Auxin response factors
- 格式:pdf
- 大小:557.80 KB
- 文档页数:8

Mol.Plant生长素信号调控拟南芥不定根形成的分子机制生长素对植物的生长发育具有重要作用。
生长素信号转导主要是通过TIR1/AFB-Aux/IAA-ARF 信号通路来完成。
拟南芥中有29 个Aux/IAA 蛋白,是生长素信号途径的抑制因子。
生长素响应因子ARF (Auxin Response Factor)是生长素信号途径的一类重要转录因子,在拟南芥中共有23个ARF转录因子,调控下游生长素响应基因的表达。
Aux/IAAs可与ARFs形成二聚体,从而抑制 ARF 行使转录调节功能【1,2】。
生长素浓度升高时,TIR1/AFB(TRANSPORT INHIBITOR1/AUXIN-SIGNALING F-BOX PROTEIN)和Aux/IAAs互作,促进Aux/IAA蛋白泛素化和26S蛋白酶复合体介导的降解,从而把 ARF 从 Aux/IAA-ARF 二聚体中释放出来,调控下游生长素响应基因的表达【3】。
有意思的是,TIR1/AFBs对相同的Aux/IAA具有不同亲和力,会引起不同的Aux/IAA的降解速率,最终导致生长素响应的多样化。
不定根(AR,Adventitious root)的发育是一个复杂的生物学过程,受多种内源激素和环境因素的调节。
生长素是不定根发生最关键的内源激素,可与其它激素互作调控不定根的发育。
瑞典科学家Catherine Bellini研究组曾在The Plant Cell发表了一篇题为Phenotypic Plasticity of Adventitious Rooting in Arabidopsis Is Controlled by Complex Regulation of AUXIN RESPONSE FACTOR Transcripts and MicroRNA Abundance的研究论文,解析了生长素在不定根发生中的作用机制。
该研究发现了一个拟南芥不定根发生的分子调控模块。


气相色谱响应因子气相色谱是一种非常常见的化学分析技术,用于测定混合物中各组分的含量。
它是一种精确、快速、重现性好的分析方法,以及其他分析技术无法替代的优点,使它在分析化学和其他领域得到了广泛的应用。
然而,该技术受到响应因子的影响,其反映物质在某种条件下将发生多大变化,从而影响测定结果的准确性。
本文将从应因子的概念和分类,分子吸光系数,响应因子的影响因素及改善措施,响应因子的校正方法等几个方面,介绍气相色谱响应因子及其影响因素,以便更好地掌握气相色谱响应因子的影响,并为正确使用气相色谱提供参考。
一、响应因子的概念和分类气相色谱响应因子( Response factor,简称RF)又称增益因子,是指某组分在测试过程中在特定条件下所反映出的增加量,它反映了检测物在某种基础水平后,所发生的变化程度。
响应因子可以分为峰态响应因子(peak response factor)和压力响应因子(pressure response factor)两类。
峰型响应因子又分为线性响应因子(linear response factor)、边坡响应因子(slope response factor)和宽度响应因子(width response factor)。
一般情况下,线性响应因子是峰态响应因子最重要的指标,它表示每单位检测物的变化量,它反映了检测物在恒定条件下测量所发生的变化程度,它也是色谱技术中定量分析结果最重要的参数之一。
二、要素:分子吸光系数分子吸光系数(Molar absorption coefficient,简称MAC)是一种物质的光学特性,它描述了某种物质在单位时间内在一定频率处发射或吸收能量的能力,它与物质的光谱特性密切相关,它可以定量反映出检测物吸收和发射光能量的能力,从而影响最终结果。
与响应因子相比,它反映的是检测物本身的光学特性,而不是受检物质的变化情况,因此,它的值可以影响最终的结果,从而影响高精度的测定结果。

普通野生稻miR160f的克隆和功能分析杨松楠;王姣;陈宗祥;田新杰;张静文;龙艳;裴新梧;袁潜华【摘要】MicroRNAs are a class of non-coding RNAs involved in post- transcriptional control of gene expression. Former study on Arabidopsis thaliana reveals that miR160 involves in root cell division and differentiation by regulating auxin response factors(ARF)thus influence the root development. This study cloned miR160f gene from common wild rice and transferred intoArabidopsis thaliana to identify its function. The results showed that over-expression of miR160f decreased the number of rosette leaves, shortened bloting time, leading to early flowering. RT-PCR showed the expressions of gene ARF10,ARF16 and ARF17 are down-regulation caused by miR160f in transgenic Arabidopsis thaliana, while the deficiency of ARF10 and ARF16 protein can inhibit root cap cell differentiation, lose control of cell division and lead to ectopic expansion of apical stem cell populations Therefore, the result demonstrates that miR160 not only influence on root development, but also may influence flowering time of common wild rice.%MicroRNAs是一类在调节基因转录后表达中起重要作用的非编码RNA。

作物学报 ACTA AGRONOMICA SINICA 2015, 41(11): 1621-1631/ISSN 0496-3490; CODEN TSHPA9E-mail: xbzw@本研究由高等学校全国优博博士学位论文作者专项(6J1153)和中央高校基本科研业务费专项(KYY201301)资助。
*通讯作者(Corresponding author): 陈赛华, E-mail: saihuachen@, Tel: 025-********第一作者联系方式: E-mail: hongxiaoxd@Received(收稿日期): 2015-04-25; Accepted(接受日期): 2015-07-20; Published online(网络出版日期): 2015-08-05. URL: /kcms/detail/11.1809.S.20150805.0926.016.htmlDOI: 10.3724/SP.J.1006.2015.01621水稻株型突变体rad-1和rad-2的鉴定与功能基因克隆牛 静 陈赛华* 赵婕妤 曾召琼 蔡茂红 周 亮 刘 喜 江 玲 万建民南京农业大学作物遗传育种与种质创新国家重点实验室, 江苏南京 210095摘 要: 株型是决定水稻等作物产量的核心因素之一, 是品种选育的重要指标。
本研究从粳稻品种Asominori 的辐射诱变中分离出2个稳定的株型突变体, rad-1和rad-2, 它们均表现出苗期弯曲生长、成熟期株高矮化、粒长变短、千粒重降低和产量下降等特征。
等位性测验结合连锁分析证实rad-1和rad-2等位, 且位于水稻第7染色体上约230 kb 的范围内。
对定位区段的序列分析后确定OsFH5为候选基因, 该基因呈现组成型表达, 编码水稻II 型成蛋白。
突变体rad-1在OsFH5基因第2个外显子上缺失8个碱基, 导致移码; 而rad-2则在第14内含子上发生单碱基的变异, 发生异常剪切。
头颈部鳞癌的研究趋势及新进展张洪瑞,苏本香,庞艳,孙月茹呼伦贝尔职业技术学院口腔教研室,内蒙古呼伦贝尔021000[摘要]头颈部鳞癌(Head and Neck Squamous Cell Carcinoma, HNSCC)为发生于口腔、咽、喉黏膜上皮的鳞癌,吸烟、酗酒与促进口腔及喉部鳞癌形成存在相关性,咽部鳞癌与人类乳头状瘤病毒感染有关。
头颈部鳞癌有多种治疗方式,特别是以分子靶向治疗为代表的药物治疗快速发展。
本文具体探讨与分析了头颈部鳞癌的流行病学状况,阐述了头颈部鳞癌的形成机制,综述了头颈部鳞癌的分子靶向治疗进展:西妥昔单抗、免疫检查点分子抑制剂、贝伐珠单抗。
[关键词]头颈部鳞癌;分子靶向治疗;免疫检查点分子抑制剂;贝伐珠单抗;西妥昔单抗[中图分类号]R739.91 [文献标识码]A [文章编号]2096-1782(2024)01(b)-0194-05 Research Trends and New Advances in Head and Neck Squamous Cell CarcinomaZHANG Hongrui, SU Benxiang, PANG Yan, SUN YueruStomatology Department, Hulunbuir Vocational Technical College, Hulunbuir, Inner Mongolia Autonomous Region, 021000 China[Abstract] Head and neck squamous cell carcinoma (HNSCC) is a squamous carcinoma that occurs in the mucosal epithelium of the oral cavity, pharynx and larynx. Smoking and alcohol abuse are associated with the formation of squa‐mous carcinoma of the oral cavity and larynx, and squamous cell carcinoma of the pharynx is associated with human papillomavirus infection. There are various treatment modalities for head and neck squamous cell carcinoma, espe‐cially the rapid development of drug therapy represented by molecular targeted therapy. This article specifically dis‐cusses and analyzes the epidemiological status of head and neck squamous cell carcinoma, describes the formation mechanism of head and neck squamous cell carcinoma, and reviews the progress of molecular targeted therapy for head and neck squamous cell carcinoma: cetuximab, immune checkpoint molecular inhibitors, and bevacizumab. [Key words] Head and neck squamous cell carcinoma; Molecular targeted therapy; Immune checkpoint molecular inhibitors; Bevacizumab; Cetuximab头颈部鳞癌(Head and Neck Squamous Cell Car‐cinoma, HNSCC)是一组起源于口腔、咽、喉的鳞状上皮细胞的异质性肿瘤,当前其发病率逐年增加,且病死率一直居高不下[1]。
