l In decision making there are many advantages in distinguishing between uncertainty, variability (random variation) and risk
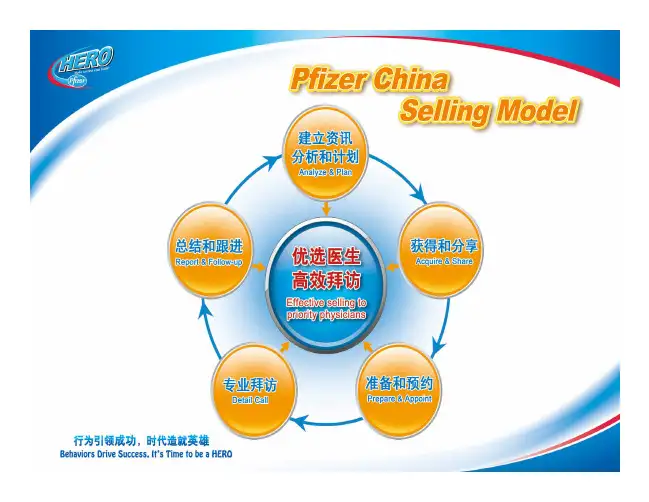
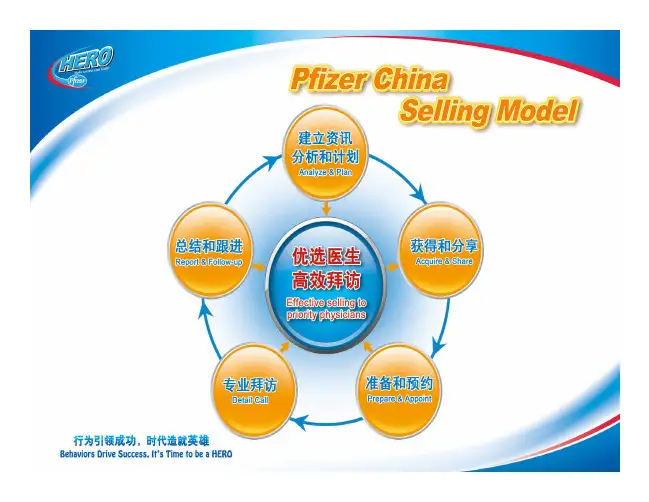
Goals and Characteristics of a Quality Decision System: Example
l Drug product manufactured at many different facilities, changes in the process, different manufactures,…
Uncertainty, Variability and Risk
l Quality – Clinical Connection
l After successful demonstration of therapeutic claim (acceptable risk-to-benefit ratio)
l Drug product manufactured for commercial distribution
l Life cycle of the product (shelf-life, exclusivity period, generic competition, post-approval changes,…)
Do not present a known or potential bioequivalence problem. Acceptable
in vitro standard
Present a known or potential bio problem. Appropriate bioequivalence


![[精华]辉瑞企业培训资料全面版](https://uimg.taocdn.com/bacddb82b84ae45c3a358cc2.webp)