最新核型多角体病资料指南PPT课件
- 格式:ppt
- 大小:1.22 MB
- 文档页数:7


核型多角体病毒对玉米草地贪夜蛾的控制作用研究
核型多角体病毒是一种常见的昆虫病毒,已经被广泛应用于农业生物防治之中。
在对玉米草地贪夜蛾进行控制方面,核型多角体病毒也被研究者广泛检测其控制作用。
通过实验室条件下的观察,研究者发现玉米草地贪夜蛾感染核型多角体病毒后,会出现明显的症状,包括食欲减退、活动力下降以及蜕皮异常等。
被感染的蛾体内还能明显观察到病毒颗粒的积累。
研究者对病毒感染蛾的生活史进行了研究。
实验发现,核型多角体病毒在玉米草地贪夜蛾体内可以快速繁殖,并且会对蛾的发育产生明显的影响。
被感染的蛾在幼虫期会出现发育迟缓和死亡率增加的情况,而在成虫期则会导致寿命缩短和繁殖力下降。
研究者还探究了核型多角体病毒对玉米草地贪夜蛾种群的控制效果。
实验数据显示,通过喷施病毒悬浮液可以有效降低蛾种群的密度。
在一定浓度下,病毒能够很快在种群中传播并导致大量蛾的死亡,从而有效控制蛾虫的数量。
研究者还通过田间试验验证了核型多角体病毒在实际生产中的应用潜力。
试验结果显示,通过喷施病毒悬浮液,可以明显降低玉米草地贪夜蛾对玉米的危害程度,减少虫害所带来的经济损失。
核型多角体病毒对玉米草地贪夜蛾的控制作用已经得到了全面的研究。
它不仅能够引起蛾的病征,而且能够在蛾体内繁殖并影响蛾的发育和繁殖。
核型多角体病毒还可以通过喷施悬浮液的方式在田间控制蛾虫的种群密度,并且具有广泛的应用潜力。
在今后的农业生产中,核型多角体病毒有望成为玉米草地贪夜蛾防治的重要手段之一。

V IROLOGICA S INICA, June 2007, 22 (3):0218-225Received: 2006-11-13, Accepted: 2007-01-30* Foundation items: 973 (2003CB114202); Programme Strategic Scientific Alliances between China and the Netherlands (2004CB720404); National Natural Fundation of China project (30630002)** Corresponding author. Tel/Fax: 86-27-87197180, E-mail: huzh@HUANG et al.Construction of the Bac-to-Bac System of Bombyx mori Nucleopolyhedroviru 219(BmNPV), are most widely used to express foreign proteins. More than a thousand genes have been cloned and expressed through recombinant AcMNPV and BmNPV, ranging from the components of transcription machinery to pharmaceutical products (1). However, the traditional preparation of recombi- nant baculovirus to express foreign genes is very time consuming, because multiple rounds of purification and amplification of recombinant viruses are needed. Recently, the newly developed Bac-to-Bac TM system of AcMNPV has overcome this drawback. The AcMNPV bacmid can autonomously replicate in E. coli as a large plasmid at a low copy number, and the recombinant virus can be generated by the site specific transposition in Escherichia coli (E. coli)and used to infect insect cells. Since this system eliminates multiple rounds of purification and amplification of virus, recombinant viruses can be selected and purified within 7-10 days.BmNPV, a member of the family Baculoviridae, is a natural pathogen of the mulberry silkworm Bombyx mori. Though the BmNPV genome is over 90% identical to the genome of AcMNPV (7), its host specificities is very narrow. Unlike AcMNPV which can infect more than 30 lepidopteran insects (2, 8), the BmNPV can only infect silkworm and its cell lines. Since it was first used to express α-interferon in 1985 (15), the BEVS of BmNPV has been used for the expression of many foreign proteins either in a cell culture system or in a insect larvae system (1).B. mori BEVS are particularly suitable for the large-scale manufacture of foreign proteins, as the protein expression using silkworm or pupae is 10- to 100-fold higher than from B. mori cells. Silkworms are safe to the environment, and easy to breed with low cost. There is a long history of raising silkworms in China. All these make B. mori BEVS one of the most optimal systems