2009年糖尿病用药市场研究报告
- 格式:pdf
- 大小:736.40 KB
- 文档页数:46

2009年中国糖尿病药物市场分析发表时间:2009年11月 9日糖尿病已成为人类第四大死因,全球每年有超过380万人死于糖尿病及其并发症。
目前全世界已有2.46亿糖尿病患者。
我国近20年来糖尿病发病率增长了5倍多,是全球增长最快的国家之一。
如此高的糖尿病发病率催生出潜力巨大的糖尿病用药市场。
糖尿病用药领域也已成为各大药企征战的重点。
2008年,全球糖尿病市场总销售额高达240亿美元,而在全球糖尿病药物中,口服药市场份额略高于胰岛素制剂。
外资产品称霸市场据华源医药电子商务数据推算,在国内4900家综合医院及近万家政府办中医院,口服糖尿病用药的购药金额均在逐年增加,且近几年在整体市场中所占份额相对稳定。
在口服糖尿病用药市场上,外企所占金额份额远远大于国内企业。
其中,拜耳医药保健有限公司最为突出,其拳头产品阿卡波糖(拜糖平)独占了30%的市场份额;在销售业绩排在前10名的企业中,三资企业占到90%,内资企业仅有北京万辉双鹤药业一家跻身前10。
造成这种市场格局很重要的原因是,口服降糖药中的明星产品基本为国外企业的专利药,虽然仿制药也层出不穷,但并未对专利药产生太大的影响;同时,专利药的推广也非常成功,牢牢控制着市场。
口服降糖药品种浅析口服糖尿病用药城市主要集中在北京和上海,两地用药金额份额超过50%。
两地用药金额最大的都是阿卡波糖。
目前国内批准使用的口服降糖药有促胰岛素分泌剂(磺酰脲类、格列奈类)和非促胰岛素分泌剂(α-糖苷酶抑制剂、双胍类、噻唑烷二酮胰岛素增敏剂)。
华源医药电子商务抽样数据涉及常用的20多个口服降糖药品种,按照用药金额份额依次降低排序为α-糖苷酶抑制剂、磺酰脲类促胰岛素分泌剂、非磺酰脲类胰岛素分泌剂、双胍类、噻唑烷二酮胰岛素增敏剂和中药降糖药,其中α-糖苷酶抑制剂在金额份额上一直领先。
近年来,非磺脲类促泌剂的销售增势最为明显,并在2008年将双胍类挤出前三。
双胍类代表药物盐酸二甲双胍作为2型糖尿病的一线用药备受市场青睐,所以从口服降糖药销售数量来看,双胍类药物仍遥遥领先。

2009年度我院降糖药物用药分析【摘要】目的总结河南省人民医院2009年降糖药的应用现状和发展趋势。
方法利用计算机网络管理系统对本院2009年各种降糖药的用药频度(DDDs)、用药金额、日治疗费用(DDC)及其排序等统计数据进行分析。
结果2009年应用的降糖药共有30个品种,其中注射剂19种,占用药金额的79.87%;口服剂型11个品种,占用药金额的20.13%;结论本院胰岛素制剂的应用占主导地位,该类药物使用合理。
【关键词】降糖药; 用药分析糖尿病是一组以慢性血糖水平增高为特征的代谢疾病群,是常见病、多发病,已成为继心血管病和肿瘤之后的第三大非传染性病症[1]。
良好的血糖控制可以减少糖尿病患者慢性并发症的发生,而降血糖药物是控制血糖的有效方法。
为了探讨临床用药的经济性、方便性和合理性,现对本院2009年降糖药物的应用情况进行统计、分析,报告如下。
1 资料与方法1.1 资料来源原始数据取自我院药库微机管理系统数据库,以2009年降糖药品出库数量和金额为基本素材,利用计算机进行数据合并、统计、排序等处理。
1.2 分析方法采用DDD分析法和金额排序法,综合分析该类药物使用情况:①限定日剂量(DDD)的确定:具体计算参照WHO药物统计中心制定的方法结合药品说明书和临床使用情况设定;②用药频度(DDDs)=总用药量/相应药品DDD 值, DDDs排序在前表示用药频率高;③药物限定日费用(DDC)=药品的销售总金额/该药品DDDs,DDC代表着该药的价格水平,表示患者应用该种药物平均的日费用;对用药总金额和DDDs 值排序,并求其比值,评价药物费用的合理程度;比值接近1.00时,表示购药与用药频度同步性良好;④采用金额排序法,统计注射用胰岛素和口服降糖药的用药金额及百分比;各类口服降糖药的构成比;各种胰岛素制剂的详细用药情况。
2 结果2009年应用的降糖药共有30个品种,其中注射剂19种,占用药金额的79.87%;口服剂型11个品种,占用药金额的20.13%。




2009DiabetesME D I C I N E SI NDE V E L O P M E N TF O RReportP R E S E N T E DB YA M E R I C A’S P H A R M A C E U T I C A L R E S E A R C H C O M P A N I E SBiopharmaceutical Companies Developing Record183 Medicines to Treat Diabetes and Related Conditions* For more information about a specific medicine in this report, please call the telephone number listed.GLP-1Emisphere Technologies type 2 diabetes Phase I (oralCedar Knolls, NJ(973 532-8000GLP-1 FC Eli Lilly type 2 diabetes Phase IIIndianapolis, IN(800 545-5979GLP-1 PEG Eli Lilly type 2 diabetes Phase IIndianapolis, IN(800 545-5979HDV-insulin Diasome Pharmaceuticals type 2 diabetes Phase II Conshohocken, PA(609 923-9443HE3286Hollis-Eden type 2 diabetes Phase II Pharmaceuticals(858 587-9333San Diego, CAHSD016Wyeth Pharmaceuticals diabetes Phase ICollegeville, PA(800 934-5556IL-1 antibody Eli Lilly type 2 diabetes Phase IIndianapolis, IN(800 545-5979INCB-13739Incyte type 2 diabetes Phase IIWilmington, DE(302 498-6700INCB-19602Incyte type 2 diabetes Phase IIWilmington, DE(302 498-6700INCB-20817Incyte type 2 diabetes Phase IIWilmington, DE(302 498-6700INGAP peptide Kinexum Metabolics type 1 diabetes, type 2 diabetes Phase II completed Harper’s Ferry, WV(304 535-3037insulin inhalation Baxter Healthcare diabetes Phase IDeerfield, IL(800 422-9837insulin inhalation MicroDose Technologies diabetes Phase I completed Monmouth Junction, NJ(732 355-2100insulin nasal spray MDRNA type 2 diabetes Phase IIBothell, WA(425 908-3601insulin oral Emisphere Technologies type 2 diabetes Phase I/IICedar Knolls, NJ(973 532-8000insulin oral Generex Biotechnology type 1 diabetes Phase III Toronto, Canada(416 364-2551insulin transdermal Dermisonics type 1 diabetes, type 2 diabetes Phase I Irvine, CA(888 401-3376INT-131InteKrin Therapeutics type 2 diabetes Phase IILos Altos, CA(650 941-5501Intesulin™Coremed diabetes Phase Iinsulin oral Lake Bluff, IL(847 772-6868ISIS 113715Isis Pharmaceuticals type 2 diabetes Phase II Carlsbad, CA(combination therapy(800 679-4747ISIS-SGLT2rx Isis Pharmaceuticals type 2 diabetes Phase I Carlsbad, CA(800 679-4747JTT-654Akros Pharma type 2 diabetes Phase IPrinceton, NJ(609 919-9570application submitted—An applica-tion for marketing has been