间苯三酚EP8.0标准--中文翻译
- 格式:doc
- 大小:61.00 KB
- 文档页数:5

第1部分化学品及企业标识化学品中文名:间硝基苯酚化学品英文名:3-nitrophenolCAS号:554-84-7分子式:C6H5NO3分子量:139.11产品推荐及限制用途:工业及科研用途。
第2部分危险性概述紧急情况概述:吞咽有害。
造成皮肤刺激。
造成严重眼损伤。
GHS危险性类别:急性经口毒性类别4皮肤腐蚀/刺激类别2严重眼损伤/眼刺激类别1标签要素:象形图:警示词:危险危险性说明:H302吞咽有害H315造成皮肤刺激H318造成严重眼损伤防范说明:•预防措施:——P264作业后彻底清洗。
——P270使用本产品时不要进食、饮水或吸烟。
——P280戴防护手套/穿防护服/戴防护眼罩/戴防护面具。
•事故响应:——P301+P312如误吞咽:如感觉不适,呼叫解毒中心/医生——P330漱口。
——P302+P352如皮肤沾染:用水充分清洗。
——P332+P313如发生皮肤刺激:求医/就诊。
——P362+P364脱掉沾染的衣服,清洗后方可重新使用——P305+P351+P338如进入眼睛:用水小心冲洗几分钟。
如戴隐形眼镜并可方便地取出,取出隐形眼镜。
继续冲洗。
——P310立即呼叫解毒中心/医生•安全储存:——无•废弃处置:——P501按当地法规处置内装物/容器。
物理和化学危险:无资料健康危害:吞咽有害。
造成皮肤刺激。
造成严重眼损伤。
环境危害:无资料第3部分成分/组成信息第4部分急救措施急救:吸入:迅速脱离现场至空气新鲜处。
保持呼吸道通畅。
如呼吸困难,给输氧。
呼吸、心跳停止,立即进行心肺复苏术。
就医皮肤接触:立即脱去污染的衣着,用甘油、聚乙二醇300至400或聚乙二醇和酒精混合液(7:3)抹洗,然后用水彻底清洗。
或用大量流动清水冲洗20~30min。
如有不适感,就医眼睛接触:立即分开眼睑,用大量流动清水或生理盐水彻底冲洗10~15min。
如有不适感,就医食入:立即给饮植物油15~30mL。
催吐。
口服活性碳,导泻。

第1部分化学品及企业标识化学品中文名:间苯二酚化学品英文名:Resorcinol分子式:C6H6O2分子量:110.11CAS号:108-46-3产品推荐及限制用途:工业及科研用途。
第2部分危险性概述紧急情况概述:吞咽有害。
造成皮肤刺激。
造成严重眼刺激。
对水生生物毒性极大。
GHS危险性类别:急性经口毒性类别4皮肤腐蚀/刺激类别2严重眼损伤/眼刺激类别2危害水生环境——急性危险类别1标签要素:象形图:警示词:警告危险性说明:H302吞咽有害H315造成皮肤刺激H319造成严重眼刺激H400对水生生物毒性极大防范说明:•预防措施:——P264作业后彻底清洗。
——P270使用本产品时不要进食、饮水或吸烟。
——P280戴防护手套/穿防护服/戴防护眼罩/戴防护面具。
——P273避免释放到环境中。
•事故响应:——P301+P312如误吞咽:如感觉不适,呼叫解毒中心/医生——P330漱口。
——P302+P352如皮肤沾染:用水充分清洗。
——P332+P313如发生皮肤刺激:求医/就诊。
——P362+P364脱掉沾染的衣服,清洗后方可重新使用——P305+P351+P338如进入眼睛:用水小心冲洗几分钟。
如戴隐形眼镜并可方便地取出,取出隐形眼镜。
继续冲洗。
——P337+P313如仍觉眼刺激:求医/就诊。
——P391收集溢出物。
•安全储存:——无•废弃处置:——P501按当地法规处置内装物/容器。
物理和化学危险:无资料健康危害:吞咽有害。
造成皮肤刺激。
造成严重眼刺激。
环境危害:对水生生物毒性极大。
第3部分成分/组成信息第4部分急救措施急救:吸入:迅速脱离现场至空气新鲜处。
保持呼吸道通畅。
如呼吸困难,给输氧。
呼吸、心跳停止,立即进行心肺复苏术。
就医皮肤接触:立即脱去污染衣物,用大量流动清水彻底冲洗,冲洗后即用浸过30%~50%酒精棉花擦洗创面至无酚味为止(注意不能将患处浸泡于酒精溶液中)。
如有条件可用数块浸有聚乙二醇(300或400)的海绵反复擦洗污染部位,至少20min。

EUROPEAN PHARMACOPOEIA 8.0TropicamideA.2-aminoethanol(ethanolamine),B.2,2′-iminodiethanol(diethanolamine),C.2,2′-(nitrosoimino)diethanol (N -nitrosodiethanolamine).01/2008:1053corrected 6.0TROMETAMOLTrometamolum C 4H 11NO 3M r 121.1[77-86-1]DEFINITION Trometamol contains not less than 99.0per cent and not more than the equivalent of 100.5per cent of aminomethylidynetri(methanol),calculated with reference to the dried substance.CHARACTERS A white or almost white,crystalline powder,or colourless crystals,freely soluble in water,sparingly soluble in alcohol,very slightly soluble in ethyl acetate.IDENTIFICATIONFirst identification:B,C .Second identification:A,B,D .A.Solution S (see Tests)is strongly alkaline (2.2.4).B.Melting point (2.2.14):168°C to 174°C.C.Examine by infrared absorption spectrophotometry(2.2.24),comparing with the spectrum obtained with trometamol CRS .D.Examine the chromatograms obtained in the test forrelated substances.The principal spot in the chromatogram obtained with test solution (b)is similar in position,colour and size to the principal spot in the chromatogram obtained with reference solution (a).TESTSSolution S .Dissolve 2.5g in carbon dioxide-free water R and dilute to 50mL with the same solvent.Appearance of solution .Solution S is clear (2.2.1)and colourless (2.2.2,Method II ).pH (2.2.3).The pH of freshly prepared solution S is 10.0to 11.5.Related substances .Examine by thin-layer chromatography (2.2.27),using silica gel G R as the coating substance.Wash the plate with methanol R before applying the solutions.Test solution (a).Dissolve 0.20g in 1mL of water R ,with heating,and dilute