for mass production of recombi- nant proteins. Several B. mori BEVS have been developed in past years (5, 10,11, 26, 27), however, the applications were limited due to the time con- suming process or low infectivity of the virus to silkworm.In this report, we described the construction of a bacmid of BmNPV, BmBacJS13, using in vivo recom- bination. To study the infectivity of the bacmid, the polyhedrin gene was inserted into the bacmid generating recombinant virus BmBacJS13-ph. The infectivity of BmBacJS13-ph was demonstrated by growth curve analysis of budded viruses and bioassay in B. mori larvae. The results indicated that BmBac- JS13-ph is a functional virus with similar infectivity to that of wt BmNPV. The results also showed that infective recombinant viruses could be generated with BmBacJS13 by site specific transposition in E. coli, indicating a functional Bac-to-Bac system of BmNPV was constructed.MATERIAL AND METHODSInsect, virus and cell lineLarvae of Bombyx mori, were reared on an artificial diet at 27℃(4) and third or fifth instars larvae were used in the experiments. BmN cells were cultured in TC-100 (JRH) insect medium supplemented with 10% fetal bovine serum (GIBCO/BRL) at 28℃ using standard techniques (17).The wt BmNPV Shaanxi strain, collected in Shaanxi province, China, were propagated on silkworm larvae (3).Construction of transfer vectorAccording to the sequence of the BmNPV T3 strain (GenBank accession number L33180), a 1.5 kb segment upstream of polyhedrin gene was amplified220 V IROLOGICA S INICA V ol.22, No 3by PCR from the wt BmNPV genome DNA using primers(5’-GGGCCGCGGGCGTAGAGATTCGAC GAAAGC-3’ and 5’-GCGCTGCAGATAATTACAA ATAGGATTGAGGCC-3’) and cloned into the Sac II and Pst I sites of pBluescriptKS II+ (Stratagene). A 1.7 kb segment downstream of the polyhedrin gene was PCR amplified using forward primer (5’-GGGG AATTCCCTGAGGTAAGCGTTAGATTCTGTGCG TTTG-3’) with Eco R I and Bsu 36I sites, and reverse primer (5’-GCGGGTACCTGACGAATCGTAAATA TGAATTCTGT -3’) with Kpn I site. The product was cloned into the plasmid containing the upstream segment to generate pKS-USR-DSR. The mini-F replicon, kanamycin resistance gene and the Tn7 target sites were cut out as an 8.6 kb Bsu 36I fragment from plasmid pBAC-Bsu 36I (18) and inserted into the Bsu 36I site of pKS-USR-DSR to generate transfer vector pBmTV (Fig. 1).Recombination and identification of the bacmid in vivoNewly molted fifth instar larvae were co-trans- fected with 20 μL DNA mixture per larva (including 0.45μg linearized plasmid pBmTV DNA, 0.15 μg wt BmNPV viral DNA and 3μL lipofectin (Invitrogen)) through subcutaneous injection (25). The haemolymph of the larvae were collected at 4 days post transfection and budded viruses (BVs) were retrieved from the supernatant of haemolymph after centrifuging. TheDNA of BVs was extracted and transformed into E. coli DH10B cells (GIBCO/BRL), and colonies were selected in the presence of kanamycin and X-Gal. The colonies