Hepa2003 JNK drives cyclin D1 expression and proliferation during liver regeneration
- 格式:pdf
- 大小:418.21 KB
- 文档页数:9

2003年度第一批排放合格车型目录(摩托车)1、重庆力帆轰达实业(集团)有限公司LF100T XGJ100T LF150-3A XGJ150-3A 机外净化器:LCLF50QT-5 XGJ50QT-5发动机:1P39FMB机外净化器:LCXGJ150-F LF150-A发动机:161FMJ机外净化器:LC(德国依米泰克公司EMITEC)XGJ150-E LF150-E发动机:162FMJ机外净化器:LC(德国依米泰克公司EMITEC)LF50QT-2A LF50QT-3 LF50QT-6发动机:1P39QMB机外净化器:LC-2A(山西利民)LF110-F LF110-G LF110-H LF110-2F LF110-2GLF110-7A LF110-8 LF110-8A LF110-9A LF110-10LF110-11发动机:1P52FMH机外净化器:LC-2(常州力扬)LF150-B LF150-2 LF150-3 LF150-4A LF150-6LF150-7发动机:162FMJ机外净化器:LC-3(常州力扬)LF150-10发动机:161MJ机外净化器:LC-3(庄信万丰)LF250发动机:2V49FMM机外净化器:LC-4(桂林利凯特)2、重庆力帆轰达实业(集团)有限公司重庆新感觉摩托车有限公司LF90 XGJ90发动机:1P47FMF机外净化器:LC-2(常州力扬精机有限公司)LF100 LF100-A LF100-B LF100-C LF100-DLF100-2 LF100-2A F100-3A LF100-3B LF100-4CLF100-5 LF100-5A LF100-6 LF100-7 LF100-8LF100-8A LF100-9 LF100-9A LF100-10 LF100-11XGJ100 XGJ100-A XGJ100-B XGJ100-C XGJ100-DXGJ100-2 XGJ100-2A XGJ100-2B XGJ100-3A XGJ100-3B XGJ100-4 XGJ100-5A XGJ100-5B XGJ100-6 XGJ100-7XGJ100-8 XGJ100-8A XGJ100-9 XGJ100-9A XGJ100-10 XGJ100-11发动机:1P50FMG机外净化器:LC-2(常州力扬精机有限公司)LF100T-2 LF100T-2D LF100T-2F XGJ100T-2发动机:1P50FMG(1P50QMG)机外净化器:LC-2A(常州力扬精机有限公司)LF110 LF110-A LF110-B LF110-C LF110-DLF110-3 LF110-E XGJ110 XGJ110-A XGJ110-B XGJ110-C XGJ110-D XGJ110-2E XGJ110-3A XGJ110-4A XGJ110-5A XGJ110-6A XGJ110-7A XGJ110-9A发动机:152FMH机外净化器:LC-2(常州力扬精机有限公司)LF110-2 XGJ110-2发动机:1P53FMH机外净化器:LC-2(常州力扬精机有限公司)LF125T-2 LF125T-2A LF125T-2B LF125T-2C LF125T-2D LF125T-2E LF125T-2F LF125T-3 LF125T-4 LF125T-5 LF125T-6 LF125T-7 LF125T-8 LF125T-9 LF125T-10 LF125T-11 LF125T-12 LF125T-13 LF125T-14 LF125T-15 LF125T-16 XGJ125T XGJ125T-2 XGJ125T-2A XGJ125T-2B XGJ125T-2C XGJ125T-2D XGJ125T-2E XGJ125T-3 XGJ125T-4XGJ125T-5 XGJ125T-6 XGJ125T-8 XGJ125T-9 XGJ125T-10 XGJ125T-16发动机:1P52QMI机外净化器:LC-3A(常州力扬精机有限公司)LF125GY LF125-B LF125-3 LF125-3A LF125-4LF125-5 LF125-6 LF125-6A LF125-7 LF125-8LF125-8B LF125-9 LF125-15 XGJ125 XGJ125-B XGJ125-E XGJ125-H XGJ125-2 XGJ125-3 XGJ125-4 XGJ125-5A XGJ125-6 XGJ125-6A XGJ125-7A XGJ125-8 XGJ125-9 XGJ125GY-A发动机:156FMI机外净化器:LC-3(常州力扬精机有限公司)LF150 LF150-F LF150-3 LF150-4 LF150-5XGJ150 XGJ150-A XGJ150-3 XGJ150-3B XGJ150-4A XGJ150-5发动机:161FMJ机外净化器:LC-3(常州力扬精机有限公司)XGJ50QT-2A XGJ50QT-3 XGJ50QT-6发动机:1P39QMB机外净化器:LC-2A(山西利民)XGJ110-E XGJ110-F XGJ110-G XGJ110-H XGJ110-7 XGJ110-8 XGJ110-8A XGJ110-9 XGJ110-11 XGJ110-10发动机:1P52FMH机外净化器:LC-2(常州力扬)XGJ150-4 XGJ150-B XGJ150-2 XGJ150-3B 发动机:162FMJ机外净化器:LC-3(常州力扬)XGJ150-10发动机:161MJ机外净化器:LC-3(庄信万丰)XGJ250发动机:2V49FMM机外净化器:LC-4(桂林利凯特)3、浙江钱江摩托股份有限公司QJ100T-5F发动机:QJ147QMG机外净化器:YR-MC100(台湾力扬工业股份有限公司)QJ125-4发动机:QJ157FMI机外净化器:YR-MA100(台湾力扬工业股份有限公司)QJ100-2 QJ100-2D QJ100-2A QJ100-2B QJ100-2C发动机:QJ150FMG-2机外净化器:YR-MC100(台湾力扬)QJ100-3 QJ100-3D QJ100-3A QJ100-3B QJ100-3C发动机:QJ150FMG机外净化器:YR-MC100(台湾力扬)QJ125-F QJ125-5 QJ125-KQJ125-P QJ125-2 QJ125-U发动机:QJ157FMI机外净化器:YR-MA100(台湾力扬)QJ50QT-8 QJ50QT-10 QJ50QT-10A QJ50QT-10B 发动机:QJ141QMB机外净化器:YR-MB100QJ100T-5 QJ100T-5A QJ100T-5B QJ100T-5CQJ100T-5D QJ100T-5E QJ100T-5G QJ100T-7QJ100T-3发动机:QJ147QMG机外净化器:YR-MC100QJ110 QJ110-A QJ110-2 QJ110-2AQJ110-2B QJ110-2C QJ110-5 QJ110-8发动机:QJ153FMH机外净化器:YR-MC100QJ125T QJ125T-A QJ125T-B QJ125T-DQJ125T-E QJ125T-G QJ125T-3A QJ125T-3BQJ125T-3C QJ125T-3D QJ125T-3E QJ125T-6QJ125T-8 QJ125T-9 QJ125T-10 QJ125T-15QJ125T-16 QJ125T-17 QJ125T-18发动机:QJ153QMI机外净化器:YR-MA100QJ150 QJ150-A QJ150-B QJ150-D 发动机:QJ162FMJ机外净化器:YR-MA100QJ250-3 QJ250-F发动机:QJ253FMM机外净化器:YR-MA100QJ100-4 QJ100-4A QJ100-4B QJ100-9 QJ100-E 发动机:QJ150FMG-3机外净化器:YR-MC100(台湾力扬)QJ110-6 QJ110-7 QJ110-10发动机:QJ153FMH-2机外净化器:YR-MC100(台湾力扬)QJ125 QJ125-A QJ125-C QJ125-DQJ125-N QJ125-3 QJ125-4A QJ125-L发动机:QJ157FMI机外净化器:YR-MA100(台湾力扬)QJ125T-5 QJ125T-4A QJ125T-4BQJ125T-12 QJ125T-12A QJ125T-11发动机:QJ153QMI-3机外净化器:YR-MA100(台湾力扬)QJ150-C QJ150-J QJ150-K发动机:QJ247FMJ机外净化器:YR-MA100(台湾力扬)QJ100T-9发动机:QJ147QMG机外净化器:YR-MC100(台湾力扬)QJ110-9发动机:QJ150FMH机外净化器:YR-MC100(台湾力扬)QJ90-2发动机:QJ147FMF机外净化器:YR-MC100(台湾力扬)QJ100-B发动机:QJ150FMG机外净化器:YR-MC100(台湾力扬)QJ100T-3 QJ100T-5H发动机:QJ147QMG机外净化器:YR-MC100 (钱江集团有限公司)QJ125T-22 QJ125T-3F QJ125T-7QJ125T-18A QJ125T-28 QJ125T-28A发动机:QJ153QMI机外净化器:YR-MA100 (钱江集团有限公司)QJ150-3A QJ150-3 QJ150-3B QJ150-M发动机:QJ162FMJ机外净化器:YR-MA100 (钱江集团有限公司)QJ250T-2发动机:QJ172MM机外净化器:YR-MA100 (钱江集团有限公司)4、中国嘉陵工业股份有限公司(集团)JH125-D(JH125D) JH125-2(JH125D-A) JH125-2A(JH125D-B) JH125-G JH125E JH125-17JH125-20 JH125-F JH125-19发动机:JL156FMI-2(156FM-2)JL156FMI-5机外净化器:CN504(英国庄信万丰)JH125-A JH125-B JH125发动机:JL156FMI(156FM)机外净化器:CN504(英国庄信万丰)JL90-A JL90-B(JD90)JL90-C发动机:JL1P47FMF-2(147FM-4)机外净化器:CN503(英国庄信万丰)JH70-B(JH7011) JH70-A(JH701) JH70-C(JH70111) JH70-J 发动机:JL1P47FMD(147FM)机外净化器:CN502(英国庄信万丰)JH70-D发动机:JL1P47FMD-5机外净化器:CN502(英国庄信万丰)JL110-7 JL110-3 JL110-4 JL110-8 JL110-9 JL110-10 发动机:JL1P50FMH(JL1P52FMH-2或JL1P50FMH-2)机外净化器:CN503JH125-16 JH125-F JH125-12 JH125-18JH125-19 JH125-5 JH125-2A(JH125D-B)发动机:JL156FMI-5机外净化器:CN504JL50QZC(CJ50ZC)JL50QT-9发动机:JL(JH)1E39QMB-2(1E39FM-3)机外净化器:MC.975.048.20JH90-A JH90-C发动机:JL1P47FMF-3(147FM-6)机外净化器:CN503JH100-C JH100 JH100-2 JH100-5 JH100-7 JH100-B 发动机:JL1P50FMG(150FM)机外净化器:CN503JH110-7机外净化器:CN503JH110-2发动机:JL152FMH-4机外净化器:CN503JH125GY(JH125LⅠ)JH125GY-A(JH125LⅡ)JH125GYJ(JH125LJ)发动机:JL156FMI-3(156FM-1)机外净化器:CN504JH150E-2(JH150T)JH150-3(JH150D-3)JH150E-B JH150-2(JH150D-2)发动机:JL161FMJ-2(161FM-2)机外净化器:CN505JL50QT-21发动机:JL1P39FMB-2机外净化器:CN502(英国庄信万丰公司)JL100-3 JL100-B JL100 JL100-4 JL100-7 发动机:JL1P50FMG-2(150FM-1)机外净化器:CN503(英国庄信万丰公司)JL50QZC-2A发动机:JL1P39QMB-2机外净化器:CN510JL50QZC-5发动机:JL1P44FMB机外净化器:CN511JL50QZC-A发动机:JL1E39QMB-2机外净化器:MC.975.048.20JH100-20A机外净化器:CN503JL110-11 JL110-12发动机:JL1P53FMH 或JL1P50FMH机外净化器:CN503JH125-31发动机:JL244FMI机外净化器:CN504JH250 JH250-2 JH250E-3发动机:JL253FMM-2机外净化器:CN506JH250E-5发动机:JL253FMM-3机外净化器:CN506JH125-27发动机:JL156FMI-5或JL156FMI-6机外净化器:CN504JH125-H发动机:JL156FMI-5机外净化器:CN5045、重庆宗申海陵机车有限责任公司ZS100-9 ZS100-2发动机:ZS150FMG-B机外净化器:ZS4IA(常州力扬精机有限公司)ZS100-16 ZS100-21 ZS100-22 发动机:ZS150FMG-2机外净化器:ZS4IA(常州力扬精机有限公司)ZS100发动机:ZS150FMG(ZS150FM-C)机外净化器:ZS4IA(常州力扬精机有限公司)ZS100-8发动机:ZS150FMG-A(ZS150FM-1D)机外净化器:ZS4IA(常州力扬精机有限公司)ZS100-4 ZS100-5 ZS100-6 ZS100-10ZS100-14 ZS100-19 ZS100-23 ZS100-12发动机:ZS150FM-1L机外净化器:ZS4IA(常州力扬精机有限公司)ZS125-2 ZS125-5 ZS125-5B ZS125-8 ZS125-9ZS125-11 ZS125-12 ZS125-13 ZS125-14 ZS125-17 ZS125-21 ZS125-22 ZS125-29 ZS125-3 ZS125-4ZS125-7 ZS125-6 ZS125-18 ZS125-19 ZS125-20 ZS125-25 ZS125-26 ZS125发动机:ZS156FMI机外净化器:ZS4IA(常州力扬精机有限公司)ZS100-23发动机:ZS150FMG-3A机外净化器:ZS4GA(常州力扬精机有限公司)ZS110-9 ZS110 ZS110-6 ZS110-10 ZS110-16 ZS110-18 ZS110-19 ZS110-20 HL110-2 HL110-3 HL110-4 HL110-5 LZX110 LZX110-6 LZX110-7 发动机:ZS150FMH机外净化器:ZS4HA(常州力扬精机有限公司)ZS125T-4 ZS125T ZS125T-2 ZS125T-3 ZS125T-5 ZS125T-7 ZS125T-8 ZS125T-9 ZS125T-10 ZS125T-11 ZS125T-12 ZS125T-13 ZS125T-15 ZS125T-16 ZS125T-18 ZS125T-22 ZS125T-23 ZS125T-24 HL125T HL125T-4 HL125T-5 HL125T-6 HL125T-7 HL125T-8 HL125T-9 HL125T-10 LZX125T LZX125T-2 LZX125T-3 LZX125T-4 LZX125T-5 LZX125T-6 LZX125T-7 LZX125T-8 LZX125T-9 LZX125T-10发动机:ZS1P52QMI机外净化器:GR7(桂林利凯特环保实业有限公司)ZS90-2 HL90 LZX90 LZX90-2发动机:ZS147FMF-A(ZS147FM-2)机外净化器:ZS4FA(常州力扬精机有限公司)ZS90发动机:ZS147FMF机外净化器:ZS4FA(常州力扬精机有限公司)ZS90-3发动机:ZS147FMF-C机外净化器:ZS4FA(常州力扬精机有限公司)ZS150 ZS150-2 ZS150-6 ZS150-8 ZS150-12 HL150 LZX150 LZX150-3 ZS150-3发动机:ZS161FMJ机外净化器:ZS4JA(常州力扬精机有限公司)HL100-8 HL100-11 HL100-13 LZX100-6LZX100-13 LZX100-14 LZX100-11发动机:ZS150FMG-B机外净化器:ZS4IA(常州力扬精机有限公司)HL125-3 HL125-6 HL125-7 HL125-8 HL125-9 HL125-10 HL125-13 LZX125 LZX125-2 LZX125-5 LZX125-5B LZX125-8 LZX125-9 LZX125-10 LZX125-11 LZX125-12 LZX125-16发动机:ZS156FMI机外净化器:ZS4IA(常州力扬精机有限公司)ZS100-4发动机:ZS150FMG(旧型号ZS150FM-1L)机外净化器:ZS4IA(常州力扬精机有限公司)ZS100-9发动机:ZS150FMG-B(旧型号ZS150FM-C)机外净化器:ZS4IA(常州力扬精机有限公司)ZS125发动机:ZS156FMI(旧型号ZS156FM-CG)机外净化器:ZS4IA(常州力扬精机有限公司)6、建设工业(集团)有限责任公司JS125 JS125-3 JS125-10JS125-12A JS125-13 JS125-12发动机:JS156FMI机外净化器:JC(德国依米泰克公司EMITEC)JY110发动机:JY1P49FMH机外净化器:JC-2(德国依米泰克公司EMITEC)JS100-8发动机:JS150FMG-4机外净化器:JC-2(德国依米泰克公司EMITEC)JS100-6A JS100-2 JS100-3 JS100-2A发动机:JS150FMG-5机外净化器:JC-4JS150-A JS150-GA JS150J-A发动机:JYM157FMJ-A机外净化器:JC-3JS100-5发动机:JS150FMG-2机外净化器:JC-57、江苏新世纪摩托车有限公司XSJ125-8 XSJ125-2H XSJ125-6 XSJ125-9HF125 HF125-A HF125-7A HF125-9AHF125-2D XSJ125-7B XSJ125-9A发动机:157FMI机外净化器:LB (德国依米泰克)XSJ150-A XSJ150-2 XSJ150-2A XSJ150-2B HF150 HF150-2A HF150-3A HF150-3 HF150-2B发动机:162FMJ机外净化器:LB (德国依米泰克)XSJ125-7A发动机:157FMI机外净化器:LB(德国依米泰克公司)XSJ150-4发动机:162FMJ机外净化器:LB(德国依米泰克公司)XSJ100-B XSJ100-3 XSJ100-2 XSJ100-2A XSJ100-2B XSJ100-7A XSJ100-7B XSJ100-7 XSJ100-8 XSJ100-10D XSJ100-5 HF100-B HF100-10D HF100-7A HF100-10A发动机:150FMG机外净化器:XSJ41A(常州力扬)XSJ110-A XSJ110-3 XSJ110-C XSJ110-2 XSJ110-7 XSJ110-8 XSJ110-6 XSJ110-5 XSJ110 XSJ110-10 HF110-9发动机:152FMH机外净化器:XSJ41B(常州力扬)XSJ125T-B XSJ125T-A XSJ125T-C XSJ125T-D XSJ125T-E XSJ125T-F XSJ125T-G XSJ125T-H XSJ125T-2 XSJ125T-3 XSJ125T-5 XSJ125T-8A XSJ125T-8B XSJ125T-10 HF125T-A HF125T-B HF125T-C发动机:152QMI机外净化器:XSJ41E(常州力扬)XSJ125T发动机:152QMI机外净化器:XSJ41E(常州力扬精机有限公司)HF250T发动机:172MM机外净化器:XSJ41B(常州力扬精机有限公司)HF250发动机:253FMM机外净化器:XSJ41B(常州力扬精机有限公司)HF150-2B发动机:247FMJ机外净化器:XSJ41B(常州力扬精机有限公司)XSJ50QT-B XSJ50QT XSJ50QT-8 XSJ50QT-9A XSJ50QT-10 XSJ50QT-3 HF50QT XSJ50QT-9 XSJ50QT-7 发动机:139QMB机外净化器:EMI100(德国依米泰克公司)XSJ125-7 XSJ125-7C XSJ125-9B发动机:157FMI机外净化器:LB(德国依米泰克公司)XSJ110—11发动机:152FMH机外净化器:XSJ41B(常州力扬)XSJ125T—10A XSJ125T—11 XSJ125T—12 发动机:152QMI机外净化器:XSJ41E(常州力扬)XSJ250—A发动机:253FMM机外净化器:XSJ41B(常州力扬)XSJ150-2C XSJ150-2D发动机:XSJ162FMJ机外净化器:LB(德国依米泰克公司)XSJ125T-12A发动机:XSJ152QMI机外净化器:XSJ41E(常州力扬精机有限公司)XSJ150-3B发动机:XSJ162FMJ机外净化器:LB(德国依米泰克)8、上海嘉陵车业有限公司JL125T-18 JL125T-5 JL125T-6 JL125T-10JL125T-28 JL125T-3A JL125T-33发动机:JL1P52QMI机外净化器:CN504(北京中和大成)JL50QT-18 JL50QT-10 JL50QT-28发动机:JL1P39QMB-2机外净化器:CN502(北京中和大成)JL100T-13发动机:JL1P50QMG机外净化器:HR-OEM-45-125 (台州市远大机械有限公司)9、天津富士达摩托车制造有限公司BT50QT-7A BT50QT-1B BT50QT-2A BT50QT-5BBT50QT-5B1 BT50QT-5BQ BT50QT-6B BT50QT-12发动机:BT1P39QMB净化器型号:BT4IA(常州力扬精机有限公司)BT100T BT100T-B BT100T-3 BT100T-4A BT100T-6 发动机:BT1P50QMG净化器型号:BT4IA(常州力扬精机有限公司)BT125TA BT125T BT125TA-B BT125T-1 BT125T-2BT125T-3 BT125T-3A BT125T-4 BT125T-5 BT125T-6BT125T-7 BT125T-8 BT125T-9 BT125T-9A BT125T-10发动机:BT1P52QMI净化器型号:BT4IA常州力扬精机有限公司BT100-12B BT100 BT100-1 BT100-2 BT100-3BT100-4 BT100-5 BT100-5B BT100-5C BT100-6BT100-7 BT100-7B BT100-8 BT100-9 BT100-10BT100-12 BT100-12A BT100-13 BT100-15 BT100-16BT100-17 BT100-18 BT100-23 BT100-24 BT100-26A BT100-28 BT100-30发动机:BT1P50FMG机外净化器:BT4IA(常州力扬精机有限公司)BT100-29 BT100-A BT100-4A BT100-7A BT100-8A BT100-9A BT100-19 BT100-20 BT100-21 BT100-22 BT100-26 BT100-27发动机:BT150FMG机外净化器:BT4IA(常州力扬精机有限公司)BT125-5C BT125 BT125A BT125GY BT125-2 BT125-2A BT125-3 BT125-3J BT125-4 BT125-5 BT125-6 BT125-6B BT125-7 BT125-8 BT125-11 BT125-12 BT125-15 BT125-16 BT125-17 BT125-19 BT125-20 BT125-22 BT125-26 BT125-18发动机:BT156FMI机外净化器:BT4IA(常州力扬精机有限公司)10、广州三雅摩托车有限公司(原北京凯特摩托车厂)KT125ZH发动机:LC156 FMI机外净化器:GR7A-M(桂林利凯特环保实业有限公司)KT100ZH-3发动机:LC150FMG-3机外净化器:GR7A-M(桂林利凯特环保实业有限公司)11、重庆银钢节能摩托车制造有限公司YG100-6 YG100-9发动机:YG1P50FMG机外净化器:YGC-01(德国依米泰克公司EMITEC)YG125-2 YG125-2A YG125-2B YG125-5发动机:YG156FMI机外净化器:YGC-02(德国依米泰克公司EMITEC)YG150-2 YG150-8发动机:YG161FMJ机外净化器:YGC-03(德国依米泰克公司EMITEC)YG100-6 YG100-3发动机:YG1P50FMG机外净化器:YGC-01(德国依米泰克公司EMITEC)YG125-2A YG125-3 YG125-6 YG125-10YG125-11发动机:YG156FMI机外净化器:YGC-02(德国依米泰克公司EMITEC)YG150-2 YG150-9发动机:YG161FMJ机外净化器:YGC-03(德国依米泰克公司EMITEC)12、浙江豪情摩托车有限公司HQ100T-10A HQ100T-10C HQ100T-10 MR100T-9发动机:HQ1P52QMG机外净化器:3060MC(常州力扬)HQ125T HQ125T-5A HQ125T-9 HQ125T-3MR125T-2 MR125T MR125T-9 MR125T-20MR125T-17 MR125T-5A BL125T BL125T-3BL125T-3C BL125T-5A BL125T-8B BL125T-9发动机:HQ152QMI机外净化器:HR-OEM-4S-125(浙江华荣)HQ150T-8 HQ150T HQ150T-2 HQ150T-6 HQ150T-6B HQ150T-7 HQ150T-8B HQ150T-9 HQ150T-10发动机:HQ157QMJ机外净化器:HR-OEM-4S-150MR150T MR150T-2 MR150T-6 MR150T-7MR150T-8 MR150T-9 MR150T-10发动机:HQ157QMJ机外净化器:HR-OEM-4S-150(浙江华荣)HQ125 HQ125-B HQ125-C HQ125-DHQ125-E HQ125-2 HQ125-2B HQ125-2C HQ125-2E HQ125-5 MR125 MR125-2MR125-2B MR125-2E MR125-5 MR125-6MR125-7 MR125-18 BL125 BL125-EBL125-2 BL125-2B BL125-15发动机:HQ157FMI机外净化器:LH-1-M-63.5HQ150 HQ150-2 HQ150-3 HQ150-4HQ150-5 HQ150-9 HQ150-10 HQ150-16MR150 MR150-2 MR150-3 MR150-4MR150-5 MR150-9 MR150-15 BL150BL150-11发动机:HQ162FMJ机外净化器:LH-1-M-63.5BL125T-4 BL125T-7 BL125T-8 BL125T-11 BL125T-15 发动机:HQ152QMI机外净化器:HR-OEM-4S-125BL150T BL150T-3发动机:HQ157QMJ机外净化器:HR-OEM-4S-15013、上海雷雅摩托车有限公司(原北京雷雅摩托车有限公司)KM125T KM125T-3 KM125T-5A K M125T-8B发动机:152QMI机外净化器:HR-OEM-4S-125(浙江华荣)LY125T-3 LY125T-4 LY125T-6 LY125T-9 LY125T-13发动机:L Y152QMI机外净化器:HR-OEM-4S-125(浙江华荣)LY125T LY125T-10 LY125T-12 LY125T-16LY125T-20 LY125T-22发动机:L Y152QMI机外净化器:LH-1-M-48LY125-2 LY125-3 LY125-3B LY125-4LY125-6 LY125-7 LY125-7A LY125-19发动机:L Y157FMI机外净化器:LH-1-M-63.5LY150 LY150-2 LY150-3 LY150-4LY150-5 LY150-6 LY150-16发动机:L Y162FMJ机外净化器:LH-1-M-63.5LY150T LY150T-A LY150T-3 LY150T-3A LY150T-5 LY150T-7 LY150T-8发动机:L Y157QMJ机外净化器:LH-1-M-48KM125T-8B KM125T KM125T-2 KM125T-3KM125T-5A KM125T-9 KM125T-15发动机型号:KM152QMI机外净化器:MJX(无锡威孚力达催化净化有限公司)KM150-3 KM150-8 KM150-12发动机型号:KM247FMJ机外净化器:MJX(无锡威孚力达催化净化有限公司)KM150-11发动机型号:KM162FMJ机外净化器:MJX(无锡威孚力达催化净化有限公司)14、中国轻骑集团日照摩托车公司XF125T-9发动机:1P52QMI机外净化器:BT4IA(常州力杨精机有限公司)15、隆鑫集团有限公司LX90 