submit-ted by the company to the Food and Drug Administration (FDA.diabetes—A chronic disease in which the body does not produce or properly use insulin, a hormone that is needed to convert sugar, starches and other food into energy needed for daily life. Symptoms may include excessive thirst, hunger, urination and weight loss. The cause of dia-betes continues to be a mystery, although both genetics and environ-mental factors such as obesity and lack of exercise appear to play roles. Type 1 diabetes, th e more severe form, results from the body’s failure to produce insulin, which “unlocks”the cells of the body, allowing glu-cose to enter and fuel them. It is estimated that 5 percent to 10 per-cent of Americans who are diag-nosed with diabetes have type 1, which requires insulin treatment. Type 2 diabetes results from insulin resistance (a condition in which the body fails to properly use insulin, combined with relative insulin defi-ciency. Most Americans who are diagnosed with diabetes have type 2, which in most cases can be con-trolled by a combination of dietary measures, weight loss, and oral medication.diabetic peripheral neuropathy—Nerve damage in the arms, hands, legs, and feet caused by diabetes. The condition develops slowly and worsens over time. Depending on the types of nerves involved, one or more signs and symptoms may be present in diabetic peripheral neu-ropathy. Sensory neuropathy results in numbness or tingling in the feetor pain or discomfort in the feet or legs, including prickly, sharp pain or burning feet. Motor neuropathyinvolves muscle weakness and lossof muscle tone in the feet and lowerlegs, loss of balance, or changes infoot shape that can lead to areas ofincreased pressure. Autonomic neu- ropathy results in dry feet and cracked skin. The loss of sensation and other problems associated with nerve damage make a patient prone to developing skin ulcers (open sores that can become infected and may not heal. This serious complica- tion of diabetes can lead to the loss of a foot, a leg, or even a life. diabetic retinopathy—The most common diabetic eye disease anda leading cause of blindness in American adults. It is caused by changes in the blood vessels of the retina, the light-sensitive tissue at the back of the eye that is necessary for good vision. In some people with diabetic retinopathy, blood vesselsmay swell and leak fluid. In others, abnormal new blood vessels grow on the surface of the retina. Over time, diabetic retinopathy, which usually affects both eyes, can worsen and cause vision loss. The condition has four stages: 1 mild nonprolifera- tive retinopathy, the earliest stage during which microaneurysms occur in the retina’s tiny blood vessels; 2 moderate nonproliferative retinopa- thy, during which some blood ves- sels that nourish the retina are blocked; 3 severe nonproliferative retinopathy, when many more blood vessels are blocked and deprive several areas of the retina with their blood supply; and 4 proliferative retinopathy, the advanced stagewhen signals sent by the retina for nourishment trigger the growth of new blood vessels, which grow along the retina and the surface of the clear, vitreous gel that fills the inside of the eye. If those thin, frag- ile blood vessels leak blood, severe vision loss and even blindness can result. Up to 45 percent of Americans diagnosed with diabetes have some stage of diabetic retinopathy. macular edema—A condition in which fluid can leak into the center of the macula, the part of the eye where sharp, straight-ahead vision occurs. The fluid makes the macula swell, thus blurring vision. It can occur at any stage of diabetic retinopathy, although it is morelikely to occur as the disease pro- gresses. About half of the people with proliferative retinopathy also have macular edema.Phase I—Safety testing and pharma- cological profiling of new drugs in small numbers of humans.Phase II—Effectiveness testing and identification of side effects of new drugs in humans.Phase