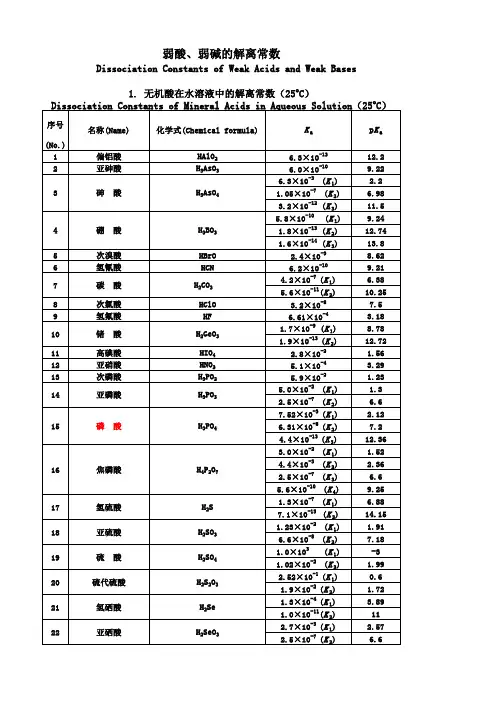
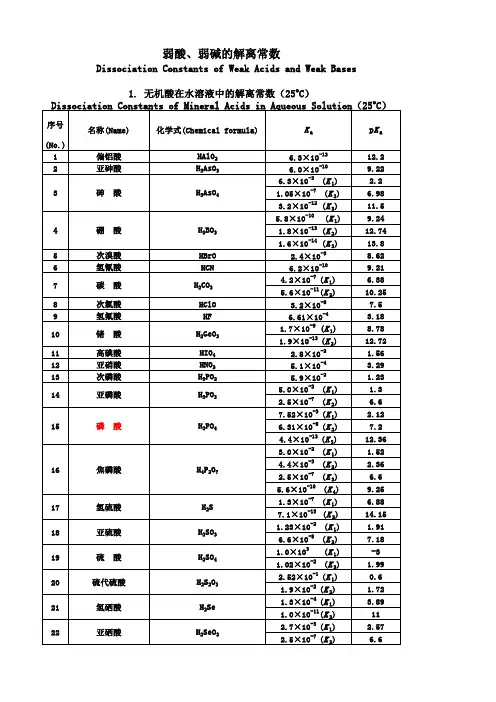
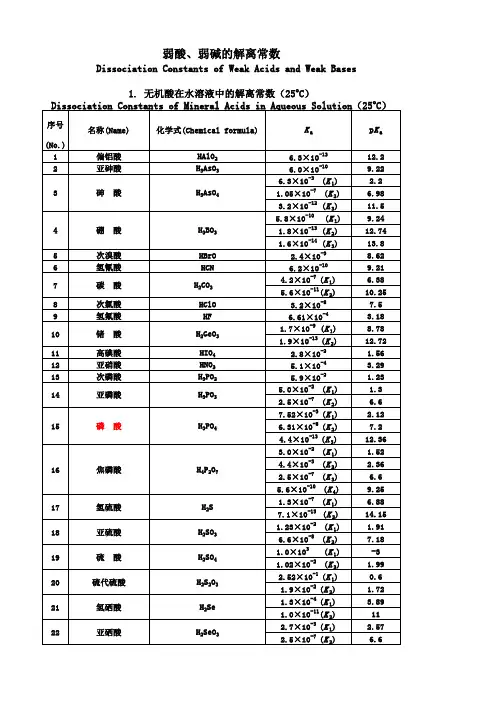
有机分子pKa列表
- 格式:pdf
- 大小:39.81 KB
- 文档页数:5

各种酸的pKa及pH值-pb的pka 各种酸的 pKa 及 pH 值 pb 的 pka在化学的世界里,酸的性质和行为是一个非常重要的研究领域。
其中,酸的解离常数(pKa)以及溶液的pH 值是描述酸性质的关键参数。
在这篇文章中,我们将深入探讨各种酸的 pKa 以及它们与 pH 值之间的关系,并特别关注一下 pb 的 pka。
首先,让我们来了解一下什么是 pKa 和 pH 值。
pKa 是酸解离常数(Ka)的负对数。
酸解离常数Ka 表示酸在溶液中解离出氢离子(H⁺)和酸根离子的程度。
Ka 值越大,说明酸的解离程度越大,酸性越强;而 pKa 值越小,酸性越强。
pH 值则是用来衡量溶液中氢离子浓度的指标。
它的定义是氢离子浓度的负对数,即 pH = logH⁺。
pH 值的范围通常在 0 到 14 之间,7为中性,小于 7 为酸性,大于 7 为碱性。
常见的无机酸如盐酸(HCl)、硫酸(H₂SO₄)和硝酸(HNO₃)都是强酸,它们在水溶液中几乎完全解离。
盐酸的 pKa 约为-63,硫酸的第一步解离 pKa 约为-3,硝酸的 pKa 约为-14。
由于它们的解离程度非常高,在计算 pH 值时,通常可以将其视为完全解离,根据其浓度直接计算氢离子浓度。
而弱酸在溶液中的解离则是一个平衡过程。
例如,乙酸(CH₃COOH)是一种常见的弱酸,其 pKa 约为 476。
这意味着在一定条件下,乙酸在溶液中只有一部分会解离出氢离子和乙酸根离子。
当我们知道乙酸的初始浓度和溶液的平衡状态时,可以通过解离平衡常数的表达式来计算溶液中的氢离子浓度,从而得出 pH 值。
再来说说磷酸(H₃PO₄),它是一种多元酸,具有三步解离。
第一步解离的 pKa 约为 212,第二步约为 721,第三步约为 1232。
多元酸的解离过程是逐步进行的,每一步的解离程度都不同,这也使得其溶液的 pH 值计算相对复杂,需要综合考虑各步解离的情况。
接下来谈谈有机羧酸,比如苯甲酸(C₆H₅COOH),其 pKa 约为42。

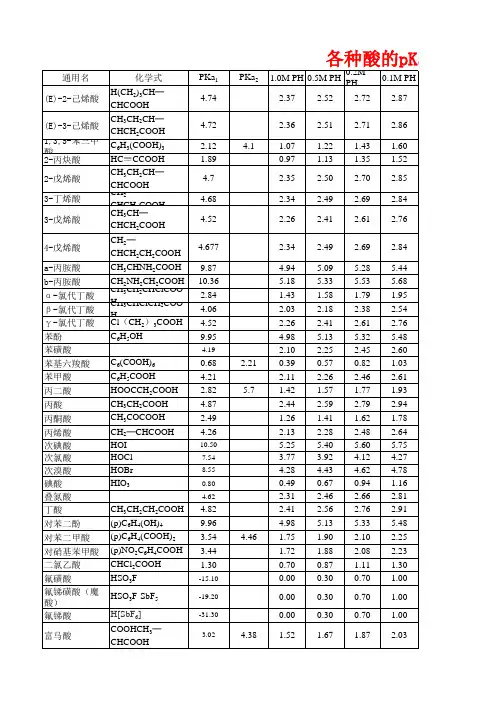
pKa ValuesINDEXInorganic2Phenazine24 Phosphates3Pyridine25 Carboxylic acids4, 8Pyrazine26Aliphatic4, 8Aromatic7, 8Quinoline27Phenols9Quinazoline27Alcohols and oxygen acids10, 11Quinoxaline27Amino Acids12Special Nitrogen Compounds28Peptides13Hydroxylamines28Nitrogen Compounds14Hydrazines28Aliphatic amines15, 17, 19Semicarbazones28 Cyanoamines16Amidoximes28Anilines17, 18, 20Thiols29 Nucleosides21Carbon Acids30,31 Special Table Heterocycles22Indicators31Acridine23References32-34 Benzoquinoline24Cinnoline23Hydantoin24Imidazole24For complex chelating agents, see also reference 77.Note. This document was compiled by W.P. Jencks and has been added to by F.H. WestheimerACIDSCompound pK Ref.H3PO2 2.0, 2.23*28H2PO4–7.21*77 AgOH 3.964HPO4_12.32*77Al(OH)311.228H3PO3 2.028As(OH)39.2228H2PO3– 6.58*77H3AsO4 2.22, 7.0, 13.028H4P2O7 1.52*77H2AsO4– 6.98*77H3 P2O7– 2.36*77 HAsO4*11.53*77H2P2O7= 6.60*77As2O304H3AsO39.22*HP2O7=9.25*77H3BO39.23*28HReO4-1.2530H2B4O7 4.0034HSCN 4.0034H2SeO3 2.6, 8.3, 2.62*28 HB4O79.0034HSeO38.3277Be(OH)2 3.74H2SeO4Strong, 2.028 HBr-9.0031HOBr8.728HSeO4 2.0034 HOCl7.53, 7.4628, 33H3SiO310.034 HClO2 2.028H2SO3 1.9, 7.0, 1.76*28, 77 HClO3-1.0028H2SO4-3.0, 1.928 HClO4 (70%)-10.0031HSO37.21*77CH3SO3H-0.631HSO4– 1.99*77 HCN9.4034H2S2O4 1.929H2CO3 6.37, 6.35*, 3.5834, 32H2Se 3.89*77 HCO310.33*HSe–11.00*77H2CrO4-0.9830H2S7.00*77 HCrO4 6.50*2, 30HS–12.92*77 HOCN 3.9234HSbO211.034 HZ 3.17*, 0.59*77HTe 5.0034H2GeO38.59, 12.7234, 78H2Te 2.64, 11.034, 78 Ge(OH)48.68, 12.728H2TeO3 2.7, 8.028HI-10.031Te(OH)6 6.2, 8.828 HOI11.028H2VO4–8.9530 HIO30.828HVO4=14.430H4IO6– 6.0034H2CrO40.7477H5IO6 1.64, 1.55, 8.2734, 28HOCN 3.7377 HMnO4-2.2530HSCN0.8577 NH3OH* 5.98*H3PO2 1.0777 NH4*9.24*77H3PO4 2.12*77 HN3 4.72*77H2S2O30.60*, 1.72*77 HNO2 3.2928H3AuO313.3, 16.078 HNO3-1.328H3GaO310.32, 11.778N2H5+7.99*77H5IO6 3.29, 6.70, 15.078H2N2O27.0534(see above!)H2N2O2–11.034H4V6O17 1.9678H2OsO512.134H2NSO3H 1.080H2O15.7noneH3O+-1.7none* Indicates a thermodynamic value.Pb(OH)2 6.48 (10.92) 4 (78)PHOSPHATES AND PHOSPHONATES CF 3- 1.16, 3.9357CCl 3- 1.63, 4.8157Phosphates NH 3+CH 2- 2.35, 5.957CompoundpKRef.(–OOCCH 2)2NH +CH 2– --, 5.5757Phosphate 1.97, 6.82, 12.555CHCl 2- 1.14, 5.6157Glyceric acid 2-phosphate 3.6, 7.153CH 2CI- 1.40, 6.3057Enolpyruvic acid 3.5, 6.453CH 2Br- 1.14, 6.5257Methyl- 1.54, 6.3155(–OOCCH 22NH +(CH 2)2- --, 6.5457Ethyl- 1.60, 6.6255CH 2I- 1.30, 6.7257n-Propyl- 1.88,6.6755NH 3+CH 2CH 2- 2.45, 7.0057n-Butyl- 1.80, 6.8455Dimethyl- 1.2955C 6H 5CH=CH- 2.00, 7.157Di-n-propyl 1.5955HOCH 2- 1.91, 7.1557Di-n-butyl- 1.7255C 6H 5NH 2+(CH 2)3- 2.1, --57Glucose-3-0.84, 5.6756C 6H 5NH(CH 2)3---, 7.1757Glucose-4-0.84, 5.6756Br(CH 2)2- 2.25, 7.357α-glycero- 1.40, 6.4454CH 3(CH 2)5CH(COO –)---, 7.557β-glycero- 1.37, 6.3454C 6H 5CH 2- 2.3, 7.55573-phosphoglyceric acid 1.42, 3.4254NH 3+(CH 2)4)- 2.55, 7.55572-phosphoglyceric acid 1.42, 3.55, 7.1peroxymonophosphoric acid 4.0569NH 3+(CH 2)5- 2.6, 7.657diphosphoglyceric acid 7.40, 7.9954NH 3+(CH 2)10---, 8.0057glyceraldehyde- 2.10, 6.7554–OOC(CH 2)10---, 8.2557dioxyacetone- 1.77,6.4554(CH 3)3SiCH 2- 3.22, 8.7057hexose di- 1.52, 6.3154fructose-6-0.97, 6.1154C 6H 5CH 2- 3.3, 8.457glucose-6-0.94, 6.1154(C 6H 5)SC- 3.85, 9.0057glucose-1- 1.10, 6.1354adenylic acid 3.8?, 6.2?54Arylphosphonic acids inosinic acid 2.4?, 6.4?542X-RC 6H 3PO 3H 2ADP 2 strong, 6.654X R ATP 3 strong, 6.654Cl 4-O 2N 1.12, 6.1457pyrophosphoric acid 0.9, 2.0, 6.6, 9.454Br 5-O 2N (a), 6.1457phosphopyruvic acid 3.5, 6.3854Cl 5-Cl (a), 6.6357creatine phosphate 2.7, 4.554Cl H 1.63, 6.9857arginine phosphate 2.8, 4.5, 9.6, 11.254Br H 1.64, 7.0057arginine 2.02, 9.0, 12.554Br 5-CH 3 1.81, 7.1557amino phosphate (-0.9), 2.8, 8.254Cl 4-NH 2--, 7.3357trimetaphosphate 2.0577CH 3O 4-O 2N 1.53, 6.9657CH 3O H 2.16, 7.7757PhosphonatesCH 3O 4-O 2N --, 8.2257H 2O 3P(CH 2)4PO 3H 2 <2, 2.75, 7.54, 8.3857HO 4-O 2N 1.22, 5.3957H 2O 3P(CH 2)3PO 3H 2 <2, 2.65, 7.34, 8.3557O 2N H 1.45, 6.7457H 2O 3PCH 2CH(CH 3)PO 3H 2 <2, 2.6, 7.00, 9.2757F H 1.64, 6.8057H 2O 3PCH 2PO 3H 2 <2, 2.57, 6.87, 10.3357I H 1.74, 7.0657Methyl- 2.3557NH 2H --, 7.2957Ethyl- 2.4357CH 3H 2.10, 7.6857n-propyl- 2.4557C 6H 5H (a), 8.1357isopropyl- 2.55, 7.7557HOOC H1.71, 9.1757n-butyl- 2.59, 8.1957isobutyl- 2.70, 8.4357**These values were obtained in 50% ethanol.s-butyl- 2.74, 8.4857(a) The compounds were not sufficiently soluble.t-butyl- 2.79, 8.8857For graphical plots of a large number of substituted phosphorus compounds see 83.neopentyl- 2.84, 8.65571,1 Dimethylpropyl- 2.88, 8.9657n-hexyl- 2.6, 7.957triphosphate8.90, 6.26, 2.3077n-dodecyl---, 8.2557tetrametaphosphate 2.7477CH 3(CH 2)5CH(COOH)- 1, --57fluorophosphate0.55, 4.856Acetic acids, substituted Phosphonates (Ref. 2)H- 4.76*20 X-H-H-NH3+-NH3+O2N- 1.68*20 X(CH2)PO3H2 2.357.1 1.85 5.35(CH3)3N+- 1.83*20 X(CH2)2PO3H2 2.457.85 2.457.00(CH3)2NH+- 1.95*20 X(CH2)4PO3H2 2.557.55CH3NH2+- 2.16*20 X(CH2)5PO3 H2 2.67.65NH3+- 2.31*20 X(CH2)6PO2H2 2.67.9CH3SO2- 2.36*20 X(CH2)10PO2H28.00NC- 2.43*20 Phosphines in acetonitrile, see ref. 89.C6H5SO2- 2.4420HO2C 2.83*20 CARBOXYLIC ACIDSAliphatic C6H5SO- 2.6620 Compound pK Ref.F- 2.6620 Acetoacetic 3.586Cl- 2.86*20 Acetopyruvic 2.61, 7.85 (enol)6Br- 2.8620 Aconitic, trans- 2.80, 4.466Cl2- 1.2920 Betaine 1.846F2- 1.2420 Citric 3.09, 4.75, 5.416Br3-0.6620 Crotonic 4.696Cl3-0.6520 Dihydroxyfumaric 1.146F3-0.23 (-0.26) (2)20 Dethylenediamine- 2.00, 2.676HONC4 3.0120 tetraacetic 6.16, 10.26F3C- 3.07*20 Formic 3.77*2N3- 3.0320 Fumaric 3.03, 4.546I- 3.1220 Glyceric 3.556C6H5O- 3.1220 Glycollic 3.826C2H5O2C- 3.3520 Glyoxylic 3.326C6H5S- 3.52*20 Homogentistic 4.406CH3O- 3.5320α-keto-β-methyl valeric 2.36NCS- 3.5820 Lactic 3.866CH3CO- 3.58*20 Maleic 1.93, 6.586Malic 3.40, 5.26C2H5O- 3.6020 Oxaloacetic (trans-enol) 2.566n-C3H7O 3.6520 +(cis-enol) 2.15, 4.066n-C4H9O 3.6620 Protocatechuic 4.486sec.