第三章理想气体的性质与过程
- 格式:ppt
- 大小:3.77 MB
- 文档页数:100





第三章理想⽓体的性质与热⼒过程第三章理想⽓体的性质和理想⽓体的热⼒过程英⽂习题1. Mass of air in a roomDetermine the mass of the air in a room whose dimensions are 4 m×5 m×6 m at 100 kPa and 25℃2. State equation of an ideal gasA cylinder with a capacity of 2.0 m 3contained oxygen gas at a pressure of 500 kPa and 25℃ initially. Then a leak developed and was not discovered until the pressure dropped to 300 kPa while the temperature stayed the same. Assuming ideal-gas behavior, determine how much oxygen had leaked out of the cylinder by the time the leak was discovered.3. Two tanks are connected by a valve. One tank contains 2 kg of carbon monoxide gas at 77oC and0.7 bar. The other tank holds 8 kg of the same gas at 27oC and 1.2 bar. The valve is opened and the gases are allowed to mix while receiving energy by heat transfer from the surrounding. The final ideal gas equilibrium temperature is 42℃ Using the model, determine (a) the final equilibrium pressure, in bar, and (b) the heat transfer for the process,in kJ.4. Electric heating of air in a houseThe electric heating systems used in many houses c o nsist of a simple duct with resistance wires. Air is heated as it flows over resistance wires. Consider a 15-kW electric system. Air enters the heating section at 100 kPa and 17oC with a volume flow rate of 150 m 3/min. If heat is lost from the air in the duct to the surroundings at a rate of 200 W, determine the exit temperature of air.C P =1.005 kJ/(kg. K).5. Evaluation of the Δu of an ideal gasAir at 300 K and 200 kPa is heated at constant pressure to 600 K. Determine the change in internal energy of air per unit mass, using (a) data from the air table, (b) the functional form of the specific heat, and (c) the average specific heat value.6. Properties of an ideal gasA gas has a density of 1.875 kg/m 3at a pressure of 1 bar and with a temperature of 15oC. A mass of 0.9 kg of the gas requires a heat transfer of 175 kJ to raise its temperature from 15oC to 250oC while the pressure of the gas remains constant. Determine (1) the characteristic gas constant of the gas, (2) the specific heat capacity of the gas at constant pressure, (3) the specific heat capacity of the gas at constant volume, (4) the change of internal energy, (5) the work transfer.7. Freezing of chicken in a boxCarbon2kg, 77oCarbon 8kg, 27oMonoxide C 0.7bar Monoxide C 1.2bar valve Tank 1Tank 2FIGURE 3-1FIGURE 3-2FIGURE 3-3A supply of 50 kg of chicken at 6℃ contained in a box is to be frozen to -18℃ in a freezer. Determine the amount of heat that needs to be removed. The latent heat of the chicken is 247 kJ/kg, and its specific heat is 3.32 kJ/kg.℃ above freezing and 1.77 kJ/kg.℃ below freezing. The container box is 1.5 kg, and the specific heat of the box material is 1.4 kJ/kg.℃. Also, the freezing temperature of chicken is -2.8℃.8. Closed- system energy balanceA rigid tank which acts as a perfect heat insulator and which has a negligible heat capacity is divided into two unequal partsA andB by a partition. Different amounts of the same ideal gas are contained in the two parts of the tank. The initial conditions of temperature T, pressure p, and total volume V are known for both parts of the tank. Find expressions for the equilibrium temperature T and pressure P reached after removal of the partition. Calculate the entropy change for A and B and the totalentropy change of the tank. Assume that Cv,m is constant,9. Thermal processes of an ideal gasAn air receiver has a capacity of 0.85 m 3and contains air