【银屑病治疗途径】【2015年IL-17在信号通路中的核心作用】Reich_et_al-2015-Experimental_Dermatology
- 格式:pdf
- 大小:931.98 KB
- 文档页数:7

Th17细胞及其分泌的IL-17在自身免疫性疾病中的作用种靖慧;解金辉【摘要】辅助性T细胞17(Th17)是一种新型T细胞亚群,能够分泌产生重要效应因子IL-17,IL-17作为炎症介质结合细胞上的IL-17受体引发包括NF-κB、MAPK 等途径在内的一系列信号传递而发挥其生物学功能,介导炎症、感染等的发生和发展.自身免疫性疾病是由于机体免疫功能紊乱,对自身抗原发生免疫反应,进而导致自身机体损伤的一种疾病,如类风湿性关节炎、系统性红斑狼疮、自身免疫性溶血性贫血等均属于常见的自身免疫性疾病.Thl7细胞及IL-17的异常表达和信号传递机制与各种自身免疫性疾病的致病机理研究成为一个热点,这为自身免疫病的防治对策提供新的认识和方案,Th17细胞及IL-17成为自身免疫性疾病一个新的治疗靶标.【期刊名称】《继续医学教育》【年(卷),期】2018(032)007【总页数】3页(P129-131)【关键词】辅助性T细胞;Th17;IL-17;自身免疫性疾病【作者】种靖慧;解金辉【作者单位】天津市血液中心免疫血液学研究室,天津 300110;天津市血液中心免疫血液学研究室,天津 300110【正文语种】中文【中图分类】R392自身免疫性疾病是机体的免疫平衡状态被打破,进而引起对自身机体的损伤的一种疾病,亦是对于外界入侵甚或自体产生的抗原发生的反应。
在参与机体免疫应答的各种免疫细胞中,CD4+T细胞作为效应T细胞发挥关键作用,Thl7细胞是一种新型的CD4+T细胞,其分泌的IL-17因子,能够作为介质调控机体的免疫调节,与其受体结合活化信号传递发挥生物学功能,调节炎症、感染等病理过程,是自身免疫病中重要的调控机制。
Th17及其分泌的IL-17表达和功能的异常与自身免疫性疾病的发生发展密切相关[1],在炎症性自身免疫病患者和动物模型中均发现IL-17的异常高表达及异常的信号传递,这与其炎症性病理机制密切相关。
现将相关研究综述如下。

药物在皮肤科疾病治疗中的新靶点近年来,药物研究领域取得了许多令人瞩目的突破,其中包括皮肤科疾病治疗。
随着对皮肤科疾病发病机制的深入研究,科学家们不断寻找和开发新的药物靶点,以帮助患者获得更好的治疗效果。
本文将探讨一些药物在皮肤科疾病治疗中的新靶点,以及它们的疗效和应用前景。
一、免疫调节剂的新靶点1.1 IL-17通路IL-17是重要的炎症因子,在多种炎症性皮肤病中起到关键作用。
目前,针对IL-17的单克隆抗体已经用于治疗银屑病和牛皮癣,取得了显著疗效。
未来的研究可以进一步寻找通过调节IL-17通路来治疗其他炎症性皮肤病的新药物靶点。
1.2 JAK-STAT通路JAK-STAT通路参与多个免疫细胞的发育和功能调控, 并在炎症性皮肤病的发病中发挥重要作用。
针对JAK-STAT通路的抑制剂已经被证明可以有效治疗苔藓样疣、银屑病等病症。
进一步研究和开发具有更高选择性的抑制剂将有助于扩大该通路在皮肤科疾病治疗中的应用范围。
二、细胞信号通路的新靶点2.1 Wnt/β-catenin信号通路Wnt/β-catenin信号通路在皮肤发育和修复过程中发挥着重要作用,并与皮肤疾病的发生有密切关联。
针对该通路的调控剂已经显示出治疗慢性创面愈合障碍和银屑病的潜力,并且正在进行更多临床研究以评估其疗效和安全性。
2.2 PI3K/Akt/mTOR信号通路PI3K/Akt/mTOR信号通路参与多种细胞的生长、增殖和存活调控,对于皮肤疾病的治疗也具有潜力。
抑制该通路可以减少炎症介质的释放和细胞凋亡,已经显示出改善银屑病和湿疹的疗效。
未来的研究将致力于发展更具选择性的药物来调控该信号通路,以减少不良反应的发生。
三、皮肤微生物群的新靶点3.1 痤疮菌群痤疮是一种常见的皮肤疾病,与皮肤微生物群的失调密切相关。
目前的治疗主要依靠外用抗生素,然而,这种治疗方法容易导致微生物耐药性。
未来的研究可以寻找和开发靶向痤疮菌群的新型治疗策略,如调整肠道菌群平衡以减少痤疮菌的生长。

白介素17在皮肤病中的研究进展作者:荚琳琳季必华来源:《科技视界》2015年第08期【摘要】IL-17作为近年来新发现的细胞因子,其来源及其在人体免疫系统中发挥的作用深受人们关注。
它是IL-23、RORγt等细胞因子作用Th17后产生。
它在系统性红斑狼疮、特应性皮炎、银屑病、白癜风等免疫相关性皮肤病中发挥着重要作用,也参与了梅毒螺旋体等病原体的慢性感染过程,与疾病活动性有一定的关系。
【关键词】IL-17;细胞因子;皮肤病白介素17(IL-17)最早是由Rouvier等人于1993年在激活的噬齿类的T细胞杂交瘤中发现的[1]。
近年来它逐步成为人们探讨的热点,尤其是在发现它参与人体固有免疫之后。
它和相关的细胞因子在免疫相关性疾病[2]和慢性感染过程[3]中发挥着重要作用,当然这其中也包括多种皮肤科疾病。
1 IL-17的来源和作用IL-17主要是由辅助性T细胞17(T helper cell 17,Th17)分泌[4],Th17在白介素23(IL-23)的作用下产生大量的IL-17[5],同时巨噬细胞、NK细胞[6]以及树突状细胞[7]都够能产生。
它可以刺激角质形成细胞、成纤维细胞及内皮细胞分泌多种炎性细胞因子和趋化因子[8]。
有研究发现银屑病患者的皮损中有大量IL-17 分泌型的树突样表皮 T 细胞(γδT细胞)浸润。
而且γδT细胞在IL-23和IL-1β联合刺激下可产生大量的IL-17。
实验发现真皮中的γδT细胞都是表达CCR6的细胞,不表达 CD27,不分泌γ干扰素,是只分泌IL-17的T细胞;在体外实验中IL-23可以刺激真皮γδT细胞增生,产生大量的IL-17;但脾脏中的γδT细胞在IL-23的作用下却无明显的增生反应[9]。
IL-17细胞因子家族包括6个成员,分别是IL-17A~F,它们通过白介素17受体(IL-17R)复合物参与炎症反应,通常所指的IL-17即IL-17A[10]。
IL-17可以诱导产生多种细胞因子和趋化因子:白介素6(IL-6)、粒细胞集落刺激因子及肿瘤坏死因子α、CXCL1、CXCL2、CCL20等。

Th17细胞及其相关因子在银屑病中西医治疗中的研究现状王英杰;刘杰;罗瑞静;武宗琴;彭勇;李淑;柴维汉(审校);李斌【摘要】目的:回顾分析近4年T h17细胞及其相关因子在银屑病中、西医治疗方面的研究进展。
方法:检索2012年~2015年期间公开发表的涉及银屑病治疗及T h17细胞及其相关因子的文献报道,经人工筛选后从西医治疗、中医治疗及物理治疗三方面进行分析,掌握目前的研究动态。
结论:不同文献报道的结论基本一致,所采用的治疗方法临床疗效确切,并能够降低T h17细胞、IL‐17、IL‐22等细胞因子的表达水平,推断其作用机制与抑制T h17细胞介导的炎症反应有关;细胞因子的表达水平与银屑病疾病严重程度呈正相关。
【期刊名称】《陕西中医》【年(卷),期】2016(000)002【总页数】3页(P251-253)【关键词】银屑病/中西医结合疗法;Th17细胞【作者】王英杰;刘杰;罗瑞静;武宗琴;彭勇;李淑;柴维汉(审校);李斌【作者单位】上海市嘉定区中医医院上海201800;上海市嘉定区中医医院上海201800;上海市嘉定区中医医院上海201800;上海市嘉定区中医医院上海201800;上海市嘉定区中医医院上海201800;上海市嘉定区中医医院上海201800;上海市嘉定区中医医院上海201800;上海市嘉定区中医医院上海201800; 上海中医药大学附属岳阳中西医结合医院上海200437【正文语种】中文【中图分类】R758.63·文献综述·主题词银屑病/中西医结合疗法Th17细胞银屑病是一种慢性易复发的自身免疫性疾病[1],既往的研究认为本病的发生可能和遗传、免疫、感染、神经经神等因素有关,其中的免疫因素是目前研究的重点之一。
辅助性T细胞17 (T helper cell 17,Th17)是一种新型的CD4+ T细胞亚群,目前Th17细胞及其细胞因子在银屑病中的作用成为研究的热点。

银屑病中Th17细胞及相关细胞因子的作用及研究进展杨建松【期刊名称】《浙江临床医学》【年(卷),期】2017(019)011【总页数】3页(P2160-2162)【作者】杨建松【作者单位】314000 蚌埠医学院【正文语种】中文银屑病俗称“牛皮癣”,是一种临床多见、反复易发、多基因缺陷的顽固性红斑鳞屑性皮肤疾病。
其发病机制尚未明确,多认为是在遗传易感、体内外等因素(感染、精神紧张、饮食及药物等)下发病或诱发加重,由Th1细胞、Th2细胞及众多细胞因子参与的免疫相关性疾病,临床表现为明显的红斑浸润,上覆有多层易刮除的干燥性银白色鳞屑,刮除后出现淡红色透亮薄膜,薄膜破溃有点状出血。
近年来研究发现,除Th1、Th2细胞及其相关细胞因子外,另一种不同于Th1、Th2分化途径的免疫细胞也参与其发病,并命名为Th17细胞,并与其他免疫细胞相互作用下参与银屑病的发生。
银屑病在遗传背景下,结合体内外环境变化而起病,发病过程中某种环节被“触发”而激活在皮肤,一连串的细胞及因子在皮肤的角化形成细胞和免疫细胞间相互起作用,多认为是Th1/Th2促炎与抗炎作用失衡,并在调节性T淋巴细胞(Treg)、干扰素、肿瘤坏死因子(TNF)、朗格汉斯巨细胞和树突状细胞分泌的细胞因子参与下,引起免疫系统失调,在皮肤表面形成红斑鳞屑并浸润。
随着对银屑病的治疗和观察,在银屑病复杂的发病机制中,一种分泌IL-17的CD4+T细胞被证实,且发现IL-17只存在于银屑病活体组织中,Th17细胞及其分泌的细胞因子参与银屑病的发病,在银屑病的免疫过程中起积极作用。
Th细胞在调节人类免疫应答中起到核心作用。
初期CD4+ T细胞可以分化为各种各样的Th细胞亚群或调节性T细胞。
在转化生长因子β(TGF-β)和IL-6、IL-1的影响下,发现不同于Th1、Th2细胞,且依赖IL-23扩增和存在的一种CD4+ T 细胞,被命名为Th17细胞。
Th17细胞及其分泌IL-17细胞因子(一种新发现的细胞因子家族的成员,包括IL-17A、IL-17B、IL-17C、IL-17D、IL-17E(IL-25)和IL-17F)在皮肤组织中存在多方面效应,可影响炎症性细胞因子、集落刺激因子和来自树突细胞的趋化因子、中性粒细胞、T淋巴细胞、单核细胞/巨噬细胞和上皮细胞等[1]。

IL-17在特应性皮炎发病中的作用机制探讨的开题报告**一、选题背景及研究意义**特应性皮炎(Atopic dermatitis,AD)是一种慢性炎症性皮肤疾病,主要表现为皮肤瘙痒、皮疹和干燥等症状,常伴随着过敏性哮喘、蕈状股癣等过敏性疾病。
AD的发病机制较为复杂,近期的研究发现,Th2细胞和IgE介导的过敏反应仅仅是AD发生发展过程的一小部分,调节性T 细胞(Treg)也参与了调节过敏反应的过程,而干扰素γ(IFN-γ)和IL-17等细胞因子的过度激活也是AD的一个重要发病机制。
IL-17是一种由Th17细胞分泌的细胞因子,参与了机体的免疫调节和炎症反应过程。
最新研究发现,IL-17在AD的发病过程中扮演了重要的角色。
在AD患者的皮肤中,IL-17浓度较高,而且皮肤病变区域的Th17细胞数量也显著增加,推测IL-17有可能导致皮肤屏障的破坏和炎症反应的加剧,同时也可能对机体免疫系统的稳定性产生负面影响。
因此,深入探究IL-17在AD发病机制中的作用机制,不仅对于AD 的病因病理研究有着重要的意义,而且还可以为开发新型的AD治疗药物提供理论基础。
**二、研究内容和方法**本研究旨在探究IL-17在AD发病机制中的作用机制,并分析其分子调控机制,方法包括以下几个部分:1.建立AD小鼠模型:使用大鼠皮下接种OVA,DNCB等化学物质或使用皮肤划破等方法建立小鼠AD模型;2.分离和培养小鼠皮肤细胞:使用酶消化等方法从小鼠皮肤组织中分离出各种不同类型的细胞,并进行培养;3. 确定IL-17的作用机制:使用Western Blotting,ELISA等方法对AD小鼠模型中IL-17的形态,结构,表达水平,分泌水平等生物学特性进行分析,同时研究IL-17对小鼠皮肤细胞的生长和分化影响机制;4. 分析IL-17的分子调控机制:通过转染、冷冻切片技术和激光共聚焦显微镜来研究IL-17的分子调控机制,特别是在细胞信号转导、表观遗传、转录因子及mRNA的稳定性等方面。

Notch信号通路在银屑病发病中的作用机制银屑病是一种常见的慢性炎症性皮肤病,其主要病理特征为角质形成细胞过度增殖,导致表皮可凋亡的角质形成细胞异常分化,并伴随皮肤炎症浸润及血管生成异常等。
银屑病病程较长、易复发且较难治愈。
目前关于银屑病的发病原因和机制尚无定论,但普遍认为与遗传和环境相关Notch 信号通路由多种分子及蛋白组成,参与多种疾病的发生过程,与银屑病的发生密切相关。
目前研究较多的是 Notch 信号通路与免疫介导机制在银屑病发病中的作用。
角质形成细胞是构成表皮结构的主要细胞,约占表皮细胞总数的 80% 。
多种皮肤肿瘤及炎症性皮肤病的发生与角质形成细胞增殖、凋亡、分化异常相关。
HaCaT 细胞是从成人皮肤中分离的永生化角质形成细胞系,其遗传特性稳定,增殖、分化特性与角质形成细胞相似,在进行实验研究时常用来替代角质形成细胞。
2014 年 4月~ 2015 年 6 月,本研究观察Notch 信号通路关键因子在银屑病皮损区皮肤中的表达情况及对HaCaT细胞增殖、凋亡及分化的影响,探讨 Notch 信号通路在银屑病发病中的作用机制。
材料与方法1.1 皮肤组织标本来源选择同期山东省中医院就诊的斑块型银屑病患者 30 例( 病例组) ,均经皮肤组织病理检查确诊。
其中男 18 例、女 12 例,年龄18 ~ 75( 32.6 ± 7. 4) 岁。
采集患者皮损区皮肤组织为病例组,同时采集距皮损区 1 cm 非皮损区皮肤组织作为对照组。
纳入标准: 皮损区近 2 周未外涂药物,近 1 个月未接受免疫抑制剂及其他药物治疗。
排除标准: 年龄< 18 岁或> 75 岁; 合并皮肤肿瘤或其他增生性皮肤病; 有心脑血管、肝、肾及造血系统等严重原发性疾病或其他情况不适合取材者。
