Opioid induced hyperalgesia clinical implications for the pain practitioner
- 格式:pdf
- 大小:98.21 KB
- 文档页数:6

1. 长期给予吗啡后突然停药,会引起痛觉过敏。
在大鼠切口痛模型中,术前六天持续皮下给予纳洛酮20mg/kg/天会显著降低大鼠后爪的痛觉过敏。
(Li X, Angst MS, Clark JD. Opioid-induced hyperalgesia and incisional pain. Anesth Analg. 2001 Jul;93(1):204-9.)2. 术中应用大剂量雷米芬太尼(0.40μg ·kg-1 ·min-1)比应用小剂量雷米芬太尼(0.05μg ·kg-1 ·min-1)更容易引起痛觉过敏,而应用小剂量氯胺酮(术前0.5mg/kg,随后5 μg ·kg-1 ·min-1,术后2 μg ·kg-1 ·min-1持续48小时)会防止痛觉过敏。
(Joly V, Richebe P, Guignard B, et al. Remifentanil-induced postoperative hyperalgesia and its prevention with small-dose ketamine. Anesthesiology. 2005 Jul;103(1):147-55.)3. 通过对大鼠腰段脊髓切片进行电生理研究发现,不含甘氨酸辅料的盐酸雷米芬太尼不能诱发NMDA受体通道的电流,而甘氨酸(雷米芬太尼制剂中的常用辅料)可以诱发NMDA 受体通道电流,而且这种通道电流可以被NMDA谷氨酸位点的特异性拮抗剂2-氨基-5-磷酸基戊酸(APV)阻断,而含有甘氨酸辅料的盐酸雷米芬太尼能够诱发与甘氨酸基本一样的NMDA受体通道电流,而且这种电流同样也可以被2-氨基-5-磷酸基戊酸(APV)阻断。
盐酸雷米芬太尼能够强化NMDA受体诱发的内向电流,而且这种强化作用可以被μ受体拮抗剂纳洛酮消除。
提示盐酸雷米芬太尼不能直接激活NMDA受体,但是可以通过阿片μ受体强化NMDA通道的作用瑞芬太尼不能直接激活NMDA受体,瑞芬太尼使用后诱导的NMDA受体激活主要通过一条含μ-阿片受体的调节通路来实现的,也就是说μ-阿片受体起着一个桥梁作用。




阿片诱发的痛觉过敏
王锦琰
【期刊名称】《中国疼痛医学杂志》
【年(卷),期】2008(014)003
【摘要】在使用阿片类药物治疗疼痛的过程中,疗效的下降往往用药理学上的耐受机制来解释(或原有病情的加重),必须加大剂量才能维持原有疗效。
最近的研究证实,阿片类药物除了产生镇痛作用之外,还能够激活体内的促伤害机制,导致机体对疼痛的敏感性增高,即阿片诱发的痛觉过敏(opioid—induced hyperalgesia,OIH)。
早先在阿片戒断反应中就观察到有痛敏出现,最新的证据表明,痛敏亦可发生于阿片治疗过程中。
也就是说,停用阿片和持续给予阿片都会造成疼痛敏感性增加,这显然是矛盾的。
【总页数】2页(P129-130)
【作者】王锦琰
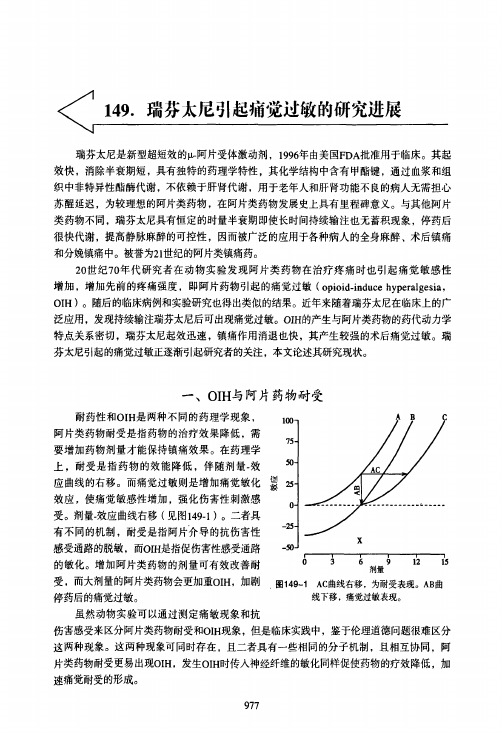
【作者单位】无
【正文语种】中文
【中图分类】R730.53
【相关文献】
1.阿片类药物诱发痛觉过敏的研究进展 [J], 韩潞潞;薛庆生;于布为
2.阿片类药物诱发痛觉过敏的药物干预 [J], 顾永丽;孙增先
3.阿片类药物诱导痛觉过敏及阿片受体拮抗剂应用的研究进展 [J], 刘艳丽;张二飞;
张俊英;胡彬
4.激活中央杏仁核代谢型谷氨酸受体1在调节大鼠阿片诱导的痛觉过敏中的作用[J], 曹波;宋雪;王总
5.去甲基化调控μ阿片受体对神经病理性疼痛痛觉过敏和吗啡耐受的影响 [J], 吴强;林静;刘民强;刘丽;何仁亮
因版权原因,仅展示原文概要,查看原文内容请购买。
PNAS:长痛不如短痛?止痛药被发现可引发慢性痛这是一个听起来让人感到匪夷所思的结果——像吗啡这样的阿片类止痛药竟然可能引发慢性痛。
最近,美国科罗拉多大学的研究人员们发现,仅仅五天的吗啡服用就可在大鼠中导致慢性痛的持续时间翻倍。
这一发表在最新一期《美国国家科学院院刊》(PNAS)上的发现无疑对人类具有重要的启示,尤其是在阿片类药物的使用乃至成瘾日益增加的今天。
吗啡结构“刚开始吗啡确实能起到明显的镇痛效果,接着这种效果将逐渐减弱直至消失——人们一贯都是这么认为的。
然而,我们的研究却显示,虽然开始能暂时缓解疼痛,但这些镇痛药从长期看反而可能增加疼痛,”文章的作者、科罗拉多大学的Peter Grace教授说道。
事实上,科学家们之前已经注意到了阿片类药物的长期使用会增加某些人对疼痛的敏感度,并将该现象成为阿片类药物引起的痛觉过敏(opioid-induced hyperalgesia,OIH)。
而在这一工作中,研究者们发现,短期的止痛药使用便可导致这一后果。
他们对接受手术后的大鼠采取了某种特定的缝合方式,使其坐骨神经遭到挤压,从而引起痛感。
十天之后,部分大鼠接受了五天的吗啡治疗,并在之后接受痛觉敏感度测试。
结果显示,没有接受吗啡的大鼠只需四周的时间恢复,即对于触觉刺激(如戳等)的痛觉敏感度下降;而服用了吗啡的大鼠需要八周才能恢复。
这一试验是在雄性大鼠身上完成的,而据研究者透露,吗啡对使雌性大鼠疼痛敏感度升高效果的持续时间则更长。
进一步的研究显示,大鼠的外周神经在手术缝合中初次受损时,会向脊髓中用于清除细胞碎片和外来微生物的免疫细胞——胶质细胞(glia cell)发出信号,使其处于“警戒”状态,包括NLRP3受体和白细胞介素-1β前体(pro-IL-1β)的上调,以便为接下来可能的不测做好准备。
“这就好比开启了脊髓的一个‘变光开关’,” Grace教授说道:“这是整个反应过程的第一步,你可能会觉得它并无大碍,但第二步就不是如此了。
(19)中华人民共和国国家知识产权局(12)发明专利申请(10)申请公布号 (43)申请公布日 (21)申请号 202011344050.3(22)申请日 2020.11.26(71)申请人 李依泽地址 300052 天津市和平区鞍山道154号申请人 张麟临(72)发明人 李依泽 张麟临 于泳浩 王国林 李晶 丁冉 董贝贝 陈怡 (74)专利代理机构 天津创信方达专利代理事务所(普通合伙) 12247代理人 李京京(51)Int.Cl.A61K 31/5517(2006.01)A61P 25/00(2006.01)(54)发明名称瑞马唑仑治疗阿片类药物诱发的术后痛觉过敏中的应用(57)摘要本发明涉及瑞马唑仑治疗阿片类药物诱发的术后痛觉过敏中的应用。
本发明证明了瑞马唑仑通过促进GABAa受体表达从而实现抑制瑞芬太尼诱发的术后痛觉过敏的作用。
本发明的研究成果提供了一种阿片类药物引起的痛觉过敏的新型治疗途径,在临床上具备良好的应用前景。
权利要求书1页 说明书6页 附图2页CN 112402428 A 2021.02.26C N 112402428A1.抑制脊髓背角神经元兴奋性的试剂在制备预防或治疗阿片类药物诱发的术后痛觉过敏的药物中的应用;优选地,所述阿片类药物是瑞芬太尼。
2.根据权利要求1所述的应用,其特征在于,所述试剂包括抑制NMDAR的GluN2B亚基膜转位的试剂、抑制AMPAR的Ser845-GluA1亚基磷酸化试剂、或促进GABAa受体表达的试剂。
3.根据权利要求2所述的应用,其特征在于,促进GABAa受体表达的试剂是瑞马唑仑。
4.根据权利要求1所述的应用,其特征在于,所述阿片类药物诱发的术后痛觉过敏包括包括阿片类药物输注后切口痛觉过敏、单纯阿片类药物输注后诱发的痛觉过敏。
5.根据权利要求4所述的应用,其特征在于,阿片类药物输注后切口痛觉过敏包括术后机械痛觉过敏和热痛觉过敏;单纯阿片类药物输注后诱发的痛觉过敏包括热痛觉过敏。