to 10mL with methanol R .Test solution (b).Dilute 1mL of test solution (a)to 10mL with methanol R .Reference solution (a).Dissolve 20mg of trometamol CRS in methanol R and dilute to 10mL with the same solvent.Reference solution (b).Dilute 1mL of test solution (a)to 100mL with methanol R .Apply to the plate 10μL of each solution.Develop over a path of 10cm using a mixture of 10volumes of diluteammonia R1and 90volumes of 2-propanol R .Dry the plateat 100°C to 105°C.Spray with a 5g/L solution of potassium permanganate R in a 10g/L solution of sodium carbonate R .After about 10min examine in daylight.Any spot in thechromatogram obtained with test solution (a),apart fromthe principal spot,is not more intense than the spot in the chromatogram obtained with reference solution (b)(1.0per cent).Chlorides (2.4.4).To 10mL of solution S add 2.5mL of dilutenitric acid R and dilute to 15mL with water R .The solution complies with the limit test for chlorides (100ppm).Heavy metals (2.4.8).Dissolve 2.0g in 10mL of water R .Neutralise the solution with hydrochloric acid R1and dilute to 20mL with water R .12mL of the solution complies withtest A for heavy metals (10ppm).Prepare the referencesolution using lead standard solution (1ppm Pb)R .Iron (2.4.9).Dissolve 1.0g in water R and dilute to 10mLwith the same solvent.The solution complies with the limit test for iron (10ppm).Loss on drying (2.2.32).Not more than 0.5per cent,determined on 1.000g by drying in an oven at 105°C.Sulfated ash (2.4.14).Not more than 0.1per cent,determined on 1.0g.Bacterial endotoxins (2.6.14):less than 0.03IU/mg,if intended for use in the manufacture of parenteral preparations without a further appropriate procedure for the removal of bacterial endotoxins.ASSAYDissolve 0.100g in 20mL of water R .Add 0.2mL of methyl red solution R .Titrate with 0.1M hydrochloric acid until the colour changes from yellow to red.1mL of 0.1M hydrochloric acid is equivalent to 12.11mg ofC 4H 11NO 3.IMPURITIES A.nitromethylidynetri(methanol).01/2011:1159TROPICAMIDETropicamidum C 17H 20N 2O 2M r 284.4[1508-75-4]DEFINITION(2RS )-N -Ethyl-3-hydroxy-2-phenyl-N -(pyridin-4-ylmethyl)propanamide.Content :99.0per cent to 101.0per cent (dried substance).CHARACTERSAppearance :white or almost white,crystalline powder.Solubility :slightly soluble in water,freely soluble in ethanol (96per cent)and in methylene chloride.IDENTIFICATION First identification:C .General Notices (1)apply to all monographs and other texts3483。

全球有机纺织品标准国际工作组全球有机纺织品标准版本:1.1目录1 原则 (3)1.1 本标准的目的 (3)1.2 范围与结构 (3)1.3 版本 (3)1.4 标识等级 (3)2 要求 (4)2.1 有机纤维生产方面的要求 (4)2.2 材料成分方面的要求 (4)2.2.1 作为“有机”或“有机转化”产品来销售、贴标或展示的产品 (4)2.2.2 作为“有机材料含量为x%的产品”或“有机转化材料含量为x%的产品”来销售、贴标或展示的产品 (4)2.3 加工方面的要求 (5)2.3.1 分隔与识别 (5)2.3.2 在各个生产阶段都禁用/限用的材料 (5)2.3.3 各加工阶段助剂和染料方面的基本要求 (6)2.3.4 纺纱 (7)2.3.5 浆纱与编织/针织 (7)2.3.6 无纺生产工艺 (7)2.3.7 预处理阶段与湿加工 (7)2.3.8 染色 (8)2.3.9 印花 (8)2.3.10 整理 (8)2.3.11 辅料方面的要求 (9)2.3.12 环境管理 (10)2.3.13 废水处理 (10)2.3.14 贮存、包装与运输 (10)2.3.15 记录保存与内部质量保证 (11)2.3.16 技术特性参数 (11)2.3.17 有机纺织品中的残留物参照值 (12)2.3.18 其它材料与辅料中的残留物参照值 (13)3 最低程度社会要求 (15)3.1 范围 (15)3.2 工作应当可以自由选择 (15)3.3 结社的自由和集体谈判的权利应当受到尊重 (15)3.4 工作环境应当安全而卫生 (15)3.5 不得使用童工 (16)3.6 应当支付最低工资 (16)3.7 工作时间不得超标 (16)3.8 不允许有歧视 (17)3.9 必须提供正式雇用 (17)3.10 不允许有粗暴或不人道对待 (17)4 质量保证体系 (18)4.1 加工与生产阶段的检查 (18)4.2 残留物试验 (18)附件 (19)其它纤维 (19)缩略词 (19)1 原则1.1 本标准的目的本标准旨在定义确保纺织品有机状态方面的要求,包括:原材料的收割、对环境和社会负责的生产、以及标识,以确保让终端消费者放心。