at a temperature of 15℃ and a pressure of 275 kN/m 3. An additional mass of 1.7 kg is pumped into the receiver. It is then left until the temperature becomes 15℃ once again. Determine (1) the new pressure of the air in the receiver, (2) the specific enthalpy of the air at 15℃ if it is assumed that the specific enthalpy of the air is zero at 0℃. Take cp=1.005 kJ/kg.K, cc=0.715 kJ/kg.K.10. Air is compressed steadily by a reversible compressor from an inlet state of 100KPa and 300K toan exit pressure of 900 kPa. Determine the compressor work per unit mass for isentropic compression with k=1.4, (1) isentropic compression with k=1.4, (2) polytropic compression with n=1.3, (3) isothermal compression, and (4) ideal two-stage compression with intercooling with a polytropic exponent of 1.3.11. A rigid cylinder contains a “floating” piston, free to mo ve within the cylinder without friction. Initially,it divided the cylinder in half, and on each side of the piston the cylinder holds 1 kg of the same ideal gas at 20oC, and 0.2 MPa . An electrical resistance heater is installed on side A of the cylinder, and it is energized slowly to P A2=P B2=0.4 MPa. If the tank and the piston are perfect heat insulators and are of negligible heat capacity, cv=0.72 kJ/(kg·K). Calculate (1)the final temperatures, volumes of A,B sides, (2)the amount of heat added to the system by the resistor. (3)the entropy changes of A,B sides, (4)the total entropy change of the cylinder.⼯程热⼒学与传热学第三章理想⽓体的性质和热⼒过程习题1 理想⽓体的c p 和c V 之差及c p 和c V 之⽐是否在任何温度下都等于⼀个常数?习题0.20.1MPa 300K 0.01m 3AMPa 300K 0.01m 3BFIGURE 3-42如果⽐热容是温度t 的单调增函数,当t 2 >t 1时平均⽐热容2121,,00t t t t c c c 中哪⼀个最⼤?哪⼀个最⼩? 3如果某种⼯质的状态⽅程式遵循T R pv g ,这种物质的⽐热容⼀定是常数吗?这种物质的⽐热容仅是温度的函数吗? 4在p-v 图上画出定⽐热容理想⽓体的可逆定容加热过程,可逆定压加热过程,可逆定温加热过程和可逆绝热膨胀过程。




工程热力学与传热学第三章 理想气体的性质与热力过程 典型问题分析一. 基本概念分析1 c p ,c v ,c p -c v ,c p /c v 与物质的种类是否有关,与状态是否有关。
2 分析此式各步的适用条件:3将满足下列要求的理想气体多变过程表示在p-v 图和T-s 图上。
(1) 工质又膨胀,又升温,又吸热的过程。
(2) 工质又膨胀,又降温,又放热的过程。
4 试分析多变指数在 1<n<k 范围内的膨胀过程特点。
二. 计算题分析理想气体状态方程式的应用 1某蒸汽锅炉燃煤需要的标准状况下,空气量为 q V =66000m 3/h ,若鼓风炉送入的热空气温度为t 1=250°C ,表压力 p g1=20.0kPa 。
当时当地的大气压力 p b =101.325kPa 。
求实际的送风量为多少?理想气体的比热容 2在燃气轮机动力装置的回热器中,将空气从150ºC 定压加热到350ºC ,试按下列比热容值计算对每公斤空气所加入的热量。
01 按真实比热容计算;02 按平均比热容表计算(附表2,3); 03 按定值比热容计算;04 按空气的热力性质表计算(附表4); 3已知某理想气体的比定容热容c v =a+bt , 其中a ,b 为常数,试导出其热力学能,焓和熵变的计算式。
理想气体的热力过程 4一容积为 0.15m 3 的储气罐,内装氧气,其初始压力 p 1=0.55MPa ,温度 t 1=38ºC 。
若对氧气加热,其温度,压力都升高。
储气罐上装有压力控制阀,当压力超过 0.7MPa 时,阀门便自动打开,dTm c dHpV U d pV d dU pdV dU WdU Q P ==+=+=+=+=)()(δδ典 型 问 题放走部分氧气,即储气罐中维持的最大压力为 0.7MPa 。
问当罐中氧气温度为 285ºC 时,对罐中氧气共加入了多少热量?设氧气的比热容为定值。
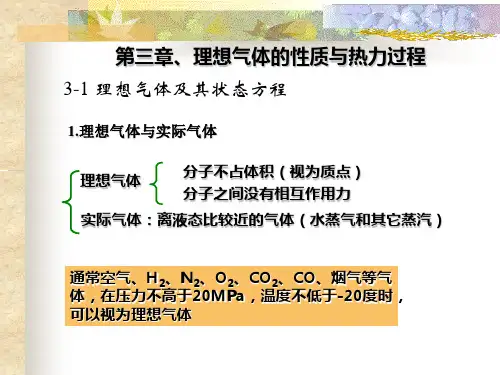
第三章 理想气体 第一节 理想气体的状态方程一、理想气体的概念理想气体是一种实际上不存在的假象气体,其分子是些弹性的,不具体积的质点,分子间相互没有作用力。
在这两点建设条件下,气体分子的运动规律极大地简化了,分子两次碰撞之间为直线运动,且弹性碰撞之间为直线运动,且弹性碰撞无能量损失。
众所周知,高温、低压的气体密度小,比体积大,若大到分子本身体积远小于其活动空间,分子间平均距离远到作用力极其微弱的状态就很接近理想气体。
因此,理想气体是气体压力趋近于零,比体积趋近于无穷大时的极限状态。
工程中常用的氧气、氮气、氢气、一氧化碳等及其混合空气、燃气、烟气等工质,在通常使用的温度、压力下都可作为理想气体处理。
不符合上述两点假设的气态物质称为实际气体。
蒸汽动力装置中采用的工质水蒸气,制冷装置的工质氟利昂蒸汽、氨蒸汽等,不能看作理想气体。
二、理想气体状态方程1、 理想气体基本定律(1) 波义耳---马略特定律:一定量的理想气体,当温度保持不变时,其压力与比体积成反比,即1122p v p v = 或 1122p V p V =(3-1)(2) 查理定律:一定量的理想气体,当比体积(或容积)保持不变时,其压力与热力学温度成正比,即1122p T p T = (3-2)(3) 盖·吕萨克定律:一定量的理想气体,当压力保持不变时,其比体积(或容积)与热力学温度成正比,即1122v T v T = 或1122V T V T =(3-3)2、 理想气体状态方程以上三个定律都是理想气体在特定条件下,状态变化的规律。
当气体的三个基本状态参数p 、v 、T 都发生变化时,克拉伯龙根据前人的实验把上述定理综合得到:112212p v p v pv c T T T==== (3-4)根据分子运动理论气体分子作用于器壁上的压力p 为2232m c p n =等式两边同乘以比体积v ,并以N ’表示1㎏气体分子数,得2232mc pv nv NvkT ==即g pv R T = (3-5)式中p 为气体的绝对压力,Pa ;v 为气体的比体积,m 3/㎏;T 为气体的热力学温度,k ;g R 称为气体常数,1/㎏·k 。