同期选取本院皮肤科留存的健康人皮肤组织样本30 例作为正常组,健康人男16 例、女14 例,年龄18 ~69( 34.2 ± 6. 8) 岁。

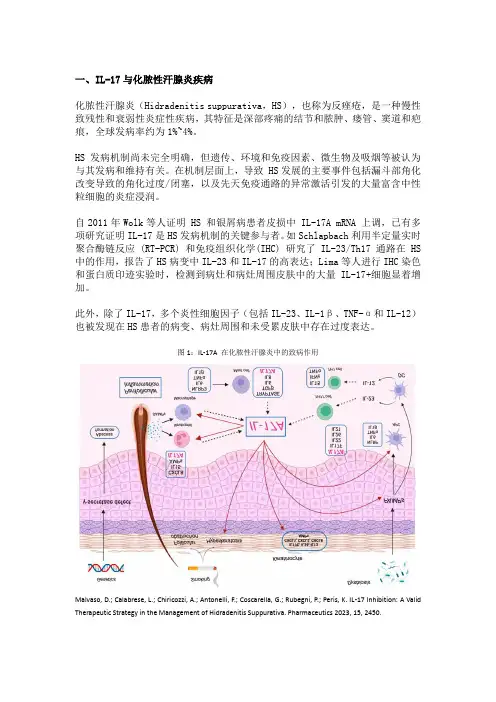
一、IL-17与化脓性汗腺炎疾病化脓性汗腺炎(Hidradenitis suppurativa,HS),也称为反痤疮,是一种慢性致残性和衰弱性炎症性疾病,其特征是深部疼痛的结节和脓肿、瘘管、窦道和疤痕,全球发病率约为1%~4%。
HS发病机制尚未完全明确,但遗传、环境和免疫因素、微生物及吸烟等被认为与其发病和维持有关。
在机制层面上,导致 HS发展的主要事件包括漏斗部角化改变导致的角化过度/闭塞,以及先天免疫通路的异常激活引发的大量富含中性粒细胞的炎症浸润。
自2011年Wolk等人证明 HS 和银屑病患者皮损中 IL-17A mRNA 上调,已有多项研究证明IL-17是HS发病机制的关键参与者。
如Schlapbach利用半定量实时聚合酶链反应 (RT-PCR) 和免疫组织化学(IHC) 研究了IL-23/Th17 通路在 HS 中的作用,报告了HS病变中IL-23和IL-17的高表达;Lima等人进行IHC染色和蛋白质印迹实验时,检测到病灶和病灶周围皮肤中的大量IL-17+细胞显着增加。
此外,除了IL-17,多个炎性细胞因子(包括IL-23、IL-1β、TNF-α和IL-12)也被发现在HS患者的病变、病灶周围和未受累皮肤中存在过度表达。
图1:IL-17A 在化脓性汗腺炎中的致病作用Malvaso, D.; Calabrese, L.; Chiricozzi, A.; Antonelli, F.; Coscarella, G.; Rubegni, P.; Peris, K. IL-17 Inhibition: A Valid Therapeutic Strategy in the Management of Hidradenitis Suppurativa. Pharmaceutics 2023, 15, 2450.截止目前,仅有靶向TNF-α和IL-17A的两款生物制剂获批用于治疗HS,分别为2015年获批的阿达木单抗(ADA)及2023年获批的司库奇尤单抗。


儿童寻常型银屑病血清中IL—17、TNF—α、MMP—9、TIMP—1的表达摘要目的探讨寻常型银屑病患儿血清白细胞介素-17(IL-17)、肿瘤坏死因子-α(TNF-α)、基质金属蛋白酶-9(MMP-9)、金属蛋白酶组织抑制剂-1(TIMP-1)的水平。
方法32例寻常型银屑病患儿(观察组)和34例健康儿童(对照组)为研究对象,比较两组血清中IL-17、TNF-α、MMP-9、TIMP-1表达水平的差异。
结果观察组血清IL-17、TNF-α、MMP-9、TIMP-1水平和MMP-9/TIMP-1比值均较对照组升高,差异均具有统计学意义(P<0.01)。
结论IL-17、TNF-α、MMP-9和TIMP-1在儿童寻常型银屑病的发病机制中起着重要作用。
关键词儿童;寻常型银屑病;白细胞介素-17;肿瘤坏死因子-α;基质金属蛋白酶-9;金属蛋白酶组织抑制剂-1Expression of serum IL-17,TNF-α,MMP-9 and TIMP-1 in children psoriasis vulgaris YANG Rui-hai,MOU Li-ping. Rizhao City Skin Disease Prevention Institution,Rizhao 276800,China【Abstract】Objective To investigate level of interleukin-17 (IL-17),tumor necrosis factor-α (TNF-α),matrix metalloproteinase-9 (MMP-9)and tissue inhibitor of matrix metalloproteinase-1 (TIMP-1)in children psoriasis vulgaris. Methods A total of 32 children with psoriasis vulgaris (observation group)and 34 healthy children (control group)were taken as study subjects. Differences of expression of serum IL-17,TNF-α,MMP-9 and TIMP-1 were compared between the two groups. Results The observation group had higher serum IL-17,TNF-α,MMP-9,TIMP-1,and ratio of MMP-9/TIMP-1 than the control group,and their differences all had statistical significance (P<0.01). Conclusion IL-17,TNF-α,MMP-9 and TIMP-1 act important roles in pathogenesis of children psoriasis vulgaris.【Key words】Children;Psoriasis vulgaris;Interleukin-17;Tumor necrosis factor-α;Matrix metalloproteinase-9;Tissue inhibitor of matrix metalloproteinase-1儿童寻常型银屑病是一种与免疫有关的慢性炎症性皮肤病,可发生于任何年龄。
芩珠凉血方对银屑病血热证患者血清 IL-17、IL-23及皮损中相关转录因子的影响向延卫;范斌;徐蓉;徐文彬;顾荻青;周蜜【摘要】OBJECTIVE To provide foundation for traditional Chinese medicine in treating psoriasis via studying the inter-vention effect of Qinzhu Liangxue Decoction on Th1 7 cells,STAT3 and RORγt of psoriasis patients.METHODS 60 cases of psoriasis vulgaris(of blood-heat syndrome)were selected for clinical research and randomly divided into treatment group(30 ca-ses) and control group(30 cases),with the normal group being 10 cases.Patients in the treatment group and control group was prescribed with oral Qinzhu Liangxue Decoction and acitretin respectively,with the treatment lasting for 1 month.Clinical effi-cacy was observed based on psoriasis area and severity index(PASI).ELISA was adopted to detect,compare and analyze chan-ges of Th1 7 cells.Expression rules of STAT3 and RORγt in the lesion tissue of psoriasis were detected by immunohistochem-istry technology.RESULTS PASI scores of both groups after treatment were lower than those before treatment,and the difference was statistically significant(P <0.05).Serum Th1 7 cytokines(IL1 7,IL23)of the two groups before treatment were higher than the normal group(P<0.05),and both IL1 7 and IL23 of these two groups went down after treatment(P <0.05). Before tr eatment,the expression of STAT3 and RORγt via immunohistochemical detection in the lesion area of both groups was higher than that of normal group(P <0.05),while it became lower aftertreatment(P <0.05).CONCULSION Qinzhu Liangxue Decoction plays an effective role in treating psoriasis by improving Th1 7 cells and regulating transcription factors STAT3 and RORγt.%目的:通过芩珠凉血方对银屑病患者血清 IL-17、IL-23及 STAT3、RORγt 干预作用的研究,为中医治疗银屑病提供依据。
Hedgehog信号通路在银屑病发病中的机制研究中
期报告
银屑病是一种常见的慢性炎症性皮肤疾病,其发病机制复杂。
近年来的研究表明,Hedgehog信号通路可能在银屑病的发病中发挥一定的作用。
本研究旨在探讨Hedgehog信号通路在银屑病发病中的机制。
本研究采用了银屑病患者皮肤组织和小鼠模型进行研究。
首先,通过实时荧光定量PCR和免疫荧光技术检测了银屑病皮肤组织和正常对照组织中Hedgehog信号通路相关基因和蛋白的表达情况。
结果显示,在银屑病皮肤组织中,Sonic Hedgehog (Shh) 和 Patched-1 (Ptch1) 的表达显著增加,而Gli1表达无明显改变。
此外,银屑病皮肤组织中还存在大量的Shh和Ptch1阳性细胞。
接着,利用小鼠模型进一步研究了Hedgehog信号通路在银屑病发病中的作用。
采用上皮细胞特异性表达Ptch1的小鼠模型,结果显示这些小鼠表现出了类似银屑病的皮肤病理特征。
进一步实验表明,Ptch1的表达可以诱导角质细胞的增殖,并促进炎性细胞浸润,并通过上调炎性细胞分泌的细胞因子和蛋白在皮肤病变中发挥作用。
综上,本研究发现Hedgehog信号通路在银屑病发病中可能通过调控角质细胞的增殖和炎性细胞的浸润作用。
这些发现为银屑病的发病机制提供了新的理解和研究思路,也为银屑病的治疗提供了新的靶点和方向。
IL-17和IL-23拮抗剂治疗银屑病研究进展邓维;张晓艳【期刊名称】《中国麻风皮肤病杂志》【年(卷),期】2018(34)7【摘要】目前美国食品药品监督局(FDA)批准用于临床的IL-17抗体拮抗剂包括secukinumab、ix-ekizumab和brodalumab,三者治疗中度至重度斑块状银屑病皮损清除均优于对照组TNF-α拮抗剂,且安全性及耐受性均较好.三种IL-23拮抗剂risankizumab,guselkumab和tildrakizumab目前均在III期临床试验中,初步临床试验结果表明三种药物治疗银屑病较对照组TNF-α拮抗剂具有更快达到皮疹改善、更高皮疹清除率和注射次数更少的优势.本文就上述六种生物制剂治疗银屑病的研究进展进行了综述.【总页数】4页(P437-440)【作者】邓维;张晓艳【作者单位】中日友好医院皮肤病与性病科,北京,100029;中日友好医院皮肤病与性病科,北京,100029【正文语种】中文【相关文献】1.激光光疗联合复方青黛胶囊治疗银屑病疗效及对血清TNF-α、IL-17、IL-23水平影响 [J], 解翠林;付曼妮;石年2.自拟凉血汤治疗寻常性银屑病患者临床疗效及对血清IL-8、IL-17、IL-23的影响分析 [J], 胡晓波;曹雯勤3.栀榆洗剂治疗寻常型银屑病疗效及对血清IL-17、IL-23水平的影响 [J], 符润娥;张颖;罗芳梅;李江4.清热养血解毒汤联合钙泊三醇倍他米松治疗寻常型银屑病血燥证及对外周血IL-17、IL-23的影响 [J], 唐娟;费良阅5.凉血活血复方治疗血热型寻常性银屑病临床疗效及对血清IL-8、IL-17、IL-23的影响 [J], 李兴军;唐沛;郭丽;毛桂华因版权原因,仅展示原文概要,查看原文内容请购买。
