HPV
- 格式:pdf
- 大小:63.35 KB
- 文档页数:9

HPV 常识HPV要点:∙ HPV (人乳头瘤病毒)是一种常见的感染皮肤和黏膜组织的病毒。
∙约有100多种HPV,其中约30种与生殖道疾病相关,主要通过性接触传播。
其中,“低危型”HPV可引起生殖道疣;而“高危型”HPV可引起宫颈癌。
∙据估计,80%的女性,一生至少会感染一次HPV。
∙虽然诸如吸烟等风险因素会增加发生宫颈癌的风险,但前提必先是感染了HPV。
∙幸运的是,大多数情况下,人体可依靠自身的免疫系统将病毒清除。
只有持续感染才会引起细胞学发生异常改变。
∙现在,可以通过HPV疫苗预防最常见的几种生殖道HPV感染。
但是,只有在女孩或年轻女性还没有发生性行为之前接种,疫苗的保护效果才能最好。
另外,现有HPV疫苗的保护作用是有限的,只是对某几种型别有保护作用,而非对所有可引发宫颈癌的高危型HPV病毒都有保护作用。
因此,定期进行妇科检查非常重要!如果您已经超过30岁,即便已经接种HPV疫苗,也应该定期进行HPV检测。
digene HPV Test 可检测13种高危型HPV,是目前临床HPV检测领域的金标准,也是宫颈癌筛查的首选方法。
是否因为感染了HPV这种性传播病毒感到尴尬或羞耻?HPV是一种嗜上皮病毒,感染HPV与感冒一样普遍。
虽然主要是通过性行为传播,但可以通过任何亲密的皮肤接触传播。
必须要了解的一点是,任何与其他人有过性接触的个体都可能感染HPV。
只要是有过性行为的人,即使只和一个曾经有过其他性伴侣的人发生性行为,也有风险感染HPV。
其中包括与同性有过性行为的女性。
很多女性从来没有听说过HPV。
很多女性可能知道细胞学检查,用以发现宫颈细胞是否异常改变。
但她们可能不知道引起宫颈细胞发生异常改变的元凶恰恰就是HPV。
女性对HPV最常见的误解是什么?很多女性没有意识到几乎每个人都会感染HPV。
感染HPV的患者经常会有诸如此类的问题:“我什么时候感染这个病毒的?”,“我的伴侣需要接受检查吗?”,“我怎样摆脱这个病毒?”。

什么是HPV病毒什么是HPV病毒?人类乳头瘤病毒(HumanPapilloma virus,简称HPV)是一种嗜皮性病毒,有高度的特异性,像乙肝病毒一样,HPV也是一种DNA 病毒。
它原是多瘤空泡病毒科的一员,1999年国际病毒分类委员会(ICTV)取消多瘤空泡病毒科,代之以乳头瘤病毒科,因此,HPV便归属此门下。
感染途径:1、性传播途径;2、密切接触;3、间接接触:通过接触感染者的衣物、生活用品、用具等;4、医源性感染:医务人员在治疗护理时防护不好,造成自身感染或通过医务人员传给患者;5、母婴传播:是由婴儿通过孕妇产道的密切接触HPV引起的临床表现:(1)皮肤低危型:包括HPV1、2、3、4、7、10、12、15等与寻常疣、扁平疣、跖疣等相关;(2)皮肤高危型:包括HPV5,8,14,,17,20,36,38与疣状表皮发育不良有关,其他还与可能HPV感染有关的恶性肿瘤包括:外阴癌、阴茎癌、肛门癌、前列腺癌、膀胱癌;(3)黏膜低危型如HPV-6,11,13,32,34,40,42,43,44,53,54等与感染生殖器、肛门、口咽部、食道黏膜;(4)黏膜高危型HPV-16、18、30、31、33、35、39与宫颈癌、直肠癌、口腔癌、扁桃体癌等。
生物学活性:HPV抵抗力强,能耐受干燥并长期保存,加热或经福尔马林处理可灭活,所以高温消毒和2%戊二醛消毒可灭活。
平时该怎样预防HPV病毒?1注意HPV的传染途径。
HPV通常通过阴道和肛门的性交传播,但也可以通过其他各种生殖器官的接触传播。
•多数HPV感染者不表现任何病症,但HPV也可能在潜伏很多年后出现病症,可能在很多年没有性生活的情况下出现HPV感染症状。
不了解这些的话,人们很容易在没有意识到的情况下传播HPV病毒。
•极少数情况下,感染HPV的孕妇可能在生产过程中将病毒传染给新生儿。
若发生此种情况,婴儿可能会在咽喉、喉头部位出现疣。
这种情况在医学中成为复发性呼吸道乳头瘤(RRP)。
人类乳突病毒(Human Papillomavirus,简称HPV)是一种嗜上皮性病毒,有高度的特异性,长期以来,已知HPV可引起人类良性的肿瘤和疣,如生长在生殖器官附近皮肤和粘膜上的人类寻常疣、尖锐湿疣以及生长在粘膜上的乳头状瘤。
像乙肝病毒一样,HPV也是一种DNA病毒。
它原是多瘤空泡病毒科的一员,1999年国际病毒分类委员会(ICTV)取消多瘤空泡病毒科,代之以乳头瘤病毒科,因此,HPV便归属此门下。
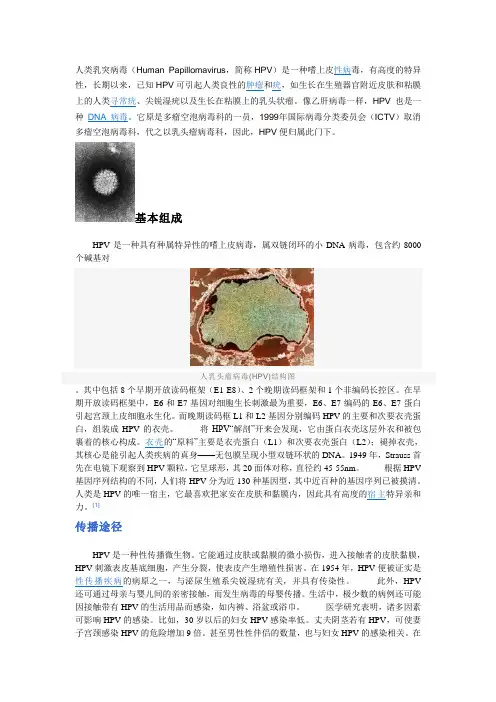
基本组成HPV是一种具有种属特异性的嗜上皮病毒,属双链闭环的小DNA病毒,包含约8000个碱基对人乳头瘤病毒(HPV)结构图。
其中包括8个早期开放读码框架(E1-E8)、2个晚期读码框架和1个非编码长控区。
在早期开放读码框架中,E6和E7基因对细胞生长刺激最为重要,E6、E7编码的E6、E7蛋白引起宫颈上皮细胞永生化。
而晚期读码框L1和L2基因分别编码HPV的主要和次要衣壳蛋白,组装成HPV的衣壳。
将HPV“解剖”开来会发现,它由蛋白衣壳这层外衣和被包裹着的核心构成。
衣壳的“原料”主要是衣壳蛋白(L1)和次要衣壳蛋白(L2);褪掉衣壳,其核心是能引起人类疾病的真身——无包膜呈现小型双链环状的DNA。
1949年,Strauss首先在电镜下观察到HPV颗粒,它呈球形,其20面体对称,直径约45-55nm。
根据HPV 基因序列结构的不同,人们将HPV分为近130种基因型,其中近百种的基因序列已被摸清。
人类是HPV的唯一宿主,它最喜欢把家安在皮肤和黏膜内,因此具有高度的宿主特异亲和力。
[1]传播途径HPV是一种性传播微生物。
它能通过皮肤或黏膜的微小损伤,进入接触者的皮肤黏膜,产生分裂,使表皮产生增殖性损害。
在1954年,HPV便被证实是性传播疾病的病原之一,与泌尿生殖系尖锐湿疣有关,并具有传染性。
此外,HPV 还可通过母亲与婴儿间的亲密接触,而发生病毒的母婴传播。
生活中,极少数的病例还可能因接触带有HPV的生活用品而感染,如内裤、浴盆或浴巾。


hpv 检测标准
HPV(人类乳头瘤病毒)检测标准通常是指用于检测人体样本中HPV感染的方法和标准。
HPV感染是一种常见的性传播疾病,与宫颈癌等多种癌症相关联。
因此,HPV检测对于宫颈癌筛查和预防具有重要意义。
以下是一些常见的HPV检测标准和方法:
1. HPV-DNA检测:这是目前最常用的HPV检测方法之一。
它通过分析样本中的DNA,检测HPV病毒的存在。
HPV-DNA检测可以使用多种技术,包括聚合酶链反应(PCR)、原位杂交(ISH)等。
2. HPV病毒亚型检测:HPV病毒有多个亚型,其中一些与宫颈癌的风险相关性较高。
因此,HPV检测通常包括对不同HPV亚型的检测和鉴定。
3. HPV RNA检测:与HPV-DNA检测相比,HPV RNA检测可以更好地区分活跃的、正在感染宿主的病毒与已经被清除的病毒。
4. HPV病毒载量检测:有些检测方法可以估计样本中HPV病毒的数量,这对评估感染的严重程度和预后具有重要意义。
HPV检测标准通常由专业医学组织或政府卫生机构制定,以确保检测方法的准确性、可靠性和临床意义。
在进行HPV检测时,必须遵循适用的标准和指南,以确保检测结果的准确性和临床意义。
1/ 1。

hpv的发展现状人乳头瘤病毒(HPV)是目前全球最常见的性传播疾病之一,它引起的感染在近几十年来一直呈现出不断增加的趋势。
本文将就HPV的发展现状进行探讨,从疫苗研发、预防与治疗、社会认知等方面进行分析。
首先,HPV疫苗的研发取得了重大突破。
目前可用的两种HPV疫苗分别是二价疫苗和四价疫苗,它们能够预防高危型别的HPV感染,从而减少宫颈癌和其他相关疾病的发生。
这些疫苗经过大规模的临床试验,证明了其对预防HPV感染和相关疾病的有效性。
随着疫苗的推广和普及,各国对于HPV感染和相关疾病的发生率都取得了显著的下降。
其次,HPV感染的预防与治疗手段也得到了不断完善。
宫颈癌筛查程序的普及和推广,使得更多的女性得以及时发现和治疗初期的宫颈癌病变,减少了其发展为晚期宫颈癌的概率。
此外,对于已经感染的HPV携带者,早期干预和治疗也成为重点研究领域。
目前,一些药物和治疗方法正在研究和开发中,旨在提高对于HPV感染的治疗效果,从而减少相关疾病的发生。
第三,社会对于HPV的认知和关注程度逐渐提高。
在过去,对于HPV的认知相对较低,许多人对于自身是否感染HPV及其相关风险了解甚少。
然而,在过去几年中,公众对于HPV感染和宫颈癌等相关疾病的认知度逐渐提高。
各国政府和医疗机构也加大了相关宣传和教育力度,通过各类媒体和健康教育活动向公众普及HPV的知识,提高人们对于HPV感染的预防和治疗的重视程度。
然而,尽管HPV的发展现状取得了长足的进展,但仍然存在许多挑战。
首先,对于HPV感染的普查和筛查仍存在一定困难。
由于HPV感染的潜伏期长、症状不明显,很多人并不清楚自己是否感染了HPV。
因此,如何提高HPV感染的普查率和筛查率仍然是一个关键问题。
其次,HPV疫苗的普及程度仍然不够。
虽然很多国家已经将HPV疫苗纳入国家免疫规划中,但在一些发展中国家,HPV疫苗的普及程度和接种率仍然较低,这可能会限制HPV感染的控制和减少相关疾病的发生。

hpv抗体原理
HPV(人乳头瘤病毒)抗体原理是指人体通过感染HPV病毒后,免疫系统会产生抗体来抵御病毒的侵袭。
HPV抗体的检测可以通过血清学方法进行,主要检测血液中是否存在特定的HPV抗体。
具体的原理是,当人体感染了HPV病毒后,病毒中的抗原(如病毒的外壳蛋白等)会被免疫系统识别,并激活机体产生特异性抗体。
这些抗体可以与HPV病毒的抗原结合,进而形成抗原抗体复合物。
这种抗原抗体复合物可以在体内进行多种免疫反应,如中和病毒、激活细胞毒性T细胞等,起到抵御病毒的作用。
在HPV抗体检测中,可以采用间接免疫荧光法(IFA)、酶联免疫吸附法(ELISA)等方法。
这些方法通过将血清样本与HPV抗原结合,再加入特定的标记物(如荧光标记或酶标记),检测标记物与抗原抗体复合物的形成情况。
根据标记物的信号强度或反应结果,可以判断血液中的HPV抗体水平,从而评估人体对HPV病毒的免疫状态。
HPV抗体检测在临床上可以用于HPV感染的早期诊断、病情评估和预后判断等。

HPV分析报告第一点:HPV的概述人乳头瘤病毒(Human papillomavirus,简称HPV)是一种常见的性传播病毒,它可以引起多种良性及恶性的生殖系统疾病。
HPV病毒具有高度的宿主特异性,主要侵犯人类皮肤和黏膜组织,其中最为人所熟知的是引起宫颈癌的病毒类型。
HPV病毒的结构相对简单,由一个双链DNA核心和蛋白质外壳组成。
这个核心含有病毒的遗传信息,是病毒复制和感染宿主细胞的关键。
外壳蛋白则负责识别并附着到宿主细胞上,进而进入细胞内部。
根据HPV的基因序列差异,可分为多种类型,其中一些类型与癌症相关,而另一些则不会导致严重疾病。
在感染HPV后,大多数人的免疫系统可以清除病毒,这个过程可能需要数月甚至数年。
然而,在一些情况下,病毒会持续存在,并可能引发细胞异常,最终导致癌症。
因此,对HPV的认知和预防至关重要。
第二点:HPV的检测与预防对于HPV的检测,目前常用的方法有细胞学检查、HPV病毒检测和HPV基因分型检测。
细胞学检查是通过检查宫颈细胞的形态变化来判断是否存在HPV感染及其引起的病变。
HPV病毒检测则直接针对病毒的存在进行检测。
而HPV基因分型检测可以识别出具体的HPV类型,这对于疾病的预防和治疗具有重要意义。
预防HPV感染的主要措施包括接种疫苗和采取安全的性行为。
HPV疫苗可以预防疫苗覆盖的HPV类型引起的宫颈癌、肛门癌、口咽癌等,是预防HPV感染的有效手段。
安全的性行为,如使用避孕套和限制性伴侣数量,也可以降低感染的风险。
此外,定期的妇科检查也是预防宫颈癌的重要措施。
通过定期检查,可以在癌症早期发现并及时治疗,提高治疗效果和生存率。
总的来说,对HPV的认知、预防和检测是保护个人健康的重要措施。
了解HPV的传播途径、感染后果以及预防方法,可以有效降低感染风险,保护自己和他人的健康。
