PIM-inhibitor-1-phosphate-SDS-MedChemExpress
- 格式:pdf
- 大小:119.08 KB
- 文档页数:5

网络出版时间:2023-11-0711:01:21 网络出版地址:https://link.cnki.net/urlid/34.1086.R.20231106.1723.010芝麻酚通过AMPK信号通路调控巨噬细胞极化减轻脂肪组织炎症刘 瞩,程明慧,张劝劝,秦 虹(中南大学湘雅公共卫生学院营养与食品卫生学教研室,湖南长沙 410078)收稿日期:2023-03-27,修回日期:2023-06-29基金项目:国家自然科学基金面上项目(No82073556)作者简介:刘 瞩(1996-),女,硕士生,研究方向:植物化学物与慢性病防治,E mail:liuzhucsu@163.com;秦 虹(1982-),女,博士,教授,博士生导师,研究方向:植物化学物与慢性病防治,通信作者,E mail:qinhong@csu.edu.cndoi:10.12360/CPB202302063文献标志码:A文章编号:1001-1978(2023)11-2082-07中国图书分类号:R 332;R329 24;R364 5;R345 57;R916 4摘要:目的 探讨芝麻酚(sesamol,SEM)对高脂饮食诱导的肥胖小鼠脂肪组织炎症的影响及对脂多糖(lipopolysaccha ride,LPS)诱导的RAW264 7巨噬细胞极化的调控分子机制。
方法 将C57BL/6J小鼠随机分成正常对照组、肥胖模型对照组和SEM干预组。
干预结束后取小鼠血清和脂肪组织,ELISA法测定小鼠血清和脂肪组织中炎症因子、抗炎因子及趋化因子的分泌水平,Westernblot法测定脂肪组织巨噬细胞M1型极化标志物的蛋白表达水平。
以LPS刺激RAW264 7巨噬细胞诱导炎症模型并用SEM进行干预,使用AMPK抑制剂compoundC进行机制探讨和验证,Westernblot法检测巨噬细胞极化标志物及AMPK信号通路相关蛋白表达水平,ELISA法检测巨噬细胞炎症相关因子。

网络出版时间:2012-09-21 08:30网络出版地址:/kcms/detail/61.1399.R.20120921.0830.001.html糖基化神经酰胺合成酶和多药耐药相关蛋白在胰腺癌中表达及其临床意义王佐正1,罗永云1,宋建军1,赵琳2(1.宁夏医科大学总医院肝胆外科,宁夏银川750004;2. 宁夏医科大学基础医学院病理系,宁夏银川,750004)摘要:目的探讨糖基化神经酰胺合成酶(GCS)及多药耐药相关蛋白(MRP)在胰腺癌组织中的表达以及临床意义。
方法采用免疫组化S-P法检测GCS、MRP在10例正常胰腺和胰腺炎及30例胰腺癌组织中的表达情况,并结合临床病理学特征进行研究。
结果GCS在胰腺癌组织中的阳性表达率为60%,在正常胰腺及胰腺炎组织中的阳性表达率为10%,GCS在胰腺癌的阳性表达率显著高于在正常胰腺和胰腺炎组织中的表达(P<0.05);MRP在胰腺癌中的阳性表达率为53.33%,在正常胰腺和胰腺炎中阳性表达率为10%,MRP在胰腺癌的阳性表达率显著高于在正常胰腺和胰腺炎组织中的表达(P<0.05)。
GCS、MRP的表达与性别、年龄、原发肿瘤的位置、肿瘤的分化程度、肿瘤是否有侵袭性、是否有淋巴结转移以及肿瘤的分期之间无显著性差异(P>0.05)。
结论GCS、MRP在胰腺癌的多药耐药性中可能占有重要地位。
其介导的耐药机制各不相同,临床检测GCS、MRP对胰腺癌化疗方案的制定具有重要的指导意义。
关键词:胰腺癌;多药耐药;糖基化神经酰胺合成酶;多药耐药相关蛋白中图分类号:R735.9 文献标志码:A 文章编号:1671-8259Expressions and clinical significance of GCS and MRP in pancreatic cancer W ANG Zuo-zheng1, LUO Yong-yun1, SONG Jian-jun1, ZHAO Lin2(1. Department of Hepatobiliary Surgery, General Hospital of Ningxia Medical University,Yinchuan 750004; 2. Department of Pathology, Ningxia Medical University, Yinchuan750004, China)ABSTRACT: Objective To study the expressions and clinical significance of glucosylceramide synthase (GCS) and multidrug-related protein (MRP) in pancreatic cancer. Methods The收稿日期:2012-07-04 修回日期:2012-08-30通讯作者:赵琳. E-mail: nxzhaolin@作者简介:王佐正(1972-),男(汉族),硕士,副主任医师,研究方向:消化系统肿瘤.E-mail: wangzuozheng2008@expressions of two proteins GCS and MRP were detected by immunohistochemistry in 30 pancreatic cancer cases and contrasted with 10 normal pancreatic tissues or pancreatitis, respectively. Results The positive staining of GCS was 60% in pancreatic cancer and 10% in normal pancreatic tissues or pancreatitis. The difference was significant using the samples of 30 pancreatic cancers and 10 normal pancreatic tissues or pancreatitis (P<0.05). The positive staining of MRP was 53.33% in pancreatic cancer and 