2014年IYPT CUPT题目(原文+中文翻译+配图)
- 格式:doc
- 大小:31.56 MB
- 文档页数:13



CUPT题⽬翻译CUPT题⽬1. Gaussian cannon ⾼斯炮A sequence of identical steel balls includes a strong magnet and lies in nonmagnetic channel. Another steel ball is rolled towards them and collides with the end ball .the ball at the opposite end of the sequence is ejected at a surprisingly high velocity .optimize the magnet’s position for the greatest effect.⼀列相同的钢球含有⼀个强磁铁并置于⽆磁通道中。
另有⼀个钢球向他们滚动并碰撞序列末尾的球。
在另⼀端末尾的球被迫以极⾼的速度离开。
请优化磁铁的位置使碰撞产⽣最佳效果。
2. Cutting the air切割空⽓When a piece of thread (e.g. nylon) is whirled around with a small mass attached to its free end, a distinct noise is emitted.study the origin of this noise and the relevant parameters.⼀段线(例如尼龙)的⾃由端系上⼀个重物,当线被旋转时会形成明显的噪声。
研究这种噪声的产⽣原因及相关参数。
3. String of beads串珠A long string of beads is released from a breaker by pulling a sufficiently long part of the chain over the edge of the breaker. Due to gravity the speed of the string increases. At a certain moment the sting no longer touches the edge of the breaker (see picture). Investigate andexplain the phenomenon.⼀长串珠⼦从烧杯⾥释放,具体释放⽅式是将⾜够长的珠链拉出烧杯的边缘,这样由于重⼒作⽤串珠的速度增⼤。


201届国际青年物理学家竞赛赛IYPIYPT T 20133年第26届国际青年物理学家竞参考翻译))题目((参考翻译题目1.自己创造如果一张纸被“手风琴式”折叠或者被卷成筒,那么它将更加难以弯曲。
使用一张单一的A4纸和少量胶水,如果需要的话,构建一座跨越280毫米间隙的桥梁。
介绍相关参数,用以描述该桥梁的强度,并优化其中的部分参数或全部参数。
2.弹性空间大球在水平拉伸膜上滚动的动态效果和它们之间明显的相互作用通常用于说明引力场。
进一步探究该系统。
在这样一个“引力场世界”确定和测量明显的“引力常数”是否可能?如果你站在地面上拿着乒乓球,并释放它,它会反弹。
如果乒乓球内含有液体,碰撞的性质会发生变化。
探究碰撞的性质如何取决于球内液体的含量和其他相关参数。
4.孤子沿水平轴等距离安装一链相似的摆,相邻的摆用轻绳相连接。
每一个摆可以绕轴旋转,但不能侧向移动(见图)。
探究沿着这样一条链的一种旋转的传播。
当各摆都经历360º旋转时,孤立波的速度是多少?一个轻球(如乒乓球),可以被向上的气流所支撑。
气流的方向可以倾斜,然而它仍然可以支撑球。
探究气流倾斜的影响,并优化该系统,得出在保持球处于稳定状态的情况下,气流倾斜的最大角度。
6.彩色塑料在明亮光线的照射下,一个透明的塑料物体(如一张空白的CD外壳)有时可以呈现各种不同的颜色(见图)。
研究和解释这种现象。
确定一下,当使用各种不同颜色的光源时,是否也可以看到这些颜色。
7.聆听光的声音将一个罐子内表面的一半涂一层锅灰,并在它的盖子上钻一个孔(见图)。
当连接交流电的灯泡发出的光线射到罐子的黑墙(锅灰层)时,可以听到明显的声音。
解释和探究这种现象。
8.喷射和薄膜喷射的细液体流对肥皂薄膜的作用(见图)。
喷射的液体流可以渗透通过薄膜或者与薄膜合并,产生有趣的形状,这取决于相关参数。
解释和探究这种相互作用,以及由此产生的形状。
9.碳麦克风一个麦克风的设计已经涉及碳颗粒的使用很多年了。

2014年普通高等学校招生全国统一考试(课标全国卷Ⅰ)理综物理第Ⅰ卷二、选择题:本题共8小题,每小题6分。
在每小题给出的四个选项中,第14~18题只有一项符合题目要求,第19~21题有多项符合题目要求。
全部选对的得6分,选对但不全的得3分,有选错的得0分。
14.在法拉第时代,下列验证“由磁产生电”设想的实验中,能观察到感应电流的是()A.将绕在磁铁上的线圈与电流表组成一闭合回路,然后观察电流表的变化B.在一通电线圈旁放置一连有电流表的闭合线圈,然后观察电流表的变化C.将一房间内的线圈两端与相邻房间的电流表连接,往线圈中插入条形磁铁后,再到相邻房间去观察电流表的变化D.绕在同一铁环上的两个线圈,分别接电源和电流表,在给线圈通电或断电的瞬间,观察电流表的变化15.关于通电直导线在匀强磁场中所受的安培力,下列说法正确的是()A.安培力的方向可以不垂直于直导线B.安培力的方向总是垂直于磁场的方向C.安培力的大小与通电直导线和磁场方向的夹角无关D.将直导线从中点折成直角,安培力的大小一定变为原来的一半16.如图,MN为铝质薄平板,铝板上方和下方分别有垂直于图平面的匀强磁场(未画出)。
一带电粒子从紧贴铝板上表面的P点垂直于铝板向上射出,从Q点穿越铝板后到达PQ的中点O,已知粒子穿越铝板时,其动能损失一半,速度方向和电荷量不变,不计重力。
铝板上方和下方的磁感应强度大小之比为()A.2B.√2C.1D.√2217.如图,用橡皮筋将一小球悬挂在小车的架子上,系统处于平衡状态。
现使小车从静止开始向左加速,加速度从零开始逐渐增大到某一值,然后保持此值,小球稳定地偏离竖直方向某一角度(橡皮筋在弹性限度内),与稳定在竖直位置时相比,小球的高度()A.一定升高B.一定降低C.保持不变D.升高或降低由橡皮筋的劲度系数决定18.如图(a),线圈ab、cd绕在同一软铁芯上。
在ab线圈中通以变化的电流。
用示波器测得线圈cd间电压如图(b)所示。

注意:题目不能只看翻译,必须充分参考原题。
1.自己创造据了解,一些电路表现出混沌行为。
构建一个具有这种属性的简单电路,并研究其行为。
2.全息照片有人认为,在一块透明塑料上划出图案可以手工制作出一张全息照片。
制作一张字母“IYPT ”的全息图并研究它是如何工作的。
3.扭曲的绳握住绳子扭它的一端。
在绳索上的某一点将形成螺旋线或圆环。
调查解释这样的现象。
4.球的声音当两个硬钢球或类似的东西被轻轻带到接触到对方,一个不寻常的“鸣叫声”。
调查解释的声音的性质。
5.载物的环在一个环的里面固定一个小重物,给环一个初始推力使其运动。
研究环的运动。
6.泡泡晶体大量非常小的相似的气泡浮在肥皂水的表面上。
气泡会自动按照一个规律的类似晶格的模式排列。
提出一种获得大小一致的的气泡的方法,并探究这种泡泡晶体的形成。
7.“罐中罐”冰箱这一个依据蒸发冷却的原理让食物保鲜的装置。
它包括一个大容器、里面的小容器。
它们之间的空间内用湿的多孔材料填充,例如沙子。
问怎么能达到最佳的散热效果?8.冻结水滴将水滴放置在冷却到-20°C左右的板上。
结冰后液滴可能会成为有锋利的顶部的圆锥状。
调查这种现象。
9.水弹有些学生不会用灌水的气球打仗,他们的水弹反弹后仍不爆裂。
调查这里的运动,变形和充满液体的气球的反弹。
在什么情况下水弹会爆裂?10.扩散系数利用显微镜按微米大小的顺序观察微粒的布朗运动。
研究扩散系数是如何取决于微粒的大小和形状的。
11.蜡烛发电厂设计一将蜡烛的火焰的热量转化成电能的装置。
调查装置的不同方面如何影响其效率。
12.冷气球由于空气逃离橡胶气球,其表面触感变得冷。
研究影响降温的参量。
作为一个函数的相关参数,气球的不同部分温度是什么?13.旋转的鞍一个球被放在旋转的鞍上。
从动力学的角度研究它,解释球不会从鞍上落下来的情形。
14.橡胶电机扭曲的橡皮筋存储着能量,例如可用于驱动飞机模型。
调查这样的能量来源的属性及其功率输出随时间的变化。
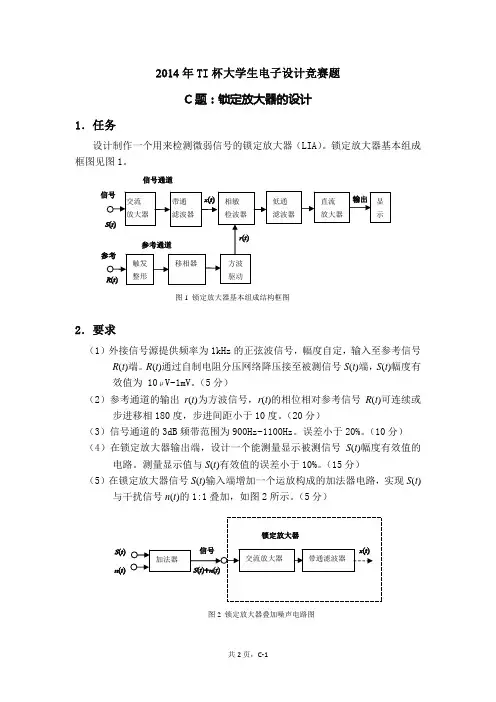
2014年TI 杯大学生电子设计竞赛题C 题:锁定放大器的设计1.任务设计制作一个用来检测微弱信号的锁定放大器(LIA)。
锁定放大器基本组成框图见图1。
2.要求(1)外接信号源提供频率为1kHz 的正弦波信号,幅度自定,输入至参考信号R (t )端。
R (t )通过自制电阻分压网络降压接至被测信号S (t )端,S (t )幅度有效值为 10μV ~1mV。
(5分)(2)参考通道的输出r (t )为方波信号,r (t )的相位相对参考信号R (t )可连续或步进移相180度,步进间距小于10度。
(20分)(3)信号通道的3dB 频带范围为900Hz ~1100Hz。
误差小于20%。
(10分) (4)在锁定放大器输出端,设计一个能测量显示被测信号S (t )幅度有效值的电路。
测量显示值与S (t )有效值的误差小于10%。
(15分)(5)在锁定放大器信号S (t )输入端增加一个运放构成的加法器电路,实现S (t )与干扰信号n (t )的1:1叠加,如图2所示。
(5分)图2 锁定放大器叠加噪声电路图S (t )n (t )图1 锁定放大器基本组成结构框图信号通道(6)用另一信号源产生一个频率为1050~2100Hz的正弦波信号,作为n(t)叠加在锁定放大器的输入端,信号幅度等于S(t)。
n(t)亦可由与获得S(t)同样结构的电阻分压网络得到。
锁定放大器应尽量降低n(t)对S(t)信号有效值测量的影响,测量误差小于10%。
(20分)(7)增加n(t)幅度,使之等于10S(t),锁定放大器对S(t)信号有效值的测量误差小于10%。
(20分)(8)其他自主发挥。
(5分)(9)设计报告。
(20分)项 目 主要内容 满分 系统方案 总体方案设计 4理论分析与计算 锁定放大器各部分指标分析与计算 6电路与程序设计 总体电路图,程序设计 4测试方案与测试结果 测试数据完整性,测试结果分析 4设计报告结构及规范性 摘要,设计报告正文的结构、图表的规范性 2总分203.说明(1)各信号输入、输出端子必须预留测量端子,以便于测量。

2014physics bowl真题中文版1.一个调频广播站用频率为99.99 X 106Hz的电磁波发射信号,以下四个选项中关于该频率书写正确的是()A.99.99KHzB.99.99MHzC.99.99GHzD.99.99NHzE.99.99THz1.在实验室中,一同学对一个物体的长度进行了6次测量结果如下:5.05cm ,5.06cm,5.07cm,5.06cm,5.07cm,5.09cm.用有效数字的规则下列哪一个选项正确表示了物体的平均长度()A.5cmB.5.06cmC.5.066 cmD.5.07cmE. 5.1cm2. 一个物体做自由落体运动,在开始下落的头3秒里该物体下落的高度为()A.10.0mB.15.0mC.30.0mD.45.0mE.90.0m3.如图所示三个质量相等的物体用三条绳子悬挂在教室天花板上保持静止,关于绳子的张力说法正确的是()A. A的张力最大B.B的张力最大C.C的张力最大ID.绳ABC中张力一样E.绳ABC的张力都为零匚平4. 一个简单的摆由摆球和轻绳组成,忽略空气阻力,可以增加单摆的周期的是()匚壬A增加摆千^质量B减少摆球质量C增加轻绳的长度D减少轻绳的长度E增加摆动的角度ZES6.MRI是一种医学诊断技术的缩写,下列哪一个是MRI的正确意思()"(A)N ledical Radio Imaging(B)M inima lly R^dioacti^'e Intel entionN Racier Iiijeotion(I>)Alapriietic Kadaoisotope Injection(E)la^netic Resoi^ance7.一辆小车从静止开始运动,在3s内速度均匀的加速到7.6m/s,在这段时间里小车运动的位移为()A.5.7MB.8.1MC.11.4MD.16.1ME.22.8M8. 一个2.5kg的物块被连接到一根理想弹簧末端上做简谐运动,物块的位置可用描述x(t)=0.20cos(8.00t+0.50) 该式里的所有量都为国际单位,下列哪个数值表示物块振动的振()A.8.00B.4.00C.1.60D.0.50E.0.209.下列哪个物理量不是标量()A力B能量C质量D速率E压强10.有两个电荷-Q和+Q被固定在x轴上,它们离原点的距离都为a,如图所示。

以下中文翻译来源于网络,仅供参考,请同学们自己准确理解原题:1.自己发明请建造一个装置——能为从米固定高度坠向坚硬地面的生鸡蛋提供安全着陆,装置必须与鸡蛋一起坠落,你能实现的装置的最小尺寸是多少?2.气膜汽笛一个简单的汽笛可以这样制造:用一根穿过一只小型容器(或杯子)底部的管子(如图)拉伸位于容器(或杯子)开口处的气膜。
经由在容器侧壁处的小孔,鼓风可以产生一个声音。
探讨相关参数如何影响声音。
3.单透镜望远镜一架望远镜可只用一个透镜制作,如果小光圈被用于替代目镜,那么透镜参数和孔会如何影响图像(如放大率、清晰度和亮度)?4.磁丘少量置于非均匀磁场中的磁性流体会形成山丘状结构,探讨这些结构的属性如何依赖于相关参数。
5.莱氏星星在莱氏效应中,置于热表面的水滴可存在几分钟。
在某些情况下,水滴会发展为振荡的星形状态。
引入不同的振荡模式并探讨它们。
6.快链条一根链条由若干相比于水平有一定角度的木棍组成,且由两根线连接(见图),垂直悬挂,然后释放。
相比于自由落体,当掉至水平地面时,链条掉落更快。
解释此现象并探讨相关参数如何影响运动。
7.螺旋水波螺旋波及其他类型的波可能出现于薄层液膜流过旋转平台,探讨这些波的类型。
8.密度可视化纹影摄影术通常用于使气体中的密度变化可视化。
安装一套纹影装备并探讨它在解决密度差异中能做多好。
9.管中球现有一根充满液体并含有一枚小球的密封透明管,此管倾斜放置且下端与马达相连,如此管沿锥形面运动。
探讨球在相关参数作用下的运动。
10.分离玻璃在两片薄玻璃之间放置一层薄薄的水,然后试着把它们分开。
探讨影响所需力的参数。
11.毛发湿度计一只简单的湿度计可仅用人的毛发制作。
探讨相关参数对其准确度和响应时间的作用。
12.扭转陀螺将轮轴固定于具有一定扭转阻力的竖直线上(见图),扭转绳子,旋转轮子并释放它,探讨此系统的动力。
13.共鸣的玻璃酒杯一部分盛满液体,暴露于扬声器的声音下会产生共振。
探讨此现象如何取决于各种参数。

46th International Chemistry OlympiadJuly 23, 2014Hanoi, Vietnam PRACTICAL EXAMINATIONCountry:Name as in passport:Student Code:Language:This booklet contains 25523 charactersGENERAL INTRODUCTIONSafety•Safety is the most important issue in the laboratory. You are expected to follow the safety rules given in the IChO regulations. Safety glasses and lab coats must be worn in laboratory ALL TIMES.•If you behave in an unsafe manner, you will receive one warning before you are asked to leave the laboratory. If required to leave due to a second warning, you will receive a score of zero for the rest practical examination.•Eating, drinking, or smoking in the laboratory or tasting a chemical is strictly forbidden.•Pipetting by mouth is strictly forbidden.•Use the labeled waste containers near you for disposal of liquids and solids. A waste container (plastic can) is also available on each bench for organic and inorganic waste.Discard used glass capillaries into a solid trash.•In case of emergency, follow the instructions given by the lab assistants. Examination Procedures•This practical examination has 28 pages for 3 practical problems. Periodic Table of Elements is at the end of this booklet. Do not attempt to separate the sheets.•You have 5 hours to complete practical problems 1, 2, and 3. You have 30 min to read through the problems before the START command is given.•DO NOT begin working on the tasks until the START command is given.•When the STOP command is given, you must stop your work on the tasks immediately.A delay in doing so may lead to your disqualification from the examination.•After the STOP command has been given, wait in your lab space. A supervisor will check your lab space. The following items should be left behind:o The practical examination booklet (this booklet),o Your chosen TLC plates in Petri dish with your student code (Problem 2).