银屑病患者血清细胞因子白细胞介素-17、半胱天冬酶-1、白细胞介素-18的变化情况徐承箴;刘伦飞【期刊名称】《中国现代医生》【年(卷),期】2017(055)014【摘要】目的探讨银屑病患者血清细胞因子白细胞介素-17(Interleukin-17,IL-17)、半胱天冬酶-1(caspase-1)、白细胞介素-18(Interleukin-18,IL-18)的变化情况.方法选择寻常型银屑病患者80例为银屑病组,健康体检者80例为对照组.酶联免疫吸附实验法测定两组血清IL-17、caspase-1、IL-18水平.结果银屑病组患者血清IL-17、caspase-1、IL-18水平分别为(215.46±19.78)pg/mL、(112.31±12.14)pg/mL、(134.25±18.85)pg/mL,对照组患者血清IL-17、caspase-1、IL-18水平分别为(142.31±7.80)pg/mL、(43.25±5.65)pg/mL、(62.97±12.13)pg/mL,两组血清IL-17、caspase-1、IL-18水平比较差异均有统计学意义(P=0.000、0.000、0.000),银屑病组患者血清IL-17、caspase-1、IL-18水平高于对照组.银屑病进展期患者血清IL-17、caspase-1、IL-18水平分别为(265.47±16.57)pg/mL、(137.56±8.93)pg/mL、(163.24±5.83)pg/mL,银屑病静止期患者血清IL-17、caspase-1、IL-18水平分别为(165.37±11.25)pg/mL、(68.73±5.46)pg/mL、(76.86±4.87)pg/mL,两组患者血清IL-17、caspase-1、IL-18水平比较差异均有统计学意义(P=0.000、0.000、0.000),银屑病进展期患者血清IL-17、caspase-1、IL-18水平高于静止期.银屑病患者血清IL-17和caspase-1、IL-18均呈正相关(r=0.673、0.742,P=0.000、0.000),血清caspase-1和IL-18呈正相关(r=0.587,P=0.000).结论寻常型银屑病患者血清IL-17、caspase-1、IL-18水平升高,IL-17、caspase-1、IL-18在寻常型银屑病的发病中具有重要作用.【总页数】4页(P5-8)【作者】徐承箴;刘伦飞【作者单位】浙江医院安吉分院浙江省安吉县人民医院皮肤科,浙江安吉 313300;浙江大学医学院附属第二医院皮肤科,浙江杭州 310003【正文语种】中文【中图分类】R758.63【相关文献】1.盐酸氨溴索对急性期慢性阻塞性肺疾病患者血清CRP、白细胞介素17及白细胞介素18的影响 [J], 吕青兰2.Ⅰ期尘肺患者血清白细胞介素12、白细胞介素12p70、白细胞介素10和白细胞介素18含量的变化 [J], 袁宝军;王冬梅;邹吉敏;赵建宏3.白细胞介素-17、-22水平变化与寻常型银屑病患者PASI评分的关联性及动态监测临床意义 [J], 刘冬梅4.白细胞介素-17A和白细胞介素-17F在卵巢上皮性癌组织中的表达情况及与患者临床特征的关系 [J], 闫美玲;张亚莉;张莉5.银屑病患者外周血白细胞介素17、白细胞介素23 mRNA的表达及与病情相关性研究 [J], 冉灵芝;曾凡杞;胡鹏飞;邹梦英因版权原因,仅展示原文概要,查看原文内容请购买。
国产白介素17A治疗银屑病,全国多中心启动临床研究随着时代的发展和医学的进步,近些年越来越多的(进口)治银药物上市,与此同时,一些国内的药企也紧跟其后,抓紧银屑病药物的研发。
查询了临床试验公示平台,找到了几个近期正在开展的临床试验,在多个临床试验中,抢眼的当属白介素类生物制剂的临床试验,例如前阵子给大家介绍的国产白细胞介素17A(IL-17A)的Ⅲ期临床试验,目前已经在全国范围内如火如荼的展开了。
今天再把这个试验项目分享给大家,有兴趣参加的银友可以根据所在地区就近选择、报名参加。
项目介绍白细胞介素17 (IL-Interleukin 17)也称为 CTLA-8 或 IL-17,属于 IL-17家族,在免疫系统中扮演着关键的协调作用。
IL-17由 Th17细胞分泌,后者的活化受IL-23的影响。
IL-23是由炎症细胞产生的促炎性细胞因子,可刺激T细胞向Th17细胞分化,并在这些细胞的存活中起着不可或缺的作用,最终导致 IL-17释放增加。
靶向IL-17A 的抗体通过抑制IL-17A-IL-17RA/IL-17RC信号通路,能有效地缓解自身免疫性疾病的症状。
在银屑病皮肤中,IL-17A被认为是通过如浸润Th17细胞、中性粒细胞和肥大细胞产生的。
通过IL-17A以及与IL-17A协同作用的其他炎性细胞因子,激活角质形成细胞,这导致中性粒细胞,淋巴细胞和骨髓细胞的进一步募集和活化,最终导致持续的局部皮肤炎症,引发银屑病表皮病变。
由角质形成细胞介导的变化包括表皮厚度增加(棘皮症),外层角质层增加(角化过度)和角质层中的细胞核保留(角化不全)。
对于银屑病患者而言,IL-17A在驱动角化细胞(皮肤细胞)过度增殖和活化方面发挥了关键作用。
参加标准•1. 年龄为18~75岁男、女性患者(包括18岁和75岁,Ib期年龄限定为18~60岁);•2. 体重指数(BMI)=体重(kg)/身高2(m2),筛选时体重指数在18~30kg/m2范围内(包括临界值);•3. 患者能够理解研究内容且自愿签署知情同意书;同时,能够并愿意按照方案要求完成研究;•4. 筛选前已确诊患有慢性斑块状银屑病至少6个月;•5. 患者适合全身性治疗。
IL-17F在银屑病皮损中的表达及意义张平;张蓉;周小勇【期刊名称】《中国皮肤性病学杂志》【年(卷),期】2010()1【摘要】目的探讨IL-17F在银屑病皮损中的表达及意义。
方法收集寻常性银屑病患者皮损及正常皮肤作为对照,荧光定量PCR法检测两种组织中IL-17F和IL-8表达的差异;IL-17F诱导人角质形成细胞系HaCaT24h后,荧光定量PCR法检测IL-8的表达变化。
结果银屑病患者皮损IL-17F表达明显高于正常组,差异有统计学意义(P均<0.01);银屑病患者皮损同时伴有IL-8的高表达;IL-17F可诱导HaCaT细胞系IL-8的表达增高。
结论IL-17F在银屑病皮损中表达上调,IL-17F可诱导HaCaT产生IL-8,这可能是IL-17F参与银屑病发生和发展的机制之一。
【总页数】3页(P13-15)【关键词】银屑病;IL-17F;IL-8;HaCaT【作者】张平;张蓉;周小勇【作者单位】武汉市第一医院皮肤科;华中科技大学医学部生物化学与分子生物学系【正文语种】中文【中图分类】R758.63【相关文献】1.T细胞、角蛋白10、Ki-67在进展期银屑病皮损和皮损边缘皮肤的表达和意义[J], 沈宏;唐旭;王一玲;许爱娥2.银屑病患者皮损中CK20、SP、S100A7的表达及临床意义 [J], 王颖3.Sprouty1、2、4在寻常型银屑病患者皮损组织中的表达及临床意义 [J], 李锦意;张婷;柳莉丹;陈永锋4.寻常型银屑病患者皮损组织中miR-124-3p表达水平及意义 [J], 翟翊然;曹丽楠;李伟栋5.肝素酶和血管内皮生长因子在进行期寻常型银屑病皮损中的表达及意义 [J], 殷文浩;金梦祝;邬万新因版权原因,仅展示原文概要,查看原文内容请购买。
Evidence that a neutrophil–keratinocyte crosstalk is an early target of IL-17A inhibition in psoriasisKristian Reich1,Kim A.Papp2,Robert T.Matheson3,John H.Tu4,Robert Bissonnette5,Marc Bourcier6, David Gratton7,Rodion A.Kunynetz8,Yves Poulin9,Les A.Rosoph10,Georg Stingl11,Wolfgang M.Bauer11,Janeen M.Salter12,Thomas M.Falk1,Norbert A.Bl€o dorn-Schlicht1,Wolfgang Hueber12,Ulrike Sommer12,Martin M.Schumacher12*,Thomas Peters12,Ernst Kriehuber12,David M.Lee12,Grazyna A. Wieczorek12,Frank Kolbinger12and Conrad C.Bleul121Dermatologikum Hamburg and SCIderm Research Institute,Hamburg,Germany;2Probity Medical Research Inc,Waterloo,ON,Canada;3Oregon Medical Research Center PC,Portland,OR,USA;4Skin Search of Rochester,Rochester,NY,USA;5Innovaderm Research Inc,Montreal, QC,Canada;6Dermatology Clinic,Moncton,NB,Canada;7International Dermatology Research,Montreal,QC,Canada;8Ultranova Skincare, Barrie,ON,Canada;9Centre de Recherche Dermatologique du Qu e bec M e tropolitain,Quebec City,QC,Canada;10North Bay Dermatology Centre, North Bay,ON,Canada;11Division of Immunology,Allergy and Infectious Diseases,Department of Dermatology,Medical University of Vienna, Vienna,Austria;12Novartis Institutes for BioMedical Research,Basel,SwitzerlandCorrespondence:Kristian Reich,Dermatologikum Hamburg,Stephansplatz5,20354Hamburg,Germany,Tel.:+4940351075-79,Fax:+4940351075-40,e-mail:kreich@dermatologikum.de*Currently employed by Firalis SAS,Huningue,France.Abstract:The response of psoriasis to antibodies targeting the interleukin(IL)-23/IL-17A pathway suggests a prominent role of T-helper type-17(Th17)cells in this disease.We examined the clinical and immunological response patterns of100subjects with moderate-to-severe psoriasis receiving3different intravenous dosing regimens of the anti-IL-17A antibody secukinumab (193mg/kg or1910mg/kg on Day1,or3910mg/kg on Days1,15and29)or placebo in a phase2trial.Baseline biopsies revealed typical features of active psoriasis,including epidermal accumulation of neutrophils and formation of microabscesses in >60%of cases.Neutrophils were the numerically largest fraction of infiltrating cells containing IL-17and may store the cytokine preformed,as IL-17A mRNA was not detectable in neutrophils isolated from active plaques.Significant clinical responses to secukinumab were observed2weeks after a single infusion, associated with extensive clearance of cutaneous neutrophils parallel to the normalization of keratinocyte abnormalities and reduction of IL-17-inducible neutrophil chemoattractants(e.g. CXCL1,CXCL8);effects on numbers of T cells and CD11c-positive dendritic cells were more delayed.Histological and immunological improvements were generally dose dependent and not observed in the placebo group.In the lowest-dose group,a recurrence of neutrophils was seen in some subjects at Week12; these subjects relapsed faster than those without microabscesses. Ourfindings are indicative of a neutrophil–keratinocyte axis in psoriasis that may involve neutrophil-derived IL-17and is an early target of IL-17A-directed therapies such as secukinumab.Abbreviations:AE,adverse event;IL,interleukin;PASI,Psoriasis Area and Severity Index;Th17,T-helper type-17cell subset;Tregs,regulatory T cells.Key words:IL-17A–neutrophils–psoriasis–secukinumabAccepted for publication25March2015IntroductionSeveral key mechanisms have been proposed as initiating and maintaining psoriasis,including the activation of dendritic cells by complexes of self-DNA and the antimicrobial peptide cathelicidin LL-37,the presentation of