were further analyzed by PCR and REN digestion. One of the positive clones, BmBacJS13 which had similar REN profiles to that wt BmNPV, was chosen for further analysis. Construction of BmBacJS13-phThe Polyhedrin gene of BmNPV was amplified by forward primer (5’-GCGGGATCCTGTCGACAAGC TCTGTCCGTTT-3’) with Bam HI (underlined), and reverse primer (5’-GGGGAATTCTTAATACGCCG GACCAGTG-3’) with Eco R I (underlined). The Bam H I site of the fragment was blunted and the fragment was inserted into Bst 1107 I and Eco R I sites of pFast bac Dual (Invitrogen) to generate pFast- DUAL-ph . The pFast bac Dual-ph was then used to produce a recombinant BmBacJS13-ph via trans- position in E. coli according to the Bac-to-Bac TM system manual. The bacmid DNAs of the recombinant were extracted and were used to transfect the BmN cells. Transfection of BmN cellsBacmid DNA of BmBacJS13-ph was isolated by methods developed for large plasmids (instruction manual of Bac-to-Bac TM system/Life Technologies). The DNA was used to transfect BmN cells with lipofectin, and genomic DNA of wt BmNPV was usedas a control. The supernatant was collected from theFig. 1. Schematic of transfer vector pBmTV . The 1.5kb upstream sequence and 1.7kb downstream sequence are homologous arms,through recombination the polh was replaced with kanamycin cassette, lacZ: attTN7: lacZ and mini-F replicon.HUANG et al.Construction of the Bac-to-Bac System of Bombyx mori Nucleopolyhedroviru 221transfected BmN cells at 96 hrs post transfection (h.p.i.) and was used to infect BmN cells. The supernatants of the infected cells were collected at 96 h.p.i and TCID50 was determined using end-point dilution method.Electron microscopeBmN cells (1×106 ) were infected with wt BmNPV or BmBacJS13-ph with a multiplicity of infection (MOI) of 5. The infected cells were harvested at 72 h.p.i. The samples were processed for electron microscopy examination as described by Van Lent et al. (22).Comparison of the growth curves between wt BmNPV and BmBacJS 13-phBmN cells were infected with BV of Bm- BacJS13-ph or wt BmNPV at an MOI of 5. At the appropriate time points post infection, the supernatants were collected and the titers were detected using the end-point dilution method, Each virus infection was repeated five times and the data were statistically analyzed with one-way ANOV A (SPSS); polyhedral inclusion body (PIB) was used as the marker during the assay.BioassayThe third instars of B. mori larvae were used in oral infection assays to determine the infectivity of Bm- BacJS13-ph and wt BmNPV. The larvae (n=50 per viral dose) were fed with artificial diet which contain- ned different concentrations of viruses (3×107 PIB/ mL, 107 PIB/mL, 3×106 PIB/mL, 106 PIB/mL, 3×105 PIB/mL and 105 PIB/mL, respectively) for twenty- four hrs. Then the larvae were transferred to fresh diet in plates and reared at 27℃to investigate the mortality. The infectivity of BmBacJS13-ph and wt BmNPV was determined by probit