Silica-Gel-CO2 Capture from Flue Gas
- 格式:pdf
- 大小:804.75 KB
- 文档页数:8

翻译部分英文原文Kinetic study of the reaction between sulfur dioxideand calcium hydroxide at low temperature in afixed-bed reactorAbstractA quantitative study of the influence of inlet sulfurdioxide concentration (600–3000 ppm),relative humidity (20–60%), reactortemperature(56–86℃)and different amounts (0–30 wt.%) ofinorganic additives(NaCl, CaCl2 andNaOH) on gas desulfurization has been carried out in acontinuous downflow fixed-bed reactor containing calcium hydroxide diluted with silica sand.Results show that the reaction rate does not depend on sulfur dioxide partial pressure (zero-order Kinetics) and that the temperature and the relative humidity have a positive influence on reactionrate. An apparent activation energy of 32 kJ/mol Ca(OH)2 has been estimated for the reaction.An empirical reaction rate equation at 71.5℃and 36.7% relative humidity that includes thetype and amount of additive is proposed. It has been found thatcalcium chloride is the bestadditive studied because it allows for a higher degree of sulfur dioxide removal. 2000 ElsevierScience B.V. All rights reserved.Keywords: Desulfurization; Sulfur dioxide; Calcium hydroxide; Kinetics; Inorganic additives1. IntroductionThe increasing concern during the last few years on the protection of the environmenthas had its influence on the design and operation of power plants, especially on thereduction of sulfur dioxide and nitrogen oxide emissions from them. They are the mainpollutants from coal and fuel-oil combustion in power plants. Both gases are responsiblefor acid rain.In USA and Europe, new power plants that use fuels with significant quantities ofsulfur have to meet severe standards to reduce these air pollutants. One of the majorproblems facing older power plants is that they were designed prior to the presentstandards for pollution control and therefore have no facilities on space to incorporatesuch controls.The technologies to control sulfur dioxide emissions can be distributed into threegroups by considering if the treatment is done before, during or after thecombustion. Itseems clear that the last group of technologies cited is the most advantageous, fromvarious points of view, for power stations which have been in operation for many years.These are called FGD technologies (Flue Gas Desulfurization),and among them, themost usedare: IDS (In-Duct Scrubbing, developed by General Electric); E-So x(developed by US EPA, Babcok and Wilcox, Ohio Coal Development Office and OhioEdison), EPRIHYPAS (Hybrid Pollution Abatement System, developed by ElectricPower Research Institute), DRAVO HALT (Hydrate Addition at Low Temperature,developed by Dravo), CONSOL COOLSIDE (developed by Consolidated CoalCom-pany)and ADVACATE(developed by Acurex and US EPA). These processes are basedon the injection of a solid sorbent plus water by spraying or injecting a slurry into theduct situated between the air preheater and the particulate collection system. Calciumhydroxide or limestone are usually used as sorbents to capture sulfur dioxide and acalcium sulfiter/sulfate mixture is obtained as the reaction product.Klingspor and Stromberg proposed a mechanism to explain the reactionbetween sulfur dioxide and calcium hydroxide or calcium carbonate in the presence ofwater vapor. According to them, when the relative humidity is low (below 20%), sulfurdioxide and water can be adsorbed on the solid surface, however, no reaction occursuntil there is at least a monolayer of water molecules adsorbed on the surface. As therelative humidity increases, less sulfur dioxide can be adsorbed on the surface becausewater adsorption on the solid occurs preferentially due to intermolecular forces. Thus,sulfur dioxide has to be absorbed on the adsorbed water, forming complexes where thesulfur atom isbound to the oxygen atom of water. This fact leads to the formation of apositive charged hydrogen atom that can combine with hydroxide or carbonate ions fromthe sorbent to form reaction intermediates and products. Experimental findings show thatthe reaction rates for lime and limestone are similar. Consequently, the complexformation SO2nH O is considered to be the rate-determining step, since all further reactions are different for the two types of sorbents. The initial rate of the process isindependent of sulfur dioxide concentration when the relative humidity is below 70%.Above this value, the reaction rate becomes gradually more and more dependent on thesulfur dioxide partial pressure. This fact can be attributed to the formation of stableconfigurations of water ligands around the sulfur dioxide molecules. Also, it has beenfound that the initial reaction rate is a very weak function of temperature but increasesexponentially with relative humidity, for both hydrated lime and limestone.Jorgensen also studied this reaction in a bench-scale sand bed reactor. Someof their conclusions point out that the calcium hydroxide conversion has a very strongdependence on relative humidity. The conversion rate is increased moderately withtemperature in agreement with activation energy of 25 kJ/mol. However, there is noclear indication of increasing conversion with increasing sulfur dioxide concentration.Ruiz-Alsop and Rochelle found that the relative humidity is the most importantvariable affecting the reaction of sulfur dioxide and calcium hydroxide. The chemicalreaction taking place at the surface of the unreacted calciumhydroxide presentszero-order kinetics in sulfur dioxide. At high relative humidity and/or high SO2concentration, the chemical reaction at the surface of the unreacted calcium hydroxidesolid controls the overall reaction rate. At low relative humidity and/or low sulfurdioxide levels, diffusion of sulfur dioxide through the solid product layer becomes therate-controlling step. The reaction rate has a weak temperature dependence. Theactivation energy of the reaction was estimated to be 12 kJ/mol.Experimental data by Krammer showed that the reaction ratedepends onthe sulfur dioxide concentration but only at low concentrations and not so obvious athigher concentrations. In contrast to other publications, they found that the influence ofSO2concentration on the reaction rate is rather linked to the conversion than to the 2relative humidity, which has a major impact on the conversion throughout the entirereaction as usually reported in literature. But they found out that the initial reaction rateseems to be independent of relative humidity and sulfur dioxide concentration, whichhad not been reported yet. They postulated that the reaction can be divided into thefollowing foursteps. During the initial stage, a chemisorption process of the sulfurdioxide on the particle surface seems to be important and the reaction rate decreasesexponentially with increasing conversion. Simultaneously, a nucleation processdominates the formation of the consecutive product layers where the reaction rateincreases with increasing relative humidity. The rate of reaction increases untilproduct layer diffusion takes over and reaction rate decreases again with conversion. Itshould be noted that only relative humidityhas an impact on product layer diffusion. Beyond a conversion of around 9%, reaction rate drops significantly, which can be dueto pore closure.Irabien consider the adsorption of sulfur dioxide on calcium hydroxideacting as a nonideal solid sorbent is the rate-limiting step. They use a parameterreferring to this nonideal behavior of the solid surface as independent of temperature butexponentially dependent on relative humidity. The authors obtained activationenergy of75 kJ/mol for the reaction.All published work thus far indicates that relative humidity has the greatest impact onthe reaction rate between sulfur dioxide and calcium hydroxide. The relative humidity isin turn correlated with the moisture content of the solids. Additives that will modify themoisture content of the calcium hydroxide solids in equilibrium with a gas phase of agiven relative humidity would then be expected to enhance the reactivity of calciumhydroxide towards sulfur dioxide in FGD processes.Organic and inorganic additives have been tested in spray dryer systems to improvethe desulfurization power of calcium hydroxide and calciumcarbonatew.It seems that inorganic hygroscopic salts such as barium, potassium, sodium and calciumchlorides and also cobalt, sodium and calcium nitrates would be the most effective ones.Some researchers also consider sodiumhydroxide as an effective additive due to itsalkaline and hygroscopic properties.Ruiz-Alsop and Rochelleindicated that deliquescence alone does notexplainthepositive effect of some salts. They contend that for an additive to be effective, it is alsonecessary that the hydroxide of the cation be very soluble, otherwise, the cation willprecipitate out as the hydroxide and the anion will form the calcium salt which could notbe hygroscopic. The effectiveness of a certain salt also depends on the relative humidity.This could be expected because when therelative humidity of the gaseous phase islower than the water activity in a saturated solution of the salt, it would not absorb waterand so, it would not enhance the calcium hydroxide reactivity. These researcherscontend that chlorides and sodium nitrate modify the properties of the product (half-hy-drated calcium sulfite) layer that is formed as the reaction takes place, therebyfacilitating the access of sulfur dioxide to unreacted calcium hydroxide, which remainsin the interior of the particle.The scope of the present work is to quantify the influence on the reaction rate ofsulfur dioxide concentration, relative humidity, temperature and type and amount ofadditive. An empirical equation, which relates the reaction rate with these variables, hasbeen obtained and an apparent activation energy value for the reaction has also beendetermined from kinetic constants at different temperatures by using the Arrhenius plot.2. Experimental sectionThis equipmentconsists of a continuous feeding and humidification system of a gaseous stream, afixed-bed reactor and an analytical system. The apparatus is operated with a personalcomputer using LabView software (NationalInstruments), which allows programmingand control of the experimental conditions, namely, nitrogen and sulfur dioxide flowrates, humidification temperature and electric resistance heating of the pipes to avoidcondensations and also provides the experimental data acquisition, in particular nitrogenand sulfur dioxide flow rates, reaction temperature, pressure, relative humidity andsulfur dioxide concentration, vs. reaction time.Simulated flue gas was obtained by mixing sulfur dioxide and nitrogen from separatecylinders in appropriate amounts using mass flow controllers Before mixing, pure nitrogenwas passed (by switching on valve 1 from thecomputer)through the humidificationsystemthat consisted of three cylindrical flasks with 200 ml of watereach submerged into a thermostatic bath. Each flask contains small glass spheres toimprove the contact between gas and water. After the humidification system, thetemperature and the relative humidity of the wet nitrogen were measured by using aVaisala HMP 235 transmitter .At the same location, the pressure was alsoMeasuredto calculate the flow rate of water vapour generated. Thewet nitrogen by-passed the reactor until the desired experimental conditionswerereached and then valve 2 was opened from the computer to allow thegaseous streamflow through the reactor. The bed was always humidified for 15 min while the sulfurdioxide analyser was set to zero. At this time, the desired flow of sulfur dioxide wasintroduced by a mass flow controller and the experiment began. Data generated duringthe experiment were stored in an EXCEL format computer file.The glass reactor, a jacketed Pyrex tube (450 mm height, 12 mm i.d.)with a porousplate to hold 1 g of dry calcium hydroxide (Probus, 99% purity and particle size smallerthan 0.05 mm in diameter)or calcium hydroxide–additive mixtures(all additives weresupplied by Fluka, 99% purity and particle size smaller than 0.05 mm in diameter) diluted with 8 g of silica sand (Merck;0.1–0.3 mm in diameter)to assure isothermaloperation and to prevent channelling due to excessive pressure drop, was thermostatedby pumping a thermal fluid (water–ethyleneglycol mixture) from an external thermostaticbath.The reacted flue gas is passed through a refrigeration systemin orderto remove water because it interferes with the SO2 analyser measurement.The output from the analyser was continu-ously collected by the computer for 1h (experiment time)and the concentration (ppm) of sulfur dioxide stored as a function of time (experimental curve). Each experiment wasconducted in the same manner except a reactive solid was substituted for the10 g ofinert silica(‘‘blank’’ experiment) to obtain a reference flow curve. The reaction rate wascalculated as SO2mol removed/h mol OH-from the area enclosed by the two curves (experimental and ‘‘blank’’). Some experiments were replicated to estimate the experimentalerror in reaction rate.3. ConclusionsIn this research, the quantitative influence of sulfur dioxide concentration, temperature,relative humidity and the type and amount of the three inorganic additives on thereaction rate between calcium hydroxide and sulfur dioxide have been determined.The SO2concentration (0–3000 ppm)was shown to have no significantinfluence on the reaction rate at a relative humidity of 38% and at 71.5℃. These results agree withthose of Ruiz-Alsop and Rochelle who indicated that sulfur dioxide concentrationdoes not influence the reaction rate at temperatures ranging from 30℃to 90℃; 17–90%relative humidity and sulfur dioxide concentration varying from 0 to 4000 ppm. Sinceour experiments are within the range of these experimental conditions, we assume thatsulfur dioxide concentration will not influence the reaction rate at our other experimentalconditions also.An empirical rate equation, which allows us to quantify the influence of temperatureand relative humidity on reaction rate has been developed and an apparent activationenergy of 32 kJ/mol Ca(OH)2 has been calculated. This value, relatively high, demonstrates the weak influence of temperature, but the reaction order of 1.2 withrespect to the relative humidity shows its strong influence on reaction rate.Three inorganic additives were tested to evaluate their quantitative influence onreaction rate. An empirical equation for each additive at 71.5℃and a relative humidityof 36.7% was developed.The kinetic rate constants for calcium chloride, sodium hydroxide and sodiumchloride were found to be respectively, 9, 5 and 0.81 times the rate constant for calciumhydroxide without any additive. The reaction orders for the weight ratio of the sameadditives were 0.6, 0.52 and y0.12, respectively. Calcium chloride is the best additivewhereas sodium chloride is an inhibitor.中文译文动力学研究与二氧化硫反应在低温和氢氧化钙在一固定床反应器摘要一个入口二氧化硫浓度(600-3000百万分之一),相对湿度(20-60%),反应器温度(影响的定量研究56-86℃)和不同的金额(0-30%重量)ofinorganic添加剂(氯化钠,氯化钙和氢氧化钠)对气体脱硫已进行acontinuous下行流了固定床反应器含有氢氧化钙与氧化硅sand.Results 稀释表明,反应速度不依赖于二氧化硫分压(零阶动力学),而温度和相对湿度对reactionrate积极的影响。

