全英文病例报告表模板
- 格式:doc
- 大小:1.05 MB
- 文档页数:17

CASEMedical Number: 682786 General informationName: Wang Runzhen Rvenue, Hankou, Hubei. Age: Forty three Tel: 82422500Sex: Female Date of admission: Jan Race: Han11st, 2001Occupation: Teacher Date of record: 11Am, Nationality: China Jan 11st, 2001Marital status: Married Complainer of history: Address:NO.38,the patient herself Hangkong Road, Jiefang Reliability: ReliableChief complaint: Right breast mass found for more than half a month.Present illness:Half a month ago, the patient suddenly felt pain in her right chest when she put up her hand. After touching it, she found a mass in her right breast, but no tendness, and the patient didn ’payt attention it. Then the pain became more and more serious, so the patient went to tumour hospital and received a pathology centesis. Her diagnosis was breast cancer. Then she came to our hospital and asked for an operation.Since onset, her appetite was good, and both her spiritedness and physical energy are normal. Defecation and urination are normal, too.Past historyOperative history: Never undergoing any operation. Infectious history: No history of severe infectious disease.Allergic history: She was not allergic to penicillin or sulfamide.Respiratory system:No history of respiratory disease. Circulatory system: No history of precordial pain. Alimentary system: No history of regurgitation. Genitourinary system: No history of genitourinary disease.Hematopoietic system: No history of anemia and mucocutaneous bleeding.Endocrine system:No acromegaly. No excessive sweats. Kinetic system:No history of confinement of limbs. Neural system:No history of headache or dizziness. Personal historyShe was born in Wuhan on Nov 19th, 1957 and almost always lived in Wuhan. She graduated from senior high school. Her living conditions were good. No bad personal habits and customs.Menstrual history: The first time when she was 14. Lasting 3 to 4 days every times and its cycle is about 30 days.Obstetrical history: Pregnacy 3 times, once nature production, abortion twice.Contraceptive history: Not clear.Family history: His parents have both died.Physical examinationT 36.4℃, P 80/min, R 20/min, BP 90/60mmHg. She is well developed and moderately nourished. Active position. The skin was not stained yellow. No cyanosis. No pigmentation. No skin eruption. Spider angioma was not seen. No pitting edema. Superficial lymph nodes were notenlarged.HeadCranium: Hair was black and well distributed. No deformities. No scars. No masses. No tenderness.Ear: Bilateral auricles were symmetric and of no masses. No discharges were found in external auditory canals. No tenderness in mastoid area. Auditory acuity was normal.Nose: No abnormal discharges were found in vetibulum nasi. Septum nasi was in midline. No nares flaring. No tenderness in nasal sinuses.Eye: Bilateral eyelids were not swelling. No ptosis. No entropion. Conjunctiva was not congestive. Sclera was anicteric. Eyeballs were not projected or depressed. Movement was normal. Bilateral pupils were round and equal in size. Direct and indirect pupillary reactions to light were existent.Mouth: Oral mucous membrane was smooth, and of no ulcer or erosion. Tongue was in midline. Pharynx was not congestive. Tonsils were not enlarged.Neck: Symmetric and of no deformities. No masses. Thyroid was not enlarged. Trachea was in midline.ChestChestwall: Veins could not be seen easily. No subcutaneous emphysema. Intercostal space was neither narrowed nor widened. No tenderness.Thorax: Symmetric bilaterally. No deformities.Breast: Symmetric bilaterally. Neither nipples nor skin were retracted. Elasticity was fine.Lungs: Respiratory movement was bilaterally symmetric with the frequency of 20/min. Thoracic expansion and tactile fremitus were symmetric bilaterally.No pleural friction fremitus. Resonance was heard during percussion. No abnormal breath sound was heard. No wheezes. No rales.Heart: No bulge and no abnormal impulse or thrills in precordial area. The point of maximum impulse was in 5th left intercostal space inside of the mid clavicular line and not diffuse. No pericardial friction sound. Border of the heart was normal. Heart sounds were strong and no splitting. Rate 80/min. Cardiac rhythm was regular. No pathological murmurs.Abdomen: Flat and soft. No bulge or depression. No abdominal wall varicosis. Gastralintestinal type or peristalses were not seen. There was not tenderness and rebound tenderness on abdomen or renal region. Liverwas not reached. Spleen was not enlarged. No masses. Fluidthrill negative. Shifting dullness negative. Borhorygmus 5/min. No vascular murmurs.Extremities: No articular swelling. Free movements ofall limbs.Neural system: Physiological reflexes were existent without any pathological ones.Genitourinary system: Not examed.Rectum: not exanedInvestigationNo.Professional Examination There are a about 3*3*2 cm mass in outer-up field of her right breast. It is hard but no tendness. It can be moved and its surface is smooth. The skin of her breast is normal. Corresponding superficial lymph nodes don’ t enlarge.History summary1.Patient was a teacher, female, 43 years old.2.Right breast mass found for more than half a month.3.No special past history.4.Physical examination showed no abnormity in lung, heart and abdoman. Information about her breast can be seen above.5.Shorting of investigation information.Impression: Breast cancer (right)Signature: He Lin(95-10033)。
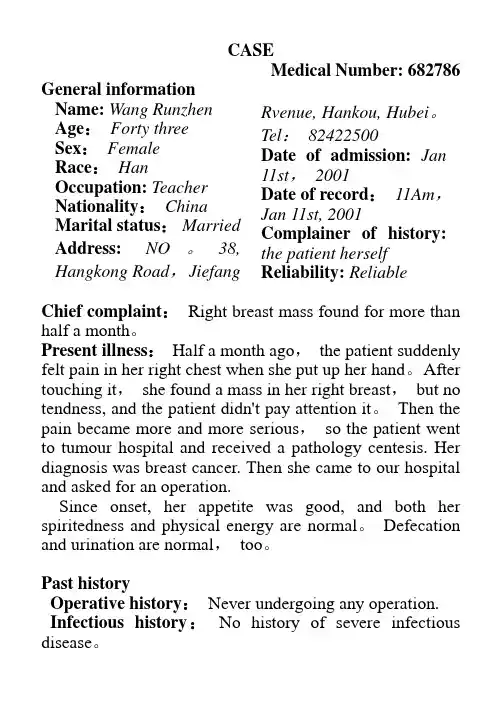
CASEMedical Number: 682786 General informationName:Wang Runzhen Age:Forty three Sex:FemaleRace:Han Occupation: Teacher Nationality:China Marital status:Married Address: NO。
38, Hangkong Road,Jiefang Rvenue, Hankou, Hubei。
Tel:82422500Date of admission:Jan 11st,2001Date of record:11Am,Jan 11st, 2001 Complainer of history: the patient herself Reliability: ReliableChief complaint:Right breast mass found for more than half a month。
Present illness:Half a month ago,the patient suddenly felt pain in her right chest when she put up her hand。
After touching it,she found a mass in her right breast,but no tendness, and the patient didn't pay attention it。
Then the pain became more and more serious,so the patient went to tumour hospital and received a pathology centesis. Her diagnosis was breast cancer. Then she came to our hospital and asked for an operation.Since onset, her appetite was good, and both her spiritedness and physical energy are normal。

病例报告英语范文深度解析与中文对照**[English Version]****Case Report: Unusual Manifestations of Acute Appendicitis in a Pediatric Patient****Abstract** This case report presents an unusual case of acute appendicitis in a 12-year-old male patient. The patient presented with atypical symptoms, making the initial diagnosis challenging. The aim of this report is to highlight the importance of clinical suspicion and thorough investigation in diagnosing uncommon presentations of common conditions.**Introduction** Acute appendicitis is a common surgical emergency, typically presenting with right lower quadrant abdominal pain, fever, and leukocytosis. However, atypical presentations are not uncommon, especially in pediatric patients. This case report describes an instance where the classic symptoms were absent, leading to delayed diagnosis.**Case Presentation** A 12-year-old male patient presented to the emergency department with a history ofvague abdominal discomfort for the past three days. Thepain was intermittent and located in the epigastric region, radiating to the back. The patient had no history of fever, vomiting, or changes in bowel habits. Physical examination revealed mild tenderness in the epigastric region, with no rebound tenderness or guarding. Laboratory tests were remarkable for a mildly elevated white blood cell count.Initial differential diagnosis included gastroenteritis, urinary tract infection, and pancreatitis. However, due to persistent abdominal discomfort and the presence of mild leukocytosis, the possibility of appendicitis was entertained. Abdominal ultrasound revealed a distended appendix with peri-appendiceal fluid, confirming the diagnosis of acute appendicitis.**Discussion** This case highlights the challenges in diagnosing acute appendicitis in pediatric patients, especially when the classic symptoms are absent. Clinicians must maintain a high index of suspicion, consideringatypical presentations, especially in children. Detailed history, thorough physical examination, and appropriatediagnostic testing are crucial in making an accurate diagnosis.**Conclusion** Acute appendicitis can present with atypical symptoms in pediatric patients, making diagnosis challenging. Clinicians should be aware of these presentations and utilize diagnostic tools such as ultrasound to aid in the prompt and accurate diagnosis of appendicitis. Prompt surgical intervention is essential to prevent complications and ensure patient recovery.**[Chinese Version]****病例报告:儿童急性阑尾炎的非典型表现****摘要** 本病例报告介绍了一名12岁男性患者的急性阑尾炎非典型表现。

2、心内科常用英文病历模板第二节心内科常用英文病历模板熟练地阅读和书写英文病历是一名临床医师需要具备的基本外语技能。
对英文病历的熟练掌握对于阅读英文文献和撰写英文论文都有很大的帮助。
本章主要介绍心内科常见疾病英文病历的格式和基本模板。
英文病历的书写格式大致与中文病历相似,主要包括以下部分:1.General information(一般情况)2.Chief complaint(主诉)3.Present illness(现病史)4.Past history(既往史)5.Personal history(个人史)6.Family history(家族史)7.Physical examination(体格检查)8.Investigation(辅助检查)9.History summary(病史特点)10.Impression(印象、初步诊断)11.Signature(签名)鉴于不同疾病的病历之间存在共性,本章按照病历的通用部分和心血管内科部分逐一进行介绍。
第一部分通用部分1. General information(一般情况)这一部分包括name(姓名),age(年龄),sex(性别),race(民族),nationality(国籍),address(地址和电话),occupation(职业),marital status(婚姻状况),date of admission(入院日期),date of record(记录日期),complainer of history(供史者)和reliability(可信度)等12项内容。
基本格式如下:Name:Liu SideAge: EightySex: MaleRace:HanNationality:China Address: NO.35, Dandong Road, Jiefang Rvenue, Hankou, Hubei. Tel: 857307523 Occupation: Retired Marital status: Married Date of admission:Aug 6th, 2001Date of record: 11Am, Aug 6th, 2001Compl ainer of history: patient’s son and wife Reliability: Reliable2. Past history(既往史)这一部分应首先总结既往一般健康状况、Operative history(手术史)、Infectious history(传染病史)、Allergic history(过敏史)等,然后对各系统健康状况进行回顾,包括Respiratory system(呼吸系统)、Circulatory system (循环系统)、Alimentary system (消化系统)、Genitourinary system(泌尿生殖系统)、Hematopoietic system(血液系统)、Endocrine system(内分泌系统)、Kinetic system(运动系统)和Neural system(神经系统)。

英文病历标准模版Patient ProfileName: Si RuihuaDepartment: ___ Power ___Sex: FemalePresent Address: Electric Power Bureau Age: 80 yearsDate of n: May 17.2003nality: Chinese XinjiangDate of Record: May 17.2003Marital Status: MarriedReliability: Reliablen: Family ___History of Allergy: None reportedChief Complaints___。
breathlessness。
and precordial pain for the last hour。
There were no precipitating factors。
and the fort could not be relieved by rest。
As a result。
she came to the hospital for help。
She did not experience syncope。
cough。
headache。
diarrhea。
or vomiting during the course of the illness。
Her appetite。
sleep。
voiding。
and stool were normal.Medical History___.______。
___ distress。
She had a heart rate of 120 beats per minute and a blood pressure of 160/90 mmHg。
Her respiratory rate was 28 breaths per minute。
and her oxygen n was 90% on room air。

Clinical Report Template for High School English BackgroundClinical reports are an important part of the healthcare profession. It is essential that healthcare professionals, including high school students studying health science, understand the fundamental structure and language used in clinical reports. This report aims to provide a template for writing a clinical report in English for high school students.IntroductionThe introduction should include the patient’s age, gender, and relevant medical history. It should also include a brief summary of the purpose of the clinical report and the main findings. It is important to use clear and concise language in this section. For example:___[Patient’s Name], a [age]-year-old [gender] with a history of [medical history], was admitted for [reason for admission]. The purpose of this clinical report is to provide an overview of their condition and to present the main findings.DiagnosisThe diagnosis section should state the patient’s diagnosis, including any relevant medical terms. It should also include a brief explanation of the diagnosis so that it is easily understood. For example:___The patient has been diagnosed with diagnosis, which is a [brief explanation of diagnosis].Clinical CourseThe clinical course section should detail the p atient’s progression throughout their hospitalisation or treatment. It should include important clinical events, such as changes in vital signs and medication administration. The objective is to give a detailed overview of the patient’s treatment and poten tial outcomes. This section should be written in a chronological order. For example:___On the third day of their hospitalisation, [Patient’s Name]’s blood pressure spiked, and they presented with [symptom]. The patient was given [medication], and their bl ood pressure was monitored closely. On the fifth day, the patient’s condition improved, and they were discharged with instructions to follow up with their primary care provider.Discussion and ConclusionThis section should summarise the main findings of the clinical report. It should also include an interpretation of the findings, a discussion of any potential complications, and recommendations for follow-up care. It is important to use appropriate medical terminology and to present information that is supported by findings in the diagnosis and clinical course sections. For example:___In conclusion, [Patient’s Name]’s diagnosis was diagnosis, and their clinical course was characterised by [important events]. While [potential complications] are a concern, [recommendations for follow-up care] are essential to a positive outcome.ReferencesIt is important to provide any references consulted in the clinical report, including any specific guidelines or clinical trials. References should be cited in the appropriate format, either in-text or in a bibliography at the end of the report.ConclusionThis template provides a basic structure for clinical reports in English for high school students. It is important to utilise clear and concise language and to utilise medical terminology appropriately. By adhering to this template, students will be able to produce clinical reports that will prove valuable to healthcare professionals.。