analysis with SPSS10.0 and LC50 values of the viruses were further compared with a two side’s z-test (21).RESULTSIdentification of the bacmid BmBacJS13After the construction of transfer vector pBmTV (Fig. 1), the pBmTV DNA was co-transfected with BmNPV DNA into newly molted fifth instar larvae. The hemolymphs of the larvae were collected 4 days post transfection, and BV DNAs were retrieved to transform E. coli DH10B cells. Colonies were chosen randomly and bacmid DNAs were digested with Hin d III. By comparison with the wt BmNPV profiles, several variations were found in different colonies (data not shown). One of the colonies, BmBacJS13, which had similar REN profiles to that of wt BmNPV, was analyzed with additional restriction enzymes. As s h o wn i n F i g.2,c o m p a r i s o n w i t h t h e wt BmNPVindicates that the Xba I profile of BmBacJS13 lacked B (19.3 kb),D (15.8 kb),G (7.6 kb) and H (6.3 kb) bands, and contained five additional bands, D+H (22.2 kb), B’ (19.0kb), G’ (9.1 kb), 4.9 kb and 1.6 kb. Due to the deletion of the polyhedron gene, the B band in wt BmNPV became a shorter band B’ in BmBacJS13. The 4.9 kb and the 1.6 kb bands were from the inserted 8.6 kb Bsu36I fragment which contains three Xba I sites. The remaining Bsu36I fragment (2.1kb) was linked with the remainder of G the fragment (7.0 kb) to generate G’(9.1 kb). The D+H band in BmBacJS13, however, was a sub-molecular band of the D and H bands in the wt BmNPV. These were the expected changes for the bacmid. Therefore BmBacJS13 was constructed correctly and was used for further investigation Biological activity of BmBacJS13-ph222 V IROLOGICA S INICA V ol.22, No 3To test the oral infectivity of BmBacJS13, the poly-hedrin gene was repaired into the bacmid, generatingBmBacJS13-ph. The BmBacJS13-ph was identified tobe correct by PCR and REN analysis (data not shown),and transfected into BmN cells to generate BVs.BmN cells were infected with BmBacJS13-ph at anMOI of 5, occlusion bodies were observed in thenucleus of cells by optical microscopy from 48 hrs p.i,and the detachment of the cells were observed at thesame time. These cytopathic effect (CPE) were similarto that of wt BmNPV infected cells. Electronmicroscopy analysis showed that the occlusion bodieswere formed in the nuclei. The polyhedra and ODVsof BmBacJS13-ph had a similar shape and size asFig. 2. Xba I digestion profiles and linearized physical maps of BmBacJS13 and wt BmNPV. A. Xba I digestion profile of BmBacJS13 and wt BmNPV. 1, λ DNA digested by Bam H I, Eco R I and Hin d III; 2, BmBacJS13; 3, wt BmNPV. B. Linearized Xba I physical maps of BmBacJS13 and wt BmNPV. The restriction sites are indicated in kb from the zero point. The genome size in kb is shown on a scale at the top. The location of polh and 8.6kb Bsu36I fragment are shown.Fig. 3. EM pictures of BmN cells infected with wt BmNPV (A, B) and BmBacJS13-ph (C, D).HUANG et al.Construction of the Bac-to-Bac System of Bombyx mori