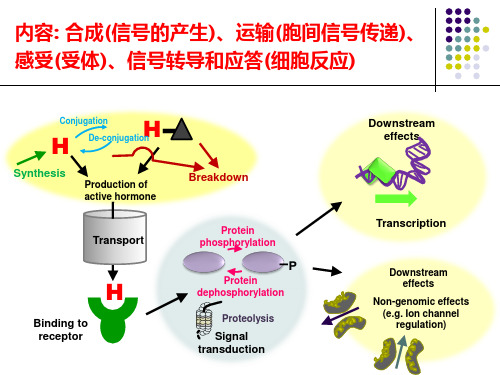
生长素受体1. 引言生长素是一种植物激素,它在调节植物生长和发育过程中起着重要作用。
而生长素受体则是植物细胞表面的蛋白质,起到接收和传递生长素信号的功能。
本文将详细介绍生长素受体的定义、结构、功能以及调控机制。
2. 定义生长素受体是指存在于植物细胞膜上的蛋白质,能够与生长素结合并转导信号,从而调节植物的生长和发育过程。
它是生长素信号传导途径中的关键组成部分。
3. 结构3.1 蛋白质组成生长素受体主要由两个部分组成:外膜域和内膜域。
外膜域通常由一个或多个结构域组成,其中包括了能够与生长素结合的结构域。
这些结构域通常包括了一个可变区域(V区)和一个保守区域(C区)。
可变区域决定了受体对不同种类的生长素的亲和力,而保守区域则在结构上保持相对稳定。
内膜域则包括了一个或多个信号转导区域,这些区域能够将生长素信号传递到细胞内部的下游信号分子。
3.2 结构多样性生长素受体在不同植物物种中存在着结构多样性。
这种多样性主要表现在外膜域的结构上。
不同植物中的生长素受体可能有不同数量的外膜域结构域,也可能有不同类型的结构域。
这种多样性使得不同植物对于生长素的响应方式也存在差异。
4. 功能4.1 生长素信号传导生长素受体在接收到生长素分子后,通过与其结合来启动信号传导过程。
当生长素分子与受体的外膜域结合时,会引发一系列的化学反应,最终将信号传递到细胞内部。
这些化学反应包括了受体构象的变化、酶活性的调节以及下游信号分子的磷酸化等。
4.2 调节植物生长和发育通过生长素受体传递的信号,植物能够调控自身的生长和发育过程。
生长素受体在根系发育中起到重要作用,能够影响根的伸长、分支和侧根的形成等过程。
生长素受体还参与了植物的光合作用、开花时间和果实发育等重要生理过程。
5. 调控机制5.1 内源激活剂内源激活剂是指能够促进生长素受体活性的化合物。
这些化合物能够与受体结合,并增强其对生长素的亲和力,从而增加信号传导效率。
一些内源激活剂包括了天然存在于植物中的化合物,如蛋白激酶、磷脂等。
植物特异性转录因子的功能及调控机制植物特异性转录因子(Plant-specific transcription factors,PSTFs)是植物中一类重要的调控因子,能够调控植物的生长发育和对外界环境的响应。
PSTFs是以不同方式反应于离子、光、水、温度和生物逆境等方面,调节植物的基因表达。
本篇文章将就植物特异性转录因子的功能及调控机制展开探讨。
一、PSTFs的功能PSTFs的功能多样,包括调节植物的生长和发育、响应逆境和调控植物的代谢等。
下面将逐一介绍。
1. 调节植物的生长和发育PSTFs参与了不同阶段的植物生长和发育过程。
例如,在植物的花器官发生中,转录因子AGAMOUS-LIKE6(AGL6)会促进花序和芽的形成;在花的器官分化阶段,APETALA2(AP2)调控花瓣和雄蕊的发育。
此外,PSTFs还参与了叶片生长和根发育的调节。
例如,GRF(Growth Regulating Factor)家族的成员在促进芽和叶的增长方面发挥了重要作用;ARF(Auxin Response Factor)家族的成员则调控了根系统的生长和分化。
2. 响应逆境植物在逆境环境下如何应对是植物学研究的一大热点。
PSTFs在这个过程中发挥了重要作用。
例如,DREB1(Dehydration-responsive element binding protein 1)调节了植物对于干旱、高盐和低温等逆境的响应。
此外,PSTFs在与植物抗病、旱灾、滞水和盐胁迫等逆境方面都具有一定的调控作用。
3. 调控植物代谢PSTFs还能调控植物的代谢,影响植物在不同环境下的适应性。
例如,在水稻中,SNAC1(Stress-responsive NAC1)家族的转录因子促进了水稻对于恶劣环境的适应性,同时也提高了水稻穗粒的产量。
二、调控机制PSTFs的表达受到多个调控机制的影响,包括转录水平和翻译后水平。
下面将对它们的调控机制分别进行介绍。
植物激素信号传导机制植物激素在调控生长发育过程中起着至关重要的作用。
植物激素通过特定的信号传导机制,调整细胞内的基因表达和蛋白质合成,最终影响植物的生长、开花、果实发育以及应对外界环境的适应性。
本文将探讨植物激素的主要类型及其信号传导机制。
一、赤霉素(gibberellin)信号传导机制赤霉素是一种重要的植物激素,参与了植物的茎长增长、种子萌发和果实发育等过程。
赤霉素信号的传导主要通过赤霉素受体和负调控子来实现。
赤霉素受体属于核受体超家族,当赤霉素结合到受体上时,受体会解离出负调控子并进入细胞核,与DNA结合,促进某些特定基因的转录。
此外,赤霉素信号还通过激活多种转录因子和底物的降解来调控细胞的代谢过程。
二、生长素(auxin)信号传导机制生长素是植物生长中重要的激素,参与了茎的伸展、根的生长以及植物对重力和光的感知。
生长素的信号传导主要通过TIR1/AFB(生长素信号感受机制的部分受体)介导的生长素介导降解途径实现。
当生长素结合到TIR1/AFB受体复合物上时,生长素与这个复合物一起结合到AUX/IAA蛋白上,导致AUX/IAA蛋白的降解。
AUX/IAA 蛋白降解后,转录因子ARF(auxin response factor)可以结合到基因的响应元件上,调控下游基因的转录。
三、脱落酸(abscisic acid)信号传导机制脱落酸是植物的重要激素,参与了植物对胁迫环境的响应、种子休眠和水分平衡等过程。
脱落酸信号传导主要通过PYR/PYL蛋白介导的抑制型拮抗机制实现。
脱落酸在存在的情况下,可以与PYR/PYL蛋白结合形成复合物。
复合物的形成使得PP2C蛋白(脱落酸信号的负调控子)失活,无法抑制SnRK2激酶的活性。
活化的SnRK2激酶可磷酸化并激活其靶标基因,从而调控脱落酸信号下游的适应性响应。
四、细胞分裂素(cytokinin)信号传导机制细胞分裂素是植物激素中的重要成员,参与了植物的细胞分裂和植物器官的形成。
Auxin response factorsTom J Guilfoyle and Gretchen HagenAuxin signaling is key to many plant growth and developmental processes from embryogenesis to senescence.Most,if not all, of these processes are initiated and/or mediated through auxin-regulated gene expression.Two types of transcription factor families are required for controlling expression of auxin response genes.One of these,the auxin response factor(ARF) family,functions by binding to auxin response elements (AuxREs)on promoters of auxin response genes,activating or repressing the auxin response genes,and recruiting a second family of transcription factors,the Aux/IAA repressors,that confer an auxin response to the genes.Recent advances have provided information on regulation of ARF gene expression, ARF roles in growth and developmental processes,and target genes regulated by ARFs.AddressesUniversity of Missouri,Department of Biochemistry,117Schweitzer Hall,Columbia,MI65211,USACorresponding author:Guilfoyle,Tom J(guilfoylet@)Current Opinion in Plant Biology2007,10:453–460This review comes from a themed issue onCell Signalling and Gene RegulationEdited by Jian-Kang Zhu and Ko Shimamoto1369-5266/$–see front matter#2007Elsevier Ltd.All rights reserved.DOI10.1016/j.pbi.2007.08.014IntroductionAuxin response factors(ARF)are transcription factors that regulate the expression of auxin response genes [1,2].ARFs bind with specificity to TGTCTC auxin response elements(AuxRE)in promoters of these genes and function in combination with Aux/IAA(auxin/indole acetic acid)repressors,which dimerize with ARF activa-tors in an auxin-regulated manner.In the last few years,a considerable amount of new information has appeared on the regulation of ARF gene/protein expression,the func-tions of ARFs in plant growth and development,the target genes controlled by ARFs,and the mechanisms by which ARFs regulate target genes.In this review,we focus on recent literature that has provided insight into roles played by ARFs in regulating a variety of plant processes and the mechanisms involved in this regulation. Modular structure