IGCSE与AS化学仪器apparatus-附加图片
- 格式:doc
- 大小:674.00 KB
- 文档页数:2

IGCSE教学大纲1.剑桥考试:May/June examination session and the October/November examination session.2.CIE :Cambridge International Examinations剑桥大学国际考试局3.IGCSE :International General Certificate of Secondary Education国际中学教育普通证书的考试4.Cambridge IGCSE Chemistry is accepted by universities and employers as proof of essential chemistry knowledge and ability. As well as a subject focus, the Chemistry syllabus enables students to:•better understand the technological world in which they live, and take an informed interest in science and scientific (科学的、系统的)developments •learn about the basic principles of Chemistry through a mix of theoretical(理论的)and practical studies•develop an understanding of the scientific skills essential for further study at A Level, skills which are useful in everyday life•learn how science is studied and practised(实践), and become aware that the results of scientific research can have both good and bad effects on individuals个体, communities团体and the environment5.Cambridge IGCSE Chemistry candidates are awarded grades ranging from A* to G. 成绩从A* 到G.6.Candidates expected to achieve grades D, E, F or G, study the Core Curriculum核心课程only and are eligible(有资格、合格的)for grades C to G. Candidates expected to achieve grade C or higher should study the Extended Curriculum延展课程, which comprises the Core and Supplement Curriculums; these candidates are eligible for all grades from A* to G.7.All candidates must enter for three papers.一、Paper1:单项选择,45分钟占总成绩的30%二、Paper2: 核心理论,1小时15分钟,占总成绩的50%Paper3:扩充理论,1小时15分钟,占总成绩的50%三、Paper4:修习,指定功课,职业培训(school-based assessment)Paper5:实验操作,1小时15分,占总成绩的20%Paper6:实验笔试(written paper),1小时,占总成绩的20%8.Assessment objectives评价目标:The three assessment objectives in Cambridge IGCSE Chemistry are:A .Knowledge with understandingStudents should be able to demonstrate(展示)knowledge and understanding in relation to:⑴ scientific phenomena, facts, laws, definitions, concepts and theories⑵ scientific vocabulary, terminology(术语)and conventions常规(including symbols, quantities and units)⑶ scientific instruments and apparatus, including techniques of operation and aspects of safety⑷ scientific quantities and their determination⑸ scientific and technological applications with their social, economic and environmental implications涉及、密切关系.考试题型:Define定义, state陈述, describe描述, explain解释or outline 概述B .Handling information and problem solvingStudents should be able, in words or using other written forms of presentation展示、陈述(i.e. Symbolic符号的, graphical图解的and numerical数字的), to:⑴ locate, select, organise and present information from a variety of sources⑵ translate information from one form to another将信息从一种形式转换到另一种形式⑶ Manipulate处理numerical and other data⑷ use information to identify patterns, report trends and draw inferences 推断、推理⑸ present reasoned explanations for phenomena, patterns and relationships⑹ make predictions and hypotheses假定、臆测⑺ solve problems, including some of a quantitative nature定量性质. predict, suggest, calculate or determineC .Experimental skills(实验技能)and investigationsStudents should be able to:⑴ know how to use techniques, apparatus and materials (including following a sequence顺序of instructions where appropriate)⑵ make and record observations, measurements and estimates评价⑶ interpret and evaluate experimental observations and data⑷ plan investigations, evaluate methods and suggest possible improvements (including the selection of techniques, apparatus and materials).9. Scheme of assessment评估方案⑴ All candidates must enter for three papers: Paper 1; either Paper 2 or Paper 3; and one from Papers 4, 5 or 6.⑵ Candidates who have only studied the Core curriculum, or who areexpected to achieve a grade D or below, should normally be entered for Paper 2.⑶ Candidates who have studied the Extended curriculum, and who are expected to achieve a grade C or above, should be entered for Paper 3.⑷ All candidates must take a practical paper, chosen from: Paper 4 (Coursework), Paper 5 (Practical Test), or Paper 6 (Alternative to Practical).10.11.Curriculum content课程内容12.Practical assessmentCriteria for assessment of experimental skills and abilities评估标准的实验技能和能力⑴ Each skill must be assessed on a six-point scale, level 6 being the highest level of achievement. Each of the skills is defined in terms of three levels of achievement at scores of 2, 4, and 6.⑵ A score of 0 is available if there is no evidence of positive achievement for a skill.⑶ For candidates who do not meet the criteria for a score of 2, a score of1 is available if there is some evidence of positive achievement.⑷ A score of 3 is available for candidates who go beyond the level defined for 2, but who do not meet fully the criteria for 4.⑸ Similarly, a score of 5 is available for those who go beyond the level defined for 4, but do not meet fully the criteria for 6.\。