•Do not leave the laboratory until you are instructed to do so by the lab assistants. •You may need to reuse some glassware during the examination. If this is the case, clean it carefully in the sink closest to you.•Replacement of chemicals and laboratory ware will be provided if necessary. Other than the first, for which you will be pardoned, each such incident will result in the loss of1 point from your 40 practical points. Refilling of wash-bottle water is permitted with noloss of points.Notes•Use only the pen provided for filling in the answer boxes. You may also use the calculator and the ruler provided. Do not use the mechanical pencil for filling in the answer boxes.•All results must be written in the appropriate areas with the working shown. Results written elsewhere will not be graded. If you need to do rough calculations, etc., use thedraft papers or the back of the sheets. All answers on the draft papers or the back of the sheets will NOT be graded .• You should take care to report answers to an appropriate number of significant figures and give the appropriate unit.• Contact a supervisor near you if you need a refreshment/toilet break. • Read the whole description of the problems before you begin•An official English version of this examination is available upon request if you require clarification.Attention:Pipetting by mouth is strictlyforbidden. Student was provided a pipettebulb. Make sure that you properly use thepipette bulb shown in Figure below.Description of three-way pipette bulb.An adapter is provided for larger pipettes. Instructions for using the thermometer 1. Press the [ON/OFF] button to display thetemperature reading in Celsius. 2. Insert the stainless steel probe (at least 5cm) in the solution to be measured. 3. Wait for display to stabilize (display valueis unchanged and stable for 3 seconds) and read the temperature on the display.4. Press the[ON/OFF]button again to turnthe thermometer off, then rinse the stainlesssteel probe with distilled water.List of chemicalsThe concentration indicated on the label is approximate. The exact values are indicated in the table.Chemical/Reagent QuantityPlacedin Labeled Safety Practical Problem 10.100 M KI solution 120 mL Glass bottle 0.1 M KI H320Solution #A1 contains KI,Na2S2O3, and starch indicator in distilled water 40 mL Glass bottle Solution #A1H314, H302,H315, H319Solution #B1 contains Fe(NO3)3, HNO3 in distilled water 40 mL Glass bottle Solution #B1H314, H315,H319, H335Solution #A2-1 contains 5.883×10–4 M Na2S2O3, KNO3, andstarch indicator in distilled water360 mL Glass bottle Solution #A2-1H314 H272 Solution #B2 contains 0.1020 MFe(NO3)3 and HNO3 in distilled water. 100 mL Glass bottle Solution #B2H314, H272,H315, H319Distilled water 1 L Glass bottle H2O (Practical Problem 1)Practical Problem 2Artemisinin 1.000 g Small bottle ArtemisininSodium borohydride, NaBH4 0.53gSmallbottleNaBH4H301-H311CH3OH 20 mL Glass bottle Methanol H225, H301n-Hexane 30mLBottlen-Hexane H225cerium staining reagent for TLC 3-5 mL Bottle Ceri reagentCH3COOH 1 mL 1.5 mL vial Acetic Acid H226, H314Ethyl acetate 5 mL Glass bottle Ethyl acetateBag of NaCl for salt bath 0.5 kg Ice bath NaCl bagCaCl2 in drying tube 5-10 g Tube CaCl2H319 Practical Problem 3~ 30 wt% H2SO4, solution inwater40 mL Bottle ~30 wt% H2SO4 H314 1.00×10–2M KMnO4, aqueoussolution50 mL Bottle ~0.01 M KMnO4,H272, H302,2.00×10-3M EDTA, aqueoussolution40 mL Bottle 2.00×10-3 M EDTA H319pH = 9-10 Buffer aqueous Solution, NH4Cl + NH340 mL Bottle pH = 9-10 BufferSolutionH302 , H319~20 wt% NaOH, aqueoussolution20 mL Plastic bottle ~20 wt% NaOH, H314 ~3 M H3PO4, solution in water 15 mL Bottle ~3 M H3PO4H314 Indicator: ETOO, solid in KCl ca. 0.5 g Plastic bottle ETOO H301List of Glassware and EquipmentsProblem Item on every working placeQuantityHotplate stirrer1 Magnetic stirring bar (seek in Kit #1)1Plastic wash bottle filled with distilled water (refill if necessary from the 1 L glass bottle of distilled water provided) 1 1-L glass beaker for inorganic waste liquid 1 250-mL conical flask for organic waste liquid1 Pipette rack with:1-mL graduated pipette5-mL graduated pipette (One for Problem 1; another labeled ‘MeOH’ for Problem 2) 10-mL graduated pipette 10-mL volumetric pipette 25-mL graduated pipette Pasteur pipette and bulb Glass spatula spoon Cleaning brushLarge glass stirring rod Glass funnel1 12 1 1 1 2 2 1 1 1 Bag of paper towels 1 Goggles1 Digital thermometer1 Three-way pipette bulb with a little rubber adapter for bigger pipettes 1 Ceramic Büchner funnel with fitted rubber bung 1 Büchner flask1 Pair of rubber gloves 1 P r a c t i c a l P r o b l e m s 1-3 One cotton glove1Practical Problem 1 (KIT # 1) Digital stop watch 1Insulating plate for the hotplate stirrer labeled I.P. 1 K I T # 1100-mL glass beaker 6 Practical Problem 2 (KIT # 2) 5-mL graduated measuring cylinder 1 50-mL graduated measuring cylinder 2 100-mL two-neck round bottom flask with plastic stopper (in ice bath) 1 100-mL conical (Erlenmeyer) flask 1 Hair dryer 1 Petri dish with cover containing 1 TLC plate, 2 capillaries in paperholder1Plastic pot for ice bath 1 Stand & clamp 1 K I T # 2TLC developing chamber with glass lid 1Replacement or extrachemicals Lab assistant’s signatureStudent’s signaturePenalty_________________ _________________ _______________________________ ______________ ____________________________ ______________ _____________________ _______ _______Tweezers 2 Metal spatula 1 Very small test tubes for TLC in container 2 Zipper store bag (containing cotton wool, round filter paper, watch glassfor Problem 2 labeled with WHITE student code) 1Empty Petri dish with cover 1 Practical Problem 3 (KIT # 3)50-mL glass beaker (for transferring EDTA and KMnO 4 solutions toburettes)225-mL burette with BLUE graduation marks 1 25-mL burette with BROWN graduation marks 1 250 mL glass beaker 2 250 mL conical flask (Erlenmeyer flask) 2 100 mL volumetric flask with stopper 2 10 mL glass graduated measuring cylinder 1 100 mL glass graduated measuring cylinder 1 Burette stand & clamp 1 Reel of pH paper 1 K I T # 3Zipper store bag (containing a large round filter paper for the glassfunnel)1Items on the tables for the common use:Electronic balance with 0.1-mg resolution (6-8 students/each)PRACTICAL EXAMINATIONCode: Question1 2 3 4 5 6 Total Examiner Mark2 4 50 2 2 10 70 Practical Problem 114 % of thetotalGradePractical Problem 1. The oxidation of iodide by iron(III) ions – a kinetic study based on the thiosulfate clock reactionClock reactions are commonly used as demonstrations by chemical educators owing to their visual appeal. Oxidation of iodide by iron(III) ions in a weakly acidic medium is a reaction that can be transformed into a clock reaction. In the presence of thiosulfate and starch, chemical changes in this clock reaction can be presented by the following equations:Fe3+(aq)+S 2O 32-(aq)[Fe(S 2O 3)]+(aq)(1)fast 2Fe 3+(aq)+3I -(aq)2Fe 2+(aq)+I 3-(aq)(2)slow I 3-(aq)+2S 2O 32-(aq)3I -(aq)+S 4O 62-(aq)(3)fast 2I 3-(aq)+starchstarch -I -5+I -(aq)(4)fastReaction (1) is a fast reversible equilibrium which occurs in the reaction mixture giving a reservoir of iron(III) and thiosulfate ions. After being produced in reaction (2), iodine in the form of triiodide ion (I 3–), is immediately consumed by thiosulfate in reaction (3). Therefore, no iodine accumulates in the presence of thiosulfate. When thiosulfate is totally depleted, the triiodide ion accumulates and it may be detected by use of starch indicator according to reaction (4).The kinetics of reaction (2) is easily investigated using the initial rates method. One has to measure the time elapsed between mixing the two solutions and the sudden color change.For the oxidation of iodide by iron(III) ions (reaction 2), the reaction rate can be defined as:3+Fe ⎡⎤⎣⎦=−d v dt(5)The initial reaction rate can then be approximated by:3+0Fe v t⎡⎤Δ⎣⎦≈−Δ (6) with Δ[Fe 3+] being the change in the concentration of iron(III) ions in the initial period of the reaction. If Δt is the time measured, then Δ[Fe 3+] is the change in iron(III) ion concentration from the moment of mixing to the moment of complete thiosulfate consumption (assume that the reaction rate does not depend on thiosulfate concentration). Therefore, from the reactions' stoichiometry it follows:3+2230Fe S O −⎡⎤⎡⎤−Δ=⎣⎦⎣⎦ (7) and consequently:22300S O v t −⎡⎤⎣⎦≈Δ(8)The initial thiosulfate concentration is constant and significantly lower than that of iron(III) and iodide ions. The above expression enables us to determine the initial reaction rate by measuring the time required for the sudden color change to take place, Δt .The rate of reaction is first order with respect to [Fe 3+], and you will determine the order with respect to [I –]. This means the initial reaction rate of reaction can be expressed as:yk v 0030]I []Fe [−+=(9)where k is the rate constant and y is the order with respect to [I –].We assume that the reaction rate does not depend on the thiosulfate concentration, and that the reaction between Fe 3+ and S 2O 32- is negligible. You have to observe carefully the color changes during the clock reaction and to determine the reaction order with respect to [I –], and the rate constant of clock reaction.Experimental Set-upInstructions for using the digital timer (stopwatch)1.Press the [MODE] button until the 00:00:00 icon is displayed.2.To begin timing, press the [START/STOP] button.3.To stop timing, press the [START/STOP] button again.4.To clear the display, press the [SPLIT/RESET] button.PRECAUTIONS¾To minimize fluctuations in temperature only use the distilled water on your bench (in the wash bottle and in the glass 1 L bottle).