电性能参数分析共29页文档
- 格式:ppt
- 大小:3.04 MB
- 文档页数:29


对电动机性能及参数的分析电动机在现代化工业生产中应用广泛,然而目前许多企业的电工人员由于业务素质与业务能力水平存在较大差异,对电动机的工作原理与性能参数的选择不甚了解,在电动机的使用与选型过程中存在不当之处,文章作者通过对电动机的工作性能与性能参数的分析,希望对现场电工人员业务水平的提高有所帮助。
标签:额定功率;效率;定子;转子引言实现机械能与电能相互轉化的旋转机械称为电机。
其中把机械能转化为电能的电机成为发电机,能够把电能转化为机械能的电机称为电动机。
电动机按照供电电源的种类不同可以划分为直流电动机与交流电动机两类,交流电动机可以分为同步电动机与异步电动机。
电动机的性能参数作为选型的重要指标,其数值的大小代表了电动机工作能力与品质的高低,应当是每位电工人员应当掌握与理解的重要参数指标。
1 电动机性能及参数的介绍1.1 直流电动机的介绍直流电动机与交流电动机相比结构复杂、价格昂贵、使用和维护的要求高。
但是直流电动机的起动转矩大,调速范围宽并且具有平滑的调速性能,因此在电车、电气机床、起重机械、电力牵引设备等方面应用广泛。
直流电动机主要有定子、转子、换向器等三部分组成,其中换向器是直流电动机所特有部件。
根据定子、转子线圈的励磁方式的不同,可以分为他励电动机、并励电动机、串励电动机与复励电动机四种。
电动机铭牌中主要参数包括电动机型号、额定功率、额定电压、额定电流、额定转速、额定效率等。
其中,额定电压(UN)是指电动机长期运行时所能承受的工作电压。
额定电流(IN)是指电机安规定的工作方式运行时,绕组允许通过的电流。
额定转速(nN)指电机在额定电压、额定电流与额定功率的情况下运行的电机转速。
额定功率(PN)是指电动机按规定的工作方式运行时所能提供的输出功率,也是电动机转轴上所输出的机械功率。
电动机由电能转化为机械能输出的过程中,由于机械传动过程的机械效率无法达到100%,不可避免的存在机械损失,因此必定存在机械效率数值。



1、各个参数之间的关系
A.在所有参数中,只有电压和电流是测量值,其他参数均是计算值。
B.Pmpp为在I-V曲线上找一点,使改点的电压乘以电流所得最大,该点对应的电压就是最大功率点电压
C.Rs
G.Rs
I.
1.
2.
3.
4.
5.正背面金属半导体接触电阻
6.外部因素影响,如探针和片子的接触等
烧结的关键就是欧姆接触电阻,也就是金属浆料与半导体材料接触处的电阻。
5则是变量电阻烧结效果的好坏直接影响Rs的最终值;
6属于外部测试因素,也会导致Rs变化
五、Rs影响因素
六、并阻Rsh组成
仅供个人学习参考
A.测试中并联电阻Rsh主要主要是由暗电流曲线推算出,主要由边缘漏电和体内漏电决定
B.边缘漏电主要由以下几个方面决定:
C.①边缘刻蚀不彻底
D.②硅片边缘污染
E.③边缘过刻
F.
G.体内漏电主要几个方面决定
H.①方阻和烧结的不匹配导致的烧穿
I.②由于铝粉的沾污导致的烧穿
J.③片源本身金属杂质含量过高导致的体内漏电
K.④工艺过程中的其他污染,如工作台板污染、网带污染、炉管污染、DI水质不合格等
八、Uoc
九、Isc
印刷铝浆太厚----查看
H.2.印
不好--印刷板间距太大--R.5.
C.2.网版参数不合格
E.4.
