Composite Titanium Dioxide Nanomaterials
- 格式:pdf
- 大小:2.36 MB
- 文档页数:37


§09030二氧化鈦Titanium Dioxide別名:Titania;CI Pigment white 6;CI (1975) No.77891;INS No. 171;CAS No. 13463-67-7。
分子式:TiO2分子量:79.881. 定義:本品可經「硫酸鹽」或「氯化物」兩種製程產之,不同製程之條件決定最終晶體為銳鈦石﹙anatase﹚或金紅石﹙rutile﹚。
採硫酸鹽製程,係利用硫酸消化鈦鐵礦﹙FeTiO3﹚或鈦礦渣後,經一系列純化步驟分離出二氧化鈦,再經水洗、煅燒及微粉化。
採氯化物製程,係利用氯氣與含鈦礦物經還原反應生成無水四氯化鈦後,直接加熱氧化或與蒸氣反應純化二氧化鈦,或者利用濃鹽酸與含鈦礦物反應生成四氯化鈦溶液,經水解純化成二氧化鈦,再經過濾、沖洗及煅燒。
為改善產品特性,二氧化鈦可能包覆著少量的鋁或矽。
2. 含量:99.0 %以上﹙以乾重計,氧化鋁及二氧化矽不予計入﹚。
3. 外觀:白色至微帶色澤粉末。
4. 溶解度:不溶於水、鹽酸、稀硫酸及有機溶劑。
在氫氟酸及熱的濃硫酸中可緩慢溶解。
5. 鑑別:本品0.5 g 加入硫酸5 mL,緩慢加熱直到硫酸冒煙後冷卻。
小心地加水稀釋至100 mL並過濾,取濾液5 mL加數滴過氧化氫試劑,立即呈現橙紅色。
6. 乾燥減重:0.5 %以下﹙105 ℃,3小時﹚。
7. 熾灼減重:1.0 %以下﹙800 ℃,以乾重計﹚。
8. 氧化鋁或二氧化矽:2 %以下(單一或共存)。
9. 酸可溶物:0.5 %以下;若含鋁或矽則在1.5 %以下。
10. 水可溶物:0.5 %以下。
11. 0.5 N鹽酸可溶物:(1)銻:2 mg/kg以下。
(2)砷:1 mg/kg以下。
(3)鎘:1 mg/kg以下。
(4)鉛:10 mg/kg以下。
12. 汞:1 mg/kg以下。
13. 分類:食品添加物第﹙九﹚類。
14. 用途:著色劑。
資料出自:.tw/foodnew/MenuThird.aspx?LanguageType=1&SecondMenuID=5&ThirdMen uID=174。

CHEMICAL INDUSTRY AND ENGINEERING PROGRESS 2017年第36卷第7期·2488·化 工 进展氧化钛纳米片材料的合成及其催化应用进展李路1,2,徐金铭2,齐世学1,黄延强2(1烟台大学化学与化工学院,山东 烟台 264005;2中国科学院大连化学物理研究所,航天催化与新材料研究室,辽宁 大连 116023)摘要:氧化钛纳米片材料为一种新兴的二维层状材料,在催化、环境、能源和电子领域引起人们广泛的关注。
本文从催化研究的角度出发,综述了氧化钛纳米片材料的结构、制备方法、金属及非金属元素的掺杂、纳米片基复合材料和其在光催化、光电催化和热催化等方面的应用进展。
分析表明氧化钛纳米片材料拥有特殊的形貌和特别的物理化学性质,通过控制材料的组成及结构变化,能够实现氧化钛纳米片材料的多种功能化。
指出氧化钛纳米片材料虽然有着优良的性能,但是在实际应用中远不能满足要求。
因此,优化合成和探索新形式的二氧化钛纳米片材料,对其表面进行改性及开发具有特殊功能纳米复合材料是解决其瓶颈的有效途径。
探索催化反应过程中的反应机理,开发氧化钛纳米片基工业应用催化剂将是今后重要的研究方向。
关键词:氧化钛纳米片;层状钛酸盐;催化;合成;纳米材料中图分类号:O611.4 文献标志码:A 文章编号:1000–6613(2017)07–2488–09 DOI :10.16085/j.issn.1000-6613.2016-2340Recent advances in titanium oxide nanosheets for catalytic applicationsLI Lu 1,2,XU Jinming 2,QI Shixue 1,HUANG Yanqiang 2(1College of Chemistry and Chemical Engineering ,Yantai University ,Yantai 264005,Shandong ,China ;2Laboratory of Catalysts and New Materials for Aerospace ,Dalian Institution of Chemical Physics ,Chinese Academy of Science ,Dalian 116023,Liaoning ,China )Abstract: As a new class 2D layered materials ,Titanium oxide nanosheets have attracted great interest inthe fields of catalysis ,environment ,energy and electronics. In this work ,we provide an overview of the recent advance of titanium oxide nanosheets on their layered structure ,synthetic methods ,doping with metals or nonmetal ,as well as their nanocomposites and applications in catalysis. Recent researches indicate that titanium oxide nanosheets with unique structure and special physical and chemical properties can achieve multiple functions by controlling their compositions and structures. Although titanium oxide nanosheets have a lot of advantages ,they are still far from practical applications. Therefore it is demanded to explore new synthesis ,doping and modification methods ,and develop new composite materials. In addition ,the reaction mechanism in the catalytic reaction process and the industrial application of titanium oxide nanosheets will be important research directions in the future. Key words :titanium oxide nanosheets ;layered titanate compounds ;catalysis ;synthesis ;nanomaterials助理研究员,从事有序介孔材料合成及表面修饰和生物质催化转化制化学品相关科研工作。
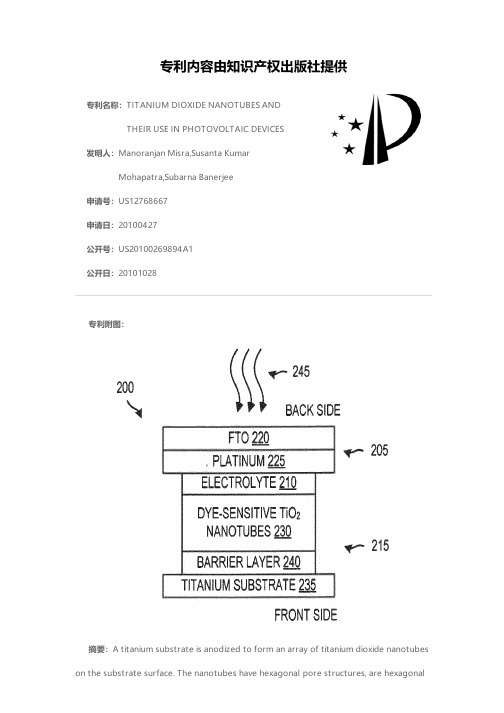
专利名称:TITANIUM DIOXIDE NANOTUBES ANDTHEIR USE IN PHOTOVOLTAIC DEVICES发明人:Manoranjan Misra,Susanta KumarMohapatra,Subarna Banerjee申请号:US12768667申请日:20100427公开号:US20100269894A1公开日:20101028专利内容由知识产权出版社提供专利附图:摘要:A titanium substrate is anodized to form an array of titanium dioxide nanotubes on the substrate surface. The nanotubes have hexagonal pore structures, are hexagonalin nature along their length and are tightly packed. The electrolyte solution used in the anodization process comprises the complexing agent Na[HEDTA]. The titanium dioxide nanotubes are formed at a rate of about 40 μm/hr. A titanium dioxide nanotube array detaches from the underlying titanium dioxide substrate by allowing the array to stand at room temperature, or by applying heat to the array. The resulting titanium dioxide membrane has a barrier layer on the back side of the membrane, which closes one end of the constituent nanotubes. The barrier layer can be removed via a chemical etch to create a membrane comprising nanotubes with open ends. The titanium dioxide membrane can be filled with a photosensitive dye and used as part of a dye sensitive photovoltaic devices.申请人:Manoranjan Misra,Susanta Kumar Mohapatra,Subarna Banerjee地址:Reno NV US,Lexington KY US,Reno NV US国籍:US,US,US更多信息请下载全文后查看。

㊀第43卷㊀第3期2024年3月中国材料进展MATERIALS CHINAVol.43㊀No.3Mar.2024收稿日期:2023-06-13㊀㊀修回日期:2023-11-12基金项目:中央高校基本科研业务费专项资金资助项目(2023CX 01021);国家自然科学基金资助项目(52301186)第一作者:穆啸楠,男,1991年生,副研究员,硕士生导师通讯作者:张洪梅,女,1979年生,教授,博士生导师,Email:zhanghm@DOI :10.7502/j.issn.1674-3962.202306010石墨烯增强钛基复合材料研究进展穆啸楠1,2,张洪梅1,2,王㊀宇1,2(1.北京理工大学材料学院,北京100081)(2.冲击环境材料技术国家级重点实验室,北京100081)摘㊀要:非连续增强钛基复合材料(DRTMCs)具有高比强度㊁低密度㊁优异的耐蚀性等诸多特性,在航空航天㊁国防工业㊁交通运输等领域具有广泛的应用前景㊂石墨烯具有良好的本征物理和力学性能,是近年来的二维碳纳米 明星 材料,被视为极具潜力的DRTMCs 纳米增强体㊂国内外研究者聚焦DRTMCs 设计与制备,突破了低温快速成型和界面改性等关键技术,初步实现了界面精细调控和微观构型,获得石墨烯在钛基体中的本征增强,制备出强塑性匹配较好的DRTMCs㊂简要综述近些年来石墨烯增强钛基复合材料的设计方法和制备工艺,探讨界面反应㊁界面结构㊁微观构型等关键因素对复合材料力学性能和失效机制的影响规律,并提出石墨烯增强钛基复合材料未来的发展方向㊂关键词:石墨烯;钛基复合材料;制备方法;界面设计;力学行为中图分类号:TB333.1+2㊀㊀文献标识码:A㊀㊀文章编号:1674-3962(2024)03-0212-10引用格式:穆啸楠,张洪梅,王宇.石墨烯增强钛基复合材料研究进展[J].中国材料进展,2024,43(3):212-221.MU X N,ZHANG H M,WANG Y.Progress on Graphene Reinforced Titanium Matrix Composites[J].Materials China,2024,43(3):212-221.Progress on Graphene Reinforced TitaniumMatrix CompositesMU Xiaonan1,2,ZHANG Hongmei1,2,WANG Yu1,2(1.School of Materials Science and Engineering,Beijing Institute of Technology,Beijing 100081,China)(2.National Key Laboratory of Science and Technology on Materials Under Shock and Impact,Beijing 100081,China)Abstract :Discontinuously reinforced titanium matrix composites (DRTMCs)have many excellent properties such ashigh specific strength,low density,superior corrosion resistance,which have become ideal candidates for aerospace,defense industry and transportation field and have a wide range of applications.Graphene,as a two-dimensional carbon nano star material with excellent intrinsic physical and mechanical properties,has been regarded as a promising nano-reinforcement for DRTMCs in recent years.Recently,domestic and foreign researchers have been focusing on solving the key issue of severe interfacial reaction caused by traditional high-temperature sintering through rapid low-temperature con-solidation as well as powder modification,established a precise interface control method for graphene-reinforced Ti compos-ites,and optimized the configuration to realize the graphene nanoplatelets intrinsic strengthening.The present work sum-marized the fabrication and design method of graphene reinforced titanium matrix composites in recent years,investigating the effects of interface reactions,interface structure and micro-configuration or other key factors on the mechanical proper-ties,failure mechanism,and prospected the development