Divisio n: Ward: Bed: Case No.Name: ______________ S ex: __________ Age: ___________ Natio n: __________ Birth Place: _______________________________ Marital Status: ___________ Work-orga nizatio n & Occupatio n: _____________________________________ Livi ng Address & Tel: ________________________________________________ Date of admissio n: ______ Date of history taken: _______ Informant: _________Chief Complaint: ___________________________________________ History of Present Illness:Past History:General Health Status: 1.good 2.moderate 3.poorDisease history :(if any, please write dow n the date of on set, brief diag no sticand therapeutic course, and the results.)Respiratory system:1. None2.Repeated pharyngeal pain3.chronic cough4.expectoration:5. Hemoptysis6.asthma7.dysp nea8.chest pa inCirculatory system:1.N one2.Palpitatio n3.exerti onal dysp nea4..cya no sis5.hemoptysis6. Edema of lower extremities7.chest pain8.s yn cope9.hypertensionDigestive system:1.None2.Anorexia3.dysphagia4.sour regurgitation5.eructation6.nausea7.Emesis8.melena9.abdominal pain 10.diarrhea11. hematemesis 12.Hematochezia 13.ja un diceUrinary system:1.N one2.Lumbar pain3.uri nary freque ncy4.uri nary urge ncy5.dysuria6.oliguria7.polyuria8.retention of urine9.ineontinence of urine10.hematuria ll.Pyuria 12.n octuria 13.puffy faceHematopoietic system:1.N one2.Fatigue3.dizz in ess4.gi ngival hemorrhage5.epistaxis6.subcuta neous hemorrhageMetabolic and endocrine system:1.None2.Bulimia3.anorexia4.hot intoleranee5.cold intoleranee6.hyperhidrosis7.Polydipsia8.amenorrhea9.tremor of hands 10.character cha nge II.Marked obesity12. marked emaciati on 13.hirsutism 14.alopecia15.Hyperpigme ntatio n 16.sexual fun cti on cha ngeNeurological system:1.N one2.Dizz in ess3.headache4.paresthesia5.hypo mn esis6. Visual disturbanee7.lnsomnia8.somnolence9.s yn cope 10.c onv ulsi on II.Disturba nee of con scious ness12.paralysis 13. vertigoReproductive system:1.No ne2.othersMusculoskeletal system:1.None2.Migrating arthralgia3.arthralgia4.artrcocele5.arthremia6.Dysarthrosis7.myalgia8.muscular atrophyInfectious Disease:1.N one2.Typhoid fever3.Dyse ntery4.Malaria 4.Schistosomiasis4. Leptospirosis 7.Tuberculosis 8.Epidemic hemorrhagic fever9.othersVaccine inoculation:1.No ne2.Yes3.Not clearVacci ne detail _________________________________________ Trauma and/or operation history: Operations:1.No ne2.YesOperati on details: ______________________________________ Traumas:1.No ne2.YesTrauma details: ________________________________________ Blood transfusion history:1.None2.Yes ( 1.Whole blood 2.Plasma3.Ingredient transfusion)Blood type: ___________ Tran sfusi on time: _________Tran sfusi on react ion1.None2.YesCli nic manifestation: ___________________________ Allergic history:1.No ne2.Yes3.Not clearallergen: _______________________________________________cli nical manifestation: ____________________________________Personal history:Custom living addres ____________________________________________ Reside nt history in en demic disease area: _________________________ Smoking: 1.No 2.YesAverage ___ p ieces per day; about yearsGivi ng-up 1.No 2.Yes (Time: _______________________ ) Drinking: 1.No 2.YesAverage ___ g rams per day; about ___ y earsGivi ng-up 1.No 2.Yes(Time: ________________________ ) Drug abuse :1.No 2.YesDrug names: ______________________________________Marital and obstetrical history:Married age: __________ y ears old Pregnancy _____________ timesLabor _______________ t imes(〔.Natural labor: ______ times 2.0perative labor: _________ times3. ___________________ Natural abortion: ______________ times4.Artificial abortion: _____ times5. _______________________ Premature labor: _________ t imes6.stillbirth _________________ times)Health status of the Mate:1.Well2.Not fineDetails: _______________________________________________ Menstrual history:Menarchal age: ______ Duration ________ day Interval _______ daysLast menstrual period: ___________ Menopausal age: ______ y ears oldAmount of flow: 1.small 2. moderate 3. large Dysmenorrheal: 1. prese nee2.abse nc M enstrual irregularity 1. No 2.YesFamily history: (especially pay atte ntio n to the in fectious and hereditary diseaserelated to the present illness)Father: l.healthy 2.ill: ________ 3.deceased cause: ____________________ Mother:1.healthy 2.ill: ________ 3.deceased cause: ____________________ Others: _______________________________________________________The an terior stateme nt was agreed by the in forma nt.Sig nature of in forma nt: Datetime:Physical ExaminationVital signs:Temperature: _____ C Blood pressure: / ______ mmHgPulse: ____ bpm (l.regular 2.irregular ) Respirati on: _ bpm (l.regular 2.irregular )General conditions:Development:I.Normal 2.Hypoplasia 3.HyperplasiaNutrition: l.good 2.moderate 3.poor 4.cachexiaFacial expression 1. no rmal 2.acute 3.chro nic other __________________ Habitus: l.asthenic type 2.sthenic type 3.ortho-thenic typePosition: l.active 2.positive pulsive 4.other ______________________ Consciousness l .clear 2.somnolence 3.confusion 4.stupor 5.slight coma6. mediate coma7.deep coma8.deliriumCooperation: 1Yes 2.No Gait: l.normal 2.abnormal _____Skin and mucosa:Color: 1.no rmal 2.pale 3.red ness 4.cya no sis 5.ja un dice 6.pigme ntatio nSkin eruption:1.No 2.Yes( type: _________ distribution: _________________ ) Subcutaneous bleeding:.no 2.yes (type: ____ distribution: _____________ ) Edema:1. no 2.yes ( locati on and degree _______________________________ ) Hair: 1.no rmal 2.abnormal(details ___________________________________ ) Temperature and moisture:no rmal cold warm dry moist dehydrati on Liverpalmar : 1.no 2.yes Spider angioma(location: ________________ ) Others: __________________________________________________________Lymph nodes: enlargement of superficial lymph node:1. no2.yesDescripti on: _______________________________________________Head:Skull size1. no rmal 2.ab no rmal (descriptio n: ________________________ ) Skull shape d. no rmal 2.abnormal(description: ________________________ ) Hair distribution :1. no rmal 2.abnormal(description: ____________________ ) Others: __________________________________________________________ Eye: exophthalmos: __________ e yelid: ___________ conjunctiva: _________ sclera: ________________ C ornea: _____________________Pupil: l.equally round and in size 2.un equal (R _____ mm L _______ mm)Pupil reflex: l.normal 2.delayed (R ___ s L __ s ) 3.abse nt (R _ L ___ )others: ______________________________________________________ Ear: Auricle l.normal 2.desformatio n (description: ______________________ ) Discharge of exter nal auditory can al:1. no 2.yes (l.left 2.right quality: ___ )Mastoid tendern ess 1.no 2.yes (l.left 2.right quality: _________________ )Disturba nee of auditory acuity:1. no 2.yes(1」eft 2.right description: ___ ) Nose: Flari ng of alae n asi :1. no 2.yes Stuffy discharge 1.no 2.yes(quality __ ) Tendern ess over para nasal sinu ses:1. no 2.yes (location: ____________ ) Mouth: Lip _____________ Mucosa ___________ Tongue ________________ Teeth:1.normal 2.Agomphiasis 3. Eurodontia 4.others: ____________________Gum :1. normal 2.ab no rmal (Descripti on ________________________ )Tonsil: __________________________ Pharynx: _____________________Sound: 1.no rmal 2.hoarse ness 3.others: ____________________________Neck:Neck rigidity 1. no 2.yes ( tra nsvers fin gers)Carotid artery: 1.normal pulsation 2.increased pulsation 3.marked distentionTrachea location: l.middle 2.deviati on (1.1eftward _______ 2.rightward _____ ) Hepatojugular vein reflux: 1. n egative 2.positiveThyroid: 1.normal 2.enlarged _______ 3.bruit (1.no 2.yes ________________ )Chest:Chest wall: 1.normal 2.barrel chest 3.prominence or retraction:(left _______ right ________ Precordial prominence _________ ) Percussion pain over sternum1.No 2.YesBreast: 1.Normal 2.ab no rmal _____________________________________Lung: Inspection: respiratory movement 1.normal 2.abnormal ___________ Palpation: vocal tactile fremitus:1. no rmal 2.ab no rmal ____________pleural rubb ing sen sati on :1. no 2.yes___________________Subcuta neous crepitus sen sati on :1. no 2.yes ____________ Percussion:1resonance 2. Hyperresonance &location ____________3 Flatness&location ________________________________4. dulln ess & location: _____________________________5. tympa ny &location: _____________________________lower border of lung: (detailed percussi on in respiratory disease)midclavicular line : R: ____ in tercostae L: ____ in tercostaemidaxillary line: R: ____ in tercostae L: ____ in tercostaescapular li ne: R: _______ in tercostae L: ____ in tercostaemoveme nt of lower borders:R: ______ c mL: _________cm Auscultation: Breath ing sound : 1.no rmal 2.ab no rmal ____________Rales:1. no 2.yes ________________________________ Heart: lnspection:Apical pulsati on: 1. normal 2.un see n 3.i ncrease 4.diffuseSubxiphoid pulsation: 1.no 2.yesLocati on of apex beat: 1. no rmal 2.shift ( _____in tercosta,dista nee away from left MCL _____ cm) Palpation:Apical pulsation:1. normal 2.lifting apex impulse 3.negative pulsationThrill:1. no 2.yes(location: __________ phase: ________________ )Percussion relative dullness border: 1.normal 2.abnormal(Dista nee betwee n An terior Medli ne and left MCL ____ cm) Auscultation: Heart rate: _ bpm Rhythm:1.regular 2.irregular ______Heart sound: 1.no rmal 2.abnormal ______________________Extra sound: 1.no 2.S3 3.® 4. opening snapP2 ____________ A _________ Pericardial frictio n soun d:1. no 2.yesMurmur: 1.no 2.yes (location ___________ p hase ____________quality ____ intensity ________ t ran smissio n _________effects of position ________________________________effects of respiration _____________________________Peripheral vascular signs1.N one2.paradoxical pulse3.pulsus alter nans4. Water hammer pulse5.capillary pulsati on6.pulse deficit7.Pistol shot sound8.Duroziezsig nAbdomen:lnspection:Shape: 1.normal 2.protuberanee 3.scaphoid 4.frog-belly Gastric patter n 1. no 2.yes In test inal pattern 1. no 2.yesAbdo minal vein varicosis 1.no 2.yes(direction: ____________ )Operatio n scarl. no 2.yes _______________________________ Palpation: l.soft 2. tensive (location: _____________________________ )Tendern ess: 1.no 2.yes(location: ______________________ )Rebo und tendern ess:1. no 2.yes(location: _____________ ) Fluctuatio n: l.prese nt 2.absce ntSuccussi on splash: 1.n egative 2.positiveLiver: ______________________________________________Percussion Liver dullness border: 1.normal 2.decreased 3.absentUpper hepatic border:Right Midclavicular Line _______ In tercostaShift dullness:1.negative 2.positive Ascites: _____________ degreePai n on percussi on in costovertebral area: 1.n egative 2.positve __ Auscultation: Bowel sounds : 1.no rmal 2.hyperperistalsis 3.hypoperistalsis4.abse nee Gurgli ng soun d:1. no 2.yesVascular bruit 1.no 2.yes (location ____________________ ) Genital organ: 1.un exam ined 2. no rmal 3.ab no rmalAnus and rectum: 1.un exam ined 2.no rmal 3.ab no rmalSpine and extremities:Spine: 1.no rmal 2.deformity (l.kyphosis 2.lo rdosis 3.scoliosis)3.Tenderness(location ____________________________ )Extremities: 1.no rmal 2.arthremia & arthrocele (location _________________ )3.A nkylosis (location _________ )4.Aropachy: 1.no 2.yes5.Muscular atrophy (location _____________________ ) Neurological system!:, normal 2.abnormal ____________________________Important examination results before hospitalizedSummary of the history: ______________________________________ Initial diagnosis: ____________________________________________Recorder:Corrector:。

英文病例报告作文模板下载温馨提示:该文档是我店铺精心编制而成,希望大家下载以后,能够帮助大家解决实际的问题。
文档下载后可定制随意修改,请根据实际需要进行相应的调整和使用,谢谢!并且,本店铺为大家提供各种各样类型的实用资料,如教育随笔、日记赏析、句子摘抄、古诗大全、经典美文、话题作文、工作总结、词语解析、文案摘录、其他资料等等,如想了解不同资料格式和写法,敬请关注!Download tips: This document is carefully compiled by theeditor. I hope that after you download them,they can help yousolve practical problems. The document can be customized andmodified after downloading,please adjust and use it according toactual needs, thank you!In addition, our shop provides you with various types ofpractical materials,such as educational essays, diaryappreciation,sentence excerpts,ancient poems,classic articles,topic composition,work summary,word parsing,copyexcerpts,other materials and so on,want to know different data formats andwriting methods,please pay attention!I went to the doctor today. I was feeling really bad. Had a headache and a stomachache.The doctor asked me a bunch of questions. Like what I ate, how much I slept.I told him about my symptoms. He said it could be something or nothing.Then he gave me some pills. Told me to take them and see how I feel.I hope I feel better soon. This sucks.Another time, I had a rash. It was itchy and red.Went to the doctor again. He looked at it and said it might be an allergic reaction.I couldn't figure out what I was allergic to. But he gave me some cream to put on.That seemed to help a little bit. But it still took a while to go away.。

英语病历模板范文Patient Identification:Date of Birth: [DOB]Sex: [Male/Female]Patient ID: [Unique Identifier]Chief Complaint:[Patient's primary concern or reason for the visit, e.g., "Severe headache for the past 3 days"]History of Present Illness:[Detailed account of the onset, duration, severity, and any associated symptoms of the current illness. Include any treatments already attempted.]Past Medical History:[List any previous medical conditions, surgeries, or hospitalizations.]Medications:[List all current medications, including dosages andfrequency.]Allergies:[Note any known allergies to medications, foods, or environmental factors.]Family Medical History:[Provide information on any significant medicalconditions in the patient's family.]Social History:[Include relevant lifestyle factors such as smoking status, alcohol consumption, exercise habits, and occupation.]Review of Systems:[Briefly summarize the patient's current state inrelation to various body systems, e.g., "No chest pain, no shortness of breath."]Physical Examination:[Record findings from the physical examination, including vital signs, general appearance, and specific observations related to the chief complaint.]Assessment:[Summarize the likely diagnosis or condition based on the information gathered.]Plan:[Outline the proposed treatment plan, including medications, referrals, follow-up appointments, and any necessary tests or procedures.]。


