食品专业英语Lesson2 Carbohydrate
- 格式:ppt
- 大小:440.00 KB
- 文档页数:20

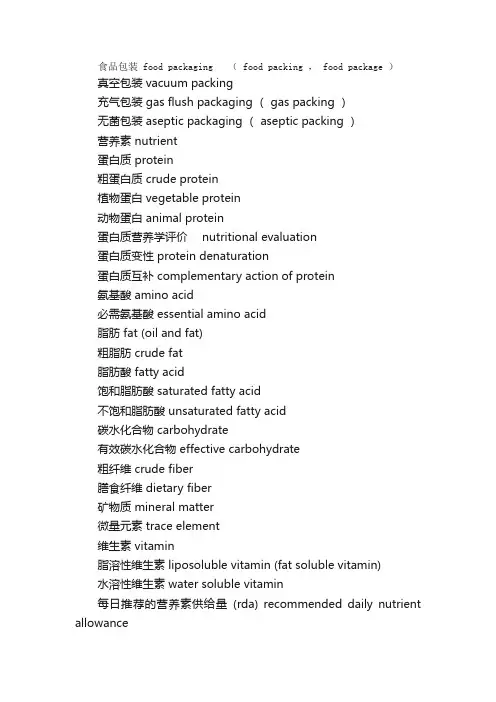
食品包装 food packaging ( food packing , food package )真空包装 vacuum packing充气包装 gas flush packaging ( gas packing )无菌包装 aseptic packaging ( aseptic packing )营养素 nutrient蛋白质 protein粗蛋白质 crude protein植物蛋白 vegetable protein动物蛋白 animal protein蛋白质营养学评价 nutritional evaluation蛋白质变性 protein denaturation蛋白质互补 complementary action of protein氨基酸 amino acid必需氨基酸 essential amino acid脂肪 fat (oil and fat)粗脂肪 crude fat脂肪酸 fatty acid饱和脂肪酸 saturated fatty acid不饱和脂肪酸 unsaturated fatty acid碳水化合物 carbohydrate有效碳水化合物 effective carbohydrate粗纤维 crude fiber膳食纤维 dietary fiber矿物质 mineral matter微量元素 trace element维生素 vitamin脂溶性维生素 liposoluble vitamin (fat soluble vitamin)水溶性维生素 water soluble vitamin每日推荐的营养素供给量(rda) recommended daily nutrient allowance水分 moisture content水分活度 water activity热量 calorie固形物 solid content可溶性固形物 soluble solid不溶性固形物 insoluble solid食品污染 food contamination生物性污染 biologic contamination化学性污染 chemical contamination放射性污染 radioactive contamination重金属 heavy metal微生物毒素 microbial toxin农药残留 residue of pesticide兽药残留 residue of veterinary drug食物中毒 food poisoning酸败 rancidity腐败 spoilage霉变 mould褐变 browning食物安全毒理学评价 toxicological evaluation for food safety 人体每日允许摄入量 (adi) acceptable daily intake (adi)食品感官检验 sensory analysis ( sensory evaluation)感官特性 organoleptic attribute食品理化检验 food physical and chemical analysis总糖 total reducing sugar还原糖 reducing sugar酸度 acidity总酸 total acid碘价 iodine value酸价 acid value过氧化值 peroxide value食品微生物学检验 food microbiological analysis 菌落总数 total plate count大肠菌群 coliform致病菌 pathogenic bacterium抗生素 antibiotic。



《食品专业英语》课程教学大纲一.课程代号:03072204二.课程名称:食品专业英语三.学分:3学分学时:52 理论学时:52 实验学时:0四.课程性质:食品科学与工程专业(本科)的公共限选课五.先修课程:食品微生物学、食品生物化学、酶学、食品工艺学、营养与卫生学、烹饪、食品分析六.教学目标、教学要求:通过本课程教学,应使学生掌握专业英语的专业词汇,掌握专业英语的翻译方法,掌握专业英语科技文献阅读的基本技能,以胜任学生未来工作和继续发展的要求。
本课程要求学生:1.重点掌握食品营养卫生学、食品微生物学、酶学、食品发酵工艺学方面的专业词汇;2.了解专业英语的基本翻译语法要点;3.掌握专业科技文献阅读的基本要求。
七.教学内容:1.Lesson 1 Nutrition (4学时)Nutrition and Food: Definition;The Nutrients;Recommended Dietry Allowances;Margin of Safety Allows for Individual Differences;Differences in Nutrient Utilization Considered.基本要求:(1)句子结构分析;(2)连词and的译法;(3)翻译要点的运用:增译和减译、词类转译、后置定语和后置定语从句的译法等。
重点:运用翻译语法要点翻译复杂句。
难点:复杂句子的结构分析。
2.Lesson 2 Carbohydrates (6学时)Sources, Types, and Terminology;Carbohydrate Composition of Food.基本要求:(1)掌握关于碳水化合物方面专业词汇的构词法,如后缀-ose, -ulose, -an和前缀-glyc等;(2)运用翻译要点翻译句子。
重点:构词法。
难点:翻译要点的运用:句子成分转译和被动语态。
3.翻译方法(8学时)(1)构词法(2)单词(3)词类转换的译法(4)句子成分转换的译法(5)被动语态的译法(6)后置定语从句的译法(7)专业英语的主要特点和翻译标准基本要求:熟悉各种翻译要点。

旗开得胜1nutrition 营养nourishment 食物,滋养品,营养情况 nutrient 营养素,营养物 odor 气味flavor 味,香味,风味,滋味;食用香料;食用香精;调料carbohydrate 碳水化合物,糖类 metabolism 新陈代谢,代谢(作用) intravenous feeding 静脉进食 vein 静脉,血管vital 生命的;生机的;维持生命所必需的 optimum .最适的macronutrient 主要营养素,常量营养素 micronutrient 微量营养ascorbic acid 抗坏血酸thiamine 硫胺素folacin 叶酸pyridoxine 吡哆醇,吡哆素,维生素B6 niacin 烟酸,尼克酸riboflavin 维生素B2,核黄素 pantothenic acid 泛酸 biotin 生物素维生素Hsodium 钠potassium 钾 phosphorus 磷lactate 分泌乳汁,喂奶,授乳.乳酸 ,乳酸盐[酯, 根]genetic 遗传学的,遗传上的,遗传基因的 infection.传染,侵染 ;传染病 ;影响,感染 chronic 长期的,慢性的 biotin.维生素H, 生物素 oxidation.氧化氧化[作用]conalbumin 伴清蛋白, 伴白蛋白 avidin 抗生物素蛋白 thiaminase. 硫胺酶 blanch 漂白, 使变白sterilization 灭菌, 消毒; 绝育消毒 asparagus 芦笋 retention 保持力 dehydrated 脱水的 snap bean 四季豆; 豆荚thaw.解冻fluorescent.荧光的 sulfur dioxide 二氧化硫amylase 淀粉酶; 液化酶; 淀粉糖化酶 carbohydrate 碳水化合物,糖类 cellulose 纤维素 fructose 果糖,左旋糖 galactose 半乳糖 glucose 葡萄糖, 右旋糖 hydrolysis 水解(作用) hemiacetal半缩醛isomers 同分异构体 lactose 乳糖 maltose 麦芽糖polymers 聚合物,多聚物 starch 淀粉 stereochemistry 立体化学 sucrose 蔗糖emulsion .乳浊液,乳胶,乳剂 fatty acid 脂肪酸triacylglycerol 甘油三酸酯三酰〔基〕甘油triglyceride 即甘油三酯 glycerol 甘油,丙三醇 phospholipid 磷脂 glyceride 甘油酯 tallow (炼制牛,羊)油脂linoleic acid 亚油酸,十八(碳)二烯-9,12-酸linolenic acid 亚麻酸,十八(碳)三烯-9.12.15-酸stearic acid 硬脂酸,十八(碳)(烷)酸 safflower 红花cholesterol 胆甾醇,胆固醇 diglyceride 甘油二酸酯 hydrogenate 氢化 pepper 胡椒; 辣椒; 花椒tactile 触觉的, 有触觉的, 能触知的 olfactory 嗅觉的neuron 神经细胞, 神经元 aldehyde 醛, 乙醛 ketone 酮 amine 胺heterocyclic 杂环的, 不同环式的 aromatic 芬芳的,芳香的 pyrazine 吡嗪, 对二氮杂苯, 胡椒嗪 threshold 阈; 阈值 chromatograph 色谱仪 isomerization 异构化(作用) lipolysis 脂解(作用)doughnut 油炸圈饼,.圆环图 hydroperoxide 氢过氧化物,过氧化氢物pentylfuran 戊基呋喃cottage scale bakery 作坊式规模的面包房plain flour 普通面粉 prove 使(面团)发起来cookery 烹任方法;烹调技术;烹调场所 bicarbonate of soda 碳酸氢钠 cream of tartar 酒石酸氢钾 baking powder 焙烤粉;发粉 feed on 以……为食;取……为养料 city water 城市给水;自来水air sifter with pneumatic conveyor 带气力输送系统的风筛机 jot 添加剂rounder 面团揉圆机;面团成圆机 the straight dough system 直接发酵面团制面包法bread improver 面包改良剂ferment in bulk 整块发酵;大容量发酵 knocked —back (发面过头后)揉紧回缩。