-C4H9O- 3.6720 Pyruvic 2.506HS- 3.67*20Tartaric + 2.99, 4.406i-C3H7O- 3.69*20 + or - 2.89, 4.406CH3S- 3.72*20 meso 3.22, 4.856i-C3H7S- 3.72*20 Vinylacetic 4.426C6H5CH2S- 3.73*20C2H5S- 3.74*20n-C3H7S- 3.77*20n-C4H9S- 3.81*20HO- 3.83*20–O3S- 4.0520(C6H5)3CS- 4.30*20C6H5- 4.31*20CH2-CH- 4.35*20* Indicates thermodynamic values.Unsaturated acids (25°)CompoundpK poundpK ref.trans-CH 3-CH=CHCO 2H 4.69*20H-CH 2CH 2CO 2H 4.88*2cis-CH 3-CH=CHCO 2H 4.44*2H-CH=CHCO 2H 4.25*2C 6H 5-CH 2CH 2CO 2H4.66*2C 6H 5CH 2CH 2CO 2H 4.66*2trans-C 6H 5-CH=CHCO 2H 4.44*2C 6H 5CH=CHCO 2H** 4.44*2m-CH 3OC 6H 4CH 2CH 2CO 2H4.65*2C 6H 5CH 2CH 2CO 2H 4.66*2C 6H 5CH=CHCO 2H**4.442m-CH 3OC 6H 4CH=CHCO 2H 4.38*2m-ClC 6H 4CH=CHCO 2H**4.29*2m-ClC 6H 4CH 2CH 2CO 2H 4.58*2Unsaturated acids, Cis- and Trans-C C R 2H R 1CO 2H C C R 2R 1HCO 2H Cis-Acid Trans-Acid R 1R 2cis-acid trans-acid Ref.H-H- 4.25*4.25*2CH 3-H- 4.44*4.69*2Cl-H- 3.323.652C 6H 5-H- 3.88*4.44*2ClC 6H 4H- 3.91 4.4126-BrC 6H 4H- 4.02 4.412CH 3-CH 3- 4.305.022C 6H 5-H- 5.26***5.58***22,4,6-(CH 3)3C 6H 2-H-6.12***5.70***2C 6H 5-CH 3- 4.98***5.98***2Dicarboxylic acids, unsaturated*Maleic 1.92, 6.232Alicyclic Dicarboxylic acidsCitraconic (Dimethylmaleic acid)2.29, 6.152cis-Caronic(1,1-dimethylcyclopropane-23-dicarboxylic acid 2.34*, 8.31*2Acetylenedicarboxylic 1.73, 4.402∆1-tetrahydrophthalic 3.01, 5.3421,2-trans-cyclopropanedicarboxylic3.65*, 5.13*2Bromomaleic 1.45, 4.622trans-caronic 3.82*, 5.32*2Bromofumaric 1.46, 3.5721,2-cis-cyclopropane-dicarboxylic3.33*, 6.47*2Chlorofumaric 1.78, 3.812Fumaric 3.02,4.382Mesaconic (Dimethylfumaric acid)**trans3.09,4.752***in 40% acetone Phthalic 2.95, 5.412*thermodynamicItaconic (1-Propene-2-3-dicarboxylic acid)3.85, 5.452Chloromaleic 1.72, 3.862AliphaticAlicyclic Dicarboxylic acidsCompound pK Ref Compound pK Ref 1,2-trans-Cyclopropane-cis-Ethyleneoxide-dicarboxylic 3.65, 5.132dicarboxylic 1.94, 3.922 trans-Ethyleneoxide-1,3-cis-Cyclobutane-dicarboxylic 1.93, 3.252dicarboxylic 4.03, 5.3121,3-trans -Cyclobutanedi-1,2-cis-Cyclopentane-carboxylic 3.81, 5.282dicarboxylic 4.37, 6.5121,2-trans-Cyyclopentane-1,3-cis-Cyclopentanedicarboxylic 3.89, 5.912dicarboxylic 4.23, 5.5321,3-trans-Cyclopentane-1,2-cisCyclohexane-dicarboxylic 4.40, 5.452dicarboxylic 4.34, 6.7621,2-trans-Cyclohexane-1,3 -cis-Cyclohexane-dicarboxylic 4.18, 5.932dicarboxylic 4.10, 5.4621,3-trans-Cyclohexane-1,4-cis-Cyclohexanedicarboxylic 4.31, 5.732di-carboxylic 4.44, 5.7921,4-trans-Cyclohexane-dicarboxylic 4.18, 5.422Dicarboxylic acids*oxalic 1.23, 4.192Succinic 4.19, 5.482 Malonic 2.83, 5.692 O-O’-Dimethyl- 3.77, 5.942 Methyl- 3.05, 5.762 (high melting)Ethyl- 2.99, 5.832 O-O’-Dimethyl- 3.94, 6.202n-propyl 3.00, 5.842 (low melting)i-propyl- 2.94, 5.882 O,O’-Diethyl- 3.63, 6.462 Dimethyl- 3.17, 6.062 (high melting)Methylethyl- 2.86, 6.412 O,O’-Diethyl- 3.51, 6.602Diethyl- 2.21, 7.292 (low melting)Ethyl-n-propyl- 2.15, 7.432Tetramethyl- 3.50, 7.282Di-n-propyl- 2.07, 7.512Glutaric 4.34, 5.422Adipic 4.42, 5.412B-Methyl 4.25, 6.222Pimelic 4.48, 5.422B-Ethyl 4.29, 6.332Suberic 4.52, 5.402B-n-Propyl 4.31, 6.392Azelaic 4.55, 5.412B,B-Dimethyl- 3.70, 6.292DL-1:2-Dichlorosuccinic 1.68, 3.1820 B,B-Methylethyl- 3.62, 6.702meso-1:2-Dichlorosuccinic 1.74, 3.2420 B,B-Diethyl- 3.62, 7.122DL-1:2-Dibromosuccinic 1.48, ----20 B,B-Di-n-propyl 3.69, 7.312meso-1:2-Dibromosuccinic 1.42, 2.9720D-Tartaric 3.03, 4.4520DL-1:2-Dimethylsuccinic 3.93, 6.0020DL-Tartaric 3.03, ----20meso-1:2-Dimethylsuccinic 3.77, 5.3620 meso-Tartaric 3.29, 4.9220*All are thermodynamic valuesAliphatic HO- 6.332Br- 6.082 Bicyclo[2.2.2]octane-1-carboxylic acids, 4-substitutedLysergic acid, etc.H- 6.752ergometrine 6.8, --2 C2H5O2C- 6.312Dihydroergometrine7.4, --2β-dihydrolysergol8.2, --2 NC- 5.902Lysergic acid7.8, 3.32C6H5O- 3.53* 3.95* 4.52*α-dihydrolysergic8.3, 3.62CH3- 3.91* 4.24* 4.34* ergometrinine7.3, --2(CH3)2CH- 4.35*α-dihydrolysergol8.3, --2(CH3)3N+- 1.37 3.45 3.436-methylergoline8.85, --2NC- 3.60* 3.55* isolysergic acid8.4, 3.42HO2C* 2.95* 3.54 3.51γ-dihydrolysergic8.6, 3.62F3C- 3.79HO- 2.98* 4.08* 4.58*I- 2.85* 3.86* Hydroxycyclohexanecarboxylic acids Cl- 2.94* 3.83* 3.99* Cyclohexanecarboxylic 4.902(CH3)3Si- 4.24* 4.27* cis-1,2 4.802C2H5O- 4.21* 4.17* 4.45* cis-1,3 4.602i-C3H7O- 4.24* 4.15* 4.68* cis-1,4 4.842n-C5H11O- 4.55* trans-1,2 4.682C6H5- 3.46*trans-1,3 4.822CH3CH2- 3.77 4.35* trans-1,4 4.682(CH3)3C- 3.46 4.28 4.40*–HO3P- 3.78 4.03 3.95 Aromaticbenzene-CO3H 4.20*2–O3S- 4.15 4.11 Anthracene-1-COOH 3.692H2N- 4.98 4.79 4.92 Anthracene-9-COOH 3.652(CH3)2N-8.42 5.10 5.03 naphthalene-2-COOH 4.172–HO3As- 4.22 Naphthalene-1-COOH 3.692–O2C- 5.41** 4.60 4.82CH3NH- 5.3 5.10 5.04 Substituted benzoic acids (ref. 2)COOH*thermodynamicfor complex chelating agents, see also ref. 84.see also page 9a for more carboxylic acids. Benzoic acid o m pOrtho-substituted benzoic acidsH- 4.20* 4.21*Benzoic acid pK Ref.O2N- 2.17* 3.45* 3.442-CH3- 3.91**2CH3CO-2-t-C4H9- 3.462CH3SO2- 3.64* 3.52*2,6-(CH3)2- 3.212CH3S-2,3,4,6-(CH3)4- 4.002HS-2,3,5,6-(CH3)4- 3.522Br- 2.85* 3.81* 4.00*2-C2H5- 3.772F- 3.27* 3.87* 4.14*CH3O- 4.09* 4.09* 4.47*2-C6H5- 3.46**2n-C3H7O- 4.24* 4.20* 4.46*2,4,6-(CH3)3- 3.432n-C4H9O- 4.25* 4.53*2,3,4,5-(CH3)4- 4.222 Benzene Polycarboxylic acids Ref. 2Acid Position of carboxyl pK I pK II pK III pK IV pK V pK VI Benzoic1 4.17*Phthalic1,2 2.98* 5.28*Isophthalic1,3 3.46* 4.46*Terephthalic1,4 3.51* 4.82*Hemimellitic1,2,3 2.80* 4.20* 5.87*Trimellitic1,2,4 2.52* 3.84* 5.20*8Trimesic 1,3,5 3.12* 3.89* 4.70*Mellophanic 1,2,3,4 2.06* 3.25* 4.73* 6.21*Prehnitic 1,2,3,5 2.38* 3.51* 4.44* 5.81*Pyromellitic1,2,4,5 1.92* 2.87* 4.49* 5.63*Benzenepentacarboxylic 1,2,3,4,5 1.80* 2.73* 3.97* 5.25* 6.46*Mellitic1.2,3,4,5,61.40*2.19*3.31*4.78*5.89*6.96**ionic strength 0.032-Methoxyethyliminodiacetic 2.2, 8.96**thermodynamic2-Methylthioethyliminodiacetic 2.1, 8.91oxalic acid* 1.25, 4.14N-n-propylaminoacetic 2.25, 10.03Carboxylic Acids Ref. 77N-2-sulfoethyliminodiacetic 1.92, 2.28, 8.16Aminomalonic acid* 3.32, 9.83α-Bromobutyric acid 2.97N-Butylaminoacetic acid 2.29, 10.07N-(carbamoylmethyl)-imino-diacetic acid2.30, 6.602-carboxyethyliminodiacetic acid2.06,3.69, 9.66Cyanomethyliminodiacetic 3.06, 4.34β-carboxymethylaminopropionic 3.61, 9.46α,β-diaminopropionic acid 1.23, 6.69α,α-diaminobutyric 1.85, 8.24, 10.44Diethylaminoacetic 2.04, 10.47Di-(carboxymethyl)-aminomethyl phosphonic acid 2.00, 2.25, 5.57, 10.76Dimethylaminoacetic 2.08, 9.80N-ethylaminoacetic 2.30, 10.10α,β-dimercaptosuccinic 2.40, 3.46, 9.44, 11.82Gluconic* 3.86β-hydroxybutyric 4.39Ethylenediamine-N,N-diacetic 5.58, 11.05α-hydroxybutyric 3.65β-hydroxypropionic 3.73N-2-hydroxyethyliminodiacetic 2.2, 8.73Iminodiacetic* 2.98, 9.893-hydroxypropyliminodiacetic 2.06, 9.24β-iodopropionic* 4.04Iminodipropionic 4.11, 9.61N-isopropylaminoacetic 2.36, 10.06Isobutyric* 4.86α-mercaptobutyric 3.53Mandelic acid 3.41N-methylaminoacetic 2.24, 10.012-MercaptoethyliminodiaceticNitrilotriacetic 3.03, 3.07, 10.