仅供个人学习参考。


126kV六氟化硫瓷柱式交流高压断路器技术规范书工程项目:广西电网公司年月目录1 总则2 使用环境条件3 技术参数和要求4 试验5 供货范围6 供方在投标时应提供的资料和技术参数7 技术资料和图纸交付进度8 运输、储存、安装、运行和维护规则9 技术服务与设计联络1 总则1.1本设备技术规范书适用于126kV瓷柱式六氟化硫交流高压断路器,它提出了该断路器的功能设计、结构、性能、安装和试验等方面的技术要求。
1.2需方在本规范书中提出了最低限度的技术要求,并未规定所有的技术要求和适用的标准,未对一切技术细则作出规定,也未充分引述有关标准和规范的条文,供方应提供一套满足本规范书和现行有关标准要求的高质量产品及其相应服务。
1.3如果供方没有以书面形式对本规范书的条款提出异议,则意味着供方提供的设备(或系统)完全满足本规范书的要求。
如有异议,不管是多么微小,都应在投标书中以“对规范书的意见和与规范书的差异(表)”为标题的专门章节加以详细描述。
本规范书的条款,除了用“宜”字表述的条款外,一律不接受低于本技术规范条款的差异。
不允许直接修改本技术规范书的条款而作为供方对本技术规范书的应答。
1.4本设备技术规范书和供方在投标时提出的“对规范书的意见和与规范书的差异(表)”经需、供双方确认后作为订货合同的技术附件,与合同正文具有同等的法律效力。
1.5供方须执行现行国家标准和行业标准。
应遵循的主要现行标准如下:GB/T 11022-1999 高压开关设备和控制设备标准的共用技术条件GB 311.1-1997 高压输变电设备的绝缘配合GB 1984-2003 交流高压断路器GB 7354-2003 局部放电测量GB/T 8905-1996 六氟化硫电气设备中气体管理和检验导则GB 4473-1996 交流高压断路器的合成试验GB/T5582-1993 高压电力设备外绝缘污秽等级GB/T 13540-1992 高压开关设备抗地震性能试验GB 50150-2006 电气装置安装工程电气设备交接试验标准DL/T593—2006 高压开关设备和控制设备标准的的共用技术要求DL/T 402-2007 交流高压断路器订货技术条件DL/T 615-1997 交流高压断路器参数选用导则GB 8923-1988 涂装前钢材表面锈蚀等级和除锈等级ISO 12944-1998 色漆和清漆-防护涂料体系对钢结构的防腐蚀保护IEC62271-100-2003 高压开关和控制设备第100部分:高压交流断路器Q/GXD 126.01-2006 电力设备交接和预防性试验规程(广西电网公司企业标准)上述标准所包含的条文,通过在本技术规范中引用而构成为本技术规范的条文。

动力电池性能参数一、电性能(1)电动势电池的电动势,又称电池标准电压或理论电压,为电池断路时正负两极间的电位差。
电池的电动势可以从电池体系热力学函数自由能的变化计算而得。
(2)额定电压额定电压(或公称电压),系指该电化学体系的电池工作时公认的标准电压。
例如,锌锰干电池为1.5V,镍镉电池为1.2V,铅酸蓄电池为2V,锂离子电池为(3)开路电压电池的开路电压是无负荷情况下的电池电压。
开路电压不等于电池的电动势。
必须指出,电池的电动势是从热力学函数计算而得到的,而电池的开路电压则是实际测量出来的。
(4)工作电压系指电池在某负载下实际的放电电压,通常是指一个电压范围。
例如,铅酸蓄电池的工作电压在2V~1.8V;镍氢电池的工作电压在1.5V~1.1V;锂离子电池的工作电压在3.6V~2.75V。
(5)终止电压系指放电终止时的电压值,视负载和使用要求不同而异。
以铅酸蓄电池为例:电动势为2.1V,额定电压为2V,开路电压接近2.15V,工作电压为2V~1.8V,放电终止电压为1.8V~1.5V(放电终止电压根据放电率的不同,其终止电压也不同)。
(6)充电电压系指外电路直流电压对电池充电的电压。
一般的充电电压要大于电池的开路电压,通常在一定的范围内。
例如,镍镉电池的充电压在1.45V~1.5V;锂离子电池的充电压在4.1V~4.2V;铅酸蓄电池的充电压在2.25V~2.5V。
(7)内阻蓄电池的内阻包括:正负极板的电阻,电解液的电阻,隔板的电阻和连接体的电阻等。
a. 正负极板电阻目前普遍使用的铅酸蓄电池正、负极板为涂膏式,由铅锑合金或铅钙合金板栅架和活性物质两部分构成。
因此,极板电阻也由板栅电阻和活性物质电阻组成。
板栅在活性物质内层,充放电时,不会发生化学变化,所以它的电阻是板栅的固有电阻。
活性物质的电阻是随着电池充放电状态的不同而变化的。
当电池放电时,极板的活性物质转变为硫酸铅(PbSO4),硫酸铅含量越大,其电阻越大。

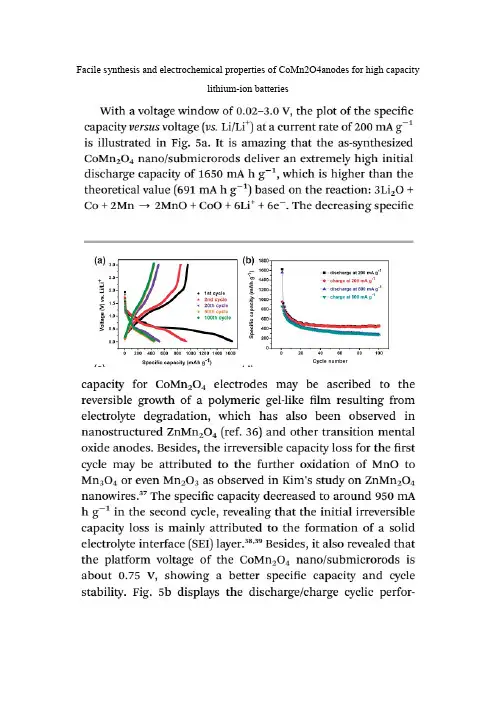
Facile synthesis and electrochemical properties of CoMn2O4anodes for high capacitylithium-ion batteriesCoMn2O4Spinel Hierarchical Microspheres Assembled with Porous Nanosheets asStable Anodes for Lithium-ion BatteriesFigure 5b shows discharge-charge curves of the electrodes made from theCoMn2O4 hierarchical microspheres at a current density of 100 mA·g-1at room temperature in a potential window between 0.01 and 3.0 V (vs. Li+/Li). It can be seen that there is a large deviation in potential between charge and discharge curves. The gap between charge and discharge curves is important, which involves the energy efficiency. The large gap may be a feature of metal oxide anode due to the polarization related to ion transfer during charge-discharge cycles. This phenomenon is often observed in many metal oxide anodes due to poor electrical conductivity, including CoMn2O4 reported previously. It is reported that the incorporation of graphene sheets can partly reduce the voltage polarization. The solution can be further studied in the future. The initial discharge and charge capacities are 1442 and 937 mAh g-1, which is higher than the theoretical value (920mAh g-1) based on the conversion reaction (see equation 5). The excess