Entresto说明书
- 格式:doc
- 大小:466.50 KB
- 文档页数:35

产小产品手册2022-2023方酶类 英纳帝斯ZYM系列酵母营养剂酵母 英纳帝斯FERM系列单宁 英纳帝斯丹诺系列多糖苹果酸乳酸发酵橡木制品氧气管理澄清剂过滤材料稳定剂硫制剂起泡酒产品系列酒厂小工具0632113820443614425526502952创造一个可持续的未来将可持续性整合到我们的商业和生产活动中,使我们能够提高运营效率, 为客户提供最佳解决方案并为社区提供支持。
了解更多新内容酵母 Q415酵母 QET , 酵母 Q RHO 16酵母 Q TD 17马克思苷F , 增强马克思苷4546果胶酶 增强特瑞0821千机酶类 英纳帝斯ZYM系列英纳帝斯通过结合对于单体酶活性的认识和酒厂的实际应用,开发了ZYM系列。
其中包含一系列不同配方的酶制剂,用于在经典和新兴应用中获得最佳效果。
7白葡萄浸渍酶芳香果胶酶MP用于浸渍白葡萄和红葡萄的微粒化酶制剂。
其含有的次要活性,能够破坏葡萄皮细胞的细胞壁和细胞膜。
这不仅能引起液泡中所含芳香族前体的溶解,而且还引起固体细胞结构中的芳香族前体的溶解。
用芳香果胶酶 MP处理过的葡萄酒具有芳香的特征,其特点是浓郁的水果香气,具有复杂性和持久性。
此外,芳香果胶酶MP有助于蛋白质稳定,从而减少皂土的添加。
应用:浸渍白葡萄和红葡萄;生产果味白葡萄酒,红葡萄酒和桃红葡萄酒;提高蛋白质稳定性用量:20-40克/吨包装规格:250克 - 1千克果胶酶特别特别为白葡萄浸渍而设计的一款具有纤维素酶和半纤维素酶等次生活性的液体果胶酶制剂。
它会导致细胞壁和细胞膜强烈快速的破坏。
这有利于芳香族前体的提取,增强了葡萄酒的品种特性,浓郁度和复杂性。
在低温冷却过程中,它可以缩短接触时间,从而降低制冷成本。
在压榨过程中,果胶酶特别在提高果汁质量的同时也能提高出汁率。
此外,该酶还有助于果汁澄清,不需要额外添加澄清酶。
应用:浸渍白葡萄用量:20-50毫升/吨包装规格:1千克果胶酶易滤具有果胶分解和β-葡聚糖酶活性的液体酶制剂。

膳魔师(江苏)家庭制品有限公司消防专员岗位说明书1、工作职责核准:审核:制作:篇二:thermos thv-2001使用说明书?thermos thv-2001使用说明书将热水或冷水装入瓶内后,务必确认关紧瓶栓,以免漏水造成烫伤。
装入过量的热开水或冷开水,会有漏水现象,请参照说明书的水量位置图。
请勿放置于靠近火源之处,以免变形。
请勿放在幼儿摸得到的地方,注意不要让儿童玩耍,会有烫伤的危险。
杯内放入热饮时,请小心烫伤。
请勿放入下述饮料:干冰、碳酸饮料、盐分的流质、牛奶、奶类饮料等。
长时间保温热茶时会变色,外出时建议使用茶包等来冲泡较为适宜。
请勿将商品放入洗碗机、烘干机、微波炉中。
避免瓶子掉落及巨大撞击,以免表面凹陷而导致保温不良等故障。
篇三:保温杯使用说明书将热水或冷水装入瓶内后,务必确认关紧瓶栓,以免漏水造成烫伤。
装入过量的热开水或冷开水,会有漏水现象,请参照说明书的水量位置图。
请勿放置于靠近火源之处,以免变形。
请勿放在幼儿摸得到的地方,注意不要让儿童玩耍,会有烫伤的危险。
杯内放入热饮时,请小心烫伤。
请勿放入下述饮料:干冰、碳酸饮料、盐分的流质、牛奶、奶类饮料等。
长时间保温热茶时会变色,外出时建议使用茶包等来冲泡较为适宜。
请勿将商品放入洗碗机、烘干机、微波炉中。
避免瓶子掉落及巨大撞击,以免表面凹陷而导致保温不良等故障。
篇四:烤箱说明书fz 61.1 ix, fz 612 c.2 ix, fz 62c.1ix 中文说明书安装!在使用产品前,请仔细阅读使用说明。
说明包含关于安全使用,安装和维护产品的重要信息。
!请保留使用说明。
位置!将包装材料放在远离儿童可及的地方。
它可能导致儿童窒息的危险。
!本设备必须由具有资格的人员,遵照设备所提供的说明书进行安装。
不正确安装可能造成人员及动物的伤害及财产的破坏。
安装设备采用合适的橱柜以保证电器正常工作。
???通风为保证良好的通风,需移去橱柜后部面板。
建议把烤箱安装在2条木条上,和安装在一个完全平整的表面上。

Azur Elite3000W70 g/min continuous steam260g steam boostSteamGlide Advanced soleplateGC5037/86Our smartest and most powerful steam ironGuaranteed no burns,with intelligent steam releasePowerful, intelligent iron for perfect results faster. Iron everything from jeans to silkwith no risk of burning thanks to OptimalTEMP technology. Advanced DynamiQsteam mode ensures the perfect amount of powerful steam when you need it*Intelligent ironing for ultimate convenienceOptimalTEMP technology: Guaranteed no burns, no settingsConvenient steam modes: DynamiQ, MAX, IONIC and OFFDynamiQ mode, intelligent steam release for perfect resultsIonic steam mode, deep ionized steam for hygienic ironingEasier and faster ironing3000 W for quick heat-up and powerful performanceTurbo steam pump pushes up to 50% more steam through fabric*Steam output up to 70g/min for faster crease removalUp to 260 g steam boost blasts stubborn creasesComfortable ironingSteamGlide Advanced soleplate, ultimate gliding & durabilityQuick Calc Release in 15s for long-lasting steam performanceAutomatic shut-off when the iron is left unattendedHighlightsOptimalTEMP technologyThanks to OptimalTEMP technology, we guarantee this iron will never cause burns to any ironable fabric and you can iron everything from jeans to silk, from linen to cashmere safely, in any order, without waiting for the temperature to adjust or pre-sorting clothes.Philips steam irons with OptimalTEMP makes your ironing easier and faster and has been tested by independent textile experts .Convenient steam modesChoose from multiple steam modes. DynamiQ mode delivers the perfect amount of steam automatically when you need it, Max mode blasts stubborn creases with powerfulcontinuous steam, IONIC steam mode withpowerful steam bursts for more hygienic ironing and OFF steam enables you switch off the steamDynamiQ steam modeThanks to DynamiQ sensor, the most advanced motion sensor used in steam irons knowsprecisely how your iron is moving and when itsstanding still. DynamiQ steam mode releases automatically perfect amount of steam when its needed during your ironing to get the ironing results faster. The steam automatically starts when your iron is moving and stopswhen you don't move for ultimate convenience and effortless ironing.Deep Ionic SteamIonic Steam mode produces deep and powerful ionic steam bursts for more hygienic ironing for your specific garment needs.3000 W for fast heat upDelivers a fast warm-up and powerfulperformance to get your ironing done quicklySteamGlide Advanced soleplateOur superior SteamGlide Advanced soleplate delivers smooth gliding performance on any fabric. Its stainless steel base is twice as hard as a aluminum, and our patented 6-layercoating with its advanced titanium layereffortlessly glides on any fabric for the fastest results.Turbo Steam powerOur built-in Turbo Steam pump delivers up to 50% more powerful continuous steam so creases disappear even quicker.Continuous steam up to 70g/minStrong and consistent steam output penetrates up to 50% more steam through fabric to remove creases faster.Steam boost up to 260 gPenetrates deeper into fabrics to easily remove stubborn creases.SpecificationsFast crease removalContinuous steam: 70 g/minIonic Deep SteamPower: 3000 WSteam boost: 260 gVertical steamEasy to useSoleplate name: SteamGlide Advanced Water tank capacity: 350 mlExtra stable heel rest Power cord length: 2.5 mDrip stopExtra large filling holeAuto shut-offScale managementDescaling and cleaning: Quick Calc ReleaseSize and weightProduct dimensions (WxHxL): 33,3 x 17,5 x13,5 cmGuarantee2 year worldwide guaranteeGreen efficiencyUser manual: 100% recycled paper* On all ironable fabrics* Compared to GC4910© 2021 Koninklijke Philips N.V.All Rights reserved.Specifications are subject to change without notice. Trademarks are the property of Koninklijke Philips N.V. or their respective owners.Issue date 2021‑02‑03 Version: 5.0.1。

HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ENTRESTO safely and effectively. See full prescribing information for ENTRESTO.ENTRESTO™ (sacubitril and valsartan) tablets, for oral useInitial U.S. Approval: 2015WARNING: FETAL TOXICITYSee full prescribing information for complete boxed warning.∙When pregnancy is detected, discontinue ENTRESTO as soon as possible. (5.1)∙Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. (5.1)----------------------------INDICATIONS AND USAGE--------------------------- ENTRESTO is a combination of sacubitril, a neprilysin inhibitor, and valsartan, an angiotensin II receptor blocker, indicated to reduce the risk of cardiovascular death and hospitalization for heart failure in patients with chronic heart failure (NYHA Class II-IV) and reduced ejection fraction. (1.1) ENTRESTO is usually administered in conjunction with other heart failure therapies, in place of an ACE inhibitor or other ARB. (1.1)-----------------------DOSAGE AND ADMINISTRATION----------------------- ∙The recommended starting dose of ENTRESTO is 49/51 mg (sacubitril/valsartan) twice-daily. Double the dose of ENTRESTO after 2 to4 weeks to the target maintenance dose of 97/103 mg (sacubitril/valsartan)twice-daily, as tolerated by the patient. (2.1)∙Reduce the starting dose to 24/26 mg (sacubitril/valsartan) twice-daily for: -patients not currently taking an angiotensin-converting enzyme inhibitor (ACEi) or an angiotensin II receptor blocker (ARB) or previously takinga low dose of these agents (2.2)-patients with severe renal impairment (2.3)-patients with moderate hepatic impairment (2.4)Double the dose of ENTRESTO every 2 to 4 weeks to the targetmaintenance dose of 97/103 mg (sacubitril/valsartan) twice-daily, astolerated by the patient. (2.2, 2.3, 2.4) ----------------------DOSAGE FORMS AND STRENGTHS--------------------- ∙Film-coated tablets (sacubitril/valsartan): 24/26 mg; 49/51 mg; 97/103 mg(3)--------------------------------CONTRAINDICATIONS----------------------------- ∙Hypersensitivity to any component. (4)∙History of angioedema related to previous ACE inhibitor or ARB therapy.(4)∙Concomitant use with ACE inhibitors. (4, 7.1)∙Concomitant use with aliskiren in patients with diabetes. (4, 7.1)------------------------WARNINGS AND PRECAUTIONS----------------------- ∙Observe for signs and symptoms of angioedema and hypotension. (5.2, 5.3) ∙Monitor renal function and potassium in susceptible patients. (5.4, 5.5)-------------------------------ADVERSE REACTIONS------------------------------ Adverse reactions occurring ≥5% are hypotension, hyperkalemia, cough, dizziness, and renal failure. (6.1)To report SUSPECTED ADVERSE REACTIONS, contact Novartis Pharmaceuticals Corporation at 1-888-669-6682 or FDA at 1-800-FDA-1088 or /medwatch.-------------------------------DRUG INTERACTIONS------------------------------ ∙Dual blockade of the renin-angiotensin system: Do not use with an ACEi, do not use with aliskiren in patients with diabetes, and avoid use with an ARB. (4, 7.1)∙Potassium-sparing diuretics: May lead to increased serum potassium. (7.2) ∙NSAIDs: May lead to increased risk of renal impairment. (7.3)∙Lithium: Increased risk of lithium toxicity. (7.4)------------------------USE IN SPECIFIC POPULATIONS----------------------- ∙Lactation: Breastfeeding or drug should be discontinued. (8.2)∙Severe Hepatic Impairment: Use not recommended. (2.4, 8.6)See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.Revised: 7/2015_______________________________________________________________________________________________________________________________________FULL PRESCRIBING INFORMATION: CONTENTS* WARNING: FETAL TOXICITY1 INDICATIONS AND USAGE1.1 Heart Failure2 DOSAGE AND ADMINISTRATION2.1 Dosing2.2 Dose Adjustment for Patients Not Taking an ACE inhibitor orARB or Previously Taking Low Doses of These Agents2.3 Dose Adjustment for Severe Renal Impairment2.4 Dose Adjustment for Hepatic Impairment3 DOSAGE FORMS AND STRENGTHS4 CONTRAINDICATIONS5 WARNINGS AND PRECAUTIONS5.1 Fetal Toxicity5.2 Angioedema5.3 Hypotension5.4 Impaired Renal Function5.5 Hyperkalemia6 ADVERSE REACTIONS6.1 Clinical Trials Experience7 DRUG INTERACTIONS7.1 Dual Blockade of the Renin-Angiotensin-Aldosterone System7.2 Potassium-Sparing Diuretics7.3 Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) IncludingSelective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors)7.4 Lithium8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy8.2 Lactation8.4 Pediatric Use8.5 Geriatric Use8.6 Hepatic Impairment8.7 Renal Impairment10 OVERDOSAGE11 DESCRIPTION12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action12.2 Pharmacodynamics12.3 Pharmacokinetics13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility13.2 Animal Toxicology and/or Pharmacology14 CLINICAL STUDIES16 HOW SUPPLIED/STORAGE AND HANDLING17 PATIENT COUNSELING INFORMATION*Sections or subsections omitted from the full prescribing information are not listed._______________________________________________________________________________________________________________________________________FULL PRESCRIBING INFORMATIONWARNING: FETAL TOXICITY• When pregnancy is detected, discontinue ENTRESTO as soon as possible (5.1)• Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus (5.1)USAGE1 INDICATIONSANDFailure1.1 HeartENTRESTO is indicated to reduce the risk of cardiovascular death and hospitalization for heart failure in patients with chronic heart failure (NYHA Class II-IV) and reduced ejection fraction.ENTRESTO is usually administered in conjunction with other heart failure therapies, in place of an ACE inhibitor or other ARB.2 DOSAGE AND ADMINISTRATION2.1 DosingENTRESTO is contraindicated with concomitant use of an angiotensin-converting enzyme (ACE) inhibitor. If switching from an ACE inhibitor to ENTRESTO allow a washout period of 36 hours between administration of the two drugs [see Contraindications (4) and Drug Interactions (7.1)].The recommended starting dose of ENTRESTO is 49/51 mg twice-daily.Double the dose of ENTRESTO after 2 to 4 weeks to the target maintenance dose of 97/103 mg twice daily, as tolerated by the patient.2.2 Dose Adjustment for Patients Not Taking an ACE inhibitor or ARB or Previously Taking Low Doses ofThese AgentsA starting dose of 24/26 mg twice-daily is recommended for patients not currently taking an ACE inhibitor or an angiotensin II receptor blocker (ARB) and for patients previously taking low doses of these agents. Double the dose of ENTRESTO every 2 to 4 weeks to the target maintenance dose of 97/103 mg twice daily, as tolerated by the patient.2.3 Dose Adjustment for Severe Renal ImpairmentA starting dose of 24/26 mg twice-daily is recommended for patients with severe renal impairment (eGFR <30mL/min/1.73 m2). Double the dose of ENTRESTO every 2 to 4 weeks to the target maintenance dose of 97/103 mg twice daily, as tolerated by the patient.No starting dose adjustment is needed for mild or moderate renal impairment.2.4 Dose Adjustment for Hepatic ImpairmentA starting dose of 24/26 mg twice-daily is recommended for patients with moderate hepatic impairment (Child-PughB classification). Double the dose of ENTRESTO every 2 to 4 weeks to the target maintenance dose of 97/103 mg twice daily, as tolerated by the patient.No starting dose adjustment is needed for mild hepatic impairment.Use in patients with severe hepatic impairment is not recommended.3 DOSAGE FORMS AND STRENGTHSENTRESTO is supplied as unscored, ovaloid, film-coated tablets in the following strengths:ENTRESTO 24/26 mg, (sacubitril 24 mg and valsartan 26 mg) are violet white and debossed with “NVR” on one side and “LZ” on the other side.ENTRESTO 49/51 mg, (sacubitril 49 mg and valsartan 51 mg) are pale yellow and debossed with “NVR” on one side and “L1” on the other side.ENTRESTO 97/103 mg, (sacubitril 97 mg and valsartan 103 mg) are light pink and debossed with “NVR” on one side and “L11” on the other side.4 CONTRAINDICATIONSENTRESTO is contraindicated:∙in patients with hypersensitivity to