最新的SQP验厂审核清单checklist及SQP评估标准
- 格式:pdf
- 大小:1.99 MB
- 文档页数:11


协作厂商质量体系评审标准
1. 评审资料预备
1) 需要提供的资料
(1) 依据此评审格式作业的自我评审结果
(2) 针对去年提出的不合格项目,提交改善方案。
(3) 针对去年提出的不合格项目,提交改善完成报告。
(4) 组织架构图,责任分配表,管理计划和作业指导书(受审方的一个产品的范例)。
将被扣各10分 => 将扣40分。
3) 如果不提交 : 第 (1),(2),(3),(4)点
2.Post management
1) 得分 : 少于450分 (90%)
- 准备和提交"改善方案&改善完成报告"
[附录: 改善(第1/第2格式]
2) 提交日期 : 改善方案 => 从评估日期开始计算5个工作日内提供
改善完成报告 => 下一个评审日期 [以上第1项的(1)~(3)点]
3) 如果不提交 : 当进行评审时将扣10分。
4) 提交方法 : 通过Mail发送给评审员。
3.稽核项目
请参考评审格式
4.判定标准
1) 总分 : 500 分(100%)
2) 判定标准 : 请参考评审格式的评分栏位。
3) 应该达到每个需求。
所有未结项目或是不合格项目应该依据实际标准进行改善。

SQP审核清单(中英文对照)1.ManagementcommitmentandcontinualimprovementFUNDAMENTA L:Thecompany"sseniormanagementshalldemonstratetheyarefullycom mittedtotheimplementationoftherequirementsoftheSupplierQual ificationProgram.Thisshallincludeprovisionofadequateresourc es,effectivecommunication,systemsofreviewandactionstakentoidentifyandeffectopportunit iesforimprovement.该公司的高级管理层应当证明他们会全力的供应商资格认证计划的要求执行。
这应包括提供足够的资源,有效的沟通,审查和改进系统和采取确定的行动效果的机会。
1.1Doesthecompany"smanagementdefineproductsafetyandqualityo bjectives?贵公司管理层确定产品的安全和质量目标?Moderate1.2Isthereviewofthemanagementsystemscarriedoutatlea stannually?是管理制度的检讨进行每年至少一次?Minor1.3Doesthereviewofthemanagementsystemsincludeevaluatio nofthefollowingitems?是否该管理系统的审查包括以下项目的评价?1.3.1Resultsofaudits(internal,secondandthirdpartyaudits);审计结果(内部,第二和第三方审计)1.3.2Followupactionsfrompreviousmanagementreviews;按照以往管理评审的跟踪措施1.3.3CustomerComplaintsandfeedback;客户投诉和反馈1.3.4Statusofpreventiveandcorrectiveactions;预防和纠正措施的状况1.3.5Processperformanceandproductconformity;过程绩效和产品的符合性1.3.6ChangesthatcouldaffecttheManagementSystems;可能影响管理体系的变化1.3.7Productsafetyandqualityobjectives;产品安全和质量目标1.3.8Riskmanagement;风险管理1.3.9Statutoryandregulatoryrequirements;法律法规要求1.3.10Resourceneeds;and资源需求,以及1.3.11Recommendationsforimprovement.改善建议Moderate1.4Arethedecisionsandactionsagreedduringthereviewco mmunicatedeffectivelytoappropriatestaff?被审查有效适当的工作人员沟通过程中的决策和行动一致?Minor1.5Aretheactionsimplementedwithintheagreedtimescales?是在商定的时间表落实的行动?Minor1.6Doesthecompany"smanagementprovidethefollowingoveral lresourcesrequiredtoimplementandimprovethequalitymanagement systemandriskassessmentplan,andtoaddresslegal,productsafety,andproductqualitymatters?请问公司的管理提供了实现和完善的质量管理体系和风险评估计划所需的下列整体资源,以解决法律,产品的安全性和产品质量问题?1.6.1personnel人员1.6.2infrastructure(e.g.,building,equipment,transportetc)基础设施(如建筑物,设备,交通工具...等)1.6.3workenvironment工作环境1.6.4financialsupport财政支持Moderate1.7Doesthecompanyhaveaprocessinplacetoidentifyoppor tunitiesforimprovements?公司是否制定一个过程,识别改进机会?Minor2RiskManagementFUNDAMENTAL:Thecompanyshallhaveaproductriskmanagementplan,basedonariskassessmentsystemwhichshallbesystematic,comprehensive,thorough,paniesmustbeawareofandmake referencetoup-to-datelegislation,productstandards,codesofpracticeanddevelopmentsinscienceortechnologythatmayi mpacttheriskconcerningtheirproductsandpackagingwheretheseex istinthecountriesofintendedsale.该公司须有产品的风险管理计划,根据风险评估制度,应是系统的,全面的,彻底的,全面实施和保持。

