PAHs欧盟资料整理规范标准2014版
- 格式:doc
- 大小:99.03 KB
- 文档页数:12

Product Safety Commission (AfPS)GS SpecificationTesting and assessment of polycyclic aromatic hydrocarbons (PAHs) in the course of awarding the GS mark- Specification pursuant to article 21(1) no. 3 of the Product Safety Act (ProdSG) –AfPS GS 2014:01 PAKState of revision: August 4th, 2014Disclaimer: G erman i s t he o riginal t ext v ersion. I n c ase o f a ny d oubt, l ack o f c larity o r a ny o ther n on clear interpretation the content of the original version is valid.Management: Federal Institute for Occupational Safety and HealthFriedrich-Henkel-Weg 1 – 2544149 DortmundTelefon: 0231/9071-0Telefax: 0231/9071-2364 '.Table of ContentsPreliminary observations (3)1 Purpose / Intention (3)2 Basics (3)3 Procedure (3)3.1 Risk assessment (3)3.2 Categorisation (4)3.3 Testing and assessment (6)4 Transitional regulations/periods (6)4.1 GS mark certificates, issued from July 1st, 2015 onwards (6)4.2 GS mark certificates, issued before July 1st, 2015 (6)4.3 Reissuing of existing GS mark certificates – exemptions (6)Annex: Testing instructions (8)1 Aim and purpose (8)2 Method (8)2.1 Brief description (8)2.2 Equipment (8)2.3 Chemicals and solutions (8)3 Preparation and execution (9)3.1 Sample preparation (9)3.2 Measuring procedure (10)3.3 Special characteristics (11)Annex: Measuring conditions for gas chromatography (for information) (12)Testing and assessment of polycyclic aromatic hydrocarbons (PAHs) in the course of awarding the GS markPreliminary observationsOn Aug. 4th 2014 the Product Safety Commission (AfPS) has assigned the requirements of PAH testing in the course of GS mark certification as specification according to art. 21 Product Safety Act (ProdSG) para. 1 no. 3. The implementation is achieved by means of this PAH document.1. Purpose / IntentionProducts (pursuant to the Product Safety Act) must comply with legal requirements to avoid health risks, e.g. art. 30 & 31 of the LFBG (Foodstuffs, Consumer Goods and Feedstuffs Code –LFGB), the Prohibition of Chemicals Ordinance, and the art. 3 of the ProdSG (Product Safety Act). This document and, in particular, the testing instructions (see Annex) specify the requirements with respect to the level of PAHs in products. In addition, the document harmonises the testing methodology for assessment by GS bodies.2. BasicsPAH contamination of materials is primarily due to the use of:- PAH contaminated softening oils in rubber and flexible (soft) plastics- PAH-contaminated carbon black as a black pigment in rubber, plastics and paints.PAH contamination has previously been detected not only in rubber but also in various types of plastic, e.g. ABS and PP, and various paints/coatings, as well as in a variety of natural materials.3. ProcedureThe GS body must take the following steps into account both in the process of awarding a new GS mark and within the framework of monitoring existing GS mark certificates:1. Risk assessment2. Categorisation3. Testing and assessment3.1 Risk assessmentThe GS body must carry out a risk assessment and, in doing so, define which relevant contact/grip and operating surfaces of the product are to be considered for testing and which are not, and must make a record of these (this means that the GS body must first specify the contact/grip and operating areas to which the requirements of the PAH document must be applied (specification of PAH relevance)). The risk assessment should be not applied, where appropriate, if the respecti ve “Erfahrungsaustauschkreis” (“Exchange of Experience Group”, EK) has already defined a procedure for the product or product group with regard to the contact/gripand operating surfaces to be tested. A reference to the EK’s definition is to be included in the documentation accordingly.Materials that cannot be accessed or that can only be accessed by using tools need not be assessed, with the exception of samples with a conspicuous odour.In principle, account must be taken of all contact/grip and operating surfaces that can come into direct contact with the skin or that can be put into the mouth during proper or foreseeable use (but not misuse).13.2 CategorisationDepending on the results of the risk assessment, the corresponding product parts are then to be categorised (see Table 1) and to be analysed on their actual PAH content according to the analysis method below. Existing test reports can be taken into account if they are compliant with the “Grundsatzbeschluss” (“principle decision”) ZEK-GB-2012-01 of the ZEK (“Central Exchange of Experience Group”) and the requirements of this PAH document. Categorisation can be dispensed with if the respective ”Erfahrungsaustauschkreis” (“Exchange of Experience Group”, EK) has already defined a categorisation of the contact/grip and operating surfaces for a product or product group. Definitions for products or product groups from the individual EKs are published on the ZLS website2 and apply from the time of publication.Table 1 presents the maximum levels of PAH in product materials, which must not be exceeded. The provisions of this document with regard to the PAH content do not apply if other legislation lays down corresponding or further requirements for the PAH content. This applies only to the material or component/assembly and not to the product as a whole. Materials and parts of the product that are not covered by other legislative provisions must be assessed within the framework of the procedure for awarding the GS mark in accordance with the requirements of the PAH document.It must be ensured that the method of testing can actually achieve the limit of quantification of 0.2 mg/kg for each individual PAH component3.At the same time, method and matrix effects, as well as the measurement uncertainty, the efficiency of extraction and losses during purification must be considered.Based on the findings of the United States Environmental Protection Agency (EPA) (according to the list in the ZEK document 04-11), the total of 18 PAHs (extended substance list of the AtAV, the predecessor committee of the AfPS) only considers PAH components whose level in the material is found to exceed 0.2 mg/kg.1 However, in order to ensure a consistent and appropriate procedure during the awarding of GS mark, it is not generally necessary to analyse all freelyaccessible surfaces. It is this document’s intention to limit the consideration to relevant contact/grip and operating surfac es. It is not expedient to test all product parts or surfaces in order “to be on the safe side”.2 Homepage of ZLS (Central Authority of the Laender for Safety):3 Example: Water-carrying parts in coffee machines that come into contact with foodstuffs (e.g. water, etc.) are subject to the Food and Feed Code legislation and are therefore excl uded from the PAH document’s field of application. However, grip surfaces on the coffee machine must still be assessed accord ing to the requirements of the PAH document.Table 1: Maximum PAH levels to be complied with for the materials in relevant contact/grip and operating surfaces that are to be categorised based on the results of the risk assessment.