trend of graphene reinforced titanium matrix composites in the further.Key words :graphene;titanium matrix composites;fabrication method;interface design;mechanical behavior1㊀前㊀言钛合金由于其优异的比强度㊁耐蚀性和高温性能,在航空航天㊁国防和汽车工业等领域拥有广泛的应用前景[1-5]㊂经过几十年的发展,钛合金力学性能发展已逼近极限,难以满足装备更为严苛的应用需求㊂钛合金的复合化成为重要的发展方向㊂非连续增强钛基复合材料㊀第3期穆啸楠等:石墨烯增强钛基复合材料研究进展(discontinuously reinforced titanium matrix composites,DRT-MCs)具有较高的模量㊁耐磨性㊁强度,与钛合金相比具有显著优势[2,6,7]㊂DRTMCs按照增强体生成方式可以分为原位自生颗粒/晶须增强DRTMCs(如通过引入碳源㊁硼源在基体内原位自生TiC㊁TiB陶瓷相)和外加颗粒增强DRTMCs(如加入SiC㊁Al2O3㊁Si3N4等陶瓷颗粒)[8,9]㊂近年来,碳纳米材料由于其超高的物理和力学性能,在增强金属综合性能方面已展现出较为突出的效果,常用的碳纳米增强体有碳纳米管(carbon nanotubes, CNTs)㊁纳米金刚石(nano diamonds,NDs)和石墨烯等㊂石墨烯纳米片(graphene nanoplatelets,GNPs)是一种具有较大比表面积,保留了大部分石墨烯优异力学(理想状态下石墨烯断裂强度~130GPa,杨氏模量~1.0TPa[8])㊁热学及电学性能的纳米碳材料,作为增强体已广泛应用于树脂㊁陶瓷和金属基复合材料㊂与传统陶瓷颗粒和晶须增强的DRTMCs相比,GNPs/Ti复合材料表现出更加优异的强度㊁塑性和抗冲击性等特性,受到国内外研究者广泛关注[9-15]㊂然而,GNPs/Ti复合材料的设计和制备始终面对三大难题:其一,GNPs在钛基体中的分散性较差;其二,由于钛具有非常高的化学活性,GNPs极易与钛基体发生严重界面反应而失去本征性能;其三, GNPs自身纳米结构的完整性较差和缺陷较多㊂以往的研究表明,CNTs和NDs作为钛基体的增强体也存在类似问题,大部分纳米碳材料在DRTMCs制备过程中通常会形成微米尺寸TiC颗粒,使复合材料的性能提升难以达到预期效果㊂对于GNPs/Ti复合材料,如何抑制GNPs的严重反应,实现界面反应精准控制,较好发挥GNPs的本征增强作用,是重要的研究方向㊂GNPs/Ti 复合材料的研究不仅对DRTMCs的发展具有重大意义,也对碳纳米相增强金属基复合材料的研发具有重要的理论指导意义㊂2㊀GNPs/Ti复合材料制备方法制备GNPs/Ti复合材料的方法有很多种,如图1所示[16-22],主要包括热压烧结㊁放电等离子烧结(spark plasma sintering,SPS)㊁微波烧结㊁热等静压㊁粉末注射成型㊁激光熔覆等㊂其中热等静压㊁金属注射成型和激光熔覆等方法流程复杂㊁成本较高,尤其是这些工艺过程是在高温环境下制备,难以实现GNPs与钛基体的反应控制㊂传统热压烧结工艺的烧结时间较长,使得GNPs与Ti几乎全部发生反应生成TiC相㊂SPS工艺具有升温速度快㊁烧结时间短㊁烧结温度低等优势,因此采用该方法制备的材料组织均匀细小且界面反应可控制[23],SPS已成为目前GNPs/Ti复合材料的优选制备方法㊂西北有色金属研究院张于胜等[24]采用SPS和热轧工艺制备出GNPs/TC4㊁石墨颗粒(graphite particles,GPs)/ TC4和氧化石墨烯(graphene oxide,GO)/TC43类钛基复合材料,研究发现由于SPS快速升降温的特点,900ħ烧结和轧制条件下所制备的复合材料碳纳米相未完全反应,其中GNPs的本征结构保留最好,所制备的GNPs/ TC4复合材料屈服强度相比TC4㊁GPs/TC4和GO/TC4分别提高了24.6%,9.22%和5.62%㊂武汉理工大学史晓亮等[25]在1100ħ㊁保温10min的条件下,制备出GNPs/TiAl合金复合材料,测试结果表明,与TiAl基体相比,GNPs使该复合材料摩擦系数降低了4倍㊁磨损率降低了4~9倍㊂哈尔滨工业大学孔凡涛等[26]对SPS制备的GNPs/Ti-47Al-2Cr-4Nb-0.3W复合材料的微观结构进行研究,发现少量添加的GNPs对基体的晶粒细化有显著效果,使平均晶粒尺寸降低了45%㊂东南大学王军等[27]采用SPS工艺制备出高致密的GNPs/TC4复合材料,研究发现晶界处均匀分布的GNPs抑制了钛合金基体中魏氏组织的形成,有利于复合材料组织细化和力学性能的提升㊂印度塔帕尔大学Sharma等[28]采用SPS工艺制备了GNPs/TC4复合材料,该复合材料纳米硬度为5.29GPa,弹性模量为119.8GPa,比烧结态TC4分别提高了68.4%和140.5%㊂四川大学杨刚等[29]在850ħ条件下制备了GNPs/Ti复合材料,并分析了其高温压缩性能,研究发现,在大电流(45000A)或等离子体局部高温作用下,碳原子加速产生活化或非晶化,破坏了GNPs 的本征结构[30]㊂北京理工大学张洪梅等[31-34]采用低温快速成型工艺制备出GNPs/Ti复合材料,有效抑制了界面反应,其中GNPs质量分数为0.1%的GNPs/Ti复合材料具有优异的拉伸强度,比纯钛提高了54.2%~70%㊂东南大学的张法明团队[27,35]对GNPs和洋葱碳增强DRT-MCs开展了系统研究,发现复合材料内部在形成了纳米/亚微米级的TiC颗粒的同时保留了部分碳纳米相,使该复合材料拉伸强度相比纯Ti提高了40%㊂此外,重庆大学刘许旸等[36]㊁西南交通大学蒋小松等[37]㊁西安交通大学刘马宝等[38]㊁北京航空材料研究院曹正等[39]㊁苏州大学陈瑶等[40]在GNPs/Ti复合材料的制备方法研究方面也取得了一定的进展,为实现GNPs在钛基体中应用提供了工艺借鉴和技术参考㊂312中国材料进展第43卷图1㊀GNPs/Ti复合材料制备方法[16-22]:(a)热压烧结,(b)放电等离子烧结,(c)微波烧结,(d)热等静压,(e)粉末注塑成型,(f)激光熔覆Fig.1㊀Preparation methods of GNPs/Ti composites[16-22]:(a)hot-pressing,(b)spark plasma sintering,(c)microwave sintering,(d)hot iso-static sintering,(e)powder injection molding,(f)laser melting deposition3㊀GNPs/Ti复合材料界面与微观构型设计相比传统陶瓷颗粒和晶须增强体,GNPs厚度仅为几至几十纳米,具有较高的比表面积(250~1000m2/g),因而GNPs/金属复合材料中的界面区域占比得到较大提高,界面作为连接基体与增强相的 桥梁 ,是GNPs/金属复合材料性能的决定因素,也是目前研究的热点㊂发展GNPs/Ti复合材料的界面设计及控制方法,优化制备工艺,揭示 界面结构-材料性能 的响应关系,已成为GNPs/Ti复合材料的重点研究方向[41,42]㊂微观构型设计可对DRTMCs综合力学性能的提升产生显著效果[31,43-45],近年来,具有特殊微观构型的DRT-MCs受到广泛关注㊂研究发现,网状结构可以细化基体晶粒,有助于基体和增强体协调变形,抑制裂纹扩展;层状结构具备很强的抗裂纹萌生与扩展能力,可以实现对裂纹钝化㊁偏转和桥接等各种韧化机制的协调,充分发挥韧化效果[46,47]㊂本文汇总了近几年国内外较为典型的GNPs/Ti复合材料界面与微观构型设计研究报道[32,36,45,48-60],如图2所示㊂粉末冶金制备GNPs/Ti复合材料涉及烧结㊁热加工和热处理等工艺过程,尽管界面处原位生成的TiC层可有效提高界面结合强度,提升GNPs载荷传递效率,但图2㊀GNPs/Ti复合材料界面与微观构型设计方法[32,36,45,48-60] Fig.2㊀Micro-configuration design and preparation methods of GNPs/Ti composites[32,36,45,48-60]同时也会破坏GNPs二维纳米结构,降低本征增强效果㊂如日本大阪大学Kondoh教授团队[61]采用SPS和热挤压工艺(挤压比为3ʒ7,挤压温度为1000ħ)制备了CNTs/ Ti复合材料,挤压后增强体分散性得到较大提高,但碳412㊀第3期穆啸楠等:石墨烯增强钛基复合材料研究进展纳米材料的本征结构损伤严重㊂奥地利研究中心Melend-ez等[62]通过粉末冶金法制备出碳纳米材料(CNTs㊁NDs)增强的DRTMCs,研究发现,碳纳米材料与基体反应生成了高含量的TiC,该复合材料虽具有高的硬度和强度,但塑(韧)性较差㊂韩国延世大学Shin等[63]在较低温度(500ħ左右)和高压力条件下烧结制备了GNPs/Ti复合材料,研究发现,GNPs与Ti基体之间基本没有界面反应,而是通过Ti-C离子键和范德华弱键结合,由于界面结合强度较弱,导致复合材料在加载条件下界面易脱粘㊂由此可知,若GNPs/Ti复合材料界面TiC相含量过低,将使得界面结合强度不够,性能提升不足;TiC相生成过量,将容易破坏GNPs的本征结构,降低复合材料塑(韧)性,引发材料脆性失效㊂因此,如何为GNPs披上一层 恰到好处 的TiC层 外衣 ,是复合材料综合性能提升的关键㊂研究人员开展了界面微结构演化和反应调控等相关工作㊂北京理工大学张洪梅等[30,31,64,65]率先研究了GNPs/Ti复合材料的界面演化机制,基于低温高压SPS预成型和快速热处理工艺制备了GNPs/Ti复合材料㊂研究发现,TiC优先在GNPs的开口边缘位置形核生长㊂如图3所示[66],在850ħ热处理条件下,随着反应时间的延长(热处理时间120~600s),界面处的TiC相形貌从初始的 颗粒孤岛 (图3a)变成 蠕虫状 的长条(图3b),最后彼此相连形成厚度约150nm 致密的 片层状 结构(图3c)㊂旋进电子衍射分析结果表明,800ħ以下热处理反应生成的TiC层无择优取向,而是由众多细小纳米TiC颗粒相连组成;850ħ以上热处理反应生成的TiC层会产生<101>和<111>织构以及生长层错[66]㊂当界面反应层达到致密状态,碳原子将主要以穿过TiC层的方式到达Ti基体,使TiC继续生长[31]㊂西安交通大学刘马宝等[38]分析了GNPs/ TC4复合材料的界面微结构演化,研究发现,GNPs/ TC4复合材料界面主要形成致密的TiC反应层(无其他碳产物生成),且反应层的致密化可明显降低碳原子的扩散速率,使GNPs的本征结构在TC4合金基体中较好地保留㊂上述研究揭示了GNPs与Ti(合金)复合材料界面元素的反应扩散行为㊁界面产物的生长特性和形貌演变规律㊂研究人员还尝试采用GNPs表面改性方式调控界面㊂如北京理工大学[64]和西北有色院[67]等研究单位采用化学镀方法在GNPs表面引入Ni㊁Cu㊁Ag等纳米颗粒层,制备了Ni@GNPs㊁Cu@GNPs和Ag@GNPs纳米复合增强体,研究发现:GNPs表面金属化可以有效延缓复合材料界面反应的进程㊂由于纳米金属层可抑制碳原子扩散,界面反应产物由粗大TiC颗粒(尺寸约几百纳米)转变为细小的TiC颗粒(尺寸约十几纳米)㊂同时,金属层转变图3㊀GNPs/Ti复合材料在850ħ热处理条件下界面TiC相形貌随时间的演化过程[66]:(a)120s,(b)300s,(c)600s Fig.3㊀Interfacial TiC morphology evolution with time of GNPs/Ti composite during heat treatment at850ħ[66]:(a)120s,(b)300s,(c)600s为金属间化合物层,提高了GNPs与钛基体的界面结合强度,并且随着金属层原子的扩散,钛基体发生相转变(如β相产生和次生α析出),进一步改变了基体微观组织形貌,使复合材料实现了 1+1>2 的GNPs㊁界面反应层和基体析出相协同增强效果,准静态拉伸性能明显优于相同工艺参数下GNPs/Ti复合材料㊂西北工业大学陈彪团队[68]通过超声分散和物理吸附的方式得到了SiC p@ GNPs纳米复合增强体,采用SPS工艺制备出SiC p@ GNPs/Ti复合材料,该复合材料耐磨性能比纯钛提高了86.8%,研究发现:GNPs表面纳米SiC p层与GNPs表面金属化的效果类似,抑制了界面反应,减少了GNPs在烧结制备过程中缺陷的形成,反应生成的少量TiC和Ti5Si3相有利于提高界面结合强度㊂综上所述,研究者在GNPs/Ti复合材料界面反应机理及界面产物生长特性等方面开展的基础研究可为GNPs/Ti复合材料的界面设计及控制提供理论依据㊂在微观构型设计方面,哈尔滨工业大学黄陆军等[46]以提高DRTMCs室温塑性和高温强度为目标,基于Hash-in-Shtrikman理论,采用晶界强韧化设计的方法,制备出TiB w增强体呈准连续三维网状分布的DRTMCs,有效提高了材料的塑(韧)性㊂东南大学张法明团队[47]采用SPS 工艺制备了具有三维网状结构的少层石墨烯(few-layer graphene,FLG)/TC4复合材料,研究了SPS工艺温度对复合材料微观组织演变㊁压缩/拉伸力学性能和摩擦学行为的影响规律,分析了强化机理㊂FLG通过低能球磨在TC4粉末表面均匀分散,并经烧结过程在复合材料晶界处原位生成三维网络分布的FLG-TiC复合增强体㊂由于FLG和TiC在网络边界的协同强化作用,该复合材料相比TC4基体综合力学性能明显提高,且磨损率显著降低㊂图4a为FLG/TiC增强TC4合金基体的微观作用机制示意图[47]㊂西北工业大学陈彪团队[69]制备了具有GNPs网状分布特征的GNPs/TC4复合材料,研究发现,网状结构512中国材料进展第43卷一定程度缓解了复合材料裂纹应力集中,微裂纹倾向于沿着网络边界和GNPs /碳化物颗粒进行形核和长大,有利于微裂纹分支和偏转㊂北京理工大学张洪梅团队[31]设计出GNPs /Ti 片状复合粉末构筑了复合材料层状结构(图4b),不仅解决了GNPs 的团聚问题,同时获得了良好的GNPs 本征增强效果,制备的层状结构GNPs /Ti 复合材料打破了传统DRT-MCs 的压缩屈服强度极限(达到~2GPa)㊂基于仿生学思想,他们设计出具有三维复合结构的GNPs-(TiB w )/Ti 界面[32-34,48](简称 三维界面 ,如图4c),显著提升了复合材料的拉伸强度(比纯Ti 基体提升200MPa),并保持优异的室温塑性(23.2%)㊂图4d 为GNPs-(TiB w )/Ti 复合材料拉伸断口形貌[32],可以看出,TiB 晶须作为桥梁连接了GNPs-TiC-Ti 的多重界面,减缓了TiC 层的断裂速度,同时抑制了GNPs 与周围基体的界面脱粘,防止TiC 层与相邻基体的变形分离,GNPs 的存在还可以桥接裂纹,进一步获得增韧效果,促进GNPs 更好地发挥其本征强化作用㊂图4㊀FLG /TC4复合材料的三维网络结构示意图和界面结合模型(a)[47],微观层状GNPs /Ti 复合材料三维微观结构及微裂纹偏转(b)[31],受苍耳植物外形启发设计的具有 三维界面 特征的GNPs-(TiB w )/Ti 复合材料(c)[34],GNPs-(TiB w )/Ti 复合材料拉伸断口的SEM 照片(d)[32]Fig.4㊀Schematics of 3D network structure and interfacial bonding model of FLG /TC4composites (a)[47],cracks deflection in micro-laminated GNPs /Ti composite (b)[31], 3D interface in GNPs-(TiB w )/Ti composite inspired by Xanthium sibiricum (c)[34],SEM image of the tensile frac-ture of GNPs-(TiB w )/Ti composites (d)[32]4㊀GNPs/Ti 