¾The heating function of the heating magnetic stirrer must be TURNED OFF (as shown in Figure 1 below) and be sure that the stirrer plate is not hot before starting your experiment. Put the insulating plate (labeled I.P.) on top of the stirrer plate for added insulation.¾Start the stopwatch as soon as the solutions #A and #B are mixed. Stop the stopwatch as soon as the solution suddenly turns dark blue.¾Magnetic stirrer bar (take it with the provided tweezers) and beakers should be washed and rinsed with distilled water and wiped dry with paper towel to reuse. General ProcedureSolution # A (containing Na2S2O3, KI, KNO3 and starch) is first placed in the beaker and is stirred using the magnetic bar. The rate of stirring is set at level 8 as indicated in Figure 1. Solution #B (containing Fe(NO3)3 and HNO3) is quickly added into solution #A and the stopwatch is simultaneously started.The time is recorded at the moment the solution suddenly turns dark blue. The temperature of the solution is recorded using the digital thermometer.Figure 1. The apparatus employed for kinetic study of the clock reaction.1.Practice run to observe the color changes-There is no need to accurately measure the volumes used in this part – just use the marks on the beaker as a guide.-Pour ca. 20 mL of solution # A1 (containing KI, Na2S2O3, and starch in water) to a 100-mL graduated beaker containing a magnetic stirrer bar. Place the beaker on top of the insulating plate on the magnetic stirrer.-Pour ca. 20 mL of solution # B1 (containing Fe(NO3)3 and HNO3 in water) in another 100 mL graduated beaker.-Quickly pour the solution # B1 into solution # A1 and start stopwatch simultaneously. Stop stopwatch when the color of the mixture changes. There is no need to record this time. Answer the following questions.Task 1.1: Write down the molecular formula of the limiting reactant for the given clock reaction.Task 1.2:What are the ions or compounds responsible for the colors observed in this experiment? Tick the appropriate box.Color CompoundPurple Fe3+[Fe(S2O3)]+ Fe2+starch-I5- I3-Dark blue Fe3+[Fe(S2O3)]+ Fe2+starch-I5- I3-2. Determination of the order with respect to [I –] (y), and the rate constant (k) In this section, Δt is determined for different initial concentrations of KI according to the table below. The experiment is repeated as necessary for each concentration of KI.Hint: Use 25 mL graduated pipette for solution #A2-1, 10 mL graduated pipette for KI, 5 mL graduated pipette for solution #B2, and one of the burettes for water (you will need to refill the burette from the wash bottle for each measurement).- Prepare 55 mL of solution # A2 in a 100 mL beaker containing a magnetic stirrer bar and place it on top of the insulating plate on the stirrer. Solution #A2 contains solution #A2-1, KI, and distilled water (see the table below for the volume of each component).- Add 5 mL of solution # B2 in another 100 mL beaker.Quickly pour prepared solution #B2 into solution #A2. Determine the time (Δt ) necessary for the color change by a stopwatch. The temperature of the solution is recorded.Task 1.3: Record the time (Δt) for each run in the table below. (You DO NOT need to fill all three columns for the runs.) For each concentration of KI, record your accepted reaction time (Δt accepted ) and temperature. You will be only graded on your values of Δt accepted and T accepted .55 mL of solution #A2 Run 1 Run 2 Run 3N o#A2-1 (mL) H 2O(mL)0.100MKI(mL)Δt (s) T (ºC) Δt (s) T (ºC) Δt (s) T (ºC) Δt accepted (s)T accepted (ºC)1 20.4 31.6 3.02 20.4 30.1 4.53 20.4 28.6 6.0 4 20.4 27.4 7.25 20.4 25.6 9.0When you are satisfied you have all the necessary data for Problem 1, before continuing further with the analysis, it is strongly recommended that you start the practical procedure for Problem 2 since there is a reaction time of one hour in that Problem.Task 1.4: Fill in the table below and plot the results in the graph.Hint: Make sure your data is graphed as large as possible in the provided space.No. 1 2 3 4 5ln([I-]0 / M) - 5.30 - 4.89 - 4.61 - 4.42 - 4.20Δt accepted (s)ln(Δt accepted / s)Task 1.5: Draw the best fit line on your graph and use this to determine the order with respect to [I–] (y).y = ………………………………Task 1.6: Complete the table below and calculate k for each of the concentrations of iodide. Report your accepted value for the rate constant, giving the appropriate unit. Remember that the order with respect to [Fe3+] is equal to one.No Δt accepted(s)[Fe3+]0(×10-3 M)[I-]0(×10-3 M)[S2O32-]0(×10-3 M)k1 5.02 7.53 10.04 12.05 15.0k accepted = ………………….Code:Task 1 2 3 45TotalExaminer Mark 35 15204 276Practical Problem 2 13 % of thetotalGradePractical Problem 2. Synthesis of a derivative of ArtemisininArtemisinin (also known as Quinghaosu) is an antimalarial drug isolated fromthe yellow flower herbArtemisia annua L., in Vietnam. This drug is highly efficaciousagainst the chloroquine-resistant Plasmodium falciparum. However, artemisinin has apoor solubility in both oil and water so that one needs to prepare its new derivatives toimprove the applicability of this drug. The reduction of artemisinin is an attractive method to synthesize new derivatives of artemisinin as shown in Scheme 1.Scheme 1In this practical exam you are going to reduce artemisinin to product P and check its purity using Thin-Layer Chromatography (TLC).Experimental Set-up- The experimental set-up is shown in Figure 2.1.- By moving the finger clamp, you can adjust the position of the two-neck round-bottom flask.41: Digital thermometer; 2: Plastic Stopper; 3: CaCl2 drying tube; 4: Ice BathFigure 2.1. Reaction system for Problem 2ProcedureStep 1. Synthesis of a Derivative of Artemisinin1.Prepare an ice bath with a temperature between –20 and –15 o C by mixing ice andsodium chloride in the plastic pot (approximate ratio of NaCl : crushed ice = 1 scoop : 3 scoops). Use the digital thermometer to monitor the temperature. Place the bath on the magnetic stirrer. Put a layer of three tissues between the bath and the stirrer.2.Connect the CaCl2 drying tube to the small neck of the round-bottom flask andclose the other neck with the plastic stopper.3.Place a magnetic stirring bar into the dry round-bottom flask and set up the reactionsystem onto the clamp-stand so that the system is immersed in the ice bath. Monitor the temperature using the digital thermometer.4.Setting aside a tiny amount (ca. 2 mg) of artemisinin for TLC analysis, open thestopper and add the 1 gram of artemisinin through the bigger neck.e the glass funnel to add 15 mL of methanol (measured using the 50-mLgraduated cylinder). Close the stopper and turn on the magnetic stirrer. (Set the magnetic stirrer to level 4). Start the stopwatch to keep track of the time.6.After ca. 5 min stirring, open the stopper and add carefully 0.53 g of NaBH4 insmall portions over 15 min using a spatula. Close the stopper in between addition.