Name: ______________ Sex: __________ Age: ___________ Nation: ___________ Birth Place: ________________________________ Marital Status:____________ Work-organization & Occupation: _______________________________________ Living Address & Tel: _________________________________________________ Date of admission: _______Date of history taken:_______ Informant:__________ Chief Complaint: ___________________________________________________History of Present Illness:__________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________Past History:General Health Status: 1.good 2.moderate 3.poorDisease history: (if any, please write down the date of onset, brief diagnosticand therapeutic course, and the results.)Respiratory system:1. None2.Repeated pharyngeal pain3.chronic cough4.expectoration:5. Hemoptysis6.asthma7.dyspnea8.chest pain_______________________________________________________________ Circulatory system:1.None2.Palpitation3.exertional dyspnea4..cyanosis5.hemoptysis6.Edema of lower extremities7.chest pain8.syncope9.hypertension _______________________________________________________________ Digestive system:1.None2.Anorexia3.dysphagia4.sour regurgitation5.eructation6.nausea7.Emesis8.melena9.abdominal pain 10.diarrhea11.hematemesis 12.Hematochezia 13.jaundice_______________________________________________________________ Urinary system:1.None2.Lumbar pain3.urinary frequency4.urinary urgency5.dysuria6.oliguria7.polyuria8.retention of urine9.incontinence of urine10.hematuria 11.Pyuria 12.nocturia 13.puffy face_______________________________________________________________ Hematopoietic system:1.None2.Fatigue3.dizziness4.gingival hemorrhage5.epistaxis6.subcutaneous hemorrhage_______________________________________________________________ Metabolic and endocrine system:1.None2.Bulimia3.anorexia4.hot intolerance5.cold intolerance6.hyperhidrosis7.Polydipsia8.amenorrhea9.tremor of hands 10.character change 11.Marked obesity12.marked emaciation 13.hirsutism 14.alopecia15.Hyperpigmentation 16.sexual function change_______________________________________________________________ Neurological system:1.None2.Dizziness3.headache4.paresthesia5.hypomnesis6. Visual disturbance7.Insomnia8.somnolence9.syncope 10.convulsion 11.Disturbance of consciousness12.paralysis 13. vertigo_______________________________________________________________ Reproductive system:1.None2.others_______________________________________________________________ Musculoskeletal system:1.None2.Migrating arthralgia3.arthralgia4.artrcocele5.arthremia6.Dysarthrosis7.myalgia8.muscular atrophy_______________________________________________________________ Infectious Disease:1.None2.Typhoid fever3.Dysentery4.Malaria 4.Schistosomiasis4.Leptospirosis 7.Tuberculosis 8.Epidemic hemorrhagic fever9.others_______________________________________________________________ Vaccine inoculation:1.None2.Yes3.Not clearVaccine detail __________________________________________ Trauma and/or operation history:Operations:1.None2.YesOperation details:_______________________________________ Traumas:1.None2.YesTrauma details:_________________________________________ Blood transfusion history:1.None2.Yes ( 1.Whole blood 2.Plasma3.Ingredient transfusion)Blood type:____________ Transfusion time:___________Transfusion reaction1.None2.YesClinic manifestation:_____________________________ Allergic history:1.None2.Yes3.Not clearallergen:________________________________________________clinical manifestation:_____________________________________Personal history:Custom living address:____________________________________________ Resident history in endemic disease area:_____________________________ Smoking: 1.No 2.YesAverage ___pieces per day; about___yearsGiving-up 1.No 2.Yes (Time:_______________________) Drinking: 1.No 2.YesAverage ___grams per day; about ___yearsGiving-up 1.No 2.Yes(Time:________________________) Drug abuse:1.No 2.YesDrug names:_______________________________________ _______________________________________________________________Marital and obstetrical history:Married age: __________years old Pregnancy ___________timesLabor _______________times(1.Natural labor: _______times 2.Operative labor: ________times3.Natural abortion: ______times4.Artificial abortion: _______times5.Premature labor:__________times6.stillbirth__________times)Health status of the Mate:1.Well2.Not fineDetails: _______________________________________________ Menstrual history:Menarchal age: _______ Duration ______day Interval ____daysLast menstrual period: ____________ Menopausal age: ____years oldAmount of flow: 1.small 2. moderate 3. largeDysmenorrheal: 1. presence 2.absence Menstrual irregularity 1. No 2.Yes Family history: (especially pay attention to the infectious and hereditary diseaserelated to the present illness)Father: 1.healthy 2.ill:________ 3.deceased cause: ___________________ Mother:1.healthy 2.ill:________ 3.deceased cause: ___________________ Others: ________________________________________________________ The anterior statement was agreed by the informant.Signature of informant: Datetime:Physical ExaminationVital signs:Temperature:______0C Blood pressure:_______/_______mmHg Pulse: _____ bpm (1.regular 2.irregular_____________________________) Respiration: ___bpm (1.regular 2.irregular____________________________) General conditions:Development: 1.Normal 2.Hypoplasia 3.HyperplasiaNutrition: 1.good 2.moderate 3.poor 4.cachexiaFacial expression: 1.normal 2.acute 3.chronic other_____________________ Habitus: 1.asthenic type 2.sthenic type 3.ortho-thenic typePosition: 1.active 2.positive pulsive 4.other_______________________ Consciousness: 1.clear 2.somnolence 3.confusion 4.stupor 5.slight coma6.mediate coma7.deep coma8.deliriumCooperation: 1Yes 2.No Gait: 1.normal 2.abnormal______Skin and mucosa:Color: 1.normal 2.pale 3.redness 4.cyanosis 5.jaundice 6.pigmentationSkin eruption:1.No 2.Yes( type: __________distribution:__________________) Subcutaneous bleeding: 1.no 2.yes (type:_______distribution:______________) Edema:1. no 2.yes ( location and degree________________________________) Hair: 1.normal 2.abnormal(details_____________________________________) Temperature and moisture: normal cold warm dry moist dehydration Liver palmar : 1.no 2.yes Spider angioma (location:________________) Others: __________________________________________________________ Lymph nodes: enlargement of superficial lymph node:1.no2.yesDescription: ________________________________________________ Head:Skull size:1.normal 2.abnormal (description:____________________________) Skull shape:1.normal 2.abnormal(description:___________________________) Hair distribution :1.normal 2.abnormal(description:______________________) Others:___________________________________________________________ Eye: exophthalmos:___________eyelid:____________conjunctiva:__________ sclera:________________Cornea:_______________________Pupil: 1.equally round and in size 2.unequal (R______mm L_______mm)Pupil reflex: 1.normal 2.delayed (R___s L___s ) 3.absent (R___L___)others:______________________________________________________ Ear: Auricle 1.normal 2.desformation (description:_______________________) Discharge of external auditory canal:1.no 2.yes (1.left 2.right quality:_____)Mastoid tenderness 1.no 2.yes (1.left 2.right quality:__________________)Disturbance of auditory acuity:1.no 2.yes(1.left 2.right description:_______) Nose: Flaring of alae nasi :1.no 2.yes Stuffy discharge 1.no 2.yes(quality______) Tenderness over paranasal sinuses:1.no 2.yes (location:_______________) Mouth: Lip______________Mucosa_____________Tongue________________ Teeth:1.normal 2. Agomphiasis 3. Eurodontia 4.others:____________________Gum :1.normal 2.abnormal (Description____________________________)Tonsil:___________________________Pharynx:_____________________Sound: 1.normal 2.hoarseness 3.others:_____________________________ Neck:Neck rigidity 1.no 2.yes (______________transvers fingers)Carotid artery: 1.normal pulsation 2.increased pulsation 3.marked distention Trachea location: 1.middle 2.deviation (1.leftward_______2.rightward______) Hepatojugular vein reflux: 1. negative 2.positiveThyroid: 1.normal 2.enlarged _______ 3.bruit (1.no 2.yes ________________) Chest:Chest wall: 1.normal 2.barrel chest 3.prominence or retraction:( left________right_________Precordial prominence__________) Percussion pain over sternum 1.No 2.YesBreast: 1.Normal 2.abnormal _______________________________________ Lung:Inspection: respiratory movement 1.normal 2.abnormal_____________ Palpation: vocal tactile fremitus:1.normal 2.abnormal _______________ pleural rubbing sensation:1.no 2.yes______________________Subcutaneous crepitus sensation:1.no 2.yes________________ Percussion:1. resonance 2. Hyperresonance &location_____________3 Flatness&location_________________________________4. dullness & location:_______________________________5.tympany &location:_______________________________lower border of lung: (detailed percussion in respiratory disease) midclavicular line : R:_____intercostae L:_____intercostaemidaxillary line: R:______intercostae L:_____intercostaescapular line: R:______intercostae L:_____intercostaemovement of lower borders:R:_______cmL:__________cm Auscultation: Breathing sound : 1.normal 2.abnormal _______________Rales:1.no 2.yes__________________________________ Heart: Inspection:Apical pulsation: 1.normal 2.unseen 3.increase 4.diffuseSubxiphoid pulsation: 1.no 2.yesLocation of apex beat: 1.normal 2.shift (______ intercosta,distance away from left MCL______cm) Palpation:Apical pulsation:1. normal 2.lifting apex impulse 3.negative pulsationThrill:1.no 2.yes(location:___________ phase:_________________)Percussion: relative dullness border: 1.normal 2.abnormalAuscultation: Heart rate:___bpm Rhythm:1.regular 2.irregular_______ Heart sound: 1.normal 2.abnormal________________________Extra sound: 1.no 2.S3 3.S4 4. opening snapP2_________ A2_________Pericardial friction sound:1.no 2.yesMurmur: 1.no 2.yes (location____________phase_____________quality______intensity________ transmission___________effects of position_________________________________effects of respiration______________________________ Peripheral vascular signs:1.None2.paradoxical pulse3.pulsus alternans4. Water hammer pulse5.capillary pulsation6.pulse deficit7.Pistol shot sound8.Duroziez signAbdomen:Inspection: Shape: 1.normal 2.protuberance 3.scaphoid 4.frog-bellyGastric pattern 1.no 2.yes Intestinal pattern 1.no 2.yesAbdominal vein varicosis 1.no 2.yes(direction:______________ )Operation scar1.no 2.yes ________________________________ Palpation: 1.soft 2. tensive (location:____________________________)Tenderness: 1.no 2.yes(location:_______________________)Rebound tenderness:1.no 2.yes(location:________________)Fluctuation: 1.present 2.abscentSuccussion splash: 1.negative 2.positiveLiver:_______________________________________________Gallbladder: __________________Murphy sign:____________Spleen:______________________________________________Kidneys:____________________________________________Abdominal mass:______________________________________Others:______________________________________________ Percussion: Liver dullness border: 1.normal 2.decreased 3.absentUpper hepatic border:Right Midclavicular Line ________IntercostaShift dullness:1.negative 2.positive Ascites:_____________degreePain on percussion in costovertebral area: 1.negative 2.positve ____ Auscultation: Bowel sounds : 1.normal 2.hyperperistalsis 3.hypoperistalsis4.absence Gurgling sound:1.no 2.yesVascular bruit 1.no 2.yes (location_____________________) Genital organ: 1.unexamined 2.normal 3.abnormalAnus and rectum: 1.unexamined 2.normal 3.abnormalSpine and extremities:Spine: 1.normal 2.deformity (1.kyphosis 2.lordosis 3.scoliosis)3.Tenderness(location______________________________)Extremities:1.normal 2.arthremia & arthrocele (location_________________)3.Ankylosis (location__________)4.Aropachy: 1.no 2.yes5.Muscular atrophy (location_______________________) Neurological system:1.normal 2.abnormal_______________________________ _____________________________________________________________________ Important examination results before hospitalized___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ Summary of the history:______________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ Initial diagnosis:_____________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________Recorder:Corrector:。
