细菌内毒素USP(清晰整齐)
- 格式:doc
- 大小:191.00 KB
- 文档页数:12

美国药典24版细菌内毒素检查法本文是USP24细菌内毒素检查法(BACTERLAL ENDOTOXINS TEST)的译文,但目前执行的标准是USP24版第二增补本(USP—NF19 Second Supplement)的细菌内毒素检查法,读者可对照学习,从中可看出USP24细菌内毒素检查的不足之处。
本文将介绍用鲎试剂检测样品内或样品上存在的细菌内毒素浓度的方法。
鲎试剂(Limulus Amebocyte,LAL)来自鲎的循环细胞,经水提取而行,制备和检验后,用于凝固法。
反应的终点是将供检样品稀释后,与平行稀释的参考内毒素标准进行直接比较得到的。
测得的内毒素含量用规定的内毒素单位表示。
鲎试剂也是用浊度法或显色法来读取数据的,这些试齐只要能满足这些选择方法的要求,就可以使用。
在做试验时,需要先做出一条标准曲线,并用该曲线计算供检样品的内毒素含量,包括样品和对照液加鲎试剂后的培育时间和在分光光度计上读取光密度时使用的波长等操作,都应该事先规定好。
如果是终点浊度法,则到达培育期后,应立刻读取读数。
而动力学检测(凶手浊度法和显色法),光密度的测定是贯穿于整个反应期间,而用于计算结果的百分数就是从这些读数中计算出来的。
在用终点法的显色法检测时,在到达事先规定的反应时间后,添加停止酶反应的试剂使反应停止后,再读取数据。
1、参考内毒素标准与对照内毒素标准参考内毒素标准(Reference standard endotoxin,RSE)是美国药典(USP)的内毒素参考标准,其效价为每瓶10 000个USP内毒素单位(EU)。
每瓶参考内毒素标准加5ml鲎试剂用水(LAL Reagent Watet),用旋转搅拌器间歇搅拌30min使其溶解,制成原液,用这一原液制作合适的系列稀释。
原液应保存于冰箱中,用于以后的稀释,但保存时间不得超过14d。
用前,用旋转搅拌器强力搅拌至少3min,每一步稀释,至少搅拌30s,然后才能用它稀释下一步。

细菌内毒素标准品
细菌内毒素是一种由细菌产生的有毒物质,它可以引起机体的
免疫反应,甚至导致严重的疾病。
因此,研究和检测细菌内毒素的
标准品具有重要意义。
在医药领域,细菌内毒素标准品被广泛应用
于药物研发、临床诊断和疾病治疗等方面。
首先,细菌内毒素标准品的研究对于药物研发具有重要意义。
许多药物的研发需要进行严格的毒性测试,以确保其安全性和有效性。
细菌内毒素标准品可以作为药物毒性测试的标准物质,帮助科
研人员评估药物的毒性水平,为药物研发提供重要参考。
其次,细菌内毒素标准品在临床诊断中也起着至关重要的作用。
许多疾病的诊断需要通过检测患者体内的细菌内毒素水平来进行判断。
因此,标准的细菌内毒素标准品可以帮助临床医生准确地诊断
疾病,为患者提供及时有效的治疗方案。
此外,细菌内毒素标准品还可以用于疾病治疗的研究中。
一些
疾病的治疗需要针对细菌内毒素进行干预,以阻断其对机体的损害。
因此,研究和生产高质量的细菌内毒素标准品对于开发新的疾病治
疗手段具有重要意义。
综上所述,细菌内毒素标准品在药物研发、临床诊断和疾病治疗中都扮演着重要的角色。
随着医学科研水平的不断提高,对细菌内毒素标准品的需求也将不断增加。
因此,我们有必要加强对细菌内毒素标准品的研究和生产,以满足医药领域对高质量标准品的需求,为保障人民健康作出更大的贡献。


细菌内毒素检测标准细菌内毒素是一种由细菌产生的有毒物质,它可以对人体和动物的健康造成严重危害。
因此,对细菌内毒素的检测是非常重要的。
目前,国际上已经建立了一系列的细菌内毒素检测标准,以确保检测结果的准确性和可靠性。
首先,细菌内毒素检测标准包括了样品的采集和处理方法。
在采集样品时,需要注意避免污染和样品损坏,同时还要确保样品的代表性。
对于不同类型的样品,比如食品、药品、环境样品等,都有相应的采集和处理方法,以保证检测结果的准确性。
其次,细菌内毒素检测标准还包括了检测方法和技术的要求。
目前常用的检测方法包括生物学检测方法、化学检测方法和免疫学检测方法等。
这些方法各有优缺点,可以根据实际情况选择合适的方法进行检测。
同时,检测设备和仪器的精密度和准确性也是检测标准中非常重要的一部分。
另外,细菌内毒素检测标准还规定了检测结果的解读和评定标准。
对于检测结果的阳性和阴性判定,以及毒素含量的测定,都有相应的标准和规定。
这些标准旨在保证检测结果的准确性和可比性,从而为食品安全、医药卫生等领域提供可靠的数据支持。
最后,细菌内毒素检测标准还包括了实验室质量控制和质量保证体系的要求。
实验室需要建立完善的质量管理体系,包括设备校准、人员培训、实验操作规范等,以确保检测结果的准确性和可靠性。
总的来说,细菌内毒素检测标准是一套系统完整的标准体系,它涵盖了样品采集、检测方法、结果解读和质量保证等方方面面。
这些标准的建立和执行,对于保障食品安全、医药卫生等领域的公共健康具有重要意义。
希望各相关单位和人员能够严格执行这些标准,确保细菌内毒素检测工作的准确性和可靠性。

BACTERIAL ENDOTOXINS TEST <85> USP35Portions of this general chapter have been harmonized with the corresponding texts ofthe European Pharmacopoeia and/or the Japanese Pharmacopoeia、Those portions that are not harmonized are marked with symbols () to specify this fact、这一章节已经与欧洲药典(EP)与日本药典(JP)统一。
没有统一的部分已经用标记出来。
The Bacterial Endotoxins Test (BET) is a test to detect or quantify endotoxins from Gram-negative bacteria using amoebocyte lysate from the horseshoe crab (Limulus polyphemus or Tachypleus tridentatus)、细菌内毒素检查法(BET)就是通过鲎的阿米巴细胞溶解产物来检测或定量来自革兰氏阴性菌的内毒素。
There are three techniques for this test: the gel-clot technique, which is based on gel formation; the turbidimetric technique, based on the development of turbidity after cleavage of an endogenous substrate; and the chromogenic technique, based on the development of color after cleavage of a synthetic peptide-chromogen complex、Proceed by any of the three techniques for the test、In the event of doubt or dispute, the final decision is made based upon the gel-clot technique unless otherwise indicated in the monograph for the product being tested、The test is carried out in a manner that avoids endotoxin contamination、细菌内毒素检查法有3种:凝胶法,基于凝胶的形成;浊度法,BACTERIAL ENDOTOXINS TEST USP32Portions of this general chapter have been harmonized with the corresponding texts of the European Pharmacopeia and/or the Japanese Pharmacopeia、Those portions that are notharmonized are marked with symbols () to specify this fact、This chapter provides a test to detect or quantify bacterial endotoxins that may be present in or on the sample of the article(s) to which the test is applied、It uses Limulus Amebocyte Lysate (LAL) obtained from the aqueous extracts of circulating amebocytes of horseshoe crab (Limulus polyphemus or Tachypleus tridentatus) which has been prepared and characterized for use as anLAL Reagent、1There are two types of techniques for this test: the gel-clot techniques, which are based on gel formation, and the photometric techniques、The latter include a turbidimetric method, which is based on the development of turbidity after cleavage of an endogenous substrate, and a chromogenic method, which is based on the development of color after cleavage of a synthetic peptide-chromogen complex、Proceed by any one of these techniques, unless otherwise indicated in the monograph、In case of dispute, the final decision is based on the gel-clot techniques, unless otherwise indicated in the monograph、In the gel-clot techniques, the reaction endpoint is determined from dilutions of the material under test in direct comparison with parallel dilutions of a reference endotoxin, and quantities of endotoxin are expressed in USP Endotoxin Units (USP-EU)、[NOTE—One USP-EU is equal to one IU of endotoxin、]Because LAL Reagents have been formulated to be used also for turbidimetric or colorimetric tests, such tests may be used to comply with the requirements、These tests require the establishment of a standard regression curve; the endotoxin content of the test material is determined by interpolation from the curve、The procedures include incubation for a preselected time of reacting endotoxin and control solutions with LAL Reagent and reading ofthe spectrophotometric light absorbance at suitable wavelengths、In the endpoint turbidimetric procedure the reading is made immediately at the end of the incubation period、In the endpoint colorimetric procedure the reaction is arrested at the end of the preselected time by the addition of an enzyme reaction-terminating agent prior to the readings、In the turbidimetric and colorimetric kinetic assays the absorbance is measured throughout the reaction period and rate values are determined from those readings、APPARATUS AND GLASSWAREDepyrogenate all glassware and other heat-stable materials in a hot-air oven using a validatedprocess、2Commonly used minimum time and temperature settings are 30 minutes at 250、If employing plastic apparatus, such as microplates and pipet tips for automatic pipetters, use only that which has been shown to be free of detectable endotoxin and not to interfere with thetest、[NOTE—In this chapter, the term “tube” includes any other receptacle such as a micro-titer well、]PREPARATION OF THE STANDARD ENDOTOXIN STOCK SOLUTION AND STANDARD SOLUTIONS The USP Endotoxin RS has a defined potency of 10,000 USP Endotoxin Units (EU) per vial、Constitute the entire contents of 1 vial of the RSE with 5 mL of LAL Reagent Water3, mixintermittently for 30 minutes, using a vortex mixer, and use this concentrate for making appropriate serial dilutions、Preserve the concentrate in a refrigerator for making subsequent dilutions for not more than 14 days、Mix vigorously, using a vortex mixer, for not less than 3 minutes before use、Mix each dilution for not less than 30 seconds before proceeding to make the next dilution、Do not store dilutions, because of loss of activity by adsorption, in the absence of supporting data to the contrary、Preparatory TestingUse an LAL Reagent of confirmed label sensitivity、The validity of test results for bacterial endotoxins requires an adequate demonstration that specimens of the article or of solutions, washings, or extracts thereof to which the test is to be applied do not of themselves inhibit or enhance the reaction or otherwise interfere with the test、Validation is accomplished by performing the inhibition or enhancement test described under each of the three techniques indicated、Appropriate negative controls are included、Validation must be repeated if the LAL Reagent source or the method of manufacture or formulation of the article is changed、Preparation of Sample SolutionsPrepare sample solutions by dissolving or diluting drugs or extracting medical devices using LAL Reagent Water、Some substances or preparations may be more appropriately dissolved, diluted, or extracted in other aqueous solutions、If necessary, adjust the pH of the solution (or dilution thereof) to be examined so that the pH of the mixture of the LAL Reagent and sample falls within the pH range specified by the LAL Reagent manufacturer、This usually applies to a product with a pH in the range of 6、0 to 8、0、The pH may be adjusted using an acid, base, or suitable buffer as recommended by the LAL Reagent manufacturer、Acids and bases may be prepared from concentrates or solids with LAL Reagent Water in containers free of detectable endotoxin、Buffers must be validated to be free of detectable endotoxin and interfering factors、DETERMINATION OF MAXIMUM VALID DILUTION (MVD)The Maximum Valid Dilution is the maximum allowable dilution of a specimen at which the endotoxin limit can be determined、It applies to injections