Opioids have been and continue to be used for the treatment of chronic pain. Evidence supports the notion that opioids can be safely administered in patients with chronic pain without the development of addiction or chemical dependency. However, over the past several years, concerns have arisen with respect to administration of opioids for the treatment of chronic pain, particularly non-cancer pain. Many of these involve legal issues with respect to diversion and prescription opioid abuse. Amongst these, opioid induced hyperalgesia (OIH) is becoming more prevalent as the population receiving opioids for chronic pain increases. OIH is a recognized complication of opioid therapy. It is a pro-nocioceptive process which is related to, but different from, tolerance. This focused review will elaborate on the neurobiological mechanisms of OIH as well as summarize the pre-clinical and clinical studies supporting the existence of OIH. In particular, the role of the excitatory neurotransmitter, N-methyl-D-aspartate appears to play a central, but not the only, role in OIH. Other mechanisms of OIH include the role of spinal dynorphins and descending facilitation from the rostral ventromedial medulla. The links between pain, tolerance, and OIH will be discussed with respect to their common neurobiology.Practical considerations for diagnosis and treatment for OIH will be discussed. It is crucial for the pain specialist to differentiate amongst clinically worsening pain, tolerance, and OIH since the treatment of these conditions differ. Tolerance is a necessary condition for OIH but the converse is not necessarily true.Office-based detoxification, reduction of opioid dose, opioid rotation, and the use of specific NMDA receptor antagonists are all viable treatment options for OIH. The role of sublingual buprenorphine appears to be an attractive, simple option for the treatment of OIH and is particularly advantageous for a busy interventional pain practice.Key words: Opioid hyperalgesia, hyperalgesia, tolerance, NMDA receptor antagonists, NMDA receptor induced hyperalgesia, spinal dynorphin induced hyperalgesia, descending facilitation and hyperalgesia, buprenorphine and hyperalgesia, opioid detoxification, office-based detoxification, complications of opioid therapy Pain Physician 2009; 12:679-684Focused ReviewOpioid Induced Hyperalgesia: Clinical Implications for the Pain PractitionerFrom: Comprehensive Pain Medicine, Pompano Beach, FL.Address correspondence:Sanford M. Silverman, MD Comprehensive Pain Medicine100 East Sample RdSuite 200Pompano Beach, FL 33064E-mail:silvpain1@ Disclaimer: T here was no external funding in thepreparation of this manuscript.Disclosure: Dr. Silverman is a speaker for Reckitt-Benckiser.Conflict of interest: None.Manuscript received:07/18/2008Revised manuscript received:9/15/2008Accepted for publication:11/4/2008Free full manuscript:Sanford M. Silverman, MDPain Physician 2009; 12:679-684 • ISSN 1533-3159For several decades, opioids have been considered integral components in the treatment of chronic pain. Evidence supportsthe notion that opioids can be safely administered to patients with chronic pain without the development of addiction or chemical dependency. Major pain and addiction organizations have endorsed this concept (1).The concept of pain sensitization from the chron-ic administration of opioids is often referred to as opioid induced hyperalgesia (OIH). There is substan-tial evidence to support the fact that this does oc-cur with chronic opioid therapy. OIH may complicate the clinical course of pain treatment in a patient re-ceiving opioids. Although the current clinical milieuPain Physician: May/June 2009: 12:679-684Since tolerance is characterized by decreasing ef-ficacy of a drug, it can be overcome by increasing the dose. However, unlike tolerance, OIH cannot be over-come by increasing dosage since OIH is a form of pain sensitization induced by the drug which occurs within the central nerve system (CNS) . Pain is worsened with increased opioid dosing and is improved by reducing or eliminating the opioid. Tolerance is a necessary con-dition for OIH, but the converse is not true. Clinically this is an important distinction