SQP、C-TPAT、GSV、SMETA、SEDEX、BSCI验厂辅导现场整改示范质量验厂、品质验厂现场整改示范1.工厂设备及工作环境1.01 下列区域是否有足够的照明:生产区、调试区、成品区、检验区、包装区及装卸区1.02 工厂是否保持清洁,在生产,加工和包装区域是否有秩序1.03 检验区是否独立,并设有检验桌及良好的通风系统1.04 工厂是否有害虫/霉菌和湿度的控制程序文件,是否有经常巡查(公司内部或第三方检查)1.05 在审核期间有没有发现窗户破损及房顶漏水可能导致产品污染1.06 工厂是否设有金属检验设备1.07 工厂是否有执行严格的利器管理程序,以保证剪刀、小刀、刀片、玻璃及断针等不会混入产品中1.08 工厂是否有效管控利器1.09 工厂是否有备用电力设备1.10 工厂是否有效防止异物混入1.11 工厂是否有效防止员工私人物品混入产品2.机器保养及设备校准2.01 工厂机器设备是否能保持清洁并运行良好2.02 机器、设备和工具是否有最近的维护/校准日期及计划日期的标识2.03 待修机器设备及生产工具是否已得到了适当标识以防止意外2.04 主要的生产工具、零部件和设备是否得到了适当清洁有序的存放,并且放在有标识的架子上,存放架是否有适当标识3.虫害控制及垃圾清理3.01 虫害是否有效控制3.02 生产垃圾如何处理4.质量管理体系4.01 工厂已建立了适合于其产品及工序的质量管理体系5.来料控制5.01 工厂是否实施物料先进先出(FIFO)体系5.02 工厂是否有进仓原物料、配件和部件的质量检验程序,作业指导书,及记录文件5.03 工厂对原料的进出数量进行了统计,并对其执行进行了有效监控及记录5.04 所需的来料测试仪器是否配备及保持在一个良好的状态5.05 所有的原物料是否有合适的标识,储存及可溯性5.06 工厂是否有文件程序和参考样品以确保来料符合规格5.07 工厂是否建立起适当的物料控制体系,以隔离不合格的原材料及避免意外污染5.08 工厂是否分离良品与不良材料,并标识所需更换的不良材料5.09 化学品和保养的物质是否妥善标识和储存,以防止污染的风险6.过程和生产控制6.01 工厂是否有专门进行产品开发的车间6.02 产品设计和开发部门是否在产品设计及开发过程中研究与应用产品安全特性,评估样式6.03 工厂是否在产品生产的各个阶段都建立了文件化的生产程序6.04 工厂是否在产品生产的各个阶段都建立了文件化的质量检验6.05 目前的生产质量是否可接受6.06 工厂是否有向工人提供足够的合格样品、首件样品、参照样品及作业指导书作为参照6.07 工厂是否有利用不合格品来向相关人员讲述常见的产品缺陷6.08 如果产品质量未能达以要求时,质量控制部门是否可追溯6.09 工厂对最终产品有没有实施100%功能性检查6.10 工厂是否有工作指引以确保产品包装是正确的6.11 包装区是否有足够的空间保证包装工序的正常运行6.12 成品箱是否保存于封锁区域,以避开阳光及雨水所造成的不良影响7.内部测试7.01 所有量规和测试设备是否有效校准7.02 工厂可获得相关的行业标准测试手册作为测试依据8.最终产品检验8.01 工厂有没有最终(也就是成品)检验程序,最终检验QC有没有工作指导书8.02 工厂的最终检验有没有做一些机械测试以确保产品的安全性8.03 最终检验QC有没有客户签样或参考样品,包装清单以及出货8.04 对于检验失败的产品,是否有在客户进行最终产品检验前进行纠正并重新检验。
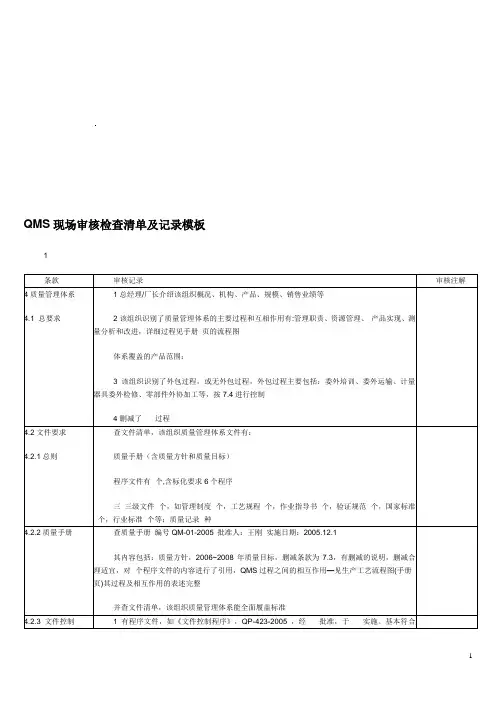
QMS现场审核检查清单及记录模板1条款审核记录审核注解4质量管理体系4.1 总要求1总经理/厂长介绍该组织概况、机构、产品、规模、销售业绩等2该组织识别了质量管理体系的主要过程和互相作用有:管理职责、资源管理、产品实现、测量分析和改进,详细过程见手册页的流程图体系覆盖的产品范围:×××3该组织识别了外包过程,或无外包过程,外包过程主要包括:委外培训、委外运输、计量器具委外检修、零部件外协加工等,按7.4进行控制4删减了×××过程4.2文件要求4.2.1总则查文件清单,该组织质量管理体系文件有:质量手册(含质量方针和质量目标)程序文件有×个,含标化要求6个程序三三级文件×个,如管理制度×个,工艺规程×个,作业指导书×个,验证规范×个,国家标准×个,行业标准×个等;质量记录×种4.2.2质量手册查质量手册编号QM-01-2005 批准人:王刚实施日期:2005.12.1其内容包括:质量方针,2006~2008年质量目标,删减条款为7.3,有删减的说明,删减合理适宜,对×个程序文件的内容进行了引用,QMS过程之间的相互作用—见生产工艺流程图(手册页)其过程及相互作用的表述完整并查文件清单,该组织质量管理体系能全面履盖标准4.2.3 文件控制1有程序文件,如《文件控制程序》,QP-423-2005 ,经×××批准,于×××实施。
基本符合标准要求2出示了公司受控文件清单,内容有名称、编号、版次、编制、审批、实施日期、备注等,从文件受控清单中抽查文件可识别文件的修改状态,如A版/0次修改,或A版/1次修改3查三份作业文件(标准/规范/作业指导书….)记录审批人和日期4查文件的发放和回收记录,记录是否发到使用部门、接受人、日期、及发放人5查文件更改记录,有批准人×××,批准日期×××,更改人×××,更改日期×××6查作废文件管理记录,作废文件保留是否有标识、是否专人保存,现场未发现作废文件7查外来文件清单:a顾客来文,b与产品有关的法律法规(国家标准、行业标准和主管部门的强制规定)8抽3个使用文件的现场,有:a现行有效版本的文件,b有控制编号9查上述2-3份文件,记录文件本身保持是否清晰、易于识别4.2.4 记录控制1有程序文件,如《记录控制程序》,QP-424-2005 ,包括记录的标识、贮存、保护、检索、保存期限和处理,从记录清单中可体现,经×××批准,于×××实施。

SQP验厂的具体要求是什么?标准是怎么样的?如何准备SQP验厂?SQP是ITS整出来的合格供应商评估计划85分以上通过,可以取得证书,有效期一年,目前DG客人对此有要求。
审核分8个部分,管理承诺与持续改进、风险评估、质量管理体系、现场与设施管理、产品控制、产品测试、过程控制与员工培训,除第二点以外都是ISO9001的要求。
风险控制是QS9000里的要求,要利用FMEA,RPN等一些基本工具。
SQP验厂的中文清单SQP 文件清单1. 组织架构图2. 责任和/ 或职责描述3. 质量体系程序(包括:质量政策、目标、质量管理体系手册和程序,以及其它流程)4. 管理层审查记录5. 内部审核文件(审核计划、报告等)6. 供应商监管文件(供应商核准程序/ 标准、已核准的供应商清单、供应商评估记录、持续表现监督等)7. 文件监管程序和记录(包括记录保管)8. 产品规格/ 要求9. 检验要求说明、可接受的标准、检验和测试报告(包括IQC 的阶段、过程中和最终检验)10. 工作要求说明/ 每项生产工序的工艺技术标准11. 生产日程安排/ 记录12. “事故”的界定和报告程序13. 产品召回程序14. 客户投诉记录15. 整改行动报告(关于事故、内部审核、投诉等)16. 追溯系统中的测试报告17. 设备维护文件(计划、程序、记录等)18. 监督和测试设备的校准(计划、程序、记录等)19. 清理日程安排和程序20. 已核准的化学品清单,附带相应的品牌/ 生产商21. 有害物管控文件(受过培训的管控人员的名单、外部有害物管控机构的联系方式、有害物管控检查记录、投饵记录,等)——》虫害控制程序22. 整个生产流程的“风险评估”记录/ 计划23. 最终产品的风险评估记录24. 产品测试步骤/ 程序25. 实验室测试报告(包括涂料、涂层和非涂料部件中的铅和重金属、硬件、标签、最终产品,等)26. 夹杂物监控记录(如:金属探测记录、金属探测器的日常敏感物检查记录,等)27. 断针处理程序(如适用的话)28. 生产前会议记录29. 程序控制计划30. 培训(程序、培训需求和记录。
