4for viral entry,both agents were able to suppress the replication of CXCR4and dual-tropic strains of HIV.Similarly,the CCR5 antagonist Maraviroc suppresses replication of HIV-1that exclusively uses CCR5for entry12and was recently approved by the FDA for combined antiretroviral therapy in treatment-experienced patients.13A combination of CCR5and CXCR4 antagonists for treatment of dual/mixed-tropic HIV infection is therefore highly desirable.Beyond its use as a coreceptor for HIV,the CXCR4 chemokine receptor has a more fundamental role in the trafficking of white blood cells,which broadly express CXCR4.14,15A member of the superfamily of G-protein coupled receptors,the interaction of CXCR4and its ligand, stromal cell-derived factor-1(SDF-1),plays a central role in the homing and retention of cells within the bone marrow microenvironment.16Consistent with these observations,ad-ministration of1to healthy volunteers caused a dose-depen-dent leukocytosis6,7that in subsequent studies was shown to include the mobilization of CD34þstem and progenitor cells suitable for hematopoietic stem cell transplantation.17-20The ability of analogues of1to mobilize progenitors correlated with their in vitro capacity to inhibit SDF-1binding to CXCR4.21Because of the need for parenteral administration, 1was developed in combination with granulocyte colony-stimulating factor(G-CSF)to mobilize hematopoietic stem cells to the peripheral blood for collection and subsequent autologous transplantation in patients with non-Hodgkin’s lymphoma(NHL)and multiple myeloma(MM).22-25Plerix-afor(1)was approved by the FDA in December2008.We have previously reported the structure-activity rela-tionships of anti-HIV bis-azamacrocycles and their transition*To whom correspondence should be addressed.Phone:617-429-7994.Fax:617-768-9809.E-mail:gary.bridger@.Ad-dress:Gary J.Bridger,Genzyme Corporation,55Cambridge Parkway,Cambridge MA02142.a Abbreviations:HIV,Human Immunodeficiency Virus;CXCR4,C-X-C chemokine receptor4;CCR5,C-C-R chemokine receptor5./jmc Published on Web12/31/2009r2009American Chemical SocietyArticle Journal of Medicinal Chemistry,2010,Vol.53,No.31251 metal complexes in detail.26-28Because of the commonstructural features between a doubly protonated cyclam(1,4,8,11-tetraazacyclotetradecane)ring present in1(at phy-siological pH)and a kinetically labile transition metal com-plex of cyclam with an overall charge ofþ2,we proposed thatboth structural motifs may bind to the CXCR4receptorthrough interactions with amino acid residues containingcarboxylate groups.29We have subsequently shown via direc-ted mutagenesis of the aspartate and glutamic acid residues inCXCR4that binding of1and related analogues to the seventransmembrane,G-protein coupled receptor is highly depen-dent upon the amino acids Asp171and Asp262,located intransmembrane region(TM)-IV and TM-VI at each end ofthe main ligand binding crevice of the receptor.30-35Mutationof either aspartic acid to aspargine significantly reduced theability of1to inhibit binding of radiolabeled stromal cellderived factor-1R(125I-Met-SDF-1R).More importantly,however,U87cells stably transfected with CD4and themutant coreceptors CXCR4[D171N]and CXCR4[D262N]were less effective at supporting infection of the CXCR4-usingHIV-1strain NL4.3compared to the wild-type receptor andthe double mutant CXCR4[D171N,D262N]completely failedas a coreceptor for HIV infection.31Correspondingly,theability of1to inhibit HIV-1infection via CXCR4[D171N]andCXCR4[D262N]was also diminished,thereby confirmingthat1binds in a region of the receptor that is critical for X4HIV-1coreceptor function.We have also reported that binding of the bis-Zn,Ni,andCu complexes of1were also dependent upon D171and D262of the receptor.36In a similar manner to1,the transitionmetal complexes were found to be less effective inhibitors of125I-Met-SDF-1R binding to the mutant receptors CXCR4-[D171N]and CXCR4[D262N]compared to the wild-typereceptor.Incorporation of Zn,Ni,or Cu into the cyclam ringsof1increased the affinity to the wild-type CXCR4receptor,but the enhancement was selectively eliminated by substitu-tion of Asp262.Supporting physiochemical evidence for theinteraction of acetate(carboxylates)with metal complexes ofazamacrocycles,including1,has been recently reported.37,38In the current study,we determine the minimum struc-tural features of1required for potent antiviral activity, leading to the identification of the single azamacrocyclic ring analogue AMD346532,33,39,40(3d)and ultimately the design of nonmacrocyclic,orally biovailable