Nucleopolyhedroviru 225those of wild type BmNPV (Fig. 3).One-step growth curve analysis indicated that BmBacJS13-ph BV had similar replication dynamics to that of wt BmNPV (Fig. 4). The titers of the two viruses were very similar during the early infection, but the titers of BmBacJS13-ph were slightly lower than that of wt BmNPV during 24 to 96 h.p.i. Statistical analysis indicated that the differences were not significant except those for 72 h.p.i..The bioassay result showed that LC50 of BmBac- JS13-ph against 3th larvae was 3.9x106 PIB/ml, which was not significantly different from of that of wt Bm- NPV (3.7x106 PIB/ml) (z=0.3276, P>0.05) (Table 1).DISCUSSIONA functional Bac-to-Bac system should possess the following characteristics: first, the bacmid should be able to autonomously replicate in the bacteria; second, a foreign gene can be inserted into the bacmid via transposition, and lastly, the backbone virus must have similar biological properties as the wt virus, in both cells and insects. Our study showed that BmBacJS13 could autonomously replicate in the bacteria, and a foreign gene (ph) was introduced into the Tn7 site ofFig. 4. Growth curves for BmBacJS13-ph and wt BmNPV. BmN cells were infected with BmBacJS13-ph or wt BmNPV at an MOI of 5. The supernatants were harvested at different times post infection and the BV titers were analyzed by EPDA. The average titer from five independent TCID50 assays were shown with the bars indicating standard errors. Table 1. LC50 of wt BmNPV and BmBacJS13-ph in early third instar B. mori larvae95% confidence limits VirusLC50(PIB/mL)Lower Upper wt BmNPV 3669441a 654072 28459424 BmBacJS13-ph3876462a 1400386 25047426aNo significant difference.BmBacJS13 with the aid of the helper plasmid. The recombinant virus BmBacJS13-ph had similar replication dynamics to that of wt BmNPV and bioassay results showed that its in vivo infectivity was similar to that of wt BmNPV. Therefore a functional Bac-to-Bac system of BmNPV has been constructed, and BmBacJS13 can be used to construct BmNPV recombinants for expressing foreign proteins. During our experiments, a similar Bac-to-Bac system of BmNPV was reported by Motohashia et al(16). In their system, a bacmid of the BmNPV T3 strain was constructed, and it could express enhanced green fluorescence gene (egfp) in larvae and pupae. As we have used a local strain from China (Shaanxi strain), it would be interesting to compare the properties between BmBacJS13 and the T3 bacmid.The Bac-to-Bac system can also be used for functional genomics studies of BmNPV. Bacmids have been widely used to delete genes by site-specific recombination in E. coli in AcMNPV and Helicoverpa armigera NPV (6, 9, 13, 12, 14, 19, 24, 23).. The target genes were deleted and subsequently repaired by transposition to determine the function of the genesin viral life cycle. Currently we are studying the functions of several BmNPV genes using BmBacJS13 and the results will be reported in the