Text A CarbohydratesMany carbohydrate s(碳水化合物)can be expressed in the form [C x(H2O)y], but this is not an accurate description of their structure. Carbohydrates are infact polyhydroxy(多羟基)aldehyde s(醛)or ketone s (酮)and the term also includes any compounds that can be hydrolyzed to form carbohydrates. The simplest carbohydrates are called sugars (or monosaccharide s(单糖)) and these may link together to form more complex carbohydrates, starches (or polysaccharide s(多糖)).The diversity of dietary carbohydrates necessitates discussion of several classes of these molecules, ranging from simple sugars to huge, branched polymers.1.MonosaccharidesMonosaccharides or simple sugars are either hexose s (6-carbon) (六碳糖)like glucose(葡萄糖), galactose(半乳糖)and fructose(果糖), or pentose s (5-carbon) (五碳糖)like ribose (核糖). These are the breakdown products of more complex carbohydrates and can be efficiently absorbed across the wall of the digestive tube(消化道)and transported into blood. Simple sugars are monosaccharides.These are characterised by hydroxyl(羟基), aldehyde and ketone group s(功能团). Monosaccharides are either aldose s(醛糖)or ketose s(酮糖). The aldo- and keto- prefix(前缀)identifies the nature of the carbonyl(羰基,羰酰)group, and the –ose suffix(后缀)designates a carbohydrate.In Fischer projection s(费歇尔投影式), if the hydroxyl group at the lowest stereogenic centre(手相中心), C5in this case, points to the right, it may be labelled a D-sugar, if it points to the left, it is an L-sugar.The number of carbon atoms is also indicated, so for example, aldohexose is the six-carbon aldehyde sugar, more commonly referred to as glucose. Intramolecular(分子内), nucleophilic addition(亲核加成)can take place between a hydroxyl and a carbonyl group of the same molecule, resulting in the formation of a cyclic hemiacetal(半缩醛).Simple monosaccharides consist of aldehyde or ketone groups and both primary and secondary hydroxyl groups, all of which are available for reaction.An alkaline(碱的,强碱的)solution of copper(铜)(Fehling's solution (斐林试剂)) oxidise s(氧化)an aldose to an aldonate(醛糖盐), and is itself reduced to copper, precipitating (沉淀)as red Cu2O.Hydrogenation(氢化作用)of fructose, and carbohydrates in general, involves addition of hydrogen(氢)to the double bond(双键)of the carbonyl group. The resulting products have an –itol suffix to denote that they are sugar alcohols.Carbohydrate hydroxyl groups form esters on reactions with acid(酸,酸的)chloride s(氯化物)or anhydride s(酐)in the presence of base(碱). All of the hydroxyl groups react.Ether s(醚,醚类)may be formed by treatment of carbohydrates with alkylhalide s(烷基卤化物)in the presence of base.2.Disaccharides(二糖)Disaccharides are simply two monosaccharides linked together by a glycosidic bond(糖苷键). The disaccharides most important in nutrition and digestion are:∙lactose(乳糖)or "milk sugar": glucose + galactose∙sucrose(蔗糖)or "table sugar": glucose + fructose∙maltose(麦芽糖): glucose + glucoseOligosaccharides are relatively short chains of monosaccharides which typically are intermediate s(中间体)in the breakdown of polysaccharides to monosaccharides.3.PolysaccharidesPolysaccharides are the most abundant dietary carbohydrate for all except very young animals. You should be familiar with three important polysaccharides, each of which is a large polymer of glucose:∙Starch(淀粉)is a major plant storage form of glucose. It occurs in two forms: alpha-amylose(α-直链淀粉), in which the glucoses are linked together in straight chains, and amylopectin, in which the glucose chains are highly branched. Except for the branch points of amylopectin, the glucose monomer s(单体)in starch are linked via(通过)alpha(1->4) glycosidic bond s(α-1,4-糖苷键), which, in the digestive tract of mammal s(哺乳动物), are hydrolyzed by amylase s(淀粉酶).∙Cellulose(纤维素)is the other major plant carbohydrate. It is the major constituent of plant cell walls, and more than half of the organic carbon on earth is found in cellulose. Cellulose is composed on unbranched, linear chains of D-glucose molecules, linked to one another by beta(1->4) glycosidic bond s(β-1,4-糖苷键), which no vertebrate has the capacity to enzymatically digest. Herbivore s(食草动物) subsist largely on cellulose, not because they can digest it themselves, but because their digestive tracts teem with microbes that produce cellulases that hydrolyze cellulose.