of ARF proteinsARFs,like many transcription factors,contain modular or portable domains that can function independently of one another.Most ARFs consist of an amino-terminal DNA-binding domain(DBD),a middle region that functions as an activation domain(AD)or repression domain(RD), and a carboxy-terminal dimerization domain(CTD)[1,2] (Figure1).The ARF DBD is classified as a plant-specific B3-type,but requires additional amino-terminal and car-boxy-terminal amino acids for efficient in vitro binding to TGTCTC AuxREs[1,3].B3-type DBDs are also found in a variety of plant transcription factors that function out-side of the auxin response pathway[1].An NMR solution structure for the RAV1(RELATED TO ABI3/VP11)B3 DBD has been determined,and homology modeling was applied to the B3DBD of ARF1[4].The B3domain of both proteins is related to the DBD found in the Eco RII restriction endonuclease and consists of a seven-stranded open b-barrel with two a-helicesflanking the barrel.The modular nature of ARF DBDs has been documented by carrying out domain swap experiments with the DBDs of an ARF repressor and an ARF activator[2].Five Arabidopsi s ARFs,ARF5-8and19,function as transcriptional activators when tested on auxin response genes in transfected protoplasts[2,5–7,8 ].The remain-der of the Arabidopsis ARFs that have been experimen-tally tested in transfected protoplasts function as transcriptional repressors[2,5].Because the tests for activation and repression with ARF family members have relied upon transient expression assays in leaf mesophyll or suspension cell culture protoplasts,it remains possible that an ARF classified as a repressor could function as an activator or an ARF classified as an activator could func-tion as a repressor in certain cell types or environments. The ARF ADs and RDs are located just carboxy-terminal to the DBDs and contain biased amino acid sequences. ARF ADs are enriched in glutamine along with serine and leucine residues,while ARF RDs are enriched in serine, proline,leucine and glycine residues[1](Figure1).The identification and portable nature of ARF ADs and RDs were demonstrated by fusing the ARF middle regions to yeast GAL4DBDs and testing the chimeric proteins in transfected protoplasts with minimal or constitutive pro-moter:GUS reporter genes containing GAL4DNA-bind-ing sites[2,5,6].All Arabidopsis ARFs,with the exception of ARF3,13,and 17,contain a CTD that is related in amino acid sequence to domains III and IV in Aux/IAA proteins[1,9 ](Figure1). While the ARF13gene and cloned cDNAs contain sequence predicted to encode a CTD[1],the CTD sequence in the mRNAs is out of frame with the sequence encoding the predicted DBD and RD[9 ].The ARF CTD is modular,based on results showing that it functions as adimerization domain in yeast two-hybrid assays with selected ARF CTDs and Aux/IAA proteins[6,10–12]as well as in protoplast transfection assays[2,5,6].ARF acti-vators with a CTD truncation activate auxin response genes constitutively in transfected protoplasts,suggesting that ARF activators are targeted to AuxREs in an auxin independent manner,that the CTD is not required for ARF DNA-binding or activation,and that an auxin response requires the ARF CTD[2,7].ARF genes in Arabidopsis and riceThe availability of annotated Arabidopsis and rice gen-omes makes it possible to compare the ARF gene num-bers and similarities between a dicot and a monocot plant. Arabidopsis contains22full-length ARF genes and one partial-length gene(ARF23)with a stop codon in its DBD [1,9 ,13](Figure1).ARF23may be a pseudogene,but if it is expressed,the ARF23gene product might function by interfering with DNA targeting of other ARF proteins,as has been suggested for some ARF mutations that are predicted to encode proteins truncated in their DBDs [14–17].The23ARF genes are distributed on allfive chromosomes,but chromosome1contains13of these genes,including a cluster of seven closely related genes (ARF12-15,20-22)as well as ARF23.The cluster of ARF genes is located near the centromere and may have originated from a recent series of tandem duplications [13].In general,Arabidopsis ARF genes fall into related sister pairs,including ARF1and2,3and4,6and8,7and 19,11and18,or triplets in the case of ARF10,16,and17 [13].Rice(Oryza sativa)contains25ARF loci(i.e.OsARF1to OsARF25going from the top of chromosome1to the bottom of chromosome12)located on10of12chromo-somes[18].OsARFs are,for the most part,related to Arabidopsis ARFs and fall into sister pairs like Arabidopsis ARFs.Interestingly,there appear to be no orthologs in rice that correspond to the ARF gene cluster on Arabi-dopsis chromosome1,suggesting the possibility that these genes were lost from rice after the divergence of mono-cots and dicots or arose in dicots or a subset of dicots following the divergence.OsARF20,which is predicted to encode a protein with two B3-type DBDs,may be a pseudogene since transcripts for this gene were not detected.Based upon the glutamine-rich middle regions in the putative OsARF