美国药典USP31(921)翻译版(下)
- 格式:docx
- 大小:44.50 KB
- 文档页数:8

美国药典-中英文对照译文美国药典中记载的辣椒碱资料辣椒碱(辣椒素)分子结构式:C18H27NO3,分子量:305.41,化学名:(反)-N-[(4-N-羟基-3-甲氧基苯基)-甲基]-8-甲基-6-壬烯基酰胺以干燥提取物计算,辣椒碱含辣椒二萜类化合物总量为标示量的90%-100%,其中辣椒素的含量达到50%以上,辣椒素和二氢辣椒素总量超过75%,其它辣椒素类化合物总量不足15%。
注意事项:小心处置辣椒碱,谨防吸入辣椒碱微粒,勿使身体接触辣椒碱。
包装贮藏:密封包装,置避光,阴凉处保存。
标示量:以辣椒二萜类化合物总百分含量表示。
美国药典参考标准:美国药典辣椒素标准规范,美国药典二氢辣椒素标准规范。
鉴别:配制1.0mg/ml辣椒碱甲醇溶液,配制符合美国药典标准的辣椒碱1.0mg/ml甲醇溶液作为对照液,分别点样于0.25mm厚硅胶、凝胶混合薄层板上,点样量为10礚,将薄层板放于乙醚-甲醇(19:1)展开剂中展开,待展开剂前沿至薄层板3/4处时将薄层板取出,晾干,用0.5% 2,6-二溴苯醌-氯化亚胺甲醇溶液喷雾显色,放于氨气中片刻,取出,鉴别色谱图:供试液主要斑点颜色(兰色)及R值与对照液主要斑点颜色(兰色)及R值一致。
熔点〈741〉: 57°-66°, 一般熔融起始温度至结束温度温差不超过5°。
干燥失重〈731〉: 置40°P2O5真空干燥器中干燥5小时,失重不超过1.0%。
灼烧残渣:≤1.0%。
辣椒素,二氢辣椒素及其它辣椒二萜类化合物含量测定:流动相:磷酸水溶液(l :1000,V/V):乙腈(600:400)混匀,0.5祄微孔滤膜滤过,脱气。
流动相视色谱行为可作适当调整。
辣椒素对照液:精密称取美国药典标准的辣椒碱适量溶于甲醇中,配制约0.1 mg/mL的辣椒甲醇溶液。
二氢辣椒素对照液:精密称取美国药典标准的辣椒碱适量溶于甲醇中,配制约0.025mg/mL的辣椒甲醇溶液。

美国药典USP31无菌检查71 STERILITY TESTS 无菌检查法Portions of this general chapter have been harmonized with the corresponding texts of the European Pharmacopeia and/or the Japanese Pharmacopeia. Those portions that are not harmonized are marked with symbols () to specify this fact.此通则的各部分差不多与欧洲药典和/或日本药典的对应部分做了和谐。
不一致的部分用符号()来标明。
The following procedures are applicable for determining whether a Pharmacopeial article purporting to be sterile complies with the requirements set forth in the individual monograph with respect to the test for sterility. Pharmacopeial articles are to be tested by the Membrane Filtration method under Test for Sterility of the Product to be Examined where the nature of the product permits. If the membrane filtration technique is unsuitable, use the Direct Inoculation of the Culture Medium method under Test for Sterility of the Product to be Examined. All devices, with the exception of Devices with Pathways Labeled Sterile, are tested using the Direct Inoculation of the Culture Medium method. Provisions for retesting are included under Observation and Interpretation of Results.下面这些步骤适用于测定是否某个用于无菌用途的药品是否符合其具体的各论中关于无菌检查的要求。

美国药典USP31无菌检查简介美国药典(United States Pharmacopeia,简称USP)是美国公认的医药行业标准组织,负责制定和发布有关医药品质量的标准和方法。
无菌检查是USP31中的一个重要章节,它主要用于评估药品、医疗器械和其他与患者直接接触的产品的无菌状态。
本文将介绍无菌检查的定义、方法和注意事项。
无菌检查的定义无菌检查是指对药品或医疗器械进行的一系列检验,以确定其是否存在可繁殖的微生物,从而判断其无菌性。
无菌状态是指在没有任何活体微生物存在的情况下。
对于注射剂、眼药水等需要直接进入体内的产品,无菌状态非常重要,以防止微生物感染和其他不良反应的发生。
无菌检查方法无菌检查的方法有多种,常用的方法包括:厌氧培养方法这种方法用于检测生长于缺氧条件下的微生物。
样品通常放置在厌氧培养基中,然后在恒温条件下培养,观察是否有菌落的形成。
高压蒸气灭菌法这种方法通过将样品暴露在高温高压的环境中,使用蒸汽来灭活可能存在的微生物。
灭菌后,用无菌培养基接种样品,并在一段时间后观察是否有菌落的形成。
过滤方法这种方法通过使用无菌过滤器来过滤样品,将可能存在的微生物滤除。
过滤后,将过滤膜接种在无菌培养基上,观察是否有菌落的形成。
光谱学方法这种方法通过使用紫外线、红外线等光谱技术来检测微生物的存在。
由于微生物具有特有的光谱特征,因此可以通过光谱仪器来进行无菌检查。
注意事项在进行无菌检查时,需要注意以下事项:检测设备的无菌性检测设备(培养皿、培养基等)在使用前应进行无菌处理,以避免外源性微生物的干扰。
常用的处理方法包括高温蒸馏、紫外线照射等。
样品的收集和处理在收集样品时,应注意避免样品与外界环境的接触,以防止样品被外源性微生物污染。
在处理样品时,应采取无菌操作,避免微生物的传播。
结果的解读在进行无菌检查后,需要对结果进行解读。
有菌落的形成通常表示样品存在微生物污染,是不符合无菌要求的。
无菌检查的结果应与相应的标准进行比较,以确定样品是否合格。

美国药典-中英文对照译文美国药典中记载的辣椒碱资料辣椒碱(辣椒素)分子结构式:C18H27NO3,分子量:305.41,化学名:(反)-N-[(4-N-羟基-3-甲氧基苯基)-甲基]-8-甲基-6-壬烯基酰胺以干燥提取物计算,辣椒碱含辣椒二萜类化合物总量为标示量的90%-100%,其中辣椒素的含量达到50%以上,辣椒素和二氢辣椒素总量超过75%,其它辣椒素类化合物总量不足15%。
注意事项:小心处置辣椒碱,谨防吸入辣椒碱微粒,勿使身体接触辣椒碱。
包装贮藏:密封包装,置避光,阴凉处保存。
标示量:以辣椒二萜类化合物总百分含量表示。
美国药典参考标准:美国药典辣椒素标准规范,美国药典二氢辣椒素标准规范。