第三点:HPV感染的治疗一旦确诊为HPV感染,治疗方法将取决于感染的类型、严重程度以及患者的个体情况。

人类乳头瘤病毒求助编辑百科名片人类乳头瘤病毒人类乳头瘤病毒(human papillomavirus,HPV)属乳头多瘤空泡病毒科(papovaviridae)乳头瘤病毒属。
易感染人类表皮和粘膜鳞状上皮,HPV感染表皮引起的增生性病变称为“疣”,感染粘膜鳞状上皮引起的增生性病变称为“乳头瘤”。
人类乳头状瘤病毒(Humanpapillomavirus,HPV)是一种嗜上皮性病毒,在人和动物中分布广泛,有高度的特异性,长期以来,已知HPV可引起人类良性的肿瘤和疣,如生长在生殖器官附近皮肤和粘膜上的人类寻常疣、尖锐湿疣以及生长在粘膜上的乳头状瘤。
自从1976年zurHansen提出HPV可能是性传播致癌因素以来,HPV感染与宫颈癌关系的研究成为肿瘤病毒病因研究的热门课题。
编辑本段感染性疾病的历史公元610年(隋代),我国着名医家巢元方撰写的《诸病源候论瘿瘤等病诸候疣目候》中记载:“疣目者,人手足边或生如豆,或如结筋,或五个或十个相连肌里,粗强于肉,谓之疣目。
”对疣目(寻常疣)的好发部位和皮损形态进行了描述。
1907年发现乳头瘤病毒是皮肤疣的病原。
1933年Shope在绵尾兔体内首次发现乳头瘤病毒(cottontial rabbit papillomavirus,CRPV),随后相继在人和各种动物中发现了乳头瘤病毒。
编辑本段病原学特征HPV为乳多空病毒科A属成员,是一类感染表皮和粘膜鳞状上皮的小DNA病毒。
直径52-55nm,无被膜,正20面体结构,表面有72个壳体。
病毒基因组是双链环状DNA分子。
具有高度种属特异性,人类皮肤角质形成细胞/黏膜鳞状上皮细胞的是其天然宿主。
具有特殊嗜上皮性,仅在一定分化程度的上皮细胞内增殖。
不经血流扩散,不产生病毒血症,也不易被免疫系统识别。
根据HPV的同源性,目前已发现120多种型别。
编辑本段HPV感染性疾病与HPV型别关系临床诊断好发部位主要HPV型别寻常疣手、足 HPV1、2 、4、7 扁平疣颜面、手、足 HPV3、10、28、41 深部掌跖疣手掌、足跖 HPV1 屠夫手部疣手 HPV7 疣状表皮发育不良面、手、足、躯干 HPV3、5、8、9、12、14、15、17-25、36-38、46、47 尖锐湿疣生殖器、肛周 HPV6、11、16、18 口腔灶性上皮增生口腔 HPV13、32 喉部乳头瘤喉部 HPV6、11、16、30 生殖器乳头瘤/癌前病变/癌 HPV16、18、31、33、35-39、45、51-52、56、58、59 (包括宫颈乳头瘤病变、宫颈癌、男性生殖器Bowen’s病、巨大尖锐湿疣、阴茎癌/肛门癌)编辑本段与HPV相关的其他肿瘤光线性角化病,皮肤鳞癌,基底细胞癌,恶性黑素瘤,脂溢性角化病,毛鞘囊肿,生乳头汗管囊腺瘤,多发性跖部表皮囊肿,硬化性苔藓,砷角化病,汗管瘤,眼部肿瘤(眼乳头状瘤、鳞状细胞癌、视网膜母细胞瘤),宫颈癌, 食管癌,肺癌。

hpv注意事项
HPV是一种常见的性传播疾病,它能引起许多健康问题,包
括生殖器疣和宫颈癌。
为了预防HPV感染和其引发的健康问题,以下是一些注意事项:
1. 接种HPV疫苗:由于HPV疫苗可以预防某些类型的HPV
感染,建议合适的人群接种疫苗。
咨询医生以确定适合您的接种计划。
2. 定期进行妇科检查:女性应该定期定期接受宫颈抹片检查,以便尽早发现宫颈癌的迹象。
3. 培养良好的性行为习惯:避免性伴侣过多,尽量避免不安全的性行为,使用安全套等方法减少感染的风险。
4. 与性伴侣保持忠诚:一个忠诚的性伴侣关系可以减少感染HPV的风险。
5. 增强免疫力:保持良好的健康习惯和生活方式,如均衡饮食,充足睡眠,适度锻炼和减少压力等,可以增强免疫力,降低感染HPV的风险。
6. 定期检查性器官:定期检查身体是否有异常,包括生殖器疣和其他HPV引起的症状。
7. 提高自我保护意识:加强对性教育的了解,学习如何正确使用避孕套,了解HPV的传播途径和预防方法,提高自我保护
意识。
8. 避免共用个人卫生用品:避免与他人共用个人卫生用品,特别是那些与生殖器接触的用品,如毛巾、衣物等,以防止传播病菌。
9. 定期进行HPV病毒检测:如果您有性行为或感觉到出现了HPV感染的症状,建议定期进行HPV病毒检测,以便早发现和治疗。
10. 寻求医学咨询和治疗: 如果出现宫颈疣、其他疣状物或任何其他与HPV感染相关的症状,及时就医并跟随医生的建议进行治疗。
总之,HPV是一种常见的传染病,但可以通过采取适当的预防措施来减少感染的风险和预防健康问题的发生。
请注意以上注意事项,并随时咨询医生以获取专业建议。

1,HPV是什么?HPV是人类乳头瘤病毒的简称,是一种传染性病毒通过接触受感染的身体部分传染。
HPV目前是根据它的致癌性与否分为了低危型和高危型,低危型的HPV不会导致宫颈癌,但是高危型的HPV可能会导致宫颈癌前病变和宫颈癌。
HPV-16和HPV-18它们造成了约70%的癌症的宫颈;(此次国内批准上市的正是这两种)HPV-6和HPV-11他们造成了约90%的生殖器疣,可以给9至45岁之间的女性接种,在加拿大,联邦政府的专家小组已经确认疫苗在男孩和年轻男子年龄9至26岁产生有效性。
2,HPV是怎么传染的?某些类型的HPV可通过性传播。
与携带HPV病毒的人进行口交、阴交或肛交,可能会感染HPV。
其中,通过阴道或肛交传播最为常见。
HPV并非只是通过性生活传播的,与携带HPV病毒的患者密切接触都可以导致女性感染HPV,甚至在儿童、无性经历的女性身上都会发现有HPV的感染存在。
较为可惜的是目前HPV病毒感染,并没有有效的治疗措施。
3,HPV疫苗有哪几种?在加拿大,有两种疫苗可以防止HPV(人乳头状瘤病毒):这是一种由默沙东公司研发的四价疫苗,商品名是“佳达修”(Gardasil4)。
可用于防治HPV16、18、6、11型病毒;(2)另外一种是由葛兰素史克(GSK)公司研发的二价疫苗“卉妍康”(Cervarix、又称希瑞适)。
该疫苗只针对HPV16和HPV18病毒的感染。
在我居住的省,使用的是HPV–4(HPV61116和18)。
这个疫苗可以几乎达到100%预防4种高危HPV病毒。
最新疫苗品种:根据加拿大癌症网站所述,加拿大最近验证了9价疫苗(Gardasil9)。
可以防止9种HPV病毒。
(HPV-6,HPV-11,HPV-16,HPV-18,HPV-31,HPV-33,HPV-45,HPV-52,HPV-58)疫苗不能防止所有类型的性传播疾病,如艾滋病毒,衣原体,或淋病。
重要的是要采取安全的性行为以及定期检查。
hpv医学名词解释
HPV 是“人乳头瘤病毒”(Human Papillomavirus) 的缩写。
它是一种小型的 DNA 病毒,属于疱疹病毒科。
HPV 可以感染人类皮肤和黏膜的上皮细胞,导致各种各样的疾病,包括尖锐湿疣、宫颈癌、肛门癌、口腔癌等。
HPV 有很多种类型,其中一些可以引起生殖器官周围的尖锐湿疣 (也称“菜花病”)。
这种病毒还可以通过血液传播到身体其他部位,引起其他疾病,例如肺炎和脑炎。
HPV 感染是一种常见的疾病,尤其是在性行为活跃的年龄段。
然而,有些人即使感染了 HPV 也不一定会出现症状。
预防 HPV 感染的最好方法是接种疫苗,以及遵守安全性行为的原则。
如果已经被 HPV 感染,则需要进行进一步的诊断和治疗以缓解症状和防止疾病恶化。
Disease Markers23(2007)273–281273 IOS PressHPV detection methodsAntoinette A.T.P.Brink,Peter J.F.Snijders and Chris J.L.M.Meijer∗VU University medical center,Department of Pathology,Section Molecular Pathology,De Boelelaan1117,1081 HV Amsterdam,The NetherlandsAbstract.Given the causal relation between a persistent high-risk human papillomavirus(hrHPV)infection and the development of high-grade cervical intraepithelial neoplasia(CIN)and cervical cancer,hrHPV testing has been advocated in addition to cytology for the detection of clinically relevant cervical lesions.HrHPV testing is thought to improve cervical screening algorithms,the management of women with cytologically equivocal smears,and the management of women treated for high grade CIN.In this chapter we discuss different methods for HPV detection and genotyping and their respective applications. Keywords:Human papillomavirus,polymerase chain reaction,reverse hybridization,hybrid capture,real-time PCR,cervical carcinoma,NASBA,DNA probes,epidemiology1.IntroductionCervical cancer is preceded by premalignant lesions termed cervical intraepithelial neoplasia(CIN).Cervi-cal cancer screening largely relies on cytological detec-tion of these premalignant lesions,which can be treated relatively easily and with minor side effects.Given the causal relation between a persistent high-risk human papillomavirus(hrHPV)infection and the development of high-grade CIN and cervical cancer,hrHPV testing has been advocated in addition to cytology for the de-tection of clinically relevant cervical lesions.HrHPV testing is thought to improve cervical screening algo-rithms[1],the management of women with cytologi-cally equivocal smears[2,3],and the management of women treated for high grade CIN[4].In this chapter we elaborate on the different methods for HPV detec-tion and their respective applications.1.1.HPV phylogenyHPVs are associated with various benign and malig-nant epithelial proliferative diseases.Over100geno-∗Corresponding author:Prof.Dr.C.J.L.M.Meijer,Dept.Pathol-ogy,VU University medical