(Caution: Adding NaBH4 rapidly causes side-reactions and overflowing). Keep stirring for 50 min. Maintain the temperature of the ice bath below –5 o C; removesome of the liquid and add more NaCl-crushed ice mixture if necessary.Cool the vial containing the 1 mL of acetic acid in the ice bath.During this waiting time, you are advised to finish calculations from Problem 1, answer the questions below, and prepare further experimental steps.7.Prepare 50 mL of ice-cold distilled water (cooled in the ice bath) in the 100 mL-conical flask. Measure ca. 20-22 mL n-hexane in the 50 mL measuring cylinder and cool it in the ice bath. After the reaction is complete, keep the reaction flask in the ice bath below 0 o C. Remove the CaCl2 tube, open the stopper, and add gradually ca. 0.5 mL of the cold acetic acid from the vial into the reaction flask until the pH is between 6 and 7. (Use the glass rod to spot the reaction mixture on to the pH paper.) With stirring, slowly add the 50 mL of ice cold water over 2 min.A white solid precipitates in the reaction flask.8.Assemble the vacuum filtration apparatus. Put a filter paper onto the Büchnerfunnel, wet the filter paper with distilled water and open the vacuum valve.Transfer the reaction mixture on to the filter, and remove the stirring bar from the reaction flask using the spatula. Wash the product three times with portions of 10 mL ice-cold water (cooled in the ice bath). Wash the product two times with portions of 10 mL ice-cold n-hexane (cooled in the ice bath). Continue to use the pump to dry the solid on the filter. After ca. 5 min, carefully transfer the dried powder on to the watch glass labeled with your code and put into the labeled Petri dish. Turn off the vacuum valve when you do not use it!Note: Your sample will be collected, dried and weighed later by the lab assistant.Task 2.1 – the recording of your yield –will be performed after the exam by the lab assistants.Step 2. TLC Analysis of the product1.Check your TLC plate before use. Unused damaged plates will be replaced uponrequest without penalty. Use the pencil to draw the start front line, and the line where the solvent front will be run to exactly as shown in Figure 2.2. Write your student code on the top of the TLC plate in pencil.Figure 2.2. Instruction of TLC plate preparation2. Dissolve ca. 1 mg of artemisinin (a spatula tip) in ca. 0.5 mL of methanol in the labeled very small test tube (use the labeled 5 mL graduated pipette). Dissolve ca. 1 mg of the product in ca. 1 mL of methanol in the labeled test tube.3. Spot the artemisinin solution and the product solution on the TLC plate using two different glass capillary spotters so the finished plate is as shown in Figure 2.2.4. Prepare the TLC developing chamber. Use the 5 mL graduated cylinder to make 5 mL of a mixture of n -hexane/ethyl acetate (7/3, v/v) as the solvent system. Pour the mixture of n -hexane/ethyl acetate into the chamber (Note: The solvent level should not reach the spots on the plate if prepared as shown). Cover and swirl the chamber and allow it to stand for 2 min.Aab R f (A )=a bCalculate SolventWatch glassDeveloping chamberTLC plateFigure 2.3. A TLC plate placed in the TLC developing chamber and instruction for R fcalculation of compound A5. Insert the TLC plate upright into the TLC developing chamber. Wait until thesolvent system reaches the pre-drawn solvent front line. (Note: You are advised to work on some question below while you wait for the TLC to run.)6. When the solvent front reaches the line, remove the TLC plate using the tweezersand then dry the solvent using the hair dryer set at level 1.7. Dip the piece of cotton wool into the cerium staining reagent, taking care not to letthe tweezers come into contact with the solution since the metal stains the plate.Carefully apply the stain to the whole TLC plate.8. Heat the TLC plate using the hair dryer set at level 2 (Attention: Do NOT set thehair dryer to COLD) until the blue spots of artemisinin and the product appear on the TLC plate.9. Ask the lab assistant to take a photo of your final TLC plate together with yourstudent code.10. Circle all the visualized spots and calculate the R f values of both artemisinin andthe product (See instruction in Fig. 2.3). Store your TLC plate in the Petri dish.Task 2.2: Fill the values of R f in Table below.R f, Artemisinin R f, Product R f Artemisinin/R f Product ---------------------- -------------------------- -------------------------- Task 2.3: Check the total number of developed spots on the TLC plate:Step 3. Identifying the reaction product PThe reduction of artemisinin leads to the formation of two stereoisomers (P). Comparing the 1H-NMR spectrum (in CDCl3) of one of these isomers with the spectrum of artemisinin shows an extra signal at δH = 5.29 ppm as a doublet, and also an extra signal as a broad singlet at δH= 2.82 ppm.Task 2.4: Suggest structure for P.(You do not need to draw the stereochemistry of the compounds).PTask 2.5:P is mixture of two stereoisomers. What is their stereochemical relationship? Check the appropriate box below.Isomers Enantiomers Diastereomers Constitutional IsomersCode: Task 1 2 3 4 567 8 9 10Total ExaminerMark 0 2522534 3 2 5 271Practical Problem 313 % of thetotalGradePractical Problem 3. Analysis of a hydrated zinc iron(II) oxalate double saltZinc iron(II) oxalate double salt is a common precursor in the synthesis of zincferrite which is widely used in many types of electronic devices due to its interesting magnetic properties. However, such double salts may exist with different compositions and different amount of water depending on how the sample was synthesized.You will analyze a pure sample of hydrated zinc iron(II) oxalate double salt (Z ) in order to determine its empirical formula.ProcedureThe concentration of the standard KMnO 4 is posted on the lab walls.Bring a clean 250 mL beaker to the lab assistant who will be waiting by the balance. You will receive a pure sample of Z for analysis. Accurately weigh between 0.7-0.8 g of the pure sample Z onto the weighing paper (m , grams). This should then be immediately quantitatively transferred into your 250 mL beaker for analysis, and its mass recorded in table below.Task 3.1: Record the mass of the sample of pure Z taken.Mass of sample, m (gram) Lab assistant’s signature---------------------------------------Analysis of Z- Using the 100 mL graduated measuring cylinder, measure ca. 30 mL of 30 wt% H 2SO 4 solution and add it into the 250-mL beaker containing your accurately weighed pure sample of Z . To speed up the dissolving of your sample you may usethe hotplate stirrer to warm up the mixture, but be careful not to boil it. You should not use the digital thermometer as the acid may damage it. After the solid has dissolved, remove the beaker from the hotplate stirrer and cool it to close to room temperature. After the solution has cooled, quantitatively transfer it into the 100 mL volumetric flask. Add distilled water up to the 100 mL–mark. We will now call this solution C.- Use an appropriately labeled beaker to transfer the standardized KMnO4 solution into the burette graduated with brown marks.- Use another appropriately labeled beaker to transfer the standardize EDTA solution into the burette graduated with blue marks.Titration with KMnO4a) Using the 5 mL graduated pipette add 5.00 mL of the solution C into a 250 mLconical flask.b) To this conical flask add about 2 mL of 30 wt% H2SO4 solution, about 3 mL of3.0 M H3PO4 solution, and about10 mL of distilled water. Heat the mixture on thehot plate stirrer until hot, but be careful not to boil it.c) Titrate the hot solution with the standardized KMnO4 solution, recording yourburette readings in the table below. At the end point of the titration, the pink color of the solution appears. Repeat the titration as desired and report your accepted volume of KMnO4 solution consumed (V1 mL) in the table.Task 3.2: Record volumes of standardized KMnO4 solution consumed(You DO NOT need to fill in the entire table)Titration No1 2 3 4Initial reading of the burette of KMnO4, mLFinal reading of the burette of KMnO4, mLConsumed volume of KMnO4,mLAccepted volume, V1 = ________ mL。
2014年普通高等学校招生全国统一考试(课标全国卷I)英语第二部分阅读理解第一节A剑桥科学节好奇心挑战赛敢于接受好奇心挑战!剑桥科学节(CSF)很高兴通知您第六届年度好奇心挑战大赛的召开。
这次大赛邀请甚至激励那些年龄在5至14岁的在校学生创作艺术品或文章,以此展示他们的好奇心以及如何在好奇心的激发下探索世界。
这次大赛激励学生们通过作画、写文章、拍照、或写诗来表达自己所好奇的事物。
要想参加这次挑战大赛,所有的艺术作品或文章都需要在2月8日星期五之前寄给剑桥艺术节,地址是剑桥区,麦斯大街265号,麻省理工博物馆,邮编02139。
参与好奇心挑战大賽并被选为获胜者的学生将在4月21日剑桥艺术节的一个特殊仪式上接受表彰。
