群勃龙.庚酸睾酮.康力龙报价单模板(中英文)
- 格式:xls
- 大小:11.01 KB
- 文档页数:2
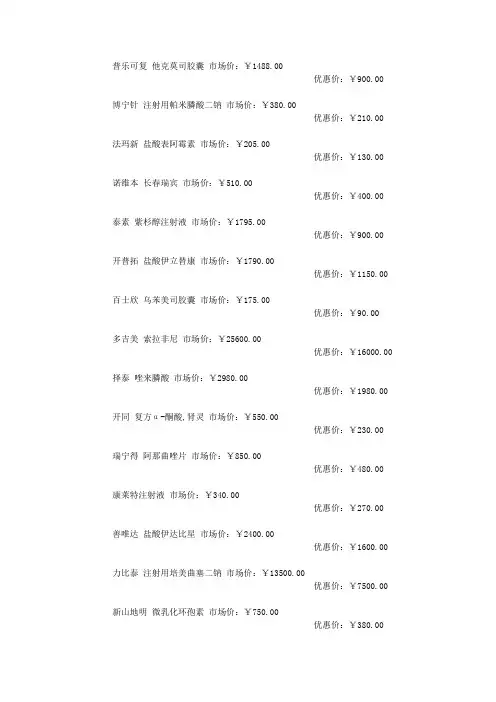
普乐可复他克莫司胶囊市场价:¥1488.00优惠价:¥900.00博宁针注射用帕米膦酸二钠市场价:¥380.00优惠价:¥210.00法玛新盐酸表阿霉素市场价:¥205.00优惠价:¥130.00诺维本长春瑞宾市场价:¥510.00优惠价:¥400.00泰素紫杉醇注射液市场价:¥1795.00优惠价:¥900.00开普拓盐酸伊立替康市场价:¥1790.00优惠价:¥1150.00百士欣乌苯美司胶囊市场价:¥175.00优惠价:¥90.00多吉美索拉非尼市场价:¥25600.00优惠价:¥16000.00择泰唑来膦酸市场价:¥2980.00优惠价:¥1980.00开同复方α-酮酸,肾灵市场价:¥550.00优惠价:¥230.00瑞宁得阿那曲唑片市场价:¥850.00优惠价:¥480.00康莱特注射液市场价:¥340.00优惠价:¥270.00善唯达盐酸伊达比星市场价:¥2400.00优惠价:¥1600.00力比泰注射用培美曲塞二钠市场价:¥13500.00优惠价:¥7500.00新山地明微乳化环孢素市场价:¥750.00优惠价:¥380.00爱必妥西妥昔单抗市场价:¥4698.00优惠价:¥2450.00泰索帝多西紫杉醇注射液市场价:¥2300.00优惠价:¥1400.00抑那通注射用缓释醋酸亮丙瑞林市场价:¥2550.00优惠价:¥1600.00赫赛汀注射用曲妥珠单抗市场价:¥25800.00优惠价:¥14500.00骁悉吗替麦考酚酯片市场价:¥770.00优惠价:¥500.00福至尔市场价:¥1680.00优惠价:¥1000.00华蟾素片市场价:¥290.00优惠价:¥100.00康士得(比卡鲁胺片)市场价:¥1596.00优惠价:¥1200.00紫龙金片市场价:¥231.00优惠价:¥120.00西黄丸北京同仁堂全国最低价格市场价:¥480.00优惠价:¥230.00参一胶囊市场价:¥248.00优惠价:¥120.00金龙胶囊市场价:¥207.00优惠价:¥110.00慈丹胶囊市场价:¥241.00优惠价:¥140.00艾瑞宁市场价:¥1200.00优惠价:¥650.00 日达仙市场价:¥1690.00优惠价:¥900.00健择市场价:¥549.00优惠价:¥400.00健脾益肾颗粒市场价:¥80.00优惠价:¥45.00金克槐耳颗粒市场价:¥160.00优惠价:¥90.00易瑞沙(英国)市场价:¥5500.00优惠价:¥4500.00易瑞沙(印度)全国最低价格市场价:¥2800.00优惠价:¥1500.00。



Package: Blister Pack (10 tablets per blister)Ingredients:- Active Ingredient: Ibuprofen (200 mg)- Inactive Ingredients: Magnesium stearate, cellulose, lactose monohydrate, pregelatinized starch, croscarmellose sodium, hypromellose, titanium dioxide, and iron oxide.Indications:Difene Tablets are a nonsteroidal anti-inflammatory drug (NSAID) used to relieve symptoms of various conditions, including:- Pain: such as headache, dental pain, menstrual cramps, arthritis, backache, and muscular pain.- Fever: to reduce feverish symptoms.- Inflammation: to reduce inflammation in conditions such as arthritis, ankylosing spondylitis, and tendinitis.Contraindications:Do not use Difene Tablets if you have any of the following conditions:- Hypersensitivity to ibuprofen or any other NSAID.- Severe kidney disease.- Active peptic ulcer or gastrointestinal bleeding.- Third trimester of pregnancy.- Severe liver disease.- Hemorrhagic diathesis.- Children under 12 years of age (unless directed by a physician).Warnings and Precautions:- Consult a healthcare professional before taking Difene Tablets if you have a history of stomach ulcers, gastrointestinal bleeding, heart disease, high blood pressure, liver or kidney disease, asthma, or if you are taking any other medications.- Do not exceed the recommended dose to avoid potential side effects.- Do not use Difene Tablets for more than 10 days unless directed by a healthcare professional.