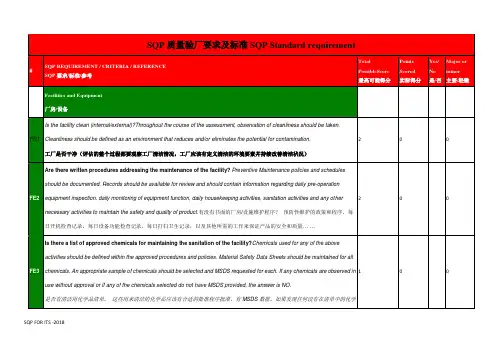
1. Senior management commitment and continual improvementCL-SQP QuestionsQuestionRisk Weighting1.1Does the company's management define product safety and quality objectives?(产品安全及质量目标的定义)Moderate 1.2Is the review of the management systems carried out at least annually?(管理系统的审核)Minor 1.3Does the company's management provide the following overall resources required toimplement and improve the quality management system and risk assessment plan,and to address legal, product safety, and product quality matters?1.3.1 personnel1.3.2 infrastructure (e.g., building, equipment, transport…etc)1.3.3 work environment1.3.4 financial supportModerate1.4Does the company have a process in place to identify opportunities forimprovements?Minor2 Risk Management2.1Legislative and Safety RequirementsCL-SQP QuestionsQuestionRisk Weighting2.1.1Is the company aware of relevant legislation, mandatory standards andindustry/customer codes of practice applicable to the product in the countries ofintended sale?(相关法律、强制标准、行业准则)Moderate2.1.2Does the company have a process in place for ensuring it is kept informed of changesto relevant legislation, standards etc?Moderate 2.2Risk Assessment (Documentation)CL-SQP QuestionsQuestionRisk Weighting2.2.1Does the company establish a product risk assessment for each product or a groupof similar products, e.g., FMEA?(产品风险评估)Moderate 2.2.2Does the product risk assessment determine the following?2.2.2.1 Possible Hazard/Risk Identification (e.g., Chemical, Physical, Regulatory);2.2.2.2 Risk level for each identified hazard/risk (e.g., Severe, High, Moderate,Slight);2.2.2.3 Whether the risk is acceptable considering the probability or likelihood andthe severity and potential consequences of the effects on consumer safety (e.g., NotAcceptable, Review & Approve, Acceptable)Moderate2.2.3Does the company conduct a process risk assessment of hazards potentiallyintroduced during the production, packaging or storage processes?(过程风险评估)Moderate2.2.4Does the process risk assessment identify the following?2.2.4.1 A list of potential risk or hazards in the production process2.2.4.2 Control points to manage the identified risk to acceptable level2.2.4.3 Accept / reject limits defined for each control2.2.4.4 Corrective action to be taken where a CCP is out of control2.2.4.5 Responsibility of Control Points2.2.4.6 Records of monitoring & reviews ModerateSupplier Qualification Program (SQP) 'Generic Hardlines' 1-day Checklist3 Management System3.2General Documentation Requirements3.10Business Continuity PlanningThe company shall have procedures in place to identify methods of ensuring business continuity in the case of major incidents/threats to a business.4. Site Standards and Facilities5. Product Control5.6 Product Transport, Storage and Distribution5.8 Product ClaimsThe company shall have procedures in place to validate any declared product information or6. Product Conformity Assessment7. Process ControlCopyright © 2010 Intertek, All Rights Reserved11。