any component∙in patients with a history of angioedema related to previous ACE inhibitor or ARB therapy [see Warnings and Precautions (5.2)]∙with concomitant use of ACE inhibitors. Do not administer within 36 hours of switching from or to an ACE inhibitor [see Drug Interactions (7.1)]∙with concomitant use of aliskiren in patients with diabetes [see Drug Interactions (7.1)].5 WARNINGS AND PRECAUTIONSToxicity5.1 FetalENTRESTO can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death.When pregnancy is detected, consider alternative drug treatment and discontinue ENTRESTO. However, if there is no appropriate alternative to therapy with drugs affecting the renin-angiotensin system, and if the drug is considered lifesaving for the mother, advise a pregnant woman of the potential risk to the fetus [see Use in Specific Populations (8.1)].5.2 AngioedemaENTRESTO may cause angioedema. In the double-blind period of PARADIGM-HF, 0.5% of patients treated with ENTRESTO and 0.2% of patients treated with enalapril had angioedema [see Adverse Reactions (6.1)]. If angioedema occurs, discontinue ENTRESTO immediately, provide appropriate therapy, and monitor for airway compromise. ENTRESTO must not be re-administered. In cases of confirmed angioedema where swelling has been confined to the face and lips, the condition has generally resolved without treatment, although antihistamines have been useful in relieving symptoms.Angioedema associated with laryngeal edema may be fatal. Where there is involvement of the tongue, glottis or larynx, likely to cause airway obstruction, administer appropriate therapy, e.g., subcutaneous epinephrine/adrenaline solution1:1000 (0.3 mL to 0.5 mL) and take measures necessary to ensure maintenance of a patent airway.ENTRESTO has been associated with a higher rate of angioedema in Black than in non-Black patients.Patients with a prior history of angioedema may be at increased risk of angioedema with ENTRESTO [see Adverse Reactions (6.1)]. ENTRESTO should not be used in patients with a known history of angioedema related to previous ACE inhibitor or ARB therapy [see Contraindications (4)].5.3 HypotensionENTRESTO lowers blood pressure and may cause symptomatic hypotension. Patients with an activated renin-angiotensin system, such as volume- and/or salt-depleted patients (e.g., those being treated with high doses of diuretics), are at greater risk. In the double-blind period of PARADIGM-HF, 18% of patients treated with ENTRESTO and 12% of patients treated with enalapril reported hypotension as an adverse event [see Adverse Reactions (6.1)], with hypotension reported as a serious adverse event in approximately 1.5% of patients in both treatment arms. Correct volume or salt depletion prior to administration of ENTRESTO or start at a lower dose.If hypotension occurs, consider dose adjustment of diuretics, concomitant antihypertensive drugs, and treatment of other causes of hypotension (e.g., hypovolemia). If hypotension persists despite such measures, reduce the dosage or temporarily discontinue ENTRESTO. Permanent discontinuation of therapy is usually not required.5.4 Impaired Renal FunctionAs a consequence of inhibiting the renin-angiotensin-aldosterone system (RAAS), decreases in renal function may be anticipated in susceptible individuals treated with ENTRESTO. In the double-blind period of PARADIGM-HF, 5% of patients in both the ENTRESTO and enalapril groups reported renal failure as an adverse event [see Adverse Reactions (6.1)]. In patients whose renal function depends upon the activity of the renin-angiotensin-aldosterone system (e.g., patients with severe congestive heart failure), treatment with ACE inhibitors and angiotensin receptor antagonists hasbeen associated with oliguria, progressive azotemia and, rarely, acute renal failure and death. Closely monitor serum creatinine, and down-titrate or interrupt ENTRESTO in patients who develop a clinically significant decrease in renal function [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)].As with all drugs that affect the RAAS, ENTRESTO may increase blood urea and serum creatinine levels in patients with bilateral or unilateral renal artery stenosis. In patients with renal artery stenosis, monitor renal function.5.5 HyperkalemiaThrough its actions on the RAAS, hyperkalemia may occur with ENTRESTO. In the double-blind period of PARADIGM-HF, 12% of patients treated with ENTRESTO and 14% of patients treated with enalapril reported hyperkalemia as an adverse event [see Adverse Reactions (6.1)]. Monitor serum potassium periodically and treat appropriately, especially in patients with risk factors for hyperkalemia such as severe renal impairment, diabetes, hypoaldosteronism, or a high potassium diet. Dosage reduction or interruption of ENTRESTO may be required [see Dosage and Administration (2.1)].6 ADVERSEREACTIONSClinically significant adverse reactions that appear in other sections of the labeling include:∙Angioedema [see Warnings and Precautions (5.2)]∙Hypotension [see Warnings and Precautions (5.3)]∙Impaired Renal Function [see Warnings and Precautions (5.4)]∙Hyperkalemia [see Warnings and Precautions (5.5)]6.1 Clinical Trials ExperienceBecause clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.In the PARADIGM-HF trial, subjects were required to complete sequential enalapril and ENTRESTO run-in periods of (median) 15 and 29 days, respectively, prior to entering the randomized double-blind period comparing ENTRESTO and enalapril. During the enalapril run-in period, 1,102 patients (10.5%) were permanently discontinued from the study, 5.6% because of an adverse event, most commonly renal dysfunction (1.7%), hyperkalemia (1.7%) and hypotension (1.4%). During the ENTRESTO run-in period, an additional 10.4% of patients permanently discontinued treatment, 5.9% because of an adverse event, most commonly renal dysfunction (1.8%), hypotension (1.7%) and hyperkalemia (1.3%). Because of this run-in design, the adverse reaction rates described below are lower than expected in practice.In the double-blind period, safety was evaluated in 4,203 patients treated with ENTRESTO and 4,229 treated with enalapril. In PARADIGM-HF, patients randomized to ENTRESTO received treatment for up to 4.3 years, with a median duration of exposure of 24 months; 3,271 patients were treated for more than one year. Discontinuation of therapy because of an adverse event during the double-blind period occurred in 450 (10.7%) of ENTRESTO treated patients and 516 (12.2%) of patients receiving enalapril.Adverse reactions occurring at an incidence of ≥5% in patients who were treated with ENTRESTO in the double-blind period are shown in Table 1.Table 1: Adverse Reactions Reported in ≥5% of Patients Treated with ENTRESTO in the Double-Blind PeriodENTRESTO (n = 4,203)% Enalapril (n = 4,229)%Hypotension 1812 Hyperkalemia 1214 Cough 913 Dizziness 65Renal failure/acute renal failure 5 5In the PARADIGM-HF trial, the incidence of angioedema was 0.1% in both the enalapril and ENTRESTO run-in periods. In the double-blind period, the incidence of angioedema was higher in patients treated with ENTRESTO than enalapril (0.5% and 0.2%, respectively). The incidence of angioedema in Black patients was 2.4% with ENTRESTO and 0.5% with enalapril [see Warnings and Precautions (5.2)].Orthostasis was reported in 2.1% of patients treated with ENTRESTO compared to 1.1% of patients treated with enalapril during the double-blind period of PARADIGM-HF. Falls were reported in 1.9% of patients treated with ENTRESTO compared to 1.3% of patients treated with enalapril.Laboratory AbnormalitiesHemoglobin and HematocritDecreases in hemoglobin/hematocrit of >20% were observed in approximately 5% of both ENTRESTO- and enalapril-treated patients in the double-blind period in PARADIGM-HF.Serum CreatinineIncreases in serum creatinine of >50% were observed in 1.4% of patients in the enalapril run-in period and 2.2% of patients in the ENTRESTO run-in period. During the double-blind period, approximately 16% of both ENTRESTO- and enalapril-treated patients had increases in serum creatinine of >50%.Serum PotassiumPotassium concentrations >5.5 mEq/L were observed in approximately 4% of patients in both the enalapril and ENTRESTO run-in periods. During the double-blind period, approximately 16% of both ENTRESTO- and enalapril-treated patients had potassium concentrations >5.5 mEq/L.INTERACTIONS7 DRUG7.1 Dual Blockade of the Renin-Angiotensin-Aldosterone SystemConcomitant use of ENTRESTO with an ACE inhibitor is contraindicated because of the increased risk of angioedema [see Contraindications (4)].Avoid use of ENTRESTO with an ARB, because ENTRESTO contains the angiotensin II receptor blocker valsartan. The concomitant use of ENTRESTO with aliskiren is contraindicated in patients with diabetes [see Contraindications (4)]. Avoid use with aliskiren in patients with renal impairment (eGFR <60 mL/min/1.73 m²).Diuretics7.2 Potassium-SparingAs with other drugs that block angiotensin II or its effects, concomitant use of potassium-sparing diuretics (e.g., spironolactone, triamterene, amiloride), potassium supplements, or salt substitutes containing potassium may lead to increases in serum potassium [see Warnings and Precautions (5.5)].7.3 Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) Including Selective Cyclooxygenase-2 Inhibitors (COX-2Inhibitors)In patients who are elderly, volume-depleted (including those on diuretic therapy), or with compromised renal function, concomitant use of NSAIDs, including COX-2 inhibitors, with ENTRESTO may result in worsening of renal function, including possible acute renal failure. These effects are usually reversible. Monitor renal function periodically.7.4 LithiumIncreases in serum lithium concentrations and lithium toxicity have been reported during concomitant administration of lithium with angiotensin II receptor antagonists. Monitor serum lithium levels during concomitant use with ENTRESTO.8 USE IN SPECIFIC POPULATIONS8.1 PregnancyRisk SummaryENTRESTO can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. In animal reproduction studies, ENTRESTO treatment during organogenesis resulted in increased embryo-fetal lethality in rats and rabbits and teratogenicity in rabbits. When pregnancy is detected, consider alternative drug treatment and discontinue ENTRESTO. However, if there is no appropriate alternative to therapy with drugs affecting the renin-angiotensin system, and if the drug is considered lifesaving for the mother, advise a pregnant woman of the potential risk to the fetus.The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.Clinical ConsiderationsFetal/Neonatal Adverse ReactionsOligohydramnios in pregnant women who use drugs affecting the renin-angiotensin system in the second and third trimesters of pregnancy can result in the following: reduced fetal renal function leading to anuria and renal failure, fetal lung hypoplasia, skeletal deformations, including skull hypoplasia, hypotension, and death.Perform serial ultrasound examinations to assess the intra-amniotic environment. Fetal testing may be appropriate, based on the week of gestation. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury. If oligohydramnios is observed, consider alternative drug treatment. Closely observe neonates with histories of in utero exposure to ENTRESTO for hypotension, oliguria, and hyperkalemia. In neonates with a history of in utero exposure to ENTRESTO, if oliguria or hypotension occurs, support blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and replacing renal function.DataAnimal DataENTRESTO treatment during organogenesis resulted in increased embryo-fetal lethality in rats at doses ≥ 49 mg sacubitril/51 mg valsartan/kg/day (≤ 0.14 [LBQ657, the active metabolite] and 1.5 [valsartan]-fold the maximum recommended human dose [MRHD] of 97/103 mg twice-daily on the basis of the area under the plasma drug concentration-time curve [AUC]) and rabbits at doses ≥ 5 mg sacubitril/5 mg valsartan/kg/day (4-fold and 0.06-fold the MRHD on the basis of valsartan and LBQ657 AUC, respectively). ENTRESTO is teratogenic based on a low incidence of fetal hydrocephaly, associated with maternally toxic doses, which was observed in rabbits at an ENTRESTO dose of ≥ 5 mg sacubitril/5 mg valsartan/kg/day. The adverse embryo-fetal effects of ENTRESTO are attributed to the angiotensin receptor antagonist activity.Pre- and postnatal development studies in rats at sacubitril doses up to 750 mg/kg/day (4.5-fold the MRHD on the basis of LBQ657 AUC) and valsartan at doses up to 600 mg/kg/day (0.86-fold the MRHD on the basis of AUC) indicate that treatment with ENTRESTO during organogenesis, gestation and lactation may affect pup development and survival.8.2 LactationRisk SummaryThere is no information regarding the presence of sacubitril/valsartan in human milk, the effects on the breastfed infant, or the effects on milk production. Sacubitril/valsartan is present in rat milk. Because of the potential for serious adverse reactions in breastfed infants from exposure to sacubitril/valsartan, advise a nursing woman that breastfeeding is not recommended during treatment with ENTRESTO.DataFollowing an oral dose (15 mg sacubitril/15 mg valsartan/kg) of [14C] ENTRESTO to lactating rats, transfer of LBQ657 into milk was observed. After a single oral administration of 3 mg/kg [14C] valsartan to lactating rats, transfer of valsartan into milk was observed.Use8.4 PediatricSafety and effectiveness in pediatric patients have not been established.8.5 Geriatric UseNo relevant pharmacokinetic differences have been observed in elderly (≥65 years) or very elderly (≥75 years) patients compared to the