future. AcknowledgmentsWe thank Prof. Just M. Vlak of Wageningen University for kindly providing pBAC-Bsu36I and223222 V IROLOGICA S INICA V ol.22, No 3Prof. Chuanxi Zhang of Zhejiang University for BmN cell line. We thank Prof. Hanzhong Wang of Wuhan Institute of Virology for technical assistance. We thank the Animal Center of Wuhan Institute of Virology for providing B. mori larvae. We also thank the grants of 973 (2003CB114202), Programme Strategic Scientific Alliances between China and the Netherlands (2004CB720404), National Natural Fundation of China project (30630002) for financial support.References1.Acharya A, Sriram S, Saehrawat S. 2002. Bombyx morinucleopolyhedrovirus: molecular biology and biotech- nological applications for large-scale synthesis of recombi- nant proteins. Curr. Sci,83: 455-465.2.Adams J R, McClintock J T.1991. Baculoviridae.Nuclearpolyhedrosis Viruses. Boca Raton, FL: CRC Press, p87-204.3.Arakawa T.2002. Promotion of nucleopolyhedrovirusinfection in larvae of the silkworm, Bombyx mori (Lepidoptera: Bombycidae) by flufenoxuron. Appl En- tomol Zool, 37: 7-11.4.Choudary P V, Kamita S G, Maeda S. 1995. Baculovirusexpression protocols. Totowa: Humana press, NJ. p243- 264.5.Deng X Z, Zhu Y D, Zhen Y, et al. 2000. Construction ofa novel BmNPV Bac to Bac system. Acta MicrobiologicaSinica, 40:155-160.(in Chinese)6.Dong C S, Li D, Long G,et al. 2005. Identification offunctional domains required for HearNPV P10 filament formation. Virology, 338: 112-120.7.Gomi S, Majima K, Maeda S.1999. Sequence analysisof the genome of Bombyx mori nucleopolyhedrovirus. J Gen Virol, 80: 1323-1337.8.Granados R R, Williams, K A. 1986. In vivo infectionand replication of baculoviruses. Boca Raton, FL: CRC Press. I: p89-108.9.Hou S W, Chen X W, Wang H Z,et al. 2002. Efficientmethod to generate homologous recombinant baculovirus genomes in E. coli. Biotechniques, 32: 783-789.10.Je Y H, Chang J H, Kim M H, et al.2001. The use ofdefective Bombyx mori nucleopolyhedrovirus genomes maintained in Escherichia coli for the rapid generation ofocclusion-positive and occlusion-negative expression vectors.Biotechnol Lett,23:1809-1817.11.Kondo A, Maeda S. 1991. Host range expansion byrecombination of the baculoviruses Bombyx mori nuclear polyhedrosis virus and Autographa californica Nuclear Polyhedrosis Virus. J Virol,65: 3625-3632.12.Lin G, Blissard G W. 2002. Analysis of an Autographacalifornica nucleopolyhedrovirus lef-11 knockout: LEF-11 is essential for viral DNA replication.J Virol, 76: 2770- 2779. 