proteins,nine OsARFs are pre-dicted to function as activators and the remainder as repressors.Regulation of ARF gene and ARF protein expressionBecause there are22Arabidopsis ARF genes that encode proteins with full-length DBDs that may recognize and compete for TGTCTC target sites in promoters of auxin response genes[1–3],it becomes interesting to determine when and where the genes are expressed and what regulates their expression.This obviously involves tran-scriptional regulation,but recent reports suggest that posttranscriptional regulation may play an important role in controlling the levels of different ARF proteins in various cells and tissues.Low resolution northern and RT-PCR analyses of seed-lings or adult plants suggest that Arabidopsis and rice ARF genes are,in general,transcribed in a wide variety of tissues and organs[3,9 ,18].An apparent exception is expression of genes within the ARF gene cluster on Arabidopsis chromosome1which appears to be restricted to embryogenesis/seed development[9 ].When higher resolution methods like ARF promoter:reporter genes or in situ hybridization are used to assess ARF gene expres-sion patterns,however,highly specific and/or dynamic patterns of gene expression have been observed for ARF1 in developingflowers[19],ARF2in developingfloral organs as well as in light-grown and dark-grown seedlings, [17,19–21],ARF3and4in developing reproductive and vegetative tissues[16,22],ARF5in developing embryos and vascular tissues[6,23,24 ,25],ARF6in developing454Cell Signalling and Gene RegulationFigure1The ARF family of transcription factors in Arabidopsis.The ARFfamily consists of five transcriptional activators(ARF5–8and19)with an AD that is enriched in glutamine(Q),serine(S),and leucine(L) [1,2,5–7,8 ].The remainder of the ARF family is thought to consist of transcriptional repressors with an RD that is usually enriched in serine (S)and in some cases proline(P),leucine(L),and/or glycine(G); however,to date,only ARF2–4and9have been shown experimentally to function as repressors and contain an active RD in plant protoplast transfection assays[2,5,7,8 ].All of the ARFs contain a conserved DBD;however,the DBD in ARF10,16,and17contains an additional 32–36residues(indicated in blue within the DBD),and ARF23 contains only a truncated DBD.ARF3,13,and17lack the conserved CTD found in most ARFs.The ARF13gene locus(Q9FX25)contains a CTD sequence similar to other genes in the chromosome1cluster [1],and full-length cDNA clones contain a complete CTD sequence, but it is out of frame,resulting in an ARF13protein that apparently lacks the CTD(At1g34170)[9].flowers[26,27],ARF7in seedlings,roots,and developing embryos[6,8 ,9 ],ARF8in seedlings and developing flowers and fruits[15,26–28],ARF12in developing seeds [9 ],ARF16in the basal region of embryos,root caps, vascular tissue of roots,and leaves[29],and ARF19in seedlings and roots[8 ,9 ,30].In some cases,the expression of ARF genes has been shown to respond to environmental or hormonal signals. ARF2,7,and19transcripts increased moderately,and ARF1transcripts decreased slightly in response to dark-induced senescence in leaves[19].Arabidopsis ARF8gene expression in seedlings increased in response to light[15], while expression of several OsARF genes was slightly downregulated by light[18].Arabidopsis ARF4,5,16, and19and rice OsARF1and23transcripts in seedlings increased slightly in response to auxin[8 ,9 ,18,25,29,31]or both auxin and ethylene for ARF19[30],while OsARF5,14, and21decreased marginally in response to auxin[18].The auxin-responsiveness of the Arabidopsis ARF19gene is thought to involve ARF7and19activators on AuxRE target sites within the ARF19promoter[8 ,9 ].Most responses of ARF genes to hormones or environmental factors that have been reported appear to be small or negligible[3,9 ],suggesting that unidentified factors must play key roles in regulating expression of these genes or regulation by hormones and environmental factors is highly specific to selected cell and tissue types or developmental programs[25].There is a growing body of information on posttranscrip-tional regulation of ARF transcript abundance by micro-RNAs(miRNA or miR)and trans-acting-small interfering RNAs(ta-siRNA).These small(i.e.21–24nucleotide) endogenous RNAs direct the cleavage of complementary mRNA targets,including a number of ARF transcripts, and target sites are widely conserved in dicots and mono-cots.ARF6and8are targets for miR167,and ARF10,16, and17are targets for miR160[32].ARF2,3and4are targets for TAS3ta-siRNAs or tasiR-ARF[33,34]. Mutations that interfere with miRNA and ta-siRNA production or target sites result in a number of dramatic phentoypes[27,29,35–38,39 ].Regulation of ARF6and8 by miR167is important for development of anthers and ovules[27].Regulation of ARF17by miR160is important for several aspects of plant growth and development,at least some of which appear to be dependent on GH3gene expression and auxin homeostasis[40,41],and regulation of ARF10and16by miR160plays a role in root