overall population [see Clinical Pharmacology (12.3)].8.6 HepaticImpairmentNo dose adjustment is required when administering ENTRESTO to patients with mild hepatic impairment (Child-Pugh A classification).The recommended starting dose in patients with moderate hepatic impairment (Child-Pugh B classification) is 24/26 mg twice daily. The use of ENTRESTO in patients with severe hepatic impairment (Child-Pugh C classification) is not recommended, as no studies have been conducted in these patients [see Dosage and Administration (2.4), Clinical Pharmacology (12.3)].Impairment8.7 RenalNo dose adjustment is required in patients with mild (eGFR 60 to 90 mL/min/1.73 m2) to moderate (eGFR 30 to 60mL/min/1.73 m2) renal impairment. The recommended starting dose in patients with severe renal impairment (eGFR <30 mL/min/1.73 m2) is 24/26 mg twice daily [see Dosage and Administration (2.3), Warnings and Precautions (5.4) and Clinical Pharmacology (12.3)].10 OVERDOSAGELimited data are available with regard to overdosage in human subjects with ENTRESTO. In healthy volunteers, a single dose of ENTRESTO 583 mg sacubitril/617 mg valsartan, and multiple doses of 437 mg sacubitril/463 mg valsartan (14 days) have been studied and were well tolerated.Hypotension is the most likely result of overdosage due to the blood pressure lowering effects of ENTRESTO. Symptomatic treatment should be provided.ENTRESTO is unlikely to be removed by hemodialysis because of high protein binding.11 DESCRIPTIONENTRESTO (sacubitril and valsartan) is a combination of a neprilysin inhibitor and an angiotensin II receptor blocker. ENTRESTO contains a complex comprised of anionic forms of sacubitril and valsartan, sodium cations, and water molecules in the molar ratio of 1:1:3:2.5, respectively. Following oral administration, the complex dissociates into sacubitril (which is further metabolized to LBQ657) and valsartan. The complex is chemically described as Octadecasodiumhexakis(4-{[(1S,3R)-1-([1,1´-biphenyl]-4-ylmethyl)-4-ethoxy-3-methyl-4-oxobutyl]amino}-4-oxobutanoate)hexakis(N-pentanoyl-N-{[2´-(1H-tetrazol-1-id-5-yl)[1,1´-biphenyl]-4-yl]methyl}-L-valinate)—water(1/15).Its empirical formula (hemipentahydrate) is C48H55N6O8Na3 2.5 H2O. Its molecular mass is 957.99 and its schematic structural formula is:ENTRESTO is available as film-coated tablets for oral administration, containing 24 mg of sacubitril and 26 mg ofvalsartan; 49 mg of sacubitril and 51 mg of valsartan; and 97 mg of sacubitril and 103 mg of valsartan. The tablet inactive ingredients are microcrystalline cellulose, low-substituted hydroxypropylcellulose, crospovidone, magnesium stearate (vegetable origin), talc, and colloidal silicon dioxide. The film-coat inactive ingredients are hypromellose, titaniumdioxide (E 171), Macrogol 4000, talc, and iron oxide red (E 172). The film-coat for the 24 mg of sacubitril and 26 mg of valsartan tablet and the 97 mg of sacubitril and 103 mg of valsartan tablet also contains iron oxide black (E 172). The film-coat for the 49 mg of sacubitril and 51 mg of valsartan tablet contains iron oxide yellow (E 172).12 CLINICAL PHARMACOLOGY12.1 Mechanism of ActionENTRESTO contains a neprilysin inhibitor, sacubitril, and an angiotensin receptor blocker, valsartan. ENTRESTOinhibits neprilysin (neutral endopeptidase; NEP) via LBQ657, the active metabolite of the prodrug sacubitril, and blocks the angiotensin II type-1 (AT 1) receptor via valsartan. The cardiovascular and renal effects of ENTRESTO in heart failure patients are attributed to the increased levels of peptides that are degraded by neprilysin, such as natriuretic peptides, by LBQ657, and the simultaneous inhibition of the effects of angiotensin II by valsartan. Valsartan inhibits the effects of angiotensin II by selectively blocking the AT 1 receptor, and also inhibits angiotensin II-dependent aldosterone release. 12.2 PharmacodynamicsThe pharmacodynamic effects of ENTRESTO were evaluated after single and multiple dose administrations in healthy subjects and in patients with heart failure, and are consistent with simultaneous neprilysin inhibition and renin-angiotensin system blockade. In a 7-day valsartan-controlled study in patients with reduced ejection fraction (HFrEF), administration of ENTRESTO resulted in a significant non-sustained increase in natriuresis, increased urine cGMP, and decreased plasma MR-proANP and NT-proBNP compared to valsartan .In a 21-day study in HFrEF patients, ENTRESTO significantly increased urine ANP and cGMP and plasma cGMP, and decreased plasma NT-proBNP, aldosterone and endothelin-1. ENTRESTO also blocked the AT 1-receptor as evidenced by increased plasma renin activity and plasma renin concentrations. In PARADIGM-HF, ENTRESTO decreased plasma NT-proBNP (not a neprilysin substrate) and increased plasma BNP (a neprilysin substrate) and urine cGMP compared with enalapril.QT Prolongation: In a thorough QTc clinical study in healthy male subjects, single doses of ENTRESTO 194 mg sacubitril/206 mg valsartan and 583 mg sacubitril/617 mg valsartan had no effect on cardiac repolarization.Amyloid-β: Neprilysin is one of multiple enzymes involved in the clearance of amyloid-β (A β) from the brain andcerebrospinal fluid (CSF). Administration of ENTRESTO 194 mg sacubitril/206 mg valsartan once-daily for 2 weeks to healthy subjects was associated with an increase in CSF A β1-38 compared to placebo; there were no changes inconcentrations of CSF A β1-40 or CSF A β1-42. The clinical relevance of this finding is unknown [see Nonclinical Toxicology(13)].Blood Pressure: Addition of a 50 mg single dose of sildenafil to ENTRESTO at steady state (194 mg sacubitril/206 mg valsartan mg once daily for 5 days) in patients with hypertension was associated with additional blood pressure (BP) reduction (~5/4 mmHg, systolic/diastolic BP) compared to administration of ENTRESTO alone.Co-administration of ENTRESTO did not significantly alter the BP effect of intravenous nitroglycerin.。

Customer Service: Email: ********************.uk• C U S T O M E R S E R V I C E • I N N O V A T I O N • M A N U F A C T U R I N G • Q U A L I T Y • T E C H N I C A L E X P E R T I S E • E X C E L L E N C E • D E S I G N •ww2T elephone: +44 (0)1772 323529 Email: ********************.ukHygienic ProductsHygienic FittingsFilters/Strainers & AccessoriesFabricationPharmaceutical High Purity Products• 3A•R JT, DIN, SMS, IDF &Clamp type available• Weld End Application:•Connections to pipework, valves and hose• M aintenance productsMaterials:• 304 and 316Lstainless steel • 0.8 standard surface fi nish • U K DairyStandard available Sizes: • ½”- 12” ODA range of high purity biopharm weld fi ttings for use inpharmaceutical industry applications.Stainless steel European unions and weld fi ttings and accessories.• Elbows • Tees • Reducers • Ferrules • Clamps • GasketsMaterials:• 316L stainless steel Sizes: • ½” – 4”Standard:• A SME Bio Processing Equipment (ASME BPE)Applications: Custom fabricated confi gurations and tubing including:• M anifolds • W all Plates • D ip Pipes • A daptors •S pecials Materials: • 316 and 304stainless steel Sizes:•½” – 12”Filters/Strainers designed to effi ciently remove particles from liquid and gases.Dixon provide a range of full fl ow, fi ne/medium strainers.Full in house CAD capability, 3D design andmanufacturing facilities to produce bespoke fabrications to meet customers’ requirements.• I n-line Strainers (short/long) • Side Entry Strainers (long)Materials:• 316L stainless steel Standard:• 3A Sanitary ID & OD(0.8Ra Max ID)Additional Accessories are available including:• Sight Glasses• Break Away Couplings • Spanners • Swivel Joints • Pipe Hangers • Gauges3T elephone: +44 (0)1772 323529 Email:********************.ukHygienic ProductsSeat & Process ValvesFeatures: • F ully Serviceable • C IPable Application:• I solation • D iversion • C ontrol • A septicMaterials:• 316L Stainless Steel Sizes:• S eat Valves; 1”- 6”• B utterfl y Valves; ½”- 4”• B all Valves, 1”- 8”• D iaphragm: ¼”- 4”Mixproof ValvesFeatures:• B ody machined from solid bar • S tandard O-rings • S ervice possible without compressed air • L ow cost ownershipCheck Valves• A ir Blow • B all• S pring Return • 3A• V arious end confi gurations availableFeatures:• Q uick disconnect clampfor quick and easy cleaning Materials:•C F-8m (316) Stainless Steel Sizes:• ½” - 4Sampling Valves Features:• V alve body from solid bar • M anual override on actuated valve •C IPable •I ncline/Angle options Application:• A septic sampling • B io-check Materials:• 304 and 316Lstainless steel Sizes:• 13mm x 1.5mm •½”- 2”Manual and automated process valves available with multiple ed in stainless steel process systems in a varietyof industries, principally Breweries & Dairies. A range of hygienic and aseptic valves used to separatenon-compatible product preventing cross ed in hygienic and industrial applicationsto prevent backfl ow.Applications:• S eparation • M anifold • D iversion• A septic version with bellows Materials:• 316L Sizes:• 1½”- 4”4T elephone: +44 (0)1772 323529 Email: ********************.ukHygienic ProductsCentrifugal PumpsTubingFeatures:•I mpeller retainer or threaded impeller nut options •S tamped volute•W et ends, motor pumpunits and carts• 3A & CIPableApplications:• U nloading • F luid transfer •C IP supply Materials:• 316L• B una, EPDM, Siliconeand FKM elastomers Sizes:• I nlet 1½”- 6”• O utlet 1½”- 4”Polished or unpolished ID/OD tubing.Usually sold in 6m lengths. Custom cut lengths available on request.Materials:• 316 and 304 stainless steel Sizes:• ½” – 8” ODStandard:• UK Dairy • A270• ASME BPEHose AssembliesHose Management & Onsite ServicesA full range of hose and hose assemblies for yourhygienic requirements:• H ygienic Food Grade Suction & Delivery Hose • S ilicone Delivery Hose • W ashdown Steam HoseOdourless and taste free, FDA approved food hose suitable for the transfer of liquid foodstuffs.Heat traced assemblies using electrical cable, steam & hot water and other special fabrications available on request.Dixon offer a hose management programme designed to help make your facilities as safe, effi cient and productive as possible.The hose management program includes the regular test and inspection of onsite hoses along with a web based documentation and management tool.Dixon-Hose Connect is a secure, user friendly web based app. It is a source to allow you to access your Hose Maintenance documentation, including Service History reports and Hose Asset Register via unique tag references and hose identifi ers, as well as training videos for your site.Used to induce fl ow or raise the pressure of a liquid/product.。


Entresto - LCZ696 产品学习简介:entresto是结合sacubitril,一脑啡肽酶抑制剂,和缬沙坦,血管紧张素Ⅱ受体阻断剂,以减少风险心力衰竭患者的心血管死亡及住院治疗慢性心力衰竭(NYHA II-IV级)和射血分数降低。
entresto通常与其他心力衰竭的联合给药治疗,在ACE抑制剂或ARB的地方。
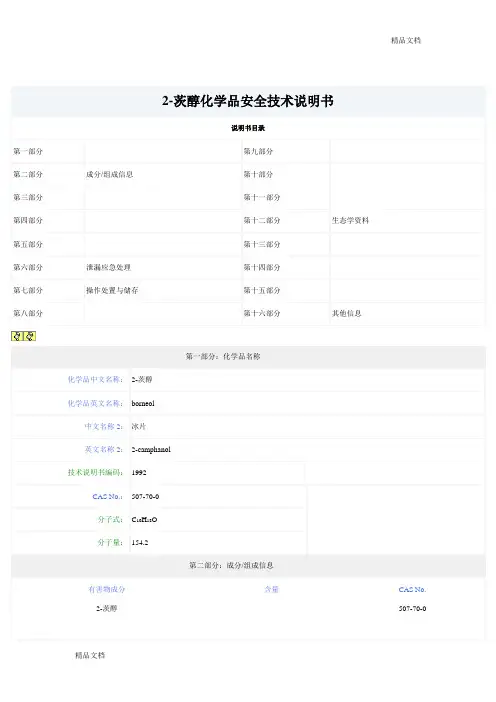
名称:Entresto研发代码:LCZ6963-(1-Biphenyl-4-ylMethyl-3-ethoxycarbonyl-1-butylcarbaMoyl)propionate-3'-Methyl-2'-(pentano yl(2'-(tetrazol-5-ylate)biphenyl-4'-ylMethyl)aMino)butyrate关键中间体:一、中文名:(R)-叔丁基(1-([1,1'-联苯]-4-基)-3-羟基丙烷-2-基)氨基甲酸酯外文名:(R)-tert-butyl (1-([1,1'-biphenyl]-4-yl)-3-hydroxypropan-2-yl)carbaMateCAS :1426129-50-1分子式:C20H25NO3分子量:327.4174用途:LCZ696中间体原研厂家:诺华下游:1012341-50-2二、中文别名:LCZ696中间体N-3 / (2R,4S)-5-(联苯-4-基)-4-[(叔丁氧基羰基)氨]-2-甲基戊酸英文名称:LCZ696 inter N-3/(2R,4S)-5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)aMino)-2-Methylpentanoic acidCAS号:1012341-50-2。

EnterokinaseMax™ (EKMax™)iiTable of ContentsTable of Contents (iii)Important Information (iv)Methods (1)Overview (1)EKMax™ Digestion (2)Appendix (6)Recipes (6)Technical Service (7)References (8)iiiImportant InformationShipping/Storage EnterokinaseMax™ and the 10X EKMax™ reaction buffer are shipped on blue iceand should be stored at -20°C.Contents EKMax™ in 50 mM potassium phosphate, pH 8.0, 500 mM NaCl and 50%glycerol, is supplied as follows:Catalog no. E180-01:Item AmountVolume EKMax™250 units, 1 unit/µl 250µl10X EKMax™ Reaction Buffer 500 mM Tris-HCl, pH 8.0 (22°C), 10 mM CaCl2, 1% Tween-20 1.5 mlCatalog no. E180-02:Item AmountVolume EKMax™1000 units, 1 unit/µl 1.0 ml10X EKMax™ Reaction Buffer 500 mM Tris-HCl, pH 8.0 (22°C), 10 mM CaCl2, 1% Tween-20 3 x 1.5 mlAdditional Materials Needed You will need to have the following materials:• 37°C water bath or heat block• Deionized water• 1.5 ml microcentrifuge tubes• SDS-PAGE apparatus and buffers (see page 4 for guidelines) • EK-Away™ Resin and buffers (Invitrogen, Catalog no. R180-01) • 15 ml polypropylene tubes (optional)• Chromatography columns (optional, Catalog no. R640-50) • Rocker or rotatorUnit Definition 1 unit is defined as the amount of EKMax™ that will digest 20 µg of BioEase™Mog1 (multicopy suppressor of GSP1, 1)fusion protein to 90% completionin 20 minutes at 37°C in 50 mM Tris-HCl, pH 8.0, 1 mM CaCl2, and0.1% Tween-20 (1X EKMax™ Buffer).For comparison purposes, 1 Invitrogen unit of EKMax™ = ~190 trypsinogenactivation units. Use this conversion only as a rough estimate of how muchEKMax™ to use. It is important to empirically determine the optimal amount ofEKMax™ to digest your fusion protein (see page 2).Limited Label License No. 62: EKMax™Enterokinase This product is sold under patent license from Genetics Institute, Inc. for Research Use Only. Licenses for commercial manufacture or use may be obtained from Genetics Institute, Inc.ivMethodsOverviewIntroduction Enterokinase is a highly specific serine protease that can be used to digest fusion proteins to release the fusion partner or tag from the desired protein. Theenzyme recognizes the sequence -(Asp)4 Lys and cleaves after the lysine residue.This cleavage sequence is present in many expression vectors available fromInvitrogen (contact Technical