CXCR4an-tagonists.11,41,42Given the growing body of evidence that the CXCR4/SDF-1interaction is involved in regulating several human malignancies,43-45CXCR4antagonists may have additional therapeutic applications in addition to HIV treatment.ChemistryAnalogues containing a single1,4,8,11-tetraazacyclotetra-decane(cyclam)ring were prepared by modifications to previously published routes26,29as shown in Scheme1.Reac-tion of the selectively protected tris-diethylphosphoramidate (Dep)cyclam ring(2a)with R,R-dibromo-p-xylene in aceto-nitrile containing potassium carbonate gave the desired bro-momethyl intermediate(2b).Reaction of the bromide with an excess of the requisite amine,followed by deprotection of the Dep-groups with a saturated solution of hydrogen bromide in acetic acid at room temperature.gave analogues3a-i as the corresponding hydrobromide salts.To prepare analogues of3d in which the cyclam ring was replaced by a series of14-membered azamacrocyclic rings,we prepared a series of selectively protected macrocyclic ring systems containing a single(unprotected)secondary amine. This approach ensures the regiochemical outcome of the reaction with a benzylic halide during final construction (as shown in Scheme6).The syntheses of appropriate pre-cursors are shown in Schemes2-5.To incorporate fluorine groups at the desired position in the macrocyclic ring,suitably fluorinated bis-electrophiles were prepared,starting from 4-oxo-heptanedioic acid diethyl ester(4)and heptane-1,4,7-triol(8)as depicted in Scheme2.Reaction of the ketone(4) with neat(diethylamino)-sulfur trifluoride46,47(DAST)at room temperature for12days gave the corresponding di-fluoro-intermediate(5)in43%yield.Reduction of the ester groups with LAH(to give the diol6),followed by derivatiza-tion with toluenesulfonyl chloride,gave the bis-electrophile (7)required for the impending macrocyclization reaction.The corresponding monofluorinated intermediate was prepared in a similar manner.Protection of the primary alcohols in8as the acetyl group using acetic anhydride gave the secondary alcohol9,which was rapidly(and virtually quantitatively) converted to the fluorinated intermediate(10)with DAST (2.0equiv)in dichloromethane.Removal of the acetyl pro-tecting groups with saturated ammonia in methanol,followed by reaction of the diol(11)with p-toluenesulfonyl chloride, Scheme1aa Reagents:(a)R,R0-dibromo-p-xylene,K2CO3,CH3CN,reflux;(b)amine,K2CO3,CH3CN,reflux;(c)HBr,acetic acid,room temp. Scheme2aa Reagents:(a)Et2NSF3(neat),room temp;(b)LAH,Et2O;(c)Ts-Cl,Et3N,CH2Cl2;(d)acetic anhydride,pyridine;(e)Et2NSF3, CH2Cl2,-78°C,then room temp;(f)NH3/MeOH,room temp;(g)Ts-Cl,Et3N,CH2Cl2.1252Journal of Medicinal Chemistry,2010,Vol.53,No.3Bridger et al.gave the desired bis-electrophile 12containing a single fluorine group.The selectively protected azamacrocyclic rings were pre-pared via directed combinatorial macrocyclization of bis-2-nitrobenzenesulfonamides 48(Ns)(15a -c ,16a -c ,18)with bis-electrophiles (7,12,17)using previously optimized condi-tions 28(Scheme 3).To incorporate a phenyl or heterocyclic ring into the macrocycle,the corresponding bis-2-nitrobenze-nesulfonamide (15a -c )was prepared from the bis-aminoethyl intermediates 28(13a -c )by reaction with nosyl chloride (Et 3N,CH 2Cl 2).Similarly,16a ,b were obtained by reac-tion of commercially available intermediates 14a ,b with nosyl chloride or in the case of 16c (X=S)by reduction of 3,30-thiodipropionitrile with BH 33Me 2S and reaction of the intermediate diamine (14c )with nosyl chloride to give 16c .Macrocyclization was accomplished by dropwise addition of a DMF solution of the bis-electrophile to a DMF solution of the bis-2-nitrobenzenesulfonamide containing Cs 2CO 3maintained at a temperature of 80°C.Standard workup,followed by purification of the crude product by column chromatography on silica gel,gave the desired macrocycles 19a -c ,20a -c ,and 21a ,b in yields of 19-55%.Reaction of theintermediates from above with HBr/acetic acid at room temperature gave 22a -c ,23a -c ,and 24a ,b ,respectively.Because of synthetic convenience,we also prepared the selectively protected “isomers”of 22a ,b and 23a in which the alternative secondary amine was available for the alkylation reaction.We reasoned that reaction of 19a ,b and 20a with approximately 1equiv of thiophenol 49(our reagent of choice for nosyl deprotections)may allow pseudoselective deprotec-tion of a single nosyl group,leaving the Dep group intact.After some optimization,we found that reaction of 19a ,b and 20a with 0.8equiv of thiophenol and