LX90-A发动机:LC147FMF机外净化器:GR7A-M(桂林利凯特环保实业有限公司)LX100-A LX100-10 LX100-2A LX100-8 LX100-9 LX100-E LX100-10A LX100-7 LX100-8A LX100-B LX100-F HS100 HS100-2 HS100-7 HS100-12 HS100-10 HS100-5 HS100-8发动机:LC150FMG-1-A或LC150FMG-1机外净化器:GR7A-M(桂林利凯特环保实业有限公司)LX100-3 LX100-3A LX100-4 LX100-3B LX100-4A HS100-3 HS100-6 HS100-4 HS100-11发动机:LC150FMG-1-B机外净化器:GR7A-M(桂林利凯特环保实业有限公司)LX110-3A LX110-3 LX110-3C LX110-3D LX110-3B LX110-4 LX110-4A HS110 HS110-2 HS110-3HS110-4 HS110-6 HS110-16 HS110-17发动机:LC152FMH机外净化器:GR7A-M(桂林利凯特环保实业有限公司)LX110-2A LX110-2 LX110 LX110-5 LX110-5ALX110-7 LX110-7A LX110-8 LX110-9 LX110-9ALX110-A LX110-C LX110-D LX110-F HS110-10HS110-11 HS110-12 HS110-13 HS110-5 HS110-7HS110-8 HS110-9 HS110-14 HS110-15 HS110-18HS110-19 HS110-20 HS110-21发动机:LC153FMH机外净化器:GR7A-M(桂林利凯特环保实业有限公司)LX125-A LX125-7 LX125-11 LX125-B LX125-3B LX125-J HS125 HS125-4 HS125-17 HS125-23 发动机:LC156FMI或LC156FMI-A机外净化器:GR7A-M(桂林利凯特环保实业有限公司)LX125-C LX125 LX125-10 LX125-10A LX125-2 LX125-2B LX125-2C LX125-2D LX125-3A LX125-4 LX125-5A LX125-6 LX125-8 LX125-8A LX125-E HS125-11 HS125-14 HS125-15 HS125-16 HS125-18 HS125-19 HS125-21 HS125-22 HS125-24 HS125-2 HS125-3 HS125-5 HS125-6 HS125-7 HS125-8 发动机:LC157FMI-A或LC157FMI机外净化器:GR7A-M(桂林利凯特环保实业有限公司)LX150-6A LX150 LX150-2 LX150-5 LX150-6LX150-A LX150-C LX150-E LX150-F LX150-2BLX150-2C LX150-7 LX150-7A LX150-8 LX150-10HS150-11 HS150-12 HS150-13 HS150-3 HS150-4HS150-5 HS150-6 HS150-7 HS150-8 HS150-9HS150 HS150-2发动机:LC161FMJ机外净化器:GR7A-M(桂林利凯特环保实业有限公司)LX150-6B LX150-H发动机:LC161FMJ机外净化器:GR7A-M(桂林利凯特环保实业有限公司)16、吉利集团有限公司JL50QT-13 JL50QT-4 JL50QT-6 JL50QT-8 JL50QT-9 JL50QT-10 JL50QT-11 JL50QT-12 JL50QT-14 JL50QT-17 JL50QT-19 JL50QT-20 JL50QT-22 JL50QT-23 JL50QT-24 JL50QT-25 JL50QT-28 JL50QT-37 JL50QT-38发动机:JL139QMB机外净化器:3060MC(常州力扬精机有限公司)JL125T-5A JL125T JL125T-2 JL125T-3 JL125T-3A JL125T-4 JL125T-5 JL125T-5B JL125T-5C JL125T-6 JL125T-7 JL125T-8 JL125T-8B JL125T-9 JL125T-10 JL125T-11 JL125T-12 JL125T-13 JL125T-14 JL125T-15 JL125T-17 JL125T-18 JL125T-19 JL125T-20 JL125T-21 JL125T-22 JL125T-23发动机:JL152QMI机外净化器:3060MC(常州力扬精机有限公司)JL150T-6 JL150T JL150T-2 JL150T-3 JL150T-3A JL150T-4 JL150T-5 JL150T-7 JL150T-8 JL150T-9 JL150T-10发动机:JL157QMJ机外净化器:3060MC(常州力扬精机有限公司)JL100-2 JL100-2E JL100-3B JL100-3D JL100-5 JL100-5A JL100-6发动机:JL150FMG机外净化器:3060MC(常州力扬精机有限公司)JL100-B JL100 JL100-A JL100-C JL100-2AJL100-2B JL100-2C JL100-2D JL100-3C JL100-4发动机:JL150FMG-2机外净化器:3060MC(常州力扬精机有限公司)JL125 JLJL125-2 JL125-2B JL125-2K JL125-3E JL125-3G JL125-3H JL125-3K JL125-4A JL125-5 JL125-6发动机:JL157FMI机外净化器:3060MC(常州力扬精机有限公司)JL150 JL150-2 JL150-2B JL150-2C JL150-3 JL150-4 JL150-5 JL150-6 JL150-7 JL150-9 JL150J发动机:JL162FMJ机外净化器:3060MC(常州力扬精机有限公司)17、金城集团有限公司JC125-17 JC125-2A JC125-11 JC125-5 JC125-12 发动机:JC157FMI-2机外净化器:10/0201E/00(台湾信通交通器材股份有限公司)JC125-17 JC125-10 JC125-7B发动机:JC156FMI机外净化器:10/0201E/00(台湾信通交通器材股份有限公司)JC100-3A JC100-8发动机:JC150FMG-4机外净化器:JM433/40/18:0:1(上海庄信万丰化工有限公司)JC150T发动机:JC157YMJ机外净化器:10/0201E/00(台湾信通交电器材股份有限公司)JC90-B发动机:JC147FMF机外净化器:JM433/40/18:0:1(上海庄信万丰化工有限公司)JC125发动机:JC157FMI机外净化器:10/0201E/00(台湾信通交通器材股份有限公司)JC50QT-10A JC50QT-5 JC50QT-8AJC50QT-11A JC50QT-12A JC50QT-15发动机:JC139QMA机外净化器:JM433/40/18:0:1(上海庄信万丰化工有限公司)JC125-17 JC125-2A JC125-11 JC125-5 JC125-12JC125-10 JC125-22发动机:JC157FMI-2机外净化器:3081MC(常州力扬精机有限公司)JC100-3A JC100-8 AX100-C发动机:JC150FMG-4机外净化器:3081MC(常州力扬精机有限公司)JC125-17 JC125-7B发动机:JC156FMI机外净化器:3081MC(常州力扬精机有限公司)JC150T发动机:JC157YMJ机外净化器:3081MC(常州力扬精机有限公司)JC90-B发动机:JC147FMF机外净化器:3081MC(常州力扬精机有限公司)JC125 JC125-15A发动机:JC157FMI机外净化器:3081MC(常州力扬精机有限公司)JC125-10发动机:JC157FMI-2机外净化器:10/0201E/00(台湾信通交通器材股份有限公司)18、台州华田摩托车有限公司JL125T-2 JL125T-4 JL125T-5 JL125T-6 JL125T-7 JL125T-8 JL125T-9 JL125T-10 JL125T-12 JL125T-13 JL125T-15 HT125T-2 HT125T-4 HT125T-5 HT125T-6 HT125T-7 HT125T-8 HT125T-9 HT125T-10 HT125T-12HT125T-13 HT125T-15 FL125T FL125T-2 FL125T-3 FL125T-4 FL125T-5 FL125T-6 FL125T-9 FL125T-10 FL125T-12 FL125T-13 FL125T-15发动机:JL152QMI或HT152QMI机外净化器:LH-1-M-63.5(上海华理环保发展有限公司)JL150发动机:JL162FMJ机外净化器:LH-1-M63.5(常州力扬精机有限公司)JL 100T-3B JL 100T-C JL 100T-5A发动机:JL147QMG机外净化器:LH-1-M63.5(常州力扬精机有限公司)JL 125T-3B JL 125T-15发动机:JL152QMI机外净化器:LH-1-M63.5(常州力扬精机有限公司)JL 150T-9 JL 150T-8 JL 150T-15 JL 150T-11发动机:JL157QMJ机外净化器:LH-1-M63.5(常州力扬精机有限公司)JL50QT-20 JL50QT-12 JL50QT-13发动机型号:JL139QMB净化器型号:LH-1-M-48(常州力扬精机有限公司)19、西藏珠峰工业股份有限公司ZF125T-7发动机:ZY154MI机外净化器:5CA1(台湾山叶机车工业股份有限公司)ZF125T-8 ZF125T-6B ZF125T-6ZF125T-11B ZF125T-12 ZF125T-3发动机:SC25AHF机外净化器:JHQ125-T(常州力扬精机有限公司)ZF125T-6B ZF125T-8 ZF125T-6 ZF125T-2ZF125T-12 ZF125T-11B ZF125T-3发动机:ZF1P52QMI机外净化器:JHQ125-T(常州力扬精机有限公司)ZF125T ZF125T-C发动机:ZF152M机外净化器:JHQ125-T(常州力扬精机有限公司)ZF125-17 ZF125-18发动机:ZF156FMI-2机外净化器:JHQ125-Q(常州力扬精机有限公司)ZF125A发动机:ZF156FMI机外净化器:JHQ125-Q(常州力扬精机有限公司)ZF100发动机:ZF150FMG机外净化器:JHQ100-W(常州力扬精机有限公司)ZF125T-15发动机:ZF1P52QMI-5机外净化器:JHQ125-T(常州力扬精机有限公司)20、重庆涪陵摩托车有限责任公司HL90-2 HL90-3发动机:ZS147FMF机外净化器:HL4FA(常州力扬精机有限公司)HL100-2 HL100-5 HL100-9 HL100-10 HL100-14 HL100-15 HL100-16 HL100-17 发动机:ZS150FMG机外净化器:HL4GA(常州力扬精机有限公司)HL100-3发动机:150FMG-3机外净化器:HL4GA(常州力扬精机有限公司)HL100-7发动机:ZS150FMG-AHL110发动机:152FMH机外净化器:HL4HB(常州力扬精机有限公司)HL125-2发动机:ZS156FMI-2机外净化器:HL4IA(常州力扬精机有限公司)HL110-6 HL110-7 HL110-8 HL110-9 HL110-10 发动机:ZS152FMH机外净化器:HL4HB(常州力扬精机有限公司)HL125-4 HL125-11 HL125-12 HL125-14HL125-15 HL125-16 HL125-17 HL125-18发动机:ZS156FMI机外净化器:HL4IA(常州力扬精机有限公司)HL150-2 HL150-3 HL150-4HL150-5 HL150-6 HL150-7发动机:ZS161FMJ机外净化器:HL4JA(常州力扬精机有限公司HL110-2 HL110-3 HL110-4 HL110-5发动机:ZS150FMH机外净化器:ZS4HA(常州力扬精机有限公司)HL125T H125T-4 HL125T-5 HL125T-6HL125T-8 HL125T-9 HL125T-10 HL125T-7发动机:ZS1P52QMI机外净化器:GR7(桂林利凯特环保实业有限公司)HL90发动机:ZS147FMF-A(ZS147FM-2)机外净化器:ZS4FA(常州力扬精机有限公司)HL150发动机:ZS161FMJHL100-8 HL100-11 HL100-13发动机:ZS150FMG-B机外净化器:ZS4IA(常州力扬精机有限公司)HL125-3 HL125-6 HL125-7 HL125-8HL125-10 HL125-13 HL125-9发动机:ZS156FMI机外净化器:ZS4IA(常州力扬精机有限公司)21、无锡鸿雁摩托车有限公司HY150 HY150-3 HY150-8 HY150-9YL150 YL150-3 YL150-8 YL150-9发动机:162FMJ机外净化器:MJX型(无锡威孚力达催化净化有限责任公司)HY125-5 HY125-7 YL125-7发动机:156FMI机外净化器:MJX型(无锡威孚力达催化净化有限责任公司)HY100-9 HY100-14 YL100-6 YL100-10发动机:150FMG机外净化器:MJX型(无锡威孚力达催化净化有限责任公司)HY125T-4 HY125T-2 HY125T-6YL125T YL125T-2 YL125T-3发动机:152QMI机外净化器:MJX型(无锡威孚力达催化净化有限责任公司)HY100-9 HY100-10 HY100-11 HY100-13 HY100-15 HY100-16 HY100-18 HY100-19 HY100-6A YL100-3A YL100-7 YL100-8 YL100-9 YL100-11 YL100-12 YL100-13 HY100-12A HY100-12 HY100-8 HY100-8A YL100-4 YL100-4A YL100-15 YL100-16 发动机:150FMG机外净化器:MJX(无锡威孚力达催化净化有限责任公司)HY125T-4 HY125T-3 HY125T-5发动机:152QMI机外净化器:MJX(无锡威孚力达催化净化有限责任公司)HY125-5 HY125-A HY125-2 HY125-3 HY125-4 HY125-6 HY125-8 HY125-9 HY125-10 HY125-11 YL125 YL125-2 YL125-3 YL125-4 YL125-5YL125-6发动机:156FMI机外净化器:MJX (无锡威孚力达催化净化有限责任公司)HY150-8 HY150-2 HY150-4 HY150-5 HY150-6 HY150-7 HY150-10 HY150-11 YL150-2 YL150-4 YL150-5 YL150-6 YL150-7 YL150-10发动机:162FMJ机外净化器:MJX (无锡威孚力达催化净化有限责任公司)22、重庆市劲隆摩托车制造有限公司JL150 JL150-6 JL150-6A JL150-7JL150-8 JL150-2B JL150-2C JL150-5JL150-A JL150-C JL150-E JL150-F发动机:161FMJ机外净化器:JLJ10(常州力扬精机有限公司)JL110-3 JL110-3A JL110-3B JL110-4JL110-4A JL110-3C JL110-3D发动机:152FMH机外净化器:JLG1A(常州力扬精机有限公司)JL100-2A JL100-A JL100-7 JL100-8 JL100-8A JL100-10 JL100-10A JL100-B JL100-E JL100-F 发动机:150FMG-1-A机外净化器:JLG1A(常州力扬精机有限公司)JL100发动机:150FMG-1-A或150FMG-1机外净化器:JLG1A(常州力扬精机有限公司)JL110-2A JL110-C JL110-5A JL110 JL110-2 JL110-5 JL110-7 JL110-7A JL110-8 JL110-9 JL110-9A JL110-A JL110-D JL110-F JL110-G 发动机:153FMH机外净化器:JLG1A(常州力扬精机有限公司)JL100-3 JL100-2B JL100-3B JL100-3AJL100-4 JL100-4A发动机:150FMG-1-B机外净化器:JLG1A(常州力扬精机有限公司)JL125发动机:157FMI或157FMI-A机外净化器:JLI10(常州力扬精机有限公司)JL125-12 JL125-2 JL125-2B JL125-2C JL125-2D JL125-3A JL125-4 JL125-5A JL125-6 JL125-8 JL125-8A JL125-10 JL125-10A JL125-C JL125-E JL125-H发动机:157FMI机外净化器:JLI10(常州力扬精机有限公司)JL70-A发动机:147FMD机外净化器:JLD10(常州力扬精机有限公司)23、海南新大洲摩托车股份有限公司XDZ50QT-13发动机:XDZ1P39QMB机外净化器:XDZ932LY(常州力扬)XDZ100T-4发动机:XDZ1P50QMG机外净化器:XDZ927KJ(台湾康捷)XDZ125T-6C XDZ125T-6D XDZ125T-6E XDZ125T-10 发动机:XDZ1P52QMI机外净化器:XDZ901LY(常州力扬)XDZ125-5A XDZ125-6 XDZ125-8发动机:XDZ157FMI机外净化器:XDZ911JM(庄信万丰)SK175发动机:SK165FMK机外净化器:SK175ZX(庄信万丰)XDZ250发动机:XDZ253FMM机外净化器:XDZ906JM(庄信万丰)24、上海新大洲摩托车有限公司XDZ50DQT发动机:XDZ1PE40QMB-4(电喷)XDZ50QT-28发动机:XDZ1P39QMB机外净化器:XDZ932LY(常州力扬)XDZ125T-2A XDZ125T-8A发动机:XDZ1P52QMI机外净化器:XDZ901LY(常州力扬)XDZ125-10发动机:XDZ157FMI机外净化器:XDZ911JM(庄信万丰)25、江门市大长江摩托车有限公司GN125 HS125J EN125 HJ125-A HJ125-AJ 发动机:F401机外净化器:HJMCHJ125-8 HJ125-2 HJ125-2A HJ125-2CHJ125-2D HJ125-7 HJ125-F HJ125-5发动机:156FMI机外净化器:HJMCHS125T HS125T-2 HJ125T-3发动机:F418或152QMI机外净化器:HJMC(常州力扬精机有限公司)GN125 HJ125-A HJ125-AJ EN125 HS125J 发动机:157FMI机外净化器:HJMC(常州力扬精机有限公司)HJ125T-2发动机:152QMI机外净化器:HJMC(常州力扬精机有限公司)26、济南轻骑摩托车股份有限公司QM125-2 QM125-2C QM125-2M QM125-2G发动机:K157FMI机外净化器:GR7A-M(桂林利凯特环保实业有限公司)QM125-10B QM125-11 QM125-7AQM125-10C QM125-6 QM125-10发动机:157FMI机外净化器:GR7A-M(桂林利凯特环保实业有限公司)QM150L-4 QM150L-2发动机:162FMJ机外净化器:GR7A-M(桂林利凯特环保实业有限公司)QM100-12 QM100-7C发动机:M150FMG-D机外净化器:GR7A-M(桂林利凯特环保实业有限公司)QM100-7 QM100-7B发动机:N151FMG机外净化器:GR7A-M(桂林利凯特环保实业有限公司)QM100-11发动机:R150FMG机外净化器:GR7A-M(桂林利凯特环保实业有限公司)QM110-4 QM110-4B发动机:R152FMHQM125T-B QM125T-A QM125T-F QM125T-C QM125T-9H QM125T-9C QM125T-9K Q M125T-9D Q M125T-9E QM125T-D发动机:P152QMI机外净化器:3060MC(常州力扬精机有限公司)QM100T-4发动机:P150QMG机外净化器:3060MC(常州力扬精机有限公司)QM125T-4 QM125T-4A QM125T-4D发动机:T151QMI机外净化器:3060MC(常州力扬精机有限公司)QM80T QM80T-A QM80T-B发动机:147QME机外净化器:3060MC(常州力扬精机有限公司)QM50QT-6 QM50QT-6B QM50QT-6C QM50QT-6D QM50QT-6E QM50QT-6H QM50QT-6K发动机:139QMB机外净化器:3060MC(常州力扬精机有限公司)QM50QT-6N QM50QT-6R发动机:139QMB机外净化器:3060MC (常州力扬精机有限公司)QM125T-9N QM125T-9S QM125T-9R发动机:P152QMI机外净化器:3060MC (常州力扬精机有限公司)QM125-2V发动机:K157FMI机外净化器:GR7A-M(桂林利凯特环保实业有限公司)QM125-10H发动机:157FMI27、中国轻骑集团有限公司湛江轻骑摩托车有限公司GS125-2 GS125-2B发动机:K157FMI机外净化器:JM433(庄信万丰集团)GS125-7 GS125-7A发动机:157FMI机外净化器:JM433(庄信万丰集团)GS125T-9E发动机:P152QMI机外净化器:JM433(庄信万丰集团)28、中国轻骑集团合肥摩托车厂KS50QT-2 KS50QT-2A KS50QT-2B发动机:D139QMB机外净化器:JM433(庄信万丰集团)KS125T KS125T-A KS125T-B KS125T-8 KS125T-10发动机:P152QMI机外净化器:JM433(庄信万丰集团)29、浙江洛嘉摩托车有限公司BG50QT BG50QT-3 BG50QT-4 LJ50QT-2发动机:LJ139QMB机外净化器:L YDS(常州力扬精机有限公司)LJ100T LJ100T-2 LJ100T-3LJ100T-4 LJ100T-5 BG100T发动机:LJ150QMG机外净化器:L YDS(常州力扬精机有限公司)BG125T LJ125T-A LJ125T-2 LJ125T-4 LJ125T-11 LJ125T-5 LJ125T-9 LJ125T LJ125T-10 LJ125T-12 发动机:LJ152QMI机外净化器:L YDS(常州力扬精机有限公司)30、浙江中南集团摩托车有限公司BD125T-C BD125T BD125T-2 BD125T-3 BD125T-6 BD125T-B BD125T-4发动机:152QMI机外净化器:HR-OEM-4S-125(浙江华荣废气净化有限公司)31、常熟市轻型摩托车厂JJ100发动机:ZS150FMG机外净化器:3060MC43×75(常州力杨精机有限公司)JJ100-3 JJ100-6发动机:ZS150FMG机外净化器:3077MC35×60(常州力杨精机有限公司)JJ125发动机:HW156FMI机外净化器:3074MC47×60(常州力杨精机有限公司)JJ125-11发动机:ZS156FMI机外净化器:3060MC43×75(常州力杨精机有限公司)JJ150-4发动机:ZS162FMJ机外净化器:3074MC47×60(常州力杨精机有限公司)FD100-6发动机:150FMG-2机外净化器:3077MCFD125发动机:ZS156FMI机外净化器:3077MCFD125T-9 FD125T-6发动机:1P52QMI机外净化器:3077MCFD125-11发动机:HW156FMI机外净化器:3077MCJJ150-5发动机:ZX162FM机外净化器:3077MCFD100发动机:HW150FMG机外净化器:3077MC(常州力扬精机公司)FD100-16发动机:ZS152FMG-2机外净化器:3077MC(常州力扬精机公司)FD150-5发动机:ZX162FM机外净化器:3077MC(常州力扬精机公司)32、惠州麦科特玛骐摩托车有限公司MCT100-12 MCT100-6 MCT100-8 MCT100-11 MCT100-15发动机:MCT 150FMG机外净化器:MCT4FGA(常州力扬精机有限公司)MCT125-5 MCT125-2 MCT125-3 MCT125-6 MCT125-7 MCT125-8 MCT125-9 MCT125-11 MCT125-16 MCT125-17 发动机:MCT 156FMI机外净化器:MCT4FIA(常州力扬精机有限公司)MCT125T-7 MCT125T-2A MCT125T-6 MCT125T-8 MCT125T-7 MCT125T-9 MCT125T-11 MCT125T-12 MCT125T-15发动机:MCT 152QMI机外净化器:MCT4QIA(常州力扬精机有限公司)33、上海美田摩托车有限公司MT150-4 MT150-5 MT150-10 MT150发动机:MT162FMJ机外净化器:LH-1-M-63.5 (浙江台州康乐轻工机械厂)MT150T MT150T-2 MT150T-3 MT150T-4 MT150T-6 MT150T-6B MT150T-7 MT150T-8B MT150T-8发动机:MT157QMJ机外净化器:HR-OEM-4S-150(浙江华荣)MT125T MT125T-5A MT125T-3 MT125T-5MT125T-9 MT125T-15 MT125T-8B发动机:MT152QMI机外净化器:HR-OEM-4S-125(浙江华荣)MT100T-10C MT100T-10 MT100T-10A MT100T-10BMT100T-13 MT100T-14 MT100T-11发动机:MT52QMG机外净化器:HR-OEM-4S-100(浙江华荣)MT125T-2 MT125T-3B MT125T-4MT125T-8 MT125T-12 MT125-6发动机:MT152QMI机外净化器:HR-OEM-4S-125(浙江华荣)HM125T HM125T-3 HM125T-4 HM125T-7 HM125T-8 HM125T-10 HM125T-6 HM125T-11 HM125T-12 HM125T-13发动机:HM152QMI机外净化器:LH-1-M-48HM125-11 HM125-13 HM125-15 HM125-16 HM125-22 HM125-17 HM125-18发动机:HM157FMI机外净化器:LH-1-M-63.5HM150-9 HM150-12 HM150-13 HM150-15 HM150-16 HM150-14发动机:HM162FMJ机外净化器:LH-1-M-63.5HM150T HM150T-2 HM150T-4 HM150T-5 HM150T-6发动机:L Y157QMJ机外净化器:LH-1-M-48。
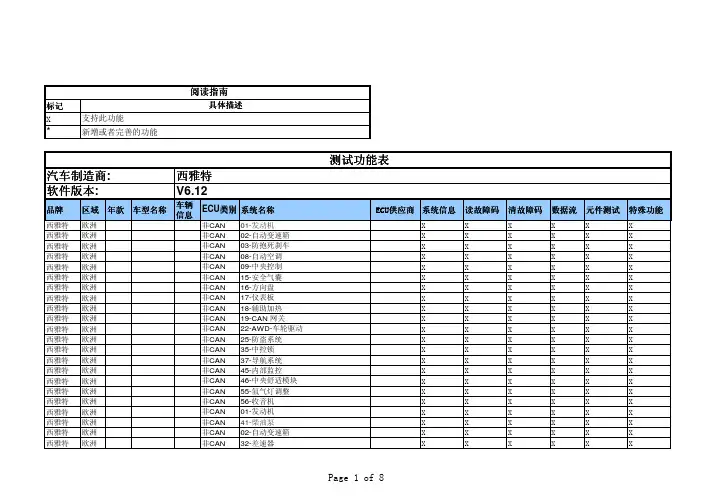