复合材料力学性能与失效机制GNPs /Ti 复合材料相比TiC(或TiB)/Ti 复合材料在力学性能上表现出更显著的强化效率,更优异的强塑性匹配,且GNPs 应用于钛合金也具有类似的增强效果[24,30,70-76]㊂选取部分具有代表性GNPs /Ti 复合材料与TiC(或TiB)/Ti 复合材料进行力学性能对比(图5a 和5b)㊂另一方面,微观构型设计表现出更突出的DRTMCs 增塑/增韧特性[30,41,58,64,68,77-97],尤其是GNPs /Ti 复合材料层状结构设计(代表成果统计见图5c)㊂进一步优化微观构型和界面微观结构,可以为GNPs /Ti 复合材料综合性能提升提供新思路与新途径㊂目前研究表明,GNPs /Ti 复合材料的强化机制主要为细晶强化㊁载荷传递㊁固溶强化㊁织构强化和Orowan 强化等(与目前已深入研究的颗粒增强金属基复合材料强化机制类似,在此不做赘述)㊂在塑(韧)性提升机制方面,除了微观构型起到的显著效果之外,界面微观结构也是影响复合材料增强和增塑(韧)的关键因素,也是微观构型发挥效果的前提㊂通过测量界面断裂韧性(如压痕实验法㊁四点弯曲实验法等[98])可以对界面特性进行评612㊀第3期穆啸楠等:石墨烯增强钛基复合材料研究进展估,但增强相尺寸为纳米尺度时,宏观的实验方法难以表征界面特性㊂研究人员采用数学模型(如等应力-等应变模型㊁剪切-滞后理论模型和粘结区模型等)对材料的宏观力学性能进行拟合,间接计算界面结合强度㊂首先对除载荷传递强化机制外的其它强化贡献进行定量计算,并利用差减法得出载荷传递作用的强化贡献,然后使用剪切-滞后强化模型倒推,间接计算出复合材料的界面结合强度[99]㊂事实上,经过拉伸变形,复合材料拉伸断口的韧窝附近或靠近增强体的区域通常会产生纳米孔洞或明显的应力集中㊂与初始状态相比,此时复合材料的界面状态已发生改变,增强体-基体的界面力学特性不能被真实反映㊂图5㊀GNPs /Ti 复合材料与传统原位自生TiC (或TiB )/Ti 复合材料性能对比:(a )拉伸强度[24,30,70-72,74-76],(b )断后延伸率[24,30,70-72,74-76],(c)不同微观构型的复合材料的归一化拉伸强度与断后伸长率分析图[30,41,58,64,68,77,85,87,89,93,95-97]Fig.5㊀Comparison of properties of GNPs /Ti composites and traditional in-situ TiC (or TiB )/Ti composites:(a )ultimate tensilestrength [24,30,70-72,74-76],(b)elongation to fracture [24,30,70-72,74-76],(c)normalized tensile strength and normalized tensile elongationof composites with different structural types [30,41,58,64,68,77,85,87,89,93,95-97]㊀㊀北京理工大学张洪梅等[44,66,100]采用微纳扩散偶实验结合动力学计算的界面精细控制方法,通过短时热处理获得不同碳-钛反应程度的GNPs /Ti 复合材料㊂在准静态及高应变率动态加载条件下开展了GNPs /Ti 复合材料力学响应行为研究,分析不同界面微观结构对GNPs 载荷传递和复合材料塑(韧)性提升的影响规律,揭示了GNPs 及界面微观结构在载荷作用下的失效机制㊂研究表明,适当的界面反应可产生GNPs-TiC 协同强韧化 效果㊂图6为准静态拉伸变形后GNPs /Ti 复合材料微观组织和断口形貌,从图中可以看出,界面附近的基体中存在Frank-read 位错源和位错环,以及大量的线段状位错线,在TiC 反应层与Ti 基体的界面处也发现了位错环以及大量位错缠结㊂TiC 反应层在载荷传递的过程中出现了多处微裂纹形核现象(如图中红色虚线所示),部分微裂纹还呈现出较大偏转㊂微裂纹的产生说明界面载荷传递的应力达到了TiC 反应层的临界断裂应力,然而TiC反应层产生的微裂纹在GNPs 两侧并非是对称的,同时GNPs 与TiC 反应层也无界面脱粘现象,因此GNPs 在载荷传递的过程中对界面反应层的裂纹扩展起到了有效地抑制作用,微裂纹如果继续扩展,则必须绕过GNPs 与TiC 的界面㊂TiC 反应层与GNPs 在复合材料拉伸过程中保持高效的协同承载能力,尽管TiC 反应层在拉伸过程中微裂纹不断形核并长大,但始终能够有效地将来自基体的加载应力传递到GNPs,随着拉伸过程的继续进行,GNPs 在到达临界断裂应力后产生撕裂或断裂,这意味着本征结构保留完好的石墨烯不仅可以起到高效的载荷传递作用,还可以有效抑制界面处微裂纹的扩展,从而同时提高复合材料的强度和塑(韧)性㊂当GNPs /Ti 复合材料处于过度反应界面状态时(即TiC 反应层过厚,GNPs 本征结构破坏严重),GNPs 将成为一种 缺陷 ㊂图7a 所示为存在严重界面反应的GNPs 和断裂TiC 层TEM 形貌照片,与图6相比,相同拉伸应712中国材料进展第43卷图6㊀拥有适当界面反应的GNPs/Ti复合材料拉伸变形后微观组织TEM照片(a)和拉伸断口SEM照片(b)[66]Fig.6㊀Microstructure TEM image(a)and fracture surface SEM images(b)of GNPs/Ti composite with suitable interface reaction de-gree under tensile deformation[66]变条件下,该界面处微裂纹的尺寸明显增大,这些微裂纹穿透了整个TiC层㊂图7b显示了断裂界面附近的GNPs非晶相,这是导致GNPs-TiC结合较弱以及微裂纹快速扩展的主要原因㊂因此,严重反应界面状态的GNPs 很难抑制裂纹的扩展,从而降低了TiC的断裂韧性(图7c)㊂也就是说,GNPs不能有效地承受来自Ti基体的载荷,GNPs本征结构消失和较大损伤也将导致粗大的TiC反应层成为普通脆性陶瓷相,使GNPs-TiC 协同强韧化 效果消失[66,101]㊂北京理工大学张洪梅团队[33,65]采用分离式霍普金森压杆结合限位环技术,研究了高应变率冲击加载条件下复合材料GNPs和TiC的力学响应行为(3000s-1)㊂图8a 所示为850ħ热轧态GNPs/Ti复合材料(适当界面反应)的动态压缩应力应变曲线㊂初始应变条件下界面处首先产生微孔洞(图8b),可以观察到微孔洞内部存在断裂图7㊀存在严重界面反应的GNPs/Ti复合材料拉伸变形后:(a)微观组织TEM照片[101],(b)微裂纹贯穿界面TEM照片[101],(c)界面处GNPs有/无非晶层界面时的失效模型[66] Fig.7㊀GNPs/Ti composite with severe reaction degree under tensile deformation:(a)TEM image of the microstructure[101],(b)TEMimage of the cracks propagation on interface[101],(c)the failuremodel of the interface without/with GNPs amorphization[66]的TiC反应层,以及处于 桥接 状态且尚未断裂的GNPs;当试样的应变为0.15时,则达到以GNPs断裂为主要现象的微裂纹形核阶段(图8c),随着应变的继续增加,微裂纹随之延伸㊁扩展和连接,最终形成横向主裂纹造成的复合材料应力塌陷,如图8d所示,可以证明在冲击环境中依然存在GNPs-TiC 协同强韧化 效应㊂图8㊀3000s-1条件下GNPs/Ti复合材料的动态压缩真应力-应变曲线(a),复合材料在不同应变下的SEM照片(b~d)[65] Fig.8㊀True stress-strain curves(3000s-1)and fracture process of the GNPs/Ti composites obtained by the stop-ring method(a),the corre-sponding SEM images at various strains(b~d)[65]㊀㊀目前,GNPs-TiC 协同强韧化 效应已随着GNPs/Ti复合材料界面反应精细可控的逐步实现,被越来越多的研究者发现㊂然而,其深层次机理研究仍不够深入,进一步探究GNPs/Ti复合材料界面微观结构关键参量与复合材料力学响应的关系(尤其是在高温㊁高应变率等极端服役条件下),需要更先进的界面表征方法,进而揭示界面特性对GNPs/Ti复合材料变形与失效机制的影响规律[78]㊂812㊀第3期穆啸楠等:石墨烯增强钛基复合材料研究进展5㊀结㊀语近些年来,国内外针对石墨烯纳米片(graphene nano-platelets,GNPs)/Ti复合材料的设计和制备取得了较大进展,但尚未形成完整的体系,许多工作有待深入研究[102-104]:(1)如何实现GNPs在钛基体中的本征增强,是设计GNPs/Ti复合材料的核心问题㊂目前该复合材料的制备工艺窗口较为狭窄,高温固结与低温反应控制仍是制备GNPs/Ti复合材料的主要矛盾,可进一步探索可靠的低温/室温成型或预成型方法;也可通过优化GNPs表面改性层种类和性质,实现界面反应的可设计与可调控㊂(2)采用材料基因工程方法设计GNPs/Ti复合材料,将实验科学和计算科学结合来深入挖掘GNPs和界面微观结构在钛基体中的作用机制,突破正向设计和精准制备技术,进一步提升GNPs/Ti复合材料的综合性能;面向国际前沿技术,开展GNPs/Ti复合材料新方法㊁新技术的研发,强化原创性基础理论㊁方法和模型的研究㊂(3)针对国家重大需求,开展面向特殊应用环境的结构-功能一体化新型钛基复合材料设计和制备研究,如高强韧抗冲击GNPs/Ti复合材料㊁轻质耐高温GNPs/Ti 复合材料㊁高强高导热GNPs/Ti复合材料等,充分利用石墨烯特殊的二维纳米结构特性,发挥出石墨烯优异的本征力学和物理性能㊂(4)目前GNPs/Ti复合材料仍处于实验室研究阶段,需加强GNPs/Ti复合材料制备向中试㊁工程化和低成本化方向发展,包括材料的制备工艺放大㊁工艺优化㊁材料质量性能的稳定性研究等㊂参考文献㊀References[1]㊀黄孝余,唐斌,李金山.铸造技术[J],2022,43(7):043.HUANG X Y,TANG B,LI J S.Foundry Technology[J],2022,43(7):043.[2]㊀雷小伟,刘甲,余巍,等.稀有金属材料与工程[J],2024,53(2):417-423.LEI X W,LIU J,YU W,et al.Rare Metal Materials and Engineering [J],2024,53(2):417-423.[3]㊀邝玮,王敏敏,李九霄,等.机械工程材料[J],2015,39(2):67-72.KUANG W,WANG M M,LI J X,et al.Materials for Mechanical Engineering[J],2015,39(2):67-72.[4]㊀黄陆军,耿林.航空材料学报[J],2014,34(4):126-138.HUANG L J,GENG L.Journal of Aeronautical Materials[J],2014, 34(4):126-138.[5]㊀赵永庆.中国材料进展[J],2010,29(5):1-9.ZHAO Y Q,Materials China[J],2010,29(5):1-9.[6]㊀WANG X,WANG L,LUO L,et al.Materials&Design[J],2017,121:335-344.[7]㊀赵永庆,葛鹏,辛社伟.中国材料进展[J],2020,39(7/8):527-534.ZHAO Y Q,GE P,XING S W.Materials China[J],2020,39(7/8): 527-534.[8]㊀LEE C,WEI X,KYSAR J W,et al.Science[J],2008,321(5887):385-388.[9]㊀魏子超,韩远飞,李劭鹏,等.航空制造技术[J],2022,65(16):104-125.WEI Z C,HAN Y F,LI S P,et al.Aeronautical Manufacuring Tech-nology[J],2022,65(16):104-125.[10]GÜLERÖ,BAG㊅C N.Journal of Materials Research and Technology[J],2020,9(3):6808-6833.[11]CHEN H,MI G,LI P,et al.Materials Letters[J],2021,291:129575.[12]DONG L L,XIAO B,JIN L H,et al.Ceramics International[J],2019,45(15):19370-19379.[13]HU Z,CHEN F,XU J,et posites Part B:Engineering[J],2018,134:133-140.[14]HU Z,TONG G,NIAN Q,et posites Part B:Engineering[J],2016,93:352-359.[15]MAO X Q,DONG L L,ZHANG Y Y,et al.Carbon[J],2024,219:118805.[16]LIU J Q,HU N,LIU X Y,et al.Nanoscale Research Letters[J],2019,14:114.[17]MU X N,ZHANG H M,CAI H N,et al.Materials Science and Engi-neering:A[J],2017,687:164-174.[18]HU Z Y,ZHANG Z H,CHENG X W,et al.Materials&Design[J],2020,191:108662.[19]APURBBA K S,SHIVANI G.JOM[J],2020,72:1211-1228.[20]CHEN H,MI G B,LI P,et al.Materials[J],2020,13:3358.[21]DEHGHAN-MANSHADI A,BERMINGHAM M J,DARGUSCH M S,et al.Powder Technology[J],2017,319:289-301.[22]YAN Q,CHEN B,LI J S.Carbon[J],2021,174:451-462.[23]WANG F C,ZHANG Z H,SUN Y J,et al.Carbon[J],2015,95:396-407.[24]DONG L L,LU J W,FU Y Q,et al.Carbon[J],2020,164:272-286.[25]XU Z S,SHI X L,ZHAI W Z,et al.Carbon[J],2014,67:168-177.[26]ZHOU H,SU Y,LIU N,et al.Materials Characterization[J],2018,138:1-10.[27]SHANG C,LIU T,ZHANG F,et posites Communications[J],2020,19:74-81.[28]SHARMA D,SINGLA V K,SINGH S.Proceedings of the Institutionof Mechanical Engineers,Part C:Journal of Mechanical Engineering Science[J],2022,236(15):8542-8551.[29]LIU J,WU M,YANG Y,et al.Journal of Alloys and Compounds[J],912。

纳米二氧化钛改性丙烯酸树脂皮革涂饰剂的研究摘要纳米二氧化钛,亦称纳米钛白粉。
从尺寸大小来说,通常产生物理化学性质显著变化的细小微粒的尺寸在100纳米以下,其外观为白色疏松粉末。
