14 group VIIA
- 格式:ppt
- 大小:3.28 MB
- 文档页数:63
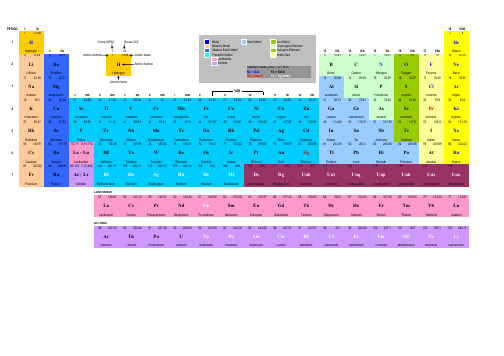
Atomic Number†Atomic WeightSymbol†Ground-State Level*Electronegativity (Pauling)Name*Density [Note]†Ionization Energy (eV)*Melting Point (°C)*Boiling Point (°C)Atomic radius (pm)[Note]Crystal Structure [Note]†Electron ConfigurationPossible Oxidation States [Note]Phase at STP†Common ConstantsSource: Absolute Zero -273.15 °CGravitation Constant 6.67428x10-11 m 3 kg -1 s -2Atomic Mass Unit 1.660539x10-27kg Molar Gas Constant8.314472 J mol -1 K -1Categories Avogadro Constant6.022142x1023 mol -1Molar Volume (Ideal Gas)0.02241410 m 3/mol Base of Natural Logarithms 2.718281828PI3.14159265358979Boltzmann constant 1.380650x10-23 J/K Planck Constant6.626069x10-34 J s Electron Mass9.10938215x10-31 kg Proton-Electron Mass Ratio 1836.152672470.5110 MeV Rydberg Constant10 973 732 m -1Electron Radius (Classical) 2.8179403x10-15 m 3.289842x1015 Hz Electron Volt 1.602176x10-19 J 13.6057 eV Elementry Charge 1.602176x10-19 C Second Radiation Constant 0.01438769 m K Faraday Constant 96 485.3399 C/mol Speed of Light in a Vacuum 299 792 458 m/s fine-structure constant 0.0072973525Speed of sound in air at STP 343.2 m/s [42]First Radiation Constant3.7417749x10-16 W m2Standard Pressure101 325 Pa{42}References:†, * (Mathematic),CRC Handbook of Chemistry and Physics 81st Edition, 2000-2001, and others18VIIIA Notes:- Density units are g/cm 3 for solids and g/L or kg/cm 3 at 0° Celsius for gases- Atomic Weight based on 12C- ( ) indicate mass number of most stable isotope - Common Oxidation States in bold- Electron Config. based on IUPAC guidelines- § indicates crystal structure is unusual or may require explanation- (m) Metallic radius, (v) Covalent radius © 2011 Vertex42 LLC. All rights reserved.Design by CnPeriodic Table of the Elements DbSg(v) 37-+1,-1LiPeriodic Table of the ElementsGROUP 1IA 1 1.0079424.00260216VIAFree Downloads at -1s 11s 11s 217VIIA0.178524.5874-259.14P e r i o d111.007940.089913.5984He1S 02.22.2-H2S 1/2H2S 1/2HydrogenHydrogenHelium2IIA 0.089913.5984(v) 37FCC +1,-1-268.93-259.14-252.87-(v) 3213IIIA 14IVA15VA-252.8709.012182510.8116236.941415.9994918.99840321012.0107714.0067820.17972S 1/2Be1S 0BG 2P°1/2C3P 0N3P 2F3.043.442.042.554S°3/2O2P°3/2Ne1S 00.981.57Gas Liquid Solid Synthetic3.98-LithiumBerylliumm u R BoronCarbonNitrogenOxygenFluorineNeon9.3227 2.468.2980 2.26p 207513.6181 1.69617.42280.911.2603 1.25114.5341 1.4294027-210.121.5645180.54134212872470Alkali Metals Noble Gas e -246.08(m) 152BCC(m) 112HCPk h (v) 82-195.79-218.3rhom.