or to solutions for parenteral administration in the form constituted or diluted for administration, or, where applicable, to the amount of drug by weight if the volume of the dosage form for administration could be varied、The general equation to determine MVD is:MVD = (Endotoxin limit × Concentration of sample solution)/()where the concentration of sample solution and are as defined below、Where the endotoxinlimit concentration is specified in the individual monograph in terms of volume (in EU per mL), divide the limit by, which is the labeled sensitivity (in EU per mL) of the LAL Reagent, to obtainthe MVD factor、Where the endotoxin limit concentration is specified in the individual monograph in terms of weight or Units of active drug (in EU per mg or in EU per Unit), multiply the limit by the concentration (in mg per mL or in Units per mL) of the drug in the solution tested or of the drug constituted according to the label instructions, whichever is applicable, and dividethe product of the multiplication by, to obtain the MVD factor、The MVD factor so obtainedis the limit dilution factor for the preparation for the test to be valid、ESTABLISHMENT OF ENDOTOXIN LIMITSThe endotoxin limit for parenteral drugs, defined on the basis of dose, is equal to K/M,4where K is the threshold human pyrogenic dose of endotoxin per kg of body weight, and M isequal to the maximum recommended human dose of product per kg of body weight in a single hour period、The endotoxin limit for parenteral drugs is specified in individual monographs in units such as EU/mL, EU/mg, or EU/Unit of biological activity、GEL-CLOT TECHNIQUESThe gel-clot techniques detect or quantify endotoxins based on clotting of the LAL Reagent in the presence of endotoxin、The concentration of endotoxin required to cause the lysate to clot under standard conditions is the labeled sensitivity of the LAL Reagent、To ensure both the precision and validity of the test, tests for confirming the labeled LAL Reagent sensitivity and for interfering factors are described under Preparatory Testing for the Gel-Clot Techniques、Preparatory Testing for the Gel-Clot TechniquesTest for Confirmation of Labeled LAL Reagent Sensitivity—Confirm the labeled sensitivity using at least 1 vial of the LAL Reagent lot、Prepare a series of two-fold dilutions of the USP EndotoxinRS in LAL Reagent Water to give concentrations of 2,, 0、5, and 0、25, where is asdefined above、Perform the test on the four standard concentrations in quadruplicate and include negative controls、The test for confirmation of lysate sensitivity is to be carried out when a new batch of LAL Reagent is used or when there is any change in the experimental conditions that may affect the outcome of the test、Mix a volume of the LAL Reagent with an equal volume (such as 0、1-mL aliquots) of one of the standard solutions in each test tube、When single test vials or ampuls containing lyophilized LAL Reagent are used, add solutions directly to the vial or ampul、Incubate the reaction mixture fora constant period according to directions of the LAL Reagent manufacturer (usually at 37 ± 1for60 ± 2 minutes), avoiding vibration、To test the integrity of the gel, take each tube in turndirectly from the incubator and invert it through about 180in one smooth motion、If a firm gelhas formed that remains in place upon inversion, record the result as positive、A result is negative if an intact gel is not formed、The test is not valid unless the lowest concentration of the standard solutions shows a negative result in all replicate tests、The endpoint is the last positive test in the series of decreasing concentrations of endotoxin、Calculate the mean value of the logarithms of the endpoint concentration and then the antilogarithm of the mean value using the following equation:Geometric Mean Endpoint Concentration = antilog (S e / f)where S e is the sum of the log endpoint concentrations of the dilution series used, and f is the number of replicate test tubes、The geometric mean endpoint concentration is the measuredsensitivity of the LAL Reagent (in EU/mL)、If this is not less than 0、5and not more than 2, the labeled sensitivity is confirmed and is used in tests performed with this lysate、Interfering Factors Test for the Gel-Clot Techniques—Prepare solutions A, B, C, and D as shown in Table 1, and perform the inhibition/enhancement test on the sample solutions at a dilution less than the MVD, not containing any detectable endotoxins, following the procedure inthe Test for Confirmation of Labeled LAL Reagent Sensitivity above、The geometric mean endpoint concentrations of solutions B and C are determined using the equation in that test、Table 1、Preparation of Solutions for the Inhibition/Enhancement Test for Gel-Clot TechniquesSolutionEndotoxinConcentration/Solution towhich Endotoxin is Added DiluentDilutionFactorInitialEndotoxinConcentrationNumber ofReplicatesA a none/sample solution ——— 4B b2/sample solution samplesolution12421440、54SolutionEndotoxinConcentration/Solution towhich Endotoxin is Added DiluentDilutionFactorInitialEndotoxinConcentrationNumber ofReplicates80、254C c2/water for BET LALReagentWater12221240、5280、252D d none/LAL Reagent Water ——— 2a Solution A: a sample solution of the preparation under test that is free of detectable endotoxins、b Solution B: test for interference、c Solution C: control for labeled LAL Reagent sensitivity、d Solution D: negative control of LAL Reagent Water、This test must be repeated when any condition that is likely to influence the test results changes、The test is not valid unless Solutions A and D show no reaction and the result of Solution C confirms the labeled sensitivity、If the sensitivity of the lysate determined in the presence of the sample solution under test of Solution B is not less than 0、5and not greater than 2, the sample solution does not containfactors which interfere under the experimental conditions used、Otherwise, the sample solution to be examined interferes with the test、If the sample under test does not comply with the test at a dilution less than the MVD, repeat the test using a greater dilution, not exceeding the MVD、The use of a more sensitive lysatepermits a greater dilution of the sample to be examined and this may contribute to the elimination of interference、Interference may be overcome by suitable treatment, such as filtration, neutralization, dialysis, or heating、To establish that the chosen treatment effectively eliminates interference without loss of endotoxins, perform the assay described below using the preparation to be examined to which USP Endotoxin RS has been added and which has been subjected to the selected treatment、Gel-Clot Limit TestThis test is used when a monograph contains a requirement for endotoxin limits、Procedure—Prepare Solutions A, B, C, and D as shown in Table 2, and perform the test on these solutions following the procedure in the Test for Confirmation of Labeled LAL Reagent Sensitivity under Preparatory Testing for the Gel-Clot Techniques、Table 2、Preparation of Solutions for the Gel-Clot Limit TestSolution*Endotoxin Concentration/Solution to which Endotoxin is Added Number of ReplicatesA none/diluted sample solution 2B2/diluted sample solution2C2/LAL Reagent Water2D none/LAL Reagent Water 2*Prepare Solution A and positive product control Solution B using a dilution not greater than the MVD and treatments as directed in the Interfering Factors Test for the Gel-Clot Techniques under Preparatory Testing for the Gel-Clot Techniques、Positive control Solutions B and C contain the standard endotoxin preparation at a concentration corresponding to twice the labeled LAL Reagent sensitivity、The negative control Solution D is LAL Reagent Water、Interpretation—The test is not valid unless both replicates of positive control Solutions B and C are positive and those of negative control Solution D are negative、The preparation under test complies with the test when a negative result is found for both tubes containing Solution A、The preparation under test does not comply with the test when a positive result is found for both tubes containing Solution A、Repeat the test when a positive result is found for 1 tube containing Solution A and a negative result for the other one、The preparation under test complies with the test when a negative result is found for both tubes containing Solution A in the repeat result、If the test is positive for the preparation under test at a dilution less than the MVD, the test may be repeated at a dilution not greater than the MVD、Gel-Clot AssayThis assay quantifies bacterial endotoxins in sample solutions by titration to an endpoint、Procedure—Prepare Solutions A, B, C, and D as shown in Table 3, and test these solutions by following the procedure in the Test for Confirmation of Labeled LAL ReagentSensitivity under