Abstract Journal of Integrative Neuroscience Topical Review Models of Dendritic and Astrocy
- 格式:pdf
- 大小:870.60 KB
- 文档页数:40

上海交通大学基础医学院高小玲课题组发表纳米递药系统脑内命运及其调控机制研究的新成果佚名【期刊名称】《上海交通大学学报(医学版)》【年(卷),期】2024(44)1【摘要】2023年12月29日,上海交通大学基础医学院药理学与化学生物学系高小玲教授联合上海中医药大学陈红专教授和加拿大多伦多大学郑岗教授,在国际权威杂志《自然·纳米技术》(Nature Nanotechnology)正式发表了题为Intracerebral fate of organic and inorganic nanoparticles is dependent on microglial extracellular vesicle function的研究论文。
该杂志同期配发Intracerebral fate of engineered nanoparticles新闻和评述。
该研究通过阐明不同纳米递药系统脑清除差异的原因,揭示小胶质细胞的细胞外囊泡(EVs)对于脑内纳米粒的清除至关重要;发现通过激活ERK1/2通路.【总页数】1页(P97-97)【正文语种】中文【中图分类】G64【相关文献】1.狄文课题组在纳米靶向治疗卵巢研究方面取得新进展上海交通大学医学院附属仁济医院研究人员构建新型纳米载药递送系统2.上海交通大学基础医学院高小玲课题组发表多功能仿生纳米结构靶向神经血管单元改善阿尔茨海默病认知障碍的新成果3.上海交通大学医学院附属瑞金医院蒙国宇课题组发表生物被膜形成机制研究成果4.上海交通大学医学院高小玲课题组提出“纳米刹车”新策略,阻断线粒体功能障碍级联反应并改善阿尔茨海默病认知功能障碍5.上海交通大学基础医学院方超课题组报道基于巨噬细胞的肿瘤靶向递药新技术因版权原因,仅展示原文概要,查看原文内容请购买。


Advances in Psychology 心理学进展, 2023, 13(10), 4743-4750Published Online October 2023 in Hans. https:///journal/aphttps:///10.12677/ap.2023.1310597近十年我国国内记忆领域的研究热点分析许奕蓉福建师范大学心理学院,福建福州收稿日期:2023年8月30日;录用日期:2023年10月17日;发布日期:2023年10月27日摘要为了从宏观上把握中国记忆领域的研究亮点和趋势,本文采用文献计量学方法,对2011~2021年发表的2176篇文献中的关键词进行分析。
通过共现和聚类分析发现,工作记忆、前瞻记忆、错误记忆和内隐记忆是近十年来中国记忆领域研究中最热门的四个研究主题。
目前的研究仍以基础研究为主,应用研究不断增多。
未来还需要对记忆机制,特别是对记忆障碍人群的研究、记忆训练的可持续性研究和跨领域合作等方面进行深入的研究。
关键词记忆,研究热点,科学知识图谱An Analysis of Hot Research Spots inMemory Field in China in Recent Ten YearsYirong XuSchool of Psychology, Fujian Normal University, Fuzhou FujianReceived: Aug. 30th, 2023; accepted: Oct. 17th, 2023; published: Oct. 27th, 2023AbstractIn order to macroscopically grasp the research highlights and trends of the memory in China, this paper used the bibliometrics methods to analyze keywords from 2176 publications published between 2011 and 2021. Based on co-occurrence and cluster analysis, this paper discovered that the working memory, prospective memory, false memory, and implicit memory are the four most popular study themes in China’s memory field research over the last ten years. The current study is still primarily based on basic research, with the number of applied studies on the rising. In the future, more research into the memory mechanisms, especially people of memory impaired, memory training sustainability research and cross domain cooperation will be required.许奕蓉KeywordsMemory, Research Hotpots, Mapping Knowledge DomainCopyright © 2023 by author(s) and Hans Publishers Inc.This work is licensed under the Creative Commons Attribution International License (CC BY 4.0)./licenses/by/4.0/1. 引言1.1. 记忆领域研究背景记忆是指在人脑中积累和保存个体经验的心理过程,与注意、思维等认知环节共同构成了人们的认知能力,对人们的学习生活和社会适应起着至关重要的作用。

Young Yun Kim的跨文化适应与传播整合理论:概念、模
型与评价
叶春丽
【期刊名称】《东南传播》
【年(卷),期】2022()9
【摘要】美国韩裔传播学者Young Yun Kim提出的跨文化适应与传播整合理论
是目前传播学角度跨文化适应研究较为完善的理论之一。
该理论整合了宏观与微观、长期和短期、问题/学习视角、同化主义和多元主义的研究成果,认为跨文化适应的核心是个体通过新文化学习和旧文化削弱实现的内在转变,其实质是个体与环境之
间的契合,而传播是驱动这一过程的关键。
该理论诞生以来在国外跨文化传播研究
中得到广泛应用,却尚未为国内新闻传播学界所熟知。
本文介绍了该理论的概念、
模型和评价,并评述了国内研究现状及其在我国传播研究中的应用前景。
【总页数】5页(P88-92)
【作者】叶春丽
【作者单位】玉溪师范学院文学院新闻系
【正文语种】中文
【中图分类】H31
【相关文献】
1.跨文化传播双维度理论中的整合策略
2.概念隐喻理论对跨文化传播的阐释力
3.助力来华留学生跨文化适应与中国文化传播——以基于“EEE”模式的来华留学生跨
文化适应服务实践为例4.顺应与概念整合理论视阈下希尼诗歌隐喻研究5.顺应与概念整合理论视阈下希尼诗歌隐喻研究
因版权原因,仅展示原文概要,查看原文内容请购买。

1.[A] selected [B] prepared [C] obliged [D] pleased2.[A] unique [B] particular [C] special [D] rare3.[A] of [B] with [C] in [D] against4.[A] subsequently [B] presently [C] previously [D] lately5.[A] Only [B] So [C] Even [D] Hence6.[A] thought [B] sight [C] cost [D] risk7.[A] advises [B] suggests [C] protests [D] objects8.[A] progress [B] fact [C] need [D] question9.[A] attaining [B] scoring [C] reaching [D] calculating10.[A] normal [B] common [C] mean [D] total11.[A] unconsciously[B] disproportionately[C] indefinitely[D] unaccountably12.[A] missions [B] fortunes [C] interests [D] careers13.[A] affirm [B] witness [C] observe [D] approve14.[A] moreover [B] therefore [C] however [D] meanwhile15.[A] given up [B] got over [C] carried on [D] put down16.[A] assessing [B] supervising [C] administering [D] valuing17.[A] development [B] origin [C] consequence [D] instrument18.[A] linked [B] integrated [C] woven [D] combined19.[A] limited [B] subjected [C] converted [D] directed20.[A] paradoxical [B] incompatible [C] inevitable [D] continuousSection II Reading ComprehensionPart ADirections:Read the following four texts. Answer the questions below each text by choosing [A], [B], [C] or [D]. Mark your answers on ANSWER SHEET 1. (40 points)Text 1While still catching up to men in some spheres of modern life, women appear to be way ahead in at least one undesirable category. “Women are particularly susceptible to developing depression and anxiety disorders in response to stress compared to men,” according to Dr. Yehuda, chief psychiatrist at New York’s Veteran’s Administration Hospital.Studies of both animals and humans have shown that sex hormones somehow affect the stress response, causing females under stress to produce more of the trigger chemicals than do males under the same conditions. In several of the studies, when stressed-out female rats had their ovaries (the female reproductive organs) removed, their chemical responsesbecame equal to those of the males.Adding to a woman’s increased dose of stress chemicals, are her increased “opportunities” for stress. “It’s not necessarily that women don’t cope as well. It’s just that they have so much more to cope with,” says Dr. Yehuda. “Their capacity for tolerating stress may even be greater than men’s,” she observes, “it’s just that they’re dealing with so many more things that they become worn out from it more visibly and sooner.”Dr. Yehuda notes another difference between the sexes. “I think that the kinds of things that women are exposed to tend to be in more of a chronic or repeated nature. Men go to war and are exposed to combat stress.Men are exposed to more acts of random physical violence. The kinds of interpersonal violence that women are exposed to tend to be in domestic situations, by, unfortunately, parents or other family members, and they tend not to be one-shot deals. The wear-and-tear that comes from these longer relationships can be quite devastating.”Adeline Alvarez married at 18 and gave birth to a son, but was determined to finish college. “I struggled a lot to get the college degree. I was living in so much frustration that that was my escape, to go to school, and get ahead and do better.” Later, her marriage ended and she became a single mother. “It’s the hardest thing to take care of a teenager, have a job, pay the rent, pay the car payment, and pay the debt.I lived from paycheck to paycheck.”Not everyone experiences the kinds of severe chronic stresses Alvarez describes. But most women today are coping with a lot of obligations, with few breaks, and feeling the strain. Alvarez’s experienc e demonstrates the importance of finding ways to diffuse stress before it threatens your health and your ability to function.21. Which of the following is true according to the first two paragraphs?[A] Women are biologically more vulnerable to stress.[B] Women are still suffering much stress caused by men.[C] Women are more experienced than men in coping with stress.[D] Men and women show different inclinations when faced with stress.22. Dr. Yehuda’s research suggests that women .[A] need extra doses of chemicals to handle stress[B] have limited capacity for tolerating stress[C] are more capable of avoiding stress[D] are exposed to more stress23. According to Paragraph 4, the stress women confront tends to be .[A] domestic and temporary[B] irregular and violent[C] durable and frequent[D] trivial and random24. The sentence “I lived from paycheck to paycheck.” (Line 5, Para. 5) shows that .[A] Alvarez cared about nothing but making money[B] Alvarez’s salary barely covered her household expense s[C] Alvarez got paychecks from different jobs[D] Alvarez paid practically everything by check25. Which of the following would be the best title for the text?[A] Strain of Stress: No Way Out?[B] Response to Stress: Gender Difference[C] Stress Analysis: What Chemicals Say?[D] Gender Inequality: Women Under StressText 2It used to be so straightforward. A team of researchers working together in the laboratory would submit the results of their research to a journal. A journal editor would then remove t he author’s names and affiliations from the paper and send it to their peers for review. Depending on the comments received, the editor would accept thepaper for publication or decline it. Copyright rested with the journal publisher, and researchers seeking knowledge of the results would have to subscribe to the journal.No longer. The Internet—and pressure from funding agencies, who are questioning why commercial publishers are making money fromgovernment–funded research by restricting access to it—is making access to scientific results a reality. The Organization for Economic Co-operation and Development (OECD) has just issued a report describing the far-reaching consequences of this. The report, by John Houghton of Victoria University in Australia and Graham Vickery of the OECD, makes heavy reading for publishers who have, so far, made handsome profits. But it goes further than that. It signals a change in what has, until now, been a key element of scientific endeavor.The value of knowledge and the return on the public investment in research depends, in part, upon wide distribution and ready access. It is big business. In America, the core scientific publishing market is estimated at between $7 billion and $11 billion. The International Association of Scientific, Technical and Medical Publishers says that there are more than 2,000 publishers worldwide specializing in these subjects. They publish more than 1.2 million articles each year in some 16,000 journals.This is now changing. According to the OECD report, some 75% of scholarly journals are now online. Entirely new business models are emerging; three main ones were identified by the report’s authors. There is the so-called big deal, where institutional subscribers pay for access to a collection of online journal titles through site-licensing agreements. There is open-access publishing, typically supported by asking the author (orhis employer) to pay for the paper to be published. Finally, there are open-access archives, where organizations such as universities or international laboratories support institutional repositories. Other models exist that are hybridsof these three, such as delayed open-access, where journals allow only subscribers to read a paper for the first six months, before making it freely available to everyone who wishes to see it. All this could change the traditional form of the peer-review process, at least for the publication of papers.26. In the first paragraph, the author discusses .