Paclitaxel_--FDA指南
- 格式:pdf
- 大小:64.71 KB
- 文档页数:2


美国FDA《联邦规章典集》(CFR)第21篇目录中文版发布时间:2010-5-11 13:44:12 发布方:奥咨达医疗器械咨询美国《联邦规章典集》(CFR)第21篇“食品与药品”总目概述:美国《联邦规章典集》(Code of Federal Regulations,CFR)第21篇“食品与药品”(Title 21―Food and Drugs)共有9卷(Volume)、3章(Chapter)、1499部(Parts)。
其中:第1―8卷第1章第1―1299部,为健康与人类服务部食品与药品管理局(Food and Drug Administration,Department of Health and Human Services)的规章;第9卷第2章第1300―1399部,为司法部毒品强制执行局(Drug Enforcement Administration,Department of Justice)的规章;第9卷第3章第1400―1499部,为毒品控制政策办公室(Office of National Drug Control Policy)的规章。
第21篇“食品与药品”(Tit le 21―Food and Drugs)的概况卷(Volume)章(Chapter)部(Parts)规制机关(Regulatory Entity)1 Ⅰ1-99 健康与人类服务部食品与药品管理局(FOOD AND DRUG ADMINISTRATION, DEPARTMENT OF HEALTH AND HUMAN SERVICES)2 100-1693 170-1994 200-2995 300-4996 500-5997 600-7998 800-12999 Ⅱ1300-1399 司法部毒品强制执行局(Drug Enforcement Administration,Department of Justice)Ⅲ1400-1499 毒品控制政策办公室(Office of National Drug Control Policy)第21篇“食品与药品”(Title 21―Food and Drugs)的章、部目录部(Part) 中译文原英文第Ⅰ章―健康与人类服务部食品与药品管理局(CHAPTER Ⅰ―FOOD AND DRUG ADMINIST RATION, DEPARTMENT OF HEALTH AND HUMAN SERVICES)第A分章―总则(SUBCHAPTER A―GENERAL)1 一般强制执行规章GENERAL ENFORCEMENT REGULATIONS2 一般行政规则与决定GENERAL ADMINISTRATIVE RULINGS AND DECISIONS3 产品管辖权PRODUCT JURISDICTION5 组织ORGANIZATION7 强制执行政策ENFORCEMENT POLICY10 行政规范与程序ADMINISTRATIVE PRACTICES AND PROCEDURES11 电子化记录;电子化签名ELECTRONIC RECORDS; ELECTRONIC SIGNATURES12 正式证据的公众听证FORMAL EVIDENTIARY PUBLIC HEARING13 在公众质询委员会前的公众听证PUBLIC HEARING BEFORE A PUBLIC BOARD OF INQUIRY14 在公众咨询委员会前的公众听证PUBLIC HEARING BEFORE A PUBLIC ADVISORY COMMITTEE15 在FDA局长前的公众听证PUBLIC HEARING BEFORE THE COMMISSIONER16 在FDA前的规制性听证REGULATORY HEARING BEFORE THE FOOD AND DRUG ADMINISTRATION17 行政罚款听证CIVIL MONEY PENALTIES HEARINGS19 行为标准与利益冲突STANDARDS OF CONDUCT AND CONFLICTS OF INTEREST20 公共信息PUBLIC INFORMATION21 隐私保护PROTECTION OF PRIVACY25 环境影响考虑ENVIRONMENTAL IMPACT CONSIDERATIONS26 药品良好制造规范报告、医疗器械质量体系核查报告以及某些医疗器械产品评价报告的互认:美国与欧共体MUTUAL RECOGNITION OF PHARMACEUTICAL GOOD MANUFACTURING PRACTICE REPORTS, MEDICAL DEVICE QUALITY SYSTEM AUDIT REPORTS, AND CERTAIN MEDICAL DEVICE PRODUCT EVALUATION REPORTS: UNITED STATES AND THE EUROPEAN COMMUNITY50 人类受试者的保护PROTECTION OF HUMAN SUBJECTS54 临床试验者的财务公开FINANCIAL DISCLOSURE BY CLINICAL INVESTIGATORS56 机构审查委员会INSTITUTIONAL REVIEW BOARDS58 对非临床实验室研究的良好实验室规范GOOD LABORATORY PRACTICE FOR NONCLINICAL LABORATORY STUDIES60 专利期恢复PATENT TERM RESTORATION70 色素添加剂COLOR ADDITIVES71 色素添加剂申请COLOR ADDITIVE PETITIONS73 免除认证的色素添加剂的列表LISTING OF COLOR ADDITIVES EXEMPT FROM CERTIFICATION74 适用认证的色素添加剂的列表LISTING OF COLOR ADDITIVES SUBJECT TO CERTIFICATION80 色素添加剂认证COLOR ADDITIVE CERTIFICATION81 用于食品、药品和化妆品的临时性色素添加剂的一般规范和一般限制GENERAL SPECIFICATIONS AND GENERAL RESTRICTIONS FOR PROVISIONAL COLOR ADDITIVES FOR USE IN FOODS, DRUGS, AND COSMETICS82 经认证的临时性列表的色素和规范的列表LISTING OF CERTIFIED PROVISIONALLY LISTED COLORS AND SPECIFICATIONS83-98 [预留的] [Reserved]99 已上市的药品、生物制品和器械的未经批准的/新的用途的信息的发布DISSEMINATION OF INFORMATION ON UNAPPROVED/NEW USES FOR MARKETED DRUGS, BIOLOGICS, AND DEVICES第B分章―用于人类消费的食品(SUBCHAPTER B―FOOD FOR HUMAN CONSUMPTION)100 总则GENERAL101 食品标识FOOD LABELING102 非标准化食品的普通的或者通常的名称COMMON OR USUAL NAME FOR NONSTANDARDIZED FOODS104 食品的营养质量指南NUTRITIONAL QUALITY GUIDELINES FOR FOODS105 特殊膳食用途的食品FOODS FOR SPECIAL DIETARY USE106 婴儿配方母乳替代食品质量控制程序INFANT FORMULA QUALITY CONTROL PROCEDURES107 婴儿配方母乳替代食品INFANT FORMULA108 紧急许可控制EMERGENCY PERMIT CONTROL109 在人类食品与食品-包装材料中的不可避免的污染物UNAVOIDABLE CONTAMINANTS IN FOOD FOR HUMAN CONSUMPTION AND FOOD-PACKAGING MATERIAL110 在制造、包装或者保存人类食品中的现行良好制造规范CURRENT GOOD MANUFACTURING PRACTICE IN MANUFACTURING, PACKING, OR HOLDING HUMAN FOOD113 装在密封容器中的热加工低酸食品THERMALLY PROCESSED LOW-ACID FOODS PACKAGED IN HERMETICALLY SEALED CONTAINERS114 酸化食品ACIDIFIED FOODS115 带壳蛋SHELL EGGS119 存在显著或者不合理风险的膳食补充剂DIETARY SUPPLEMENTS THAT PRESENT A SIGNIFICANT OR UNREASONABLE RISK120 危害分析与关键控制点(HACCP)体系HAZARD ANALYSIS AND CRITICAL CONTROL POINT (HACCP) SYSTEMS123 鱼与渔业产品FISH AND FISHERY PRODUCTS129 饮用水加工与装瓶PROCESSING AND BOTTLING OF BOTTLED DRINKING WATER130 食品标准:总则FOOD STANDARDS: GENERAL131 乳与奶油MILK AND CREAM133 乳酪与相关乳酪产品CHEESES AND RELATED CHEESE PRODUCTS135 冷冻点心FROZEN DESSERTS136 烘焙产品BAKERY PRODUCTS137 谷物粉与相关产品CEREAL FLOURS AND RELATED PRODUCTS139 通心粉与面条产品MACARONI