that has obvious rami-fications with respect to continued use of opioids in a given patient.B asic s cience e vidence of o pioid i nduced H yperalgesiaMao (6) has documented the occurrence of OIH in laboratory animals. The commonly employed test is to examine the responses of rats, specifically the paw, to withdrawal tests in response to noxious stimuli af-ter receiving multiple boluses or a continuous infusion of opioid. A comparison of dose response effects are measured before and after administration of an opi-oid. With intrathecal morphine administration there is progressive reduction in baseline nocioceptive pain thresholds (7). Similar findings have been seen in rats receiving fentanyl boluses (8) and in animals receiving repeated heroin administration (9). These preclinical studies support the concept that there can be sensitiza-tion to pain with concurrent administration of opioids.c linical e vidence of o pioid i nduced H yperalgesiaSupporting evidence has shown that OIH occurs clinically outside the laboratory, and is seen after intra-operative remifentanil infusion, resulting in decreased opioid efficacy (10). Significant pain reduction has been demonstrated in patients who have been detoxified from high dose opioids (11). When challenged with cold pressor tests, opioid addicts maintained on meth-adone demonstrated increased pain sensitivity (12). There have also been a host of experimental studies in human volunteers and anecdotal reports of increased pain sensitivity induced or observed with the concomi-tant use of opioids. These studies and the mechanisms of OIH have been extensively reviewed (13).n euroBiological M ecHanisMs for o pioid i nduced H yperalgesiaThere are many proposed mechanisms for OIH in-cluding 3 areas which will be discussed:emphasizes physician monitoring of addiction, abuse, and diversion, OIH is often overlooked as a potential complication of opioid therapy.As earlier as the nineteenth century, OIH was ob-served in patients receiving morphine for pain. It was recognized that a potent analgesic such as morphine could actually result in an increase in pain and was observed by Albutt in 1870:“At such times I have certainly felt it a great re-sponsibility to say that pain, which I know is an evil, is less injurious than morphia, which may be an evil. Here experience is needed. Does morphia tend to encourage the very pain it pretends to relieve?“…..in the cases in question, I have much reason to suspect that a reliance upon hypodermic mor-phia only ended in that curious state of perpetu-ated pain” (2).This article is a focused review of OIH neurobiol-ogy and its clinical implications for the pain practitioner. Practical guidelines are suggested for the clinical man-agement of OIH.T olerance v ersus p ain s ensiTizaTionTolerance is a pharmacologic concept. It occurs when there is a progressive lack of response to a drug thus requiring increased dosing. Tolerance can occur with a variety of drugs including opioids (3,4).Tolerance may not only develop to the analge-sia provided by opioids but also develop undesirable side effects which are seen with opioid administra-tion such as pruritis, nausea, sedation, and respiratory depression.Sensitization to pain occurs in several areas of the nervous system involving the transmission of pain. Pe-ripheral mechanisms have been well documented with respect to neural injury involving mediators of inflam-mation. This is known as primary hyperalgesia and is seen clinically with peripheral nerve injuries. Second-ary hyperalgesia, on the other hand, occurs “down-stream” from the initiating nocioceptive stimulus and peripheral injury. In the spinal cord, wide dynamic range neurons become sensitized through a variety of mechanisms which may be mediated