III—Extensive clinical trials in humans to verify effectiveness and monitor adverse reactions of new drugs.polyneuropathy—A diffuse periph- eral nerve disorder that is bilaterally symmetrical and thus not confined to the distribution of a single nerve or a single limb. Electrodiagnostictests are done to classify the nerve structures involved, their distribu- tion, and the severity of the disorder in order to help identify the cause. Some polyneuropathies, such as those caused by lead toxicity or tick bite, affect primarily motor fibers. Others, such as those caused by diabetes or AIDS, affect primarily sensory fibers. Diabetic neuropathiescan also affect cranial nerves.T he U.S. system of new drug approvals is perhaps the most rigorous in the world.It takes 10-15 years, on average, for an experimental drug to travel from lab to U.S. patients, according to the Tufts Center for the Study of Drug Development, based on drugs approved from 1994 through 1998. Only five in 5,000 compounds that enter preclinical testing make it to human testing. And only one of those five is approved for sale.On average, it costs a company $1.3 billion to get one new medicine from the laboratory to U.S. patients, according to a 2007 study by the Tufts Center for the Study of Drug Development.Once a new compound has been identified in the laboratory, medicines are developed as follows: Preclinical Testing. A pharmaceutical company con -ducts laboratory and animal studies to show biological activity of the compound against the targeted disease, and the compound is evaluated for safety.Investigational New Drug Application (IND.After completing preclinical testing, a company files an IND with the U.S. Food and Drug Administration (FDA to begin to test the drug in people. The IND becomes effective if FDA does not disapprove it within 30 days. The IND shows results of previous experiments; how, where and by whom the new studies will be conducted; the chemical structure of the compound; how it is thought to work in the body; any toxic effects found in the animal studies; and how the compound is man -ufactured. All clinical trials must be reviewed and approved by the Institutional Review Board (IRB where the trials will be conducted. Progress reports on clinical trials must be submitted at least annually to FDA and the IRB.Clinical Trials, Phase I.These tests involve about 20 to 100 normal, healthy volunteers. The tests study a drug’s safety profile, including the safe dosage range. The studies also determine how a drug is absorbed, distributed, metabolized, and excreted as well as the duration of its action.Clinical Trials, Phase II.In this phase, controlled trials of approximately 100 to 500 volunteer patients (people with the disease assess a drug’s effectiveness.Clinical Trials, Phase III.This phase usually involves 1,000 to 5,000 patients inc linics and hospitals. Physicians monitor patients closely to confirm efficacy and identify adverse events.New Drug Application (NDA/Biologic License Application (BLA.Following the completion of all three phases of clinical trials, a company analyzes all of the data and files an NDA or BLA with FDA if the data successfully demonstrate both safety and effec -tiveness. The applications contain all of the scientific information that the company has gathered. Applications typically run 100,000 pages or more. The average review time for the 24 new therapeutics approved by the FDA in 2008 was 17.8 months.Approval.Once FDA approves an NDA or BLA, the new medicine becomes available for physicians to prescribe. A company must continue to submit periodic reports to FDA, including any cases of adverse reactions and appropriate quality-control records. For some medicines, FDA requires additional trials (Phase IV to evaluate long-term effects.Discovering and developing safe and effective new medicines is a long, difficult, and expensive process. Pharmaceutical companies invested an estimated $65.2 billion in research and development in 2008.