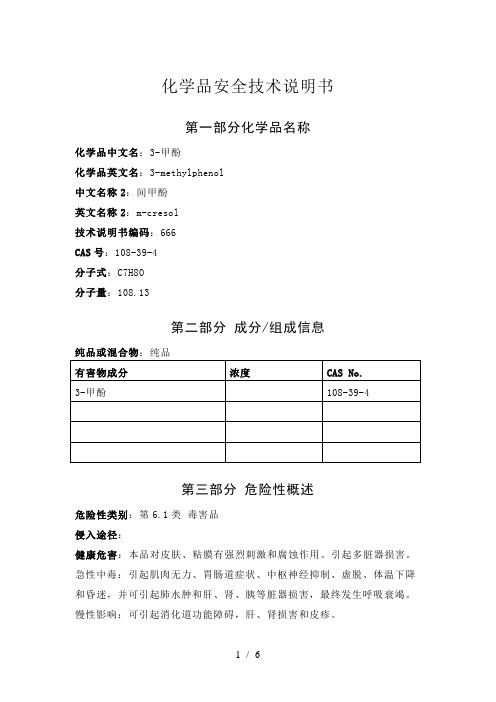
化学品安全技术说明书第一部分化学品名称化学品中文名:3-甲酚化学品英文名:3-methylphenol中文名称2:间甲酚英文名称2:m-cresol技术说明书编码:666CAS号:108-39-4分子式:C7H8O分子量:108.13第二部分成分/组成信息纯品或混合物:纯品第三部分危险性概述危险性类别:第6.1类毒害品侵入途径:健康危害:本品对皮肤、粘膜有强烈刺激和腐蚀作用。
引起多脏器损害。
急性中毒:引起肌肉无力、胃肠道症状、中枢神经抑制、虚脱、体温下降和昏迷,并可引起肺水肿和肝、肾、胰等脏器损害,最终发生呼吸衰竭。
慢性影响:可引起消化道功能障碍,肝、肾损害和皮疹。
环境危害:对环境有危害,对水体可造成污染。
燃爆危险:本品可燃,高毒,具腐蚀性、强刺激性,可致人体灼伤。
第四部分急救措施皮肤接触:立即脱去污染的衣着,用甘油、聚乙烯乙二醇或聚乙烯乙二醇和酒精混合液 (7:3)抹洗,然后用水彻底清洗。
或用大量流动清水冲洗至少15分钟。
就医。
眼睛接触:立即提起眼睑,用大量流动清水或生理盐水彻底冲洗至少15分钟。
就医。
吸入:迅速脱离现场至空气新鲜处。
保持呼吸道通畅。
如呼吸困难,给输氧。
如呼吸停止,立即进行人工呼吸。
就医。
食入:立即给饮植物油15~30mL。
催吐。
就医。
第五部分消防措施危险特性:遇明火、高热可燃。
有害燃烧产物:一氧化碳、二氧化碳。
灭火方法:消防人员须佩戴防毒面具、穿全身消防服,在上风向灭火。
灭火剂:雾状水、泡沫、干粉、二氧化碳、砂土。
第六部分泄漏应急处理应急处理:迅速撤离泄漏污染区人员至安全区,并进行隔离,严格限制出入。
切断火源。
建议应急处理人员戴自给正压式呼吸器,穿防毒服。
不要直接接触泄漏物。
尽可能切断泄漏源。
防止流入下水道、排洪沟等限制性空间。
小量泄漏:用砂土、干燥石灰或苏打灰混合。
大量泄漏:构筑围堤或挖坑收容。
用泡沫覆盖,降低蒸气灾害。
用泵转移至槽车或专用收集器内,回收或运至废物处理场所处置。

重金属方法A供试溶液:12ml待测水溶液,2ml pH为3.5的缓冲溶液,混合后加1.2ml的硫代乙酰胺试液,立即混合。
对照溶液:10ml的标准铅溶液(1ppm or 2ppm Pb), 2ml pH为3.5的缓冲溶液,2ml的待测液,混合后加1.2ml的硫代乙酰胺试液,立即混合。
空白溶液:10ml的水,2ml pH为3.5的缓冲溶液,2ml的测试溶液。
混合后加1.2ml的硫代乙酰胺试液,立即混合,同空白溶液比较,对照溶液显浅棕色。
2分钟后,供试的溶液颜色不得比对照溶液深。
方法B用含最少量水的溶剂(例如含15%水的二氧杂环乙烷或含15%水的丙酮)溶解规定量的供试品,制成待测液供试溶液:12ml待测液, 2ml pH为3.5的缓冲溶液,混合后加1.2ml的硫代乙酰胺试液,立即混合。
对照溶液:10ml的标准铅溶液(1ppm or 2ppm Pb), 2ml pH为3.5的缓冲溶液, 2ml的待测液,混合后加1.2ml的硫代乙酰胺试液,立即混合。
空白溶液:10ml的水,2ml pH为3.5的缓冲溶液,2ml的测试溶液。
混合后加1.2ml的硫代乙酰胺试液,立即混合,同空白溶液比较,对照溶液显浅棕色。
2分钟后,供试溶液的颜色不得比对照溶液深。
供试溶液:规定量(不超过2g)的待检测物质置于坩埚内,加4ml的250g/l 的硫酸镁溶液(稀硫酸溶解硫酸镁),玻璃棒搅拌混和,小心加热。
如果混合物还是液体,则在水浴中蒸发使其干燥。
连续加热灼烧,灼烧温度不超过800℃,直到获得白色或灰白色的残渣。
取出,冷却后以稀硫酸润湿残渣,加热蒸发后继续灼烧,灼烧的总时间不能超过2小时。
取出,冷却。
如法制取2份残渣,分别加入5ml稀盐酸,0.1ml的酚酞试液,然后滴加氨水,直到粉红色出现。
冷却,滴加冰醋酸至颜色消失,颜色消失后再多加0.5ml冰醋酸。
必要时过滤,并洗涤残渣。
加水稀释至20ml,制成待测液。
取12ml该待测液,加2ml pH3.5的缓冲溶液,混和,加1.2ml硫代乙酰胺试液,立即混合,制成供试溶液。
埃索美拉唑镁三水合物C34H36MgN6O6S2,3H2O M r 767.2[217087-09-7]化学名:镁双[5-甲氧基-2-[(S)-[(4-甲氧基-3,5-二甲基吡啶-2-基)甲基]亚磺酰基]-1H-苯并咪唑-1-基]三水合物。
含量:98.0-102.0%(无水物)性状外观:白色或类白色粉末,微有引湿性。
溶解性:微溶于水,易溶于甲醇,在庚烷中几乎不溶。
鉴别完成其中一组试验:A,B,C;A,B,E;B,C,D;B,D,E。
A.比旋光度(2.2.7):- 155。
~ - 137。
溶解0.250g到甲醇中,并用相同的溶剂稀释至25.0 mLB.红外吸收分光光度法(2.2.24)。
比较:埃索美拉唑镁三水合物对照品。
C.原子吸收光谱法(2.2.23)镁检查项下,测试溶液在285.2nm处显示最大吸收D.对映体纯度(见检查项下)。
E:根据硫酸盐灰分的测试程序(2.4.14),点燃约0.5g被检查物质。
残留物用10mL水溶解,该溶液2mL应显示镁的反应(2.3.1)。
检查吸光度 (2.2.25):≤0.20 (440 nm)用甲醇溶解0.500g,并稀释至25.0 mL,通过膜过滤器过滤该溶液(标称孔径0.45微米)。
有关物质液相色谱法(2.2.29)。
使用标准程序。
使用新鲜配制的溶液。
供试品溶液用流动相溶解3.5mg被测物质,并稀释至25.0ml。
对照溶液(a)。
用流动相溶解1mg奥美拉唑对照品和1mg奥美拉唑杂质D对照品,并稀释至10.0ml。
对照溶液(b)用流动相溶解3mg奥美拉唑峰识别用对照品(含杂质E)并稀释至20.0ml。
对照溶液(c)用流动相稀释1.0ml供试品溶液至100.0ml,再用流动相稀释此溶液1.0ml 至10.0ml。
色谱柱:规格: 4.6*125固定相:辛烷基硅烷键合硅胶(C8 5μm)流动相:混合27体积的乙腈和73倍体积的磷酸氢二钠溶液(1.4g/ L用磷酸调节pH至7.6)流速: 1 mL/min.紫外分光光度检测器: 280 nm.进样: 40 μL.运行时间:埃索美拉唑保留时间的5倍。
⾊氨酸欧洲药典EP8.0Tryptophan EUROPEAN PHARMACOPOEIA8.0Reference solution .Dissolve 25.0mg of trypsin BRP in 0.001M hydrochloric acid and dilute to 25.0mL with the same acid.Store the solutions at 0-5°C.Warm 1mL of each solution to about 25°C over 15min and use 50µL of each solution for each titration.Carry out the titration in an atmosphere of nitrogen.Transfer 10.0mL of 0.0015M borate buffer solution pH 8.0R to the reaction vessel and,while stirring,add 1.0mL of a freshly prepared 6.86g/L solution of benzoylarginine ethyl ester hydrochloride R .When the temperature is steady at 25.0±0.1°C (after about 5min)adjust to pH 8.0exactly with 0.1M sodium hydroxide .Add 50µL of the test solution and start a timer.Maintain at pH 8.0by the