水的净化(Water Purification)
- 格式:doc
- 大小:15.00 KB
- 文档页数:2



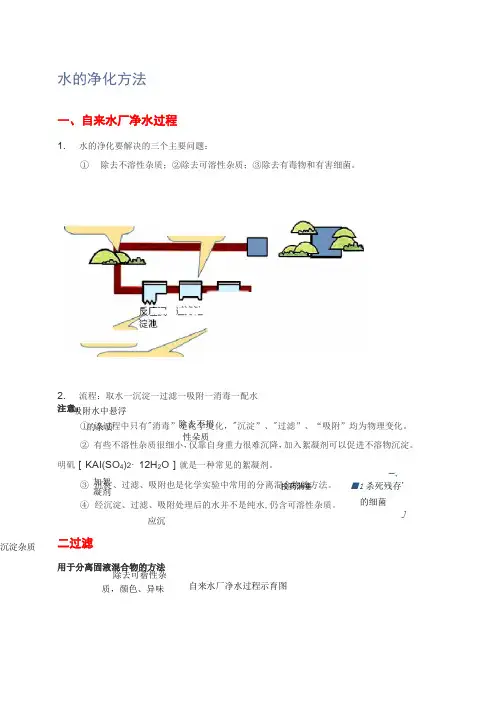
取水口■"W 1声附泄蓿水池击打用户而【1水的净化方法一、自来水厂净水过程1.水的净化要解决的三个主要问题:① 除去不溶性杂质;②除去可溶性杂质;③除去有毒物和有害细菌。
2.流程:取水一沉淀一过滤一吸附一消毒一配水 注意: ① 该过程中只有"消毒”是化学变化,"沉淀”、"过滤”、“吸附”均为物理变化。
② 有些不溶性杂质很细小,仅靠自身重力很难沉降,加入絮凝剂可以促进不溶物沉淀。
明矶[KAI(SO 4)2・12H 2O ]就是一种常见的絮凝剂。
③ 沉淀、过滤、吸附也是化学实验中常用的分离混合物的方法。
④ 经沉淀、过滤、吸附处理后的水并不是纯水,仍含可溶性杂质。
二过滤用于分离固液混合物的方法应沉沉淀杂质自来水厂净水过程示育图吸附水中悬浮的杂质加絮凝剂除去可蓿性杂质,颜色、异味一、■1杀死残存' 的细菌 J除去不招性朵质投药消垂操作要点:一贴、二低、三靠说明:一贴:滤纸紧贴漏斗内壁;二低:滤纸边缘低于漏斗边缘,液面低于滤纸边缘;三靠:倾倒滤液的烧杯尖嘴紧靠玻璃棒(目的:防止液体溅出),玻璃棒紧靠三层滤纸处(目的:防止戳破滤纸),漏斗下端管口紧靠烧杯内壁(目的:防止滤液溅出)。
注意:过滤只能除去不溶性杂质,无法除去可溶性杂质。
三、吸附活性炭具有疏松多孔的结构,在净水过程中起吸附作用,该过程为物理变化。
用具有吸附作用的固体过滤,不仅可以滤去不溶性杂质,还可以吸附一些可溶性杂质,除去颜色和气味。
这是市场上出售的使用活性炭的净水器的净水原理。
制取蒸馏水的简易装置四、蒸馏用于分离液体混合物的方法原理:利用液体混合物中各组分沸点不同,使低沸点组分先蒸发,再冷凝,以分离整个组分。
蒸馏得到的水称为蒸馏水,是净化程度较高的水。
实验室常用的蒸馏装置—制度计爪蕪气蒸懦装置KiElfl 水口人水口活性炭净水器示意图简易净水器示意图河水 注意:水的净化方法通常包括沉淀、过滤、吸附和蒸馏。






【关键字】大学英语作文水的净化(Water Purification)水的净化(water purification)the provision of safe water necessitates one of the rnajor expenditures of manpower and revenue in our modern cities. the purification of water is basically a two-step or three-step process carried out under the strict supervision of public health scientists and engineers. as the first step, natural water from the least contaminated source is allowed to stand in large reservoirs, where most of the mud, clay, and silt settle out; this is called "sedimentation". often in water with high mud content, lime and aluminum sulfate are added to the water in the settling reservoirs. these chemicals react in the water to form aluminum hydroxide, which settles slowly andcarries much of the suspended material, including most of carries much of the suspended material, including most of the bacteria, to the bottom of the reservoirs. as the second step, the water is filtered through beds of sand and grovel, which remove other impurities and chemicals in it. during or after filtration, chemicals are ordinarily added to the water to kill