potassium carbonate in DMF (or acetonitrile)gave the precursors 25and 26a ,b in manageable,albeit modest yields (20-50%)following col-umn purification on silica gel (Scheme 4).Finally,the inter-mediates 27a ,b and 28(Scheme 5)were synthesized as recently described by palladium(0)catalyzed coupling of organozinc iodide reagents with bromopyridines.50Having completed the series of selectively protected aza-macrocycles,we proceeded to completion of the desired analogues by straightforward installation of the right-hand portion containing the aminomethyl pyridine moiety.As shown in Scheme 6,this was accomplished in all cases by direct alkylation of the available secondary amine of the macrocycle with the benzylic chlorides 34a ,b .Intermediate 34a was prepared in four steps from 4-bromomethyl benzoic acid methyl ester (29)and 2-aminomethylpyridine (31):con-version of 31to the 2-nitrobenzenesulfonamide 32,followed by alkylation with the benzyl bromide 30(obtained by reduc-tion of 29with DIBAL-H)gave the desired alcohol 33.As previously reported,28reaction of benzylic alcohols such as 33with methanesulfonyl chloride gave the chloride 34a rather than the corresponding mesylate,presumably via in situ nucleophilic substitution of the initially formed mesylate with chloride.Intermediate 34b (Scheme 6)containing a Dep-protecting group was prepared by an alternative synthesisScheme 3aaReagents:(a)Ns-Cl,Et 3N,CH 2Cl 2;(b)Cs 2CO 3,DMF,80°C;(c)HBr(g),AcOH,room temp.Scheme4Scheme5Article Journal of Medicinal Chemistry,2010,Vol.53,No.31253(procedures in Supporting Information).Alkylation of the available secondary amine of the macrocycles with 34a (or 34b )in CH 3CN in the presence of K 2CO 3gave the penultimate intermediates 35a -n .Deprotection of the nosyl groups with thiophenol and K 2CO 3in DMF gave the free base of the desired analogues,which in the vast majority of cases were converted to the corresponding hydrobromide salts.For analogues derived from the macrocyclic precursors 25and 26a ,b ,the intermediates isolated prior to the deprotection also contained a residual Dep group in addition to nosyl groups.For compound 45,we found that conversion to the hydro-bromide salt using a saturated solution of HBr in acetic acid resulted in concomitant deprotection of the remaining Dep group to obtain compound 45.For compounds 44and 46,the residual Dep group was removed prior to nosyl deprotection and salt formation.The thioether analogue 41a was also used to prepare the corresponding sulfoxide and sulfone analogues for antiviral evaluation as shown in Scheme 7.Initially,we globally protected the amino groups of 41a with Boc and subjected this intermediate to oxidation with oxone in MeOH 51at -10°C to give a mixture of the sulfoxide and sulfone that were separated by column chromatography on silica gel.However,while deprotection of the Boc groups with simulta-neous conversion to the hydrobromide salt proceeded without incident for the sulfone (to give 41c ),we found that deprotec-tion of the corresponding sulfoxide led to substantial reduc-tion and hence recovery of the starting analogue 41a .To overcome this problem,the sulfoxide was synthesized by direct oxidation of 41a with 1equiv of oxone in MeOH to give 41b in a 21%isolated yield and was subsequently tested as the free base in antiviral assays.Finally,we prepared a short series of analogues containing a carbon atom in place of a tertiary nitrogen group at the ring junction.To economize on the number of synthetic steps,weelected to synthesize the dimesylate 54(Scheme 8),an inter-mediate that could be commonly used for the synthesis of multiple analogues via macrocylization with the bis-2-nitro-benzenesulfonamide precursors already in our possession (namely 15a ,16a ,b from Scheme 3).Intermediate 54was prepared from the commercially available starting material bromo-p -tolunitrile via a double one-carbon homologation of the malonate 51,followed by derivatization to gave the requisite bis-methanesulfonate 54.Macrocyclizations of 54with bis-sulfonamides 15a and 16a ,b were performed as described above.Deprotection of the nosyl groups followed by conversion to the corresponding hydrobromide salts gave analogues 56and 58a ,b .DiscussionHaving previously established the optimum ring size and distance between the amines of both aliphatic andScheme 6a aReagents:(a)DIBAL-H,CH 2Cl 2;(b)Ns-Cl,Et 3N,CH 2Cl 2;(c)K 2CO 3,CH 3CN,60°C;(d)Ms-Cl,Et 3N,CH 2Cl 2;(e)K 2CO 3,CH 3CN,80°C;(f)R =Ns:thiophenol,K 2CO 3,DMF,or R =Dep:HBr(g),AcOH,room temp.Scheme 