* Wording “short-term repetitive skin contact” from supplement to REACH annex XVII no. 50 (REGULATION (EU) No 1272/2013)3.3 Testing and assessmentThe testing instructions found in the annex describe the steps of sample preparation, extraction of the PAHs, purification of the extract, identification and quantification; these must be applied uniformly by all laboratories carrying out testing.The GS body assesses the test results and decides whether the GS mark can be awarded in compliance with the other requirements.4. Transitional regulations/periodsFrom July 1st 2015 (issue date of the GS mark certificate), it is compulsory to apply this document when awarding the GS mark to products.The document ZEK 01.4-08 will cease to be valid from June 30th 2015.Since testing for PAH levels in products constitutes an overarching requirement for almost all of the members of all of the ”Erfahrungsaustauschkreise” (“Exchange of Experience Groups”), the following procedure is defined:4.1 GS mark certificates, issued from July 1st 2015 onwards(incl. ongoing procedures that are concluded after July 1st 2015)Compulsory application of this GS specification from July 1st 2015 onwards (exception: see 4.3).4.2 GS mark certificates, issued before July 1st2015Existing GS mark certificates initially remain valid.Within the framework of regular checks to monitor the manufacturing process (at the latest within one year or, in cases where the regular monitoring period is two years, within two years), the requirements under no. 3 of the ZEK (“Central Exchange of Experience Group”) document with regard to the risk assessment must be taken into account, regardless of whether the product was found in the manufacturing facility or not. If, in the process, it is found that the corresponding requirements are not met, the GS mark certificate must be withdrawn immediately. T he ZEK ”Grundsatzbeschluss” (“principle decision”) ZEK-GB-2006-01 must be complied with.4.3 Reissuing of existing GS mark certificates – exemptionsFor the reissuing of an existing GS mark certificate, immediate consideration is not required in the following circumstances:If the company name is changed, new GS mark certificates are usually issued. However, since the product does not change in terms of construction or other properties, and the reissuing of the GS mark certificate is more or less a pure formality, it is not necessary to consider the requirements of the PAH decision until the time of the check which must be performed for the purposes of monitoring the manufacturing process.(Please note: The reissuing of the GS mark certificate does not alter the previously defined periods for carrying out checks on the product’s manufacturing process.)The same applies by analogy when the holder of the GS mark certificate changes address provided none of the product’s properties change and the product does not r equire an additional safety check.The above procedure can also be applied in relation to duplicate certificates (also referred to as OEM certificates). In such cases, a review pursuant to the requirements of the PAH document must strictly be carried out by the time of the next product-manufacturing check according to the periods already defined in the “main certificate” or, at the latest, by December 28th 2015. Monitoring intervals beyond this date are not permissible in such cases.With regard to the OE M certificates and therefore also the “main certificates”, the PAH document – as specified – must be applied by December 28th 2015 at the latest.Annex: Testing instructionsHarmonised method for the determination of polycyclic aromatic hydrocarbons (PAHs) in polymers1 Aim and purposeDetermination of polycyclic aromatic hydrocarbons (PAHs) in polymer samples.2 Method2.1 Brief description2.1.1 Standard methodA representative partial sample is taken of the material and cut up into pieces with a maximum size of 2–3 mm using scissors, wire cutters, etc. Then, 500 mg of the sample is weighed into a container and extracted with 20 ml of toluene (to which an internal standard has been added) for 1 h at 60 °C in an ultrasonic bath. An aliquot is taken from the extract once it has cooled down to room temperature. In the case of polymers (e.g. plastics or rubber products) for which matrix problems arise during the analysis, an additional purification step is carried out using column chromatography. Quantification is performed on a gas chromatograph with a mass-selective detector (GC/MSD) using the SIM method.2.1.2 Method for insufficient quantitiesIf the total mass of material to be analysed is less than 500 mg, one should proceed as follows: Identical materials from the product can be combined and considered as one sample. However, additional product specimens must not be used.If less than 50 mg of material is available for individual samples, these are not tested.If the available mass of chopped-up material is between 50 mg and 500 mg, the sample must be tested according to 2.1.1 and the quantity of toluene converted or adapted in proportion. The actual mass of the sample is to be recorded in the test report accordingly.2.2 Equipment•Ultrasonic bath with a minimum power of 200 W and a bath area of 706 cm2, corresponding to 0.28 W/cm2, without a basket and with an internal or external thermostat•Gas chromatograph with a mass-selective detector2.3 Chemicals and solutions2.3.1 Chemicals•Toluene•Internal standardso Standard 1: Naphthalene-d8o Standard 2: Pyrene-d10 or anthracene-d10 or phenanthrene-d10o Standard 3: Benzo[a]pyrene-d12 or perylene-d12 or triphenylbenzeneAt least three internal standards must be used; these are added to the extraction solvent (toluene).