(v) 77hex(v) 75-188.12-248.59-182.9-219.640003550 -0.535 5.3917 1.848[He] 2s 1[He] 2s 2Alkaline EarthMetalsHalogens m e m e /m p [He] 2s 2 2p 1-(v) 73[He] 2s 2 2p 2[He] 2s 2 2p 3[He] 2s 2 2p 4[He] 2s 2 2p 5-(v) 69-(v) 71[He] 2s 2 2p 6+1+2me c 2R ∞+3+2,4,-4+2,3,4,5,-2,-3-2-1031122.9897701224.3050Transition Metals Non Metalsr0R ∞c 1839.9481530.973611632.065Na2S 1/2Mg1S 01735.4531326.9815381428.0855Si3P 0P4S°3/2eV R ∞hc Al2P°3/22.583.16Ar1S 00.93 1.31Rare Earth Metalse ch/k 1.611.902.19-SodiumMagnesiumF cAluminumSilicon PhosphorusSulfurChlorineArgon5.1391 1.7387.6462Poor Metals Metalloidsa2.710.4867 1.9610.36003.2145.9858 2.338.1517 1.8232P°1/2S3P 2Cl11IB 12IIB (m) 1436VIB 7VIIB 8VIII 9VIII §(v) 102FCO12.9676 1.78415.759697.7288365010902p hc 2660.32280.5115.21444.72-101.525191414290044.2-34.04-189.3-185.80.968(v) 99FCC(v) 111cubic(v) 106-(v) 97-[Ne] 3s 1[Ne] 3s 2[Ne] 3s 2 3p 1[Ne] 3s 2 3p 2[Ne] 3s 2 3p 3[Ne] 3s 2 3p 4[Ne] 3s 2 3p 5[Ne] 3s 2 3p 6+1+2+3+2,4,-4+3,4,5,-3+2,4,6,-2+1,3,5,7,-1010VIII (m) 186BCC(m) 160HCP3IIIB 4IVB 5VB 41939.09832063.3875984240.078K2S 1/22350.94152451.99612144.9559102247.8674.507 6.82810.856 4.3407 1.55 6.1132 2.985 6.5615 6.11 6.74627.14 6.7665+2,3,6Ni3F 4Cu1.881.912S 1/2Zn1S 02758.9332002858.69342554.9380492655.845312963.5463065.409Ti3F 2V4F 3/2Ca1S 0Sc2D 3/2Fe5D 4Co1.83Cr7S 3Mn6S 5/24F 9/2Ge3P 0As1.812.014S°3/2Se3P 2Br2.182.552P°3/2Kr79.9043683.7983374.921603478.9669.7233272.64351S 00.82 1.00 1.361.54 1.631.66 1.552.963PotassiumCalciumScandiumTitaniumVanadiumChromiumManganeseIronGalliumGermaniumArsenicSeleniumCobaltNickelCopperZincBromineKryptonGa1.901.652P°1/28.97.88108.9087.63987.477.43407.8747.9024 5.904 5.9993 5.3237.89948.927.72647.149.3942 3.1211.8138 3.7513.99965.7279.7886 4.8199.7524328719103407190714841541283016682861149529271455267112462061153890729.762204938.329131084.622927419.53685-7.359-157.362820817614221-153.22(m) 227BCC(m) 197FCC(m) 162HCP(m) 147HCP(m) 134§cubic(m) 126BCC(m) 125BCC(m) 128BCC(m) 127FCC(m) 134§hex(m) 135HCP(m) 124FCC(m) 128rhom.