cap formation[29].The regulation of ARF3and4targets by TAS3ta-siRNAs is required for proper leaf development [38,42]and juvenile to adult phase changes or hetero-blasty[36,37,39 ].It is not obvious why some ARF transcripts,like ARF3 and4,are targeted by ta-siRNAs while others like ARF6, 8,10,16,and17are targeted by miRNAs.One possibility is that miRNAs may be restricted to cells in which they are expressed or to nearby cells where they regulate expression of ARFs locally,whereas ta-siRNAs might travel through the plant to cells or tissues where they regulate ARFs at a distance or throughout the plant(e.g.in juvenile to adult phase changes)[37,38].It is also not entirely clear why posttranscriptional regulation of ARF mRNA levels seems to be at least as important as tran-scriptional regulation of ARF genes.One suggestion is that cleavage of ARF transcripts by miRNAs and ta-siRNAs would provide for rapid removal of the ARF transcription factors from cells immediately following signaling events[43].Direct targeting of ARF mRNAs for destruction would presumably result in more immedi-ate clearance of ARF transcription factors from cells than shutting down transcription and allowing the mRNAs to decay by normal pathways.Two additional modes of ARF posttranscriptional regu-lation have been reported.In one case,several ARF mRNAs contain upstream open reading frames(uORFs) that are located in the50leader sequence[28,44].The uORFs in ARF3and5have been shown to impede translation of the major ORF in transfected protoplasts and in an in vitro translation system[44].In a second case, ARF2protein levels were regulated positively by light and negatively by ethylene,and the decrease in ARF2 protein levels in response to ethylene was mediated by the ubiquitin–proteasome pathway[20].Roles of ARFs in growth and development Most of what is known about the roles that ARFs play in plant growth and development has been revealed by studies on arf mutants.Classical genetic approaches led to the identification of mutations for arf2,which have defects in apical hook formation in etiolated seedlings and have increased seed size[17,20],arf3/ettin(ett),which have a loss of abaxial identity in the gynoecium[22],arf5/ monopteros(mp),which show defects in embryo devel-opment and vascular tissue formation[23],arf7/nonphoto-tropic hypocotyl4(nph4),which have defects in hypocotyl tropisms and resistance to auxin and ethylene[14,30], arf8,which uncouple fruit development from fertilization [28],and arf19,which show insensitivity to auxin and ethylene[30].Screens for T-DNA insertion mutations have identified mutations in at least18of the Arabidopsis ARF genes,but few of these have distinctive growth or developmental phenotypes beyond those genes ident-ified by forward genetic approaches[8 ,9 ,15,19–21,26,29].Double mutants for ARF sister pairs[13]generally have much stronger phenotypes than the single mutants, suggesting that related ARFs have somewhat redundant roles in Arabidopsis.arf2single mutants have delayed flowering,leaf senescence,andfloral abscission as well as defective apical hook formation,and in arf1arf2double Auxin Response Factors Guilfoyle and Hagen455mutants,these phenotypes are enhanced[9 ,19,20]. While arf3single mutants only display defects in abaxial identity of the gynoecium,arf3arf4double mutants have reduced abaxial identity in all lateral organs,including leaves[16].Embryo patterning and vasculature defects observed with arf5mutants are enhanced in arf5arf7 double mutants[6].Both arf6and arf8single mutants have delayedflower maturation and reduced fertility, while arf6arf8double mutants haveflower arrested de-velopment before bud opening and are completely infer-tile[26].arf7and arf19single mutants have slightly reduced adventitious and lateral root numbers,but arf7 arf19double mutants have strikingly reduced numbers of adventitious and lateral roots[8 ,9 ].While no phenotypic defects were reported for arf10or arf16single mutants [9 ],arf10arf16double mutants have root cap defects and abnormal root gravitropism[29].Target genes for ARF transcription factors Extensions of the phenotypic analysis of arf mutants described above have been directed at identifying the genes that ARFs target to initiate or maintain growth and developmental processes.Identification of potential tar-get genes that are under the direct transcriptional control of ARF transcription factors has involved a number of methods,including differential hybridization,protoplast transfection assays,transcript profiling using microarrays of auxin-induced genes,as well as microarrays from auxin signaling mutants including arf and aux/iaa mutants [6,7,8 ,9 ,14,15,21,26,29,40,41,45–51,52 ].From these analyses,a number of genes that are either induced or repressed have been identified.Within these gene sets, those containing the AuxRE sequences TGTCTC or GAGACA in their promoter regions have been of particu-lar interest,because these represent potential binding sites for the ARF proteins.Microarray analysis,northern