by neurotrans-mitters such as calcitonin gene-related peptide, Va-soactive Intestinal Peptide (VIP), Dynorphin (DYN), Cholcystokinin (CCK), Neuropeptide Y (NPY), and N-methyl-D-aspartate (NMDA) (5).Opioid Induced Hyperalgesia• The central glutaminergic system• Spinal dynorphins• Descending facilitationc enTral g luTaMinergic s ysTeMThe majority of studies examining the mecha-nisms of OIH involve the systemic administration of opioids (6,7). The excitatory neurotransmitter NMDA plays a central role in the development of OIH. The current data suggest that opioid induced desensiti-zation (pharmacological tolerance) and sensitization (OIH), while distinct processes, may share common cel-lular mechanisms in part mediated through activation of the central glutamatergic system (7).The role of NMDA can be summarized as follows: 1. NMDA receptors become activated and when in-hibited, prevent the development of tolerance and OIH (14-16).2. The glutamate transporter system is inhibited,therefore increasing the amount of glutamate available to NMDA receptors (17).3. Calcium regulated intracellular Protein Kinase C islikely a link between cellular mechanisms of toler-ance and OIH (16,18,19).4. Cross talk of neural mechanisms of pain and toler-ance may exist (20,21).5. Prolonged morphine administration induces neu-rotoxicity via NMDA receptor mediated apoptotic cell death in the dorsal horn (22).r ole of s pinal d ynorpHinSpinal dynorphin plays an important role in OIH in that levels have shown increases with continuous infu-sions of mu receptor agonists. These increased levels lead to the release of spinal excitatory neuropeptides such as calcitonin gene-related peptide from primary afferents (23). OIH is therefore a pro-nociceptive pro-cess facilitated by increasing the synthesis of excitato-ry neuropeptides and their release upon peripheral nocioceptive stimulation (7).r ole of d escending f aciliTaTionDescending facilitation influence on OIH may be seen through several mechanisms. Subsets of neurons (on and off cells) within the rostral ventromedial medul-la (RVM) have a unique response to opioids (24,25). Their activities may facilitate spinal nocioceptive processing (26). In addition, lesioning of the descending pathway to the spinal cord (dorsal lateral funiculus) prevents the increase seen in excitatory neuropeptides (23).Clearly several distinct neurobiological mecha-nisms may exist for OIH. However, determining which mechanism(s) predominate in any given patient has important implications for the pain practitioner. More studies are needed to examine the interactions be-tween the glutaminergic system, spinal dynorphin, and descending facilitation.o pioid i nduced H yperalgesia andc linical p racTiceAs shown, lack of efficacy may be seen with the administration of opioids for chronic pain. Common solutions to this include opioid rotation, reduction of the administered dose, or detoxification. However, a major dilemma faces the pain practitioner. Is the lack of efficacy seen as a result of tolerance or OIH? The challenge is to distinguish between the 2 since the treatment of each is quite different. In addition, the clinician must be able to distinguish between OIH and clinical exacerbation of preexisting pain.Several features of OIH may be helpful in differ-entiating between it and increases in preexisting pain. OIH will exacerbate a preexisting painful condition and therefore will increase pain intensity above the preexistent pain levels. However, further disease pro-gression needs to be ruled out which would increase pain. The practitioner must also consider additional increased pain resulting from increased activity or de-mand (often referred to as pseudotolerance). Further-more, OIH typically produces diffuse