Service for more information). Genes cloned intothe multiple cloning site of these vectors express recombinant N-terminal fusionproteins. The native proteins can be released from the N-terminal fusion peptideor protein by digesting with enterokinase.EnterokinaseMax™ (EKMax™) is a specially prepared recombinant enzyme,consisting of the catalytic subunit of the holoenzyme. This subunit is expressedand purified from the yeast, Pichia pastoris, yielding an enzyme with higherspecific activity. This results in more efficient cleavage using less enzyme.Description of Enterokinase Enterokinase (enteropeptidase EC 3.4.21.9) is the physiological activator of trypsinogen and a serine protease that exhibits specificity for the sequence (Asp)4 Lys (Anderson et al., 1977). The bovine holoenzyme is a heterodimer consisting of a 115 kDa structural subunit and a 35 kDa catalytic subunit. The larger subunit acts as a membrane anchor and positions the catalytic subunit on the luminal side of the brush border membrane. The catalytic subunit is homologous to other serine proteases and is inhibited by chemical modification of the serine and histidine active site residues (Grant and Hermon-Taylor, 1977; Light and Liepnieks, 1979; Maroux et al., 1971).Specificity of Enterokinase It has been proposed that the active center of enterokinase possesses a distinctive cationic subsite that binds -(Asp)4. Enterokinase is highly specific and tolerates very few changes to its recognition site. If the ionic charge of the recognition site is preserved, enterokinase will recognize the site, but the rate of hydrolysis of the peptide bond will be reduced (Light and Janska, 1989). The four aspartyl residues act as a signal for enterokinase cleavage. It has been reported that with only three aspartyl residues the rate of hydrolysis is reduced. Two aspartyl residues preceding the lysyl residue are the minimum number of acidic residues needed to maintain specificity (Maroux et al., 1971). Non-specific cleavage by enterokinase may occur in the cases described above, but this is usually alleviated by reducing the amount of enzyme used.Expression of the Recombinant Catalytic Subunit EKMax™ is a clone of the catalytic subunit of enterokinase (LaVallie et al., 1993) expressed in the yeast Pichia pastoris. EKMax™ is secreted into the medium, purified, and migrates at 43 kDa on an SDS-PAGE gel. The calculated molecular weight of the protein is 26.3 kDa, but it contains three sites for asparagine-linked glycosylation. The apparent molecular weight of 43 kDa is consistent with previous observations (LaVallie et al., 1993) and is assumed to be because ofN-linked glycosylation.1EKMax™ DigestionIntroduction You will need to have pure or partially pure fusion protein. This sectiondescribes how to digest the fusion protein with EKMax™ to cleave your proteinfrom the fusion partner. This requires setting up a series of pilot reactions todetermine empirically the best conditions for digestion. The efficiency ofcleavage of EKMax™ will differ with each fusion protein. Also, if you areaccustomed to using EK3 (Biozyme), it is still necessary to test differentconcentrations of EKMax™ to determine the right amount for complete digestion.The table below outlines the steps needed to digest your fusion protein andobtain pure protein.Stage Description1 Obtain purified fusion protein at a concentration of > 0.1 mg/ml2 Dialyze (if necessary) into 1X EKMax™ Buffer3 Set up pilot reactions using different amounts of EKMax™ and digestovernight4 Assay reactions on an SDS-PAGE gel and analyze5 Optimize digestion conditions by adjusting amount of enzyme or thetemperature as needed6 Scale-up digestions to produce more of your native protein7 Purify your native protein away from the fusion partner andEKMax™Additional Materials Needed You will need to have the following materials:• 37°C water bath or heat block• Deionized water• 1.5 ml microcentrifuge tubes• SDS-PAGE apparatus and buffers (see page 4 for guidelines) • EK-Away™ Resin and buffers (Invitrogen, Catalog no. R180-01) • 15 ml snap-cap polypropylene tubes (optional)•Chromatography columns (optional, Catalog no. R640-50)• Rocker or rotatorImportantWe have found that EKMax™ will digest fusion proteins bound to theirrespective affinity columns, releasing the desired protein and leaving the fusionpartner bound to the column. For methods to digest Xpress™ fusions bound toProBond™ and thioredoxin fusion proteins in situ on ThioBond™, contactTechnical Service (see page 7).continued on next page2Obtain Purified Fusion Protein Purify at least 120 µg of your fusion protein using your system of choice following the manufacturer's instructions.• To purify thioredoxin fusion proteins, refer to the ThioBond™ manual included with the ThioBond™ resin or contact Invitrogen for information. • To purify Xpress™ fusion proteins, refer to the ProBond™ Purification System manual or contact Invitrogen for informationNote: Both manuals may be downloaded from .EKMax™ will digest fusion proteins in crude cell lysates. Note however, that you will lose your fusion tag and will have to develop a separate protocol for purification of your protein.Dialysis of the Fusion Protein It may be necessary to dialyze your fusion protein against 1X EKMax™ buffer (see Recipes, page 6) before digesting it with EKMax™. EKMax™ is inhibited by high ionic strength. 250 mM NaCl reduces EKMax™ activity to 75% of normal and 2 M NaCl almost completely inhibits enzyme activity (Barratti et al., 1973). Also, EKMax™ is known to be inhibited by > 2 M urea, > 20 mMβ-mercaptoethanol (β-ME), >0.1% SDS, > 50 mM imidazole, and pH values below 6 and above 9.Use the table below to determine if you need to dialyze your fusion protein prior to digestion with EKMax™.If your purified protein ....Then ......contains > 2 M urea, > 250 mM NaCl,> 20 mM -ME, 0.1% SDS, or > 50 mMimidazoledialyze to remove the inhibitorsis in a buffer where the pH is lowerthan 6 or higher than 9dialyze to adjust the pH to between 6and 9.is free from inhibitors and the pH isbetween 6 and 9do not dialyze. Proceed straight toPreparation of Pilot Reactions, below.Recommendation If you are not sure whether you should dialyze your protein or not, dialyze asmall volume of your protein solution and test both dialyzed and undialyzedsamples in a pilot EKMax™ digestion.continued on next page3Preparation of Pilot Reactions You will need at least 120 µg of your fusion protein for the pilot reactions. The ratio of enzyme to fusion protein to achieve complete digestion may vary depending on the protein expressed. It is very important to use only the minimal amount of EKMax™ necessary to completely digest the fusion protein. Excess EKMax™ may cause non-specific cleavage of your fusion protein in some cases.1. To determine the optimal units of EKMax™ needed for complete digestion,use five different amounts of EKMax™ (4 units, 1 unit, 0.1 unit, 0.01 unit,and 0.001 unit). For 4 units of EKMax™, use 4 µl of undiluted EKMax™. Use 1X EKMax™ buffer to make serial 10-fold dilutions of the enzyme.2. Set up 6 reactions, including a reaction without EKMax™ to control forproteases in your protein solution:Fusion Protein 20 µg10X EKMax™ Buffer 3 µlEKMax™1-4 µlDeionized Water to 29 µl(use 30 µl for the no EKMax™ control)Final Volume 30 µl3. Mix well and incubate at 37°C overnight (~16 hours). If your protein isunstable and degrades at 37°C, try incubation at 22°C, 16°C, or +4°C. It is not necessary to increase the time of digestion to compensate for the decrease in temperature.4. Prepare and load 1-20 µl on an SDS-PAGE gel (see next section). For westernblotting, load ~1 µg of your fusion protein, and for Coomassie-stained gels, load ~10 µg.If you wish to digest your fusion protein in a crude lysate, be sure to dialyze toremove any inhibitors of EKMax™. Set up your pilot reactions as described aboveto determine the amount of EKMax™ needed to digest your fusion protein.SDS-PAGEAnalysisUse an SDS-PAGE gel that will allow you to differentiate between undigested anddigested fusion protein. The type of gel and concentration of acrylamide dependson the size of your fusion protein and the fusion partner. Some fusion partners willbe too small to resolve conveniently (i.e. the Xpress™ tag). You should be able todistinguish the removal of the fusion partner from your protein, either byCoomassie-staining or by using antibody detection methods. Choose the dilutionof EKMax™ that gives you complete digestion of your recombinant fusion protein.Recommendationfor ThioredoxinFusion ProteinsA Tricine gradient gel may be necessary to visualize the 14.6 kDa thioredoxinfusion partner (Schagger and von Jagow, 1987). Alternatively, a western blot usingthe Anti-Thio™ Antibody (Catalog no. R920-25) may be used to detect theaccumulation of the thioredoxin fusion partner and/or subsequent loss of signalfrom the native protein.continued on next page4Recommendation for Xpress™Fusion Proteins Since the Xpress™ peptide is less than 4 kDa, a shift in the size of your protein may be undetectable. A western blot using the Anti-Xpress™ Antibody (Catalog no.R910-25) or the Anti-Xpress™-HRP Antibody (Catalog no. R911-25) may be necessary to visualize the cleavage and removal of the Xpress™ fusion peptide from fusion proteins. Perform a western blot and look for the loss of the signal from your protein. The Xpress™ peptide is so small, it may not transfer well to nitrocellulose or nylon, making it difficult to detect.Optimizing EKMax™ Cleavage In some cases, increasing the calcium chloride concentration to 10 mM or the amount of Tween-20 to 1% in the digestion reaction increases the activity of EKMax™. There is a possibility that EKMax™ may recognize sites that are similar to its recognition site; however, the rate of hydrolysis will be reduced. This is usually alleviated by decreasing the amount of EKMax™.Scale-Up of EKMax™ Reaction After you have optimized the EKMax™ reaction, you may scale up your digestion reaction in a linear manner. You may need a concentrated solution of fusion protein in order to scale up your digestion. Use standard ultrafiltration methods to concentrate your protein solution.Removal of EKMax™After digestion, EKMax™ may be removed by affinity chromatography on soybean trypsin inhibitor (STI) resin. For easy removal of EKMax™, EK-Away™Resin (Catalog no. R180-01) is available from Invitrogen. EK-Away™ Resin consists of soybean trypsin inhibitor immobilized on 4% beaded agarose. For a protocol to use EK-Away™ Resin, refer to the EK-Away™ manual. The manual is available at or contacting Technical Service (see page 7).Removal of Fusion Partners Fusion partners may be removed by the same affinity resin as was used to purify the fusion protein. For protocols to remove the Xpress™ tag or the thioredoxin fusion partner after EKMax™ digestion, contact Technical Service (see page 7). For other fusion proteins, consult the manufacturer of your particular system.5Appendix Recipes10X EKMax™Reaction Buffer 500 mM Tris-HCl, pH 8.010 mM CaCl21% Tween-20 (v/v)1. For 1 liter, dissolve 60.5 g Tris base in 950 ml deionized water.2. Adjust pH to 8.0 with concentrated HCl.3. Add 1.47 g CaCl2-2H2O and 10 ml Tween-20 and mix.4. Adjust the volume to 1 liter. Store at room temperature.6Technical ServiceVisit the Invitrogen website at for:• Technical resources, including manuals, vector maps and sequences,application notes, MSDSs, FAQs, formulations, citations, handbooks, etc.• Complete technical service contact information• Access to the Invitrogen Online Catalog• Additional product information and special offersContact Us For more information or technical assistance, call, write, fax, or email. Additional international offices are listed on our website (). Corporate Headquarters:Invitrogen Corporation1600 Faraday AvenueCarlsbad, CA 92008 USATel:176****7200Tel(TollFree):180****6288Fax:176****6500E-mail: ***************************Japanese Headquarters:Invitrogen JapanLOOP-X Bldg. 6F3-9-15, KaiganMinato-ku, Tokyo 108-0022Tel: 81 3 5730 6509Fax: 81 3 5730 6519E-mail: *********************European Headquarters:Invitrogen LtdInchinnan Business Park3 Fountain DrivePaisley PA4 9RF, UKTel: +44 (0) 141 814 6100Tech Fax: +44 (0) 141 814 6117E-mail: ***********************MSDS MSDSs (Material Safety Data Sheets) are available on our website at/msds.Limited Warranty Invitrogen is committed to providing our customers with high-quality goods and services. Our goal is to ensure that every customer is 100% satisfied with ourproducts and our service. If you should have any questions or concerns about anInvitrogen product or service, contact our Technical Service Representatives.Invitrogen warrants that all of its products will perform according tospecifications stated on the certificate of analysis. The company will replace, freeof charge, any product that does not meet those specifications. This warrantylimits Invitrogen Corporation’s liability only to the cost of the product. Nowarranty is granted for products beyond their listed expiration date. No warrantyis applicable unless all product components are stored in accordance withinstructions. Invitrogen reserves the right to select the method(s) used to analyze aproduct unless Invitrogen agrees to a specified method in writing prior toacceptance of the order.Invitrogen makes every effort to ensure the accuracy of its publications, butrealizes that the occasional typographical or other error is inevitable. ThereforeInvitrogen makes no warranty of any kind regarding the contents of anypublications or documentation. If you discover an error in any of our publications,please report it to our Technical Service Representatives.Invitrogen assumes no responsibility or liability for any special, incidental,indirect or consequential loss or damage whatsoever. The above limitedwarranty is sole and exclusive. No other warranty is made, whether expressedor implied, including any warranty of merchantability or fitness for a particularpurpose.continued on next pageReferencesAnderson, L. E., Walsh, K. A., and Neurath, H. (1977). Bovine Enterokinase. Purification, Specificity, and Some Molecular Properties. Biochemistry 16, 3354-3360.Barratti, J., Maroux, S., and Louvard, D. (1973). Effect of Ionic Strength and Calcium Ions on the Activation of Trypsinogen by Enterokinase. A Modified Test for the Quantitative Evaluation of the Enzyme. Biochem. Biophys. ACTA 321, 632-638.Grant, D. A. W., and Hermon-Taylor, J. (1977). Hydrolysis of Artificial Substrates by Enterokinase and Trypsin and the Development of a Sensitive Specific Assay for Enterokinase in Serum. Biochem J. 155, 243-254.LaVallie, E. R., Rehemtulla, A., Racie, L. A., Diblasio, E. A., Ferenz, C., Grant, K. L., Light, A., and McCoy, J. M. (1993). Cloning and Functional Expression of a cDNA Encoding the Catalytic Subunit of Bovine Enterokinase. J. Biol. Chem. 268, 23311-23317.Light, A., and Janska, H. (1989). Enterokinase (enteropeptidase): Comparative Aspects. TIBS 14, 110-112. Light, A., and Liepnieks, J. J. (1979). The Preparation and Purification of Bovine Enterokinase. J. Biol.Chem. 254, 1677-1683.Maroux, S., Baratti, J., and Desnuelle, P. (1971). Purification and Specificity of Porcine Enterokinase. J.Biol. Chem. 246, 5031-5039.Schagger, H., and von Jagow, G. (1987). Tricine-Sodium dodecyl sulfate-Polyacrylamide Gel Electrophoresis for the Separation of Proteins in the Range from 1 to 100 kDa. Anal. Biochem. 166, 368-379.©1998-2006 Invitrogen Corporation. All rights reserved.For research use only. Not intended for any animal or human therapeutic or diagnostic use.。