‘Sharing Miracles’ Television Program Features Inspirational Stories of Real Diabetes Patients S haring Miracles—a 30-minute public affairs television program—tells the compelling and inspirational stories of real patients. Two recent episodes focused on patients who suffer from diabetes— Leave It to Beaver star Jerry Mathers and professional basketball star Dominique Wilkins. Mathers is an American icon, best known for his portrayal of the mischievous Theodore “Beaver” Cleaver. Currently shown on TV Land and in countries throughout the world, Leave It to Beaver has made JerryMathers one of the best-known actors in television history. D espite Beaver’s seemingly perpetual youth, life caught up with Mathers when he was diagnosed with diabetes. Originally faced with a life expectancy of three to five years, he has kept his disease at bay over the last 10 years. Speaking of his experience on Sharing Miracles, Mathers says, “You have to take control of your diabetes; you can’t let it take control of you.” Now, Mathers works tirelessly as a patient advocate, using his celebrity to help spread the word to others. Hall-of-Famer Dominique Wilkins is one of the greatest players in the history of the National Basketball Association (NBA. Wilkins is a nine-time NBA all-star, league scoring champion, and the winner of the NBA’s slam dunk contest. But today, in addition to fame, he also lives with diabetes, a disease that took the life of his father and grandfather, and has afflicted other members of his family. “Once I found out what my problem was, I changed my diet, and I started to exercise and take my medication…I didn’t want what happened to my father to happen to me, and I wasn’t going to let diabetes beat me,” Wilkins says. “For me, going through what I’ve been through with diabetes made me realize that I have to be a champion in helping try to eradicate this disease.” Previous episodes of Sharing Miracles have featured Academy Award-winning actress Marcia Gay Harden, a breast cancer advocate; Grammy Award-winning country music superstar Naomi Judd, who overcame hepatitis C; Emmy Award-winning actor Joey Pantoliano, who suffers from clinical depression; Super Bowl Champion and former Pittsburgh Steelers running back Jerome Bettis, who has asthma; two-time NCAA tournament-winning University of Connecticut basketball coach Jim Calhoun, who has overcome cancer three times; Emmy-nominated former star of Family Ties Meredith Baxter, who survived breast cancer; Olympic Gold Medal winners Mark Spitz (high cholesterol, Bruce Jenner (attention deficit disorder, and Greg Louganis (HIV; syndicated television talk show host Montel Williams, who suffers from multiple sclerosis; pop icon and Broadway star Deborah Gibson, who has suffered from devastating anxiety attacks; and Pro Football Hall-of-Famer and Super Bowl Champion Len Dawson, who survived prostate cancer. Sharing Miracles is produced by PhRMA’s Communica tions & PublicAffairs Department and airs on Sunday mornings on more than 300 television stations across the United States, reaching more than 50 million households. The show’s corresponding Web site, , is an interactive forum for people to relate their own personal stories of hope and survival. Every patient’s battle is unique, but the collective power of shared experiences can offer great help and courage to others who are fighting for their lives. You can find out where Sharing Miracles is showing in your area and order free DVDs of the show on the web site or by calling 202-835-3460. You can also view the show in it’s entirety at anytime on our web site,. New Medicines. New Hope.® Pharmaceutical Research and Manufacturers of America 950 F Street, NW Washington, DC 20004 | | | | 5/09。

2009~2012年我院抗糖尿病药物用药分析
李磊
【期刊名称】《齐齐哈尔医学院学报》
【年(卷),期】2013(034)015
【摘要】目的了解我院降糖药临床使用情况和变化趋势,探讨降糖药的合理使用.方法采用金额排序、DDDs排序的分析方法对我院2009~2012年抗糖尿病药物进行统计、分析.结果 4年来我院降糖药使用金额逐年上升,用药金额与用药频度基本呈正相关性.双胍类、磺脲类及胰岛素类药连续4年为临床主要使用的降糖药.二甲双胍、格列吡嗪、普通胰岛素、诺和灵30R等为一线用药.结论我院降糖药使用是合理的.