addition of 0.1M sodium hydroxide ,the tip of the microburette being immersed in the solution;note the volume added every 30s.Follow the reaction for 8min.Calculate the volume of 0.1M sodium hydroxide used per second.Carry out a titration in the same manner using the reference solution and calculate the volume of 0.1M sodium hydroxide used per second.Calculate the activity in microkatals per milligram using the following expression:m =mass of the substance to be examined,in milligrams;m ′=mass of trypsin BRP ,in milligrams;V =volume of 0.1M sodium hydroxide used per second by the test solution;V ′=volume of 0.1M sodium hydroxide used per second by the reference solution;A=activity of trypsin BRP ,in microkatals per milligram.STORAGEIn an airtight container,protected from light,at a temperature of 2°C to 8°C.LABELLING The label states:–the activity in microkatals per milligram;–for the amorphous substance,that it is hygroscopic.01/2009:1272corrected 7.0TRYPTOPHANTryptophanumC 11H 12N 2O 2M r 204.2[73-22-3]DEFINITION(S )-2-Amino-3-(1H -indol-3-yl)propanoic acid.Content :98.5per cent to 101.0per cent (dried substance).CHARACTERSAppearance :white or almost white,crystalline or amorphous powder.Solubility :sparingly soluble in water,slightly soluble inethanol (96per cent).It dissolves in dilute solutions of mineral acids and alkali hydroxides.IDENTIFICATIONFirst identification:A,B.Second identification:A,C,D.A.Speci?c optical rotation (see Tests).B.Infrared absorption spectrophotometry (2.2.24).Preparation :discs.Comparison :tryptophan CRS .C.Examine the chromatograms obtained in the test for ninhydrin-positive substances.Results :the principal spot in the chromatogram obtained with test solution (b)is similar in position,colour and size to the principal spot in the chromatogram obtained with reference solution (a).D.Dissolve about 20mg in 10mL of water R .Add 5mL of dimethylaminobenzaldehyde solution R6and 2mL of hydrochloric acid R1.Heat on a water-bath.A purple-blue colour develops.TESTSAppearance of solution .The solution is clear (2.2.1)and not more intensely coloured than reference solution BY6(2.2.2,Method II ).Dissolve 0.1g in 1M hydrochloric acid and dilute to 10mL with the same acid.Speci?c optical rotation (2.2.7):?30.0to ?33.0(dried substance).Dissolve 0.25g in water R ,heating on a water-bath if necessary,and dilute to 25.0mL with the same solvent.Ninhydrin-positive substances .Thin-layer chromatography (2.2.27).Solvent mixture :glacial acetic acid R ,water R (50:50V/V ).Test solution (a).Dissolve 0.10g of the substance to be examined in the solvent mixture and dilute to 10mL with the solvent mixture.Test solution (b).Dilute 1mL of test solution (a)to 50mL with the solvent mixture.Reference solution (a).Dissolve 10mg of tryptophan CRS in the solvent mixture and dilute to 50mL with the solvent mixture. Reference solution (b).Dilute 5mL of test solution (b)to 20mL with the solvent mixture.Reference solution (c).Dissolve 10mg of tryptophan CRS and 10mg of tyrosine CRS in the solvent mixture and dilute to25mL with the solvent mixture.Plate :TLC silica gel plate R .Mobile phase :glacial acetic acid R ,water R ,butanol R (20:20:60V/V/V ).Application :5µL.Development :over a path of 15cm.Drying :in air.Detection :spray with ninhydrin solution R and heat at 100-105°C for 15min.System suitability :reference solution (c):–the chromatogram