- Avoid alcohol consumption while taking Difene Tablets.- Use caution when driving or operating machinery as Difene Tablets may cause drowsiness or dizziness in some individuals.- Difene Tablets may interact with certain medications, including anticoagulants, corticosteroids, and diuretics. Consult a healthcare professional if you are taking any of these medications.Dosage:The recommended dosage of Difene Tablets is as follows:- Adults and children over 12 years of age: 1 tablet every 4 to 6 hours as needed, not to exceed 6 tablets in 24 hours.- Elderly patients: Adjust dosage based on renal function and healthcare professional advice.How to Use:- Swallow the tablet whole with a glass of water.- Do not chew or crush the tablet.- Take Difene Tablets with or without food.Side Effects:The following side effects may occur while taking Difene Tablets:- Gastrointestinal: Nausea, vomiting, stomach pain, heartburn, indigestion, diarrhea, constipation, and ulcers.- Hematologic: Increased risk of bleeding and bruising.- Dermatologic: Skin rash, itching, and hives.- Cardiovascular: Increased blood pressure, heart failure, and myocardial infarction.- Central nervous system: Dizziness, headache, and drowsiness.Overdose:If an overdose is suspected, contact a healthcare professional immediately. Symptoms of an overdose may include severe stomach pain, vomiting, bleeding, and kidney damage.Storage:- Store Difene Tablets at room temperature (15°C to 30°C or 59°F to 86°F).- Keep the blister pack tightly closed when not in use.- Protect from light and moisture.- Do not use after the expiration date.Manufactured by:[Manufacturing Company Name][Address][City, State, ZIP Code]Please read this leaflet carefully before taking Difene Tablets. If you have any questions or concerns, consult your healthcare professional.---Note: This is a fictional product and the information provided here is for illustrative purposes only. The actual dosage, contraindications, warnings, and side effects may vary based on the specific product andits labeling. Always consult the product's official labeling and a healthcare professional before use.。

litron laboratories 价目表
【原创版】
目录
1.介绍 Litron Laboratories
2.Litron Laboratories 的价目表概述
3.价目表中的具体项目和价格
4.对价目表的评价和建议
正文
Litron Laboratories 是一家致力于提供高质量实验室设备和服务
的公司。
他们的产品和服务涵盖了实验室的各个方面,包括实验设备、实验室家具、实验室消耗品等。
为了方便客户了解和选择他们的产品和服务,Litron Laboratories 提供了一份详细的价目表。
这份价目表概述了 Litron Laboratories 提供的所有产品和服务,包括实验设备、实验室家具和实验室消耗品。
在实验设备方面,他们提供了各种类型的实验室设备,如光学显微镜、电子显微镜、离心机等。
在实验室家具方面,他们提供了各种类型的实验室家具,如实验台、储物柜、通风柜等。
在实验室消耗品方面,他们提供了各种类型的实验室消耗品,如试剂、实验器材等。