analysis,and protoplast transfection assays with arf7/nph4mutants,suggest that ARF7plays a major role in regulating a wide variety of early/primary auxin response genes in Arabidopsis seed-lings[7,8 ,9 ,14].In microarray analyses with arf7and arf7 arf19mutants,most of the responsive genes appeared to be activated by ARF7,but a small number appeared to be repressed,implying that ARF7could function as both an activator and repressor[9 ].This type of microarray analysis with arf mutants does not,however,distinguish direct from indirect effects or whether a gene is or is not a direct target of an ARF transcription factor,making it difficult to conclude whether or how an ARF transcription factor functions on a potential target gene.ARF5and19 appear to be involved in activating expression of some of the same Aux/IAA genes(e.g.IAA1and19)that are activated by ARF7[6,8 ,9 ,45].Infloral tissues,arf6 arf8double mutants display reduced expression of a SAUR(small auxin-up RNA)clade(SAUR62–SAUR67) of auxin response genes,as well as a subset of Aux/IAA genes,suggesting that ARF6and8may activate at least some auxin response genes in reproductive organs[26].A subset of the auxin-inducible GH3genes(e.g.GH3.5and GH3.6)are candidate targets for ARF8and17,and these genes are thought to be activated by ARF8and repressed by ARF17[15,40,41].Expression of the GH3.6gene is also down regulated in an arf7mutant and further reduced in an arf7arf19double mutant[9 ],suggesting that this auxin response gene could be a target for at least three ARF activators and one ARF repressor.That different ARFs can potentially target the same auxin response genes in planta is consistent with results using protoplast transfection assays[2,5,7,8 ]and in vitro binding of ARF DBDs to the same TGTCTC AuxREs[3].A few of the plant-specific ASYMMETRIC LEAVES2/ LATERAL ORGAN BOUNDARIES(ASL2/LOB)gene family members have been identified as putative ARF targets in rice[51]and Arabidopsis[9 ,52 ].Results with ASL2/LOB genes in Arabidopsis provide the most convin-cing evidence to date that a specific ARF(i.e.ARF7) regulates expression of selected auxin response genes [52 ].These results showed that ARF7binds to TGTCTC AuxREs in the promoters of two ASL2/LOB genes in vitro,and that a steroid hormone-inducible form of ARF7in an arf7arf19mutant line of Arabidopsis activated the expression of the ASL2/LOB genes in an auxin-dependent manner.While providing strong sup-port for ARF7regulating the expression of the two ASL2/ LOB genes in Arabidopsis,these results fall short of demonstrating direct regulation of the two genes by ARF7,and proof will require chromatin immunoprecipi-tation(ChIP)or some other assay that demonstrates ARF7resides on AuxREs in the promoters of the genes when they are activated in response to auxin. Molecular mechanisms involved in ARFs’regulation of gene expressionARF transcription factors by themselves do not appear to regulate genes in response to auxin,but require the association of Aux/IAA repressors for an auxin response [1,2](Figure2).Neither ARF targeting to AuxREs through the ARF DBD nor activation of genes through the ARF AD seems to be responsive to auxin[2,5].Most, if not all,of an auxin response is dependent on the ARF CTD[2,5,7].This,of course,is not surprising since Aux/ IAA repressors can dimerize with ARF CTDs to bring about repression of auxin-response genes.In light of the evidence that Aux/IAA repressors are rapidly degraded in an auxin dose-dependent manner upon their recruitment to TIR1(TRANSPORT INHIBITOR RESISTANT1) or TIR1-like auxin receptors[53],the auxin response appears to be mediated mostly,if not exclusively,via association and dissociation of Aux/IAA repressors from ARF activators that reside on auxin response gene pro-moters.On the surface,auxin signaling to activate genes appears to be streamlined and simple,involving only456Cell Signalling and Gene Regulationauxin transport into cells,auxin binding to TIR1recep-tors,recruitment of Aux/IAA repressors to auxin-occupied TIR1receptors,subsequent destruction of Aux/IAA repressors by the ubiquitin–proteasome pathway,and the resulting activation/derepression of auxin response genes through ARF activators.Although surprisingly uncomplicated mechanistically, based on the above model,regulation of auxin response gene expression must be highly complex because of the large family of ARF proteins that might compete for AuxRE target sites as well as the potential interactions of ARFs with themselves and with the similarly large family of Aux/IAA repressors(i.e.29family members in Arabidopsis)(Figure2).It remains unclear whether Aux/ IAA repressors interact with ARF repressors or whether ARF repressors interact with ARF activators to regulate gene expression in planta.Results from yeast two-hybrid and plant protoplast assays suggest,however,that ARF repressor-Aux/IAA repressor and ARF repressor-ARF activator interactions are much weaker than ARF activator-Aux/IAA repressor interactions and ARF activa-tor-ARF activator