-2.14, 8.17, 10.792-PhosphonoethyliminodiaceticMethyliminodiacetic 2.81, 10.181.95,2.45, 6.54, 10.46*ThermodynamicPHENOLSCompound pK pound pK Ref. Chromotropic acid 5.36, 15.66Resorcinol--, 9.15 (30o)50o-Methoxyphenol--, 9.9350p-Methoxyphenol--, 10.1650 o-Hydroxybenz-3-Hydroxyanthran-aldehyde7.9550ilic acid10.09, 5.20512-Amino-4,5 dimethyl-2-Aminophenolphenol hydrochloride10.4 5.2851hydrochloride9.99, 4.86514,5-dihydroxybenzene-1,3 disulphonic acid7.6612.6eKojic acid9.4077Phenol o m p Phenol o m pH-9.95*9.94*O2N-7.23*8.35*7.14* (CH3)3N+-7.4288OCH- 6.798.007.66CH3SO2-9.337.83NC-8.61**7.95CH3CO-9.198.05CH3O2C-8.47*C2H5O2C-8.50*n-C4H9O2C-8.47*C3H5CH2O2C-8.41*I-9.17*Br-8.42*9.11*9.34*Cl-8.48*9.02*9.38*F-8.81*9.28*9.95*CH3S-9.539.53HO-9.489.449.96HOCH2-9.92*9.83*9.82*CH3-10.28*10.08 10.19*C2H5-10.29.910.0CH3O-9.939.6510.20H2N-9.719.8710.30-O2C-9.94*9.39*-O3S-9.299.03--O3P-10.29.9--O3As8.37 C6H5-9.939.599.51NO- 6.35**2-Chloro-4-Nitro- 5.42792-Nitro-4-Chloro- 6.4679* Thermodynamic**Reference 52ALCOHOLS and other OXYGEN ACIDSAlcoholsCompound pK pound pK Ref. Choline13.96C3F7•CH(C2F5)•OH 10.4865 Chloral hydrate9.66, 11.061(C3F7)2CH•OH10.5265 Trifluoroethanol12.562Carbonium ionsCF3CH2OH11.4, 12.4363CF3CH(OH)CH311.863Triphenylmethanols in H2SO4 HC1O4 HNO3refCF3CH2(CH3)3OH12.43104,4,4-Trimethoxy.82. .82 .8066C3F7CH2OH11.4**634,4’-Dimethoxy-1.24-1.14-1.1166(C3F7)2CHOH10.6**634-Methoxy-3.40-3.59-3.4166HCCCH2OH13.55644-Methyl-5.41-5.6766C(CH2OH))414.1644-Trideuteriomethyl- 5.43 5.6766HOCH2CHOHCH2OH 4.4643,3’,3”-Trimethyl- 6.35-5.9566HOCH2CH2OH14.7764Unsubstituted triphenyl-CH3CCH2OH14.8264methanol- 6.63-6.89 6.6066CH3OH15.54644,4;,4;-Trichloro- 7.74-8.0166 CH2=CHCH2OH15.52644-Nitro-9.15-9.7666 H2O15.7464CCl3CH2OH 11.8***CH3CH2OH1664CF3CH2OH 11.3***Substituent effects for ionization of RCH2OHRCCl-312.24,11.8064,65CF3-12.3764CHF2CH2-12.7464Hydroxamic acidsCHCl2-12.8964Furo-8.4572CHEC-13.5564Glycine7.4072H2Cl-14.3164Hippuro-8.8072CH3CCH2-14.864iso Nicotin7.8572HOCH215.164p-Methylbenz-8.9072H-15.564Nicotin-8.3072CH2=CH-15.564Nicotin-methiodide 6.4672CH3-(extrap)(15.9)64m-Nitrobenz-8.0772CF3C(CH3)2OH11.664Picolin8.5072HOCH2CF2CH2OH1164Pyrimidine-2-carbox-7.8872Primary alcohols=R•CH2•OH and Salicyl-7.4372Secondary alcohols in 50% alcohol Tropo-9.0972C2F511.3565C4F911.3565C5F1111.3765C7F1511.3565Other oxygen acidsCHF212.0065Trimethylamine-n-oxide 4.618CF2Cl11.6365Dimethylglyoxime12.8477CHF2CF211.3465(50% dioxane)CHF2 • (CF2)211.3565O-methyl ether12.9277CF3 • CH212.765Tropolone12a77CF3 • (CH2)212.965 α-Bromotropolone 6.95a77CF3 • CHMe • OH11.2865Acetald hydrate13.4891C3F7 • CHMe • OH11.3865Formald hydrate13.2991C3F7CHEt • OH11.3765C3F7CHPr • OH11.3765C3F7 • CH(CF3) • OH10.4665a50% dioxane***50 aquaeous ethanolOTHER OXYGEN ACIDSHydroxamic acids Aceto-9.4068Compound pK Ref.n-Butyro-9.4868Pyridine oxidesn-Butyro-9.00684-Aminopyridine 1-oxide 3.6967p-Methoxybenzo-9.19684-Dimethylaminopyridine 1-oxide3.8867N-Hydroxyphthalimide 7.00, 6.1071, 72Salicylo 7.32684-Dimethylaminopyridine 1-oxide3.8867Benzo-8.8868p-Chlorobenzo-9.59684-Dimethylamino-1-methoxypyridinium perchlorate >1167α-Naphtho-~7.768Propiono-9.46682-Methylaminopyridine 1-oxide 2.61672-Amino-1-methoxypyridinium perchlorate12.467Oximes4-Hydroxypyridine 1-oxide 2.4567Benzophenone oxime 11.3184-Methoxypyridine 1-oxide 2.0567Diethyl ketoxime 12.6181-Methoxypyridi-4-one 2.5767Isonitrosoacetylacetone (INAA) 7.4762-Hydroxypyridine 1-oxide -0.8675-Methyl-1,2,3-cyclohexanetrione-1,3-dioxime8.3762-Ethoxypyridine 1-oxide 1.18671-Methoxypyrid-2-one -1.3Acetophenone oxime 11.48184-Methylaminopyridine 1-oxide 3.8567Acetoxime 11.42184-Amino-1-methoxypyridinium perchlorate>1167Isonitrosoacetone (INA) 8.376Salicyclaldoxime (SA)9.2762-Aminopyridine 1-oxide 2.67671,2,3-Cyclohexanetrionetrioxime 8.0762-Dimethylaminopyridine 1-oxide2.27675-Methyl-1,2,3-cyclohexane-trionetrioxime8.0762-Methylamino-1-methoxypyridinium toluene-p-sulphonate >11674-Benzyloxypyridine 1-oxide 1.9967Oxygen acids1-Benzyloxypyrid-4-one 2.5867sulfinic acids 2-Methoxypyridine 1-oxide 1.2367p-Toluene- 1.99731-Benzyloxypyrid-2-one -1.767p-Chlorobenzene-73p-Nitrobenzene-73Pyridine 1-oxides p-Bromobenzene- 1.8973RpK Ref.m-Nitrobenzene- 1.88734-CH 3 1.2947Benzene-1.84,2.16733-CH 3 1.0847Peroxyacids3,4-(CH)4 1.0147Peroxymonosulfuric 9.4693-COOC 4H 90.0347Acetic 8.2704-NO 2-1.747n-Butyric 8.2703-NH 2 1.4747Formic 7.170H0.7947Propionic 8.1703-COOH 0.0947peroxydiphosphoric 5.18, 7.8854-COOH-0.4847peroxymonophosphoric 4.8590Peroxides ROOH (Ref. 70)H CH 3C 2H 5iso-C 3H 7tert-C 4H 9iso-C 4H 911.611.511.812.112.812.8Oximesref. 93Pyridine-2-aldoxime heptiodide 8.00benzoquinoline mon- 6.25Pyridine-4-aldoxime methiodide 8.503-pyridine-1,2-ethanedione-2-oxime methiodide7.20Pyridine-4-aldoxime pentiodide 8.504-Pyridine-1,2-ethanedione-2-oxime methiodide7.1O-Methyltyrosine ethyl ester 7.3122 octopine 13, 1.368.776Pyridine-2-aldoxime methiodide8.0Phenylglyoxald-8.3 2.40Pyridine-4-aldoxime dodeciodide8.5Phenylalanine 1.839.136 Pyridine-3-alkoxime methiodide9.22-Pyrrolidoone-5-carboxylic acid (glucamicacid) 3.32Hydroxamic acids ref. 93Serine 2.219.156 D-Lysine-7.93Threonine 2.6310.436 N-phenylnicotino-8.00N-Trimethyl tyrosine9.7521 Chloroaceto-8.40Tyrosine 10.07, 2.209.11 Formo-8.65Urocanic acid 5.8 3.5p-Chlorophenoxyaceto-8.75Valine 2.329.626 p-Hydroxybenzo-8.93β-Alanine 3.6010.196 p-Methoxybenzo-9.00γ-Aminobutyric acid 4.2310.436 N-Phenylbenzo-9.15Arginine 12.48 2.179.046 o-Aminobenzo-9.17Asparagine 2.028.86 L-Tyrosine9.20Azaserine8.556 L-Lysine7.9Canavanine7.40, 9.2511.50 (?)6 p-Nitrobenzo-8.0Creatine 2.6711.026 p-Aminobenzo-9.3Cysteine 10.78 1.718.336 L-Lacti-9.33,4-DihydroxyphenylalaninePropiono-9.49.88, 2.368.686 Phthalo-9.411.68Indole-3-aceto-9.5Glutamine 2.179.136 Cyclohexano-9.7Histamine 5.09.76 Hexano-9.7β-Hydroxyglutamic 2.099.206acid 4.18Amino Acids Hydroxyproline 1.929.736 Compound pK Ref.Leucine 2.369.606-COOH-NH3Methionine 2.289.21 Alanine 2.359.6961-Methylhistidine 6.48, 1.698.856α-Aminobutyric acid 2.559.60Norleucine 2.399.766α-Aminoisobutyric 2.3610.216Norvaline 2.369.766 Argininosuccinic >12, 1.629.586Ornithine 1.718.6962.70, 4.2610.76 Aspartic acid 2.09,3.869.826Proline 1.9910.606 Canaline10.3, 9.2011.6 (?)6Sarcosine 2.2310.016 Creatinine4.849.26Taurine 1.58.746 Cystine 1.657.856Thiolhistidine <1.5, 11.42.269.856 1.848.476 Diidotyrosine 6.48, 2.127.826Tryptophan 2.389.396 Glutamic acid 2.19, 4.259.676Tyrosine ethyl ester 7.339.8022 Glycine 2.349.66PeptidesHistidine 6.0, 1.829.176Anserine 7.0 2.659.56 Carnosine 6.83--9.516Hydroxylsine 2.138.626Cystinyldiglycine 3.12 6.3669.67 3.12 6.95 Isoleucine 2.369.686Glycylglycine 3.06 8.13 Lysine 2.188.956Gly-gly-gly 3.267.912310.53Glycylproline 2.848.556 O-Methyl tyrosine9.2721Aspartyl histi- 2.457.98dine 6.82 3.02Gly-gly-gly-gly 3.057.7523 Diglycylcystine 2.717.946Lysyl-lysine (L,L) 3.017.536 Glutathione 9.12 2.128.66610.0511.013.53Compound-COOH-NH2-NH2-NH2-NH2Ref. Gly•Ala (L) or (D) 3.178.2327 Ala•Gly (L) or (D) 3.168.2427 Gly•Ala•Ala (LL) 3.388.1027 Gly•Ala•Ala (LD) 3.308.1727 Ala•Ala•OH (DD) 3.308.1427 Ala•Ala•OH (LD) 3.128.3027 H•Ala•Ala•Ala•OH (3L) 3.398.0327 H•Ala•Ala•Ala•OH (LLD) 3.378.0527 H•Ala-Ala-Ala•OH (LDL) 3.318.1327 H•Ala-Ala-Ala•OH (DLL) 3.378.0627 H-Ala-Ala-Ala•OH (3D) 3.398.0627 H•Ala-Ala-Ala-Ala•OH (4L) 3.427.9427 H•Ala-Ala-Ala-Ala•OH (LLDL) 3.247.9327 H•Ala-Ala-Ala-Ala•OH (LDLL) 3.227.9927 H•Ala-Ala-Ala-Ala•OH (DLLL) 3.427.9927 H•Lys-Ala•OH (LL) 3.227.6210.7027 H•Lys-Ala•OH (LD) 3.007.7410.6327 H•Ala-Lys-Ala•OH (3L) 3.157.6510.3027 H•Ala-Lys-Ala•OH (LDL) 3.337.9710.3627 H•Ala-Lys-Ala•OH (LLD) 3.297.8410.4927 H•Ala-Lys-Ala-Ala•OH (4L) 3.588.0110.5827 H•Ala-Lys-Ala•OH (LDLL) 3.328.0110.3727 H•Ala-Lys-Ala-Ala-Ala•OH (5L) 3.537.7510.3527 H•Ala-Lys-Ala-Ala-Ala•OH (LDLLL) 3.307.8510.2927 H•Lys-Lys•OH (LL) 3.017.5310.0511.0127 H•Lys-Lys•OH (LD) 2.857.539.9210.9827 H•Lys-Lys•OH (3L) 3.087.349.8010.5411.3227 H•Lys-Lys-Lys•OH (LDL) 2.917.299.7910.5411.4227 H•Lys-Lys-Lys•OH (LDD) 2.947.149.6010.3811.0927 Compound pK ref.Glutathione 3.59, 8.75, 9.6577Glycylserine8.2377Glycylleucine8.1377Leucylglycine7.9677Glycylisoleucine7.9677Leucylglycylglycine7.6677Glycylphenylalanine8.2877Glycyltyrosine8.2277Benzylglutamic acid 3.49, 4.9977Glycyltryptophane8.0477Glutathione, oxidized 3.15, 4.03, 8.57, 9.5477Alanylalanine (LL) 3.308.1492Alanylalanine (LD) 3.128.3092Lysylalanine (LL) 3.227.6210.7092Lysylalanine (LD) 3.007.7410.6392Leucyltyrosine (LL) 