T hermo Scientific Precision Water BathsThe ultimate in performance and reliability2Precision is compact – Save valuable bench space with a smaller footprint on general purpose baths, compared to previous models.Precision is effortless – Automatically preheat and turn off your bath for efficient work scheduling with new auto-on and auto-off timers.Precision is intuitive – Simplify parameter setting and monitoring with new icon-based controller interface.Precision is safe – Protect your work with safety features including audible alarm, adjustable digital over-temperature protection, low-level detection and high-temperature cut-offs.Precision is everywhere – Access worldwide service and support when you need it, for added peace of mind. Additionally, global voltage input allows water baths to be used almost anywhere in the world for easy ordering.Precision Water BathsA new generation to support your scienceAll Precision Water Baths come with a Thermo Scientific rubber duck for approximately $1.99, included in the total price.For decades, Thermo Scientific ™ Precision ™ Water Baths have brought outstanding performance and reliability to laboratories worldwide. Now they are setting a new standard in laboratory water baths, with enhanced designs and added features to help simplify workflows and maximize productivity.With their rugged construction and advanced microprocessortechnology, Precision Water Baths are a smart choice for your smart lab.3A pplicationsThermo Scientific Precision Water BathsColiformShakingDubnoffBacteriological Examinations •••••Coagulation Tests •••Coliform Determinations •••Copper Strip Corrosion Tests •••Crude Oil Studies ••Cytochemistry •••Dialysis•Demulsibility Studies ••Enzyme Studies••Electrophoresis Gel Destaining •Environmental Studies •••••Food Processing QC ••••Genetic Studies ••••Hormone Studies ••••Immunological Research••••Incubation for Microbiological Assays ••••Incubation for Microcentrifugation Tubes ••Melting Agar •Metallurgical Analysis ••••Molecular Biology ••••Protein Analysis ••Radioactive Isotope ••••Radiochemistry •••Serological Research ••••Thawing••••Thawing Cryopreservation Vials •Tissue Culture Research ••••Virology Research ••••Warming Reagents ••Water Quality Research•••••4Precision General Purpose Water Baths are rugged, high performance baths that are designed to maintain water temperature from ambient to 100°C. Ideal for a wide range of lab applications, capacities range from 2L 1 to 28L,including shallow models. Over-temperature safety circuitry is designed to prevent thermal runaway, while new auto-on and auto-off timers allow you to optimize operation schedules. Benefit from outstanding chemical andcorrosion resistance with epoxy powder-coated exterior, and easily clean the chamber with its seamless stainless-steel interior.Additional features:• Smaller footprint, compared to previous models, frees up valuable benchtop space• Advanced microprocessor controller is designed for extended functionality• Protect your work with audible alarms• Conveniently save commonly used settings with four temperature presets• Baths come with clear polycarbonate gable cover, diffuser tray, drain hose and rubber duck• UL Listed and CE Marked; US FDA Class I Medical Device• Includes a setting for thermal beads 2Enhanced hinge designsupports open lid configuration andeasy lid removal.Help preventbath damage and overheating withlow-fluid protection.Simplify operation with quickdisconnect drain and concealed drain hose on 10, 20, 28 Land dual models.Icon-based graphical display for easy operation and monitoring.¹ 2L is designed to maintain water temperature from ambient to 90°C.Thermal beads do not provide the same performance as water; see manual for additional instructions.5GP 02 Water BathGP 2S Water BathGP 05 Water BathGP 10 Water BathGP 20 Water BathGP 28 Water BathGP 15D Water BathPrecision General Purpose Water Bath SpecificationsModelCat. No.Chamber Capacity Temp. Range Temperature Stability/Uniformity @37°C*Work Area (L x W x H)in. (mm)Overall Dimensions without Cover(L x W x H) in. (mm)Global Voltage**Heater Output †GP 02TSGP02 2 liter Amb. to 90°C ±0.1°C / ±0.2°C 5.4 x 6.1 x 5.9(138 x 155 x 150)9.1 x 7.8 x 9.2(230 x 199 x 233)100-115V/200-230V, 50/60Hz 200W GP 2S TSGP2S 2 liter (Shallow)Amb. to 100°C ±0.1°C / ±0.2°C 6 x 11.8 x 2.6 (153 x 300 x 65)9.7 x 14 x 9.1(246 x 355 x 232)100-115V/200-230V,50/60Hz 300W GP 05TSGP05 5 liter Amb. to 100°C ±0.1°C / ±0.2°C 6.1 x 11.8 x 5.9(154 x 300 x 150)9.7 x 14 x 9.1(246 x 355 x 232)100-115V/200-230V,50/60Hz 300W GP 10TSGP1010 liter Amb. to 100°C ±0.1°C / ±0.2°C 11.9 x 13 x 5.9(301 x 330 x 150) 15.5 x 15.1 x 9.2(393 x 383 x 233)100-115V/200-230V,50/60Hz 800W GP 20TSGP2020 liter Amb. to 100°C ±0.1°C / ±0.2°C 11.7 x 19.7 x 5.9(297 x 500 x 150)15.4 x 21.8 x 9.2(392 x 555 x 233)100-115V/200-230V,50/60Hz 1200W GP 28TSGP2828 liter Amb. to 100°C ±0.1°C / ±0.2°C 11.7 x 19.7 x 7.9 (297 x 500 x 200)15.4 x 21.8 x 11.1(392 x 555 x 282)100-115V/200-230V,50/60Hz 1200W GP 15DTSGP15D5 liter & 10 liter (Dual)Amb. to 100°C±0.1°C / ±0.2°CSee TSGP05 & TSGP1015.4 x 23.1 x 9.2(392 x 587 x 233)100-115V/200-230V,50/60Hz300W & 800W*Uniformity and stability tests were performed with cover installed and ambient controlled to ±1°C.** GP models come with N5-15 Plug.† Heater output at 120V and 240V.The GP 02 and GP 2S units may not reach 90°C and the GP 05 units may not reach 100°C when the supply voltage is 100VAC. The maximum Temperature value mentioned in the above table for each units canbe achieved only when the supply voltage is 120/240VAC.6Accessories for Precision General Purpose Water BathsStainless Steel Gable Cover GPSSL02GPSSL05GPSSL05GPSSL10GPSSL20GPSSL20GPSSL05and GPSSL10Concentric Ring Cover –––1546230Q 1546231Q 1546231Q –Polycarbonate Gable CoverTSGPACL02TSGPACL05TSGPACL05TSGPACL10TSGPACL20TSGPACL20TSGPACL05 and TSGPACL10Stainless Steel Petri Dish Rack ––––31661833166183–Stainless Steel Test Tube Rack ––––31616013161601–Nalgene Test Tube Half Rack White 36 x 13 mm 5972–0013TC5972–0013TC 5972–0013TC 5972–0013TC 5972–0013TC 5972–0013TC 5972–0013TC Nalgene Test Tube Half Rack White 36 x 16 mm –5972-0016TC 5972-0016TC 5972-0016TC 5972-0016TC 5972-0016TC 5972-0016TC Nalgene Test Tube Half Rack White 20 x 20 mm –5972-0020TC 5972-0020TC 5972-0020TC 5972-0020TC 5972-0020TC 5972-0020TC Nalgene Test Tube Half Rack White 16 x 25 mm –5972-0025TC 5972-0025TC 5972-0025TC 5972-0025TC 5972-0025TC 5972-0025TC Nalgene Test Tube Half Rack White 9 x 30 mm –5972-0030TC 5972-0030TC 5972-0030TC 5972-0030TC 5972-0030TC 5972-0030TC Nalgene Test Tube Full Rack Red 72 x 13 mm _5970-0513TC5970-0513TC 5970-0513TC 5970-0513TC 5970-0513TC 5970-0513TC Nalgene Test Tube Full Rack Red 72 x 16 mm __5970-0516TC 5970-0516TC 5970-0516TC 5970-0516TC 5970-0516TC Nalgene Test Tube Full Rack Red 40 x 20 mm __5970-0520TC 5970-0520TC 5970-0520TC 5970-0520TC 5970-0520TC Nalgene Test Tube Full Rack Red 40 x 25 mm __5970-0525TC5970-0525TC 5970-0525TC 5970-0525TC 5970-0525TC Nalgene Test Tube Full Rack Red 24 x 30 mm ___5970-0530TC 5970-0530TC 5970-0530TC 5970-0530TC Hand Pump102391102391102391102391102391102391102391Replacement Diff user Tray102352102353102353102354102355102355102353 &102354Nalgene Unwire Test Tube Half RackThermo Scientifi c™ Nalgene™ Unwire™ test tube racks are ideal for use in all types of water baths. They are designed not to fl oat or fade color.Learn more at: thermofi/nalgeneracksPrecision Circulating Water Baths are an ideal choice when temperature uniformity and control are particularly critical, as when working with enzymes or in serological applications. Available in three different models, these high performance baths range in capacity 19L, 35L, and 89L. The advanced temperature controller provides ±0.05°C uniformity at 70°C and stability of ±0.1°C with stainless steel gable cover.Additional features:• Achieve enhanced temperature uniformity with perimeter-directed water flow• Easily clean and maintain bath with coil-free internal design• Optimize scheduling with auto-on and auto-off timers • Accommodate taller labware with new hinged lid and extended height• Help prevent bath overheating and damage with low-fluid protection• Easily operate and monitor with icon-based graphical display• Protect your work with audible alarms• Baths include stainless steel gable covers, diffuser tray, and rubber duck• UL Listed and CE Marked; US FDA Class I Medical DeviceCIR 89 Water Bath CIR 19 Water Bath CIR 35 Water BathPrecision Circulating Water Bath SpecificationsModel Cat. No.ChamberCapacity TemperatureRangeTemperature Stability/Uniformity @37°C*Work Area(L x W x H)in. (mm)Overall Dimensionswithout Cover(L x W x H) in. (mm)GlobalVoltage**HeaterOutput†CIR 19TSCIR1919 Liter Amb. + 5°C to100°C±0.1°C / ±0.05°C 12 x 15.3 x 7.6(305 x 387 x 192)15.5 x 24.9 x 9.8(394 x 632 x 249)100-115V/200-230V, 50/60Hz1200WCIR 35TSCIR3535 Liter Amb. + 5°C to100°C±0.1°C / ±0.05°C 12 x 27.3 x 7.6(305 x 692 x 192)15.5 x 36.9 x 9.8(394 x 938 x 249)100-115V/200-230V, 50/60Hz1500WCIR 89TSCIR8989 Liter Amb. + 5°C to100°C±0.1°C / ±0.05°C 19 x 36 x 9.5(483 x 914 x 241)21.5 x 45.7 x 11.8(546 x 1160 x 300)100-115V/200-230V, 50/60Hz1500W*Uniformity and stability tests were performed with cover installed and ambient controlled to ±1°C.** GP models come with N5-15 Plug.† Heater output at 120V and 240V.78Precision Coliform Water Baths are designed specifically for fecal coliform determination. Advanced controller, featuring LCD readout for easy operation and monitoring, is factory preset to 35.0, 41.5, 44.5 and 45.5°C.Additional features:• Perimeter-directed water flow for enhanced temperature uniformity• Easily clean and maintain bath with coil-free design • Optimize scheduling with auto-on and auto-off timers • Help prevent bath overheating and damage with low-fluid protection• Easily operate and monitor with icon-based graphical display• Accommodate taller labware with new extended-height, hinged lid• Protect your work with audible alarms• Bath includes stainless steel gable cover, diffuser tray, and rubber duck• Quickly scroll through factory presets 35.0, 41.5, 44.5 and 45.5°C with the push of a button• UL Listed and CE Marked; US FDA Class I Medical DeviceCOL 19 Water BathCOL 35 Water BathPrecision Coliform Water Bath SpecificationsCat. No.Chamber Capacity Temperature Range Temperature Stability/Uniformity @37°C*Work Area (L x W x H)in. (mm)Overall Dimensions without Cover(L x W x H) in. (mm)Global Voltage**Heater Output †COL 19TSCOL1919 Liter 35.0, 41.5, 44.5 and 45.5°C ±0.1°C / ±0.05°C 12 x 15.3 x 7.6(305 x 387 x 192)15.5 x 24.9 x 9.8(394 x 632 x 249)1100-115V/200-230V, 50/60Hz 1200W COL 35TSCOL3535 Liter35.0, 41.5, 44.5 and 45.5°C±0.1°C / ±0.05°C12 x 27.3 x 7.6 (305 x 692 x 192)15.5 x 36.9 x 9.8(394 x 938 x 249)1100-115V/200-230V, 50/60Hz1500W*Uniformity and stability tests were performed with cover installed and ambient controlled to ±1°C.** GP models come with N5-15 Plug.