Preparatory Testing for the Gel-Clot Techniques、Table 3、Preparation of Solutions for the Gel-Clot AssaySolutionEndotoxinConcentration/Solution towhich Endotoxin is Added DiluentDilutionFactorInitial EndotoxinConcentrationNumber ofReplicatesA a none/sample solution LALReagentWater1 — 22 — 24 — 28 — 2B b2/sample solution — 122C c2/LAL Reagent Water LALReagentWater12221240、52SolutionEndotoxinConcentration/Solution towhich Endotoxin is Added DiluentDilutionFactorInitial EndotoxinConcentrationNumber ofReplicates80、252D d none/LAL Reagent Water ——— 2a Solution A: a sample solution under test at the dilution, not to exceed the MVD, with which the Interfering Factors Test for the Gel-Clot Techniques was completed、Subsequent dilution of the sample solution must not exceed the MVD、Use LAL Reagent Water to make dilution series of four tubes containing the sample solution under test at concentrations of 1, ½, ¼, and1/8 relative to the dilution with which the Interfering Factors Test for the Gel-Clot Techniques was completed、Other dilutions may be used as appropriate、b Solution B: Solution A containing standard endotoxin at a concentration of 2(positive product control)、c Solution C: two series of 4 tubes of LAL Reagent Water containing the standard endotoxin at a concentration of 2,, 0、5, and 0、25, respectively、d Solution D: LAL Reagent Water (negative control)、Calculation and Interpretation—The test is not valid unless the following conditions are met: (1) both replicates of negative control Solution D are negative; (2) both replicates of positive product control Solution B are positive; and (3) the geometric mean endpoint concentration of Solution Cis in the range of 0、5to 2、To determine the endotoxin concentration of Solution A, calculate the endpoint concentration for each replicate series of dilutions by multiplying each endpoint dilution factor by、Theendotoxin concentration in the sample is the geometric mean endpoint concentration of the replicates (see the formula given in the Test for Confirmation of Labeled LAL Reagent Sensitivity under Preparatory Testing for the Gel-Clot Techniques)、If the test is conducted with a diluted sample solution, calculate the concentration of endotoxin in the original sample solution by multiplying by the dilution factor、If none of the dilutions of the sample solution ispositive in a valid assay, report the endotoxin concentration as less than(if the diluted samplewas tested, less than times the lowest dilution factor of the sample、) If all dilutions are positive, the endotoxin concentration is reported as equal to or greater than the greatest dilution factor multiplied by(e、g、, initial dilution factor times 8 times in Table 3)、The article meets the requirements of the test if the concentration of endotoxin is less than that specified in the individual monograph、PHOTOMETRIC TECHNIQUESThe turbidimetric method measures increases in turbidity、Depending on the test principle used, this technique is classified as either endpoint-turbidimetric or kinetic-turbidimetric、The endpoint-turbidimetric technique is based on the quantitative relationship between the concentration of endotoxins and the turbidity (absorbance or transmission) of the reaction mixture at the end of an incubation period、The kinetic-turbidimetric technique is a method to measure either the onset time needed to reach a predetermined absorbance of the reaction mixture or the rate of turbidity development、The chromogenic method measures the chromophore released from a suitable chromogenic peptide by the reaction of endotoxins with the LAL Reagent、Depending on the test principle employed, this technique is classified as either endpoint-chromogenic or kinetic-chromogenic、The endpoint-chromogenic technique is based on the quantitative relationship between the concentration of endotoxins and the release of chromophore at the end of an incubation period、The kinetic-chromogenic technique is a method to measure either the onset time needed to reach a predetermined absorbance of the reaction mixture or the rate of color development、All photometric tests are carried out at the incubation temperature recommended by the LAL Reagent manufacturer, which is usually 37 ± 1、Preparatory Testing for the Photometric TechniquesTo assure the precision or validity of the turbidimetric and chromogenic techniques, preparatory tests are conducted to verify that the criteria for the standard curve are valid and that the sample solution does not inhibit or enhance the reaction、Revalidation for the test method is required when conditions that are likely to influence the test result change、Verification of Criteria for the Standard Curve—Using the Standard Endotoxin Solution, prepare at least three endotoxin concentrations to generate the standard curve、Perform the test using at least three replicates of each standard endotoxin concentration according to the manufacturer's instructions for the LAL Reagent (with regard to volume ratios, incubation time, temperature, pH, etc、)、If the desired range in the kinetic methods is greater than two logs, additional standards should be included to bracket each log increase within the range of the standard curve、The absolute value of the correlation coefficient, |r|, must be greater than or equal to 0、980 for the range of endotoxin concentrations indicated by the manufacturer of the LAL Reagent、Interfering Factors Test for the Photometric Techniques—Select an endotoxin concentration at or near the middle of the endotoxin standard curve、Prepare Solutions A, B, C, and D as shown in Table 4、Perform the test on Solutions A, B, C, and D at least in duplicate following the instructions for the LAL Reagent used (with regard to volume of sample and LAL Reagent, volume ratio of sample to LAL Reagent, incubation time, etc、)、Table 4、Preparation of Solutions for the Inhibition/Enhancement Test for PhotometricTechniquesSolution Endotoxin ConcentrationSolution to whichEndotoxin is AddedNumber ofReplicatesA a none sample solution not less than 2B b middle concentration of the standardcurvesample solution not less than 2C c at least 3 concentrations (lowestconcentration is designated) LAL Reagent Water each not lessthan 2D d none LAL Reagent Water not less than 2a Solution A: the sample solution may be diluted not to exceed MVD、b Solution B: the preparation under test at the same dilution as Solution A, containing added endotoxin at a concentration equal to or near the middle of the standard curve、c Solution C: the standard endotoxin at the concentrations used in the validation of the method described in Verification of Criteria for the Standard Curve under Preparatory Testing for the Photometric Techniques(positive control series)、d Solution D: LAL Reagent Water (negative control)、Calculate the mean recovery of the added endotoxin by subtracting the mean endotoxin concentration in the solution (if any) from that containing the added endotoxin、In order to be considered free of interfering factors under the conditions of the test, the measured concentration of the endotoxin added to the sample solution must be within 50% to 200% of the known added endotoxin concentration after subtraction of any endotoxin detected in the solution without added endotoxin、When the endotoxin recovery is out of the specified ranges, the interfering factors must be removed as described in the Interfering Factors Test for the Gel-ClotTechniques under Preparatory Testing for the Gel-Clot Techniques、Repeating the Interfering Factors Test for the Gel-Clot Techniques validates the treatment、Procedure for the Photometric TechniquesFollow the procedure described in the Interfering Factors Test for the Photometric Techniques under Preparatory Testing for the Photometric Techniques、Calculation for the Photometric TechniquesCalculate the endotoxin concentration of each of the replicates of test Solution A using the standard curve generated by positive control series C、The test is not valid unless the following conditions are met: (1) the results of control series C comply with the requirements for validation defined under Verification of Criteria for the Standard Curve under Preparatory Testing for the Photometric Techniques;(2) the endotoxin recovery, calculated from the concentration found in Solution B after subtracting the endotoxin concentration found in Solution A is within 50 to 200%; and (3) the result of negative control series D does not exceed the limit of the blank value required in the description of the LAL Reagent used、Interpretation of Results from the Photometric TechniquesIn photometric assays, the preparation under test complies with the test if the mean endotoxin concentration of the replicates of Solution A, after correction for dilution and concentration, is less than the endotoxin limit for the product、1LAL Reagent reacts with some-glucans in addition to endotoxins、Some preparations that are treated will not react with-glucans and must be used for samples that contain glucans、2For a validity test of the procedure for inactivating endotoxins, see Dry-HeatSterilization under Sterilization and Sterility Assurance of Compendial Articles1211、Use an LAL Reagent having a sensitivity of not less than 0、15 Endotoxin Unit per mL、3Sterile Water for Injection or other water that shows no reaction with the specific LAL Reagent with which it is to be used, at the limit of sensitivity of such reagent、4K is 5 USP-EU/kg for any route of administration other than intrathecal (for which K is 0、2 USP-EU/kg body weight)、For radiopharmaceutical products not administered intrathecally theendotoxin limit is calculated as 175/V, where V is the maximum recommended dose in mL、For intrathecally administered radiopharmaceuticals, the endotoxin limit is obtained by the formula 14/V、For formulations (usually anticancer products) administered on a per square meter of body surface, the formula is K/M, where K= 5 EU/kg and M is the (maximum dose/m2/hour × 1、80 m2)/70 Kg、。