CASEMedical Number: 682786General informationName: Wang RunzhenAge: Forty threeSex: FemaleRace: HanOccupation: TeacherNationality: ChinaMarital status: MarriedAddress: NO.38, Hangkong Road, Jiefang Rvenue, Hankou, Hubei. Tel: 82422500Date of admission: Jan 11st, 2001Date of record: 11Am, Jan 11st, 2001Complainer of history: the patient herselfReliability: ReliableChief complaint: Right breast mass found for more than half a month.Present illness: Half a month ago, the patient suddenly felt pain in her right chest when she put up her hand. After touching it, she found a mass in her right breast, but no tendness, and the patient didn’t pay attention it. Then the pain became more and more serious, so the patient went to tumour hospital and received a pathology centesis. Her diagnosis was breast cancer. Then she came to our hospital and asked for an operation.Since onset, her appetite was good, and both her spiritedness and physical energy are normal. Defecation and urination are normal, too.Past historyOperative history: Never undergoing any operation.Infectious history: No history of severe infectious disease.Allergic history: She was not allergic to penicillin or sulfamide.Respiratory system: No history of respiratory disease.Circulatory system: No history of precordial pain.Alimentary system: No history of regurgitation.Genitourinary system: No history of genitourinary disease.Hematopoietic system: No history of anemia and mucocutaneous bleeding.Endocrine system: No acromegaly. No excessive sweats.Kinetic system: No history of confinement of limbs.Neural system: No history of headache or dizziness.Personal historyShe was born in Wuhan on Nov 19th, 1957 and almost always lived in Wuhan. She graduated from senior high school. Her living conditions were good. No bad personal habits and customs.Menstrual history: The first time when she was 14. Lasting 3 to 4 days every times and its cycle is about 30 days.Obstetrical history: Pregnacy 3 times, once nature production, abortion twice.Contraceptive history: Not clear.Family history: His parents have both died.Physical examinationT 36.4℃, P 80/min, R 20/min, BP 90/60mmHg. She is well developed and moderately nourished. Active position. The skin was not stained yellow. No cyanosis. No pigmentation. No skin eruption. Spider angioma was not seen. No pitting edema. Superficial lymph nodes were not enlarged.HeadCranium: Hair was black and well distributed. No deformities. No scars. No masses. No tenderness. Ear: Bilateral auricles were symmetric and of no masses. No discharges were found in external auditory canals. No tenderness in mastoid area. Auditory acuity was normal.Nose: No abnormal discharges were found in vetibulum nasi. Septum nasi was in midline. No nares flaring. No tenderness in nasal sinuses.Eye: Bilateral eyelids were not swelling. No ptosis. No entropion. Conjunctiva was not congestive. Sclera was anicteric. Eyeballs were not projected or depressed. Movement was normal. Bilateral pupils were round and equal in size. Direct and indirect pupillary reactions to light were existent.Mouth: Oral mucous membrane was smooth, and of no ulcer or erosion. Tongue was in midline. Pharynx was not congestive. Tonsils were not enlarged.Neck: Symmetric and of no deformities. No masses. Thyroid was not enlarged. Trachea was in midline. ChestChestwall: Veins could not be seen easily. No subcutaneous emphysema. Intercostal space was neither narrowed nor widened. No tenderness.Thorax: Symmetric bilaterally. No deformities.Breast: Symmetric bilaterally. Neither nipples nor skin were retracted. Elasticity was fine.Lungs: Respiratory movement was bilaterally symmetric with the frequency of 20/min. Thoracic expansion and tactile fremitus were symmetric bilaterally. No pleural friction fremitus. Resonance was heard during percussion. No abnormal breath sound was heard. No wheezes. No rales.Heart: No bulge and no abnormal impulse or thrills in precordial area. The point of maximum impulse was in 5th left intercostal space inside of the mid clavicular line and not diffuse. No pericardial friction sound. Border of the heart was normal. Heart sounds were strong and no splitting. Rate 80/min. Cardiac rhythm was regular. No pathological murmurs.Abdomen: Flat and soft. No bulge or depression. No abdominal wall varicosis. Gastralintestinal type or peristalses were not seen. There was not tenderness and rebound tenderness on abdomen or renal region. Liver was not reached. Spleen was not enlarged. No masses. Fluidthrill negative. Shifting dullnessnegative. Borhorygmus 5/min. No vascular murmurs.Extremities: No articular swelling. Free movements of all limbs.Neural system: Physiological reflexes were existent without any pathological ones.Genitourinary system: Not examed.Rectum: not exanedInvestigationNo.Professional ExaminationThere are a about 3*3*2 cm mass in outer-up field of her right breast. It is hard but no tendness. It can be moved and its surface is smooth. The skin of her breast is normal. Corresponding superficial lymph nodes don’t enlarge.History summary1. Patient was a teacher, female, 43 years old.2. Right breast mass found for more than half a month.3. No special past history.4. Physical examination showed no abnormity in lung, heart and abdoman. Information about her breast can be seen above.5. Shorting of investigation information.Impression: Breast cancer (right)Signature: xxx。
Division: __________ Ward: __________ Bed: _________ Case No. ___________ Name: ______________ Sex: __________ Age: ___________ Nation: ___________ Birth Place: ________________________________ Marital Status:____________ Work-organization & Occupation: _______________________________________ Living Address & Tel: _________________________________________________ Date of admission: _______Date of history taken:_______ Informant:__________Chief Complaint: ___________________________________________________ History of Present Illness:___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________Past History:General Health Status: 1.good 2.moderate 3.poorDisease history: (if any, please write down the date of onset, brief diagnosticand therapeutic course, and the results.)Respiratory system:1. None2.Repeated pharyngeal pain3.chronic cough4.expectoration:5. Hemoptysis6.asthma7.dyspnea8.chest pain_______________________________________________________________ Circulatory system:1.None2.Palpitation3.exertional dyspnea4..cyanosis5.hemoptysis6.Edema of lower extremities7.chest pain8.syncope9.hypertension _______________________________________________________________ Digestive system:1.None2.Anorexia3.dysphagia4.sour regurgitation5.eructation6.nausea7.Emesis8.melena9.abdominal pain 10.diarrhea11.hematemesis 12.Hematochezia 13.jaundice_______________________________________________________________ Urinary system:1.None2.Lumbar pain3.urinary frequency4.urinary urgency5.dysuria6.oliguria7.polyuria8.retention of urine9.incontinence of urine10.hematuria 11.Pyuria 12.nocturia 13.puffy face_______________________________________________________________ Hematopoietic system:1.None2.Fatigue3.dizziness4.gingival hemorrhage5.epistaxis6.subcutaneous hemorrhage_______________________________________________________________ Metabolic and endocrine system:1.None2.Bulimia3.anorexia4.hot intolerance5.cold intolerance6.hyperhidrosis7.Polydipsia8.amenorrhea9.tremor of hands 10.character change 11.Marked obesity12.marked emaciation 13.hirsutism 14.alopecia15.Hyperpigmentation 16.sexual function change_______________________________________________________________ Neurological system:1.None2.Dizziness3.headache4.paresthesia5.hypomnesis6. Visual disturbance7.Insomnia8.somnolence9.syncope 10.convulsion 11.Disturbance of consciousness12.paralysis 13. vertigo_______________________________________________________________ Reproductive system:1.None2.others_______________________________________________________________ Musculoskeletal system:1.None2.Migrating arthralgia3.arthralgia4.artrcocele5.arthremia6.Dysarthrosis7.myalgia8.muscular atrophy_______________________________________________________________ Infectious Disease:1.None2.Typhoid fever3.Dysentery4.Malaria 4.Schistosomiasis4.Leptospirosis 7.Tuberculosis 8.Epidemic hemorrhagic fever9.others_______________________________________________________________ Vaccine inoculation:1.None2.Yes3.Not clearVaccine detail __________________________________________ Trauma and/or operation history:Operations:1.None2.YesOperation details:_______________________________________ Traumas:1.None2.YesTrauma details:_________________________________________ Blood transfusion history:1.None2.Yes ( 1.Whole blood 2.Plasma3.Ingredient transfusion)Blood type:____________ Transfusion time:___________Transfusion reaction1.None2.YesClinic manifestation:_____________________________ Allergic history:1.None2.Yes3.Not clearallergen:________________________________________________clinical manifestation:_____________________________________Personal history:Custom living address:____________________________________________ Resident history in endemic disease area:_____________________________ Smoking: 1.No 2.YesAverage ___pieces per day; about___yearsGiving-up 1.No 2.Yes (Time:_______________________) Drinking: 1.No 2.YesAverage ___grams per day; about ___yearsGiving-up 1.No 2.Yes(Time:________________________) Drug abuse:1.No 2.YesDrug names:_______________________________________ _______________________________________________________________ Marital and obstetrical history:Married age: __________years old Pregnancy ___________timesLabor _______________times(1.Natural labor: _______times 2.Operative labor: ________times3.Natural abortion: ______times4.Artificial abortion: _______times5.Premature labor:__________times6.stillbirth__________times)Health status of the Mate:1.Well2.Not fineDetails: _______________________________________________ Menstrual history:Menarchal age: _______ Duration ______day Interval ____daysLast menstrual period: ____________ Menopausal age: ____years oldAmount of flow: 1.small 2. moderate 3. largeDysmenorrheal: 1. presence 2.absence Menstrual irregularity 1. No 2.Yes Family history: (especially pay attention to the infectious and hereditary diseaserelated to the present illness)Father: 1.healthy 2.ill:________ 3.deceased cause: ___________________ Mother:1.healthy 2.ill:________ 3.deceased cause: ___________________ Others: ________________________________________________________ The anterior statement was agreed by the informant.Signature of informant: Datetime:Physical ExaminationVital signs:Temperature:______0C Blood pressure:_______/_______mmHg Pulse: _____ bpm (1.regular 2.irregular_____________________________) Respiration: ___bpm (1.regular 2.irregular____________________________) General conditions:Development: 1.Normal 2.Hypoplasia 3.HyperplasiaNutrition: 1.good 2.moderate 3.poor 4.cachexiaFacial expression: 1.normal 2.acute 3.chronic other_____________________ Habitus: 1.asthenic type 2.sthenic type 3.ortho-thenic typePosition: 1.active 2.positive pulsive 4.other_______________________ Consciousness: 1.clear 2.somnolence 3.confusion 4.stupor 5.slight coma6.mediate coma7.deep coma8.deliriumCooperation: 1Yes 2.No Gait: 1.normal 2.abnormal______Skin and mucosa:Color: 1.normal 2.pale 3.redness 4.cyanosis 5.jaundice 6.pigmentationSkin eruption:1.No 2.Yes( type: __________distribution:__________________) Subcutaneous bleeding: 1.no 2.yes (type:_______distribution:______________) Edema:1. no 2.yes ( location and degree________________________________) Hair: 1.normal 2.abnormal(details_____________________________________) Temperature and moisture: normal cold warm dry moist dehydration Liver palmar : 1.no 2.yes Spider angioma (location:________________) Others: __________________________________________________________ Lymph nodes: enlargement of superficial lymph node:1.no2.yesDescription: ________________________________________________ Head:Skull size:1.normal 2.abnormal (description:____________________________) Skull shape:1.normal 2.abnormal(description:___________________________) Hair distribution :1.normal 2.abnormal(description:______________________) Others:___________________________________________________________ Eye: exophthalmos:___________eyelid:____________conjunctiva:__________ sclera:________________Cornea:_______________________Pupil: 1.equally round and in size 2.unequal (R______mm L_______mm)Pupil reflex: 1.normal 2.delayed (R___s L___s ) 3.absent (R___L___)others:______________________________________________________ Ear: Auricle 1.normal 2.desformation (description:_______________________) Discharge of external auditory canal:1.no 2.yes (1.left 2.right quality:_____)Mastoid tenderness 1.no 2.yes (1.left 2.right quality:__________________)Disturbance of auditory acuity:1.no 2.yes(1.left 2.right description:_______) Nose: Flaring of alae nasi :1.no 2.yes Stuffy discharge 1.no 2.yes(quality______) Tenderness over paranasal sinuses:1.no 2.yes (location:_______________) Mouth: Lip______________Mucosa_____________Tongue________________ Teeth:1.normal 2. Agomphiasis 3. Eurodontia 4.others:____________________Gum :1.normal 2.abnormal (Description____________________________)Tonsil:___________________________Pharynx:_____________________Sound: 1.normal 2.hoarseness 3.others:_____________________________ Neck:Neck rigidity 1.no 2.yes (______________transvers fingers)Carotid artery: 1.normal pulsation 2.increased pulsation 3.marked distention Trachea location: 1.middle 2.deviation (1.leftward_______2.rightward______) Hepatojugular vein reflux: 1. negative 2.positiveThyroid: 1.normal 2.enlarged _______ 3.bruit (1.no 2.yes ________________) Chest:Chest wall: 1.normal 2.barrel chest 3.prominence or retraction:( left________right_________Precordial prominence__________) Percussion pain over sternum 1.No 2.YesBreast: 1.Normal 2.abnormal _______________________________________ Lung:Inspection: respiratory movement 1.normal 2.abnormal_____________ Palpation: vocal tactile fremitus:1.normal 2.abnormal _______________ pleural rubbing sensation:1.no 2.yes______________________Subcutaneous crepitus sensation:1.no 2.yes________________ Percussion:1. resonance 2. Hyperresonance &location_____________3 Flatness&location_________________________________4. dullness & location:_______________________________5.tympany &location:_______________________________lower border of lung: (detailed percussion in respiratory disease) midclavicular line : R:_____intercostae L:_____intercostaemidaxillary line: R:______intercostae L:_____intercostaescapular line: R:______intercostae L:_____intercostaemovement of lower borders:R:_______cmL:__________cm Auscultation: Breathing sound : 1.normal 2.abnormal _______________Rales:1.no 2.yes__________________________________ Heart: Inspection:Apical pulsation: 1.normal 2.unseen 3.increase 4.diffuseSubxiphoid pulsation: 1.no 2.yesLocation of apex beat: 1.normal 2.shift (______ intercosta,distance away from left MCL______cm) Palpation:Apical