(v) 116§hex(v) 114§BCO(v) 122§cubic(v) 119BCO(v) 110-[Ar] 4s 1[Ar] 4s 2[Ar] 3d 1 4s 2[Ar] 3d 2 4s 2[Ar] 3d 3 4s 2[Ar] 3d 5 4s 1[Ar] 3d 5 4s 2+2,3,4,6,7[Ar] 3d 10 4s 2[Ar] 3d 10 4s 2 4p 1[Ar] 3d 10 4s 2 4p 2[Ar] 3d 10 4s 2 4p 3[Ar] 3d 6 4s 2[Ar] 3d 7 4s 2[Ar] 3d 8 4s 2[Ar] 3d 10 4s 1+2,3+1,2[Ar] 3d 10 4s 2 4p 4[Ar] 3d 10 4s 2 4p 5[Ar] 3d 10 4s 2 4p 6+1+2+3+2,3,4+2,3,4,5053785.46783887.623988.90585+2+34091.2244192.90638+2,4,6,-2+1,5,-1+2,4+3,5,-3+2,3+2,344101.0745102.905504295.9443(98)48112.41149114.8184654131.293Rb2S 1/2Sr1S 0Y2D 3/2Zr3F 2Nb6D 1/2Mo7S 31.602.16Tc6S 5/2Ru5F 51.91S 01.931.69In2P°1/2Sn3P 01.781.96106.4247107.868252127.6053126.9044750118.71051121.760Sb4S°3/2Te3P 22.052.10I2P°3/2Xe1S 02.662.60RubidiumStrontiumYttriumZirconium0.820.95 1.221.33IodineXenon2.20Rh4F 9/2Pd1S 02.282.20Ag2S 1/2CdIndiumTinAntimonyTelluriumRhodiumPalladiumSilverCadmium1.532 4.17712.63 5.6949 4.472 6.21738.57 6.758910.287.0924NiobiumMolybdenumTechnetiumRuthenium12.457.458912.0238.336911.57.2812.377.36057.31 5.78647.317.343910.497.57628.658.9938 4.9410.4513 5.912.12986.6978.6084 6.249.00961526334518554409-111.8-108630.631587449.519886.511 6.633939.31688777138221574265233441502477474426234639961.782162321.07767196436951554.92963113.7184.3156.62072231.932602(m) 146BCC(m) 139BCC(m) 180HCP(m) 160HCP(m) 134FCC(m) 137FCC(m) 136HCP(m) 134HCP(m) 167§tetra.(v) 141§tetra.(m) 144FCC(m) 151§hex(v) 133BCO(v) 130-(v) 138§rhom.(v) 135hex[Kr] 4d 4 5s 1[Kr] 4d 5 5s 1[Kr] 4d 5 5s 2[Kr] 4d 7 5s 1[Kr] 5s 1[Kr] 5s 2[Kr] 4d 1 5s 2[Kr] 4d 2 5s 2[Kr] 4d 10 5s 2 5p 1[Kr] 4d 10 5s 2 5p 2[Kr] 4d 10 5s 2 5p 3[Kr] 4d 10 5s 2 5p 4[Kr] 4d 8 5s 1[Kr] 4d 10[Kr] 4d 10 5s 1[Kr] 4d 10 5s 2[Kr] 4d 10 5s 2 5p 5[Kr] 4d 10 5s 2 5p 6(m) 248BCC(m) 215FCC+1+2+3+4+3,5+2,3,4,5,6+4,7+2,3,4,6,872178.49+3+2,4+3,5,-3+2,4,6,-2+2,3,4+2,4+1+283208.9803884(209)655132.9054556137.327Lanthanide Series73180.947974183.84+1,5,7,-1077192.21778195.07875186.20776190.2381204.383382207.279196.9665580200.5985(210)86(222)2.33 2.02Hf3F 2Ta4F 3/2Cs2S 1/2Ba1S 0Os5D 4Ir4F 9/2W5D 0Re6S 5/2PtPo3P 2At2P°3/22.0 2.2Rn1S 00.790.89 1.3 1.52.36 1.92.2 2.2-3D 3Au2S 1/22.28 2.54Hg1S 0Tl2P°1/221.62Pb3P 0Bi4S°3/2CesiumBariumHafniumTantalumTungstenRheniumOsmiumIridiumPlatinumBismuthPoloniumAstatineRadonGoldMercuryThalliumLead13.31 6.825116.657.54961.879 3.8939 3.51 5.211722.618.438222.658.967019.257.864021.027.833513.53410.437511.85 6.108221.098.958819.39.22559.1968.414--11.347.41679.787.28559.7310.748528.446717271870223346033017545830335012246644283422555531865596-38.83356.7330414731768.338251064.182856254962302-327.461749271.31564-71-61.7(m) 265BCC(m) 222BCC(m) 159HCP(m) 146BCC(m) 135HCP(m) 136FCC(m) 139BCC(m) 137HCP(m) 151§rhom.