热力学activity coefficientazeotropecomputational chemistry dissolutionenthalpyentropyenvironmentequation of stateequilibriumexergygasificationhomogenizationkinetic theoryphase changephase equilibriasolubilitysolutionstatistical thermodynamics thermodynamic properties thermodynamicsthermodynamics processvapor liquid equilibria vaporizationviscosity流体力学与传递现象aerosolagglomerationaggregationbubblecirculating fluidized bed coalescencecomplex fluidscompressorcomputational fluid dynamics,CFD condensationconvectiondiffusionemulsionsevaporationflowflow regimesfluid mechanicsfluidizationfluidized-bedfoulingfractalsgas-liquid flowgranulationheat conductionheat transferhydrodynamics hydrothermallaminar flowliquefactionmass transfermesoscalemicrochannelsmicrofluidicsmicroreactormicroscalemixingmomentum transfermoving bedmultiphase flowmultiscalenon-Newtonian fluids packed bedparticleparticle formationparticle processing particle size distribution PIVpneumatic conveyingporous mediapumpradiationrheologysinteringsolid mechanicstransitiontransporttransport processestwo-phase flowvoidage催化、动力学与反应工程activationattritionautocatalysisbubble column reactor catalysiscatalystcatalyst activation catalyst supportchemical reactionchemical reactors deactivationdynamicselectrochemistry electrolysiselectrolytesexplosionsfixed-bedhydrogenationkinetic modelingkineticsmoleclar sievesmonolithmultiphase reaction multiphase reactor nonlinear dynamics oxidationpartial oxidation photochemistryradicalreactionreaction engineering reaction kineticsreactorsresidence time distribution riserSCRstatic mixersteady statestirred vesselsupporttrickle-bed reactor turbulenceturbulent flowzeolite分离工程absorptionadsorbentsadsorptionbatchwisebinary mixtureblendbubble columncentrifugation chromatographycolumncrystallizationdesalinationdesorptiondistillationextractionextrusionfilmfiltrationflotationflue gasgranular flowgranular materialsion exchangeleachingmembranesnanofiltrationnucleationpermeabilitypermeationpervaporationprecipitationpurificationreactive distillation sedimentationsegregationselectivityseparationsolvent extractionsolventssorbentssupercritical carbon dioxide supercritical fluid supercritical water suspensionsultrafiltration过程系统工程与过程安全algorithmchaoscomputer simulationcontrolDEMdiscrete element modeling dispersiondynamic modelingdynamic simulation economicsformulationgas holdupglobal optimization integrationmodel reductionmodel-predictive control Monte Carlo simulation neural networksoptimal designoptimizationparameter estimation parameter identification principal component analysis process controlprocess systemsproduct designproduct engineering systems engineering transient response表面与界面工程colloidcorrosionelectro-osmosis electrophoresisgelsinterfaceinterfacial rheology interfacial tension surfacesurfactants生物化工aerationaerobicanaerobicantibodybiocatalysisbiochemical engineering biodieselbioenergybiofilmbiofuelb iological engineering biomassbiomedical engineering biomolecular engineering bioprocessbioreactors bioseparation biotechnology biotemplatingcell biologycell engineeringdialysisDNAdownstream processing enzymefermentationgenetic algorithm immobilization metabolism microstructuremolecular biology monoclonal antibody morphologypeptidepharmaceuticalspopulation balance population balance equations proteinprotein denaturationprotein refoldingprotein stabilitysynthetic biologytissue engineeringvirus-like particle能源、资源与环境工程CO2 capturecoagulationcoal combustioncokingdegradationdepositiondrainagefuelfuel cellsgreenhouse gasHDShydratehydrogen productionmethanenatural gaspetroleumpollutionpyrolysisrecoveryreductionregenerationremediationrenewable energy sequestrationslurrysoft solidssolar energysustainabilitysyngaswaste treatmentwaste waterwind energy材料与产品工程complexescompositeselasticityelectronic materials fabricationfood processingionic liquidsmouldingnanomaterials nanoparticles nanostructure nanotechnologypolymer processing polymerization polymerspowder technology powderspreparationsilicasize distribution synthesis其他activated carbon alcoholalkanealuminaaqueous solution benzenecarbon dioxidecarbon monoxide chemical analysis chemical processes crushingdesigndistributionsdryingdustexperimental validation foamgashydrocarbonsHydrogenhydrolysisimaginginstability instrumentation manufacture mathematical modeling measurementmechanical properties microelectronics mixturesmodelMoldingmolecular engineering molecular simulation molecular synthesis NMRNumerical analysisnumerical simulation organic compounds prediction productionreactivitysafetyscale-upsimulationstability tomography活度系数共沸(混合)物计算化学溶解焓熵环境状态方程平衡火用气化均化(作用)动力学理论相变相平衡溶解性溶液统计热力学热力学性质热力学热力学过程汽液平衡汽化黏度气溶胶团聚聚集(作用)气泡循环流化床聚结复杂流体压缩机计算流体力学凝结对流扩散乳液蒸发流动流域流体力学流态化流化床结垢分形气液两相流造粒热传导传热增湿流体动力学水热层流液化传质介尺度微通道微流体学微反应器微尺度混合动量传递移动床多相流多尺度非牛顿流体填充床粒子粒子形成粒子处理加工粒度分布粒子图像测速气力输送多孔介质泵辐射流变学烧结固体力学过渡传递传递过程两相流空隙率活化(作用)磨损自催化鼓泡反应器催化(作用)催化剂催化剂活化催化剂载体化学反应化学反应器失活动态学电化学电解电解质爆炸固定床加氢动力学模型动力学分子筛整体器件多相反应多相反应器非线性动力学氧化部分氧化光化学自由基反应反应工程反应动力学反应器停留时间分布上升管选择催化还原静态混合器稳态搅拌容器载体滴流床反应器湍动湍流沸石吸收吸附剂吸附(作用)间歇式二元混合物混合鼓泡塔离心分离色谱塔器结晶脱盐解吸,脱附蒸馏萃取挤出膜过滤浮选烟道气颗粒物料离子交换浸取膜纳滤成核渗透率渗透渗透蒸发沉淀纯化反应精馏沉降离析选择性分离溶剂萃取溶剂吸附剂超临界二氧化碳超临界流体超临界水悬浮系超滤算法混沌计算机模拟控制动力效应模型分立元件建模分散,分散系动态建模动态仿真经济公式化气含率整体优化集成模型简化模型预测控制蒙特卡罗模拟神经网络优化设计优化参数估值参数识别主元分析过程控制过程系统产品工程系统工程瞬态响应胶体腐蚀电渗透电泳凝胶界面界面流变学界面张力表面表面活性剂曝气需氧厌氧抗体生物催化生化工程生物柴油生物能源生物膜生物燃料生物工程生物质生物医学工程生物分子工程生物过程生物反应器生物分离生物技术生物模板细胞生物学细胞工程透析脱氧核糖核酸下游加工过程酶发酵遗传算法固定化代谢显微结构分子生物学单克隆抗体形态学肽药物种群平衡公式蛋白质蛋白质变性蛋白质复性蛋白质稳定性合成生物学组织工程学病毒样颗粒二氧化碳捕集混凝煤燃烧焦化降解沉积物排水燃料燃料电池温室气体加氢脱硫水合物制氢甲烷天然气石油污染热解回收还原再生修复再生能源存埋浆料半固体太阳能可持续性合成气废物处理废水风能配合物复合材料弹性电子材料加工制造食品加工离子液体模制纳米粒子纳米结构纳米技术聚合物加工聚合聚合物粉体技术粉体制备二氧化硅粒度分布合成活性炭醇烷烃氧化铝水溶液苯二氧化碳一氧化碳化学分析化学过程粉碎设计分布干燥尘埃实验验证泡沫气体碳氢化合物氢水解成像不稳定性仪器,仪表制造数学模拟测量机械性能微电子学混合物模型模塑分子工程分子模拟分子合成核磁共振数值分析有机化合物预测生产活性安全放大模拟稳定性成像。


收稿日期:2009-03-30;修回日期:2009-07-10作者简介:赵毅(1956—),男,河北秦皇岛人,环境科学与工程学院院长,教授,博士生导师,从事燃煤大气污染控制方面的研究。
E -mail:zhaoyi9515@脱除烟气中的SO 2,脱硝主要为选择性催化还原法(SCR )和选择性非催化还原法(SNCR )法。
若燃煤电厂采用分级处理汞,不仅投资额大,占用空间,而且不适合老电厂的改造。
因此,如何利用现有的烟气治理设备达到同时脱汞的目的是当前研究的热点。
1基于FF 或ESP 的吸附剂喷入技术烟气中的汞主要有3种形式:气态汞(Hg 0)、二价汞(Hg 2+)和颗粒态汞(Hg p),其中,气态汞占总汞的79%以上。
电除尘器只能去除被飞灰吸附的Hg p,因此传统的电除尘器对汞的去除效果并不明显(低于20%)。
基于FF 或ESP 的吸附剂喷入技术是国外目前较广泛应用的汞排放控制技术。
常用的吸附剂有如下几种。
1.1活性炭活性炭吸收剂最初用于垃圾焚烧炉的汞排放控活性炭其表面含有S 、Cl 、Br 等元素。
在室温下,两者都能发生物理和化学吸附,因此改性活性炭具有更高的捕集效率。
研究表明[1],由于化学活性炭表面固着Br 、Cl 元素,该元素比炭先一步与汞接触,从而增加了炭表面上发生汞化学吸附的几率,阻止了被吸附在活性炭表面的汞再次蒸发逸出,因而大幅提高了汞的吸附效率(可达95%)。
同时,化学活性炭对汞的吸附受到浸泡浓度、反应时间、实验温度、活性炭的特性等因素的影响。
虽然活性炭吸附剂在烟气脱汞方面具有很高的效率,但其价格昂贵,进一步的大规模工业化应用受到了经济方面的制约,因此,开发利用廉价高效的替代物则成为当前研究的热点。
1.2飞灰通过飞灰的吸收、吸附作用可除去烟气中一定量的汞。
提高飞灰含碳量有利于提高脱汞率,但有关研究证实,二者不呈线性关系。
飞灰中残炭表面的含氧官能团(C =O )有利于汞的氧化和化学吸附,但汞第42卷中国电力环境保护与炭的作用并不是一般意义上的化学键,而是介于化学键与范德华力之间的氢键,所以很难用简单的化学反应来描述其吸附机理。

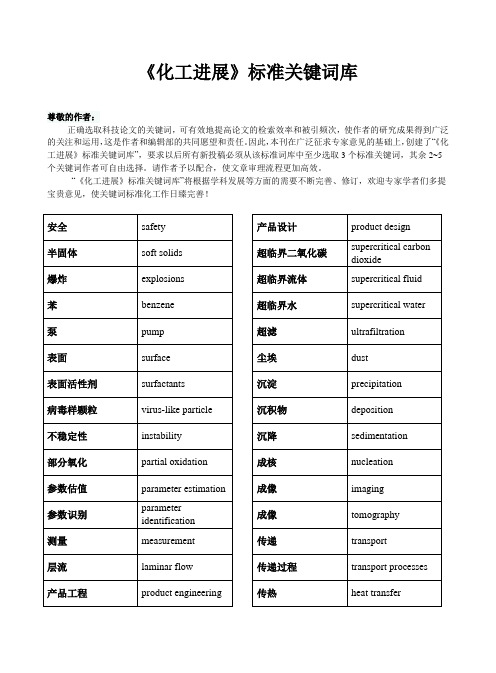
《化工进展》标准关键词库尊敬的作者:正确选取科技论文的关键词,可有效地提高论文的检索效率和被引频次,使作者的研究成果得到广泛的关注和运用,这是作者和编辑部的共同愿望和责任。
因此,本刊在广泛征求专家意见的基础上,创建了“《化工进展》标准关键词库”,要求以后所有新投稿必须从该标准词库中至少选取3个标准关键词,其余2~5个关键词作者可自由选择。
请作者予以配合,使文章审理流程更加高效。
“《化工进展》标准关键词库”将根据学科发展等方面的需要不断完善、修订,欢迎专家学者们多提宝贵意见,使关键词标准化工作日臻完善!安全safety半固体soft solids爆炸explosions苯benzene泵pump表面surface表面活性剂surfactants病毒样颗粒virus-like particle不稳定性instability部分氧化partial oxidation参数估值parameter estimation参数识别parameter identification测量measurement层流laminar flow产品工程product engineering 产品设计product design超临界二氧化碳supercritical carbondioxide超临界流体supercritical fluid 超临界水supercritical water 超滤ultrafiltration尘埃dust沉淀precipitation沉积物deposition沉降sedimentation成核nucleation成像imaging成像tomography传递transport传递过程transport processes 传热heat transfer传质mass transfer纯化purification醇alcohol催化(作用)catalysis催化剂catalyst催化剂活化catalyst activation催化剂载体catalyst support萃取extraction存埋sequestration代谢metabolism单克隆抗体monoclonal antibody 弹性elasticity蛋白质protein蛋白质变性protein denaturation 蛋白质复性protein refolding蛋白质稳定性protein stability滴流床反应器trickle-bed reactor 电化学electrochemistry电解electrolysis电解质electrolytes电渗透electro-osmosis 电泳electrophoresis电子材料electronic materials 动力效应模型DEM动力学kinetics动力学理论kinetic theory动力学模型kinetic modeling动量传递momentum transfer 动态仿真dynamic simulation 动态建模dynamic modeling 动态学dynamics对流convection多尺度multiscale多孔介质porous media多相反应multiphase reaction 多相反应器multiphase reactor 多相流multiphase flow二氧化硅silica二氧化碳carbon dioxide二氧化碳捕集CO2 capture二元混合物binary mixture发酵fermentation反应reaction反应动力学reaction kinetics反应工程reaction engineering 反应精馏reactive distillation 反应器reactors放大scale-up非牛顿流体non-Newtonian fluids非线性动力学nonlinear dynamics 废水waste water废物处理waste treatment沸石zeolite分布distributions分离separation分立元件建模discrete element modeling分散,分散系dispersion 分形fractals分子工程molecular engineering分子合成molecular synthesis 分子模拟molecular simulation 分子筛moleclar sieves分子生物学molecular biology 粉碎crushing粉体powders粉体技术powder technology 风能wind energy浮选flotation辐射radiation腐蚀corrosion复合材料composites复杂流体?complex fluids干燥drying公式化formulation共沸(混合)物azeotrope鼓泡反应器bubble columnreactor鼓泡塔bubble column固定床fixed-bed固定化immobilization固体力学solid mechanics光化学photochemistry过程控制process control过程系统process systems过渡transition过滤filtration焓enthalpy合成synthesis合成气syngas合成生物学synthetic biology 核磁共振NMR化学反应chemical reaction 化学反应器chemical reactors 化学分析chemical analysis 化学过程chemical processes 还原reduction环境environment回收recovery混沌chaos混合blend混合mixing混合物mixtures混凝coagulation活度系数activity coefficient 活化(作用)activation活性reactivity 活性碳activated carbon火用exergy机械性能mechanicalproperties集成integration挤出extrusion计算化学computationalchemistry计算机模拟computer simulation 计算流体力学computational fluiddynamics,CFD加工制造fabrication加氢hydrogenation加氢脱硫HDS甲烷methane间歇式batchwise浆料slurry降解degradation胶体colloid焦化coking搅拌容器stirred vessel结垢fouling结晶crystallization解吸,脱附desorption介尺度mesoscale界面interface界面流变学interfacial rheology 界面张力interfacial tension 浸取leaching经济economics静态混合器static mixer聚合polymerization聚合物polymers聚合物加工polymer processing 聚集(作用)aggregation聚结coalescence均化(作用)homogenization抗体antibody颗粒过程particulate processes 颗粒流granular flow颗粒物料granular materials 可持续性sustainability空隙率voidage控制control扩散diffusion 离析segregation离心分离centrifugation离子交换ion exchange离子液体ionic liquids粒度分布particle sizedistribution粒度分布size distribution 粒子particle粒子图像测速PIV粒子形成particle formation 两相流two-phase flow 流变学rheology流动flow流化床fluidized-bed流态化fluidization流体动力学hydrodynamics 流体力学fluid mechanics 流域flow regimes煤燃烧coal combustion 酶enzyme蒙特卡罗模拟Monte Carlosimulation模拟simulation模塑Molding模型model模型简化model reduction模型预测控制model-predictive control模制moulding膜film膜membranes磨损attrition纳滤nanofiltration 纳米材料nanomaterials 纳米技术nanotechnology 纳米结构nanostructure 纳米粒子nanoparticles 黏度viscosity凝胶gels凝结condensation 排水drainage泡沫foam配合物complexes平衡equilibrium曝气aeration 气含率gas holdup气化gasification气力输送pneumaticconveying气泡bubble气溶胶aerosol气体gas气液两相流gas-liquid flow 汽化vaporization汽液平衡vapor liquidequilibria氢Hydrogen燃料fuel燃料电池fuel cells热传导heat conduction 热解pyrolysis热力学thermodynamics 热力学过程thermodynamicsprocess热力学性质thermodynamicproperties溶剂solvents溶剂萃取solvent extraction 溶解dissolution溶解性solubility溶液solution乳液emulsions色谱chromatography 熵entropy上升管riser烧结sintering设计design神经网络neural networks 渗透permeation渗透率permeability渗透蒸发pervaporation 生产production生化工程biochemical engineering生物柴油biodiesel生物催化biocatalysis 生物反应器bioreactors 生物分离bioseparation生物分子工程biomolecular engineering生物工程biological engineering生物过程bioprocess生物技术biotechnology 生物模板biotemplating生物膜biofilm生物能源bioenergy生物燃料biofuel生物医学工程biomedicalengineering生物质biomass失活deactivation石油petroleum实验验证experimentalvalidation食品加工food processing数学模拟mathematicalmodeling数值分析Numerical analysis 数值模拟numerical simulation 水合物hydrate水解hydrolysis水热hydrothermal水溶液aqueous solution瞬态响应transient response算法algorithm塔器column太阳能solar energy肽peptide碳氢化合物hydrocarbons 天然气natural gas 填充床packed bed停留时间分布residence time distribution统计热力学statistical thermodynamics透析dialysis湍动turbulence湍流turbulent flow 团聚agglomeration 脱盐desalination脱氧核糖核酸DNA烷烃alkane微尺度microscale微电子学microelectronics 微反应器microreactor微流体学microfluidics微通道microchannels 温室气体greenhouse gas 稳定性stability稳态steady state 污染pollution吸附(作用)adsorption吸附剂adsorbents吸附剂sorbents吸收absorption系统工程systems engineering 细胞工程cell engineering细胞生物学cell biology下游加工过程downstreamprocessing显微结构microstructure相变phase change相平衡phase equilibria形态学morphology修复remediation需氧aerobic悬浮系suspensions选择催化还原SCR选择性selectivity循环流化床circulating fluidizedbed压缩机compressor烟道气flue gas厌氧anaerobic氧化oxidation氧化铝alumina药物pharmaceuticals液化liquefaction一氧化碳carbon monoxide 仪器,仪表instrumentation移动床moving bed遗传算法genetic algorithm 优化optimization优化设计optimal design有机化合物organic compounds 预测prediction载体support再生regeneration再生能源renewable energy 造粒granulation增湿humidification 蒸发evaporation蒸馏distillation整体器件monolith整体优化global optimization 酯化esterification制备preparation制氢hydrogen production 制造manufacture种群平衡population balance 种群平衡公式population balanceequations主元分析principal componentanalysis状态方程equation of state自催化autocatalysis自由基radical组织工程学tissue engineering。