pain, less defined in quality, which extends to other areas of distribution from the preexisting pain. OIH is not unlike the pain experienced during opioid withdrawal, since the neu-robiology of both is similar (7).OIH has been demonstrated clinically by inducing changes in pain threshold, tolerability, and distribution pattern in opioid-maintained former addicts (12).Finally, if the preexisting pain is undertreated or if pharmacologic tolerance exists, then an increase in opioid dose will result in reduction of pain. Conversely, OIH would be worsened with increasing opioid dose. o pioid i nduced H yperalgesia T reaTMenT s TraTegiesThe pain practitioner has several options when confronted with a demonstrated lack of opioid efficacy. Rational polypharmacy to include non-opioid medica-tion should be utilized when treating any patient with intractable pain. This strategy helps minimize the dose of opioid used, thus reducing the possibility of sidePain Physician: May/June 2009: 12:679-684effects and therefore, OIH. Neuropathic pain tends to preferentially respond to non-opioid medications such as antidepressants and anticonvulsants. Rotation to a different class of opioid may yield improvement in an-algesia. Interventional pain management can reduce the need for pharmacotherapy altogether. Behavioral management can accomplish similar goals.However, if these options are not feasible, then the practitioner is faced with several choices to diag-nose and treat OIH:1. Increase the dose of opioid and evaluate for in-creased efficacy (tolerance).2. Reduce or eliminate the opioid and evaluate(OIH).3. Utilize opioids with unique properties that maymitigate OIH.4. Utilize specific agents that are NMDA receptorantagonists.The third option has become particularly attrac-tive with the use of methadone and buprenorphine. Methadone, although a pure mu receptor agonist, has properties that may prevent or reduce OIH (27). It is a racemic mixture in which the d-isomer is an NMDA receptor antagonist. Methadone also displays incom-plete cross-tolerance properties unique from other mu receptor agonists which may create a niche role for it in the treatment of OIH and other forms of intractable pain, especially neuropathic pain. Anecdotal reports exist of patients who have been thought to have OIH and been treated with combinations of option 2 and option 3 — i.e. reducing the dose of opioid (by 40 – 50%) and adding “low-dose” methadone (28).Buprenorphine has been used to treat chronic pain (29). It is a partial opioid agonist with antago-nist properties which has been used for decades in anesthesia and for the treatment of pain. The IV/IM formulation (Buprenex) is available in the US for the treatment of pain and in Europe is available as a trans-dermal preparation. Most recently, it has been used for the treatment of opioid dependence in its sublin-gual form (Suboxone, Subutex).Buprenorphine has been shown to be intermedi-ate in its ability to induce pain sensitivity in patients maintained on methadone and control patients not taking opioids (12). Buprenorphine showed an en-hanced ability to treat hyperalgesia experimentally induced in volunteers compared to fentanyl (30). In addition, spinal dynorphin, a known kappa receptor agonist, increases during opioid administration, thus contributing to OIH. Buprenorphine is a kappa recep-tor antagonist. For these reasons, buprenorphine may be unique in its ability to treat chronic pain and pos-sibly OIH.p racTical c onsideraTionsThe treatment of OIH can be time-consuming and at times, impractical. Weaning patients from high dose opioids usually requires time and patience (for both the physician and patient). While reducing the opioid dose, patients may experience transient increases in pain or