(m) 170HCP(m) 139FCC(m) 144FCC-§cubic--(m) 175FCC(v) 146§rhom.(v) 145-[Xe] 6s 1[Xe] 6s 2[Xe] 4f 14 5d 2 6s 2[Xe] 4f 14 5d 3 6s 2[Xe] 4f 14 5d 4 6s 2[Xe] 4f 14 5d 5 6s 2[Xe] 4f 14 5d 6 6s 2[Xe] 4f 14 5d 7 6s 2[Hg] 6p 2[Hg] 6p 3[Hg] 6p 4[Hg] 6p 5[Xe] 4f 14 5d 9 6s 1[Xe] 4f 14 5d 10 6s 1[Xe] 4f 14 5d 10 6s 2[Hg] 6p 1[Hg] 6p 6+1+2+4+5+2,3,4,5,6+2,4,6,7,-1+2,3,4,6,8+2,3,4,6+2,4+3,5+2,4+1,3,5,7,-1+1,3+1,2+1,3+2,4787(223)88Fr2S 1/2Francium(226)Actinide Series104(261)Ra1S 0Rf3F 2 ?RadiumRutherfordium- 4.07275 5.2784 6.0 ?+1+2+4107(264)108(277)105(262)106(266)111(272)112(285)109(268)110(281)118115116(292)HsMt117113114(289)UuhUusUutUuq UupDsRg DubniumSeaborgiumBohriumHassiumUuo0.70.9Ununtrium Ununquadium UnunpentiumUnunhexiumMeitnerium Darmstadtium RoentgeniumCoperniciumUnunseptiumUnunoctiumBh--7001737---BCC[Rn] 7s 1[Rn] 7s 2[Rn] 5f 14 6d 2 7s 2 ?59140.9076560144.2461(145)162.50067164.9303270173.0471174.96768167.25969168.9342157138.9055La2D 3/21.10Lanthanum62150.3663151.964A c t i n i d e s8958140.116Cerium L a n t h a n i d e s6664157.2565158.92534Ce1G°4Pr4I°9/21.121.13Nd 5I 4Pm6H°5/21.14-Sm7F 0Eu8S°7/21.17-Gd9D°2Tb6H°15/21.20-Dy5I 8Ho4I°15/21.221.23Er3H 6Tm2F°7/21.241.25Yb1S 0Lu2D 3/2-1.27ThuliumYtterbiumEuropiumGadoliniumTerbiumDysprosiumHolmiumErbiumPraseodymium NeodymiumPromethiumSamarium5.5827.353 5.6437 5.244Lutetium6.146 5.5769 6.689 5.5387 6.64 5.86388.551 5.93898.7955.67047.901 6.14988.219 6.1843 6.57 6.25429.8416.02159.066 6.10779.321 5.425992034647983360931329010213100110015271313325013563000107218038221196166327001497286815455.4737.01 5.52507.264§hex (m) 181§hex (m) 18319508193230141225671474HCP (m) 180§hex (m) 1803402(m) 187§hex (m) 182FCC (m) 182HCP (m) 178HCP (m) 176BCC (m) 180HCP (m) 177HCP (m) 176FCC (m) 174HCP (m) 176HCP (m) 176HCP [Xe] 5d 1 6s 2[Xe] 4f 1 5d 1 6s 2[Xe] 4f 3 6s 2[Xe] 4f 4 6s 2[Xe] 4f 5 6s 2[Xe] 4f 6 6s 2[Xe] 4f 7 6s 2[Xe] 4f 7 5d 1 6s 2[Xe] 4f 9 6s 2+2,3+3[Xe] 4f 10 6s 2[Xe] 4f 11 6s 2[Xe] 4f 12 6s 2[Xe] 4f 13 6s 2+3+3,4+3,4+3+3+2,3+3,4+3+3+3[Xe] 4f 14 6s 2[Xe] 4f 14 5d 1 6s 2+2,3+2,3+3(227)90232.038191231.035992238.028995(243)96(247)93(237)94(244)103(262)U5L°6101(258)102(259)99(252)100(257)Ac2D 3/2Th3F 