特邀发言人也将为学生们颁发奖品。
获奖作品将出版成书。
同时也将展出学生们的参赛作品并给学生们发放奖品。
参与比赛的学生家人也可以参加庆典活动,并且庆典活动提供早午餐。
3月10日至3月15日期间,将通知每位获奖者有关闭幕式和好奇心挑战大赛庆典活动的具体流程和细节。
活动指南以及其他相关信息可登陆http:// . 进行查询。
B旅鸽曾经在美国大部分地区的上空飞翔过,其数量多得令人难以置信。
18世纪到19世纪的文字记载中曾经描述过成群旅鸽飞行时遮天蔽日长达好几个小时的场景。
据计算,旅鸽数量最多时达30 多亿,占美国鸟类数量总和的24%至40%,甚至可能是当时全世界数量最多的鸟类。
即使是在1870年,旅鸽数量已然减少时,也有人在辛辛那提附近看到有1英里宽、320英里长(大约515千米)覆盖面积的旅鸽群。
不幸的是,可能正是旅鸽巨大的数量导致了它们种群的灭亡。
在旅鸽数量极为庞大的地区,人们认为旅鸽很多,可以源源不断地供应,因此成千上万的旅鸽被捕杀。
商业猎杀者用谷物把它们吸引到小的开阔地,一旦等到旅鸽走过去进食,便向它们洒下大网,这样一次可以捕捉到几百只旅鸽。
这些旅鸽被船运到大城市,接着又被卖到餐厅。
在19世纪的最后几十年,由于美国对木材的需要,人们伐掉了旅鸽曾经筑巢的阔叶林,成群的旅鸽被驱散并被迫飞往更远的北部,那里的低温和春季风暴导致它们数量下降。
46th International Chemistry OlympiadJuly 25, 2014Hanoi, VietnamTHEORETICAL EXAMINATION WITH ANSWER SHEETS GRADINGCountry:Name as in passport:Student Code:Language:GENERAL INTRODUCTIONYou have additional 15 minutes to read the whole set.This booklet is composed of 9 problems. You have 5 hours to fulfill the problems. Failure to stop after the STOP command may result in zero points for the current task.Write down answers and calculations within the designated boxes. Give your work where required.Use only the pen and calculator provided.The draft papers are provided. If you need more draft paper, use the back side of the paper. Answers on the back side and the draft papers will NOT be marked.There are 52 pages in the booklet including the answer boxes, Cover Sheet and Periodic Table.The official English version is available on demand for clarification only.Need to go to the restroom – raise your hand. You will be guided there.After the STOP signal put your booklet in the envelope (do not seal), leave at your table. Do not leave the room without permission.Physical Constants, Units, Formulas and EquationsAvogadro's constant N A = 6.0221 × 1023 mol–1Universal gas constant R = 8.3145 J·K–1·mol–1Speed of light c = 2.9979 × 108 m·s–1Planck's constant h= 6.6261 × 10–34 J·sStandard pressure p° = 1 bar = 105 PaAtmospheric pressure 1 atm = 1.01325 × 105 Pa = 760 mmHg Zero of the Celsius scale 273.15 KMass of electron m e = 9.1094 × 10–31kg1 nanometer (nm) = 10–9 m ; 1 angstrom (Å) = 10–10 m1 electron volt (eV) = 1.6022 × 10–19 J = 96485 J·mol–1Problem 1. Particles in a box: polyenesIn quantum mechanics, the movement of π electrons along a neutral chain ofconjugated carbon atoms may be modeled using the ‘particle in a box’ method. The energy of the π electrons is given by the following equation:2228mLh n E n = where n is the quantum number (n = 1, 2, 3, …), h is Planck’s constant, m is the mass of electron, and L is the length of the box which may be approximated by L = (k + 2)×1.40 Å (k being the number of conjugated double bonds along the carbon chain in the molecule). A photon with the appropriate wavelength λ may promote a π electron from the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO). An approximate semi-empirical formula based on this model which relates the wavelength λ, to the number of double bonds k and constant B is as follows:λ (nm) = B )12()2(2++×k k Equation 11. Using this semi-empirical formula with B = 65.01 nm calculate the value of the wavelength λ (nm) for octatetraene (CH 2 = CH – CH = CH – CH = CH – CH = CH 2).Code:Question 1 2 3 4 5 Total Examiner Mark 3 7 6 4 7 27 Theoretical Problem 1 5.0 % of thetotalGrade2.Derive Equation 1 (an expression for the wavelength λ (nm) corresponding to the transfer of an electron from the HOMO to the LUMO) in terms of k and the fundamental constants, and hence calculate theoretical value of the constant B calc..3. We wish to synthesize a linear polyene for which the excitation of a π electron from the HOMO to the LUMO requires an absorption wavelength of close to 600 nm. Using your expression from part 2, determine the number of conjugated double bonds (k) in this polyene and give its structure. [If you did not solve Part 2, use the semi-empirical Equation 1 with B = 65.01 nm to complete Part 3.]Thus, k = 15.So, the formula of polyene is:CH 2 = CH – (CH = CH)13 – CH = CH 22 points4. For the polyene molecule found in Part 3, calculate the difference in energy between the HOMO and the LUMO, ΔE , (kJ·mol –1).In case Part 3 was not solved, take k = 5 to solve this problem.5. The model for a particle in a one-dimensional box can be extended to a three dimensional rectangular box of dimensions L x , L y and L z , yielding the following expression for the allowed energy levels:⎟⎟⎠⎞⎜⎜⎝⎛++=2222222,,8z z y y x x n n n L n L n L n m h E zy xThe three quantum numbers n x , n y , and n z must be integer values and are independentof each other.5.1 Give the expressions for the three different lowest energies, assuming that the box is cubic with a length of L .Levels with the same energy are said to be degenerate. Draw a sketch showing all the energy levels, including any degenerate levels, that correspond to quantum numbers having values of 1 or 2 for a cubic box.Problem 2. Dissociating Gas CycleDininitrogen tetroxide forms an equilibrium mixture with nitrogen dioxide:N 2O 4(g) ⇌ 2NO 2(g)1.00 mole of N 2O 4 was put into an empty vessel with a fixed volume of 24.44 dm 3.The equilibrium gas pressure at 298 K was found to be 1.190 bar. When heated to 348 K, the gas pressure increased to its equilibrium value of 1.886 bar. 1a. Calculate ∆G 0 of the reaction at 298K, assuming the gases are ideal.1b. Calculate ∆H 0 and ∆S 0 of the reaction, assuming that they do not change significantly with temperature.Code: Question 1a 1b 2 3 TotalExaminerMark12 8 3 10 33Theoretical Problem 2 5.0 % of thetotalGrade∆S 0∆G 0348 = - 4.07 kJ = ∆H – 348∆S (1) ∆G 0298 = 4.72 kJ = ∆H – 298∆S (2) (2) - (1) → ∆S = 0.176 kJ·mol –1·K –1 ∆H 0∆H 0 = 4.720 + 298 × 0.176 = 57.2 (kJ·mol –1)4pts 4ptsIf you cannot calculate ∆H 0, use ∆H 0 = 30.0 kJ·mol –1 for further calculations.The tendency of N 2O 4 to dissociate reversibly into NO 2 enables its potential use in advanced power generation systems. A simplified scheme for one such system is shown below in Figure (a). Initially, "cool" N 2O 4 is compressed (1→2) in a compressor (X ), and heated (2→3). Some N 2O 4 dissociates into NO 2. The hot mixture is expanded (3→4) through a turbine (Y ), resulting in a decrease in both temperatureand pressure. The mixture is then cooled further (4→1) in a heat sink (Z ), to promote the reformation of N 2O 4. This recombination reduces the pressure, thus facilitates the compression of N 2O 4 to start a new cycle. All these processes are assumed to take place reversibly.out(a)To understand the benefits of using reversible dissociating gases such as N 2O 4, we will focus on step 3 → 4 and consider an ideal gas turbine working with 1 mol of air (which we assume to be an inert, non-dissociating gas). During the reversible adiabatic expansion in the turbine, no heat is exchanged .2. Give the equation to calculate the work done by the system w(air) during the reversible adiabatic expansion for 1 mol of air during stage 3 → 4. Assume that C v,m(air) (the isochoric molar heat capacity of air) is constant, and the temperature changes from T3 to T4.∆U = q + w; work done by turbine w(air)=-w 1 ptq = 0, thus w(air) = ∆U = C v,m(air)[T3-T4] 2 pts3.Estimate the ratio w(N2O4)/w(air), in which w(N2O4) is the work done by the gas during the reversible adiabatic expansion process 3 → 4 with the cycle working with 1 mol of N2O4, T3 and T4 are the same as in Part 2. Take the conditions at stage 3 to be T3 = 440 K and P3 = 12.156 bar and assume that:(i) the gas is at its equilibrium composition at stage 3;(ii) C v,m for the gas is the same as for air;(iii) the adiabatic expansion in the turbine takes place in a way that the composition of the gas mixture (N2O4 + NO2) is unchanged until the expansion is completed.Oxidation number of Ag1 : ……….+1 Oxidation number of Ag2 : ……… +3 2 pointsCode: Question 1 2 3 4 Total ExaminerMarks 8 14 2 12 36 Theoretical Problem 3 9.0 % of the totalGrade1c.What is the coordination number of O atoms in the lattice of A?The coordination number of O atoms =……… 3 1 point1d.How many Ag I and Ag III bond to one O atom in the lattice of A?Number of Ag I = (1)Number of Ag III = ……. 