10% in normal pancreatic tissues or pancreatitis. The difference was significant using the samples of 30 pancreatic cancer and 10 normal pancreatic tissues or pancreatitis (P<0.05). The positive expression of GCS and MRP did not show significant correlation with clinical findings including sex, age, tumor location, degree of differentiation, aggressiveness, lymph rode metastasis or TNM stage of tumors (P>0.05). Conclusion The expressions of GCS and MRP are significantly higher in pancreatic cancer than in normal pancreatic tissues or pancreatitis. GCS and MRP may play an important role in multidrug resistance in pancreatic cancer, but their mechanisms are different. To examine GCS and MRP in clinic has an important guiding significance in determining chemotherapy strategy for pancreatic cancer.KEY WORDS: pancreatic cancer; multidrug resistance; glucosylceramide synthase (GCS); multidrug-related protein (MRP)目前化疗是胰腺癌辅助治疗的重要手段和方法之一,但效果较差,主要原因是肿瘤细胞对化疗药物的耐药,肿瘤细胞的耐药是多种耐药基因参与的结果。

Hereditas (Beijing) 2020年3月, 42(3): 287―295 收稿日期: 2019-12-31; 修回日期: 2020-02-12基金项目:国家自然科学基金项目(编号:81520108023),中国医学科学院医学与健康科技创新工程项目(编号:2019-I2M-1-003,2016-I2M-3-007),北京市科技新星项目(编号:Z171100001117017)资助[Supported by the National Natural Science Foundation of China (No. 81520108023), CAMS Innovation Fund for Medical Sciences (Nos. 2019-I2M-1-003, 2016-I2M-3-007), Beijing Nova Program (No.Z171100001117017)]作者简介: 王迪,硕士研究生,专业方向:肿瘤遗传学。
E-mail: 156********@ 通讯作者:郝佳洁,博士,副研究员,研究方向:肿瘤遗传学。
E-mail: hjj8173@王明荣,博士,研究员,研究方向:肿瘤遗传学。
E-mail: wangmr2015@DOI: 10.16288/j.yczz.19-334网络出版时间: 2020/2/28 11:15:42URI: /kcms/detail/11.1913.r.20200227.0958.002.html研究报告PAI-1过表达促进食管鳞癌细胞的侵袭和迁移王迪,杨荔艳,刘奏,余竟,张敏杰,张钰,蔡岩,徐昕,郝佳洁, 王明荣国家癌症中心/国家肿瘤临床医学研究中心, 中国医学科学院北京协和医学院肿瘤医院肿瘤精准医学研究中心, 分子肿瘤学国家重点实验室,北京 100021摘要: 食管癌是常见的恶性肿瘤之一。
由SERPINE1基因编码的纤溶酶原激活物抑制因子 1 (plasminogenactivator inhibitor-1, PAI-1)已被报道在多种类型癌症患者的肿瘤组织中存在高表达并参与癌症进展。
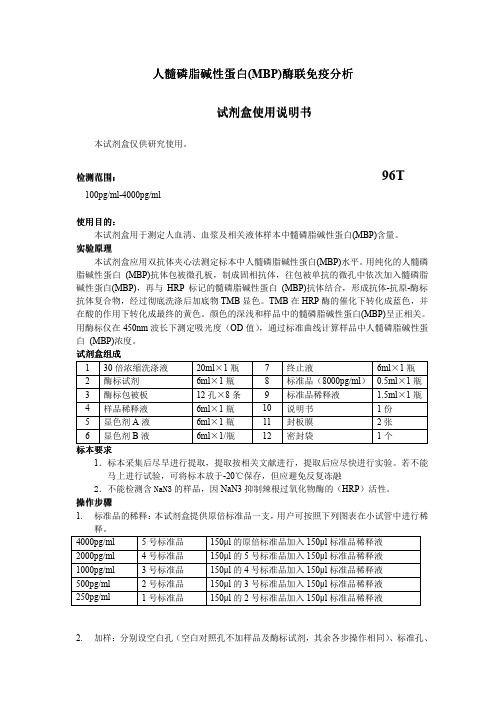
人髓磷脂碱性蛋白(MBP)酶联免疫酶联免疫分析分析分析试剂试剂盒使用说明书盒使用说明书盒使用说明书本试剂盒仅供研究使用。
检测范围检测范围:: 96T100pg/ml-4000pg/ml使用目的使用目的::本试剂盒用于测定人血清、血浆及相关液体样本中髓磷脂碱性蛋白(MBP)含量。
实验原理本试剂盒应用双抗体夹心法测定标本中人髓磷脂碱性蛋白(MBP)水平。
用纯化的人髓磷脂碱性蛋白 (MBP)抗体包被微孔板,制成固相抗体,往包被单抗的微孔中依次加入髓磷脂碱性蛋白(MBP),再与HRP 标记的髓磷脂碱性蛋白 (MBP)抗体结合,形成抗体-抗原-酶标抗体复合物,经过彻底洗涤后加底物TMB 显色。
TMB 在HRP 酶的催化下转化成蓝色,并在酸的作用下转化成最终的黄色。
颜色的深浅和样品中的髓磷脂碱性蛋白(MBP)呈正相关。
用酶标仪在450nm 波长下测定吸光度(OD 值),通过标准曲线计算样品中人髓磷脂碱性蛋白 (MBP)浓度。
试剂盒组成 1 30倍浓缩洗涤液 20ml ×1瓶 7 终止液 6ml ×1瓶 2 酶标试剂 6ml ×1瓶 8 标准品(8000pg/ml )0.5ml ×1瓶 3 酶标包被板 12孔×8条 9 标准品稀释液 1.5ml ×1瓶 4 样品稀释液 6ml ×1瓶 10 说明书 1份 5 显色剂A 液 6ml ×1瓶 11 封板膜 2张 6显色剂B 液6ml ×1/瓶12密封袋1个标本标本要求要求1.标本采集后尽早进行提取,提取按相关文献进行,提取后应尽快进行实验。
若不能马上进行试验,可将标本放于-20℃保存,但应避免反复冻融2.不能检测含NaN3的样品,因NaN3抑制辣根过氧化物酶的(HRP )活性。
操作步骤1. 标准品的稀释:本试剂盒提供原倍标准品一支,用户可按照下列图表在小试管中进行稀释。