AND NOODLE PRODUCTS145 罐装水果CANNED FRUITS146 罐装水果汁CANNED FRUIT JUICES150 水果黄油、果冻、防腐剂以及相关产品FRUIT BUTTERS, JELLIES, PRESERVES, AND RELATED PRODUCTS152 水果馅饼FRUIT PIES155 罐装蔬菜CANNED VEGETABLES156 蔬菜汁VEGETABLE JUICES158 冷冻蔬菜FROZEN VEGETABLES160 蛋与蛋制品EGGS AND EGG PRODUCTS161 鱼与有壳的水生动物FISH AND SHELLFISH163 可可制品CACAO PRODUCTS164 树坚果与花生制品TREE NUT AND PEANUT PRODUCTS165 饮料BEVERAGES166 人造黄油MARGARINE168 增甜剂与餐桌糖浆SWEETENERS AND TABLE SIRUPS169 食品敷料与调味料FOOD DRESSINGS AND FLAVORINGS170 食品添加剂FOOD ADDITIVES171 食品添加剂申请FOOD ADDITIVE PETITIONS172 允许直接加入用于人类消费食品的食品添加剂FOOD ADDITIVES PERMITTED FOR DIRECT ADDITION TO FOOD FOR HUMAN CONSUMPTION173 在用于人类消费的食品中允许的次直接的食品添加剂SECONDARY DIRECT FOOD ADDITIVES PERMITTED IN FOOD FOR HUMAN CONSUMPTION174 间接食品添加剂:总则INDIRECT FOOD ADDITIVES: GENERAL175 间接食品添加剂:胶粘剂与涂层的组分INDIRECT FOOD ADDITIVES: ADHESIVES AND COMPONENTS OF COATINGS176 间接食品添加剂:纸与纸板组分INDIRECT FOOD ADDITIVES: PAPER AND PAPERBOARD COMPONENTS177 间接食品添加剂:聚合体INDIRECT FOOD ADDITIVES: POLYMERS178 间接食品添加剂:辅剂、生产助剂和消毒剂INDIRECT FOOD ADDITIVES: ADJUVANTS, PRODUCTION AIDS, AND SANITIZERS 179 在食品生产、加工和处理中的辐照IRRADIATION IN THE PRODUCTION, PROCESSING AND HANDLING OF FOOD180 在额外试验期间临时在食品或者在与食品接触中被允许的食品添加剂FOOD ADDITIVES PERMITTED IN FOOD OR IN CONTACT WITH FOOD ON AN INTERIM BASIS PENDING ADDITIONAL STUDY181 先前核准的食品配料PRIOR-SANCTIONED FOOD INGREDIENTS182 一般认为安全的物质SUBSTANCES GENERALLY RECOGNIZED AS SAFE184 被确认为一般认为安全的直接食品物质DIRECT FOOD SUBSTANCES AFFIRMED AS GENERALLY RECOGNIZED AS SAFE 186 被确认为一般认为安全的间接食品物质INDIRECT FOOD SUBSTANCES AFFIRMED AS GENERALLY RECOGNIZED AS SAFE 189 禁止用于人类食品的物质SUBSTANCES PROHIBITED FROM USE IN HUMAN FOOD190 膳食补充剂DIETARY SUPPLEMENTS191-199 [预留的] [Reserved]第C分章―药品:总则(SUBCHAPTER C―DRUGS: GENERAL)200 总则GENERAL201 标识LABELING202 处方药广告PRESCRIPTION DRUG ADVERTISING203 处方药销售PRESCRIPTION DRUG MARKETING205 对批发处方药销售商颁发州执照的指南GUIDELINES FOR STATE LICENSING OF WHOLESALE PRESCRIPTION DRUG DISTRIBUTORS206 人用固体口服剂型药品的印码IMPRINTING OF SOLID ORAL DOSAGE FORM DRUG PRODUCTS FOR HUMAN USE207 药品生产者的登记与商业销售的药品的列表REGISTRATION OF PRODUCERS OF DRUGS AND LISTING OF DRUGS IN COMMERCIAL DISTRIBUTION208 处方药的药物治疗指导MEDICATION GUIDES FOR PRESCRIPTION DRUG PRODUCTS210 制造、加工、包装或者保存药品的现行良好制造规范;总则CURRENT GOOD MANUFACTURING PRACTICE IN MANUFACTURING, PROCESSING, PACKING, OR HOLDING OF DRUGS; GENERAL211 对完成的药品的现行良好制造规范CURRENT GOOD MANUFACTURING PRACTICE FOR FINISHED PHARMACEUTICALS216 药房配药PHARMACY COMPOUNDING225 对含药饲料的现行良好制造规范CURRENT GOOD MANUFACTURING PRACTICE FOR MEDICATED FEEDS226 对A型含药物品的现行良好制造规范CURRENT GOOD MANUFACTURING PRACTICE FOR TYPE A MEDICATED ARTICLES 250 对特殊人用药品的特殊要求SPECIAL REQUIREMENTS FOR SPECIFIC HUMAN DRUGS290 管制的药品CONTROLLED DRUGS299 药品;正式名称与已确定的名称DRUGS; OFFICIAL NAMES AND ESTABLISHED NAMES第D分章―人用药品(SUBCHAPTER D―DRUGS FOR HUMAN USE)300 总则GENERAL310 新药NEW DRUGS312 试验用新药申请INVESTIGATIONAL NEW DRUG APPLICATION314 为FDA批准上市新药的申请APPLICATIONS FOR FDA APPROVAL TO MARKET A NEW DRUG315 诊断用放射性药品DIAGNOSTIC RADIOPHARMACEUTICALS316 罕见病药ORPHAN DRUGS320 生物利用度与生物等效性要求BIOAVAILABILITY AND BIOEQUIVALENCE REQUIREMENTS328 含有酒精的预期用于口部摄入的非处方药品OVER-THE-COUNTER DRUG PRODUCTS INTENDED FOR ORAL INGESTION THAT CONTAIN ALCOHOL330 一般认为安全与有效以及不错误标识的非处方人用药品OVER-THE-COUNTER (OTC) HUMAN DRUGS WHICH ARE GENERALLY RECOGNIZED AS SAFE AND EFFECTIVE AND NOT MISBRANDED331 用于非处方的人类使用的抗酸产品ANTACID PRODUCTS FOR OVER-THE-COUNTER (OTC) HUMAN USE332 用于非处方的人类使用的抗胃肠气胀产品ANTIFLATULENT PRODUCTS FOR OVER-THE-COUNTER HUMAN USE333 用于非处方的人类使用的局部抗菌药品TOPICAL ANTIMICROBIAL DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE335 用于非处方的人类使用的止泻药品ANTIDIARRHEAL DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE336 用于非处方的人类使用的止吐药品ANTIEMETIC DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE338 用于非处方的人类使用的帮助夜间睡眠的药品NIGHTTIME SLEEP-AID DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE340 用于非处方的人类使用的兴奋药品STIMULANT DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE341 用于非处方的人类使用的感冒、咳嗽、过敏症药、支气管扩张以及平喘药品COLD, COUGH, ALLERGY, BRONCHODILATOR, AND ANTIASTHMATIC DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE343 用于非处方的人类使用的内服的止痛、退热以及抗风湿药品INTERNAL ANALGESIC, ANTIPYRETIC, AND ANTIRHEUMATIC DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE344 用于非处方的人类使用的局部的耳部药品TOPICAL OTIC DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE346 