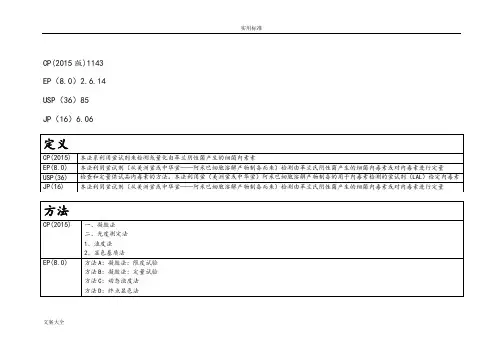
CP(2015 版)1143 EP (8.0) 2.6.14 USP( 36) 85JP( 16) 6.06仅在最低浓度的标准溶液的所有平行管的检查结果均为阴性的情况下,试验方为有效。
反应终点浓度指系列递减的内毒素浓度中最后一个呈阳性结果的浓度。
将终点浓度取对数,计算它们的平均值,再将平均值的结果取反对数,最后的表达式如下:终点浓度的几何平均值=lg-1(工e/f)工e =所用系列溶液的终点浓度的对数值的和f=平行管的数量反应终点的浓度的几何平均值即为鲎试剂灵敏度的测量值(IU/ml )。
当终点浓度的几何平均值在0.5入至2.0入之间时,可判定受试鲎试剂的标示灵敏度为入,可用于内毒素的检查。
(ii )干扰因素试验开展该项实验的目的是检查供试品溶液中反应的增强因素或阻抑因素的存在。
按表2.6.14.-1 制备溶液A B、C D。
供试品的稀释度不得超过MVD且供试品溶液不能检查出内毒素,具体操作见(1)预备试验的(i )鲎试剂标示灵敏度的复核试验项。
表 2614-1人=经检查无内毒素的溶液工e为所用系列溶液的终点浓度的对数值的和;f为平行管的数量。
反应终点的几何平均值即为LAL试剂的标示灵敏度(单位为EU/mL。
当终点浓度的几何平均值在0.5入至2.0入之间,可判定受试 LAL试剂的标示灵敏度为入。
凝胶法的干扰因素试验一一按表1制备溶液A B C D,使用的供试品溶液应为未检验出内毒素且不超过MVD勺溶液,按LAL试剂灵敏度复核试验项下操作。
用检查法给出的公式计算溶液B、C的反应终点的几何平均值。
示灵敏度范围内时,试验方有效。
计算溶液B的鲎试剂灵敏度,如果值在 [0.5入,2.0入]间,可判定供试品溶液在该浓度下无干扰作用;反之则判定供试品溶液对试验能形成干扰。
若供试品溶液在小于 MVD的稀释倍数下对试验有干扰,应将供试品溶液进行不超过MVD勺进一步稀释,再重复干扰试验。
使用灵敏度更高如溶液的平行管的检查结果均为阳性时:- 如供试品的稀释倍数为 MVD供试品不符合规定。

细菌内毒素标准品细菌内毒素(Endotoxin)是一种存在于细菌细胞壁内的毒素,当细菌死亡或破裂时,内毒素会释放出来,引起人体免疫系统的异常反应,甚至导致严重的炎症反应。
因此,细菌内毒素的检测和标准化非常重要,以确保药品和医疗器械的安全性和有效性。
细菌内毒素标准品是用于内毒素检测的标准物质,通常是从大肠杆菌等细菌中提取纯化得到的。
它的主要作用是作为内毒素检测的标准,用于验证检测方法的准确性和灵敏度,确保检测结果的可靠性。
此外,细菌内毒素标准品还被广泛应用于药品、医疗器械、生物制品等领域的质量控制和研发过程中。
在选择细菌内毒素标准品时,需要考虑其纯度、稳定性、活性等因素。
首先,标准品的纯度必须高,不含其他杂质,以确保检测结果的准确性。
其次,标准品的稳定性也非常重要,它需要能够长期保存而不失活或降解,以确保长期的实验可靠性。
最后,标准品的活性必须稳定,能够准确反映实际样品中内毒素的含量,以确保检测结果的可比性和可靠性。
细菌内毒素标准品的制备通常包括以下几个步骤,首先,从细菌中提取内毒素,并进行初步纯化;其次,通过柱层析、凝胶过滤等方法进一步纯化内毒素,去除杂质;最后,对纯化得到的内毒素进行活性测定和稳定性测试,并进行适当的配制和包装。
制备好的标准品需要经过严格的质量控制和验证,确保其符合相关的标准和规定。
在使用细菌内毒素标准品进行内毒素检测时,需要严格按照操作规程进行,避免污染和误差的产生。
同时,还需要配合合适的检测方法和仪器设备,确保检测结果的准确性和可靠性。
此外,定期对标准品进行验证和比对也是非常重要的,以确保其长期稳定性和可靠性。
细菌内毒素标准品在医药、生物制品等领域具有广泛的应用前景,随着医疗技术的不断发展和完善,对内毒素的检测要求也越来越高。
因此,对细菌内毒素标准品的研发和生产提出了更高的要求,希望能够不断提高其质量和性能,为医药和生物制品的质量控制提供更可靠的保障。
总之,细菌内毒素标准品在内毒素检测中起着至关重要的作用,它的质量和性能直接影响着检测结果的准确性和可靠性。

REAGENTS AND TEST SOLUTIONS 试剂和测试溶液Amoebocyte Lysate— A lyophilized product obtained from the lysate of amoebocytes (white blood cells) from the horseshoe crab (Limulus polyphemus or Tachypleus tridentatus). This reagent refers only to a product manufactured in accordance with the regulations of the competent authority. [NOTE—Amoebocyte Lysate reacts to some-glucans in addition to endotoxins. Amoebocyte Lysate preparations that do not react to glucans are available: they are prepared by removing the G factor reacting to glucans from Amoebocyte Lysate or by inhibiting the G factor reacting system of Amoebocyte Lysate and may be used for endotoxin testing in the presence of glucans. ] 变形细胞溶解液—由鲎的〔白血细胞〕变形细胞溶解液制得的冻干产品。
该试剂仅指那些按照相关的监管机构的法规要求制得的产品。
[注:变形细胞溶解液除了与内毒素反响外,还会与某些-葡聚糖反响。
可以制成不与-葡聚糖发生反响的变形细胞溶解液制品:可通过从变形细胞溶解液中去除会与葡聚糖反响的G因子或抑制变形细胞溶解液的G因子反响系统来制得该制品,这些制品可用于在存在葡聚糖时进行内毒素检测。


ment can be a serious source of bias. In general, the rejection of measurements solely on the basis of their relative magnitudesis a procedure that should be used sparingly.Each suspected potency measurement, or outlier, may be tested against the following criterion. This criterion is based on the variation within a single group of supposedly equivalent measurements from a normal distribution. On average, it will reject a valid observation once in 25 trials or once in 50 trials. Designate the measurements in order of magnitude from y1to yN, wherey1is the candidate outlier, and N is the number of measurements in the group. Compute the relative gap by using Table A2-1, Test for Outlier Measurements, and the formulas below:When N = 3 to 7:G1= (y2− y1)/(yN− y1)When N = 8 to 10:G2= (y2− y1)/(yN − 1− y1)When N = 11 to 13:G3= (y3− y1)/(yN −1− y1)If G1, G2, or G3, as appropriate, exceeds the critical value in Table A2-1, Test for Outlier Measurements, for the observed N, there is a statistical basis for omitting the outlier measurement(s).Table A2-1. Test for Outlier MeasurementsIn samples from a normal population, gaps equal to or larger than the following values of G1, G2, and G3 occur with a probability P = 0.01, when outlier measurements can occur only at one end; or with P = 0.02, when they may occur at either end.N34567G10.9870.8890.7810.6980.637N8910G20.6810.6340.597N111213G30.6740.6430.617EXAMPLEEstimated potencies of sample in log scale = 1.561, 1.444, 1.517, 1.535.Check lowest potency for outlier:G1= (1.517 − 1.444)/(1.561 − 1.444) = 0.624<0.889Therefore 1.444 is not an outlier.Check highest potency for outlier:G1= (1.561 − 1.535)/(1.561 − 1.444) = 0.222<0.889Therefore 1.561 is not an outlier.Outlier potencies should be marked as outlier values and excluded from the assay calculations. NMT one potency can be excluded as an outlier.á85ñ BACTERIAL ENDOTOXINS TEST♦Portions of this general chapter have been harmonized with the corresponding texts of the European Pharmacopoeia and/or the Japanese Pharmacopoeia. Those portions that are not harmonized are marked with symbols (♦♦) to specify this fact.♦The Bacterial Endotoxins Test (BET) is a test to detect or quantify endotoxins from Gram-negative bacteria using amoebo-cyte lysate from the horseshoe crab (Limulus polyphemus or Tachypleus tridentatus).There are three techniques for this test: the gel-clot technique, which is based on gel formation; the turbidimetric technique, based on the development of turbidity after cleavage of an endogenous substrate; and the chromogenic technique, based on the development of color after cleavage of a synthetic peptide-chromogen complex. Proceed by any of the three techniques for the test. In the event of doubt or dispute, the final decision is made based upon the gel-clot limit test unless otherwise USP 40Biological Tests / á85ñ Bacterial Endotoxins Test 163indicated in the monograph for the product being tested. The test is carried out in a manner that avoids endotoxin contami-nation.APPARATUSDepyrogenate all glassware and other heat-stable materials in a hot air oven using a validated process.♦1♦ A commonly used minimum time and temperature is 30 min at 250°. If employing plastic apparatus, such as microplates and pipet tips for auto-matic pipetters, use apparatus that is shown to be free of detectable endotoxin and does not interfere in the test. [NOTE —In this chapter, the term “tube” includes any other receptacle such as a microtiter well.]REAGENTS AND TEST SOLUTIONSAmoebocyte LysateA lyophilized product obtained from the lysate of amoebocytes (white blood cells) from the horseshoe crab (Limulus polyphe-mus or Tachypleus tridentatus ). This reagent refers only to a product manufactured in accordance with the regulations of the competent authority. [NOTE —Amoebocyte Lysate reacts to some b -glucans in addition to endotoxins. Amoebocyte Lysate prepa-rations that do not react to glucans are available: they are prepared by removing the G factor reacting to glucans from Amoe-bocyte Lysate or by inhibiting the G factor reacting system of Amoebocyte Lysate and may be used for endotoxin testing in the presence of glucans.]Water for Bacterial Endotoxins Test (BET)Use Water for Injection or water produced by other procedures that shows no reaction with the lysate employed, at the de-tection limit of the reagent.Lysate TSDissolve Amoebocyte Lysate in Water for BET , or in a buffer recommended by the lysate manufacturer, by gentle stirring.Store the reconstituted lysate, refrigerated or frozen, according to the specifications of the manufacturer.PREPARATION OF SOLUTIONSStandard Endotoxin Stock SolutionA Standard Endotoxin Stock Solution is prepared from a USP Endotoxin Reference Standard that has been calibrated to the current WHO International Standard for Endotoxin. Follow the specifications in the package leaflet and on the label for prepa-ration and storage of the Standard Endotoxin Stock Solution . Endotoxin is expressed in Endotoxin Units (EU). [N OTE —One USP Endotoxin Unit (EU) is equal to one International Unit (IU) of endotoxin.]Standard Endotoxin SolutionsAfter mixing the Standard Endotoxin Stock Solution vigorously, prepare appropriate serial dilutions of Standard Endotoxin Solu-tion , using Water for BET . Use dilutions as soon as possible to avoid loss of activity by adsorption.Sample SolutionsPrepare the Sample Solutions by dissolving or diluting drugs using Water for BET . Some substances or preparations may be more appropriately dissolved, or diluted in other aqueous solutions. If necessary, adjust the pH of the solution to be examined (or dilution thereof) so that the pH of the mixture of the lysate and Sample Solution falls within the pH range specified by the lysate manufacturer, usually 6.0–8.0. The pH may be adjusted by use of an acid, base, or suitable buffer as recommended by the lysate manufacturer. Acids and bases may be prepared from concentrates or solids with Water for BET in containers free of detectable endotoxin. Buffers must be validated to be free of detectable endotoxin and interfering factors.