标记X*品牌区域年款车型名称车辆信息ECU 类别系统名称ECU ECU供应商供应商系统信息读故障码清故障码数据流元件测试特殊功能西雅特欧洲非CAN 01-发动机XX X X X X 西雅特欧洲非CAN 02-自动变速箱X X X X X X 西雅特欧洲非CAN 03-防抱死刹车X X X X X X 西雅特欧洲非CAN 08-自动空调X X X X X X 西雅特欧洲非CAN 09-中央控制X X X X X X 西雅特欧洲非CAN 15-安全气囊X X X X X X 西雅特欧洲非CAN 16-方向盘X X X X X X 西雅特欧洲非CAN 17-仪表板X X X X X X 西雅特欧洲非CAN 18-辅助加热X X X X X X 西雅特欧洲非CAN 19-CAN 网关X X X X X X 西雅特欧洲非CAN 22-AWD-车轮驱动X X X X X X 西雅特欧洲非CAN 25-防盗系统X X X X X X 西雅特欧洲非CAN 35-中控锁X X X X X X 西雅特欧洲非CAN 37-导航系统X X X X X X 西雅特欧洲非CAN 45-内部监控X X X X X X 西雅特欧洲非CAN 46-中央舒适模块X X X X X X 西雅特欧洲非CAN 55-氙气灯调整X X X X X X 西雅特欧洲非CAN 56-收音机X X X X X X 西雅特欧洲非CAN 01-发动机X X X X X X 西雅特欧洲非CAN 41-柴油泵X X X X X X 西雅特欧洲非CAN 02-自动变速箱X X X X X X 西雅特欧洲非CAN32-差速器XXXXXX汽车制造商:软件版本:西雅特V6.12测试功能表阅读指南具体描述支持此功能新增或者完善的功能西雅特欧洲非CAN11-发动机 II X X X X X X 西雅特欧洲非CAN51-电子驱动X X X X X X 西雅特欧洲非CAN12-离合器X X X X X X 西雅特欧洲非CAN21-发动机 III X X X X X X 西雅特欧洲非CAN61-电瓶调节X X X X X X 西雅特欧洲非CAN22-AWD车轮驱动X X X X X X 西雅特欧洲非CAN31-其它发动机X X X X X X 西雅特欧洲非CAN71-电瓶充电X X X X X X 西雅特欧洲非CAN03-防抱死刹车X X X X X X 西雅特欧洲非CAN14-电控悬挂X X X X X X 西雅特欧洲非CAN15-安全气囊X X X X X X 西雅特欧洲非CAN58-辅助燃油箱X X X X X X 西雅特欧洲非CAN13-自动距离控制X X X X X X 西雅特欧洲非CAN24-防滑系统X X X X X X 西雅特欧洲非CAN55-氙气灯调整X X X X X X 西雅特欧洲非CAN68-雨刮系统X X X X X X 西雅特欧洲非CAN23-制动助力器X X X X X X 西雅特欧洲非CAN34-水平控制X X X X X X 西雅特欧洲非CAN65-轮胎气压X X X X X X 西雅特欧洲非CAN1C-水平感知系统X X X X X X 西雅特欧洲非CAN43-辅助制动X X X X X X 西雅特欧洲非CAN44-辅助转向X X X X X X 西雅特欧洲非CAN53-驻车制动X X X X X X 西雅特欧洲非CAN54-后扼流器X X X X X X 西雅特欧洲非CAN64-稳定系统X X X X X X 西雅特欧洲非CAN35-中控锁X X X X X X 西雅特欧洲非CAN06-乘客位座椅记忆X X X X X X 西雅特欧洲非CAN08-自动空调X X X X X X 西雅特欧洲非CAN42-驾驶位电动门X X X X X X 西雅特欧洲非CAN45-内部监控X X X X X X 西雅特欧洲非CAN16-方向盘X X X X X X 西雅特欧洲非CAN18-辅助加热X X X X X X 西雅特欧洲非CAN52-乘客位电动门X X X X X X 西雅特欧洲非CAN63-驾驶侧辅助进入X X X X X X 西雅特欧洲非CAN26-自动天窗X X X X X X 西雅特欧洲非CAN28-后部空调X X X X X X 西雅特欧洲非CAN62-右后门控制X X X X X X西雅特欧洲非CAN73-乘客侧辅助进入X X X X X X 西雅特欧洲非CAN36-驾驶位座椅记忆X X X X X X 西雅特欧洲非CAN38-电动天窗X X X X X X 西雅特欧洲非CAN72-右后门控制X X X X X X 西雅特欧洲非CAN7D-辅助加热X X X X X X 西雅特欧洲非CAN46-中央舒适模块X X X X X X 西雅特欧洲非CAN48-驾驶侧后座椅控制X X X X X X 西雅特欧洲非CAN78-右车门滑动系统X X X X X X 西雅特欧洲非CAN66-乘客侧后座椅控制X X X X X X 西雅特欧洲非CAN0D-左车门滑动系统X X X X X X 西雅特欧洲非CAN05-访问/起动授权系统X X X X X X 西雅特欧洲非CAN17-仪表板X X X X X X 西雅特欧洲非CAN57-电视调谐器X X X X X X 西雅特欧洲非CAN29-左大灯X X X X X X 西雅特欧洲非CAN25-防盗系统X X X X X X 西雅特欧洲非CAN27-后头部控制X X X X X X 西雅特欧洲非CAN67-音量控制X X X X X X 西雅特欧洲非CAN39-右大灯X X X X X X 西雅特欧洲非CAN75-远程信息处理X X X X X X 西雅特欧洲非CAN37-导航系统X X X X X X 西雅特欧洲非CAN77-车载电话X X X X X X 西雅特欧洲非CAN49-自动大灯X X X X X X 西雅特欧洲非CAN56-收音机X X X X X X 西雅特欧洲非CAN47-音响系统X X X X X X 西雅特欧洲非CAN09-中央电器X X X X X X 西雅特欧洲非CAN59-牵引支架保护X X X X X X 西雅特欧洲非CAN76-驻车辅助X X X X X X 西雅特欧洲非CAN19-CAN 网关X X X X X X 西雅特欧洲非CAN69-拖车X X X X X X 西雅特欧洲非CAN07-头部控制X X X X X X 西雅特欧洲非CAN1D-驾驶员身份认证系统X X X X X X 西雅特欧洲非CAN0E-多媒体播放机 1X X X X X X 西雅特欧洲非CAN4E-右后头部控制X X X X X X 西雅特欧洲非CAN0F-数字收音机X X X X X X 西雅特欧洲非CAN2D-对讲机系统X X X X X X 西雅特欧洲非CAN1E-多媒体播放机 2X X X X X X 西雅特欧洲非CAN5E-头部控制:天窗X X X X X X西雅特欧洲非CAN2F-数字电视X X X X X X 西雅特欧洲非CAN3D-特殊功能X X X X X X 西雅特欧洲非CAN2E-多媒体播放机 3X X X X X X 西雅特欧洲非CAN6E-头部控制:天窗X X X X X X 西雅特欧洲非CAN2F-数字电视X X X X X X 西雅特欧洲非CAN4D-日期调整X X X X X X 西雅特欧洲非CAN3E-多媒体播放机 4X X X X X X 西雅特欧洲非CAN7E-头部控制:仪表板X X X X X X 西雅特欧洲非CAN4F-中央电器 II X X X X X X 西雅特欧洲CAN01-发动机 X X X X X X西雅特欧洲CAN 01-发动机(EV_ECM20TFS8K2907115Q)X X X X X X西雅特欧洲CAN 01-发动机(EV_ECM12TFS02103F906070)X X X X X X西雅特欧洲CAN 01-发动机(EV_ECM12TFS02103F906070A)X X X X X X西雅特欧洲CAN 01-发动机(EV_ECM12TFS02103F906070BC)X X X X X X西雅特欧洲CAN 01-发动机(EV_ECM12TFS02103F906070D)X X X X X X西雅特欧洲CAN 01-发动机(EV_ECM12TFS02103F906070G_AU35)X X X X X X西雅特欧洲CAN 01-发动机(EV_ECM12TFS02103F906070G_VW36)X X X X X X西雅特欧洲CAN 01-发动机(EV_ECM12TFS02103F906070M)X X X X X X西雅特欧洲CAN 01-发动机(EV_ECM12TFS02103F906070T_AU35)X X X X X X西雅特欧洲CAN 01-发动机(EV_ECM12TFS02103F906070T_VW36)X X X X X X西雅特欧洲CAN 01-发动机(EV_ECM14TSI03C906027AD)X X X X X X西雅特欧洲CAN 01-发动机(EV_ECM14TSI03C906027AE)X X X X X X西雅特欧洲CAN41-柴油泵 X X X X X X 西雅特欧洲CAN02-自动变速箱 X X X X X X 西雅特欧洲CAN02-自动变速箱(EV_TCMVL381) X X X X X X 西雅特欧洲CAN32-差速器 X X X X X X 西雅特欧洲CAN11-发动机 II X X X X X X 西雅特欧洲CAN51-电子驱动 X X X X X X 西雅特欧洲CAN12-离合器 X X X X X X 西雅特欧洲CAN21-发动机 III X X X X X X 西雅特欧洲CAN61-电瓶调节 X X X X X X 西雅特欧洲CAN22-AWD-四轮驱动 X X X X X X 西雅特欧洲CAN31-其它发动机 X X X X X X 西雅特欧洲CAN71-电瓶充电器 X X X X X X 西雅特欧洲CAN03-防抱死刹车 X X X X X X 西雅特欧洲CAN03-防抱死刹车(2009,2010款) X X X X X X 西雅特欧洲CAN14-电控悬挂 X X X X X X 西雅特欧洲CAN74 底盘控制 X X X X X X 西雅特欧洲CAN42-驾驶位电动门 X X X X X X 西雅特欧洲CAN13-自动距离控制 X X X X X X 西雅特欧洲CAN24-防滑系统 X X X X X X 西雅特欧洲CAN15-安全气囊 X X X X X X西雅特欧洲CAN 15-安全气囊(EV_AirbaVW10BPAVW250)X X X X X X西雅特欧洲CAN 15-安全气囊(EV_AirbaVW10SMEVW360)X X X X X X西雅特欧洲CAN 15-安全气囊(EV_AirbaAU10SMEAU485)X X X X X X西雅特欧洲CAN 15-安全气囊(EV_AirbaAU10SMEAU416)X X X X X X西雅特欧洲CAN 15-安全气囊(EV_AirbaECUVWAUDI010)X X X X X X西雅特欧洲CAN52-乘客位电动门 X X X X X X 西雅特欧洲CAN23-制动助力器 X X X X X X 西雅特欧洲CAN34-水平控制 X X X X X X 西雅特欧洲CAN55-氙气灯调整 X X X X X X 西雅特欧洲CAN62-右后门控制 X X X X X X 西雅特欧洲CAN43-辅助制动 X X X X X X 西雅特欧洲CAN44-辅助转向 X X X X X X 西雅特欧洲CAN65-轮胎气压 X X X X X X 西雅特欧洲CAN72-右后门控制 X X X X X X 西雅特欧洲CAN53-驻车制动 X X X X X X 西雅特欧洲CAN54-后扼流器 X X X X X X 西雅特欧洲CAN58-辅助燃油箱 X X X X X X 西雅特欧洲CAN78-右车门滑动系统 X X X X X X 西雅特欧洲CAN04 转向角 X X X X X X 西雅特欧洲CAN64-稳定系统 X X X X X X 西雅特欧洲CAN68-雨刮系统 X X X X X X 西雅特欧洲CAN0D-左车门滑动系统 X X X X X X 西雅特欧洲CAN35-中控锁 X X X X X X 西雅特欧洲CAN35-中控锁(2009,2010款) X X X X X X 西雅特欧洲CAN06-带记忆的乘客座椅X X X X X X 西雅特欧洲CAN08-自动空调 X X X X X X 西雅特欧洲CAN08-自动空调(2009,2010款) X X X X X X 西雅特欧洲CAN1C-水平感知系统 X X X X X X 西雅特欧洲CAN45-内部监控 X X X X X X 西雅特欧洲CAN16-方向盘 X X X X X X 西雅特欧洲CAN16-方向盘(2009,2010款) X X X X X X 西雅特欧洲CAN18-辅助加热 X X X X X X 西雅特欧洲CAN3C-车道改变 X X X X X X西雅特欧洲CAN63-驾驶侧辅助进入 X X X X X X 西雅特欧洲CAN26-自动天窗 X X X X X X 西雅特欧洲CAN28-后部空调 X X X X X X 西雅特欧洲CAN4C-轮胎压力 II X X X X X X 西雅特欧洲CAN73-乘客侧辅助进入 X X X X X X 西雅特欧洲CAN36-带记忆的驾驶位座椅X X X X X X 西雅特欧洲CAN38-电动天窗 X X X X X X 西雅特欧洲CAN5C-车道保持 X X X X X X 西雅特欧洲CAN7D-辅助加热 X X X X X X 西雅特欧洲CAN46-中央舒适模块 X X X X X X 西雅特欧洲CAN48-驾驶侧后座椅控制 X X X X X X 西雅特欧洲CAN6C-.备份Cam X X X X X X 西雅特欧洲CAN0B-二次空气加热 X X X X X X 西雅特欧洲CAN66-乘客侧后座椅控制X X X X X X 西雅特欧洲CAN05-访问/起动授权系统X X X X X X 西雅特欧洲CAN07-头部控制 X X X X X X 西雅特欧洲CAN57-电视调谐器 X X X X X X 西雅特欧洲CAN29-左大灯 X X X X X X 西雅特欧洲CAN25-防盗系统 X X X X X X 西雅特欧洲CAN25-防盗系统(2009,2010款) X X X X X X 西雅特欧洲CAN17-仪表板 X X X X X X 西雅特欧洲CAN17-仪表板(2009,2010款) X X X X X X西雅特欧洲CAN 17-仪表板(EV_Kombi_UDS_VDD_RM09)X X X X X X西雅特欧洲CAN67-音量控制 X X X X X X 西雅特欧洲CAN39-右大灯 X X X X X X 西雅特欧洲CAN75-远程信息处理 X X X X X X 西雅特欧洲CAN27-后头部控制 X X X X X X 西雅特欧洲CAN77-车载电话 X X X X X X 西雅特欧洲CAN49-自动大灯 X X X X X X 西雅特欧洲CAN56-收音机 X X X X X X 西雅特欧洲CAN37-导航系统 X X X X X X 西雅特欧洲CAN09-中央电器 X X X X X X 西雅特欧洲CAN59-牵引支架保护 X X X X X X 西雅特欧洲CAN76-驻车辅助 X X X X X X 西雅特欧洲CAN47-音响系统 X X X X X X西雅特欧洲CAN19-CAN 网关 X X X X X X 西雅特欧洲CAN69-拖车 X X X X X X 西雅特欧洲CAN1D-驾驶员身份认证系统X X X X X X 西雅特欧洲CAN0E-多媒体播放机 1 X X X X X X 西雅特欧洲CAN4E-右后头部控制 X X X X X X 西雅特欧洲CAN0F-数字收音机 X X X X X X 西雅特欧洲CAN2D-对讲机系统 X X X X X X 西雅特欧洲CAN1E-多媒体播放机 2 X X X X X X 西雅特欧洲CAN5E-头部控制:天窗 X X X X X X 西雅特欧洲CAN1F 固定调谐器 X X X X X X 西雅特欧洲CAN3D-特殊功能 X X X X X X 西雅特欧洲CAN2E-多媒体播放机 3 X X X X X X 西雅特欧洲CAN6E-头部控制:天窗 X X X X X X 西雅特欧洲CAN2F-数字电视 X X X X X X 西雅特欧洲CAN4D-数据传送器 X X X X X X 西雅特欧洲CAN3E-多媒体播放机 4 X X X X X X 西雅特欧洲CAN7E-头部控制:仪表板X X X X X X 西雅特欧洲CAN4F-中央电器 II X X X X X X 西雅特欧洲CAN5D-运行 X X X X X X 西雅特欧洲CAN5F-信息选择 X X X X X X 西雅特欧洲CAN6D-电动行李箱盖 X X X X X X 西雅特欧洲CAN6F-中央舒适模块II X X X X X X 西雅特欧洲专家功能仪表保养归零X 西雅特欧洲专家功能气囊复位X 西雅特欧洲专家功能读取PIN码(二代防盗来自防盗盒)X 西雅特欧洲专家功能读取PIN码(二代防盗来自仪表板)X 西雅特欧洲专家功能读取PIN码(三代防盗来自发动机)X 西雅特欧洲专家功能里程表调整(Passat 1.8 GSi)X。

SIMATICET 200SPF-TM ServoDrive 设备手册Siemens AG Division Digital Factory Postfach 48 48 90026 NÜRNBERG A5E47579551-ABⓅ 04/2020 本公司保留更改的权利Copyright © Siemens AG 2020.保留所有权利法律资讯警告提示系统为了您的人身安全以及避免财产损失,必须注意本手册中的提示。
人身安全的提示用一个警告三角表示,仅与财产损失有关的提示不带警告三角。
警告提示根据危险等级由高到低如下表示。
危险表示如果不采取相应的小心措施,将会导致死亡或者严重的人身伤害。
警告表示如果不采取相应的小心措施,可能导致死亡或者严重的人身伤害。
小心表示如果不采取相应的小心措施,可能导致轻微的人身伤害。
注意表示如果不采取相应的小心措施,可能导致财产损失。
当出现多个危险等级的情况下,每次总是使用最高等级的警告提示。
如果在某个警告提示中带有警告可能导致人身伤害的警告三角,则可能在该警告提示中另外还附带有可能导致财产损失的警告。
合格的专业人员本文件所属的产品/系统只允许由符合各项工作要求的合格人员进行操作。
其操作必须遵照各自附带的文件说明,特别是其中的安全及警告提示。
由于具备相关培训及经验,合格人员可以察觉本产品/系统的风险,并避免可能的危险。
按规定使用 Siemens 产品请注意下列说明:警告Siemens产品只允许用于目录和相关技术文件中规定的使用情况。
如果要使用其他公司的产品和组件,必须得到Siemens推荐和允许。
正确的运输、储存、组装、装配、安装、调试、操作和维护是产品安全、正常运行的前提。
必须保证允许的环境条件。
必须注意相关文件中的提示。
商标所有带有标记符号 ® 的都是 Siemens AG的注册商标。
本印刷品中的其他符号可能是一些其他商标。
若第三方出于自身目的使用这些商标,将侵害其所有者的权利。

肿瘤内科出科测试题含参考答案一、单选题(共100题,每题1分,共100分)1、原发性肺腺癌通常免疫组化表现为:A、CK7+,CK20+B、CK7+,CK20-C、CK7-,CK20+D、CK7-,CK20-E、以上的不是正确答案:B2、左半结肠癌典型症状:A、腹部肿块B、消瘦C、贫血D、肠梗阻及便血E、腹痛正确答案:D3、下列哪些非小细胞肺癌患者术后辅助化疗获益最少:A、ⅠA期B、ⅠB期C、ⅡA期D、ⅡB期E、ⅢA期正确答案:A4、皮肤恶性黑色素瘤的病因目前唯一有证据的是:A、刀割、绳勒等局部刺激B、有大量普通痣或发育异常的痣C、有皮肤癌家族史D、激光和冷冻E、过度紫外线照射正确答案:E5、晚期肺鳞癌一线治疗疾病进展后,二线治疗宜选下列哪一方案:A、培美曲塞B、多西他赛C、培美曲塞+顺铂D、贝伐单抗+多西他赛E、长春瑞滨+顺铂正确答案:B6、易累积手和足的软组织肉瘤A、上皮样肉瘤B、滑模肉瘤C、横纹肌肉瘤D、PNET正确答案:A7、导致宫颈癌发病的最重要原因是:A、性传播疾病史B、多个性伴侣C、使用避孕药D、性交年龄过早E、人乳头瘤病毒的持续感染正确答案:E8、抗肿瘤药物局部渗漏引起组织反应或坏死以及栓塞性静脉炎属A、远期局部反应B、远期全身反应C、近期局部反应D、近期全身反应E、以上都是正确答案:C9、胃癌术后辅助化疗不包括如下药物A、紫杉醇B、替吉奥C、5FuD、希罗达E、奥沙利铂正确答案:A10、(2)超声胃镜证实胃窦部病变累及肌层,病理是MALT淋巴瘤,下列哪项不是恰当的治疗()A、抗HP治疗;B、累及野放疗;C、手术;D、美罗华免疫化疗正确答案:C11、不符合早期胃癌的的描述为A、可呈隆起性病变B、可多中心发生C、可呈凹陷性病变D、不伴淋巴结转移E、癌组织局限于粘膜和粘膜下层正确答案:D12、肺癌TNM分期(第7版)中,下列哪种情况属于T2:A、原发肿瘤最大径>3cm,≤7cmB、累及主支气管,距离隆突≥2cmC、肿瘤累及脏层胸膜D、产生肺段或肺叶不张或阻塞性肺炎E、以上都是正确答案:E13、阿片类药物的不良反应持续存在的是A、瘙痒B、尿潴留C、嗜睡D、呕吐E、便秘正确答案:E14、内分泌治疗的药物包括以下哪些?A、芳香化酶抑制剂(AI)、雄激素和雌激素、孕激素B、LH-RH类似物、抗雌激素、芳香化酶抑制剂(AI)、雄激素和雌激素、孕激素C、LH-RH类似物、抗雌激素、芳香化酶抑制剂(AI)D、LH-RH类似物、抗雌激素、芳香化酶抑制剂(AI)、孕激素E、抗雌激素、芳香化酶抑制剂(AI)、雄激素和雌激素、孕激素正确答案:B15、下列哪项说法不正确:A、所有的患者都需要伊马替尼辅助治疗B、中,高危患者需要伊马替尼辅助治疗C、R1切除的患者需要接受伊马替尼辅助治疗D、伴有kit外显子11突变的患者应接受伊马替尼辅助治疗正确答案:D16、病例五.患者、男、73岁,2008年11月出现间歇性无痛肉眼血尿,无尿频、尿急、尿痛,于2008-11-24行TURB术,术后病理:高级别浸润性尿路上皮癌Ⅲ级。

AutomotiveAerospaceElectricalTruckHydraulics1Powering business worldwideNext generation transportationEaton is driving the development of newtechnologies –from hybriddrivetrains and emission control systems to advanced engine components –that reduce fuel consumption and emissions in trucks and cars.Higher expectationsWe continue to expand our aerospace solutions andservices to meet the needs of new aviation platforms,including the high-flying light jet and very light jet markets.Building on our strengths Our hydraulics businesscombines localised service and support with an innovative portfolio of fluid powersolutions to answer the needs of global infrastructure projects,including locks,canals and dams.Powering Greener Buildings and BusinessesEaton’s Electrical Group is a leading provider of powerquality,distribution and control solutions that increase energy efficiency and improve power quality,safety and reliability.Our solutions offer a growing portfolio of “green”products and services,such as energy audits and real-time energy consumption monitoring.Eaton’s Uninterruptible Power Supplies (UPS),variable-speed drives and lighting controls help conserve energy and increase efficiency.Eaton delivers the power inside hundreds of products that are answering the demands of today’s fast changing world.We help our customers worldwide manage the power they need for buildings,aircraft,trucks,cars,machinery and entire businesses.And we do it in a way that consumes fewer resources.Eaton’s range ofSF6free switchgearfor Medium VoltageEaton Corporation is a worldwide leader in thedesign, manufacture, and sale of safe, reliableand high-performance medium voltage powerdistribution equipment in accordance with IEC,ANSI and GB / DL standardsComplete Global Medium Voltage Switchgear SolutionsEaton,a premier leader in designing and manufacturing powerdistribution and protection equipment in the electrical industry,offers a comprehensive range of medium voltage(MV)solutionsto meet the needs of virtually every application.From productsthat feature cutting-edge design that allow for easy access,maintenance and space savings,to arc-resistant products thatenhance safety,Eaton’s medium voltage solutions provide avariety of products for every need.Additionally,Eaton’s globalservice network provides maximum customer support in allregions of the world.As one of the few completely vertically integrated and diversifiedindustrial manufacturers in the world,Eaton designs not only MVassemblies,but also the key components that comprise the MVsolutions–from steel housing and circuit breaker compartmentsto vacuum interrupters,circuit breakers,bus systems and fuses.Eaton’s MV heritage,strengthened by acquisitions such asWestinghouse DCBU,Cutler Hammer,MEM and Holec,hasresulted in breakthrough MV technologies and numerousinternational patents over the years.Part of Eaton’s complete electrical PowerChain Solutions–which help businesses minimize risks while realizing greaterreliability,cost efficiencies,capital utilization and safety–Eaton’s medium voltage equipment meets all applicablestandards and certifications such as IEC,NEMA/ANSI,GB / DL,UL,IEEE,KEMA and CSA.When it comes to medium voltage solutions,you can trust theone name with a long history of proven performance:Eaton.3XIRIA Plus is the name of Eaton's new solid insulated RMU for smart grid applications. The system is characterised by its high level of operational safety and is suitable for applications up to 12kV.The XIRIA Plus RMU is designedaround Eaton's proven vacuuminterrupters, which require nomaintenance and are certified for10,000 operation cycles.All live parts in the available panels aresingle pole insulated. The usedmaterials are shaped specifically toprovide optimum insulation combinedwith excellent thermal characteristics.In addition, the insulation is configuredto provide effective control over electricfields around the used components,thereby minimizing any risk of internalarcing.Within the XIRIA Plus panels both theprimary parts and the mechanisms arehoused in a fully enclosed housingwhich protects the whole systemagainst environmental influences. Theuse of vacuum interrupters and solidinsulation means that the XIRIA Plus isenvironmentally friendly. Thesetechnologies ensure that this system