具有抗紫外线、抗菌、自洁净、抗老化功效,可用于化妆品、功能纤维、塑料、油墨、涂料、油漆、精细陶瓷等领域。
本课题用超声波和分散剂协同分散金红石晶型纳米二氧化钛粉体,进行了分散剂的浓度为5%的选择,通过悬浮液的稳定性的比较最终确定较佳的分散工艺:在纳米TiO2条件下,分散剂六偏磷酸钠浓度0.3%、超声分散功率为540W,超声分散时间为30min。
向四口烧瓶中加入水、十二烷基硫酸钠、OP-10、丙烯酸。
搅拌15min(可先预先加热油浴到42℃)加入主单体(甲基丙烯酸甲酯,丙烯酸丁酯)总量的20%,乳化45min。
升温到75℃,开始滴加引发剂,1小时加完。
加剩余主单体同时在另一滴管中加N-羟基丙烯酰胺、丙烯酸,1小时同时加完,然后加引发剂,30min加完,加冷凝水。
74-76℃保温2h。
降温到50℃,加氨水调pH值(7~8),制得丙烯酸树脂乳液,与纳米二氧化钛共混,制得改性后的乳液,应用于皮革,发现皮革耐黄变性增强,吸水率变化不大,物理机械性能及耐干湿擦强度均得到提高。
,分散,丙烯酸树脂,皮革涂饰剂关键词:纳米TiO2Nano-titanium Dioxide Modified Acrylic Resin Leather FinishingStudiesABSTRACTTitanium dioxide, also known as nano-titanium dioxide. From the size, it typically produces a significant change in physical and chemical properties of the fine particle size of 100 nanometers or less, the appearance of loose powder is white. With anti-ultraviolet, antibacterial, self-cleaning, anti-aging effects, can be used in cosmetics, functional fibers, plastics, inks, coatings, paints, fine ceramics and other fields.The subject ultrasonic and dispersant coordinated distributed rutile titanium dioxide powders were dispersant selected through comparison of the stability of the suspension to finalize a better dispersion process: In the nano-TiO2 concentration of 5% of the conditions , a dispersing agent concentration of 0.3% sodium hexametaphosphate, ultrasonic dispersing power of 540W, ultrasonic dispersion time was 30min.Into a four-necked flask was added water, sodium lauryl sulfate, OP-10, acrylic acid. Stirring 15min (may be pre-heated oil bath to 42 ℃) added to the main monomer (methyl methacrylate, butyl acrylate) 20% of total, emulsifying 45min. Heated to 75 ℃, began dropping initiator 1 hour addition was complete. Plus another while remaining dropper main monomer added N-hydroxy acrylamide, acrylic acid, 1 hour, while the addition was complete, then adding the initiator, 30min addition was complete, add condensate. 74-76 ℃ insulation 2h. Cooled to 50 ℃, adding ammonia to adjust pH (7-8) to give acrylic resin emulsion, blended with titanium dioxide, to obtain the modified emulsion used in leather, leather found enhanced resistance to yellowing, water absorption change small, physical and mechanical properties and resistance to wet and dry rubbing strength are improved.KEY WORDS: nano- TiO2,dispersion,acrylic resin,leather finishing2目录摘要 (1)ABSTRACT (2)1.1纳米材料和纳米科学技术 (5)1.2纳米二氧化钛的特性及应用 (6)1.2.1杀菌功能 (6)1.2.2防紫外线功能 (6)1.2.3光催化功能 (7)1.2.4纳米Ti02的颜色效应 (7)1.3纳米材料的分散 (8)1.3.1纳米材料团聚的原因 (8)1.3.2常用的分散方法 (8)1.4纳米复合材料 (9)1.4.1纳米复合材料的制备 (9)1.4.2纳米二氧化钛丙烯酸树脂复合涂饰剂 (10)1.5本课题的提出及研究的主要内容 (10)2.氧化钛水分散的研究 (12)2.1试验药品和仪器 (12)2.1.1试验药品 (12)2.1.2实验仪器 (12)2.2试验 (12)2.3结果与讨论 (13)2.4小结 (13)3.1试验药品及仪器 (14)3.1.1试验药品 (14)3.1.2试验仪器 (14)3.2实验过程 (14)3.2.1软丙烯酸树脂的合成 (14)3.3检测软丙烯酸酯 (15)3.3.1吸水率的测定 (15)3.3.2固含量的检测 (15)4 纳米Ti02改性丙烯酸树脂涂饰剂及应用研究 (16)4.1试验药品和仪器 (16)4.1.1试验药品 (16)4.1.2试验仪器 (17)4.2.1软性丙烯酸树脂的制备 (17)4.3检测 (17)4.3.1改性乳液检测 (17)4.3.2改性乳液薄膜性能检测 (17)4.3.3在皮革上的应用试验检测 (18)4.4结果与讨论 (19)4.4.1复合乳液相关结果与讨论 (19)4.4.2在皮革上的应用试验 (21)4.5小结 (23)5结论 (24)致谢 (25)参考文献 (26)41. 文献综述1.1纳米材料和纳米科学技术纳米(符号为nm)是长度单位,原称毫微米,就是10-9米(10亿分之一米),即10-6毫米(100万分之一毫米)。
第49卷第9期2021年5月广州化工Guangzhou Chemical IndustryVol. 49 No. 9May. 2021高钛渣提钛制备纳米二氧化钛及其光催化性能的研究**基金项目:沈阳医学院科技发展基金(No : 20191026) ; 2019辽宁省教育厅科学研究一般项目(N 。
: 201902);沈阳医学院创新创业训练计划(N 。
: 20209034)D通讯作者:王凯(1978-),女,讲师,主要从事纳米材料光催化性能研究。
王小禾,王 凯,隋丽丽,董微,常红,吴 園,莫大森(沈阳医学院,辽宁沈阳110034)摘 要:以高钛渣为原料,采用浓硫酸焙烧法得到硫酸氧钛溶液,水热法制备偏钛酸进行高温锻烧,制备不同晶型组成的纳米二氧化钛产品,以亚甲基蓝为降解对象,检测不同熾烧温度下纳米二氧化钛产品的光催化性能。
在254 nm 波长的光照下,对亚甲基蓝溶液的光催化降解实验结果表明:纳米Ti()2对亚甲基蓝有一定的降解活性,650 t 锻烧得到的二氧化钛产品对亚甲基蓝 的光催化降解活性最高。
关键词:高钛渣;二氧化钛;光催化;亚甲基蓝中图分类号: X52文献标志码:A 文章编号:1001 -9677(2021)09-0064-03Photocatalytic Performance of Nanometer TiO 2 Preparedfrom High Titanium Slag *WANGXiao-he, WANG Kai, SUI Li-li, DONG Wei, CHANG Hong, WU Nan, MO Da-sen(Shenyang Medical College , Liaoning Shenyang 110034, China)Abstract : Using high titanium slag as raw material , titanium oxysulfate solution was obtained by roasting withconcentrated sulfuric acid. Metatitanic acid was prepared by hydrothermal method and calcined at high temperature toprepare nanotitanium dioxide products with different crystal form& The photocatalytic activity was tested of nano titanium dioxide products with different calcination temperatures and methylene blue as degradation object. The photocatalyticdegradation under UV irradiation at k = 254 nm showed thatnano TiO 2 had a certain degradation activity to Methylene blue. The photocatalytic activity of titanium dioxide products calcined at 650 P was the highest.Key words : high titanium slag ; titanium dioxide ; photocatalysis ; methylene blueTiOz 具有廉价、稳定、无毒、光催化活性较高等特点,被广泛应用于有机污染物的降解。
一种纳米氮化钛基复合材料及其制备方法和应用嘿,咱今儿来聊聊一种特别牛的东西——纳米氮化钛基复合材料!这玩意儿可厉害了,就像一个小小的超级英雄,有着大大的能量。
你想想看啊,纳米级别的东西,那得多小啊,小到咱肉眼都看不见。
可就是这么小的玩意儿,却能发挥出巨大的作用。
纳米氮化钛基复合材料呢,就像是一个由各种厉害角色组成的团队。
先来说说它的制备方法吧。
这可不是随随便便就能搞出来的,得有专门的技术和工艺。
就好像做饭一样,得有合适的食材、调料,还得掌握好火候,才能做出美味的菜肴。
制备这种复合材料也是一样,得精确地控制各种条件,让不同的材料完美地结合在一起。
这可不是一件容易的事儿,但科学家们就是有办法做到!他们就像是神奇的魔法师,能把这些材料变成厉害的纳米氮化钛基复合材料。
那它都有啥应用呢?哎呀呀,那可多了去了。
比如说在电子领域,它能让电子设备变得更小巧、更强大。
这就好比给电子设备装上了超级引擎,跑得更快、更稳。
在医疗领域呢,它说不定能帮助医生更好地治疗疾病,就像给医生配备了更厉害的武器。
还有在材料科学领域,它能让材料的性能大幅提升,就像给材料打了一针兴奋剂。
你说这纳米氮化钛基复合材料是不是很神奇?它就像是隐藏在科技世界里的宝藏,等待着我们去挖掘、去利用。
说不定未来的某一天,我们生活中的方方面面都离不开它了呢!咱再想想,要是没有这些厉害的复合材料,我们的生活得少了多少乐趣和便利啊。
没有更强大的电子设备,我们怎么愉快地刷手机、玩游戏?没有更好的医疗手段,病人得多遭多少罪啊?所以说啊,这纳米氮化钛基复合材料的研究和发展可太重要了。
它就像一颗正在发芽的种子,虽然现在还小小的,但未来有着无限的可能。
谁知道它会给我们带来什么样的惊喜呢?也许有一天,我们会发现,它已经改变了我们的整个世界!这难道不让人兴奋吗?难道不让人对未来充满期待吗?反正我是觉得特别神奇,特别期待!这纳米氮化钛基复合材料,绝对是科技领域的一颗闪亮明星啊!。
Titanium Dioxide Nanomaterials:Synthesis,Properties,Modifications,andApplicationsXiaobo Chen*and Samuel S.Mao†Lawrence Berkeley National Laboratory,and University of California,Berkeley,California94720Received March27,2006Contents1.Introduction28912.Synthetic Methods for TiO2Nanostructures28922.1.Sol−Gel Method28922.2.Micelle and Inverse Micelle Methods28952.3.Sol Method28962.4.Hydrothermal Method28982.5.Solvothermal Method29012.6.Direct Oxidation Method29022.7.Chemical Vapor Deposition29032.8.Physical Vapor Deposition29042.9.Electrodeposition29042.10.Sonochemical Method29042.11.Microwave Method29042.12.TiO2Mesoporous/Nanoporous Materials29052.13.TiO2Aerogels29062.14.TiO2Opal and Photonic Materials29072.15.Preparation of TiO2Nanosheets29083.Properties of TiO2Nanomaterials29093.1.Structural Properties of TiO2Nanomaterials29093.2.Thermodynamic Properties of TiO2Nanomaterials29113.3.X-ray Diffraction Properties of TiO2Nanomaterials29123.4.Raman Vibration Properties of TiO2Nanomaterials29123.5.Electronic Properties of TiO2Nanomaterials29133.6.Optical Properties of TiO2Nanomaterials29153.7.Photon-Induced Electron and Hole Propertiesof TiO2Nanomaterials29184.Modifications of TiO2Nanomaterials29204.1.Bulk Chemical Modification:Doping29214.1.1.Synthesis of Doped TiO2Nanomaterials29214.1.2.Properties of Doped TiO2Nanomaterials29214.2.Surface Chemical Modifications29264.2.1.Inorganic Sensitization29265.Applications of