† Heater output at 120V and 240V.9Stainless Steel Petri Dish Rack 31661833166183316618331661833166183Stainless Steel Test Tube Rack 31616013161601316160131616013161601Nalgene Test Tube Half Rack White 36 x 13 mm 5972–0013TC5972–0013TC 5972–0013TC 5972–0013TC5972–0013TC Nalgene Test Tube Half Rack White 36 x 16 mm –5972-0016TC 5972-0016TC –5972-0016TC Nalgene Test Tube Half Rack White 20 x 20 mm –5972-0020TC 5972-0020TC –5972-0020TC Nalgene Test Tube Half Rack White 16 x 25 mm –5972-0025TC 5972-0025TC –5972-0025TC Nalgene Test Tube Half Rack White 9 x 30 mm –5972-0030TC 5972-0030TC –5972-0030TC Nalgene Test Tube Full Rack Red 72 x 13 mm _5970-0513TC5970-0513TC –5970-0513TC Nalgene Test Tube Full Rack Red 72 x 16 mm __5970-0516TC –5970-0516TC Nalgene Test Tube Full Rack Red 40 x 20 mm __5970-0520TC –5970-0520TC Nalgene Test Tube Full Rack Red 40 x 25 mm __5970-0525TC –5970-0525TC Nalgene Test Tube Full Rack Red 24 x 30 mm __5970-0530TC –5970-0530TC Replacement Diffuser Tray 316008316007316006316008316007Quick Drain Kit09824609824609824609824609824610W hat is the ideal capacity of a Precision General Purpose, Circulating and Coliform Water Bath?Below are recommended quantities of beakers, flasks and test tube racks to best fit into each model.Beakers and Flasks 50 mL 133121818151224661224125 mL 122910101192136921250 mL 122488661232612500 mL 0224666410214101000 mL1331241024135972-0013TC 363672722162882882882164321152216432165972-0016TC 3607272144216216216144360648144360205972-0020TC 200404080160160120120240480120240255972-0025TC 1603232649696969616033696160305972-0030TC 901818367272545410825254108135970-0513TC 72072722162882882882164321152216432165970-0516TC 7207272144216216216144360648144360205970-0520TC 4004040120160160160120240480120240255970-0525TC 40000808080808016028080160305970-0530TC24242448969672721442887214411T hermo Scientific Precision Shaking Water BathsPrecision Shaking Water Baths support a range of sensitive life science and QA/QC applications, from warming fragile reagents to tissue culturing and genetics sequencing. Easily clean and maintain your bath with coil-free interior. Precision Shaking Water Baths are available in 15 L and 27 L capacities. The Precision Shaking Water Bath shallow form has a capacity of 15 L with a removable tray that provides a depth of 3.5 in (8.9 cm) for use with smaller sample containers.Precision Dubnoff Shaking Water Baths are designed specifi cally for applications that require your samples to be incubated in a controlled atmosphere. This bath has 15 L capacity and a depth of 3.5 in (8.9 cm). One large and two small gassing hoods are included with each bath, along with the gable cover.Additional features:• Easily clean and maintain bath with coil-free design • Optimize scheduling with auto-on and auto-off timers • Help prevent bath overheating and damage with low-fl uid protection and audible alarms• Conveniently save commonly used settings with four temperature and shaking speed presets• Easily operate and monitor with icon-based graphical display• Accommodate taller labware with new hinged lid and extended height• Baths come with stainless steel gable cover, shaking tray, and rubber duck• Adjustable shaking speed from 30 to 200 oscillations per minute – features next-generation shaker motor • UL Listed and CE Marked; US FDA Class I Medical DeviceSWB 27 Water BathPrecision Shaking Water Bath Specifi cationsModel Cat. No.ChamberCapacity TemperatureRange Temperature Stability/Uniformity @37°C*Work Area (L x W x H)in. (mm)Overall Dimensions without Cover(L x W x H) in. (mm)Global Voltage**Heater Output †SWB 15TSSWB1515 Liter Amb. + 5°C to 100°C ±0.1°C / ±0.05°C 11.5 x 12 x 6.5(292 x 305 x 165)15.5 x 24.9 x 9.8(394 x 632 x 249)100-115V/200-230V, 50/60Hz 1200W SWB 27TSSWB2727 Liter Amb. + 5°C to 100°C ±0.1°C / ±0.05°C 11.5 x 24 x 6.5(292 x 610 x 165)15.5 x 36.9 x 9.8(394 x 938 x 249)100-115V/200-230V, 50/60Hz 1500W SWB 15S TSSWB15S 15 Liter (Shallow)Amb. + 5°C to 100°C ±0.1°C / ±0.05°C 11.5 x 12 x 3.5(292 x 305 x 89)15.5 x 24.9 x 9.8(394 x 632 x 249)100-115V/200-230V, 50/60Hz 1200W DUB 15TSDUB1515 Liter (Dubnoff )Amb. + 5°C to 100°C±0.1°C / ±0.05°C11.5 x 12 x 3.5(292 x 305 x 89)15.5 x 24.9 x 9.8(394 x 632 x 249)100-115V/200-230V, 50/60Hz1200W*Uniformity and stability tests were performed with cover installed and ambient controlled to ±1°C. Fits in bath opening (L x W x H) on 15 L shaking baths 12 x 15.3 x 7.6 in.(305 x 387 x 193 mm) and on 27 L shaking baths 12 x 27.3 x 7.6 in. (305 x 692 x 193 mm).** GP models come with N5-15 Plug.† Heater output at 120V and 240V.SWB 15 Water BathSWB 15S Water BathDUB 15 Water Bath12P recision Shaking Water Bath AccessoriesAccessories for Precision Shaking Water BathsDescriptionTSSWB15TSSWB27TSSWB15S TSDUBB15S Large Gassing HoodPermits control over the atmosphere surrounding the sample. Also improves temperatureuniformity and reduces energy consumption. Each bath will accommodate 1 large hood or 2 small hoods. Measures: 11.21 x 11.35 in. (285 x 288 mm)––31626403162640Small Gassing HoodPermits control over the atmosphere surrounding the sample. Also improves temperature uniformity and reduces energy consumption. Each bath will accommodate 1 large hood or 2 small hoods. Measures: 5.6 x 11.35 in. (142 x 288 mm)––316263931626390.5 mL Microfuge Tube Rack*Secure various size microfuge tubes to the bath platform. Requires one fastener. Measures: 5 x 4 x 1–1/2 in. (127 x 101 x 39 mm)31661843166184316618431661841.0 mL Microfuge Tube Rack*Secure various size microfuge tubes to the bath platform. Requires one fastener. Measures: 5 x 4 x 1–1/2 in. (127 x 101 x 39 mm)3166185316618531661853166185Test Tube Tray 13–25 mm (holds 10 tubes)Tray containing a number of prearranged test tube clips. Does not require additional hardware.Measures 5 x 10.2 in. (127 x 259 mm)3161597316159731615973161597Flask Tray 25 mL (holds 18 fl asks)Tray containing a number of prearranged fl ask clips. Does not require additional hardware. Measures 5 x 10.2 in. (127 x 259 mm)3161599316159931615993161599Flask Tray 50mL (holds 10 fl asks)Tray containing a number of prearranged fl ask clips. Does not require additional hardware. Measures 5 x 10.2 in. (12.7 x 25.9 cm)3166228316622831662283166228High Wall Tray – Small Hold large objects (11.25 x 12.5 x 7.5 in.)3164716–31647163164716High Wall Tray – Large Hold large objects (11.25 x 24 x 7.5 in.)–3164717––Test Tube Clip 13 mm-25mm*Secure various size test tubes to the bath platform. Stainless Steel. Each requires one fastener.3166216316621631662163166216Bath Capacity20482020Flask Clips – 25 mL*Secure 25mL fl asks to the bath platform. Stainless Steel. Each requires one fastener.3166227316622731662273166227Bath Capacity20482020Flask Clips – 50 mL*Secure 50mL fl asks to the bath platform. Stainless Steel. Each requires one fastener.3166198316619831661983166198Bath Capacity15361515Flask Clips – 125 mL*Secure 125mL fl asks to the bath platform. Stainless Steel. Each requires one fastener.3166221316622131662213166221Bath Capacity92499Flask Clips – 250 mL*Secure 250mL fl asks to the bath platform. Stainless Steel. Each requires one fastener.3166566316656631665663166566Bath Capacity61466Flask Clips – 500 mL*Secure 500mL fl asks to the bath platform. Stainless Steel. Each requires one fastener.3166199316619931661993166199Bath Capacity41244Flask Clips – 1000 mL*Secure 1000mL fl asks to the bath platform. Stainless Steel. Each requires one fastener.3166200316620031662003166200Bath Capacity2522Fasteners (packs of 25)One required for each test tube clip and fl ask clip 3166189316618931661893166189Quick Drain Kit Quick Disconnect drain insert for easier draining, comes drain insert, drain tap, and hose.098246098246098246098246*Requires Fasteners (Part Number 3166189)All water baths and accessories currently not available in North America.Test Tube TrayFlask TraySmall Gassing HoodMicrofuge Tube RackHigh Wall TrayFlask Clips13What is the ideal capacity of a Precision Shaking Water Bath?Flasks and Test Tubes* Quantity of each flask clip, test tube clip and fastener that fits in each shaking water bath.All water baths and accessories currently not available in North America.Below are recommended quantities of flasks and test tubes to best fit in each model.F ind out more at /precisionbathsThis product is intended for General Laboratory Use. It is the customer’s responsibility to ensure that the performance of the product is suitable for customer’s specific use or application. © 2015-2021 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific and its subsidiaries unless otherwise specified. BRTCPRECISION COL015960 06210820。

Philips PerfectCare 7000 SeriesSteam generator ironMax 7.5 bar pressure Up to 500 g steam boost1.8L detachable watertank Carry lockPSG7028/36Fast and powerfulwith large detachable water tankPerfectCare 7000 Series is designed for your comfort and convenience. Easy to handle with detachable water tank and powerful steam to achieve great results, faster. We guarantee no burns, on all ironable garments thanks to OptimalTEMP.OptimalTEMP technology, no settings required•Guaranteed no burns•No settings to changeExtra convenience•Automatic shut off when iron is left unattended•Carry lock for safe and easy transport•Large 1.8L detachable water tank for easy refilling•Light weight and comfortable iron to handleFast & powerful•Easy and efficient descaling system for lasting performance•Powerful steam for ultimate crease removal•SteamGlide Advanced soleplate, ultimate gliding & durabilityHighlightssafe and easy auto shut off The automatic shut off function automaticallyswitches off your steam generator iron if it hasnot been used for a few minutes. This savesenergy and gives you peace of mind.Safety carry lockLock your iron securely to the base station foreasy carrying around the house and to reducethe risk of accidentally touching the hotsoleplate.Easy De-CalcRegular descaling protects your iron, extendsits lifetime, and ensures the best steam performance. Our exclusive Easy De-Calc system collects limescale continuously with an indicator light to tell you when it needs emptying. Simply remove the plug and let the water and scale particles flow out.Guaranteed no burns Even if you’re multi-tasking or get distracted, you’ll never burn your clothes. Thanks to our OptimalTEMP technology, we promise this steam generator iron will never burn any ironable fabric. You can even leave it resting face down on your clothes or ironing board. No burns, no shine. Guaranteed.No settings to change Save a step in your weekly ironing routine. You won’t need to separate fabrics or change settings and wait for the temperature to change anymore. Thanks to OptimalTEMP, you iron everything from your denim jeans to delicate silks without adjusting the temperature. It does it all for you automatically, and immediately.Ultra-powerful steam When you need to tackle tough creases with ease, rely on our continuous steam to do the hard work for you. Watch those creases meltaway when you use an extra boost of steam where you need it. And it’s perfect when you want to steam vertical curtains or refresh hanging clothes.1.8L detachable water tank 1.8-liter transparent tank gives you up to 2 hours of continuous use. When the water tank is empty, you will be reminded by the indicator light and you can refill easily at any time under the tap through the large filling door.SteamGlide Advanced soleplate Our superior SteamGlide Advanced soleplate delivers smooth gliding performance on any fabric. Its stainless steel base is twice as hard as a aluminum, and our patented 6-layer coating with its advanced titanium layer effortlessly glides on any fabric for the fastest results.Light weight iron The iron is amazingly light and comfortable to handle, gliding easily and reducing wrist strain. Its minimal weight also makes it easy and effective for vertical steaming of curtains and hanging clothes.Issue date 2023-09-04 Version: 4.4.1© 2023 Koninklijke Philips N.V.All Rights reserved.Specifications are subject to change without notice. Trademarks are the property of Koninklijke Philips N.V. or their respective owners.SpecificationsStorage•Cord storage: Velcro fixGeneral specifications•Hose storage: Compartment•Safety carry Lock•Auto shut-offEasy to use•Water tank capacity: 1800 ml•Safe on all ironable fabrics: Even delicates like silk •Heat up time: 2 minute(s)•Soleplate gliding performance: 5 stars •Soleplate name: SteamGlide Advanced •Detachable water tank•Cord freedom (swivel): 180 degree cord freedom •Refill any time during use•Power cord length: 1.65 m•Drip Stop•Hose length: 1.7 m•Ready to use: Light indicator•Soleplate scratch resistance: 5 stars•Water low indicatorGuarantee•2 year worldwide guaranteeTechnology•OptimalTEMP technology•Cyclonic steam chamber •For all ironable fabrics•No burns•No temperature settings neededFast crease removal•Pressure: Max 7.5 bar•Power: 2100 W•Continuous steam: Up to 120 g/min•Steam boost: Up to 500 g•Vertical steam•Voltage: 220-240 V•Ready to use: 2 minute(s)•Steam-on-demandGreen efficiency•Energy saving*: 30 %•Product packaging: 100% recycable•User manual: 100% recycled paperScale management•Descaling and cleaning: Easy De-calc•Descaling reminder: LightSize and weight•Weight of iron: 0.8 kg•Packaging dimensions (WxHxL): 31.5 x 31.5x47.5 cm•Product dimensions (WxHxL): 24 x 27.5 x 42 cm•Total weight with packaging: 5.3 kg•Weight of iron + base: 3.85 kg*up to 30% energy savings based on IEC 603311, compared toFastCare Compact in max. temp。
核准日期:2017年7月26日修改日期:沙库巴曲缬沙坦钠片说明书请仔细阅读说明书并在医师指导下使用【药品名称】通用名称:沙库巴曲缬沙坦钠片商品名称:诺欣妥®(Entresto®)英文名称:Sacubitril Valsartan Sodium Tablets汉语拼音:Shakubaqu Xieshatan Na Pian【成份】本品活性成份:沙库巴曲缬沙坦钠化学名称:十八钠六(4-{[(1S,3R)-1-([1,1’-联苯基)-4-基甲基)-4-乙氧基-3-甲基-4-氧代丁基]氨基}-4-氧代丁酸) 六(N-戊酰基-N-{[2’-(1H-四氮唑-5-基)联苯-4-基]甲基}-L-缬氨酸)-水(1/15)化学结构式:分子式:C24H28NNaO5・C24H27N5Na2O3・2½ H2O分子量:957.99【性状】本品为紫白色椭圆形薄膜衣片,一面凹刻有“LZ”字样,另一面凹刻有“NVR”字样(50mg规格)、或淡黄色椭圆形薄膜衣片,一面凹刻有“L1”字样,另一面凹刻有“NVR”字样(100mg规格)、或淡粉色椭圆形薄膜衣片,—面凹刻有“L11”字样,另一面凹刻有“NVR”字样(200mg规格)。
【适应症】用于射血分数降低的慢性心力衰竭(NYHA Ⅱ-Ⅳ级,LVEF ≤40%)成人患者,降低心血管死亡和心力衰竭住院的风险。
沙库巴曲缬沙坦钠片可代替血管紧张素转化酶抑制剂(ACEI)或血管紧张素Ⅱ受体拮抗剂(ARB),与其他心力衰竭治疗药物(例如:β受体阻断剂、利尿剂和盐皮质激素拮抗剂)合用。
【规格】以沙库巴曲缬沙坦计:(1)50mg(沙库巴曲24mg/缬沙坦26mg);(2)100mg(沙库巴曲49mg/缬沙坦51mg);(3)200mg(沙库巴曲97mg/缬沙坦103mg)。
【用法用量】本品可以与食物同服,或空腹服用(参见【药代动力学】)。
由于与ACE抑制剂合用时存在血管性水肿的潜在风险,禁止本品与ACEI合用。