pulsation:1. normal 2.lifting apex impulse 3.negative pulsationThrill:1.no 2.yes(location:___________ phase:_________________)Percussion: relative dullness border: 1.normal 2.abnormalAuscultation: Heart rate:___bpm Rhythm:1.regular 2.irregular_______ Heart sound: 1.normal 2.abnormal________________________Extra sound: 1.no 2.S3 3.S4 4. opening snapP2_________ A2_________Pericardial friction sound:1.no 2.yesMurmur: 1.no 2.yes (location____________phase_____________quality______intensity________ transmission___________effects of position_________________________________effects of respiration______________________________ Peripheral vascular signs:1.None2.paradoxical pulse3.pulsus alternans4. Water hammer pulse5.capillary pulsation6.pulse deficit7.Pistol shot sound8.Duroziez signAbdomen:Inspection: Shape: 1.normal 2.protuberance 3.scaphoid 4.frog-bellyGastric pattern 1.no 2.yes Intestinal pattern 1.no 2.yesAbdominal vein varicosis 1.no 2.yes(direction:______________ )Operation scar1.no 2.yes ________________________________ Palpation: 1.soft 2. tensive (location:____________________________)Tenderness: 1.no 2.yes(location:_______________________)Rebound tenderness:1.no 2.yes(location:________________)Fluctuation: 1.present 2.abscentSuccussion splash: 1.negative 2.positiveLiver:_______________________________________________Gallbladder: __________________Murphy sign:____________Spleen:______________________________________________Kidneys:____________________________________________Abdominal mass:______________________________________Others:______________________________________________ Percussion: Liver dullness border: 1.normal 2.decreased 3.absentUpper hepatic border:Right Midclavicular Line ________IntercostaShift dullness:1.negative 2.positive Ascites:_____________degreePain on percussion in costovertebral area: 1.negative 2.positve ____ Auscultation: Bowel sounds : 1.normal 2.hyperperistalsis 3.hypoperistalsis4.absence Gurgling sound:1.no 2.yesVascular bruit 1.no 2.yes (location_____________________) Genital organ: 1.unexamined 2.normal 3.abnormalAnus and rectum: 1.unexamined 2.normal 3.abnormalSpine and extremities:Spine: 1.normal 2.deformity (1.kyphosis 2.lordosis 3.scoliosis)3.Tenderness(location______________________________)Extremities:1.normal 2.arthremia & arthrocele (location_________________)3.Ankylosis (location__________)4.Aropachy: 1.no 2.yes5.Muscular atrophy (location_______________________) Neurological system:1.normal 2.abnormal_______________________________ _____________________________________________________________________ Important examination results before hospitalized___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ Summary of the history:______________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ Initial diagnosis:_____________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________Recorder:Corrector:。
HUAZHONG UNIVERSITY OF SCIENCE AND TECHNOLOGY TONGJI MEDICAL COLLEGE ACCESSORY TONGJI HOSPITALHospitalization Records for None-operation Division Division: __________ Ward: __________ Bed: _________ Case No. ___________Name: ______________ Sex: __________ Age: ___________ Nation: ___________ Birth Place: ________________________________ Marital Status:____________ Work-organization & Occupation: _______________________________________ Living Address & Tel: _________________________________________________ Date of admission: _______Date of history taken:_______ Informant:__________ Chief Complaint: ___________________________________________________History of Present Illness:___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________Past History:General Health Status: 1.good 2.moderate 3.poorDisease history: (if any, please write down the date of onset, brief diagnosticand therapeutic course, and the results.)Respiratory system:1. None2.Repeated pharyngeal pain3.chronic cough4.expectoration:5. Hemoptysis6.asthma7.dyspnea8.chest pain_______________________________________________________________ Circulatory system:1.None2.Palpitation3.exertional dyspnea4..cyanosis5.hemoptysis6.Edema of lower extremities7.chest pain8.syncope9.hypertension_______________________________________________________________ Digestive system:1.None2.Anorexia3.dysphagia4.sour regurgitation5.eructation6.nausea7.Emesis8.melena9.abdominal pain 10.diarrhea11.hematemesis 12.Hematochezia 13.jaundice_______________________________________________________________ Urinary system:1.None2.Lumbar pain3.urinary frequency4.urinary urgency5.dysuria6.oliguria7.polyuria8.retention of urine9.incontinence of urine10.hematuria 11.Pyuria 12.nocturia 13.puffy face_______________________________________________________________ Hematopoietic system:1.None2.Fatigue3.dizziness4.gingival hemorrhage5.epistaxis6.subcutaneous hemorrhage_______________________________________________________________ Metabolic and endocrine system:1.None2.Bulimia3.anorexia4.hot intolerance5.cold intolerance6.hyperhidrosis7.Polydipsia8.amenorrhea9.tremor of hands 10.character change 11.Marked obesity12.marked emaciation 13.hirsutism 14.alopecia15.Hyperpigmentation 16.sexual function change_______________________________________________________________ Neurological system:1.None2.Dizziness3.headache4.paresthesia5.hypomnesis6. Visual disturbance7.Insomnia8.somnolence9.syncope 10.convulsion 11.Disturbance of consciousness12.paralysis 13. vertigo_______________________________________________________________ Reproductive system:1.None2.others_______________________________________________________________Musculoskeletal system:1.None2.Migrating arthralgia3.arthralgia4.artrcocele5.arthremia6.Dysarthrosis7.myalgia8.muscular atrophy_______________________________________________________________ Infectious Disease:1.None2.Typhoid fever3.Dysentery4.Malaria 4.Schistosomiasis4.Leptospirosis 7.Tuberculosis 8.Epidemic hemorrhagic fever9.others_______________________________________________________________ Vaccine inoculation:1.None2.Yes3.Not clearVaccine detail __________________________________________ Trauma and/or operation history:Operations:1.None2.YesOperation details:_______________________________________ Traumas:1.None2.YesTrauma details:_________________________________________ Blood transfusion history:1.None2.Yes ( 1.Whole blood 2.Plasma3.Ingredient transfusion)Blood type:____________ Transfusion time:___________Transfusion reaction1.None2.YesClinic manifestation:_____________________________ Allergic history:1.None2.Yes3.Not clearallergen:________________________________________________clinical manifestation:_____________________________________Personal history:Custom living address:____________________________________________ Resident history in endemic disease area:_____________________________ Smoking: 1.No 2.YesAverage ___pieces per day; about___yearsGiving-up 1.No 2.Yes (Time:_______________________) Drinking: 1.No 2.YesAverage ___grams per day; about ___yearsGiving-up 1.No 2.Yes(Time:________________________) Drug abuse:1.No 2.YesDrug names:_______________________________________ _______________________________________________________________Marital and obstetrical history:Married age: __________years old Pregnancy ___________timesLabor _______________times(1.Natural labor: _______times 2.Operative labor: ________times3.Natural abortion: ______times4.Artificial abortion: _______times5.Premature labor:__________times6.stillbirth__________times)Health status of the Mate:1.Well2.Not fineDetails: _______________________________________________ Menstrual history:Menarchal age: _______ Duration ______day Interval ____daysLast menstrual period: ____________ Menopausal age: ____years oldAmount of flow: 1.small 2. moderate 3. largeDysmenorrheal: 1. presence 2.absence Menstrual irregularity 1. No 2.Yes Family history: (especially pay attention to the infectious and hereditary diseaserelated to the present illness)Father: 1.healthy 2.ill:________ 3.deceased cause: ___________________ Mother:1.healthy 2.ill:________ 3.deceased cause: ___________________ Others: ________________________________________________________ The anterior statement was agreed by the informant.Signature of informant: Datetime:Physical ExaminationVital signs:Temperature:______0C Blood pressure:_______/_______mmHg Pulse: _____ bpm (1.regular 2.irregular_____________________________) Respiration: ___bpm (1.regular 2.irregular____________________________) General conditions:Development: 1.Normal 2.Hypoplasia 3.HyperplasiaNutrition: 1.good 2.moderate 3.poor 4.cachexiaFacial expression: 1.normal 2.acute 3.chronic other_____________________ Habitus: 1.asthenic type 2.sthenic type 3.ortho-thenic typePosition: 1.active 2.positive pulsive 4.other_______________________ Consciousness: 1.clear 2.somnolence 3.confusion 4.stupor 5.slight coma6.mediate coma7.deep coma8.deliriumCooperation: 1Yes 2.No Gait: 1.normal 2.abnormal______Skin and mucosa:Color: 1.normal 2.pale 3.redness 4.cyanosis 5.jaundice 6.pigmentationSkin eruption:1.No 2.Yes( type: __________distribution:__________________) Subcutaneous bleeding: 1.no 2.yes (type:_______distribution:______________) Edema:1. no 2.yes ( location and degree________________________________) Hair: 1.normal 2.abnormal(details_____________________________________) Temperature and moisture: normal cold warm dry moist dehydration Liver palmar : 1.no 2.yes Spider angioma (location:________________) Others: __________________________________________________________ Lymph nodes: enlargement of superficial lymph node:1.no2.yesDescription: ________________________________________________ Head:Skull size:1.normal 2.abnormal (description:____________________________) Skull shape:1.normal 2.abnormal(description:___________________________) Hair distribution :1.normal 2.abnormal(description:______________________) Others:___________________________________________________________ Eye: exophthalmos:___________eyelid:____________conjunctiva:__________ sclera:________________Cornea:_______________________Pupil: 1.equally round and in size 2.unequal (R______mm L_______mm)Pupil reflex: 1.normal 2.delayed (R___s L___s ) 3.absent (R___L___)others:______________________________________________________ Ear: Auricle 1.normal 2.desformation (description:_______________________) Discharge of external auditory canal:1.no 2.yes (1.left 2.right quality:_____)Mastoid tenderness 1.no 2.yes (1.left 2.right quality:__________________)Disturbance of auditory acuity:1.no 2.yes(1.left 2.right description:_______) Nose: Flaring of alae nasi :1.no 2.yes Stuffy discharge 1.no 2.yes(quality______) Tenderness over paranasal sinuses:1.no 2.yes (location:_______________) Mouth: Lip______________Mucosa_____________Tongue________________ Teeth:1.normal 2. Agomphiasis 3. Eurodontia 4.others:____________________Gum :1.normal 2.abnormal (Description____________________________)Tonsil:___________________________Pharynx:_____________________Sound: 1.normal 2.hoarseness 3.others:_____________________________ Neck:Neck rigidity 1.no 2.yes (______________transvers fingers)Carotid artery: 1.normal pulsation 2.increased pulsation 3.marked distention Trachea location: 1.middle 2.deviation (1.leftward_______2.rightward______) Hepatojugular vein reflux: 1. negative 2.positiveThyroid: 1.normal 2.enlarged _______ 3.bruit (1.no 2.yes ________________)Chest:Chest wall: 1.normal 2.barrel chest 3.prominence or retraction:( left________right_________Precordial prominence__________) Percussion pain over sternum 1.No 2.YesBreast: 1.Normal 2.abnormal _______________________________________ Lung:Inspection: respiratory movement 1.normal 2.abnormal_____________ Palpation: vocal tactile fremitus:1.normal 2.abnormal _______________pleural rubbing sensation:1.no 2.yes______________________Subcutaneous crepitus sensation:1.no 2.yes________________ Percussion:1. resonance 2. Hyperresonance &location_____________3 Flatness&location_________________________________4. dullness & location:_______________________________5.tympany &location:_______________________________lower border of lung: (detailed percussion in respiratory disease)midclavicular line : R:_____intercostae L:_____intercostaemidaxillary line: R:______intercostae L:_____intercostaescapular line: R:______intercostae L:_____intercostaemovement of lower borders:R:_______cmL:__________cm Auscultation: Breathing sound : 1.normal 2.abnormal _______________Rales:1.no 2.yes__________________________________ Heart: Inspection:Apical pulsation: 1.normal 2.unseen 3.increase 4.diffuseSubxiphoid pulsation: 1.no 2.yesLocation of apex beat: 1.normal 2.shift (______ intercosta,distance away from left MCL______cm) Palpation:Apical pulsation:1. normal 2.lifting apex impulse 3.negative pulsationThrill:1.no 2.yes(location:___________ phase:_________________)Percussion: relative dullness border: 1.normal 2.abnormalAuscultation: Heart rate:___bpm Rhythm:1.regular 2.irregular_______Heart sound: 1.normal 2.abnormal________________________Extra sound: 1.no 2.S3 3.S4 4. opening snapP2_________ A2_________Pericardial friction sound:1.no 2.yesMurmur: 1.no 2.yes (location____________phase_____________quality______intensity________ transmission___________effects of position_________________________________effects of respiration______________________________Peripheral vascular signs:1.None2.paradoxical pulse3.pulsus alternans4. Water hammer pulse5.capillary pulsation6.pulse deficit7.Pistol shot sound8.Duroziez signAbdomen:Inspection: Shape: 1.normal 2.protuberance 3.scaphoid 4.frog-bellyGastric pattern 1.no 2.yes Intestinal pattern 1.no 2.yesAbdominal vein varicosis 1.no 2.yes(direction:______________ )Operation scar1.no 2.yes ________________________________ Palpation: 1.soft 2. tensive (location:____________________________)Tenderness: 1.no 2.yes(location:_______________________)Rebound tenderness:1.no 2.yes(location:________________)Fluctuation: 1.present 2.abscentSuccussion splash: 1.negative 2.positiveLiver:_______________________________________________Gallbladder: __________________Murphy sign:____________Spleen:______________________________________________Kidneys:____________________________________________Abdominal mass:______________________________________Others:______________________________________________ Percussion: Liver dullness border: 1.normal 2.decreased 3.absentUpper hepatic border:Right Midclavicular Line ________IntercostaShift dullness:1.negative 2.positive Ascites:_____________degreePain on percussion in costovertebral area: 1.negative 2.positve ____ Auscultation: Bowel sounds : 1.normal 2.hyperperistalsis 3.hypoperistalsis4.absence Gurgling sound:1.no 2.yesVascular bruit 1.no 2.yes (location_____________________) Genital organ: 1.unexamined 2.normal 3.abnormalAnus and rectum: 1.unexamined 2.normal 3.abnormalSpine and extremities:Spine: 1.normal 2.deformity (1.kyphosis 2.lordosis 3.scoliosis)3.Tenderness(location______________________________)Extremities:1.normal 2.arthremia & arthrocele (location_________________)3.Ankylosis (location__________)4.Aropachy: 1.no 2.yes5.Muscular atrophy (location_______________________) Neurological system:1.normal 2.abnormal_______________________________ _____________________________________________________________________Important examination results before hospitalized___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ Summary of the history:______________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ Initial diagnosis:_____________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________Recorder:Corrector:。