shows 2clearly separated spots.Limit :test solution (a):–any impurity :any spot,apart from the principal spot,is not more intense than the principal spot in the chromatogram obtained with reference solution (b)(0.5per cent).Impurity A and other related substances .Liquidchromatography (2.2.29).Prepare the standard,test and reference solutions immediately before use.Buffer solution pH 2.3.Dissolve 3.90g of sodium dihydrogen phosphate R in 1000mL of water R .Add about 700mL of a2.9g/L solution of phosphoric acid R and adjust to pH 2.3with the same acid solution.Solvent mixture :acetonitrile R ,water R (10:90V/V ).3490See the information section on general monographs (cover pages)EUROPEAN PHARMACOPOEIA 8.0TryptophanStandard solution .Dissolve 10.0mg of N-acetyltryptophan R in the solvent mixture and dilute to 100.0mL with the solvent mixture.Dilute 2.0mL of this solution to 100.0mL with the solvent mixture.Test solution (a).Dissolve 0.10g of the substance to be examined in the solvent mixture and dilute to 10.0mL with the solvent mixture.Test solution (b).Dissolve 0.10g of the substance to beexamined in the standard solution and dilute to 10.0mL with the standard solution.Reference solution (a).Dissolve the contents of a vial of 1,1′-ethylidenebistryptophan CRS (impurity A)in 1.0mL of the solvent mixture.Reference solution (b).Dissolve the contents of a vial of 1,1′-ethylidenebistryptophan CRS (impurity A)in 1.0mL of the standard solution.Reference solution (c).Dilute 0.5mL of reference solution (a)to 5.0mL with the solvent mixture.Column :–size :l =0.25m,?=4.6mm;–stationary phase :octadecylsilyl silica gel for chromatography R (5µm);–temperature :40°C.Mobile phase :–mobile phase A :acetonitrile R ,buffer solution pH 2.3(115:885V/V );–mobile phase B :acetonitrile R ,buffer solution pH 2.3(350:650V/V );Time (min)Mobile phase A (per cent V/V )Mobile phase B (per cent V/V )0-1010010-45100→00→10045-65100Flow rate :0.7mL/min.Detection :spectrophotometer at 220nm.Injection :20µL of test solutions (a)and (b)and reference solutions (b)and (c).Retention time :tryptophan =about 8min;N -acetyltrypto-phan =about 29min;impurity A =about 34min.System suitability :–resolution :minimum 8.0between the peaks due toN -acetyltryptophan and impurity A in the chromatogram obtained with reference solution (b);if necessary,adjust the time programme for the elution gradient (an increase in the duration of elution with mobile phase A produces longer retention times and a better resolution);–signal-to-noise ratio :minimum 15for the principal peak in the chromatogram obtained with reference solution (c);–symmetryfactor :maximum 3.5for the peak due toimpurity A in the chromatogram obtained with reference solution (b).–in the chromatogram obtained with test solution (a)there is no peak with the same retention time as N -acetyltryptophan (in such case correct the area of the N -acetyltryptophan peak).Limits :test solution (b):–impurity A :not more than 0.5times the area of the principal peak in the chromatogram obtained with reference solution (c) (10ppm);–sum of impurities with a retention time less than that of tryptophan :not more than 0.6times the area of the peak due to N -acetyltryptophan in the chromatogram obtained with reference solution (b)(100ppm);–sum of impurities with a retention time greater than that of tryptophan and up to 1.8times the retention time