[A] the background information of journal editing[B] the publication routine of laboratory reports[C] the relations of authors with journal publishers[D] the traditional process of journal publication27. Which of the following is true of the OECD report?[A] It criticizes government-funded research.[B] It introduces an effective means of publication.[C] It upsets profit-making journal publishers.[D] It benefits scientific research considerably.28. According to the text, online publication is significant in that .[A] it provides an easier access to scientific results[B] it brings huge profits to scientific researchers[C] it emphasizes the crucial role of scientific knowledge[D] it facilitates public investment in scientific research29. With the open-access publishing model, the author of a paper is required to .[A] cover the cost of its publication[B] subscribe to the journal publishing it[C] allow other online journals to use it freely[D] complete the peer-review before submission30. Which of the following best summarizes the text?[A] The Internet is posing a threat to publishers.[B] A new mode of publication is emerging.[C] Authors welcome the new channel for publication.[D] Publication is rendered easily by online service.Text 3In the early 1960s Wilt Chamberlain was one of the only three players in the National Basketball Association (NBA) listed at over seven feet. If he had played last season, however, he would have been one of 42. The bodies playing major professional sports have changed dramatically over the years, and managers have been more than willing to adjust team uniforms to fit the growing numbers of bigger, longer frames.The trend in sports, though, may be obscuring an unrecognized reality: Americans have generally stopped growing. Though typically about two inches ta ller now than 140 years ago, today’s people—especially those born to families who have lived in the U.S. for many generations—apparently reached their limit in the early 1960s.And they aren’t likely to get any taller. “In the general population today, at t his genetic, environmental level, we’ve pretty much gone as far as we can go,” says anthropologist WilliamCameron Chumlea of Wright State University. In the case of NBA players, their increase in height appears to result from the increasingly common practice of recruiting players from all over the world.Growth, which rarely continues beyond the age of 20, demands calories and nutrients—notably, protein—to feed expanding tissues. At the start of the 20th century, under-nutrition and childhood infections got in the way. But as diet and health improved, children and adolescents have, on average, increased in height by about an inch and a half every 20 years, a pattern known as the secular trend in height. Yet according to the Centers for Disease Control and Prevention, average height—5'9" for men, 5'4" for women—hasn’t really changed since 1960.Genetically speaking, there are advantages to avoiding substantial height. During childbirth, larger babies have more difficulty passing through the birth canal. Moreover, even though humans have been upright for millions of years, our feet and back continue to struggle with bipedal posture and cannot easily withstand repeated strain imposed by oversize limbs. “There are some real constraints that are set by the genetic architecture of the individual organism,” says anthropologist William Leonard of Northwestern University.Genetic maximums can change, but don’t expect this to happen soon. Claire C. Gordon, senior anthropologist at the Army Research Center in Natick, Mass., ensures that 90 percent of the uniforms and workstations fit recruits without alteration. She says that, unlike those for basketball, the length of military uniforms has not changed for some time. And if you need to predict human height in the near future to design a piece of equipment, Gordon says that by and large, “you could use today's data and feel fairly confident.”31. Wilt Chamberlain is cited as an example to .[A] illustrate the change of height of NBA players[B] show the popularity of NBA players in the U.S.[C] compare different generations of NBA players[D] assess the achievements of famous NBA players32. Which of the following plays a key role in body growth according to the text?[A] Genetic modification.[B] Natural environment.[C] Living standards.[D] Daily exercise.33. On which of the following statements would the author most probably agree?[A] Non-Americans add to the average height of the nation.[B] Human height is conditioned by the upright posture.[C] Americans are the tallest on average in the world.[D] Larger babies tend to become taller in adulthood.34. We learn from the last paragraph that in the near future .[A] the garment industry will reconsider the uniform size[B] the design of military uniforms will remain unchanged[C] genetic testing will be employed in selecting sportsmen[D] the existing data of human height will still be applicable35. The text intends to tell us that .[A] the change of human height follows a cyclic pattern[B] human height is becoming even more predictable[C] Americans have reached their genetic growth limit[D] the genetic pattern of Americans has alteredText 4In 1784, five years before he became president of the United States, George Washington, 52, was nearly toothless. So he hired a dentist to transplant nine teeth into his jaw—having extracted them from the mouths of his slaves.That’s a far different image from the cherry-tree-chopping George most people remember from their history books. But recently,many historians have begun to focus on the role slavery played in the lives of the founding generation. They have been spurred in part by DNA evidence made available in 1998, which almost certainly proved Thomas Jefferson had fathered at least one child with his slave Sally Hemings. And only over the past 30 years have scholars examined history from the bottom up. Works of several historians reveal the moral compromises made by the nation’s early leaders and the fragile nature of the country’s infancy. More significant, they argue that many of the Founding Fathers knew slavery was wrong—and yet most did little to fight it.More than anything, the historians say, the founders were hampered by the culture of their time. While Washington and Jefferson privately expressed distaste for slavery, they also understood that it was part of the political and economic bedrock of the country they helped to create.For one thing, the South could not afford to part with its slaves. Owning slaves was “like having a large bank account,” says Wiencek, auth or of An Imperfect God: George Washington, His Slaves, and the Creation of America. The southern states would not have signed the Constitution without protections for the “peculiar institution,” including a clause that counted a slave as three fifths of a man for purposes of congressional representation.And the statesmen’s political lives depended on slavery. The three-fifths formula handed Jefferson his narrow victory in the presidential election of 1800 by inflating the votes of the southern states in the Electoral College. Once in office, Jefferson extended slavery with the Louisiana Purchase in 1803; the new land was carved into 13 states, including three slave states.Still, Jefferson freed Hemings’s children—though not Hemings herself or his approximately 150 other slaves. Washington, who had begun to believe that all men were created equal after observing the bravary of the black soldiers during the Revolutionary War, overcame the strong opposition of his relatives to grant his slaves their freedom in his will. Only a decade earlier, such an act would have required legislative approval in Virginia.36. George Washington’s dental surgery is mentioned to .[A] show the primitive medical practice in the past.[B] demonstrate the cruelty of slavery in his days.[C] stress the role of slaves in the U.S. history.[D] reveal some unknown aspect of his life.37. We may infer from the second paragraph that .[A] DNA technology has been widely applied to history research.[B] in its early days the U.S. was confronted with delicate situations.[C] historians deliberately made up some stories of Jefferson’s life.[D] political compromises are easily found throughout the U.S. history.38. What do we learn about Thomas Jefferson?[A] His political view changed his attitude towards slavery.[B] His status as a father made him free the child slaves.[C] His attitude towards slavery was complex.[D] His affair with a slave stained his prestige.39. Which of the following is true according to the text?[A] Some Founding Fathers benefit politically from slavery.[B] Slaves in the old days did not have the right to vote.[C] Slave owners usually had large savings accounts.