【总页数】3页(P2276-2278)
【作者】李磊
【作者单位】221002 徐州市第一人民医院药剂科
【正文语种】中文
【相关文献】
1.我院2012~2014年抗糖尿病药物用药分析 [J], 董洪明
2.我院2009-2012年门诊抗精神病药用药分析 [J], 李焕芬;王来海;蒋立新
3.我院抗糖尿病药物用药分析 [J], 张桂芳;张金凤;田绍丽;滑翔
4.2010年我院口服抗糖尿病药物用药分析 [J], 吴杏梅
5.我院药房抗糖尿病药物的用药分析 [J], 周娟
因版权原因,仅展示原文概要,查看原文内容请购买。

2009~2012年住院患者口服抗糖尿病的药物分析目的了解我院抗糖尿病药物的使用情况,为临床合理用药提供一定的参考。
方法采用金额排序法和频度分析法(DDDS)抽取我院于2009年~2012年收治的住院患者口服抗糖尿病药物的应用情况,并进行统计分析。
结果经过统计结果分析显示,口服抗糖尿病用药金额呈现逐年增长的趋势。
住院患者口服抗糖尿病药物的用药频度(DDDs)中,二甲双胍的用药频度(DDDS)最高,说明深受患者所欢迎。
同时,在4年内的用药频度(DDDS)连续上升的药物分别是二甲双胍、阿卡波糖、吡格列酮、瑞格列奈以及格列吡嗪。
4年内用药频度(DDDS)出现连续下降的药物分别是格列本脲和格列硅酮两种药物。
结论本院口服抗糖尿病药物的使用基本合理,安全性高、依从性好、价格适中,深受临床医师和住院患者的欢迎。
标签:住院患者;糖尿病;口服;药物糖尿病(Diabetes)是一种临床较为常见的疾病,多由胰岛素分泌缺陷或者胰岛素作用障碍所致[1]。
作为一种以高血糖为特征的代谢性疾病,如果患者没有得到及时有效的治疗,则易出现多种较为严重的并发症,如肾脏病变、血管病变等,甚至会危及患者的生命[2]。
临床治疗方法大多数是对症治疗,将患者的高血糖恢复至正常水平,以减少相关并发症的发生[3]。
目前,糖尿病的基本治疗多以药物为主,其中口服抗糖尿病药物占据多数。
本文采用金额排序法和频度分析法(DDDS)抽取我院于2009年~2012年收治的住院患者口服抗糖尿病药物的应用情况,并进行统计分析,为临床合理用药提供一定的指导。
1资料与方法1.1一般资料所有数据均来源于我院计算机系统,收集2009年~2012年住院患者口服抗糖尿病药用药数据,包括药品的种类、药品的名称、药品的规格以及药品的用药金额等。
1.2方法本次研究中主要采用金额排序法和用药频度(DDDs)分析法。
其中,金额排序法主要用于统计并比较2009年~2010年我院住院患者口服抗糖尿病药物用药的金额。

2006~2009年我院治疗糖尿病药物的用药分析摘要】目的:评价我院糖尿病药的应用情况与发展趋势。
方法:统计我院06~09年糖尿病药物用药总金额及糖尿病药物各品种的用药金额和作药频度分别进行前10位的排序。
结果与结论:治疗糖尿病药物的用药金额在我院用药金额总金额中所占的比例较稳定,且用药金额逐年增长。
【关键词】治疗糖尿病药物;日均费用;分析;DDDS;发展趋势【中图分类号】R425【文献标识码】A【文章编号】1005-0515(2011)03-0147-01近年来,有多种治疗糖尿病药物应用于临床治疗,特别是治疗2-型糖尿病的新药层出不穷。
不过最近(meteanaysis)[1]得出这样结论:广为应用于的第二代降糖药物磺脲类和二甲双胍对血糖的控制和降低心血管疾病危险率的效应,不亚于近年来出现的各类新药,也就是说,广为人知的老药不仅在价格上较新药便宜,其控制血糖和血脂的疗效敢不逊色于新药。
我院是粤西地区大型的糖尿病专科医院,通过对本院2006~2009年治疗糖尿病药物的应用进行统计,分析,并了解该类药物的用药情况和趋势。
1资料和方法1.1资料来源于我院药库2006~2009年的购药数据,药品数据包括通用名、商品名、购药金额、及采购数量等。
1.2统计我院2006~2009年治疗糖尿病用药总金额及各品种的用药金额,用药频度,与日均费用,并对治疗糖尿病药物用金额和用药频度进行前10位的排序。
1.3用药频度(DDDS)以约定日剂量(DDD)为标准计算,用药频度(DDDS)。
DDD是指用于成人的可达到主要治疗目的的药物平均日剂量。
本文以《中国药典》(2005版)及《新编药物学》(第15版)[2]规定的日剂量为准,其中DDDS=某药的总用药剂量/该药的DDD(同一药物因剂型不同而DDD值不同,需分别计算DDDS后再相加,即为该药的总DDDS)。
日均费用=某药的总费用/该药的DDDS。