2Pa4K 11/2Np6L 11/2Pu7F 097(247)98(251)FmBk6H°15/2Cf5I 8Am8S°7/2Cm9D°21S 0Lr1.31.33H 6Md1.31.281.31.31.32F°7/2No1.3Es1.34I°15/2- 5.973813.51 5.991420.45 6.265719.816 6.026011353927PlutoniumAmericiumCuriumBerkelium2P°1/2 ?1.11.31.51.381.36CaliforniumEinsteiniumFermiumMendelevium-ActiniumThorium ProtactiniumUraniumNeptuniumNobelium Lawrencium1050320017504820157240006444000640323010.07 5.1711.724 6.306715.37 5.8919.05 6.1941- 4.9 ?14.78 6.197915.1 6.28171050-900-1176201113453110827-827-860-1527-1627-- 6.58- 6.65- 6.42- 6.50--[Rn] 6d 1 7s 2[Rn] 6d 2 7s 2[Rn] 5f 2 6d 1 7s 2[Rn] 5f 3 6d 1 7s 2[Rn] 5f 4 6d 1 7s 2[Rn] 5f 6 7s 2[Rn] 5f 9 7s 2[Rn] 5f 10 7s 2[Rn] 5f 11 7s 2[Rn] 5f 12 7s 2-FCC(m) 179FCC (m) 163§tetra (m) 156BCP (m) 173HCP (m) 174HCP (m) 155SO (m) 159§mono.[Rn] 5f 7 7s 2[Rn] 5f 7 6d 7s 2--(m) 170hex -hex [Rn] 5f 13 7s 2[Rn] 5f 14 7s 2[Rn] 5f 14 7s 2 7p ?+3+4+4,5+3,4,5,6+3,4,5,6+3,4,5,6+3,4,5,6+3+2,3+2,3+3+3,4+3+3+3------。

1. The Elements and The Periodic Table元素和周期表The number of protons in the nucleus of an atom is referred to as the atomic number, or proton number, Z. The numbers of electrons in an electrically neutral atom is also equal to the atomic number, Z. The total mass of an atom is determined very nearly by the total number of protons and neutrons in its nucleus. This total is called the mass of the number, A. The number of neutrons in an atom, the neutron number, is given by the quantity A-Z.refer to sb. [sth.] as 称某人(物)为be determined by 由…确定原子核中质子的数目称为原子序数,或者质子数,以Z表示。
电中性原子中电子的数目也等于原子序数Z。
经测定,原子的总质量与原子核中质子与中子的总数差不多。
(几乎相同)(或者说原子的总质量几乎可以由原子核中质子与中子的总数确定。
)这个总数叫质量数,以A表示。
因此,原子中的质子的数目,质子数,可以定量地由A-Z给出。
即原子中质子数=A-ZThe term element refers to a pure substance with atoms all kinds of a single kind. To the chemist the “kind” of an atom is specified by its atomic number, since this is the property that determines its chemical behavior. At present all the atoms from Z=1 to Z=107 are known; there are 107 chemical elements. Each chemical element has been given a name and a distinctive symbol. For most elements the symbol is simply the abbreviated form of the English name consisting of one or two letters,for example:元素这个术语指的是仅仅由同一种类的原子组成的物质。

Restricted Substance Testing Service國際無鹵(Halogen Free)規範及趨勢介紹2007/11/28ShinJyh,Chen陳新智RSTS E&E Technical CenterMulti-chemical Lab,UTIS Lab,Taipei,Taiwan綜合化學實驗室,超微量工業安全實驗室簡報大綱⏹⏹⏹1886⏹氟是一種極具腐蝕性的淡黃色雙原子氣體素,也是很強的氧化劑。