COMMENTARIESPressure Swing AdsorptionIntroductionPressure swing adsorption(PSA)is a very versatile technology for separation and purification of gas mix-tures.Some of the key industrial applications include (a)gas drying,(b)solvent vapor recovery,(c)fraction-ation of air,(d)production of hydrogen from steam-methane reformer(SMR)and petroleum refinery off-gases,(e)separation of carbon dioxide and methane from landfill gas,(f)carbon monoxide-hydrogen sepa-ration,(g)normal isoparaffin separation,and(h)alcohol dehydration.There are several hundred thousand PSA units operating around the world servicing these and other applications.In fact,PSA has become the state-of-the-art separation technology for application areas a-d listed above,and the sizes of these units range from very small(∼300SCFD units for the production of90% O2from air for personal medical use)to very large(∼100 MMSCFD units for the production of99.999+%hydro-gen from an SMR).Many of these processes are de-scribed in the published books and review papers on the subject.1-13The growth in the research and development of PSA technology has been phenomenal since the first U.S. patent on the subject,authored by C.W.Skarstrom,was granted in1960.14A recent survey showed that∼600 U.S.patents on PSA were issued in the application areas a,c,and d alone during1980-2000,while the number of published papers with PSA as the keyword exceeded800during the period of1970-2000.15The concept of PSA for gas separation is relatively simple.Certain components of a gas mixture are selectively adsorbed on a microporous-mesoporous solid adsorbent at a relatively high pressure by contacting the gas with the solid in a packed column of the adsorbent in order to produce a gas stream enriched in the less strongly adsorbed components of the feed gas. The adsorbed components are then desorbed from the solid by lowering their superincumbent gas-phase par-tial pressures inside the column so that the adsorbent can be reused.The desorbed gases are enriched in the more strongly adsorbed components of the feed gas.No external heat is generally used for desorption.Many different nomenclatures are used to describe these concepts.A PSA process carries out the adsorption step at a superambient pressure,and the desorption is achieved at a near-ambient pressure level.A vacuum swing adsorption(VSA)process undergoes the adsorp-tion step at a near-ambient pressure level,and the desorption is achieved under vacuum.A pressure-vacuum swing adsorption(PVSA)process utilizes the benefits of both concepts.Although simple in concept, a practical PSA/VSA process can be fairly complex because it involves a multicolumn design where the adsorbers operate under a cyclic steady state using a series of sequential nonisothermal,nonisobaric,and non-steady-state steps.These include adsorption,de-sorption,and a multitude of complementary steps which are designed to control the product gas purity and recovery and to optimize the overall separation perfor-mance.9The research trends are to(a)produce purer products at higher recovery,(b)lower adsorbent inven-tory and energy of separation,and(c)increase the scale of application at a lower overall cost.Unique PSA cycles are also designed to simulta-neously produce two pure products from a multicompo-nent feed gas(e.g.,O2and N2from air,CO2and H2from SMR off-gas,and CO2and CH4from landfill gas)as well as to produce a product gas containing a component which is not initially present in the feed gas(ammonia synthesis gas from SMR off-gas).These examples dem-onstrate the wide flexibility of PSA process designs.10,16,17 The key reasons for such growth in this technology are as follows:18(a)An extra degree of thermodynamic freedom for describing the adsorption process introduces immense flexibility in PSA process design as compared with other conventional separation tools such as distillation,ex-traction,or absorption.(b)Numerous microporous-mesoporous families of adsorbents(new or modified)like activated carbons, zeolites,aluminas,silica gels,and polymeric sorbents exhibiting different adsorptive properties for separation of gas mixtures(equilibria,kinetics,and heats)are available.(c)The optimum marriage between a material and a process in designing the PSA separation scheme pro-motes innovations.(d)Many PSA process paths can be designed for the same separation objectives.A good example of items a-d listed above can be found in the area of air fractionation by adsorption. Approximately390U.S.patents were issued on the subject during the last20years.15The processes are designed to(a)separately produce∼23-95%O2from air,(b)separately produce98-99.99+%N2from air,and (c)simultaneously produce∼90+%O2and99+%N2 from air.Many different zeolites having different ther-modynamic selectivities and capacities for adsorption of N2over O2are employed,and the processes are tailor-made to fit the zeolite properties in order to produce the specific product demands for cases a and c.11Many different carbon molecular sieves having different ki-netic selectivities and capacities for adsorption of O2 over N2are employed for case b with appropriate process designs for controlling the N2product purity and recovery.19,20These processes use many different de-signs of PSA,VSA,or PVSA cycles and operating conditions in order to achieve the final goals.A key success in this area has been the lowering of the specific power(<10KWD/ton)of O2production(∼90+%)from air below that of the conventional cryogenic distillation route by the use of LiX zeolite as the N2selective1389Ind.Eng.Chem.Res.2002,41,1389-139210.1021/ie0109758CCC:$22.00©2002American Chemical SocietyPublished on Web02/09/2002adsorbent,21,22while retaining the traditional advantage of lower capital cost for a PSA/VSA process.This opens up the door for increasing the sizes of O2-PSA processes beyond today’s standards.Parallel developments can be cited in the other application areas.9,10,12,13It is expected that this trend will continue in the future.New PSA cycles using old or new adsorbents(preferably tailor-made for each other)will continue to be developed in the existing and new application areas.Good combina-tions of PSA processes and adsorbents are needed for bulk separation of N2and CH4at high pressures, separation of dilute O2from Ar,and bulk separation of propane-propylene mixtures.Emerging ConceptsSeveral very interesting PSA process developments are emerging.They include(a)rapid PSA cycles,(b) novel PSA adsorber designs,(c)sorption-enhanced reac-tion processes(SERP),and(d)high-temperature PSA (HTPSA)cycles.(a)The rapid PSA processes are designed to increase the productivity of the processes by an order of magni-tude by using total process cycle times of seconds instead of minutes as in the case of the conventional PSA cycles.This is achieved by simply running a conventional cycle faster using novel hardware(e.g., rotary valves)23or by changing both the adsorber and the process cycle designs.11However,separation ef-ficiency and performance may be compromised(lower product purity or recovery)because of time limitations in incorporating the complementary cyclic steps and limitations caused by adsorption kinetics.24(b)Faster PSA cycles are often limited by the hydro-dynamic constraints(gas maldistribution,adsorbent fluidization,column pressure drop,etc.),which limit gas flow rates through packed beds.Radial bed adsorbers where the adsorbent is placed between two concentric cylinders and gas flows radially through the packed section may alleviate many of these problems.These adsorbers,however,are more expensive.Many such designs have been proposed and developed which allow faster cycles,higher gas throughputs at lower pressure drops,and complete absence of fluidization.25,26An interesting possibility will be the development of a PSA cycle using a rotary bed adsorber which has been successfully employed for practicing thermal swing adsorption concepts.27(c)The SERP is a hybrid concept where an equilib-rium-controlled reaction is carried out in the presence of an adsorbent which selectively removes one of the undesired reaction products from the reaction zone,thus increasing the yield and the rate of formation of another desired product by Le Chatelier’s principle.The adsor-bent is then regenerated periodically using the prin-ciples of PSA.The concept has also been called pressure swing reactor.28Very recently,a novel process called SERP-H2was demonstrated in a pilot-scale unit for production of CO-and CO2-free(<40ppm)H2by SMR using a CO2selective chemisorbent and a SMR catalyst inside the reactor.29,30The primary impurity in the90+ mol%H2product(dry basis)was unreacted CH4.A high conversion of CH4to H2at a much lower reaction temperature than the conventional SMR process could be achieved.The concept may be useful for direct production of CO-free H2in a fuel cell application. (d)A HTPSA(∼200°C)process was developed for sequestering and recovering CO2from a hot,wet waste gas.31This process also used a chemisorbent for CO2 (∼10-20mol%in feed),and it recovered∼80%of feed CO2as an essentially pure CO2product(dry basis).The concept may be very attractive for controlling green-house effects by removing CO2from a hot and wet flue gas without cooling the gas and removing the water. PSA Process DesignDespite such growth in the practical applications of this technology,the design and optimization of a PSA system still largely remains an experimental effort.A priori design of a practical PSA system that can guarantee the commercial specifications without the use of supporting data from a bench-or pilot-scale process rig may not yet be feasible for the following two reasons. First,most of the practical PSA processes are fairly complex,involving a number of sequential but interact-ing unsteady-state cycle steps.It may be possible to formulate a rigorous mathematical framework(model) to describe such processes,but it is usually expensive and time-consuming to solve such models with the accuracy and reliability needed for industrial design.It requires repeated numerical solutions of a set of coupled nonlinear partial differential equations in the time and space domain with different initial and boundary condi-tions defining the steps of the process for any given cycle until a cyclic steady-state solution is achieved.The computation time for such calculations is often prohibi-tive,and model simplification becomes imperative.10 Second,fundamental understanding of the multicom-ponent gas-solid interactions(thermodynamic and kinetic)that govern the performance of a PSA adsorber is very limited because of their complexity.An accurate description of these interactions must be available before the mathematical process model can be solved.10 It is essential that these interactions are known under all conditions of temperature,pressure,and composition prevailing in a PSA adsorber during all steps of the process.Because these conditions may vary widely in a practical PSA process,the measurement and correlation of such a massive volume of data are often impractical. One needs to predict these interactions from a minimum source of experimental data for the system of interest. Although much progress has been made in this area, the state of the art is often not adequate for satisfying the general design needs.A common practice is to develop a simplified and specific model for the PSA process of interest and use simplistic descriptions(models or empirical)of the gas-solid interactions for the relevant system,to evaluate approximately the effects of the design variables on the performance of the PSA process and finally obtain a crude optimum design.The effort is always closely tied to experimental verification and fine-tuning by measur-ing actual process data from a pilot plant.Such models have often proved to be very useful for process optimiza-tion and screening new ideas.10The partial differential equations to be solved are the coupled mass,momentum,and heat(both gas and adsorbed phases)conservation equations describing the state of the adsorber for each step of the PSA process. The key input data for solving these equations are(i) the multicomponent adsorption equilibria,(ii)the multicomponent adsorptive/desorptive mass-transfer characteristics,and(iii)the multicomponent isosteric heats of adsorption for the system of interest.1390Ind.Eng.Chem.Res.,Vol.41,No.6,2002There lies the crux of the problem.The design engineer must be able to predict these input data from a limited source of experimental data because the range of conditions(pressure,temperature,and gas composi-tion)encountered within the adsorber of a practical PSA process can be immense.Although numerous efforts have been made in the past30years to develop techniques to predict multi-component adsorption characteristics(thermodynamic and kinetic)from pure gas adsorption characteristics of the components,these models have met with only limited success.10,32The state of the art does not allow a priori selection of these methods for the system of interest without extensive experimental