[D] Slavery was regarded as a peculiar institution.40. Washington’s decision to free slaves originated from his .[A] moral considerations.[B] military experience.[C] financial conditions.[D] political stand.Part BDirections:In the following text, some segments have been removed. For Questions 41-45, choose the most suitable one from the list A-G to fit into each ofthe numbered blanks. There are two extra choices, which do not fit in any of the blanks. Mark your answers on ANSWER SHEET 1. (10 points)The time for sharpening pencils, arranging your desk, and doing almost anything else instead of writing has ended. The first draft will appear on the page only if you stop avoiding the inevitable and sit, stand up, or lie down to write. (41)_______________.Be flexible. Your outline should smoothly conduct you from one point to the next, but do not permit it to railroad you. If a relevant and important idea occurs to you now, work it into the draft. (42) _______________. Grammar, punctuation, and spelling can wait until you revise. Concentrate on what you are saying. Good writing most often occurs when you are in hot pursuit of an idea rather than in a nervous search for errors.(43) _______________. Your pages will be easier to keep track of that way, and, if you have to clip a paragraph to place it elsewhere, you will not lose any writing on either side.If you are working on a word processor, you can take advantage of its capacity to make additions and deletions as well as move entire paragraphs by making just a few simple keyboard commands. Some software programs can also check spelling and certain grammatical elements in your writing. (44) _______________. These printouts are also easier to read than the screen when you work on revisions.Once you have a first draft on paper, you can delete material that is unrelated to your thesis and add material necessa ry to illustrate your points and make your paper convincing. The student who wrote “The A&P as a Stateof Mind” wisely dropped a paragraph that questioned whether Sammy displays chauvinistic attitudes toward women. (45) _______________.Remember that your initial draft is only that. You should go through the paper many times—and then again—working to substantiate and clarify your ideas. You may even end up with several entire versions of the paper. Rewrite. The sentences within each paragraph should be related to a single topic. Transitions should connect one paragraph to the next so that there are no abrupt or confusing shifts. Awkward or wordy phrasing or unclear sentences and paragraphs should be mercilessly poked and prodded into shape.[A] To make revising easier, leave wide margins and extra space between lines so that you can easily add words, sentences andcorrections. Write on only one side of the paper.[B] After you have already and adequately developed the body of your paper, pay particular attention to the introductory and concluding paragraphs. It’s probably best to write the introduction last, after you know precisely what you are introducing. Concluding paragraphs demand equal attention because they leave the reader with a final impression.[C] It’s worth remembering, however, that though a clean copy fresh off a printer may look terrible, it will read only as well as the thinking and writing that have gone into it. Many writers prudently store their data on disks and print their pages each time they finish a draft to avoid losing any material because of power failures or other problems.[D] It makes no difference how you write, just so you do. Now that you have developed a topic into a tentative thesis, you can assemble your notes and begin to flesh out whatever outline you have made.[E] Although this is an interesting issue, it has nothing to do with the thesis, which explains how the setting influences Sammy’s decision to quit his job. Instead of including that paragraph, she added one that d escribed Lengel’s crabbed response to the girls so that she could lead up to the A & P “policy” he enforces.[F] In the final paragraph about the significance of the setting in “A&P” the student brings together the reasons Sammy quit his job by referring t o his refusal to accept Lengel’s store policies.[G] By using the first draft as a means of thinking about what you want to say, you will very likely discover more than your notes originally suggested. Plenty of good writers don’t use outlines at all but discover ordering principles as they write. Do not attempt to compose a perfectly correct draft the first time around.Part CDirections:Read the following text carefully and then translate the underlined segments into Chinese. Your translation should be written neatly on ANSWER SHEET 2. (10 points)In his autobiography,Darwin himself speaks of his intellectualpowers with extraordinary modesty. He points out that he always experienced much difficulty in expressing himself clearly and concisely, but (46)he believes that this very difficulty may have had the compensating advantage of forcing him to think long and intently about every sentence, and thus enabling him to detect errors in reasoning and in his ownPart A51. Directions:You have just come back from Canada and found a music CDin your luggage that you forgot to return to Bob, your landlord there. Write him a letter to1) make an apology, and2) suggest a solution.You should write about 100 words on ANSWER SHEET 2.Do not sign your own name at the end of the letter. Use “Li Ming” instead.Do not write the address. (10 points)Part B52. Directions:Write an essay of 160-200 words based on the following drawing. In your essay, you should1) describe the drawing briefly,2) explain its intended meaning, and then3) give your comments.You should write neatly on ANSHWER SHEET 2. (20 points)2023年全国硕士硕士招生考试英语(一)答案详解Section I Use of English一、文章总体分析这是一篇议论文。

期刊全称JCR期刊简称ISSN所属小类ACM COMPUTING SURVEYS ACM COMPUT SURV0360-0300C OMPUTER SC ACM Transactions on Intelligent Systems and Te ACM T INTEL SYST TEC2157-6904C OMPUTER SC ACM Transactions on Intelligent Systems and Te ACM T INTEL SYST TEC2157-6904C OMPUTER SC ACS Applied Materials & Interfaces ACS APPL MATER INTER1944-8244M ATERIALS S ACS Applied Materials & Interfaces ACS APPL MATER INTER1944-8244N ANOSCIENC ACS Macro Letters ACS MACRO LETT2161-1653P OLYMER SCI ACS Nano ACS NANO1936-0851M ATERIALS S ACS Nano ACS NANO1936-0851C HEMISTRY, MACS Nano ACS NANO1936-0851N ANOSCIENCACS Nano ACS NANO1936-0851C HEMISTRY, P Acta Biomaterialia ACTA BIOMATER1742-7061M ATERIALS S Acta Biomaterialia ACTA BIOMATER1742-7061E NGINEERING ADVANCES IN APPLIED MECHANICS ADV APPL MECH0065-2156E NGINEERING ADVANCES IN APPLIED MECHANICS ADV APPL MECH0065-2156M ECHANICS Advanced Energy Materials ADV ENERGY MATER1614-6832M ATERIALS S Advanced Energy Materials ADV ENERGY MATER1614-6832E NERGY & FU Advanced Energy Materials ADV ENERGY MATER1614-6832P HYSICS, CON Advanced Energy Materials ADV ENERGY MATER1614-6832P HYSICS, APP Advanced Energy Materials ADV ENERGY MATER1614-6832C HEMISTRY, P ADVANCED FUNCTIONAL MATERIALS ADV FUNCT MATER1616-301X M ATERIALS S ADVANCED FUNCTIONAL MATERIALS ADV FUNCT MATER1616-301X C HEMISTRY, M ADVANCED FUNCTIONAL MATERIALS ADV FUNCT MATER1616-301X N ANOSCIENC ADVANCED FUNCTIONAL MATERIALS ADV FUNCT MATER1616-301X P HYSICS, CON ADVANCED FUNCTIONAL MATERIALS ADV FUNCT MATER1616-301X P HYSICS, APP ADVANCED FUNCTIONAL MATERIALS ADV FUNCT MATER1616-301X C HEMISTRY, P Advanced Healthcare Materials ADV HEALTHC MATER2192-2640M ATERIALS SADVANCED MATERIALS ADV MATER0935-9648M ATERIALS S ADVANCED MATERIALS ADV MATER0935-9648C HEMISTRY, M ADVANCED MATERIALS ADV MATER0935-9648N ANOSCIENC ADVANCED MATERIALS ADV MATER0935-9648P HYSICS, CON ADVANCED MATERIALS ADV MATER0935-9648P HYSICS, APP ADVANCED MATERIALS ADV MATER0935-9648C HEMISTRY, PANNU REV BIOMED ENG1523-9829E NGINEERING ANNUAL REVIEW OF BIOMEDICAL ENGINEAnnual Review of Chemical and Biomolecular En ANNU REV CHEM BIOMOL1947-5438E NGINEERING Annual Review of Chemical and Biomolecular En ANNU REV CHEM BIOMOL1947-5438C HEMISTRY, A Annual Review of Food Science and Technology A NNU REV FOOD SCI T1941-1413F OOD SCIENCANNU REV MATER RES1531-7331M ATERIALS S ANNUAL REVIEW OF MATERIALS RESEARCAPPLIED ENERGY APPL ENERG0306-2619E NGINEERING APPLIED ENERGY APPL ENERG0306-2619E NERGY & FU BIOMATERIALS BIOMATERIALS0142-9612M ATERIALS S BIOMATERIALS BIOMATERIALS0142-9612E NGINEERING BIORESOURCE TECHNOLOGY BIORESOURCE TECHNOL0960-8524E NERGY & FU BIORESOURCE TECHNOLOGY BIORESOURCE TECHNOL0960-8524A GRICULTURA BIORESOURCE TECHNOLOGY BIORESOURCE TECHNOL0960-8524B IOTECHNOLO BIOSENSORS & BIOELECTRONICS BIOSENS BIOELECTRON0956-5663E LECTROCHE BIOSENSORS & BIOELECTRONICS BIOSENS BIOELECTRON0956-5663C HEMISTRY, A BIOSENSORS & BIOELECTRONICS BIOSENS BIOELECTRON0956-5663N ANOSCIENC BIOSENSORS & BIOELECTRONICS BIOSENS BIOELECTRON0956-5663B IOTECHNOLO BIOSENSORS & BIOELECTRONICS BIOSENS BIOELECTRON0956-5663B IOPHYSICS BIOTECHNOLOGY ADVANCES BIOTECHNOL ADV0734-9750B IOTECHNOLO Biotechnology for Biofuels BIOTECHNOL BIOFUELS1754-6834E NERGY & FU Biotechnology for Biofuels BIOTECHNOL BIOFUELS1754-6834B IOTECHNOLO CARBON CARBON0008-6223M ATERIALS S CARBON CARBON0008-6223C HEMISTRY, P CHEMISTRY OF MATERIALS CHEM MATER0897-4756M ATERIALS S CHEMISTRY OF MATERIALS CHEM MATER0897-4756C HEMISTRY, PCOMPR REV FOOD SCI F1541-4337F OOD SCIENC COMPREHENSIVE REVIEWS IN FOOD SCIENCOMPUT-AIDED CIV INF1093-9687E NGINEERING COMPUTER-AIDED CIVIL AND INFRASTRUCCOMPUTER-AIDED CIVIL AND INFRASTRUCCOMPUT-AIDED CIV INF1093-9687C OMPUTER SCCOMPUT-AIDED CIV INF1093-9687C ONSTRUCTIO COMPUTER-AIDED CIVIL AND INFRASTRUCCOMPUT-AIDED CIV INF1093-9687T RANSPORTA COMPUTER-AIDED CIVIL AND INFRASTRUCCRITICAL REVIEWS IN BIOTECHNOLOGY CRIT REV BIOTECHNOL0738-8551B IOTECHNOLOCRIT REV FOOD