•External standard: 18 PAH substances according to Table 1 or those listed under no. 3.2, as a mix or individually•Petroleum ether•Silica gel•Sodium sulfate2.3.2 Calibration solutionsThe concentrations of the calibration solutions must be chosen so that a three-point calibration covers a working range of 0.1 to 10 mg/kg in the samples. This corresponds to a concentration range of 2.5 to 250 ng/ml in the calibration solutions.3 Preparation and execution3.1 Sample preparationA representative partial sample is taken of the material. The fragments produced by chopping up the samples to be analysed (using scissors, wire cutters, pliers, etc.) should have a maximum size of 2 – 3 mm.3.1.1 Extraction500 mg of the sample is placed in a glass ”Bördelglas” (“vial”). To this 20 ml of tolue ne, previously amended with internal standards, are added. The sample is then extracted for 1 h in the ultrasonic bath at a temperature of 60 °C throughout. For this purpose, the vials are placed or suspended in the ultrasonic bath without using a basket. The vials are then removed, the extract is left to cool to room temperature and shaken briefly, and an aliquot is taken from the extract and measured either directly or following dilution with toluene.3.1.2 Column chromatography extraction stepFor some polymers (e.g. plastic or rubber products), especially those that dissolve well in toluene under the described extraction conditions, it is necessary to clean the extract using adsorption chromatography on silica gel.For this purpose, a clean-up column with “Hahnschliff” (“stopcock”) (approx. 220 mm x 15 mm) is filled with glass wool, 4 g of silica gel and 1 cm of sodium sulfate.The silica gel is deactivated beforehand by adding 10% water (the corresponding volume of water is added to the silica gel in a glass flask, and the mixture is homogenised on the rotaryevaporator for 1 h at standard pressure and room temperature. The silica gel can then be stored in the sealed glass flask at room temperature).The packed column is conditioned with 10 ml of petroleum ether.The aliquot of toluene extract is then evaporated to a volume of approx. 1 ml on the rotary evaporator and poured into the column. The pointed flask is rinsed out with approx. 20 ml of eluent, which is then also transferred to the clean-up column. Elution is performed with 50 ml of petroleum ether. The collected petroleum ether eluate is amended with 1 ml of toluene and evaporated to a volume of approx. 1 ml under a nitrogen stream (e.g. on the TurboVap). This is then made up to a defined volume with toluene, and the extract is analysed by GC-MS.3.2 Measuring procedureThe method of determination to be applied is gas chromatography with a mass-selective detector in the SIM mode.The following 18 PAHs are to be determined:•Naphthalene•Acenaphthylene•Acenaphthene•Fluorene•Phenanthrene•Anthracene•Fluoranthene•Pyrene•Chrysene•Benzo[a]anthracene•Benzo[b]fluoranthene•Benzo[k]fluoranthene•Benzo[j]fluoranthene•Benzo[a]pyrene•Benzo[e]pyrene•Indeno[1,2,3-cd]pyrene•Dibenzo[a,h]anthracene•Benzo[g,h,i]perylene3.2.1 Measuring conditions for gas chromatographyThe equipment parameters (temperatures, columns, mass traces) may be chosen by the individual laboratory or are determined by the analytes.3.2.2 AnalysisInternal standards: at least three internal standards must be used. For these three standards, the internal standards and the correction ranges are defined as followed:Parameter Internal standards with recommended reference•Naphthalene Naphthalene-d8•Acenaphthylene Pyrene-d10 or anthracene-d10 or phenanthrene-d10 •Acenaphthene Pyrene-d10 or anthracene-d10 or phenanthrene-d10 •Fluorene Pyrene-d10 or anthracene-d10 or phenanthrene-d10 •Phenanthrene Pyrene-d10 or anthracene-d10 or phenanthrene-d10 •Anthracene Pyrene-d10 or anthracene-d10 or phenanthrene-d10 •Fluoranthene Pyrene-d10 or anthracene-d10 or phenanthrene-d10•Pyrene Pyrene-d10 or anthracene-d10 or phenanthrene-d10•Benzo[a]anthracene Pyrene-d10 or anthracene-d10 or phenanthrene-d10 •Chrysene Pyrene-d10 or anthracene-d10 or phenanthrene-d10•Benzo[b]fluoranthene Benzo[a]pyrene-d12 or perylene-d12 or triphenylbenzene •Benzo[k]fluoranthene Benzo[a]pyrene-d12 or perylene-d12 or triphenylbenzene •Benzo[j]fluoranthene Benzo[a]pyrene-d12 or perylene-d12 or triphenylbenzene •Benzo[a]pyrene Benzo[a]pyrene-d12 or perylene-d12 or triphenylbenzene •Benzo[e]pyrene Benzo[a]pyrene-d12 or perylene-d12 or triphenylbenzene •Indeno[1,2,3-cd]pyrene Benzo[a]pyrene-d12 or perylene-d12 or triphenylbenzene •Dibenzo[a,h]anthracene Benzo[a]pyrene-d12 or perylene-d12 or triphenylbenzene •Benzo[g,h,i]perylene Benzo[a]pyrene-d12 or perylene-d12 or triphenylbenzene•External calibration: for each individual PAH, at least a three-point calibration must be carried out with reference to the internal standardisation set out above. A working range of 0.1 to 10 mg/kg is recommended here.•Concentrations above the calibration range can be determined by diluting the extract.3.2.3 Limit of quantificationThe limit of quantification for material samples is 0.2 mg/kg per parameter.3.3 Special characteristicsBecause it is relatively volatile compared to the other 17 PAHs, naphthalene constitutes a parameter that is hard to assess in products that come into contact with the skin. Experience from the testing institutes indicates that it is possible to identify instances of both naphthalene depletion in materials and secondary contamination. The result obtained for naphthalene therefore only ever reflects the test specimen’s current situation at the time of measurement.Annex: Measuring conditions for gas chromatography (for information) Injected volume: 1 µl pulsed splitlessColumn: HT8 25m, ID 0.22mm, film thickness: 0.25µmInjector temperature: 280°CTransfer-line temperature: 260°CInitial temperature: 50°CInitial time: 2 min Heatingrate: 11°C/min Finaltemperature: 320°C Finaltime: 8 min3,418 D-naphthalene8,186 D-phenanthrene23,182 D-benzo[a]pyrene3,459 Naphthalene5,586 Acenaphthylene5,845 Acenaphthene6,634 Fluorene8,235 Phenanthrene8,337 Anthracene11,217 Fluoranthene11,914 Pyrene16,830 Benzo(a)anthracene16,982 Chrysene21,860 Benzo(b+j)fluoranthene21,964 Benzo(k)fluoranthene23,055 Benzo(e)pyrene23,302 Benzo(a)pyrene27,974 Indeno(ghi)perylene28,121 Dibenzo(ah)anthracene28,549 Benzo(ghi)perylene。

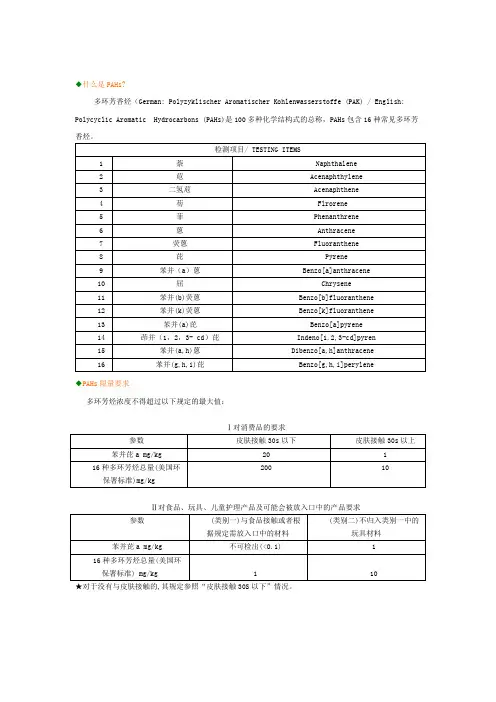
◆什么是PAHs?多环芳香烃(German: Polyzyklischer Aromatischer Kohlenwasserstoffe (PAK) / English: Polycyclic Aromatic Hydrocarbons (PAHs)是100多种化学结构式的总称,PAHs包含16种常见多环芳香烃。
◆PAHs限量要求多环芳烃浓度不得超过以下规定的最大值:Ⅰ对消费品的要求Ⅱ对食品、玩具、儿童护理产品及可能会被放入口中的产品要求★对于没有与皮肤接触的,其规定参照“皮肤接触30S以下”情况。
◆多环芳烃(PAHs)来源:有机物的不完全燃烧,煤/油/气/烟草/烤肉,木炭,原油,木馏油,焦油,药物,染料,塑料,橡胶,农药,发动机,发电机产生PAHs◆多环芳烃(PAHs)可能存在的材料:木炭,原油,木馏油,焦油(天然),药物,染料,塑料,橡胶,农药(人为),润滑油,脱膜剂,电容电解液,矿物油,柏油(人为),杀虫剂、杀菌剂、蚊香、吸烟、汽油阻凝剂(人为)其它◆多环芳香烃(PAHs)可能存在的具体物料:电线/插头、塑料手柄、塑料包装箱、橡胶手柄、有异味塑料、橡胶产品◆多环芳香烃(PAHs)的法规要求:欧盟国家76/769/EEC/German:LMBG/美国USEPA,中国GB,GB/T,GHZ◆多环芳香烃(PAHs)的危害:强致癌物质,损伤生殖系统,易导致皮肤癌,肺癌,上消化道肿瘤,动脉硬化,不育症◆卤素(F/Cl/Br/I)是什么?化学周期表中第VIII族元素包括氟、氯、溴、碘、砹,合称卤素。
其中砹为放射性元素,在产品中几乎不存在,前四种元素在产品中尤其在聚合物材料中以有机物形式存在。
目前用于产品中的卤素化合物主要有:PBB、PBDE、TBBP-A、PCB、六溴十二烷、三溴苯酚、短链氯化石蜡;另有用于冷冻剂、隔热材料臭氧破坏物质CFCs、HCFCs、HFCs等。
◆卤素(F/Cl/Br/I)的危害性:在塑料等聚合物产品中添加卤素用以提高燃点,优点是燃点比普通聚合物高,燃点大概在300度,燃烧时会散发出卤化物气体,,并迅速吸收氧气,从而使火熄灭。


物质:甲醛
对象禁止供货时期1 级各式使用于销售产品本体或其包装等用途,如扬声器、机架本体或栈板等木工产
品,或其他非木质材料制成的产品等。
立即禁止备注:栈板之甲醛限值不受本标准规范.