∗基金项目:北京市医院管理局重点医学专业发展计划项目(编号:ZYLX201610)作者单位:250000山东省淄博市妇幼保健院内一科(许海苗,郑瑞琦);首都医科大学附属北京佑安医院感染消化内科(丁惠国)第一作者:许海苗,女,40岁,医学硕士,副主任医师㊂E-mail: xhm124320301@ ㊃非酒精性脂肪性肝病㊃匹伐他汀联合多烯磷脂酰胆碱治疗非酒精性脂肪性肝炎患者疗效研究∗许海苗,郑瑞琦,丁惠国㊀㊀ʌ摘要ɔ㊀目的㊀探讨应用匹伐他汀联合多烯磷脂酰胆碱治疗非酒精性脂肪性肝炎(NASH)患者的临床疗效㊂方法㊀2020年1月~2021年8月我院收治的46例NASH患者,其中观察组应用匹伐他汀联合多烯磷脂酰胆碱治疗24例,对照组应用多烯磷脂酰胆碱治疗22例,均治疗6个月㊂常规检测血清总胆固醇(TC)㊁甘油三酯(TG)㊁高密度脂蛋白胆固醇(HDL-C)和低密度脂蛋白胆固醇(LDL-C)水平,计算胰岛素抵抗指数(HOMA-IR),采用ELISA法检测血清肿瘤坏死因子-α(TNF-α)㊁白介素-10(IL-10)和超敏C反应蛋白(hs-CRP)水平,采用放射免疫法检测血清胰岛素样生长因子1(IGF-1)和脂联素水平㊂结果㊀治疗后,观察组血清TC㊁TG和LDL-C水平分别为(4.7ʃ0.5)mmol/L㊁(2.1ʃ0.4)mmol/L 和(2.9ʃ0.5)mmol/L,显著低于对照组ʌ分别为(5.8ʃ0.4)mmol/L㊁(4.5ʃ0.6)mmol/L和(4.3ʃ0.4)mmol/L,P<0.05ɔ,而血清HDL-C水平为(1.8ʃ0.3)mmol/L,显著高于对照组ʌ(1.0ʃ0.3)mmol/L,P<0.05ɔ;观察组血清ALT㊁AST和GGT水平分别为(53.5ʃ6.9)U/L㊁(45.2ʃ5.6)U/L和(66.7ʃ3.9)U/L,均显著低于对照组ʌ分别为(59.4ʃ6.7)U/L㊁(49.1ʃ5.5) U/L和(79.3ʃ3.7)U/L,P<0.05ɔ;观察组HOMA-IR为(2.2ʃ0.4),显著小于对照组ʌ(2.7ʃ0.5),P<0.05ɔ,而血清脂联素和IGF-1水平分别为(11.9ʃ0.8)mg/L和(0.4ʃ0.1)μg/L,显著高于对照组ʌ分别为(9.2ʃ0.8)mg/L和(0.2ʃ0.1)μg/L, P<0.05ɔ;观察组血清TNF-α㊁IL-10和hs-CRP水平分别为(3.5ʃ0.5)ng/L㊁(27.5ʃ5.2)ng/L和(4.1ʃ0.8)mg/L,与对照组ʌ分别为(3.6ʃ0.4)ng/L㊁(26.9ʃ4.9)ng/L和(4.2ʃ0.9)mg/Lɔ比,差异无统计学意义(P>0.05)㊂结论㊀联合应用匹伐他汀和多烯磷脂酰胆碱治疗NASH患者可有效降低血脂水平,帮助改善肝功能指标,可能与减轻了胰岛素抵抗有关㊂㊀㊀ʌ关键词ɔ㊀非酒精性脂肪性肝炎;匹伐他汀;多烯磷脂酰胆碱;治疗㊀㊀DOI:10.3969/j.issn.1672-5069.2023.03.013㊀㊀Short-term efficacy of pitavastatin and polyene phosphatidyl choline combination in the treatment of patients with nonalcoholic steatohepatitis㊀Xu Haimiao,Zheng Ruiqi,Ding Huiguo.Department of Internal Medicine,Maternal and Child Health Hospital,Zibo250000,Shandong Province,China㊀㊀ʌAbstractɔ㊀Objective㊀The aim of this study was to investigate the short-term clinical efficacy of pitavastatin and polyene phosphatidyl choline combination in the treatment of patients with nonalcoholic steatohepatitis(NASH).Methods㊀46patients with NASH were enrolled in our hospital between January2020and August2021,and were divided randomly into control(n=22) and observation group(n=24),receiving polyene phosphatidyl choline or pitavastatin and polyene phosphatidyl choline combination treatment for six months.Serum total cholesterol(TC),triglyceride(TG),high-density lipoprotein cholesterol(HDL -C)and low-density lipoprotein cholesterol(LDL-C)levels were routinely detected,and the homeostasis model assessment-insulin resistance index(HOMA-IR)was calculated.Serum tumor necrosis factor-α(TNF-α),interleukin-10(IL-10)and high-sensitivity C-reactive protein(hs-CRP)levels were detected by ELISA.Serum ALT,AST andγ-glutamyltransferase (GGT)levels were obtained.Serum insulin-like growth factor1(IGF-1)level was detected by radioimmunoassay.Results㊀At the end of the six month treatment,serum TC,TG and LDL-C levels in the combination group were(4.7ʃ0.5)mmol/L,(2.1ʃ0.4)mmol/L and(2.9ʃ0.5)mmol/L,all