capacities could be associated with the decomposition of the electrolyte at low voltages generating a solid electrolyte interphase (SEI) layer and the further lithium storage via interfacial charging at the metal/Li2O interface. The large irreversible capacity loss of the first cycle can be attributed to the phenomenon that the formed SEI film can not completely decompose during the first charge.Figure 5c shows the discharge/charge capacity versus cycle number for the electrodes made from the CoMn2O4 hierarchical microspheres at a current of 100 mAh g-1. It is interesting to observe that the discharge capacity decreases only slightlybefore 36 cycles. After that, more interestingly, an unusual phenomenon that the discharge capacity gradually increases to 900 mAh g-1 can be seen. This capacity is very close to the theoretical value of a fully reversible conversion reaction (920 mAh g-1). Generally, after charge and discharge at a low current, the electrolyte penetrates into inner part of active materials. In our case, the current density of 100 mAh g-1 is not very high. So, to some extent, the above situation may also happen, making inner part of CoMn2O4 also involved in the conversion reactions,and then results in the increase of capacity. On the other hand, the phenomenon that the discharge capacity gradually increases also attributes to progressive generation of electro-chemistry active polymeric gel-like films. Not all the surface layer is covered on the first discharge. The internal surface within the pores is more difficult to access. It would appear that the polymer layer builds-up slowly, over a number of cycles. Of course, the specific reasons need to be studied further. After 65 cycles, this discharge capacity can be retained at 894 mAh g-1, corresponding to 94.9% of the second discharge capacity (942 mAh g-1), indicating excellent cycling stability. These above results are better than those in previous reports. It has been reported that CoMn2O4 hollow microcubes achieved a capacity of 624 mAh g-1 with retention of about 75.5% at a current of 200 mAh g-1after 50 cycles, while CoMn2O4 powders only exhibited a capacity of 515 mAh g-1 with retention of about 64% at a current of 69 mAh g-1 after 50 cycles.Double-Shelled CoMn2O4 Hollow Microcubes as High-Capacity Anodes forLithium-Ion BatteriesThe as-prepared double-shelled CoMn2O4 hollow microcubes are then tested as anodes for LIBs. Representative chargedischarge profiles of the electrode made of the double-shelled CoMn2O4hollow microcubes at a current density of 200 mA g−1are shown in Figure 4b. The initial discharge and charge capacities are 1282 and 806 mA g−1, respectively. The irreversible capacity loss of the first cycle can be attributed to the formation of the SEI film. Interestingly, even the initial charge capacity is much higher than the theoretical value (691 mA g−1) based on the oxidation of metallic Co and Mn nanoparticles to CoO and MnO respectively: 3Li2O +Co +2Mn ↔2MnO +CoO + 6Li++ 6e−. There are two possible reasons for this discrepancy. Firstly, it may be attributed to the reversible formation/dissolution of polymeric gel-like film resulted from electrolyte degradation, which has also been observed in nanostructured ZnMn2O4[27]and other transition metal oxides. [28]Secondly, it may also be contributed from the further oxidation of MnO to Mn3O4 or even Mn2O3 as observed in Kim’s study on ZnMn2O4 nanowires.