7aaReagents:(a)oxone,MeOH,-10°C;(b)(Boc)2O,THF;(c)HBr(g),AcOH,room temp.Scheme 8aaReagents:(a)NaH,R -bromo-tolunitrile,THF;(b)LiAlH 4,THF;(c)Ns-Cl,Et 3N,CH 2Cl 2;(d)2-picolyl chloride,Et 3N,K 2CO 3,KBr,CH 3CN,reflux;(e)Ms-Cl,Et 3N,CH 2Cl 2;(f)cetyltrimethyammonium bromide,NaCN,benzene,H 2O,reflux;(g)conc HCl/AcOH (4:1),reflux;(h)BH 3.Me 2S,THF;(i)Ms-Cl,Et 3N,CH 2Cl 2;(j)Cs 2CO 3,DMF,80°C;(k)thiophenol,K 2CO 3,CH 3CN (or DMF),40°C.1254Journal of Medicinal Chemistry,2010,Vol.53,No.3Bridger et al.pyridine-fused bis-tetraazamacrocycles required for potent X4anti-HIV activity,we designed a series of compounds to address the question of structural redundancy.The prototype bis-macrocycle 1has a center of symmetry and contains eight amino groups,of which four are positively charged at phy-siological pH.In the current study,we aimed to answer two specific questions:(1)Are all four positive charges required for potent anti-HIV activity?(2)On a per ring basis,what are the minimum structural requirements for activity?Assuming that the structural requirements are not iden-tical for both rings of 1,we reasoned that the simplest replacement for a single tetraaza-macrocyclic ring would be a pseudo diamine-segment,representing the first two amino groups of the macrocyclic ring from the point of attachment at the benzylic position.A judicious choice of “diamine”would also reduce the overall charge to þ1.Having previously established that the optimum distance between the first two amino groups was a two-carbon unit,we prepared a series of aminomethyl-substituted analogues in which the second amino group was a substituent upon an aromatic ring or part of a heterocyclic ring.In either case,the second p K a would be sufficiently low to prevent a second protonation at physiological pH.The compounds were tested for their ability to inhibit replication of HIV-1III B in MT-4cells,a strain of HIV-1that uses exclusively CXCR4for fusion and viral entry into target cells.The results are shown in Table 1.Compared to 1,the introduction of a benzylamine group (3a )in place of the azamacrocyclic ring substantially reduced anti-HIV potency,although the compound remained active at submicromolar concentrations.The concentration of 3a re-quired to inhibit HIV-1replication by 50%(the EC 50)was 0.49μM,which was approximately 100-fold higher than the 50%inhibitory concentration of 1.Aromatic amino groups at the 2-position (3b )or 4-position (3c )did not affect antiviral potency.Both 3b ,c exhibited comparable EC 50’s to the un-substituted benzyl group (3a ).However,we observed a sub-stantial increase in anti-HIV potency when the benzyl group was replaced by a pyridyl group (3d ).Compound 3d exhibited a 50%inhibitory concentration of 0.009μM,which was only ca.2-fold higher than the EC 50of 1.Furthermore,the 50%cytotoxic concentration (CC 50)of compound 3d in MT-4cells was greater than 112μM.Thus 3d exhibits a selectivity index of greater than 12000.The positional specificity of the pyridine-N in 3d was also examined.Replacement of the 2-pyridyl group with the 3-pyridyl (3e )or 4-pyridyl (3f )group had a detrimental effect on anti-HIV potency.For example,the EC 50’s of analogues 3e ,f were approximately 3orders of magnitude higher than the concentration of 3d required to inhibit HIV-1replication by 50%(the EC 50’s of 3e and 3f were 8.470and 9.977μM,respectively).Methylation of the amine in 3d (to give 3g )or extension of the connectivity to an aminoethyl pyridine group (to give 3h )also adversely affected the anti-HIV potency.Finally,we replaced the pyridine moiety with a comparable heterocycle of lower p K a than pyridine,namely the pyrazine group (3i ).Perhaps not surprisingly,the antiviral potency of analogue 3i was approximately comparable to the benzyl analogue 3a ,which did not contain a vicinal heterocycle nitrogen atom.With the optimized “right-hand”replacement for the aza-macrocycle ring of 1fixed as the 2-aminomethyl pyridine group,we then turned our attention to the “left-hand”ring.Needless to say,the mandatory synthesis of the symmetrical analogue in which both rings were replaced by a 2-amino-methyl pyridine group turned out to be a predictably fruitless exercise (EC 50was >250μM,data not shown).We therefore focused on systematically replacing individual amine groups of the left ring.As shown in Table 2,we first prepared an analogue in which the [14]aneN 4(cyclam)ring had been replaced by the optimized and equally suitable,py[iso -14]-aneN 4ring (to give compound 36).Consistent with the structure -activity relationship of py[iso -14]aneN 4bis-azama-crocycles,compound 