♦1 For a validity test of the procedure for inactivating endotoxins, see Dry-Heat Sterilization under Sterilization and Sterility Assurance of Compendial Articles á1211ñ.Use Lysate TS having a sensitivity of not less than 0.15 Endotoxin Unit per mL.♦164 á85ñ Bacterial Endotoxins Test / Biological Tests USP 40DETERMINATION OF MAXIMUM VALID DILUTION (MVD)The maximum valid dilution is the maximum allowable dilution of a specimen at which the endotoxin limit can be deter-mined. Determine the MVD from the following equation:MVD = (endotoxin limit × concentration of Sample Solution)/(l)Endotoxin LimitThe endotoxin limit for parenteral drugs, defined on the basis of dose, equals K/M♦2♦, where K is a threshold pyrogenic dose of endotoxin per kg of body weight, and M is equal to the maximum recommended bolus dose of product per kg of body weight. When the product is to be injected at frequent intervals or infused continuously, M is the maximum total dose admin-istered in a single hour period. The endotoxin limit for parenteral drugs is specified in the individual monograph in units such as EU/mL, EU/mg, EU/Unit of biological activity, etc.Concentration of Sample Solutionmg/mL: in the case of endotoxin limit specified by weight (EU/mg);Units/mL: in the case of endotoxin limit specified by unit of biological activity (EU/Unit);mL/mL: when the endotoxin limit is specified by volume (EU/mL).l: the labeled sensitivity in the Gel-Clot Technique (EU/mL) or the lowest concentration used in the standard curve for the Turbidimetric Technique or Chromogenic Technique.GEL-CLOT TECHNIQUEThe gel-clot technique is used for detecting or quantifying endotoxins based on clotting of the lysate reagent in the pres-ence of endotoxin. The minimum concentration of endotoxin required to cause the lysate to clot under standard conditions is the labeled sensitivity of the lysate reagent. To ensure both the precision and validity of the test, perform the tests for confirm-ing the labeled lysate sensitivity and for interfering factors as described in Preparatory Testing, immediately below.Preparatory TestingTEST FOR CONFIRMATION OF LABELED LYSATE SENSITIVITYConfirm in four replicates the labeled sensitivity, l, expressed in EU/mL of the lysate prior to use in the test. The test for confirmation of lysate sensitivity is to be carried out when a new batch of lysate is used or when there is any change in the test conditions that may affect the outcome of the test. Prepare standard solutions having at least four concentrations equivalent to 2l, l, 0.5l, and 0.25l by diluting the USP Endotoxin RS with Water for BET.Mix a volume of the Lysate TS with an equal volume (such as 0.1-mL aliquots) of one of the Standard Endotoxin Solutions in each test tube. When single test vials or ampuls containing lyophilized lysate are used, add solutions directly to the vial or am-pul. Incubate the reaction mixture for a constant period according to the directions of the lysate manufacturer (usually at37±1° for 60±2 min), avoiding vibration. To test the integrity of the gel, take each tube in turn directly from the incubator, and invert it through about 180° in one smooth motion. If a firm gel has formed that remains in place upon inversion, record the result as positive. A result is negative if an intact gel is not formed. The test is considered valid when the lowest concentra-tion of the standard solutions shows a negative result in all replicate tests.The endpoint is the smallest concentration in the series of decreasing concentrations of standard endotoxin that clots the lysate. Determine the geometric mean endpoint by calculating the mean of the logarithms of the endpoint concentrations of the four replicate series and then taking the antilogarithm of the mean value, as indicated in the following formula:geometric mean endpoint concentration = antilog (S e/f)where S e is the sum of the log endpoint concentrations of the dilution series used, and f is the number of replicate test tubes. The geometric mean endpoint concentration is the measured sensitivity of the lysate (in EU/mL). If this is not less than 0.5l and not more than 2l, the labeled sensitivity is confirmed and is used in tests performed with this lysate.♦2K is 5 USP-EU/kg of body weight for any route of administration other than intrathecal (for which K is 0.2 USP-EU/kg of body weight). For radiopharmaceutical products not administered intrathecally, the endotoxin limit is calculated as 175 EU/V, where V is the maximum recommended dose in mL. For intrathecally ad-ministered radiopharmaceuticals, the endotoxin limit is obtained by the formula 14 EU/V. For formulations (usually anticancer products) administered on a per square meter of body surface, the formula is K/M, where K = 100 EU/m2 and M is the maximum dose/m2.♦USP 40Biological Tests / á85ñ Bacterial Endotoxins Test 165TEST FOR INTERFERING FACTORSUsually prepare solutions (A–D) as shown in Table 1, and perform the inhibition/enhancement test on the Sample Solutions at a dilution less than the MVD, not containing any detectable endotoxins, operating as described for Test for Confirmation of Labeled Lysate Sensitivity . The geometric mean endpoint concentrations of Solutions B and C are determined using the formula described in the Test for Confirmation of Labeled Lysate Sensitivity . The test for interfering factors must be repeated when any condition changes that is likely to influence the result of the test.Table 1. Preparation of Solutions for the Inhibition/Enhancement Test for Gel-Clot TechniquesSolution Endotoxin Concentration/Solution to Which Endotoxin Is Added Diluent Dilution Factor Endotoxin Concentration NumberofReplicates A a None/Sample Solution ———4B b 2l /Sample Solution Sample Solution 12l 4 21l 4 40.5l 4 80.25l 4C c 2l /Water for BET Water for BET 12l 2 21l 240.5l 280.25l 2D d None/Water for BET ———2a Solution A : A Sample Solution of the preparation under test that is free of detectable endotoxins.bSolution B : Test for interference.c Solution C : Control for labeled lysate sensitivity.dSolution D : Negative control of Water for BET.The test is considered valid when all replicates of Solutions A and D show no reaction and the result of Solution C confirmsthe labeled sensitivity.If the sensitivity of the lysate determined in the presence of Solution B is not less than 0.5l and not greater than 2l , theSample Solution does not contain factors that interfere under the experimental conditions used. Otherwise, the Sample Solution to be examined interferes with the test.If the sample under test does not comply with the test at a dilution less than the MVD, repeat the test using a greater dilu-tion, not exceeding the MVD. The use of a more sensitive lysate permits a greater dilution of the sample to be examined, and this may contribute to the elimination of interference.Interference may be overcome by suitable treatment such as filtration, neutralization, dialysis, or heating. To establish that the chosen treatment effectively eliminates interference without loss of endotoxins, perform the assay described above using the preparation to be examined to which Standard Endotoxin has been added and which has then been submitted to the chosen treatment.Limit TestPROCEDUREPrepare Solutions A, B, C, and D as shown in Table 2, and perform the test on these solutions following the procedure above for Preparatory Testing , Test for Confirmation of Labeled Lysate Sensitivity .Table 2. Preparation of Solutions for the Gel-Clot Limit TestSolution *Endotoxin Concentration/Solution to WhichEndotoxin Is Added Number of ReplicatesA None/Diluted Sample Solution 2B 2l /Diluted Sample Solution 2C 2l /Water for BET 2D None/Water for BET 2* Prepare