2 2 points1e.Predict the magnetic behaviour of A. Check the appropriate box below.S2O82-(aq) + 2Ag+(aq) + 2H2O (l) 2SO42-(aq) + Ag I Ag III O2 (s) + 4H+(aq)1 point2. Among the silver oxides which have been crystallographically characterized, the most surprising is probably that compound A is not a Ag II O. Thermochemical cycles are useful to understand this fact. Some standard enthalpy changes (at 298 K) are listed:Atom Standard enthalpyof formation(kJ·mol–1)1st ionization(kJ·mol–1)2nd ionization(kJ·mol–1)3rd ionization(kJ·mol–1)1st electronaffinity(kJ·mol–1)2nd electronaffinity(kJ·mol–1)Cu(g) 337.4 751.7 1964.1 3560.2Ag(g) 284.9 737.2 2080.2 3367.2O(g)249.0 -141.0844.0Compounds ΔH o f (kJ ·mol –1) Ag I Ag III O 2 (s) –24.3 Cu II O (s) –157.3The relationship between the lattice dissociation energy (U lat ) and the lattice dissociation enthalpy (ΔH lat ) for monoatomic ion lattices is: nRT U H lat lat +=Δ, where n is the number of ions in the formula unit.2a. Calculate U lat at 298 K of Ag I Ag III O 2 and Cu II O. Assume that they are ionic compounds. U lat of Ag I Ag III O 2 Calculations:ΔH lat (Ag I Ag III O 2) = 2 ΔH o f (O 2-) + ΔH o f (Ag +) + ΔH o f (Ag 3+) –ΔH o f (Ag I Ag III O 2) = (2×249 – 2 × 141 + 2 × 844) + (284.9 + 737.2) + (284.9 + 737.2 + 2080.2 + 3367.2 ) – (–24.3)= +9419.9 (kJ·mol –1)U lat (Ag I Ag III O 2) = ΔH lat (Ag I Ag III O 2) – 4RT= + 9419.9 – 10.0 = + 9409.9 (kJ·mol –1)3 points(no penalty if negative sign)U lat of Cu II OCalculations for: U lat of Cu II OΔH lat (Cu II O) = ΔH o f (O 2–) + ΔH o f (Cu 2+) – ΔH o f (Cu II O)= (249 – 141 + 844) + (337.4 + 751.7 + 1964.1) – (–157.3)= 4162.5 (kJ ·mol –1)U lat (Cu II O) = ΔH lat (Cu II O) – 2RT = 4162.5 – 5.0 = 4157.5 (kJ ·mol –1)3 points(no penalty if negative sign)If you can not calculate the U lat of Ag I Ag III O 2 and Cu II O, use following values forfurther calculations: U lat of Ag I Ag III O 2 = 8310.0 kJ·mol –1; U lat of Cu II O = 3600.0 kJ·mol –1.The lattice dissociation energies for a range of compounds may be estimated using this simple formula:311C ⎟⎟⎠⎞⎜⎜⎝⎛×=m lat V UWhere: V m (nm 3) is the volume of the formula unit and C (kJ·nm·mol –1) is an empirical constant which has a particular value for each type of lattice with ions of specified charges.The formula unit volumes of some oxides are calculated from crystallographic data as the ratio between the unit cell volume and the number of formula units in the unit cell and listed as below:Oxides V m (nm 3)Cu II O 0.02030 Ag III 2O 3 0.06182 Ag II Ag III 2O 4 0.089852b. Calculate U lat for the hypothetical compound Ag II O. Assume that Ag II O and Cu II O have the same type of lattice, and that V m (Ag II O) = V m (Ag II Ag III 2O 4) – V m (Ag III 2O 3).2c. By constructing an appropriate thermodynamic cycle or otherwise, estimate the enthalpy change for the solid-state transformation from Ag II O to 1 mole of Ag I Ag III O 2. (Use U lat Ag II O = 3180.0 kJ·mol -1 and U lat Ag I Ag III O 2 = 8310.0 kJ·mol -1 if you cannot calculate U lat Ag II O in Part 2b).2Ag IIO (s)Ag I Ag III O 2(s)2Ag 2+(g)+2O 2-(g)Ag +(g)+Ag 3+(g)+2O 2-(g)H rxn2U lat (AgO)+4RT-U lat (Ag I Ag III O)-4RTIE 3(Ag)-IE 2(Ag)Calculations:ΔH rxn = 2U lat (Ag II O) + 4RT + IE 3 – IE 2 – U lat (Ag I Ag III O 2) – 4RT= 2 × 3733.6 + 3367.2 – 2080.2 – 9409.9= – 655.7 (kJ/mol) or - 663.0 kJ/mol using given U lat values 4 pts2d. Indicate which compound is thermodynamically more stable by checking the appropriate box below.3. When Ag I Ag III O 2 is dissolved in aqueous HClO 4 solution, a paramagnetic compound (B ) is first formed then slowly decomposes to form a diamagnetic compound (C ). Given that B and C are the only compounds containing silver formed in these reactions, write down the equations for the formation of B and C .For B :Ag I Ag III O 2 (s) + 4 HClO 4 (aq) 2Ag(ClO 4)2 (aq) + 2 H 2O (l) 1 pointFor C : 4Ag(ClO 4)2 (aq) + 2 H 2O (l)4 AgClO 4 (aq) + 4 HClO 4 (aq) + O 2 (g) 1 point4. Oxidation of Ag+ with powerful oxidizing agents in the presence of appropriate ligands can result in the formation of high-valent silver complexes. A complex Z is synthesized and analyzed by the following procedures:An aqueous solution containing 0.500 g of AgNO3 and 2 mL of pyridine (d = 0.982 g/mL) is added to a stirred, ice-cold aqueous solution of 5.000 g of K2S2O8. The reaction mixture becomes yellow, then an orange solid (Z) is formed which has a mass of 1.719 g when dried.Elemental analysis of Z shows the mass percentages of C, H, N elements are38.96%, 3.28%, 9.09%, respectively.A 0.6164 g Z is added to aqueous NH3. The suspension is boiled to form a clear solution during which stage the complex is destroyed completely. The solution is acidified with excess aqueous HCl and the resulting suspension is filtered, washed and dried (in darkness) to obtain 0.1433 g of white solid (D). The filtrate is collected and treated with excess BaCl2 solution to obtain 0.4668 g (when dry) of white precipitate (E).4a.Determine the empirical formula of Z and calculate the percentage yield in the preparation.4b. Ag (IV) and Ag (V) compounds are extremely unstable and found only in few fluorides. Thus, the formation of their complexes with organic ligands in water can be discounted. To confirm the oxidation number of silver in Z, the effective magnetic moment (µeff ) of Z was determined and found to be 1.78 BM. Use the spin only formula to determine the number of unpaired electrons in Z and the molecular formula of Z. (Z contains a mononuclear complex with only one species of Ag and only one type of ligand in the ligand sphere.)4c. Write down all chemical equations for the preparation of Z, and its analysis.Formation of Z:2Ag+(aq) + 8Py (l) + 3S2O82–(aq) 2[Ag II(Py)4](S2O8) (s) + 2SO42–(aq) 2 ptsDestruction of Z with NH3:[Ag II(Py)4](S2O8) (s) + 6NH3(l) [Ag(NH3)2]+(aq) + ½ N2(g) + 2SO42-(aq)+3NH4+ + 4Py (l) 2 pts(aq)(All reasonable N –containing products and O2 are acceptable)Formation of D:[Ag(NH3)2]+(aq) + 2H+(aq) + Cl– (aq) AgCl (s) + 2NH4+(aq) 1 ptFormation of E:Ba2+(aq) + SO42– (aq) BaSO4(s)1ptProblem 4. Zeise’s Salt1. Zeise's salt, K[PtCl 3C 2H 4], was one of the first organometallic compounds to bereported. W. C. Zeise, a professor at the University of Copenhagen, prepared this compound in 1827 by reacting PtCl 4 with boiling ethanol and then adding potassium chloride (Method 1). This compound may also be prepared by refluxing a mixture of K 2[PtCl 6] and ethanol (Method 2). The commercially available Zeise's salt is commonly prepared from K 2[PtCl 4] and ethylene (Method 3).1a. Write balanced equations for each of the above mentioned preparations of Zeise's salt, given that in methods 1 and 2 the formation of 1 mole of Zeise’s salt consumes 2 moles of ethanol.PtCl 4 + 2 C 2H 5OH → H[PtCl 3C 2H 4] + CH 3CH=O + HCl + H 2O H[PtCl 3C 2H 4] + KCl → K[PtCl 3C 2H 4] + HClK 2[PtCl 6] + 2 C 2H 5OH → K[PtCl 3C 2H 4] + CH 3CH=O + KCl + 2 HCl + H 2O K 2[PtCl 4] + C 2H 4 → K[PtCl 3C 2H 4] + KCl1pt for each (2 pts if the first two reactions combined), total of 4 pts1b. Mass spectrometry of the anion [PtCl 3C 2H 4]– shows one set of peaks with mass numbers 325-337 au and various intensities.Calculate the mass number of the anion which consists of the largest natural abundance isotopes (using given below data).Code: Question 1a 1b 2a 3a 3b 3c Total ExaminerMark 4 1 10 2 6 4 27Theoretical Problem 4 4.0 % of the totalGradeIsotopePt 19278Pt 19478Pt 19578Pt 19678Pt 19878C 126C136Natural abundance,% 0.8 32.9 33.8 25.3 7.2 75.8 24.2 98.9 1.1 99.99Calculations:195 + 3×35 + 2×12 + 4×1 = 328 1 pt2. Some early structures proposed for Zeise’s salt anion were:In structure Z1, Z2, and Z5 both carbons are in the same plane as dashed square. [You should assume that these structures do not undergo any fluxional process byinterchanging two or more sites.]2a. NMR spectroscopy allowed the structure for Zeise’s salt to be determined as structure Z4. For each structure Z1-Z5, indicate in the table below how many hydrogen atoms are in different environments, and how many different environments of hydrogen atoms there are, and how many different environments of carbon atoms there are?StructureNumber of differentenvironments of hydrogen Number of differentenvironments of carbonZ121pt 21 ptZ22 1pt 21 ptZ321pt 21 ptZ41 1pt 11 ptZ52 1pt 11 pt3. For substitution reactions of square platinum(II) complexes, ligands may be arranged in order of their tendency to facilitate substitution in the position trans to themselves (the trans effect). The ordering of ligands is:CO , CN- , C2H4 > PR3 , H- > CH3- , C6H5- , I- , SCN- > Br- > Cl- > Py > NH3 > OH- , H2OIn above series a left ligand has stronger trans effect than a right ligand.Some reactions of Zeise’s salt and the complex [Pt2Cl4(C2H4)2] are given below.3a.Draw the structure of A, given that the molecule of this complex has a centre of symmetry, no Pt-Pt bond, and no bridging alkene.Structure of A2 pt3b.Draw the structures of B, C, D, E, F and G.B1 ptCPtCl NH2C6H5Cl1 ptD1 ptE1 ptF1 ptG1 pt3c.Suggest the driving force(s) for the formation of D and F by choosing one or more of the following statements (for example, i and ii):i) Formation of gasii) Formation of liquidiii) Trans effectiv) Chelate effectStructure D FDriving force(s) i iii and iv2 pts 2 ptsProblem 5. Acid-base Equilibria in WaterA solution (X) contains two weak monoprotic acids (those having one acidicproton); HA with the acid dissociation constant of K HA = 1.74 × 10–7, and HB with the acid dissociation constant of K HB = 1.34 × 10–7. The solution X has a pH of 3.75.1. Titration of 100 mL solution X requires 100 mL of 0.220 M NaOH solution for completion.Calculate the initial (total) concentration (mol·L –1) of each acid in the solution X . Use reasonable approximations where appropriate. [K W = 1.00 × 10–14 at 298 K.]HAH HBH OH Code:Question 1 2 3 4 TotalExaminer Mark 6 4 4 6 20 Theoretical Problem 5 6.5 % of thetotalGrade2. Calculate the pH of the solution Y which initially contains 6.00×10-2 M of NaA and 4.00×10-2 M of NaB.Solution:Solution Y contains NaA 0.06 M and NaB 0.04 M. The solution is basic, OH– was produced from the reactions:NaA + H 2O HA + OH–K b,A = K w/K HA = 5.75 ×10-8NaB + H 2O HB + OH– K b,B = K w/K HB = 7.46 ×10-8H 2O H+ + OH–K w = 1.00 10-14and we have:3. Adding large amounts of distilled water to solution X gives a very (infinitely) dilute solution where the total concentrations of the acids are close to zero. Calculate the percentage of dissociation of each acid in this dilute solution.Solving the equation gives: α = 0.573- The percentage of dissociation of HA = 65.5 %- The percentage of dissociation of HB = 57.3 % 2 points4. A buffer solution is added to solution Y to maintain a pH of10.0. Assume no change in volume of the resulting solution Z.Calculate the solubility (in mol·L–1) of a subtancce M(OH)2 in Z, given that the anions A– and B– can form complexes with M2+:M(OH)2 M2+ + 2OH–K sp = 3.10 ×10-12M2+ + A– [MA]+K 1= 2.1 × 103[MA]+ + A– [MA 2] K2 = 5.0 × 102M2+ + B– [MB]+K’1 = 6.2 × 103[MB]+ + B– [MB 2] K’2 = 3.3 × 102MO H[MB][MBSolve this equation: [A -] = 8.42× 10 –3 M Substitute this value into Eq. 3 and Eq. 4:[MA +] = 0.651 × [A –] = 5.48 × 10 –3 M [MA 2] = 325.5 × [A –]2 = 2.31 × 10 –2 MSimilarly, [B –]total = 0.04 M][92.1][1010.3102.6]][[][432'1−−−−++×=××××==B B B M K MB Eq. 6222'2'12][3.634]][[][−−+×==B B M K K MB Eq.7[B –]total = [B -] + [MB +] + 2 × [MB 2] = 0.04 M Eq. 8 2ptsSubstitute Eq. 6 and Eq. 7 into Eq. 8: [B –] + 1.92 × [B –] + 2 × 634.3 × [B –]2 = 0.04 Solve this equation: [B –] = 4.58 × 10–3 M Substitute this value into Eq. 6 and Eq. 7: [MB +] = 1.92 ×[B –] = 8.79 × 10 –3 M [MB 2] = 634.3 ×[B –]2 = 1.33 × 10–2 MThus, solubility of M(OH)2 in Z is s’s’ = 3.10×10 – 4 + 5.48×10 – 3 + 2.31×10 – 2 + 8.79 × 10 – 3+ 1.33 ×10 – 2 = 5.10×10 – 2 M Answer: Solubility of M(OH)2 in Z = 5.10×10 – 2 M. 2 pointsProblem 6. Chemical KineticsThe transition-metal-catalyzed amination of aryl halides has become one of the mostpowerful methods to synthesize arylamines. The overall reaction for the nickel-catalyzed amination of aryl chloride in basic conditions is:in which NiLL’ is the nickel complex catalyst. The reaction goes through several steps in which the catalyst, reactants, and solvent may be involved in elementary steps.6a. To determine the reaction order with respect to each reactant, the dependence of the initial rate of the reaction on the concentrations of each reagent was carried out with all other reagents present in large excess. Some kinetic data at 298 K are shown in the tables below. (Use the grids if you like)Code: Question 6a6b 6c 6d 6e Total ExaminerMarks 6 8 4 12 2 32 Theoretical Problem 6 7.0 % of thetotalGradeDetermine the order with respect to the reagents assuming they are integers. -Order with respect to [ArCl] = = 1-Order with respect to [NiLL’] = = 1-Order with respect to [L’] = = -1 6pts6b. To study the mechanism for this reaction, 1H, 31P, 19F, and 13C NMR spectroscopy have been used to identify the major transition metal complexes in solution, and the initial rates were measured using reaction calorimetry. An intermediate, NiL(Ar)Cl, may be isolated at room temperature. The first two steps of the overall reaction involve the dissociation of a ligand from NiLL’ (step 1) at 50 o C, followed by the oxidation addition (step 2) of aryl chloride to the NiL at room temperature (rt):Using the steady state approximation, derive an expression for the rate equation for the formation of [NiL(Ar)Cl].(4 pts for rate calculation)The next steps in the overall reaction involve the amine (RNH2) and t BuONa. To determine the order with respect to RNH2 and t BuONa, the dependence of the initial rates of the reaction on the concentrations of these two reagents was carried with the other reagents present in large excess. Some results are shown in the tables below.6c . Determine the order with each of these reagents, assuming each is an integer. (Use the grids if you like)- Order with respect to [NaO t Bu] = 0 2 pts- Order with respect to [RNH 2] = 02 ptsDuring a catalytic cycle, a number of different structures may be involved which include the catalyst. One step in the cycle will be rate-determining.A proposed cycle for the nickel-catalyzed coupling of aryl halides with amines is as follows:6d. Use the steady-state approximation and material balance equation to derive the rate law for d[ArNHR]/dt for the above mechanism in terms of the initial concentration of the catalyst [NiLL’]0 and concentrations of [ArCl], [NH 2R], [NaO t Bu], and [L’].NiLLNiLLApply the steady-state approximation to the concentrations for the intermediates:[NiL][L’] + k [NiL(Ar)HNR] (Equation 1) 1pt(Equation 2) 1pt6e.Give the simplified form of the rate equation in 6d assuming that k1 is very small. d[ArNHR]/dt = - d[ArCl]/dt =k2[ArCl] [NiL] = k1k2 [ArCl][NiLL’]0 / k-1[L’] (i.e. consistent with all the orders of reaction as found in the beginning) 2 ptsProblem 7. Synthesis of Artemisinin(+)-Artemisinin, isolated from Artemisia annua L.(Qinghao, Compositae ) is a potent antimalarial effective against resistant strains of Plasmodium . A simple route for the synthesis of Artemisinin is outlined below.First, pyrolysis of (+)-2-Carene broke the cyclopropane ring forming, among other products, (1R )-(+)-trans -isolimonene A (C 10H 16), which then was subjected to regioselective hydroboration using dicyclohexylborane to give the required alcohol B in 82% yield as a mixture of diastereoisomers. In the next step, B was converted to the corresponding γ,δ-unsaturated acid C in 80% yield by Jones’ oxidation.7a. Draw the structures (with stereochemistry) of the compounds A-C .A B CMeMeHHO4 pts (2 pts if wrong stereochemistry) 4 pts 4 ptsCode: Question 7a 7b 7c 7d 7e 7f Total ExaminerMark128 8 12 12 12 64Theoretical Problem 7 8.0 % of thetotalGradeThe acid C was subjected to iodolactonization using KI, I2 in aqueous. NaHCO3solution to afford diastereomeric iodolactones D and E (which differ in stereochemistry only at C3 ) in 70% yield.7b. Draw the structures (with stereochemistry) of the compounds D and E.The acid C was converted to diastereomeric iodolactones D and E (epimeric at the chiralcenter C3). Look at the number-indicated in the structure F in the next step.D E4 pts 4ptsThe iodolactone D was subjected to an intermolecular radical reaction with ketoneX using tris(trimethylsilyl)silane (TTMSS) and AIBN (azobisisobutyronitrile) in acatalytic amount, refluxing in toluene to yield the corresponding alkylated lactone F in72% yield as a mixture of diastereoisomers which differ only in stereochemistry at C7along with compound G (~10%) and the reduced product H, C10H16O2(<5%).7c. Draw the structures (with stereochemistry) of compound H and the reagent X.Because alkylated lactone F is known, we can deduce the reagent X as methyl vinylketone. H is the reduced product of D.X H2 pts 6 ptsThe keto group of F reacted with ethanedithiol and