Document No.: SQP-D02 Issue Date: 25 Nov 2011 Issue No.: 00 Page 1 of 1©2011 Intertek, All Rights ReservedThe Intertek Group is the owner of the copyright in the material and intellectual know-how presented. No parts in this material maybe reproduced, adapted or distributed outside of your company without the written consent of the Intertek Group other than to the extent necessary to view the material.SQP Document List1. Organization chart2. Responsibility and/or job description,3. Quality System Procedures (e.g., quality policy, objectives, manual and procedures for theQuality Management System and other processes)4. Management review records5. Internal audit documents (audit plan, report, etc.)6. Supplier Control documents (supplier approval procedure / criteria, list of approval supplier list, supplier evaluation records, on-going performance monitoring, etc.)7. Document control procedure and records (including record keeping)8. Product specifications/requirements9. Inspection Instructions, acceptance criteria and inspection & testing reports (including thestages of IQC, In-process and Final inspection)10. Work instructions / workmanship standards for each manufacturing process11. Production schedules/records12. Procedure for defining and reporting of “incident”13. Product recall procedure14. Customer complaints records15. Corrective action reports (related to incident, internal audit, complaint, etc)16. Test records on Traceability system17. Equipment maintenance documents (plan, procedure, record, etc)18. Calibration of monitoring & measuring devices (plan, procedures, records, etc)19. Cleaning schedule and procedure20. List of Approved Chemicals with Corresponding Brands / Manufacturers21. Pest control documents (list of trained pest control staff, contract with external pest controlagency, pest control inspection record, bait documentation, etc)22. Record / plan for “Risk Assessment” of the entire manufacturing processes23. Risk assessment records of final product24. Product testing procedure/program25. Laboratory test reports (including lead and heavy metals content in paints, coatings and non-paint components, hardware, labels, final product, etc).26. Monitoring records of foreign body detectors (e.g. metal detection records, daily sensitivitychecking records of metal detectors…etc)27. Broken needle procedure & records (if applicable)28. Pre-production meetings records29. Process Control Plan30. Training (procedure, training needs & records)SQP文件清单1. 组织架构图2. 责任和 / 或职责描述3. 质量体系程序 (包括:质量政策、目标、质量管理体系手册和程序,以及其它流程)4. 管理层审查记录5. 内部审核文件 (审核计划、报告等)6. 