Solution A and the positive product control Solution B using a dilution not greater than the MVD and treatments as described for the Test for Interfering Factors in Preparatory Testing . The positive control Solutions B and C contain the Standard Endotoxin Solution at a concentration corresponding to twice the la-beled lysate sensitivity. The negative control Solution D consists of Water for BET.166 á85ñ Bacterial Endotoxins Test / Biological Tests USP 40INTERPRETATIONThe test is considered valid when both replicates of Solutions B and C are positive and those of Solution D are negative. When a negative result is found for both replicates of Solution A, the preparation under test complies with the test. When a positive result is found for both replicates of Solution A, the preparation under test does not comply with the test.When a positive result is found for one replicate of Solution A and a negative result is found for the other, repeat the test. In the repeat test, the preparation under test complies with the test if a negative result is found for both replicates of Solution A. The preparation does not comply with the test if a positive result is found for one or both replicates of Solution A. However, if the preparation does not comply with the test at a dilution less than the MVD, the test may be repeated using a greater dilu-tion, not exceeding the MVD.Quantitative TestPROCEDUREThe test quantifies bacterial endotoxins in Sample Solutions by titration to an endpoint. Prepare Solutions A, B, C, and D as shown in Table 3, and test these solutions by following the procedure in Preparatory Testing, Test for Confirmation of Labeled Lysate Sensitivity.Table 3. Preparation of Solutions for the Gel-Clot AssaySolutionEndotoxin Concentration/Solution to Which EndotoxinIs Added DiluentDilutionFactorEndotoxinConcentrationNumberofReplicatesA a None/Sample Solution Water for BET1—22—24—28—2B b2l/Sample Solution—12l2C c2l/Water for BET Water for BET12l221l240.5l280.25l2D d None/Water for BET———2a Solution A: Sample Solution under test at the dilution, not to exceed the MVD, with which the Test for Interfering Factors was completed. Subsequent dilution of the Sample Solution must not exceed the MVD. Use Water for BET to make a dilution series of four tubes containing the Sample Solution under test at concentra-tions of 1, 1/2, 1/4, and 1/8 relative to the concentration used in the Test for Interfering Factors. Other dilutions up to the MVD may be used as appropriate.b Solution B: Solution A containing standard endotoxin at a concentration of 2l (positive product control).c Solution C: Two replicates of four tubes of Water for BET containing the standard endotoxin at concentrations of 2l, l, 0.5l, and 0.25l, respectively.d Solution D: Water for BET (negative control).CALCULATION AND INTERPRETATIONThe test is considered valid when the following three conditions are met: (1) Both replicates of negative control Solution D are negative; (2) Both replicates of positive product control Solution B are positive; and (3) The geometric mean endpoint con-centration of Solution C is in the range of 0.5l to 2l.To determine the endotoxin concentration of Solution A, calculate the endpoint concentration for each replicate by multiply-ing each endpoint dilution factor by l. The endotoxin concentration in the Sample Solution is the endpoint concentration of the replicates. If the test is conducted with a diluted Sample Solution, calculate the concentration of endotoxin in the original Sample Solution by multiplying by the dilution factor. If none of the dilutions of the Sample Solution is positive in a valid assay, report the endotoxin concentration as less than l (if the diluted sample was tested, report as less than l times the lowest dilu-tion factor of the sample). If all dilutions are positive, the endotoxin concentration is reported as equal to or greater than the greatest dilution factor multiplied by l (e.g., initial dilution factor times eight times l in Table 3).The preparation under test meets the requirements of the test if the concentration of endotoxin in both replicates is less than that specified in the individual monograph.USP 40Biological Tests / á85ñ Bacterial Endotoxins Test 167PHOTOMETRIC QUANTITATIVE TECHNIQUESTurbidimetric TechniqueThis technique is a photometric assay measuring increases in reactant turbidity. On the basis of the particular assay principle employed, this technique may be classified as either an endpoint-turbidimetric assay or a kinetic-turbidimetric assay. The end-point-turbidimetric assay is based on the quantitative relationship between the concentration of endotoxins and the turbidity (absorbance or transmission) of the reaction mixture at the end of an incubation period. The kinetic-turbidimetric assay is a method to measure either the time (onset time) needed to reach a predetermined absorbance or transmission of the reaction mixture, or the rate of turbidity development. The test is carried out at the incubation temperature recommended by the ly-sate manufacturer (which is usually 37±1°).Chromogenic TechniqueThis technique is an assay to measure the chromophore released from a suitable chromogenic peptide by the reaction of endotoxins with lysate. On the basis of the particular assay principle employed, this technique may be classified as either an endpoint-chromogenic assay or a kinetic-chromogenic assay. The endpoint-chromogenic assay is based on the quantitative re-lationship between the concentration of endotoxins and the release of chromophore at the end of an incubation period. The kinetic-chromogenic assay is a method to measure either the time (onset time) needed to reach a predetermined absorbance of the reaction mixture, or the rate of color development. The test is carried out at the incubation temperature recommendedby the lysate manufacturer (which is usually 37±1°).Preparatory Testing To assure the precision or validity of the turbidimetric and chromogenic techniques, preparatory tests are conducted to veri-fy that the criteria for the standard curve are valid and that the sample solution does not interfere with the test. Validation for the test method is required when conditions that are likely to influence the test result change.ASSURANCE OF CRITERIA FOR THE STANDARD CURVEThe test must be carried out for each lot of lysate reagent. Using the Standard Endotoxin Solution, prepare at least three en-dotoxin concentrations within the range indicated by the lysate manufacturer to generate the standard curve. Perform the as-say using at least three replicates of each standard endotoxin concentration according to the manufacturer's instructions for the lysate (volume ratios, incubation time, temperature, pH, etc.). If the desired range is greater than two logs in the kinetic methods, additional standards should be included to bracket each log increase in the range of the standard curve. The abso-lute value of the correlation coefficient, r , must be greater than or equal to 0.980 for the range of endotoxin concentrations set up.TEST FOR INTERFERING FACTORSSelect an endotoxin concentration at or near the middle of the endotoxin standard curve. Prepare Solutions A, B, C, and D as shown in Table 4. Perform the test on Solutions A, B, C, and D at least in duplicate, according to the instructions for the lysate employed, for example, concerning volume of Sample Solution and Lysate TS, volume ratio of Sample Solution to Lysate TS,incubation time, etc.Table 4. Preparation of Solutions for the Inhibition/Enhancement Test for Photometric TechniquesSolutionEndotoxin Concentration Solution to WhichEndotoxin Is Added Number of Replicates A a None Sample Solution Not less than 2B b Middle concentration of the standard curve Sample Solution Not less than 2C c At least three concentrations (lowest concentration is des-ignated l )Water for BET Each not less than 2D d None Water for BET Not less than 2a Solution A : The Sample Solution may be diluted not to exceed MVD.b Solution B : The preparation under test at the same dilution as Solution A, containing added endotoxin at a concentration equal to or near the middle of the standard curve.c Solution C : The standard endotoxin at the concentrations used in the validation of the method described for Assurance of Criteria for the Standard Curve under Preparatory Testing (positive controls).d Solution D : Water for BET (negative control).The test is considered valid when the following conditions are met.168 á85ñ Bacterial Endotoxins Test / Biological Tests USP 401.The absolute value of the correlation coefficient of the standard curve generated using Solution C is greater than or equalto 0.980.2.The result with Solution D does not exceed the limit of the blank value required in the description of the lysate reagentemployed, or