鉴别:配制1.0mg/ml辣椒碱甲醇溶液,配制符合美国药典标准的辣椒碱1.0mg/ml甲醇溶液作为对照液,分别点样于0.25mm厚硅胶、凝胶混合薄层板上,点样量为10礚,将薄层板放于乙醚-甲醇(19:1)展开剂中展开,待展开剂前沿至薄层板3/4处时将薄层板取出,晾干,用0.5% 2,6-二溴苯醌-氯化亚胺甲醇溶液喷雾显色,放于氨气中片刻,取出,鉴别色谱图:供试液主要斑点颜色(兰色)及R值与对照液主要斑点颜色(兰色)及R值一致。
熔点〈741〉: 57°-66°, 一般熔融起始温度至结束温度温差不超过5°。
干燥失重〈731〉: 置40°P2O5真空干燥器中干燥5小时,失重不超过1.0%。
灼烧残渣:≤1.0%。
辣椒素,二氢辣椒素及其它辣椒二萜类化合物含量测定:流动相:磷酸水溶液(l :1000,V/V):乙腈(600:400)混匀,0.5祄微孔滤膜滤过,脱气。
流动相视色谱行为可作适当调整。
辣椒素对照液:精密称取美国药典标准的辣椒碱适量溶于甲醇中,配制约0.1 mg/mL的辣椒甲醇溶液。
二氢辣椒素对照液:精密称取美国药典标准的辣椒碱适量溶于甲醇中,配制约0.025mg/mL的辣椒甲醇溶液。

Many Pharmacopeial articles either are hydrates or contain water in adsorbed form. As a result, the determination of the water content is important in demonstrating compliance with the Pharmacopeial standards. Generally one of the methods given below is called for in the individual monograph, depending upon the nature of the article. In rare cases, a choice is allowed between two methods. When the article contains water of hydration, the Method I (Titrimetric), the Method II (Azeotropic), or the Method III (Gravimetric) is employed, as directed in the individual monograph, and the requirement is given under the heading Water.很多药典物品要么是水合物,要么含有处于吸附状态的水。
因此,测定水分含量对于证实与药典标准的符合性是很重要的。
通常,在具体的各论中会根据该物品的性质,要求使用下面若干方法中的一个。
偶尔,会允许在2个方法中任选一个。
当该物品含有水合状态的水,按照具体各论中的规定,使用方法I(滴定测量法)、方法II(恒沸测量法)、或方法III(重量分析法),这个要求在标题水分项下给出。
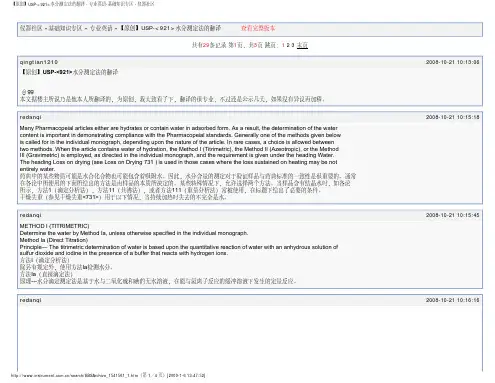
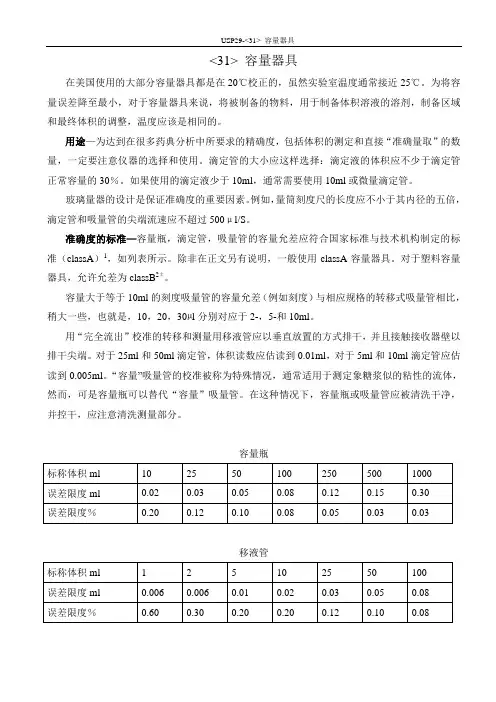
USP29-<31> 容量器具<31> 容量器具在美国使用的大部分容量器具都是在20℃校正的,虽然实验室温度通常接近25℃。
为将容量误差降至最小,对于容量器具来说,将被制备的物料,用于制备体积溶液的溶剂,制备区域和最终体积的调整,温度应该是相同的。
用途—为达到在很多药典分析中所要求的精确度,包括体积的测定和直接“准确量取”的数量,一定要注意仪器的选择和使用。
滴定管的大小应这样选择:滴定液的体积应不少于滴定管正常容量的30%。
如果使用的滴定液少于10ml,通常需要使用10ml或微量滴定管。
玻璃量器的设计是保证准确度的重要因素。
例如,量筒刻度尺的长度应不小于其内径的五倍,滴定管和吸量管的尖端流速应不超过500μl/S。
准确度的标准—容量瓶,滴定管,吸量管的容量允差应符合国家标准与技术机构制定的标准(classA)1,如列表所示。
除非在正文另有说明,一般使用classA容量器具。
对于塑料容量器具,允许允差为classB2±。
容量大于等于10ml的刻度吸量管的容量允差(例如刻度)与相应规格的转移式吸量管相比,稍大一些,也就是,10,20,30µl分别对应于2-,5-和10ml。
用“完全流出”校准的转移和测量用移液管应以垂直放置的方式排干,并且接触接收器壁以排干尖端。
对于25ml和50ml滴定管,体积读数应估读到0.01ml,对于5ml和10ml滴定管应估读到0.005ml。
“容量”吸量管的校准被称为特殊情况,通常适用于测定象糖浆似的粘性的流体,然而,可是容量瓶可以替代“容量”吸量管。
在这种情况下,容量瓶或吸量管应被清洗干净,并控干,应注意清洗测量部分。
容量瓶移液管USP29-<31>容量器具第2页共2页滴定管1. 参看“玻璃量器检定”,N.B.S.Circ.602,April 1,1959, NTISCOM-73-10504,国家技术信息2. 参看ASTME288,Fed.Spec.NNN-F-289,和ISO标准384。

美国药典USP31-NF26无菌检查法《71》.doc71 STERILITY TESTS 无菌检查法Portions of this general chapter have been harmonized with the corresponding texts of the European Pharmacopeia and/or the Japanese Pharmacopeia. Those portions that are not harmonized are marked with symbols () to specify this fact.此通则的各部分已经与欧洲药典和/或日本药典的对应部分做了协调。
不一致的部分用符号()来标明。
The following procedures are applicable for determining whether a Pharmacopeial article purporting to be sterile complies with the requirements set forth in the individual monograph with respect to the test for sterility. Pharmacopeial articles are to be tested by the Membrane Filtration method under Test for Sterility of the Product to be Examined where the nature of the product permits. If the membrane filtration technique is unsuitable, use the Direct Inoculation of the Culture Medium method under Test for Sterility of the Product to be Examined. All devices, with the exception of Devices with Pathways Labeled Sterile, are tested using the Direct Inoculation of the Culture Medium method. Provisions for retesting are included under Observation and Interpretation of Results.下面这些步骤适用于测定是否某个用于无菌用途的药品是否符合其具体的各论中关于无菌检查的要求。