∙Glycogen(糖原)is the third large polymer of glucose and is the major animal storage carbohydrate. Like starch, the glucose molecules in glycogen are linked together by alpha(1->4) glycosidic bonds.Text B LipidsLipids are a family of naturally occurring compounds grouped together on the basis of their relative insolubility in water and solubility in nonpolar solvents. These are naturally occurring molecules that can be isolated from cells and tissues by extraction using nonpolar organic solvents.There are two classes of lipids:i) those with ester linkages that can be hydrolyzed, for example, fats and waxesii) those without ester linkages and so cannot be hydrolyzed, for example, cholesterol and steroids.Fats and oils are triacylglycerols. They are triesters of glycerol (propane-1,2,3-triol) with three long chain carboxylic acids. Reaction with aqueous sodium hydroxide yields glycerol and the three fatty acids.Fatty acids are usually unbranched and contain an even number of carbon atoms, usually from 12 to 20. If there are any double bonds, they tend to have cis geometry. Fats containing one double bond are monounsaturated and those with more than one double bond are polyunsaturated. Saturated fats have a uniform shape that allows them to pack together in a crystal lattice. Unsaturated fats have double bonds that introduce kinks into the hydrocarbon chain making crystal formation more difficult. This is why saturated fats have higher melting points and are solids at room temperature compared to unsaturated fats that tend to be liquids (oils).The most abundant lipid in olive oil is glyceryl trioleate, a triester formed from glycerol and oleic acid, a mono-unsaturated fatty acid with a cis double bond in the middle of the C18 chain.Waxes are mixtures of esters of long chain carboxylic acids and long chain alcohols. The carboxylic acids usually have an even number of carbon atoms, ranging from 16 to 36, and the long chain alcohols also have an even number of carbon atoms, ranging from 24-36.脂质脂质是一类不溶于水,但溶于非极性溶剂的天然化合物。

营养与食品卫生专业词汇2 Ccachexia 恶病质cadaverine 尸胺cadmium 镉cadmium carbonate 碳酸镉cadmium chlorate 氯酸镉cadmium hydroxide 氢氧化镉cadmium nitrate 硝酸镉cadmium sulfide 硫化镉caffeine 咖啡碱calciferol 钙化醇calcitonin,CT 降钙素calcium 钙caloric quotient 热能系数calorie 卡(路里)calorifacient 生热的,产热的calorimeter 测热计,热量计calorimetry 测热法Camplobacter fetus subsp.jejuni 胎儿弯曲菌空肠亚种Camplobacterjejuni 空肠弯曲菌Candida 假丝酵母,念珠菌属cannabidiol 大麻二酚cannabinol 大麻酚cannabis seed oil poisining 大麻子油中毒canned food 罐头食品capsanthin 辣椒红caramel 酱色,焦糖carbamate 氨基甲酸酯carbohydrate 碳水化物carbonic anhydrase 碳酸酐酶carcinogen 致癌物carcinogenicity test 致癌试验carithamine 红花黄色素carnitine 肉毒碱casein 酪蛋白cassava 木薯catabolism 分解代谢cation exchange resins 阳离子交换树脂cell transformation test 细胞转化试验cellulose 纤维素ceramics 陶瓷ceruloplasmin 血浆铜蓝蛋白cesium 铯chemical expansion 化学性膨胀(罐头) chemical engineering ,chemical industry,chemical technolog 化工chemical pollution 化学污染chemical prevention 化学防治Chlorella 小球藻属chlorinated hydrocarbon,organochlorine 氯化烃,有机氯chlorogenic acid 绿原酸cholesterol 胆固醇cholecal ciferol 维生素D3,胆钙化醇choline esterase 胆碱酯酶chromium 铬chromosome 染色体chromosome abberation 染色体畸变chromosome aberration assay test 染色体畸变分析试齄chronic mercury poisoning 慢性汞中毒chronic toxicity test 慢性毒性试验chylomicron 乳糜微粒cigarette 香烟Citreoviridin 黄绿青霉素citrinin 桔青霉素Cladosporium 枝孢属clam 蛤Clavus secalinus 麦角Claviceps purpurea Tulasne 麦角菌cleaner 洗涤剂cleaning 净化cleavage of phosphide ester linkage 磷酯键裂解cleft palate 腭裂Clitocybe 杯伞属Clostridium 芽胞杆菌属Clostridium botulinum 内毒杆菌Clostridium butylicum 丁酸杆菌Clostridium perfringens 产气荚膜杆菌coagulant 凝固剂coagulase 凝固酶cobalt 钴cobalt chloride 氯化钻coffee 咖啡colchicine 秋水仙碱cold frozen 冷冻cold press 冷榨cold sterilization 冷灭菌cold storage 冷藏coliform group 大肠菌群colitap 大肠菌快速检验纸片coli test 大肠杆菌试验colliod 胶体colloid system 胶体系统colonization factor antigen,CFA 定居因子抗原complementary action 互补作用concentrated milk 浓缩奶concentration factor 浓集系数condensed milk 炼乳congenital mercury poisoning 先天性汞中毒conglycinin 伴大豆球蛋白conjunctival dryness 结膜干燥contaminants in food 食品污染物contaminants of biological origin 生物性污染物contaminants of chemical origin 化学性污染物contaminants of radioactive origin 放射性污染物contamination 污染control organ of food hygiene 食品卫生监督机构control system of food hygiene 食品卫生监督制copper 铜corn cockle seed 麦仙翁籽corn soybean milk (CSM) 玉米大豆乳coronary heart disease 冠心病cowmilk 牛乳Coxsackie virus 柯沙奇病毒crayfish 蝲蛄critical porion 敏感期cumulative coefficient 蓄积系数cumulative toxicity index 蓄积毒性系数cumulative toxicity test 蓄积毒性试验Curcuma longa 姜黄curcumin 姜黄素β-cyanoalanine β-氰丙氨酸cyanogenetic glycoside plant poisoning 含氰甙植物中毒cyanogenetic glycoside 含氰甙cyanomethemoglobin 氰化正铁血红蛋白cycasin 苏铁素cyclamate 环己氨基磺酸盐cyclic N-nitroso compound 环状N-亚硝基化台钧cyclochlorotine 环氯素cysteine 半胱氨酸cystine 胱氨酸cytochrome 细胞色素cytochrome oxidase 细胞色素氧化酶Ddaily dietary allowance 每日膳食供给量Datura stramonium L. 