center,PO Box7057,1007MB Amster-dam,The Netherlands.Tel.:+31204444070;Fax:+31204442964; E-mail:cjlm.meijer@vumc.nl.types of HPV are recognized.An HPV isolate is de-scribed as a novel type if the nucleotide sequence of its E6,E7and L1genes differs more than10%from that of any other HPV type.Based on tropism,a distinction can be made between cutaneous HPV types that infect the epidermis,and mucosal HPV types that infect the epithelia of the anogenital or the aerodigestive tract. In vitro studies showed that a group of phylogenet-ically related mucosal HPV types have oncogenic po-tential:their E6and E7proteins interfere with cell cy-cle regulation by mediating degradation of p53and pRb proteins,respectively[5,6].Exactly these HPV types are an important causal factor in carcinogenesis of the uterine cervix and are therefore designated“high-risk”(hr)[7].Epidemiological studies have shown that these hrHPVs include types16,18,31,33,35,39,45,51,52, 56,58,59,68,73and82,and probably also types26, 53and66[8].In the phylogenetic tree of HPV types, hrHPVs cluster in the groups designated A5,A6,A7, A9and A11[9].Non-oncogenic or low-risk genital HPVs(lrHPVs)are phylogenetically distant from the hrHPVs.They include HPV6,11,40,42,43,44,54, 61,70,72,81,and CP6108.Their E6and E7pro-teins are less competent in interfering with p53[10] and pRb[11]functions than the E6and E7proteins of hrHPVs.LrHPVs can cause benign proliferations such as condylomata acuminata(genital warts).For a number of mucosal HPV types,the risk has not beenISSN0278-0240/07/$17.00 2007–IOS Press and the authors.All rights reserved274 A.A.T.P.Brink et al./HPV detection methodsdetermined yet[8].However,some of them could be considered as potentially oncogenic due to their phylo-genetic relationship with known hrHPV types.For ex-ample HPV type67,which initially was isolated from a vaginal intraepithelial neoplasia(VIN)[12],clusters in the A9group that contains only hrHPVs such as HPV16[9].1.2.The need for HPV genotypingMany HPV tests rely on cocktails of probes repre-senting13or14of the most common hrHPV types. It has been suggested that the added value of less fre-quent hrHPV types to such probe cocktails would be small,probably irrelevant for screening programs,and resulting in a substantial decrease in screening speci-ficity[13,14].However,the increasing need to distin-guish individual hrHPV types is illustrated by sever-al current developments in thefield of HPV research. Firstly,there is growing evidence that certain hrHPV types confer increased risks for high-grade CIN and cervical carcinomas.This is discussed in more detail in the next paragraph.Secondly,20–30%of HPV in-fections involve multiple HPV types and in those cas-es typing is necessary to determine the contribution of the individual HPV types.Thirdly,there is an increas-ing interest in prophylactic HPV vaccination(reviewed in[15]),which entails studies of geographical distri-bution of HPV types and their associated diseases as a basis for prophylactic vaccination programs.Once vaccination has started,HPV typing remains necessary to monitor the changes in prevalence of the type(s) represented in the vaccines as a measure for vaccine efficacy.HPV genotyping may also have some advantages and perhaps some prognostic value in monitoring of women who have been treated for CIN3or cervical cancer,although the treatment itself does not depend on the typing result.Demonstrating the same hrHPV type in the post-treatment specimen as in the primary CIN3lesion may indicate a recurrent hrHPV infection due to incomplete removal of the lesion or disability of the respective woman to clear a particular hrHPV type[16,17]and might require more intense follow-up or more aggressive treatment.Finally,the number of known HPV types is still in-creasing.Although novel HPV types are often isolated from malignancies,their oncogenic potential can only be deduced from a combination of in vitro and epi-demiological studies,the latter of which should include typing.1.3.HrHPV types and risk of high-grade cervicallesionsWe showed that in the Dutch screened popula-tion[18],HPV types16and33are more preva-lent in women with a cytological reading of moderate dyskaryosis or worse and underlying CIN2or worse, than in women with normal cytology.This suggests that infection with these types confers an increased risk for development of high-grade cervical lesions[19]. Moreover,HPV type distribution in women with cer-vical carcinoma as compared with cytologically nor-mal women showed that HPV18is mainly a risk factor for the development of adenocarcinoma(AdCa)and its precursor adenocarcinoma in situ(ACIS),whereas HPV16is associated with both squamous cell carcino-ma(SCC)and AdCa[20].In line with the abovemen-tionedfindings,an increased risk posed by HPV16and HPV18for cervical(pre)cancers of the squamous and adeno-histotypes was shown in a prospective screen-ing cohort of20810women followed for up to10 years[21].In addition,an increased risk for cervi-cal precancer posed by HPV16but not by HPV18was shown in a prospective trial of women with equivocal or mild cervical abnormalities[22].A likely expla-nation for increased prevalence of HPV18in