it is less than the endotoxin detection limit of the lysate reagent employed.Calculate the mean recovery of the added endotoxin by subtracting the mean endotoxin concentration in the solution, if any (Solution A, Table 4), from that containing the added endotoxin (Solution B, Table 4). In order to be considered free of factors that interfere with the assay under the conditions of the test, the measured concentration of the endotoxin added to the Sample Solution must be within 50%–200% of the known added endotoxin concentration after subtraction of any endo-toxin detected in the solution without added endotoxin.When the endotoxin recovery is out of the specified range, the Sample Solution under test is considered to contain interfer-ing factors. Then, repeat the test using a greater dilution, not exceeding the MVD. Furthermore, interference of the Sample Solution or diluted Sample Solution not to exceed the MVD may be eliminated by suitable validated treatment such as filtration, neutralization, dialysis, or heat treatment. To establish that the chosen treatment effectively eliminates interference without loss of endotoxins, perform the assay described above, using the preparation to be examined to which Standard Endotoxin has been added and which has then been submitted to the chosen treatment.Test ProcedureFollow the procedure described for Test for Interfering Factors under Preparatory Testing, immediately above.CalculationCalculate the endotoxin concentration of each of the replicates of Solution A, using the standard curve generated by the positive control Solution C. The test is considered valid when the following three requirements are met.1.The results of the control Solution C comply with the requirements for validation defined for Assurance of Criteria for theStandard Curve under Preparatory Testing.2.The endotoxin recovery, calculated from the concentration found in Solution B after subtracting the concentration of en-dotoxin found in Solution A, is within the range of 50%–200%.3.The result of the negative control Solution D does not exceed the limit of the blank value required in the description of thelysate employed, or it is less than the endotoxin detection limit of the lysate reagent employed.InterpretationIn photometric assays, the preparation under test complies with the test if the mean endotoxin concentration of the repli-cates of Solution A, after correction for dilution and concentration, is less than the endotoxin limit for the product.á87ñ BIOLOGICAL REACTIVITY TESTS, IN VITROThe following tests are designed to determine the biological reactivity of mammalian cell cultures following contact with the elastomeric plastics and other polymeric materials with direct or indirect patient contact or of specific extracts prepared from the materials under test. It is essential that the tests be performed on the specified surface area. When the surface area of the specimen cannot be determined, use 0.1 g of elastomer or 0.2 g of plastic or other material for every mL of extraction fluid. Exercise care in the preparation of the materials to prevent contamination with microorganisms and other foreign matter. Three tests are described (i.e., the Agar Diffusion Test, the Direct Contact Test, and the Elution Test).1 The decision as to which type of test or the number of tests to be performed to assess the potential biological response of a specific sample or extract depends upon the material, the final product, and its intended use. Other factors that may also affect the suitability of a sample for a specific use are the polymeric composition; processing and cleaning procedures; contacting media; inks; adhe-sives; absorption, adsorption, and permeability of preservatives; and conditions of storage. Evaluation of such factors should be made by appropriate additional specific tests before determining that a product made from a specific material is suitable for its intended use. Materials that fail the in vitro tests are candidates for the in vivo tests described in Biological Reactivity Tests, In Vivo á88ñ.1 Further details are given in the following publications of the American Society for Testing and Materials, 1916 Race St., Philadelphia, PA 19103: Standard test method for agar diffusion cell culture screening for cytotoxicity, ASTM Designation F 895-84; Standard practice for direct contact cell culture evaluation of materi-als for medical devices, ASTM Designation F 813-83.USP 40Biological Tests / á87ñ Biological Reactivity Tests, In Vitro 169。
CP(2015版)1143 EP(8.0)2.6.14 USP(36)85
JP(16)6.06
文案大全
文案大全
文案大全
文案大全
文案大全
文案大全
文案大全
文案大全
文案大全
文案大全
文案大全
文案大全
文案大全
文案大全
文案大全
文案大全
文案大全
文案大全
文案大全
文案大全
文案大全
文案大全
文案大全
文案大全
文案大全
文案大全
文案大全
小结:
细菌内毒素检测法,CP、EP、USP、JP中所描述的检测方法一致,包括种类、操作基本相同。
EP、USP、JP中有关于鲎试剂的介绍,CP中没有对这方面进行描述。
检测方法EP、USP、JP比CP描述的更加详细,例如鲎试剂的反应原理、供试品溶液表示方法等。
文案大全。
细菌内毒素标准品
细菌内毒素是一种由细菌产生并释放到其周围环境中的毒素,它对人类和动物的健康造成了严重威胁。
因此,对细菌内毒素进行研究和监测显得至关重要。
而在进行这项工作时,使用标准品是非常必要的。
细菌内毒素标准品是指已知浓度和纯度的内毒素样品,它们通常用于实验室研究、产品质量控制和药物研发等领域。
对于细菌内毒素标准品的选择和使用,有一些重要的事项需要注意。
首先,选择合适的细菌内毒素标准品是至关重要的。
不同类型的细菌产生的内毒素可能具有不同的生物活性和致病性,因此在选择标准品时,需要根据具体的研究目的和应用领域来进行选择。
同时,标准品的纯度和稳定性也是需要考虑的因素,只有具有高纯度和稳定性的标准品才能够确保实验结果的准确性和可靠性。
其次,正确的使用细菌内毒素标准品也是非常重要的。
在进行实验或者监测时,需要严格按照标准品的使用说明来操作,确保使用的标准品符合实验要求。
同时,在使用过程中需要注意安全,避免接触到内毒素对人体造成危害。
此外,定期对细菌内毒素标准品进行质量控制也是必不可少的。
定期检测标准品的纯度、稳定性和活性,确保标准品的质量符合要求。
只有在质量得到保证的情况下,才能够保证实验结果的准确性
和可靠性。
综上所述,细菌内毒素标准品在细菌内毒素研究和监测中起着
非常重要的作用。
正确选择和使用标准品,定期进行质量控制,是
确保研究结果准确可靠的关键步骤。
希望通过对细菌内毒素标准品
的正确使用和管理,能够更好地推动细菌内毒素研究的进展,保障
人类和动物的健康安全。
细菌内毒素标准品细菌内毒素(Endotoxin)是一类存在于细菌细胞壁内的毒素,主要由脂多糖组成,是一种糖脂复合物。
它们是一种强烈的免疫原,能够引起机体免疫系统的异常激活,导致炎症反应,严重时甚至会引发败血症等严重疾病。
因此,细菌内毒素的检测和标准化显得尤为重要。
细菌内毒素标准品是用于内毒素检测的标准物质,它的制备和使用对于保障内毒素检测的准确性和可靠性具有重要意义。
细菌内毒素标准品的制备需要严格控制各项工艺参数,确保其纯度、活性和稳定性。
同时,在使用细菌内毒素标准品时,也需要严格按照标准操作规程进行操作,以确保测试结果的准确性和可比性。
细菌内毒素标准品的制备通常包括以下几个步骤,首先是选择合适的内毒素来源,通常选择肠杆菌等革兰氏阴性细菌作为内毒素的来源;其次是提取内毒素,这一步需要采用适当的提取方法,如热水提取法、酸碱提取法等;然后是内毒素的纯化和富集,这一步需要采用凝胶过滤、离子交换层析等手段;最后是内毒素的定量和活性测定,这一步需要采用适当的生物学或化学方法进行内毒素含量和活性的测定。
在使用细菌内毒素标准品进行内毒素检测时,需要严格按照操作规程进行操作,同时还需要注意以下几个方面,首先是标准品的储存和稳定性,标准品需要在规定的条件下储存,避免受到光、热、潮湿等因素的影响;其次是标准品的稀释和配制,需要按照规定的方法和比例进行稀释和配制,以确保测试结果的准确性;最后是测试条件的控制,包括试剂的质量控制、仪器的校准和环境条件的控制等,这些都对测试结果具有重要影响。
细菌内毒素标准品的制备和使用对于内毒素检测具有重要意义,它不仅可以用于内毒素检测方法的验证和比对,还可以用于药物研发、医疗器械检测等领域。
因此,我们需要加强对细菌内毒素标准品的研究和开发,提高其质量和稳定性,为内毒素检测提供更可靠的支持。
内毒素检测是用于检测环境中存在的内毒素或内源性毒素的过程,通常涉及食品、制药、医疗设备、生物制品等领域。
内毒素是一种细菌产生的毒素,它们可能对生命体产生有害影响。
以下是一些内毒素检测环境要求的标准和最佳实践:实验室环境:内毒素检测通常需要在洁净的实验室环境中进行,以防止外部污染。
实验室应具备适当的温度和湿度控制,以确保试剂和样本的稳定性。
设备和仪器:内毒素检测需要使用高精度的仪器和设备,以确保准确的结果。
仪器应定期维护和校准,以保持其性能。
人员培训:实验室工作人员需要接受专门培训,了解内毒素检测的原理和操作程序。
他们应该熟悉生物安全操作和废物处理程序,以减少风险。
样本采集和处理:样本采集应该在洁净和合规的环境中进行,以避免外部污染。
样本应迅速采集和处理,以防止内毒素的降解。
质控:内毒素检测需要使用质控样本来验证方法的准确性和可重复性。
实验室应建立质控程序,以确保结果的可信度。
数据记录和管理:数据应仔细记录,包括样本信息、检测方法、结果等。
数据应以安全的方式存储和管理,以确保机密性和完整性。
标准操作程序(SOPs):实验室应拥有详细的SOPs,以规范内毒素检测的流程和步骤。
SOPs应定期审查和更新,以反映最新的最佳实践和法规。
法规和标准:内毒素检测应遵守相关的法规和标准,例如美国药典(USP)或其他国家和地区的相关法规。
这些法规和标准通常规定了检测方法、限量标准和质控要求。
内毒素检测的要求和标准可能因国家、行业和应用领域而异。
因此,在进行内毒素检测时,需要查阅适用的法规和标准,并确保实验室符合这些要求。
此外,与内毒素检测相关的最佳实践也可以帮助确保准确性和可靠性。
USP 内毒素标准品性质特点简介
内毒素标准品简介
内毒素是存在于病原体如细菌内的天然化合物,具有潜在的毒性。
一般来说,内毒素不同干外毒素,活的细菌是不会分泌可溶性的内毒素的。
内毒素是细菌的结构成分,当细菌被溶解时而被细菌释放出来。
内毒素标准品1235503(10,000USP内毒素单位)(需要冷运)
内毒素是致热性的。
手提瓶及其内装物要格外小心避免污染。
这种材料含有聚乙二醇,一种刺激性物质。
内市表标准品1235503这一小瓶含有10.000个USP内毒表单位,用丰USP 细首内毒表检查、营营<85>服幕不要你审在5ml的|A试剂水中重建整个药瓶的含量,将溶液储存在冰箱中,14天内使用。
在冰箱里储存未打开的Vial。
内毒素标准品特点
内毒素不是蛋白质,因此非常耐热。
在100℃的高温下加热1小时也不会披破坏,只有在160℃的温度下加热2到4个小时,或用强碱,强酸或强氧化剂加温煮沸30分钟才能破坏它的生物活性。
与外毒素不同之处在于;内毒素不能被稀甲醛溶液脱去毒性成为类毒素;把内毒素注射到机体内虽可产生一定量的特异免疫产物(称为抗体),但这种抗体抵消内毒素毒性的作用微弱。
细菌内毒素标准品标定1.试剂和样品鲎试剂(生产厂家:湛江安度斯生物有限公司,批号:1112202,灵敏度:0.125EU/ml,规格:0.1 ml/支)细菌内毒素检查用水(生产厂家:湛江安度斯生物有限公司,批号:1106130,规格:5 ml/支)细菌内毒素工作标准品(P=160EU/支,生产厂家:中国药品生物制品检定所批号:150601-201174)细菌内毒素国家标准品(10000 EU/支,生产厂家:中国药品生物制品检定所批号:150600-200707)2.操作步骤2.1细菌内毒素国家标准品稀释、细毒内毒素工作标准品稀释将细菌内毒素国家标准品用1ml细菌内毒素检查用水溶解,在旋涡混合仪上混合15分钟,然后制备成4个浓度的标准内毒素溶液,即0.25 EU/ml、0.125 EU/ml、0.0625 EU/ml和0.03 EU/ml备用,每稀释一步均应在旋涡混合器上混合30秒。
具体稀释步骤如下:100008.12.0108.12.015.15.00.25110.125110.0625110.03待复核的细菌内毒素工作标准品标示效价为160 EU,用1ml细菌内毒素检查用水溶解,在旋涡混合仪上混合15分钟,然后制备成4个浓度的内毒素溶液,即640倍(0.25 EU/ml)、1280倍(0.125 EU/ml)、2560倍(0.0625 EU/ml)和5120倍(0.03 EU/ml)备用,每稀释一步均应在旋涡混合器上混合30秒。
具体稀释步骤如下:1608.12.0165.05.084.12.015.15.00.25110.125110.0625110.03以上细菌内毒素国家标准品和细菌内毒素工作标准品需同时进行。
2.2加样取规格为0.1ml/支的鲎试剂36支,轻弹瓶壁,使粉末落入瓶底,用砂轮在瓶颈轻轻划痕,70%异丙醇棉球擦拭后启开备用,防止玻璃屑落入瓶内。
每支加入0.1ml检查用水溶解,轻轻转动瓶壁,使内容物充分溶解,避免产生气泡将准备好的鲎试剂取其中18(管)放在试管架上,排成5列,其中4列4支(管),1列2支(管),4支(管)4列每列每支分别加入0.1ml的2λ、λ、0.5λ和0.25λ的内毒素标准溶液;另2支(管)加入0.1ml检查用水。
BACTERIAL ENDOTOXINS TEST <85> USP35Portions of this general chapter have been harmonized with the corresponding texts ofthe European Pharmacopoeia and/or the Japanese Pharmacopoeia. Those portions that are not harmonized are marked with symbols () to specify this fact.这一章节已经和欧洲药典(EP)与日本药典(JP)统一。
没有统一的部分已经用标记出来。