蔓陀萝day blindness 昼盲(症)daylily 黄花莱,金针莱21-day survival index 21天哺育成活率2,4-D;2,4-dichlorophenoxyacetic acid 2,4-D; 2,4-二氯苯氧醋酸DDVP;dichlorvos;dichlorphos 敌敌畏dealkylation 脱烷基化作用deamination 脱氨基作用Debaryomyces 德巴利氏酵母属decimal reduction time (DRT) 90%递减时间diarrhea 腹泻difference between individuals 个体差异delayed neurotoxicity 迟发性神经毒作用delayed neurotoxicity test 迟发神经毒性试验delta-BHC δ-六六六Demeton,systox 内吸磷Density of energy 热能密度Density of nutrient 营养素密度dental plaque 斑牙deoxyadenosylcobalamin 腺苷钴胺酸Deoxynivalenol (DON) 脱氧雪腐镰刀菌醇descendant 子系体,后代Deuteromycetes (Imperfect fungi) 半知茵纲(不完全菌纲) dhurrin 蜀黍氰甙diabetes mellitus,DM 糖尿病diabetic ketoacidosis 糖尿病酮症酸中毒diacetoxyscirpenol 二醋酸熏草镰刀菌烯醇dialycerides 甘油二脂Diazinon 二嗪农dibenz(a,h)anthracene,DBA 二苯并(a,h)蒽dichloro-diphenyltrichloroethane;1,1,1-trichloro-2, 2-bis(P-chlorophenyl)ethan 滴滴涕(DDT) dientamoeba fragilis 脆双核阿米巴diet 膳食,饮食dietary 食谱,饮食的dietary history 饮食习惯dietary fiber 膳食纤维dietary guidelines 膳食指南dietary recall 膳食回顾dietary reference values,DRVs 膳食参考量difference between species 种间差异digestibility 消化率digestion 消化dihydrochalcone 二氢查耳酮dihydronivalenone 二氢雪腐烯酮dihydrosafrole 二氢黄樟素1,25-dihydroxycholecalciferol 1,25-二羟维生素D37,12-dimethylbenz(a)anthracene;7,12-DMBA 7,12-二甲基苯并(a)蒽dioxin 二噁英direct calorimetry 直接测热法discrimination factor 差异系数diterpene 双萜dithion 畜虫磷diverticulosis 憩室病DNA repair synthesis test DNA修复合成试验domestic animal 家畜dominant lethal test 显性致死试验dopamine-β-hydroxylase 多巴胺-β-羟化酶dose-effect(dose-response) relationship 剂量效应(反应)关系drogs 废渣dried bean curd 豆腐干Drosophila melanogaster recessive lethal test 果蝇隐性致死试验Drosophila recessive lethal assay 果蝇隐性致死试验duodenum 十二指肠dwarfism 矮小,侏儒症dystrophic hyperkeratosis 营养不良性角化过度。


extraction 提取(法),萃取(法),抽提法underlying 英[,ʌndə'laiiŋ]adj. 1.根本的, 基础的2.含蓄的, 潜在的,隐含的 3. 表面下的;下层的aldehyde ['ældihaid]醛aldoses['ældəus]醛糖ketone['ki:təun]酮ketoses['ki:təus]酮糖hemiacetal [,hemi'æsitæl] 半缩醛prevail vi.①胜(过),优胜,成功,奏效②流行,盛行,普遍~on 说服,劝说,诱导cyclic环状的designate vt.①指明,指出,标示②指定,选派③把…叫做,称呼append vt.①附加②贴上,挂上trioses ['traiəus]丙糖tetroses ['tetrəus]丁糖pentoses ['pentəus]戊糖hexoses ['heksəus]已糖levulose ['levjuləus]左旋糖,果糖hexulose ['heksjələus] 已酮糖,asymmetric[,æsɪ'metrɪk]不对称D-glycerol - D-甘油moiety ['mɔɪɪti:]n.①一半(尤用于法律方面);约一半②组成部分;一份(尤指政府给告密者的一份报酬) ③(部落的两个基本分支中)一个分支enolize使烯醇化enolase['i:nə,leis]烯醇化酶enolization[,enəlai'zeiʃən]烯醇化作用alkaline['ælkəlaɪn]碱的,强碱的alkali n ['ælkəlaɪ]碱,强碱monosaccharide [,mɔnəu'sækəraid]单糖disaccharide [dai'sækəraid]双糖condense冷凝,缩合,凝缩,浓缩oligo-and-polysaccharides 低聚糖-多聚糖oligo- 少,缺乏,[专]寡,低glucan ['ɡlu:kən] 葡聚糖(=glucosan['ɡlu:kəsæn]) fructan ['fræktən] 果聚糖inulin ['injulin]菊粉inulase['injuleis]菊粉酶phosphorylate['fɔsfərileit]磷酸化phosphorylase磷酸化酶nucleotide[' 'nju: kli:ə,taɪd]核苷酸nucleotidase 核苷酶nucleoside核苷nucleoprotein核蛋白derivative [di'rivətiv] 衍生物glycose单糖glycosides ['ɡlaikə,said] 糖苷,配糖物glycosan聚糖glycan ['ɡlaikæn] n.多糖,聚糖,多聚糖glyconic acids糖酸glyceric acids甘油酸glycuronic acid 糖醛酸homoglycan均聚糖hexosan['heksəsæn]已聚糖galactan[ɡə'læktin] 半乳聚糖mannan['mænæn]甘露聚糖pentosan['pentəsæn]戊聚糖heteroglycan [,hetərə'ɡlaikən] 杂聚糖diheteroglycan双杂聚糖aldaric acid 醛糖二酸tartaric [tɑ:'tærik]acid酒石酸reducing function还原性功能团alginpl藻酸algin ['ældʒin]①藻朊,海藻素[增稠剂] ②褐藻胶,藻酸,藻朊酸alginic a. ~acid 藻朊酸exudate ['eksjudeit]渗出物(液),流出物(液) mucopolysaccharide 粘多糖mucosity [mju:'kɔsiti]n.粘(性)mucus n.[生](由粘膜分泌的)粘液alcohol ['ælkə,hɔl]①醇,醇元②乙醇,酒精alditol ['ælditɔl] 多羟糖醇,醛醇triitol丙糖醇glycerol['glɪsə,rɔ:l]甘油,丙三醇sorbitol['sɔ:bitəl]山梨醇affinity [ə'fɪnɪti:]①亲合性②亲合势③爱好饮料(一种饮料名称)heptitol庚糖醇perseitol甘露庚糖醇octitol辛糖醇avocado[,ævə'kɑ:dəʊ]锷梨condensed N-acetylated amino sugar 缩聚N-乙酰氨基糖glycoprotein糖蛋白chitin ['kaitin] 壳多糖,甲壳质,几丁质deoxy deoxy- 脱氧gum ①植物胶,(树)胶②松脂③[复]胶质软糖mucilage ['mju:silidʒ]①粘浆,粘液②粘胶glucosinolate芥子油苷glycoside ['ɡlaikə,said] n.配醣,配糖类thioglycoside硫代糖苷thiol['θaiəul] (mercaptan [mə'kæptæn] ) 硫醇thioctic acid硫辛酸thioglucoside硫葡糖苷cyclitols环多醇myoinositol[,maiəui'nəusitɔl] 肌醇myogen ['maiədʒen] n.肌浆蛋白myology [mai'ɔlədʒi]n. 肌肉学phytic acid 植酸reductone 还原酮deplete[di'pli:t] vt.①(部分或全部地)弄空,使空虚,耗尽…的精力(或资源等) ②[医]减少…的体液,放去…的血date 海枣,枣椰子green pea豌豆green bean青刀豆raffinose['ræfinəus]棉籽糖stachyose['stækiəus]水苏糖verbascose ['və:bəskəus] 毛蕊花糖postmortem [pəʊst'mɔ:təm]宰后depolymerize [di:'pɔliməraiz] 解聚bouillon ['bu:jɔŋ]汤,羹(用肉,鸡等煮成) broth [brɔ:θ]肉汤(微生物的营养培养基) skim撇去,撇出(浮在上面的油脂,泡沫等)skim milk 脱脂乳legume ['leɡju:m]①荚(果);②豆科植物③豆荚,豆cruciferae[kru'sɪfə,ri] 十字花科malt [mɔ:lt] ①麦芽;②麦芽酒,啤酒nitrile['naitrail]腈goitrin甲状腺肿素goitrigenic甲状腺肿病源cauliflower花椰菜brussel sprouts 抱子甘蓝,北京牙菜turnip芜菁rutabagas芜菁甘蓝radish小萝卜cyanogenetic生氰, 能产生氰化物的cyanide氰化合物cyanidin花青素glycoside苷maceration 浸透,泡软,浸渍(作用) manioc ['mæniɔk] n.树薯cassava [kə'sɑ:və] n.木薯saponin皂角苷,皂素,皂缀糖steroid甾族化合物,类固醇triterpenoid [trai'tə:pinɔid] n. 三萜系化物asparagus石刁柏,芦笋,龙须菜yam薯蓣,山药,甘薯,山芋alfalfa紫花苜蓿[美],苜蓿。