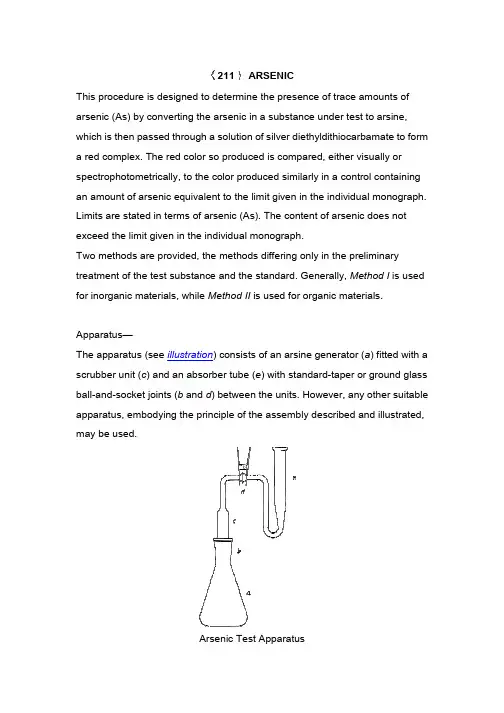
This procedure is designe arsenic (As) by converting which is then passed throu a red complex. The red co spectrophotometrically, to an amount of arsenic equi Limits are stated in terms exceed the limit given in th Two methods are provided treatment of the test subst for inorganic materials, whApparatus—The apparatus (see illustra scrubber unit (c) and an ab ball-and-socket joints ( apparatus, embodying the may be used. 211ARSENICsigned to determine the presence of trace amo erting the arsenic in a substance under test to through a solution of silver diethyldithiocarbam ed color so produced is compared, either visu ly, to the color produced similarly in a control c equivalent to the limit given in the individual m erms of arsenic (As). The content of arsenic do n in the individual monograph.ovided, the methods differing only in the prelim substance and the standard. Generally, Metho s, while Method II is used for organic materlustration) consists of an arsine generator (an absorber tube (e) with standard-taper or grb and d) between the units. However, any ot g the principle of the assembly described andArsenic Test ApparatusArsenic Trioxide Stock So previously dried at 105 hydroxide solution (1 in 5) solution with 2 N sulfuric a recently boiled and cooledStandard Arsenic Solution Solution to a 1000-mL volu add recently boiled and co Arsenic Solution contains solution in an all-glass conStandard Preparation—generator flask, and dilute Test Preparation— Unless transfer to the generator fl calculated by the formula:in which L is the arsenic lim 35 mL.Procedure— Treat the Sta as follows. Add 20 mL of 7 mL of stronger acid stanno mix. Allow to stand at room tube (c) with two pledgets acetate solution, freed from vacuum at room temperatuic trioxide,n 5 mL of sodium tralize theacid, then addxide Stockuric acid, thenh mL of Standard s). Keep thisution into amonograph, bstanceute with water toparation similarly iodide TS, 0.5 pyl alcohol, and the scrubber saturated leadd dried inthe two pledgets.Lubricate the joints (b and d) with a suitable stopcock grease designed for use with organic solvents, and connect the scrubber unit to the absorber tube (e). Transfer 3.0 mL of silver diethyldithiocarbamate TS to the absorber tube. Add 3.0 g of granular zinc (No. 20 mesh) to the mixture in the flask, immediately connect the assembled scrubber unit, and allow the evolution of hydrogen and the color development to proceed at room temperature for 45 minutes, swirling the flask gently at 10-minute intervals. Disconnect the absorber tube from the generator and scrubber units, and transfer the absorbing solution to a 1-cm absorption cell. Any red color produced by the Test Preparation does not exceed that produced by the Standard Preparation. If necessary or desirable, determine the absorbance at the wavelength of maximum absorbance between 535 and 540 nm, with a suitable spectrophotometer or colorimeter, using silver diethyldithiocarbamate TS as the blank.Interfering Chemicals— Metals