36proved to be a potent inhibitor of HIV-1replication,exhibiting an EC 50of 0.001μM,that is,around 9-fold and 4-fold lower,respectively,than the con-centration of 3d or 1required to inhibit viral replication by 50%.Although the pyridine-N of the macrocyclic ring in 36was previously found to be critical for high antiviral potency,we reasoned that a precise determination of the pyridine-N contribution to potency could help redesign a less basic pounds 37and 38were then prepared to answer this question.Both analogues 37,containing a phenyl replacement and 38,containing an “exocyclic”pyridine fused group,retained reasonable anti-HIV potency (the EC 50’s of 37and 38were 0.040and 0.104μM,respectively)but were at least 40-to 100-fold less potent than analogue 36.So what role does the pyridine group play?At physiological pH,the overall charge of the py[iso -14]-aneN 4ring in 36is also þ2(in a similar manner to cyclam 52)and the likely protonation sequence is indicated in Figure 1A,based on the sequence reported by Delgado et al.53for similar 14-membered tetraazamacrocyclic rings contain-ing pyridine.Presumably,the secondary amino groups are predominantly protonated and the overall structure is stabi-lized by intramolecular hydrogen bond interactions from the adjacent hydrogen-bond acceptors,the pyridine and tertiary benzylic amine groups (while minimizing the elec-trostatic repulsion of two positive charges in a confined macrocyclic ring).This is confirmed by a conformational analysis of 36on B3LYP/6-31G*level followed by single point energy calculations.In the energetically most stable ring conformation (LMP2/6-311þG*þZPE),the pyridine nitro-gen forms two six-membered intramolecular hydrogen bond interactions with the two adjacent protonated nitrogens as shown in Figure 2.Potential five-membered intramolecular hydrogen bond interactions are formed with the tertiary amine.Table 1.Antiviral Activity of Single RingAzamacrocyclesnR 1R 2HIV-1(III B )EC 50(μM)MT-4cells CC 50(μM)3a 1H Ph0.4911603b 1H 2-amino-Ph 1.825243c 1H 4-amino-Ph 0.7172273d 1H 2-pyridine 0.009>1123e 1H 3-pyridine 8.470373f 1H 4-pyridine 9.977>2793g 1Me 2-pyridine 0.416383h 2H 2-pyridine 49.135>1103I 1H5-Me-pyrazine1.8957810.004>421ArticleJournal of Medicinal Chemistry,2010,Vol.53,No.31255The stabilization provided by this “shared”protonated structure could account for the high basicity of azamacrocyc-lic rings,as suggested by Kimura et al.54It did not seem unreasonable,therefore,that a potential role of the pyridine group is the contribution of a single intramolecular hydrogen-bond,which locks the conformation of the protonated aza-macrocyclic ring in manner that is beneficial to antiviral potency.To test this hypothesis,we prepared a series of analogues (depicted in Figure 1B,data in Table 2)in which the fused aromatic group had been removed and replaced by an aliphatic group,in some cases containing a hydrogen-bondacceptor at the key position “x,”the position occupied by the pyridine nitrogen in compound 36.Consistent with the hydrogen-bonding hypothesis,the alkyl analogue 39exhibited an anti-HIV potency that was compar-able to the phenyl and exocyclic pyridine analogues 37and 38(the EC 50’s of 37and 39,were 0.040and 0.043μM,re-spectively).This result categorically rules out the possibility that the conformational restrictions imposed by the fused aromatic groups in compounds 37,38were even partially responsible for the high potency of 36.However,incorpora-tion of a hydrogen-bond acceptor at position x (Figure 1B)in some cases restored activity comparable to 36.For example,the oxygen analogue 40exhibited an EC 50that was only 4-fold higher than the concentration of 36required to inhibit HIV-1replication by 50%(the EC 50of 40was 0.004μM).The corresponding thioether analogue 41a exhibited an EC 50of 0.013μM,which is approximately 3-fold higher than com-pound 40.Although the antiviral potency of the thioether analogue 41a compared to the ether analogue 41is greater than one would predict from the strength of the hydrogen-bond acceptor acceptor capabilities (thioether groups are considerably weaker H-bond acceptors than the oxygen inTable 2.Antiviral Activity of Single RingAzamacrocyclesFigure 1.Proposed hydrogen-bond structure of protonated aza-macrocycles.1256Journal of Medicinal Chemistry,2010,Vol.53,No.3Bridger et al.40),this result can be reconciled by considering the nature of the H-bond required;a six-membered intramolecular H-bond constrained by the macrocyclic ring (Figure 2).With the thioether compound 41a in hand,we also