putative auto-antigens to T lympho-cytes,and the release of pro-inflammatory mediators such as interleukin(IL)-23,tumor necrosis factor-a(TNF-a)and IL-17A, leading to the activation of keratinocytes,which close the loop by producing antimicrobial peptides such as LL-37,chemoattractants and b-defensins(1–3).A central element in current disease models is the T-helper type-17cell subset(Th17).Consistent with this concept,IL-23(believed to mediate the crosstalk between den-dritic and Th17cells)and IL-17A(a key effector cytokine released from Th17cells)have emerged as attractive targets for the devel-opment of new therapies for psoriasis.The clinical efficacy of ustekinumab–an anti-p40monoclonal antibody that blocks IL-12 and IL-23–and antibodies directed against IL-17A and the IL-17 receptor A chain has been taken as evidence that the IL-23/Th17axis is indeed central to the pathophysiological cascade of psoriasis (4–9).More recently,inflammatory cells other than Th17–in par-ticular,neutrophils,a numerically important component of the infiltrate in active psoriasis–have also been suggested as sources of IL-17A(10,11).IL-17A not only contributes to the epidermal abnormalities typical of psoriasis,but also induces the expression of chemokines in keratinocytes such as GRO-a(CXCL1)and IL-8 (CXCL8),which orchestrate the recruitment of neutrophils to psoriatic lesions(12).To further dissect the role of different sources of IL-17A in psoriasis,this study investigated serial biopsies taken from active plaques at Baseline and treatment Weeks2and12during a phase 2trial in which subjects with moderate-to-severe psoriasis received 1of3induction regimens with the IL-17A-selective antibody secukinumab.Two weeks after a single intravenous infusion of secukinumab,IL-17-containing neutrophils had almost completely disappeared parallel to a striking normalization of psoriatic epider-mal changes and reduction of keratinocyte-derived,IL-17-inducibleneutrophil chemoattractants.Ourfindings suggest that IL-17A is not only part of an adaptive immune circuit involving specific T-cell subsets,but also part of an innate axis between neutrophils and keratinocytes that serves as an early target of anti-IL-17A antibodies.Materials and methodsSubjects and study designA total of130subjects were enrolled at14sites in the United States and Canada between December2008and July2009.Sub-jects aged18–65years were eligible if they had chronic plaque psoriasis for≥6months,coverage of≥10%of their body surface area with plaques,an Investigator’s Global Assessment score≥3 (scale of0–5)and a Psoriasis Area and Severity Index(PASI) score≥12.The study consisted of a12-week induction period and a follow-up period up to Week56after thefirst dose of study drug.Eligible subjects were randomly assigned to receive1of3 regimens of intravenous secukinumab(AIN457;Novartis Pharma-ceuticals,Basel,Switzerland),a fully human immunoglobulin-G1j monoclonal antibody selective for IL-17A,or placebo in a3:3:3:1 ratio(details regarding sample size calculation,randomization and blinding are provided in the Supporting Information).There were low-and mid-single-dose cohorts who received secukinumab3 and10mg/kg,respectively,infused on Day1(with placebo administered on Day15and Day29)and a high-dose cohort who received three infusions of secukinumab10mg/kg at2-week inter-vals.Infusions were given over2h.The primary objectives were to compare the change from Baseline in PASI score at Week12 between cohorts and to determine the proportions of subjects who did not relapse at any time through Week56.Secondary efficacy endpoints included the proportions of sub-jects with≥50%,≥75%and≥90%improvements from Baseline in PASI(PASI50/PASI75/PASI90),and changes in Investigator’s Glo-bal Assessment and Dermatology Life Quality Index scores.One study site with30subjects was terminated prematurely because of data-quality concerns;the efficacy and safety data for100subjects (excluding those from the terminated site)are presented in this analysis.The study was conducted according to the Declaration of Helsinki.The study protocol and all amendments were approved by the central independent ethics committees or institutional review boards in the participating countries.All study subjects provided written informed consent for their participation.The full study protocol is available from the sponsor( identifier NCT00805480;date of registration:5December2008). RNA extraction,NanoString nCounterâand quantitative reverse-transcription–polymerase chain reaction gene expression analysis of skin biopsiesFour-millimetre punch biopsies were obtained from a representa-tive psoriatic plaque at Baseline and from the same plaque at Weeks 2and12.All100subjects included here had Baseline biopsies taken and biopsies from Weeks2and12were available from almost all subjects for analysis(Fig.S1).One part of each biopsy was immedi-ately embedded in optimal cutting temperature compound(Tissue-TekâO.C.T.TM Compound,Sakura Finetek,Alphen aan den Rijn, the Netherlands),stored atÀ70°C and later processed for RNA extraction,while the other part wasfixed in paraformaldehyde and used for histology and immunohistochemistry.All biopsies were handled and analysed by personnel blinded to treatment and time points.RNA was isolated using the RNeasy Fibrous Tissue Mini Kit (Qiagen NV,Venlo,the Netherlands)as described in the Support-ing Information.To analyse a broader set of mRNAs with high sensitivity,a subset of samples was processed with the nCounter Prep Station and Digital Analyzer and tested with a custom-designed nCounter Gene Expression CodeSet Maestro(NanoString Technologies,Seattle, WA,USA)containing probes for180psoriasis-related transcripts, nine candidate reference transcripts for normalization and two gender control transcripts.Probe sequences for genes reported in this study are shown in Table S1;further details of the methodology and the control quantitative reverse-transcription-polymerase chain reaction performed for IL17A and IFNG are given in the Supporting Information.Immunohistochemistry