testing of the method.This obviously defeats the purpose.Currently, an extensive experimental database is needed(equilib-ria and kinetic)for the design of each separation system of interest.The quality of simulation of a PSA process perfor-mance using a process model largely depends on the detailedness of the model and the accuracy of the input data.33The results can be extremely sensitive to small errors(say(10-15%)in the input data for many process designs because these models often act as amplifiers of errors.On the other hand,many industrial PSA processes require very stringent product specifica-tions.For example,a PSA process for production of H2 from SMR off-gas must have a product H2purity of 99.999+mol%containing less than10ppm of CO x impurities.Furthermore,a difference of(2%in the estimation of the product H2recovery from the feed gas can make or break the economics of a process design for a medium-sized unit(∼50MMSCFD H2).These critical industrial demands are not often appreciated, and a large volume of published literature on PSA models fails to seriously evaluate the quality of the model and the input data by comparing the model calculations with actual experimental process data only qualitatively.Nature of the ProblemThe problem at hand is by no means simple.The adsorbents of practical use,such as activated carbons, aluminas,silica gels,and zeolites,can be very hetero-geneous microporous solids.The first three types of adsorbent contain intricate networks of interconnected micro-and mesopores of various shapes and sizes,which give rise to a nonuniform distribution of gas-solid and gas-gas interaction fields within the adsorbent mass. Heterogeneity in these adsorbents is also caused by differences in the chemical nature of the surfaces at different parts of the adsorbent mass.The pore struc-tures of the zeolites may be ideally well-defined and uniform,but energetic heterogeneity for adsorption is introduced by lattice defects,the presence of hydrated and nonhydrated ion species of one or more kinds at different locations within the framework,the presence of trace moisture within the pore structure,nonuniform hydrolysis of the zeolite framework during regeneration, the existence of distributed Si/Al ordering of the frame-work,etc.34Another problem is introduced by the dissimilar nature of the adsorbate molecules in a gas mixture (sizes,shapes,polarities,etc.).This can severely com-plicate their interactions with the heterogeneous porous solid surfaces and with each other inside the pores of the adsorbent.It is apparent that realistic and accurate estimation of single-component or multicomponent gas-solid or gas-gas interactions within such distributed energy fields inside the adsorbent pores(which often remains unknown)will be difficult,if not impossible.Even the current experimental methods to characterize the pore structures of real heterogeneous microporous solids are very crude and full of uncertainty.The challenge,therefore,is to develop methods for quantitative characterization of adsorbent heterogeneity and to accurately predict multicomponent gas adsorp-tion characteristics(equilibria,kinetics,and heats) using a limited data source.The models,however,must also take into account dissimilar adsorbate properties and lateral interactions between the adsorbates at higher coverages.The models must also be analytical in order to be practically useful.This area should be a subject of serious research by adsorption scientists for many years to come because the problem is complex and success will not be easy.Unfortunately,the topic is being ignored in the recent years.There is also a desperate need to generate and compile a multicomponent gas adsorption database.A large volume of pure gas and some binary gas adsorp-tion equilibrium data are currently available in the literature,35but multicomponent gas equilibrium data are rare.The situation is identical for gas adsorption kinetics data.36The data for heats of adsorption are only emerging.37-39These data will be needed to test the reliability of the theoretical models.In conclusion,it can be said that the development of practical PSA processes for gas separation has been extremely successful despite the fact that an“in-depth”understanding of the complex physicochemical phenom-enon governing this technology may be lacking.Much more basic research is needed before a priori design of PSA processes can be made with acceptable accuracy and confidence.A judicious balance between experi-mental process development and theoretical designs using process models will continue to be the state of the art in the near future.Literature Cited(1)Ruthven,D.M.Principles of Adsorption and Adsorption Processes;John Wiley:New York,1984.(2)Yang,R.T.Gas Separation by Adsorption Processes;But-terworth:London,1987.(3)Suzuki,M.Adsorption Engineering;Kodansha:Tokyo, 1990.(4)Ruthven,D.M.;Farooq,S.;Knaebel,K.S.Pressure Swing Adsorption;VCH Publishers:New York,1994.(5)Crittenden,B.;Thomas,W.J.Adsorption Technology and Design;Butterworth-Heinemann:Oxford,U.K.,1998.(6)Keller,G.E.;Anderson,R.A.;Yon,C.M.Adsorption.In Handbook of Separation Process Technology;Rousseau,R.W.,Ed.; John Wiley:New York,1987;Chapter12.(7)Humphrey,J.L.;Keller,G. E.Adsorption.Separation Process Technology;McGraw-Hill:New York,1997;Chapter4.(8)Sircar,S.Adsorption.In The Engineering Handbook;Dorf, R.C.,Ed.;CRC Press:Boca Raton,FL,1996;Chapter59.(9)Sircar,S.Pressure Swing Adsorption Technology.In Ad-sorption Science and Technology;Rodrigues,A.E.,et al.,Eds.; Kluwer Academic Publishers:Dordrecht,The Netherlands,1989; pp285-321.(10)Sircar,S.Pressure Swing Adsorption:Research Needs in Industry.Proceedings of Third International Conference on Fun-damentals of Adsorption,Sonthofen,Germany,1989;Mersmann, A.B.,Scholl,S.E.,Eds.;AIChE:New York,1989;pp815-843.Ind.Eng.Chem.Res.,Vol.41,No.6,20021391(11)Sircar,S.;Rao,M.B.;Golden,T.C.Fractionation of Air by Zeolites.In Studies in Surface Science and Catalysis;Dab-rowski,A.,Ed.;Elsevier Science:New York,1998;Vol.120A,pp 395-423.(12)Sircar,S.;Rao,M.B.;Golden,T.C.Drying Gases and Liquids by Activated Alumina.In Adsorption on New and Modified Inorganic Solvents;Dabrowski,A.,Tertykh,V.A.,Eds.;Studies in Surface Science and Catalysis;Elsevier Science:New York, 1996;Vol.99,pp629-646.(13)Sircar,S.;Golden,T.C.Sep.Sci.Technol.2000,35,667.(14)Skarstrom,C.W.U.S.Patent2,944,627,1960.(15)Sircar,S.Adsorption2000,6,359.(16)Sircar,S.Sep.Sci.Technol.1990,25,1087.(17)Fuderer,A.U.S.Patent4,375,363,1983.(18)Sircar,S.Adsorpt.Sci.Technol.2001,19,347.(19)Knoblauch,K.;Heimbach,H.;Harder, B.U.S.Patent 4,548,799,1985.(20)Lemcoff,N.C.;Gmelin,R.C.U.S.Patent5,176,722,1993.(21)Hirano,S.Dynamic Adsorption Properties of Li Ion-Exchanged Zeolite Adsorbents.In Fundamentals of Adsorption; Kaneko,K.,Ed.;2001;Vol.7,in press.(22)Kirner,J.F.U.S.Patent5,268,023,1993.(23)Keefer,B.G.;Doman,D.G.WIPO International Publica-tion No.W097/39821,1997.(24)Sircar,S.;Hanley,B.F.Adsorption1995,1,313.(25)Poteau,M.;Eteve,S.U.S.Patent5,232,479,1993.(26)Smolarek,J.;Leavitt,F.W.;Nowobilski,J.J.;Bergsten,E.;Fassbaugh,J.H.U.S.Patent5,759,242,1998.(27)Hirose,T.;Kuma,T.Honeycomb Rotor Continuous Ad-sorber for Solvent Recovery and Dehumidification.2nd Korea-Japan Symposium on Separation Technology,1990.(28)Vaporciyan,G.G.;Kadlec,R.H.AIChE J.1987,33,1334.(29)Sircar,S.;Hufton,J.R.;Nataraj,S.U.S.Patent6,103,143, 2000.(30)Waldron,W.E.;Hufton,J.R.;Sircar,S.AIChE J.2001, 47,1477.(31)Sircar,S.;Golden,C.M.A.U.S.Patent6,322,612,2001.(32)Sircar,S.AIChE J.1995,41,1135.(33)Hartzog,D.G.;Sircar,S.Adsorption1995,1,133.(34)Sircar,S.Adsorption Technology s A Versatile Separation Tool.In Separation Technology s The Next Ten Years;Garside,J., Ed.;Institute of Chemical Engineers:Rugby,Warwickshire,U.K., 1994;pp47-72.(35)Valenzuela,D.P.;Myers,A.L.Adsorption Equilibrium Data Handbook;Prentice-Hall:Englewood Cliffs,NJ,1989.(36)Ka¨rger,J.;Ruthven,D.M.Diffusion in Zeolites and Other Porous Solids;John Wiley:New York,1992.(37)Dunne,J.;Rao,M.B.;Sircar,S.;Gorte,R.J.;Myers,A.L. Langmuir1997,13,4333.(38)Cao,D.V.;Sircar,S.Ind.Eng.Chem.Res.2001,40,156.(39)Cao,D.V.;Sircar,S.Adsorption2001,7,73.Shivaji SircarAir Products and Chemicals,Inc.,Allentown,Pennsylvania18195IE01097581392Ind.Eng.Chem.Res.,Vol.41,No.6,2002。
Enhancement of CO2Adsorption and CO2/N2Selectivity on ZIF-8via Postsynthetic ModificationZhijuan ZhangSchool of Chemistry and Chemical Engineering,South China University of Technology,Guangzhou,510640,P.R.ChinaDept.of Chemistry and Chemical Biology,Rutgers University,Piscataway,New Jersey,08854Shikai Xian,Qibin Xia,Haihui Wang,and Zhong LiSchool of Chemistry and Chemical Engineering,South China University of Technology,Guangzhou,510640,P.R.ChinaJing LiDept.of Chemistry and Chemical Biology,Rutgers University,Piscataway,New Jersey,08854DOI10.1002/aic.13970Published online January11,2013in Wiley Online Library()Imidazolate framework ZIF-8is modified via postsynthetic method using etheylenediamine to improve its adsorption per-formance toward CO2.Results show that the BET surface area of the modified ZIF-8(ED-ZIF-8)increases by39%,and its adsorption capacity of CO2per surface area is almost two times of that on ZIF-8at298K and25bar.H2O uptake on the ED-ZIF-8become obviously lower compared to the ZIF-8.The ED-ZIF-8selectivity for CO2/N2adsorption gets significantly improved,and is up to23and13.9separately at0.1and0.5bar,being almost twice of those of the ZIF-8. The isosteric heat of CO2adsorption(Q st)on the ED-ZIF-8becomes higher,while Q st of N2gets slightly lower com-pared to those on the ZIF-8Furthermore,it suggests that the postsynthetic modification of the ZIF-8not only improves its adsorption capacity of CO2greatly,but also enhances its adsorption selectivity for CO2/N2/H2O significantly. V C2013American Institute of Chemical Engineers AIChE J,59:2195–2206,2013Keywords:ZIF-8,modification,adsorption/gas,isosteric heat of adsorption,selectivityIntroductionCO2has often been cited as the primary anthropogenicgreenhouse gas(GHG)as well as the leading culprit inglobal climate change.The development of a viable carboncapture and sequestration technology(CCS),is therefore,ascientific challenge of the highest order.1–4Currently,a vari-ety of methods,such as membrane separation,chemicalabsorption with solvents,and adsorption with solid adsorb-ents,have been proposed to sequester CO2from thefluegases of power plant.Thereinto,the adsorption is consideredto be one of the most promising technologies for capturingCO2fromflue gases because of their easy control,low oper-ating and capital costs,and superior energy efficiency.5–7Many adsorbents have been investigated for CO2adsorptionincluding activated carbons,zeolites,hydrotalcites and metaloxides.8–14However,although some zeolite materials havebeen claimed to be most adequate for CO2separation fromflue streams,it is difficult to regenerate them without signifi-cant heating which leads to low productivity and greatexpense.15,16Recently,metal-organic frameworks(MOFs)haveattracted great attention and present a promising platform forthe development of next-generation capture materialsbecause of their high capacity for gas adsorption and tunablepore surfaces that can facilitate highly selective binding ofCO2.17–26To optimize a MOF for a particular application,itis important to be able to tailor its pore metrics and function-ality in a straightforward fashion.However,tailoring MOFsmaterials by modifying their textural properties(e.g.,surfacearea and pore volume)and surface chemistry(acid–baseproperties,functional groups)for adsorption application isstill a difficult task.27Many researchers have given insightinto modification of the MOF materials so as to develop newand better adsorbents.Strategies reported include ligandfunctionalization,24,28–39framework interpenetration,22,23introduction of alkali-metal cations,40–42control of poresize32,43–47and incorporation of open metal sites(OMSs).39,48–51However,because of the instability underconditions for the synthesis of MOFs or the competitivereaction with some framework components,it may be diffi-cult for certain functional groups to incorporate into MOFsusing aforementioned strategies.Another strategy for gener-ating desired functionalities in MOFs is the postsynthesis Additional Supporting Information may be found in the online version of thisarticle.Correspondence concerning this article should be addressed to Z.Li atcezhli@.V C2013American Institute of Chemical EngineersAIChE Journal2195June2013Vol.59,No.6modification of preconstructed,robust precursor MOFs.52–55 For example,An et al.33demonstrated that postsynthetic exchange of extra-framework cations within anionic bio-MOF-156can be used as a means to systematically modify its pore dimensions and metrics.Farha et al.57synthesized a series of cavity modified MOFs by replacing coordinated sol-vents with several different pyridine ligands.They found that a p-(CF3)NC5H4-modified MOF showed considerable improvements in the CO2/N2selectivities compared to the parent framework.46Long and his coworkers58previously reported the grafting of ethylenediamine(en)within a water-stable MOF H3[(Cu4Cl)3(BTTri)8](CuBTTri),and found that the en modified sample had more greater attraction of CO2 at low pressures and the CO2/N2selectivity also increased over the entire pressure range measured.More recently, Long and coworkers59incorporated the N,N0-dimethylethyle-nediamine(mmen)into the CuBTTri MOF,and showed that the CO2uptake was drastically enhanced.Zhang et al.60 reported that after ZIF-8was modified by ammonia impreg-nation,the surface basicity was