用于非处方的人类使用的肛肠药品ANORECTAL DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE347 用于非处方的人类使用的皮肤保护药品SKIN PROTECTANT DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE348 用于非处方的人类使用的外部的止痛药品EXTERNAL ANALGESIC DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE349 用于非处方的人类使用的眼科药品OPHTHALMIC DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE350 用于非处方的人类使用的止汗药品ANTIPERSPIRANT DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE352 用于非处方的人类使用的遮光药品SUNSCREEN DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE [STAYED INDEFINITELY]355 用于非处方的人类使用的防龋药品ANTICARIES DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE357 用于非处方的人类使用的其他内服药品MISCELLANEOUS INTERNAL DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE358 用于非处方的人类使用的其他外用药品MISCELLANEOUS EXTERNAL DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE361 一般认为安全与有效以及不错误标识的处方人用药品:用于研究的药品PRESCRIPTION DRUGS FOR HUMAN USE GENERALLYRECOGNIZED AS SAFE AND EFFECTIVE AND NOT MISBRANDED: DRUGS USED IN RESEARCH369 在用于非处方销售的药品与器械上关于警告的解释性声明INTERPRETATIVE STATEMENTS RE WARNINGS ON DRUGS AND DEVICES FOR OVER-THE-COUNTER SALE370-499 [预留的] [Reserved]第E分章―动物药品、饮料和相关产品(SUB CHAPTER E―ANIMAL DRUGS, FEEDS, AND RELATED PRODUCTS)500 总则GENERAL501 动物食品标识ANIMAL FOOD LABELING502 非标准化的动物食品的普通的或通常的名称COMMON OR USUAL NAMES FOR NONSTANDARDIZED ANIMAL FOODS509 在动物食品与食品-包装材料中的不可避免的污染物UNAVOIDABLE CONTAMINANTS IN ANIMAL FOOD AND FOOD-PACKAGING MATERIAL510 新动物药NEW ANIMAL DRUGS511 作为试验用途的新动物药NEW ANIMAL DRUGS FOR INVESTIGATIONAL USE514 新动物药申请NEW ANIMAL DRUG APPLICATIONS515 含药饲料厂执照MEDICATED FEED MILL LICENSE520 口服剂型的新动物药ORAL DOSAGE FORM NEW ANIMAL DRUGS522 植入或者注射剂型的新动物药IMPLANTATION OR INJECTABLE DOSAGE FORM NEW ANIMAL DRUGS524 眼科和局部剂型的新动物药OPHTHALMIC AND TOPICAL DOSAGE FORM NEW ANIMAL DRUGS526 乳房内的剂型INTRAMAMMARY DOSAGE FORMS529 某些其他剂型的新动物药CERTAIN OTHER DOSAGE FORM NEW ANIMAL DRUGS530 在动物中的特别标签药品使用EXTRALABEL DRUG USE IN ANIMALS556 在食品中新动物药残留的容许量TOLERANCES FOR RESIDUES OF NEW ANIMAL DRUGS IN FOOD558 用于动物饲料的新动物药NEW ANIMAL DRUGS FOR USE IN ANIMAL FEEDS564 [预留的] [Reserved]570 食品添加剂FOOD ADDITIVES571 食品添加剂申请FOOD ADDITIVE PETITIONS573 在动物饲料与饮用水中允许的食品添加剂FOOD ADDITIVES PERMITTED IN FEED AND DRINKING WATER OF ANIMALS579 在动物饲料和宠物食品的生产、加工和处理中的辐照IRRADIATION IN THE PRODUCTION, PROCESSING, AND HANDLING OF ANIMAL FEED AND PET FOOD582 一般认为安全的物质SUBSTANCES GENERALLY RECOGNIZED AS SAFE584 在动物饲料与饮用水中被确认为一般认为安全的食品物质FOOD SUBSTANCES AFFIRMED AS GENERALLY RECOGNIZED AS SAFE IN FEED AND DRINKING WATER OF ANIMALS589 禁止用于动物食品或者饲料的物质SUBSTANCES PROHIBITED FROM USE IN ANIMAL FOOD OR FEED590-599 [预留的] [Reserved]第F分章―生物制品(SUB CHAPTER F―BIOLOGICS)600 生物制品:总则BIOLOGICAL PRODUCTS: GENERAL601 颁发执照LICENSING606 对血液与血液组分的现行良好制造规范CURRENT GOOD MANUFACTURING PRACTICE FOR BLOOD AND BLOOD COMPONENTS607 对人类血液与血液制品的制造者的机构登记与产品列表ESTABLISHMENT REGISTRATION AND PRODUCT LISTING FOR MANUFACTURERS OF HUMAN BLOOD AND BLOOD PRODUCTS610 普通生物制品标准GENERAL BIOLOGICAL PRODUCTS STANDARDS630 对血液、血液组分和血液衍生物的一般要求GENERAL REQUIREMENTS FOR BLOOD, BLOOD COMPONENTS, AND BLOOD DERIVATIVES640 对人类血液和血液制品的附加标准ADDITIONAL STANDARDS FOR HUMAN BLOOD AND BLOOD PRODUCTS660 对用于实验室检测的诊断物质的附加标准ADDITIONAL STANDARDS FOR DIAGNOSTIC SUBSTANCES FOR LABORATORY TESTS680 对其他产品的附加标准ADDITIONAL STANDARDS FOR MISCELLANEOUS PRODUCTS第G分章―化妆品(SUBCHAPTER G―COSMETICS)700 总则GENERAL701 化妆品标识COSMETIC LABELING710 化妆品机构的自愿登记VOLUNTARY REGISTRATION OF COSMETIC PRODUCT ESTABLISHMENTS720 化妆品配料构成声明的自愿存档VOLUNTARY FILING OF COSMETIC PRODUCT INGREDIENT COMPOSITION STATEMENTS740 化妆品警告声明COSMETIC PRODUCT WARNING STATEMENTS741-799 [预留的] [Reserved]第H分章―医疗器械(SUBCHAPTER H―MEDICAL DEVICES)800 总则GENERAL801 标识LABELING803 医疗器械报告MEDICAL DEVICE REPORTING806 医疗器械;改正与移动的报告MEDICAL DEVICES; REPORTS OF CORRECTIONS AND REMOVALS807 对器械的制造者与首次进口者的机构登记与器械列表ESTABLISHMENT REGISTRATION AND DEVICE LISTING FOR MANUFACTURERS AND INITIAL IMPORTERS OF DEVICES808 对州和地方医疗器械要求的联邦优先权的豁免EXEMPTIONS FROM FEDERAL PREEMPTION OF STATE AND LOCAL MEDICAL DEVICE REQUIREMENTS809 人用体外诊断产品IN VITRO DIAGNOSTIC PRODUCTS FOR HUMAN USE810 医疗器械召回权MEDICAL DEVICE RECALL AUTHORITY812 试验用器械豁免INVESTIGATIONAL DEVICE EXEMPTIONS813 [预留的] [Reserved]814 医疗器械的上市前批准PREMARKET APPROVAL OF MEDICAL DEVICES820 质量体系规章QUALITY SYSTEM REGULATION821 医疗器械跟踪要求MEDICAL DEVICE TRACKING REQUIREMENTS822 