TiO2Nanomaterials29295.1.Photocatalytic Applications29295.1.1.Pure TiO2Nanomaterials:FirstGeneration29305.1.2.Metal-Doped TiO2Nanomaterials:Second Generation29305.1.3.Nonmetal-Doped TiO2Nanomaterials:Third Generation 29315.2.Photovoltaic Applications29325.2.1.The TiO2Nanocrystalline Electrode inDSSCs29325.2.2.Metal/Semiconductor Junction SchottkyDiode Solar Cell29385.2.3.Doped TiO2Nanomaterials-Based SolarCell29385.3.Photocatalytic Water Splitting29395.3.1.Fundamentals of Photocatalytic WaterSplitting2939e of Reversible Redox Mediators2939e of TiO2Nanotubes29405.3.4.Water Splitting under Visible Light29415.3.5.Coupled/Composite Water-SplittingSystem29425.4.Electrochromic Devices29425.4.1.Fundamentals of Electrochromic Devices29435.4.2.Electrochromophore for an ElectrochromicDevice29435.4.3.Counterelectrode for an ElectrochromicDevice29445.4.4.Photoelectrochromic Devices29455.5.Hydrogen Storage29455.6.Sensing Applications29476.Summary29487.Acknowledgment29498.References29491.IntroductionSince its commercial production in the early twentiethcentury,titanium dioxide(TiO2)has been widely used as apigment1and in sunscreens,2,3paints,4ointments,toothpaste,5etc.In1972,Fujishima and Honda discovered the phenom-enon of photocatalytic splitting of water on a TiO2electrodeunder ultraviolet(UV)light.6-8Since then,enormous effortshave been devoted to the research of TiO2material,whichhas led to many promising applications in areas ranging fromphotovoltaics and photocatalysis to photo-/electrochromicsand sensors.9-12These applications can be roughly dividedinto“energy”and“environmental”categories,many of whichdepend not only on the properties of the TiO2material itselfbut also on the modifications of the TiO2material host(e.g.,with inorganic and organic dyes)and on the interactions ofTiO2materials with the environment.An exponential growth of research activities has been seenin nanoscience and nanotechnology in the past decades.13-17New physical and chemical properties emerge when the sizeof the material becomes smaller and smaller,and down to*Corresponding author.E-mail:XChen3@.†E-mail:SSMao@.2891 Chem.Rev.2007,107,2891−295910.1021/cr0500535CCC:$65.00©2007American Chemical SocietyPublished on Web06/23/2007the nanometer scale.Properties also vary as the shapes of the shrinking nanomaterials change.Many excellent reviews and reports on the preparation and properties of nanomaterials have been published recently.6-44Among the unique proper-ties of nanomaterials,the movement of electrons and holes in semiconductor nanomaterials is primarily governed by the well-known quantum confinement,and the transport proper-ties related to phonons and photons are largely affected by the size and geometry of the materials.13-16The specific surface area and surface-to-volume ratio increase dramati-cally as the size of a material decreases.13,21The high surface area brought about by small particle size is beneficial to many TiO 2-based devices,as it facilitates reaction/interaction between the devices and the interacting media,which mainly occurs on the surface or at the interface and strongly depends on the surface area of the material.Thus,the performance of TiO 2-based devices is largely influenced by the sizes of the TiO 2building units,apparently at the nanometer scale.As the most promising photocatalyst,7,11,12,33TiO 2mate-rials are expected to play an important role in helping solvemany serious environmental and pollution challenges.TiO 2also bears tremendous hope in helping ease the energy crisis through effective utilization of solar energy based on photovoltaic and water-splitting devices.9,31,32As continued breakthroughs have been made in the preparation,modifica-tion,and applications of TiO 2nanomaterials in recent years,especially after a series of great reviews of the subject in the 1990s.7,8,10-12,33,45we believe that a new and compre-hensive review of TiO 2nanomaterials would further promote TiO 2-based research and development efforts to tackle the environmental and energy challenges we are currently facing.Here,we focus on recent progress in the synthesis,properties,modifications,and applications of TiO 2nanomaterials.The syntheses of TiO 2nanomaterials,including nanoparticles,nanorods,nanowires,and nanotubes are primarily categorized with the preparation method.The preparations of mesopo-rous/nanoporous TiO 2,TiO 2aerogels,opals,and photonic materials are summarized separately.In reviewing nanoma-terial synthesis,we present a typical procedure and repre-sentative transmission or scanning electron microscopy images to give a direct impression of how these nanomate-rials are obtained and how they normally appear.For detailed instructions on each synthesis,the readers are referred to the corresponding literature.The structural,thermal,electronic,and optical properties of TiO 2nanomaterials are reviewed in the second section.As the size,shape,and crystal structure of TiO 2nanomate-rials vary,not only does surface stability change but also the transitions between different phases of TiO 2under pressure or heat become size dependent.The dependence of X-ray diffraction patterns and Raman vibrational spectra on the size of TiO 2nanomaterials is also summarized,as they could help to determine the size to some extent,although correlation of the spectra with the size of TiO 2nanomaterials is not straightforward.The review of modifications of TiO 2nanomaterials is mainly limited to the research related to the modifications of the optical properties of TiO 2nanoma-terials,since many applications of TiO 2nanomaterials are closely related to their optical properties.TiO 2nanomaterials normally are transparent in the visible light region.By doping or sensitization,it is possible to improve the optical sensitiv-ity and activity of TiO 2nanomaterials in the visible light region.Environmental (photocatalysis and sensing)and energy (photovoltaics,water splitting,photo-/electrochromics,and hydrogen storage)applications are reviewed with an emphasis on clean and sustainable energy,since the increas-ing energy demand and environmental pollution create a pressing need for clean and sustainable energy solutions.The fundamentals and working principles of the TiO 2nanoma-terials-based devices are discussed to facilitate the under-standing and further improvement of current and practical TiO 2nanotechnology.2.Synthetic Methods for TiO 2Nanostructures2.1.Sol −Gel MethodThe sol -gel method is a versatile process used in making various ceramic materials.46-50In a typical sol -gel process,a colloidal suspension,or a sol,is formed from the hydrolysis and polymerization reactions of the precursors,which are usually inorganic metal salts or metal organic compounds such as metal plete polymerization and loss of solvent leads to the transition from the liquid sol into a solid gel phase.Thin films can be produced on a piece ofDr.Xiaobo Chen is a