greatly increased and there-fore the CO2uptake was enhanced.Park et al.61reported a postsynthetic reversible incorporation of organic linkers3,6-di(4-pyridyl)-1,2,4,5-tetrazine(bpta)into SNU-30 [Zn2(TCPBDA)(H2O)2]Á30DMFÁ6H2O through single-crystal-to-single-crystal transformations,and found that the desol-vated SNU-310exhibited enhanced selective adsorption of CO2over N2.Xiang et al.62incorporated the CNTs into HKUST-1,and then modified it with Li1.The results showed that the hybrid Li@CNT@[Cu3(btc)2],which is formed by the combination of Li doping and CNT incorpora-tion,having an enhancement of CO2uptakes by about 305%.However,to this date,no work has been reported out on the postsynthetic modification of ZIF-8to enhance its functionality.In this work,the postsynthetic modification of the ZIF-8is proposed to prepare a novel adsorbent with higher CO2 adsorption capacity and CO2/N2selectivity.The postsyn-thetic modification of the ZIF-8crystals would be carried out by using ethylenediamine treatment.Then the surface groups of the modified ZIF-8samples(ED-ZIF-8)would be characterized.Single-component isotherms of CO2and N2 on the modified ZIF-8samples would be measured sepa-rately.Furthermore,the CO2/N2selectivity is estimated by using IAST on the basis of single-component isotherms of CO2and N2.The influence of the textural structures and sur-face chemistry of the original and modified ZIF-8samples on their adsorption capacities for CO2and selectivity of CO2/N2would be discussed and reported here.This informa-tion will be valuable for selecting appropriate adsorbents for CO2capture process.Methods and MaterialsMaterials and instrumentsZinc nitrate hexahydrate(Zn(NO3)2Á6H2O,98%,extra pu-rity)and2-methylimidazole(H A MeIM)(99%purity)were purchased from J&K Chemicals.N,N A Dimethylacetamide (DMF)was purchased from Qiangshen Chemicals Co.,Ltd. of Jiangshu(Jiangshu,China),and it was further purified by 4A molecular sieve to eliminate the water.Maganetic suspension balance RUBOTHERM was sup-plied by Germany.Its precision was0.000001g.ASAP 2010sorptometer was supplied by Micromeritics Co., Norcross,GA,USA.AdsorbentsSynthesis of ZIF-8was performed following the reported procedures63with a few modifications.First,a solid mixture of zinc nitrate hexahydrate Zn(NO3)2Á6H2O(0.956g, 3.2 mmol)and2-methylimidazole(H A MeIM)(0.24g, 3.4 mmol)was dissolved in70mL of DMF solvent.The mixture was quickly transferred to a100mL autoclave and sealed. Second,the autoclave was heated at a rate of5K/min to 413K in a programmable oven and held at this temperature for24h under autogenous pressure by solvothermal synthe-sis,followed by cooling at a rate of0.3K/min to room tem-perature.Third,after removal of mother liquor from the mix-ture,chloroform(40mL)was added to the autoclave.The as-synthesized ZIF-8crystals were then isolated byfiltration. Colorless polyhedral crystals were collected from the upper layer,washed with DMF(10mL33),and dried at383K overnight.To further remove the guest species from the framework and prepare the evacuated form of ZIF-8crystals for modifi-cation and gas-sorption analysis,the as-synthesized ZIF sam-ples were immersed in methanol at ambient temperature for 48h,and evacuated at ambient temperature for5h,and sub-sequently at an elevated temperature673K for2h. Postsynthetic modification of adsorbentsThe as-synthesized ZIF-8crystals(labeled as ZIF-8)were dried at383K for24h for postsynthetic modification.The subsequent treatment applied to the modification of ZIF-8crystals consists of the following steps:The modified ZIF-8sample(labeled as ED-ZIF-8)was synthesized using ethylenediamine as a linker.In a typical procedure,the ZIF-8sample was added to30%ethylenediamine solution and then the mixture was placed in a stainless high-pressure autoclave.The autoclave was heated in an oven at416K for 1h and then381K for6h.The light yellow product wasfil-tered and washed with deionized water.Finally,the sample was dried at383K overnight.Characterization of adsorbentsThe specific surface area and pore volume of original ZIF-8and modified ZIF-8crystals were measured on a Micrometrics gas adsorption analyzer ASAP2010instrument equipped with commercial software for calculation and analysis.Powder X-ray diffraction data were collected using a D8 advance h-2h diffractometer(Bruker)in reflectance Bragg-Brentano geometry employing Cu K a line focused radiation with40kV voltages and40mA current.The X-ray scanning speed was set at2 /min and a step size of0.02 in2h.A Jade5XRD pattern processing software(MDI,Inc.,Liver-more,CA)was used to analyze the XRD data collected on the ZIF-8samples.The surface organic molecules were analyzed by taking FTIR spectra on a Bruker550FTIR instrument equipped with a diffuse reflectance accessory that included a reaction cell.Data acquisition was performed automatically using an interfaced computer and a standard software package.The samples were dried in vacuo at423K prior to mixing with KBr powder.The samples were run in ratio mode allowing for subtraction of a pure KBr baseline.The sample chamber2196DOI10.1002/aic Published on behalf of the AIChE June2013Vol.59,No.6AIChEJournalwas kept purged with nitrogen during the entire experiment.The spectrometer collected 64spectra in the range of 400–4,000cm 21,with a resolution of 4cm 21.CO 2and N 2adsorption measurementsThe CO 2and N 2adsorption-desorption isotherms at 298K,308K,318K,and 328K were obtained on a RUBO-THERM magnetic suspension balance.The initial activa-tion of the modified sample was carried out at 423K for 12h in a vacuum environment.He (ultra-high purity,U-sung)was used as a purge gas in this study.The adsorp-tion processes were carried out using high purity CO 2and N 2(99.999%)gas.A feed flow rate of 60mL/min of CO 2,40mL/min of N 2and 30mL/min of He,respec-tively,were controlled with the mass flow controllers (MFC)to the sample chamber.Both adsorption and de-sorption experiments were conducted at the same tempera-ture.The temperature of the sorption chamber can be adjusted and maintained constant by an internal tempera-ture sensor.However,the pressure can be changed step-wise through the gas flow rate.Typically,there are four steps for finishing determination of an isotherm of CO 2or N 2by using Rubotherm magnetic suspension balance.These detail steps are shown by the operation manual of Rubotherm maganetic suspension balance.H 2O adsorption measurementsThe water adsorption measurements were conducted on a computer-controlled DuPont Model 990TGA.The partial pressure of water was varied by changing the blending ratios of water-saturated nitrogen and pure nitrogen gas streams.Before measurement,the modified ZIF-8samples were acti-vated at 423K for 6h.Results and DiscussionStructure and pore characterizationFigure 1exhibits the adsorption-desorption isotherms of N 2at 77K on the two samples ZIF-8and ED-ZIF-8.It can be seen that both samples show type-I behavior,indicating they are microporous in nature.Table 1lists structure param-eters of the two samples.These data indicate that the BET surface area and micropore volume of the ED-ZIF-8sample are significantly higher than those of the original ZIF-8sam-ple,with an increase of $39%and 35.6%,respectively.Yaghi and his coworkers reported a pore volume of 0.66cm 3/g for ZIF-8from the single crystal structure.For the ZIF-8sample,the total pore volume is calculated to be 0.54cm 3/g,because part of the pores might be blocked.However,after the postsynthetic modification,the blocked pores were reopened,and at meanwhile,some new pores were formed.64,65Thus,the total pore volume of the ED-ZIF-8sample is greatly improved.Figure 2shows the powder X-ray diffraction (PXRD)pat-tern of the modified ZIF-8sample.It can be seen that the main peaks of the modified ZIF-8sample are very clear,and similar to those of the original ZIF-8sample,indicating that the integrity of the modified ZIF-8sample maintains well af-ter the postsynthetic modification.However,for a deep look-ing,it can be found that the major peaks of ED-ZIF-8all shifted to the left side (low-angle area)a little bit,which means after modification,the lattice distance increased.In order to obtain information concerning changes in the surface groups,FTIR experiments were carried out to char-acterize the samples.Figure 3a shows the FTIR spectra of the original ZIF-8and the ED-ZIF-8sample.It is noticed that the spectra for the two samples show high similarities,and the main peaks of both ZIF-8samples match well with the published FTIR spectra for the ZIF-8.However,someTable 1.Porous Structure Parameters of the Modified ZIF-8CrystalsSample BET surface area (m 2.g 21)Langmuir surface area (m 2.g 21)Micropore volume(cm 3.g 21)Total pore volume (cm 3.g 21)Micropore diameter (nm)Mesopore diameter (nm)ZIF-8102513520.450.540.352 4.43ED-ZIF-8142818970.610.750.5444.53Figure 1.N 2adsorption-desorption isotherms of ZIF-8and ED-ZIF-8samples.[Color figure can be viewed in the online issue,which is available at .]Figure2.PXRD patterns of ZIF-8and ED-ZIF-8samples.[Color figure can be viewed in the online issue,which is available at .]AIChE Journal June 2013Vol.59,No.6Published on behalf of the AIChE DOI 10.1002/aic2197differences are also observed.For example,the spectrum of the ED-ZIF-8sample is different from that of the ZIF-8sam-ple in that (1)as shown in Figure 3b there is a new peak at 3381cm 21which is assigned to N A H group appeared on the spectrum of the ED-ZIF-8sample,suggesting some N A H groups have been introduced on the surfaces of the sample ED-ZIF-8,and (2)a peak at 3626cm 21assigned to O A H of the adsorbed H 2O is present in the spectrum of the ZIF-8sample,which is absent in the spectrum of the ED-ZIF-8sample,as shown in Figure 3b.CO 2and N 2adsorption isothermsFor comparison,Figure 4shows the isotherms of CO 2on the ZIF-8and ED-ZIF-8samples.It is visible that the amount adsorbed of CO 2increases as temperature decreases.This suggests that the adsorption of CO 2is mainly physical adsorption.More importantly,it is found that the ED-ZIF-8sample had higher CO 2adsorption capacities compared to the ZIF-8sample,indicating that the adsorption capacities of the modified ZIF-8toward CO 2are greatly improved,nearly being twice as much as the ZIF-8.One of the reasons is that the surface area (BET)of the ED-ZIF-8increases by 39%,as indicated in Table 1.The other reason is that adsorptioncapacity per unit surface area of the ED-ZIF-8for CO 2increases due to an introduction of N A H groups by postsyn-thetic modification.To further understand that,Figure 4a and 4b are separately transferred into Figure 5a and 5b in which the equilibrium uptakes of CO 2based on unit surface area (BET)of the two samples are plotted as a function of CO paring Figure 5b and Figure 5a shows that the CO 2uptake per surface area (BET)of the ED-ZIF-8is obvi-ously higher than that of the ZIF-8,which is mainly ascribed to the introduction of N A H groups,as shown in Figure 3.Figure 6a and 6b show the N 2adsorption isotherms on the two samples.It is visible that the N 2uptakes on the modified ZIF-8samples are slightly higher than that on the ZIF-8due to its larger surface area and pore volume after modification.However,after Figure 6a and 6b are converted into Figure 7a and 7b in which the equilibrium uptakes of N 2based on unit surface area of the two samples are plotted as a function of pressure,it is found from Figure 7that the equilibrium uptakes of N 2per surface area of the ED-ZIF-8are slightly lower than that of the ZIF-8,which means that ED-ZIF-8sample has less affinity toward N 2than ZIF-8sample.This will be helpful to enhance the adsorption selectivity for CO 2/N 2.Figure 3.a.FTIR spectra of the modified ZIF-8crystalsbetween 4,000–400cm 21;b.FTIR spectra of the modified ZIF-8crystals between 4000–2,400cm 21.[Color figure can be viewed in the online issue,which is available at .]Figure 4.a.Isotherms of CO 2on the ZIF-8sample withdifferent temperatures; b.isotherms of CO 2on the ED-ZIF-8sample with different temperatures.[Color figure can be viewed in the online issue,which is available at .]2198DOI 10.1002/aicPublished on behalf of the AIChE June 2013Vol.59,No.6AIChEJournalMultiple cycles of CO 2adsorption-desorption on the ED-ZIF-8To evaluate the regeneration performance of the modified sample or the reversibility of CO 2adsorption on the modi-fied sample,the experiments of multiple cycles of CO 2adsorption-desorption on the ED-ZIF-8were performed in the Rubotherm system at 298K.For adsorption process,the adsorption pressure were targeted for 25bar;while for de-sorption process the system pressure was targeted for 1mbar,and then the desorption system was quickly depressur-ized by using vacuum pumping.Figure 8shows the variation curve of the amounts adsorbed of CO 2on the ED-ZIF-8dur-ing four consecutive cycles of CO 2adsorption-desorption experiments at 298K.It was visible clearly that during the desorption,the amounts adsorbed of CO 2on the ED-ZIF-8sample decreased sharply with time,and then reached a very low content,about 2.21wt %of residual CO 2which was present on the sample after desorption at 1mbar.The effi-ciency of CO 2desorption was nearly up to 98%over the entire four circles.It indicated further that CO 2adsorption was reversible with very little accumulation of irreversible bound CO 2on the ED-ZIF-8framework.In addition,it wasalso observed from Figure 8that the curves representing the cycles of CO 2adsorption-desorption experiments were very similar,suggesting that adsorption and desorption properties of the sample ED-ZIF-8for CO 2were stable or repeatable.It also proved that the pressure swing was effective in strip-ping adsorbed CO 2from the ED-ZIF-8.H 2O adsorption isothermsFigure 9shows the water isotherms on the modified ZIF-8samples at 298K.The water uptake on the ED-ZIF-8sample is less than that on the ZIF-8sample,indicating that the sur-face of the modified sample became more hydrophobic com-pared to the ZIF-8sample.It also means that the interaction of the water molecule with the modified sample became weaker as compared to that with the ZIF-8.Ideal adsorbed solution theory (IAST)selectivity of CO 2/N 2The ideal adsorbed solution theory (IAST)developed by Myers and Praunitz 66provides an effective method to predict the adsorption selectivity and the adsorption equilibrium of gas mixtures from the isotherms of the pure components.Figure 5.a.Isotherms of CO 2on the ZIF-8samplebased on unit surface area;b.isotherms of CO 2on the ED-ZIF-8sample based on unit surface area.