在价目表中,每个项目都详细列出了其价格。
例如,他们的光学显微镜价格从 1000 美元到 5000 美元不等,电子显微镜价格从 5000 美元到 20000 美元不等,离心机价格从 500 美元到 2000 美元不等。
这些价格都是根据产品的质量和性能来设定的,旨在为客户提供最好的产品和服务。
总的来说,Litron Laboratories 的价目表提供了一份清晰、详细的产品和服务清单,方便客户了解和选择他们的产品和服务。
然而,这份价目表也有一些不足之处。
例如,有些项目的价格较高,可能会让一些客户
望而却步。

任某辉、张葳生产、销售假药一审刑事判决书被告人任某辉被告人张葳被告人曾某深圳市龙华区人民检察院以深龙华检刑诉〔2019〕38号起诉书指控被告人任某辉、张葳、曾某犯生产、销售假药罪,于2019年1月8日向本院提起公诉。
本院适用普通程序,依法组成合议庭,于2020年5月6日作出(2019)粤0309刑初151号刑事判决书。
宣判后,被告人张葳不服,提出上诉。
广东省深圳市中级人民法院于2020年7月15日以(2020)粤03刑终1136号刑事裁定书裁定撤销(2019)粤0309刑初151号刑事判决,发回本院重审。
本院受理后,依法重新组成合议庭于2021年3月4日公开开庭审理了本案。
深圳市龙华区人民检察院指派检察员罗淑芳出庭支持公诉,被告人任某辉及其辩护人罗晓春、被告人张葳及其辩护人骆建军、韦敏杰、被告人曾某及其辩护人林塭丰、白峻到庭参加诉讼。
现已审理终结。
公诉机关指控:2014年11月至2018年6月,被告人任某辉在未取得营业执照和药品经营许可证的情况下,购进价值1920000元疑似假药康力龙、宝丹酮的材料、半成品、包装材料等,再进行配制、包装、分装,然后通过网络及微信朋友圈对外销售,收取货款共计2920000元。
其中任某辉于2017年4月至2017年10月24日,多次销售“伟哥”、“西力士”、“克伦特罗”、“康力龙”给被告人张葳,共计收取货款12500元。
2016年6月至2018年5月,被告人张葳在未取得营业执照和药品经营许可证的情况下,通过网络购进疑似假药的材料、半成品、包装材料、成品等,再进行配制、包装、分装,然后通过网络及微信朋友圈对外销售。
其中张葳在2017年4月至10月24日,通过微信向被告人任某辉购买12500元的“伟哥”、“西力士”、“克伦特罗”、“康力龙”,向被告人曾某购买11580元的疑似假药。
张葳向被告人曾某销售疑似假药HGH等共计187060元,另通过网络、微信朋友圈销售“克伦特罗”、“T3”、“康力龙”、“HGH”、“HCG”等共计376219元。

UnitedHealthcare ® Community PlanZulresso ® (Brexanolone)Policy Number : CS2023D0080J Effective Date : November 1, 2023 Instructions for UseTable of Contents Page Application ..................................................................................... 1 Coverage Rationale ....................................................................... 1 Applicable Codes .......................................................................... 2 Background .................................................................................... 2 Clinical Evidence ........................................................................... 2 U.S. Food and Drug Administration ............................................. 3 References ..................................................................................... 3 Policy History/Revision Information ............................................. 3 Instructions for Use ....................................................................... 3 This Medical Benefit Drug Policy does not apply to the states listed below; refer to the state-specific policy/guideline, if noted: StatePolicy/GuidelineFloridaRefer to the state’s Medicaid clinical policyIndiana Zulresso ® (Brexanolone) (for Indiana Only)Kansas None LouisianaRefer to the state’s Medicaid clinical policy North CarolinaNone OhioZulresso ® (Brexanolone) (for Ohio Only)TexasRefer to the drug specific criteria found within the Texas Medicaid Provider Procedures