pre-pared the sulfoxide (41b )and sulfone (41c )analogues by direct oxidation of 41a .We reasoned that the oxygen atoms of the sulfoxide and sulfone are stronger H-bond acceptors than the sulfur atom of 41a and may consequently improve the anti-HIV potency.However,both 41b and 41c were considerably weaker antiviral agents,exhibiting 50%effective concentra-tions for inhibition of HIV-1replication that were at least 79-fold higher than the EC 50of 41a (the EC 50’s of 41b and 41c were 0.485and 11.878μM,respectively).The precise reason for the poor antiviral activity exhibited by analogues 41b ,c was unclear;although the sulfoxide and sulfone are more sterically demanding than the thioether and could induce a ring conformation that is detrimental to antiviral activity,we could not rule out the possibility that the H-bond acceptor oxygen is now “one-bond”outside of the ring,and the intramolecular H-bond itself induces an unfavorable confor-mation (a seven-membered ring H-bond in 41b ,c (Figure 2)compared to a six-membered in 41a ).To complete this series of compounds therefore,we decided to introduce the fluoro and difluoro substituents at position x (Figure 1B).Several reports have demonstrated that the fluoro group can partici-pate as an acceptor for intramolecular H-bonds,particularly within highly constrained ring structures.55-57This is also confirmed by our calculations,as shown in Figure 2.The fluoro (43)and difluoro (42)analogues were also attractive substituents for two other reasons:(1)the substituents would be situated at the fourth carbon from the adjacent amine group,thereby minimizing the affect on p K a ;(2)in a similar manner to the sulfoxide and sulfone,the H-bond acceptor would be one-bond outside of the macrocyclic ring.However in this case,because the fluorine atom in C -F groups is isostructural with hydrogen,a negative effect of the fluoro substituents on antiviral activity can only be attributed to an inappropriately positioned H-bond rather than steric requirements (that is,in the absence of an H-bond,we would expect the fluoro or difluoro analogues to exhibit an EC 50comparable to the methylene analogue 39).In antiviral test-ing,the fluoro (43)and difluoro (42)analogues displayed EC 50’s that were greater than 20-fold higher than the methy-lene analogue 39(the EC 50’s of 39,42,and 43were 0.043,0.920,and 1.239μM,respectively),confirming the negative consequences of an incorrectly positioned hydrogen-bond (Figure 2).Next,we focused on the sequence of aliphatic amine groups in the macrocyclic ring required for potent antiviral activity.By straightforward synthetic manipulation of our collection of ring systems,we prepared the structural isomers of analo-gues 36,37,and 39in which the side-chain (R,in Table 2)was connected to the alternative secondary amine group to give compounds 44,45,and 46.In antiviral testing,analogue 44was substantially less potent than its corresponding regioi-somer 39:the EC 50of 44was 11.131μM,which was approxi-mately 260-fold higher than the EC 50of 39.A similar loss of antiviral potency was observed with the phenyl analogue 46and its isomer 37(the EC 50’s of 46and 37were 14.106and 0.040μM,respectively).Interestingly,the loss of antiviral potency with the pyridine-fused isomer 45compared to 36was significant but not as substantial;the EC 50of 45was 0.063μM,around 60-fold higher than the concentration of 36required to inhibit HIV-1replication by 50%.There was a possibility,therefore,that while the “tri-aza”ring configura-tion required for potent antiviral activity is clearlyrepresentedFigure 2.Lowest energy conformations of compounds 36,40,41c ,and 42.View from top on a plane defined by three nitrogens and X (see Figure 1).Dashed lines indicate hydrogen bond interactions:the hydrogen bond acceptors in 36and 40are in one plane with the three nitrogens.This is not the case for 41c and 42.Bond angles:36:—(N 333H -N þ)=140.5°,122.4°,102.1°,108.4°.40:—(O 333H -N þ)=135.1°,141.5°;—(N 333H -N þ)=104.6°,102.8°.41c :—(O 333H -N þ)=112.8°,112.8°;—(N 333H -N þ)=108.2°,108.0°.42:—(F 333H -N þ)=142.2°,142.2°;—(N 333H -N þ)=114.7°,114.7°.。

AChR和MuSK抗体阳性的MG患者不同临床特点比较和治疗方案的选择发布时间:2022-12-09T02:29:00.856Z 来源:《医师在线》2022年24期作者:刘晓青1,张亚丽2 [导读] 目的:分析乙酰胆碱受体(AChR)和肌肉特异性受体酪氨酸激酶(MuSK)抗体阳性重症肌刘晓青1,张亚丽21内蒙古医科大学研究生院;内蒙古自治区呼和浩特市;010000 2内蒙古医科大赤峰临床医学院;内蒙古自治区赤峰024000【摘要】目的:分析乙酰胆碱受体(AChR)和肌肉特异性受体酪氨酸激酶(MuSK)抗体阳性重症肌无力(MG)患者的临床特点和治疗方案选择。
方法:回顾性分析2016年9月~2021年2月上海华山医院及本院收治153例不同类型MG患者的人口学特征、临床特点、治疗方案选择及转归情况。
结果:D组患者女性比例高于A组(P<0.05);D组患者以眼外肌麻痹为首发症状的比例类高于M组(P<0.05);D组患者呼吸肌和颈肌受累比例低于M组;D组患者MGFA分级为ⅢB~Ⅴ的比例低于M组,肌无力危象比例高于A组(P<0.05);服用溴吡斯的明后,D组症状改善比例低于A组,不良反应发生比例高于A组(P<0.05)。