or salts of metals, such as chromium, cobalt, copper, mercury, molybdenum, nickel, palladium, and silver, may interfere with the evolution of arsine. Antimony, which forms stibine, produces a positive interference in the color development with silver diethyldithiocarbamate TS; when the presence of antimony is suspected, the red colors produced in the two silver diethyldithiocarbamate solutions may be compared at the wavelength of maximum absorbance between 535 and 540 nm, with a suitable colorimeter, since at this wavelength the interference due to stibine is negligible.METHOD IINOTES—(1) Caution—Some substances may react with explosive violence when digested with hydrogen peroxide. Exercise safety precautions at all times. (2) If halogen-containing compounds are present, use a lower temperature while heating the test specimen with sulfuric acid, avoid boiling the mixture,and add the hydrogen pero loss of trivalent arsenic.(3) If the test substance re sulfuric acid before heating in 2), and add a few drops Standard Preparation—generator flask, add 2 mL percent hydrogen peroxide mixture to strong fuming, c to strong fumes. Repeat th any traces of hydrogen pe Test Preparation— Unless transfer to a generator flas by the formula:in which L is the arsenic lim beads, and digest in a fum temperature not exceeding may be necessary to wet s added should not exceed hydrogen peroxide, allowin between drops. Add the fir order to prevent a rapid re excessive. When the reac occasionally to prevent the heating unit. Maintain oxid adding small quantities of mixture turns brown or dar is destroyed, gradually rais n peroxide with caution, before charring begins nic.ce reacts too rapidly and begins charring with eating, use instead 10 mL of cooled dilute sulf rops of the hydrogen peroxide before heating — Pipet 3.0 mL of Standard Arsenic Solution 2 mL of sulfuric acid, mix, and add the total am roxide used in preparing the Test Preparation ing, cool, add cautiously 10 mL of water, and eat this procedure with another 10 mL of wate en peroxide. Cool, and dilute with water to 35 m nless otherwise directed in the individual mon or flask the quantity, in g, of the test substance 3.0 / Lnic limit in ppm. Add 5 mL of sulfuric acid and a fume hood, preferably on a hot plate and at eding 120, until charring begins. (Additional s wet some specimens completely, but the tota ceed 10 mL.) Cautiously add, dropwise, 30 per llowing the reaction to subside and again hea the first few drops very slowly with sufficient m pid reaction. Discontinue heating if foaming be reaction has abated, heat cautiously, rotating nt the specimen from caking on glass exposed n oxidizing conditions at all times during the dig es of the hydrogen peroxide solution wheneve or darkens. Continue the digestion until the org ly raising the temperature of the hot plate unti egins, to prevent g with 5 mL of e sulfuric acid (1 eating.ution into aal amount of 30 ation . Heat the and again heat water to remove o 35 mL. monograph, tance calculated d and a few glass nd at aonal sulfuric acid e total volume 30 percent n heatingent mixing, in ng becomes ating the flask posed to the he digestion by never thehe organic