research engineer at The University of California at Berkeley and a Lawrence Berkeley National Laboratory scientist.He obtained his Ph.D.Degree in Chemistry from Case Western Reserve University.His research interests include photocatalysis,photovoltaics,hydrogen storage,fuel cells,environmental pollution control,and the related materials and devices development.Dr.Samuel S.Mao is a career staff scientist at Lawrence Berkeley National Laboratory and an adjunct faculty at The University of California at Berkeley.He obtained his Ph.D.degree in Engineering from The University of California at Berkeley in 2000.His current research involves the development of nanostructured materials and devices,as well as ultrafast laser technologies.Dr.Mao is the team leader of a high throughput materials processing program supported by the U.S.Department of Ener-gy.2892Chemical Reviews,2007,Vol.107,No.7Chen andMaosubstrate by spin-coating or dip-coating.A wet gel will form when the sol is cast into a mold,and the wet gel is converted into a dense ceramic with further drying and heat treatment.A highly porous and extremely low-density material called an aerogel is obtained if the solvent in a wet gel is removed under a supercritical condition.Ceramic fibers can be drawn from the sol when the viscosity of a sol is adjusted into a proper viscosity range.Ultrafine and uniform ceramic powders are formed by precipitation,spray pyrolysis,or emulsion techniques.Under proper conditions,nanomaterials can be obtained.TiO 2nanomaterials have been synthesized with the sol -gel method from hydrolysis of a titanium precusor.51-78This process normally proceeds via an acid-catalyzed hydrolysis step of titanium(IV)alkoxide followed by condensa-tion.51,63,66,79-91The development of Ti -O -Ti chains is favored with low content of water,low hydrolysis rates,and excess titanium alkoxide in the reaction mixture.Three-dimensional polymeric skeletons with close packing result from the development of Ti -O -Ti chains.The formation of Ti(OH)4is favored with high hydrolysis rates for a medium amount of water.The presence of a large quantity of Ti -OH and insufficient development of three-dimensional polymeric skeletons lead to loosely packed first-order particles.Polymeric Ti -O -Ti chains are developed in the presence of a large excess of water.Closely packed first-order particles are yielded via a three-dimensionally devel-oped gel skeleton.51,63,66,79-91From the study on the growth kinetics of TiO 2nanoparticles in aqueous solution using titanium tetraisopropoxide (TTIP)as precursor,it is found that the rate constant for coarsening increases with temper-ature due to the temperature dependence of the viscosity of the solution and the equilibrium solubility of TiO 2.63Second-ary particles are formed by epitaxial self-assembly of primary particles at longer times and higher temperatures,and the number of primary particles per secondary particle increases with time.The average TiO 2nanoparticle radius increases linearly with time,in agreement with the Lifshitz -Slyozov -Wagner model for coarsening.63Highly crystalline anatase TiO 2nanoparticles with different sizes and shapes could be obtained with the polycondensation of titanium alkoxide in the presence of tetramethylammonium hydroxide.52,62In a typical procedure,titanium alkoxide is added to the base at 2°C in alcoholic solvents in a three-neck flask and is heated at 50-60°C for 13days or at 90-100°C for 6h.A secondary treatment involving autoclave heating at 175and 200°C is performed to improve the crystallinity of the TiO 2nanoparticles.Representative TEM images are shown in Figure 1from the study of Chemseddine et al.52A series of thorough studies have been conducted by Sugimoto et ing the sol -gel method on the formation of TiO 2nanoparticles of different sizes and shapes by tuning the reaction parameters.67-71Typically,a stock solution of a 0.50M Ti source is prepared by mixing TTIP with triethanolamine(TEOA)([TTIP]/[TEOA])1:2),followed The diluted with ashape controller solution °C for 1day and at 140°C for3days.The pH of the solutioncan be tuned by adding HClO 4or NaOH solution.Amines are used as the shape controllers of the TiO 2act as surfactants.amines include TEOA,and triethyl-enetetramine.The morphology of the TiO 2nanoparticles changes from cuboidal to ellipsoidal at pH above 11with TEOA.The TiO 2into ellipsoidal above pH 9.5with diethylenetriamine with a higher aspect ratio than that with TEOA.Figure 2shows representative TEM images of the TiO 2nanoparticles under different initial pH conditions with the shape control of TEOA at [TEOA]/[TIPO])2.0.Secondary amines,such as diethylamine,and tertiary amines,such as trimethylamine and triethylamine,act as complexing agents of Ti(IV)ions promote the ratios.The shape of the TiO 2nanoparticle can also be tuned from round-cornered cubes to sharp-edged cubes with sodium oleate and sodium stearate.70The shape control is attributed to the tuning of the growth rate of the different crystal planes of TiO 2nanoparticles by the to these planes under different pH conditions.70A prolonged heating time below 100°C for the as-prepared be used to avoid the the TiO 2nano-during the crystallization process.58,72By heating amorphous TiO 2in ana-2with average particle sizes between 7and 50nm can be obtained,as reported by Zhang and Banfield.73-77Much effort has been exerted to achieve highly crystallized and narrowly dispersed TiO 2nanoparticles using the sol -gel method with other modifications,such as a semicontinuous reaction method by Znaidi et al.78and a two-stage mixed method and a continuous reaction method by Kim et al.53,54By a combination of the sol -gel method and an anodic alumina membrane (AAM)template,TiO 2nanorods have dipping porous into a boiled TiO 2sol followed processes.92,93In a typical experiment,a TiO 2sol solution is prepared by mixing TTIP dissolved in ethanol with a solution containing water,acetyl acetone,and ethanol.AAM is immersed into the boiled it is dried in air and calcined at 400°C for 10h.The AAM template is removed in a 10wt %H 3PO 4aqueous solution.The calcination temperature can be used to control the crystal phase of the TiO 2nanorods.At low temperature,anatase nanorods can be obtained,while at high temperature rutile nanorods can be obtained.The pore size of the AAM template can be used to control the size of these TiO 2nanorods,which typically range from 100to 300nm in diameter and several micrometers in length.Appar-ently,the size distribution of the final TiO 2nanorods is largely controlled by the size distribution of the pores of the AAM template.In order to obtain smaller and mono-sized TiO 2nanorods,it is necessary fabricate high-quality AAM templates.Figure 3shows a for TiO 2nanorods fabricated with this method.Normally,the TiO 2nanorods are composed of small TiO 2nanoparticles or electrophoretic deposition of