供应商监管文件 (供应商核准程序 / 标准、已核准的供应商清单、供应商评估记录、持续表现监督等)7. 文件监管程序和记录 (包括记录保管)8. 产品规格 / 要求9. 检验要求说明、可接受的标准、检验和测试报告 (包括IQC的阶段、过程中和最终检验)10. 工作要求说明 / 每项生产工序的工艺技术标准11. 生产日程安排 / 记录12. “事故”的界定和报告程序13. 产品召回程序14. 客户投诉记录15. 整改行动报告 (关于事故、内部审核、投诉等)16. 追溯系统中的测试报告17. 设备维护文件 (计划、程序、记录等)18. 监督和测试设备的校准 (计划、程序、记录等)19. 清理日程安排和程序20. 已核准的化学品清单,附带相应的品牌 / 生产商21. 有害物管控文件 (受过培训的管控人员的名单、外部有害物管控机构的联系方式、有害物管控检查记录、投饵记录,等)22. 整个生产流程的“风险评估”记录 / 计划23. 最终产品的风险评估记录24. 产品测试步骤 / 程序25. 实验室测试报告 (包括涂料、涂层和非涂料部件中的铅和重金属、硬件、标签、最终产品,等)26. 夹杂物监控记录 (如:金属探测记录、金属探测器的日常敏感物检查记录,等)27. 断针处理程序 (如适用的话)28. 生产前会议记录29. 程序控制计划30. 培训 (程序、培训需求和记录)Supplier Qualification Program (SQP) Assessment CriteriaSection 1 - Management Commitment and Continual ImprovementAssesses the degree to which a company’s management is committed to providing adequate assessment resources, effective communication, systems of review that identify actions taken and opportunities for improvement.Section 2 - Risk Management SystemsThe company shall have management systems for assuring product safety, legality and quality. (Applies basic risk assessment principles)♦Legislative and Safety Requirements - the company must be aware of and make reference to up-to-date legislation, product standards, codes of practice and developments in science or technology that may impact the risk concerning their products and packaging in the countries of intended sales.♦Risk Assessment - the company shall have risk management plan for product and production processes, based on a risk assessment system which is systematic, comprehensive, thorough, fully implemented and maintained.♦Risk Assessment Verification - the company shall conduct the verification of risk assessment by competent person. Section 3 - Quality Management SystemsThe company shall develop, document and implement an effective quality management system, and address the following areas: ♦Policy Statement♦Control of Document - All documents, records and data impacting the management of product safety, legality and quality are present and effectively controlled♦Control of Records♦Specifications♦Responsibility and Authority - clearly defined and documented organizational structure♦Internal Audit♦Purchasing, Supplier & Sub-contractor Approval and Performance Monitoring♦Customer Property - customer property (including intellectual property) should be subject to controls♦Corrective and Preventive Action - procedures to record, investigate, analyse and correct cause(s) of non-conforming products or failure(s) to meet standards, specifications and procedures♦Identification & Traceability - a system to identify and trace product lots including raw materials, components and packaging materials for all phases of the production process (receipt of materials to product dispatch) ♦Incident, Product Withdrawal and Product Recall - a plan and system to effectively manage product withdrawal and product recall processes♦Business Continuity Planning - plan for identifying methods that ensure business continuity in the event of major