ManualZulresso (brexanolone) is proven and medically necessary for the treatment of postpartum depression in patients who meet all of the following criteria :Diagnosis of major depressive disorder (MDD) according to the current DSM (i.e., DSM-5), by a mental health professional; andOnset of current depressive episode was during the third trimester or within 4 weeks postpartum; andCurrent depressive episode is considered moderate to severe based on a standardized, validated tool; andPatient has not previously received Zulresso (brexanolone) for the current postpartum depressive episode from the most recent pregnancy (within 6 months); andPatient has not previously received Zurzuvae (zuranolone) for the current postpartum depressive episode from the most recent pregnancy (within 6 months); andThe provider and/or the provider’s healthcare setting is certified in the Zulresso REMS program, with ability to support onsite continuous monitoring; andBrexanolone is dosed in accordance with the United States Food and Drug Administration (FDA)-approved labeling; and Commercial Policy • Zulresso ® (Brexanolone)Approval is for a single 60-hour infusionThe following list(s) of procedure and/or diagnosis codes is provided for reference purposes only and may not be all inclusive. Listing of a code in this policy does not imply that the service described by the code is a covered or non-covered health service. Benefit coverage for health services is determined by federal, state, or contractual requirements and applicable laws that may require coverage for a specific service. The inclusion of a code does not imply any right to reimbursement or guarantee claim payment. Other Policies and Guidelines may apply.HCPCS Code DescriptionJ1632 Injection, brexanolone, 1 mgDiagnosis Code DescriptionF53.0 Postpartum depressionBrexanolone is a neuroactive steroid gamma-aminobutyric acid (GABA) A receptor positive modulator, that is chemically identical to endogenous allopregnanolone.Meltzer-Brody et al. assessed brexanolone as a treatment for moderate to severe postpartum depression (PPD) in two double-blind, randomized, placebo-controlled, phase 3 trials.2 Women in the trial were 18-45 years of age, 6 months post-partum or less at screening, and diagnosed with PPD with a Hamilton Rating Scale for Depression (HAM-D) score of ≥ 26 and 20-25 for study 1 and study 2, respectively. Study participants were randomly assigned to receive either brexanolone 90 μg/kg per hr. (BRX90), brexanolone 60 μg/kg per hr. (BRX60), or matching placebo for a single 60-hour infusion in study 1. In study 