上市后监视POSTMARKET SURVEILLANCE860 医疗器械分类程序MEDICAL DEVICE CLASSIFICATION PROCEDURES861 性能标准制定程序PROCEDURES FOR PERFORMANCE STANDARDS DEVELOPMENT862 临床化学与临床毒理学器械CLINICAL CHEMISTRY AND CLINICAL TOXICOLOGY DEVICES864 血液学与病理学器械HEMATOLOGY AND PATHOLOGY DEVICES866 免疫学与微生物学器械IMMUNOLOGY AND MICROBIOLOGY DEVICES868 麻醉学器械ANESTHESIOLOGY DEVICES870 心血管器械CARDIOVASCULAR DEVICES872 牙科器械DENTAL DEVICES874 耳、鼻和咽器械EAR, NOSE, AND THROAT DEVICES876 胃肠病学-泌尿学器械GASTROENTEROLOGY-UROLOGY DEVICES878 普通与整形外科器械GENERAL AND PLASTIC SURGERY DEVICES880 普通医院与个人使用器械GENERAL HOSPITAL AND PERSONAL USE DEVICES882 神经学器械NEUROLOGICAL DEVICES884 产科与妇科学器械OBSTETRICAL AND GYNECOLOGICAL DEVICES886 眼科器械OPHTHALMIC DEVICES888 矫形外科器械ORTHOPEDIC DEVICES890 内科学器械PHYSICAL MEDICINE DEVICES892 放射学器械RADIOLOGY DEVICES895 禁止的器械BANNED DEVICES898 电极铅线与患者电缆的性能标准PERFORMANCE STANDARD FOR ELECTRODE LEAD WIRES AND PATIENT CABLES第I分章―乳房造影质量标准法(SUBCHAPTER I―MAMMOGRAPHY QUALITY STANDA RDS ACT)900 乳房造影法MAMMOGRAPHY第J分章―放射学的健康(SUBCHAPTER J―RADIOLOGICAL HEALTH)1000 总则GENERAL1002 记录与报告RECORDS AND REPORTS1003 缺陷与未能守法的通报NOTIFICATION OF DEFECTS OR FAILURE TO COMPLY1004 电子产品的回购、修理或者置换REPURCHASE, REPAIRS, OR REPLACEMENT OF ELECTRONIC PRODUCTS1005 电子产品的进口IMPORTATION OF ELECTRONIC PRODUCTS1010 电子产品的性能标准:总则PERFORMANCE STANDARDS FOR ELECTRONIC PRODUCTS: GENERAL1020 电离辐射发生产品的性能标准PERFORMANCE STANDARDS FOR IONIZING RADIATION EMITTING PRODUCTS1030 微波与射电频率发生产品的性能标准PERFORMANCE STANDARDS FOR MICROWAVE AND RADIO FREQUENCY EMITTING PRODUCTS1040 发光产品的性能标准PERFORMANCE STANDARDS FOR LIGHT-EMITTING PRODUCTS1050 声波、次声波和超声波发生产品的性能标准PERFORMANCE STANDARDS FOR SONIC, INFRASONIC, AND ULTRASONIC RADIATION-EMITTING PRODUCTS第K分章―[预留的](SUBCHAPTER K―[RESERVED])第L分章―根据由食品与药品管理局行政执行的某些其他法的规章(SUBCHAPTER L―REGULATIONS UNDER CERTAIN OTHER ACTS ADMINISTERED BY THE FOOD AND DRUG ADMINISTRATION)1210 根据《联邦进口乳法》的规章REGULATIONS UNDER THE FEDERAL IMPORT MILK ACT1230 根据《联邦腐蚀性毒物法》的规章REGULATIONS UNDER THE FEDERAL CAUSTIC POISON ACT1240 传染病的控制CONTROL OF COMMUNICABLE DISEASES1250 州际运输卫生INTERSTATE CONVEYANCE SANITATION1251-1269 [预留的] [Reserved]1270 预期用于移植的人体组织HUMAN TISSUE INTENDED FOR TRANSPLANTATION1271 人体细胞、组织以及细胞的和基于组织的产品HUMAN CELLS, TISSUES, AND CELLULAR AND TISSUE-BASED PRODUCTS 1272-1299 [预留的] [Reserved]第Ⅱ章―司法部毒品强制执行局(CHAPTER Ⅱ―DRUG ENFORCEMENT ADMINISTRATION, DEPARTMENT OF JUSTICE)1300 定义DEFINITIONS1301 管制物质的制造者、分销者和调剂者的登记REGISTRATION OF MANUFACTURERS, DISTRIBUTORS, AND DISPENSERS OF CONTROLLED SUBSTANCES1302 对管制物质的标识与包装要求LABELING AND PACKAGING REQUIREMENTS FOR CONTROLLED SUBSTANCES1303 定额QUOTAS1304 登记者的记录与报告RECORDS AND REPORTS OF REGISTRANTS1305 令的格式ORDER FORMS1306 处方PRESCRIPTIONS1307 杂项MISCELLANEOUS1308 管制物质的表SCHEDULES OF CONTROLLED SUBSTANCES1309 表I化学品的制造者、分销者、进口者和出口者的登记REGISTRATION OF MANUFACTURERS, DISTRIBUTORS, IMPORTERS AND EXPORTERS OF LIST I CHEMICALS1310 列入表的化学品和某些机器的记录与报告RECORDS AND REPORTS OF LISTED CHEMICALS AND CERTAIN MACHINES 1311 [预留的] [Reserved]1312 管制物质的进口与出口IMPORTATION AND EXPORTATION OF CONTROLLED SUBSTANCES1313 前体与必要化学品的进口与出口IMPORTATION AND EXPORTATION OF PRECURSORS AND ESSENTIAL CHEMICALS1314-1315 [预留的] [Reserved]1316 行政职能、规范和程序ADMINISTRATIVE FUNCTIONS, PRACTICES, AND PROCEDURES第Ⅲ章―毒品控制政策办公室(CHAPTER Ⅲ―Office of National Drug Control Policy)1400 [预留的] [Reserved]1401 信息的公众可及性PUBLIC AVAILABILITY OF INFORMATION1402 强制性解密审查MANDATORY DECLASSIFICATION REVIEW1403 对给予州和地方政府资金和合作协议的统一行政要求UNIFORM ADMINISTRATIVE REQUIREMENTS FOR GRANTS AND COOPERATIVE AGREEMENTS TO STATE AND LOCAL GOVERNMENTS1404 政府范围的排除与暂停(非获得)GOVERNMENTWIDE DEBARMENT AND SUSPENSION (NONPROCUREMENT)1405 对无毒品工作场所的政府范围的要求(财政援助)GOVERNMENTWIDE REQUIREMENTS FOR DRUG-FREE WORKPLACE (FINANCIAL ASSISTANCE)1406-1499 [预留的] [Reserved]。



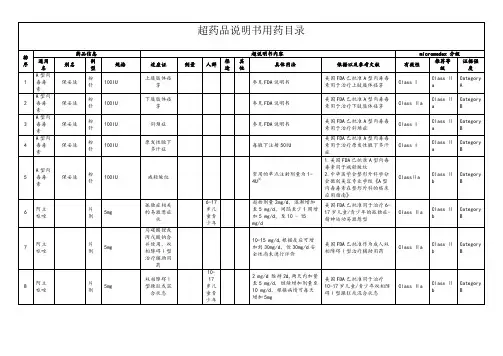