Lesson 2 CarbohydratesThe food scientist has a many-sided interest in carbohydrates. He is concerned with their amounts in various foods, availability (nutritional and economic), methods of extraction and analysis, commercial forms and purity, nutritional value, physiological effects, and functional properties in foods. Understanding their functional properties in processed foods requires not only knowledge of the physical and chemical properties of isolated carbohydrates, but also knowledge of the reactions and interactions that occur in situs between carbohydrates and other food constituents and the effects of these changes upon food quality and acceptance. This is a tall order for knowledge. Because processing affects both nutritional and esthetic values of food, knowledge of the changes that carbohydrates undergo during milling, cooking, dehydration, freezing, and storage is especially important.Students are advised to study the fundamental chemistry underlying useful carbohydrates properties. Of service will be an understanding of the association of polar molecules through hydrogen bonding, ionic effects, substituent effects, chelation with inorganic ions, complexing with lipids and proteins, and decomposition reaction. This background will provide an understanding of properties that affect the texture and acceptance of processed foods (e.g., solubility, hygroscopicity, diffusion, osmosis, viscosity, plasticity, and flavor production), properties that enable the formation or high quality pastries, gels, coatings, confections, and reconstitutable dehydrated and frozen foods.Ability to predict what changes in functional properties are likely to ensue from incorporating various types of carbohydrates into processed foods is a practical goal of the food scientist. Such forecasting requires either a wealth of experience with trial-and-error methods or a deep knowledge of carbohydrate properties as related to structure—perhaps both. However, scientific knowledge of cause and effect is highly respected when it shortens industrial development time.Source, Types, and TerminologyThe layman‟s conception of carbohydrates generally involves only the sugars and starches of foods—those that generate calories and fat. The food chemist knows many other types that are ingested.Because most people enjoy the sweetness of sugars and the mouth feel of cooked starches, they become familiar by association with table sugar (sucrose), invert sugar(hydrolyzed sucrose), corn syrup sugars (D-glucose and maltose), milk sugar (lactose), and the more starchy foods. These carbohydrates are nutritionally available; i .e., they are digested (hydrolyzed to component monosaccharides) and utilized by the human body. Carbohydrates of dietary fiber (cellulose, hemicelluse, pentosans, and pectic substances), in contrast, tend to be overlooked because they are largely unavailable. Digestive enzymes do not hydrolyze them significantly; nevertheless, they may be quite important for human health.The carbohydrates of natural and processed foods are divided into available and unavailable types. The available carbohydrates vary in degrees of absorption and utilization depending upon quantities ingested, accompanying food types, and human differences in complements of defective enzymes and intestinal transport mechanisms. Malabsorption difficulties and adverse physiological effects are known for all the available carbohydrates but gelatinized starches give little or no trouble.It is important to realize that in ruminants the unavailable and most abundant polysaccharide cellulose is partially hydrolyzed to the same highly available sugar that starch provides upon digestion; i.e. D-glucose. Grazing animals do it through the cellulases generated by the microorganisms of their rumen. Cellulose is, therefore, a contributing source of valuble animal protein. T he efficiency and economics of the ruminant‟s conversion of cellulose to nutrients probably can improve upon by food chemists. Development of cellulases that are stable outside the cells of microorganisms enables the culturing of fungi and with yeasts on cellulose hydrolyzates. Fungi (e.g., mushrooms) can produce protein with the biological value of animal protein. The conversion of cellulose wastes to animal feed and human food is an intriguing prospect for limiting environmental pollution and for feeding the world‟s expending population.Carbohydrates were first named according to their natural sources; e.g., beet sugar, cane sugar, grape sugar, malt