2结果2.12006~2009年治疗糖尿病药物的购药金额及购进药物总金额(见表1)2.22006~2009年治疗及辅助治疗糖尿病药物金额前十名药品排序。

糖尿病患者论文:09年某区域市场糖尿病患者口服用药情况分析健康是一个人一生最大的财富,拥有健康的身体才是每个人生活幸福、快乐的前提。
随着人民生活水平的提高,患有糖尿疾病人数不断增加。
据世界卫生组织的统计数字显示:目前全世界至少有1.8亿多人患有糖尿病,在我国将近有3000--4000万左右人患有糖尿病。
根据世界卫生组织的分类原则,糖尿病因病因分为四种类型:1 ⅰ型糖尿病是由于胰岛β细胞破坏,导致胰岛素绝对缺乏。
2 ⅱ型糖尿病是由于胰岛素抵抗为主伴胰岛素分泌不足;或胰岛素分泌不足为主或不伴胰岛素抵抗。
此类约占所有糖尿病人的90%以上。
3 特异性糖尿病4 营养不良相关性糖尿病ⅰ型糖尿病患者必须使用胰岛素治疗,又称作胰岛素依赖性糖尿病;ⅱ型糖尿病患者又称作非胰岛素依赖性糖尿病,主要服以下四类药品,其作用机理分别是:磺酰脲类降糖药:刺激胰岛β细胞释放胰岛素:双胍类降糖药:1.增加周围组织对胰岛素的敏感性,增加胰岛素介导的葡萄糖利用. 2. 增加非胰岛素依赖的组织对葡萄糖的利用。
3. 抑制肝糖原异生作用,降低肝糖输出。
4. 抑制肠壁细胞摄取葡萄糖。
5. 抑制胆固醇的生物合成和贮存,降低血甘油三酯、总胆固醇水平。
α-葡萄糖苷抑制剂降糖药:主要在肠道内竞争性抑制葡萄糖苷酶,降低多糖及蔗糖分解生成葡萄糖,减少并延缓葡萄糖的吸收,可降低餐后血糖。
胰岛素增敏剂降糖药:主要是通过提高胰岛素敏感性而控制血糖水平除以上四类药品外,口服降糖药还有中成药及辅助降糖药,如胰激肽原酶肠溶片、六味地黄丸等。
针对某区域市场09年销售的糖尿病类药品进行统计分析,情况如下:从表一来看,除去辅助降糖药外,09年降糖药销量排行榜依次为磺酰脲类——二甲双胍类——胰岛素增敏剂——α-葡萄糖苷抑制剂——中成药;销售金额排行榜依次为磺酰脲类——胰岛素增敏剂——α-葡萄糖苷抑制剂——二甲双胍类——中成药。
由此发现磺酰脲类降糖药因价格便宜,质量稳定,临床用药时间长,而成为糖尿病患者的首选,销量和销售额都位居排行榜第一。
·预防医学·2009 2012年我院抗糖尿病药物用药分析李磊【摘要】目的了解我院降糖药临床使用情况和变化趋势,探讨降糖药的合理使用。
方法采用金额排序、DDDs排序的分析方法对我院2009 2012年抗糖尿病药物进行统计、分析。
结果4年来我院降糖药使用金额逐年上升,用药金额与用药频度基本呈正相关性。
双胍类、磺脲类及胰岛素类药连续4年为临床主要使用的降糖药。
二甲双胍、格列吡嗪、普通胰岛素、诺和灵30R等为一线用药。
结论我院降糖药使用是合理的。
【关键词】降糖药;DDDs;应用分析糖尿病是一种由于机体不能正常释放或利用胰岛素,使血中葡萄糖水平不适当升高而导致的一种疾病。
如果不进行及时有效的治疗将会导致许多严重的并发症:神经、血管、眼睛、肾脏病变,严重者危及生命。
随着人们生活方式的改变,糖尿病的患病率呈上升趋势,2010年糖尿病和糖尿病前期的患病率分别为9.7%和15.5%,以此推算,中国现有糖尿病患者达9240万例,居全球之首[1]。
为了更好地发挥抗糖尿病药物的临床作用,预测这类药物的发展趋势,对我院2009 2012年糖尿病药使用情况进行分析,以探讨近4年我院糖尿病药物临床用药的经济学评价。
一、材料和方法1.数据来源:2009 2012年降糖药的出库数量和金额数据均出自我院药库计算机管理系统数据库。
包括药品名称、规格、单价、数量、金额等。
2.分析方法:采用金额排序法、DDDs排序法,进行分类统计分析。
①限定日剂量(DDD)根据WHO ATC/DDD Index2008的推荐值、《新编药物学》(第16版)[2]结合药品说明书来确定;②用药频度(DDDs)=药品年消耗总量/该药品DDD值。
DDDs表明了该药品的使用频度,值越大使用频度越大,反之则越小;③对总的购药金额、DDDs进行排序分析。
二、结果统计2009 2012年我院糖尿病品种、销售金额、年DDDs,计算费用年增长率,DDDs年增长率,结果见表1,各类降糖药的用药频度DDDs及其排序见表2,各类降糖药的用药金额及其排序见表3,各年度DDDs前10位药物及其DDC见表4。
我院2009~2013年口服降糖药用药分析目的分析我院2009~2013年近5年来降糖药用药情况,为临床合理用药提供参考。