EPA Reg. No. 84009-27EPA Est. No. 084840-LA-001RM031017A 1117/0822(03)No Root. No Weed. No Problem.▪Kills grass and weeds▪Kills over 130 types of weeds (as listed) ▪Covers up to 6,300 sq ftKEEP OUT OF REACH OF CHILDRENWARNINGSee back panel booklet for additional precautionary statements.ACTIVE INGREDIENT:Glyphosate, isopropylamine salt ................ 41.0%OTHER INGREDIENTS ........................................... 59.0%TOTAL: .....................................................................100.0%ConcentrateWeed & Grass Killer41% GlyphosateDIRECTIONS FOR USEt is a violation of Federal law to use this product in a manner inconsistent with its labeling. READ ENTIRE LABEL. USE O NLY ACCO RDING TO LABEL INSTRUCTIONS.WHEN TO USEUse anytime weeds are actively growing. Most treated weeds usually show initial symptoms within 2–4 days and complete kill in 1 to 2 weeks. Larger more established weeds may take up to 4 weeks for complete kill. For best results, apply on a warm sunny day when daytime temperature is above 60° and no rainfall is forecast for 24 hours. Warm, sunny weather will speed up weed control. Apply only when air is calm to prevent drift to desirable plants. Reapply if it rains within 2 hours after application. Rainfall or watering 2 hours after application will not wash away effectiveness. Hard-to-control weeds (such as bermudagrass) may require a repeat application if they regrow. Do not make applications for spot weed control in lawns since Compare-N-Save Concentrate Weed & Grass Killer 41% Glyphosate kills all green plants, including lawn grass.Kills:Weeds and grassesUse on:Patios, walkways or drivewaysAround fruits, vegetables, flowers, shrubs and trees Along fencesIn Large Areas:For lawn replacementFor garden replacementWHERE TO USEFor Weed Control: Use along fences, paths, patios, sidewalks and driveways, around trees, shrubs, and ornamental plantings and flower beds, around buildings, and in brick and gravel walkways. Use to trim and edge landscape areas. Do NOT use for spot weed control in lawns since this product kills all green plants, including lawn grass.Spot Spraying: Use on actively growing weeds in and around flower beds, ornamental trees, fruit and nut trees, grapevines, shrubs, fences, driveways and walkways. Spray when air is calm to prevent drift to desirable plants. Make sure this product does not contact the leaves, green stems, or exposed roots of plants you don’t want to kill. If necessary, use cardboard or plastic to shield plants. Compare-N-Save Concentrate Weed & Grass Killer 41% Glyphosate will not move in or on the soil to untreated plants. If this product is used to control weeds around fruit or nut trees or grapevines, allow 21 days before eating the fruit or nuts.Landscaping: Use to prepare areas for planting of ornamentals, trees, shrubs, desert landscapes, rock gardens, flower beds or similar plantings. Treated areas can be replanted 1 day after treatment.Lawn Renovation:• Use this product to kill existing weeds and grasses including the old lawn.• For best results, apply in Spring or Fall, when daytime temperatures are atleast 60°.• Under dry conditions, water lawn every other day for a week before usingthis product.• Do not mow for 7 days before or after treatment.• After 7 days, prepare the soil for planting by raking or rototilling the lawn. Rake up and remove loosened thatch or debris.• Mix in starter fertilizer and other amendments, if needed. Level the soil.• Apply seed according to directions on seed package, or install sod. Grass seed must have good contact with soil to germinate and grow.• Keep area moist for 2 weeks to establish new lawn, then water as needed. Flowerbed and Garden Plot Preparations: Use this product to kill weeds, grasses and brush before planting flowers, fruits, vegetables or herbs.• If soil is dry, water before application and 2 to 3 days after application.• Apply evenly to treatment area.• Wait 1 to 3 days after last application before planting flowers, trees and shrubs. Brush Control: Use when brush is green and growing. If plants are taller than 5 feet, cut back and spray regrowth. Brush sprayed in the fall may not be fully controlled until the following season. Hard-to-control species such as Blackberry, Kudzu, or Poison Oak may require a second application.Stump Treatment: Stumps can be treated to prevent regrowth. For best results, treat when the vegetation is actively growing and within 5 minutes of being cut down.• Cut the stump close to the soil surface.• Drive 4–5 nail holes into the stump.• Flood the stump with undiluted product.This treatment will control or suppress many types of woody brush trees, such as alder, blue gum, eucalyptus, madrone, oak, giant reed, saltcedar, sweetgum, and tan oak.To Kill Vines:• If vine is growing up a pole, fences, or mature tree trunk, cut vine to a height of3 to4 feet and spray to thoroughly cover remaining vine.• If vine is climbing a shrub or immature (green) tree trunk, cut vines at base and spray its regrowth. Shield shrubs and green bark from spray drift with a sheet (piece) of cardboard or plastic.HOW TO USEIMPORTANT: Compare-N-Save Concentrate Weed & Grass Killer 41% Glyphosate is an all-purpose weed and grass killer which will kill almost all plants contacted.f necessary, use cardboard or plastic to shield desirable plants. f plants are accidentally sprayed, rinse off immediately with water. Spray when air is calm to prevent drift to desirable plants. Spray to evenly wet the weed.To apply this product use:• Plastic, aluminum or stainless steel sprayer• Hand-trigger sprayerRESTRICTION: Do not use or store solutions of this product in galvanized steel or unlined steel sprayers, or apply through any type of irrigation system or with a sprinkling can.Rinse sprayer and flush all sprayer components with water 3 times after use. Spray rinse water on bare soil or gravel. After very thorough cleaning, sprayer may be used to apply other products.Use the following table to determine the amount of Compare-N-Save Concentrate Weed & Grass Killer 41% Glyphosate you need.Close cap tightly after use.APPLICATORMIXING INSTRUCTIONSAREA TOTREAT General WeedControlAnnualsTough Weed ControlPerennials or BrushLawn RenovationTank SprayerUse 1½ oz (3 Tbs)Compare-N-SaveConcentrate Weed& Grass Killer 41%Glyphosate in 1 galof waterUse 2½ oz (5 Tbs)Compare-N-SaveConcentrate Weed& Grass Killer 41%Glyphosate in 1 galof water300 sq ft (30ft x 10 ft)Hand-trigger Sprayers Use 2 tsp in 24 ozof waterUse 1 Tbs in 24 ozof waterUse for spottreatmentor singleweeds1 teaspoon = (tsp) 0.5 fl oz = 1 Tablespoon (Tbs) = 3 tsp 1 fl oz = (2 Tbs)For best results use mixture within 2 weeks.CONTROLS WEEDS AND GRASSES INCLUDING: Weed Control (Annuals)• Annual Bluegrass • Annual Ryegrass • Barnyardgrass • Beggarweed • Black Medic • Blue Mustard • Blue Toadflax • Brassbuttons • Bromegrass • Bur Clover • Buttercup • Chickweed • Common Groundsel • Common Plantain • Crabgrass• CreepingBeggarweed• DiffuseLovegrass• Dog Fennel• EveningPrimrose• Fall Panicum• Fiddleneck• Field Pennycress• Field Sandbur• Filaree• Florida Pusley• Foxtail• Garden Spurge• Goosegrass• Henbit• Horseweed/marestail• Knotweed• Lambsquarters• Little Bitter Cress• London Rocket• Maiden Cane• Mallow• Mayweed• MouseearChickweed• PennsylvaniaSmartweed• Prickly Lettuce• Prostrate Spurge• Puncture Vine• RedrootPigweed• Sandspur• Shepherdspurse• Smooth Cat’s Ear• SmoothPigweed• Sowthistle• Spoiled Spurge• Sprangletop• Tansy Mustard• Tansy Ragwort• Teaweed• Texas Panicum• Tumble Mustard• Witchgrass• Wild MustardTough Weed Control (Perennials, Brush)• Alder• Artichoke Thistle • Bahiagrass • Bentgrass • Bermudagrass • Blackberries • Broadleaf Plantain• Canada Thistle • Cattail• Ceanothus • Cherry species • Cocklebur • Cogongrass • Common Mullien• Common Ragweed• Coyote Brush • Creeping Bentgrass • Creeping Charlie • Curly Dock • Dandelion• Dallisgrass• Dewberry• Elderberry• False Dandelion• Fescue species• Field Bindweed(WildMorningglory)• Guineagrass• Honeysuckle• Horsenettle• Horseradish• Iceplant• Johnsongrass• KentuckyBluegrass• Kikuyugrass• Knapweed• Kudzu• Lantana• Milkweed• Multiflora Rose• Nightshadesilverleaf• Nimblewill• Nutsedge• Oak species• Oldenlandia• Orchardgrass• Oxalis• Pampasgrass• Pennywort• PerennialRyegrass• Poison Hemlock• Poison Oak• Poison Ivy• Primrose• Purple Nutsedge• Quackgrass• Quaking Aspen• Ragweed• Raspberry• Red Clover• SmoothBromegrass• SmoothBentgrass• St.Augustinegrass• Sumac• Tall Fescue• Timothy• Torpedo• Trumpetcreeper• Vaseygrass• Virginia Creeper• White Clover• Whitetop• Wild Barley• Wild Oats• Wild SweetPotato• Willow• Yellow Nutgrass• YellowStarthistle• ZoysiaFor information regarding specific weeds controlled by this productcall (985) 386-6042.RE-SEEDING AND REPLANTING INSPRAYED AREASWHEN TO REPLANT• All ornamental flowers, trees and shrubs may be planted 1 day after application.• The following berries may be planted 1 day after application:∙Blackberry ∙Blueberry ∙Boysenberry ∙Cranberry∙Currant∙Dewberry∙Elderberry∙Gooseberry∙Huckleberry∙Loganberry∙Olallieberry∙Raspberry(Black and Red)• The following fruits, vegetables and herbs may be planted 1 day after application:∙Artichoke (Jerusalem)∙Beans (all)∙Beet Greens ∙Broccoli∙Brussel Sprouts ∙Cabbage (all)∙Carrot ∙Cauliflower∙Celery∙Chard (Swiss)∙Chicory∙Collards∙Endive∙Horseradish∙Kale∙Kohlrabi∙Leek∙Lentils∙Lettuce∙Mustard Greens∙Okra∙Onion∙Rape Greens∙Parsley∙Parsnips∙Potato∙Turnip∙Yams• The following fruits, vegetables and herbs may be planted 3 days after application:∙Corn∙Cucumber ∙Eggplant ∙Garlic ∙Gourds∙Melons (all)∙Peas (all)∙Peppers (all)∙Pumpkin∙Squash(summer andsummer)∙Tomatillo∙Tomato (byseed)∙Watercress• Lawn grasses may be planted 7 days after application.• Tomatoes from transplant and unlisted fruits, vegetables and herbs may be planted 30 days after application.STORAGE AND DISPOSAL PESTICIDE STORAGE: Store in original container in a safe place away from direct sunlight.PESTICIDE DISPOSAL: Nonrefillable container. Do not reuse or refill this container.If empty: Place in trash or offer for recycling if available.If partly filled: Call your local solid waste agency for disposal instructions. Never place unused product down any indoor or outdoor drain. NOTICE: To the extent consistent with applicable law, buyer assumes all responsibility for safety and use not in accordance with directions. Roundup is a trademark of Monsanto Technology LLC.031017A 1117/0822(03)P E E L B A C K B O O K H E R E / J A L E A Q U Í A B R I RManufactured for:Ragan and Massey, LLC. 101 Ponchatoula Parkway Ponchatoula, LA 70454 Compare-N-Save Concentrate Weed & Grass Killer 41% GlyphosateKEEP OUT OF REACH OF CHILDRENWARNINGSee Inside Booklet for Directions for Use & Storage and Disposal.PRECAUTIONARY STATEMENTSHAZARDS TO HUMANS AND DOMESTIC ANIMALSWARNINGCauses substantial but temporary eye injury. Do not get in eyes or on clothing. Wear safety glasses. Harmful if inhaled. Avoid breathing spray mist. Wash thoroughly with soap and water after handling and before eating, drinking, chewing gum, or using tobacco. Remove and wash contaminated clothing before reuse.People and pets may enter treated areas after spray has dried.ENVIRONMENTAL HAZARDSTo protect the environment, do not allow pesticide to enter or run off into storm drains, drainage ditches, gutters or surface waters. Applying this product in calm weather when rain is not predicted for the next 24 hours will help to ensure that wind or rain does not blow or wash pesticide off the treatment area. Rinsing application equipment over the treated area will help avoid run off to water bodies or drainage systems.PHYSICAL OR CHEMICAL HAZARDSSpray solutions of this product may be mixed, stored and applied using stainless steel, fiberglass, plastic or plastic-lined steel containers. DO NOT MI X, STORE OR APPLY THI S PRODUCT OR SPRAY SOLUTIONS OF THIS PRODUCT IN GALVANIZED STEEL OR UNLINED STEEL (EXCEPT STAINLESS STEEL) CONTAINERS OR SPRAY TANKS Compare•N•Save® is a registered trademark of Tangi•Pac, L.L.C.EPA Reg. No. 84009-27 EPA Est. No. 084840-LA-001FIRST AIDIF IN EYES:• Hold eye open and rinse slowly and gently for 15–20 minutes.• Remove contact lenses, if present, after the first 5 minutes, thencontinue rinsing.• Call a poison control center or doctor for treatment advice.IF INHALED:• Move person to fresh air.• If person is not breathing, call 911 or an ambulance, then giveartificial respiration, preferably mouth to mouth if possible.• Call a poison control center or doctor for treatment advice.Emergency Medical Information• Have the product container or label with you when calling a poison control center or doctor, or going for treatment.• For medical emergencies call your poison control center at 1-800-222-1222.• This product is identified as COMPARE-N-SAVE CONCENTRATE WEED & GRASS KILLER 41% GLYPHOSATE, EPA Reg. No. 84009-27.。