sugar, milk sugar, cornstarch, liver glycogen, and sweet corn glycogen. Trivial names were then formed, in English terminology, often from a prefix related to the source followed by the suffix “-ose” to denote carbohydrate. Names arising in this way, for example, are fructose, maltose, lactose, xylose, and cellulose. These short, well-established names are stillcommonly used. They furnish no information on the chemical structures, however, so a definitive carbohydrate nomenclature has been developed. From the definitive names, structural formulas can be written. Some of the terms involved in the definitive nomenclature are explained in the following paragraphs.The simple sugars (monosaccharides) are basically aliphatic polyhydroxy aldehydes and ketones: HOCH2- (CHOH) n-CHO and HOCH2- (CHOOH) n-1-C-O-Ch2OH, called “aldoses” and “ketoses,” respectively. However, it must be understood that cyclic hemiacetals of those open-chain forms prevail in solids and at equilibrium in solutions. In the definitive nomenclature, the suffix “ose” is appended to prefixes denoting the number of carbon atoms in the monosaccaride; e.g. trioses (n=1), tetroses (n=2), pentoses (n=3), hexoses (n=4) to distinguish aldoses from ketoses, ketoses are designated as”-uloses.” Thus, the simplest ketose, HOCH2-C:O-CH2OH, is a triulose; the most common ketose, D-fructose (levulose), is a hexulose. To designate the configurations of hydroxyl groups on the asymmetric carbon atoms of monosaccharides, the prefixes D and L are used together with prefixes derived from the trivial sugar names (e.g., D-glycero-, L-arabino-, D-xylo-) followed by pentose, hexose, hexulose, etc.As open-chain hydroxy aldehydes and hydroxyl ketones, the monosaccharides are very reactive. They readily enolize in alkaline solutions to reduce ions such as Cu2+and Fe(CN)63-. Therefore, they are called “reducing sugars”. Plants protect the reactive monosaccharides for transport and storage by condensing them with loss of water, into less reactive sugars, e.g., D-glucose and D-fructose, are condensing in such a way that their reactive functions are lost to form the disaccharide no reducing sugar, sucrose. The less reactive sucrose is then transported to all parts of the plant for enzymin syntheses of oligo-and polysaccharides. From thousands or more D-glucose moieties of sucrose the glucans, starch and cellulose, are built. From the D-fructose moiety of sucrose, fructans such as inulin are assembled. Other polysaccharides are formed from other sugar, which rose by enzymic transformations of phosphorylated hexoses and sugar nucleotides.The prefix “glyc,” is used in a generic sense to designate sugars and their derivatives; e.g., glycoses, glycosides, glycosans, glyconic, glyceric, and glycuronic acids. The generic name for polysaccharides is “glycan s”. Homoglycans are composed of single monosaccharide; for example,the D-glucans, cellulose and starch, release only D-glucose by hydrolysis. Other homoglycans (e.g., the hexosans, D-galactan and D-mannan, and the pentosans, L-arabinan and D-xylan) are uncommon in nature. Heteroglycans, composed of two or more different monosaccharides, are widely distributed than the homoglycans that are not glucans. Galactomnnans, glucomammans, arabinogalactans, and arabinoxylans are common diheteroglycans(composed of two sugars).the glycans prevail over free glycoses in natural foods.The reducing sugars are readily oxidized. Mild oxidation of aldoses yields aldonic acids, HOCH2-(CHOH)n-COOH; e.g., gluconic acid(n=4). Oxidation of both ends of the aldose molecule yields aldaric acids, HOOC-(CHOH)n-COOH; e.g., tartaric acid(n=2). Oxidation of the terminal CH2OH group of hexoses without oxidation of the reducing function (protected) produces hexuronic acids, HOOC-(CHOH)-CHO. The hexuronic acids are common monosaccharide constituents of many heteroglycans. For example, they are found in acidic hemicelluloses, pectic substances, algin and exudate gums, and the mucopolysaccharides of mammalian tissues. Penturonic acids have not been found in nature.Reduction of aldoses or ketoses yield sugar alcohols, properly called …alditols,” HOCH2-(CHOH)n-CH2OH. T he suffix “-itol “ is applied to the trivial prefixes to denote different alditols; e.g., D-glucitol, D-mannitol, xylitol. The triitol, glyceritol (by common usage, glycerol, n=1), is the alditol moiety of fats. Glycerol and D-glucitol(sorbitol) are acceptable and useful food additives because they are glucogenic and keep foods moist by virtue of their strong affinity for