of N-acetyltryptophan :not more than 1.9times the area of the peak due to N -acetyltryptophan in the chromatogram obtained with reference solution (b)(300ppm);–disregard limit:0.02times the area of the peak due to N -acetyltryptophan in the chromatogram obtained with reference solution (b);disregard the peak due to N -acetyltryptophan.Chlorides (2.4.4):maximum 200ppm.Dissolve 0.25g in 3mL of dilute nitric acid R and dilute to 15mL with water R .The solution,without any further addition of nitric acid,complies with the test.Sulfates (2.4.13):maximum 300ppm.Dissolve 0.5g in a mixture of 5volumes of dilute hydrochloric acid R and 25volumes of distilled water R ,and dilute to 15mL with the same mixture of solvents.Ammonium (2.4.1,Method B ):maximum 200ppm,determined on 0.10g.Prepare the standard using 0.2mL of ammonium standard solution (100ppm NH 4)R .Iron (2.4.9):maximum 20ppm.In a separating funnel,dissolve 0.50g in 10mL of dilute hydrochloric acid R .Shake with 3quantities,each of 10mL,of methyl isobutyl ketone R1,shaking for 3min each time.To the combined organic layers add 10mL of water R and shake for3min.Examine the aqueous layer.Heavy metals (2.4.8):maximum 10ppm.2.0g complies with test D.Prepare the reference solution using 2mL of lead standard solution (10ppm Pb)R .Loss on drying (2.2.32):maximum 0.5per cent,determined on 1.000g by drying in an oven at 105°C.Sulfated ash (2.4.14):maximum 0.1per cent,determined on 1.0g.ASSAYDissolve 0.150g in 3mL of anhydrous formic acid R .Add 30mL of anhydrous acetic acid R .Titrate with 0.1M perchloric acid ,using 0.1mL of naphtholbenzein solution R as indicator.1mL of 0.1M perchloric acid is equivalent to 20.42mg of C 11H 12N 2O 2.STORAGEProtected from light.IMPURITIESA.3,3′-[ethylidenebis(1H -indole-1,3-diyl)]bis[(2S )-2-aminopropanoic]acid (1,1′-ethylidenebistryptophan),B.(S )-2-amino-3-[(3RS )-3-hydroxy-2-oxo-2,3-dihydro-1H -indol-3-yl]propanoic acid (dioxyindolylalanine), General Notices (1)apply to all monographs and other texts3491Tuberculin for human use,old EUROPEAN PHARMACOPOEIA8.0C.R=H:(S)-2-amino-4-(2-aminophenyl)-4-oxobutanoicacid(kynurenine),E.R=CHO:(S)-2-amino-4-[2-(formylamino)phenyl]-4-oxobutanoic acid(N-formylkynurenine),D.(S)-2-amino-3-(5-hydroxy-1H-indol-3-yl)propanoic acid(5-hydroxytryptophan),F.(S)-2-amino-3-(phenylamino)propanoic acid(3-phenylaminoalanine),G.(S)-2-amino-3-(2-hydroxy-1H-indol-3-yl)propanoic acid(2-hydroxytryptophan),H.R=H:(3RS)-1,2,3,4-tetrahydro-9H-β-carboline-3-carboxylic acid,I.R=CH3:1-methyl-1,2,3,4-tetrahydro-9H-β-carboline-3-carboxylicacid,J.R=CHOH-CH2-OH:(S)-2-amino-3-[2-[2,3-dihydroxy-1-(1H-indol-3-yl)propyl]-1H-indol-3-yl]propanoic acid,K.R=H:(S)-2-amino-3-[2-(1H-indol-3-ylmethyl)-1H-indol-3-yl]propanoicacid,L.1-(1H-indol-3-ylmethyl)-1,2,3,4-tetrahydro-9H-β-carboline-3-carboxylic acid.01/2008:0152TUBERCULIN FOR HUMAN USE,OLD Tuberculinum pristinum ad usum humanum DEFINITIONOld tuberculin for human use consists of a?ltrate,concentrated by heating,containing the soluble products of the culture and lysis of one or more strains of Mycobacterium bovis and/or Mycobacterium tuberculosis that is capableof demonstrating a delayed hypersensitivity in an animal sensitised to micro-organisms of the same species.Old tuberculin for human use in concentrated form is a transparent,viscous,yellow or brown liquid. PRODUCTIONGENERAL PROVISIONSThe production of old