incidents/threats to a business.Supplier Qualification Program (SQP) Assessment Criteria♦Customer Focus♦Complaint HandlingSection 4 - Site and Facilities ManagementThe site and the facilities must be maintained and managed so as to prevent or minimize contamination and assure the production of safe and legal finished products. Areas of focus include:♦Site Location and Perimeter♦Factory Layout, Product Flow and Segregation♦Staff Facilities - such facilities must be designed and operated so that they sufficiently minimise all risk of product contamination♦Cleaning and Hygiene Practices♦Waste/Waste Disposal - systems for the collection, collation and disposal of waste material♦Pest Control - controls and practices for minimizing the risk of pest infestationSection 5 – Product ControlThe company shall demonstrate effective control of its products to ensure safety, legality and quality including the following areas: ♦Reference Samples (pre-production and production) - procedures in place for the selection, handling, storage, approval and use of reference samples♦Chemical Control - chemical composition of products and chemicals used in the manufacture or processing of products shall be identified, monitored and recorded as required by legislation in the country of sale and / or manufacture ♦Product Packaging Materials♦Control of non-conforming materials - non-conforming materials, components and products shall be clearly identified, labelled, quarantined, investigated and documented♦Special Handling - handling requirements shall be in place for specific materials♦Product Transport, Storage and Distribution♦Stock Control and Product Release – procedures shall be in place to prevent release of finished product unless all agreed procedures have been followedSection 6 – Product Testing and Product Claims♦Product Testing – the company shall have a suitable, sufficient and validated testing program to ensure the safe, legal production of products that meet required quality standards.♦Product Claims – the company shall validate any declared product information or claims made regarding its products and monitor compliance with such claims necessary.Supplier Qualification Program (SQP) Assessment CriteriaSection 7 – Process ControlThe company shall demonstrate effective control of all operations undertaken, to ensure product safety, legality and quality – as well as ensure that the processes and equipment employed are capable of producing consistently safe and legal product with the desired quality characteristics. The following areas shall be addressed:Generic Hardline♦Control of Operations - ensure processes and equipment employed are capable of producing consistently safe and legal product with the desired quality characteristics♦Control of Incoming Components and Raw Materials♦In-Process and Final Inspections - to assure delivery of safe, legal product of the required quality♦Foreign Body Detection and Control♦Calibration and Control of Measuring and Monitoring