The Bacterial Endotoxins Test (BET) is a test to detect or quantify endotoxins from Gram-negative bacteria using amoebocyte lysate from the horseshoe crab (Limulus polyphemus or Tachypleus tridentatus).细菌内毒素检查法(BET)是通过鲎的阿米巴细胞溶解产物来检测或定量来自革兰氏阴性菌的内毒素。
There are three techniques for this test: the gel-clot technique, which is based on gel formation; the turbidimetric technique, based on the development of turbidity after cleavage of an endogenous substrate; and the chromogenic technique, based on the development of color after cleavage of a synthetic peptide-chromogen complex. Proceed by any of the three techniques for the test. In the event of doubt or dispute, the final decision is made based upon the gel-clot technique unless otherwise indicated in the monograph for the product being tested. The test is carried out in a manner that avoids endotoxin contamination.细菌内毒素检查法有3种:凝胶法,基于凝胶的形成;浊度法,BACTERIAL ENDOTOXINS TEST USP32Portions of this general chapter have been harmonized with the corresponding texts of the European Pharmacopeia and/or the Japanese Pharmacopeia. Those portions that are notharmonized are marked with symbols () to specify this fact.This chapter provides a test to detect or quantify bacterial endotoxins that may be present in or on the sample of the article(s) to which the test is applied. It uses Limulus Amebocyte Lysate (LAL) obtained from the aqueous extracts of circulating amebocytes of horseshoe crab (Limulus polyphemus or Tachypleus tridentatus) which has been prepared and characterized for use as anLAL Reagent.1There are two types of techniques for this test: the gel-clot techniques, which are based on gel formation, and the photometric techniques. The latter include a turbidimetric method, which is based on the development of turbidity after cleavage of an endogenous substrate, and a chromogenic method, which is based on the development of color after cleavage of a synthetic peptide-chromogen complex. Proceed by any one of these techniques, unless otherwise indicated in the monograph. In case of dispute, the final decision is based on the gel-clot techniques, unless otherwise indicated in the monograph.In the gel-clot techniques, the reaction endpoint is determined from dilutions of the material under test in direct comparison with parallel dilutions of a reference endotoxin, and quantities of endotoxin are expressed in USP Endotoxin Units (USP-EU).[NOTE—One USP-EU is equal to one IU of endotoxin.]Because LAL Reagents have been formulated to be used also for turbidimetric or colorimetric tests, such tests may be used to comply with the requirements. These tests require the establishment of a standard regression curve; the endotoxin content of the test material is determined by interpolation from the curve. The procedures include incubation for a preselected time of reacting endotoxin and control solutions with LAL Reagent and reading of the spectrophotometric light absorbance at suitable wavelengths. In the endpoint turbidimetric procedure the reading is made immediately at the end of the incubation period. In the endpoint colorimetric procedure the reaction is arrested at the end of the preselected time by the addition of an enzyme reaction-terminating agent prior to the readings. In the turbidimetric and colorimetric kinetic assays the absorbance is measured throughout the reaction period and rate values are determined from those readings.APPARATUS AND GLASSWAREDepyrogenate all glassware and other heat-stable materials in a hot-air oven using a validatedprocess.2Commonly used minimum time and temperature settings are 30 minutes at 250. If employing plastic apparatus, such as microplates and pipet tips for automatic pipetters, useonly that which has been shown to be free of detectable endotoxin and not to interfere with the test.[NOTE—In this chapter, the term “tube” includes any other receptacle such as a micro-titer well.]PREPARATION OF THE STANDARD ENDOTOXIN STOCK SOLUTION AND STANDARD SOLUTIONS The USP Endotoxin RS has a defined potency of 10,000 USP Endotoxin Units (EU) per vial. Constitute the entire contents of 1 vial of the RSE with 5 mL of LAL Reagent Water3, mixintermittently for 30 minutes, using a vortex mixer, and use this concentrate for making appropriate serial dilutions. Preserve the concentrate in a refrigerator for making subsequent dilutions for not more than 14 days. Mix vigorously, using a vortex mixer, for not less than 3 minutes before use. Mix each dilution for not less than 30 seconds before proceeding to make the next dilution. Do not store dilutions, because of loss of activity by adsorption, in the absence of supporting data to the contrary.Preparatory TestingUse an LAL Reagent of confirmed label sensitivity.The validity of test results for bacterial endotoxins requires an adequate demonstration that specimens of the article or of solutions, washings, or extracts thereof to which the test is to be applied do not of themselves inhibit or enhance the reaction or otherwise interfere with the test. Validation is accomplished by performing the inhibition or enhancement test described under each of the three techniques indicated. Appropriate negative controls are included. Validation must be repeated if the LAL Reagent source or the method of manufacture or formulation of the article is changed.Preparation of Sample SolutionsPrepare sample solutions by dissolving or diluting drugs or extracting medical devices using LAL Reagent Water. Some substances or preparations may be more appropriately dissolved, diluted, or extracted in other aqueous solutions. If necessary, adjust the pH of the solution (or dilution thereof) to be examined so that the pH of the mixture of the LAL Reagent and sample falls within the pH range specified by the LAL Reagent manufacturer. This usually applies to a product with a pH in the range of 6.0 to 8.0. The pH may be adjusted using an acid, base, or suitable buffer as recommended by the LAL Reagent manufacturer. Acids and bases may be prepared from concentrates or solids with LAL Reagent Water in containers free of detectable endotoxin. Buffers must be validated to be free of detectable endotoxin and interfering factors.DETERMINATION OF MAXIMUM VALID DILUTION (MVD)The Maximum Valid Dilution is the maximum allowable dilution of a specimen at which the endotoxin limit can be determined. It applies to injections or to solutions for parenteral administration in the form constituted or diluted for administration, or, where applicable, to theamount of drug by weight if the volume of the dosage form for administration could be varied. The general equation to determine MVD is:MVD = (Endotoxin limit × Concentration of sample solution)/()where the concentration of sample solution and are as defined below. Where the endotoxin limit concentration is specified in the individual monograph in terms of volume (in EU per mL), divide the limit by, which is the labeled sensitivity (in EU per mL) of the LAL Reagent, to obtainthe MVD factor. Where the endotoxin limit concentration is specified in the individual monograph in terms of weight or Units of active drug (in EU per mg or in EU per Unit), multiply the limit by the concentration (in mg per mL or in Units per mL) of the drug in the solution tested or of the drug constituted according to the label instructions, whichever is applicable, and dividethe product of the multiplication by, to obtain the MVD factor. The MVD factor so obtained is the limit dilution factor for the preparation for the test to be valid.ESTABLISHMENT OF ENDOTOXIN LIMITSThe endotoxin limit for parenteral drugs, defined on the basis of dose, is equal to K/M,4where K is the threshold human pyrogenic dose of endotoxin per kg of body weight, and M isequal to the maximum recommended human dose of product per kg of body weight in a single hour period.The endotoxin limit for parenteral drugs is specified in individual monographs in units such as EU/mL, EU/mg, or EU/Unit of biological activity.GEL-CLOT TECHNIQUESThe gel-clot techniques detect or quantify endotoxins based on clotting of the LAL Reagent in the presence of endotoxin. The concentration of endotoxin required to cause the lysate to clot under standard conditions is the labeled sensitivity of the LAL Reagent. To ensure both the precision and validity of the test, tests for confirming the labeled LAL Reagent sensitivity and for interfering factors are described under Preparatory Testing for the Gel-Clot Techniques.Preparatory Testing for the Gel-Clot TechniquesTest for Confirmation of Labeled LAL Reagent Sensitivity—Confirm the labeled sensitivity using at least 1 vial of the LAL Reagent lot. Prepare a series of two-fold dilutions of the USP EndotoxinRS in LAL Reagent Water to give concentrations of 2,, 0.5, and 0.25, where is as defined above. Perform the test on the four standard concentrations in quadruplicate and。