3.467.8410.0992Leucyltyrosine (DL) 3.128.3810.3592Lysyllysine (LD) 2.857.539.9292NITROGEN COMPOUNDSAliphatic Amines pK ref.Ammonia9.211n-Propyl-10.531 Primary Amines Trimethylsilymethyl-10.961β-Alanine ester9.131CH3ONH2 4.6012 Allylamine-9.692Allyl-9.491 Benzyl9.341γ-Amino-n-butyric acid ester 9.711n-Butyl-10.591sec-Butyl-10.561t-Butyl-10.551Cyclohexyl-10.641 Cyclohexylmethyl-10.491β-difluoroethyl-7.521 Ethanol-9.501Ethyl10.631 Ethylenedi-9.98, 7.521, 77Glycine ester7.751 Hydrazine8.101Hydroxyl- 5.971 Isopropyl-10.631Methoxy- 4.601 Methyl-10.621neo-Pentyl-10.211 Phenylamyl-10.492δ-Phenylbutyl10.402β-Phenylethyl-9.831γ-Phenylpropyl-10.201Triethylenedi-8.8*?X XNH3+XCH2NH3+X(CH2)2NH3+X(CH2)3NH3+X(CH2)4NH3+X(CH2)5NH3+ref. H-9.25*10.64*10.67*10.58*10.61*10.63*2 HF2C-7.52RO2C-7.759.139.7110.15*10.372 HO- 5.96*9.50*C6H5- 4.58*9.37*9.83*10.20*10.39*10.49*2 H2N-8.12*9.98*10.65*10.84*11.05*2 H2C=CH-9.69CH3-10.64*10.67*10.58*10.61*10.63*10.64*2 X-H-NH3+-CO2–-SO3–-PO3–2X-NH3+9.25*-.88110.25X(CH2)2NH3+10.649.77 5.7510.8X(CH2)2NH3+10.6710.199.2010.8X(CH2)4NH3+10.619.3110.7710.6510.9X(CH2)5NH3+10.639.7410.7510.9511.0X(CH2)8NH3+10.6510.10X(CH2)10NH3+10.6411.3511.25X(CH2)3NH3+10.588.5910.4310.05Secondary amines Di-n-butyl-11.251 Dimethyl-10.641Diisobutyl-10.501Di-n-propyl-11.001α-Ethylpyrroline7.432 Diisopropyl-11.051α-Benzylpyrroline-7.082t-Butylcyclohexyl-11.2312-Methylpiperidine10.992α-Cyclohexylpyrroline7.952α-Cyclohexylpyrrolidine10.802α-(p-Tolyl)pyrroline7.592α-(p-Tolyl)pyrrolidine10.012α-Ethylpyrrolidine10.432N,O-dimethylhydroxylamine 4.7512α-Benzylpyrrolidine10.362Acetanilide+0.614N-methylhydroxylamine 5.9612*thermodynamic valueDiethyl-10.981Aliphatic Amines Methyl-β-diethylamino-ethyl-sulfide 1,2-Iminoethane 7.9871,2-Dimethyl-∆2-pyrroline 11.942cis-2,3-Iminobutane 8.7271-methyl-2-n-butyl-∆2-pyrroline 11.901,2-Imino-2-methylpropane 8.6171-Ethyl-2-methyl-∆2-pyrroline 11.9221,2-Iminobutane 8.2971-n-Butyl-2-methyl-∆2-pyrroline 11.902trans-2,3-Iminobutane 8.6971,2-Dimethyl-∆2-tetrahydropyridine11.572Secondary Amines N-Ethyl derivative of: 1,2-Imino-ethane7.937Allylmethyl-10.111Benzylethyl-9.681Trans-2,3-Iminobutane 9.477Morpholine 8.361Trimethylhydroxylamine 3.6512N-Benzoylpiperazine 7.781Dimethylethyl-9.991Di-sec-butyl-11.011Triethyl-10.651N-Methylmethoxyamine 4.751Dimethyl-n-butyl-10.021Pyrolidine 11.271Dimethyl-isopropyl-10.3011-Tosylpiperazine 7.39Dimethyl-t-butyl-10.521Benzylmethyl-9.581Tri-n-butyl-10.891Piperidine 11.221Diallylmethyl-8.791N-Carbethoxypiperazin 8.2811-n-Propylpiperidine 10.482Dietrimethylsilylmethyl-11.40110.110.15Diallyl-9.2919.8--5N-Methylhydroxyl- 5.9611,2-Dimethylpyrrolidine 10.262Trimethyleneimine 11.2911-Methyl-2-n-butylpyrrolidin 10.242Cis-2,6-dimethyl-piperidine 10.9231-Ethyl-2-methylpyrrolidine 10.6421-n-Butyl-2-methylpyrrolidine 10.4321-Ethyl-2-methylpyrrolidine 10.7021,2-Iminobutane 8.187Tertiary amines cis-2,3-Iminobutane 8.567Trimethyl-9.761N-dimethylhydroxylamine 5.2012Dimethyldiethyl-10.291Allyldimethyl 8.781Dimethyl-n-propyl-9.9911,2-Dimethylpiperidine 10.262Dimethyl-isobutyl-9.9111-Ethyl-2-methyl-∆2-tetrahydropyridine11.572Dimethyl-sec-butyl-10.401Tri-n-propyl-10.651Triallyl-8.311N-Allylpiperidine 9.6921-Diethylamino-hexane-thiol-(6)Cyanoamines2-Amino-2-cyanopropane 5.39N-piperidine-CH 2CN 4.558β-Isopropylaminopropionitrile 8.09Et 2NCN -2.08β-Diethylaminopropionitrile 7.69Et 2N(CH 2)2CN 7.658Et 2NCH 2CN 4.558Et 2N(CH 2)4CN 10.088Et 2N(CH 2)3CN 9.298Et 2NC(CH 3)2CN 9.138Et 2N(CH 2)5CN 10.468EtN(CH 2CN)2-0.68HN(CH 2CN)20.28EtN(CH 2CH 2CN)2 4.558HN(CH 2CH 2CN)2 5.268H 2NCH 2CN 5.348N(CH 2CH 2CN)3 1.18N-Amphetamine-(CH 2)2-CN 7.238N-piperidine-C(CH 3)2CN 9.228N-Norcodeine-(CH 2)2CN 5.688N-Methamphetamine-(CH 2)2CN 6.958Dimethylcyanimide 1.29Methyl cyanamide 1.29Diethylcyanimide 1.29Ethyl cyanamide 1.29Aminoacetonitrile 5.39Cyanamide 1.19Diethylaminoacetonitrile 4.59Dimethylaminoacetonitrile 4.29β-Aminopropionitrile7.79CF3CH2NHCH3 6.0510β-Dimethylaminopropionitrile7.09Phenylethylaminesβ,β"-Dicyanodiethylamine 5.292-phenylethylamine9.7811 For complex chelating agents of aliphatic amines,see also ref. 77.N-methyl-2-(3,4-dihydroxyphenyl)-ethylamine8.7811N-methyl-2-phenyl10.3111 Fluoro-substituted aminesEpinephrine8.5511 CF3CH2NH2 5.710Arterenol8.5511 CF3CH2N(CH3)2 4.7510R2R1CHCH2NHR4R3ref. 11R1R2R3R4pKH H H H9.78H H OH H8.90H OH OH H8.81OH H OH H8.67H OH H H9.22OH OH H H8.93OH OH OH H8.58H H H CH310.31H H OH CH39.31H OH OH CH28.62OH H OH CH38.89H OH H CH39.36OH OH H CH38.78OH OH OH CH38.55Ring amines and imines (in 80% methyl cellosolve) (ref. 2)Pentamethylene9.99Cyclotridecyl9.63 Hexamethylene10.00Cyclotetradecyl9.54 Heptamethylene9.77Cyclopentadecyl9.54 Octamethylene9.39Cycloheptadecyl9.57 Nonamethylene9.14Cyclooctadecyl9.54 Decamethylene9.04Undecamethylene9.14Amines otherDodecamethylene9.31Dimeoone 5.2318 Tridecamethylene9.35Phthalimide8.3018 Tetradecamethylene9.35Nitrourea 4.5718 Hexadecamethylene9.29Nitrourethane 3.2818 Heptadecamethylene9.27Diphenylthiocarbazone 4.56 Cyclohexyl9.82β,β,β"-Triaminotriethylamine Cycloheptyl9.998.42, 9.44, 10.1387 CyclooctylCyclononyl9.95Anilines Ref. 2Cyclodecyl9.85MonosubstitutedCycloundecyl9.71Substituent o m p Cyclododecyl9.62H- 4.62* 4.64* 4.58*。

pKa ValuesINDEXInorganic2Phenazine24 Phosphates3Pyridine25 Carboxylic acids4, 8Pyrazine26Aliphatic4, 8Aromatic7, 8Quinoline27Phenols9Quinazoline27Alcohols and oxygen acids10, 11Quinoxaline27Amino Acids12Special Nitrogen Compounds28Peptides13Hydroxylamines28Nitrogen Compounds14Hydrazines28Aliphatic amines15, 17, 19Semicarbazones28 Cyanoamines16Amidoximes28Anilines17, 18, 20Thiols29 Nucleosides21Carbon Acids30,31 Special Table Heterocycles22Indicators31Acridine23References32-34 Benzoquinoline24Cinnoline23Hydantoin24Imidazole24For complex chelating agents, see also reference 77.Note. This document was compiled by W.P. Jencks and has been added to by F.H. WestheimerACIDSCompound pK Ref.H3PO2 2.0, 2.23*28H2PO4–7.21*77 AgOH 3.964HPO4_12.32*77Al(OH)311.228H3PO3 2.028As(OH)39.2228H2PO3– 6.58*77H3AsO4 2.22, 7.0, 13.028H4P2O7 1.52*77H2AsO4– 6.98*77H3 P2O7– 2.36*77 HAsO4*11.53*77H2P2O7= 6.60*77As2O304H3AsO39.22*HP2O7=9.25*77H3BO39.23*28HReO4-1.2530H2B4O7 4.0034HSCN 4.0034H2SeO3 2.6, 8.3, 2.62*28 HB4O79.0034HSeO38.3277Be(OH)2 3.74H2SeO4Strong, 2.028 HBr-9.0031HOBr8.728HSeO4 2.0034 HOCl7.53, 7.4628, 33H3SiO310.034 HClO2 2.028H2SO3 1.9, 7.0, 1.76*28, 77 HClO3-1.0028H2SO4-3.0, 1.928 HClO4 (70%)-10.0031HSO37.21*77CH3SO3H-0.631HSO4– 1.99*77 HCN9.4034H2S2O4 1.929H2CO3 6.37, 6.35*, 3.5834, 32H2Se 3.89*77 HCO310.33*HSe–11.00*77H2CrO4-0.9830H2S7.00*77 HCrO4 6.50*2, 30HS–12.92*77 HOCN 3.9234HSbO211.034 HZ 3.17*, 0.59*77HTe 5.0034H2GeO38.59, 12.7234, 78H2Te 2.64, 11.034, 78 Ge(OH)48.68, 12.728H2TeO3 2.7, 8.028HI-10.031Te(OH)6 6.2, 8.828 HOI11.028H2VO4–8.9530 HIO30.828HVO4=14.430H4IO6– 6.0034H2CrO40.7477H5IO6 1.64, 1.55, 8.2734, 28HOCN 3.7377 HMnO4-2.2530HSCN0.8577 NH3OH* 5.98*H3PO2 1.0777 NH4*9.24*77H3PO4 2.12*77 HN3 4.72*77H2S2O30.60*, 1.72*77 HNO2 3.2928H3AuO313.3, 16.078 HNO3-1.328H3GaO310.32, 11.778N2H5+7.99*77H5IO6 3.29, 6.70, 15.078H2N2O27.0534(see above!)H2N2O2–11.034H4V6O17 1.9678H2OsO512.134H2NSO3H 1.080H2O15.7noneH3O+-1.7none* Indicates a thermodynamic value.Pb(OH)2 6.48 (10.92) 4 (78)PHOSPHATES AND PHOSPHONATES CF 3- 1.16, 3.9357CCl 3- 1.63, 4.8157Phosphates NH 3+CH 2- 2.35, 5.957CompoundpKRef.