SCI1040-8398F OOD SCIENC CRITICAL REVIEWS IN FOOD SCIENCE ANDCRIT REV FOOD SCI1040-8398N UTRITION & CRITICAL REVIEWS IN FOOD SCIENCE ANDCURRENT OPINION IN BIOTECHNOLOGY CURR OPIN BIOTECH0958-1669B IOCHEMICAL CURRENT OPINION IN BIOTECHNOLOGY CURR OPIN BIOTECH0958-1669B IOTECHNOLOCURR OPIN SOLID ST M1359-0286M ATERIALS S CURRENT OPINION IN SOLID STATE & MATCURR OPIN SOLID ST M1359-0286P HYSICS, CON CURRENT OPINION IN SOLID STATE & MATCURRENT OPINION IN SOLID STATE & MATCURR OPIN SOLID ST M1359-0286P HYSICS, APP ELECTROCHEMISTRY COMMUNICATIONS E LECTROCHEM COMMUN1388-2481E LECTROCHEIEEE Communications Surveys and Tutorials IEEE COMMUN SURV TUT1553-877X T ELECOMMUN IEEE Communications Surveys and Tutorials IEEE COMMUN SURV TUT1553-877X C OMPUTER SC IEEE Industrial Electronics Magazine IEEE IND ELECTRON M1932-4529E NGINEERING IEEE SIGNAL PROCESSING MAGAZINE IEEE SIGNAL PROC MAG1053-5888E NGINEERINGIEEE TRANSACTIONS ON EVOLUTIONARY IEEE T EVOLUT COMPUT1089-778X C OMPUTER SCIEEE TRANSACTIONS ON EVOLUTIONARY IEEE T EVOLUT COMPUT1089-778X C OMPUTER SC IEEE TRANSACTIONS ON FUZZY SYSTEMS IEEE T FUZZY SYST1063-6706E NGINEERING IEEE TRANSACTIONS ON FUZZY SYSTEMS IEEE T FUZZY SYST1063-6706C OMPUTER SC IEEE TRANSACTIONS ON INDUSTRIAL ELE IEEE T IND ELECTRON0278-0046E NGINEERING IEEE TRANSACTIONS ON INDUSTRIAL ELE IEEE T IND ELECTRON0278-0046I NSTRUMENT IEEE TRANSACTIONS ON INDUSTRIAL ELE IEEE T IND ELECTRON0278-0046A UTOMATIONIEEE Transactions on Industrial Informatics IEEE T IND INFORM1551-3203E NGINEERING IEEE Transactions on Industrial Informatics IEEE T IND INFORM1551-3203C OMPUTER SC IEEE Transactions on Industrial Informatics IEEE T IND INFORM1551-3203A UTOMATION IEEE Transactions on Neural Networks and LearnIEEE T NEUR NET LEAR2162-237X E NGINEERINGIEEE T NEUR NET LEAR2162-237X C OMPUTER SC IEEE Transactions on Neural Networks and LearnIEEE Transactions on Neural Networks and LearnIEEE T NEUR NET LEAR2162-237X C OMPUTER SCIEEE T NEUR NET LEAR2162-237X C OMPUTER SC IEEE Transactions on Neural Networks and LearnIEEE TRANSACTIONS ON PATTERN ANALY IEEE T PATTERN ANAL0162-8828E NGINEERINGIEEE TRANSACTIONS ON PATTERN ANALY IEEE T PATTERN ANAL0162-8828C OMPUTER SC IEEE TRANSACTIONS ON POWER ELECTRO IEEE T POWER ELECTR0885-8993E NGINEERING IEEE Transactions on Smart Grid IEEE T SMART GRID1949-3053E NGINEERINGIEEE T THZ SCI TECHN2156-342X E NGINEERING IEEE Transactions on Terahertz Science and TechIEEE T THZ SCI TECHN2156-342X O PTICSIEEE Transactions on Terahertz Science and TechIEEE T THZ SCI TECHN2156-342X P HYSICS, APP IEEE Transactions on Terahertz Science and TechIEEE WIRELESS COMMUNICATIONS IEEE WIREL COMMUN1536-1284T ELECOMMUN IEEE WIRELESS COMMUNICATIONS IEEE WIREL COMMUN1536-1284E NGINEERING IEEE WIRELESS COMMUNICATIONS IEEE WIREL COMMUN1536-1284C OMPUTER SCIEEE WIRELESS COMMUNICATIONS IEEE WIREL COMMUN1536-1284C OMPUTER SCInternational Journal of Neural Systems INT J NEURAL SYST0129-0657C OMPUTER SC INTERNATIONAL JOURNAL OF PLASTICITYINT J PLASTICITY0749-6419M ATERIALS SINT J PLASTICITY0749-6419E NGINEERING INTERNATIONAL JOURNAL OF PLASTICITYINTERNATIONAL JOURNAL OF PLASTICITYINT J PLASTICITY0749-6419M ECHANICS INTERNATIONAL MATERIALS REVIEWS INT MATER REV0950-6608M ATERIALS S JOURNAL OF CATALYSIS J CATAL0021-9517E NGINEERINGJOURNAL OF CATALYSIS J CATAL0021-9517C HEMISTRY, P JOURNAL OF HAZARDOUS MATERIALS J HAZARD MATER0304-3894E NGINEERING JOURNAL OF HAZARDOUS MATERIALS J HAZARD MATER0304-3894E NGINEERING JOURNAL OF HAZARDOUS MATERIALS J HAZARD MATER0304-3894E NVIRONMEN Journal of Materials Chemistry A J MATER CHEM A2050-7488M ATERIALS S Journal of Materials Chemistry A J MATER CHEM A2050-7488E NERGY & FU Journal of Materials Chemistry A J MATER CHEM A2050-7488C HEMISTRY, P Journal of Materials Chemistry B J MATER CHEM B2050-750X M ATERIALS S Journal of Materials Chemistry C J MATER CHEM C2050-7526M ATERIALS S Journal of Materials Chemistry C J MATER CHEM C2050-7526P HYSICS, APP JOURNAL OF MEMBRANE SCIENCE J MEMBRANE SCI0376-7388P OLYMER SCI JOURNAL OF MEMBRANE SCIENCE J MEMBRANE SCI0376-7388E NGINEERING JOURNAL OF NANOBIOTECHNOLOGY J NANOBIOTECHNOL1477-3155N ANOSCIENC JOURNAL OF NANOBIOTECHNOLOGY J NANOBIOTECHNOL1477-3155B IOTECHNOLO JOURNAL OF POWER SOURCES J POWER SOURCES0378-7753E LECTROCHE JOURNAL OF POWER SOURCES J POWER SOURCES0378-7753E NERGY & FU Journal of Statistical Software J STAT SOFTW1548-7660C OMPUTER SC Journal of Statistical Software J STAT SOFTW1548-7660S TATISTICS & LAB ON A CHIP LAB CHIP1473-0197C HEMISTRY, M LAB ON A CHIP LAB CHIP1473-0197N ANOSCIENC LAB ON A CHIP LAB CHIP1473-0197B IOCHEMICAL mAbs MABS-AUSTIN1942-0862M EDICINE, RE MACROMOLECULAR RAPID COMMUNICAT MACROMOL RAPID COMM1022-1336P OLYMER SCIMACROMOLECULES MACROMOLECULES0024-9297P OLYMER SCIMATERIALS SCIENCE & ENGINEERING R-R MAT SCI ENG R0927-796X M ATERIALS S MATERIALS SCIENCE & ENGINEERING R-R MAT SCI ENG R0927-796X P HYSICS, APP Materials Today MATER TODAY1369-7021M ATERIALS S MEDICAL IMAGE ANALYSIS MED IMAGE ANAL1361-8415E NGINEERING MEDICAL IMAGE ANALYSIS MED IMAGE ANAL1361-8415R ADIOLOGY, MEDICAL IMAGE ANALYSIS MED IMAGE ANAL1361-8415C OMPUTER SC MEDICAL IMAGE ANALYSIS MED IMAGE ANAL1361-8415C OMPUTER SCMETABOLIC ENGINEERING METAB ENG1096-7176B IOTECHNOLOMIS QUARTERLY MIS QUART0276-7783C OMPUTER SCMOL NUTR FOOD RES1613-4125F OOD SCIENC MOLECULAR NUTRITION & FOOD RESEARCMRS BULLETIN MRS BULL0883-7694M ATERIALS S MRS BULLETIN MRS BULL0883-7694P HYSICS, APP Nano Energy NANO ENERGY2211-2855M ATERIALS S Nano Energy NANO ENERGY2211-2855N ANOSCIENC Nano Energy NANO ENERGY2211-2855P HYSICS, APP Nano Energy NANO ENERGY2211-2855C HEMISTRY, P NANO LETTERS NANO LETT1530-6984M ATERIALS S NANO LETTERS NANO LETT1530-6984C HEMISTRY, M NANO LETTERS NANO LETT1530-6984N ANOSCIENC NANO LETTERS NANO LETT1530-6984P HYSICS, CON NANO LETTERS NANO LETT1530-6984P HYSICS, APP NANO LETTERS NANO LETT1530-6984C HEMISTRY, P Nano Research NANO RES1998-0124M ATERIALS S Nano Research NANO RES1998-0124N ANOSCIENC Nano Research NANO RES1998-0124P HYSICS, APP Nano Research NANO RES1998-0124C HEMISTRY, P Nano Today NANO TODAY1748-0132M ATERIALS S Nano Today NANO TODAY1748-0132C HEMISTRY, M Nano Today NANO TODAY1748-0132N ANOSCIENC Nanomedicine-Nanotechnology Biology and Med NANOMED-NANOTECHNOL1549-9634N ANOSCIENC Nanomedicine-Nanotechnology Biology and Med NANOMED-NANOTECHNOL1549-9634M EDICINE, RE Nanoscale NANOSCALE2040-3364M ATERIALS S Nanoscale NANOSCALE2040-3364C HEMISTRY, M Nanoscale NANOSCALE2040-3364N ANOSCIENC Nanoscale NANOSCALE2040-3364P HYSICS, APP NATURE BIOTECHNOLOGY NAT BIOTECHNOL1087-0156B IOTECHNOLO NATURE MATERIALS NAT MATER1476-1122M ATERIALS S NATURE MATERIALS NAT MATER1476-1122P HYSICS, CON NATURE MATERIALS NAT MATER1476-1122P HYSICS, APP NATURE MATERIALS NAT MATER1476-1122C HEMISTRY, PNature Nanotechnology NAT NANOTECHNOL1748-3387M ATERIALS SNature Nanotechnology NAT NANOTECHNOL1748-3387N ANOSCIENC NPG Asia Materials NPG ASIA MATER1884-4049M ATERIALS S PROCEEDINGS OF THE IEEE P IEEE0018-9219E NGINEERING Polymer Reviews POLYM REV1558-3724P OLYMER SCIPROGRESS IN ENERGY AND COMBUSTION PROG ENERG COMBUST0360-1285E NGINEERING PROGRESS IN ENERGY AND COMBUSTION PROG ENERG COMBUST0360-1285E NGINEERINGPROGRESS IN ENERGY AND COMBUSTION PROG ENERG COMBUST0360-1285E NERGY & FU PROGRESS IN ENERGY AND COMBUSTION PROG ENERG COMBUST0360-1285T HERMODYNA PROGRESS IN MATERIALS SCIENCE PROG MATER SCI0079-6425M ATERIALS S PROGRESS IN PHOTOVOLTAICS PROG PHOTOVOLTAICS1062-7995M ATERIALS S PROGRESS IN PHOTOVOLTAICS PROG PHOTOVOLTAICS1062-7995E NERGY & FUPROGRESS IN PHOTOVOLTAICS PROG PHOTOVOLTAICS1062-7995P HYSICS, APP PROGRESS IN QUANTUM ELECTRONICS PROG QUANT ELECTRON0079-6727E NGINEERING PROGRESS IN SURFACE SCIENCE PROG SURF SCI0079-6816P HYSICS, CON PROGRESS IN SURFACE SCIENCE PROG SURF SCI0079-6816C HEMISTRY, P RENEWABLE & SUSTAINABLE ENERGY RE RENEW SUST ENERG REV1364-0321E NERGY & FU SMALL SMALL1613-6810M ATERIALS S SMALL SMALL1613-6810C HEMISTRY, M SMALL SMALL1613-6810N ANOSCIENC SMALL SMALL1613-6810P HYSICS, CON SMALL SMALL1613-6810P HYSICS, APP SMALL SMALL1613-6810C HEMISTRY, P Soft Matter SOFT MATTER1744-683X M ATERIALS S Soft Matter SOFT MATTER1744-683X P OLYMER SCI Soft Matter SOFT MATTER1744-683X P HYSICS, MUL Soft Matter SOFT MATTER1744-683X C HEMISTRY, PSOL ENERG MAT SOL C0927-0248M ATERIALS S SOLAR ENERGY MATERIALS AND SOLAR CSOL ENERG MAT SOL C0927-0248E NERGY & FU SOLAR ENERGY MATERIALS AND SOLAR CSOL ENERG MAT SOL C0927-0248P HYSICS, APP SOLAR ENERGY MATERIALS AND SOLAR CTRENDS IN BIOTECHNOLOGY TRENDS BIOTECHNOL0167-7799B IOTECHNOLO TRENDS IN FOOD SCIENCE & TECHNOLOG T RENDS FOOD SCI TECH0924-2244F OOD SCIENCACM TRANSACTIONS ON GRAPHICS ACM T GRAPHIC0730-0301C OMPUTER SCACM TRANSACTIONS ON MATHEMATICAL ACM T MATH SOFTWARE0098-3500C OMPUTER SC ACM TRANSACTIONS ON MATHEMATICAL ACM T MATH SOFTWARE0098-3500M ATHEMATIC ACTA MATERIALIA ACTA MATER1359-6454M ATERIALS S ACTA MATERIALIA ACTA MATER1359-6454M ETALLURGY ADVANCES IN BIOCHEMICAL ENGINEERIN ADV BIOCHEM ENG BIOT0724-6145B IOTECHNOLO AICHE JOURNAL AICHE J0001-1541E NGINEERING ANNALS OF BIOMEDICAL ENGINEERINGANN BIOMED ENG0090-6964E NGINEERING ANNUAL REVIEW OF INFORMATION SCIEN ANNU REV INFORM SCI0066-4200C OMPUTER SCAPPL MICROBIOL BIOT0175-7598B IOTECHNOLO APPLIED MICROBIOLOGY AND BIOTECHNOAPPLIED SOFT COMPUTING APPL SOFT COMPUT1568-4946C OMPUTER SC APPLIED SOFT COMPUTING APPL SOFT COMPUT1568-4946C OMPUTER SC APPLIED SPECTROSCOPY REVIEWS APPL SPECTROSC REV0570-4928S PECTROSCOP APPLIED SPECTROSCOPY REVIEWS APPL SPECTROSC REV0570-4928I NSTRUMENT APPLIED SURFACE SCIENCE APPL SURF SCI0169-4332M ATERIALS S APPLIED SURFACE SCIENCE APPL SURF SCI0169-4332P HYSICS, CON APPLIED SURFACE SCIENCE APPL SURF SCI0169-4332P HYSICS, APP APPLIED SURFACE SCIENCE APPL SURF SCI0169-4332C HEMISTRY, P APPLIED THERMAL ENGINEERING APPL