[Color figure can be viewed in the online issue,which is available at .]Figure 6.a.Isotherms of N 2on the ZIF-8sample atdifferent temperatures;b.isotherms of N 2on the ED-ZIF-8sample at different temperatures.[Color figure can be viewed in the online issue,which is available at .]AIChE JournalJune 2013Vol.59,No.6Published on behalf of the AIChE DOI 10.1002/aic2199Previous work reported that the IAST can accurately predict gas mixture adsorption in a number of zeolites and MOF materials.10,48,67–70The IAST assumes that the adsorbed mixture is an ideal solution at constant spreading pressure and temperature,where all the components in the mixture conform to the rule analogous to Raoult’s law,and the chemical potential of the adsorbed solution is considered equal to that of the gas phase at equilibrium.From the IAST,the spreading pressure p is given byp 0i ðp 0i Þ5RT A ðp 0iqd ln p (1)p Ã5p A 5ðp 0i 0q i dp (2)Where A is the specific surface area of the adsorbent,p andp *are the spreading pressure and the reduced spreading pres-sure,separately.p 0i is the gas pressure of component i corre-sponding to the spreading pressure p of the gas mixture.At a constant temperature,the spreading pressure of single component is the samep Ã15p Ã25…5p Ãn 5p(3)For binary adsorption of component 1and 2,the IASTrequiresy 1p t 5x 1p 1ð12y 1Þp t 5ð12x 1Þp 2(4)Where y 1and x 1denote the molar fractions of component 1in the gas phase and in the adsorbed phase,respectively.p t is the total gas pressure,p 1and p 2are the pressures of com-ponent 1and 2at the same spreading pressure as that of the mixture,respectively.Adsorption selectivity in a binary mixture of component 1and 2is defined asS 125x 1x 2 y 2y 1 (5)For the application of IAST to predict adsorption separa-tion selectivity,the following two conditions are necessary:good quality adsorption data of each single component;and excellent curve fitting model for such data.48,71,72In order to perform the integrations of Eqs.(1)and (2)required by IAST,the single-component isotherms should be fitted by a proper isotherm model.In practice,several meth-ods are available.In this work,it is found that the dual-site Langmuir-Freundlich (DSLF)equation can be successful to fit this set of adsorption data.The dual-site Langmuir-Freundlich model can be expressed as followsq 5q m ;13b 1p 1=n 111b 1p 11q m ;23b 2p 1=n 211b 2p 2(6)Where p is the pressure of the bulk gas at equilibrium with the adsorbed phase (kPa),q m,1,q m,2are the saturation capaci-ties of sites 1and 2(mmol/g),b 1and b 2are the affinity coefficients of sites 1and 2(1/kPa),and n 1and n 2are the deviations from an ideal homogeneous surface.Figure 10shows a comparison of the model fits and the isotherm data.It is visible that the DSLF model can be applied favorably for fitting experimental data of CO 2and N 2adsorption.Table 2presents the fitting parameters ofFigure 7.a.Isotherms of N 2on the ZIF-8sample basedon unit surface area;b.isotherms of N 2on the ED-ZIF-8sample based on unit surface area.[Color figure can be viewed in the online issue,which is available at .]Figure 8.Recycle runs of CO 2adsorption-desorptionon the ED-ZIF-8at 298K and 25bar for adsorption and 1mbar for desorption.[Color figure can be viewed in the online issue,which is available at .]2200DOI 10.1002/aicPublished on behalf of the AIChE June 2013Vol.59,No.6AIChEJournalDSLF equation as well as the correlation coefficients (R 2).Examination of the data shows that this DSLF model is able to fit the adsorption data well since the correlation coeffi-cients R 2are up to 0.9997.In this work,the equilibrium adsorption data of single component CO 2as well as N 2are available,and the DSLF model can fit the experimental isotherms of CO 2and N 2adsorption very well.Therefore,the DSLF model can be combined with the ideal adsorbed solution theory (IAST)to predict the mixture adsorption isotherms and calculate the selectivities of the two samples for CO 2/N 2adsorption.Figure 11a and 11b present,respectively,the adsorption isotherms predicted by IAST for equimolar mixtures of CO 2/N 2in the samples ZIF-8and ED-ZIF-8as a function of total bulk pressure.It can be seen that CO 2is preferentially adsorbed over N 2on the two samples because of stronger interactions between CO 2and the ZIF-8sample,and the amount adsorbed of N 2is much lower in the mixtures than that in single-component adsorption because of competition adsorption from CO 2,which adsorbs more strongly.Figure 12shows the IAST-predicted selectivities of the two samples for equimolar CO 2and N 2mixtures at 298K as a function of total bulk pressure.It can be seen that the adsorption selectivity of the two samples for CO 2/N 2dropped with an increase in the pressure.More importantly,Figure 9.H 2O adsorption isotherms on the modifiedZIF-8samples at 298K.[Color figure can be viewed in the online issue,which is available at .]Table 2.The Fitting Parameters of the Dual-site Langmuir-Freundlich Equations for the Pure Isotherms of CO 2and N 2at 298KZIF-8ED-ZIF-8CO 2N 2CO 2N 2R 20.99970.99990.99970.9999q m,1(mmol/g)27.2527.8748.8828.32q m,2(mmol/g) 2.122 1.919 4.672 1.847b 1(atm 21)0.015330.0011700.012590.001388b 2(atm 21)0.0068950.026090.029480.02504n 1 1.6000.7875 1.4040.7704n 20.32440.96340.44300.8671Figure 10.DSLF fitting of the CO 2and N 2isotherms onZIF-8and ED-ZIF-8at 298K.[Color figure can be viewed in the online issue,which is available at .]Figure11.a.The IAST -predicted isotherm forequimolar CO 2/N 2mixtures of the ZIF-8sample at 298K as a function of total bulk pressure; b.the IAST -predicted isotherm for equimolar CO 2/N 2mixtures of the ED-ZIF-8sample at 298K as a function of total bulk pressure.[Color figure can be viewed in the online issue,which is available at .]AIChE Journal June 2013Vol.59,No.6Published on behalf of the AIChE DOI 10.1002/aic2201the adsorption selectivity of CO 2/N 2on the sample ED-ZIF-8is always higher than that on the sample ZIF-8,especially in the low-pressure region.For example,at 0.1and 0.5bar,the selectivity of the sample ED-ZIF-8for CO 2/N 2were up to 23and 13.9separately,which is almost twice of those of the sample ZIF-8.Figure 13a and 13b show,respectively,the IAST-pre-dicted selectivities of the samples ZIF-8and ED-ZIF-8for CO 2/N 2at different mixture compositions and different pres-sures.It is noticed that the selectivity increases rapidly as the gas-phase mole fraction of N 2approaches unity.For example,at yN 250.9,a typical feed composition of flue gas,high selectivities are obtained.Even at yN 250.5,the selectivity of the ED-ZIF-8for CO 2/N 2is in the range of 6–24,much higher than those on the ZIF-8sample and many other MOF samples such as ZIF-7030,ZIF-6830and MOF-508b.73This property is very important since some separa-tion processes could be operated at low pressures,such as vacuum swing adsorption (VSA),which could be extremely efficient by using the sample ED-ZIF-8because its selectiv-ity increases dramatically with decreasing pressure.Ideal adsorbed solution theory (IAST)selectivity of CO 2/N 2/H 2OThe major challenge of CO 2capture from power plant flue gas wastes is the separation of CO 2/N 2.In addition,competition adsorption of water molecule must be taken into account,because these flue gas wastes are usually saturated with certain amount of water (5–7%by volume)for the industrial postcombustion processes.Thus,for real industrial use of adsorbents,the effect of water on CO 2/N 2selectivity is another crucial factor that needs to be considered and evaluated.Here,the IAST was adopted to evaluate the ter-nary mixture CO 2/N 2/H 2O adsorption on the modified ZIF-8samples.First,the experimental isotherms of water on the modified ZIF-8samples at 298K were fitted using the DSLF model.Table 3presents the fitting parameters of DSLF equation as well as the correlation coefficients.It can be seen that theDSLF model fits the H 2O adsorption on both samples very well.Second,the DSLF model was combined with the ideal adsorbed solution theory (IAST)to predict the mixture adsorption isotherms,and then calculate the selectivities of the two samples for CO 2/N 2adsorption.Figure 14shows the predicted isotherms of ternary mix-ture CO 2/N 2/H 2O on the modified ZIF-8samples at 298K.It can be observed that in comparison with the ZIF-8,after modification,the CO 2adsorption capacity of the ED-ZIF-8in the ternary mixture obviously increased,and its N 2adsorption capacity somewhat increased,which made CO 2/N 2adsorption selectivity of the ED-ZIF-8increase.More importantly,its water adsorption capacity in the ternary mix-ture became lower compared to the ZIF-8,and it was also lower than the single component water uptake.It means the competition adsorption of H 2O in the ternary mixture was weakened on the surfaces of the ED-ZIF-8sample.Figure 12.The IAST -predicted selectivity for equimolarCO 2and N 2at 298K as a function of total bulk pressure.[Color figure can be viewed in the online issue,which is available at .]Figure 13.a.The IAST predicted selectivities atdifferent mixture compositions and different pressures for the ZIF-8sample at 298K;b.the IAST predicted selectivities at different mixture compositions and different pressures for the ED-ZIF-8sample at 298K.[Color figure can be viewed in the online issue,which is available at .]2202DOI 10.1002/aicPublished on behalf of the AIChE June 2013Vol.59,No.6AIChEJournal。
Development of Silica-Gel-Supported PolyethylenimineSorbents for CO 2Capture from Flue GasZhonghua ZhangEMS Energy Institute and Dept.of Energy and Material Engineering,Pennsylvania State University,209AcademicProjects Building,University Park,PA 16802School of Chemical and Environmental Engineering,China University of Mining and Technology (Beijing),Beijing 100083,ChinaXiaoliang Ma,Dongxiang Wang,and Chunshan SongEMS Energy Institute and Dept.of Energy and Material Engineering,Pennsylvania State University,209AcademicProjects Building,University Park,PA 16802Yonggang WangSchool of Chemical and Environmental Engineering,China University of Mining and Technology (Beijing),Beijing 100083,ChinaDOI 10.1002/aic.12771Published online November 17,2011in Wiley Online Library ().In order to reduce the sorbent preparation cost and improve its volume-based sorption capacity,the use of an inexpensive and commercially available silica gel was explored as a support to prepare a solid polyethylenimine sorbent (PEI/SG)for CO 2capture from flue gas.The effects of the pore volume and particle size of the silica gels,molecular weight of polyethylenimine and amount of polyethylenimine loaded,sorption temperature and moisture in the flue gas on the CO 2sorption capacity of PEI/SG were examined.The sorption performance of the developed PEI/SG was evaluated by using a thermogravimetric analyzer and a fixed-bed flow sorption system in comparison with the SBA-15-supported polyethylenimine sorbent (PEI/SBA-15).The best PEI/SG sorbent showed a mass-based CO 2sorption capacity of 138mg-CO 2/g-sorbent,which is almost the same as that of PEI/SBA-15.In addition,the PEI/SG gave a high volume-basedsorption capacity of 83mg-CO 2/cm 3-sorbent,which is higher than that of PEI/SBA-15by a factor of 2.6.VC 2011American Institute of Chemical Engineers AIChE J,58:2495–2502,2012Keywords:carbon dioxide,CO 2capture,flue gas,polyethylenimine,silica gel,sorptionIntroductionThe influence of greenhouse gases on global climate has attracted great attention with respect to developing strategies for the reduction of carbon dioxide (CO 2)emissions.1CO 2capture and separation from the flue gas have been considered as a key option for greenhouse gas control.2,3,4Among various approaches reported for CO 2capture,the amine scrubbing process is currently more favored technology for CO 2capture on industrial scale.5,6,7However,this process has some prob-lems,including high-energy consumption,low-absorption/desorption rate,degradation of amine solution in the process,and corrosion of the amine solution.In comparison with the liquid amine scrubbing technique,the solid-supported amine sorbents have shown some significant advantages,including high capacity,no or less corrosion,higher sorption/desorption rate and lower energy consumption.Currently,there are two types of the solid-supportedamine sorbents according to their preparation methods.The sorbents of type 1are prepared through the formation of the covalent bonds between the amine-containing compounds and the surface of the solid support,such as SBA-12,8SBA-15,9–13SBA-16,14,15MCM-41,16and MCM-48.17A signifi-cant advantage of this type of the sorbents is less loss and migration of the amine-containing compounds from the surface in the sorption-desorption cycles due to the strong covalent