water. Pentitols(n=3) and hexitols(n=4) are found in small amount in many fruits, vegetables and mushrooms. The heptitol, perseitol (n=5), and an octitol have been isolated from avocados. Some aditols are nutritionally available; others are not.Other types of carbohydrates found in food are the condensed N-acetylated amino sugars of mucopolysaccharides, glycoproteins, and chitin; the condense deoxy sugars of gum, mucilages, and nucleotides; glcosides (sugars condensed with nonsugars); glucosinolates (toxic thioglycosides); cyclitols (myoinositol, phytic acid); and reductone, L-ascorbic acid.Complex carbohydrates, such as cellulose and hemicellulose, are largely indigestible, as are a number of oligosaccharides, certain other carbohydrates, gums, and fibrous matter found in foods of plant origins.Carbohydrate Composition of FoodsDieticians need more exact information on the carbohydrate compassion of foods. Food pressers also make practical use of carbohydrate composition data. For example, the reducing sugar content of fruits and vegetables that are to be dehydrated or processed with heat is frequently an indicator of the extent of nonenzymic browing that can expected during processing and storage. The possible hydrolysis of sucrose to reducing sugars during processing also is to be considered .the natural changes in carbohydrate composition that occur during maturation and post harvest ripening of plant foods is therefore of particular interest to food chemists.Citrus fruits, which normally ripen on the tree and contain no starch, undergo little change in carbohydrate composition following harvest. However, in fruit that are picked before complete ripening (e.g., apples, bananas, pears), much of the stored starch is converted to sugars as ripening process. The reducing sugar content of potatoes also increases during cold storage. According to the activity of endogenous invertase during the sun drying of grapes and dates, sucrose is converted to D-glucose and D-fructose; accordingly, the color of the dried products is deepened by nonenzymic browning reactions.Green peas, green beans, and sweet corn are picked before maturity to obtain succulent textures and sweetness. Later the sugars would be converted to polysaccharides, water would be lost, and tough textures would develop. In soybean, which is allowed to mature completely before harvest, the starch reserve is depleted as sucrose and galactosyl sucroses (raffinose, stachyose, verbascose, etc.) are form in the malting of cereal grains, rapid conversions of reserve carbohydrate to sugars occur as enzymes are strongly activated.In foods of animal origin, postmortem activity of enzymes must be considered when carbohydrate composition data is obtained. The glycogen of animal tissues, especially liver is rapidly depolymerized to D-glucose after slaughter, and immediate deep freezing is required to preserve the glycogen. Mammalian internal organs, such as liver, kidney, and brains also eggs and shellfish, provide small amount of D-glucose in the diet .Red fresh meats contain only traces of available carbohydrate (D-glucose, D-fructose, and D-ribose) and these are extracted into bouillons and broths. Dairy products provide the main source of mammalian carbohydrate. Whole cow‟s milk contains about 4.9% carbohydrates an d dried skim milk contains over 50% lactose.Examination of food composition tables shows that in general, cereals are highest in starch content and lowest in sugars. Fruit are highest in free sugars and lowest in starch .On a dry basis, the edible portions of fruit usually contain 80-90% carbohydrate. Legumes occupy intermediate portion with regard to starch and are high in unavailable carbohydrate.Glycosides of many types are widely distributed in plants. Certain biologically active thioglucosides, pr operly called “glucosinolates”, are found in significant amount in crucifers. Mustard oils, nitriles, and goitrins are released by enzymic hydrolysis. Their suspected goitrogenic in humans have been investigated, but the amount of glucosnolates normally consumed in food such as fresh cabbage (300-1000ppm), cauliflower, Brussels sprouts, turning, rutabagas, and radishes are not now believed to cause adverse physiological effects. Cyanogenetic glycosides, which release hydrogen cyanide by enzymic hydrolysis under certain condition of vegetable maceration, are known to be sources of acute toxicity in certain animal feeds; however they are not active in the customary foods of developed countries. Certain foreign varieties of lima beans and manioc root (cassava) may yield up to 0.3% hydrogen cyanide by weight, but lima beans distributed in the United States yield less than 0.02%. Saponins, the surface-active glycosides of steroids and triterpenoids, are found in low concentrations in tealeaves, spinach, asparagus, beets sugar beet (0.3%), yams, soybeans (0.5%), alfalfa (2-3%), and peanuts and other legumes.。