invasive carcinoma but not in high-grade precursor lesions,is an association of HPV18with a cytopathological effect high in the endocervical canal that is likely to be missed by cytology.Thesefindings strongly suggest that in particular women who are HPV16or HPV18positive should be monitored very closely,even if their smears are cyto-logically normal.2.HPV detection methodsBecause HPV cannot be cultured,all HPV tests cur-rently in use rely on the detection of viral nucleic acids.HPV detection methods can be divided in target-amplification methods and signal-amplification meth-ods.In the following section,technical aspects of the different currently available HPV detection methods are described.2.1.Target amplification techniques2.1.1.Consensus primer polymerase chain reaction(PCR)In most of the PCR-based HPV detection systems,a broad spectrum of HPV types is amplified by consen-A.A.T.P.Brink et al./HPV detection methods275sus primers,followed by detection with type-specific probes.The consensus primers may be degenerate as in the MY09/11[23]and CPI/II[24]systems.Al-ternatively,they may contain mismatches that are ac-cepted under low-stringency PCR conditions as in the GP5+/6+system[25],they may contain inosine residues at ambiguous base positions such as in the IU/IWDO[26]and SPF[27]primers,or sets of over-lapping primers as is the case in the PGMY[28]and Amplicor[29]systems.2.1.2.Detection and/or genotyping of consensus PCRproductsFor group-specific detection of HPVs without high-resolution typing,an enzyme immuno assay(EIA)can be applied conveniently using cocktails of e.g.HR-or LR-HPV probes[30,31].Typing of PCR products was traditionally done by means of dot blotting or Southern blotting and hy-bridization with type-specific oligonucleotides.More recently,reverse hybridization techniques were intro-duced.These methods rely on the hybridization of la-belled consensus PCR products to HPV-type specific oligos immobilized onfilters.Examples are reverse line blot(RLB)analysis following MY09/11[23]or GP5+/6+[25]consensus PCR,or a line probe assay (LiPa)following SPF PCR[27].Detection of the hy-bridized PCR product is done by a colorimetric reac-tion[23,27]or by chemiluminescence[25],the latter al-lowing repeated usage of the samefilter[25].Instead of filters,glass microarrays of HPV type-specific probes can also be used[32].Recently,a quantitative and high-throughput method was developed[33]based on Luminex suspension array technology.This method re-lies on detection of consensus primer PCR(GP5+/6+) products with type-specific oligonucleotide probes cou-pled tofluorescence-labelled polystyrene beads and al-lows detection of up to100different HPV types simul-taneously.Two non-hybridization typing methods following consensus PCR are sequence analysis of the PCR prod-uct[34,35]and restriction fragment length polymor-phism(RFLP)analysis[36].RFLP implies the diges-tion of consensus PCR products with restriction en-donucleases,and comparison of the digestion pattern with those of known HPV types.These techniques are useful if unknown types of HPV are present in the spec-imens,but they have several drawbacks as compared with hybridization methods.For example,RFLP and sequence analysis are not suitable for the detection of infections with multiple HPV types:these will usually give an uninterpretable mix-up of digestion/sequence patterns.In addition,these two methods are less suit-able for high-throughput analyses because they are rel-atively laborious.Finally,RFLP and sequence analysis are less sensitive than hybridization methods because more PCR product is needed to generate a positive sig-nal.As opposed to typing after consensus PCR,it is also possible to type during the reaction,in real time.Var-ious real-time PCR techniques are available(see also Section3.2.2),the best known being molecular bea-cons,thefluorescent5’exonuclease assay(e.g.Taq-man)andfluorescence energy resonance transfer assays (e.g.LightCycler).At present the only real-time assay that allows con-sensus HPV-PCR with simultaneous typing is a molec-ular beacons assay[37].However,the multiplicity of this technique is limited because the current genera-tion of real-time thermocyclers do not allow for more than six differentially labelled probes.Hence,for typ-ing of multiple HPV infections,reverse hybridization methods are preferred over real-time assays.2.1.3.Type-specific PCRIf one is interested in a particular HPV type,type-specific PCR can be applied(described in[25,38] among others).Care should be taken when designing primers,because they may still react with other types if chosen in well-conserved regions.Confirmation of the specificity of type-specific PCRs,as with consensus primer PCRs,can be done by(regular or reverse)filter hybridization or by EIA,but also in real-time[39–41].A great advantage of real-time PCR assays is the pos-sibility to quantify the HPV in the specimen.Several studies have shown that the amount of hrHPV present in a cervical smear(the“viral load”)as measured by real-time PCR is predictive for the presence or develop-ment of high-grade cervical lesions[42–45];reviewed in[46].However,to obtain reliable quantification data