and immunofluorescence Epidermal thickness and parakeratosis,as well as staining of Ki67, CD11c,CD3,IL-17,myeloperoxidase,and mast cell tryptase,were evaluated on paraffin-embedded,haematoxylin/eosin-stained sec-tions,alone or in combination with immunohistochemistry using a prospectively defined semi-quantitative scoring system on digi-tally scanned images(AxioVision SE64Rel.4.8;Carl Zeiss Micros-copy,Oberkochen,Germany;Fig.S2).Results were confirmed by automated digital imaging of selected sections.Immunohisto-chemical stainings were performed according to the manufac-turer’s instructions,using the Dako REAL TM Detection System, alkaline phosphatase/RED,rabbit/mouse(Dako,Glostrup,Den-mark)in an automated staining system(Dako Autostainer Plus, Dako).Double immunofluorescence stainings of IL-17vs tryptase and myeloperoxidase,respectively,were performed manually. Slides were mounted with ProLongâGold Antifade Mountant with DAPI(Life Technologies,Grand Island,NY,USA).Image acquisition was performed on an LSM700confocal microscope (Carl Zeiss Microscopy).Lists of antibodies and procedural details are provided in the Supporting Information.Analysis of peripheral blood T cells and isolated peripheral blood and skin leucocyte subsetsSurface markers on peripheral T-lymphocyte subsets and the stim-ulated expression of selected cytokines were assessed byflow cytometry.Percentages of Th17,Th1and regulatory T cells(Tregs) were determined as described in the Supporting Information.Data acquisition was performed on a BD FACSCanto TM II(Becton, Dickinson and Company,Franklin Lakes,NJ,USA).Leucocyte subsets were also isolated from peripheral blood and psoriatic skin samples and analysed by quantitative polymerase chain reaction as described in the Supporting Information.StatisticsEfficacy and pharmacodynamic parameters were evaluated in all sub-jects who received≥1dose of study medication and had no major protocol deviations that could affect these parameters;safety was eval-uated in all subjects who received≥1dose of study medication.The change from Baseline in PASI score in each secukinumab group com-pared with the placebo group was evaluated using the Wilcoxon rank-sum test.The proportions of subjects who did not relapse in the secukinumab1910and3910mg/kg groups compared with the 193mg/kg group were evaluated using Fisher’s exact test.Subjects lost to follow-up were considered relapsed on the day of thefirst visit without available PASI data.The relapse time of subjects who did not relapse during the complete course of the clinical trial was set to 56weeks(end of study).The significance of the difference betweenthese subject groups was assessed using a2-sided Wilcoxon rank-sum test.P-values corrected for ties are reported.NanoString data were log2-transformed.For assessment of the dose and time effect on the investigated transcripts,means of the log2-transformed counts and standard errors of the means were plotted.ResultsDuring a12-week induction period,subjects received either one infusion with secukinumab3or10mg/kg,respectively,on Day1or 3infusions of secukinumab10mg/kg on Days1,15and29or respective placebo infusions and were followed up to Week56as described.Subjects across all groups had a mean Baseline PASI of approximately19.Details of the study design and the Baseline char-acteristics of included subjects are shown in Fig.S3and Table S2, respectively.By Week12and by Week56,9(9%)and38(38%)of the subjects had discontinued from the trial,respectively(Fig.S1). EfficacyAll3secukinumab dose regimens resulted in statistically signifi-cant improvements in mean PASI scores compared with placebo at Week12(Fig.1a).The PASI responses were rapid,being detectable by1–2weeks after dosing and reaching maximum improvement by6–8weeks.Consistently,large percentages of sub-jects achieved PASI responses at Week12in each of the active treatment groups,with the highest percentage of responders in the secukinumab3910mg/kg group(82.6%and75.9%for PASI75 and PASI90,respectively),followed by the1910mg/kg group (75.0%and54.2%)and the193mg/kg group(40%and10%; Fig.1b,c).More than80%of subjects in all secukinumab groups showed improved Investigator’s Global Assessment scores by Week 2,with these improvements continuing through Week12,and the mean Dermatology Life Quality Index decreased in all3secukinu-mab groups vs the placebo group(data not shown).Median times to relapse(defined as the time from thefirst day of dosing to the day when≥50%of the maximal PASI response was lost)were24.2weeks in the secukinumab193mg/kg group,28.4weeks in the1910mg/kg group and40.1weeks in the3910mg/kg group.In the3910mg/kg group,about half of the subjects maintained their treatment response through Week 40(58.6%,48.3%and27.8%relapse free at Weeks36,40and56, respectively).SafetyThe safetyfindings during this56-week trial were consistent with previous observations for secukinumab in similar populations (8,9).The rates of subjects reporting adverse events(AEs)were higher in the secukinumab treatment groups(66.7%,86.2%and 83.9%with193,1910and3910mg/kg,respectively)than in the placebo group(30.0%).Ten serious AEs were reported in seven subjects.Serious AEs included tibia fracture,fibula fracture, pancreatitis(four AEs in two subjects),myocardial infarction, worsening of psoriasis,angina and worsening of coronary artery disease.In this study,all serious AEs occurred in subjects on secu-kinumab,and none were considered related to study drug by the investigator.One serious AE(pancreatitis,3910mg/kg cohort) resulted in discontinuation of study drug.One case of mild oral candidiasis was reported with the3910mg/kg dose.There were no serious infectious AEs,no cases of neutropenia and no signifi-cant effects on peripheral T helper–lymphocyte subsets(Th1, Th17),except for a small increase in CD25high/CD127low/Foxp3+ regulatory T cells in the highest-dose group at Week12(4.9%at screening vs5.8%at Week12;P=0.0002)(Fig.S4).Impact of anti-IL-17A treatment on epidermal andinflammatory changes in psoriatic lesionsThere was a striking