any remaining harmful bacteria. chlorine is one of the most common chemicals used for this purpose. a third step taken by some rmnicipalities is adding to the water other beneficial chemicals such as fluoride to make tooth enamel hard, and soda ash to make the water itself soft. the water purification process,carried out with little variation from one large city to another, is perhaps the biggest factor in the prevention of major outbreaks of disease in this country.文档从互联网中收集,已重新整理排版,word版本支持修改!。

知识点二水的净化purification1、水的净化(1)沉降静置沉降——直接把水静置,让悬浮杂质沉降下来吸附沉降——加入絮凝剂(明矾)能与水作用生成胶状物质,adsorption[æd'sɔ:pʃən](2)过滤filter [filtə]目的:分离固液混合物仪器:玻璃仪器(烧杯、漏斗、玻璃棒)、铁架台(带铁圈)、滤纸beaker['bi:kə]funnel['fʌnl] filter paper实验装置:操作要领:一贴、二低、三靠一贴:滤纸紧贴漏斗内壁。
二低:滤纸边缘低于漏斗边缘;液面低于滤纸边缘。
三靠:烧杯口紧靠玻璃棒;玻璃棒下端紧靠三层滤纸;漏斗下端紧靠烧杯内壁。
注意:过滤filter中玻璃棒的作用——引流溶解dissolve中玻璃棒的作用——搅拌,加速溶解蒸发evaporation中玻璃棒的作用——搅拌,防止液体因局部受热而飞溅(3)吸附adsorption[æd'sɔ:pʃən]加入活性炭active carbon。
由于活性炭具有疏松多孔的结构,能吸附一些可溶性的杂质,除去臭味,吸附颜色(物理变化)(4)投药杀毒(Cl2)投药杀死水中的细菌(5)蒸馏distillation[,disti'leiʃən]净化程度最高的净化方法沸石:防止暴沸。
冷却水:下口进,上口出2、硬水和软水(1)定义硬水:含较多可溶性钙、镁化合物的水软水:不含或含较少可溶性钙、镁化合物的水(2)鉴别:用肥皂水来鉴别硬水和软水。
加肥皂水,观察泡沫多,浮渣少的为软水;泡沫少,浮渣多的为硬水。
(3)转化(生活中)煮沸boiling、蒸馏distillation(工业上)硬水软水3、简易净水装置注意:1、小卵石、石英砂、膨松棉的作用是过滤水中的固体杂质2、活性炭的作用:吸附杂质3、经过简易净水装置处理后的水属于混合物mixture ['mikstʃə]【例题1】(08北京中考18)下列实验基本操作中,正确的是A.倾倒液体B.过滤C.加热液体D.熄灭酒精灯变式练习1(10宣武一模6)下列实验操作中正确的是A.熄灭酒精灯B.滴加液体C.过滤D.稀释浓硫酸【例题2】(07北京中考14)下列叙述中不正确的是A.硬水易生水垢B.软水与肥皂作用不易起泡沫C.地球上可利用的淡水资源是有限的D.生活污水的任意排放会造成水体的污染变式练习2(09海淀二模15)我们的生活离不开水。
中考化学考点之水的净化一、水的净化1.纯水与天然水的区别2.自来水的净化自来水厂净化过程(如图所示)可表示为:取水加絮凝剂反应沉淀池过滤池活性炭吸附池清水池消毒配水泵用户。
(1)加絮凝剂:明矾可作絮凝剂。
明矾溶于水后生成的胶状物对杂质有吸附性,使杂质沉降达到净水的目的。
(2)过滤:可以除去液体中混有的固体杂质。
(3)吸附:活性炭具有吸附作用,不仅可以吸附一些不溶性的杂质和一些可溶性的杂质,还可以除去异味和色素。
(4)消毒:用氯气(Cl2)作消毒剂可以杀死水中的细菌,用漂白粉[主要成分是次氯酸钙Ca(ClO)2]也可以消毒杀菌。
3.活性炭的净水作用活性炭是具有疏松多孔结构的单质碳,对气体、蒸气或固体小颗粒具有强大的吸附能力,广泛地应用于净化某些气体或液体。
活性炭净化水,不仅可以滤去其中的不溶性物质,还可以吸附一些溶解的杂质、去除臭味。
活性炭净水器如图所示。
4.常见水的净化方法(1)静置:使不溶性大颗粒物质沉降。
(2)吸附:加入明矾,形成的胶状物对悬浮杂质吸附沉降;加入活性炭,利用其吸附性除去有色杂质和臭味。
(3)过滤:除去水中不溶性杂质的方法。
(4)蒸馏:净化程度较高,除去溶于水的杂质,使硬水软化。