isa conservational alternative toswitchgear systems using SulfurHexafluoride (SF6) gas for insulation. Thecost of ownership is also significantlyreduced, as no regular testing of gaspressure or other routine maintenance isneeded and there is no high end-of-lifecost associated with ultimately disposingof the equipment.With a compact design and a provisionfor cable connection from the front, theXIRIA Plus system is economical in itsuse of valuable floor space, and easy toaccommodate in even the most restrict-ed environments.When it comes to the safety of theoperating personnel the XIRIA Plusdesign leaves nothing to chance.All parts are fully enclosed by an internalarc tested safe metal housing.The panels in the system are providedwith direct visible indication of theintegrated earthing and ON/OFF-positionby means of inspection windows in thefront.XIRIA PlusSolid insulated RMU for smart grid application4XIRIA PlusSafe reliable and efficient solutions for all applications in the medium voltage secondary distribution network.Smart grid readinessDesigned to integrate solutions for sensing, monitoring and remote control for feeder automation and load management purposes.Safe in use•Visible isolation by means of inspection windows in the front •Compartments protected against penetration of objects •Capacitive voltage detection system for verification of safe isolation from supply•Logical mechanical and electrical interlocks prevent misoperationEnvironmentally friendly•Minimized number of components•Environmental-friendly design with respect to the materials used•No use of SF6-gas for switching and insulation•Minimal number of transition points in primary design enable low energy loss during operation•Only re-usable and / or recyclable materials usedUser friendly•Cable connection and user interfaces for operation on the same front side of the panel•Ergonomic cable connection height• A customized low voltage compartment is optional •Clear and simple straightforward operation panelsLow total cost of ownershipLow initial costs due to:•Panels with only 350mm or 450mm width depending on function•Cable connection from the front / wall standing arrangement No costs during service due to:•Robust design with minimum number of parts (routine tested in factory)•Long-life solid insulated components as insulation medium •Maintenance free vacuum circuit-breaker and loadbreak switch •Primary parts and mechanism installed in a fully sealed for life enclosed housing•No SF6 pressure checksLow end of life disposal cost due to:•Vacuum switching technology•Solid insulation with air as isolating medium •Recycling or re-use of materialsOperation•Complete design certified in accordance with GB standards • Arc fault tested according GB 3906-2006• Quality assurance in accordance with ISO 9001• Single pole insulated primary parts within one compartment • Primary parts and mechanism in sealed for life fully enclosed housing• Configurations up to 6 functions in one tank• Field extensible for projectsrequiring more than six functions3-position disconnector All panels are equipped with a disconnector positioned in the same sealed for life enclosure as the circuit-breaker. The disconnector consists of three shafts connected to the busbars or earthing points. Since it is mechanically interlocked the disconnector can only be operated when the circuit breaker is in the open position.•Manual-operated switch with 3-position (service / isolated / earthed)•Maintenance free•Housed in sealed for life enclosure•Auxiliary contacts for service / isolated / earthed•Position indication by means of inspection windows and mechanical indicators•Mechanically interlocked with the vacuum circuit-breaker3-All entocan5Main constructionVacuum technology features•Eaton has an unsurpassed leadership in vacuum technology supported by a strong heritage of innovation from companies such as Westinghouse and Holec•Pioneers in vacuum technology for over 90 years. First vacuum interrupter supplied at 15kV-12kA in 1967•Eaton was the first one to develop and patent copper-chromium alloy content for contacts and center shields•Our vacuum interrupters for contactor applications can perform up to 2.5 million mechanical operations•More than 5 million units delivered worldwide, operating safely and reliably in all types of networks, medium voltage applications and environments•High end certified supplier to almost all major electrical manufacturers worldwideSolid insulation system•More than 60 years experience in solid insulation technology with hundreds ofthousands of functions successfully operating worldwide in all kinds of environment without any ageing concern•Full encapsulation capability in the 1990’s •High thermal conductivity •High electrical resistivity •Low moisture absorption•High creepage current resistance •High mechanical strength•Optimized electrical field control•Fully insulated busbars to prevent any phase to phase and phases to earth faults •Optional extensible busbarsVisible isolation by means of inspection windows in the frontWhen carrying out operational actions and work on the cables, it is vital to have unambiguous status indications. When it comes to the safety of operating personnel Eaton leaves nothing to chance. That is why the XIRIA Plus design is fitted with directly visible isolation by means of inspection windows in the front which makes the isolating distance between the cable and busbar system directly visible. A visible, shortcircuit proof earthing can take place via the load-break switch orcircuit-breaker.Capacitive voltage detection system for verification of safe isolation from supplyEach panel type within the XIRIA Plus family is equipped with a standard three phase Voltage Detection System for voltage testing. The VDS shows the operator if the panel is isolated from supply or not. Logical mechanical and electrical interlocks prevent incorrect operationWithin the XIRIA Plus design misoperation by an operator is prevented by using different interlocks. The interlocks are mechanical and electrical. For example electrical and mechanical interlocks prevent operation of the change-over switch when the circuit-breaker is switched on. All mechanical interlocks are constructed in such a way that they directly block the mechanism. Switching to service position is only possible with closed cable compartmentAs standard, the door of the cable compartment can only be opened when thecircuit-breaker is in the earthed position. After the door isremoved it is possible to switchoff the circuit-breaker for cabletesting. Switching on to serviceposition is only possible withthe door positioned back again.Compartments protectedagainst penetration of objectsWithin the XIRIA Plus design itis not possible to accidentallypenetrate the switchgear bypart of a body or a tool.All high voltage compartmentsare designed according to IP65degree.Smooth contemporary designAll compartments of the XIRIAPlus panels are designed insuch a way that the system issafe to touch from the outside.By using a smooth and smartdesign it is not possible for theoperator to injure himself bymoving parts or by parts thatstick out of the switchgearwhen moving in front of theswitchgear.Routine testsVarious prescribed routine testsare carried out during theproduction of the switchgear.To assure quality, all processesare in accordance with ISO9001. This means that at everystage of production thecomponents, circuit-breakersand current transformers areinspected for correctfunctionality. When the entireinstallation has beenassembled, a thorough visualinspection is carried out,together with mechanical,functional and electrical checks.Philosophy on internal arcsEaton always puts extra focuson creating safe switchgear foroperators at all times. One ofthe biggest potential threats tooperators is an internal arc inswitchgear.Engineers therefore did every -thing necessary in design andconstruction to prevent internalarcs.Eaton supports the philosophythat it is best to avoid internalarcs than to cure, in line withthe relevant standardGB3906-2006. Within the XIRIAPlus design a double preventionphilosophy is used. Firstly, thedesign is constructed in such away that an internal arc isprevented. In the unlikely casethat an internal arc could occur,the XIRIA Plus is equipped toprovide maximum safety to theoperator, and to control andminimise damage to the rest ofthe switchgear and room.Safe in useThe XIRIA Plus design contains some special features that guarantee theoperator to work safely with the different panel types.What you see is what youget!67One of the key strategic initiatives of Eaton is to provide environ-mentally friendly products. Eaton achieves this by looking at the total product chain, from design to dismantling and recycling.The optimal situation is that for each phase there is no damageto the environment and at the end, all materials can be re-used again in the same product (the Cradle-to-Cradle principle). The product chain can be divided into four main blocks. These blocks are the design (materials used) of the product, the assemblyof the product, the usage phase of the product and finally the dismantling of the product.Environmentally friendlyLike all Eaton medium voltage switchgear,XIRIA Plus is designed to be an environmentally friendly product throughout the whole value chain.With respect to the design of switchgear,the vision "the less number of components the better"applies.This because every part must be manu -factured and therefore impacts on the environment.Next,applies the affect of different materials on the environment. Use of minimized number of componentsXIRIA Plus is designed to use the minimum of materials and resources,without affecting the strength of the system. For example,Eaton reduced the number of components dramatically,compared to conventional switchgear,by using a simple spring charging mechanism and integrated compartments.This also ensures straight forwardassembly with low labor cost.Materials with no/lessimpact on the environment Eaton selects materials with care.It is essential that they are safe for personnel and the environment -not just during use,but at the end of service life too.Within XIRIA Plus a combination of solid (cast resin)insulation and air as insulation medium is used.The solid insulationtechnology,in combination with electrical field calculations,provides a very compact,environmentally friendly design for the switchgear.As theswitching medium,vacuum technology is used within the interrupters of the XIRIA Plus circuit-breakers.XIRIA Plus can be completely recycled at the end of its life.No use of SF 6gas for insulation or switching Emissions switchgear contribute of SF 6-gas from significantly to the threat of the greenhouse effect and associated climate change.SF 6is on the list of greenhouse gasses in the Kyoto protocol.SF 6is the most potent of the six main greenhouse gasses,with a Global Warming Potential (GWP)of 23,000.Eaton made a fundamental choice not to use SF as 6a switching and insulation medium for medium voltage equipment. The main reason for not using anySF 6in medium voltage equip -ment was the complexity of the treatment required for the toxicity of the gasses that have been in contact with an arc,and the need for additional safety measures when used in public locations such asresidential areas and shopping centers.Besides the energy sources,special focus was placed on the efficient use of material during assembly.For example,sheet steel plates are cut with as little waste material as possible.Residual material is used within other product components.Environmentally friendly designEfficient use of materialsTo prevent energy loss by the system itself,XIRIA Plus uses a minimum number of primary change-over points.All the available change-over points use optimal surface contacts and by this,prevent extra energy losses over these points.Minimal energy loss during operationDuring dismantling,XIRIA Plus switchgear is demounted into parts and thereafter categorized per material.Next the parts will be recycled or re-used.Because XIRIA uses no SF 6,there is no loss of this gas during dismantling of the switchgear.Re-use or recycling of materialsBecause XIRIA Plus is designed for a lifetime of at least 30years,the system needs no energy usage for maintenance activities during this long period.Due to the green insulation and switching technology,there is also no leakage of the SF 6-gas during its lifetime and no need for extra maintenance activities on SF 6pressure checks.No service checks on site8Features and benefitsThe benefit of a sealed for life tankA “sealed for life” steel enclosure contains all primary parts and driving mechanisms • Maintenance free • Internal arc proofThe benefit of a compact design•Minimal floor space •Low building costs •Easy to install•Up to 6 feeders in one tankExtensibility• Safe and reliable field extensible solution for projects requiring more than six functions •Epoxy extensible busbar bushing •Single phase insulating•Self-pluggable contacts design •Easy to install on siteProduct range for highest flexibility in use•Flexible combination to build configurations up to 6 functions in one tank.