妇科英文病历模板篇一:妇产科英文模板CASEMedical Number: 756943General informationName: Yue Jun-rongAge: Forty- two years old Sex: FemaleRace: Han Occupation: UnemploymentNationality: ChinaMarital status: Married Address : Xiaochang county of Xiaogan cityin Hubei. Tel: 4835963Date of admission: Feb.27th, 2003Date of record: 3pm, Feb.27th, 2003 Complainer of history: the patient herselfReliability: ReliableChief complaint: The patient was found “myoma ofuterus” over two years ago and menometrorrhagia for 5 months.Present illness: In 1999, the patient was found “myoma ofuterus” in a physical examination. But she had nothing1uncomfortable and her catamenia was normal. She used some Chinese traditional medicine. About 5 months ago, she found the cycle of her catamenia was shorten from 30 days to 20 days and the period lasted from 2 days to 4 days. She felt no pain and the quantity was normal. She was accepted in our hospital and her diagnosis was “subserous myoma of uterus”.Since onset, her appetite was good, and both her spiritedness and physical energy are normal. Defecation and urination are normal, too.Past historyOperative history: Never undergoing any operation.Infectious history: No history of severe infectious disease.Allergic history: She was not allergic to penicillin or sulfamide.Respiratory system: No history of respiratory disease.Circulatory system: No history of precordial pain.Alimentary system: No history of regurgitation.Genitourinary system: No history of genitourinary disease. Hematopoietic system: No history of anemia andmucocutaneous bleeding. Endocrine system: Noacromegaly. No excessive sweats.2Kinetic system: No history of confinement of limbs.Neural system: No history of headache or dizziness.Personal historyShe was born in Hubei on July 16th, 1956 and almost always lived in Wuhan. She graduated from senior high school. Her living conditions were good. No bad personal habits and customs.Menstrual history: The first time when she was 14. Lasting 2 days every times and its cycle is about 30 days.Obstetrical history: Pregnacy 3 times, once nature production, induced abortion twice.Contraceptive history: Not clear.Family history: His parents are both alive.Physical examinationT 36.8?, P 80/min, R 20/min, BP 120/80mmHg. She is well developed and moderately nourished. Active position. The skin was not stained yellow. No cyanosis. No pigmentation. No skin eruption. Spider angioma was not seen. No pitting edema. Superficial lymph nodes were not enlarged.HeadCranium: Hair was black and well distributed. No deformities. No scars. No masses. No tenderness.3Ear: Bilateral auricles were symmetric and of no masses. No discharges were found in external auditory canals. No tenderness in mastoid area. Auditory acuity was normal.Nose: No abnormal discharges were found in vetibulum nasi. Septum nasi was in midline. No nares flaring. No tenderness in nasal sinuses.Eye: Bilateral eyelids were not swelling. No ptosis. No entropion. Conjunctiva was not congestive. Sclera was anicteric. Eyeballs were not projected or depressed. Movement was normal. Bilateral pupils were round and equal in size. Direct and indirect pupillary reactions to light were existent.Mouth: Oral mucous membrane was smooth, and of no ulcer or erosion. Tongue was in midline. Pharynx was not congestive. Tonsils were not enlarged.Neck: Symmetric and of no deformities. No masses. Thyroid was not enlarged. Trachea was in midline.ChestChestwall: Veins could not be seen easily. No subcutaneous emphysema. Intercostal space was neither narrowed nor widened. No tenderness.Thorax: Symmetric bilaterally. No deformities.4Breast: Symmetric bilaterally. Neither nipples nor skin were retracted. Elasticity was fine.Lungs: Respiratory movement was bilaterally symmetric with the frequency of 20/min. Thoracic expansion and tactile fremitus were symmetric bilaterally. No pleural friction fremitus. Resonance was heard during percussion. No abnormal breath sound was heard. No wheezes. No rales.Heart: No bulge and no abnormal impulse or thrills in precordial area. The point of maximum impulse was in 5th left intercostal space inside of the mid clavicular line and not diffuse. No pericardialfriction sound. Border of the heart was normal. Heart sounds were strong and no splitting. Rate 80/min. Cardiac rhythm was regular. Nopathological murmurs.Abdomen: Flat and soft. No bulge or depression. No abdominal wall varicosis. Gastralintestinal type or peristalses were not seen. There was not tenderness and rebound tenderness on abdomen or renal region. Liver was not reached. Spleen was not enlarged. No masses. Fluidthrill negative. Shifting dullness negative. Borhorygmus 5/min. No vascular murmurs.Extremities: No articular swelling. Free movements of all5limbs.Neural system: Physiological reflexes were existent without any pathological ones. Genitourinary system: Not examed.Rectum: not exanedInvestigationBlood-Rt: Hb 127g/l RBC 3.93T/l WBC 3.9G/lUrine-Rt: SG 1.070 pH 6.0B-ultrasound: 1. subserous myoma of uterus2. position of loop is normalHepatic function: NormalPT & APTT: NormalProfessional ExaminationPudendum: Married typeVagina: unobstructed, secretion is excessive, white and ropy.Os of cervix: No bleeding, slight anabrosis.Body of uterus: Big like a fist of man, hard and its surface is smooth.Others: NormalHistory summary1. Patient was female, 45 years old2. The patient was found “myoma of uterus” over two6year ago and menometrorrhagia for 5 months..3. No special past history.4. Physical examination showed no abnormity in lung, heart and abdoman. Professional examination can been seen above.5. investigation information: see aboveImpression: subserous myoma of uterusSignature: He Lin (95-10033)来源:杨帆| 分享(7) | 浏览(49)篇二:妇产科英文病历Inpatient HistoryName: Yali ZhouSex: FemaleAge: 38year Ward: No.8 Bed: No.816 Marital status: Married Birthplace: ShanghaiNationality: Han Provider: Patient, reliable. Record date: 2005-12-13G & O History: GW: 30+5weeks, G2P0, LMP: 2005-5-10; EDC: 2006-2-17Chief Complaint: G2P0, GW: 30+5weeks. This patient presents hypertension for 3 months, and systemic edema for 2 weeks.7History of Present illness:The patient had regular menses previously. LMP: 2005-5-10; EDC:2006-2-17. Uric HcG test was positive after 40 days of amenorrhea. Fetal movements were felt in 4 months’ gestation. In 12+2weeks’ gestation, the patient’s blood pressure was found 160/90 mmHg when shecompleted her first ante-partum examination in Hospital of Women and Children’s Health in Huangpu District. Therewas no symptoms at that time, and she didn’t take anytreatment. Half a month ago, she presented edema on both the lower extremities, which expanded to the whole body gradually. She came to our hospital on Dec, 5th and took her sencond ante-partum examination. The bp was200/160mmHg, and uric protein(++) on dipstick test. She has occasional headaches, but no epigastric pain, no visual disturbances, no oliguria, no nausea or vomiting, no thoracic pain. She was admitted on 2005-12-6.After admission, she appears clear, with a good appetite, good sleeping, and normal urination and defecation.Past history: Patient denies history of hepatitis and tuberculosis. No history of allergies. Vaccinated regularly. Past medical history is uemarkable. Surgical history denied.8No history of severe trauma and transfusion.Review of systems:Respiratory system: No history of chronic cough or breathlessness. No hemoptysis or dyspnea.Cardiovascular system: No precordial pain. No palpation. No syncope. For details see present history.Gastroentestinal system: No history of chronic abdominal pain and diarrhea; No nausea or vomiting; No hematemesis and blood stool.Endocrinic system: No polydipsia or polyphasia or polyuria. No sudden change of character and intelligence.Hematologic system: No bruises or abnormal hemorrhage. No recurrent oral ulcer and gingival bleeding.Genitourinary system: No decreased libido; No vaginal dryness or vaginal bleeding; History of STD denied; No urinary frequency. No precipitant urination or dysuria. No hematuria or proteinuria.Neuropsychiatric system: No convulsion or anesthesia. No headaches. No abnormal orientation. No deterioration of memory or intelligence.Locomotor system: No arthralgia, no muscular atrophies or dystrophies.9Personal History:Born and grown up in Shanghai. Patient denied history of tobacco or alcohol use.Marital and Childbearing history: Married. 0-0-1-0; She had an abortion in 3 months’ gestation in Dec., 2004. Birthcontrol has been instructed.Family history: The patient’s Mother and a sister sufferedfrom hypertension. No family history of DM or stroke. No family history of nervous or mental diseases.Physical ExaminationT: 37? P: 89/minR: 20/minBP: 180/120mmHgGeneral appearance: Patient is a 38 years old female who appears pleasant, in no apperant distress, given her age, well developed andwell nourished. Oriented to person, place and time.Lymph nodes: Not enlarged.Skin: No jaundice or rashes. No cyanosis and bruises. No edema.Head: Skull and scalp normal. No tenderness. No loss of hair.Eyes: No edema in eyelids, no ptosis, no conjunctivalcongestion.Width of palpebral fissures is normal. No10jaundice. Pupil’s size and shape is normal. Corneal is clear. No exophthalmos.Ears: Auditory acuity is excellent. No ear purulent discharge.Nose: Shape is normal. No obstruction. No deviation of nasal septum.Mouth: No lips herpes. No cyanosis. No gums pyorrhea and bleeding. No tongue deviation. Tonsils not enlarged.Neck: Her neck is soft. Trachea is midline. No thyroid abnormality was found. Neck vein was not distended.Chest: Contour is normal. No sternum tenderness. The breasts are bilaterally symmetrical. No tenderness and mass.Lung:Inspection: Respiration regular. Degree of expansion is symmetry.Plapation: Tactile fremitus symmetrical.Percussion: extensive resonance to percussion.Ausculation: Clear to ausculation with no rubs noted.Heart:Inspection: No abnormal pulsation or retraction.Plapation: The apex beat can be felt in the 5th intercostal space 1 cm inside of the left mid-clavicular line.11Percussion: The border of cardiac is not enlarged.Ausculation: The heart sounds were of good quality and the rhythm was regular.Radial pulse is normal.Abdomen:Inspection: Universial abdominal bulge. Dilated veins observed.Palpation: Soft. Liver and spleen is not enlarged. Nontender. Murphy’s sign is negative. For details seeobstetric examination.Percussion: No shifting dullness. The upper border of the liver is in the 5th intercostal space.Ausculation: Bowl sound clear. 4/min.Spine and extremities: Severe edema in both lower extremities. No clubbed finger. No disorder of the movement of axial and appendicular bones.Reflex: Symmetrical, equal without pathological responses. Babinski sign and Kernig sign and hoffmann sign are all negative.Obstetric examinationPatient appears pleasant, given her age, well developed and12well nourished. No jaundice. No enlarged lymph nodes.Fetus: Abdominal girth: 93cm; height of fundus: 29cm; estimated fetal weight: 1600g; fetal position: LOA; point of fetal heart tone: ; fetal heart rate: 148/min; FM: active.Pelvis: 24-17-19-9 cm.Anorectal examination: fetal presentation: N/A; sincipital presentation: N/A; fetal membrane: not ruptured. Amniotic fluid: N/A;Flexion of knee: active.Laboratory and special examinationthDec. 6, Blood Rt: Hb: 121g/L; PLT 136×10e9Urine Rt: uric protein(++); occlude blood: (+++)Dec. 7th, Fetal Ultrasound: BPD: 78mm; HC: 259mm; AC: 238mm; FL:51mm; HL:49mm. fetal presentation: head; Position of placenta: right wall of uterus.Thickness of placenta: 23mm. Degree of placental maturity: ?; fetal heartbeat and fetal movement seen; amniotic fluid: 64mm. There is no hematocoelia or ascites. The lower edge of placenta is1323mm from thecervix.Umbilical A: P2: 0.87; R2: 0.59; S/D: 2.46.Fetal heart rate: 145/minDec. 8th, 24h uric protein: 7.5gDec.10th, serum potassium: 3.9mmol/LScr: 86umol/LALT: 25U/L ; AST: 30U/LFeatures of the case:1. Female, 38years old, G2P0, GW: 30+5weeks.2. This patient presents hypertension for 3 months, andsystemic edema for 2 weeks.3. PE: BP: 180/120mmHg. Obstetric exam: Fetus: Abdominal girth: 93cm; height offundus: 29cm; estimated fetal weight: 1600g; fetal position:LOA; point of fetal hearttone: ; fetal heart rate: 148/min; FM: active.Pelvis: 24-17-19-9 cm.Flexion of knee: active.4. Laboratory and special exam:Dec. 6th, Blood Rt: Hb: 121g/L; PLT 136×10e9Urine Rt: uric protein(++); occlude blood: (+++)14Dec. 7th, Fetal Ultrasound: BPD: 78mm; HC: 259mm; AC: 238mm; FL:51mm;Degree of placental maturity: ?; fetal heartbeat and fetalmovementseen; amniotic fluid: 64mm.Umbilical A: P2: 0.87; R2: 0.59; S/D: 2.46.Fetal heart rate: 145/minDec. 