TiO 2colloidal suspensions into 2nanowire arrays can be obtained.94In a typical procedure,in ethanol at room temperature,with deionized water and ethanol 2-3with nitric acid.is used as the anode,and an AAM with attached to Cu foil is used as the cathode.A TiO 2sol is deposited into the pores of the AMM under a voltage of 2-5V and annealed at 500°C for 24h.After the AAM a 5wt %NaOH solution,isolated TiO 2nanowires are obtained.In order toTitanium Dioxide Nanomaterials Chemical Reviews,2007,Vol.107,No.72893fabricate TiO 2nanowires instead of nanorods,an AAM with long pores is a must.TiO 2nanotubes can also be obtained using the sol -gel method by templating with an AAM 95-98and other organic compounds.99,100For example,when an AAM is used as the template,a thin layer of TiO 2sol on the wall of the pores of the AAM is first prepared by sucking TiO 2sol into the pores of the AAM and removingit under vacuum;TiO 2nanowires are obtained after the sol is fully developed and the AAM is removed.In the procedure by Lee and co-workers,96a TTIP solution was prepared by mixing TTIP with 2-propanol and 2,4-pentanedione.the AAM was dipped into thisFigure 1.TEM images of TiO 2nanoparticles prepared by hydrolysis of Ti(OR)4in the presence of tetramethylammonium hydroxide.Reprinted with permission from Chemseddine,A.;Moritz,T.Eur.J.Inorg.Chem.1999,235.Copyright 1999Wiley-VCH.Figure 2.TEM images of uniform anatase TiO 2nanoparticles.Reprinted from Sugimoto,T.;Zhou,X.;Muramatsu,A.J.Colloid Interface Sci.2003,259,53,Copyright 2003,with permission from Elsevier.2894Chemical Reviews,2007,Vol.107,No.7Chen and Maosolution,it was removed from the solution and placed under vacuum until the entire volume of the solution was pulled through the AAM.The AAMhydrolyzed by water vapor over a HCl solution for 24h,and then calcined ina furnace at 673K for 2h and cooled to room temperaturetemperature ramp of2°C/h.Pure TiO 2nanotubes were dissolved in a 6M NaOH solution for several minutes.96Alternatively,TiO 2nanotubes could be obtained by coating the AAM membranes at 60°C for a certain period of time (12-48h)4under pH )2.1and removing the AAM after TiO 2nanotubes were developed.97Figure 4shows a typical SEM image of the 2array from the AAM template.97In another scheme,a ZnO nanorod array on a glass substrate can be used as a template to fabricate TiO 2nanotubes with the sol -gel method.101Briefly,TiO 2sol isdeposited on a ZnO nanorod template by dip-coating with a slow withdrawing speed,then dried at 100°C for 10min,and heated at 550°C for 1h in air to obtain ZnO/TiO 2nanorod arrays.The ZnO nanorod template is etched-up by immersing the ZnO/TiO 2nanorod arrays in a dilute hydro-chloric acid aqueous solution to obtain TiO 2nanotube arrays.Figure 5shows a typical SEM image of the TiO 2nanotube array with the ZnO nanorod array template.The TiO 2nanotubes inherit the uniform hexagonal cross-sectional shape and the length of 1.5µm and inner diameter of 100-of the ZnO nanorod template.As the concentration of the TiO 2sol is constant,well-aligned TiO 2nanotube arrays can only be obtained from an optimal dip-coating cycle number in the range of 2-3cycles.A dense porous TiO 2thick film with holes is obtained number further increases.The heating rate is critical to the formation of TiO 2nanotube arrays.When the heating rate is extra rapid,e.g.,above 6°C min -1,the TiO 2coat will easily crack and flake off from the ZnO nanorods due to great tensile stress the TiO 2coat and the ZnO 2film with loose,porous nanostructure is obtained.2.2.Aggregates of surfactant molecules dispersed in a liquid the surfactant concentration exceeds the critical micelle concentration (CMC).The CMC free solution in equilibrium with surfactants in aggregated form.In micelles,the hydrophobic hydrocarbon chains of the surfactants are and the hydro-the surfactants are oriented toward the medium.The concentration of lipid present in solution determines the self-organization molecules of surfactants and lipids.The lipids form a single layer on the liquid surface and are dispersed in solution below the CMC.The lipids organize in spherical micelles at the first CMC (CMC-I),into elongated pipes at the second CMC (CMC-II),and into stacked lamellae of pipes at the lamellar point (LM or CMC-III).The CMC depends on the chemical composition,mainly on the ratio of the head area and the tail length.Reverse micelles are formed in nonaqueous the hydrophilic headgroups are directed toward of the micelles while the hydrophobic groups areFigure 3.TEM image of anatase nanorods and a single nanorod composed of small TiO 2nanoparticles or nanograins (inset).Reprinted from Miao,L.;Tanemura,S.;Toh,S.;Kaneko,K.;Tanemura,M.J.Cryst.Growth 2004,264,246,Copyright 2004,with permission from Elsevier.Figure 4.SEM image of TiO 2nanotubes prepared from the AAO template.Reprinted with permission from Liu,S.M.;Gan,L.M.;Liu,L.H.;Zhang,W.D.;Zeng,H.C.Chem.Mater.2002,14,1391.Copyright 2002American Chemical Society.Figure 5.SEM of a TiO 2nanotube array;the inset shows the ZnO nanorod array template.Reprinted with permission from Qiu,J.J.;Yu,W.D.;Gao,X.D.;Li,X.M.Nanotechnology 2006,17,4695.Copyright 2006IOP Publishing Ltd.Titanium Dioxide Nanomaterials Chemical Reviews,2007,Vol.107,No.72895directed outward toward the nonaqueous media.There is no obvious CMC for reverse micelles,because the number of aggregates is usually small and they are not sensitive to the surfactant concentration.Micelles are oftenglobular roughly spherical in shape,but ellipsoids,cylinders,and bilayers are also possible.The shape of amicelle is a functionmolecular geometry of its surfactant molecules and surfactant concentration,tem-perature,pH,and ionic strength.Micelles and inverse micelles are commonly employed to synthesize TiO 2nanomaterials.102-110A statistical experi-mental design method conducted by Kim et al.to for the preparation of TiO 2nanoparticles.103The values of H 2O/surfactant,H 2O/titanium precursor,ammonia concentration,feed rate,and reaction temperature were significant TiO 2nanoparticle size and size distribution.Amorphous TiO 2nanoparticles with diameters of 10-20nm were synthesized and converted to the anatase phase at 600°C and to the more thermodynamically stable rutile phase at 900°C.Li et al.developed TiO 2nanoparticles with the chemical reactions between TiCl 4solution and ammonia in a reversed micro-emulsion system consisting of cyclohexane,poly(oxyethyl-5ether,and poly(oxyethylene)9nonyle phenol ether.104The produced amorphous TiO 2nanoparticles transformed into anatase when heated at temperatures from 200to 750°C and into rutile at temperatures higher than 750°Agglomeration and growth also occurred at elevated Shuttle-like crystalline TiO 2nanoparticles were synthesized by Zhang et al.with hydrolysis of titanium tetrabutoxide in the presence of acids (hydrochloric acid,nitric acid,sulfuric acid,and phosphoric