reduction in histological correlates of psoriat-ic epidermal pathology[i.e.epidermal thickness,parakeratosis (Figs2a,b and3),acanthosis and numbers of Ki67-positive pro-liferating epidermal cells(Fig.S6a,b)]as early as Week2.Simi-larly,mRNA levels for keratin-16and desmocollin-2–two keratinocyte markers upregulated in lesional skin–were already significantly reduced at Week2(Fig.2h).By Week12,all epider-mal markers had returned to normal levels,particularly in the high-dose cohort.The most prominentfinding with regard to the lesional inflam-matory infiltrate was that epidermal microabscesses and dermal neutrophils identified by myeloperoxidase staining–which were present in65%and76%of Baseline biopsies,respectively–had almost entirely cleared by Week2(Figs2c,d and3a).In contrast, numbers of T lymphocytes and CD11c-positive dendritic cells decreased more slowly,and elevated levels of T lymphocytes and dendritic cells persisted at Week12(Fig.2e,f).Thus,the kinetics of the effects of secukinumab on neutrophils paralleled the rapid epidermal improvement,in contrast to the more steady improve-ment observed for T lymphocytes and dendritic cells.Immunohistochemistry and double immunofluorescence label-ling for IL-17and myeloperoxidase with a widely used affinity-purified polyclonal IL-17antibody(10,11,13)revealed that epider-mal neutrophils were the numerically predominant cell type con-taining IL-17in active plaques.Blocking experiments with recombinant human IL-17A and IL-17F confirmed that the anti-IL-17polyclonal antibody primarily detects IL-17A,with some possible cross-reactivity with IL-17F(see Supporting Information and Fig.S5).IL-17was detected in Munro’s microabscesses(accumula-tions of neutrophils in the stratum corneum)and spongiform pus-tules of Kogoj(accumulations of neutrophils in the stratum spinosum),as well as in single neutrophils in the dermis(Fig.3b). Small subsets of lesional T cells(CD3positive)and dermal mast cells(tryptase positive;Fig.S5)were also found to contain IL-17,in(a)(b)(c)Figure1.Psoriasis Area and Severity Index(PASI)response rates over time in subjects receiving secukinumab or placebo.(a)%change in PASI from Baseline.(b)≥75%reduction from Baseline in PASI(PASI75).(c)≥90%reduction from Baselinein PASI(PASI90).(a)(b)(c)(d)Figure 3.Representative immunohistochemistry of skin biopsies from psoriatic lesions and analysis of IL17A expression in peripheral blood and skin leucocyte subsets.(a)Baseline evaluation of IL-17in a subject from the secukinumab 1910mg/kg cohort shows prominent staining in epidermal Munro’smicroabscesses (oval outline)and spongiform pustules of Kogoj (arrow,top row).Epidermal IL-17staining was cleared at Week 2,but still evident in dermal T cells and mast cells at Week 12(middle and bottom rows).(Control sections are shown in Fig.S5.)(b)Immunofluorescence double labelling for IL-17(green)andmyeloperoxidase (MPO;red)with DAPI counterstain confirming IL-17staining of neutrophils in Munro’s microabscesses (top row),spongiform pustules of Kogoj (arrowhead,bottom left)and neutrophils in dermal vessels (arrow,bottom right).(c)Levels of IL-17A,CD3E,CD1c and proteinase-3(PR3;to identify neutrophils)mRNA in peripheral blood cells of subjects with psoriasis (n =4,top row)andhealthy donors (n =3,bottom row).IL-17A mRNA was not detected in more than 95%pure granulocyte fractions (Gran),but observed at low levels in Tlymphocytes.Quantification cycle (Cq)for b 2-microglobulin (B2M)between 19and 24for all samples shown;not detectable:Cq >45.(d)Levels of IL-17A,CD3E,CD1c,MPO (to identify cutaneous neutrophils),and tryptase (to identify cutaneous mast cells)mRNA in cells isolated from psoriatic plaques.One representative experiment of 4is shown.Samples were run in duplicate for each probe,and quantification was based on DD Cq calculations.Arithmetic means Æstandard deviation of different donors (a)and of triplicate determinations of individual samples (b)are shown.DC,dendritic cell;Mono,mononuclear;PSOR,psoriasis.(a)(b)(c)(d)(e)(f)(g)(h)Figure 2.Changes in epidermal and inflammatory parameters in skin lesions during treatment with secukinumab or placebo (a –f;data from all patients).(a –f)Mean values for (a)epidermal thickness in mm (normal 0.1–0.2mm),(b)parakeratosis,(c)epidermal microabscesses of neutrophils,(d)numbers of myeloperoxidase(MPO)-positive neutrophils,(e)CD3-positive T cells and (f)CD11c-positive dendritic cells.Means for (b–f)derived from semi-quantitative scoring system [see theMaterials and methods section and Fig.S2;normal =0,except for CD3and CD11c (0–1)].Solid lines indicate mean changes in Psoriasis Area and Severity Index (PASI).(g,h)Mean log 2-transformed NanoString counts of expression levels of (g)inflammatory cytokines and (h)epidermal markers in the same biopsies.BL,Baseline;PBO,placebo;all graph titles in g)and h)indicate genes,e.g.CXCL8,DSC2,IFNG ,KRT16,TNF indicate genes encoding IL-8,desmocollin-2,IFN-c ,keratin-16,TNF-a .Error bars are Æstandard error of the mean.line with earlierfindings(10,11).Unlike neutrophils,however,mast cells remained essentially unchanged in number in response to IL-17A blockade(Fig.3a,S6c).IL-17A mRNA could be detected in T cells,but not in mast cells or neutrophils by quantitative real-time polymerase chain reaction analysis of sorted cell preparations from psoriatic lesions, and IL-17A mRNA was also absent in peripheral blood granulo-cytes isolated from subjects with psoriasis(Fig.3c,d).Quantifi-cation of cytokine mRNA in skin biopsies showed significant reductions of IL-17A and IL-17F mRNA by Week2,but only limited effects on TNF-a mRNA levels(Fig.2g),confirming ear-lierfindings(4,14).Consistent with the known effects of IL-17 on epidermal chemokine production(12),blockade with secu-kinumab rapidly reduced the mRNA expression of keratinocyte-derived neutrophil chemoattractants such as GRO-a(CXCL1) and IL-8(CXCL8)by Week2(Fig.2h).No consistent effects on histological or molecular parameters were observed in the placebo group.In light of the rapid clearance of neutrophils after infusion