8th, 24h uric protein: 7.5gDec.10th, serum potassium: 3.9mmol/LScr: 86umol/LALT: 25U/L ; AST: 30U/LDiagnosis and differential diagnosis:Diagnosis: 1. Severe pre-eclampsia. This patient is a 38-year-old woman, who presents with hypertension and edema. Pre-eclampsia is hypertension associated with proteinuria and edema, occurring primarily in nulliparas after the 20th gestational week and most frequently near term. Other clinical findings of the patient include uric protein (++),etc. These lead to the diagnosis of pre-eclampsia, which feature the clinic status of the latter. The patient has (1) blood pressure 180/120mmHg(160/110mmHg);15(2)proteinuria(++) on dipstick testing and 7.5g (5g)in a 24-hour period. Conclusively, she can be classified as severe pre-eclampsia.Pre-eclampsia is a multisystemic syndrome, primary investigations reveal that she has occasional headaches, but no epigastric pain, no visual disturbances, no oliguria, no nausea or vomiting, no thoracic pain, indicating that there are no many complications at present. Further evaluations are indispensable, which requires more careful investigations.2. Chronic essential hy pertension. The patient’shypertension began from the 12w of gestation, which indicate thatshe has chronic hypertension. Besides, she has a family history of hypertension. After all, she doesn’tpresent severe complaints when her blood pressure were as high as 200/160mmHg. All these lead to the diagnosis of chronic hypertension. To confirm the diagnosis, the blood pressure after delivery should be evaluated.Differential diagnosis: 1. chronic essential hypertension associated with pregnancy. Essential hypertension associated with pregnancy can also cause a very high blood pressure. However, given the age,proteinuria and edema are possibly not complications of hypertension, indicating that she has16superimposed pre-eclampsia. Besides, the symptoms of proteinuria and edema are temporally associated with gestation.2. Chronic hypertension due to renal disease. This includes chronic hypertension due to interstitial nephritis, chronic glomerulonephritis, SLE, diabetic glomerulosclerosis, and so on. In these occasions, the patient would also possibly present hypertension, proteinuria and edema, but her proteinuria was found recently and she didn’t have any symptoms associated with renal diseases previously. In addition, her serum creatinine is in the normal scale (Scr: 86umol/L), which contradicts the hypothesis that she has a renal disease. So the diagnosis of chronic hypertension due to renal disease is not considered at present.Further investigations and treatments:1. Close observation and monitoring, plus quick evaluation: daily weighing; q4-6h monitoring of blood pressure; daily monitoring ofprotein in urine; Regular liver and kidney function testing; Ultrasound of the abdomen; Fetus heartbeat monitor; Conduct ophthalmoscopy examination to evaluat e the severity of the patient’s condition; Conduct PT,APTT, FDP, 3P test to evaluate the coagulant function.172. Rests: Lie in bed on left side.3. Magnesium sulfate administration with close observation offlexion of knee, respiratory rate and urine.4. Control hypertension with Labetalol or Nitroglycerin. The goal of bp control is diastolic pressure《110mmHg andMAP《140mmHg.5. Administer furosemide to control edema.6. Cautious evaluation of the maternal and fetal complications and take action correspondingly. Severe maternal complications include edema of the brain, pulmonary edema; DIC; HELLP syndrome; renal failure. Indicative symptoms include headache, epigastric pain, visual disturbances, oliguria, nausea and vomiting, thoracic pain, etc.7. Use corticosteroids to accelerate fetal lung maturity.8. Delivery. In an effort to reduce perinatal morbidity and mortality, delivery should be delayed. If the patient develops into the following conditions: 1.Blood pressure consistently higher than 100mmHg diastolic in a 24h period or confirmed higher than 110mmHg; 2. Rising serum creatinine; 3. Persistent severe headache; 4. epigastric pain; 4. abnormal liver function tests; 5: Thrombocytopnia; 6: HELLP18syndrome; 7: Eclampsia; 8: Pulmonary edema; 9: Abnormal antepartum fetal heart rate testing; 10: SGA fetus with failure to grow on serial ultrasound examinations.Clinic diagnosis: 1. Severe pre-eclampsia2. Chronic essential hypertension Signiture: /Jacky Luo 篇三:妇科英文病历CASEMedical Number: 756943General informationName: Yue Jun-rongAge: Forty- two years oldSex: FemaleRace: HanOccupation: UnemploymentNationality: ChinaMarital status: MarriedAddress: Xiaochang county of Xiaogan city in Hubei. Tel: 4835963 Date of admission: Feb.27th, 2003 Date of record: 3pm, Feb.27th, 2003 Complainer of history: the patient herself Reliability: Reliable Chief complaint: The patient was found “myoma ofuterus” over two years ago and menometrorrhagia for 519months.Present illness: In 1999, the patient was found “myoma ofuterus” in a physical examination. But she had nothing uncomfortable and her catamenia was normal. She used some Chinese traditional medicine. About 5 months ago, she found the cycle of her catamenia was shorten from 30 days to 20 days and the period lasted from2 days to 4 days. She felt no pain and the quantity was normal. She was accepted in our hospital and her diagnosis was “subserous myoma of uterus”.Since onset, her appetite was good, and both her spiritedness and physical energy are normal. Defecation and urination are normal, too.Past historyOperative history: Never undergoing any operation.Infectious history: No history of severe infectious disease.Allergic history: She was not allergic to penicillin or sulfamide. Respiratory system: No history of respiratory disease.Circulatory system: No history of precordial pain.Alimentary system: No history of regurgitation. Genitourinary system: No history of genitourinary disease.20Hematopoietic system: No history of anemia and mucocutaneous bleeding.Endocrine system: No acromegaly. No excessive sweats.Kinetic system: No history of confinement of limbs.Neural system: No history of headache or dizziness.Personal historyShe was born in Hubei on July 16th, 1956 and almost always lived in Wuhan. She graduated from senior high school. Her living conditions were good. No bad personal habits and customs.Menstrual history: The first time when she was 14. Lasting 2 days every times and its cycle is about 30 days.Obstetrical history: Pregnacy 3 times, once nature production, induced abortion twice.Contraceptive history: Not clear.Family history: His parents are both alive.Physical examinationT 36.8?, P 80/min, R 20/min, BP 120/80mmHg. She is well developed and moderately nourished. Active position. The skin was not stained yellow. No cyanosis. No pigmentation. No skin eruption. Spider angioma was not seen. No pitting edema. Superficial lymph nodes were not21enlarged. HeadCranium: Hair was black and well distributed. No deformities. No scars. No masses. No tenderness.Ear: Bilateral auricles were symmetric and of no masses. No discharges were found in external auditory canals. No tenderness in mastoid area. Auditory acuity was normal.Nose: No abnormal discharges were found in vetibulum nasi. Septum nasi was in midline. No nares flaring. No tenderness in nasal sinuses.Eye: Bilateral eyelids were not swelling. No ptosis. No entropion.Conjunctiva was not congestive. Sclera was anicteric. Eyeballs were not projected or depressed. Movement was normal. Bilateral pupils wereround and equal in size. Direct and indirect pupillary reactions tolight were existent.Mouth: Oral mucous membrane was smooth, and of no ulcer or erosion. Tongue was in midline. Pharynx was not congestive. Tonsils were not enlarged.Neck: Symmetric and of no deformities. No masses. Thyroid was not enlarged. Trachea was in midline.ChestChestwall: Veins could not be seen easily. No subcutaneous22emphysema. Intercostal space was neither narrowed nor widened. No tenderness.Thorax: Symmetric bilaterally. No deformities.Breast: Symmetric bilaterally. Neither nipples nor skin were retracted. Elasticity was fine.Lungs: Respiratory movement was bilaterally symmetric with the frequency of 20/min. Thoracic expansion and tactile fremitus were symmetric bilaterally. No pleural friction fremitus. Resonance was heard during percussion. No abnormal breath sound was heard. No wheezes. No rales.Heart: No bulge and no abnormal impulse or thrills in precordial area. The point of maximum impulse was in 5th left intercostal space inside of the mid clavicular line and not diffuse. No pericardialfriction sound. Border of the heart was normal. Heart sounds were strongand no splitting. Rate 80/min. Cardiac rhythm was regular. Nopathological murmurs.Abdomen: Flat and soft. No bulge or depression. No abdominal wall varicosis. Gastralintestinal type or peristalses were not seen. Therewas not tenderness and rebound tenderness on abdomen or renal region. Liver was not reached. Spleen was not enlarged. No masses. Fluidthrill23negative. Shifting dullness negative. Borhorygmus 5/min. No vascular murmurs. Extremities: No articular swelling. Free movements of all limbs.Neural system: Physiological reflexes were existent without anypathological ones.Genitourinary system: Not examed.Rectum: not exanedInvestigationBlood-Rt: Hb 127g/l RBC 3.93T/l WBC 3.9G/lUrine-Rt: SG 1.070 pH 6.0B-ultrasound: 1. subserous myoma of uterus2. position of loop is normalHepatic function: NormalPT & APTT: NormalProfessional ExaminationPudendum: Married typeVagina: unobstructed, secretion is excessive, white and ropy.Os of cervix: No bleeding, slight anabrosis.Body of uterus: Big like a fist of man, hard and its surface is smooth. Others: Normal24History summary1. Patient was female, 45 years old2. The patient was found “myoma of uterus” over twoyear ago and menometrorrhagia for 5 months..3. No special past history.4. Physical examination showed no abnormity in lung, heart and abdoman. Professional examination can been seen above.5. investigation information: see aboveImpression: subserous myoma of uterusSignature: He Lin (95-10033)25。
CASE REPORT FORM TEMPLATEVersion: 6.0 (8 November 2012)PROTOCOL: [INSERT PROTOCOL NUMBER][INSERT PROTOCOL TITLE]Participant Study Number:Study group:BASELINE DATAGeneral Instructions for Completion of the Case Report Forms (CRF)Completion of CRFs• A CRF must be completed for each study participant who is successfully enrolled (received at least one dose of study drug)•For reasons of confidentiality, the name and initials of the study participant should not appear on the CRF. General•Please print all entries in BLOCK CAPITAL LETTERS using a black ballpoint pen.•All text and explanatory comments should be brief.•Answer every question explicitly; do not use ditto marks.•Do not leave any question unanswered. If the answer t o a question is unknown, write “NK” (Not Known). If a requested test has not been done, write “ND” (Not Done). If a question is not applicable, write “NA” (Not Applicable).•Where a choice is requested, cross (X) the appropriate response.Dates and Times•All date entries must appear in the format DD-MMM-YYYY e.g. 05-May-2009. The month abbreviations are as follows:January = Jan May = May September = SepFebruary = Feb June = Jun October = OctMarch = Mar July = Jul November = NovApril = Apr August = Aug December = DecIn the absence of a precise date for an event or therapy that precedes the participant’s inclusion into the study,a partial date may be recorded by recording “NK” in the fields that are unknown e.g. where the day and month are not clear, the following may be entered into the CRF: N K N K 2 0 0 9DD MMM YYYY•All time entries must appear in 24-hour format e.g. 13:00. Entries representing midnight should be recorded as 00:00 with the date of the new day that is starting at that time.Correction of Errors•Do not overwrite erroneous entries, or use correction fluid or erasers.•Draw a straight line through the entire erroneous entry without obliterating it.•Clearly enter the correct value next to the original (erroneous) entry.•Date and initial the correction.Protocol Number: Page 1 of 15PARTICIPANT INFORMATIONParticipant NumberStudy Group ______________________________________________ Study Site (Health Centre Name) ______________________________________________Inclusion/exclusion criteria*Patient must meet all criteria to eligible for the studyMet all 1.Not met* 2.Date of Informed ConsentD D M M M Y Y Y YDate of Birth D D M M M Y Y Y YOr estimated age_______Gender 1Male2FemalePregnant 1.Yes2. No9. Unknown If pregnant, Estimated Gestational Age ___________weeksDate of EnrolmentD D M M M Y Y Y YHad malaria in the last 28 days 1. Yes2. No9. Unknown Had antimalarial in the last 28 days 1. Yes2. No9. UnknownBASELINE SYMPTOMSFever (in last 24 hours) 1. Yes2. No Duration: _______ days Dizziness 1. Yes2. No Duration: _______ days Headache 1. Yes2. No Duration: _______ days Nausea 1. Yes2. No Duration: _______ days Anorexia 1. Yes2. No Duration: _______ days Vomiting 1. Yes2. No Duration: _______ days Diarrhoea 1. Yes2. No Duration: _______ days Abdominal pain 1. Yes2. No Duration: _______ days Itching 1. Yes2. No Duration: _______ days Skin rash 1. Yes2. No Duration: _______ days Urticaria 1. Yes2. No Duration: _______ days Joint pain 1. Yes2. No Duration: _______ days Muscle pain 1. Yes2. No Duration: _______ days Palpitations 1. Yes2. No Duration: _______ days Dyspnoea 1. Yes2. No Duration: _______ days Hearing problem 1. Yes2. No Duration: _______ days Confusion 1. Yes2. No Duration: _______ days Visual blurring 1. Yes2. No Duration: _______ days Fatigue 1. Yes2. No Duration: _______ days Other symptom: ____________________ Duration: _______ days Other symptom: ____________________ Duration: _______ days Other symptom: ____________________ Duration: _______ daysMEDICATION HISTORY (within the last 7 days)- Make multiple copies of this page if requiredMedication Name(write NK if unknown)Start Date Stop Date______________________________D D M M M Y Y Y Y D D M M M Y Y Y Y OR 1 Unknown OR 1 Ongoing______________________________D D M M M Y Y Y Y D D M M M Y Y Y Y OR 1 Unknown OR 1 Ongoing______________________________D D M M M Y Y Y Y D D M M M Y Y Y Y OR 1 Unknown OR 1 Ongoing______________________________D D M M M Y Y Y Y D D M M M Y Y Y Y OR 1 Unknown OR 1 Ongoing______________________________D D M M M Y Y Y Y D D M M M Y Y Y Y OR 1 Unknown OR 1 Ongoing______________________________D D M M M Y Y Y Y D D M M M Y Y Y Y OR 1 Unknown OR 1 Ongoing______________________________D D M M M Y Y Y Y D D M M M Y Y Y Y OR 1 Unknown OR 1 Ongoing______________________________D D M M M Y Y Y Y D D M M M Y Y Y Y OR 1 Unknown OR 1 Ongoing______________________________D D M M M Y Y Y Y D D M M M Y Y Y Y OR 1 Unknown OR 1 Ongoing______________________________D D M M M Y Y Y Y D D M M M Y Y Y Y OR 1 Unknown OR 1 Ongoing______________________________D D M M M Y Y Y Y D D M M M Y Y Y Y OR 1 Unknown OR 1 Ongoing______________________________D D M M M Y Y Y Y D D M M M Y Y Y Y OR 1 Unknown OR 1 Ongoing______________________________D D M M M Y Y Y Y D D M M M Y Y Y Y OR 1 Unknown OR 1 OngoingSIGNIFICANT MEDICAL HISTORY (within the past 5 years)- Make multiple copies of this page if requiredDoes the participant have a history of any background/concomitant conditions/symptoms according to the following schedule? 