THERM ENG1359-4311E NGINEERINGAPPLIED THERMAL ENGINEERING APPL THERM ENG1359-4311M ECHANICS APPLIED THERMAL ENGINEERING APPL THERM ENG1359-4311E NERGY & FU APPLIED THERMAL ENGINEERING APPL THERM ENG1359-4311T HERMODYNAARCH COMPUT METHOD E1134-3060E NGINEERING ARCHIVES OF COMPUTATIONAL METHODSARCH COMPUT METHOD E1134-3060C OMPUTER SC ARCHIVES OF COMPUTATIONAL METHODSARCH COMPUT METHOD E1134-3060M ATHEMATIC ARCHIVES OF COMPUTATIONAL METHODSARTIFICIAL INTELLIGENCE ARTIF INTELL0004-3702C OMPUTER SC AUSTRALIAN JOURNAL OF GRAPE AND WI AUST J GRAPE WINE R1322-7130F OOD SCIENC AUSTRALIAN JOURNAL OF GRAPE AND WI AUST J GRAPE WINE R1322-7130H ORTICULTUR AUTOMATICA AUTOMATICA0005-1098E NGINEERINGAUTOMATICA AUTOMATICA0005-1098A UTOMATION BIOCHEMICAL ENGINEERING JOURNAL BIOCHEM ENG J1369-703X E NGINEERING BIOCHEMICAL ENGINEERING JOURNAL BIOCHEM ENG J1369-703X B IOTECHNOLO BIODEGRADATION BIODEGRADATION0923-9820B IOTECHNOLO Biofabrication BIOFABRICATION1758-5082M ATERIALS S Biofabrication BIOFABRICATION1758-5082E NGINEERING Bioinspiration & Biomimetics BIOINSPIR BIOMIM1748-3182M ATERIALS S Bioinspiration & Biomimetics BIOINSPIR BIOMIM1748-3182E NGINEERING Bioinspiration & Biomimetics BIOINSPIR BIOMIM1748-3182R OBOTICS Biointerphases BIOINTERPHASES1934-8630M ATERIALS S Biointerphases BIOINTERPHASES1934-8630B IOPHYSICSBIOMASS & BIOENERGY BIOMASS BIOENERG0961-9534E NERGY & FU BIOMASS & BIOENERGY BIOMASS BIOENERG0961-9534A GRICULTURA BIOMASS & BIOENERGY BIOMASS BIOENERG0961-9534B IOTECHNOLO Biomechanics and Modeling in Mechanobiology B IOMECH MODEL MECHAN1617-7959E NGINEERINGBiomechanics and Modeling in Mechanobiology B IOMECH MODEL MECHAN1617-7959B IOPHYSICS Biomedical Materials BIOMED MATER1748-6041M ATERIALS S Biomedical Materials BIOMED MATER1748-6041E NGINEERING BIOMEDICAL MICRODEVICES BIOMED MICRODEVICES1387-2176E NGINEERING BIOMEDICAL MICRODEVICES BIOMED MICRODEVICES1387-2176N ANOSCIENC BIOTECHNIQUES BIOTECHNIQUES0736-6205B IOCHEMICAL BIOTECHNIQUES BIOTECHNIQUES0736-6205B IOCHEMISTR BIOTECHNOLOGY AND BIOENGINEERING BIOTECHNOL BIOENG0006-3592B IOTECHNOLO BIOTECHNOLOGY PROGRESS BIOTECHNOL PROGR8756-7938B IOTECHNOLO BIOTECHNOLOGY PROGRESS BIOTECHNOL PROGR8756-7938F OOD SCIENC BMC BIOTECHNOLOGY BMC BIOTECHNOL1472-6750B IOTECHNOLO BUILDING AND ENVIRONMENT BUILD ENVIRON0360-1323E NGINEERING BUILDING AND ENVIRONMENT BUILD ENVIRON0360-1323E NGINEERING BUILDING AND ENVIRONMENT BUILD ENVIRON0360-1323C ONSTRUCTIO CELLULOSE CELLULOSE0969-0239M ATERIALS SCELLULOSE CELLULOSE0969-0239M ATERIALS SCELLULOSE CELLULOSE0969-0239P OLYMER SCICEMENT & CONCRETE COMPOSITES CEMENT CONCRETE COMP0958-9465M ATERIALS S CEMENT & CONCRETE COMPOSITES CEMENT CONCRETE COMP0958-9465C ONSTRUCTIOCEMENT AND CONCRETE RESEARCH CEMENT CONCRETE RES0008-8846M ATERIALS SCEMENT AND CONCRETE RESEARCH CEMENT CONCRETE RES0008-8846C ONSTRUCTIO CHEMICAL ENGINEERING JOURNAL CHEM ENG J1385-8947E NGINEERING CHEMICAL ENGINEERING JOURNAL CHEM ENG J1385-8947E NGINEERING CHEMICAL ENGINEERING RESEARCH & DE CHEM ENG RES DES0263-8762E NGINEERING CHEMICAL ENGINEERING SCIENCE CHEM ENG SCI0009-2509E NGINEERING CHEMOMETRICS AND INTELLIGENT LABO CHEMOMETR INTELL LAB0169-7439C HEMISTRY, A CHEMOMETRICS AND INTELLIGENT LABO CHEMOMETR INTELL LAB0169-7439C OMPUTER SC CHEMOMETRICS AND INTELLIGENT LABO CHEMOMETR INTELL LAB0169-7439M ATHEMATICCHEMOMETRICS AND INTELLIGENT LABO CHEMOMETR INTELL LAB0169-7439S TATISTICS & CHEMOMETRICS AND INTELLIGENT LABO CHEMOMETR INTELL LAB0169-7439I NSTRUMENT CHEMOMETRICS AND INTELLIGENT LABO CHEMOMETR INTELL LAB0169-7439A UTOMATIONCIRP ANN-MANUF TECHN0007-8506E NGINEERING CIRP ANNALS-MANUFACTURING TECHNOLCIRP ANN-MANUF TECHN0007-8506E NGINEERING CIRP ANNALS-MANUFACTURING TECHNOLCOMBUSTION AND FLAME COMBUST FLAME0010-2180E NGINEERING COMBUSTION AND FLAME COMBUST FLAME0010-2180E NGINEERING COMBUSTION AND FLAME COMBUST FLAME0010-2180E NGINEERING COMBUSTION AND FLAME COMBUST FLAME0010-2180E NERGY & FU COMBUSTION AND FLAME COMBUST FLAME0010-2180T HERMODYNA COMMUNICATIONS OF THE ACM COMMUN ACM0001-0782C OMPUTER SC COMMUNICATIONS OF THE ACM COMMUN ACM0001-0782C OMPUTER SCCOMMUNICATIONS OF THE ACM COMMUN ACM0001-0782C OMPUTER SCCOMPOS PART A-APPL S1359-835X M ATERIALS S COMPOSITES PART A-APPLIED SCIENCE ANCOMPOS PART A-APPL S1359-835X E NGINEERING COMPOSITES PART A-APPLIED SCIENCE ANCOMPOSITES PART B-ENGINEERING COMPOS PART B-ENG1359-8368M ATERIALS S COMPOSITES PART B-ENGINEERING COMPOS PART B-ENG1359-8368E NGINEERING COMPOSITES SCIENCE AND TECHNOLOGY COMPOS SCI TECHNOL0266-3538M ATERIALS SCOMPOSITE STRUCTURES COMPOS STRUCT0263-8223M ATERIALS S COMPUTERS & CHEMICAL ENGINEERING COMPUT CHEM ENG0098-1354E NGINEERINGCOMPUTERS & CHEMICAL ENGINEERING COMPUT CHEM ENG0098-1354C OMPUTER SC COMPUTERS & EDUCATION COMPUT EDUC0360-1315C OMPUTER SC COMPUTER METHODS IN APPLIED MECHA C OMPUT METHOD APPL M0045-7825E NGINEERING COMPUTER METHODS IN APPLIED MECHA C OMPUT METHOD APPL M0045-7825M ECHANICS COMPUTER METHODS IN APPLIED MECHA C OMPUT METHOD APPL M0045-7825M ATHEMATICCONSTR BUILD MATER0950-0618M ATERIALS S CONSTRUCTION AND BUILDING MATERIALCONSTR BUILD MATER0950-0618E NGINEERING CONSTRUCTION AND BUILDING MATERIALCONSTR BUILD MATER0950-0618C ONSTRUCTIO CONSTRUCTION AND BUILDING MATERIALCORROSION SCIENCE CORROS SCI0010-938X M ATERIALS S CORROSION SCIENCE CORROS SCI0010-938X M ETALLURGY CORROSION CORROSION0010-9312M ATERIALS S CORROSION CORROSION0010-9312M ETALLURGYDATA MIN KNOWL DISC1384-5810C OMPUTER SC DATA MINING AND KNOWLEDGE DISCOVEDATA MIN KNOWL DISC1384-5810C OMPUTER SC DATA MINING AND KNOWLEDGE DISCOVEDENTAL MATERIALS DENT MATER0109-5641M ATERIALS S DENTAL MATERIALS DENT MATER0109-5641D ENTISTRY, O DESALINATION DESALINATION0011-9164E NGINEERING DESALINATION DESALINATION0011-9164W ATER RESOU DYES AND PIGMENTS DYES PIGMENTS0143-7208M ATERIALS S DYES AND PIGMENTS DYES PIGMENTS0143-7208E NGINEERINGDYES AND PIGMENTS DYES PIGMENTS0143-7208C HEMISTRY, AELECTROCHIMICA ACTA ELECTROCHIM ACTA0013-4686E LECTROCHE Electronic Materials Letters ELECTRON MATER LETT1738-8090M ATERIALS S ENERGY AND BUILDINGS ENERG BUILDINGS0378-7788E NGINEERING ENERGY AND BUILDINGS ENERG BUILDINGS0378-7788C ONSTRUCTIOENERGY AND BUILDINGS ENERG BUILDINGS0378-7788E NERGY & FU ENERGY CONVERSION AND MANAGEMEN E NERG CONVERS MANAGE0196-8904M ECHANICS ENERGY CONVERSION AND MANAGEMEN E NERG CONVERS MANAGE0196-8904E NERGY & FUENERGY CONVERSION AND MANAGEMEN E NERG CONVERS MANAGE0196-8904T HERMODYNA ENERGY CONVERSION AND MANAGEMEN E NERG CONVERS MANAGE0196-8904P HYSICS, NUC ENERGY & FUELS ENERG FUEL0887-0624E NGINEERINGENERGY & FUELS ENERG FUEL0887-0624E NERGY & FU ENERGY JOURNAL ENERG J0195-6574E NERGY & FU ENERGY ENERGY0360-5442E NERGY & FU ENERGY ENERGY0360-5442T HERMODYNA ENVIRONMENTAL MODELLING & SOFTWA ENVIRON MODELL SOFTW1364-8152E NGINEERING ENVIRONMENTAL MODELLING & SOFTWA ENVIRON MODELL SOFTW1364-8152E NVIRONMEN ENVIRONMENTAL MODELLING & SOFTWA ENVIRON MODELL SOFTW1364-8152C OMPUTER SC EVOLUTIONARY COMPUTATION EVOL COMPUT1063-6560C OMPUTER SCEVOLUTIONARY COMPUTATION EVOL COMPUT1063-6560C OMPUTER SC FLUID PHASE EQUILIBRIA FLUID PHASE EQUILIBR0378-3812E NGINEERING FLUID PHASE EQUILIBRIA FLUID PHASE EQUILIBR0378-3812T HERMODYNAFLUID PHASE EQUILIBRIA FLUID PHASE EQUILIBR0378-3812C HEMISTRY, P FOOD ADDITIVES AND CONTAMINANTS FOOD ADDIT CONTAM A1944-0049T OXICOLOGY FOOD ADDITIVES AND CONTAMINANTS FOOD ADDIT CONTAM A1944-0049F OOD SCIENC FOOD ADDITIVES AND CONTAMINANTS FOOD ADDIT CONTAM A1944-0049C HEMISTRY, A FOOD AND BIOPRODUCTS PROCESSING FOOD BIOPROD PROCESS0960-3085E NGINEERING FOOD AND BIOPRODUCTS PROCESSING FOOD BIOPROD PROCESS0960-3085B IOTECHNOLO FOOD AND BIOPRODUCTS PROCESSING FOOD BIOPROD PROCESS0960-3085F OOD SCIENC FOOD CHEMISTRY FOOD CHEM0308-8146F OOD SCIENCFOOD CHEMISTRY FOOD CHEM0308-8146N UTRITION & FOOD CHEMISTRY FOOD CHEM0308-8146C HEMISTRY, A FOOD AND CHEMICAL TOXICOLOGY FOOD CHEM TOXICOL0278-6915T OXICOLOGY FOOD AND CHEMICAL TOXICOLOGY FOOD CHEM TOXICOL0278-6915F OOD SCIENC FOOD CONTROL FOOD CONTROL0956-7135F OOD SCIENC FOOD HYDROCOLLOIDS FOOD HYDROCOLLOID0268-005X F OOD SCIENC FOOD HYDROCOLLOIDS FOOD HYDROCOLLOID0268-005X C HEMISTRY, A FOOD MICROBIOLOGY FOOD MICROBIOL0740-0020B IOTECHNOLO FOOD MICROBIOLOGY FOOD MICROBIOL0740-0020F OOD SCIENC FOOD MICROBIOLOGY FOOD MICROBIOL0740-0020M ICROBIOLOG FOOD QUALITY AND PREFERENCE FOOD QUAL PREFER0950-3293F OOD SCIENC FOOD RESEARCH INTERNATIONAL FOOD RES INT0963-9969F OOD SCIENC FUEL FUEL0016-2361E NGINEERING FUEL FUEL0016-2361E NERGY & FU Fuel Cells FUEL CELLS1615-6846E LECTROCHE Fuel Cells FUEL CELLS1615-6846E NERGY & FUFUEL PROCESSING TECHNOLOGY FUEL PROCESS TECHNOL0378-3820E NGINEERINGFUEL PROCESSING TECHNOLOGY FUEL PROCESS TECHNOL0378-3820E NERGY & FUFUEL PROCESSING TECHNOLOGY FUEL PROCESS TECHNOL0378-3820C HEMISTRY, AFUTURE GENER COMP SY0167-739X C OMPUTER SC FUTURE GENERATION COMPUTER SYSTEMGEOTHERMICS GEOTHERMICS0375-6505G EOSCIENCES GEOTHERMICS GEOTHERMICS0375-6505E NERGY & FU GOLD BULLETIN GOLD BULL0017-1557M ATERIALS S GOLD BULLETIN GOLD BULL0017-1557C HEMISTRY, I GOLD BULLETIN GOLD BULL0017-1557C HEMISTRY, P HOLZFORSCHUNG HOLZFORSCHUNG0018-3830M ATERIALS S HOLZFORSCHUNG HOLZFORSCHUNG0018-3830F ORESTRY HUMAN-COMPUTER INTERACTION HUM-COMPUT INTERACT0737-0024C OMPUTER SC HUMAN-COMPUTER INTERACTION HUM-COMPUT INTERACT0737-0024C OMPUTER SC HYDROMETALLURGY HYDROMETALLURGY0304-386X M ETALLURGY IEEE COMMUNICATIONS MAGAZINE IEEE COMMUN MAG0163-6804T ELECOMMUNIEEE COMMUNICATIONS MAGAZINE IEEE COMMUN MAG0163-6804E NGINEERING IEEE Computational Intelligence Magazine IEEE COMPUT INTELL M1556-603X C OMPUTER SC IEEE CONTROL SYSTEMS MAGAZINE IEEE CONTR SYST MAG1066-033X A UTOMATION IEEE ELECTRON DEVICE LETTERS IEEE ELECTR DEVICE L0741-3106E NGINEERING IEEE Journal of Photovoltaics IEEE J PHOTOVOLT2156-3381M ATERIALS S IEEE Journal of Photovoltaics IEEE J PHOTOVOLT2156-3381E