DNA extraction is usually necessary,thus increasing the work load.2.1.4.mRNA amplificationSeveral recent studies have shown that hrHPV test-ing can also be done through detection of the viral mR-NA.The most relevant transcripts to look for are those encoding the viral oncoproteins E6and E7.It is hy-pothesized that the presence of viral E6/E7mRNA in a cervical smear has a better positive predictive value for high-grade cervical lesions than the presence of viral DNA,because the E6/E7mRNA represents an active276 A.A.T.P.Brink et al./HPV detection methodsinfection with cell-transforming potential whereas the viral DNA may also be present in clinically irrelevant conditions.Detection of viral mRNA in cervical smears can be done by reverse-transcriptase(RT-)PCR[47]or by nu-cleic acid sequence-based amplification(NASBA)[48]. For the latter assay type,a commercially available sys-tem was recently developed that detects E6/E7tran-screening and triage of women with smears showing minor cellular abnormalities.An alternative method relying on hrHPV transcrip-tion is the detection of HPV-human fusion transcripts that arise from hrHPV genomes integrated into the host cell genome.This method was designated“am-plification of papillomavirus oncogene transcripts”(APOT)[54].Although this method is rather laborious and there-fore not suitable for large-scale hrHPV testing,it can give information that is not obtained using other mR-NA amplification techniques.On the one hand,it re-veals viral integration,an event that is specific for a real precancerous lesion as opposed to a productive viral infection[55].On the other hand,it allows the iden-tification of patient-specific fusion transcripts.This may be useful in monitoring of women who have been treated for high-grade lesions,because it will help to determine whether a high-grade lesion occurring after treatment is a recurrence or even a metastasis of the original lesion[56].2.2.Signal-amplification techniques2.2.1.Liquid-phase signal amplification techniques The best known technique in this category is the commercially available,FDA-approved and clinically validated Hybrid Capture2(HC2)method[57].This method uses a mixture of full-length RNA probes repre-senting hrHPV types16,18,31,33,35,39,45,51,52, 56,58,59,and68.Prior to the test,the clinical samples are heat-alkaline-denatured.Hybridization of one or more of the probes to HPV DNA present in the samples is detected by peroxidase-labelled antibodies that rec-ognize the RNA/DNA hybrid,and visualized by chemi-luminescence.The analytical sensitivity of this method is1pg/ml of cloned HPV16DNA,which corresponds to approximately105HPV16genome copies.This sen-sitivity is lower than that of most target-amplification methods.Because a mixture of probes is used,HC2is at present not suitable for high-resolution typing.Some cross reactivity of the HC2probes with HPV types not represented in the probe mix,including non-oncogenic HPVs,has been described[58].2.2.2.Morphological signal-amplification techniques In addition to the abovementioned methods,hrH-PV detection can be performed by DNA in situ hy-bridization(ISH)to cytological slides[59,60]and his-tological preparations[61].This can be achieved by fluorescent detection[61]or colored substrate deposi-tion and brightfield microscopy.The relatively small size of the HPV genome(7.8kb)and thus of the probe precludes direct detection of hybrids in case of low viral genome copy numbers and therefore some type of signal amplification is generally necessary.A commercially available HPV ISH system uses an indi-rect biotin-streptavidin method(Ventana Inform HPV) which at present lacks sufficient sensitivity to detect high-grade cervical lesions[60].Alternatively,tyra-mide signal amplification(TSA),also known as catal-ysed reporter deposition(CARD)can be used,both in fluorescent[61,62]and brightfield[62]applications. Also for the CARD method,a commercially available system exists(Dako GenPoint).CARD greatly en-hances sensitivity,but in general HPV ISH is too labo-rious to be used in high-throughput HPV testing.3.Clinical specimens for HPV detectionThe kind of clinical specimens available will deter-mine the choice of the HPV detection method.Most PCR methods mentioned above can be done with any type of clinical material,including formalin-fixed, paraffin-embedded tissue and Pap-stained archival smears.The recent focus on liquid-based cytology (LBC)for cervical cancer screening has initiated nu-merous studies evaluating the feasibility of different HPV detection methods on cell suspensions destined for LBC,or cell suspensions remaining after LBC preparation.Some of the pros and cons of different types of clinical specimens are discussed below.A.A.T.P.Brink et al./HPV detection methods2773.1.Clinical specimen types suitable for targetamplification methods3.1.1.Specimen types for PCR-based methodsPCR-based HPV DNA detection methods can gen-erally be applied to all kinds of clinical specimens, provided the nucleic acids contained within are not heavily degraded or cross-linked and no putative PCR-inhibiting substances(anic solvents)are present.Notably,the nucleic acids in formalin-fixed, paraffin-embedded(FFPE)tissue