tuberculin is based on a seed-lot system. The production method shall have been shown to yield consistently old tuberculin of adequate potency and safety in man.A batch of old tuberculin,calibrated in InternationalUnits by the method described under Assay and for which adequate clinical information is available as to its activity in man,is set aside to serve as a reference preparation.The International Unit is the activity of a stated quantity ofthe International Standard.The equivalence in International Units of the International Standard is stated by the World Health Organization.SEED LOTSThe strains of mycobacteria used shall be identi?ed by historical records that include information on their origin and subsequent manipulation.The working seed lots used to inoculate the media for the production of a concentrated harvest shall not have undergone more than4subcultures from the master seed lot.Only seed lots that comply with the following requirements may be used for propagation.Identi?cation.The species of mycobacterium of the master and working seed lots is identi?ed.Bacterial and fungal contamination.Carry out the test for sterility(2.6.1),using10mL for each medium.The workingseed lot complies with the test for sterility except for the presence of mycobacteria.PROPAGATION AND HARVESTThe bacteria are grown in a liquid medium which may be a glycerolated broth or a synthetic medium.Growth must betypical for the strain.The culture is inactivated by a suitable method,such as treatment in an autoclave(121°C for not lessthan30min)or in?owing steam at a temperature not lessthan100°C for at least1h.The culture liquid,from whichthe micro-organisms may or may not have been separatedby?ltration,is concentrated by evaporation,usually toone-tenth of its initial volume.The preparation is free fromlive mycobacteria.The concentrated harvest is shown tocomply with the test for mycobacteria(2.6.2)before additionof any antimicrobial preservative or other substance thatmight interfere with the test.Phenol(5g/L)or anothersuitable antimicrobial preservative that does not give rise tofalse positive reactions may be added.Only a concentrated harvest that complies with the following requirements may be used in the preparation of the?nal bulk tuberculin.pH(2.2.3).The pH of the concentrated harvest is6.5to8.Glycerol.Where applicable,determine the glycerol contentof the concentrated harvest.The amount is within the limits approved for the particular product.3492See the information section on general monographs(cover pages)。
欧洲药典7.0DefinitionPurifiedproteinobtainedeitherbypartialacidhydrolysis(typeA),partialalkalinehydrolysis(typeB)orenzymatic hydrolysis of collagen fromanimals(including fish and poultry); it may also be a mixture of different types. The hydrolysis leads to gelling or non-gelling product grades. Both product grades are covered by this monograph. Gelatin described in this monograph is not suitable for parenteral administration or for other special purposes.定义通过酸性水解(A型),部分碱性水解(B型)从动物(包括鱼,家畜)获得的蛋白质通过水解从而得到凝胶或非凝胶类型。
两种产品类型都包含在本药典中。
本药典中的明胶不适用于注射给药或用于其他用途。
形态特征外观:淡黄色或者淡棕黄色,通常为透明的片状,碎片、颗粒状或者粉末水溶性:一般不容易常用有机溶剂,凝胶类型在冷水中膨胀。
等电子点是明胶不同用途的相对参考质量指标:A型明胶Ph 6.0~9.5, B型明胶pH4.7~5.6, 对于不同用途的明胶pH范围可略微不同.不同类型的明胶透明度,色泽不同。