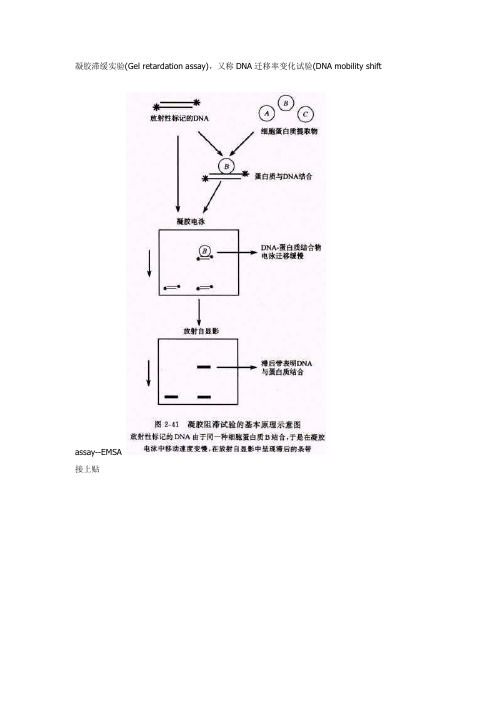
凝胶滞缓实验(Gel retardation assay),又称DNA迁移率变化试验(DNA mobility shiftassay--EMSA接上贴如《现代分子生物学实验技术》该书中就有详细介绍说明,高等教育出版社出版,卢圣栋主编。
若找不到该书,一般有关分子生物的实验技术书上都会有介绍。
总的说来,EMSA不是太好做,目前有两种方法,一种是有放射性同位素标记的,还有一种是不用同位素的,采用的化学发光的方法。
因为它的影响因素很多,有抗体的浓度,一些离子的强度和实验的条件等等,如果要做出好的EMSA的结果需要摸条件。
我们采用的是化学的方法,总的来说没有同位素的方法经典。
感觉一般的书介绍的都比较粗略,最好查阅相关要作的分子已发表papae的实验条件,在此基础上再不断的摸索。
分子克隆第三版上也有相关的知识。
如果要买公司的试剂盒的话,可在网上查阅相关的信息。
以上仅为我之拙见。
推荐一个很好的EMSA试剂相关网站,可以参考。
另外,现在有新的方法定量检测活性转录因子含量,类似于ELISA的方法,只是板子上包被的是寡核苷酸,操作很简单,效果也比较好,可以参考。
promega 公司有专门做NF-kB活性的试剂盒,名字叫E3300,效果不错。
我参考了一些分子生物学技术参考书总结了该实验的protocol(包括核蛋白的提取过程),供您参考如下:实验材料及方法一、细胞核蛋白质的提取试剂和溶液缓冲液A 缓冲液B10mmol/L HEPES-KOH Ph7.9 20mmol/L HEPES-KOH Ph7.91.5mmol/L MgCL2 1.5mmol/L MgCL210mmol/L KCL 420mmol/L NaCL0.5mmol/L DTT 0.2mmol/L EDTA0.2mmol/L PMSF 25% Glycerol0.5mmol/L DTT0.2mmol/L PMSF操作步骤1.去细胞培养液,用PBS(含0.05mmol/LPMSF)洗两次,用刮板将细胞刮下,计数细胞,收集于适当体积离心管,用少量PBS冲洗平皿一并吸入离心管。

IGCSE CHEMISTRY化学带图解单词(实验仪器部分)1 particle 微粒粒子2 solids 固体3 collide 碰撞4 random motion 不规则运动5 diffusion 扩散作用6 melting point 熔点7 water vapour 水蒸气水汽8 boiling point 沸点9 heating curve 加热曲线10 melting 融化11 expand 膨胀12 lattice 晶格13 evaporating 蒸发作用的14 evaporation 蒸发15 pressure 压强16 temperature 温度17 volume 体积18 a closed container 封闭的容器1 stopwatch 秒表2 measuring cylinder 量筒3 burette 滴定管4 pipette 移液管吸移管5 thermometer 温度计6 purity 纯度7 flask 烧瓶8 test tube 试管9 beaker 烧杯10 condenser 冷凝器11 cuvette 比色杯12 evaporating basin 蒸发皿13 funnel 漏斗14 dropper 滴管1 compressed 被压缩的2 diffuse 扩散3 relative molecular mass 相对分子质量(化学式中个院子的相对原子质量的总和)4 cooling curve 冷却曲线5 mixture 混合物6 dissolved 溶解的7 solution 溶液8 solute 溶质9 solvent 溶剂10 sparingly soluble 微溶的11 limewater 石灰水12 insoluble 不溶的13 saturated 饱和的14 more soluble 更易溶15 volatile 挥发性的16 pure 纯的17 impurity 杂质18 residue 废料残渣19 filtrate 滤液20 crystallisation 结晶结晶作用21 distilled water 蒸馏水22 refine 提纯23 ethanol 乙醇24 chromatogram 色谱25 identify 鉴定26 chromatography 色层分析纸27 locating agent 显色剂28 ninhydrin 宁希德林, (水合)茚三酮29 amino acid 氨基酸30 stationary phase 固定相31 mobile phase 流动相32 chemical means 化学方法1 nucleus 原子核2 element 元素3 periodic Table 周期表4 atomic mass units 原子量单位5 proton number 质子数s neutron number 中子数7 nucleon number 核数8 isotopes 同位素9 radioactive 放射性的10 decays 衰变11 radiation 辐射放射12 radiation sickness 辐射病13 geiger counter 盖革计数器14 tracers 示踪剂15 radiotherapy 放射性治疗16 period number 周期数17 valency electrons 价电子18 group number 族数19 unreactive 化学惰性的20 alchemists 炼金术师21 elixir of life 仙丹22 gold 金23 chemists 化学家24 scanning tunneling microscope 扫描隧道电子显微镜25 cathode 阴极26 radioactivity 放射现象放射性27 alpha particles a粒子28 beta particles B粒子29 model 模型30 non-metals 非金属31 chemical properties 化学性质32 chemical change 化学变化33 compound 化合物34 word equation 文学方程式35 reverse change 逆向变化。