13.Lin G, Blissard G W. 2002. Analysis of an Autographacalifornica multicapsid nucleopolyhedrovirus lef-6-null virus: LEF-6 is not essential for viral replication but appears to accelerate late gene transcription. J Virol, 76: 5503-5514.14.Lung O, Westenberg M, Vlak J M, et al. 2002.Pseudotyping Autographa californica multicapsid nucleo- polyhedrovirus (AcMNPV): F proteins from group II NPVs are functionally analogous to AcMNPV GP64. J Virol, 76: 5729-5736.15.Maeda S, Kawai M, Obinata H, et al. 1985. Productionof human alpha-interferon in silkworm using a baculovirus.Nature, 315:592-594.16.Motohashia T, Shimojimab T, Fukagawac T, et al. 2005.Efficient large-scale protein production of larvae and pupae of silkworm by Bombyx mor i nuclear polyhedrosisvirus bacmid system. Biochem Biophys Res Commun, 326: 564-56917.O'Reilly D R, Miller L K, Luckov V A. 1992.Baculovirus Expression Vectors. A Laboratory Manual.New York: Oxford University Press, NY. p216-229.18.Pijlman G P, Dortmans J F M, Vermeesch A M G,et al.2002. Pivotal roal of the non-hr prigin of DNA replication in the genesis of defective interfering baculovirus. J Virol, 76: 5605-5611.19.Smith G E, Fraser M J, Summers M D. 1983. Molecularengineering of the Autographa californica nuclear polyhed- rosis virus genome: deletion mutations within the polyhe- drin gene. J Virol, 46: 584-593.20.Smith G E, Summers M D, Fraser M J. 1983.Production of human beta interferon in insect cells infected with a baculovirus expression vector. Mol Cell Biol, 3: 2156-2165.21.Snedecor G W, Cochran, W G. 1989. Statistical methods.8th Edition, Iowa: Iowa State University Press, p503.22.van Lent J W M, Groenen J T M, Klingge-Roode E C,et al. 1990. Localization of the 34 kDa polyhedron224HUANG et al.Construction of the Bac-to-Bac System of Bombyx mori Nucleopolyhedroviru 225envelope protein in Spodoptera frugiperda cells infected with Autographa californica nuclear polyhedrosis virus.Arch Virol, 111: 103-114.23.Wang H Z, Deng F, Pijlman G P, et al.2003. Cloning ofbiologically active genomes from a Helicoverpa armigera single-nucleocapsid nucleopolyhedrovirus isolate by usinga bacterial artificial chromosome. Virus Research, 97: 57-63.24.Wu D, Deng F, Sun X L, et al. 2005. Functional analysisof FP25K of Helicoverpa armigera single nucleocapsid nucleopolyhedrovirus. J Gen Virol, 86: 2439-2444.25.Wu X F, Cao C P, Kumar V S, et al. 2004. An innovativetechnique for inoculating recombinant baculovirus into the silkworm Bombyx mori using lipofectin. Research in Microbiology, 155: 462-466.26.Wu X F, Cao C P, Xu Y X, et al. 2004. Construction of ahost range-expanded hybrid baculovirus of BmNPV and AcMNPV, and knockout of cysteinase gene for more efficient expression. Science in China Ser. C Life Science, 47: 406-415.27.Wu X F, Yang G Z, Hu J X. 1998. A recombinantrescue linearizable BmNPV baculovirus BmBacPAK [Patent]. China Patent: application No. 98110963.2.。


核型多角体病毒杀虫剂摘要: 随着化学农药问题的日益严重,昆虫病毒防治害虫是生物防治的一种有效方法。
本文主要介绍核型多角体病毒的形态特征、杀虫机理、核型多角体病毒的改造以及病毒杀虫剂的生产与应用。
关键词:核型多角体病毒,杀虫剂,生产与应用病毒杀虫剂是利用昆虫病毒的生命活动来控制那些直接和间接对人类和环境造成危害的昆虫。
昆虫体内普遍存在病毒,已发现的有1600多种,主要包括核型多角体病毒、颗粒体病毒、质型多角体病毒、昆虫痘病毒和非包涵体病毒,其寄主昆虫主要属于鳞翅目,少数属于膜翅目、双翅目、鞘翅目和脉翅目。
昆虫的幼虫感染病毒后容易死亡;成虫感染后不易死亡,但成为带毒者后对植物的危害会降低。
利用昆虫病毒来控制农林害虫和卫生害虫优点有:1、宿主特异性高,能杀灭害虫而不影响害虫的天敌,因此,引起害虫的再猖獗与次要害虫数量上升等的可能性较小;2、不会污染环境,对人畜安全;3、后效作用明显;4、昆虫病毒制剂生产容易、使用方便、成本低廉、适于推广。
(一)核型多角体病毒的形态特征1.1形态核型多角体病毒(NPV)含包涵体,为多角体病毒Polyhedra。