bonds between the amine-containing compounds and the support surface.However,this kind of the sorbents usually shows relatively lower CO 2adsorption capacity due to the limitation of the amine groups on the surface,although a significant progress has been made recently.The preparation method for these sorbents is also relatively com-plex,resulting in higher cost for the sorbent preparation.The sorbents of type 2are prepared by loading the amine-containing polymer or oligomer,such as polyethylenimine (PEI)or oligoethylenimine,on the nanoporous materials,such as KIT-6,18SBA-15,19–21and MCM-41,22–27using a simple wet-impregnation method on the basis of the ‘‘molec-ular basket’’sorbent (MBS)concept proposed first by XuCorrespondence concerning this article should be addressed to X.Ma at mxx2@ and C.Song at csong@.VC 2011American Institute of Chemical Engineers AIChE Journal 2495August 2012Vol.58,No.8et al.22–25and further developed by others.21,28,29The signifi-cant advantages of these sorbents are that the sorbents can be prepared relatively easily by loading a large amount of CO2-philic polymer/oligomer on various porous materials, and are able to offer a sorption capacity higher than 140mg-CO2/g-sorbent,as the method is easy to increase and control amount of the amines loaded.However,most porous materials,such as MCM-41,SBA-15and KIT-6,reported currently in literature for preparation of these solid amine sorbents are expensive and commercially unavailable.For using MCM-41and SBA-15as supports,it was estimated that the expense of the support materials accounts for more than90%of the total sorbent preparation cost,which results in a preparation cost higher than$700/kg.35Therefore,using an inexpensive support material can significantly reduce the preparation cost of the sorbent.Furthermore,poor hydrother-mal stability of the mesoporous silica molecular sieves30 may cause the degradation of the sorbents within the sorp-tion/desorption cycles.The volume-based sorption capacity of these sorbents is also lower due to low-packing density of the sorbents.All these limit the practical application of these solid amine sorbents in the large-scale CO2capture from flue gas.Currently,a great deal of attention has been paid to development of the inexpensive solid amine adsorbents/sorb-ents,such as amine-treated Zeolite13X,31glassfiber,32,33 and activated carbon.34,35It is highly desired to develop a solid amine sorbent with high performance and low cost for the large-scale CO2capture from the power stationflue gas. Silica gel is a porous material,which has been produced on a large scale with a price less than$40/kg,and used widely in industry.Recently,Zhu et al.reported their devel-opment of the solid amine sorbent by loading polyethyleni-mine onto silica gels,but the CO2sorption capacity was only46.9mg-CO2/g-sorbent at a CO2partial pressure of 4.88atm.36The objective of this study is to explore the use of inexpensive and commercially available silica gel as a support to prepare the silica-gel-supported polyethylenimine sorbent(PEI/SG)with high-sorption performance and low cost for CO2capture fromflue gas.The effects of the silica gel pore volume,silica gel particle size,polyethylenimine molecular weight and loading amount on the CO2sorption capacity were examined to determine the best PEI/SG for-mulation.The PEI/SG was evaluated in a thermogravimetric analyzer(TGA)and afixed-bedflow sorption system in comparison with the SBA-15-supported polyethylenimine sorbent(PEI/SBA-15)reported in our previous study.21 ExperimentalMaterialsVarious silica gel samples with pore volume of0.68,0.75 and 1.15cm3/g,and particle size of33–75,75–150,and 250–425l m,respectively,were purchased from Aldrich, and used as supports for preparation of the PEI/SG samples. Polyethylenimine(PEI)with an average molecular weight (Mn)of423,25,000,and50,000g/mol,respectively,was purchased from Aldrich.Methanol with a purity of99.8% was also purchased from Aldrich for using as a solvent in preparation of PEI/SG.Preparation of PEI/SG samplesThe PEI/SG samples were prepared by a wet-impregnation method.17,18In a typical preparation,about1.0g of PEI was dissolved in20cm3of methanol under stirring for 60min to make a PEI/methanol solution,and then desired amount of the silica gel after vacuum drying at108 C was added to the PEI/methanol solution.The slurry was continuously stirred at room temperature for about5h,allowing the meth-anol in the slurry to be evaporated.After methanol was mainly evaporated,the sample was further dried in a vacuum oven at40 C,1kPa for12h,and then was sealed in a bottle before use.The PEI/SG samples with different PEI loading amount were denoted as PEI(x)/SG,where x is the weight percentage of PEI-loading amount in the sorbent. Evaluation of CO2sorption/desorption performanceThe CO2sorption/desorption performance of the prepared sorbents was evaluated by using TGA(TA Instruments).A typical analysis is described as below:About15mg of the sorbent was placed at the TGA sample pan.The sample wasfirst heated from room temperature to100 C at a rate of10 C/min under a pure N2flow with aflow rate of 100cm3/min,and was kept at100 C for60min to remove the remained solvent,the sorbed moisture and CO2from the sample.Then,the sample was cooled down to the desired sorption temperature and kept at this temperature for20min. The sorption was started by switching theflow gas from pure N2to pure CO2at the sameflow rate and hold at the sorption temperature for40min for CO2sorption.After the sorption, theflow gas was switched back to the pure N2and the temper-ature was increased to100 C for50min for desorption.The sorption capacity of the sorbent was calculated according to the mass change during sorption.The CO2sorption performance of some samples was also evaluated in afixed-bedflow sorption system.A schematic diagram of the system is shown in Figure1.The sorbent was placed in a stainless column with150mm length and 7mm inner dia.The column was set in a furnace with a temperature controller.The gas was controlled by a mass flow controller andflowed up through the column.The con-centration of CO2was monitored by an on-line SRI861 C gas chromatography(GC).A typical evaluation procedure is described below:About5.8cm3sorbent sample was packed into the column.The sorbent in the column was heated to 100 C and hold at this temperature overnight under an ultra-high pure heliumflow at aflow rate of50cm3/min to remove the sorbed moisture and/or CO2in the sorbent. Then,the column was cooled to a desired sorption tempera-ture,such as75 C,under the same heliumflow.The sorp-tion was started by switching the helium gas to a simulated flue gas with15v%CO2,4.5v%O2in N2at aflow rate of 20cm3/min.After saturation of the sorbent,the simulated flue gas was switched to the ultrahigh pure helium gas at a flow rate of100cm3/min,and the sorbent bed temperature was increased to100 C and kept at this temperature for 240min for desorption.The CO2concentration in the efflu-ent was measured by using the on-line GC with an interval of2.5min.The sorption capacity was calculated on the basis of the breakthrough curve,as described in our previous study.29Design of orthogonal tests for optimization of PEI/SG formulationMany factors,including pore volume and particle size of silica gel,molecular weight of PEI,and amount of PEI loaded,can affect the sorption capacity of PEI/SG.In order to optimize the PEI/SG formulation,a design of orthogonal tests was used to improve the experimental efficiency.2496DOI10.1002/aic Published on behalf of the AIChE August2012Vol.58,No.8AIChE JournalThe factors and levels for the orthogonal tests are listed in Table 1.There were four factors with three levels for each factor:pore volume of silica gel (0.68,0.75,and 1.15cm 3/g),particle size of silica gel (33–75,75–150,250–425l m),PEI loading percentage (40,50,and 60wt %),and molecular weight of PEI (423,25,000,and 50,000g/mol).Characterization of Silica Gel and PEI/SGThe Brunauer-Emmett-Teller surface area (S BET ),pore volume and pore size of the samples were measured by the N 2adsorption-desorption at 77K using the Micromeritics ASAP 2020surface area and porosity analyzer.The pore volume of the materials was calculated on the basis of the adsorption amount of nitrogen at the relative pressure of P/P 0¼0.995.The pore size was estimated according to the BJH pore size distribution curve.Thermal stability of PEI,silica gel and PEI(x)/SG samples was evaluated by using a TA Q600-SDT TGA-DSC thermal gravimeter.About 15mg of the sample was heated at a ramp rate of 10 C/min from room temperature to 600 C under an N 2flow at 100cm 3/min.The scanning electron microscopy (SEM)images were obtained on a FEI Quanta 200environmental scanning electron microscope operating at 20kV.Results and DiscussionOptimization of PEI/SG formulationMany factors may affect the sorption capacity of PEI/SG,including the pore volume and particle size of silica gel,molecular weight and loading amount of PEI.In order to optimize the PEI/SG formulation,the orthogonal test design was used for improving the efficiency of experiments.Theorthogonal test design with a L 9(34)array is shown in Table 2,which reduces the total test number from 34(i.e.,81)to 9.The CO 2sorption capacity of the prepared sorbents was measured using TGA at 75 C.The results of the test and the extreme difference analysis are also listed in Table 2,in which K ij is the average CO 2sorption capacity under the level i for investigating variable j ,and R j is the differ-ence between the max{K j }and min{K j }.Comparison of K ij values for each factor indicates that in the variant ranges examined in this study increase of the pore volume,decrease of the particle size and reduction of the molecular weight benefit the increase of the CO 2capacity significantly,while the change in the PEI loading in a range from 40to 60wt %has only a little effect on the CO 2capacity.According to the K ij values,the best combination of the parameters for prepa-ration of PEI/SG are 1.15cm 3/g pore volume;33–75l m particle size;423g/mol PEI molecular weight and 50wt %PEI loading.A PEI/SG sample was prepared on the basis of the suggested parameters,and the measured CO 2sorption capacity of this sample (PEI(50)/SG)was 131.1mg-CO 2/g-sorbent,which is close to the capacity of PEI(50)/SBA-15reported in our previous study.21Table 1.Factors and Levels for Orthogonal TestFactorLevel 123Pore volume,cm 3/g 0.680.75 1.15Particle size,l m 33–7575–150250–425PEI loading,wt %405060Molecular weight (Mn),g/mol 4232500050000Figure 1.Schematic diagram of the fixed-bed flow system for evaluating CO 2sorption-desorption performance.[Color figure can be viewed in the online issue,which is available at.]Table 2.Orthogonal Design of Experiments and Results*CO 2sorption capacity of sorbents (unit is mg-CO 2/g-sorbent).AIChE Journal August 2012Vol.58,No.8Published on behalf of the AIChE DOI 10.1002/aic2497Effect of PEI loadingIn order to further examine the effect of PEI loading,a se-ries of PEI/SG samples were prepared by using a silica gel with 1.15cm 3/g pore volume and 33–75l m particle size,and PEI with an average molecular weight of 423g/mol at different PEI loading of 10,30,50,and 60wt %,respec-tively.The surface area,pore volume and pore-size distribu-tion of the prepared PEI/SG samples were measured by the nitrogen adsorption/desorption.Figure 2shows the pore vol-ume and surface area of the PEI/SG samples as a function of PEI loading amount.The pore volume and the surface area of the silica gel support were 1.03cm 3/g and 260m 2/g,respectively.With increase of the PEI loading,both the pore volume and the surface area of PEI/SG decreased continu-ously.The pore volume and surface area of PEI(50)/SG were 0.17cm 3/g and 35m 2/g,respectively.When the PEI loading amount increased to 60wt %,almost no pore volume and surface area were measurable.Since the pore volume of the silica gel is 1.03cm 3/g and the density of the PEI liquid is about 1.0g/cm 3,the theoretical maximum PEI loading amount in the pores of the silica gel is about 50.7wt %,if assuming that all loaded PEI is put into the pores of the silica gel,and the PEI density does not change after loading the PEI into pores of the silica gel.When PEI loading is over 50wt %,for example PEI(60)/SG,the pores in the silica gel are completely filled by PEI,resulting in almost no measurable pore volume and no measurable sur-face area,as shown in Figure 2.The excessive PEI may be coated on the external surface of the silica gel particles,leading to the agglomeration of the silica gel particles.Figure 3showed the pore-size distribution of PEI/SG sam-ples with different PEI loading,which clearly indicates that the loaded PEI was filled into the pores and resulted in the significant reduction of the pore volume and surface area.Interestingly,it was found that the increase of the PEI load-ing amount reduced the pore volume,but not significantly changed the pore size.The similar phenomenon was also found in our previous study of PEI/SBA-15.21SEM images of silica gel,PEI(10)/SG,PEI(50)/SG,and PEI(60)/SG are shown in Figure 4.The images clearly show that almost no PEI or very little of PEI was observed on the external surface of the particles in PEI(10)/SG,PEI(50)/SG,while some PEI coated on the external surface of PEI(60)/SG particles was observed,leading to the partial agglomera-tion of the particles.The SEM results further confirm that the most of PEI was filled into the silica gel pores in PEI(50)/SG sample.The effect of PEI loading amount on the CO 2sorption capacity at 75 C was further