Contains Nonbinding RecommendationsDraft Guidance on PaclitaxelThis draft guidance, once finalized, will represent the Food and Drug Administration's (FDA's) current thinking on this topic. It does not create or confer any rights for or on any person and does not operate to bind FDA or the public. You can use an alternative approach if the approach satisfies the requirements of the applicable statutes and regulations. If you want to discuss an alternative approach, contact the Office of Generic Drugs.Active Ingredient: PaclitaxelForm/Route: Suspension/InjectableRecommended Studies: 2 studies1. Type of study: Bioequivalence study with pharmacokinetic (PK) endpointsDesign: Single-dose, two-way crossover, fasting, in vivoStrength: 100 mg/vial (260 mg/m2 dose administered in 30 minutes)Subjects: Breast cancer patients after failure of combination chemotherapy formetastatic disease or relapse within 6 months of adjuvant chemotherapyAdditional comments:a.Submission of a Bio Investigational New Drug Application (Bio-IND) isrequired prior to the conduct of a bioequivalence in vivo study for a cytotoxicdrug product such as paclitaxel (see 21 CFR § 320.31).b.The pivotal bioequivalence study should be conducted using test productmanufactured on the proposed commercial scale.c.If the patient’s health status prevents fasting, the sponsor may provide a non-high-fat diet during the proposed study provided that both study periods areconducted under same conditions.d.If the patient’s health status necessitates a dose reduction or any change in therecommended 260 mg/m2 dose administered in 30 minutes, they are to bewithdrawn from the study.e.Patients must have baseline neutrophil counts ≥ 1500 cells/mm3; Patients whoexperience a severe hypersensitivity reaction to ABRAXANE should not berechallenged with the drug; Frequent peripheral blood counts are to beperformed; Prior therapy should have included an anthracycline unlessclinically contraindicated; Female patients should be nonpregnant and non-lactating; Women of childbearing potential should be advised to avoidbecoming pregnant while receiving paclitaxel injectable suspension, and menshould be advised not to father a child while receiving paclitaxel injectablesuspension.f.The use of antiemetic prophylaxis is acceptable provided that the patientreceives the same prophylaxis in both periods of the study.Analytes to measure: unbound and total paclitaxel in plasmaBioequivalence based on (90% CI): AUC and Cmax for unbound and total paclitaxel________________________________________________________________________ Recommended Sep 201212.Type of study: In Vitro Particle Size DistributionDesign: In vitro bioequivalence study on at least three lots of both test andreference productsStrength: 100 mg/vialAdditional comments: NoneParameters to measure: D10, D50, and D90Bioequivalence based on (95% CI): Population bioequivalence based on D50 and span(D90-D10)/D50 or polydispersity index________________________________________________________________________As per 21 CFR § 314.94(a)(9)(iii), the proposed parenteral drug product should bequalitatively (Q1) and quantitatively (Q2) the same as the corresponding reference listeddrug product. In addition, firms are recommended to obtain assurance from OGD that thetest product has the same in-vitro characteristics as those of reference listed drug productprior to conducting any bioequivalence study for submission. Additional in vitrocharacterization are recommended to demonstrate the sameness between the test andreference products in terms of particle morphology, particle size, surface potential,paclitaxel crystallinity, fraction of free and bound paclitaxel or albumin in reconstitutedsuspension, nature of bond between paclitaxel and albumin, and in vitro release kinetics.In addition, albumin, the only excipient in the final product, is critical to the formulation.The characterization of the oligomeric status of albumin in both the albumin excipientand the final drug product is also recommended. The in vitro characterization tests arerecommended to be conducted on three batches of the ANDA and RLD products (at leastone ANDA batch should be produced by the commercial scale process).________________________________________________________________________Waiver request of in vivo testing: Not applicableIn Vitro Release Testing Method: The applicants are encouraged to explore methods tocharacterize in vitro release.Recommended Sep 2012 2。

天然抗癌药物紫杉醇的研究进展吴秀兰;贾艳;朱波;周杰【摘要】紫杉醇是一种二萜类化合物,具有独特的抗癌活性.近年来围绕紫杉醇所进行的高水平研究越来越多.综述了紫杉醇来源的研究进展,主要涉及化学合成和生物合成方法,并对其发展前景进行展望.【期刊名称】《中国野生植物资源》【年(卷),期】2014(033)005【总页数】5页(P42-45,60)【关键词】紫杉醇;来源;生物合成【作者】吴秀兰;贾艳;朱波;周杰【作者单位】正大天晴药业集团股份有限公司,江苏南京210023;正大天晴药业集团股份有限公司,江苏南京210023;正大天晴药业集团股份有限公司,江苏南京210023;正大天晴药业集团股份有限公司,江苏南京210023【正文语种】中文【中图分类】R2841 紫杉醇介绍紫杉醇(Paclitaxel,商品名Taxol) 被用于治疗卵巢癌、乳腺癌、肺癌以及Kaposi’s 肉瘤的治疗,同时也在研究与其他抗癌药物联用的治疗方法,是获得FDA批准的第一个来自天然植物的化学药物[1]。