significantly lower than[(5.8ʃ0.4)mmol/L,(4.5ʃ0.6)mmol/L and(4.3ʃ0.4) mmol/L,respectively,P<0.05],while serum HDL-C level was(1.8ʃ0.3)mmol/L,significantly higher than[(1.0ʃ0.3)mmol/L,P<0.05]in the control;serum ALT,AST and GGTlevels were(53.5ʃ6.9)U/L,(45.2ʃ5.6)U/L and(66.7ʃ3.9)U/L,all significantly lower than[(59.4ʃ6.7)U/L,(49.1ʃ5.5)U/L and(79.3ʃ3.7)U/L,respectively,P<0.05]in the control;the HOMA-IR was(2.2ʃ0.4),muchlower than[(2.7ʃ0.5),P<0.05],while serum adiponectinand IGF-1levels were(11.9ʃ0.8)mg/L and(0.4ʃ0.1)μg/L,both significantly higher than[(9.2ʃ0.8)mg/L and(0.2ʃ0.1)μg/L,respectively,P<0.05]in the control group;serum TNF-α,IL-10and hs-CRP levels were(3.5ʃ0.5)ng/L,(27.5ʃ5.2)ng/L and(4.1ʃ0.8)mg/L,all not significantly different as compared to[(3.6ʃ0.4)ng/L,(26.9ʃ4.9)ng/L and(4.2ʃ0.9)mg/L]in the control group(P>0.05).Conclusion㊀The administration of pitavastatin and polyene phosphatidyl choline combination in the treatment of patients with NASH could reduce blood lipid levels and improve liver function normalization,which might be related to the reduction of insulin resistance.㊀㊀ʌKey wordsɔ㊀Nonalcoholic steatohepatitis;Pitavastatin;Polyene phosphatidyl choline;Therapy㊀㊀非酒精性脂肪性肝炎(nonalcoholic steatohepatitis,NASH)是指非乙醇因素引发的以肝细胞内脂肪蓄积为特征的慢性肝脏炎症性疾病[1]㊂NASH的发病机制尚未完全明确,一般认为主要可能与胰岛素抵抗(IR)和游离脂肪酸增加等引起的糖脂代谢紊乱有关,故改善机体糖脂代谢,减轻IR是治疗NASH的基本原则[2,3]㊂多烯磷脂酰胆碱是临床常用的护肝药,可置换人体内源性磷脂,并可修复肝细胞膜损伤,近年来国内外均有指南推荐其作为治疗NASH的辅助药物[4]㊂有报道指出,多烯磷脂酰胆碱治疗可减轻NASH患者肝组织脂肪蓄积,但部分患者对治疗应答仍较差[5]㊂他汀类药物是临床上常用的降脂药物,对肝脂肪变性等具有一定的治疗作用,可在一定程度上改善机体血脂代谢紊乱,而匹伐他汀在治疗NASH方面尚需要进一步探究㊂本研究应用匹伐他汀联合多烯磷脂酰胆碱治疗NASH 患者,观察了短期临床疗效,现报道如下㊂1㊀资料与方法1.1病例来源㊀2020年1月~2021年8月我院诊治的NASH患者46例,男26例,女20例;年龄为32~ 55岁,平均年龄为(42.8ʃ4.6)岁;体质指数(BMI)为27.4~37.9kg/m2,平均为(29.9ʃ2.1)kg/m2㊂符合非酒精性脂肪性肝病防治指南中有关NASH的诊断标准[6]㊂排除标准:①肝硬化;②胆道感染;③肾功能异常;④妊娠或哺乳期妇女;⑤合并病毒性肝炎㊁自身免疫性肝病㊁酒精性肝病和家族遗传性高脂血症㊂将患者分成两组,两组性别㊁年龄和肝功能损害程度等一般资料比较无显著性差异,具有可比性(P>0.05)㊂患者签署知情同意书,本研究经我院医学伦理委员会审核通过㊂1.2治疗方法㊀给予对照组患者多烯磷脂酰胆碱[赛诺菲(北京)制药有限公司,国药准字H20059010]2粒口服,3次/d;观察组在此治疗的基础上给予匹伐他汀(信立泰药业股份有限公司,国药准字H20193061)2mg口服,1次/d,饭后服用㊂两组均连续治疗观察6个月㊂1.3血生化和炎性因子检测㊀应用美国贝克曼库尔特有限公司生产的AU5800型全自动生化仪及其配套试剂检测血浆总胆固醇(total cholesterol,TC)㊁甘油三酯(triglyceride,TG)㊁低密度脂蛋白胆固醇(lowdensity lipoprotein-cholesterol,LDL-C)㊁高密度脂蛋白胆固醇(high density lipoprotein-cholesterol,HDL-C)和血糖指标,计算胰岛素抵抗指数(homeostasis model assessment-insulin resistance index,HOMA-IR) =空腹胰岛素ˑ空腹血糖/22.5;常规检测血清丙氨酸氨基转移酶(alanine aminotransferase,ALT)㊁天冬氨酸氨基转移酶(aspartate aminotransferase,AST)㊁谷氨酰转肽酶(γ-glutamyltransferase,GGT)水平;采用ELISA法检测血清肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)㊁白介素-10(interleukin-10,IL-10)㊁超敏C反应蛋白(hypersensitive C-reactive pro-tein,hs-CRP)水平(试剂盒由宁波美康生物科技股份有限公司提供);采用放射免疫法检测血清胰岛素样生长因子1(insulin-like growth factor1,IGF-1)和脂联素水平㊂1.4统计学处理㊀应用IBM SPSS