(–OOCCH 2)2NH +CH 2– --, 5.5757Phosphate 1.97, 6.82, 12.555CHCl 2- 1.14, 5.6157Glyceric acid 2-phosphate 3.6, 7.153CH 2CI- 1.40, 6.3057Enolpyruvic acid 3.5, 6.453CH 2Br- 1.14, 6.5257Methyl- 1.54, 6.3155(–OOCCH 22NH +(CH 2)2- --, 6.5457Ethyl- 1.60, 6.6255CH 2I- 1.30, 6.7257n-Propyl- 1.88,6.6755NH 3+CH 2CH 2- 2.45, 7.0057n-Butyl- 1.80, 6.8455Dimethyl- 1.2955C 6H 5CH=CH- 2.00, 7.157Di-n-propyl 1.5955HOCH 2- 1.91, 7.1557Di-n-butyl- 1.7255C 6H 5NH 2+(CH 2)3- 2.1, --57Glucose-3-0.84, 5.6756C 6H 5NH(CH 2)3---, 7.1757Glucose-4-0.84, 5.6756Br(CH 2)2- 2.25, 7.357α-glycero- 1.40, 6.4454CH 3(CH 2)5CH(COO –)---, 7.557β-glycero- 1.37, 6.3454C 6H 5CH 2- 2.3, 7.55573-phosphoglyceric acid 1.42, 3.4254NH 3+(CH 2)4)- 2.55, 7.55572-phosphoglyceric acid 1.42, 3.55, 7.1peroxymonophosphoric acid 4.0569NH 3+(CH 2)5- 2.6, 7.657diphosphoglyceric acid 7.40, 7.9954NH 3+(CH 2)10---, 8.0057glyceraldehyde- 2.10, 6.7554–OOC(CH 2)10---, 8.2557dioxyacetone- 1.77,6.4554(CH 3)3SiCH 2- 3.22, 8.7057hexose di- 1.52, 6.3154fructose-6-0.97, 6.1154C 6H 5CH 2- 3.3, 8.457glucose-6-0.94, 6.1154(C 6H 5)SC- 3.85, 9.0057glucose-1- 1.10, 6.1354adenylic acid 3.8?, 6.2?54Arylphosphonic acids inosinic acid 2.4?, 6.4?542X-RC 6H 3PO 3H 2ADP 2 strong, 6.654X R ATP 3 strong, 6.654Cl 4-O 2N 1.12, 6.1457pyrophosphoric acid 0.9, 2.0, 6.6, 9.454Br 5-O 2N (a), 6.1457phosphopyruvic acid 3.5, 6.3854Cl 5-Cl (a), 6.6357creatine phosphate 2.7, 4.554Cl H 1.63, 6.9857arginine phosphate 2.8, 4.5, 9.6, 11.254Br H 1.64, 7.0057arginine 2.02, 9.0, 12.554Br 5-CH 3 1.81, 7.1557amino phosphate (-0.9), 2.8, 8.254Cl 4-NH 2--, 7.3357trimetaphosphate 2.0577CH 3O 4-O 2N 1.53, 6.9657CH 3O H 2.16, 7.7757PhosphonatesCH 3O 4-O 2N --, 8.2257H 2O 3P(CH 2)4PO 3H 2 <2, 2.75, 7.54, 8.3857HO 4-O 2N 1.22, 5.3957H 2O 3P(CH 2)3PO 3H 2 <2, 2.65, 7.34, 8.3557O 2N H 1.45, 6.7457H 2O 3PCH 2CH(CH 3)PO 3H 2 <2, 2.6, 7.00, 9.2757F H 1.64, 6.8057H 2O 3PCH 2PO 3H 2 <2, 2.57, 6.87, 10.3357I H 1.74, 7.0657Methyl- 2.3557NH 2H --, 7.2957Ethyl- 2.4357CH 3H 2.10, 7.6857n-propyl- 2.4557C 6H 5H (a), 8.1357isopropyl- 2.55, 7.7557HOOC H1.71, 9.1757n-butyl- 2.59, 8.1957isobutyl- 2.70, 8.4357**These values were obtained in 50% ethanol.s-butyl- 2.74, 8.4857(a) The compounds were not sufficiently soluble.t-butyl- 2.79, 8.8857For graphical plots of a large number of substituted phosphorus compounds see 83.neopentyl- 2.84, 8.65571,1 Dimethylpropyl- 2.88, 8.9657n-hexyl- 2.6, 7.957triphosphate8.90, 6.26, 2.3077n-dodecyl---, 8.2557tetrametaphosphate 2.7477CH 3(CH 2)5CH(COOH)- 1, --57fluorophosphate0.55, 4.856Acetic acids, substituted Phosphonates (Ref. 2)H- 4.76*20 X-H-H-NH3+-NH3+O2N- 1.68*20 X(CH2)PO3H2 2.357.1 1.85 5.35(CH3)3N+- 1.83*20 X(CH2)2PO3H2 2.457.85 2.457.00(CH3)2NH+- 1.95*20 X(CH2)4PO3H2 2.557.55CH3NH2+- 2.16*20 X(CH2)5PO3 H2 2.67.65NH3+- 2.31*20 X(CH2)6PO2H2 2.67.9CH3SO2- 2.36*20 X(CH2)10PO2H28.00NC- 2.43*20 Phosphines in acetonitrile, see ref. 89.C6H5SO2- 2.4420HO2C 2.83*20 CARBOXYLIC ACIDSAliphatic C6H5SO- 2.6620 Compound pK Ref.F- 2.6620 Acetoacetic 3.586Cl- 2.86*20 Acetopyruvic 2.61, 7.85 (enol)6Br- 2.8620 Aconitic, trans- 2.80, 4.466Cl2- 1.2920 Betaine 1.846F2- 1.2420 Citric 3.09, 4.75, 5.416Br3-0.6620 Crotonic 4.696Cl3-0.6520 Dihydroxyfumaric 1.146F3-0.23 (-0.26) (2)20 Dethylenediamine- 2.00, 2.676HONC4 3.0120 tetraacetic 6.16, 10.26F3C- 3.07*20 Formic 3.77*2N3- 3.0320 Fumaric 3.03, 4.546I- 3.1220 Glyceric 3.556C6H5O- 3.1220 Glycollic 3.826C2H5O2C- 3.3520 Glyoxylic 3.326C6H5S- 3.52*20 Homogentistic 4.406CH3O- 3.5320α-keto-β-methyl valeric 2.36NCS- 3.5820 Lactic 3.866CH3CO- 3.58*20 Maleic 1.93, 6.586Malic 3.40, 5.26C2H5O- 3.6020 Oxaloacetic (trans-enol) 2.566n-C3H7O 3.6520 +(cis-enol) 2.15, 4.066n-C4H9O 3.6620 Protocatechuic 4.486sec.-C4H9O- 3.6720 Pyruvic 2.506HS- 3.67*20Tartaric + 2.99, 4.406i-C3H7O- 3.69*20 + or - 2.89, 4.406CH3S- 3.72*20 meso 3.22, 4.856i-C3H7S- 3.72*20 Vinylacetic 4.426C6H5CH2S- 3.73*20C2H5S- 3.74*20n-C3H7S- 3.77*20n-C4H9S- 3.81*20HO- 3.83*20–O3S- 4.0520(C6H5)3CS- 4.30*20C6H5- 4.31*20CH2-CH- 4.35*20* Indicates thermodynamic values.Unsaturated acids (25°)CompoundpK poundpK ref.trans-CH 3-CH=CHCO 2H 4.69*20H-CH 2CH 2CO 2H 4.88*2cis-CH 3-CH=CHCO 2H 4.44*2H-CH=CHCO 2H 4.25*2C 6H 5-CH 2CH 2CO 2H4.66*2C 6H 5CH 2CH 2CO 2H 4.66*2trans-C 6H 5-CH=CHCO 2H 4.44*2C 6H 5CH=CHCO 2H** 4.44*2m-CH 3OC 6H 4CH 2CH 2CO 2H4.65*2C 6H 5CH 2CH 2CO 2H 4.66*2C 6H 5CH=CHCO 2H**4.442m-CH 3OC 6H 4CH=CHCO 2H 4.38*2m-ClC 6H 4CH=CHCO 2H**4.29*2m-ClC 6H 4CH 2CH 2CO 2H 4.58*2Unsaturated acids, Cis- and Trans-C C R 2H R 1CO 2H C C R 2R 1HCO 2H Cis-Acid Trans-Acid R 1R 2cis-acid trans-acid Ref.H-H- 4.25*4.25*2CH 3-H- 4.44*4.69*2Cl-H- 3.323.652C 6H 5-H- 3.88*4.44*2ClC 6H 4H- 3.91 4.4126-BrC 6H 4H- 4.02 4.412CH 3-CH 3- 4.305.022C 6H 5-H- 5.26***5.58***22,4,6-(CH 3)3C 6H 2-H-6.12***5.70***2C 6H 5-CH 3- 4.98***5.98***2Dicarboxylic acids, unsaturated*Maleic 1.92, 6.232Alicyclic Dicarboxylic acidsCitraconic (Dimethylmaleic acid)2.29, 6.152cis-Caronic(1,1-dimethylcyclopropane-23-dicarboxylic acid 2.34*, 8.31*2Acetylenedicarboxylic 1.73, 4.402∆1-tetrahydrophthalic 3.01, 5.3421,2-trans-cyclopropanedicarboxylic3.65*, 5.13*2Bromomaleic 1.45, 4.622trans-caronic 3.82*, 5.32*2Bromofumaric 1.46, 3.5721,2-cis-cyclopropane-dicarboxylic3.33*, 6.47*2Chlorofumaric 1.78, 3.812Fumaric 3.02,4.382Mesaconic (Dimethylfumaric acid)**trans3.09,4.752***in 40% acetone Phthalic 2.95, 5.412*thermodynamicItaconic (1-Propene-2-3-dicarboxylic acid)3.85, 5.452Chloromaleic 1.72, 3.862AliphaticAlicyclic Dicarboxylic acidsCompound pK Ref Compound pK Ref 1,2-trans-Cyclopropane-cis-Ethyleneoxide-dicarboxylic 3.65, 5.132dicarboxylic 1.94, 3.922 trans-Ethyleneoxide-1,3-cis-Cyclobutane-dicarboxylic 1.93, 3.252dicarboxylic 4.03, 5.3121,3-trans -Cyclobutanedi-1,2-cis-Cyclopentane-carboxylic 3.81, 5.282dicarboxylic 4.37, 6.5121,2-trans-Cyyclopentane-1,3-cis-Cyclopentanedicarboxylic 3.89, 5.912dicarboxylic 4.23, 5.5321,3-trans-Cyclopentane-1,2-cisCyclohexane-dicarboxylic 4.40, 5.452dicarboxylic 4.34, 6.7621,2-trans-Cyclohexane-1,3 -cis-Cyclohexane-dicarboxylic 4.18, 5.932dicarboxylic 4.10, 5.4621,3-trans-Cyclohexane-1,4-cis-Cyclohexanedicarboxylic 4.31, 5.732di-carboxylic 4.44, 5.7921,4-trans-Cyclohexane-dicarboxylic 4.18, 5.422Dicarboxylic acids*oxalic 1.23, 4.192Succinic 4.19, 5.482 Malonic 2.83, 5.692 O-O’-Dimethyl- 3.77, 5.942 Methyl- 3.05, 5.762 (high melting)Ethyl- 2.99, 5.832 O-O’-Dimethyl- 3.94, 6.202n-propyl 3.00, 5.842 (low melting)i-propyl- 2.94, 5.882 O,O’-Diethyl- 3.63, 6.462 Dimethyl- 3.17, 6.062 (high melting)Methylethyl- 2.86, 6.412 O,O’-Diethyl- 3.51, 6.602Diethyl- 2.21, 7.292 (low melting)Ethyl-n-propyl- 2.15, 7.432Tetramethyl- 3.50, 7.282Di-n-propyl- 2.07, 7.512Glutaric 4.34, 5.422Adipic 4.42, 5.412B-Methyl 4.25, 6.222Pimelic 4.48, 5.422B-Ethyl 4.29, 6.332Suberic 4.52, 5.402B-n-Propyl 4.31, 6.392Azelaic 4.55, 5.412B,B-Dimethyl- 3.70, 6.292DL-1:2-Dichlorosuccinic 1.68, 3.1820 B,B-Methylethyl- 3.62, 6.702meso-1:2-Dichlorosuccinic 1.74, 3.2420 B,B-Diethyl- 3.62, 7.122DL-1:2-Dibromosuccinic 1.48, ----20 B,B-Di-n-propyl 3.69, 7.312meso-1:2-Dibromosuccinic 1.42, 2.9720D-Tartaric 3.03, 4.4520DL-1:2-Dimethylsuccinic 3.93, 6.0020DL-Tartaric 3.03, ----20meso-1:2-Dimethylsuccinic 3.77, 5.3620 meso-Tartaric 3.29, 4.9220*All are thermodynamic valuesAliphatic HO- 6.332Br- 6.082 Bicyclo[2.2.2]octane-1-carboxylic acids, 4-substitutedLysergic acid, etc.H- 6.752ergometrine 6.8, --2 C2H5O2C- 6.312Dihydroergometrine7.4, --2β-dihydrolysergol8.2, --2 NC- 5.902Lysergic acid7.8, 3.32C6H5O- 3.53* 3.95* 4.52*α-dihydrolysergic8.3, 3.62CH3- 3.91* 4.24* 