[29]However, no peaks corresponding to the Mn2+/Mn3+ redox couple can be observed in the CV curves in our study. Thus, the more plausible origin for the extra reversible capacity in the present study is the reversible formation/dissolution of polymeric gel-like film.Figure 4c shows the discharge capacity as a function of cycle number at current densities of 200 and 800 mA g−1in the voltage range of 0.01–3.0 V vs.Li/Li+. Discharge capacities obtained for the first and second cycles at 200 mA g−1 are 1282 and 827 mA g−1, respectively. From the second cycle onwards, the discharge capacity only decreases slightly. After 50 cycles at 200 mA g−1, a high discharge capacity of 624 mA g−1is still retained, corresponding to 75.5% of the second discharge capacity. Even at a high current density of 800 mA g−1, a discharge capacity of 406 mA g−1 is retained after 50 cycles.Previously, the electrochemical properties of sub-micrometer sized CoMn2O4 particles have been reported, where a reversible capacity of about 515mA g−1 with retention of about 64% after 50 cycles at a current density of 69 mA g−1is achieved.[27]To the best of our knowledge, this is the first report on utilizing CoMn2O4 hollow structures as an anode material for LIBs. A much higher specific capacity with enhanced capacity retention is achieved in the present study through engineering the microstructure. More specifically, the superior electrochemical performance of our CoMn2O4 material can be attributed to the following two factors: (1) The nanometer-sized subunits not only make the conversion reaction more feasible but also allow the reversible formation/dissolution of polymeric gel-like film at the surfaceof the active material, both of which contribute to the high specific capacity.[30,31](2) The hollow interior and the porosity in the shells can buffer the large volume change of anodes based on the conversion reaction during the repeated Li+insertion/extraction, thus alleviating the pulverization problem and enhancing the cycling performance.Electrochemical properties of yolk–shell and hollow CoMn2O4powders directly prepared by continuous spray pyrolysis as negative electrode materials for lithium ionbatteries3The cycling performances of the CoMn2O4powders prepared from spray solutions with different concentrations of sucrose are shown in Fig. 4c. The Y1 and Y2 samples had high discharge capacities of 519 and 573 mAh g 21 after 40 cycles, respectively. However, the H1 and H2 samples had low discharge capacities of 418 and 276 mAh g 21 after 40 cycles, respectively. The H1, Y1 and Y2 samples had good cycle performances at a high discharge rate of 800 mA g 21.On the Other hand,the H2 sample had poor cycle performances. The CoMn2O4 powders had high Coulombic efficiencies above 97% from the second cycles irrespective of their morphologies.Facile preparation of ZnMn2O4hollow microspheres as high-capacity anodes for lithium-ionbatteries†Hydrothermal synthesis and characterization of MnCo2O4in the low-temperature hydrothermal process: Their magnetism and electrochemical properties。