NERGY & FU IEEE Journal of Photovoltaics IEEE J PHOTOVOLT2156-3381P HYSICS, APPIEEE J SEL AREA COMM0733-8716T ELECOMMUN IEEE JOURNAL ON SELECTED AREAS IN COIEEE J SEL AREA COMM0733-8716E NGINEERING IEEE JOURNAL ON SELECTED AREAS IN COIEEE JOURNAL OF SELECTED TOPICS IN QUIEEE J SEL TOP QUANT1077-260X E NGINEERINGIEEE J SEL TOP QUANT1077-260X O PTICSIEEE JOURNAL OF SELECTED TOPICS IN QUIEEE J SEL TOP QUANT1077-260X P HYSICS, APP IEEE JOURNAL OF SELECTED TOPICS IN QUIEEE JOURNAL OF SOLID-STATE CIRCUITS IEEE J SOLID-ST CIRC0018-9200E NGINEERINGIEEE J-STSP1932-4553E NGINEERING IEEE Journal of Selected Topics in Signal ProcessIEEE NETWORK IEEE NETWORK0890-8044T ELECOMMUN IEEE NETWORK IEEE NETWORK0890-8044E NGINEERING IEEE NETWORK IEEE NETWORK0890-8044C OMPUTER SC IEEE NETWORK IEEE NETWORK0890-8044C OMPUTER SC IEEE PHOTONICS TECHNOLOGY LETTERS IEEE PHOTONIC TECH L1041-1135E NGINEERING IEEE PHOTONICS TECHNOLOGY LETTERS IEEE PHOTONIC TECH L1041-1135O PTICSIEEE PHOTONICS TECHNOLOGY LETTERS IEEE PHOTONIC TECH L1041-1135P HYSICS, APP IEEE Photonics Journal IEEE PHOTONICS J1943-0655E NGINEERING IEEE Photonics Journal IEEE PHOTONICS J1943-0655O PTICSIEEE Photonics Journal IEEE PHOTONICS J1943-0655P HYSICS, APPIEEE ROBOT AUTOM MAG1070-9932R OBOTICS IEEE ROBOTICS & AUTOMATION MAGAZINIEEE ROBOT AUTOM MAG1070-9932A UTOMATION IEEE ROBOTICS & AUTOMATION MAGAZINIEEE Transactions on Affective Computing IEEE T AFFECT COMPUT1949-3045C OMPUTER SC IEEE Transactions on Affective Computing IEEE T AFFECT COMPUT1949-3045C OMPUTER SC IEEE TRANSACTIONS ON ANTENNAS AND IEEE T ANTENN PROPAG0018-926X T ELECOMMUN IEEE TRANSACTIONS ON ANTENNAS AND IEEE T ANTENN PROPAG0018-926X E NGINEERINGIEEE T AUTOMAT CONTR0018-9286E NGINEERING IEEE TRANSACTIONS ON AUTOMATIC CONIEEE T AUTOMAT CONTR0018-9286A UTOMATION IEEE TRANSACTIONS ON AUTOMATIC CONIEEE Transactions on Biomedical Circuits and SyIEEE T BIOMED CIRC S1932-4545E NGINEERINGIEEE T BIOMED CIRC S1932-4545E NGINEERING IEEE Transactions on Biomedical Circuits and SyIEEE T BIO-MED ENG0018-9294E NGINEERING IEEE TRANSACTIONS ON BIOMEDICAL ENGIEEE TRANSACTIONS ON BROADCASTING IEEE T BROADCAST0018-9316T ELECOMMUN IEEE TRANSACTIONS ON BROADCASTING IEEE T BROADCAST0018-9316E NGINEERINGIEEE T CIRCUITS-I1549-8328E NGINEERING IEEE TRANSACTIONS ON CIRCUITS AND SYIEEE TRANSACTIONS ON CONTROL SYSTE IEEE T CONTR SYST T1063-6536E NGINEERING IEEE TRANSACTIONS ON CONTROL SYSTE IEEE T CONTR SYST T1063-6536A UTOMATION IEEE Transactions on Cybernetics IEEE T CYBERNETICS2168-2267C OMPUTER SC IEEE Transactions on Cybernetics IEEE T CYBERNETICS2168-2267C OMPUTER SC IEEE TRANSACTIONS ON ELECTRON DEVICIEEE T ELECTRON DEV0018-9383E NGINEERINGIEEE T ELECTRON DEV0018-9383P HYSICS, APP IEEE TRANSACTIONS ON ELECTRON DEVICIEEE T ENERGY CONVER0885-8969E NGINEERING IEEE TRANSACTIONS ON ENERGY CONVERIEEE T ENERGY CONVER0885-8969E NERGY & FU IEEE TRANSACTIONS ON ENERGY CONVERIEEE T GEOSCI REMOTE0196-2892I MAGING SCIE IEEE TRANSACTIONS ON GEOSCIENCE ANDIEEE T GEOSCI REMOTE0196-2892G EOCHEMIST IEEE TRANSACTIONS ON GEOSCIENCE ANDIEEE T GEOSCI REMOTE0196-2892E NGINEERING IEEE TRANSACTIONS ON GEOSCIENCE ANDIEEE T GEOSCI REMOTE0196-2892R EMOTE SENS IEEE TRANSACTIONS ON GEOSCIENCE ANDIEEE Transactions on Human-Machine Systems I EEE T HUM-MACH SYST2168-2291C OMPUTER SC IEEE Transactions on Human-Machine Systems I EEE T HUM-MACH SYST2168-2291C OMPUTER SC IEEE TRANSACTIONS ON IMAGE PROCESSI IEEE T IMAGE PROCESS1057-7149E NGINEERING IEEE TRANSACTIONS ON IMAGE PROCESSI IEEE T IMAGE PROCESS1057-7149C OMPUTER SCIEEE T INFORM THEORY0018-9448E NGINEERING IEEE TRANSACTIONS ON INFORMATION THIEEE TRANSACTIONS ON INFORMATION THIEEE T INFORM THEORY0018-9448C OMPUTER SC IEEE TRANSACTIONS ON INTELLIGENT TR IEEE T INTELL TRANSP1524-9050E NGINEERING。

第42卷第4期2020年4月染整技术Textile Dyeing and Finishing JournalVol.42 No.4Apr.2020阿尔茨海默病老人针织智能安全监护服装的设计许黛芳、赵卫国2(1.嘉兴学院设计学院,浙江嘉兴314001;2.浙江理工大学浙江省服装工程技术研究中心,杭州310018)摘要智能安全监护服装是近年来智能服装设计研究的热点。
提出了目前阿尔茨海默病老人智能安全监护服装设计中普遍存在的问题,即设计理论模式不成熟、可穿戴设备体积过大以及与服装融合性差。
为了解决设计过 程中的关键性问题,从系统设计、服装造型结构设计、面料设计、工艺设计4个维度分析以石墨烯柔性传感器和功能模块为核心的智能服装,设计了具有A-GPS、跌倒检测、健康监测和语音提醒功能的阿尔茨海默病老人针织智能安全监护服装,解决了传统阿尔茨海默病老人安全监护服装设计中的诸多弊端。
关键词阿尔茨海默病;针织;智能;安全监护;服装设计中图分类号:TS941.7 文献标志码:A 文章编号:1005-9350(2020)04-0051-06Design of knitted intelligent safety monitoring clothing for theelderly with Alzheimer's diseaseX U Daifang, Z H A O Weiguo(1.College of Design, Jiaxing University, Jiaxing 314001, C h in a; 2. Zhejiang Clothing Engineering TechnologyResearch Center, Zhejiang S c i-T e rh University, Hangzhou 310018, China)Abstract Intelligent security monitoring clothing had become a hot topic in the research of intelligent clothing design in recent years. At present, the existing problems in the intelligent safety monitoring clothing design for the elderly with A lzheimer's disease were put forward, for example, the design theoretical model was not mature, wearable devices were too large in size, and the integration with clothing was poor. In order to solve the key problems in the process of design, in te lligent clothing with graphene flexible sensor and function module as the core were analyzed from four dimension of system design, structure design, fabrics design and process design. The knitted intelligent safety monitoring clothes for the elderly with Alzheim er's disease with A-G P S, falling detection, health monitoring and voire remind funrlion was designed. It solved many problems in the design of traditional safety monitoring rlothing for the elderly with Alzheim er's disease.Key words Alzheim er's disease; knitted; intelligent; safety monitoring; clothing design在大数据背景下,服装穿着的安全监护引起了人 们的高度重视。

Journal Data Filtered By: Selected JCR Year: 2013 Selected Editions: SCIE,SSCIRank FULL JOURNAL TITLE71364OR-A QUARTERLY JOURNAL OF OPERATIONS RESEARCH8747AAOHN JOURNAL8747AAOHN JOURNAL3832AAPG BULLETIN1058AAPS JOURNAL3987AAPS PHARMSCITECH10656AATCC REVIEW7605ABACUS-A JOURNAL OF ACCOUNTING FINANCE AND BUSINESS STUDIES4123ABDOMINAL IMAGING10799ABHANDLUNGEN AUS DEM MATHEMATISCHEN SEMINAR DER UNIVERSITAT H 5573ABSTRACT AND APPLIED ANALYSIS9948ACADEMIA-REVISTA LATINOAMERICANA DE ADMINISTRACION3054ACADEMIC EMERGENCY MEDICINE1347ACADEMIC MEDICINE3000ACADEMIC PEDIATRICS5681ACADEMIC PSYCHIATRY3277ACADEMIC RADIOLOGY307ACADEMY OF MANAGEMENT ANNALS653ACADEMY OF MANAGEMENT JOURNAL3193ACADEMY OF MANAGEMENT LEARNING & EDUCATION2021ACADEMY OF MANAGEMENT PERSPECTIVES275ACADEMY OF MANAGEMENT REVIEW2376ACCIDENT ANALYSIS AND PREVENTION8123ACCOUNTABILITY IN RESEARCH-POLICIES AND QUALITY ASSURANCE7061ACCOUNTING AND BUSINESS RESEARCH7718ACCOUNTING AND FINANCE6293ACCOUNTING AUDITING & ACCOUNTABILITY JOURNAL7757ACCOUNTING HORIZONS3219ACCOUNTING ORGANIZATIONS AND SOCIETY2989ACCOUNTING REVIEW32ACCOUNTS OF CHEMICAL RESEARCH6528ACCREDITATION AND QUALITY ASSURANCE6188ACI MATERIALS JOURNAL6915ACI STRUCTURAL JOURNAL980ACM COMPUTING SURVEYS7547ACM JOURNAL ON EMERGING TECHNOLOGIES IN COMPUTING SYSTEMS6290ACM SIGCOMM COMPUTER COMMUNICATION REVIEW8673ACM SIGPLAN NOTICES9917ACM TRANSACTIONS ON ALGORITHMS6517ACM TRANSACTIONS ON APPLIED PERCEPTION8813ACM TRANSACTIONS ON ARCHITECTURE AND CODE OPTIMIZATION7770ACM TRANSACTIONS ON AUTONOMOUS AND ADAPTIVE SYSTEMS8567ACM TRANSACTIONS ON COMPUTATIONAL LOGIC8712ACM TRANSACTIONS ON COMPUTER SYSTEMS8924ACM TRANSACTIONS ON COMPUTER-HUMAN INTERACTION7940ACM TRANSACTIONS ON DATABASE SYSTEMS9234ACM TRANSACTIONS ON DESIGN AUTOMATION OF ELECTRONIC SYSTEMS8308ACM TRANSACTIONS ON EMBEDDED COMPUTING SYSTEMS1157ACM TRANSACTIONS ON GRAPHICS7426ACM TRANSACTIONS ON INFORMATION AND SYSTEM SECURITY5497ACM TRANSACTIONS ON INFORMATION SYSTEMS206ACM TRANSACTIONS ON INTELLIGENT SYSTEMS AND TECHNOLOGY8912ACM TRANSACTIONS ON INTERNET TECHNOLOGY6076ACM TRANSACTIONS ON KNOWLEDGE DISCOVERY FROM DATA1493ACM TRANSACTIONS ON MATHEMATICAL SOFTWARE7571ACM TRANSACTIONS ON MODELING AND COMPUTER SIMULATION7205ACM TRANSACTIONS ON MULTIMEDIA COMPUTING COMMUNICATIONS AND 7694ACM TRANSACTIONS ON PROGRAMMING LANGUAGES AND SYSTEMS9878ACM TRANSACTIONS ON RECONFIGURABLE TECHNOLOGY AND SYSTEMS 4930ACM TRANSACTIONS ON SENSOR NETWORKS4907ACM TRANSACTIONS ON SOFTWARE ENGINEERING AND METHODOLOGY 8869ACM TRANSACTIONS ON STORAGE4517ACM TRANSACTIONS ON THE WEB9069ACOUSTICAL PHYSICS10100ACOUSTICS AUSTRALIA11446ACROSS LANGUAGES AND CULTURES457ACS APPLIED MATERIALS & INTERFACES292ACS CATALYSIS564ACS CHEMICAL BIOLOGY914ACS CHEMICAL NEUROSCIENCE1396ACS COMBINATORIAL SCIENCE588ACS MACRO LETTERS1692ACS MEDICINAL CHEMISTRY LETTERS137ACS NANO11471ACS SUSTAINABLE CHEMISTRY & ENGINEERING1029ACS SYNTHETIC BIOLOGY10592ACSMS HEALTH & FITNESS JOURNAL8310ACTA ACUSTICA UNITED WITH ACUSTICA9437ACTA ADRIATICA10562ACTA AGRICULTURAE SCANDINAVICA SECTION A-ANIMAL SCIENCE8481ACTA AGRICULTURAE SCANDINAVICA SECTION B-SOIL AND PLANT SCIENCE 9767ACTA ALIMENTARIA2837ACTA ANAESTHESIOLOGICA SCANDINAVICA8205ACTA APPLICANDAE MATHEMATICAE9797ACTA ARITHMETICA7627ACTA ASTRONAUTICA3551ACTA ASTRONOMICA3255ACTA BIOCHIMICA ET BIOPHYSICA SINICA5162ACTA BIOCHIMICA POLONICA11360ACTA BIOETHICA11360ACTA BIOETHICA8402ACTA BIOLOGICA CRACOVIENSIA SERIES BOTANICA8997ACTA BIOLOGICA HUNGARICA494ACTA BIOMATERIALIA5717ACTA BIOTHEORETICA9056ACTA BOTANICA BRASILICA9642ACTA BOTANICA CROATICA10739ACTA BOTANICA GALLICA8618ACTA BOTANICA MEXICANA9010ACTA CARDIOLOGICA7486ACTA CARDIOLOGICA SINICA8163ACTA CARSOLOGICA7346ACTA CHIMICA SINICA7649ACTA CHIMICA SLOVENICA7563ACTA CHIROPTEROLOGICA9837ACTA CHIRURGIAE ORTHOPAEDICAE ET TRAUMATOLOGIAE CECHOSLOVACA 9683ACTA CHIRURGICA BELGICA9430ACTA CHROMATOGRAPHICA8950ACTA CIRURGICA BRASILEIRA9797ACTA CLINICA CROATICA3298ACTA CRYSTALLOGRAPHICA SECTION A3246ACTA CRYSTALLOGRAPHICA SECTION B-STRUCTURAL SCIENCE9163ACTA CRYSTALLOGRAPHICA SECTION C-CRYSTAL STRUCTURE COMMUNICA 316ACTA CRYSTALLOGRAPHICA SECTION D-BIOLOGICAL CRYSTALLOGRAPHY 8961ACTA