BF3•Et2O in dichloromethane(DCM) at 0o C to afford two diastereomers: thioketal lactones I and J in nearlyquantitative yield (98%). The thioketalization facilitated the separation of the majorisomer J in which the thioketal group is on the opposite face of the ring to the adjacentmethyl group.7d.Draw the structures (with stereochemistry) of the compounds I and J.The keto group of lactone F reacted with ethanedithiol and BF3·Et2O in dichloromethaneto afford thioketal lactones, I and the major isomer J.I J6 pts (3 pts if I and J are swapped) 6 pts (3 pts if I and J are swapped)The isomer J was further subjected to alkaline hydrolysis followed byesterification with diazomethane providing hydroxy methyl ester K in 50% yield. Thehydroxy methyl ester K was transformed into the keto ester L using PCC (P yridiumC hloro C hromate) as the oxidizing agent in dichloromethane (DCM).A two-dimensional NMR study of the compound L revealed that the twoprotons adjacent to the newly-formed carbonyl group are cis to each other andconfirmed the structure of L.7e. Draw the structures (with stereochemistry) of the compounds K and L .Hydrolysis followed by esterification of J provided hydroxy ester K .Oxidation of the hydroxy group in K by PCC resulted in the keto ester L in which two protons adjacent to the carbonyl group are cis-oriented.K L6 pts 6 ptsThe ketone L was subjected to a Wittig reaction with methoxymethyl triphenylphosphonium chloride and KHMDS (P otassium H exa M ethyl D i S ilazid - a strong, non-nucleophilic base) to furnish the required methyl vinyl ether M in 45% yield. Deprotection of thioketal using HgCl 2, CaCO 3 resulted in the key intermediate N (80%). Finally, the compound N was transformed into the target molecule Artemisinin by photo-oxidation followed by acid hydrolysis with 70% HClO 4.LMN323231. O 2, h υ4。
1 1 v 0 v 0 min 0 0 min 第 31 届全国部分地区大学生物理竞赛试卷与解答2014.12.071. 将地球半径R 、自转周期T 、地面重力加速度g 取为已知量,则人造地球同步卫星的轨道半径=gT 242R 3R ,轨道速度相对第一宇宙速度的比值=4 2R T 2g 62. 如图所示,水平桌面上静放着质量为M ,内半径为R 的半球面形薄瓷碗,碗的底座与桌面间无摩擦。
将质量为m 的小滑块在图示的碗边位置从静止释放,随后将会无摩 擦地沿碗的内表面滑下。
小滑块到达最低位置时, 它相对桌面的速度大小为3M 2mm g 。
M3. 如图所示,长l 的轻细杆两端连接质量相同的小球A 、B ,开始时细杆处于竖直方位, A 下端 B 球距水平地面高度记为 h 。
某时刻让 B 球具有水平朝右初速度 (其大小 l2v 0v 0 2 ),其上方 A 球具有水平朝右初速度2。
假设而后A 、 B 同时着地,则h 可 B 取的最小值h =2 gl 4v 2l 8v 2,取h 时,B 从开始运动到着地过程中其水平位移s h4. 两个测量者A 和B ,各自携带频率同为1000Hz 的声波波源。
设 A 静止,B 以10m / s 的速度朝着A 运动,已知声速为340m / s ,不考虑人体的反射,则 A 接收到的拍频A 拍= 30 Hz (请保留 2 位有效数字),B 接收到的拍频B 拍= 29 Hz(请保留 2 位有效数字)。
图 25. 如图 1 所示,3 个相同的匀质球体以相同的水平初速度v 0 平图 1抛出去。
其中球 1 抛出时无自转, 球 2、球 3 抛出时有自转,自转方向已在图 1 中示出,自转角速度值 0 相同且较大。
球 1 抛出后,落地前球心的一段31运动轨道如图 2 长方形内一段曲线所示, 2试在该长方形区域内定性画出球 2、球 3落地前各自球心的一段运动轨道。
(球 2、球 3 球心在图 2 中的初始位置, 可不受图 1 所示位置限制。
ROBLEM A: The Keep-Right-Except-To-Pass Rule问题A:除非超车否则靠右行驶的交通规则In countries where driving automobiles on the right is the rule (that is, USA, China and most other countries except for Great Britain, Australia, and some former British colonies), multi-lane freeways often employ a rule that requires drivers to drive in the right-most lane unless they are passing another vehicle, in which case they move one lane to the left, pass, and return to their former travel lane.在一些汽车靠右行驶的国家(比如美国,中国等等),多车道的高速公路常常遵循以下原则:司机必须在最右侧驾驶,除非他们正在超车,超车时必须先移到左侧车道在超车后再返回。
Build and analyze a mathematical model to analyze the performance of this rule in light and heavy traffic. You may wish to examine tradeoffs between traffic flow and safety, the role of under- or over-posted speed limits (that is, speed limits that are too low or too high), and/or other factors that may not be explicitly called out in this problem statement. Is this rule effective in promoting better traffic flow? If not, suggest and analyze alternatives (to include possibly no rule of this kind at all) that might promote greater traffic flow, safety, and/or other factors that you deem important.建立数学模型来分析这条规则在低负荷和高负荷状态下的交通路况的表现。
注意:题目不能只看翻译,必须充分参考原题。
Problem No. 1 “Invent yourself”It is known that some electrical circuits exhibit chaotic behaviour. Build a simple circuit with such a property, and investigate its behaviour.1.自己创造据了解,一些电路表现出混沌行为。
构建一个具有这种属性的简单电路,并研究其行为。
Problem No. 2 “Hologram”It is argued that a hologram can be hand made by scratching a piece of plastic. Produce such a ‘hologram’ with the letters ‘IYPT’ and investigate how it works.2.全息照片有人认为,在一块透明塑料上划出图案可以手工制作出一张全息照片。
制作一张字母“IYPT ”的全息图并研究它是如何工作的。
Problem No. 3 “Twisted rope”Hold a rope and twist one end of it. At some point the rope will form a helix or a loop. Investigate and explain the phenomenon.3.扭曲的绳握住绳子扭它的一端。
在绳索上的某一点将形成螺旋线或圆环。
调查解释这样的现象。
Problem No. 4 “Ball sound”When two hard steel balls, or similar, are brought gently into contact with each other,an unusual ‘chirping’ sound may be produced. Investigate and explain the nature of the sound.4.球的声音当两个硬钢球或类似的东西被轻轻带到接触到对方,一个不寻常的“鸣叫声”。
调查解释的声音的性质。
Problem No. 5 “Loaded hoop”Fasten a small weight to the inside of a hoop and set the hoop in motion by giving it an initial push. Investigate the hoop’s motion.5.载物的环在一个环的里面固定一个小重物,给环一个初始推力使其运动。
研究环的运动。
Problem No. 6 “Bubble crystal”A large number of very small, similar air bubbles float on the surface of a soapy liquid.The bubbles will arrange themselves into a regular pattern similar to a crystalline lattice. Propose a method to obtain bubbles of a consistent size, and investigate the formation of such a bubble crystal.6.泡泡晶体大量非常小的相似的气泡浮在肥皂水的表面上。
气泡会自动按照一个规律的类似晶格的模式排列。
提出一种获得大小一致的的气泡的方法,并探究这种泡泡晶体的形成。
Problem No. 7 “Pot-in-pot refrigerator”The ‘pot-in-pot refrigerator’ is a device that keeps food cool using the principle of evaporative cooling. It consists of a pot placed inside a bigger pot with the space between them filled with a wet porous material, e.g. sand. How might one achieve the best cooling effect?7.“罐中罐”冰箱这一个依据蒸发冷却的原理让食物保鲜的装置。
它包括一个大容器、里面的小容器。
它们之间的空间内用湿的多孔材料填充,例如沙子。
问怎么能达到最佳的散热效果?Problem No. 8 “Freezing droplets”Place a water droplet on a plate cooled down to around -20 °C. As it freezes, the shape of the droplet may become cone-like with a sharp top. Investigate this effect.8.冻结水滴将水滴放置在冷却到-20°C左右的板上。
结冰后液滴可能会成为有锋利的顶部的圆锥状。
调查这种现象。
Problem No. 9 “Water bombs”Some students are ineffective in water balloon fights as the balloons they throw rebound without bursting. Investigate the motion, deformation, and rebound of aballoon filled with fluid. Under what circumstances does the balloon burst?9.水弹有些学生不会用灌水的气球打仗,他们的水弹反弹后仍不爆裂。
调查这里的运动,变形和充满液体的气球的反弹。
在什么情况下水弹会爆裂?Problem No. 10 “Coefficient of diffusion”Using a microscope, observe the Brownian motion of a particle of the order of micrometre in size. Investigate how the coefficient of diffusion depends on the size and shape of the particle.10.扩散系数利用显微镜按微米大小的顺序观察微粒的布朗运动。
研究扩散系数是如何取决于微粒的大小和形状的。
Problem No. 11 “Candle Power Plant”Design a device that converts the heat of a candle flame into electrical energy. Investigate how different aspects of the device affect its efficiency.11.蜡烛发电厂设计一将蜡烛的火焰的热量转化成电能的装置。
调查装置的不同方面如何影响其效率。
Problem No. 12 “Cold balloon”As air escapes from an inflated rubber balloon, its surface becomes cooler to the touch. Investigate the parameters that affect this cooling. What is the temperature of various parts of the balloon as a function of relevant parameters?12.冷气球由于空气逃离橡胶气球,其表面触感变得冷。
研究影响降温的参量。
作为一个函数的相关参数,气球的不同部分温度是什么?Problem No. 13 “Rotating saddle”A ball is placed in the middle of a rotating saddle. Investigate its dynamics and explain the conditions under which the ball does not fall off the saddle.13.旋转的鞍一个球被放在旋转的鞍上。
从动力学的角度研究它,解释球不会从鞍上落下来的情形。
Problem No. 14 “Rubber motor”A twisted rubber band stores energy and can be used to power a model aircraft for example. Investigate the properties of such an energy source and how its power output changes with time.14.橡胶电机扭曲的橡皮筋存储着能量,例如可用于驱动飞机模型。
调查这样的能量来源的属性及其功率输出随时间的变化。
Problem No. 15 “Oil stars”If a thick layer of a viscous fluid (e.g. silicone oil) is vibrated vertically in a circular reservoir, symmetrical standing waves can be observed. How many lines of symmetry are there in such wave patterns? Investigate and explain the shape and behaviour of the patterns.15.油星星如果一层厚厚的粘性流体(如硅油)在一个圆形的水槽里上下振动,可以观察到对称驻波。
在这样的波图案中有多少条对称的线?研究并解释图案的形状和行为。
Problem No. 16 “Magnetic brakes”When a strong magnet falls down a non-ferromagnetic metal tube, it will experience a retarding force. Investigate the phenomenon.16.磁力刹车当一个强磁铁从非铁磁性金属管内降下来时,它会经历一个阻滞力,研究这个现象。