ElettrologiaTrasporto della carica e correnteElettrolisiDETERMINAZIONE DELLA COSTANTE DI FARADAY.UE3020700 07/15 UDBASI GENERALIViene chiamata elettrolisi la separazione di un legame chimico per effetto della corrente elettrica. Il processo di conduzione elettrica è collegato a una separazione di materiali in cui la carica trasportata Q e la quantità di materiale separata n sono proporzionali l’una all’altra. La costante di proporzionalità viene definita costante di Faraday F ed è una costante naturale universale.Più precisamente, nella proporzionalità tra la carica Q e il numero di mole n della quantità di materiale separato deve essere considerata anche la valenza z degli ioni separati. Vale:(1) Q F n z =⋅⋅È quindi possibile determinare la costante di Faraday misu-rando, a valenza nota, la carica Q e il numero di mole n di un processo elettrolitico.Nell’esperimento, dall’acqua viene prodotta per elettrolis i una determinata quantità di idrogeno e di ossigeno. Per determi-nare la carica Q trasportata nel processo si registra l'anda-mento temporale della corrente I (t ) determinando Q per inte-grazione:(2) ()Q I t dt =⎰.Il numero di mole n H degli ioni di idrogeno separati viene determinato in base al volume di idrogeno V H2 raccolto a temperatura ambiente θ e pressione esterna p . A tale scopo occorre tuttavia tenere presente che l’idrogeno viene raccolto in forma molecolare e che per ogni molecola di idrogeno rac-colta sono stati separati due ioni di idrogeno. Dall’equazione di stato del gas ideale deriva pertanto:(3) T R V p n ⋅⋅⋅=H2H 2 Kmol J3148⋅=,R : costante dei gas universale.Per via della bassa conducibilità dell'acqua distillata, per l'elet-trolisi dell'acqua viene utilizzato acido solforico diluito nella concentrazione di 1 mol/l.Fig. 1: Apparecchio di scomposizione dell'acqua di Hofmann.ELENCO DEGLI STRUMENTI1 apparecchio discomposizione dell'acqua di Hofmann U14332 10028991 alimentatore CC 0 − 20 V, 0 − 5 A U33020 1003311/2 1 multimetro digitale con accumulatore U118241 10086311 set di 15 cavi per esperimenti, 75 cm U13800 1002840 Dotazione supplementare necessaria:1 termometro ad asta stabile U16115 10030131 barometro aneroide F U29948 1010232 Acido solforico, 1 mol/lNORME DI SICUREZZAL'acido solforico diluito ha un effetto irritante su cutee occhi.∙Indossare occhiali protettivi (integrali), guanti protettivi in neoprene o vinile e un camice da laboratorio.∙In caso di contatto con gli occhi, sciacquare immediata-mente con abbondante acqua e consultare il medico. MONTAGGIOMessa in funzione dell'apparecchio di scomposizione dell'acqua di Hofmann∙Montare l'apparecchio di scomposizione dell'acqua di Hofmann seguendo le istruzioni per l'uso e sistemandolo se necessario in un contenitore idoneo.∙Fissare quindi il recipiente di livello alla massima altezza possibile dell'asta di supporto (Fig. 1). Sfilare il più possi-bile l'anello dal manicotto.∙Aprire i due rubinetti smerigliati collocati sui tubi di raccol-ta del gas.∙Versare circa 200 ml di acido solforico diluito (1 mol/l) dal flacone di conservazione in un becher adatto.∙Trasferire con cautela l'acido solforico diluito versandolo dal becher nel recipiente di livello. A tale scopo introdurre leggermente il beccuccio del becher nell'apertura del re-cipiente di livello. Durante la fase di riempimento, abbas-sare gradualmente l'altezza del recipiente di livello fino a quando i tubi di raccolta del gas non sono completamen-te pieni. Al termine della procedura di riempimento, l'al-tezza del recipiente di livello dovrà essere tale per cui la colonna di liquido nel recipiente stesso e nei due tubi di raccolta del gas sia in primo luogo alla medesima altezzae in secondo luogo all'altezza dei rubinetti smerigliati (Fig.2 in alto).∙Collegare il polo negativo dell’alimentatore a corrente continua all'elettrodo del tubo di raccolta del gas sinistro e, tra di essi, inserire in serie un multimetro per la misu-razione della corrente (Fig. 2 in alto). Collegare il polo positivo dell’alimentatore CC all'elettrodo del tubo di ra c-colta del gas destro.Fig. 2: Rappresentazione schematica della disposizione per la misurazione all'inizio (in alto) e alla fine (in basso) dellamisurazione stessa.Installazione del software del multimetro digitaleNon va utilizzato il software fornito in dotazione con il multi-metro digitale, bensì una versione più attuale.∙Non collegare ancora al computer.∙Scaricare il software (file .rar) qui:www.3bscientific.de/datenbank/download/Software-for-1008631.zip∙Estrarre il file .rar in un'apposita cartella.∙ Eseguire "setup.exe" e seguire le istruzioni.Messa in funzione del multimetro digitale∙Collegare il multimetro digitale al computer utilizzando il cavo ottico USB fornito.∙Accendere il multimetro digitale premendo simultanea-mente il tasto "PC-LINK". Sul display del multimetro digi-tale compare in alto a sinistra "PC-LINK". Alla prima ac-censione, il computer dovrebbe riconoscere automatica-mente come nuovo hardware e installare il multimetro di-gitale.∙Avviare il software. Premere "Pausa" nella barra del menu. In basso a sinistra appare il messaggio "Registra-zione arrestata – Fare clic su Pausa per continuare".∙Premere nella barra del menu "Collegamento", sotto "Apparecchio:" selezionare il multimetro portatile 3415 e confermare con "OK".∙Nella barra del menu premere un'altra volta "Collegamen-to" e, alla voce "Collega", selezionare la porta COM corri-spondente. Poiché "Pausa" è attivo (v. sopra), il multime-tro digitale non parte ancora con la registrazione dei valo-ri misurati.∙Nella barra del menu premere "Avanzate", poi "Interval-lo…". Nella finestra pop-up che si apre ("Imposta interval-lo"), sotto "Valore" registrare 00:00:01, che significa che viene rilevato un valore di misurazione al secondo. ESECUZIONEPreparazione∙Chiudere i due rubinetti smerigliati.∙Accendere l'alimentatore CC, impostare la tensione mas-sima U0 = 20 V (I ≈ 0,75 A) e far funzionare l'elettrolisi per circa 5 minuti senza registrare i valori di misurazione rela-tivi alla saturazione del liquido con il gas.∙Staccare la tensione U0.∙Aprire con cautela i due rubinetti smerigliati di modo che dal tubo di raccolta del gas sinistro esca H2e da quello destro O2 e che si ripristini il volume di liquido originale. Misurazione∙Richiudere i due rubinetti smerigliati.∙Accendere la tensione U0= 20 V.∙Osservare la generazione di gas nel tubo di raccolta del gas H2 (polo negativo) dell'apparecchio di scomposizione dell'acqua e far scorrere verso il basso il recipiente di li-vello di modo che la colonna di liquido presente all'interno di quest'ultimo e nel tubo di raccolta del gas H2 rimanga alla medesima altezza (Fig. 2 in basso).Non appena la colonna di liquido raggiunge la tacca dei 5 ml:∙Premere "Pausa" nella barra del menu del software per avviare la registrazione dei valori di misurazione. La cor-rente aumenta molto lentamente circa 5 mA/min. Non appena la colonna di liquido raggiunge la tacca dei 25 ml:∙Nella barra del menu del software premere un'altra volta "Pausa" per interrompere la registrazione dei valori di mi-surazione e togliere la tensione U0.∙Nella barra del menu del software, premere "File" e con "Salva…" salvare i dati di misurazione in formato csv (per Excel®).∙Misurare la temperatura ambiente θe la pressione atmo-sferica p annotando entrambi i valori.ESEMPIO DI MISURAZIONEVolume iniziale V1: 5 cm3 Volume finale V2: 25 cm3 Temperatura ambiente θ: 27,5°C Pressione atmosferica p:1012 hPaFig. 3: Rappresentazione dei valori di misurazione registrati con il multimetro digitale in Excel® (estratto dal filecsv).ANALISI∙ Aprire il file csv con i valori di misurazione della corrente in funzione del tempo ad es. in Excel® (Fig. 3).∙ Convertire i valori di tempo nella colonna "DateTime" nel formato "Numero" con 5 posizioni decimali.∙ Sottrarre da tutti i valori nella colonna "DateTime" il valore della prima cella.∙Moltiplicare tutti i valori nella colonna "DateTime" per 86400 s, quindi convertire nel formato "Numero" con 0 posizioni decimali. I valori di tempo ora sono espressi insecondi (Fig. 4).Fig. 4: Rappresentazione dei valori di misurazione registraticon il multimetro digitale in Excel® (estratto dal file csv) dopo l'adeguamento del formato data-tempo.∙ Riportare graficamente i valori misurati per la corrente(colonna "Value") rispetto al tempo in secondi (colonna "DateTime") (Fig. 5).501001502002500200400600800I / mAt / sFig. 5: Dipendenza della corrente dal tempo.La corrente aumenta nel tempo in maniera pressoché lineare. ∙Adattare una retta I (t ) = m ·t + k ai punti di misurazione: (4) 5A 6,010s 0,7554Am k -=⋅=.∙Determinare la carica Q in base a (2) come segue (t max = 214 s, v. Fig. 4):(5)()maxmaxmaxmax02max max 0()12163t t t t Q I t dt m t k dtm t dt k dt m t k t As==⋅+=⋅+=⋅⋅+⋅=⎰⎰⎰⎰. ∙Calcolare il volume di idrogeno: (6) 3H22120cm V V V =-=. ∙Calcolare la temperatura assoluta: (7) 273K 300,5K T =θ+=.Per la valenza degli ioni di idrogeno vale z H = 1. Dalle equazioni (1) e (3) si ottiene quindi l’equazione condizionale per la costa n-te di Faraday(8) H2H H222R T R TF Q Q p V n p V ⋅⋅=⋅=⋅⋅⋅⋅⋅⋅.Con le grandezze calcolate in (5), (6) e (7) e la pressione atmo-sferica misurata p si ottiene infine il valore(9) 43J8,324300,5KAs mol K 163As 10,11021012hPa 20cm molF ⋅⋅=⋅=⋅⋅⋅. Il valore risultante dalla misurazione coincide fino al 5% con ilvalore di letteratura F = 9,6 · 104 As/mol.A titolo di confronto, può essere determinato anche il volume V O2 dell’ossigeno raccolto. Esso sarà pari solo alla metà del volume di idrogeno, in quanto per ogni molecola di acqua scissa si separano due ioni di idrogeno e uno di ossigeno. Pertanto la valenza degli ioni di ossigeno sarà z O = 2.INFORMAZIONI AGGIUNTIVESi riscontrano errori sistematici associati soprattutto a solu-zione dell'ossigeno nell'elettrolita, bolle di gas trattenute sullepareti di vetro, aumento della temperatura dell'elettrolita e delgas in seguito al passaggio di corrente.L'ossigeno atomico separato con l'elettrolisi reagisce in partecon la formazione di acido persolforico. La quantità di ossige-no raccolta è pertanto leggermente inferiore rispetto allaquantità di ossigeno espulsa. Per questo, per effettuare lavalutazione si prende la quantità di idrogeno.3B Scientific GmbH, Rudorffweg 8, 21031 Amburgo, Germania, 。
Entresto(sacubitril和缬沙坦[valsartan])使用说明书2015年第一版批准日期:2015年7月7日;公司:Novartis Pharmaceuticals CorporationFDA的药品评价和研究中心心血管和肾病产品部主任说:“心力衰竭是成年死亡和残疾主要原因,”“治疗可帮助有心衰人们活得更长和享受更积极的生命。
”优先审评和快速通道指定/drugsatfda_docs/label/2015/207620Orig1s000lbl.pdf处方资料重点这些重点不包括安全和有效使用ENTRESTO所需所有资料。
请参阅ENTRESTO完整处方资料。
ENTRESTO™(sacubitril和缬沙坦[valsartan])片,为口服使用美国初次批准:2015适应证和用途ENTRESTO是sacubitril,一种脑啡肽酶[neprilysin]抑制剂,和缬沙坦,一种血管紧张素II受体阻断剂组合复方,适用于在有慢性心力衰竭(NYHA类别II-IV)患者中减低对心力衰竭心血管死亡和住院的风险和减少射血分数。
(1.1)ENTRESTO是通常与其他心力衰竭治疗结合,代替一种ACE抑制剂或其他血管紧张素II受体阻断剂[ARB]。
(1.1)剂量和给药方法⑴ENTRESTO的推荐起始剂量是49/51 mg(sacubitril/缬沙坦)每天2次。
当患者耐受,2至4周后加倍ENTRESTO剂量至目标维持剂量97/103 mg(sacubitril/缬沙坦)每天2次。
(2.1)⑵对以下患者减低起始剂量至24/26 mg(sacubitril/缬沙坦)每天2次:①患者当前未服用一种血管紧张素-转化酶抑制剂(ACEi)或一种血管紧张素II受体阻断剂(ARB)或以前用低剂量这些药物(2.2)②有严重肾受损患者(2.3)③有中度肝受损患者(2.4)当被患者耐受时,每2至4周加倍ENTRESTO的剂量至目标维持剂量97/103 mg(sacubitril/缬沙坦)每天2次。
(2.2,2.3,2.4)剂型和规格膜-包衣片(sacubitril/缬沙坦):24/26 mg; 49/51 mg; 97/103 mg(3)禁忌证⑴对任何组分超敏性。
(4)⑵与以前ACE抑制剂或血管紧张素II受体阻断剂[ARB]治疗相关的血管水肿病史。
(4)⑶与ACE抑制剂同时使用。
(4,7.1)⑷在有糖尿病患者中同时使用与阿利吉仑[aliskiren]。
(4,7.1)警告和注意事项⑴观察对血管水肿和低血压的体征和症状。
(5.2,5.3)⑵在易感患者中监视肾功能和钾。
(5.4,5.5)不良反应发生≥5%不良反应是低血压,高钾血症,咳嗽,眩晕,和肾衰竭。
(6.1)报告怀疑不良反应,联系Novartis制药公司电话1-888-669-6682或FDA电话1-800-FDA-1088或/medwatch. 药物相互作用⑴肾素-血管紧张素系统的双重阻断:不要与一种ACEi使用,在患者有糖尿病不要与阿利吉仑使用,和避免与一种血管紧张素II受体阻断剂[ARB]使用。
(4,7.1)⑵保钾利尿剂:可能导致血清钾增加。
(7.2)⑶NSAIDs:可能导致肾受损的风险增加。
(7.3)⑷锂:增加锂毒性的风险。
(7.4)在特殊人群中使用⑴哺乳:应终止哺乳喂养或药物。
(8.2)⑵严重肝受损:建议不使用。
(2.4,8.6)完整处方资料1 适应证和用途1.1 心力衰竭ENTRESTO适用于对有慢性心力衰竭(NYHA类别II-IV)患者心力衰竭减低心血管死亡及住院的风险和减低射血分数。
ENTRESTO通常与其他心力衰竭治疗结合,替代一种ACE抑制剂或其他ARB。
2 剂量和给药方法2.1 给药ENTRESTO是禁忌与一种血管紧张素-转化酶(ACE)抑制剂同时使用。
如从一种ACE抑制剂转换至ENTRESTO允许这两种药物给药间一个36小时冲洗期[见禁忌证(4)和药物相互作用(7.1)]。
ENTRESTO的推荐起始剂量是49/51 mg每天2次。
当2至4周后患者耐受时加倍ENTRESTO剂量至目标维持剂量97/103 mg每天2次。
2.2 对没有用一种ACE抑制剂或ARB或以前用这些药物低剂量患者剂量调整对当前没有用一种ACE抑制剂或一种血管紧张素II受体阻断剂(ARB)和对患者以前用这些药物低剂量患者建议24/26 mg每天2次的起始剂量。
当患者耐受时每2至4周加倍ENTRESTO剂量至目标维持剂量97/103 mg每天2次。
2.3 对严重肾受损剂量调整建议对有严重肾受损(eGFR <30 mL/min/1.73 m2)患者的起始剂量24/26 mg每天2次。
当患者耐受时每2至4周加倍ENTRESTO剂量至目标维持剂量97/103 mg每天2次。
对轻度或中度肾受损无需起始剂量调整。
2.4 对肝受损剂量调整建议对有中度肝受损(Child-Pugh B分类)患者的起始剂量24/26 mg每天2次。
当患者耐受时每2至4周加倍ENTRESTO剂量至目标维持剂量97/103 mg每天2次。
对轻度肝受损无需起始剂量调整。
建议有严重肝受损患者不使用。
3 剂型和规格ENTRESTO以无刻痕[unscored],椭圆形,膜包衣片以下列规格供应:ENTRESTO 24/26 mg,(sacubitril 24 mg和缬沙坦26 mg)为紫白色和在一侧凹陷有“NVR”和另一侧“LZ”。
ENTRESTO 49/51 mg,(sacubitril 49 mg和缬沙坦51 mg)是淡黄色和一侧凹陷有“NVR”和另一侧“L1”。
ENTRESTO 97/103 mg,(sacubitril 97 mg和缬沙坦103 mg)是浅粉红色和一侧凹陷有“NVR”和另侧“L11”。
4 禁忌证以下患者禁忌ENTRESTO:●在对任何组分超敏性患者●有与以前ACE抑制剂或ARB治疗相关血管水肿病史患者[见警告和注意事项(5.2)]●与ACE抑制剂的同时使用。
转换从或至一个ACE抑制剂36小时内不要给予[见药物相互作用(7.1)]●在有糖尿病患者中同时使用阿利吉仑[见药物相互作用(7.1)].5 警告和注意事项5.1 胎儿毒性当给予妊娠妇女ENTRESTO可能致胎儿危害。
在妊娠的第二和第三个三个月期间使用作用在肾素-血管紧张素系统药物减低胎儿肾功能和增加胎儿和新生儿患病率和死亡。
当检测到妊娠,考虑另外药物治疗和终止ENTRESTO。
但是,如对治疗没有适当替代影响肾素-血管紧张素系统药物,和如考虑药物挽救母亲生命,忠告妊娠妇女对胎儿潜在风险[见在特殊人群中使用(8.1)]。
5.2 血管水肿ENTRESTO可能致血管水肿.。
在PARADIGM-HF试验双盲期,用ENTRESTO治疗患者0.5%和用依那普利治疗患者[enalapril]0.2%有血管水肿[见不良反应(6.1)]。
如发生血管水肿,立即终止ENTRESTO,提供适当治疗,和监视对气道损害。
ENTRESTO必须不再给予。
确证的血管水肿情况中其中肿胀曾被局限于面和唇,情况一般地无治疗,虽然抗组胺药在缓解症状中曾有用。
血管水肿伴随喉头水肿可能是致命的。
其中牵连舌,声门或咽喉,可能致气道阻塞,给予适当治疗,如,皮下肾上腺素[epinephrine/adrenaline]溶液1:1000(0.3 mL至0.5 mL)和需要采取措施确保患者气道的维持。
在黑种人中比非黑种人患者ENTRESTO曾伴随较高血管水肿发生率。
有既往血管水肿病史患者用ENTRESTO可能处于血管水肿的风险增高[见不良反应(6.1)]。
在有已知与既往ACE抑制剂或ARB治疗相关血管水肿病史患者不应使用ENTRESTO[见禁忌证(4)]。
5.3 低血压ENTRESTO降低血压和可能致症状性低血压。
有一个活化的肾素-血管紧张素系统患者,例如容积- 和/或盐-耗竭患者(如,那些正在用高剂量利尿剂治疗),是处于更大风险。
在PARADIGM-HF的双盲期,18%的用ENTRESTO治疗患者和12%的用依那普利治疗患者报道低血压作为一种不良事件[见不良反应(6.1)],在两个治疗臂报道约1.5%患者低血压为严重不良事件。
ENTRESTO给药前或在一个较低剂量开始时纠正容积或盐耗竭。
如发生低血压,考虑利尿剂,同时抗高血压药的剂量调整,和治疗低血压的其他原因(如,低血容量)。
如尽管这类措施低血压持续,减低剂量或暂时地终止ENTRESTO。
通常不需要永久终止治疗。
5.4 受损的肾功能作为抑制肾素-血管紧张素-醛固酮系统(RAAS)的后果,在易感个体用ENTRESTO治疗可以预期肾功能减低。
在PARADIGM-HF试验的双盲期中,在ENTRESTO和依那普利组都有5%的患者报道肾衰竭作为一种不良事件[见不良反应(6.1)]。
在患者其肾功能依赖于肾素-血管紧张素-醛固酮系统的活性(如,患者有严重充血性心力衰竭),用ACE抑制剂和血管紧张素受体拮抗剂治疗曾伴随少尿,渐进氮质血症和,罕见地,急性肾衰竭和死亡。
严密监视血清肌酐,和在患者发生临床上显著肾功能减低滴定下调整或中断ENTRESTO[见在特殊人群中使用(8.7)和临床药理学(12.3)]。
如同所有影响RAAS药物,在有双侧或单侧肾动脉狭窄患者ENTRESTO可能增加血尿素和血清肌酐水平。
在有肾动脉狭窄患者,监视肾功能。
5.5 高钾血症通过其对RAAS的作用,用ENTRESTO可能发生高钾血症。
PARADIGM-HF试验的双盲期中,12%的用ENTRESTO治疗患者和14%的用依那普利治疗患者报道高钾血症作为一个不良事件[见不良反应(6.1)]。
定期地监视血清钾和适当地治疗,尤其是在在有对高钾血症风险因子患者例如严重肾受损,糖尿病,醛固酮减少症,或一个高钾膳食。
可能需要剂量减少或中断ENTRESTO[见剂量和给药方法(2.1)]。
6 不良反应在说明书的其他节中出现临床上显著不良反应包括:●血管水肿[见警告和注意事项(5.2)]●低血压[见警告和注意事项(5.3)]●受损的肾功能[见警告和注意事项(5.4)]●高钾血症[见警告和注意事项(5.5)]6.1 临床试验经验因为临床试验是在广泛不同情况下进行的,临床试验观察到不良反应率不能与另一种药临床试验发生率直接比较而且可能不反映实践中观察到的发生率。
在PARADIGM-HF试验中,进入随机化双盲期比较ENTRESTO和依那普利前,受试者被要求分别完成顺序依那普利和ENTRESTO磨合期(中位)15和29天。
依那普利磨合期时,1,102例患者(10.5%)被永久地从研究终止,5.6%因为一种不良事件,大多数常是肾功能不全(1.7%),高钾血症(1.7%)和低血压(1.4%)。