2, BRX90 or placebo was infused as a single 60-hour infusion. The primary efficacy endpoint was the change from baseline in the 17-item HAM-D total score at 60 hours. This was assessed in all patients who started infusion of brexanolone or placebo, had a valid HAM-D baseline assessment, and had at least one post-baseline HAM-D assessment. The trials are NCT02942004 (study 1) and NCT02942017 (study 2). In study 1, at 60 hours, the least-squares (LS) mean reduction in HAM-D total score from baseline was 19.5 points (SE 1.2) in the BRX60 group and 17.7 points (1.2) in the BRX90 group compared with 14.0 points (1.1) in the placebo group [difference -5.5 (95% CI -8.8 to -2.2), p = 0.0013 for the BRX60 group; -3.7 (95% CI -6.9 to -0.5), p = 0.0252 for the BRX90 group]. In study 2, at 60 hours, the LS mean reduction in HAM-D total score from baseline was 14.6 points (SE 0.8) in the BRX90 group compared with 12.1 points (SE 0.8) for the placebo group [difference -2.5 (95% CI -4.5 to -0.5), p = 0·0160]. The authors conclude that brexanolone for PPD resulted in significant and clinically meaningful reductions in HAM-D total score at 60 hours compared with placebo, with rapid onset of action and durable treatment response during the study period. The authors conclude that results suggest that brexanolone injection is a novel therapeutic drug for PPD that has the potential to improve treatment options for women with this disorder.Brexanolone safety, tolerability, and pharmacokinetics were evaluated in a multicenter, open-label study in 20 patients aged 15 to 17 years diagnosed with PPD and were comparable to those in adult patients with PPD.1Professional SocietiesThe American College of Obstetricians and Gynecologists (ACOG) has published a clinical practice guideline with recommendations on treatment and management of perinatal mental health conditions including depression. ACOG recommends consideration of brexanolone administration in the postpartum period for moderate-to-severe perinatal depression with onset in the third trimester or within 4 weeks postpartum. The decision to use brexanolone should balance the benefits (e.g., rapid onset of action) with the risks and challenges (e.g., limited access, high cost, lack of data supporting safety with breastfeeding, requirement for inpatient monitoring during the infusion, lack of efficacy data beyond 30 days).