核型多角体直径:0.5~15微米(μm=μ=10-6米)病毒粒子直径:26~70毫微米,长200~400毫微米(nm=mμm=10-9米)粒子杆状,核含双股RNA被壳螺旋状。
不同的昆虫形态有所不同,如黄地老虎核多角体病毒(AsNPV)多角体大多呈六边形。
大小一般为 l.7—2.6um,为多粒包埋类型,每个病毒束内有2—7个棱衣壳,以3—4个最多见。
核衣壳为杆状,有的稍有弯曲,大小约为308nm × 52nm[3]。
扁刺蛾核型多角体病毒(TsNPV) 在透射电镜下观察多角体平面观量不规则的四边形,五边形以及少量六边形。
多角体大小不均一,为0.5~1.05um,平均直径为0.59um±0.13um。
多角体在弱戚中作用 15分钟左右时,可看到多角体内的病毒粒子随机分布,大小均一,并包埋于多角体的空膜中。


核型多角体病的综合防治近两年来,核型多角体病(即血液型脓病)在我县春、秋大蚕期频频暴发,并且发病面积大、速度快、蚕农损失重,有部分蚕农绝收,影响到蚕茧茧质,给我县蚕桑生产带来了一定的负面影响。
核型多角体病是病毒病,该病毒对不良环境包括消毒剂有较强的抵抗力,室温下存活2~3年。
蚕儿感病一般表现为皮肤紧张发亮,环节肿胀,常爬行于蚕匾四周,狂躁爬行,血液混浊呈乳白色.该病属于亚急性必传染病.蚕一旦感染后,温度高则病程短,发病快,健康壮蚕经4~6d即死亡,是养蚕消毒的主要对象。
1 核型多角体病频发的原因(1)气候因子。
如2004年晚秋期间,我县榔桥镇李园村晚秋蚕1—4龄几乎都在高温下饲养,5龄期(10月5~7日)又遇低温阴雨,平均温度仅为17.1℃,降雨0.5~6.6mm。
为给蚕加温,大多数蚕农关闭门窗,以致空气不流通,10月6一10日连续3d高温闷热,外温达31℃巳高温不仅直接导致蚕体抗病力下降,还能构成对脓病的诱发,特别是对5龄起蚕的诱发率最高,环境中只要有少量的病毒微量感染,即可导致大面积发病。
我县属亚热带,皖南山区,一般养蚕期间气温高,蚕座高温闷热,易造成蚕体虚弱。
(2)前期消毒不彻底。
我县蚕农沿用传统养蚕方法,重视蚕中消毒轻养蚕前消毒,基本不做“回山”消毒工作,并且消毒方法也不正确,目前蚕农为减少投入,有的用开水消毒,甚至没有消毒这个概念。
(3)不重视桑园病虫害的防治,桑园虫口密度大,桑叶虫口多,造成野外昆虫和家蚕交叉感染。
(4)缺乏良好饲养条件,大部分蚕户没有专用蚕室,人与蚕同处一室多,用药不当或用药剂量不足都不能有效地控制该病的蔓延,也是造成脓病暴发的一个因素,加上蚕室和住房相连及农户相互串门,互相交流养蚕情况,观看对方蚕,更增加传染机会。
(5)劳动力不足,青壮年外出务工,在家养蚕的大部分以老年人居多,老人养蚕老办法、老习惯,轻消毒,重养蚕。
如我们在下村指导蚕农进行蚕桑生产时,发现有的蚕农在大蚕期遇到低温时,在地铺上覆盖塑料薄膜,关闭门窗,天晴温度升高时又不及时去除塑料薄膜,造成蚕座小气候环境差,不适合大蚕期“大蚕风中养”的生理特性,容易造成蚕脓病的发生,在发现蚕病后,不能有针对性的用药治疗,如包合大和村竟然将医用“阿托品”来给蚕治病,榔桥李园村有些蚕农用青毒素治病等,延误了蚕病及早治疗的好时期,也促进了蚕病的发生。



同安钮夜蛾核型多角体病毒初步研究李奕震1,陈志云1,2 ,关健超1(1.华南农业大学林学院,广州510642;2.中山市林业局,广东中山528403)摘要: 对在林间导致同安钮夜蛾(Anua indiscriminata Moore)幼虫死亡的一种病原物进行了分离和鉴定,并对该病原物进行室内毒力测定,结果表明,该病原物为核型多角体病毒,初定名为同安钮夜蛾核型多角体病毒(AiNPV)。
其对同安钮夜蛾幼虫的致死浓度LC50、LC90分别为3.4×104和3.8×107PIB/mL。
关键词: 同安钮夜蛾;核型多角体病毒;桉树中图分类号:文献标识码:APreliminary Studies on Nuclear Polyhedrosis Virus of Anua indiscriminataLI Yi-Zhen1,CHEN Zhi-Yun1,2,GUAN Jian-Chao1(1.College of Forestry,South China Agricultural University,Guangzhou510642,China; 2.Forestry Bureau ofZhongshan City, Zhongshan, Guangdong 528403, China)Abstract:Isolation and identification of a pathogen from dead larvae of A. indiscriminate, and toxicity determination of the pathogen were done in the laboratory. The results showed that the pathogen was a NPV,denominated as A. indiscriminata nuclear polyhedrosis virus (AiNPV),and its LC50 and LC90 against the larvae were 3.4×104 and 3.8×107PIB/mL,respectively.Key words:Anua indiscriminata ;nuclear polyhedrosis virus;eucalyptus同安钮夜蛾是林果的一种害虫(吴荣宗,1981),以往曾对其在果树上的生物学特性进行过研究(Fujii et al.,1981),但至于其自身所带的病毒未被发现和报导。
核型多角体病毒对玉米草地贪夜蛾的控制作用研究
核型多角体病毒(nucleopolyhedrovirus,NPV)是一类以双链DNA为基因组的病毒,广泛存在于自然界中。
研究表明,NPV对玉米草地贪夜蛾(Spodoptera frugiperda)有着显著的控制作用。
玉米草地贪夜蛾是一种广泛分布于热带和亚热带地区的农业害虫。
其幼虫是玉米、大豆等农作物的重要害虫。
传统的控制方法主要采用化学农药,但长期的使用已经引发了许多问题,如农药抗性的产生和环境污染等。
寻找一种高效、环境友好的生物防治手段迫在眉睫。
通过一系列实验研究,发现NPV通过直接接触和经口摄入的方式感染和杀死玉米草地贪夜蛾幼虫。
病毒感染后,玉米草地贪夜蛾幼虫的活动力明显下降,食欲减退,并迅速出现病征。
在病征出现后,病毒会在玉米草地贪夜蛾体内迅速繁殖,最终导致害虫的死亡。
在这个过程中,NPV通过释放大量的病毒颗粒继续感染其他幼虫,从而实现对玉米草地贪夜蛾的传播和控制。
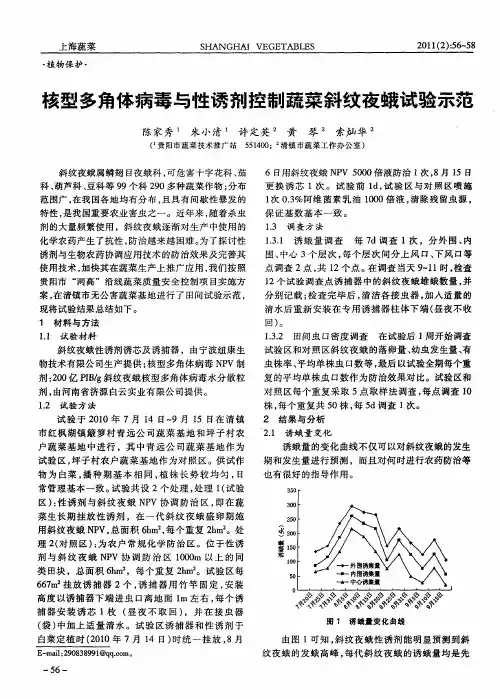
研究还发现环境因素对NPV的传播和控制作用有着重要影响。
温度、湿度和光照等因素都会影响NPV的繁殖和传播速度。
在适宜的环境条件下,NPV的感染和传播速度较快,从而更加有效地控制玉米草地贪夜蛾。