CRYSTALLOGRAPHICA SECTION F-STRUCTURAL BIOLOGY AND CRYSTA 4606ACTA CYTOLOGICA890ACTA DERMATO-VENEREOLOGICA8895ACTA DERMATOVENEROLOGICA CROATICA1188ACTA DIABETOLOGICA10855ACTA ENDOCRINOLOGICA-BUCHAREST8924ACTA ENTOMOLOGICA MUSEI NATIONALIS PRAGAE7675ACTA ETHOLOGICA9957ACTA GEODAETICA ET GEOPHYSICA8364ACTA GEODYNAMICA ET GEOMATERIALIA7940ACTA GEOGRAPHICA SLOVENICA-GEOGRAFSKI ZBORNIK7675ACTA GEOLOGICA POLONICA5103ACTA GEOLOGICA SINICA-ENGLISH EDITION5237ACTA GEOPHYSICA3771ACTA GEOTECHNICA9600ACTA GEOTECHNICA SLOVENICA6796ACTA HAEMATOLOGICA7639ACTA HERPETOLOGICA4036ACTA HISTOCHEMICA5751ACTA HISTOCHEMICA ET CYTOCHEMICA11387ACTA HISTRIAE8254ACTA ICHTHYOLOGICA ET PISCATORIA9896ACTA INFORMATICA11135ACTA LINGUISTICA HUNGARICA1039ACTA MATERIALIA1740ACTA MATHEMATICA9913ACTA MATHEMATICA HUNGARICA8680ACTA MATHEMATICA SCIENTIA9809ACTA MATHEMATICA SINICA-ENGLISH SERIES9993ACTA MATHEMATICAE APPLICATAE SINICA-ENGLISH SERIES5584ACTA MECHANICA8708ACTA MECHANICA SINICA8473ACTA MECHANICA SOLIDA SINICA7963ACTA MEDICA OKAYAMA10523ACTA MEDICA PORTUGUESA9083ACTA METALLURGICA SINICA9770ACTA METALLURGICA SINICA-ENGLISH LETTERS6238ACTA METEOROLOGICA SINICA7789ACTA MICROBIOLOGICA ET IMMUNOLOGICA HUNGARICA 11432ACTA MICROSCOPICA11379ACTA MONTANISTICA SLOVACA7355ACTA NATURAE2969ACTA NEUROBIOLOGIAE EXPERIMENTALIS3952ACTA NEUROCHIRURGICA8807ACTA NEUROLOGICA BELGICA2609ACTA NEUROLOGICA SCANDINAVICA195ACTA NEUROPATHOLOGICA8548ACTA NEUROPSYCHIATRICA3480ACTA OBSTETRICIA ET GYNECOLOGICA SCANDINAVICA 8282ACTA OCEANOLOGICA SINICA5467ACTA ODONTOLOGICA SCANDINAVICA3806ACTA OECOLOGICA-INTERNATIONAL JOURNAL OF ECOLOGY 11300ACTA OECONOMICA6847ACTA OF BIOENGINEERING AND BIOMECHANICS1163ACTA ONCOLOGICA2482ACTA OPHTHALMOLOGICA4884ACTA ORNITHOLOGICA2582ACTA ORTHOPAEDICA8969ACTA ORTHOPAEDICA BELGICA9050ACTA ORTHOPAEDICA ET TRAUMATOLOGICA TURCICA 11079ACTA ORTOPEDICA BRASILEIRA6804ACTA OTO-LARYNGOLOGICA5003ACTA OTORHINOLARYNGOLOGICA ITALICA3804ACTA PAEDIATRICA4146ACTA PALAEONTOLOGICA POLONICA6909ACTA PARASITOLOGICA10595ACTA PAULISTA DE ENFERMAGEM10595ACTA PAULISTA DE ENFERMAGEM5751ACTA PETROLOGICA SINICA6633ACTA PHARMACEUTICA2509ACTA PHARMACOLOGICA SINICA8777ACTA PHYSICA POLONICA A6794ACTA PHYSICA POLONICA B7486ACTA PHYSICA SINICA3446ACTA PHYSICA SLOVACA7285ACTA PHYSICO-CHIMICA SINICA4734ACTA PHYSIOLOGIAE PLANTARUM884ACTA PHYSIOLOGICA7958ACTA PHYSIOLOGICA HUNGARICA8364ACTA POLITICA8250ACTA POLONIAE PHARMACEUTICA8548ACTA POLYMERICA SINICA9517ACTA POLYTECHNICA HUNGARICA5936ACTA PROTOZOOLOGICA515ACTA PSYCHIATRICA SCANDINAVICA515ACTA PSYCHIATRICA SCANDINAVICA2738ACTA PSYCHOLOGICA5315ACTA RADIOLOGICA7563ACTA REUMATOLOGICA PORTUGUESA11084ACTA SCIENTIAE VETERINARIAE9225ACTA SCIENTIARUM POLONORUM-HORTORUM CULTUS8605ACTA SCIENTIARUM-AGRONOMY9579ACTA SCIENTIARUM-TECHNOLOGY5874ACTA SOCIETATIS BOTANICORUM POLONIAE6856ACTA SOCIOLOGICA6020ACTA THERIOLOGICA2467ACTA TROPICA9648ACTA VETERINARIA BRNO7691ACTA VETERINARIA HUNGARICA5184ACTA VETERINARIA SCANDINAVICA11154ACTA VETERINARIA-BEOGRAD6584ACTA VIROLOGICA5508ACTA ZOOLOGICA10619ACTA ZOOLOGICA ACADEMIAE SCIENTIARUM HUNGARICAE10132ACTA ZOOLOGICA BULGARICA7890ACTAS ESPANOLAS DE PSIQUIATRIA6071ACTAS UROLOGICAS ESPANOLAS11112ACTES DE LA RECHERCHE EN SCIENCES SOCIALES9787ACTION RESEARCH9917ACUPUNCTURE & ELECTRO-THERAPEUTICS RESEARCH4267ACUPUNCTURE IN MEDICINE9482AD HOC & SENSOR WIRELESS NETWORKS3576AD HOC NETWORKS9464ADANSONIA6390ADAPTED PHYSICAL ACTIVITY QUARTERLY6060ADAPTIVE BEHAVIOR6060ADAPTIVE BEHAVIOR754ADDICTION754ADDICTION451ADDICTION BIOLOGY6655ADDICTION RESEARCH & THEORY2603ADDICTIVE BEHAVIORS2603ADDICTIVE BEHAVIORS5984ADICCIONES5984ADICCIONES8250ADMINISTRATION & SOCIETY1366ADMINISTRATION AND POLICY IN MENTAL HEALTH AND MENTAL HEALTH SE 9566ADMINISTRATION IN SOCIAL WORK6903ADMINISTRATIVE LAW REVIEW2688ADMINISTRATIVE SCIENCE QUARTERLY7073ADSORPTION SCIENCE & TECHNOLOGY4112ADSORPTION-JOURNAL OF THE INTERNATIONAL ADSORPTION SOCIETY 7764ADULT EDUCATION QUARTERLY9453ADVANCED COMPOSITE MATERIALS128ADVANCED DRUG DELIVERY REVIEWS94ADVANCED ENERGY MATERIALS3300ADVANCED ENGINEERING INFORMATICS4776ADVANCED ENGINEERING MATERIALS170ADVANCED FUNCTIONAL MATERIALS682ADVANCED HEALTHCARE MATERIALS76ADVANCED MATERIALS10660ADVANCED MATERIALS & PROCESSES8341ADVANCED NONLINEAR STUDIES11471ADVANCED OPTICAL MATERIALS4377ADVANCED POWDER TECHNOLOGY9004ADVANCED ROBOTICS10808ADVANCED STEEL CONSTRUCTION519ADVANCED SYNTHESIS & CATALYSIS637ADVANCES IN AGRONOMY1670ADVANCES IN ANATOMIC PATHOLOGY193ADVANCES IN ANATOMY EMBRYOLOGY AND CELL BIOLOGY6266ADVANCES IN APPLIED CERAMICS9183ADVANCES IN APPLIED CLIFFORD ALGEBRAS7316ADVANCES IN APPLIED MATHEMATICS8511ADVANCES IN APPLIED MATHEMATICS AND MECHANICS5854ADVANCES IN APPLIED MECHANICS2971ADVANCES IN APPLIED MICROBIOLOGY7571ADVANCES IN APPLIED PROBABILITY5703ADVANCES IN ASTRONOMY4943ADVANCES IN ATMOSPHERIC SCIENCES1397ADVANCES IN ATOMIC MOLECULAR AND OPTICAL PHYSICS2329ADVANCES IN BIOCHEMICAL ENGINEERING-BIOTECHNOLOGY4096ADVANCES IN BOTANICAL RESEARCH9065ADVANCES IN CALCULUS OF VARIATIONS880ADVANCES IN CANCER RESEARCH1052ADVANCES IN CARBOHYDRATE CHEMISTRY AND BIOCHEMISTRY261ADVANCES IN CATALYSIS9427ADVANCES IN CEMENT RESEARCH3199ADVANCES IN CHEMICAL PHYSICS7053ADVANCES IN CHILD DEVELOPMENT AND BEHAVIOR3597ADVANCES IN CHRONIC KIDNEY DISEASE10260ADVANCES IN CLINICAL AND EXPERIMENTAL MEDICINE861ADVANCES IN CLINICAL CHEMISTRY232ADVANCES IN COLLOID AND INTERFACE SCIENCE7764ADVANCES IN COMPLEX SYSTEMS4606ADVANCES IN COMPUTATIONAL MATHEMATICS9405ADVANCES IN COMPUTERS6682ADVANCES IN CONDENSED MATTER PHYSICS7127ADVANCES IN DATA ANALYSIS AND CLASSIFICATION8585ADVANCES IN DIFFERENCE EQUATIONS7874ADVANCES IN DIFFERENTIAL EQUATIONS402ADVANCES IN ECOLOGICAL RESEARCH8535ADVANCES IN ELECTRICAL AND COMPUTER ENGINEERING5052ADVANCES IN ENGINEERING SOFTWARE767ADVANCES IN EXPERIMENTAL SOCIAL PSYCHOLOGY552ADVANCES IN GENETICS10372ADVANCES IN GEOMETRY10260ADVANCES IN GEOPHYSICS2175ADVANCES IN HEALTH SCIENCES EDUCATION2175ADVANCES IN HEALTH SCIENCES EDUCATION2057ADVANCES IN HETEROCYCLIC CHEMISTRY2300ADVANCES IN HIGH ENERGY PHYSICS8891ADVANCES IN IMAGING AND ELECTRON PHYSICS523ADVANCES IN IMMUNOLOGY2403ADVANCES IN INORGANIC CHEMISTRY2209ADVANCES IN INSECT PHYSIOLOGY8132ADVANCES IN LIFE COURSE RESEARCH643ADVANCES IN MARINE BIOLOGY7235ADVANCES IN MATERIALS SCIENCE AND ENGINEERING9171ADVANCES IN MATHEMATICAL PHYSICS5299ADVANCES IN MATHEMATICS8473ADVANCES IN MATHEMATICS OF COMMUNICATIONS9324ADVANCES IN MECHANICAL ENGINEERING6915ADVANCES IN MEDICAL SCIENCES5320ADVANCES IN METEOROLOGY475ADVANCES IN MICROBIAL PHYSIOLOGY7389ADVANCES IN NURSING SCIENCE7389ADVANCES IN NURSING SCIENCE676ADVANCES IN NUTRITION198ADVANCES IN OPTICS AND PHOTONICS1246ADVANCES IN ORGANOMETALLIC CHEMISTRY841ADVANCES IN PARASITOLOGY4162ADVANCES IN PHYSICAL ORGANIC CHEMISTRY57ADVANCES IN PHYSICS5692ADVANCES IN PHYSIOLOGY EDUCATION1193ADVANCES IN POLYMER SCIENCE3145ADVANCES IN POLYMER TECHNOLOGY1151ADVANCES IN PROTEIN CHEMISTRY AND STRUCTURAL BIOLOGY 3715ADVANCES IN QUANTUM CHEMISTRY4407ADVANCES IN SKIN & WOUND CARE4407ADVANCES IN SKIN & WOUND CARE5691ADVANCES IN SPACE RESEARCH9254ADVANCES IN STRATEGIC MANAGEMENT-A RESEARCH ANNUAL 8786ADVANCES IN STRUCTURAL ENGINEERING2196ADVANCES IN THE STUDY OF BEHAVIOR3975ADVANCES IN THEORETICAL AND MATHEMATICAL PHYSICS 2608ADVANCES IN THERAPY10471ADVANCES IN VIBRATION ENGINEERING1257ADVANCES IN VIRUS RESEARCH2077ADVANCES IN WATER RESOURCES1983AEOLIAN RESEARCH9079AEQUATIONES MATHEMATICAE5845AEROBIOLOGIA10253AERONAUTICAL JOURNAL2241AEROSOL AND AIR QUALITY RESEARCH1618AEROSOL SCIENCE AND TECHNOLOGY11391AEROSPACE AMERICA6718AEROSPACE SCIENCE AND TECHNOLOGY5901AESTHETIC PLASTIC SURGERY3380AESTHETIC SURGERY JOURNAL8235AEU-INTERNATIONAL JOURNAL OF ELECTRONICS AND COMMUNICATIONS 10991AFFILIA-JOURNAL OF WOMEN AND SOCIAL WORK11243AFINIDAD7968AFRICA7659AFRICA SPECTRUM4629AFRICAN AFFAIRS11286AFRICAN AND ASIAN STUDIES8589AFRICAN DEVELOPMENT REVIEW-REVUE AFRICAINE DE DEVELOPPEMENT 7824AFRICAN ENTOMOLOGY8402AFRICAN HEALTH SCIENCES9324AFRICAN INVERTEBRATES9324AFRICAN JOURNAL OF AQUATIC SCIENCE6718AFRICAN JOURNAL OF ECOLOGY6397AFRICAN JOURNAL OF HERPETOLOGY11223AFRICAN JOURNAL OF LIBRARY ARCHIVES AND INFORMATION SCIENCE6490AFRICAN JOURNAL OF MARINE SCIENCE8057AFRICAN JOURNAL OF PSYCHIATRY8057AFRICAN JOURNAL OF PSYCHIATRY10078AFRICAN JOURNAL OF RANGE & FORAGE SCIENCE9017AFRICAN JOURNAL OF TRADITIONAL COMPLEMENTARY AND ALTERNATIVE M 11233AFRICAN NATURAL HISTORY11173AFRICAN STUDIES7473AFRICAN ZOOLOGY11154AFRICANA LINGUISTICA1365AGE1659AGE AND AGEING5412AGEING & SOCIETY287AGEING RESEARCH REVIEWS3197AGGRESSION AND VIOLENT BEHAVIOR2922AGGRESSIVE BEHAVIOR2922AGGRESSIVE BEHAVIOR3975AGING & MENTAL HEALTH3975AGING & MENTAL HEALTH447AGING CELL6129AGING CLINICAL AND EXPERIMENTAL RESEARCH3788AGING MALE5917AGING NEUROPSYCHOLOGY AND COGNITION680AGING-US11422AGRARFORSCHUNG SCHWEIZ11130AGREKON9261AGRIBUSINESS9261AGRIBUSINESS6718AGRICULTURAL AND FOOD SCIENCE4623AGRICULTURAL AND FOREST ENTOMOLOGY1063AGRICULTURAL AND FOREST METEOROLOGY6364AGRICULTURAL ECONOMICS6364AGRICULTURAL ECONOMICS10323AGRICULTURAL ECONOMICS-ZEMEDELSKA EKONOMIKA10323AGRICULTURAL ECONOMICS-ZEMEDELSKA EKONOMIKA10328AGRICULTURAL HISTORY10328AGRICULTURAL HISTORY7607AGRICULTURAL SCIENCES IN CHINA2580AGRICULTURAL SYSTEMS2790AGRICULTURAL WATER MANAGEMENT5270AGRICULTURE AND HUMAN VALUES5270AGRICULTURE AND HUMAN VALUES1569AGRICULTURE ECOSYSTEMS & ENVIRONMENT10452AGRO FOOD INDUSTRY HI-TECH10372AGROCHIMICA11389AGROCIENCIA11471AGROECOLOGY AND SUSTAINABLE FOOD SYSTEMS5687AGROFORESTRY SYSTEMS1983AGRONOMY FOR SUSTAINABLE DEVELOPMENT4670AGRONOMY JOURNAL9553AI COMMUNICATIONS9056AI EDAM-ARTIFICIAL INTELLIGENCE FOR ENGINEERING DESIGN ANALYSIS AN 9324AI MAGAZINE5997AIAA JOURNAL11446AIBR-REVISTA DE ANTROPOLOGIA IBEROAMERICANA2361AICHE JOURNAL369AIDS1475AIDS AND BEHAVIOR3058AIDS CARE-PSYCHOLOGICAL AND SOCIO-MEDICAL ASPECTS OF AIDS/HIV 