matter e until fumes ofsulfur trioxide are copiously evolved, and the solution becomes colorless or retains only a light straw color. Cool, add cautiously 10 mL of water, mix, and again evaporate to strong fuming, repeating this procedure to remove any trace of hydrogen peroxide. Cool, add cautiously 10 mL of water, wash the sides of the flask with a few mL of water, and dilute with water to 35 mL. Procedure— Proceed as directed for Procedure under Method I.Interfering Chemicals— See Interfering Chemicals under Method I.Auxiliary Information— Staff Liaison : Kahkashan Zaidi, Ph.D., Senior Scientist Expert Committee : (GC05) General Chapters 05USP31–NF26 Page 131Phone Number : 1-301-816-8269本过程用于确定微量的后将其通过二乙基二硫代氨觉对照或者风光光度计两种限量的砷盐的红色。

921WATER DETERMINATION水分测定Many Pharmacopeial articles either are hydrates or contain water in adsorbed form. As a result, the determination of the water content is important in demonstrating compliance with the Pharmacopeial standards. Generally one of the methods given below is called for in the individual monograph, depending upon the nature of the article. In rare cases, a choice is allowed between two methods. When the article contains water of hydration, the Method I (Titrimetric), the Method II (Azeotropic), or the Method III (Gravimetric) is employed, as directed in the individual monograph, and the requirement is given under the heading Water.很多药典物品要么是水合物,要么含有处于吸附状态的水。
因此,测定水分含量对于证实与药典标准的符合性是很重要的。
通常,在具体的各论中会根据该物品的性质,要求使用下面若干方法中的一个。
偶尔,会允许在2个方法中任选一个。
当该物品含有水合状态的水,按照具体各论中的规定,使用方法I(滴定测量法)、方法II(恒沸测量法)、或方法III(重量分析法),这个要求在标题水分项下给出。

氨苄西林钠Ampicillin Sodium(USP31-NF26第1420页)C16H18N3NaO4S 371.39按无水物计算,氨苄西林钠的效价以氨苄西林(C16H19N3O4S)计,每1mg 应为845µg~988µg。
[包装和贮存]贮存在密闭容器中。
[标贴] 本品用于制备注射剂时,标贴上应注明本品无菌或本品在制备注射剂过程中须经进一步的处理。
[USP标准物质] <11> USP氨苄西林RS、USP氨苄西林钠RS、USP内毒素RS。
[鉴别] A:红外光吸收图谱<179M>。
B:显钠盐反应<191>。
[结晶性] <695> 应符合要求。
(注-如为冻干粉则可不检此项)[pH] <791> 取本品,加水制成每1mL中含10.0mg氨苄西林的溶液,振摇使溶解,pH值应为8.0~10.0。
[水分] <921> 不得过2.0%。
方法Ⅰ。
[二甲基苯胺] <223> 应符合要求。
内标溶液、对照品溶液、供试品溶液的制备方法如下:内标溶液:取N,N-二乙基苯胺75mg,加1mol/L盐酸溶液使溶解,用水逐步稀释制成每1mL中约含30µg的溶液。
对照品溶液:取N,N-二甲基苯胺50.0mg,精密称定,置50mL量瓶中,加1mol/L盐酸溶液25mL振摇混匀后,用水稀释至刻度,摇匀,精密量取2mL,置100mL量瓶中,用水稀释至刻度,摇匀,精密量取1mL,置适宜的离心管中,精密加入1.25mol/L氢氧化钠溶液、内标溶液和环己烷各1mL,强烈振摇1分钟然后离心,取上清液,作为对照品溶液。
供试品溶液:取本品1.0g,精密称定,置适宜的离心管中,加1.25mol/L 氢氧化钠溶液2mL,振摇使溶解,精密加入内标溶液1mL和环己烷1mL,强烈振摇1分钟然后离心,取上清液,作为供试品溶液。
色谱系统(见色谱法<621>):气相色谱仪应配有火焰离子化检测器和2mm×2m的填充有上涂3%液相G3的硅烷化S1A的色谱柱。
621CHROMATOGRAPHY色谱法INTRODUCTION介绍This chapter defines the terms and procedures used in chromatography and provides general information. Specific requirements for chromatographic procedures for drug substances and dosage forms, including adsorbent and developing solvents, are given in the individual monographs.此章节定义了色谱法中用到的术语和步骤,并提供了通用信息。
对于原料药和成药的色谱步骤的具体要求,包括吸附剂和展开溶剂,在具体各论中给出。
Chromatography is defined as a procedure by which solutes are separated by a dynamic differential migration process in a system consisting of two or more phases, one of which moves continuously in a given direction and in which the individual substances exhibit different mobilities by reason of differences in adsorption, partition, solubility, vapor pressure, molecular size, or ionic charge density. The individual substances thus separated can be identified or determined by analytical procedures.色谱法是应用溶质在两相或多相系统中的差速迁移来进行分离的技术,其中一相持续地向特定方向移动,而由于物质在吸附性、分配、溶解性、气体压力、分子大小、或离子电荷密度上的差异,会显示出不同的移动性。