最初,紫杉醇是从短叶紫杉(Taxus brevifolia)的树皮中分离获得,这种紫杉主要分布在北美西北太平洋区域从北加拿大到阿拉斯加的原生森林中。
短叶紫杉生长极其缓慢且不易繁殖,一棵直径22 cm、高度9 m的树大约有125年树龄,其树皮极薄,只有大约0.3~0.6 cm厚,这样的一棵树可以得到大约2kg树皮。
紫杉醇必须从新鲜砍伐剥取的树皮中提取。
从砍伐树木,收集紫杉树皮到分离萃取出紫杉醇,消耗极大的人力物力财力。
30吨干树皮可以得到大约100 g紫杉醇,花费大约150万美元。
因为紫杉醇极难得到,所以在研究初期对紫杉醇的研究进度也极其缓慢。
因此从植物中提取紫杉醇代价太高,而且会对生态环境造成破坏。
迄今为止已经探索出化学半合成法、化学全合成法、植物细胞培养或内生菌培养等多种方法获得紫杉醇[1-3]。
2 紫杉醇的理化性质紫杉醇为白色结晶性粉末,不溶于水,易溶于氯仿、丙酮等有机溶剂。


基于美国FDA不良事件数据库的注射用紫杉醇(白蛋白结合型) 不良反应信号挖掘作者:王郁薇蒙龙刘箫来源:《中国药房》2021年第03期摘要目的:利用美國FDA不良事件报告系统(FAERS)数据库挖掘注射用紫杉醇(白蛋白结合型)的药品不良反应信号,为其临床安全合理用药提供参考。
方法:采用报告比值比(ROR)法对美国FDA公共数据开放项目(Open-FDA)数据库中于2004年1月1日-2019年12月31日上报的的注射用紫杉醇(白蛋白结合型)的不良事件进行数据挖掘,分析不良事件涉及的人口学特征、不良反应构成和信号。
结果:注射用紫杉醇(白蛋白结合型)的不良事件报告数分别为1 659 例,其中女性(1 169例,占70.5%)多于男性(345例,占20.8%);年龄主要在45~64岁(519例,占31.3%)。
该药的不良反应信号主要集中在神经系统、血液及淋巴系统、胃肠系统、肝胆系统、呼吸系统、胸及纵隔系统和全身性不良反应。
分析发现了药品说明书未记载的阳性不良反应信号20 个,包括白细胞减少、淋巴细胞减少、黄斑水肿、腹痛、吞咽困难、寒战、黄疸、肝衰竭、肝硬化、尿路感染、脓性分泌物、射血分数降低、低钙血症、低钾血症、低钠血症、骨痛、面瘫、精神状态变化、鼻出血、肺不张等,其中淋巴细胞减少、黄斑水肿、精神状态改变并未记录在该药的药品说明书中,其他则为药品说明书中已记录的不良反应的具体表现。
结论:临床应用注射用紫杉醇(白蛋白结合型)时,除药品说明书中已提到的不良反应外,还应密切关注其神经毒性、淋巴细胞变化并定期进行眼部与精神状态监测,以避免因不良反应导致的停药或造成患者器官损害。
关键词注射用紫杉醇(白蛋白结合型);美国 FDA 不良事件报告系统;不良事件;药品不良反应;信号挖掘中图分类号 R979.1 文献标志码 A 文章编号 1001-0408(2021)03-0328-06DOI 10.6039/j.issn.1001-0408.2021.03.13ABSTRACT OBJECTIVE: To utilize ADR signal of Paclitaxel for injection (albumin-bound type) by using FDA adverse event reporting system (FAERS), and to provide reference for rational use of drugs in the clinic. METHODS: The reporting odds ratio (ROR) method was used for data mining of adverse events (AEs) related to Paclitaxel for injection (albumin-bound type)reported by FDA public data program (Open-FDA) during Jan. 1st, 2004-Dec. 31th, 2019. The demographic characteristics, constituents and signals of ADR were analyzed. RESULTS: A total of 1 659 AEs were identified for Paclitaxel for injection (albumin-bound type). The female (1 169 cases, 70.5%) was more than the male (345 cases, 20.8%). The age was mainly 45-64 years old(519 cases, 31.3%). ADR signal mainly involved nerve system, blood and lymphatic system, gastrointestinal system, hepatobiliary system, respiratory system, thoracic and mediastinal system and general ADR. Twenty positive ADR signals which were not recorded in the drug instructions were found in the study, mainly including leucopenia, lymphopenia, macular edema, abdominal pain, dysphagia, shivering, jaundice, liver failure, cirrhosis, urinary tract infection, purulent secretion, decreased ejection fraction, hypocalcemia, hypokalemia,hyponatremia, bone pain, facial paralysis, mental state change, epistaxis, atelectasis. Among them, lymphopenia, macular edema and mental state changes were not recorded in the drug instructions, while others were the specific manifestations of ADR recorded in the drug instructions. CONCLUSIONS: In the clinical application of Paclitaxel for injection (albumin-bound type),in addition to ADR mentioned in the drug instructions, great importance should be closely paid to neurotoxicity, lymphocyte changes, regular eye monitoring and mental state monitoring, so as to avoid drug withdrawal or organ injury induced by ADR.KEYWORDS Paclitaxel for injection (albumin-bound type); FDA adverse event reporting system; Adverse events; ADR; Signal mining紫杉醇是一种抗微管剂,是一种可通过促进微管的组装和稳定来抑制细胞分裂的细胞毒性藥物[1],其对包括非小细胞肺癌和乳腺癌在内的多种恶性肿瘤的治疗具有重要临床价值[2]。


紫杉醇的研究紫杉醇(taxol)是继阿霉素和顺铂后最热点的新抗癌药,已于1992年底被美国FDA批准作为抗晚期癌症的新药上市。
紫杉醇在肿瘤的治疗药物中代表了一类新的、独特的抗微管药物。
它的抗癌机制与其它的抗癌药不同。
它的主要作用是通过促进极为稳定的微管聚集,并阻止微管正常的生理性解聚,从而导致癌细胞的死亡,并抑制其组织的再生。
我国每年至少有30万乳腺癌和卵巢癌的患者,因此我国进行紫杉醇的开发研究,其社会效益和经济效益极其可观。
本文将就目前国内外紫杉醇研究的现状作一综述。
紫杉醇研发过程年代进展1958 NCI开始大规模植物药研发筛选1967 发现紫杉醇抗癌活性1968 从红豆杉中分离出紫杉醇1971 完成结构鉴定1979 发表作用机制1983 临床Ⅰ试验1985 临床II期1991 临床III期1992 FDA批准上市紫杉醇,英文名称Paclitaxel,别名泰素,紫素,特素,化学名称5β,20-环氧-1,2α,4,7β,10β,13α-六羟基紫杉烷-11-烯-9-酮-4,10-二乙酸酯-2-苯甲酸酯-13[(2’R,3’S)-N-苯甲酰-3-苯基异丝氨酸酯],分子量853.92,分子式C47H51NO14。
1简介结构式【产品名称】紫杉醇【英文名称】Paclitaxel【产品别名】泰素,紫素,特素【化学名称】5β,20-环氧-1,2α,4,7β,10β,13α-六羟基紫杉烷-11-烯-9-酮-4,10-二乙酸酯-2-苯甲酸酯-13[(2’R,3’S)-N-苯甲酰-3-苯基异丝氨酸酯] 【分子式】C47H51NO14【分子量】853.92【CA S NO】33069-62-4【产品来源】为红豆杉科植物红豆杉的干燥根、枝叶以及树皮。