are only amplified in a reliable manner when PCR products are generated that are smaller than150bp due tofixative-induced cross links[63].On the other hand,this type of speci-men generally does not contain large amounts of PCR-inhibiting substances,as demonstrated by the fact that a relatively crude extract can be used in the PCR without laborious extraction protocols[64].As opposed to FFPE material,cervical smears col-lected in LBC media may show excellent preservation of cell morphologyand integrity of nucleic acids.How-ever,to achieve this preservation they usually contain relatively high amounts of organic solvents which will inhibit the PCR and need to be removed by careful ex-traction.An exception are smears collected in Digene Universal Collection Medium(UCM)[65,66],which can be used directly in the PCR after a simple dilution and freeze-thaw protocol(Hesselink et al.,submitted). Pap-stained archival cervical smears can also be used for PCR purposes,but they also need extraction of nu-cleic acids to remove remnants of dyes.In addition, the nucleic acids in this type of specimen may also be degraded due tofixation artefacts.For all above-mentioned samples,careful analysis of the nucleic acid quality is recommended to exclude false negative HPV test results due to DNA degradation and/or PCR inhi-bition.This can be achieved e.g.by a PCR specific for a human single-copy gene[67].For most reliable PCR results,it is recommended to standardize the amount of input DNA in the PCR.It is self-evident that this can only be done using purified nucleic acids.3.1.2.Specimen types for mRNA amplificationmethodsUntil recently,hrHPV mRNA testing on cervical smears was hampered by the lack of suitable collection media.The poor RNA quality of samples collected in solutions such asPBS precluded transcription anal-ysis.However,use of LBC is increasing and this is accompanied by the introduction of preservation media PreservCyt LBC medium are of sufficient quality for HPV RT-PCR[68]and NASBA analysis[69]. Alternatively,cervical smears could be collected in a dedicated RNA-preservation buffer[51],but in gen-eral these contain chaotropic salts such as guanidinium isothiocyanate and thus destroy cellular morphology. When hrHPV mRNA is to be detected in cervical biopsy specimens,these should preferably be snap-frozen to guarantee mRNA integrity.However,several groups claim successful mRNA extraction from FFPE material(reviewed in[70]).Provided the use of proper quality controls,this is of course a very interesting source of material,especially for retrospective studies or in situations where snap-freezing is not an option.3.2.Clinical specimen types suitable forsignal-amplification methodsThe Digene HC2method is FDA-approved for use on cervical smears and cervical biopsies collected in Digene’s Sample Transport Medium(STM).In addi-tion,the FDA-approval of HC2includes use on cer-vical smears collected in Cytyc’s ThinPrep medium. However,because this medium preserves cell integrity as opposed to STM,ThinPrep smears require an addi-tional pre-treatment to obtain full cell lysis and release of nucleic acids[71].With some modifications of the standard protocol,HC2can also be used on the residual material of cervical smears collected in SurePathfluid (i.e.the suspension that remains after the LBC slides have been prepared)[72–74].An intermediate between the commercially available LBC media that preserve cell morphology but require pre-treatment steps prior to HC2,and Digene’s STM that does not require pre-treatment but destroys cell morphology,is Digene Universal Collection Medium (UCM).This medium can be used for sample trans-port[66],does not require additional steps prior to HC2 analysis,and because it preserves cell morphology it also allows LBC[65].4.Considerations regarding test sensitivity andspecificity4.1.Considerations for primary screening and triage In general,the analytical sensitivities of HPV tests depend on the test system(target amplification ver-278 A.A.T.P.Brink et al./HPV detection methodssus signal amplification).The sensitivity of the HC2 method is approximately5,000copies of the HPV genome per reaction well according to the manufac-turer.PCR-based techniques have a much higher ana-lytical sensitivity than HC2,with a slight variation be-tween the methods(reviewed in[46]).For most PCR assays,less than100to1000HPV genome equivalents in a reaction tube are sufficient to generate a positive PCR signal and to enable typing.Despite these high analytical sensitivities,currently available hrHPV tests miss a subset of high-grade CIN lesions[75,76].This may be due to several factors. For PCR-based methods,modification or loss of primer binding sites due to viral integration or naturally oc-curring sequence variants may be a problem.For HC2 where whole-genome probes are used this is generally not an issue,but then the lower analytical sensitivity of HC2might preclude detection of lesions with low viral loads.The ideal hrHPV test used in primary screen-ing should combine the high analytical sensitivity of PCR assays with the possibility to detect all configu-rations and variations of the hrHPV