CHARACTERSAppearance: faintly yellow or light yellowish-brown, solid,usually occurring as translucent sheets, shreds, granules or powder. Solubility: practically insoluble in common organic solvents gelling grades swell in cold water and give on heating a colloidal solution which on cooling forms a more or less firm gel. The isoelectric point is a relevant quality parameter for use of gelatin in different applications: for type A gelatin it is typically between pH 6.0 and pH 9.5 and for type B gelatin is typically between pH 4.7 and pH 5.6. These ranges cover a variety of different gelatins and for specific applications a narrower tolerance is usually applied.Different gelatins form aqueous solutions that vary in clarity and colour. For a particular application, a suitable specification for clarity and colour is usually applied.IDENTIFICATIONA. To 2 mL of solution S (see Tests) add 0.05 mL of coppersulfate solution R. Mix and add 0.5 mL of dilute sodiumhydroxide solution R. A violet colour is produced.B. To 0.5 g in a test-tube add 10 mL of water R. Allow tostand for 10 min, heat at 60 °C for 15 min and keep thetube upright at 0 °C for 6 h. Invert the tube; the contentsimmediately flow out for non-gelling grades and do not flowout immediately for gelling grades.鉴别A 在2mlS溶液(见下文试验)中添加0.05ml硫酸铜。
无水间苯三酚
分子式:C6H6O3
分子量:126.1
CAS号:[108-73-6]
命名:1,3,5-苯三醇
含量:99-101%(以干物质计)
性状
外观:白色或类白色粉末。
溶解性:微溶于水,溶于乙醇(96%),几乎不溶于二氯甲烷。
鉴别
A.红外吸收分光光度法(2.2.24)。
比较:无水间苯三酚对照品。
B.薄层色谱法(2.2.27)。
供试溶液:取本品0.20 g溶解于甲醇R中并稀释至10ml。
标准溶液:取标准品0.20 g溶解于甲醇R中并稀释至10ml。
薄层板:硅胶板F254。
流动相:无水甲酸R:正己烷R:醋酸乙酯R:2:37.5: 62.5( V / V / V)。
点样量:10μl。
展开距离:超过板的2/3。
检测:波长254 nm的紫外线灯下。
结果:供试品溶液在薄层色谱中移动距离及斑点大小与对照溶液基本一致。
C.干燥失重法(见试验)。
试验
溶液S:取本品2.5 g溶解在乙醇(96 %)中并稀释到25ml。
溶液外观:溶液S是澄明的(2.2.1),与对照溶液相比,颜色不得更深。
pH (2.2.3):4.0-6.0。
取溶液S 10ml,加新沸放冷的水稀释至100ml。
有关物质 液相色谱法(2.2.29)。用前配制溶液并避光保存。
流动相溶液:流动相B,流动相A(10:90,V/V)
供试品溶液:取50mg样品溶解于流动相溶液,并用流动相溶液稀释至10ml。
对照溶液(a):取1ml供试溶液用流动相溶液稀释到100ml,从中取出1ml再用
流动相溶液稀释至10ml。
对照溶液(b):分别取间苯二酚R(杂质B)、水合间苯三酚R(杂质D)、连苯
三酚R(杂质A)6mg溶解于10ml的流动相溶液中,加入2ml的供试品溶液,
用流动相溶液稀释至20ml,再从中取1ml用流动相溶液稀释至50ml。
对照溶液(c):取4mg连苯三酚R(杂质A)、水合间苯三酚R(杂质D)、1,2,4-
苯三醇(杂质E)、2,6-二氯酚(杂质I)、4-氯间苯二酚(杂质K)、3,5-二氯苯胺
(杂质L),溶解于10.0ml的流动相溶液并稀释至20.0ml。
对照溶液(d):取10mg样品溶解于10ml的流动相溶液中,加入1ml的对照溶
液(c),用流动相溶液稀释至20ml。
柱:
--尺寸:l=0.25m, Ø = 4.0 mm
--固定相:十八烷基硅胶键合硅胶,5μm
流动相
--流动相A:1.36 g/L的磷酸二氢钾溶液(用磷酸调至pH 3)
--流动相B:乙腈R
流速:1ml/min
检测:检测波长265nm
进样:分别进样20μl的供试品溶液,对照溶液(a),对照溶液(b),对照溶液
(d)
杂质鉴定:用对照溶液(d)的色谱图确定杂质A,D,E,I,K和L峰。
间苯三酚相对保留时间(R = about 12 min):
杂质 E = about 0.7;
杂质 A = about 0.9;
杂质 D = about 1.3;
杂质 B = about 1.35;
杂质 K = about 1.5;
杂质 I = about 1.8;
杂质 L = about 2.0。
系统适应性:对照溶液(b):
分辨率:杂质和间苯三酚间最小分辨率为2.5;杂质D和B间最小分辨率为4。
限度:
--校正因子:对于峰面积的计算,使用下列校正因子计算相应的杂质峰面积:
杂质A = 0.6;杂质D = 0.2;杂质E = 0.7;杂质I = 0.6;杂质K = 0.6; 杂质L =
0.4;
--杂质A, D, E, I, K, L:每种杂质的峰面积均不得超过对照溶液(b)的主峰面积
的1.5倍(0.15%);
--未知杂质:每个杂质的峰面积均不得超过对照溶液(a)主峰面积(0.10%);
--总杂质:不得超过对照溶液(a)主峰面积的3倍(0.3%);
--忽视限度:对照溶液(a)主峰面积的0.5倍(0.05%)。
氯化物(2.4.4):最大限度200ppm。
将2.5ml溶液S用水稀释至15ml。
硫酸盐 (2.4.13):最大限度500 ppm。
将5ml溶液S用水稀释至15ml。
重金属 (2.4.8):最大限度20 ppm。
混合溶剂:水R,乙醇(96%)R(10:90,V/V)。
0.500g符合鉴别H的样品。
制备标准溶液:采用1 mL铅标准溶液(10 ppm Pb)R。
干燥失重(2.2.32):不得超过1.0%,取1.000克本品置于105℃烤箱中干燥。
炽灼残渣(2.4.14):1.0g样品的遗留残渣不得超过1.0%。
测定
溶解0.500g样品于50ml水R中,用1 M氢氧化钠滴定,确定终点电位(2.2.20)。
每1ml的1 M氢氧化钠溶液,相当于63.05mg C6H6O3。
储存
避光储存。
杂质
已知杂质:A,D,E,I,K,L。
其它可检出杂质(下述物质,如果有足够的水平,是由一个或其他的试验检测。
由总验收标准限定,因此不需要单独识别这些杂质,见5.10 药用物质中杂质的
控制):B,O。
A.1,2,3-苯三酚 (连苯三酚)
B.1,3-苯二酚 (间苯二酚)
D. 2,3',4,5',6-五羟基联苯(水合间苯三酚)
E.1,2,4-苯三酚
I.2,6-二氯苯酚
K.4-氯-1,3-苯二酚(4-氯间苯二酚)
L.3.5 -二氯苯胺
O.4,6-二氯间苯二酚