of secukinumab,which occurred in parallel to the improvement of epidermal changes and clinical signs of psoriasis,it was speculated that reoccurrence of these cells may contribute to early clinical relapse.Thefinding that epidermal and dermal neutrophils,as well as neutrophil-attracting chemokines,became detectable in the low-dose cohort in about a third of the Week-12biopsies in asso-ciation with an increase in clinical disease activity between Weeks 10and12supported this hypothesis(Figs1and2c,d,h).Indeed, subjects in the low-dose group with detectable epidermal microab-scesses at Week12had a shorter time to relapse than those with-out epidermal neutrophils(14.0vs28.0weeks;P=0.04;Table1). Thus,the early clinical response to secukinumab was linked to the disappearance of cutaneous neutrophils,while their reoccurrence preceded the clinical relapse observed when the effect of a single induction dose of the drug decreased.DiscussionIn this study,the intravenous treatment of subjects with moder-ate-to-severe plaque psoriasis with the anti-IL-17A antibody secu-kinumab was used as a model to better understand disease mechanisms and to further dissect the response to IL-17A-targeting therapies.Treatment with secukinumab led to dose-dependent improvements of psoriasis during a12-week period,with PASI75 (PASI90)response rates of40%(10%),75%(54.2%)and82.6% (75.9%)achieved in the low-,mid-and high-dose cohorts,respectively.Particularly in the high-dose cohort,this effect was long-lasting(no relapse in half of the patients through Week40with the last dose received at Week4).The clinical efficacy and safety profile observed was consistent with thefindings of two phase3trials[FIXTURE (Full year Investigative eXamination of secukinumab vs eTanercept Using two dosing Regimens to determine Efficacy in psoriasis)and ERASURE(Efficacy of Response And Safety of twofixed secUkinumab REgimens in psoriasis)]using subcutaneous doses of secukinumab (15).In combination with the results of other trials exploring anti-bodies directed against IL-17A(5)or the IL-17receptor A chain (7),these studies document the potential of IL-17blockade as a new therapeutic approach in moderate-to-severe plaque psoriasis.Current concepts used to explain the efficacy of anti-IL-17ther-apies favour a T-lymphocytic immune response and the crosstalk between dendritic cells and Th17cells as the driving elements in psoriasis(2).Th17cells produce,among other cytokines,different members of the IL-17family,such as IL-17A and IL-17F,which are overexpressed in active plaques(16),and their differentiation and maturation is thought to critically depend on IL-23(17). Within this paradigm,the principal interpretation of the high lev-els of clinical improvement of psoriasis in response to treatment with ustekinumab(which targets the p40molecule shared by IL-12and IL-23),as well as of the emerging data indicating even higher response rates for antibodies against IL-17A(blocking IL-17A homodimers and IL-17A/F heterodimers)and IL-17receptor A(potentially blocking IL-17A,IL-17AF,IL-17F,IL-17C and IL-17E),has been that these therapies antagonize major effector cytokines related to the IL-23/Th17axis(18).Until now,this view has remained largely unchallenged despite the identification of other cellular sources of IL-17,in particular mast cells and neu-trophils(10,11,19).Neutrophils have not been a primary focus of recent models of psoriasis pathophysiology,although they repre-sent a numerically dominant and characteristic component of the inflammatory infiltrate in this disease.In fact,the epidermal accu-mulation of neutrophils and formation of Munro’s microabscesses are histological hallmarks of psoriasis and likely reflect the pres-ence of innate immune mechanisms within the psoriasis inflam-matory cascade.Based on the investigation of large numbers of biopsies,the present study confirms earlierfindings of smaller studies (10,11,19)that the number of IL-17-containing T cells in active psoriasis is small(<10%of CD3-positive cells)and that neutro-phils,especially when Munro’s microabscesses are present,are the numerically dominant cell type with IL-17detectable by immuno-histochemistry.There has been some discussion of whether neutrophils only release preformed IL-17or actually synthesize IL-17mRNA and protein in the skin.In the present study,IL-17A mRNA was detected in T cells,but not in neutrophils isolated from psoriatic plaques(Fig.3d);thus,thesefindings would favour an IL-17pro-tein storage model.Expression of IL-17A mRNA in conjunction with ROR c t co-expression and release of IL-17by extracellular trap formation was,however,observed in epidermal neutrophils in a recent investigation of2models of human skin inflammation (20).The ability of neutrophils to produce IL-17A mRNA and protein under control of ROR c t in response to IL-6and IL-23, both of which are overexpressed in psoriatic plaques(21),was also demonstrated in another study investigating human andmouseneutrophils(22).Based on these differentfindings,it is not fully clear at present whether IL-17found in cutaneous neutrophils in active psoriasis is newly synthesized in loco or is contained pre-formed in neutrophils entering the skin,similar to what has been observed for other neutrophil-derived mediators such as TNF-a (23,24).While available data from other studies and the data pre-sented here suggest that neutrophils are a relevant source of IL-17 in psoriasis,further work is required to clarify whether,and at which stage of their development,or under which conditions,the synthesis of IL-17in neutrophils actually occurs.Based on ourfindings on the kinetics of changes in T cells, CD11c-positive dendritic cells,neutrophils and keratinocytes,as well as the expression of key cytokines and chemoattractive medi-ators following treatment with secukinumab,some relevant con-clusions can be drawn:The most prominent observation2weeks after a single infusion of the anti-IL-17A antibody secukinumab was a significant reduction in psoriatic epidermal abnormalities (hyperparakeratosis,acanthosis