•Any combination of load break switches and circuit-breakers can be placed in a 2- 3- 4- 5 or 6 panel unit.Smart grid readinessAutomation upgrading •Remote close/open•Auxiliary contacts for each position local or remote indications•Measuring CT and current signal Option•Trip indicator with auxiliary contacts •Fault indicator •Current meterLoad break switch “C”Fuse-switch combination“F”Circuit breaker “T”Bus coupler “B”Metering panel “M1”Direct connection“D”Busbar PT panel “M2”Disconnector PT panel “Cv”Cable connectionPT panel “PT”OptionwConfiguration information LBS function, type C panelStandard630A vacuum LBSThree-position disconnector Voltage indicator630A bushing Options Motor operation Fault indicator Current meterType D panelStandardVoltage indicator630A bushingPadlock for cable compartment cover Options Fault indicator Current meter910Fuse-switch function, type F panelThe guide for fuse selectionGeneral type XRN-T/12Fuse selection and transformer application Rated voltage (12kV)Preferred type SDLDJ SF(K)LDJRated voltage (kV)1212Rated fuse current (A)3.15、 6.3、 7.5、 10、 16、 20、 25、 31.5、 4050、 63、 80100、 125Transformer rated capacity (kVA)Fuse rated current (A)Length A (mm)292292292Diameter D (mm)516676The fuse dimensionStandard 630A vacuum LBS Three-position disconnector Voltage indicator Type C bushing Fuse blown indicationOptions Motor operation Fault indicator Current meter Fuse Type A bushingFuse striker:Medium type (according GB15166.2, alternating current switch-fuse combinations).50100125160200250315400500630800100012506.31016162025324050638010012511CB function, type T panelTLF protection for circuit breaker function design• Time limit fuse “TLF” protection is an alternativefor the standard electronic protection relay.• It ensures tripping of the circuit-breaker through a patented electronic circuit in the event of phase short-circuit and earth fault currents • High reliability • Compact design• Fully enclosed housing• Easy accessible fuses on the front • Easy selection of current setting• Compliance with ENA specification 12-6 issue 1:1973• Fully certifiedProtection CT Type WIC1-WE2WIC1-W2WIC1-W3Primary current scope 16-56A 16-56A 32-112AType WIC1-W4WIC1-W5WIC1-W6Primary current scope 64-224A 128-448A 256-896AOptionsProtection relay and CT WIC1-2PE (Standard)WIC1-1PE (Optional)Motor operation Fault indicator Current meterWI1-SZ5 trip indicator with auxiliary switches WIC1-PC2 adapter for relay settingStandard630A vacuum CBThree Voltage indicator 630A bushingWI1-position disconnector -SZ4 trip indicatorType B panel Type M1 panelStandardTwo 0.2s single phase metering PTs Two 0.2s single phase metering CTs The 500mm height low voltage compartment OptionsMoisture sensor and heater Electrical locking of energized cable compartment cover Voltage meterA A A StandardVoltage indicator630A LBS630A bushingThree-position disconnectorOptions630A CBMotor operation1213GeneralRated voltageImpulse withstand voltagePower frequency withstand voltage Rated frequencyInternal arc classification (IAC)Degree of protection in serviceDegree of protection with doors/covers open Ambient air temperature range Busbar systemRated normal currentRated short-time withstand current Rated peak withstand current Load break switches Rated normal currentRated short-circuit making current Rated short-time withstand current Rated cable charging breaking current Mechanical endurance classMechanical endurance class as 3-position disconnector Electrical endurance class Circuit-breakersRated normal current Rated breaking currentRated short-circuit making currentRated capacitive switching current class Rated cable charging breaking current Mechanical endurance classMechanical endurance class as 3-position disconnector Electrical endurance classRated short-time withstand current Mechanism type Fuse-switch panel Rated normal currentMax. rated current of the optional fuse Rated breaking currentRated short-circuit making current Rated transfer currentFor others, please contact local Eaton sales representative.ItemXIRIA Plus ratingskV kV kV-1m Hz kA-s°C A kA-s kA A kA kA-s AA kA kA AkA-sA A kA kA ARatings1295 (phase to phase/earth), 110 (Isolation gap)42 (phase to phase/earth), 48 (Isolation gap)50AFLR 20-1IP4X IP2X -25 - +4063020-4, 25-250, 6363050, 6320-4, 25-231.5M2 10000 x M1 3000 x E2 100 x6302050C231.5M2 10000 x M1 3000 x E220-4O - 180S - CO - 180S - CO 100125501253150XIRIA Plus designed to GB standardsXIRIA Plus compiles with the following standards:GB/T 11022-2011 Common specifications for high-voltage switchgear and controlgearGB 3804-2004 High voltage alternating-current switches for rated voltages above 3.6kV and up to and including 40.5kVGB 311.1-1997 Insulation co-ordination for high voltage transmission and distribution equipmentGB 1985-2004 High-voltage alternating-current disconnectors and earthing switchesGB 3906-2006 Alternating-current metal-enclosed switchgear and controlgear for rated voltages above 3.6 kV and up to and including 40.5 kV GB 1984-2003 High-voltage alternating-current circuit breakersGB 16926-2009 High-voltage alternating current switch-fuse combinations14XIRIA Plus dimensionType C panel dimensionType F panel dimensionType T panel dimensionMetering panel and extension dimension (C+M+F)Example for combination dimension Width Depth Height=25mm+350mm+450nn+350mm+25mm =1200mm =720mm =1400mmCFT block type=35mm+420mm+750mm+500mm+35mm =1740mm =720mm=1400mm(M1=1900mm)1400168635720351400298420(Extensible type )635720350(Block type )420(Extensible type )350(Block type )351400148500(Extensible type )370720750500420140019003535450(Block type )2515CFC floor planCable type :3X1 35-630MM2Cable cone type :C630A C (Load-break switch)Cable type :3X1 16-95MM2Cone type :A200AF (fuse combination unit)Pressure relief16CTC floor planPressure reliefCable type :3X1 35-630MM2Cable cone type :C630A C (Load-break switch)Cable type :3X1 35-630MM2Cable cone type :C630A T (Circuit break)17Electrical powermanagement by EatonFoundation for successElectrical power.The most significant and pervasive energy source on earth. It runs businesses, fuels innovations and keeps the lights on.When the power system is not designed or managed properly, it compromises success, resulting in lower productivity and increased costs.Eaton takes the complexity out of power management with industry-leading innovation, expert services and holistic solutions.And our customers realize powerful benefits: improved reliability,increased efficiency and enhancedsafety.Customer criticalIf it’s critical to our customers,it’s critical to us.In fact,we view it all as mission critical.ExpertiseWith unparalleled knowledge of power management across industries,we provide the know-how for every application.SupportOur people makethe difference.Support is not just an extra benefit;it’s at the heart of how we do business.For energy challenges big and small, if it matters to you, it matters to us. Our missionis to ensure your success, however you define it.++全球商业动力之源提供动力。

SPECIFICATIONSProduct SeriesComponent Type Motion Control Mounting, MotorBrakeFood GradeConnector, Motor EndKeyed ShaftFeedback TypeFeedback Resolution Feedback ProtocolSpeed, RatedSpeed, MaxMagnet Stack LengthFrame SizeVoltage ClassShaft SealSpecial / CustomOutput Torque, Continuous Output Torque, Peak Compatible Drive Series'Rated Power, ContinuousRotor InertiaOperating Temperature, Max Operating Temperature, Min MPL Low InertiaRotary Servo MotorFlange Mount, MetricNo Y / NNoSpeedTec DIN (Type M7)Yes Y / NAbsolute Single-turn typeSin/Cos, 128 cycles/rev resolutionHiperface protocol7000rpm7000rpm20 = 50.8mmFrame 15 / Frame 063 = 63mm size200V ACNo Y / NNo0.49Nm, continuous1.58NmKinetix 5500 (Bul. 2198)Kinetix 6200 / 6500 (Bul. 2094)Kinetix 6000 (Bul. 2094)Kinetix 300 (Bul. 2097)Kinetix 350 (Bul. 2097)Kinetix 2000 (Bul. 2093)Kinetix 7000 (Bul. 2099)Ultra 3000 (Bul. 2098)compatible0.27kW continuous1.3E-05kg m², rotor40°C max0°C min·Continuous stall torque of 0.26 to 163Nm (2.3 to 1440lb-in.)·Peak torque of 0.77 to 278Nm (6.8 to 2460lb-in.)·Integral 24V brake option·Absolute multi-turn and single-turn high resolution, incremental encoder and resolver feedback options·Low-profile, field-reversible motor connectors for minimal servo motor impact on machine design·DIN connector versions allow flexible orientation of connectors and use of a single cable family with all MP-Series motorsRepresentative Photo Only(actual product may vary based on configuration selections)MP SERIES MPL 240V SERVO MOTOR,0.49 N-M,7000 RPM1300 NHP NHP | .au | 0800 NHP NHP | MPLA1520UEJ72AA DatasheetNHP Electrical Engineering ProductsOperating Temperature, MinStorage Temperature, MaxStorage Temperature, MinRelative humidity, minRelative Humidity, MaxHumidity typeWeightIP RatingDetails, IP RatingShock Acceleration (Max.)Shock Duration (Max.)Vibration Acceleration (Max.)Vibration Frequency, Operational (Max.)0°C min70°C max-30°C min5%RH95%RHNon-condensing1.2kgIP50IP66IP50 minimum, without shaft seal; IP66 with optional shaft seal and use of environmentally sealed cable connectors20g6ms2.5g2000HzREFERENCESInstallation Guide:-User Manual:-Manufacturer Datasheet:-Manufacturer Catalogue & Product Selection:-Supplier Declaration of Conformity:-IECEx Certificate-1300 NHP NHP | .au | 0800 NHP NHP | MPLA1520UEJ72AA DatasheetNHP Electrical Engineering Products。

TUBE-TECH CL1BCompressorDESCRIPTION.The TUBE-TECH compressor CL1B differs from many other compressors,in that the gain-reduction element is made from a non-semiconductor element,which in itself has a very low harmonic distortion and none of the non-linearity problems involved when using most semiconductor elements.Furthermore there is no long-term degradation of the element thus giving it almost infinite life.This element is placed after the input-transformer of the compressor and followed by an all tube-based amplifier with a gain of-∞dB to+30dB.Thus the signal is not fed through any semiconductor circuitry on its way to the output.The amplifier consists of two tubes(valves)in push-pull configuration(one ECC83as thepre-amp and phase splitter,and one ECC82as the output stage),and an output transformer. The power supply for the pre-amp and phase splitter are stabilized and the heaters of both tubes(valves)are fed with a stabilized DC voltage.The whole amplifier(including input and output transformer)and the power supplies are placed on one PC-board.Both input and output are balanced(600Ω)and fully floating.The in/out key switches the compressor in and out without clicks.THE SIDECHAIN:The side chain is the only part of the compressor that contains semiconductors.They are used for three reasons:First they do not affect the sound reproduction,second they have a high slew rate,which is of importance for the performance of the compressor and third they don't take up much room.It contains two J-FET quad op-amps,one npn-transistor and one FET-transistor,which handles the signal for the gain-reduction element.The compressor contains two time constants circuits:1.Fixed attack and release times2.Variable attack and release timesThe attack/release select switch makes it possible to use these two circuits separately or combine their functions.This gives a feature not normally obtained in other compressors:In the combined(fix./man.)state the attack-and release controls makes it possible toobtain a complex release-time slope.(See page4)(980112)COMPRESSOR INTERCONNECTION:The side chain sockets for interconnection of several compressors are located on the rear panel.A switch(BUS SELECT)on the front selects which compressors are interconnected,and on which bus they are connected.If you e.g.have10compressors in a rack,you can select compressor1,5,7and8on bus1,and compressor2,3,6and9on bus2,leaving compressor4 and6in the off position.Compressors1,5,7,8are now interconnected and all four will perform the exact same compression.This applies to compressor2,3,6and9as pressor4and6are independent.The interconnection implies,that the unit,which performs the most compression,is controlling the others.To choose which one you want to control,select the attack/release time,the threshold and the ratio on that unit,and turn the threshold fully counter clockwise on the reminding compressors. It is of course possible to have all the interconnected compressors control each other simultaneously.NB:Remember to set the ratio control and the gain control in the same position on the "slaves".Otherwise the stereo image could be shifted during compression.Theattack/release-control on the slaves will have no effect.The input/output capability of the side chain-circuit allows up to ten compressors to be linked together.They are connected in parallel with a standard1/4"stereo jack/-jack cord(tip:bus1,ring:bus 2).The two jack socket on the rear panel is connected in parallel and both are input/output.