mild withdrawal which can exacerbate pain. The hyperalgesic effect may not be mitigated until a certain critical dose of opioid is reached. Patients of-ten become frustrated and managing the appropriate dose reductions often requires multiple office visits. This can be extremely impractical in a managed care environment. In the author’s experience, many pa-tients simply give up and seek to resume opioid ther-apy elsewhere.Breaking the cycle of pain and hyperalgesia (in some cases opioid dependence and addiction) is an attractive course of action for the interventional pain specialist. Interventional pain management seeks to isolate or block pain input from specific nocicep-tive points in the nervous system. This usually pro-vides rapid diagnostic information and often results in therapeutic improvement. Medically supervised withdrawal with sublingual buprenorphine also pro-vides a rapid, safe, and effective treatment for opi-oid dependence, thus breaking the cycle of pain and hyperalgesia.Sublingual buprenorphine has recently been ap-proved for the treatment of opioid dependence and through its use, a physician can provide rapid and ef-ficient medically supervised withdrawal from opioids in an office setting (31). Office-based detoxification is itself an “interventional medical technique” for the treatment of this complex patient population suffering from pain, chemical dependency, and OIH. Resolution of OIH usually follows quickly during the maintenance phase with buprenorphine. Office-based detoxification provides a means to resolve OIH with-out the frustrations mentioned earlier.Methadone can be used to treat OIH. As stated earlier, it may be more effective for treatment of neuropathic pain. Converting a patient to metha-done offers a controlled method of weaning from opioids. The pharmacologic rationale for the use ofOpioid Induced Hyperalgesiamethadone is its ability to relieve withdrawal. Since methadone has a relatively long half-life (24 to 36 hours), there are fewer variations in plasma levels compared to short acting opioids such as oxycodone and hydrocodone. It is these properties which have made methadone the standard for the treatment of opioid dependence in the U.S. for the past 4 decades (32,33). As long as methadone is not used to treat opioid dependence, any physician can use it for the treatment of pain, and subsequently OIH (34). How-ever, methadone itself can also cause OIH (35), which may also limit its role.Ketamine has been used to treat OIH and as an ad-juvant to opioid therapy for the treatment of chronic pain (33-39). It is an NMDA receptor antagonist and has known intrinsic analgesic properties. Clonidine is an alpha 2 agonist and has been used for the treat-ment of neuropathic pain, post-operative pain, and severe cancer pain (39-41). It is also widely used to treat the symptoms of opioid withdrawal. Clonidine has also been shown to produce paradoxical pain hypersensitivity in rats (42). Since OIH and the pain from opioid withdrawal share many similarities (both in quality and neurochemistry), it is conceivable that clonidine may be useful in the treatment of OIH.c onclusionAs with any therapy, side effects and complica-tions can occur. An exit strategy should exist when utilizing opioids to treat chronic pain because of the potential complications in managing these patients such as opioid dependence, addiction, and abuse. OIH is a less recognized side effect of chronic opioid ther-apy. However, it is becoming more prevalent as the number of patients receiving opioids for chronic pain increases (43). OIH should be considered in the dif-ferential when opioid therapy fails. Prior to institut-ing treatment with opioids, OIH should be addressed with patients as part of a comprehensive