在常溫下,它幾乎能和所有的元素化合,並產生大量的熱能。
⏹1774⏹具有強氧化性,所以可以作為一種廉價的消毒劑,一般的自來水就採用它來消毒,同時氯氣也可用於紙漿和棉布的漂白⏹1824年法國⏹溴是唯一在室溫下為液體的非金屬元素,紅棕色蒸氣⏹常溫下為紅色發煙液體,化學性質較碘活潑,但不比氯活潑,能與許⏹1811⏹碘在常溫下是紫色的固體。
⏹碘的用途⏹砈像金屬,活性較碘低,固態,具放射性⏹1940年初次被合成的,可用α粒子轟擊鉍地衰變成⏹prEN14582Oxygenmethod⏹IEC61189-2Test methodsinterconnection鹵素含量測試方法比較法規編號prEN14582Method.ABS EN14582prEN14582Method.BASTM E442-91鹵素測試方法⏹IEC61189-2法主要針對⏹BS EN樣品燃燒/鹼液吸收HBr、HCl鹼液吸收IC儀器分析數據審核正式報告Optional產品應用及規範要求⏹IECReinforced and unclad woven laminated sheetsIPCadopted halogen-freeOther⏹acceptable⏹A testPBDEOther⏹All partsrequirements.⏹Homogenous揮發性有機化合物∙溶劑/助劑∙表面清洗劑∙冷煤–半揮發性有機化合物∙含溴化合物,作為––⏹含溴/氯化合物電子廢料、塑膠產品主要添加物質⏹燃燒時亦會產生酸性氣體HeatFuelAirFireH e a t t r a n s f e rMixing of fuel and airH ea t t r an sf e rF l am eR e t a r d a n tF l a m eR e t a rd a n t阻燃劑分類⏹無機類⏹含溴有機類常見耐燃劑種類DecaBDE十溴聯苯醚HBCD六溴環十二烷TPP磷酸三苯酯TribromoPhenol三溴酚無機類地區阻燃劑阻燃劑產品及應用Reactive Bisphenol ATypeBFR productgroup含鹵化合物使用範圍鹵素項目阻燃劑產品及應用Substance阻燃劑產品及應用Substance四溴雙酚Tetrabromobisphenol-A⏹TBBPA阻燃劑主要用於電路板⏹主要為反應型阻燃劑,因低成本、與電路板相關零件相容性佳,因而電子電機類產品禁用No.EU directive176/769/EEC電子電機類產品禁用No.1無鹵素材料對產業影響⏹傳統PCB⏹銅箔基板廠商主打完全無鹵素的環保材料,主要將鹵素系難燃無鹵替代性材料使用⏹FR4(epoxyresin/glass∙製程參數改變Information阻燃劑種類⏹不含溴(Non-Brominated ⏹絕大部分的對無鹵規範之因應建立規範•了解買家無鹵規範•訂定產品規範/因應時程Halogen Free 管控系統替代材料•功能性考量•成本考量產品了解•了解鹵素存在範圍•測試計劃Halogen Free無鹵產品管控系統。

完整版)化学专业英语Teaching Material for Scientific EnglishI。
Naming of XXX1.Naming of XXXThe English word for both "元素" and "单质" is "element"。
To distinguish een the two。
"free element" may be used when emphasizing "单质"。
Therefore。
the English names for XXX are the same。
The following are the names of elements that are also names of free elements:Group IA:XXXXXXSodiumGroup IIA:XXXMagnesiumGroup IIIA: Boron AluminumGroup IVA: Carbon Silicon GermaniumGroup VA: Nitrogen PhosphorusGroup VIA: Oxygen Sulfur XXXXXXPoloniumGroup VIIA: Fluorine Chlorine Bromine IodineXXXGroup 0: XXXNeon ArgonXXX Xenon RadonGroup IA: Potassium CalciumGroup IIA:RubidiumCesiumFranciumGroup IIIA:GalliumIndiumXXXGroup IVA:ArsenicXXXXXXXXXLead2.Naming of CompoundsCompounds are named from left to right according to their chemical formula。
第十一章第VIIA族,卤素 Group 7, the halogens第一节卤素 The halogens学习目标 Learning objectives:∙元素周期表中第VIIA族元素的原子半径有何变化趋势,存在这种趋势的原因?∙元素周期表中第VIIA族元素的电负性有何变化趋势,存在这种趋势的原因?大纲参考:3.2.5第VIIA族元素位于元素周期表的右手边,由非金属元素组成。