标准值(排放浓度):
胶合木质材料标准值(排放浓度):依木制品类型分二阶段管制排放浓度
说明:
第一阶段:Phase 1 (简称P1)
第二阶段:Phase 2 (简称P2)
HWPW-VC:Hardwood plywood—Veneer Core,单板芯之硬木胶合板
HWPW-CC:Hardwood plywood—Composite Core,复合芯之硬木胶合板
PB:ParticleBoard,粒片板
MDF:Medium density fiberboard,中密度纤维板
Thin MDF:最大厚度小于8mm 之MDF
(二) 其它非胶合木质材料排放浓度:
于12m3、1m3、或者0。
0225m3 之气密试验腔体中试验,于空气中的浓度在0。
1ppm (0。
124mg/m3)
以下(依照EN 717-1)
量测方法:
(一) 胶合木质材料:
(1)穿孔器测试法-EN 120 (PB,MDF, thin MDF)
(2)Chamber 测试法-ASTM E 1333-96(2002)
(3) 干燥器测试法-JIS A 1460
(二)其它非胶合木质材料:密闭腔体测试法-EN 717—1
甲醛︰不得检出。

关于欧盟和德国限制使用PAHs物质的规定欧洲议会及欧盟理事会已于2005年11月16日在法国斯特拉斯堡签署了2005/69/EC指令并于同年12月9日发布。
该指令主要针对轮胎和添加油中的多环芳烃(PAHs)作出指示。
在轮胎的制造过程中,由于使用了含有多种多环芳烃的添加油,产品中或多或少含有此类多环芳烃化合物。
.苯并芘(BaP)是其中的一个典型代表。
普遍认为,该类化合物具有致癌性、致突变性及生殖系统毒害性。
毒性、生态毒性及环境科学委员会(CSTEE)经科学研究证实,多环芳烃类化合物对人类健康确实有害。
有鉴于此,在1976年7月27日颁布的相关指令76/769/EEC 基础上,欧洲议会及欧盟理事会签发了该指令。
该指令适用范围为添加油和轮胎(客车轮胎、轻型和重型货车轮胎、农用车轮胎及摩托车轮胎),指令将对2010年1月1日后生产或翻新的轮胎同样有效。
指令规定直接投入市场的添加油或用于制造轮胎的添加油应符合以下技术参数:苯并芘(BaP)含量应低于1 mg/kg,同时8种PAHs(BaP,BeP,BaA,CHR,BbFA,BjFA,BkFA,DBAhA)总含量应低于10 mg/kg。
基于新型低PAHs轮胎的安全性测试及道路湿滑性测试尚需假以时日,该指令的起始有效日定为2010年1月1日。
而此类测试方法将由欧洲标准化委员会(CEN)或国际标注化组织(ISO)制订。
该指令还指示,欧盟各成员国应当于2006年12月29日之前制订相应的法律、规章及管理文件,其起始有效期应为2010年1月1日德国政府最新规定:所有在德国出售的电动工具必须经过检验其中不含有过量的PAHs,要进入德国市场的电器产品必须通过专业的检验机构的检测。
一、 PAHs简介PAHs,学名多环芳烃。
是石油、煤等燃料及木材、可燃气体在不完全燃烧或在高温处理条件下所产生的一类有害物质,通常存在于石化产品、橡胶、塑胶、润滑油、防锈油、不完全燃烧的有机化合物等物质中,是环境中重要致癌物质之一,PAHs主要包括16种同类物质。

PAHS是什么标准PAHS是一种常用于评估产品材料中有害物质的标准。
PAHS是多环芳烃的缩写,多环芳烃是指由若干个苯环组成的有机化合物。
这些化合物在自然界中广泛存在,包括石油、煤矿等资源中,同时也是一些工业生产活动的副产品。
然而,多环芳烃中的某些化合物被发现具有致癌和致突变作用,对人类和环境具有潜在的危害。
因此,为了保护消费者和环境的安全,制定了PAHS标准来限制产品材料中多环芳烃的含量。
PAHS标准可以应用于各种产品领域,如玩具、电子产品、汽车零部件等。
通过检测和限制这些产品中多环芳烃的含量,可以降低人与产品接触后潜在的风险。
PAHS标准通常根据不同国家和地区的法规要求而制定,例如欧盟、美国、日本等。
这些标准主要关注多环芳烃中的苯并[a]芘、苯并拟啶、苯并[a]蒽等化合物,这些化合物被认为具有较高的毒性和潜在的致癌作用。
对于企业来说,遵循PAHS标准是确保产品符合法规要求的重要一环。
通过建立良好的供应链管理和材料控制体系,企业可以确认其所使用的原材料符合PAHS标准,并监测产品的生产过程,确保产品在各个环节都符合相关要求。
此外,还可以通过第三方机构的检测和认证,获得相应的PAHS合规证书,提高产品的市场竞争力。
消费者也可以通过关注产品是否符合PAHS标准来选择安全的产品。
购买符合PAHS标准的产品可以减少暴露于有害物质的风险,特别是对于儿童和孕妇等特殊人群来说,选择符合安全标准的产品尤为重要。
尽管PAHS标准对于保护人类和环境的安全起到了积极的作用,但还存在一些挑战和改进的空间。
首先,标准的制定需要根据科学研究的进展进行更新,以确保标准与最新的科学认知保持一致。
其次,对于不同产品的PAHS限制是有差异的,需要根据产品的特点和接触风险进行细化,并与其他相关标准相互协调。
总之,PAHS标准是一种重要的评估产品材料中有害物质的标准。
它对于保护人类健康和环境安全起到了积极的作用。
企业和消费者都应该重视PAHS标准,遵守相关法规要求,并通过审慎的选择和购买来确保产品的安全性。


欧盟多环芳香烃标准
欧盟对多环芳香烃(PAHs)等有害物质的标准主要包括以下两个方面:
1.REACH法规:REACH(Registration, Evaluation,
Authorisation and Restriction of Chemicals)是欧洲联盟制定的一项化学品法规,旨在确保化学品的安全使用。
在REACH法规中,对一系
列化学品,包括多环芳香烃,设置了注册、评估和限制的要求。
这包括
限制某些物质的使用和排放,以减少其对环境和人体的潜在危害。
2.食品接触材料标准:针对食品接触材料,欧洲食品安全局
(EFSA)和欧洲联盟委员会设定了相关标准。
特别是对于食品接触材料中可能存在的多环芳香烃,有特定的限量要求,确保食品接触材料不会
引起对人体健康的危害。
在具体的产品和应用领域,欧盟可能还会发布一些具体的标准和法规,以限制或监管多环芳香烃的使用。
因此,具体的标准可能会因应用领域和产品类型而有所不同。
在了解相关标准时,建议查阅欧盟委员会的官方网站或欧盟成员国的国家标准机构的网站,以获取最新和具体的法规和标准信息。

2005/69/EC多环芳香烃(PAHS)指令中文版090624(上)2009-12-01 09:51欧洲议会和欧盟理事会第 2005/69/EC 号指令 2005 年 11 月 16 日欧盟理事会第76/769/EEC 号指令《关于统一各成员国有关限制销售和使用某些危害物质及制品的法律法规和管理条例》的第 27 次修订 ( 填充油和轮胎中的多环芳香烃 ) ( 技术翻译:刘金云校对:王小平 ) 欧洲议注意到建立欧洲共同体的条约,特别是其中第95条,注意到欧盟委员会的提案,注意到欧洲经济与社会委员会的意见,按照欧洲共同体条约第251条所制订的程序行事,鉴于:1) 制作轮胎时使用了填充油,其中可能含有各种浓度的多环芳香烃(PAHs),这些PAHs并非故意添加的。
在制作过程中,PAHs会同橡胶基质混合在一起。
因此,在成品中,会含有各种浓度的PAHs2) 苯并(a)芘(BaP)可以作为存在PAHs的定性的和定量的标记。
BaP和其它的PAHs被归为“致癌的、诱导有机体突变和生殖毒性”的物质。
由于这些PAHs 的存在,多种填充油也被归到致癌的、诱导有机体突变和生殖毒性的物质。
3) PAHs 对健康造成的负面影响,毒性、生态毒性和环境科学委员会( CSTEE )已经证实了这一科学发现。
4) 应当尽量减少 BaP 和其它的的PAHs被释放到环境中。
为了对人类健康和环境提供高质量的保护,为了对PAHs的年度总释放量作出努力,因此,在生产轮胎时,有必要禁止使用含高浓度PAHs的填充油和其混合物作为填充油,并禁止这些产品投放市场。
减少PAHs的释放量在1979年发布的《长期的、跨国界的持久有机污染物质对空气污染的公约》的《1998议定书》中所要求的。
5) 欧盟理事会于1976年7月27日发布的第76/769/EEC号指令《关于统一各成员国有关限制销售和使用某些危害物质及制品的法律法规和管理条例》也需作相应修订。
6) 本指涵盖了客车轮胎、轻卡车和重卡车轮胎,农用轮胎和摩托车轮胎,与其它的欧盟条款的要求并不相违背。
欧盟6P标准2010-08-25 15:296p标准1、RoHS六项限用物质检测标准测试欧盟《关于在电子电气设备中限制使用某些有害物质的第2002/95/EC号指令(RoHS指令),要求从2006年7 月1 日起,各成员国应确保在投放于市场的电子和电气设备中限制使用铅、汞、镉、六价铬、多溴联苯和多溴二苯醚六种有害物质。
RoHS中对六种有害物规定的上限浓度:镉(Cd):<100ppm 铅(Pb):<1000ppm 汞(Hg):<1000ppm 六价铬(Cr6+):<1000ppm多溴联苯(PBBs):<1000ppm多溴联笨醚(PBDEs):<1000ppm2、多环芳烃(PAHs)检测标准测试欧盟2005/69/EC指令规定直接投放市场的添加油或用于制造轮胎的添加油应符合以下技术参数:苯并吡(BaP)含量不得超过1mg/kg,同时16种PAH的总含量应低于10mg/kg。
德国对电动工具等产品也加强了PAHs的管控要求。
美国EPA标准还要求对16种PAH进行检测。
3、邻苯二甲酸酯检测标准测试欧盟第2005/84/EC号指令要求所有玩具或儿童护A理用品的塑料所含的DEHP、DBP、及BBP浓度超过0.1%的不得在欧盟市场出售;对可放进口中的玩具及儿童护理塑料中所含的具另三种邻苯二甲酸盐(DINP、DIDP及DNOP)进行限制,浓度不得超过0.1%。
1000PPM4、玩具EN71-3八大重金属检测ASTMF963测试EN71-3标准规定了玩具中八种可溶性金属(Cd、Pb、Hg、Cr、Ba、Se、As、Sb)的溶出量限制。
EN71-3八大重金属溶出量测试限量值:Sb (锑)( < 60 ppm )As (砷)(< 25 ppm)Ba (钡)(< 1000 ppm)Cd (镉)(< 75 ppm)Cr (铬)(< 60 ppm)Pb (铅)(< 90 ppm)Hg (汞)(< 60 ppm)Se (硒)(< 500 ppm)5、包装材料重金属检测标准指令测试94/62/EC指令要求所有流通于欧洲市场的包装及其材料中的镉、铅、汞及六价铬四种物质含量总和不得超过100ppm。
pahs新标准
PAHS(多环芳烃)是一类广泛存在于环境和工业产品中的有机污染物。
为确保人类健康和环境保护,各国和国际组织制定了一系列关于PAHS的标准和法规。
最新的PAHS标准主要包括以下几个方面。
1.欧盟REACH法规:欧盟对PAHS的管理主要依据REACH法规(化学品注册、评估、授权和限制法规)。
根据REACH法规,生产商和进口商需要对PAHS进行注册、提交风险评估报告,并采取措施确保化学品的安全使用。
2.美国加州Proposition65:美国加州proposition65法规要求企业确保其产品中PAHS的含量低于规定的限量。
该法规适用于各类产品,如纺织品、玩具、化妆品等。
3.国际标准ISO14129:国际标准ISO14129《橡胶和塑料制品中多环芳烃的测定》规定了测定橡胶和塑料制品中PAHS含量的方法。
该标准旨在帮助企业更好地控制和监测产品中的PAHS含量。
4.中国GB36246-2018:我国发布了GB36246-2018《玩具中多环芳烃的测定》标准,规定了玩具中PAHS的测定方法。
此外,我国还针对纺织品、皮革制品等其他产品制定了相应的PAHS检测标准。
需要注意的是,各国和地区的PAHS标准可能会随着科
学研究和技术发展而不断更新。
在实际应用中,企业需关注最新标准要求,确保产品符合相关法规要求,保障消费者健康和环境保护。
同时,也有助于提高企业自身的管理和技术水平,提升产品质量。
pahs含量管控标准
根据不同国家和地区的法规和标准,对于pahs(多环芳烃)含量的管控标准也不尽相同。
以下是一些常见的pahs含量管控标准:
1. 欧盟标准:
- REACH法规:根据注册、评估、授权和限制化学物质法规,在欧盟市场上销售的化学制品(包括pahs)必须进行注册和评估,并受到特定限制。
- EN 71-3:玩具标准,规定了玩具产品中pahs物质的限值和测试方法。
2. 美国标准:
- CPSC(Consumer Product Safety Commission):对于儿童玩具和儿童用品,限制了pahs的含量。
- FDA(Food and Drug Administration):对食品接触材料(如食品包装)中pahs的含量有限制。
3. 中国标准:
- GB 21456-2014:化妆品安全技术规范,限制了化妆品中pahs的含量。
- GB/T 31903-2015:玩具安全标准,规定了玩具产品中pahs物质的限值和测试方法。