Statistics24.0软件进行统计学分析,对正态分布的计量资料以(xʃs)表示,采用独立t检验㊂计数资料的比较采用x2检验,P<0.05被认为差异有统计学意义㊂2㊀结果2.1两组血脂水平比较㊀治疗前,两组血浆TC㊁TG㊁HDL-C和LDL-C水平比较无显著性差异(P> 0.05);治疗后,观察组血浆TC㊁TG和LDL-C水平显著低于对照组(P<0.05),而血浆HDL-C水平显著高于对照组(P<0.05,表1)㊂2.2两组肝功能指标比较㊀治疗前,两组血清ALT㊁AST和GGT水平比较无显著性差异(P>0.05);治疗后,观察组血清ALT㊁AST和GGT水平显著低于对照组(P<0.05,表2)㊂2.3两组胰岛功能指标比较㊀治疗前,两组HOMA-IR㊁血清脂联素和IGF-1水平比较无显著性差异(P>0.05);治疗后,观察组HOMA-IR显著低于对照组(P<0.05),而血清脂联素和IGF-1水平显著高于对照组(P<0.05,表3)㊂2.4两组血清炎性因子水平比较㊀治疗后,两组血清TNF-α和hs-CRP水平下降,血清IL-10水平上升,但两组间比较无显著性差异(P>0.05,表4)㊂表1㊀两组血脂水平(mmol/L,xʃs)比较例数TC TG HDL-C LDL-C 观察组治疗前24 5.9ʃ0.9 4.3ʃ0.50.9ʃ0.1 4.6ʃ0.7治疗后24 4.7ʃ0.5① 2.1ʃ0.4① 1.8ʃ0.3① 2.9ʃ0.5①对照组治疗前22 5.8ʃ0.8 4.4ʃ0.5 1.0ʃ0.2 4.7ʃ0.5治疗后22 5.8ʃ0.4 4.5ʃ0.6 1.0ʃ0.3 4.3ʃ0.4㊀㊀与对照组比,①P<0.05表2㊀两组肝功能指标(U/L,xʃs)比较例数ALT AST GGT 观察组治疗前2481.9ʃ6.181.6ʃ5.191.5ʃ4.5治疗后2453.5ʃ6.9①45.2ʃ5.6①66.7ʃ3.9①对照组治疗前2280.7ʃ6.582.9ʃ5.392.8ʃ4.2治疗后2259.4ʃ6.749.1ʃ5.579.3ʃ3.7㊀㊀与对照组比,①P<0.05表3㊀两组胰岛功能指标(xʃs)比较例数HOMA-IR脂联素(mg/L)IGF-1(μg/L)观察组治疗前24 3.7ʃ0.69.1ʃ0.90.2ʃ0.1治疗后24 2.2ʃ0.4①11.9ʃ0.8①0.4ʃ0.1①对照组治疗前22 3.8ʃ0.59.3ʃ0.60.2ʃ0.1治疗后22 2.7ʃ0.59.2ʃ0.80.2ʃ0.1㊀㊀与对照组比,①P<0.05表4㊀两组血清炎性因子水平(xʃs)比较例数TNF-α(ng/L)IL-10(ng/L)hs-CRP(mg/L)观察组治疗前24 5.5ʃ0.719.2ʃ3.9 4.9ʃ0.7治疗后24 3.5ʃ0.527.5ʃ5.2 4.1ʃ0.8对照组治疗前22 5.4ʃ0.819.0ʃ3.7 5.1ʃ0.8治疗后22 3.6ʃ0.426.9ʃ4.9 4.2ʃ0.93㊀讨论NASH是由于肝脏脂质堆积过多造成的以肝细胞脂肪变性为主要特征的肝脏病变引起的疾病㊂相关研究指出,NASH患者肝细胞脂肪酸代谢异常,造成脂肪酸堆积,导致血脂代谢紊乱[8,9]㊂多烯磷脂酰胆碱是一种具有促进膜流动性作用的磷脂,能减少过氧化氢的生成量,抑制胶原的生成,使得溶酶体功能恢复正常,改变凋亡受体基因表达[10]㊂相关研究指出,多烯磷脂酰胆碱可将中性脂肪和胆固醇转化而降低机体血脂水平,但仅此药改善患者脂代谢紊乱的效果较差[11],故需在此基础上给予降脂药联合治疗㊂匹伐他汀是第三代他汀类药物,具有降脂效果好㊁生物利用度高等优点,可促进LDL的清除,降低血浆LDL-C和总TG水平[12]㊂本研究结果显示,治疗后观察组血清TC㊁TG和LDL-C水平显著低于对照组,而血清HDL-C水平显著高于对照组,表明联合药物治疗可降低机体血脂水平,其原因在于应用匹伐他汀可高效阻碍人肝细胞对胆固醇的合成和分泌[13]㊂临床资料显示,胰岛素可抑制游离脂肪酸氧化,使得肝脏内游离脂肪酸利用减少[14],故胰岛素抵抗与NASH的发生有关㊂胰岛素抵抗发生主要与脂肪细胞体积增大㊁脂肪更新障碍有关,而胰岛素抵抗可影响肝脏脂肪酸摄入,两者相互影响可加重病情的严重程度[15,16],故减轻机体胰岛素抵抗对于该疾病的治疗至关重要,而联合治疗可改善机体血脂代谢紊乱,因而收到了较好的治疗效果㊂本研究发现,治疗后观察组HOMA-IR显著小于对照组,而血清脂联素和IGF-1水平显著高于对照组,提示联合治疗可改善患者胰岛素抵抗,主要是因为匹伐他汀可通过影响脂肪CIC-3氯通道介导降脂作用,改善了胰岛素抵抗㊂多烯磷脂酰胆碱中的主要成分为人体无法合成的细胞膜构成成分㊂相关报道指出,应用多烯磷脂酰胆碱治疗可增加蛋白合成,降低肝细胞脂肪浸润[17]㊂临床资料也显示,应用多烯磷脂酰胆碱可促进肝细胞再生,起到恢复肝功能的作用[18,19]㊂我们在临床实践中发现,单独应用多烯磷脂酰胆碱对部分NASH患者治疗疗效不理想㊂本研究发现,治疗后观察组血清ALT㊁AST和GGT水平显著低于对照组,提示在NASH患者给予匹伐他汀联合多烯磷脂酰胆碱治疗可起到改善肝功能的作用,可能与这种联合能降低机体血脂水平,减少肝细胞内脂质堆积有关[20,21]㊂另外,本研究结果显示,治疗后两组血清TNF-α㊁IL-10和hs-CRP水平均较治疗前有显著性的变化,表明两种治疗方法均可降低机体的炎症反应水平,但经进一步分析发现,两组血清炎症因子水平无显著性差异,我们推测本组NASH患者总体炎症反应不显著,因而对治疗应答也不明显㊂只有严重的NASH或存在多种合并症的患者,才可能出现明显的机体炎症反应㊂综上所述,应用匹伐他汀联合多烯磷脂酰胆碱治疗NASH患者可减轻胰岛素抵抗,降低血脂水平,最终起到改善肝功能的作用㊂但本研究观察时间短,并未涉及饮食和运动对研究的可能干扰,需要进一步观察㊂ʌ参考文献ɔ[1]Zhang Y,Li J,Liu H.Correlation between the thyroid hormonelevels and nonalcoholic fatty liver disease in type2diabetic patients with normal thyroid function.BMC Endocr Disord,2022,22(1): 144-149.