方法通过医院信息管理系统,对2009~2013年我院口服降糖药的销售金额、用药频度(DDDs)、日均费用(DDDc)等进行回顾性分析。
结果我院近5年来口服降糖药销售金额排序前2位的是磺酰脲类、胰岛素增敏药;罗格列酮的销售金额始终位居第1位;DDDs值排名位居第1位的是二甲双胍,而DDC排名最低;其中瑞格列奈的用药金额排序和DDDs排序同步性较好。
结论我院的口服降糖药使用基本合理,新药用量呈上升趋势,质优价廉的降糖药在临床应用中占优势。
[Abstract] Objective To investigate and analyze the application of oral hypoglycemic agents from 2009 to 2013 in our hospital,and provide guidance for rational clinical medication. Methods Depending on the original data from “the management system of the drug stock”,the data of oral hypoglycemic agents were analyzed retrospectively in respect of the consumption sum,DDDs and drug daily cost (DDC)etc. Results Sulfonylureas and insulin sensitizer ranked the top two places in respect of the consumption sum in our hospital during 2009-2013. Among the insulin sensitizer,rosiglitazone sales amount dominated the first place on the list of consumption sum. Metformin ranked at the front in respect of DDDs and its DDC is the lowest among of them during the five years. The consumption sum of repaglinide has grown in step with DDDs. Conclusion The utilization of oral hypoglycemic agents in our hospital was rational on the whole. The consumption quantity of new drugs showed an upward trend;the kinds of good quality and low price assumed a dominant place in clinical application.[Key words] Oral hypoglycemic agents;Analysis of drug use;DDDs;DDC;Consumption sum糖尿病(diabotes mellitus,DM)是一组以长期血糖水平增高为特征的代谢疾病群。
2009-2011年我院降糖药物应用分析摘要】目的了解和分析我院降糖药物的使用情况和发展趋势,为临床用药提供参考。
方法以我院2009-2011年降糖药物的出库记录为依据,采用金额排序法和频度分析法,分析降糖药物的年消耗金额,各类药物消耗金额排序和用药频度排序。
结果三年内降糖药物销售金额逐年上升,但降糖药物销售金额占药品总销售金额的比例平衡;胰岛素的销售金额排在第一位,其中诺和灵30R笔芯销售金额连续3年位居首位,磺酰脲类格列美脲的用药频度连续2年位居首位。
结论我院使用的降糖药以胰岛素类、磺酰脲类、胰岛素增敏剂为主,临床对磺酰脲类、胰岛素增敏剂的选择倾向大。