acid)in NP-5(Igepal CO-520)-room temperature.110The particle size of the TiO 2nanoparticles were largely controlled by the reaction condi-tions,and the key factors affecting the formation of rutile at room temperature included the acidity,the type of acid used,and the microenvironment of the reverse micelles.Ag-glomeration of the particles occurred with prolonged reaction times and increasing the [H 2O]/[NP-5]and [H 2O]/[Ti-(OC 4H 9)4]ratios.When suitable acid was applied,round TiO 2nanoparticles could also be obtained.Representative TEM images of the shuttle-like and round-shaped TiO 2nanopar-ticles are In the study carried out by Lim et al.,TiO 2nanoparticles were prepared by the controlled hydrolysis of TTIP in reverse micelles formed in CO 2with the surfactants ammonium carboxylate perfluoropolyether (PFPECOO -4+ethyl methacrylate-block-1H,1H,2H,2H-perfluorooctyl meth-acrylate)(PDMAEMA-b-PFOMA).106It was found that the crystallite size prepared in the presence of reverse micelles increased as either the water to surfactant or the precursor to The TiO 2nanomaterials prepared with the above micelle and reverse micelle methods normally have amorphous structure,and calcination is usually necessary in order to induce high crystallinity.However,this process usually leads to the growth and agglomeration of TiO 2nanoparticles.The crystallinity of TiO 2nanoparticles initially (synthesized by controlled hydrolysis of titanium alkoxide in reverse micelles in a hydrocarbon solvent)could be improved by annealing in the presence of the micelles at temperatures considerably lower than those required for the traditional calcinationtreatment in the solid state.108This procedure could produce crystalline TiO 2nanoparticles with unchanged physical dimensions and minimal agglomeration and allows the preparation of highly crystalline TiO 2nanoparticles,as shown in Figure 7,from the study of Lin et al.1082.3.Sol MethodThe sol method here refers to the nonhydrolytic sol -gel processes and usually involves the reaction of titanium chloride with a variety of different oxygen donor molecules,Figure 6.TEM images of the shuttle-like and round-shaped (inset)TiO 2nanoparticles.From:Zhang,D.,Qi,L.,Ma,J.,Cheng,H.J.Mater.Chem.2002,12,3677(/10.1039/b206996b).s Reproduced by permission of The Royal Society of Chemistry.Figure 7.HRTEM images of a TiO 2nanoparticle after annealing.Reprinted with permission from Lin,J.;Lin,Y.;Liu,P.;Meziani,M.J.;Allard,L.F.;Sun,Y.P.J.Am.Chem.Soc.2002,124,11514.Copyright 2002American Chemical Society.TiX 4+Ti(OR)4f 2TiO 2+4RX (1)TiX 4+2ROR f TiO 2+4RX(2)2896Chemical Reviews,2007,Vol.107,No.7Chen and MaoThe condensation between Ti -Cl and Ti-OR leads to the formation of Ti -O -Ti bridges.Thealkoxide groups can be provided by titanium alkoxides orby reaction of the titanium chloride with alcohols or ethers.In the method by Trentler andColvin,119a metal alkoxide was rapidly injected into the hot solution of titanium halide mixed with trioctylphosphine oxide (TOPO)heptadecane at 300°C dry inert gas protection,completed alkyl substituents including rate of R,while average particle sizes were relatively unaffected.Variation of X yielded a clear trend in average particle size,but without a discernible trend in reaction rate.Increased nucleophilicity resulted in Average sizes ranged from 9.2nm for TiF 4to 3.8nm for TiI 4.The amount of passivating agent influenced the chemistry.was slower and resulted in smaller particles,while reactions without TOPO were much quicker and yielded mixtures of brookite,and anatase with average particle sizes nm.Figure 8shows typical TEM images of TiO 2nanocrystals developed by Trentler et al.119In the method used by Niederberger and Stucky,111TiCl 4was slowly added to anhydrous benzyl alcohol vigorous stirring at 40-150°C for 1-21days in the reaction vessel.The precipitate was calcinated at 450°C for 5h after thoroughly washing.The reaction between TiCl 4and benzyl alcohol was found suitable for the synthesis of highly crystalline anatase phase TiO 2nanoparticles with nearly uniform size and shape at very low temperatures,such as 40°C.The particle size could be selectively adjusted in the range of 4-8nm with the appropriate thermal conditions and a proper choice of the and titanium tetrachloride.The particle growth depended strongly on temperature,and lowering the titanium tetrachloride led to a considerable 111Surfactants have been widely used in the preparation of a variety of nanoparticles with good size distribution and Adding different surfactants acetic acid and acetylacetone,can help synthesize monodispersed TiO 2nanoparticles.120,121For example,Scolan and Sanchez found that monodisperse nonaggregated TiO 2nanoparticles in the 1-5nm range were obtained through hydrolysis of titanium butoxide in the presence of acetylacetone and p -toluenesulfonic acid at 60°C.120The dispersed in water -alcohol or alcohol solutions at concentrations higher than 1M without aggregation,which is attributed to the complexation of the surface by acetylacetonato ligands hybrid made with acetylacetone,p -toluenesulfonic acid,and wa-ter.120With the aid of surfactants,different sized and shaped TiO 2nanorods can be synthesized.122-130For example,the growth of high-aspect-ratio anatase TiO 2nanorods has been reported by Cozzoli and co-workers by controlling the hydrolysis process of TTIP in 122-126,130Typically,TTIP was added into 80-100°C under inert gas protection (nitrogen flow)and stirred for 5min.A 0.1-2M aqueous base solution was then rapidly injected and kept at with stirring.The bases employed included organic amines,such as trimethylamino-N-oxide,trimethylamine,tetramethylammonium hydroxide,tetrabut-ylammonium hydroxyde,triethylamine,and tributylamine.precursor with the carboxylic acid,the hydrolysis rate of titanium alkoxide (in 4-6h)crystal-lization in mild conditions was promoted with the use of suitable catalysts (tertiary amines or quaternary ammonium hydroxides).A kinetically overdriven growth mechanism led 2Typical TEM images of the TiO 2nanorods are shown in Figure 9.123Recently,Joo et al.127and Zhang et al.129reported similar procedures in obtaining TiO 2nanorods without the use of catalyst.Briefly,a mixture of TTIP and OA was used to generate OA complexes of titanium at 80°C 1-octadecene.Figure 8.TEM image of TiO 2nanoparticles derived from reaction of TiCl 4and TTIP in TOPO/heptadecane at 300°C.The inset shows a HRTEM image of a single particle.Reprinted with permission from Trentler,T.J.;Denler,T.E.;Bertone,J.F.;Agrawal,A.;Colvin,V.L.J.Am.Chem.Soc.1999,121,1613.Copyright 1999American Chemical Society.Figure 9.TEM of TiO 2nanorods.The inset shows a HRTEM of a TiO 2nanorod.Reprinted with permission from Cozzoli,P.D.;Kornowski,A.;Weller,H.J.Am.Chem.Soc.2003,125,14539.Copyright 2003American Chemical Society.Titanium Dioxide Nanomaterials Chemical Reviews,2007,Vol.107,No.72897。