4.34* ergometrinine7.3, --2(CH3)2CH- 4.35*α-dihydrolysergol8.3, --2(CH3)3N+- 1.37 3.45 3.436-methylergoline8.85, --2NC- 3.60* 3.55* isolysergic acid8.4, 3.42HO2C* 2.95* 3.54 3.51γ-dihydrolysergic8.6, 3.62F3C- 3.79HO- 2.98* 4.08* 4.58*I- 2.85* 3.86* Hydroxycyclohexanecarboxylic acids Cl- 2.94* 3.83* 3.99* Cyclohexanecarboxylic 4.902(CH3)3Si- 4.24* 4.27* cis-1,2 4.802C2H5O- 4.21* 4.17* 4.45* cis-1,3 4.602i-C3H7O- 4.24* 4.15* 4.68* cis-1,4 4.842n-C5H11O- 4.55* trans-1,2 4.682C6H5- 3.46*trans-1,3 4.822CH3CH2- 3.77 4.35* trans-1,4 4.682(CH3)3C- 3.46 4.28 4.40*–HO3P- 3.78 4.03 3.95 Aromaticbenzene-CO3H 4.20*2–O3S- 4.15 4.11 Anthracene-1-COOH 3.692H2N- 4.98 4.79 4.92 Anthracene-9-COOH 3.652(CH3)2N-8.42 5.10 5.03 naphthalene-2-COOH 4.172–HO3As- 4.22 Naphthalene-1-COOH 3.692–O2C- 5.41** 4.60 4.82CH3NH- 5.3 5.10 5.04 Substituted benzoic acids (ref. 2)COOH*thermodynamicfor complex chelating agents, see also ref. 84.see also page 9a for more carboxylic acids. Benzoic acid o m pOrtho-substituted benzoic acidsH- 4.20* 4.21*Benzoic acid pK Ref.O2N- 2.17* 3.45* 3.442-CH3- 3.91**2CH3CO-2-t-C4H9- 3.462CH3SO2- 3.64* 3.52*2,6-(CH3)2- 3.212CH3S-2,3,4,6-(CH3)4- 4.002HS-2,3,5,6-(CH3)4- 3.522Br- 2.85* 3.81* 4.00*2-C2H5- 3.772F- 3.27* 3.87* 4.14*CH3O- 4.09* 4.09* 4.47*2-C6H5- 3.46**2n-C3H7O- 4.24* 4.20* 4.46*2,4,6-(CH3)3- 3.432n-C4H9O- 4.25* 4.53*2,3,4,5-(CH3)4- 4.222 Benzene Polycarboxylic acids Ref. 2Acid Position of carboxyl pK I pK II pK III pK IV pK V pK VI Benzoic1 4.17*Phthalic1,2 2.98* 5.28*Isophthalic1,3 3.46* 4.46*Terephthalic1,4 3.51* 4.82*Hemimellitic1,2,3 2.80* 4.20* 5.87*Trimellitic1,2,4 2.52* 3.84* 5.20*8Trimesic 1,3,5 3.12* 3.89* 4.70*Mellophanic 1,2,3,4 2.06* 3.25* 4.73* 6.21*Prehnitic 1,2,3,5 2.38* 3.51* 4.44* 5.81*Pyromellitic1,2,4,5 1.92* 2.87* 4.49* 5.63*Benzenepentacarboxylic 1,2,3,4,5 1.80* 2.73* 3.97* 5.25* 6.46*Mellitic1.2,3,4,5,61.40*2.19*3.31*4.78*5.89*6.96**ionic strength 0.032-Methoxyethyliminodiacetic 2.2, 8.96**thermodynamic2-Methylthioethyliminodiacetic 2.1, 8.91oxalic acid* 1.25, 4.14N-n-propylaminoacetic 2.25, 10.03Carboxylic Acids Ref. 77N-2-sulfoethyliminodiacetic 1.92, 2.28, 8.16Aminomalonic acid* 3.32, 9.83α-Bromobutyric acid 2.97N-Butylaminoacetic acid 2.29, 10.07N-(carbamoylmethyl)-imino-diacetic acid2.30, 6.602-carboxyethyliminodiacetic acid2.06,3.69, 9.66Cyanomethyliminodiacetic 3.06, 4.34β-carboxymethylaminopropionic 3.61, 9.46α,β-diaminopropionic acid 1.23, 6.69α,α-diaminobutyric 1.85, 8.24, 10.44Diethylaminoacetic 2.04, 10.47Di-(carboxymethyl)-aminomethyl phosphonic acid 2.00, 2.25, 5.57, 10.76Dimethylaminoacetic 2.08, 9.80N-ethylaminoacetic 2.30, 10.10α,β-dimercaptosuccinic 2.40, 3.46, 9.44, 11.82Gluconic* 3.86β-hydroxybutyric 4.39Ethylenediamine-N,N-diacetic 5.58, 11.05α-hydroxybutyric 3.65β-hydroxypropionic 3.73N-2-hydroxyethyliminodiacetic 2.2, 8.73Iminodiacetic* 2.98, 9.893-hydroxypropyliminodiacetic 2.06, 9.24β-iodopropionic* 4.04Iminodipropionic 4.11, 9.61N-isopropylaminoacetic 2.36, 10.06Isobutyric* 4.86α-mercaptobutyric 3.53Mandelic acid 3.41N-methylaminoacetic 2.24, 10.012-MercaptoethyliminodiaceticNitrilotriacetic 3.03, 3.07, 10.-2.14, 8.17, 10.792-PhosphonoethyliminodiaceticMethyliminodiacetic 2.81, 10.181.95,2.45, 6.54, 10.46*ThermodynamicPHENOLSCompound pK pound pK Ref. Chromotropic acid 5.36, 15.66Resorcinol--, 9.15 (30o)50o-Methoxyphenol--, 9.9350p-Methoxyphenol--, 10.1650 o-Hydroxybenz-3-Hydroxyanthran-aldehyde7.9550ilic acid10.09, 5.20512-Amino-4,5 dimethyl-2-Aminophenolphenol hydrochloride10.4 5.2851hydrochloride9.99, 4.86514,5-dihydroxybenzene-1,3 disulphonic acid7.6612.6eKojic acid9.4077Phenol o m p Phenol o m pH-9.95*9.94*O2N-7.23*8.35*7.14* (CH3)3N+-7.4288OCH- 6.798.007.66CH3SO2-9.337.83NC-8.61**7.95CH3CO-9.198.05CH3O2C-8.47*C2H5O2C-8.50*n-C4H9O2C-8.47*C3H5CH2O2C-8.41*I-9.17*Br-8.42*9.11*9.34*Cl-8.48*9.02*9.38*F-8.81*9.28*9.95*CH3S-9.539.53HO-9.489.449.96HOCH2-9.92*9.83*9.82*CH3-10.28*10.08 10.19*C2H5-10.29.910.0CH3O-9.939.6510.20H2N-9.719.8710.30-O2C-9.94*9.39*-O3S-9.299.03--O3P-10.29.9--O3As8.37 C6H5-9.939.599.51NO- 6.35**2-Chloro-4-Nitro- 5.42792-Nitro-4-Chloro- 6.4679* Thermodynamic**Reference 52ALCOHOLS and other OXYGEN ACIDSAlcoholsCompound pK pound pK Ref. Choline13.96C3F7•CH(C2F5)•OH 10.4865 Chloral hydrate9.66, 11.061(C3F7)2CH•OH10.5265 Trifluoroethanol12.562Carbonium ionsCF3CH2OH11.4, 12.4363CF3CH(OH)CH311.863Triphenylmethanols in H2SO4 HC1O4 HNO3refCF3CH2(CH3)3OH12.43104,4,4-Trimethoxy.82. .82 .8066C3F7CH2OH11.4**634,4’-Dimethoxy-1.24-1.14-1.1166(C3F7)2CHOH10.6**634-Methoxy-3.40-3.59-3.4166HCCCH2OH13.55644-Methyl-5.41-5.6766C(CH2OH))414.1644-Trideuteriomethyl- 5.43 5.6766HOCH2CHOHCH2OH 4.4643,3’,3”-Trimethyl- 6.35-5.9566HOCH2CH2OH14.7764Unsubstituted triphenyl-CH3CCH2OH14.8264methanol- 6.63-6.89 6.6066CH3OH15.54644,4;,4;-Trichloro- 7.74-8.0166 CH2=CHCH2OH15.52644-Nitro-9.15-9.7666 H2O15.7464CCl3CH2OH 11.8***CH3CH2OH1664CF3CH2OH 11.3***Substituent effects for ionization of RCH2OHRCCl-312.24,11.8064,65CF3-12.3764CHF2CH2-12.7464Hydroxamic acidsCHCl2-12.8964Furo-8.4572CHEC-13.5564Glycine7.4072H2Cl-14.3164Hippuro-8.8072CH3CCH2-14.864iso Nicotin7.8572HOCH215.164p-Methylbenz-8.9072H-15.564Nicotin-8.3072CH2=CH-15.564Nicotin-methiodide 6.4672CH3-(extrap)(15.9)64m-Nitrobenz-8.0772CF3C(CH3)2OH11.664Picolin8.5072HOCH2CF2CH2OH1164Pyrimidine-2-carbox-7.8872Primary alcohols=R•CH2•OH and Salicyl-7.4372Secondary alcohols in 50% alcohol Tropo-9.0972C2F511.3565C4F911.3565C5F1111.3765C7F1511.3565Other oxygen acidsCHF212.0065Trimethylamine-n-oxide 4.618CF2Cl11.6365Dimethylglyoxime12.8477CHF2CF211.3465(50% dioxane)CHF2 • (CF2)211.3565O-methyl ether12.9277CF3 • CH212.765Tropolone12a77CF3 • (CH2)212.965 α-Bromotropolone 6.95a77CF3 • CHMe • OH11.2865Acetald hydrate13.4891C3F7 • CHMe • OH11.3865Formald hydrate13.2991C3F7CHEt • OH11.3765C3F7CHPr • OH11.3765C3F7 • CH(CF3) • OH10.4665a50% dioxane***50 aquaeous ethanolOTHER OXYGEN ACIDSHydroxamic acids Aceto-9.4068Compound pK Ref.n-Butyro-9.4868Pyridine oxidesn-Butyro-9.00684-Aminopyridine 1-oxide 3.6967p-Methoxybenzo-9.19684-Dimethylaminopyridine 1-oxide3.8867N-Hydroxyphthalimide 7.00, 6.1071, 72Salicylo 7.32684-Dimethylaminopyridine 1-oxide3.8867Benzo-8.8868p-Chlorobenzo-9.59684-Dimethylamino-1-methoxypyridinium perchlorate >1167α-Naphtho-~7.768Propiono-9.46682-Methylaminopyridine 1-oxide 2.61672-Amino-1-methoxypyridinium perchlorate12.467Oximes4-Hydroxypyridine 1-oxide 2.4567Benzophenone oxime 11.3184-Methoxypyridine 1-oxide 2.0567Diethyl ketoxime 12.6181-Methoxypyridi-4-one 2.5767Isonitrosoacetylacetone (INAA) 7.4762-Hydroxypyridine 1-oxide -0.8675-Methyl-1,2,3-cyclohexanetrione-1,3-dioxime8.3762-Ethoxypyridine 1-oxide 1.18671-Methoxypyrid-2-one -1.3Acetophenone oxime 11.48184-Methylaminopyridine 1-oxide 3.8567Acetoxime 11.42184-Amino-1-methoxypyridinium perchlorate>1167Isonitrosoacetone (INA) 8.376Salicyclaldoxime (SA)9.2762-Aminopyridine 1-oxide 2.67671,2,3-Cyclohexanetrionetrioxime 8.0762-Dimethylaminopyridine 1-oxide2.27675-Methyl-1,2,3-cyclohexane-trionetrioxime8.0762-Methylamino-1-methoxypyridinium toluene-p-sulphonate >11674-Benzyloxypyridine 1-oxide 1.9967Oxygen acids1-Benzyloxypyrid-4-one 2.5867sulfinic acids 2-Methoxypyridine 1-oxide 1.2367p-Toluene- 1.99731-Benzyloxypyrid-2-one -1.767p-Chlorobenzene-73p-Nitrobenzene-73Pyridine 1-oxides p-Bromobenzene- 1.8973RpK Ref.m-Nitrobenzene- 1.88734-CH 3 1.2947Benzene-1.84,2.16733-CH 3 1.0847Peroxyacids3,4-(CH)4 1.0147Peroxymonosulfuric 9.4693-COOC 4H 90.0347Acetic 8.2704-NO 2-1.747n-Butyric 8.2703-NH 2 1.4747Formic 7.170H0.7947Propionic 8.1703-COOH 0.0947peroxydiphosphoric 5.18, 7.8854-COOH-0.4847peroxymonophosphoric 4.8590Peroxides ROOH (Ref. 70)H CH 3C 2H 5iso-C 3H 7tert-C 4H 9iso-C 4H 911.611.511.812.112.812.8Oximesref. 