genome,including integrated genomes.Because the intact E6/E7region of the hrHPV genome is necessary for cellular trans-formation[77]and is invariably retained upon integra-tion[78],a PCR system detecting this region would be the method of choice[79].It is self-evident that ampli-fication primers should then be chosen in such a way that they cover all known HPV genomic variations. On the other hand,a very sensitive hrHPV test con-sequently has a low clinical specificity,and as a pri-mary screening tool it would result in a substantial in-crease in referrals for colposcopy and repeat smears.In the Dutch screening population,approximately5.0%of the women is hrHPV DNA-positive by the GP5+/6+ system[18].Because even women with hrHPV DNA-positive normal cytology have a210times greater risk to develop CIN3compared to women with hrHPV negative normal cytology[80],all5.0%hrHPV DNA-positive women would need follow-up.However,only 10%of them will actually develop CIN3[81].This calls for additional stratification of women who have a positive hrHPV DNA test,which can be achieved for example by viral load analysis or HPV mRNA analysis (see Sections2.1.3and2.1.4).4.2.Considerations for situations not involving triage For several research questions,clinical specificity of the hrHPV test is not an issue as it is in population-based screening and triage.Methods with high analytical sensitivity are especially suitable for studies in which any HPV infection should be detected,irrespective of its clinical relevance.Examples are epidemiological studies,and studies to determine vaccination efficacy. It is self-evident that HPV genotyping is desired in such studies.5.ConclusionsAs seen in the previous paragraphs,the aim of the study and the clinical material available will determine the choice of the HPV detection method.In general, consensus PCR followed by reverse hybridization is very sensitive and gives the most extensive typing infor-mation for many kinds of clinical specimens,including those containing multiple infections.In general,it is to be expected that future amplifica-tion systems will become faster and more automated. References[1] A.T.Lorincz and R.M.Richart,Human Papillomavirus DNATesting as an Adjunct to Cytology in Cervical Screening Pro-grams,Arch Pathol Lab Med127(2003),959–968.[2]J.Cuzick,A.Szarewski,H.Cubie et al.,Management of wom-en who test positive for high-risk types of human papillo-mavirus:the HART study,Lancet362(2003),1871–1876. [3] A.G.Bais,M.Rebolj,P.J.Snijders et al.,Triage using HPV-testing in persistent borderline and mildly dyskaryotic smears: Proposal for new guidelines,Int J Cancer116(2005),122–129.[4]G.D.Zielinski,A.G.Bais,T.J.Helmerhorst et al.,HPV testingand monitoring of women after treatment of CIN3:review of the literature and meta-analysis,Obstet Gynecol Surv59 (2004),543–553.[5]M.Scheffner,B.A.Werness,J.M.Huibregtse,A.J.Levineand P.M.Howley,The E6oncoprotein encoded by humanpapillomavirus types16and18promotes the degradation of p53,Cell63(1990),1129–1136.[6]N.Dyson,P.M.Howley,K.Munger and E.Harlow,The hu-man papilloma virus-16E7oncoprotein is able to bind to the retinoblastoma gene product,Science243(1989),934–937.[7] F.X.Bosch,A.Lorincz,N.Munoz,C.J.Meijer and K.V.Shah,The causal relation between human papillomavirus and cervi-cal cancer,J Clin Pathol55(2002),244–265.[8]N.Munoz,F.X.Bosch,S.de Sanjose et al.,Epidemiologicclassification of human papillomavirus types associated with cervical cancer,N Engl J Med348(2003),518–527.[9] E.M.de Villiers,C.Fauquet,T.R.Broker,H.U.Bernard andH.zur Hausen,Classification of papillomaviruses,Virology324(2004),17–27.[10] B.A.Werness,A.J.Levine and P.M.Howley,Association ofhuman papillomavirus types16and18E6proteins with p53, Science248(1990),76–79.[11]J.R.Gage,C.Meyers and F.O.Wettstein,The E7proteins ofthe nononcogenic human papillomavirus type6b(HPV-6b) and of the oncogenic HPV-16differ in retinoblastoma protein binding and other properties,J Virol64(1990),723–730.A.A.T.P.Brink et al./HPV detection methods279[12]Y.Kirii and T.Matsukura,Nucleotide sequence and phylo-genetic classification of human papillomavirus type67,Virus Genes17(1998),117–121.[13]N.Munoz,F.X.Bosch,X.Castellsague et al.,Against whichhuman papillomavirus types shall we vaccinate and screen?The international perspective,Int J Cancer111(2004),278–285.[14]M.Schiffman,M.J.Khan,D.Solomon et al.,A study of theimpact of adding HPV types to cervical cancer screening and triage tests,J Natl Cancer Inst97(2005),147–150.[15] D.A.Galloway,Papillomavirus vaccines in clinical trials,Lancet Infect Dis3(2003),469–475.[16] A.H.Beskow,M.Moberg and U.B.Gyllensten,HLA class IIallele control of HPV load in carcinoma in situ of the cervix uteri,Int J Cancer117(2005),510–514.[17] A.H.Beskow and U.B.Gyllensten,Host genetic control ofHPV16titer in carcinoma in situ of the cervix uteri,Int J Cancer101(2002),526–531.[18]N.W.J.Bulkmans,L.Rozendaal,P.J.Snijders et al.,POBAS-CAM,a population-based randomised controlled trial for im-plementation of high-risk HPV testing in cervical screening;Design,methods and baseline data of44,102women,Int JCancer110(2003),94–101.