informed consent/agreement.r eferences1. American Society of Addiction Medicine(ASAM). Definitions Related to the Useof Opioids for the Treatment of Pain: AConsensus Document from the Amer-ican Academy of Pain Medicine, theAmerican Pain Society, and the Amer-ican Society of Addiction Medicine.American Academy of Pain Medicine,Glenview, IL, 2001.2. Albutt C. On the abuse of hypoder-mic injections of morphia. Practitioner1870; 5:327-331.3. American Psychiatric Association. Diag-nostic and Statistical Manual of MentalDisorders, 4th Edition (DSM-IV). Ameri-can Psychiatric Press. Washington, DC,1994.4. American Society of Addiction, Princi-ples of Addiction Medicine, 3rd edition,Medicine, 2003, pp 3-16.5. Yaksh Tl, Malmberg AB. Central phar-macology of nociceptive transmission.In Wall P, Melzack (eds): Textbook ofPain, 3rd ed, 1994; pp. 165-200.6. Mao J. Opioid-induced abnormal painsensitivity: Implications in clinical opi-oid therapy. Pain 2002; 100:213-217. 7. Mao J, Price D, Mayer D. Mechanisms ofhyperalgesia and morphine tolerance:A current view of their possible interac-tions. Pain 1995; 62:259-274.8. Celerier E, Rivat C, Jun Y, Laulin JP, Larch-er A, Reynier P, Simonnet G. Long-last-ing hyperalgesia induced by fentanyl inrats: Preventive effect of ketamine. An-esthesiology 2000; 92:465-472.9. Celerier E, Laulin JP, Corcuff JB, Le MoalM, Simonnet G. Progressive enhance-ment of delayed hyperalgesia inducedby repeated heroin administration: Asensitization process. J Neurosci2001;21:4074-4080.10. Guignard B, Bossard AE, Coste C, Ses-sler DI, Lebrault C, Alfonsi P, FletcherD, Chauvin M. Acute opioid tolerance:Intraoperative remifentanil increasespostoperative pain and morphine re-quirement. Anesthesiology2000; 93:409-417.11. Baron, MJ, McDonald PW. Significantpain reduction in chronic pain patientsafter detoxification from high dose opi-oids. J Opioid Manag 2006; 2:277-282.12. Compton P, Charuvastra VC, Ling W.Pain intolerance in opioid-maintainedformer opiate addicts: Effect of long-acting maintenance agent. Drug and Al-cohol Dependence 2001; 63:139-146.13. Angst MS, Clark JD. Opioid-induced Hy-peralgesia: A qualitative systematic re-view. Anesthesiology2006; 104:570-587.14. Trujillo KA, Akil H. Inhibition of mor-phine tolerance and dependence by theNMDA receptor antagonist MK-801. Sci-ence 1991; 251:85-87.15. Marek P, Ben Eliyahu S, Gold M, Liebe-skind JC. Excitatory amino acid antago-nists (kynurenic acid and MK-801) atten-uate the development of morphine tol-erance in the rat. Brain Research 1991;547:77-81.16. Mao J, Price DD, Mayer DJ. Thermal hy-peralgesia in association with the de-velopment of morphine tolerance inrats: Roles of excitatory amino acid re-ceptors and protein kinase C. J Neuro-sci 1994; 14:2301-2312.17. Mao J, Sung B, Ji RR, Lim G. Chronic mor-phine induces downregulation of spinalglutamate transporters: Implications inmorphine tolerance and abnormal painsensitivity. J Neurosci2002; 22:8312-8323.18. Narita M, Mizoguchi H, Nagase H, Su-zuki T, Tseng LF. Involvement of spinalprotein kinase C gamma in the atten-uation of opioid-mu-receptor mediat-ed G-protein activity after chronic in-trathecal administration of [D-Ala2,N-Mephe4,Gly-Ol5] enkephalin. J Neurosci2001; 21:3715-3720.19. Zeitz KP, Malmberg AB, Gilbert H, Bas-Pain Physician: May/June 2009: 12:679-684ceptive modulatory systems in periaq-ueductal gray and spinal cord with ros-tral ventromedial medulla. Neurosci-ence 1992; 47:863-871.27. Davis A, Inturrisi C. d-Methadone blocksmorphine tolerance and N-Methyl-D-as-partate-induced. The Journal of Phar-macology and Experimental Therapeu-tics 1999; 289:1048-1053.28. Vorobeychik Y, Chen L, Bush MC, MaoJ. Improved opioid analgesic effect fol-lowing opioid dose reduction. Pain Med2008; 9:724-727.29.Johnson RE, Fudala PJ, Payne R. Bu-prenorphine: Considerations for painmanagement. J Pain Symptom Man-ag2005; 29:297-326.30. Koppert W, Ihmsen H, Korber N, Wehr-fritz A, Sittl R, Schmelz M. Different pro-files of buprenorphine induced anal-gesia and antihyperalgesia in a humanpain model. Pain 2005; 118:15-22. 