【规格含量】99.6%【物理性质】白色结晶体粉末。
无臭,无味。
不溶于水,易溶于氯仿、丙酮等有机溶剂。
鉴别:a.红外吸收:红外光谱图中的主要吸收带与对照品一致。
b.HPLC鉴别:在含量检测中,检测制备的色谱图中主峰的保留时间与标准制备色谱图中主峰的保留时间一致。
基于下一代测序技术的传染病诊断设备:微生物鉴定及抗生素抗性和毒力标志物的检测工业和食品药品管理局工作人员指南草案Document issued on: May 13, 2016U.S. Department of Health and Human ServicesFood and Drug AdministrationCenter for Devices and Radiological HealthOffice of In Vitro Diagnostics and Radiological HealthDivision of Microbiology Devices目录I.简介 (1)II.背景 (1)A.传染病NGS Dx设备的系统方法 (2)B.FDA-ARGOS: FDA监管级微生物序列数据库 (2)III.范围 (3)IV.利弊分析 (5)V.设备描述 (5)A.预期用途 (5)B.测试方法学 (6)C.辅助试剂 (7)D.控制 (8)(1)阴性对照 (8)(2)阳性对照 (9)(3)内部对照 (9)E.试验结果和报告的解释 (10)VI.设备验证 (10)A.预分析因素 (11)(1)样本收集和处理 (11)(2)样本准备和测序 (12)(3)测序、化学过程和数据收集 (12)(4)数据储存 (13)(5)临床决策要求 (13)B.感染性疾病NGS Dx设备性能指标 (13)(1)感染性疾病NGS Dx设备数据集 (14)(2)测序策略 (14)(3)用于靶向鉴定的参考序列和所选的靶标 (14)(4)临床识别信息学工序流程 (15)(5)减法原理 (15)(6)质量控制 (15)(7)测序及序列比对 (15)(8)污染分析 (15)(9)感染性疾病NGS Dx设备应当提供从样本到结果的周期 (16)(10)数据存储 (16)C.分析性能 (17)(1)检测极限 (17)(2)包容性 (17)(3)干扰物质 (18)(4)精确(可重现性及可重复性) (19)(5)携带及交叉污染 (19)(6)稳定性 (19)(7)其他分析性实验 (19)D.仪器和软件 (19)E.临床评估 (21)(1)阴性百分比一致性评价 (21)(2)阳性百分比一致性评价 (22)(3)数据展示 (23)(4)样本研究和样本类型 (23)Ⅶ设备调试 (24)基于下一代测序技术的传染病诊断设备:微生物鉴定及抗生素抗性和毒力标志物的检测工业和食品药品管理局工作人员指南草案I.简介美国食品药品监督管理局(FDA)发布该指南草案是为工业和机构工作人员提供建议,以研究建立基于下一代测序的用于分析传染病临床表现特征的微生物鉴定及抗生素耐药性和毒力标志的检测设备(以下简称“传染病NGS Dx设备”)。
第六章紫杉醇的生产工艺6.1 概述6.1.1 紫杉醇类药物1、紫杉醇紫杉醇(Paclitaxel,Taxol®)的化学名称为5β,20-环氧-1β,2α,4α,7β,13α-五羟基-紫杉-11-烯-9-酮-4-乙酸酯-2-苯甲酸酯-10-乙酰基-13-[(2′R,3′S) -N-苯甲酰基-3′-苯基异丝氨酸酯]。
紫杉醇具有复杂的化学结构,属三环二萜类化合物,整个分子由三个主环构成的二萜核和一个苯基异丝氨酸侧链组成(图6-1)。
分子中有11个手性中心和多个取代基团。
图6-1 紫杉醇的化学结构2、多烯紫杉醇多烯紫杉醇(多西他赛,Docetaxel,Taxotere®,图6-2)是一种紫杉醇类似物,两者仅在母环10位和侧链上3'位上的取代基略有不同。
3、临床应用紫杉醇被誉为是随机筛选药物的成功范例。
紫杉醇具有独特的抗癌机制,其作用靶点是有丝分裂和细胞周期中至关重要的微管蛋白。
紫杉醇与β微管蛋白N端第31位氨基酸和217-231位结合,能促进微管蛋白聚合而形成稳定的微管,并抑制微管的解聚,将细胞周期阻断于G2/M期,从而抑制了细胞的有丝分裂,最终导致癌细胞的死亡。
多烯紫杉醇与紫杉醇有相同的作用机制,但抑制微管解聚、促进微管二聚体聚合成微管的能力是紫杉醇的两倍。
紫杉醇1992年12月被美国FDA批准用于治疗晚期卵巢癌。
1998年和1999年,FDA又分别批准半合成紫杉醇与顺铂联合使用作为治疗晚期卵巢癌和非小细胞肺癌的一线用药。
2004年FDA批准白蛋白修饰的紫杉醇,降低毒性,提高疗效。
6.1.2 紫杉醇的生产工艺路线1、天然提取工艺路线紫杉醇的来源最初以天然提取为主,主要是从由红豆杉属(Taxus)植物的树皮中分离得到。
2、生物合成工艺目前,已有前体饲喂、添加诱导子、两相培养等技术在红豆杉细胞培养中相继使用。
在进一步研究紫杉醇的生物合成途径与调控、规模反应器放大规律的基础上,提高生产率,增加经济可行性,使得细胞培养过程被认为是相当有潜力的路线。
FDA基因检测用药的指南基因检测用药是一种个体化医疗策略,它通过检测患者的基因信息,为医生提供个性化的用药指导。
FDA(美国食品药品监督管理局)在这一领域发挥着重要的作用,其制定的指南对于基因检测用药的规范和安全性评估至关重要。
下面将详细介绍FDA在基因检测用药方面的指南。
首先,FDA在基因检测用药方面制定的指南包括了整个流程的规范。
从检测方法的选择到报告的解释和使用,FDA都提供了详细的指导。
在基因检测方法选择方面,FDA要求科研和开发人员要选择经过严格验证和认证的方法,以确保检测结果的准确性和可靠性。
在报告的解释和使用方面,FDA要求提供清晰、详细的结果解读,包括对药物相互作用、适应症、剂量调整等方面的指导。
其次,FDA的指南还强调了基因检测用药的安全性评估。
基因检测用药涉及到个体化用药和新兴的治疗策略,因此其安全性评估尤为重要。
FDA要求科研和开发人员应开展临床实验以评估基因检测用药的安全性和有效性。
在药物治疗方面,FDA要求进行临床试验来评估不同基因型患者的用药反应和不良反应风险。
在基因检测方法方面,FDA要求开发人员评估基因检测方法的准确性、重复性和可靠性,并提供相关数据以支持其使用。
此外,FDA的指南还涉及基因检测用药的数据分析和解读。
基因检测用药的数据分析复杂且具有个体差异性,因此需要科研和开发人员具备相关的专业知识和经验。
FDA要求科研和开发人员在数据分析和解读过程中要严格遵守标准化的操作程序,并提供相关的技术规范和标准。
此外,FDA还鼓励科研和开发人员进行数据共享和合作,以促进基因检测用药领域的发展和进步。
最后,为了保障基因检测用药的准确性和可靠性,FDA还制定了相关的质量控制标准。
基因检测用药是一项高科技的医疗技术,需要严格的质量控制来确保结果的准确性和可靠性。
FDA要求科研和开发人员对基因检测用药的每个环节进行质量控制,并保留相关的质量控制记录和数据。
此外,FDA还对基因检测用药的设备和试剂品质量标准进行了规定,以确保基因检测用药的质量和安全性。
紫杉醇(paclitaxel,商品名为Taxol)是从红豆杉属植物中分离出来的一种二萜类化合物,1992年底被美国食口与药物管理局(FDA)批准作为抗晚期癌症的新药上市。
从天然红豆杉属植物中提取紫杉醇的工作十分复杂,难度较大。
其主要原因是:紫杉醇在植株体内的含量大低,最高含量不到万分之二;存在大量的紫杉醇类似物。
这些类似物的化学结构和性质均和紫杉醇相近,致使紫杉醇与它们的分离十分困难,尤其是与cephalomannine的分离。
国内外学者围绕紫杉醇的提取与纯化开展了大量卓有成效的工作。
1 溶剂萃取法溶剂萃取法常用于紫杉醇的粗提阶段。
紫杉醇的粗提阶段又可分为初级萃取和次级萃取。
在这两级萃取过程中,溶剂的选择对于精提产物的质量和过程经济性具有重要影响。
初级萃取和次级萃取一般采用的溶剂系统不同。
各个时期的研究者对这两个过程的溶剂系统的研究结果已有详细的总结。
最近、日本学者对紫杉醇提取的溶剂种类进行了详细的研究,结果表明:在乙酸乙酯、乙醚、乙腈、丙酮、甲醇、已烷、异丙醇、乙酸乙酯-甲醇、乙酸乙酯-二氯甲烷、乙酸乙酯-丙酮、乙酸乙酯-乙醚等溶剂中,以乙酸乙酯-丙酮(1:1)混和溶剂提取的效果最好,所得浸膏仅为植物干重的7.70%,紫杉醇的含量高达浸膏的0.084%,而用甲醇提取所得浸膏为植物干重的20.98%,紫杉醇的含量为浸膏的0.027%,尚需要多次抽提才能得到紫杉醇含量较高的浸膏。
现在看来利用乙酸乙酯-丙酮(1:1)一次便可以使紫杉醇提取量高于以往常用溶剂所能得到的量,这就为后序的分离纯化工作带来很大的方便,由于乙酸乙酯-丙酮(1:1)的价格与甲醇的价格相当,且可回收再利用,因此,这一提取方法的经济性较为合理。
在初级萃取过程中引入超声技术,可大大缩短初级萃取过程的时间。
例如Xu采用甲醇-二氯甲烷(95.5)作初级溶剂,所需萃取时间约为35-60min。
在溶剂系统不变的情况下,将原料与溶剂的混和物进行超声振荡,萃取达到平衡的时间缩短到仅5min,与此对比,Hoke等人采用纯甲醇作为初级溶剂,无超声振荡,所需时间长达16-48h。