1 Yes 2 NoIf Yes, detail in the table below and reference the ICD10 system codehttp://apps.who.int/classifications/apps/icd/icd10online/Code Title Code Title1 Certain infectious and parasitic diseases 12 Diseases of the skin and subcutaneous tissue2 Neoplasms 13 Diseases of the musculoskeletal system and connective tissue3 Diseases of the blood and blood-forming organs andcertain disorders involving the immune mechanism14 Diseases of the genitourinary system4 Endocrine, nutritional and metabolic diseases 15 Pregnancy, childbirth and the puerperium5 Mental and behavioural disorders 16 Certain conditions originating in the perinatal period6 Diseases of the nervous system 17 Congenital malformations, deformations and chromosomal abnormalities7 Diseases of the eye and adnexa 18 Symptoms, signs and abnormal clinical and laboratory findings, not elsewhere classified8 Diseases of the ear and mastoid process 19 Injury, poisoning and certain other consequences of external causes9 Diseases of the circulatory system 20 External causes of morbidity and mortality10 Diseases of the respiratory system 21 Factors influencing health status and contact with health services11 Diseases of the digestive system 22 Codes for special purposes SIGNIFICANT MEDICAL HISTORY (within the past 5 years)Code Condition/Symptom Onset Date Stop DateD D M M M Y Y Y Y D D M M M Y Y Y YOR 1 Unknown OR 1 OngoingD D M M M Y Y Y Y D D M M M Y Y Y YOR 1 Unknown OR 1 OngoingD D M M M Y Y Y Y D D M M M Y Y Y YOR 1 Unknown OR 1 OngoingD D M M M Y Y Y Y D D M M M Y Y Y YOR 1 Unknown OR 1 OngoingD D M M M Y Y Y Y D D M M M Y Y Y YOR 1 Unknown OR 1 OngoingBASELINE PHYSICAL EXAMINATION – PART 1 Weight . kg Height. cmTemperature . C Method of RecordingHeart ratebpm Axillary Tympanic Rectal Oral1234Respiratory rate bpmBlood pressure/ mmHgHepatomegaly 1. Yes2. Nocm If yes, size:Splenomegaly 1. Yes2. Nocm If yes, size:Normal Abnormal Specify if abnormalCentral Nervous System 1.2.________________________________________Cardiovascular System 1.2.________________________________________Respiratory System 1.2.________________________________________Gastrointestinal System 1.2.________________________________________Skin 1.2.________________________________________Joints 1.2.________________________________________BASELINE PHYSICAL EXAMINATION – PART 2Danger signs or features of severe malaria?(If no symptoms tick box on the right. Otherwise complete list below)No symptoms2YesNoNotKnownC l i n i c a l m a n i f e s t a t i o n sImpaired consciousness1299Prostration1 2 99Multiple convulsions1 2 99Respiratory distress (metabolic acidotic) 1 2 99Circulatory collapse 1 2 99Jaundice 1 2 99Haemoglobinuria 1 2 99Abnormal bleeding1 2 99Pulmonary oedema (radiological)1 2 99L a b o r a t o r y f i n d i n g sHypoglycaemia (blood glucose <2.2 mmol/l or <40 mg/dl 1 2 99Acidosis (plasma bicarbonate <15 mmol/l) 1 2 99Severe anaemia (Hb < 5g/dl or haematocrit <15%) 1 2 99Hyperparasitaemia (>4% in non-immune patients) 1 2 99Hyperlactataemia (venous lactic acid >5 mmol/l)1 2 99Renal impairment (serum creatinine above normal range for age)12 99HAEMATOLOGYParticipant NumberHAEMATOLOGY – make multiple copies of this page if requiredD a y 0DateTime 24hrHb (g/dL)Hct (%)WBC (109/L)D D M M M Y Y Y YH H : M M... Neutrophils (%) Lymphocytes (%) Monocytes (%) Eosinophils (%) Platelets (109/L).-.... D a y _DateTime 24hr Hb (g/dL) Hct (%) WBC (109/L)D D M M M Y Y Y YH H : M M... Neutrophils (%) Lymphocytes (%) Monocytes (%) Eosinophils (%) Platelets (109/L).-.... D a y _DateTime 24hr Hb (g/dL) Hct (%) WBC (109/L)D D M M M Y Y Y YH H : M M... Neutrophils (%) Lymphocytes (%) Monocytes (%) Eosinophils (%) Platelets (109/L).-....D a y _DateTime 24hr Hb (g/dL) Hct (%) WBC (109/L)D D M M M Y Y Y YH H : M M... Neutrophils (%) Lymphocytes (%) Monocytes (%) Eosinophils (%) Platelets (109/L).-....D a y _DateTime 24hr Hb (g/dL) Hct (%) WBC (109/L)D D M M M Y Y Y YH H : M M... Neutrophils (%) Lymphocytes (%) Monocytes (%) Eosinophils (%) Platelets (109/L).-....D a y _DateTime 24hr Hb (g/dL) Hct (%) WBC (109/L)D D M M M Y Y Y YH H : M M... Neutrophils (%) Lymphocytes (%) Monocytes (%) Eosinophils (%) Platelets (109/L). - .... D a y _DateTime 24hr Hb (g/dL) Hct (%) WBC (109/L)D D M M M Y Y Y YH H : M M... Neutrophils (%) Lymphocytes (%) Monocytes (%) Eosinophils (%) Platelets (109/L). - ....PHYSICAL EXAMINATIONParticipant NumberPHYSICAL EXAMINATION: DAY_ – make multiple copies of this page if requiredDateD D M M M Y Y Y YTime HH : M MWeight.kgHeight.cmTemperature.CMethod of RecordingHeart ratebpmAxillaryTympanicRectalOral1234Respiratory ratebpmBlood pressure/mmHepatomegaly 1. Yes 2. No cm If yes, size: Splenomegaly1. Yes2. NocmIf yes, size:Danger signs or features of severe malaria?(If no symptoms tick box on the right. Otherwise complete list below)No symptoms2YesNoNot KnownC l i n i c a l m a n i f e s t a t i o n sImpaired consciousness1299Prostration1 2 99Multiple convulsions1 2 99Respiratory distress (metabolic acidotic) 1 2 99Circulatory collapse 1 2 99Jaundice 1 2 99Haemoglobinuria 1 2 99Abnormal bleeding1 2 99Pulmonary oedema (radiological)1 2 99L a b o r a t o r y f i n d i n g sHypoglycaemia (blood glucose <2.2 mmol/l or <40 mg/dl 1 2 99Acidosis (plasma bicarbonate <15 mmol/l) 1 2 99Severe anaemia (Hb < 5g/dl or haematocrit <15%) 1 2 99Hyperparasitaemia (>4% in non-immune patients) 1 2 99Hyperlactataemia (venous lactic acid >5 mmol/l)1 2 99Renal impairment (serum creatinine above normal range for age)12 99Are there new symptoms or is the patient showing a worsening from baseline ?1. Yes2. NoSYMPTOM CHECKParticipant NumberSYMPTOM CHECK – make multiple copies of this page if requiredD a y _DateFeverDizziness HeadacheNauseaAnorexiaVomitingDiarrhoeaAbdominal painItchingD D M M M Y Y Y YYes ☐ No☐Yes ☐ No☐Yes ☐ No☐Yes ☐ No☐ Yes ☐ No☐ Yes ☐ No☐Yes ☐ No☐Yes ☐ No☐Yes ☐ No☐Time 24hr Skin rashUrticariaJoint painMuscle pain PalpitationsDyspnoeaHearing problemConfusionVisual blurringFatigueH H : M MYes ☐ No☐Yes ☐ No☐Yes ☐ No☐ Yes ☐ No☐ Yes ☐ No☐Yes ☐ No☐Yes ☐ No☐Yes ☐ No☐ Yes ☐ No☐ Yes ☐ No☐D a y _DateFeverDizziness HeadacheNauseaAnorexiaVomitingDiarrhoeaAbdominal painItchingD D M M M Y Y Y YYes ☐ No☐Yes ☐ No☐Yes ☐ No☐Yes ☐ No☐ Yes ☐ No☐ Yes ☐ No☐Yes ☐ No☐Yes ☐ No☐Yes ☐ No☐Time 24hr Skin rashUrticariaJoint painMuscle pain PalpitationsDyspnoeaHearing problemConfusionVisual blurringFatigueH H : M MYes ☐ No☐Yes ☐ No☐Yes ☐ No☐Yes ☐ No☐Yes ☐ No☐Yes ☐ No☐Yes ☐ No☐Yes ☐ No☐ Yes ☐ No☐Yes ☐ No☐D a y _DateFeverDizziness HeadacheNauseaAnorexiaVomitingDiarrhoeaAbdominal painItchingD D M M M Y Y Y YYes ☐ No☐Yes ☐ No☐Yes ☐ No☐ Yes ☐ No☐ Yes ☐ No☐ Yes ☐ No☐Yes ☐ No☐Yes ☐ No☐Yes ☐ No☐Time 24hr Skin rashUrticariaJoint pain Muscle pain PalpitationsDyspnoeaHearing problemConfusionVisual blurringFatigueH H : M MYes ☐ No☐Yes ☐ No☐ Yes ☐ No☐ Yes ☐ No☐ Yes ☐ No☐Yes ☐ No☐Yes ☐ No☐Yes ☐ No☐ Yes ☐ No☐ Yes ☐ No☐PARASITEMIAParticipant NumberPARASITEMIA – make multiple copies of this page if requiredD a y _Date smear takenTime smear takenMalaria species(Record counts for different species on separate rows) Parasite count Units (tick one)☐ /1000RBC ☐ /200WBC ☐ /500WBC ☐ /µLGametocytesGametocytecountUnits (tick one)☐ /1000RBC☐ /200WBC ☐ /500WBC☐ /µLPCR/DNA sample collected?D D M M M Y Y Y YH H : M MPFPVPOPMYesNoYesNo1234121 2D a y _Date smear takenTime smear takenMalaria species(Record counts for different species on separate rows)Parasite countUnits (tick one)☐ /1000RBC ☐ /200WBC ☐ /500WBC ☐ /µLGametocytesGametocytecountUnits (tick one)☐ /1000RBC☐ /200WBC ☐ /500WBC☐ /µLPCR/DNA sample collected?D D M M M Y Y Y YH H : M MPFPVPOPMYesNoYesNo1234121 2D a y _Date smear takenTime smear takenMalaria species(Record counts for different species on separate rows)Parasite countUnits (tick one)☐ /1000RBC ☐ /200WBC ☐ /500WBC ☐ /µLGametocytesGametocytecountUnits (tick one)☐ /1000RBC☐ /200WBC ☐ /500WBC☐ /µLPCR/DNA sample collected?D D M M M Y Y Y YH H : M MPFPVPOPMYesNoYesNo1234121 2D a y _Date smear takenTime smear takenMalaria species(Record counts for different species on separate rows)Parasite countUnits (tick one)☐ /1000RBC ☐ /200WBC ☐ /500WBC ☐ /µLGametocytesGametocytecountUnits (tick one)☐ /1000RBC☐ /200WBC ☐ /500WBC☐ /µLPCR/DNA sample collected?D D M M M Y Y Y YH H : M MPFPVPOPMYesNoYesNo1234121 2CASE REPORT FORM TEMPLATEMOLECULAR GENOTYPE Participant NumberPHARMACOKINETIC PROFILEDate Time Sample numberD D M M M Y Y Y Y H H :M MB00D D M M M Y Y Y Y H H :M MB01D D M M M Y Y Y Y H H :M MB02D D M M M Y Y Y Y H H :M MB03D D M M M Y Y Y Y H H :M MB04D D M M M Y Y Y Y H H :M MB05D D M M M Y Y Y Y H H :M MB06D D M M M Y Y Y Y H H :M MB07D D M M M Y Y Y Y H H :M MB08D D M M M Y Y Y Y H H :M MB09D D M M M Y Y Y Y H H :M MB10D D M M M Y Y Y Y H H :M MB11D D M M M Y Y Y Y H H :M MB12STUDY DRUG ADMINISTRATIONParticipant NumberSTUDY DRUG ADMINSTRATION –make multiple copies of this page if requiredStudy drug Dose Treatmentobserved?Date of dose Time of dose Vomited?Time ofvomitRetreatment?RetreatmentdoseTime ofretreatment___________ ______Yes ☐No ☐D D M M M Y Y Y Y H H :M MYes ☐No ☐H H :M MYes ☐No ☐______H H :M M___________ ______Yes ☐No ☐D D M M M Y Y Y Y H H :M MYes ☐No ☐H H :M MYes ☐No ☐______H H :M M___________ ______Yes ☐No ☐D D M M M Y Y Y Y H H :M MYes ☐No ☐H H :M MYes ☐No ☐______H H :M M___________ ______Yes ☐No ☐D D M M M Y Y Y Y H H :M MYes ☐No ☐H H :M MYes ☐No ☐______H H :M M___________ ______Yes ☐No ☐D D M M M Y Y Y Y H H :M MYes ☐No ☐H H :M MYes ☐No ☐______H H :M M___________ ______Yes ☐No ☐D D M M M Y Y Y Y H H :M MYes ☐No ☐H H :M MYes ☐No ☐______H H :M M___________ ______Yes ☐No ☐D D M M M Y Y Y Y H H :M MYes ☐No ☐H H :M MYes ☐No ☐______H H :M M___________ ______Yes ☐No ☐D D M M M Y Y Y Y H H :M MYes ☐No ☐H H :M MYes ☐No ☐______H H :M M___________ ______Yes ☐No ☐D D M M M Y Y Y Y H H :M MYes ☐No ☐H H :M MYes ☐No ☐______H H :M M___________ ______Yes ☐No ☐D D M M M Y Y Y Y H H :M MYes ☐No ☐H H :M MYes ☐No ☐______H H :M M___________ ______Yes ☐No ☐D D M M M Y Y Y Y H H :M MYes ☐No ☐H H :M MYes ☐No ☐______H H :M MCONCOMITANT MEDICATIONSParticipant NumberCONCOMITANT MEDICATIONS – make multiple copies of this page if requiredMedicationnameFormulation Dose Units Frequency Route Date started Date stopped Ongoing? Indication___________ __________________________ D D M M M Y Y Y Y D D M M M Y Y Y Y Yes ☐No ☐______________________ __________________________ D D M M M Y Y Y Y D D M M M Y Y Y Y Yes ☐No ☐______________________ __________________________ D D M M M Y Y Y Y D D M M M Y Y Y Y Yes ☐No ☐______________________ __________________________ D D M M M Y Y Y Y D D M M M Y Y Y Y Yes ☐No ☐______________________ __________________________ D D M M M Y Y Y Y D D M M M Y Y Y Y Yes ☐No ☐______________________ __________________________ D D M M M Y Y Y Y D D M M M Y Y Y Y Yes ☐No ☐______________________ __________________________ D D M M M Y Y Y Y D D M M M Y Y Y Y Yes ☐No ☐______________________ __________________________ D D M M M Y Y Y Y D D M M M Y Y Y Y Yes ☐No ☐______________________ __________________________ D D M M M Y Y Y Y D D M M M Y Y Y Y Yes ☐No ☐______________________ __________________________ D D M M M Y Y Y Y D D M M M Y Y Y Y Yes ☐No ☐______________________ __________________________ D D M M M Y Y Y Y D D M M M Y Y Y Y Yes ☐No ☐___________ADVERSE EVENTSParticipant NumberADVERSE EVENTS – make multiple copies of this page if requiredAdverse event nameIntensity 1 Mild 2 Moderate 3 SevereIf SAE specify: 1Death2Life-threatening3Persistent or symptomatic disability or incapacity 4Hospitalisation or prolongation of hospitalisation 5Congenital anomaly or birth defect6Other important medical eventOnset Date D D M M M Y Y Y YEnd Date D D M M M Y Y Y Y OR Ongoing at the end of studyTherapy 1None3Other 2Drug4Drug and otherAction Taken with Study Drug 1Dose unchanged4Drug withdrawn99Not Known2Dose reduced5 Dose increased3Drug temporarily interruptedOutcome 1Recovered3Recovering with sequelae5Fatal 2Recovering 4Continuing 99Not KnownRelationship to Study drug 1Certain4Unlikely2Probable5Not related3Possible6UnclassifiedFINAL STUDY OUTCOMEParticipant NumberFINAL STUDY OUTCOMESubject has completed the study?1Completiondate :D D M M M Y Y Y YIf NOT completed specify last follow up date:D D M M M Y Y Y YReason not completed: (Tick only one box)1Significant non-compliance2Drug-related AE3Treatment failure4Consent withdrawn5Lost to follow-up6Other (specify) ____________________Remarks:Investigator's Statement: I have reviewed the data recorded in this CRF and confirm that the data are complete and accurateInvestigator (Full name): _________________________________________Investigator Signed? 1Signature Date: D D M M M Y Y Y Y。