(STRONG RECOMMENDATION, MODERATE-QUALITY EVIDENCE)This section is to be used for informational purposes only. FDA approval alone is not a basis for coverage.Zulresso is indicated for the treatment of postpartum depression (PPD) in patients 15 years and older.Zulresso is only available through a restricted program under a REMS called the Zulresso REMS due to the risk of excessive sedation or sudden loss of consciousness that can result in serious harm.Important requirements of the Zulresso REMS include the following:Healthcare facilities must enroll in the program and ensure that Zulresso is only administered to patients who are enrolled in the Zulresso REMSPharmacies must be certified with the program and must only dispense Zulresso to healthcare facilities who are certified inthe Zulresso REMSPatients must be enrolled in the Zulresso REMS prior to administration of ZulressoWholesalers and distributors must be registered with the program and must only distribute to certified healthcare facilities and pharmacies1.Zulresso [package insert]. Cambridge, MA: Sage Therapeutics; June 2022.2.Meltzer-Brody S, Colquhoun H, Riesenberg R, Epperson CN, Deligiannidis KM, Rubinow DR, Li H, Sankoh AJ, Clemson C,Schacterle A, Jonas J, Kanes S. Brexanolone injection in post-partum depression: two multicentre, double-blind,randomised, placebo-controlled, phase 3 trials. Lancet. 2018 Sep 22;392(10152):1058-1070.3.AAmerican Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. 2013. Washington,DC. Pages 451-459.4.Treatment and Management of Mental Health Conditions During Pregnancy and Postpartum: ACOG Clinical PracticeGuideline No. 5. Obstet Gynecol. 2023;141(6):1262-1288. doi:10.1097/AOG.0000000000005202.Date Summary of Changes11/01/2023 Coverage Rationale•Replaced references to “brexanolone” with “Zulresso (brexanolone)”•Revised coverage criteria:o Added criterion requiring the patient has not previously received Zurzuvae (zuranolone) for thecurrent postpartum depressive episode from the most recent pregnancy (within 6 months)o Replaced criterion requiring “onset of current depressive episode was during the third trimester and 4 weeks postpartum” with “onset of current depressive episode was during the thirdtrimester or within 4 weeks postpartum”Supporting Information•Updated Clinical Evidence and References sections to reflect the most current information•Archived previous policy version CS2022D0080IThis Medical Benefit Drug Policy provides assistance in interpreting UnitedHealthcare standard benefit plans. When deciding coverage, the federal, state, or contractual requirements for benefit plan coverage must be referenced as the terms of the federal, state, or contractual requirements for benefit plan coverage may differ from the standard benefit plan. In the event of a