Contains Nonbinding RecommendationsDraft Guidance on PaclitaxelThis draft guidance, once finalized, will represent the Food and Drug Administration's (FDA's) current thinking on this topic. It does not create or confer any rights for or on any person and does not operate to bind FDA or the public. You can use an alternative approach if the approach satisfies the requirements of the applicable statutes and regulations. If you want to discuss an alternative approach, contact the Office of Generic Drugs.Active Ingredient: PaclitaxelForm/Route: Suspension/InjectableRecommended Studies: 2 studies1. Type of study: Bioequivalence study with pharmacokinetic (PK) endpointsDesign: Single-dose, two-way crossover, fasting, in vivoStrength: 100 mg/vial (260 mg/m2 dose administered in 30 minutes)Subjects: Breast cancer patients after failure of combination chemotherapy formetastatic disease or relapse within 6 months of adjuvant chemotherapyAdditional comments:a.Submission of a Bio Investigational New Drug Application (Bio-IND) isrequired prior to the conduct of a bioequivalence in vivo study for a cytotoxicdrug product such as paclitaxel (see 21 CFR § 320.31).b.The pivotal bioequivalence study should be conducted using test productmanufactured on the proposed commercial scale.c.If the patient’s health status prevents fasting, the sponsor may provide a non-high-fat diet during the proposed study provided that both study periods areconducted under same conditions.d.If the patient’s health status necessitates a dose reduction or any change in therecommended 260 mg/m2 dose administered in 30 minutes, they are to bewithdrawn from the study.e.Patients must have baseline neutrophil counts ≥ 1500 cells/mm3; Patients whoexperience a severe hypersensitivity reaction to ABRAXANE should not berechallenged with the drug; Frequent peripheral blood counts are to beperformed; Prior therapy should have included an anthracycline unlessclinically contraindicated; Female patients should be nonpregnant and non-lactating; Women of childbearing potential should be advised to avoidbecoming pregnant while receiving paclitaxel injectable suspension, and menshould be advised not to father a child while receiving paclitaxel injectablesuspension.f.The use of antiemetic prophylaxis is acceptable provided that the patientreceives the same prophylaxis in both periods of the study.Analytes to measure: unbound and total paclitaxel in plasmaBioequivalence based on (90% CI): AUC and Cmax for unbound and total paclitaxel________________________________________________________________________ Recommended Sep 201212.Type of study: In Vitro Particle Size DistributionDesign: In vitro bioequivalence study on at least three lots of both test andreference productsStrength: 100 mg/vialAdditional comments: NoneParameters to measure: D10, D50, and D90Bioequivalence based on (95% CI): Population bioequivalence based on D50 and span(D90-D10)/D50 or polydispersity index________________________________________________________________________As per 21 CFR § 314.94(a)(9)(iii), the proposed parenteral drug product should bequalitatively (Q1) and quantitatively (Q2) the same as the corresponding reference listeddrug product. In addition, firms are recommended to obtain assurance from OGD that thetest product has the same in-vitro characteristics as those of reference listed drug productprior to conducting any bioequivalence study for submission. Additional in vitrocharacterization are recommended to demonstrate the sameness between the test andreference products in terms of particle morphology, particle size, surface potential,paclitaxel crystallinity, fraction of free and bound paclitaxel or albumin in reconstitutedsuspension, nature of bond between paclitaxel and albumin, and in vitro release kinetics.In addition, albumin, the only excipient in the final product, is critical to the formulation.The characterization of the oligomeric status of albumin in both the albumin excipientand the final drug product is also recommended. The in vitro characterization tests arerecommended to be conducted on three batches of the ANDA and RLD products (at leastone ANDA batch should be produced by the commercial scale process).________________________________________________________________________Waiver request of in vivo testing: Not applicableIn Vitro Release Testing Method: The applicants are encouraged to explore methods tocharacterize in vitro release.Recommended Sep 2012 2。