921 WATER DETERMINATION水分测定很多药典物品要么是水合物,要么含有处于吸附状态的水。
因此,测定水分含量对于证实与药典标准的符合性是很重要的。
通常,在具体的各论中会根据该物品的性质,要求使用下面若干方法中的一个。
偶尔,会允许在2个方法中任选一个。
当该物品含有水合状态的水,按照具体各论中的规定,使用方法I (滴定测量法)、方法II(恒沸测量法)、或方法III(重量分析法),这个要求在标题水分项下给出。
The heading Loss on drying (see ) is used in those cases where the loss sustained on heating may be not entirely water.在加热时的持续失重可能不全是水分的情况下,使用标题干燥失重(见干燥失重<731>)。
METHOD I (TITRIMETRIC) 方法I(滴定测量法)Determine the water by , unless otherwise specified in the individual monograph.除非具体各论中另有规定,使用方法Ia来测定水分。
Method Ia (Direct Titration) 方法Ia(直接滴定)Principle— The titrimetric determination of water is based upon the quantitative reaction of water with an anhydrous solution of sulfur dioxide and iodine in the presence of a buffer that reacts with hydrogen ions.原理:水分的滴定法检测是基于水与二氧化硫的无水溶液以及存在于缓冲液中与氢离子反应的碘之间的定量反应。
制药用水(USP31-NF28附录)在生产、加工、配制药品(Compendial articles)时,水是最为广泛使用的物质、原料或成份。
这就要对这类水进行微生物质量控制。
因为在水的纯化、贮存和分配过程中,水中的微生物可能发生增殖。
如水是用在最终成品,这些微生物或其代谢产物必然会引起不良结果。
如水用在生产药物早期阶段,以及作为制备各种不同类别纯水的进水,则必须符合环境保护局(Environmental Protection Agency EPA)发布的国家基本饮用水规定(National Primary Drinking Water Regulations NPDWR)(40CFR141)。
欧洲共同体或日本的有关饮用水规定也可适用。
这些规定保证水中不存在大肠杆菌(coliforms)。
如经确定该菌来自粪便,则可预示或标示可能有粪便源的其他微生物,包括病毒,对人可能是致病的。
另一方面,符合国家饮用水标准并不排除有其他微生物的存在。
这类微生物对公众卫生健康不是一件重大事件,但是如果存在的话,可能对药物或制剂产品构成危害或被认为不应有的。
由于这些原因,药用水有许多不同级别。
水的类别饮用水(Drinking Water)——在药学中未有专论涉及饮用水这一品种,但必须符合EPA NPDWR的质量标准或者符合欧共体或日本的相类似的规定。
饮用水可来自不同的水源,包括公用水生产设施,私人供水设施(如井),或者是混合供水源。
在化学合成的早期阶段以及早期阶段的药品生产设备的清洗可用饮用水,饮用水是生产药用水的规定水源,饮用水的质量可随季节变化而变化,药用水的生产工序设计时必须考虑这一特点。
纯水(Purified Water)——纯水(用USP纯水)是生产法定制剂的一个辅料,用于某些设备的清洗;以及用于一些原料药的制备。
纯水必须符合离子和有机化学纯度的规定要求而且必须防止微生物的增殖。
纯水的制备是用饮用水作为进水的,是用单元操作方法,如去离子化、蒸馏、离子交换、反渗透、过滤或其他合适的方法纯在常规条件下生产,贮存和循环的纯水系统是易于形成粘着的微生物膜,这种膜可能是出水中活的微生物或内毒素量超过标准的根源。
Method Ib (Residual Titration) 方法Ib(残留滴定)Principle— See the information given in the section Principle under Method Ia. In the residual titration, excess Reagent is added to the test specimen, sufficient time is allowed for the reaction to reach completion, and the unconsumed Reagent is titrated with a standard solution of water in a solvent such as methanol. The residual titration procedure is applicable generally and avoids the difficulties that may be encountered in the direct titration of substances from which the bound water is released slowly.原理:见方法Ia项下原理部分给出的信息。
在残留滴定中,额外的试剂被加入到供试样品中,为反应的完成留下了充分的时间,并且将未消耗掉的试剂与水和某种溶剂(例如,甲醇)的标准溶液一起滴定。
残留滴定程序通常是可行的,并避免了可能在直接滴定该物质过程中遇到的困难,这些物质中被束缚水分释放得很缓慢。
Apparatus, Reagent, and Test Preparation— Use Method Ia.仪器、试剂、供试配制液:同方法Ia。
Standardization of Water Solution for Residual Titration— Prepare a Water Solution by diluting 2 mL of water with methanol or other suitable solvent to 1000 mL. Standardize this solution by titrating 25.0 mL with the Reagent, previously standardized as directed under Standardization of the Reagent. Calculate the water content, in mg per mL, of the Water Solution taken by the formula:用于残留滴定的水溶液的标准化:以甲醇或其他适当溶剂将2mL水稀释至1000mL,以配制水溶液。
美国药典USP31 71 无菌检查法中文版美国药典USP31-NF26无菌检查法《71》.doc71 STERILITY TESTS 无菌检查法此通则的各部分已经与欧洲药典和/或日本药典的对应部分做了协调。