[2]Pavlov AI,Ivolgin AF,Katenko SV,et al.Diagnostics and treat-ment of non-alcoholic fatty liver disease with concomitant asthenic syndrome.Ter Arkh,2021,93(8):890-896.[3]Du F,Huang R,Lin D,et al.Resveratrol improves liver steatosisand insulin resistance in non-alcoholic fatty liver disease in associa-tion with the gut microbiota.Front Microbiol,2021,12(1):611323.[4]Tian DD,Gastecki M,Lynch KD,et al.Differential effects of sily-marin on pitavastatin disposition in rodent models of simple fatty liver versus nonalcoholic steatohepatitis:A natural product-disease -drug interaction.FASEB J,2019,33(S1):e814. [5]Gerges SH,Wahdan SA,Elsherbiny DA,et al.Non-alcoholicfatty liver disease:An overview of risk factors,pathophysiological mechanisms,diagnostic procedures,and therapeutic interventions.Life Sci,2021,271(Suppl17):119220.[6]中华医学会肝病学分会脂肪肝和酒精性肝病学组,中国医师协会脂肪肝专家委员会.非酒精性脂肪性肝病防治指南(2018年版).实用肝脏病杂志,2018,21(2):177-186.[7]郑瑞丹,陆伦根,孟家榕,等.非酒精性脂肪性肝病的临床及病理学特征.中华肝脏病杂志,2006,14(6):449-452. [8]Dennis BB,Sallam S,Perumpail BJ,et al.Management of cardio-metabolic complications in patients with nonalcoholic fatty liver dis-ease:A review of the literature with recommendations.J Clin Gas-troenterol,2021,55(9):747-756.[9]Wei S,Yu X.Efficacy of resveratrol supplementation on liver en-zymes in patients with non-alcoholic fatty liver disease:A systematic review and plement Ther Med, 2021,57(3):102635.[10]Zhao B,Li GP,Peng JJ,et al.Pitavastatin combined with ezetimi-be treatment was an effective approach to non-IRA lesion of ST-segment elevation myocardial infarction patients with primary percu-taneous coronary intervention.Curr Pharm Biotechnol,2021,22(4):549-556.[11]王丽娟,朱兵兵,胡敏华,等.匹伐他汀与阿托伐他汀治疗NASH患者疗效比较.实用肝脏病杂志,2020,23(2):215-218. [12]魏秀琴,闫晓霞,石国荣,等.阿托伐他汀联合硫普罗宁治疗NASH患者疗效初步研究.实用肝脏病杂志,2021,24(1):47-50.[13]Chen LW,Lin CS,et al.Pitavastatin exerts potent anti-inflamma-tory and immunomodulatory effects via the suppression of AP-1sig-nal transduction in human T cells.Int J Mol Sci,2019,20(14):3534.[14]Lüchtenborg C,Niederhaus B,Brügger B,et al.Lipid profiles offive essential phospholipid preparations for the treatment of nonalco-holic fatty liver disease:A comparative study.Lipids,2020,55(3):271-278.[15]吕春卉,李大炜,王小洁,等.二甲双胍联合辛伐他汀治疗NASH合并2型糖尿病患者疗效研究.实用肝脏病杂志,2022, 25(1):50-53.[16]冯曲波,颜婷,伍国林.复方二氯醋酸二异丙胺联合门冬氨酸鸟氨酸治疗老年性NASH的效果研究.中华老年多器官疾病杂志,2022,21(4):82-286.[17]Alamri AS,Alhomrani M,Alsanie WF,et al.Prevalence and pre-dictors of non-alcoholic fatty Liver disease in tertiary care hospital of Taif,Saudi Arabia:A retrospective study.Saudi J Biol Sci, 2021,28(9):4921-4925.[18]Rodriguez AD,NC García,Lvarez AF,et al.The reina study:Effects on safety of pitavastatin treatment in dyslipidemic patients.Atherosclerosis,2020,315(11):e198.