31. Helm S, Trescot A, Colson J, Sehgal N,Silverman S. Opioid antagonists, partialagonists, and agonists/antagonists:The role of office-based detoxification.Pain Physician 2008; 11:225-235.32. Dole VP, Narcotic addiction, physicaldependence and relapse. N Engl J Med1972; 286:988-992.33. Kosten TR, Current pharmacotherapiesfor opioid dependence. Psychopharma-cology Bulletin 1990; 26:69-74.34. Heit HA, Covington E, Good PA. DearDEA. Pain Med 2004; 5:303-308.35. Davis MP, Shaiova LA, Angst M. Whenopioids cause pain. Journal of ClinicalOncology 2007; 25:4497-4498.36. Bell R, Eccleston C, Kalso E. Ketamine asan adjuvant to opioids for cancer pain.Cochrane Database Systematic Review2003; CD003351.37. McQueen AL, Baroletti SA. Adjuvantketamine analgesia for the manage-ment of cancer pain. Annals Pharmaco-therapy 2002; 36:1614-1619.38. Mercadante S, Villari P, Ferrera P. Burstketamine to reverse opioid tolerance incancer pain. Journal of Pain and Symp-tom Management 2003; 25:302-305.39. Kingery WS. A critical review of con-trolled clinical trials for peripheral neu-ropathic pain and complex regional painsyndromes. Pain 1997; 73:123-139. 40. Sites BD, Beach M, Biggs R, Rohan C,Wiley C, Rassias A. Gregory J, FanciulloG. Intrathecal clonidine added to a bu-pivacaine-morphine spinal anestheticimproves postoperative analgesia fortotal knee arthroplasty. Anesth Analg2003; 96:1083-1088.41. Tumber PS, Fitzgibbon DR. The controlof severe cancer pain by continuousintrathecal infusion and patient con-trolled intrathecal analgesia with mor-phine, bupivacaine and clonidine. Pain1998; 78:217-220.42. Quartilho A, Mata HP, Ibrahim MM, Van-derah TW, Ossipov MH, Lai J, Porreca F,Malan TP Jr. Production of paradoxicalsensory hypersensitivity by alpha2-ad-renoreceptor agonist. Anesthesiology2004; 100:1538-1544.43. Trescot AM, Helm S, Hansen H, Be-nyamin R, Glaser SE, Adlaka R, PatelS, Manchikanti L. Opioids in the man-agement of chronic non-cancer pain:An update of American Society of theInterventional Pain Physicians (ASIPP)Guidelines. Pain Physician2008; 11:S12-S16.baum AI. Reduced development of tol-erance to the analgesic effects of mor-phine and clonidine in PKC gamma mu-tant mice. Pain 2002; 94:245-253. 20. Mao J. NMDA and opioid receptors:Their interactions in anti-nociception,tolerance and neuroplasticity. BrainRes Rev 1999; 30:289-304.21. Mao J, Price DD, Mayer DJ. Experimen-tal mononeuropathy reduces the anti-nociceptive effects of morphine: Impli-cations for common intracellular mech-anisms involved in morphine toler-ance and neuropathic pain. Pain 1995;61:353-364.22. Mao J, Sung B, Ru-Rong J, Grewo L.Neuronal apoptosis associated withmorphine tolerance: Evidence for anopioid-induced neurotoxic mechanism.J Neurosci 2002; 22:7650-7661.23. Gardell LR, Wang R, Burgess SE, Ossi-pov MH, Vanderah TW, Malan Jr. TP, LaiJ, Porreca F. Sustained morphine expo-sure induces a spinal dynorphin-de-pendent enhancement of excitatorytransmitter release form primary affer-ent fibers. J Neurosci 2002; 22:6747-6755.24. Barbaro NM, Heinricher MM, Fields HL.Putative pain modulating neurons inthe rostral ventral medulla: Reflex-re-lated activity predicts effects of mor-phine. Brain Research1986; 366:203-210.25. Heinricher MM, Morgan MM, FieldsHL. Direct and indirect actions of mor-phine on medullary neurons that mod-ulate nociception. Neuroscience 1992;48:533-543.26. Morgan MM, Heinricher MM, Fields HL.Circuitry linking opioid-sensitive noci-。