第VIIA族单质是双原子分子,包括 F2、Cl2、Br2、I2和At2,这些单质称为卤素。
物理性质 Physical properties气态卤素外观差别很大,如图1所示。
室温条件下,氟气是淡黄色气体,氯气是黄绿色气体,溴气是红棕色液体,而碘是黑色固体。
随着卤素在元素周期表内排列位置越靠下,卤素颜色越来越深、越来越浓。
卤素都有一种“室内游泳池”的气味。
氟气的物理性质没有代表性,原因在于与其他卤素单质内部化学键力量强度相比,F-F单键之间的力量非常弱。
氟原子很小,造成非成键的电子之间存在排斥力,因为这些电子之间靠得太近:Repulsion 排斥力氟气、氯气、溴气和碘的物理性质如表2所示。
提示Hint卤素这个词指的是形成盐的物质。
卤素能与多种金属发生反应,形成氟化物、氯化物、溴化物和碘盐。
图1气体卤素,依次排列为:氟气、氯气、溴气和碘。
表1氟气、氯气、溴气和碘的键能表中的红色箭头指示了这些变化趋势很清晰,可解释如下:原子的大小Size of atoms随着卤素在元素周期表内排列位置越靠下,卤素原子越大,原因在于与上一种元素相比,每种元素都多一层电子,如表2所示。
表2 第VIIA族元素氟气-碘的物理性质Chlorine 氯 bromine 溴 iodine碘图2 随着卤素在周期表内的位置越靠下,外壳距离原子核越远。
电负性 Electronegativity电负性可衡量原子吸引电子的能力,也可以称为电子密度,是指在一个共价键内吸引电子朝向自身原子的能力(如章节3.3所示)。
专利名称:Use as a chemical compound and those ion conductors which were induced fromLa2Mo2O9发明人:フランソワ グーテノワール,フィリップ ラコル申请号:JP特願2001-575504(P2001-575504)申请日:20010405公开号:JP特表2003-530291(P2003-530291A)A公开日:20031014专利内容由知识产权出版社提供摘要:The invention concerns novel compounds derived from La2Mo2O9 and their use as ion conductors. The compounds of the invention have formula (1): A2-x A'x B2-y B'y O9-z+delta X z in which A is at least one trivalent element selected from trivalent rare earths, trivalent bismuth, trivalent antimony and trivalent arsenic; A' is at least one monovalent element selected from alkalis; or a divalent element selected from alkaline-earths, tin, lead, samarium, europium, erbium, thulium and ytterbium; or a quadrivalent element selected from thorium, uranium, group IVA elements, cerium, praseodymium and terbium; B is at least one hexavalent element from groups VIA, VIIA, VIII and group VIB with the exception of oxygen; B' is at least one element selected from lithium, sodium, magnesium, calcium, scandium, yttrium, rare earths with atomic numbers 63 to 71, elements from groups IVA to IIB with an oxidation number of less than 6, aluminium III, silicon IV, gallium III, germanium IV, indium III, tin IV, phosphorus V, antimony V and bismuth V; X is selected from sulphur, fluorine and chlorine; where 0<=x<2; 0<=y<2;0<=z<=3; and with the condition that when A is lanthanum and B is tungsten or molybdenum, at least one of x, y or z is different from 0; the compounds having a cationiccubic or pseudo-cubic beta-SnWO4 type lattice.申请人:ロディア テレ ラレ,サントル ナスィオナル ド ラ ルシェルシュ スィアンティフィク地址:フランス国 エフ17041 ラ ロシェル、リュ シェフ ド ベ、26、ゾヌ アンデュストリエル,フランス国 エフ75016 パリ、リュ ミシェル アンジェ、3国籍:FR,FR代理人:倉内 基弘 (外1名)更多信息请下载全文后查看。