and hyperproliferation),together with strongly decreased mRNA expression levels of keratinocyte-derived chemokines GRO-a(CXCL1)and IL-8(CXCL8)and an almost complete clearance of epidermal IL-17-positive neutroph-ils.Secukinumab may therefore interfere with the influx of neu-trophils into psoriatic lesions indirectly by abrogating the effect of IL-17A on keratinocytes and other cells(e.g.endothelial cells) involved in neutrophil recruitment(25).In addition,secukinumab may target direct effects of IL-17A on neutrophil survival and acti-vation,as previously described(22,26).Early normalization of the epidermal microarchitecture could help explain the magnitude of the clinical improvement observed at Week2,with~60%of subjects treated with the highest dose of secukinumab achieving a PASI75response at that time point;a similar response pattern was observed for the anti-IL-17A antibody ixekizumab(5).It should also be noted that there was a substantial early reduction of mRNA expression levels of IFN c,and especially IL-17A and F,but not of TNF-a mRNA(Fig.2g).As we could detect IL-17A mRNA only in T cells isolated from psoriatic lesions,which are also regarded as the main source of IFN c,a possible explanation is an early inhibition of T-cell cytokine production by anti-IL-17A treatment.At Week12,secukinumab had also reduced the number of lesional CD11c-positive dendritic cells and T cells similar to what has been proposed to coincide with morefinal disease resolution during treatment with other targeted therapies for psoriasis(3). Following the early effects on epidermal changes,neutrophil influx and cytokine synthesis,this normalizing effect of secukinumab on relevant cells and mediators of adaptive skin immunity likely con-tributes to the observed sustainability of the clinical response,with disease control maintained in many subjects for>30weeks after the last infusion of the drug.Taken together,the results of the present study strengthen the view that neutrophils are a potential source of IL-17in psoriasis and newly identify these cells as an early cellular target of the novel class of IL-17-directed therapies.Although blockade of IL-8 and neutrophil apheresis have shown some effects in pustular vari-ants of psoriasis(27,28),it is unlikely that targeting of neutrophils or single neutrophil-chemotactic factors alone will be a successful therapeutic approach in plaque-type psoriasis.We propose a model(Fig.S7)in which the early response to anti-IL-17A antibodies such as secukinumab involves the interruption of a neutrophil–keratinocyte crosstalk in which IL-17derived from T cells,and potentially neutrophils,stimulates the epidermal pro-duction of chemokines that,in turn,orchestrate the further influx of neutrophils into psoriatic lesions,while the full and long-term clinical response is associated with the reduction of lesional den-dritic cells and T cells.The observed rapid epidermal clearance of neutrophils is considered to be an important element of IL-17A inhibition in psoriasis as these cells–through the release of medi-ators such as TNF-a,LL-37and IL-17A–perpetuate and enhance the abnormal defense programme characteristic of the disease (29–31).AcknowledgementsThis trial and publication were funded by Novartis Pharma AG,Basel, Switzerland.Editorial assistance was provided by BioScience Communica-tions,New York,NY,and supported by Novartis.Technical assistance was provided by Melanie Ceci,Aur e lie Seguin and Tiziana Valensise of Novartis Institutes for Biomedical Research,Basel,and by Martina Bresch,Beyhan Ertas and Martina Schroeder of the Dermatologikum Hamburg.Conflict of interestKR has served as a consultant or paid speaker for,or participated in clini-cal trials sponsored by,AbbVie,Amgen,Biogen-Idec,Celgene,Centocor, Covagen,Forward Pharma,GlaxoSmithKline,Janssen-Cilag,Leo,Lilly,Me-dac,MSD,Novartis,Pfizer,Takeda and Vertex.KAP has received grants and has consulted and served as an investigator for AbbVie,Amgen,Astel-las,Biogen-Idec,Celgene,Centocor,Eli Lilly,Forward Pharma,Fujisawa, GlaxoSmithKline,Janssen,Kyowa-Kirin,Leo,MSD,Novartis(outside the submitted work),Pfizer and Takeda.RTM has received grants/clinical trial stipends from Novartis.JHT served as a clinical investigator for Novartis during conduct of this study.RB received grants from Novartis during the conduct of this study and has received grants,personal fees and non-financial support from AbbVie,Amgen,Astellas,Celgene,Eli Lilly,Janssen, Pfizer and Tribute.MB has served as a clinical trial sponsor for Amgen,Eli Lilly and Novartis.DG has served as a clinical trial investigator for Novar-tis.RAK is a member of an advisory board for Novartis and several other pharmaceutical companies.YP has received grants from AbbVie,Amgen, Celgene,Eli Lilly,Janssen,Merck,Pfizer and Novartis(outside the submit-ted work).LAR,WMB,TMF and NAB-S declare no conflict of interests. GS has received grants/clinical trial payments from Janssen,MSD and Novartis(unrelated to secukinumab).JMS,US,TP,EK,GAW,FK and CCB are full-time employees of Novartis.WH and DML are full-time employees of and own stock in Novartis.MMS was a full-time employee of Novartis at the time the study was conducted and the manuscript prepared.Author contributionsKR,JMS,WH,DML,FK and CCB contributed to the study design.KR, KAP,RTM,JHT,RB,MB,DG,RAK,YP,LAR,GS,WMB,TMF,NAB-S, US,TP,EK and GAW contributed to data acquisition.TMF,US,MMS, TP,EK,GAW,FK and CCB contributed to data analysis.KR,TMF,US, MMS,TP,EK,DML,GAW,FK and CCB contributed to data interpreta-tion.KR,FK and CCB contributed to the writing of the manuscript.All authors provided critical review of the draft manuscript and approved submission of thefinal manuscript for publication.Ethical considerationsThe study was conducted according to the Declaration of Helsinki. The study protocol and all amendments were approved by the central independent ethics committees or institutional review boards in the partici-pating countries.All study subjects provided written informed consent for their participation.。