结论:双抗体阳性MG患者性别分布、发病年龄、骨骼肌受累均、服用溴吡斯的明后效果与MuSK抗体阳性患者类似,但双抗体阳性MG患者病情严重程度介于其他两类型之间。
【关键词】重症肌无力;抗乙酰胆碱受体抗体;抗肌肉特异性酪氨酸激酶抗体Comparison of different clinical characteristics and selection of treatment for AChR and MuSK antibody positive MG patients Liu Xiaoqing1 Zhang Yali2 1Graduate School of Inner Mongolia Medical University;Hohhot, Inner Mongolia;010000 2 Chifeng Clinical Medical College of Inner Mongolia Medical University;024000?【Abstract】:Objective To analyze the clinical characteristics and treatment options of patients with acetylcholine receptor (AChR) and muscle-specific receptor tyrosine kinase (MuSK) antibody-positive myasthenia gravis (MG).Methods The demographic characteristics, clinical characteristics, treatment options and outcomes of 153 patients with different types of MG admitted to our hospital from September 2016 to February 2021 were retrospectively analyzed.Results The proportion of women in group D was higher than that in group A (P<0.05); the proportion of patients with external ophthalmoplegia as the first symptom in group D was higher than that in group M (P<0.05); the proportion of patients with respiratory and cervical muscles in group D was lower In group M; the proportion of patients with MGFA grade IIIB-V in group D was lower than that in group M, and the proportion of myasthenic crisis was higher than that in group A (P<0.05); after taking pyridostigmine, the proportion of symptom improvement in group D was lower than In group A, the incidence of adverse reactions was higher than that in group A (P<0.05).Conclusion The gender distribution, age of onset, and skeletal muscle involvement of double-antibody-positive MG patients were similar to those of MuSK antibody-positive patients after taking pyridostigmine, but the disease severity of double-antibody-positive MG patients was between the other two types.【Keywords】Myasthenia gravis;anti-acetylcholine antibody;anti-muscle specific kinase antibody Fund program: Supported by Inner Mongolia Autonomous Region 重症肌无力(myasthenia gravis, MG)[1]是一种由自身抗体介导T淋巴细胞辅助、补体共同参与的累及神经肌肉接头的自身免疫性疾病。

肾意义单克隆免疫球蛋白血症的诊治单克隆免疫球蛋白血症 (monoclonal gammopathy of undeter mined significanees,MGUS)指浆细胞异常单克隆增殖并在血清中产生M蛋白的一组疾病。
疾病进展可累及肾脏。
2012年,国际肾脏病与单克隆免疫丙种球蛋白病研究组[1]将MGUS疾病中因单克隆浆细胞异常增殖分泌M蛋白所致的肾脏损害命名为“肾意义单克隆免疫球蛋白血症”(monoclonal gammopathy of renal significance,MGRS) 。
1.发病机制、风险分层评估MGRS直接证据是肾组织中发现MIg。
[2]M蛋白可通过经典途径及旁路激活途径影响肾组织。
[3,4]见图1。
美国Mayo临床医学中心认为需从临床变化特点和流行病学,围绕血清蛋白电泳进行风险分层评估,血清游离轻链比值异常(<0.26或>1.65)、高M蛋白水平(>15g/L)和非IgG型MGUS是疾病进展的3个危险因素。
[5]均无危险因素为低危组,每6个月进行随访并复查血清蛋白电泳、血常规、血肌酐、血清钙,如处于稳定状态,可每2-3年复查,当提示为浆细胞恶性肿瘤症状出现时则需密切随访,国际骨髓瘤工作组[6]也是如此建议。
有其中1个危险因素的患者为中危组,具有全部危险因素的则是高危组,20年的疾病进展风险率分别为21%、58%,这类患者一经确诊除要行血液学实验室相关检查外,还需行骨骼影像学检查、骨髓细胞遗传学、骨髓荧光原位杂交检测,以后每年复查以上项目。
[6]图1.肾意义单克隆免疫球蛋白血症分类Fig1.The classification of monoclonal gammopathy of renalsignificance2.MGRS的诊断MGRS临床表现可缺如也可多样,缺乏特异性,肾脏损害可以血尿、蛋白尿等尿检异常为主,也可呈肾炎或肾病、肾功能综合征不全、终末期肾病等表现,以尿检异常、肾功能不全常见。

2020骨髓增生异常综合征的治疗挑战与新策略(全文)骨髓增生异常综合征(MDS)是一组极具异质性的、来源于造血干细胞的肿瘤性疾病,精准诊断和精准分层尤为重要。
通过骨髓穿刺活检、细胞遗传学检测和二代测序(NGS)分子图谱对体细胞突变进行检测是精准诊断的标准方法,虽然获得这些结果可能具有挑战性,但对患者的个体化治疗至关重要。
美国国立卫生研究院的数据显示,在社区的实践中,只有不足50%的患者进行了细胞遗传学和分子生物学检查。
目前,主要使用修订的国际预后评分系统,整合分子数据并考虑患者相关因素,对MDS患者进行危险分层。
根据这些信息,患者可分为两大类:低危组或高危组。
近期,美国李莫菲特癌症研究中心Rami S. Komrokji教授分别对高危和低危MDS的治疗经验与挑战进行了分享,小编整理如下,供血液科医生参考。
高危MDS患者面临的挑战和新策略异基因造血干细胞移植是高危MDS患者的重要治疗方法,且能否移植取决于患者的疾病状态、功能状态和合并症。
对于高危患者,去甲基化药物(HMA)阿扎胞苷或地西他滨的标准治疗仅对不足50%的患者有效,仅使20%的患者完全缓解(平均持续时间为1年)。
真实世界数据显示,高危MDS患者采用HMA标准疗法的平均生存期约为1.0-1.5年。
因此,即将接受移植的患者,以及由于合并症或功能状态不佳而不能接受移植的患者,迫切需要可以提高缓解率的治疗方法。
高危患者目前尚无HMA治疗失败后的标准治疗,预后通常较差。
因此,建议此类患者检查二代测序图谱中任何可识别的体细胞突变(如IDH1或IDH2),或者考虑临床试验。
如果患者转化为急性髓系白血病(AML),可考虑强化化疗,包括CPX-351(脂质体化的柔红霉素/阿糖胞苷[5:1]复合物)。
研究数据表明,在HMA治疗失败后使用BCL-2抑制剂维奈克拉作为“补充”治疗策略可能会使患者缓解,并能为患者接受移植提供机会,但这种策略仍需进一步研究观察。
APR-246是首创小分子药物,可以再激活突变型p53,恢复其功能并触发癌细胞凋亡。

表观遗传组学—肿瘤精准治疗的新靶标闫文姬;郭明洲【摘要】以肿瘤遗传学为基础的个体化治疗已对肿瘤的临床治疗产生影响,例如用于治疗EGFR基因突变肺腺癌的酪氨酸激酶抑制剂吉非替尼,但只有少部分患者具有这种遗传学改变.表观遗传在各种肿瘤中均发生异常改变,且在一定条件下表观遗传改变可以逆转,因此,以表观遗传学为基础的精准治疗将更具有发展和应用前景.已有研究发现表观遗传改变可作为化疗药的敏感性标志物,如MGMT甲基化是脑胶质瘤对烷化剂敏感的标志物,CHFR甲基化是胃癌对多西他赛耐药的标志物等.表观遗传药物也已用于临床,如DNA甲基转移酶抑制剂decitabine和组蛋白去乙酰化酶抑制剂SAHA已用于肿瘤的治疗.目前,许多针对表观遗传调控关键分子的靶向药物正在进行临床试验.联合应用不同的表观遗传药物或化疗药可能达到更好的效果.【期刊名称】《胃肠病学和肝病学杂志》【年(卷),期】2015(024)005【总页数】3页(P491-493)【关键词】表观遗传学;精准治疗【作者】闫文姬;郭明洲【作者单位】中国人民解放军总医院消化内科,北京100853;中国人民解放军总医院消化内科,北京100853【正文语种】中文【中图分类】Q751 个体化治疗和精准治疗的概念个体化医学(personalized medicine)不是一个新概念,早在19 世纪,Stepanov 认为“医生是治疗患者而不是治疗疾病,即使针对同样一个疾病,每一个患者都需要根据其身体状况而进行针对性的治疗”[1]。
早在2008 年,Collins[2]就预测在6 ~8 年内,基于人类基因组的个体化治疗将会取得实质性的进展。