需要注意的是,不同用途的产品和不同国家/地区的标准可能存在差异,具体的标准要根据实际情况和当地法规来确定。
因此,针对具体产品的pahs含量管控,建议参考当地相关法规和标准。
PAHs多环芳烃的详细介绍PAHs介绍多环芳烃(PAHs)是指具有两个或两个以上苯环的一类有机化合物。
多环芳烃是分子中含有两个以上苯环的碳氢化合物,包括萘、蒽、菲、芘等 150余种化合物。
英文全称为polycyclic aromatic hydrocarbon,简称PAHs。
有些多环芳烃还含有氮、硫和环戊烷,常见的多环芳烃具有致癌作用的多环芳烃多为四到六环的稠环化合物。
国际癌研究中心(IARC)(1976年)列出的94种对实验动物致癌的化合物。
其中15种属于多环芳烃,由于苯并a芘是第一个被发现的环境化学致癌物,而且致癌性很强,故常以苯并(a)芘作为多环芳的代表,它占全部致癌性多环芳烃1%-20%。
PAHS的用途与危害多并最终儿童行为的结果联系起来。
研究发现,接触到更高水平的PAH伴有焦虑/抑郁症的儿童年龄6至7比那些与低暴露水平的24%更高的分数。
发现有婴幼儿在他们的脐带血PAH水平升高46%更可能比那些脐带血中PAH水平低,最终得分高的焦虑/抑郁量表。
主要成分多环芳烃(PAHs)主要的十八种化合物为:萘、苊烯、苊、芴、菲、蒽、荧蒽、芘、苯并(a)蒽、屈、苯并(b)荧蒽、苯并(k)荧蒽、苯并(a)芘、茚并(1,2,3-cd)芘、二苯并(a,h)蒽和苯并(g,h,i)苝、1-甲基奈、2-甲基萘。
目前确定的PAHs常见的24种同类物质主要包括:Polycyclic Aromatic Hydrocarbons CASREACh EPAOEKO-TEX100GS MarkBenzo[a]pyrene苯骈 (a) 芘 (BaP) 50-32-8 X X X X Benzo[e]pyrene苯并[e]芘(BeP) 192-97-2 X X Benzo[a]anthracene苯骈 (a) 蒽 (BaA) 56-55-3 X X X X Dibenzo[a,h]anthracene二苯骈 (a,h),蒽 (DBA)53-70-3 X X X X Benzo[b]fluoranthene苯骈 (b) 萤蒽 (BbFA)205-99-2 X X X X Benzo[j]fluoranthene苯并[J]荧(BiFA) 205-82-3 X X Benzo[k]fluoranthene苯骈 (k) 萤蒽 (BkF)207-08-9 X X X X Chrysene屈 (CHR) 218-01-9 X X X Acenaphthene苊 (ANA) 83-32-9 X X X Acenaphthylene苊烯 (ANY) 208-96-8 X X X Anthracene蒽 (ANT) 120-12-7 SVHC X X X Benzo[ghi]perylene苯骈 (g,h,i) 苝 (BP191-24-2 X X XE)Fluoranthene萤蒽 (FLT) 206-44-0 X X XFluorene芴 (FLU) 86-73-7 X X XIndeno[1,2,3-cd]pyrene茚苯 (1,2,3-Cd)193-39-5 X X X 芘 (IPY)Naphthalene萘 (NAP) 91-20-3 X X XPhenanthrene菲 (PHE) 85-01-8 X X XPyrene芘 (PYR) 129-00-0 X X X27208-37XCyclopenta[c,d]pyrene环戊烯(c,d)芘-3Dibenzo[a,e]pyrene二苯并(a,e)芘192-65-4 XDibenzo[a,h]pyrene二苯并(a,h)芘189-64-0 XDibenzo[a,i]pyrene二苯并[a,i]芘189-55-9 XDibenzo[a,l]pyrene二苯并(a,l)芘191-30-0 X2381-21-X1-Methylpyrene1-甲基芘7多环芳烃(PAHs)的污染源有自然源和人为源两种。
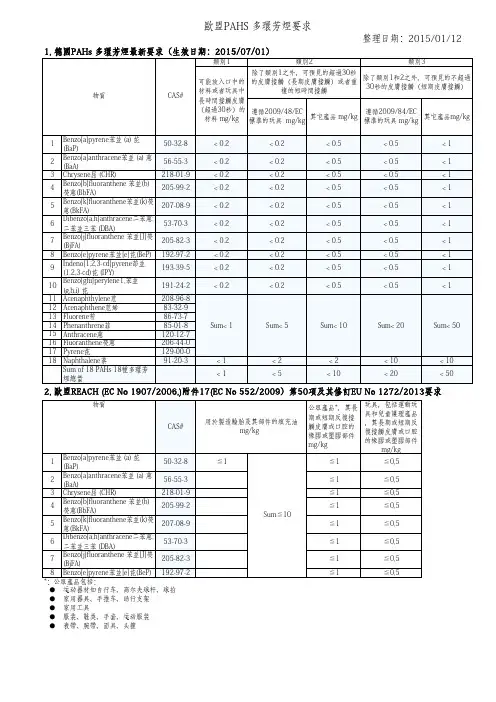
德国TUV BFR以及Stiftung Warentest共同讨论后一致同意PHAs允许存在的最大限值如下表所示:与皮肤接触时间检测项目a苯并芘16 种多环芳烃总量(EPA低于或等于30秒20mg/kg200mg/kg 超过30 秒1mg/kg10mg/kg与皮肤接触时间敏感性产品绝不能检出(1)终端消费者所接触的商品,其材料中所允许的多环芳烃的最大含量是根据2005年2月8日在柏林召开的专家会仪上讨论决定的。
2)检测方法的检测限为0.1mg/kg。
3)玩具、婴儿的用具以及食物容器等都属于敏感性产品。
欧盟2005 年发布的《关于多环芳香烃指令》欧盟2005年发布的《关于多环芳香烃指令》(PAHs指令),限制包含苯并芘(Bap在内的16种PAHs的使用。
基于已经发生的在德国港口发现的进口产品PAHs 超标事实,德国安全技术认证中心经验交流办公室( ZLS-ATAV 规定 从2008年4月1日起,所有GS 标志认证机构将加测 PAHs 测试的产品将无法获得 GS 认证而顺利进入德国。
合物的统称,主要来源于焦油炼油中的残留,存在于原油, 料,塑料,橡胶,润滑油,防锈油,脱膜剂,汽油阻凝剂,油,柏油等石化产品中,还存在于农药,木炭,杀菌剂,蚊香等日常化学产品 中。
在含有橡胶部件的物品,特别是使用回收轮胎部件 /材料的产品中,可能含有高量的PAHs 多环芳烃化合物具有致癌性、致突变性和生殖系统毒害性。
16 种 PAHs 分别是:1 Nap hthale ne 萘 2 Ace nap hthyle ne 苊烯 3 Ace nap hthe ne 苊 4Fluorene 芴 5 Phenanthrene 菲 6 Anthracene 蒽 7 Fluoranthene 荧蒽 8Pyrene 芘 9 Benzo (a )anthracene 苯并(a )蒽 10 Chrysene 屈 11Benzo (b )fluoranthene 苯并(b )荧蒽 12 Benzo (k )fluoranthene 苯并(k )荧蒽 13Benzo (a )pyrene 苯并(a )芘 14I nde no (1,2,3-cd ) pyre ne 茚苯(1,2,3-cd )芘 15Dibe nzo (a,h )a nthrace ne 二苯并(a,n )蒽 16Benzo (g,hi )perylene 苯并(ghi )北(二萘嵌苯)按照 ZLS-ATAV 勺要 求,16PP AHs 的限值如下:参数BaP 16项PAHs 总和一类与食物接触的材料或三岁以下孩童会放入口 中的物品和玩具不得检测到*(v0.2mg/)不得检测到*(<0.2mg/)二类塑料,经常 性和皮肤接触的部件,接触时间会超过 30秒的部件,以及一类中未规范的玩具 1 mg/kg 10 mg/kg 三类塑料,偶尔性接触的部件,即与皮肤接触时间少于 30秒 的部件,或与皮肤没有接触的部件 20mg/kg 200 mg/kgPAHs 指令()及德国GS 认证对PAHs 的要求欧盟2005年发布的《关于多环芳香烃指令》(PAHs 指令),限制包含苯 并芘(Bap 在内的16种PAHs 的使用。
Product Safety Commission (AfPS)GS SpecificationTesting and assessment of polycyclic aromatic hydrocarbons (PAHs) in the course of awarding the GS mark- Specification pursuant to article 21(1) no. 3 of the Product Safety Act (ProdSG) –AfPS GS 2014:01 PAKState of revision: August 4th, 2014Disclaimer: G erman i s t he o riginal t ext v ersion. I n c ase o f a ny d oubt, l ack o f c larity o r a ny o ther n on clear interpretation the content of the original version is valid.Management: Federal Institute for Occupational Safety and HealthFriedrich-Henkel-Weg 1 – 2544149 DortmundTelefon: 0231/9071-0Telefax: 0231/9071-2364Table of ContentsPreliminary observations (3)1 Purpose / Intention (3)2 Basics (3)3 Procedure (3)3.1 Risk assessment (3)3.2 Categorisation (4)3.3 Testing and assessment (6)4 Transitional regulations/periods (6)4.1 GS mark certificates, issued from July 1st, 2015 onwards (6)4.2 GS mark certificates, issued before July 1st, 2015 (6)4.3 Reissuing of existing GS mark certificates – exemptions (6)Annex: Testing instructions (8)1 Aim and purpose (8)2 Method (8)2.1 Brief description (8)2.2 Equipment (8)2.3 Chemicals and solutions (8)3 Preparation and execution (9)3.1 Sample preparation (9)3.2 Measuring procedure (10)3.3 Special characteristics (11)Annex: Measuring conditions for gas chromatography (for information) (12)Testing and assessment of polycyclic aromatic hydrocarbons (PAHs) in the course of awarding the GS markPreliminary observationsOn Aug. 4th 2014 the Product Safety Commission (AfPS) has assigned the requirements of PAH testing in the course of GS mark certification as specification according to art. 21 Product Safety Act (ProdSG) para. 1 no. 3. The implementation is achieved by means of this PAH document.1. Purpose / IntentionProducts (pursuant to the Product Safety Act) must comply with legal requirements to avoid health risks, e.g. art. 30 & 31 of the LFBG (Foodstuffs, Consumer Goods and Feedstuffs Code –LFGB), the Prohibition of Chemicals Ordinance, and the art. 3 of the ProdSG (Product Safety Act). This document and, in particular, the testing instructions (see Annex) specify the requirements with respect to the level of PAHs in products. In addition, the document harmonises the testing methodology for assessment by GS bodies.2. BasicsPAH contamination of materials is primarily due to the use of:- PAH contaminated softening oils in rubber and flexible (soft) plastics- PAH-contaminated carbon black as a black