Devices (for purposes of monitoring product safety, quality and legality) - shall be identified and calibrated to a recognized national or international standard♦Equipment & Tooling Maintenance♦Final Product Packing and ControlGarments♦Sample Preparation, Pattern & Marker♦Pre-production Activity♦Control of Incoming Components and Raw Materials♦Spreading, Cutting and Bundling♦Knitting♦Embroidery / Appliqué♦Printing♦Fusing♦Sewing♦Linking♦Washing♦Mending and Stitching♦Attachment♦Finishing and Pressing♦Final Inspections - to assure delivery of safe, legal product of the required quality♦Metal Detection and Control♦Final Packing♦Final Audit♦Calibration and Control of Measuring and Monitoring Devices (for purposes of monitoring product safety, quality and legality) - shall be identified and calibrated to a recognized national or international standardSupplier Qualification Program (SQP) Assessment CriteriaToys♦Pre-production Activity♦Control of Incoming Components and Raw Materials♦Molding (Injection molding, Blow molding, Insert molding, Roto cast molding, Diecast molding, Vacuum Forming, etc) ♦Die Cutting for Fabric/Rigid Plastic/PVC Sheet or Laminates, etc.♦Forming and Stamping♦Decoration (Spray Decoration, Coating, Tempo, Hand Painting, Printing)♦Sonic Welding Process♦Gluing Process♦Assembly (Manual/Automated)♦Cutting♦Sewing / Hair Rooting♦Attachment (e.g., eyes, noses, buttons, snaps or other metal press fasteners)♦Stuffing♦Metal Detection and Control♦Final Inspections - to assure delivery of safe, legal product of the required quality♦Final Product Packing and Control♦Calibration and Control of Measuring and Monitoring Devices (for purposes of monitoring product safety, quality and legality) - shall be identified and calibrated to a recognized national or international standardFootwear♦Footwear Manufacturing - Sample Development Activity♦Pre-production Activity (Footwear)♦Shoe Sole Bonding Test Process♦Wear Test Process♦Control of Incoming Components and Raw Materials (Footwear)♦Cutting♦Preparation / Secondary Processing♦Stitching♦Injection Molding♦Bottoming♦Assembly Operation – Lasting♦Autoclave Process (Vulcanizing)♦Finishing♦Final Inspections (Footwear)Supplier Qualification Program (SQP) Assessment Criteria♦Metal Detection and Control♦Final Packing♦Storage♦'Lasts' Management♦Equipment & Tooling Maintenance (Footwear)♦Calibration and Control of Measuring and Monitoring Devices (for purposes of monitoring product safety, quality and legality) - shall be identified and calibrated to a recognized national or international standardSection 8 – Personnel Training and CompetencyThe company shall ensure that personnel performing work affecting product safety, legality and quality are demonstrably competent to carry out their activity, as a result of training, work experience and / or qualification.Americas Asia EMEAElma Isakovic Samuel Lau Catherine BeareTel: +1 732 394 5367 Tel: +852 3760 6334 Tel: +44 78 7237 9094elma.isakovic@ u@ catherine.beare@。