[19]Hoffmann U,Lu MT,Olalere D,et al.Rationale and design of themechanistic substudy of REPRIEVE:effects of pitavastatin on coro-nary artery disease and inflammatory biomarkers.Am Heart J, 2019,212(6):1-12.[20]Lei X,Zhang J,Xu Q,et al.Exploring the efficacy and safety of poly-ene phosphatidylcholine for treatment of drug-induced liver injury using the Roussel Uclaf causality assessment method:a propensity score matching comparison..J Int Med Res,2021,49(8):1-9. [21]吕璐,平凡,李玉秀.他汀类药物治疗NASH获益及风险.中华内分泌代谢杂志,2021,37(11):1042-1048.(收稿:2022-07-11)(本文编辑:刘波)。
· 糖尿病与并发症 ·糖尿病新世界 2023年12月糖尿病新世界 DIABETES NEW WORLD己酮可可碱联合甲钴胺治疗糖尿病周围神经病变的疗效及对其神经症状的影响解洋,张开凤,袁园丹徒区人民医院内分泌科,江苏镇江 212100[摘要] 目的 分析比较己酮可可碱联合甲钴胺治疗糖尿病周围神经病变的疗效及对其神经症状的影响。
方法 选取2022年1—9月镇江市丹徒区人民医院内分泌科收治的100例2型糖尿病周围神经病患者作为研究对象,以随机数字表法分为常规治疗组(n =50,血栓通/丹参酮治疗)和联合治疗组(n =50,己酮可可碱联合甲钴胺注射液治疗)。
比较两组患者治疗后的临床有效率,同时对比其治疗前后的神经症状评分(Neurological Symptom Score, NSS )及神经缺损评分(Neurological Disability Score, NDS )。
结果 治疗前,两组患者的NDS 、NSS 评分差异无统计学意义(P 均>0.05)。
治疗后,两组的NDS 、NSS 评分均出现下降,且联合治疗组患者的评分明显低于常规治疗组;此外,联合治疗组患者用药后的临床有效率高于常规治疗组,差异有统计学意义(P 均<0.05)。
结论 针对2型糖尿病周围神经病变患者给予己酮可可碱联合甲钴胺治疗可有效提高其临床疗效,同时能在一定程度上改善患者的神经症状及神经缺损情况。
[关键词] 己酮可可碱;甲钴胺;糖尿病周围神经病变;神经症状[中图分类号] R587.2 [文献标识码] A [文章编号] 1672-4062(2023)12(b )-0182-04Effect of Pentoxifylline Combined with Mecobalamine on Diabetic Periph⁃eral Neuropathy and Its Neurological SymptomsXIE Yang, ZHANG Kaifeng, YUAN YuanDepartment of Endocrinology, Dantu District People's Hospital, Zhenjiang, Jiangsu Province, 212100 China[Abstract ] Objective To analyze and compare the efficacy of pentoxifylline combined with mecobalamine in the treat‑ment of diabetic peripheral neuropathy and its influence on neurological symptoms. Methods A total of 100 patients with type 2 diabetic peripheral neuropathy admitted to the Endocrinology Department of Dantu District People's Hos‑pital of Zhenjiang City from January to September 2022 were selected as the study objects, and were divided into con‑ventional treatment group (n =50, thromboxone/tanshinone treatment) and combined treatment group (n =50, pentoxifyl‑line combined with mecobalamine injection treatment) by random number table method. The clinical effective rate of the two groups after treatment was compared, and Neurological Symptom Score (NSS) and Neurological DisabilityScore (NDS) before and after treatment were compared. Results Before treatment, there was no significant difference in NDS and NSS scores between the two groups (both P >0.05). After treatment, NDS and NSS scores of both groups de‑creased, and the scores of combined treatment group were significantly lower than that of conventional treatment group; In addition, the clinical effective rate of combined treatment group was higher than that of conventional treat‑ment group, and the differences were statistically significant (all P <0.05). Conclusion For patients with type 2 dia‑betic peripheral neuropathy, the combination therapy of pentoxifylline and mecobalamine can effectively improve theclinical efficacy, and can improve the neurological symptoms and nerve defects of patients to a certain extent.