conflict, the federal, state, or contractual requirements for benefit plan coverage govern. Before using this policy, please check the federal, state, or contractual requirements for benefit plan coverage. UnitedHealthcare reserves the right to modify itsPolicies and Guidelines as necessary. This Medical Benefit Drug Policy is provided for informational purposes. It does not constitute medical advice.UnitedHealthcare may also use tools developed by third parties, such as the InterQual® criteria, to assist us in administering health benefits. The UnitedHealthcare Medical Benefit Drug Policies are intended to be used in connection with the independent professional medical judgment of a qualified health care provider and do not constitute the practice of medicine or medical advice.。
群勃龙(醋酸去甲雄三烯醇酮)群勃龙被许多运动员视为最棒的类固醇,它是市场上作用最为广泛的品种之一,关于群勃龙还有许多未解之谜,相信在将来我们会对这种化合物有更深的了解。
群勃龙(醋酸去甲雄三烯醇酮)TrenboloneAcetate是兽用类固醇,群勃龙(去甲雄三烯醇酮)激素最早是在二十世纪六十年代晚期出现的,醋酸去甲雄三烯醇酮这个版本的商品名是Finajet和Finaject。
但是这并不是唯一的一种群勃龙,唯一的一种人医用群勃龙是法国Negma Laboratories生产的环己甲基碳酸群勃龙,商品名Parabolan,已经在1997年停产了。
醋酸群勃龙的生产商Hoechst-Roussel在八十年代末关停了兽用药生产线,但是这个时期他们又推出了Finaplix pellets,这是一种皮下植入的含有醋酸去甲雄三烯醇酮化合物的小球,这玩意儿生产出来是给待屠宰的牲畜短期增加瘦体重的,它在这一领域获得了巨大成功,现在已经成了畜牧业不可缺少的一部分。
好多运动员也买了Finaplix pellets 自己做成针剂来用。
多年来竞技运动圈里把上文中的兽用药做成注射剂是非常常见的,现在大多数地下实验室也都开始生产注射用醋酸去甲雄三烯醇酮了。
群勃龙在出状态阶段的作用是无与伦比的,把其他几种类固醇叠加使用也没有群勃龙猛,它还是最好的非赛季类固醇之一,增围增力效果都非常好,还不会像其他传统类固醇那样存水存脂。
群勃龙种类有很多,最受运动员欢迎的还是醋酸版本,它更容易维持峰值,血药浓度也更稳定,功效也更好。
群勃龙的功能和性质:(CYCLE购买定制咨询微信468034130 )醋酸群勃龙(Trenbolone Acetate)是一种19-nor类固醇。
这类类固醇是指睾酮在第十九位丢失了一个碳原子的结构变化。
群勃龙和代卡-多乐宝灵Deca Durabolin(癸酸诺龙Nandrolone Decanoate)都属于这一类。
事实上群勃龙就是诺龙的一个变式。
诸城外贸有限责任公司兽药采购招标报价单1. 麻杏石甘口服液[矩阵文本题]2. 麻杏石甘颗粒[矩阵文本题]3. 双黄连口服液[矩阵文本题]4. 双黄连可溶性粉[矩阵文本题]5. 银黄可溶性粉[矩阵文本题]6. 板青颗粒[矩阵文本题]7. 肝胆口服液[矩阵文本题]8. 驱球止痢合剂[矩阵文本题]9. 三味拳参口服液[矩阵文本题]10. 清瘟解毒口服液[矩阵文本题]11. 清解合剂[矩阵文本题]12. 桑仁清肺口服液[矩阵文本题]13. 七清败毒颗粒[矩阵文本题]14. 柴胡口服液[矩阵文本题]15. 银翘散[矩阵文本题]16. 单硫酸卡那霉素可溶性粉[矩阵文本题]17. 地克珠利颗粒[矩阵文本题]18. 癸氧喹酯溶液[矩阵文本题]19. 盐酸沃尼妙林预混剂[矩阵文本题]20. 复方磺胺嘧啶混悬液[矩阵文本题]21. 恩诺沙星溶液[矩阵文本题]22. 注射用头孢噻呋钠[矩阵文本题]23. 注射用头孢噻呋[矩阵文本题]24. 复方磺胺嘧啶混悬液[矩阵文本题]25. 地美硝唑预混剂[矩阵文本题]26. 恩诺沙星注射液[矩阵文本题]27. 复方戊二醛溶液[矩阵文本题]28. 马立克疫苗[矩阵文本题]29. 1B4/91+Ma5+clone30[矩阵文本题]30. 球虫苗[矩阵文本题]31. 关节炎活苗[矩阵文本题]32. 新流腺[矩阵文本题]33. 新支二联活[矩阵文本题]34. "H5+H7油苗重组禽流感病毒(H5+H7)三价灭活疫苗(H5N1 Re-11株+Re-12株,H7N9 H7-Re3株"[矩阵文本题]35. 法氏囊[矩阵文本题]36. MG活苗[矩阵文本题]37. 鸡痘[矩阵文本题]38. 传鼻灭活苗[矩阵文本题]39. 关节炎油苗[矩阵文本题]40. 肿头活苗[矩阵文本题]41. "MG+MS油苗鸡滑液支原体灭活疫苗(YBF-MS1株)鸡毒支原体灭活疫苗"[矩阵文本题]42. 传喉活苗[矩阵文本题]43. "新支流(H9)油鸡新城疫、传染性支气管炎二联活疫苗(La Sota株+H120株)"[矩阵文本题]44. 鸡痘+脑炎活苗[矩阵文本题]45. "减蛋综合征(鸡新城疫病毒(La Sota株)、传染性支气管炎病毒(M41株)、减蛋综合征病毒(AV127株)三联灭活疫苗"[矩阵文本题]46. "新支法关四联油鸡新城疫、传染性支气管炎、传染性法氏囊病、病毒性关节炎四联活疫苗(La Sota株+M41株+S-VP2蛋白+AV2311株)"[矩阵文本题]47. 