2015 年1月30 日美国总统奥巴马宣布加速启动精准医学(precision medicine)的研究计划(/precisionmedicine)[3]。
在《Toward Precision Medicine》书中即引入了精准医学的概念,建议按照不同患者的分子水平改变对疾病进行更准确的分类、诊断和针对性的治疗[4]。
www.thelancet.com/neurology Vol 14 October 2015 1023ReviewLancet Neurol 2015; 14: 1023–36Department of Clinical Medicine, University of Bergen, Bergen, Norway (Prof N E Gilhus MD);
Department of Neurology, Haukeland University Hospital, Bergen, Norway (Prof N E Gilhus); and Department of Neurology, Leiden University Medical Center, Leiden, Netherlands (Prof J J Verschuuren MD)
Correspondence to:Prof Nils Erik Gilhus, Department of Neurology, Haukeland University Hospital, 5021 Bergen, Norwaynils.gilhus@helse-bergen.no
Myasthenia gravis: subgroup classifi cation and therapeutic strategies
Nils Erik Gilhus, Jan J VerschuurenMyasthenia gravis is an autoimmune disease that is characterised by muscle weakness and fatigue, is B-cell mediated, and is associated with antibodies directed against the acetylcholine receptor, muscle-specifi c kinase (MUSK), lipoprotein-related protein 4 (LRP4), or agrin in the postsynaptic membrane at the neuromuscular junction. Patients with myasthenia gravis should be classifi ed into subgroups to help with therapeutic decisions and prognosis. Subgroups based on serum antibodies and clinical features include early-onset, late-onset, thymoma, MUSK, LRP4, antibody-negative, and ocular forms of myasthenia gravis. Agrin-associated myasthenia gravis might emerge as a new entity. The prognosis is good with optimum symptomatic, immunosuppressive, and supportive treatment. Pyridostigmine is the preferred symptomatic treatment, and for patients who do not adequately respond to symptomatic therapy, corticosteroids, azathioprine, and thymectomy are fi rst-line immunosuppressive treatments. Additional immunomodulatory drugs are emerging, but therapeutic decisions are hampered by the scarcity of controlled studies. Long-term drug treatment is essential for most patients and must be tailored to the particular form of myasthenia gravis.
IntroductionDysfunction at the neuromuscular junction underlies several disorders that are characterised by skeletal muscle weakness usually involving some but not all muscle groups. Genetic forms of these disorders are termed congenital myasthenic syndromes. Some toxins, like botulinum toxin and curare, can cause neuromuscular dysfunction; acquired antibody-mediated forms include autoimmune and neonatal myasthenia gravis, Lambert–Eaton myasthenic syndrome, and neuromyotonia.Myasthenia gravis forms the largest disease group of neuromuscular junction disorders and is caused by pathogenic autoantibodies to components of the postsynaptic muscle endplate (fi gure 1).1–4 Fluctuations in
severity of muscle weakness are typical. Some, but not all, muscles are aff ected and not necessarily symmetrically. Increased weakness with continued muscle activity represents a diagnostic clue for myasthenia gravis, but these clinical features can vary. Patients with myasthenia gravis should be classifi ed into subgroups, with implications for diagnosis, optimum therapy, and prognosis. In myasthenia gravis guidelines and consensus reports, subgrouping is recommended,1–5 but exact
defi nitions vary and new subgroups are emerging as a result of increased knowledge. As this subgrouping takes into account myasthenia gravis autoantibodies, epidemiology, clinical presentation, and comorbidities, the subgroups are discussed after these sections in this Review. For a few patients, subgrouping is not possible owing to insuffi cient precise information, including suboptimum autoantibody testing and pathological changes of the thymus below the detection threshold of imaging.Autoantibodies against the acetylcholine receptor (AChR), muscle-specifi c kinase (MUSK), and lipoprotein-related protein 4 (LRP4) are well established as sensitive and specifi c diagnostic markers and pathogenic factors, and these autoantibodies are instrumental for subgrouping patients with myasthenia gravis. A
prerequisite for optimum diagnosis and treatment, therefore, is access to autoantibody testing.1–5
With modern immunosuppressive, symptomatic, and supportive treatments, the prognosis for patients with myasthenia gravis is good. Most patients with mild-to-moderate symptoms will obtain full remission or substantial improvement. Full remission is rare in severe cases, some variation over time is common, and steady progression is unusual. Daily life functions of individuals with myasthenia gravis are not, or only modestly, aff ected and life expectancy is not reduced.6 Long-term drug