[Key words ] Pentoxifylline; Mecobalamine; Diabetic peripheral neuropathy; Neurological symptom DOI :10.16658/ki.1672-4062.2023.24.182[作者简介] 解洋(1987-),男,本科,主治医师,研究方向为内分泌学。
氨基聚乙二醇-1,2-二硬脂酰甘油磷酰乙醇胺的合成脂质体是一种定向药物载体,属于靶向给药系统的一种新剂型。
脂质体对机体毒副作用小,其脂质双分子层与生物膜有较大的相似性与组织相溶性,易于被组织吸收。
脂质体包裹药物为物理过程,不改变药物分子结构,当药物被包裹后可降低药物毒性,减小药物使用量,具有缓释和控释作用,因此,脂质体靶向给药可以大大提高药物的疗效[1, 2]。
甲氧基聚乙二醇-1, 2-二硬脂酰甘油磷酰乙醇胺 (mPEG-DSPE) 是制备脂质体的重要辅料之一,它一端是甲氧基聚乙二醇(mPEG),具有亲水性,另一端是脂肪酸甘油酯,具有亲脂性。
不同于天然磷脂,mPEG-DSPE属于合成磷脂,具有确定的化学成分。
经过mPEG修饰后脂质体的柔顺性和亲水性显著增强,通过单核巨噬细胞系统吞噬,减少脂质体脂膜与血浆蛋白的相互作用,延长循环时间,形成了长循环脂质体[3, 4]。
将抗体或配体结合在mPEG的末端,既可保持长循环,又可保持对靶体的识别[5, 6]。
mPEG-DSPE在脂质体研究方面应用极为广泛,国内外需求量在逐年增加,此类产品市售价格昂贵,所以,开发新的mPEG-DSPE合成方法有着广阔的市场前景。
虽然国内外对mPEG-DSPE 的应用研究已较为深入,但其合成方法报道的较少。
有文献[7]报道通过mPEG与三光气反应进行制备,但该路线步骤多,三光气毒性较大,反应收率较低,给以后的放大生产带来不便。
本文探究了一种新的合成方法,以1, 2-二硬脂酸甘油酯为原料,将其与三氯氧磷及乙醇胺反应,合成关键中间体1, 2-二硬脂酰甘油磷酰乙醇胺 (DSPE) 后,再与mPEG2000反应,制备mPEG2000-DSPE (图式1)。
该方法具有操作简单、收率高、三废少等优点,有较好的应用前景。
1 实验部分1.1 仪器与试剂上海精密科学仪器SGW X-4显微熔点测试仪;Bruker Vector 22 (4000~400 cm-1) 红外光谱仪;Varian Mercury Plus 300型核磁共振谱仪 (CDCl3为溶剂,TMS作内标);Mariner电喷雾质谱仪。
摘要慢性伤口是目前全球伤口疾病中的一个很严峻的问题,对人类生活造成了严重的影响。
由细菌和其他微生物感染的慢性伤口是导致并发症的主要因素之一,研发具有抗菌性的水凝胶敷料是提高伤口愈合效率的有效方法。
通常抗菌水凝胶是将水凝胶作为载体,封载抗菌药物或银纳米颗粒。
抗菌药物的滥用会引起耐药性的影响,而银离子的生物安全性一直存疑。
因此,研发出一种新型具有抗菌效果的多功能水凝胶敷料具有重要意义。
本文采用自由基聚合的合成方法,将多巴胺(DA) 、肝素(HP)、抗菌剂2-丙烯酰氧基-乙基-N,N-二甲基-6-溴化铵季铵盐(AEDMHA)和丙烯酰胺(AM)有效地结合在高分子网络中,制备出一种既能满足创口对敷料多种物理性能的需求,还可以实现抗菌性能且生物相容性良好的复合水凝胶敷料。
将制得的水凝胶通过系列的理化性能表征和生物学性能表征,得到以下结果:(1)通过简单的自由基聚合制备得到聚多巴胺-肝素/聚丙烯酰胺水凝胶,该水凝胶制备方法简便,反应条件温和。
制备得到的水凝胶具有组织粘附性,物理机械性能良好,吸水性能和保持水分能力好。
(2)将制备得到的聚多巴胺-肝素/聚丙烯酰胺水凝胶进行抗菌实验,发现对革兰氏阴性菌-大肠杆菌(E.coil)与革兰氏阳性菌-金黄色葡萄球菌(S.aureus)均具有一定的抗菌效果,且抗细菌粘附性能良好;对该水凝胶进行细胞毒性评价,其对小鼠3T3成纤维细胞无毒性,生物相容性良好。
(3)成功合成2-丙烯酰氧基-乙基-N,N-二甲基-6-溴化铵季铵盐(AEDMHA);再将AEDMHA加入到聚多巴胺-肝素/聚丙烯酰胺凝胶前驱液体系中,用以增强原凝胶体系的抗菌效果,通过自由基聚合制得聚多巴胺-肝素/季铵盐/聚丙烯酰胺水凝胶,该凝胶也具有组织粘附性、物理机械性能好、吸水性能好的优点。
(4)将制备得到的聚多巴胺-肝素/季铵盐/聚丙烯酰胺水凝胶进行抗菌实验,发现对E.coil与S.aureus均具有抗菌效果;对该水凝胶进行细胞毒性评价,其对小鼠3T3成纤维细胞有轻度毒性,生物相容性较好。
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Oct.-04-2018Print Date:Oct.-04-20181. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :PIM inhibitor 1 (phosphate)Catalog No. :HY-101870BCAS No. :2088852-47-31.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:NoneFormula:C26H29F3N5O7PMolecular Weight:611.51CAS No. :2088852-47-34. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2018 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。