pigment in rubber, plastics and paints.PAH contamination has previously been detected not only in rubber but also in various types of plastic, e.g. ABS and PP, and various paints/coatings, as well as in a variety of natural materials.3. ProcedureThe GS body must take the following steps into account both in the process of awarding a new GS mark and within the framework of monitoring existing GS mark certificates:1. Risk assessment2. Categorisation3. Testing and assessment3.1 Risk assessmentThe GS body must carry out a risk assessment and, in doing so, define which relevant contact/grip and operating surfaces of the product are to be considered for testing and which are not, and must make a record of these (this means that the GS body must first specify the contact/grip and operating areas to which the requirements of the PAH document must be applied (specification of PAH relevance)). The risk assessment should be not applied, where appropriate, if the respective “Erfahrungsaustauschkreis” (“Exchange of Experience Group”, EK) has already defined a procedure for the product or product group with regard to the contact/gripand operating surfaces to be tested. A reference to the EK’s definition is to be included in the documentation accordingly.Materials that cannot be accessed or that can only be accessed by using tools need not be assessed, with the exception of samples with a conspicuous odour.In principle, account must be taken of all contact/grip and operating surfaces that can come into direct contact with the skin or that can be put into the mouth during proper or foreseeable use (but not misuse).13.2 CategorisationDepending on the results of the risk assessment, the corresponding product parts are then to be categorised (see Table 1) and to be analysed on their actual PAH content according to the analysis method below. Existing test reports can be taken into account if they are compliant with the “Grundsatzbeschluss” (“principle decision”) ZEK-GB-2012-01 of the ZEK (“Central Exchange of Experie nce Group”) and the requirements of this PAH document. Categorisation can be dispensed with if the respective ”Erfahrungsaustauschkreis” (“Exchange of Experience Group”, EK) has already defined a categorisation of the contact/grip and operating surfaces fo r a product or product group. Definitions for products or product groups from the individual EKs are published on the ZLS website2 and apply from the time of publication.Table 1 presents the maximum levels of PAH in product materials, which must not be exceeded. The provisions of this document with regard to the PAH content do not apply if other legislation lays down corresponding or further requirements for the PAH content. This applies only to the material or component/assembly and not to the product as a whole. Materials and parts of the product that are not covered by other legislative provisions must be assessed within the framework of the procedure for awarding the GS mark in accordance with the requirements of the PAH document.It must be ensured that the method of testing can actually achieve the limit of quantification of 0.2 mg/kg for each individual PAH component3.At the same time, method and matrix effects, as well as the measurement uncertainty, the efficiency of extraction and losses during purification must be considered.Based on the findings of the United States Environmental Protection Agency (EPA) (according to the list in the ZEK document 04-11), the total of 18 PAHs (extended substance list of the AtAV, the predecessor committee of the AfPS) only considers PAH components whose level in the material is found to exceed 0.2 mg/kg.1 However, in order to ensure a consistent and appropriate procedure during the awarding of GS mark, it is not generally necessary to analyse all freelyaccessible surfaces. It is this document’s intention to limit the consideration to relevant contact/grip and operating surfaces. It is n ot expedient to test all product parts or surfaces in order “to be on the safe side”.2 Homepage of ZLS (Central Authority of the Laender for Safety): http://www.zls-muenchen.de3 Example: Water-carrying parts in coffee machines that come into contact with foodstuffs (e.g. water, etc.) are subject to the Food and Feed Code legislation and are therefore excluded from the PAH document’s field of application. However, grip surfaces on the coffee machine must still be assessed according to the requirements of the PAH document.Table 1: Maximum PAH levels to be complied with for the materials in relevant contact/grip and operating surfaces that are to be categorised based on the results of the risk assessment.* Wording “short-term repetitive skin contact” from supplement to REACH annex XVII no. 50 (REGULATION (EU) No 1272/2013)3.3 Testing and assessmentThe testing instructions found in the annex describe the steps of sample preparation, extraction of the PAHs, purification of the extract, identification and quantification; these must be applied uniformly by all laboratories carrying out testing.The GS body assesses the test results and decides whether the GS mark can be awarded in compliance with the other requirements.4. Transitional regulations/periodsFrom July 1st 2015 (issue date of the GS mark certificate), it is compulsory to apply this document when awarding the GS mark to products.The document ZEK 01.4-08 will cease to be valid from June 30th 2015.Since testing for PAH levels in products constitutes an overarching requirement for almost all of the members of all of the ”Erfahrungsaustauschkreise” (“Exchange of Experience Groups”), the following procedure is defined:4.1 GS mark certificates, issued from July 1st 2015 onwards(incl. ongoing procedures that are concluded after July 1st 2015)Compulsory application of this GS specification from July 1st 2015 onwards (exception: see 4.3).4.2 GS mark certificates, issued before July 1st2015Existing GS mark certificates initially remain valid.Within the framework of regular checks to monitor the manufacturing process (at the latest within one year or, in cases where the regular monitoring period is two years, within two years), the requirements under no. 3 o f the ZEK (“Central Exchange of Experience Group”) document with regard to the risk assessment must be taken into account, regardless of whether the product was found in the manufacturing facility or not. If, in the process, it is found that the corresponding requirements are not met, the GS mark certificate must be withdrawn immediately. The ZEK ”Grundsatzbeschluss” (“principle decision”) ZEK-GB-2006-01 must be complied with.4.3 Reissuing of existing GS mark certificates – exemptionsFor the reissuing of an existing GS mark certificate, immediate consideration is not required in the following circumstances:If the company name is changed, new GS mark certificates are usually issued. However, since the product does not change in terms of construction or other properties, and the reissuing of the GS mark certificate is more or less a pure formality, it is not necessary to consider the requirements of the PAH decision until the time of the check which must be performed for the purposes of monitoring the manufacturing process.(Please note: The reissuing of the GS mark certificate does not alter the previously defined periods for carrying out checks on the product’s manufacturing process.)The same applies by analogy when the holder of the GS mark certificate changes address provided none of the product’s properties change and the product does not require an additional safety check.The above procedure can also be applied in relation to duplicate certificates (also referred to as OEM certificates). In such cases, a review pursuant to the requirements of the PAH document must strictly be carried out by the time of the next product-manufacturing check according to the periods already defined in the “main certificate” or, at the latest, b y December 28th 2015. Monitoring intervals beyond this date are not permissible in such cases.With regard to the OEM certificates and therefore also the “main certificates”, the PAH document – as specified – must be applied by December 28th 2015 at the latest.Annex: Testing instructionsHarmonised method for the determination of polycyclic aromatic hydrocarbons (PAHs) in polymers1 Aim and purposeDetermination of polycyclic aromatic hydrocarbons (PAHs) in polymer samples.2 Method2.1 Brief description2.1.1 Standard methodA representative partial sample is taken of the material and cut up into pieces with a maximum size of 2–3 mm using scissors, wire cutters, etc. Then, 500 mg of the sample is weighed into a container and extracted with 20 ml of toluene (to which an internal standard has been added) for 1 h at 60 °C in an ultrasonic bath. An aliquot is taken from the extract once it has cooled down to room temperature. In the case of polymers (e.g. plastics or rubber products) for which matrix problems arise during the analysis, an additional purification step is carried out using column chromatography. Quantification is performed on a gas chromatograph with a mass-selective detector (GC/MSD) using the SIM method.2.1.2 Method for insufficient quantitiesIf the total mass of material to be analysed is less than 500 mg, one should proceed as follows: Identical materials from the product can be combined and considered as one sample. However, additional product specimens must not be used.If less than 50 mg of material is available for individual samples, these are not tested.If the available mass of chopped-up material is between 50 mg and 500 mg, the sample must be tested according to 2.1.1 and the quantity of toluene converted or adapted in proportion. The actual mass of the sample is to be recorded in the test report accordingly.2.2 Equipment•Ultrasonic bath with a minimum power of 200 W and a bath area of 706 cm2, corresponding to 0.28 W/cm2, without a basket and with an internal or external thermostat•Gas chromatograph with a mass-selective detector2.3 Chemicals and solutions2.3.1 Chemicals•Toluene•Internal standardso Standard 1: Naphthalene-d8o Standard 2: Pyrene-d10 or anthracene-d10 or phenanthrene-d10o Standard 3: Benzo[a]pyrene-d12 or perylene-d12 or triphenylbenzeneAt least three internal standards must be used; these are added to the extraction solvent (toluene).