饮食科普类作文英语Title: The Fundamentals of Nutrition: A Comprehensive Guide。
Introduction:Nutrition is the cornerstone of a healthy lifestyle, influencing our overall well-being and longevity. With the abundance of information available, it's crucial to understand the fundamentals of nutrition to make informed choices about our diet. This article will delve into the essential aspects of nutrition, covering macronutrients, micronutrients, dietary guidelines, and common misconceptions.Macronutrients:Macronutrients are the primary components of our diet, providing the energy and building blocks necessary for bodily functions. They include carbohydrates, proteins, andfats.1. Carbohydrates:Carbohydrates are the body's primary source of energy, particularly for high-intensity activities. They are foundin foods like grains, fruits, vegetables, and legumes. It's essential to choose complex carbohydrates, such as whole grains and vegetables, over simple sugars to maintainstable blood sugar levels and promote sustained energy.2. Proteins:Proteins are crucial for building and repairing tissues, supporting immune function, and synthesizing hormones and enzymes. Good sources of protein include lean meats, poultry, fish, eggs, dairy products, legumes, nuts, and seeds. It's important to consume a variety of protein sources to ensure adequate intake of essential amino acids.3. Fats:Fats play a vital role in maintaining cell structure, insulating organs, and absorbing fat-soluble vitamins. Healthy fat sources include avocados, nuts, seeds, olive oil, fatty fish, and coconut oil. While fats are calorie-dense, opting for unsaturated fats over saturated and trans fats can help reduce the risk of heart disease and promote overall health.Micronutrients:Micronutrients are essential vitamins and minerals that are required in smaller quantities but are equallyimportant for various physiological functions.1. Vitamins:Vitamins are organic compounds that play critical roles in metabolism, immune function, and overall health. Theycan be obtained from a diverse diet rich in fruits, vegetables, whole grains, lean proteins, and dairy products. Examples include vitamin A, vitamin C, vitamin D, vitamin E, and the B-complex vitamins.2. Minerals:Minerals are inorganic elements essential for bone health, fluid balance, nerve function, and muscle contraction. Common minerals include calcium, magnesium, potassium, iron, zinc, and selenium. Consuming a balanced diet with plenty of fruits, vegetables, whole grains, and nuts/seeds ensures adequate mineral intake.Dietary Guidelines:Incorporating the following dietary guidelines into your lifestyle can promote optimal health and well-being:1. Eat a variety of foods from all food groups to ensure a diverse nutrient intake.2. Limit processed foods, sugary beverages, and excessive salt intake.3. Practice portion control to maintain a healthyweight.4. Stay hydrated by drinking plenty of water throughout the day.5. Be mindful of portion sizes and listen to yourbody's hunger and fullness cues.6. Balance energy intake with physical activity to support overall health and weight management.Common Misconceptions:Despite the wealth of information available, several misconceptions about nutrition persist:1. Myth: All fats are bad for you.Fact: While saturated and trans fats should be limited, unsaturated fats are essential for health and should be included in moderation.2. Myth: Carbohydrates are the enemy of weight loss.Fact: Choosing complex carbohydrates and controlling portion sizes can support weight loss and overall health.3. Myth: Supplements can replace a healthy diet.Fact: While supplements can be beneficial for addressing specific nutrient deficiencies, they should not be used as a substitute for a balanced diet rich in whole foods.Conclusion:Understanding the fundamentals of nutrition is essential for making informed choices about our diet and promoting optimal health and well-being. By focusing on macronutrients, micronutrients, following dietary guidelines, and dispelling common misconceptions, we can cultivate a balanced and nutritious eating pattern that supports our overall health for years to come.。