examined by TGA and the results are shown in Figure 5.The silica gel alone gave a CO 2adsorption capacity of 5.1mg-CO 2/g-sor-bent,indicating that there is also a weaker interaction between CO 2and the silica gel at 75 C.When PEI loading amount increased from zero to 50wt %,the CO 2sorption capacity increased to the maximum of 131.1mg-CO 2/g-sor-bent.After this point,the CO 2sorption capacity decreased with increase of the PEI loading amount.This trend is simi-lar to the result observed in our previous study of the PEI/SBA-15samples.22It is clearly shown that loading of PEI on the silica gel increases the CO 2sorption capacity signifi-cantly.This is because PEI contains a great number of the potential CO 2-philic sites and the silica gel makes these sites accessible through improving the dispersion of PEI.When the PEI loading is higher than 50wt %,the excessive PEI is coated on the external surface of the silica gel particles,resulting in the agglomeration of the particles,as shown in Figure 4d,and,thus,the number of the accessible sorption sites is reduced.The efficiency of the amine groups in the loaded PEI,which is defined as a molar ratio of the sorbed CO 2to the amine groups in the sorbent,for each PEI/SG sample was calculated,and the results are also shown in Figure 5.This figure shows the maximum value of 0.28mol-CO 2/mol-amine at 40wt %PEI loading (PEI(40)/SG).Below 40wt %PEI loading,the amine efficiency increases with increas-ing PEI loading.This is probably because the interaction of the amine groups in PEI with silanol groups on the silica gel surface,resulting in the consumption of a part of the amine groups.When the PEI loading is higher than 40wt %,increase of the PEI loading results in reduction of the percentage of the accessible amine sites due to increase of the diffusion resistance of CO 2into the bulk of PEI in the pores,especially in the case when the agglomeration is formed,as shown in Figure 4d.Effect of temperature on the CO 2sorption capacityEffect of the sorption temperature on the sorption capacity of PEI(50)/SG was examined using TGA with pure CO 2gasFigure 2.Effect of different PEI loading on pore volumeand BET surface area of PEI/SG samples.[Color figure can be viewed in the online issue,which is available at .]Figure 3.The pore-size distributionsof silica gelbefore and after PEI loading.[Color figure can be viewed in the online issue,which is available at .]2498DOI 10.1002/aicPublished on behalf of the AIChE August 2012Vol.58,No.8AIChEJournalat25,50,65,75,85and100 C,respectively.The results are shown in Figure6.At25 C,the CO2sorption capacity of PEI(50)/SG was85.6mg-CO2/g-sorbent.With increasing temperature,the CO2sorption capacity became higher and reached the highest capacity of131.1mg-CO2/g-sorbent at 75 C.Continuously increasing temperature from75to 100 C,the sorption capacity decreased to124.5mg-CO2/g-sorbent.The best capacity was obtained at75 C,similar to the results observed for PEI(50)/MCM-4122and PEI(50)/ SBA-15.21The results indicate that at the temperature range from25to75 C,the diffusion of CO2in the PEI may be a sorption rate-control step.Consequently,elevating tempera-ture results in increase of the number of accessible amine sites in the sorbent,and,thus,increases the CO2sorption capacity.When the temperature is higher than75 C,the thermodynamics may play a more important role,thus, resulting in decrease of the sorption capacity with increasing temperature.Effect of moisture on CO2sorption capacityEffect of moisture on the CO2sorption capacity of PEI(50)/SG was examined in thefixed-bedflow system using a simulatedflue gas with15v%CO2,4.5v%O2in N2.About 3.0v%of H2O was added to the simulated flue gas by bubbling the gas through water,as shown in Figure1.The sorption breakthrough curves of PEI(50)/SG in the absence/presence of moisture are shown in Figure7.The breakthrough capacity of PEI(50)/SG was138mg-CO2/g-sorbent in the absence of moisture,while in the presence of moisture in theflue gas the breakthrough capacity of PEI(50)/SG increased to185mg-CO2/g-sorbent,which is corresponding to an amine efficiency of0.36mol-CO2/mol-amine.This result confirms that the presence of moisture improves the sorption capacity of the supportedPEI Figure5.Effect of different PEI loading on the CO2sorption capacity and amine efficiency.The data were from TGA at75 C with pure CO2gas.[Colorfigure can be viewed in the online issue,which isavailable at.]Figure4.SEM images of(a)silica gel,(b)PEI(10)/SG,(c)PEI(50)/SG,and(d)PEI(60)/SG.AIChE Journal August2012Vol.58,No.8Published on behalf of the AIChE DOI10.1002/aic2499sorbents,which was first found in our previous study for CO 2sorption on PEI(50)/MCM-41.23Regenerability and stability of PEI(50)/SGIn addition of the high CO 2capacity and selectivity,regeneration and stability of sorbent in cycles are crucial for practical application of the sorbent,as they are directly related to the cost of the sorption/desorption process.In this study,desorption was performed by switching the flue gas to pure N 2gas.The 10sorption-desorption cycles of PEI(50)/SG were conducted by using TGA.The sorption was at 75 C and the desorption was at 75and 100 C,respectively,for comparison.The measured sorption capacities as a func-tion of the cycle number are shown in Figure 8.For desorp-tion at 100 C,a slight decrease of the sorption capacity with increasing cycle number was observed.The sorption capacity decreased from 131mg-CO 2/g-sorb for the fresh sorbent to 116mg-CO 2/g-sorb for the regenerated sorbent after 10cycles.The sorption capacity was reduced by about 11.6%after 10cycles.For desorption at 75 C,a lessdecrease of the sorption capacity with increasing cycle num-ber was observed.The sorption capacity of the regenerated sorbent after 10cycles was 125mg-CO 2/g-sorb,indicating that 95.4%of the sorption capacity was recovered after 10cycles.The results suggest that desorption at 75 C is better than that at 100 C for keeping the sorption capacity in the cycles.The detailed analysis of the sorption capacity as a function of the cycle number further shows that the degrada-tion of the sorbent became insignificant with increasing cycle number.For example in the case of desorption at 75 C,almost no change in the capacity was observed at the last 5cycles.The loss of the CO 2sorption capacity in the first couple of cycles may be due to the evaporation of ethyleni-mine oligomers with lower molecular weight.Further inves-tigation on it is necessary to clarify the degradation reason.The thermal stability of the silica gel,PEI and PEI/SG with different PEI loading were also examined by using TGA.Figure 9shows the weight loss of the samples as a function of temperature in N 2.The silica gel was stableatFigure 6.Effect of sorption temperatures on CO 2sorption capacity.The data were from TGA with pure CO 2gas.Figure 7.Breakthrough curves of PEI(50)/SBA-15andPEI(50)/SG for CO 2capture from the simu-lated flue gas (15v%CO 2,4.5v%O 2in N 2)in presence/absence of moisture at 75 C and 207h 21GHSV in the fixed-bed flow sorption system.[Color figure can be viewed in the online issue,which is available at.]Figure 8.Recovery percentage of the CO 2capacity as afunction of sorption-desorption cycle number.Data were from TGA at 75 C with pure CO 2gas for sorp-tion and desorption at 75and 100 C,respectively,with pure N 2gas for desorption.[Color figure can be viewed in the online issue,which is available at wileyonlinelibrary.com.]Figure 9.Stability of PEI and PEI/SG with different PEIloading.Data were from TGA with pure N 2gas at a flow rate of 100cm 3/min and a ramp rate of 10 C/min.[Color figure can be viewed in the online issue,which is available at .]2500DOI 10.1002/aic Published on behalf of the AIChE August 2012Vol.58,No.8AIChE Journalthe temperature less than 600 C with only mass loss of 3.9wt %.The PEI lost about 1.0wt %at 100 C,which is probably due to the moisture and other low-boiling com-pounds in PEI.PEI began to lose the weight significantly at above 120 C,and a sharp weight loss was observed at 300 C for decomposition of PEI.When the temperature increased to above 375 C,the weight of PEI sample became zero,indicating that PEI decomposed and/or evaporated completely.PEI/SG samples began to lose the weight around 120 C and ended the loss of the weight around 375 C with the sharp weight loss at 300 C,similar to the pure PEI.It indicates that there is almost no change in the thermal stabil-ity of PEI after loading PEI on the silica gel,and PEI(50)/SG should be stable at a temperature less than 120 C.Comparison of PEI(50)/SG with PEI(50)/SBA-15PEI(50)/SBA-15,which was prepared by loading 50wt %PEI on a mesoporous molecular sieve of SBA-15,has been developed in our previous study.21,29In order to compare the sorption capacity of PEI(50)/SG with PEI(50)/SBA-15,both were evaluated at the same conditions by using both the TGA and the fixed-bed flow sorption system.The break-through curves of PEI(50)/SBA-15at 75 C with the simulated flue gas (15v%CO 2,4.5v%O 2in N 2)in the presence/absence of moisture are also shown in Figure 7.The sorption capacities measured for the two sorbents in dif-ferent tested systems are shown in Figure 10for comparison.On the basis of the TGA results,the mass-based CO 2sorp-tion capacity of PEI(50)/SG is 131mg-CO 2/g-sorbent,which is less than,but very close to that (136mg-CO 2/g-sorbent)of PEI(50)/SBA-15.The mass-based CO 2sorption capacity of PEI(50)/SG measured in the fixed-bed flow system is 138mg-CO 2/g-sorbent,which is almost the same as that 140mg-CO 2/g-sorbent of PEI(50)/SBA-15.It indicates that the mass-based sorption capacity of PEI(50)/SG is compara-ble with that of PEI(50)/SBA-15.In addition,the volume-based sorption capacity of PEI(50)/SG and PEI(50)/SBA-15is 83and 32mg-CO 2/cm 3-sorbent,respectively,on the basis of the tests in the fixed-bed flow system.The higher vol-ume-based capacity of PEI(50)/SG is due to its higher pack-ing density (0.60g/cm 3)than that (0.23g/cm 3)of PEI(50)/SBA-15by 173%.This difference in the packing density results in the greater volume-based sorption capacity of PEI(50)/SG than that of PEI(50)/SBA-15by a factor of 2.6.In comparison with the result from Zhu et al.,36the CO 2capacity (86mg-CO 2/g-sorbent)of PEI(50)/SG developed in this study is higher than that (46mg-CO 2/g-sorbent)of the best PEI-modified silica gel sorbent prepared by Zhu et al.by more than 85%at the same sorption temperature (25 C).The significantly higher capacity of PEI(50)/SG can be attributed to the optimization of PEI/SG formulation in this study.Summary and ConclusionsA new type of MBS with high capacity and low cost has been developed by loading PEI on a commercial silica gel.The effects of the silica gel pore volume,silica gel particle size,PEI molecular weight and PEI loading amount on the CO 2sorption capacity were examined for optimizing the PEI/SG formulation.It was found that in the parameter range examined in this study the large pore volume and small par-ticle size of silica gel,low-molecular-weight of PEI and 50wt %PEI loading are better for preparation of high per-formance PEI/SG sorbent.The best PEI/SG sorbent,PEI(50)/SG,was obtained by loading 50wt %PEI with an average molecular weight of 423g/mol on a silica gel with a pore volume of 1.15cm 3/g and a particle size of 33–75l m,which gave a mass-based CO 2sorption capacity of 138mg-CO 2/g-sorbent,almost the same as that of PEI(50)/SBA-15.In addition,PEI(50)/SG gave a high volume-based sorption capacity of 83mg/cm 3,which is higher than that of PEI(50)/SBA-15by a factor of 2.6due to the former has a higher packing density than the latter.The result indicates that the silica-gel-based MBS is promising for large-scale CO 2capture from flue gas due to its high sorption capacity,especially the high volume-based capacity,low-preparation cost and commercial availability of the silica gel.However,there are still challenges for the practical application of this sorbent for CO 2capture from flue gas,including the stabil-ity,regeneration method and the mass and heat transfers in the scale up of the process.AcknowledgmentsThis work was supported by U.S.Department of Energy,National Energy Technology Laboratory (NETL)through DOE Grants:DE-FE0000458.The China Scholarship Council is gratefully acknowledged for a scholarship to support Zhonghua Zhang at the Pennsylvania State University.Literature Cited1.Cox PM,Betts RA,Jones CD,Spall SA,Totterdell IJ.Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model.Nature .2000;408:184–187.2.Sheppard MC,Socolow RH.Carbon-Constrained World by Rapid Commercialization of Carbon Capture and Sequestration.AIChE J .2007;53:3022–3028.3.Song CS.Global challenges and strategies for control,conversion and utilization of CO 2for sustainable development involving energy,catalysis,adsorption and chemical processing.Catalysis Today .2006;115:2–32.4.Haszeldine RS.Carbon capture and storage:How green can black be?Science .2009;325:1647–1652.5.Rao AB,Rubin ES.A Technical,economic,and environmental assessment of amine-based co 2capture technology for power plant greenhouse gas control.Environ Sci Technol .2002;36:4467–4475.6.Rochelle GT.Amine Scrubbing for CO 2Capture.Science .2009;325:1652–1654.Figure 10.The CO 2mass-based and volume-basedsorption capacities of PEI(50)/SBA-15and PEI(50)/SG measured in TGA with pure CO 2gas and the fixed-bed flow system with the simulated gas (15v%CO 2,4.5v%O 2in N 2)both at 75 C.[Color figure can be viewed in the online issue,which is available at .]AIChE JournalAugust 2012Vol.58,No.8Published on behalf of the AIChE DOI 10.1002/aic 2501。