•External standard: 18 PAH substances according to Table 1 or those listed under no. 3.2, as a mix or individually•Petroleum ether•Silica gel•Sodium sulfate2.3.2 Calibration solutionsThe concentrations of the calibration solutions must be chosen so that a three-point calibration covers a working range of 0.1 to 10 mg/kg in the samples. This corresponds to a concentration range of 2.5 to 250 ng/ml in the calibration solutions.3 Preparation and execution3.1 Sample preparationA representative partial sample is taken of the material. The fragments produced by chopping up the samples to be analysed (using scissors, wire cutters, pliers, etc.) should have a maximum size of 2 – 3 mm.3.1.1 Extraction500 mg of the sample is placed in a glass ”Bördelglas” (“vial”). To this 20 ml of toluene, previously amended with internal standards, are added. The sample is then extracted for 1 h in the ultrasonic bath at a temperature of 60 °C throughout. For this purpose, the vials are placed or suspended in the ultrasonic bath without using a basket. The vials are then removed, the extract is left to cool to room temperature and shaken briefly, and an aliquot is taken from the extract and measured either directly or following dilution with toluene.3.1.2 Column chromatography extraction stepFor some polymers (e.g. plastic or rubber products), especially those that dissolve well in toluene under the described extraction conditions, it is necessary to clean the extract using adsorption chromatography on silica gel.For this purpose, a clean-up column with “Hahnschliff” (“stopcock”) (approx. 220 mm x 15 mm) is filled with glass wool, 4 g of silica gel and 1 cm of sodium sulfate.The silica gel is deactivated beforehand by adding 10% water (the corresponding volume of water is added to the silica gel in a glass flask, and the mixture is homogenised on the rotaryevaporator for 1 h at standard pressure and room temperature. The silica gel can then be stored in the sealed glass flask at room temperature).The packed column is conditioned with 10 ml of petroleum ether.The aliquot of toluene extract is then evaporated to a volume of approx. 1 ml on the rotary evaporator and poured into the column. The pointed flask is rinsed out with approx. 20 ml of eluent, which is then also transferred to the clean-up column. Elution is performed with 50 ml of petroleum ether. The collected petroleum ether eluate is amended with 1 ml of toluene and evaporated to a volume of approx. 1 ml under a nitrogen stream (e.g. on the TurboVap). This is then made up to a defined volume with toluene, and the extract is analysed by GC-MS.3.2 Measuring procedureThe method of determination to be applied is gas chromatography with a mass-selective detector in the SIM mode.The following 18 PAHs are to be determined:•Naphthalene•Acenaphthylene•Acenaphthene•Fluorene•Phenanthrene•Anthracene•Fluoranthene•Pyrene•Chrysene•Benzo[a]anthracene•Benzo[b]fluoranthene•Benzo[k]fluoranthene•Benzo[j]fluoranthene•Benzo[a]pyrene•Benzo[e]pyrene•Indeno[1,2,3-cd]pyrene•Dibenzo[a,h]anthracene•Benzo[g,h,i]perylene3.2.1 Measuring conditions for gas chromatographyThe equipment parameters (temperatures, columns, mass traces) may be chosen by the individual laboratory or are determined by the analytes.3.2.2 AnalysisInternal standards: at least three internal standards must be used. For these three standards, the internal standards and the correction ranges are defined as followed:Parameter Internal standards with recommended reference•Naphthalene Naphthalene-d8•Acenaphthylene Pyrene-d10 or anthracene-d10 or phenanthrene-d10 •Acenaphthene Pyrene-d10 or anthracene-d10 or phenanthrene-d10 •Fluorene Pyrene-d10 or anthracene-d10 or phenanthrene-d10 •Phenanthrene Pyrene-d10 or anthracene-d10 or phenanthrene-d10 •Anthracene Pyrene-d10 or anthracene-d10 or phenanthrene-d10 •Fluoranthene Pyrene-d10 or anthracene-d10 or phenanthrene-d10•Pyrene Pyrene-d10 or anthracene-d10 or phenanthrene-d10•Benzo[a]anthracene Pyrene-d10 or anthracene-d10 or phenanthrene-d10 •Chrysene Pyrene-d10 or anthracene-d10 or phenanthrene-d10•Benzo[b]fluoranthene Benzo[a]pyrene-d12 or perylene-d12 or triphenylbenzene •Benzo[k]fluoranthene Benzo[a]pyrene-d12 or perylene-d12 or triphenylbenzene •Benzo[j]fluoranthene Benzo[a]pyrene-d12 or perylene-d12 or triphenylbenzene •Benzo[a]pyrene Benzo[a]pyrene-d12 or perylene-d12 or triphenylbenzene •Benzo[e]pyrene Benzo[a]pyrene-d12 or perylene-d12 or triphenylbenzene •Indeno[1,2,3-cd]pyrene Benzo[a]pyrene-d12 or perylene-d12 or triphenylbenzene •Dibenzo[a,h]anthracene Benzo[a]pyrene-d12 or perylene-d12 or triphenylbenzene •Benzo[g,h,i]perylene Benzo[a]pyrene-d12 or perylene-d12 or triphenylbenzene•External calibration: for each individual PAH, at least a three-point calibration must be carried out with reference to the internal standardisation set out above. A working range of 0.1 to 10 mg/kg is recommended here.•Concentrations above the calibration range can be determined by diluting the extract.3.2.3 Limit of quantificationThe limit of quantification for material samples is 0.2 mg/kg per parameter.3.3 Special characteristicsBecause it is relatively volatile compared to the other 17 PAHs, naphthalene constitutes a parameter that is hard to assess in products that come into contact with the skin. Experience from the testing institutes indicates that it is possible to identify instances of both naphthalene depletion in materials and secondary contamination. The result obtained for naphthalene therefore only ever reflects the test specimen’s current situation at the time of measurement.Annex: Measuring conditions for gas chromatography (for information) Injected volume: 1 µl pulsed splitlessColumn: HT8 25m, ID 0.22mm, film thickness: 0.25µmInjector temperature: 280°CTransfer-line temperature: 260°CInitial temperature: 50°CInitial time: 2 min Heatingrate: 11°C/min Finaltemperature: 320°C Finaltime: 8 min3,418 D-naphthalene8,186 D-phenanthrene23,182 D-benzo[a]pyrene3,459 Naphthalene5,586 Acenaphthylene5,845 Acenaphthene6,634 Fluorene8,235 Phenanthrene8,337 Anthracene11,217 Fluoranthene11,914 Pyrene16,830 Benzo(a)anthracene16,982 Chrysene21,860 Benzo(b+j)fluoranthene21,964 Benzo(k)fluoranthene23,055 Benzo(e)pyrene23,302 Benzo(a)pyrene27,974 Indeno(ghi)perylene28,121 Dibenzo(ah)anthracene28,549 Benzo(ghi)perylene。