不一致的部分用符号()来标明。
下面这些步骤适用于测定是否某个用于无菌用途的药品是否符合其具体的各论中关于无菌检查的要求。
只要其性质许可,这些药品将使用供试产品无菌检查法项下的膜过滤法来检测。
如果膜过滤技术是不适合的,则使用在供试产品无菌检查法项下的培养基直接接种法。
除了具有标记为无菌通道的设备之外,所有的设备均须使用培养基直接接种法进行检测。
在结果的观测与理解项下包含了复验的规定。
由于无菌检查法是一个非常精确的程序,在此过程中程序的无菌状态必须得到确保以实现对结果的正确理解,因此人员经过适当的培训并取得资质是非常重要的。
无菌检查在无菌条件下进行。
为了实现这样的条件,试验环境必须调整到适合进行无菌检查的方式。
为避免污染而采取的特定预防措施应不会对任何试图在检查中发现的微生物产生影响。
通过在工作区域作适当取样并进行适当控制,来定期监测进行此试验的工作条件。
这些药典规定程序自身的设计不能确保一批产品无菌或已经灭菌。
这主要是通过灭菌工艺或者无菌操作程序的验证来完成。
当通过适当的药典方法获得了某物品中微生物污染的证据,这样获得的结果是该物品未能达到无菌检验要求的结论性证据,即便使用替代程序得到了不同的结果也无法否定此结果。
如要获得关于无菌检验的其他信息,见药品的灭菌和无菌保证<1211>按照下面描述的方法配制实验用培养基;或者使用脱水培养基,只要根据其制造商或者分销商说明进行恢复之后,其能够符合好氧菌、厌氧菌、霉菌生长促进试验的要求即可。
使用经过验证的工艺对培养基进行灭菌操作。
下面的培养基已经被证实适合进行无菌检查。
巯基醋酸盐液体培养基主要用于厌氧菌的培养。
但其也用于检测好氧菌。
大豆酪蛋白消化物培养基适合于培养霉菌和好氧菌。
美国药典USP31 71 无菌检查法中文版美国药典USP31-NF26无菌检查法《71》.doc71 STERILITY TESTS 无菌检查法此通则的各部分已经与欧洲药典和/或日本药典的对应部分做了协调。
不一致的部分用符号()来标明。
下面这些步骤适用于测定是否某个用于无菌用途的药品是否符合其具体的各论中关于无菌检查的要求。
只要其性质许可,这些药品将使用供试产品无菌检查法项下的膜过滤法来检测。
如果膜过滤技术是不适合的,则使用在供试产品无菌检查法项下的培养基直接接种法。
除了具有标记为无菌通道的设备之外,所有的设备均须使用培养基直接接种法进行检测。
在结果的观测与理解项下包含了复验的规定。
由于无菌检查法是一个非常精确的程序,在此过程中程序的无菌状态必须得到确保以实现对结果的正确理解,因此人员经过适当的培训并取得资质是非常重要的。
无菌检查在无菌条件下进行。
为了实现这样的条件,试验环境必须调整到适合进行无菌检查的方式。
为避免污染而采取的特定预防措施应不会对任何试图在检查中发现的微生物产生影响。
通过在工作区域作适当取样并进行适当控制,来定期监测进行此试验的工作条件。
这些药典规定程序自身的设计不能确保一批产品无菌或已经灭菌。
这主要是通过灭菌工艺或者无菌操作程序的验证来完成。
当通过适当的药典方法获得了某物品中微生物污染的证据,这样获得的结果是该物品未能达到无菌检验要求的结论性证据,即便使用替代程序得到了不同的结果也无法否定此结果。
如要获得关于无菌检验的其他信息,见药品的灭菌和无菌保证<1211>按照下面描述的方法配制实验用培养基;或者使用脱水培养基,只要根据其制造商或者分销商说明进行恢复之后,其能够符合好氧菌、厌氧菌、霉菌生长促进试验的要求即可。
使用经过验证的工艺对培养基进行灭菌操作。
下面的培养基已经被证实适合进行无菌检查。
巯基醋酸盐液体培养基主要用于厌氧菌的培养。
但其也用于检测好氧菌。
大豆酪蛋白消化物培养基适合于培养霉菌和好氧菌。
Method Ib (Residual Titration) 方法Ib(残留滴定)Principle— See the information given in the section Principle under Method Ia. In the residual titration, excess Reagent is added to the test specimen, sufficient time is allowed for the reaction to reach completion, and the unconsumed Reagent is titrated with a standard solution of water in a solvent such as methanol. The residual titration procedure is applicable generally and avoids the difficulties that may be encountered in the direct titration of substances from which the bound water is released slowly.原理:见方法Ia项下原理部分给出的信息。
在残留滴定中,额外的试剂被加入到供试样品中,为反应的完成留下了充分的时间,并且将未消耗掉的试剂与水和某种溶剂(例如,甲醇)的标准溶液一起滴定。
残留滴定程序通常是可行的,并避免了可能在直接滴定该物质过程中遇到的困难,这些物质中被束缚水分释放得很缓慢。
Apparatus, Reagent, and Test Preparation— Use Method Ia.仪器、试剂、供试配制液:同方法Ia。
Standardization of Water Solution for Residual Titration— Prepare a Water Solution by diluting 2 mL of water with methanol or other suitable solvent to 1000 mL. Standardize this solution by titrating 25.0 mL with the Reagent, previously standardized as directed under Standardization of the Reagent. Calculate the water content, in mg per mL, of the Water Solution taken by the formula:用于残留滴定的水溶液的标准化:以甲醇或其他适当溶剂将2mL水稀释至1000mL,以配制水溶液。
使用此前已经按照试剂的标准化项下规定进行过标准化的试剂,对25mL此溶液进行滴定,从而对其进行标准化。
按照下面的公式,计算此水溶液中的水分含量(单位mg/mL):V F/25,in which V' is the volume of the Reagent consumed, and F is the water equivalence factor of the Reagent. Determine the water content of the Water Solution weekly, and standardize the Reagent against it periodically as needed.其中,V'是消耗掉的试剂,F是试剂的水平衡因子。
每周测定水溶液的水分含量,并据此根据需要定期对试剂进行标准化。