此外,水中还含有细菌、病毒,可使用消毒剂如通入氯气等杀菌消毒。
净化效果:吸附、沉淀、过滤和蒸馏,其中单一操作净化程度最高的是蒸馏。
综合运用时,按“吸附沉淀过滤蒸馏”的顺序操作,净化效果更好。
5.简易净水器简易净水器中小卵石、石英砂和蓬松棉起过滤作用,活性炭具有疏松多孔的结构,因此具有吸附作用。
石英砂与小卵石的位置不能颠倒,其原因是若石英砂与小卵石的位置对换,不溶性固体会堵塞石英砂的空隙,造成过滤受阻。
装置由上至下过滤孔越来越小,净化效果好。
纱布的主要作用是隔离物品。
二、过滤过滤是把液体与固体分离开的一种方法,是一种常用的分离混合物的物理方法。
1.过滤器的准备如图所示,取一张圆形滤纸,对折两次,然后打开,使滤纸成为圆锥形(一边为一层滤纸,另一边为三层滤纸),放入漏斗内,使滤纸紧贴漏斗内壁,使滤纸边缘略低于漏斗口,用少量水润湿滤纸使滤纸与漏斗壁之间没有气泡。
水的净化(Water Purification)
水的净化(Water Purification)水的净化(Water Purification) 水的净化(water purification)
the provision of safe water necessitates one of the rnajor expenditures of manpower and revenue in our modern cities. the purification of water is basically a two-step or three-step process carried out under the strict supervision of public health scientists and engineers. as the first step, natural water from the least contaminated source is allowed to stand in large reservoirs, where most of the mud, clay, and silt settle out; this is called sedimentation . often in water with high mud content, lime and aluminum sulfate are added to the water in the settling reservoirs. these chemicals react in the water to form aluminum hydroxide, which settles slowly and carries much of the suspended material, including most of carries much of the suspended material, including most of the bacteria, to the bottom of the reservoirs. as the second step, the water is filtered through beds of sand and grovel, which remove other impurities and chemicals in it. during or after filtration, chemicals are ordinarily added to the water to kill
any remaining harmful bacteria. chlorine is one of the most common chemicals used for this purpose. a third step taken by some rmnicipalities is adding to the water other beneficial chemicals such as fluoride to make tooth enamel hard, and soda ash to make the water itself soft. the water purification process, carried out with little variation from one large city to another, is perhaps the biggest factor in the prevention of major outbreaks of disease in this country.。