(980112)CONTROLS:GAIN:The gain control is used to"make up"for the gain loss,which takes place when the unit is compressing.It is placed after the gain-reduction circuitand therefore has no influence on the threshold setting.The gain-control iscontinuously variable from off to+30dB.RATIO:The ratio control varies the ratio by which the input signal is compressed.If the ratio selected is to2:1,and the input signal increases10dB,theoutput signal is only increased by5db.The ratio control is continuouslyvariable from2:1to10:1.THRESHOLD:The threshold is the point where the compressor begins its action.It isdefined as the point where the gain is reduced by1dB.The threshold is continuously variable from+20dBU to-40dBU. METER:The VU-meter switch has three positions:1.Input The meter is reading the level at the input socket.pressionThe VU-meter is reading gain reduction.Its rest position is"0VU",and the amount ofcompression is shown as a decreasing deflection indB.3.Output The VU-meter is reading the level at the output socket."0VU"is equivalent to+4dBU.NB:Leave the meter switch in position compression as it mightintroduce distortion if left in the input or output position.IN/OUT:This leverswitch switches the compressor in and out of the signal path.The out position bypasses the entire compressor.ATTACK:The attack control chooses how fast/slow the compressor responds to an increase in the input signal.The attack control is continuously variable from0.5to300milliseconds. RELEASE:The release control chooses how fast/slow the compressor responds to a decrease in the input signal.The release control is continuously variable from0,05to10seconds.(980112)ATTACK/RELEASE SELECT:This switch selects how the compressor reacts to an increase(attack)ordecrease(release)of the input signal.There are three settings of the switch:1.Fixed.Attack time:1msecRelease time:50msec2.Manual.Attack time:from0.5msec to300msecRelease time:from0.05sec to10sec3.Fix/man.This setting combines the release times of fixed and manualmode.The attack time is as in the fixed mode.The fix/man mode always has a fast attack,but it is possible to obtain a release time depending on the input signal,e.g.get a fast release when the peak disappears,then superseded shortly thereafter by the release time selected by the release control.From the time the peak disappears,until the selected release time takes over,is dependent upon the setting of the attack control.That is,the attack control changes function from a pure attack control,to a control of delay with the same time range.The more CW the attack control is turned,the longer time before the release controltakes over.The more CCW the attack control is turned,the shorter time before the release control takes over.This function is valid only if the time of the peak is shorter than the setting of the attack control. If the peak of the program is longer than the setting of the attack control,or if the attack control has reached the full CCW position,it will respond as in the manual mode.The fix/man mode acts as an automatic release function with a constant fast attack time and fast release time for short peaks and a longer release times for longer peaks.This setting is mainly intended for use on program material(overall compression).BUS SELECT:Interconnects several compressors on bus1or bus2.If the compressor is left in the off position,it works entirely independently.(980112)SUGGESTED APPLICATIONSOFTUBE-TECH COMPRESSOR CL1BIn the following,you will find suggestions on various applications of the TUBE-TECH compressorCL1B.They are given as a convenient guide to enable you to familiarise yourself with the different aspects of using the compressor.We have not mentioned specific settings of gain and threshold as they are dependent upon input levels.Instead we have specified how much compression in dB,we feel,is needed for the various examples.OVERALL COMPRESSION:FINAL MIXCOMPRESSION NEEDED:3-4dBAttack/release select:Fix/manAttack:2o'clockRelease:10o'clockRatio:9o'clockSTANDARD COMPRESSION:BASS,PIANO,GUITAR,KEYBOARDS AND VOCALSCOMPRESSION NEEDED:4-5dBAttack/release select:ManualAttack:2o'clockRelease:10o'clockRatio:10-2o'clockHEAVY COMPRESSION ON INSTRUMENTS:LINE GUITAR AND PIANOCOMPRESSION NEEDED:10dBAttack/release select:ManualAttack:7o'clockRelease:1o'clockRatio:3o'clockCOMPRESSION OF DRUMS:SNARE AND BASS DRUMCOMPRESSION NEEDED:2-3dBAttack/release select:FixedRatio:9-12o'clock(980112)ADJUSTMENT PROCEDURE:CAUTION:Before making any adjustment let the unit heat-up at least15min.Observe that the offset-voltage measured at the side chain jack socket,when the THRESHOLD is off,is not greater than+/-15mV DC in both position"fixed"and "manual".(tip is bus1and ring is bus2).If the voltage exceeds this value,replace either IC1or IC2.THE GRE SHALL BE MARKED BETWEEN1.225-1.285ADJUSTMENT OF BASIC GAIN:1)Apply a signal of1kHz,-30,0dBU into the input of the compressor.2)Turn the GAIN-control fully clockwise.3)Set the RATIO-control at2:14)Adjust the pre-set GAIN(located on amp/psu PCB)to an output-reading of0,0dBU.ADJUSTMENT OF COMPRESSION TRACKING:1)Turn the THRESHOLD-control fully counter-clockwise.2)Set the RATIO-control at2:1.3)Set the BUS-select-switch at1.4)Apply a signal of1kHz,0,0dBU into the input of the compressor.5)Adjust the GAIN-control to an output-reading of0,0dBU.6)Apply a DC-voltage of+250,0mV into the side chain jack socket(tip)and observe thatthe output level has dropped to-10,0dB.7)If this is not the case,adjust the level with P2(P1)*,to obtain a drop of exactly-10,0dB. *The trimpots in parenthesis refers to PCB870316-0,1,2(980810)ADJUSTMENT OF THE VU METER READING"COMPRESSION":1)Turn the THRESHOLD-control fully counter-clockwise.2)Switch the METER-selector to Compression.3)Set the RATIO-control at2:14)Apply a signal of1kHz,0,0dBU into the input of the compressor.5)Adjust the GAIN-control to an output-reading of0,0dBU.6)Adjust P4(P2)*until the meter is reading0VU.7)Apply a DC-voltage of+250,0mV into the side chain jack socket and observe that theoutput level has dropped to-10,0dBU.If this is not the case,adjust the compressiontracking(see above)8Adjust P3until the meter is reading-10,0VU.9)Remove the DC-voltage from the side chain jack socket.10)Repeat step6-9.NB:The VU-meter accuracy should be within+/-0,5dB when reading compression. ADJUSTMENT OF THE RELEASE CONTROL:1)Set the METER switch in position compression.2)Set the attack/release SELECT switch in position manual.3)Apply a signal of1kHz,0,0dBU into the input of the compressor.4)Adjust the THRESHOLD-control to a reading of-10VU of the VU-meter5)Set the ATTACK-control at fast.6)Set the RELEASE-control at slow.7)Switch off the1kHz and observe that the VU meter moves to0VU in approx.10sec.8)If this is not the case,adjust P1(P5)*,to obtain a release time of approximately10sec. *The trimpots in parenthesis refers to PCB870316-0,1,2(950119)Over view of the sidechain PCBPCB870316-0,1,2P2P3P1P50VU-10VU-10dB Rel.10Sec.PCB870316-3P4P3P2P10VU-10VU-10dB Rel10Sec.101115TECHNICAL SPECIFICATIONS CL1B:Input impedance:600OhmsOutput impedance:<60OhmsFrequency-response:5Hz-25kHz+0.5/-3dB Distortion THD@40Hz:0dBU:<0,15%10dBU:<0,15%maximum output(1%THD):+26,0dBUmaximum input(1%THD):+21,0dBUNoise Rg=200Ohm:Output Gain0dB+30dB Unweighted-85,0dBU-75,0dBUCCIR468-3-75,0dBU-65,0dBUCMRR@10KHz<-60dBGain:off to+30dBCompressorRatio:2:1to10:1Threshold:off to-40dBUAttack:0,5mS to300mSRelease:0,05S to10STracking between interconnected compressors:(0to30dB compression):<+/-1dBTubesECC821ECC831DimensionsHeight:3units132m m/5,2”Width:483m m/19”Depth:170m m/6,7”WeightNet:4,1Kg/9,0lbsShipping:5,9Kg/13,0lbsPower requirements@115V/230V AC,50-60Hz30-40WAll specifications at RL=600Lydkraft reserves the right to alter specifications without prior notice(051018jgp)。

安全技术说明书页: 1/10 巴斯夫安全技术说明书按照GB/T 16483编制日期 / 本次修订: 01.04.2023版本: 8.0日期/上次修订: 06.09.2021上次版本: 7.0日期 / 首次编制: 15.11.2005产品: 蓖麻油聚烃氧酯35 ELProduct: Kolliphor® EL(30554032/SDS_GEN_CN/ZH)印刷日期 03.12.20231. 化学品及企业标识蓖麻油聚烃氧酯35 ELKolliphor® EL推荐用途和限制用途: 药用辅料公司:巴斯夫(中国)有限公司中国上海浦东江心沙路300号邮政编码 200137电话: +86 21 20391000传真号: +86 21 20394800E-mail地址: **********************紧急联络信息:巴斯夫紧急热线中心(中国)+86 21 5861-1199巴斯夫紧急热线中心(国际):电话: +49 180 2273-112Company:BASF (China) Co., Ltd.300 Jiang Xin Sha RoadPu Dong Shanghai 200137, CHINA Telephone: +86 21 20391000Telefax number: +86 21 20394800E-mail address: ********************** Emergency information:Emergency Call Center (China):+86 21 5861-1199International emergency number: Telephone: +49 180 2273-1122. 危险性概述纯物质和混合物的分类:对水环境的急性危害: 分类3巴斯夫安全技术说明书日期 / 本次修订: 01.04.2023版本: 8.0产品: 蓖麻油聚烃氧酯35 ELProduct: Kolliphor® EL(30554032/SDS_GEN_CN/ZH)印刷日期 03.12.2023 标签要素和警示性说明:危险性说明:H402对水生生物有害。

c-Jun-N-Terminal Kinase Drives Cyclin D1Expression and Proliferation During Liver Regeneration Robert F.Schwabe,1,2Cynthia A.Bradham,1,2Tetsuya Uehara,1,2Etsuro Hatano,1,2Brydon L.Bennett,3Robert Schoonhoven,4and David A.Brenner1,2The c-Jun-N-terminal kinase(JNK)pathway is strongly activated after partial hepatectomy(PH),but its role in hepatocyte proliferation is not known.In this study,JNK activity wasblocked with the small molecule inhibitor JNK SP600125in vivo and in vitro as shown bya reduction of c-Jun phosphorylation,AP-1DNA binding activity,and c-jun messengerRNA(mRNA)expression.SP600125inhibited proliferating cell nuclear antigen(PCNA)expression,cyclin D1mRNA and protein expression and reduced mitoticfigures after PH.Survival was reduced significantly3days after PH in SP600125-treated versus vehicle-treated rats(3of11vs.8of9,P<.01).In epidermal growth factor(EGF)-treated primarycultures of rat hepatocytes,SP600125decreased3H-thymidine uptake,cyclin D1mRNAand protein expression,and inhibited the EGF-induced transcription of a cyclin D1pro-moter-driven reporter gene.The defective regeneration and the decreased survival inSP600125-treated rats did not result from a major increase in apoptosis as shown by normallevels of caspase3activity and only slight increases in apoptoticfigures.In conclusion,ourdata show that JNK drives G0to G1transition in hepatocytes and that cyclin D1is adownstream target of the JNK pathway during liver regeneration.(H EPATOLOGY2003;37:824-832.)T wo-thirds partial hepatectomy(PH)of the liver results in the rapid and potent activation of mul-tiple cellular signaling pathways inducing a pro-liferative response of hepatocytes,which allows the liver to regain its original size and cell mass within7to10days.1,2 Although c-Jun-N-terminal kinase(JNK)is strongly ac-tivated within minutes after PH,3,4the function of JNK activation in liver regeneration has not yet been deter-mined.The JNK/c-Jun pathway is a critical component of the proliferative response and induces G0to G1cell cycle progression in many cell types.5Fibroblasts derived from c-Jun null mice have an impaired proliferation and reduced activity of cyclin D1–dependent kinases.6Fibro-blasts derived from JNK1Ϫ/ϪJNK2Ϫ/Ϫmice have a defect in proliferation that resembles the defect of c-Jun Ϫ/Ϫfibroblast andfibroblasts with a mutated c-Jun.6-8The cyclin D1gene has emerged as an important target for the JNK/c-Jun pathway in driving proliferation.The cyclin D1promoter contains an activating protein1 (AP-1)site and ectopic expression of either c-fos or c-Jun induces cyclin D1messenger RNA(mRNA),9which in turn is critical in driving G0to G1cell cycle progression in many cell types including hepatocytes.10During liver regeneration,the activation of JNK is preceded by the release of tumor necrosis factor␣(TNF-␣).Inhibition of TNF-␣or inactivation of the TNF receptor(TNFR)1 lead to defects in liver regeneration and increased mortal-ity after PH and is associated with a reduced JNK activa-tion.3,11TNF-␣does not act as a strong mitogen itself, but may prime hepatocytes for the proliferative action of growth factors such as epidermal growth factor(EGF), hepatocyte growth factor(HGF),and transforming growth factor␣through mechanisms that potentially in-volve AP-1,STAT3,C/EBP,and nuclear factorB (NF-B).2,12In this study,we determined the role of JNK in hepa-tocyte proliferation during liver regeneration and in EGF-Abbreviations:PH,partial hepatectomy;JNK,c-Jun-N-terminal kinase;AP-1, activating protein1;mRNA,messenger RNA;TNF,tumor necrosis factor;EGF, epidermal growth factor;HGF,hepatocyte growth factor;NF-B,nuclear factorB;PCNA,proliferating cell nuclear antigen;PCR,polymerase chain reaction;IL, interleukin;DMSO,dimethyl sulfoxide.From the1Departments of Medicine,2Biochemistry and Biophysics,4Environ-mental Sciences and Engineering,University of North Carolina at Chapel Hill, Chapel Hill,NC;and3Signal Research Division,Celgene Corporation,San Diego,CA.Received August8,2002;accepted January6,2003.Supported in part by grants DK-34987and GM41804from the National Institutes of Health.Address reprint requests to:David A.Brenner,M.D.,University of North Caro-lina,Department of Medicine,CB#7038,Chapel Hill,NC27599.E-mail: dab@;fax:919-966-7468.Copyright©2003by the American Association for the Study of Liver Diseases. 