【关键词】降糖药物用药金额【中图分类号】R952 【文献标识码】A 【文章编号】2095-1752(2012)24-0351-02Analysis of Hypoglycemic Drug Application in the Funan People's from 2009-2011 Xing Zi-hua(FuNanXian People's hospital)【Absteact】Objective:To investigate the status quo and tendency of the application of hypoglycemic drugs in our hospital for clinical reference of rational use of hypoglycemic drugs.Methods:The number of drug categories,the total consumption sum,individual consumption sum of each category of hypoglycemic drugs in our hospital 2009-2011 were analyzed and sorted statistically.[6] Results:The consumptions sum of hypoglycemic drugs increased annually while its percentage in the total consumption sum of drugs remained the same over the past threeyears.Among the hypoglycemic drugs ,insulin rankde the first in terms of consumption sum with Novolin30R Penfill the most for the past three years.The DDDs of sulfonylurea kept ranking the first for the past twoyears.Conclusion:lnsulin,inhibitor,sulfonylurea and insulin sensitizer were the major ones in use of hypoglycemic drugs in the hospital.Meanwhile,the apparent increase of consumption sum of sulfonylurea and insulin sensitizer implies that these drugs would assume a dominant place in clinical application in the near future in our hospital. 【Key words】 Hypoglycemic Drugs Consumption Sum DDDs糖尿病(diabetes)是由遗传因素、免疫功能紊乱、微生物感染及其毒素、自由基毒素、精神因素等等各种致病因子作用于机体导致胰岛素功能紊乱、胰岛素抵抗等而引起的糖、蛋白质、脂肪、水、电解质等一系列代谢紊乱综合征。
我院2007-2009年降糖药利用分析
宋秀君;王德印;王胜利
【期刊名称】《中国执业药师》
【年(卷),期】2011(8)2
【摘要】目的:了解我院降糖药的使用情况及发展趋势.方法:对我院2007-2009年降糖药的销售金额、用药频度等进行统计、分析.结果:降糖药销售金额逐年上升,降糖药销售金额占药品总销售金额的比例逐年减小,胰岛素类销售金额增长较快,诺和灵30R的销售金额及DDDs排序连续3年位居第一.在口服降糖药中,非磺脲类促胰岛素分泌剂的销售前景较好.结论:降糖药的需求量逐年增加,胰岛素类、非磺脲类促胰岛素分泌剂、α-葡萄糖苷酶抑制剂、磺酰脲类是我院主要降糖药.
【总页数】3页(P8-10)
【作者】宋秀君;王德印;王胜利
【作者单位】内蒙古林业总医院临床药剂部,内蒙古,牙克石,022150;呼伦贝尔市人民医院办公室,海拉尔,021000;呼伦贝尔市圣力医药有限公司,海拉尔
【正文语种】中文
【相关文献】
1.我院2007-2009年口服降糖药处方的经济学及药学评价 [J], 邹明智
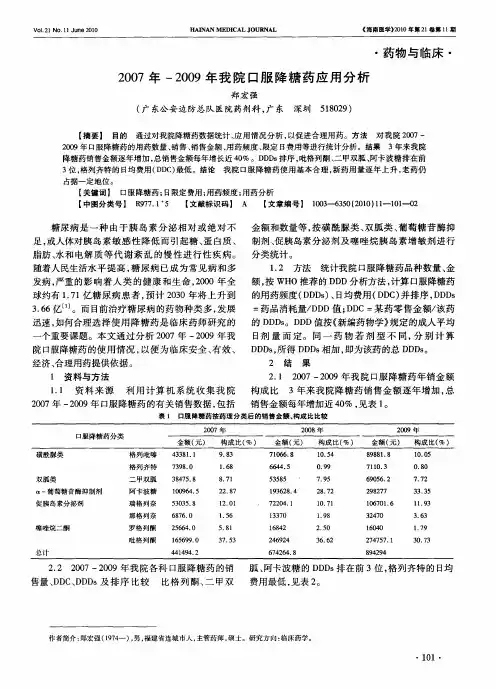
2.我院2007-2009年口服降糖药应用分析 [J], 魏钰茜;林辉
3.2007-2009年我院口服降糖药应用情况分析 [J], 钱华;李志军;吕颖
4.2007-2009年我院口服降糖药物的用药分析 [J], 王霞; 马亮英
5.我院2007-2009年口服降糖药应用情况调查分析 [J], 庄丽;许文举
因版权原因,仅展示原文概要,查看原文内容请购买。