新支二联活+IB4/91[矩阵文本题]48. 新流油苗[矩阵文本题]49. H9油苗[矩阵文本题]50. 滑液囊支原体(MS)[矩阵文本题]51. 新支二联活苗[矩阵文本题]52.新支二联活苗[矩阵文本题]53.H5+H7油苗[矩阵文本题]54.新流油苗[矩阵文本题]55.新流法油苗[矩阵文本题]56.新流腺油苗[矩阵文本题]57. 法氏囊苗[矩阵文本题]58.Ⅳ苗[矩阵文本题]59. 鸡痘苗[矩阵文本题]60. 转移因子口服液[矩阵文本题]61. 白细胞介素-2[矩阵文本题]62. I型鸭肝炎病毒卵黄抗体[矩阵文本题]63. 鸭坦布苏病毒病灭活疫苗[矩阵文本题]64. 鸡新城疫、禽流感(H9亚型)二联灭活疫苗[矩阵文本题]65. 鸭瘟活疫苗(细胞苗)[矩阵文本题]66. 酸化剂[矩阵文本题]67. 干扰素[矩阵文本题]68. 大肠杆菌噬菌体[矩阵文本题]69. 葡萄糖氧化酶[矩阵文本题]70. 复合维生素[矩阵文本题]71. 新易泰[矩阵文本题]72. 达易克[矩阵文本题]73. 唯可舒[矩阵文本题]74. 开口宝[矩阵文本题]75. 美立安[矩阵文本题]76. 畅安泰[矩阵文本题]77. 感易安[矩阵文本题]78. 畅可舒[矩阵文本题]79. 唯长安[矩阵文本题]80. 易安舒[矩阵文本题]81. 禽乐宝[矩阵文本题]82. 沙立宁[矩阵文本题]83. 诺易健[矩阵文本题]84. 强澸清[矩阵文本题]85. 维多利康[矩阵文本题]86. 信易安[矩阵文本题]87. 控易泰[矩阵文本题]88. 安乐康[矩阵文本题]89. 成长2020[矩阵文本题]90. 长健2020[矩阵文本题]91. 速安平[矩阵文本题]92. 速安平2[矩阵文本题]93. 克球为安[矩阵文本题]94. 克霉为安[矩阵文本题]95. 康泰来[矩阵文本题]96. 喜益安[矩阵文本题]97. 多糖宝[矩阵文本题]98. 硫酸粘菌素[矩阵文本题]99. 盐酸多西环素[矩阵文本题]100. 替米考星(碱)[矩阵文本题]101. 盐酸大观霉素[矩阵文本题]102. 10%海南霉素预混剂[矩阵文本题]103. 45%延胡索酸泰妙菌素[矩阵文本题]104. 50%浓戊二醛溶液[矩阵文本题]105. 酒石酸泰万菌素[矩阵文本题]106. 氟苯尼考[矩阵文本题]107. 硫酸庆大霉素[矩阵文本题]108. 盐酸林可霉素[矩阵文本题]109. 硫酸安普霉素[矩阵文本题]110. 乳酸环丙沙星[矩阵文本题]111. 硫酸新霉素[矩阵文本题]112. 阿莫西林[矩阵文本题]113. 硫氰酸红霉素[矩阵文本题]114. 氨苄西林钠[矩阵文本题]115. 免疫宝[矩阵文本题]116. 食品葡萄糖[矩阵文本题]117. 麦芽糊精[矩阵文本题]118. 薄荷粗提物[矩阵文本题]119. 杨树花粗提物[矩阵文本题]120. 基本信息[矩阵文本题] *。
联系人/Attention:电话/TEL:单价(元)/Unit Price(RMB)金额(元)/Price(RMB)单价(元)/Unit Price(RMB)金额(元)/Price(RMB)供应商/Supplier :签字/Signature:盖章/Stamp:日期/Date:1产品大小/Part Size (mm)产品重量/Part Weight(g)数据文件名/Drawing No.模具供应商报价单Mold Quotation Form外形尺寸/Mold Size (mm)模具重量/Mold Weight(Kg)模具寿命/Longevity模具名称/Mold Specification型腔数量/Cav.产品零件号/Part No.进气堵盖30万加工材料费/MachiningMaterial Cost 材料名称/Material材料牌号/Specification尺寸/Size(mm×mm×mm)重量/Weight(Kg)模架/Mold Base型芯/Core型腔/Cavity电极/Electrode 斜顶/Lifter单价(元)/Unit Price(RMB)金额(元)/Price(RMB)调质/Tempered 其它/Other 滑块/Slide热处理/HeatTreatment 热处理名称/Item重量/Weight(Kg)氮化/Nitriding淬火/Hardened装配材料费/AssemlyMaterial Cost 装配件/Item规格型号/Specification 品牌/Supplier 数量/Number 顶杆/Ejection Pin顶管/Ejection Sleeve水管接头/Connector 热流道/Hot Runner标准件/Standard Components油缸/Hydraulic Cylinder温控器/Temp Controller设计费/DesignCost 名称/Item工时(小时)/Hour 单价(元)/Unit Price(RMB)金额(元)/Price(RMB)扫描测绘/Scanning 结构设计/CADCAE分析/CAE加工费/ManufacturingCost 名称/Item工时(小时)/Hour 单价(元)/Unit Price(RMB)金额(元)/Price(RMB)一般机床/Machining数控机床/CNC电火花/EDM线切割/W.C.钳工/Fitting三坐标测量型腔/Cavity其它/Other皮纹三坐标测量产品/Part其它费用/Other Fee 费用名称/Item费用计算说明/Descripition 金额(元)/Price(RMB)试模费/Trial Fee运输费/Freight Fee 管理费/Managing Fee 利润/Profit税收/Tex模具总价(元)/Mold Price(RMB):0.00小计(元)/Subtotal(RMB)0.00小计(元)/Subtotal(RMB)0.00小计(元)/Subtotal(RMB)0.00小计(元)/Subtotal(RMB)0.00。