[19]N.W.Bulkmans,M.C.Bleeker,J.Berkhof et al.,Prevalence oftypes16and33is increased in high-risk human papillomaviruspositive women with cervical intraepithelial neoplasia grade2 or worse,Int J Cancer117(2005),177–181.[20]S.Bulk,J.Berkhof,N.W.Bulkmans et al.,Preferential risk ofHPV16for squamous cell carcinoma and of HPV18for ade-nocarcinoma of the cervix compared to women with normalcytology in The Netherlands,Br J Cancer94(2006),171–175.[21]M.J.Khan,P.E.Castle,A.T.Lorincz et al.,The elevated10-year risk of cervical precancer and cancer in women with human papillomavirus(HPV)type16or18and the possibleutility of type-specific HPV testing in clinical practice,J Natl Cancer Inst97(2005),1072–1079.[22]P.E.Castle,D.Solomon,M.Schiffman and C.M.Wheeler,Human papillomavirus type16infections and2-year absoluterisk of cervical precancer in women with equivocal or mild cytologic abnormalities,J Natl Cancer Inst97(2005),1066–1071.[23]P.E.Gravitt,C.L.Peyton,R.J.Apple and C.M.Wheeler,Geno-typing of27human papillomavirus types by using L1consen-sus PCR products by a single-hybridization,reverse line blot detection method,J Clin Microbiol36(1998),3020–3027. [24]L.M.Tieben,J.ter Schegget,R.P.Minnaar et al.,Detection ofcutaneous and genital HPV types in clinical samples by PCR using consensus primers,J Virol Methods42(1993),265–279.[25] A.J.van den Brule,R.Pol,N.Fransen-Daalmeijer et al.,GP5+/6+PCR followed by reverse line blot analysis enablesrapid and high-throughput identification of human papillo-mavirus genotypes,J Clin Microbiol40(2002),779–787. [26]L.Gregoire,M.Arella,J.Campione-Piccardo and W.D.Lancaster,Amplification of human papillomavirus DNA se-quences by using conserved primers,J Clin Microbiol27(1989),2660–2665.[27] B.Kleter,L.J.van Doorn,L.Schrauwen et al.,Developmentand clinical evaluation of a highly sensitive PCR-reverse hy-bridization line probe assay for detection and identification of anogenital human papillomavirus,J Clin Microbiol37(1999), 2508–2517.[28]P.E.Gravitt,C.L.Peyton,T.Q.Alessi et al.,Improved ampli-fication of genital human papillomaviruses,J Clin Microbiol38(2000),357–361.[29]J.Monsonego,J.M.Bohbot,G.Pollini et al.,Performance ofthe Roche AMPLICOR human papillomavirus(HPV)test inprediction of cervical intraepithelial neoplasia(CIN)in women with abnormal PAP smear,Gynecol Oncol99(2005),160–168.[30]M.V.Jacobs,P.J.Snijders,A.J.van den Brule et al.,A generalprimer GP5+/GP6(+)-mediated PCR-enzyme immunoassaymethod for rapid detection of14high-risk and6low-risk human papillomavirus genotypes in cervical scrapings,J Clin Microbiol35(1997),791–795.[31] B.Kleter,L.J.van Doorn,J.ter Schegget et al.,Novel short-fragment PCR assay for highly sensitive broad-spectrum de-tection of anogenital human papillomaviruses,Am J Pathol 153(1998),1731–1739.[32]N.H.Cho,H.J.An,J.K.Jeong et al.,Genotyping of22humanpapillomavirus types by DNA chip in Korean women:com-parison with cytologic diagnosis,Am J Obstet Gynecol188 (2003),56–62.[33]M.Schmitt,I.G.Bravo,P.J.Snijders et al.,Bead-based multi-plex genotyping of human papillomaviruses,J Clin Microbiol 44(2006),504–512.[34]O.Forslund,A.Antonsson,P.Nordin,B.Stenquist and B.G.Hansson,A broad range of human papillomavirus types de-tected with a general PCR method suitable for analysis of cu-taneous tumours and normal skin,J Gen Virol80(Pt9)(1999),2437–2443.[35]V.Shamanin,M.Glover,C.Rausch et al.,Specific types ofhuman papillomavirus found in benign proliferations and car-cinomas of the skin in immunosuppressed patients,CancerRes54(1994),4610–4613.[36]O.Lungu,T.C.Wright Jr.and S.Silverstein,Typing of hu-man papillomaviruses by polymerase chain reaction amplifi-cation with L1consensus primers and RFLP analysis,MolCell Probes6(1992),145–152.[37]K.Szuhai,E.Sandhaus,S.M.Kolkman-Uljee et al.,A novelstrategy for HPV detection and genotyping with SybrGreen and Molecular Beacon PCR,Am J Pathol159(2001),1651–1660.[38]J.M.Walboomers,M.V.Jacobs,M.M.Manos et al.,Humanpapillomavirus is a necessary cause of invasive cervical cancer worldwide,J Pathol189(1999),12–19.[39] A.Josefsson,K.Livak and U.Gyllensten,Detection and quan-titation of human papillomavirus by using thefluorescent5’exonuclease assay,J Clin Microbiol37(1999),490–496. [40] A.T.Hesselink,A.J.van den Brule,Z.M.Groothuismink etal.,Comparison of three different PCR methods for quanti-fying human papillomavirus type16DNA in cervical scrape specimens,J Clin Microbiol43(2005),4868–4871.[41]M.Moberg,I.Gustavsson and U.Gyllensten,Real-time PCR-based system for simultaneous quantification of human papil-lomavirus types associated with high risk of cervical cancer,J Clin Microbiol41(2003),3221–3228.[42] A.M.Josefsson,P.K.Magnusson,N.Ylitalo et al.,Viral loadof human papilloma virus16as a determinant for developmentof cervical carcinoma in situ:a nested case-control study, Lancet355(2000),2189–2193.[43]N.Ylitalo,P.Sorensen,A.M.Josefsson et al.,Consistent highviral load of human papillomavirus16and risk of cervical carcinoma in situ:a nested case-control study,Lancet355 (2000),2194–2198.[44]M.van Duin,P.J.Snijders,H.F.Schrijnemakers et al.,Hu-man papillomavirus16load in normal and abnormal cervical scrapes:an indicator of CIN II/III and viral clearance,Int J Cancer98(2002),590–595.。