0270-9139/03/3704-0016$30.00/0doi:10.1053/jhep.2003.50135824treated primary hepatocytes by blocking JNK with the small molecule inhibitor SP600125.13Inhibition of JNK resulted in a diminished proliferative response as shown by a decrease of proliferating cell nuclear antigen(PCNA) expression and mitoticfigures.Cyclin D1was a transcrip-tional target of JNK and may mediate its pro-proliferative effects in hepatocytes.Materials and MethodsSP600125Treatment in Animals Undergoing PH. All animal studies were approved by the University of North Carolina Ethics Board.Male Sprague-Dawley rats (200-225g)were subcutaneously injected with either SP600125(a gift from Celgene Corporation,San Diego, CA)at a dose of6mg/kg or the corresponding volume(6 mL/kg)of PPCES vehicle(30%PEG-400/20%polypro-pylene glycol/15%Cremophor EL/5%ethanol/30%sa-line).One hour later,animals were anesthetized with ketamine and two-thirds PH was performed according to Anderson and Higgins as previously described.14Animals were killed at various time points after PH and the rem-nant liver was snap frozen orfixed in4%paraformalde-hyde.For all24-and60-hour PH and survival experiments,treatment groups(i.e.,vehicle or SP600125 treated)consisted of at least4rats.Hepatocyte Isolation and SP600125Treatment of Cultured Hepatocytes.Hepatocytes were isolated from male Sprague-Dawley rats by collagen perfusion as de-scribed previously.15Hepatocytes were plated in6-well plates(0.5ϫ106cells/well)coated with rat type I collagen in Waymouth’s medium containing10%fetal bovine se-rum,0.1mmol/L insulin,and0.1mmol/L dexametha-sone in a humidified atmosphere at37°C and5%CO2. After3hours,the culture was washed with phosphate-buffered saline and changed to hormonally defined medium containing0.1mmol/L insulin,2mmol/L L-glutamine,5mg/mL transferrin,1nmol/L selenium, and10nmol/L free fatty acids in RPMI basal medium. For cyclin D1expression and3H-thymidine incorpora-tion studies,hepatocytes were plated in Williams E media containing5mmol/L pyruvate and0.1mmol/L insulin. Two hours before EGF(20ng/mL)treatment,hepato-cytes were pretreated with SP600125at a concentration of20mol/L or0.1%dimethyl sulfoxide(DMSO). Western Blot Analysis.Snap frozen liver samples or cultured hepatocytes were lysed as previously described.16 Acrylamide gels were loaded with50to100g protein. Protein transfer onto nitrocellulose membranes was checked by Ponceau S.Blots were incubated with either anti–cyclin D1antibody(Santa Cruz Biotechnology, Santa Cruz,CA)anti–phospho-c-Jun(Santa Cruz Bio-technology),anti–phospho-STAT3(Cell Signaling,Bev-erly,MA),or␣-phospho Erk antibody(New England Biolabs,Beverly,MA)at a dilution of1:1,000for1to3 hours followed by incubation with goat-anti-mouse sec-ondary antibody at1:1,000for1hour and chemilumi-nescent detection.For the confirmation of equal loading, blots were reprobed with anti-tubulin primary antibody (Oncogene Science,Cambridge,MA)or anti-actin pri-mary antibody(ICN,Costa Mesa,CA).Northern Blot Analysis.Twenty micrograms of RNA was separated by gel electrophoresis on2.2mol/L formaldehyde1%agarose gels,transferred to nylon mem-branes,and hybridized with32P-labeled probes for c-jun as previously described.17Reverse-Transcription Polymerase Chain Reaction. RNA was prepared from frozen liver tissue by the Trizol method.One microgram of RNA was reverse transcribed as previously described.16One microliter of the reverse-transcription reaction was subjected to polymerase chain reaction(PCR)for either cyclin D1,-actin,or interleu-kin6(IL-6).Cyclin D1was amplified for28cycles using 5Ј-GCG AAG TGG AGA CCA TCC G-3Јsense and 5Ј-GTC CAC ATC TCG GAC GTC G-3Јantisense primer at a concentration of1mol/L in10mmol/L Tris (pH9.2),35mmol/L MgCl2,75mmol/L KCl,and100g/mL bovine serum albumin.IL-6was amplified for40 cycles in10mmol/L Tris(pH8.3),50mmol/L KCl,and 1.5mmol/L MgCl2using previously described primers.18-actin was amplified for28cycles as previously de-scribed.16Electrophoretic Mobility Shift Assay.Nuclear ex-tracts were prepared as described.16Five micrograms of nuclear protein were incubated with100pg of32P labeled probe containing the AP-1consensus site in buffer con-taining10mmol/L HEPES pH7.8,2mmol/L MgCl2,50 mmol/L KCl,1mmol/L dithiothreitol,0.1mmol/L ethylenediaminetetraacetic acid,20%glycerol,single-stranded oligonucleotide(25g/mL),and poly dI/dC (25g/mL)for15minutes on ice. Immunohistochemistry.Liver tissue wasfixed in4% paraformaldehyde for20hours.PCNA expression was detected by immunostaining using monoclonal anti-PCNA primary antibody(DAKO,Carpinteria,CA)at a concentration of50g/mL for10minutes,the DAKO Envision system(DAKO),and3.3-diaminobenzidine substrate as previously described.19PCNA expression was quantified using Bioquant TCW98software(Biometrics, Nashville,TN)and an Olympus microscope(Melville, NY).3H-Thymidine Incorporation Assay.Two hours af-ter plating,hepatocytes were pretreated with20mol/L SP600125or DMSO for2hours and then treated withHEPATOLOGY,Vol.37,No.4,2003SCHWABE ET AL.82520ng/mL EGF(R&D Systems,Minneapolis,MN).Two Ci3H-thymidine were added18hours before subject-ing the cells to precipitation with10%trichloroacetic acid.Cells were lysed in0.2N NaOH and counted in a scintillation counter(Beckman-Coulter,Fullerton,CA). TNF-␣Enzyme-Linked Immunosorbent Assay. Whole-liver tissue was lysed in25mmol/L HEPES(pH 7.4)containing0.1%CHAPS(Sigma),5mmol/L MgCl2,1.3mmol/L ethylenediaminetetraacetic acid,1 mmol/L EGTA,protease and phosphatase inhibitors. Cleared lysates were measured by enzyme-linked immu-nosorbent assay(R&D Systems)at a1:5dilution accord-ing to the manufacturer’s description and adjusted to total protein concentration.Detection of Apoptosis.Apoptosis was quantified by counting nuclei with an apoptotic morphology as previ-ously described.14Caspase3–like activity was determined by incubating whole-cell extracts with amino-4-triflu-oromethyl coumarin-DEVD as previously described.16 Reporter Gene Assays.To assess the effects of SP600125on cyclin D1transcription,hepatocytes were transfected with eitherϪ964CD1Luc,or Ϫ964mtCD1luc.9For transfection,2.5g of plasmid DNA,0.5g of Renilla-luc Tk plasmid,and2.5L transfect F1reagent(Targeting Systems,San Diego,CA) were added to the cells in OptiMEM media for2hours. After12hours,the cells were treated with EGF for16 hours.Luciferase activity was determined on a2010lu-minometer(Analytical Luminescence,San Diego,CA) and adjusted to the internal Renilla-luc control. Statistics.A paired Student’s t test was performed us-ing Microsoft Excel(Redmond,WA)and a P level less than.01was considered statistically significant.For sur-vival studies after PH,the Wilcoxon test was performed and a P value of.01was used as criterion of statistical significance.ResultsSP600125Inhibits JNK Activity After PH and inCultured Hepatocytes.To analyze the effects of SP600125on JNK activity,rats were treated with SP600125or vehicle1hour before PH and killed1hour after PH,which corresponded to the peak of JNK activity after PH.4Because SP600125acts as a reversible inhibitor of JNK and may be washed out in JNK activity assays,we analyzed signaling events downstream of JNK activation. SP600125specifically inhibited c-Jun phosphorylation (Fig.1A)and c-Jun mRNA induction and blunted the induction of AP-1DNA binding after PH(Fig.1B),but did not affect signal transducer and activator of(STAT)3 phosphorylation(Fig.1A).In cultured hepatocytes,EGF rapidly induced the phosphorylation of c-Jun,which was almost completely blocked by SP600125(Fig.1C). SP600125specifically blocked JNK and did not influence TNF-␣–induced IB degradation,EGF-induced Akt ac-tivation(unpublished data),and EGF-induced Erk phos-phorylation(Fig.1C),which is consistent with previous studies in other cell types.13,20To exclude that the effects of SP600125after PH were caused mainly by blocking the release of the cytokines that are required for normal liver regeneration,we determined the effects of SP600125 on IL-6and TNF-␣after PH.Hepatic levels of IL-6 mRNA and TNF-␣protein were not influencedby Fig.1.SP600125inhibits JNK activity in the regenerating liver and in cultured hepatocytes.(A)The effect of SP600125on JNK activity after PH was determined by Western blot analysis of the JNK target c-Jun in vehicle-and SP600125-treated rats(upper panel).To show the speci-ficity of SP600125,STAT3phosphorylation was analyzed after PH in vehicle-and SP600125-treated rats(lower panel).(B)The induction of c-jun mRNA and AP-1DNA binding activity was examined by Northern blot and electrophoretic mobility shift assays,respectively,1hour after 70%PH in untreated,vehicle-treated,and SP600125-treated rats.(C) Hepatocytes were pretreated with SP600125(20mol/L)for2hours followed by EGF(20nmol/L)treatment for20minutes.Phosphorylation of c-Jun and Erk were determined by Western blot analysis.(D)IL-6 mRNA was determined by reverse-transcription PCR in livers from vehicle-or SP600125-treated rats before or after PH and compared with levels of -actin.RNA from Rat1fibroblasts served as a positive control for IL-6.(E)TNF-␣levels from whole-liver extracts from vehicle-or SP600125-treated rats were determined by enzyme-linked immunosorbent assays before PH,30minutes,and90minutes after PH.826SCHWABE ET AL.HEPATOLOGY,April2003SP600125(Fig.1D and E).The decrease of intrahepatic TNF-␣seen after PH most likely was caused by the re-lease of preformed TNF-␣and loss of intracellular stor-ages.SP600125Inhibits Proliferation After PH.To ad-dress the speci fic role of JNK in liver regeneration,we analyzed the proliferative response after PH in the pres-ence or absence of SP600125.As expected,vehicle-treated rats showed a strong increase in the number of PCNA-positive nuclei 24hours after PH (12.3%Ϯ7%,n ϭ4)compared with rats before PH (0.15%Ϯ0.12%,Fig.2A).In contrast,SP6-treated rats showed a marked decrease in the number of PCNA-positive nuclei (1.14%Ϯ0.22%,n ϭ4)that signi ficantly differed from vehicle-treated rats (Student ’s t test,P value Ͻ.01)and was nearly as low as those of livers before PH.After 60hours there was a further increase in PCNA expression in vehicle-treated animals (31.7%Ϯ2.9%)and at this time point,SP600125-treated animals also showed a signi ficant per-centage of PCNA-positive nuclei (21.0%Ϯ7.1%)that was reduced only slightly in comparison with the vehicle control (Fig.2A).Mitotic figures were low in both treat-ment groups 24hours after PH.Sixty hours after PH there was a high percentage of mitotic hepatocytes in ve-hicle-treated animals,but almost no mitotic figures in SP600125-treated hepatocytes (Fig.2B).These findings suggest that JNK exerts an important role in promoting G0exit and cell cycle progression in hepatocytes after PH.SP600125Inhibits Cyclin D1Expression After PH.To further investigate the functional links between JNK activation and proliferation,we analyzed the ex-pression of the G1cell cycle regulator cyclin D1that contains an AP-1site in its promoter.9Cyclin D1ex-pression is suf ficient to drive hepatocytes to enter the cell cycle.10In vehicle-treated animals,cyclin D1pro-tein expression was strongly up-regulated 24hours af-ter PH.In contrast,SP600125-pretreated animals showed a marked reduction in cyclin D1protein ex-pression after 24hours (Fig.3A).To determine whether JNK exerted its effect on cyclin D1through a transcriptionally regulated mechanism as suggested by the AP-1site in its promoter,we analyzed cyclin D1mRNA levels by reverse-transcription PCR.Cyclin D1mRNA was induced 24hours after PH in vehicle-treated animals (Fig.3B).This induction was reduced in SP600125-treated animals in comparison with vehi-cle treated animals (Fig.3B),but to a slightly lesser degree than cyclin D1protein levels,whereas -actin mRNA levels were not affected,suggesting that JNK controls cyclin D1expression mainly through a tran-scriptionally regulatedpathway.Fig.2.SP600125inhibits PCNA expression and mitosis in the regen-erating liver.(A)PCNA expression in vehicle-and SP600125-treated rats was examined 24and 60hours af-ter PH by immunohistochemistry as described in the Materials and Methods section and quantified us-ing image analysis software.Statis-tical significance was determined by the t test.