93Pyridine-2-aldoxime heptiodide 8.00benzoquinoline mon- 6.25Pyridine-4-aldoxime methiodide 8.503-pyridine-1,2-ethanedione-2-oxime methiodide7.20Pyridine-4-aldoxime pentiodide 8.504-Pyridine-1,2-ethanedione-2-oxime methiodide7.1O-Methyltyrosine ethyl ester 7.3122 octopine 13, 1.368.776Pyridine-2-aldoxime methiodide8.0Phenylglyoxald-8.3 2.40Pyridine-4-aldoxime dodeciodide8.5Phenylalanine 1.839.136 Pyridine-3-alkoxime methiodide9.22-Pyrrolidoone-5-carboxylic acid (glucamicacid) 3.32Hydroxamic acids ref. 93Serine 2.219.156 D-Lysine-7.93Threonine 2.6310.436 N-phenylnicotino-8.00N-Trimethyl tyrosine9.7521 Chloroaceto-8.40Tyrosine 10.07, 2.209.11 Formo-8.65Urocanic acid 5.8 3.5p-Chlorophenoxyaceto-8.75Valine 2.329.626 p-Hydroxybenzo-8.93β-Alanine 3.6010.196 p-Methoxybenzo-9.00γ-Aminobutyric acid 4.2310.436 N-Phenylbenzo-9.15Arginine 12.48 2.179.046 o-Aminobenzo-9.17Asparagine 2.028.86 L-Tyrosine9.20Azaserine8.556 L-Lysine7.9Canavanine7.40, 9.2511.50 (?)6 p-Nitrobenzo-8.0Creatine 2.6711.026 p-Aminobenzo-9.3Cysteine 10.78 1.718.336 L-Lacti-9.33,4-DihydroxyphenylalaninePropiono-9.49.88, 2.368.686 Phthalo-9.411.68Indole-3-aceto-9.5Glutamine 2.179.136 Cyclohexano-9.7Histamine 5.09.76 Hexano-9.7β-Hydroxyglutamic 2.099.206acid 4.18Amino Acids Hydroxyproline 1.929.736 Compound pK Ref.Leucine 2.369.606-COOH-NH3Methionine 2.289.21 Alanine 2.359.6961-Methylhistidine 6.48, 1.698.856α-Aminobutyric acid 2.559.60Norleucine 2.399.766α-Aminoisobutyric 2.3610.216Norvaline 2.369.766 Argininosuccinic >12, 1.629.586Ornithine 1.718.6962.70, 4.2610.76 Aspartic acid 2.09,3.869.826Proline 1.9910.606 Canaline10.3, 9.2011.6 (?)6Sarcosine 2.2310.016 Creatinine4.849.26Taurine 1.58.746 Cystine 1.657.856Thiolhistidine <1.5, 11.42.269.856 1.848.476 Diidotyrosine 6.48, 2.127.826Tryptophan 2.389.396 Glutamic acid 2.19, 4.259.676Tyrosine ethyl ester 7.339.8022 Glycine 2.349.66PeptidesHistidine 6.0, 1.829.176Anserine 7.0 2.659.56 Carnosine 6.83--9.516Hydroxylsine 2.138.626Cystinyldiglycine 3.12 6.3669.67 3.12 6.95 Isoleucine 2.369.686Glycylglycine 3.06 8.13 Lysine 2.188.956Gly-gly-gly 3.267.912310.53Glycylproline 2.848.556 O-Methyl tyrosine9.2721Aspartyl histi- 2.457.98dine 6.82 3.02Gly-gly-gly-gly 3.057.7523 Diglycylcystine 2.717.946Lysyl-lysine (L,L) 3.017.536 Glutathione 9.12 2.128.66610.0511.013.53Compound-COOH-NH2-NH2-NH2-NH2Ref. Gly•Ala (L) or (D) 3.178.2327 Ala•Gly (L) or (D) 3.168.2427 Gly•Ala•Ala (LL) 3.388.1027 Gly•Ala•Ala (LD) 3.308.1727 Ala•Ala•OH (DD) 3.308.1427 Ala•Ala•OH (LD) 3.128.3027 H•Ala•Ala•Ala•OH (3L) 3.398.0327 H•Ala•Ala•Ala•OH (LLD) 3.378.0527 H•Ala-Ala-Ala•OH (LDL) 3.318.1327 H•Ala-Ala-Ala•OH (DLL) 3.378.0627 H-Ala-Ala-Ala•OH (3D) 3.398.0627 H•Ala-Ala-Ala-Ala•OH (4L) 3.427.9427 H•Ala-Ala-Ala-Ala•OH (LLDL) 3.247.9327 H•Ala-Ala-Ala-Ala•OH (LDLL) 3.227.9927 H•Ala-Ala-Ala-Ala•OH (DLLL) 3.427.9927 H•Lys-Ala•OH (LL) 3.227.6210.7027 H•Lys-Ala•OH (LD) 3.007.7410.6327 H•Ala-Lys-Ala•OH (3L) 3.157.6510.3027 H•Ala-Lys-Ala•OH (LDL) 3.337.9710.3627 H•Ala-Lys-Ala•OH (LLD) 3.297.8410.4927 H•Ala-Lys-Ala-Ala•OH (4L) 3.588.0110.5827 H•Ala-Lys-Ala•OH (LDLL) 3.328.0110.3727 H•Ala-Lys-Ala-Ala-Ala•OH (5L) 3.537.7510.3527 H•Ala-Lys-Ala-Ala-Ala•OH (LDLLL) 3.307.8510.2927 H•Lys-Lys•OH (LL) 3.017.5310.0511.0127 H•Lys-Lys•OH (LD) 2.857.539.9210.9827 H•Lys-Lys•OH (3L) 3.087.349.8010.5411.3227 H•Lys-Lys-Lys•OH (LDL) 2.917.299.7910.5411.4227 H•Lys-Lys-Lys•OH (LDD) 2.947.149.6010.3811.0927 Compound pK ref.Glutathione 3.59, 8.75, 9.6577Glycylserine8.2377Glycylleucine8.1377Leucylglycine7.9677Glycylisoleucine7.9677Leucylglycylglycine7.6677Glycylphenylalanine8.2877Glycyltyrosine8.2277Benzylglutamic acid 3.49, 4.9977Glycyltryptophane8.0477Glutathione, oxidized 3.15, 4.03, 8.57, 9.5477Alanylalanine (LL) 3.308.1492Alanylalanine (LD) 3.128.3092Lysylalanine (LL) 3.227.6210.7092Lysylalanine (LD) 3.007.7410.6392Leucyltyrosine (LL) 3.467.8410.0992Leucyltyrosine (DL) 3.128.3810.3592Lysyllysine (LD) 2.857.539.9292NITROGEN COMPOUNDSAliphatic Amines pK ref.Ammonia9.211n-Propyl-10.531 Primary Amines Trimethylsilymethyl-10.961β-Alanine ester9.131CH3ONH2 4.6012 Allylamine-9.692Allyl-9.491 Benzyl9.341γ-Amino-n-butyric acid ester 9.711n-Butyl-10.591sec-Butyl-10.561t-Butyl-10.551Cyclohexyl-10.641 Cyclohexylmethyl-10.491β-difluoroethyl-7.521 Ethanol-9.501Ethyl10.631 Ethylenedi-9.98, 7.521, 77Glycine ester7.751 Hydrazine8.101Hydroxyl- 5.971 Isopropyl-10.631Methoxy- 4.601 Methyl-10.621neo-Pentyl-10.211 Phenylamyl-10.492δ-Phenylbutyl10.402β-Phenylethyl-9.831γ-Phenylpropyl-10.201Triethylenedi-8.8*?X XNH3+XCH2NH3+X(CH2)2NH3+X(CH2)3NH3+X(CH2)4NH3+X(CH2)5NH3+ref. H-9.25*10.64*10.67*10.58*10.61*10.63*2 HF2C-7.52RO2C-7.759.139.7110.15*10.372 HO- 5.96*9.50*C6H5- 4.58*9.37*9.83*10.20*10.39*10.49*2 H2N-8.12*9.98*10.65*10.84*11.05*2 H2C=CH-9.69CH3-10.64*10.67*10.58*10.61*10.63*10.64*2 X-H-NH3+-CO2–-SO3–-PO3–2X-NH3+9.25*-.88110.25X(CH2)2NH3+10.649.77 5.7510.8X(CH2)2NH3+10.6710.199.2010.8X(CH2)4NH3+10.619.3110.7710.6510.9X(CH2)5NH3+10.639.7410.7510.9511.0X(CH2)8NH3+10.6510.10X(CH2)10NH3+10.6411.3511.25X(CH2)3NH3+10.588.5910.4310.05Secondary amines Di-n-butyl-11.251 Dimethyl-10.641Diisobutyl-10.501Di-n-propyl-11.001α-Ethylpyrroline7.432 Diisopropyl-11.051α-Benzylpyrroline-7.082t-Butylcyclohexyl-11.2312-Methylpiperidine10.992α-Cyclohexylpyrroline7.952α-Cyclohexylpyrrolidine10.802α-(p-Tolyl)pyrroline7.592α-(p-Tolyl)pyrrolidine10.012α-Ethylpyrrolidine10.432N,O-dimethylhydroxylamine 4.7512α-Benzylpyrrolidine10.362Acetanilide+0.614N-methylhydroxylamine 5.9612*thermodynamic valueDiethyl-10.981Aliphatic Amines Methyl-β-diethylamino-ethyl-sulfide 1,2-Iminoethane 7.9871,2-Dimethyl-∆2-pyrroline 11.942cis-2,3-Iminobutane 8.7271-methyl-2-n-butyl-∆2-pyrroline 11.901,2-Imino-2-methylpropane 8.6171-Ethyl-2-methyl-∆2-pyrroline 11.9221,2-Iminobutane 8.2971-n-Butyl-2-methyl-∆2-pyrroline 11.902trans-2,3-Iminobutane 8.6971,2-Dimethyl-∆2-tetrahydropyridine11.572Secondary Amines N-Ethyl derivative of: 1,2-Imino-ethane7.937Allylmethyl-10.111Benzylethyl-9.681Trans-2,3-Iminobutane 9.477Morpholine 8.361Trimethylhydroxylamine 3.6512N-Benzoylpiperazine 7.781Dimethylethyl-9.991Di-sec-butyl-11.011Triethyl-10.651N-Methylmethoxyamine 4.751Dimethyl-n-butyl-10.021Pyrolidine 11.271Dimethyl-isopropyl-10.3011-Tosylpiperazine 7.39Dimethyl-t-butyl-10.521Benzylmethyl-9.581Tri-n-butyl-10.891Piperidine 11.221Diallylmethyl-8.791N-Carbethoxypiperazin 8.2811-n-Propylpiperidine 10.482Dietrimethylsilylmethyl-11.40110.110.15Diallyl-9.2919.8--5N-Methylhydroxyl- 5.9611,2-Dimethylpyrrolidine 10.262Trimethyleneimine 11.2911-Methyl-2-n-butylpyrrolidin 10.242Cis-2,6-dimethyl-piperidine 10.9231-Ethyl-2-methylpyrrolidine 10.6421-n-Butyl-2-methylpyrrolidine 10.4321-Ethyl-2-methylpyrrolidine 10.7021,2-Iminobutane 8.187Tertiary amines cis-2,3-Iminobutane 8.567Trimethyl-9.761N-dimethylhydroxylamine 5.2012Dimethyldiethyl-10.291Allyldimethyl 8.781Dimethyl-n-propyl-9.9911,2-Dimethylpiperidine 10.262Dimethyl-isobutyl-9.9111-Ethyl-2-methyl-∆2-tetrahydropyridine11.572Dimethyl-sec-butyl-10.401Tri-n-propyl-10.651Triallyl-8.311N-Allylpiperidine 9.6921-Diethylamino-hexane-thiol-(6)Cyanoamines2-Amino-2-cyanopropane 5.39N-piperidine-CH 2CN 4.558β-Isopropylaminopropionitrile 