Procedure— Where the individual monograph specifies that the water content is to be determined by Method Ib, transfer 35 to 40 mL of methanol or other suitable solvent to the titration vessel, and titrate with the Reagent to the electrometric or visual endpoint. Quickly add the Test Preparation, mix, and add an accurately measured excess of the Reagent. Allow sufficient time for the reaction to reach completion, and titrate the unconsumed Reagent with standardized Water Solution to the electrometric or visual endpoint. Calculate the water content of the specimen, in mg, taken by the formula:步骤:当具体各论中规定用方法Ib测定水分含量时,将35至40mL甲醇或其他适当溶剂转移至该滴定容器,并用试剂滴定至测电法或视觉观察的终点。
快速加入供试配制品,混匀,并加入精确称量的额外试剂。
留下充分的时间以使该反应完成,使用标准化的水溶液对未消耗的试剂进行滴定至测电法或视觉观察的终点。
按照下面的公式,计算样品中的水分含量(单位mg):F(X'XR),in which F is the water equivalence factor of the Reagent; X' is the volume, in mL, of the Reagent added after introduction of the specimen; X is the volume, in mL, of standardized Water Solution required to neutralize the unconsumed Reagent; and R is the ratio, V'/25 (mL Reagent/mL Water Solution), determined from the Standardization of Water Solution for Residual Titration.其中,F是试剂的水平衡因子;X'是在放入样品后加入的试剂体积(单位mL);X是用于中和未消耗试剂所必需的已标准化水溶液的体积(单位mL);R是通过用于残留滴定的水溶液的标准化来测定的,V /25的比值(mL试剂/mL水溶液)。
Method Ic (Coulometric Titration) 方法Ic(库仑滴定)Principle— The Karl Fischer reaction is used in the coulometric determination of water. Iodine, however, is not added in the form of a volumetric solution but is produced in an iodide-containing solution by anodic oxidation. The reaction cell usually consists of a large anode compartment and a small cathode compartment that are separated by a diaphragm. Other suitable types of reaction cells (e.g., without diaphragms) may also be used. Each compartment has a platinum electrode that conducts current through the cell. Iodine, which is produced at the anode electrode, immediately reacts with water present in the compartment. When all the water has been consumed, an excess of iodine occurs, which usually is detected electrometrically, thus indicating the endpoint. Moisture is eliminated from the system by pre-electrolysis. Changing the Karl Fischer solution after each determination is not necessary since individual determinations can be carried out in succession in the same reagent solution.A requirement for this method is that each component of the test specimen is compatible with the other components, and no side reactions take place. Samples are usually transferred into the vessel as solutions by means of injection through a septum. Gases can be introduced into the cell by means of a suitable gas inlet tube. Precision in the method is predominantly governed by the extent to which atmospheric moisture is excluded from the system; thus, the introduction of solids into the cell is not recommended, unless elaborate precautions are taken, such as working in a glove-box in an atmosphere of dry inert gas. Control of the system may be monitored by measuring the amount of baseline drift. This method is particularly suited to chemically inert substances like hydrocarbons, alcohols, and ethers. In comparison with the volumetric Karl Fischer titration, coulometry is a micro-method.原理:库仑滴定法水分测定应用了卡尔·费休反应的原理。