(B)Mitotic figures are indicated by arrows in hematoxylin-eosin–stained section 60hours after PH.Mitotic figures were counted in 20fields of animals killed after 24and 60hours after PH.HEPATOLOGY,Vol.37,No.4,2003SCHWABE ET AL.827SP600125Inhibits Hepatocyte Proliferation and Cyclin D1Expression in Cultured Hepatocytes.The complete mitogen EGF signi ficantly increases c-Jun phosphorylation in cultured primary rat hepatocytes (Fig.1C)and drives cell cycle progression in cultured hepato-cytes,thereby mimicking some of the pro-proliferative signaling occurring during PH.4,21To determine whether JNK is critical for EGF-induced proliferation in cultured hepatocytes,we examined the effect of SP600125on 3H-thymidine incorporation and cyclin D1expression.EGF induced a 2-fold increase in 3H-thymidine incorporation after 24hours,and a 6-fold increase after 48hours (Fig.4A).When preincubated with SP600125,3H-thymidine uptake decreased by 48%(24-hour time point,P Ͻ.01)and 67%(48-hour time point,P Ͻ.01)in the EGF-treated hepatocytes and also was lower in the absence of EGF.To test whether cyclin D1transcription was inhib-ited by SP600125in vitro ,we transfected hepatocytes with a reporter gene containing the Ϫ964cyclin D1pro-moter linked to fire fly luciferase (Ϫ964CD1Luc)or the same reporter gene with a mutated AP-1site at Ϫ954(Ϫ964mtCD1Luc).9EGF induced the expression of this reporter gene 2-fold (Fig.4B).Pretreatment with SP600125reduced the transcription of this reporter gene in EGF-treated hepatocytes by 40%but did not affect the internal control Tk-driven renilla luciferase.The reduced luciferase activity was comparable with the level observedin cells transfected with Ϫ964mtCD1Luc,which lacks the AP-1binding site in its promoter (Fig.4B).To further con firm this data,we analyzed cyclin D1mRNA and protein expression in EGF-treated hepatocytes in the presence or absence of SP600125.Both cyclin D1mRNA and protein levels were induced after 48hours of EGF treatment,and pretreatment with SP600125strongly re-duced the levels of cyclin D1mRNA and protein (Fig.4C and D).Inhibition of JNK Decreases Survival After PH.Previous studies have shown that the absence of critical signaling molecules such as c-Jun or TNF-receptor 1in-hibits liver regeneration and decreases survival after PH and is associated with increased microvesicular steato-sis.11,22In the present study,JNK inhibitiondecreasedFig.3.SP600125inhibits cyclin D1expression after PH.(A)Cyclin D1protein expression was determined by Western blot analysis in SP600125-or vehicle-treated rats 24hours after PH.Cyclin D1expres-sion was quantified using NIH Image software (Bethesda,MD)and normalized to a band from the Coomassie stained blot.(B)Cyclin D1mRNA and -actin mRNA were detected by reverse-transcription PCR in either SP600125-or vehicle-treated rats 24hours after PH.Cyclin D1and -actin bands were quantified by densitometry.Shown is the average of the ratio between cyclin D1and -actin mRNA in each treatmentgroup.Fig.4.SP600125inhibits 3H-thymidine incorporation and cyclin D1transcription in cultured hepatocytes.(A)3H-thymidine incorporation was measured in hepatocytes in the presence or absence of EGF (20nmol/L)in either SP600125-treated (20mol/L)or DMSO-treated (0.1%)hepa-tocytes after 24and 48hours of treatment.Statistical significance was determined by the t test.(B)Cyclin D1–dependent transcription was determined by reporter gene assay.Hepatocytes were transfected with Ϫ964CD1luc or Ϫ964mutCD1luc and treated with EGF (20nmol/L)after SP600125(20mol/L)or DMSO (0.1%)pretreatment as de-scribed in the Materials and Methods section.Statistical significance was determined by the t test.(C)Cyclin D1and -actin mRNA levels were determined by PCR 48hours after EGF (20nmol/L)stimulation in the presence or absence of SP600125(20mol/L).(D)Cyclin D1and actin protein levels were determined by Western blot analysis 48hours after EGF (20nmol/L)stimulation in the presence or absence of SP600125(20mol/L).828SCHWABE ET AL.HEPATOLOGY,April 2003survival 3days after PH (Fig.5A,vehicle 89%vs.SP60012527%,P ϭ.01,Wilcoxon test)and SP600125-treated animals had elevated transaminase levels 60hours after PH (Fig.5B,P Ͻ.01).SP600125-treated animals showed a high degree of microvesicular steatosis 2to 3days after PH (Fig.5C).However,vehicle-treated animals also showed microvesicular steatosis,albeit to a lesser de-gree.To determine whether JNK blockade induced an increase in mortality due to higher rates of apoptosis as shown in animals with defective NF-B and inducible nitric oxide synthase in previous studies,14,23we quanti-fied apoptotic figures in hematoxylin-eosin –stained liver sections of SP600125-and vehicle-treated animals.In ad-dition,caspase 3–like activity was measured in extracts from these livers.In both vehicle-and SP600125-treated animals,the numbers of apoptotic hepatocytes were low.Twenty-four hours after PH,apoptotic figures were ele-vated slightly in SP600125-treated animals,but at all other time points the number of apoptotic figures wassimilar (Fig.5D).These findings were con firmed by the low caspase 3–like activity after PH in both SP600125-and vehicle-treated animals after 24and 60hours,whereas caspase 3–like activity was highly elevated in hepatocytes treated with the agonistic Jo2antibody (Fig.5E).Thus,the reduced hepatocyte proliferation in SP600125-treated animals after PH did not appear to be caused by an increase in hepatocyte apoptosis.DiscussionIn the adult liver,hepatocytes are long-lived and rarely undergo proliferation,yet they retain a remarkable ability to proliferate.1This allows the liver to regain its original size within 7to 10days after 70%PH and quickly restore function.This regenerative response is initiated by a series of signaling events that allow the hepatocyte to enter the cell cycle and undergo several rounds of proliferation.JNK activation is one of the earliest signals to bedetectedFig. 5.JNK inhibition increases mortality after PH.(A)Rats were pre-treated with SP600125or vehicle 1hour before PH.Survival was moni-tored over the following 3days and is shown as a Kaplan-Meier graph.Statistical significance was deter-mined by the Wilcoxon test.(B)Rats were pretreated with SP600125or vehicle 1hour before PH.The levels of aspartate and alanine amino-transferases were determined 60hours after PH.Statistical signifi-cance was determined by the t test.(C)Sections from SP600125-or ve-hicle-treated animals 60hours after PH were stained with hematoxylin-eosin.(D)Rats were pretreated with SP600125or vehicle 1hour before PH and livers were harvested 24or 60hours after PH.Cells with typical apoptotic morphology were counted in 20ϫ200fields in 2different animals per treatment group.The number of apoptotic cells was di-vided by the total number of paren-chymal cells and is expressed as percentage.(E)Caspase 3activity was determined by incubating cell extracts from livers harvested 24and 60hours after PH with the caspase 3substrate AFC-DEVD for 2hours.Mouse hepatocytes treated with the agonistic Jo2antibody served as positive control.Caspase 3activity is shown as AFC release (pmol)per g protein extract after 2hours of incubation.HEPATOLOGY,Vol.37,No.4,2003SCHWABE ET AL.829after PH,but the function of JNK in liver regeneration remains largely unknown.4Several agonists may induce JNK during liver regeneration:(1)TNF-␣is an impor-tant mediator of early-phase JNK activation after PH as shown by the reduced JNK activation and AP-1binding activity in rats injected with antibodies to TNF-␣and mice with inactivated TNFR1.3,11TNF-␣acts as a comi-togen that primes hepatocytes for the pro-proliferative effects of hepatic mitogens including EGF and HGF through the activation of NF-B,AP-1,STAT3,and the release of IL-6,but alone is incapable to induce hepato-cyte proliferation.3,24,25However,the relative role of these pathways in driving proliferation after PH is unclear and some factors such as NF-B may only play a minor role.26 (2)EGF and HGF are believed to play a key role in liver regeneration and both induce JNK activation and AP-1–dependent gene transcription in cultured hepatocytes.4,27 The merging of2crucial pro-proliferative signaling cas-cades(i.e.,cytokines and growth factors)at the level of JNK points toward a role for JNK in driving proliferation during liver regeneration.This hypothesis is supported by studies showing JNKs to be an important regulator of cell proliferation in other cell types.8,28JNKs phosphorylate the N-terminal domain of c-Jun,increase its transactiva-tion,and thereby up-regulate AP-1–dependent transcrip-tion.Additionally,JNKs phosphorylate the transcription factors ATF2and JunD.These factors homodimerize and heterodimerize with other AP-1components to induce AP-1–dependent transcription and the up-regulation of many genes that are critically involved in cell proliferation and survival.5JNK Activity Is Required for Hepatocyte Prolifera-tion.The liver contains JNK1and JNK2,which are be-lieved to fulfill similar functions,but it does not contain the neuronal form JNK3.Because JNK1Ϫ/Ϫ/JNK2Ϫ/Ϫmice are not viable,29,30our study used a pharmacologic approach to study the role of JNKs in liver regeneration. The small molecule JNK inhibitor SP600125blocks both JNK1and JNK2in a highly specific manner13and effi-ciently inhibited JNK activity in the liver and cultured hepatocytes in our study.Blocking JNK activity with SP600125inhibited the normal proliferative response af-ter PH,indicating that JNKs are essential components of the cellular machinery that drives hepatocytes to enter the cell cycle after PH.SP600125-treated animals showed a blocked G1/S transition,as apparent by the decreased expression of PCNA and by the reduction of the levels of cyclin D1,which is predominantly expressed during G1. SP600125additionally reduced mitoticfigures after PH and3H-thymidine incorporation in cultured hepatocytes. At later time points,SP600125-treated animals had sim-ilar expression levels of PCNA,indicating the absence of early-phase JNK activity still allows hepatocytes to enter the cell cycle,albeit in a delayed manner.JNK Activation Drives Cyclin D1Expression.Sev-eral of the AP-1regulated genes are critical regulators of cell growth,among them cyclin D1and PCNA.9,31The importance of cyclin D1as a critical downstream target of JNK is suggested by the impaired transcription of cyclin D1and the reduced activity of cyclin D1–dependent ki-nases in c-junϪ/Ϫmouse embryonicfibroblasts.6,32In hepatocytes,cyclin D1plays a crucial role in driving pro-liferation as recently shown by a study in which cyclin D1 overexpression per se was sufficient to promote hepato-cyte replication.10Moreover,it has been shown that cy-clin D1is the most prominently up-regulated type D cyclin after PH and in response to mitogenic stimuli and plays a critical role in the driving cells through the G1 restriction point.33Our study found a strong reduction of cyclin D1protein and mRNA expression in SP600125-treated animals after PH.Similar results were obtained in cultured hepatocytes in which cyclin D1mRNA and pro-tein levels as well as the transcription of a cyclin D1–promoter-driven reporter gene were decreased by SP600125in EGF-treated hepatocytes.Cyclin D1ex-pression in the liver may be regulated at the posttranscrip-tional level.34,35In our study,however,cyclin D1was regulated mainly at the transcriptional level through a JNK-dependent pathway,suggesting that cyclin D1is an important mediator of pro-proliferative effects of JNK.In conjunction with results from previous studies,our data indicates that the growth factorϩTNF-␣3JNK3 AP-13cyclin D1pathway is crucial in driving prolifer-ation during liver regeneration.JNK Blockade Decreases Survival After PH.Inac-tivation of important signaling pathways such as TNF-␣and IL-6impairs liver regeneration and decreases survival after PH.11,25In our study,SP600125significantly re-duced survival3days after PH.SP600125-treated ani-mals showed a higher degree of microvesicular steatosis similar to mice with inactivated TNFR1or c-jun.11,22 However,vehicle-treated animals,also showed increased microvesicular steatosis in our study,presumably due to components in the drug vehicle that may exacerbate the low degree of microvesicular steatosis occurring normally after PH.11Animals that survived day3after PH showed a slightly delayed but ultimately normal liver regeneration with a slightly decreased liver-to-body-weight ratio at day 3and a normal liver-to-body-weight ratio at day5(data not shown).These data are consistent with other studies in which the blockade of single signaling pathways caused a significant delay of liver regeneration and increased mortality,but an ultimately normal regeneration in sur-viving animals,most likely due to compensatory effects of830SCHWABE ET AL.HEPATOLOGY,April2003。