8.09Et 2NCN -2.08β-Diethylaminopropionitrile 7.69Et 2N(CH 2)2CN 7.658Et 2NCH 2CN 4.558Et 2N(CH 2)4CN 10.088Et 2N(CH 2)3CN 9.298Et 2NC(CH 3)2CN 9.138Et 2N(CH 2)5CN 10.468EtN(CH 2CN)2-0.68HN(CH 2CN)20.28EtN(CH 2CH 2CN)2 4.558HN(CH 2CH 2CN)2 5.268H 2NCH 2CN 5.348N(CH 2CH 2CN)3 1.18N-Amphetamine-(CH 2)2-CN 7.238N-piperidine-C(CH 3)2CN 9.228N-Norcodeine-(CH 2)2CN 5.688N-Methamphetamine-(CH 2)2CN 6.958Dimethylcyanimide 1.29Methyl cyanamide 1.29Diethylcyanimide 1.29Ethyl cyanamide 1.29Aminoacetonitrile 5.39Cyanamide 1.19Diethylaminoacetonitrile 4.59Dimethylaminoacetonitrile 4.29β-Aminopropionitrile7.79CF3CH2NHCH3 6.0510β-Dimethylaminopropionitrile7.09Phenylethylaminesβ,β"-Dicyanodiethylamine 5.292-phenylethylamine9.7811 For complex chelating agents of aliphatic amines,see also ref. 77.N-methyl-2-(3,4-dihydroxyphenyl)-ethylamine8.7811N-methyl-2-phenyl10.3111 Fluoro-substituted aminesEpinephrine8.5511 CF3CH2NH2 5.710Arterenol8.5511 CF3CH2N(CH3)2 4.7510R2R1CHCH2NHR4R3ref. 11R1R2R3R4pKH H H H9.78H H OH H8.90H OH OH H8.81OH H OH H8.67H OH H H9.22OH OH H H8.93OH OH OH H8.58H H H CH310.31H H OH CH39.31H OH OH CH28.62OH H OH CH38.89H OH H CH39.36OH OH H CH38.78OH OH OH CH38.55Ring amines and imines (in 80% methyl cellosolve) (ref. 2)Pentamethylene9.99Cyclotridecyl9.63 Hexamethylene10.00Cyclotetradecyl9.54 Heptamethylene9.77Cyclopentadecyl9.54 Octamethylene9.39Cycloheptadecyl9.57 Nonamethylene9.14Cyclooctadecyl9.54 Decamethylene9.04Undecamethylene9.14Amines otherDodecamethylene9.31Dimeoone 5.2318 Tridecamethylene9.35Phthalimide8.3018 Tetradecamethylene9.35Nitrourea 4.5718 Hexadecamethylene9.29Nitrourethane 3.2818 Heptadecamethylene9.27Diphenylthiocarbazone 4.56 Cyclohexyl9.82β,β,β"-Triaminotriethylamine Cycloheptyl9.998.42, 9.44, 10.1387 CyclooctylCyclononyl9.95Anilines Ref. 2Cyclodecyl9.85MonosubstitutedCycloundecyl9.71Substituent o m p Cyclododecyl9.62H- 4.62* 4.64* 4.58*。


pKa值是一个物质的酸性或碱性强弱的指标,是定义为该物质在溶液中从酸性形式转化为碱性形式或者从碱性形式转化为酸性形式时所需的溶液pH值。
通过pKa值,我们可以了解到溶液中该物质的离子化程度,从而对其在溶液中的化学性质有更深入的理解。
2羟基丁酸和2氧亚基丁酸是两种在生物体内具有重要生物学功能的有机酸。
它们在生物体内通过不同的离子化状态发挥着不同的生物活性,因此研究它们的pKa值对于了解它们在生物体内的作用机制具有重要意义。
1. 2羟基丁酸的pKa值2羟基丁酸(2-hydroxybutanoic acid),也称丙酮酸(alpha-hydroxybutyric acid),是一种重要的代谢产物,在人体内主要起能量供给作用。
它的pKa值决定了其在生物体内的离子化状态,从而影响其在生物学过程中的作用方式。
研究表明,2羟基丁酸的pKa值约为4.5-5.0,这意味着在生物体内pH约为7.4的情况下,2羟基丁酸主要呈现为离子态。
这种离子态的2羟基丁酸能够参与到酮体代谢途径中,通过与乙酰辅酶A结合形成乙酰乙酸,进而参与三羧酸循环并提供能量。
了解并理解2羟基丁酸的pKa值对于深入了解其在生物代谢过程中的作用机制至关重要。
2. 2氧亚基丁酸的pKa值2氧亚基丁酸(2-oxobutanoic acid)是丁酮酸(alpha-ketobutyric acid)的另一种名称,它也是一种重要的代谢产物,参与到生物体内的能量代谢过程中。
它的pKa值同样对于了解其在生物体内的生物活性具有重要意义。
研究表明,2氧亚基丁酸的pKa值约为2.0-2.4,比2羟基丁酸的pKa值要低得多。
低pKa值意味着在生物体内,2氧亚基丁酸更容易以其酸性形式存在。
在生物体内,2氧亚基丁酸可以通过与辅酶A结合形成乙醇辅酶A,进而参与到糖酵解途径中,为生物体提供能量。
3. 研究意义通过比较和了解2羟基丁酸和2氧亚基丁酸的pKa值,我们可以发现它们在生物代谢途径中的不同作用方式。

部分酸的PKa值砷酸H3AsO4 PKa=2.2 7.00 11.50 亚砷酸HAsO2PKa=9.22硼酸H3BO3 PKa=9.24焦硼酸H2B4O7PKa=4 9碳酸H2CO3 PKa=6.38 10.21氢氰酸HCN PKa=9.21铬酸H2CrO4PKa=0.74 6.50氢氟酸HF PKa=3.18亚硝酸HNO2PKa=3.29过氧化氢H2O2PKa=11.75磷酸H3PO4PKa=2.12 7.2 12.36 焦磷酸H4P2O7 PKa=1.52 2.36 6.60 9.25 亚磷酸H3PO3 PKa=1.3 6.60氢硫酸H2S PKa1=7.05 18.15硫酸H2SO4 PKa2=1.99亚硫酸H2SO3 PKa=1.9 7.20偏硅酸H2SiO3PKa=9.77 11.8甲酸HCOOH PKa=3.74乙酸CH3COOH PKa=4.74一氯乙酸CH2ClCOOH PKa=2.86二氯乙酸CHCl2COOH PKa=1.3三氯乙酸Cl3COOH PKa=0.64氨基乙酸盐+NH3CH2COOH PKa=2.35 9.6 乳酸CH3CHOHCOOH PKa=3.86苯甲酸C6H5COOH PKa=4.21草酸H2C2O4PKa=1.22 4.19d-酒石酸HOOCHOHC-CHOHCOOH PKa=3.04 4.37 邻-苯二甲酸HOOC-Ph-COOH PKa=2.95 5.41 柠檬酸HOOCOHC-(CH2COOH)2PKa=3.13 4.76 6.4 苯酚C6H5OH PKa=9.95乙二胺四乙酸H6-EDTA2+ PKa=0.9 1.6 2.0 2.67 6.16 10.26 铵离子NH4+PKa=9.26联铵离子+H3NNH3+PKa=8.48羟铵离子NH3+OH PKa=5.96甲胺离子CH3NH3+ PKa=10.62乙胺离子C2H5NH3+PKa=10.75二甲胺离子(CH3)2NH2+PKa=10.07二乙胺离子(C2H5)2NH2+PKa=11.11乙醇胺离子HOCH2CH2NH3+PKa=9.50三乙醇胺离子(HOCH2CH2)3NH+ PKa=7.76六亚甲基四胺离子(CH2)6NH+ PKa=5.15乙二胺离子+H3NCH2CH2NH3+PKa1=6.85 PKa2=9.93部分有机酸甲酸HCOOH PKa=3.77 乙酸CH3COOH PKa=4.74 丙酸CH3CH2COOH PKa=4.87 丁酸CH3CH2CH2COOH PKa=4.82阿尔法氯代丁酸CH3CH2CHClCOOH PKa=2.84 贝塔氯代丁酸CH3CHClCH2COOH PKa=4.06 伽马氯代丁酸ClCH2CH2CH2COOH PKa=4.52 氯乙酸ClCH2COOH PKa=2.86 二氯乙酸(Cl)2CHCOOH PKa=1.26 三氯乙酸(Cl)3CCOOH PKa=0.64 氟乙酸FCH2COOH PKa=2.66 氯乙酸ClCH2COOH PKa=2.86 溴乙酸BrCH2COOH PKa=2.86 碘乙酸ICH2COOH PKa=3.21。

有机化学中的取代基效应在绪论中,我们提到了有机化合物可以按分子内的官能团进行分类。
官能团的性质在较大程度上决定了这类化合物的理化性质。
例如,羟基化合物一般都可以电离出质子,显示出酸性。
醇类化合物一般属于弱酸性物质,其酸性比水还弱。
酚类化合物的酸性则比水的明显要强,羧酸的酸性更强,属于中等强度的酸。
磺酸是一类酸性与硫酸相当的强酸。
几个代表性羟基化合物的pKa如下所示:羟基化合物(CH3)3COH CH3OH H2O C6H5OH CH3COOH CF3COOH C6H5SO3H pKa 18 17 15.74 9.99 4.76 0.23 -6.5很显然,不同类型的羟基化合物所呈现的酸性强度存在巨大的差别。
那么,究竟是何种因素导致酸性的这种巨大差异呢?第一章中已经述及,一种物质的酸性强弱取决于其电离出质子的能力大小,而质子的电离能力又取决于O–H键的键能和极性大小。
因此,上述pKa 值的差异说明,与羟基氧原子相连的基团的性质对O–H键的键能和极性产生了很大的影响。
为了更好地了解这些基团的影响,本章将系统地对这些基团影响官能团性质的方式进行介绍。
第一节共价键的极性与诱导效应有机化合物的基本结构是由碳氢所组成的。
由于碳原子和氢原子的电负性非常接近,分别为2.2和2.1,它们形成共价键时,成键的共用电子对在碳原子和氢原子核外出现的几率十分接近,这种共价键的极性很低,称为非极性共价键。
除了C–H键以外,常见的非极性共价键还包括C–C、C=C和C≡C键等。
形成共价键的两个原子若电负性差别较大,那么成键电子对出现在电负性大的原子核周围的几率会大于出现在电负性小的原子核周围的几率,这样就使得该共价键呈现极性。
常见的极性共价键包括C–X(碳卤键)、C–O、C=O、C–N、O–H、N–H、C=N、C≡N、N=O等。
一、诱导效应的定义共价键极性的产生会进一步影响分子内其它原子核周围的电子云密度分布情况。
以正丙烷分子为例,它属于非极性分子,其分子内各碳原子周围的电子云密度基本相同。
pKa(酸度系数)详细资料大全1.酸度系数。
2.cAMP依赖蛋白激酶,简称激酶A(PKA——protein kinase A)。
cAMP发挥各种效应主要是通过激活它来实现的。
它的重要作用是使某些基因调节蛋白磷酸化,进而激活特定的基因转录。
3.药理学名词。
4.物理学中名词。
基本介绍•中文名称:pKa•外文名称:protein kinase A•别名:激酶A•成分:蛋白激酶•作用:使某些基因调节蛋白磷酸酸度系数,概述,酸碱度关系,影响因素,重要性,一般物质的值,蛋白激酶 A,药理学中的PKa,物理学中的PKA,酸度系数概述酸度系数,又名酸离解常数,代号K a值,在化学及生物化学中,是指一个特定的平衡常数,以代表一种酸离解氢离子的能力。
该平衡状况是指由一种酸(HA)中,将氢离子(即一粒质子)转移至水(H2O)。
水的浓度([H 2O])是不会在系数中显示的。
离解的化学反应为:HA+H 2O≒A - +H 3O +平衡状况亦会以氢离子来表达,反映出酸质子理论:平衡常数的方程式为:由于在不同的酸这个常数会有所不同,所以酸度系数会以常用对数的加法逆元,以符号p K a,来表示:一般来说,较大的K a值(或较小的p K a值)代表较强的酸,这是由于在同一的浓度下,离解的能力较强。
利用酸度系数,可以容易的计算酸的浓度、共轭碱、质子及氢氧离子。
如一种酸是部份中和,K a 值是可以用来计算出缓冲溶液的pH值。
在亨德森-哈塞尔巴尔赫方程亦可得出以上结论。
酸碱度关系由于HA与A的电离作用就等同于水的自我离子化,酸度系数与碱度系数的积就相等于水的离解常数(K w),在25℃下即1.0 × 10 -14。
由于K a与K b的积是一常数,较强的酸即代表较弱的共轭碱;较弱的酸,则代表较强的共轭碱。
影响因素作为一个平衡常数,酸度系数K a是以反应物与化合物,更准确的应是质子化状态(AH)与脱质子化状态(A)的自由能差ΔG°来计算。
第五章 有机化合物的酸碱性酸碱是化学中的重要概念,从广义的角度讲,多数的有机化学反应都可以被看作是酸碱反应。
因此,酸碱的概念在有机化学中有着重要的应用,在学习有机化学的时候,学习与了解有机化合物的酸碱性是十分必要的。
5.1 Brönsted 酸碱理论1923年,为了克服S. A. Arrehenius 依据电离学说,所提出的水溶液中酸碱理论的不足,丹麦的J. N. Brönsted 和英国的J. M. Lowry 分别独立地提出了新的酸碱理论。
该理论给出的酸碱定义为:凡是能给出质子的任何物质(分子或离子),叫做酸;凡是能接受质子的任何物质,叫做碱。
简言之,酸是质子的给予体,碱是质子的接受体。
因此,Brönsted 酸碱理论又称为质子酸碱理论。
依据Brönsted 酸碱理论,酸给出质子后产生的碱,称之为酸的共轭碱;碱接受质子生成的物质就是它的共轭酸。
即:酸碱 +质子CH3CO 2H CH 3CO 2- + H +C2H 5OHC 2H 5O - + H +可以看出,CH 3CO 2H 给出质子是酸,生成的CH 3CO 2―则是碱。
这样的一对酸碱,称为共轭酸碱对。
C 2H 5OH 和C 2H 5O ―也是如此。
酸、碱的电离可以看作是两对酸碱的反应过程。
例如:CH3CO 2H + H 2OCH 3CO 2- + H 3O +酸1 + 碱2碱1 + 酸2H2O + CH 3NH 2OH - + CH 3NH 3+醋酸在水中的电离,CH 3CO 2H 给出一个质子是酸,H 2O 接受一个质子为碱。
这里,CH 3CO 2H/CH 3CO 2―与H 2O/H 3O +分别是两个共轭酸碱对。
但是,甲胺在水中电离时,H 2O 给出一个质子是酸,CH 3NH 2接受一个质子为碱。
H 2O/OH ―与CH 3NH 2/CH 3NH 3+分别是两个共轭酸碱对。
由此可见, Brönsted 理论中的酸碱概念是相对的。
pka的计算例子pKa是表示酸碱强弱的指标,是酸碱性常数的负对数。
在化学中,pKa值的计算是非常重要的,它可以用来预测化合物的酸碱性质,以及在化学反应中的反应速率和平衡位置。
下面将列举一些pKa的计算例子,以帮助读者更好地理解和应用这一概念。
1. 甲酸(HCOOH)和乙酸(CH3COOH)的pKa值分别为3.75和4.76。
这意味着在等浓度下,甲酸的酸性比乙酸强,其pKa值越小,酸性越强。
2. 醋酸(CH3COOH)和水(H2O)的pKa值分别为4.76和15.7。
由于水的pKa值远高于醋酸,可以得出结论,醋酸是一个较强的酸,而水是一个较弱的酸。
3. 胺是一类含有氮原子的有机化合物,它们可以作为碱。
例如,乙胺(CH3CH2NH2)的pKa值为10.6,而二甲基胺((CH3)2NH)的pKa值为10.8。
这表明二甲基胺比乙胺稍微弱碱一些。
4. 碳酸根离子(HCO3-)是碳酸(H2CO3)的共轭碱。
碳酸的pKa值为6.35,而碳酸根离子的pKa值为10.33。
这意味着在碱性条件下,碳酸会脱去一个质子形成碳酸根离子。
5. 氨基酸是一类化合物,它们同时含有酸性和碱性基团。
例如,天冬氨酸的pKa值为2.09和9.47,而丙氨酸的pKa值为2.34和9.88。
这说明天冬氨酸在酸性条件下更容易失去质子,而丙氨酸在碱性条件下更容易得到质子。
6. 苯胺(C6H5NH2)是一种芳香胺,它的pKa值为4.6。
这表明苯胺在酸性条件下可以失去一个质子,形成苯胺阳离子。
7. 氯酸(HClO3)和硝酸(HNO3)是两种常见的无机酸。
它们的pKa 值分别为-1.8和-1.3,这说明它们是非常强的酸。
8. 羟胺(NH2OH)是一种含氮的有机化合物,它的pKa值为9.50。
这表明在碱性条件下,羟胺可以得到一个质子,形成羟胺阳离子。
9. 酚是一类化合物,它们含有一个或多个羟基(-OH)。
对苯二酚(C6H4(OH)2)的pKa值为9.99,这说明在碱性条件下,对苯二酚可以失去一个质子。