interactions[2,6].Furthermore,it is difficult to visualize the consequences of ARF repressor-Aux/IAA repressor interactions on an auxin response gene. It seems more likely that ARF repressors may compete with ARF activators in a concentration-dependent manner for AuxRE binding sites in promoters,and if an ARF repressor is bound to an AuxRE,repression would occur independently of Aux/IAA repressors,providing another level of gene regulation that would be independent of auxin.In any case,there would still befive ARF activators that could potentially interact with29Aux/IAA repressors as well as with themselves to regulate auxin-responsive gene expression in Arabidopsis(Figure2).Elucidating the in planta targeting of ARFs to AuxREs and the interactions among the ARFs and Aux/IAA repressors at cellular resol-ution is a major challenge for the future and will require experimental approaches similar to those recently carried out with ARF5and IAA12[24 ].Molecular mechanisms involved in the activation or repression of genes by ARFs or repression by Aux/IAA proteins are almost entirely unknown.There are a couple of candidate co-regulators that may interact with ARFs or Aux/IAA repressors in regulating expression of auxin response genes.PICKLE(PKL)-mediated chromatin remodeling has been implicated for genes activated by ARF7and19by bringing about repression through IAA14 [54].The PKL protein is predicted to be a component of an ATP-dependent chromatin-remodeling complex, which may be associated with histone deacetylases and function as a co-repressor.At this point,however,how PKL functions in Arabidopsis is unknown.SEUSS(SEU) appears to function by directly interacting with ARF3in promotingfloral organ growth and patterning[55].SEU is related to a family of transcriptional co-regulators that,at least in some cases,function in complexes as co-repres-sors or co-activators.Because SEU might function in either activation or repression,it is possible that by binding to ARF3,SEU could activate rather than repress genes targeted by ARF3.It will be interesting in future studies to determine whether chromatin modifying com-plexes(e.g.histone acetylases,histone deacetylases, Auxin Response Factors Guilfoyle and Hagen457Figure2Models for transcriptional activation and repression by ARFs.The ARFs are color-coded for the DBD,AD/RD,and CTD as in Figure1.An Aux/ IAA repressor(G)is color-coded for the RD domain I(red),the stability domain II(blue),and the dimerization domains III and IV (color-coded the same as ARF CTDs).ARF activator monomers can target and activate auxin response genes containing TGTCTC AuxREs (a)[1,2,7].ARF activators might dimerize via their CTDs(b)or bind as dimers to AuxRE target sites(c)to potentiate activation of auxin response genes[1,3].Like ARF activators,ARF repressor monomers can target and repress auxin response genes(d).It is possible that ARF repressors can dimerize via their CTDs(e)or bind as dimers to AuxRE target sites(f)to potentiate repression of auxin response genes.ARF activators are thought to dimerize with Aux/IAA repressors via their CTDs under conditions where auxin concentrations are low,resulting in repression of auxin response genes(g)[1,2,57].When auxin concentrations are elevated,the Aux/IAA repressors are destroyed by the ubiquitin–proteasome pathway[53],resulting in activation/ derepression(a)of auxin response genes in a dose-dependent manner [1,2,57].Which particular event occurs(a through g)could be determined by the concentrations of different ARF and Aux/IAA proteins in cells,as well as different affinities of ARFs for AuxRE target sites and different affinities of ARF and Aux/IAA proteins for one another.In the diagrams,green arrows represent activation and red lines with an X represent repression.The level of activation or repression is represented by the thickness of the green arrow or red line.The auxin response gene promoter is represented as a thin black line,and the ORF is represented as a thick black line.histone methyltransferases)and/or ATP-dependent chro-matin remodeling complexes that function as co-activa-tors or co-repressors are associated with promoters of auxin response genes and whether this association is affected by auxin.ConclusionsIn the past few years,forward and reverse genetic approaches have revealed a wealth of information on roles played by ARFs in plant growth and development.A number of candidate genes that are regulated by ARFs and may function in these growth and developmental processes have been identified experimentally or pre-dicted from data mining(i.e.mining genes with TGTCTC or GAGACA AuxREs in their promoters).To date,how-ever,not a single gene has been definitively shown to be targeted and directly regulated by a specific ARF.Further experiments like ChIP assays will be required to prove that a specific ARF targets an AuxRE in the promoter of a candidate gene.It is possible that additional cis-elements in auxin-responsive promoters are required for targeting ARFs to AuxREs[10]and that not all TGTCTC or GAGACA sequences in known auxin-responsive promo-ters or other uncharacterized promoters are AuxREs[51]. Furthermore,it is not clear how degenerate the consensus TGTCTC sequence can be and still bind ARFs in planta to function as an AuxRE.While a model for auxin-regulated gene expression proposes interactions among ARF and Aux/IAA proteins to bring about activation and repression on auxin response genes[1,2],it is possible that additional levels of regu-lation occur off the genes.For example,ARF-ARF, ARF-Aux/IAA,and Aux/IAA-Aux/IAA interactions or interactions of ARFs and Aux/IAA proteins with other factors might occur that sequester or prevent specific ARFs or Aux/IAA proteins from reaching their gene target sites.On the other hand,ARFs might directly interact with other transcription activators or repressors in regu-lating the expression of auxin response genes[56].In addition,the specificity of interactions among ARF and Aux/IAA proteins is not well understood,because most of the interactions that have been measured were carried out under unphysiological conditions.Interactions among ARF and Aux/IAA proteins in planta,whether on or off genes,may be less promiscuous than suggested from yeast two-hybrid or in vitro binding assays.ARF activators,as well as repressors,could function independently in regulating auxin response gene expres-sion if present at high concentrations relative to Aux/IAA repressors(i.e.ARF–ARF dimerization out competing ARF–Aux/IAA dimerization on auxin response genes) (Figure2).This type of regulation,which might result from relatively high expression levels of ARFs compared to Aux/IAA proteins in cells[6,24 ],would be expected to be auxin-independent.Auxin independent repression of auxin response genes might also be conferred through Aux/IAA repressors that contain functional repression and dimerization domains,but lack a functional domain II(i.e. the domain involved in targeting the repressors for degra-dation in an auxin-dependent manner),like Arabidopsis IAA20,30,32,and34.Determining the range of mech-anisms involved in regulating the targeting of ARFs and their partner Aux/IAA repressors to AuxREs and in reg-ulating their activities is an area open for exploration. AcknowledgementThe authors’research is supported by the National Science Foundation Grant Number IOB0550417.References and recommended readingPapers of particular interest,published within the annual period of review,have been highlighted as:of special interestof outstanding interest1.Guilfoyle TJ,Hagen G:Auxin response factors.J Plant GrowthReg2001,10:281-291.2.Tiwari SB,Hagen G,Guilfoyle TJ:The roles of auxin responsefactor domains in auxin-responsive transcription.Plant Cell2003,15:533-543.3.Ulmasov T,Hagen G,Guilfoyle TJ:Dimerization and DNAbinding of auxin response factors.Plant J1999,19:309-319. 4.Yamasaki K,Kigawa T,Inoue M,Tateno M,Yamasaki T,Yabuki T,Aoki M,Seki E,Matsuda T,Tomo Y et al.:Solution structure of the B3DNA binding domain of the Arabidopsis cold-responsive transcription factor RAV1.Plant Cell2004,16:3448-3459.5.Ulmasov T,Hagen G,Guilfoyle TJ:Activation and repression oftranscription by auxin response factors.Proc Nat Acad Sci U S A 1999,96:5844-5849.6.Hardtke CS,Ckurshumova W,Vidaurre DP,Singh SA,Stamatiou G,Tiwari SB,Hagen G,Guilfoyle TJ,Berleth T:Overlapping and non-redundant functions of the Arabidopsis auxin response factors MONOPTEROS andNONPHOTOTROPIC HYPOCOTYL4.Development2004,131:1089-1100.7.Wang S,Tiwari SB,Hagen G,Guilfoyle TJ:AUXIN RESPONSEFACTOR7restores the expression of auxin-responsive genes in mutant Arabidopsis leaf mesophyll protoplasts.Plant Cell2005,17:1979-1993.8.Wilmoth JC,Wang S,Tiwari SB,Joshi AD,Hagen G,Guilfoyle TJ, Alonso JM,Ecker JR,Reed JW:NPH4/ARF7and ARF19promote leaf expansion and auxin-induced lateral root formation.Plant J2005,43:118-130.T-DNA knockouts for Arabidopsis ARF7and ARF19were identified in this study,and phenotypes of the single and double mutants indicated that ARF7and ARF19cooperate in promoting lateral and adventitious root formation and leaf expansion.Results also indicated that ARF7and ARF19activators regulate expression of the ARF19gene and a subset of auxin response genes.9.Okushima Y,Overvoorde PJ,Arima K,Alonso JM,Chan A,Chang C,Ecker JR,Hughes B,Lui A,Nguyen D et al.:Functional genomic analysis of the AUXIN RESPONSE FACTOR genefamily members in Arabidopsis thaliana:unique andoverlapping functions of ARF7and ARF19.Plant Cell2005,17:444-463.The authors identified T-DNA insertions in18of the23ARF genes in Arabidopsis,but only a few of the knockouts had developmental defects. The arf7arf19double mutant showed a dramatic reduction in lateral root formation,unlike the single mutants that showed only small defects in lateral root formation.Global gene expression analysis with arf7and arf19 single and double mutants suggested that ARF7plays a major role and ARF19a lesser role in regulating a variety of auxin response genes in young seedlings.458Cell Signalling and Gene Regulation。