4786AIDS EDUCATION AND PREVENTION1263AIDS PATIENT CARE AND STDS1263AIDS PATIENT CARE AND STDS2576AIDS RESEARCH AND HUMAN RETROVIRUSES3806AIDS RESEARCH AND THERAPY991AIDS REVIEWS4533AIP ADVANCES4960AIR QUALITY ATMOSPHERE AND HEALTH9464AIRCRAFT ENGINEERING AND AEROSPACE TECHNOLOGY8747AJAR-AFRICAN JOURNAL OF AIDS RESEARCH8747AJAR-AFRICAN JOURNAL OF AIDS RESEARCH2658AJIDD-AMERICAN JOURNAL ON INTELLECTUAL AND DEVELOPMENTAL DISAB11373AKTUELLE RHEUMATOLOGIE10530AKTUELLE UROLOGIE7232ALCHERINGA3371ALCOHOL3250ALCOHOL AND ALCOHOLISM3250ALCOHOL AND ALCOHOLISM5113ALCOHOL RESEARCH & HEALTH11471ALCOHOL RESEARCH-CURRENT REVIEWS1476ALCOHOLISM-CLINICAL AND EXPERIMENTAL RESEARCH65ALDRICHIMICA ACTA8555ALEA-LATIN AMERICAN JOURNAL OF PROBABILITY AND MATHEMATICAL STA 960ALGAL RESEARCH-BIOMASS BIOFUELS AND BIOPRODUCTS8895ALGEBRA & NUMBER THEORY9410ALGEBRA AND LOGIC10575ALGEBRA COLLOQUIUM9069ALGEBRA UNIVERSALIS9405ALGEBRAIC AND GEOMETRIC TOPOLOGY8111ALGEBRAS AND REPRESENTATION THEORY8969ALGORITHMICA3777ALGORITHMS FOR MOLECULAR BIOLOGY533ALIMENTARY PHARMACOLOGY & THERAPEUTICS8909ALLELOPATHY JOURNAL4563ALLERGOLOGIA ET IMMUNOPATHOLOGIA10443ALLERGOLOGIE438ALLERGY1441ALLERGY AND ASTHMA PROCEEDINGS1685ALLERGY ASTHMA & IMMUNOLOGY RESEARCH8190ALLGEMEINE FORST UND JAGDZEITUNG2641ALPINE BOTANY6095ALTERNATIVE THERAPIES IN HEALTH AND MEDICINE10108ALTERNATIVES1221ALTEX-ALTERNATIVES TO ANIMAL EXPERIMENTATION2205ALZHEIMER DISEASE & ASSOCIATED DISORDERS59ALZHEIMERS & DEMENTIA1324ALZHEIMERS RESEARCH & THERAPY11371AMA-AGRICULTURAL MECHANIZATION IN ASIA AFRICA AND LATIN AMERICA 1806AMBIO10462AMBIX5974AMEGHINIANA7290AMERICAN ANNALS OF THE DEAF6185AMERICAN ANTHROPOLOGIST5320AMERICAN ANTIQUITY8567AMERICAN BANKRUPTCY LAW JOURNAL11401AMERICAN BEE JOURNAL7084AMERICAN BEHAVIORAL SCIENTIST10067AMERICAN BIOLOGY TEACHER4498AMERICAN BUSINESS LAW JOURNAL7616AMERICAN CERAMIC SOCIETY BULLETIN9669AMERICAN CRIMINAL LAW REVIEW1687AMERICAN ECONOMIC JOURNAL-APPLIED ECONOMICS2394AMERICAN ECONOMIC JOURNAL-ECONOMIC POLICY1904AMERICAN ECONOMIC JOURNAL-MACROECONOMICS4280AMERICAN ECONOMIC JOURNAL-MICROECONOMICS1482AMERICAN ECONOMIC REVIEW2902AMERICAN EDUCATIONAL RESEARCH JOURNAL4103AMERICAN ETHNOLOGIST3876AMERICAN FAMILY PHYSICIAN9746AMERICAN FERN JOURNAL769AMERICAN HEART JOURNAL5518AMERICAN HISTORICAL REVIEW8364AMERICAN INDIAN AND ALASKA NATIVE MENTAL HEALTH RESEARCH 5251AMERICAN JOURNAL OF AGRICULTURAL ECONOMICS5251AMERICAN JOURNAL OF AGRICULTURAL ECONOMICS5037AMERICAN JOURNAL OF ALZHEIMERS DISEASE AND OTHER DEMENTIAS 6442AMERICAN JOURNAL OF AUDIOLOGY2582AMERICAN JOURNAL OF BIOETHICS2582AMERICAN JOURNAL OF BIOETHICS2557AMERICAN JOURNAL OF BOTANY1016AMERICAN JOURNAL OF CANCER RESEARCH1374AMERICAN JOURNAL OF CARDIOLOGY3045AMERICAN JOURNAL OF CARDIOVASCULAR DRUGS2297AMERICAN JOURNAL OF CHINESE MEDICINE2467AMERICAN JOURNAL OF CLINICAL DERMATOLOGY9400AMERICAN JOURNAL OF CLINICAL HYPNOSIS339AMERICAN JOURNAL OF CLINICAL NUTRITION2316AMERICAN JOURNAL OF CLINICAL ONCOLOGY-CANCER CLINICAL TRIALS 1772AMERICAN JOURNAL OF CLINICAL PATHOLOGY3512AMERICAN JOURNAL OF COMMUNITY PSYCHOLOGY9199AMERICAN JOURNAL OF COMPARATIVE LAW4498AMERICAN JOURNAL OF CRITICAL CARE6465AMERICAN JOURNAL OF DENTISTRY5037AMERICAN JOURNAL OF DERMATOPATHOLOGY4912AMERICAN JOURNAL OF DRUG AND ALCOHOL ABUSE4912AMERICAN JOURNAL OF DRUG AND ALCOHOL ABUSE10295AMERICAN JOURNAL OF ECONOMICS AND SOCIOLOGY4533AMERICAN JOURNAL OF EDUCATION6049AMERICAN JOURNAL OF EMERGENCY MEDICINE4414AMERICAN JOURNAL OF ENOLOGY AND VITICULTURE651AMERICAN JOURNAL OF EPIDEMIOLOGY6909AMERICAN JOURNAL OF EVALUATION9199AMERICAN JOURNAL OF FAMILY THERAPY8649AMERICAN JOURNAL OF FORENSIC MEDICINE AND PATHOLOGY216AMERICAN JOURNAL OF GASTROENTEROLOGY2256AMERICAN JOURNAL OF GERIATRIC PHARMACOTHERAPY1305AMERICAN JOURNAL OF GERIATRIC PSYCHIATRY1305AMERICAN JOURNAL OF GERIATRIC PSYCHIATRY6134AMERICAN JOURNAL OF HEALTH BEHAVIOR4026AMERICAN JOURNAL OF HEALTH PROMOTION3043AMERICAN JOURNAL OF HEALTH-SYSTEM PHARMACY1340AMERICAN JOURNAL OF HEMATOLOGY5327AMERICAN JOURNAL OF HOSPICE & PALLIATIVE MEDICINE3617AMERICAN JOURNAL OF HUMAN BIOLOGY3617AMERICAN JOURNAL OF HUMAN BIOLOGY161AMERICAN JOURNAL OF HUMAN GENETICS1395AMERICAN JOURNAL OF HYPERTENSION4533AMERICAN JOURNAL OF INDUSTRIAL MEDICINE2807AMERICAN JOURNAL OF INFECTION CONTROL5277AMERICAN JOURNAL OF INTERNATIONAL LAW483AMERICAN JOURNAL OF KIDNEY DISEASES3108AMERICAN JOURNAL OF MANAGED CARE3108AMERICAN JOURNAL OF MANAGED CARE4351AMERICAN JOURNAL OF MATHEMATICS3346AMERICAN JOURNAL OF MEDICAL GENETICS PART A1518AMERICAN JOURNAL OF MEDICAL GENETICS PART B-NEUROPSYCHIATRIC GE 1286AMERICAN JOURNAL OF MEDICAL GENETICS PART C-SEMINARS IN MEDICAL 3987AMERICAN JOURNAL OF MEDICAL QUALITY572AMERICAN JOURNAL OF MEDICINE5581AMERICAN JOURNAL OF MENS HEALTH2267AMERICAN JOURNAL OF NEPHROLOGY1191AMERICAN JOURNAL OF NEURORADIOLOGY5429AMERICAN JOURNAL OF NURSING5429AMERICAN JOURNAL OF NURSING1012AMERICAN JOURNAL OF OBSTETRICS AND GYNECOLOGY4636AMERICAN JOURNAL OF OCCUPATIONAL THERAPY992AMERICAN JOURNAL OF OPHTHALMOLOGY5010AMERICAN JOURNAL OF ORTHODONTICS AND DENTOFACIAL ORTHOPEDICS 4790AMERICAN JOURNAL OF ORTHOPSYCHIATRY4790AMERICAN JOURNAL OF ORTHOPSYCHIATRY6391AMERICAN JOURNAL OF OTOLARYNGOLOGY752AMERICAN JOURNAL OF PATHOLOGY4513AMERICAN JOURNAL OF PERINATOLOGY5905AMERICAN JOURNAL OF PHARMACEUTICAL EDUCATION2478AMERICAN JOURNAL OF PHYSICAL ANTHROPOLOGY2478AMERICAN JOURNAL OF PHYSICAL ANTHROPOLOGY3426AMERICAN JOURNAL OF PHYSICAL MEDICINE & REHABILITATION7675AMERICAN JOURNAL OF PHYSICS1193AMERICAN JOURNAL OF PHYSIOLOGY-CELL PHYSIOLOGY964AMERICAN JOURNAL OF PHYSIOLOGY-ENDOCRINOLOGY AND METABOLISM 1149AMERICAN JOURNAL OF PHYSIOLOGY-GASTROINTESTINAL AND LIVER PHYSI 996AMERICAN JOURNAL OF PHYSIOLOGY-HEART AND CIRCULATORY PHYSIOLOG 981AMERICAN JOURNAL OF PHYSIOLOGY-LUNG CELLULAR AND MOLECULAR PH 1297AMERICAN JOURNAL OF PHYSIOLOGY-REGULATORY INTEGRATIVE AND COM 1486AMERICAN JOURNAL OF PHYSIOLOGY-RENAL PHYSIOLOGY2475AMERICAN JOURNAL OF POLITICAL SCIENCE6982AMERICAN JOURNAL OF POTATO RESEARCH872AMERICAN JOURNAL OF PREVENTIVE MEDICINE3169AMERICAN JOURNAL OF PRIMATOLOGY107AMERICAN JOURNAL OF PSYCHIATRY107AMERICAN JOURNAL OF PSYCHIATRY7845AMERICAN JOURNAL OF PSYCHOLOGY901AMERICAN JOURNAL OF PUBLIC HEALTH901AMERICAN JOURNAL OF PUBLIC HEALTH2235AMERICAN JOURNAL OF REPRODUCTIVE IMMUNOLOGY139AMERICAN JOURNAL OF RESPIRATORY AND CRITICAL CARE MEDICINE957AMERICAN JOURNAL OF RESPIRATORY CELL AND MOLECULAR BIOLOGY 3079AMERICAN JOURNAL OF RHINOLOGY & ALLERGY2119AMERICAN JOURNAL OF ROENTGENOLOGY1047AMERICAN JOURNAL OF SCIENCE976AMERICAN JOURNAL OF SOCIOLOGY4365AMERICAN JOURNAL OF SPEECH-LANGUAGE PATHOLOGY4365AMERICAN JOURNAL OF SPEECH-LANGUAGE PATHOLOGY725AMERICAN JOURNAL OF SPORTS MEDICINE2663AMERICAN JOURNAL OF SURGERY756AMERICAN JOURNAL OF SURGICAL PATHOLOGY4760AMERICAN JOURNAL OF THE MEDICAL SCIENCES6172AMERICAN JOURNAL OF THERAPEUTICS1550AMERICAN JOURNAL OF TRANSLATIONAL RESEARCH409AMERICAN JOURNAL OF TRANSPLANTATION2129AMERICAN JOURNAL OF TROPICAL MEDICINE AND HYGIENE5798AMERICAN JOURNAL OF VETERINARY RESEARCH4740AMERICAN JOURNAL ON ADDICTIONS11198AMERICAN LABORATORY7750AMERICAN LAW AND ECONOMICS REVIEW7497AMERICAN MALACOLOGICAL BULLETIN10366AMERICAN MATHEMATICAL MONTHLY8673AMERICAN MIDLAND NATURALIST3322AMERICAN MINERALOGIST4401AMERICAN MUSEUM NOVITATES809AMERICAN NATURALIST1093AMERICAN POLITICAL SCIENCE REVIEW7308AMERICAN POLITICS RESEARCH542AMERICAN PSYCHOLOGIST7332AMERICAN REVIEW OF PUBLIC ADMINISTRATION8524AMERICAN SCIENTIST876AMERICAN SOCIOLOGICAL REVIEW10667AMERICAN SPEECH7322AMERICAN STATISTICIAN7525AMFITEATRU ECONOMIC1207AMINO ACIDS11435AMME IDARESI DERGISI6129AMPHIBIA-REPTILIA2486AMYLOID-JOURNAL OF PROTEIN FOLDING DISORDERS2343AMYOTROPHIC LATERAL SCLEROSIS AND FRONTOTEMPORAL DEGENERATIO 7914ANADOLU KARDIYOLOJI DERGISI-THE ANATOLIAN JOURNAL OF CARDIOLOGY 10925ANADOLU PSIKIYATRI DERGISI-ANATOLIAN JOURNAL OF PSYCHIATRY2744ANAEROBE1091ANAESTHESIA4912ANAESTHESIA AND INTENSIVE CARE7980ANAESTHESIST7402ANAIS BRASILEIROS DE DERMATOLOGIA7332ANAIS DA ACADEMIA BRASILEIRA DE CIENCIAS11241ANALELE STIINTIFICE ALE UNIVERSITATII AL I CUZA DIN IASI-SERIE NOUA-M 10770ANALELE STIINTIFICE ALE UNIVERSITATII OVIDIUS CONSTANTA-SERIA MATE 8090ANALES DE PEDIATRIA9079ANALES DE PSICOLOGIA9079ANALES DE PSICOLOGIA7159ANALES DEL JARDIN BOTANICO DE MADRID9031ANALES DEL SISTEMA SANITARIO DE NAVARRA9031ANALES DEL SISTEMA SANITARIO DE NAVARRA9913ANALOG INTEGRATED CIRCUITS AND SIGNAL PROCESSING9073ANALYSES OF SOCIAL ISSUES AND PUBLIC POLICY7192ANALYSIS & PDE4799ANALYSIS AND APPLICATIONS10471ANALYSIS MATHEMATICA1057ANALYST785ANALYTICA CHIMICA ACTA1262ANALYTICAL AND BIOANALYTICAL CHEMISTRY8904ANALYTICAL AND QUANTITATIVE CYTOLOGY AND HISTOLOGY2845ANALYTICAL BIOCHEMISTRY4044ANALYTICAL CELLULAR PATHOLOGY468ANALYTICAL CHEMISTRY6838ANALYTICAL LETTERS3590ANALYTICAL METHODS5113ANALYTICAL SCIENCES9778ANASTHESIOLOGIE & INTENSIVMEDIZIN10259ANASTHESIOLOGIE INTENSIVMEDIZIN NOTFALLMEDIZIN SCHMERZTHERAPIE 7984ANATOMIA HISTOLOGIA EMBRYOLOGIA4716ANATOMICAL RECORD-ADVANCES IN INTEGRATIVE ANATOMY AND EVOLUTI 7516ANATOMICAL SCIENCE INTERNATIONAL4701ANDEAN GEOLOGY5960ANDROLOGIA11471ANDROLOGY1382ANESTHESIA AND ANALGESIA414ANESTHESIOLOGY154ANGEWANDTE CHEMIE-INTERNATIONAL EDITION821ANGIOGENESIS2730ANGIOLOGY5563ANGLE ORTHODONTIST3965ANIMAL1701ANIMAL BEHAVIOUR9240ANIMAL BIODIVERSITY AND CONSERVATION8717ANIMAL BIOLOGY8567ANIMAL BIOTECHNOLOGY10185ANIMAL CELLS AND SYSTEMS2282ANIMAL COGNITION2453ANIMAL CONSERVATION3263ANIMAL FEED SCIENCE AND TECHNOLOGY3032ANIMAL GENETICS6620ANIMAL PRODUCTION SCIENCE4558ANIMAL REPRODUCTION SCIENCE6546ANIMAL SCIENCE JOURNAL7634ANIMAL SCIENCE PAPERS AND REPORTS5727ANIMAL WELFARE10855ANKARA UNIVERSITESI VETERINER FAKULTESI DERGISI4870ANNALEN DER PHYSIK8496ANNALES ACADEMIAE SCIENTIARUM FENNICAE-MATHEMATICA7831ANNALES BOTANICI FENNICI8396ANNALES D ENDOCRINOLOGIE9837ANNALES DE BIOLOGIE CLINIQUE10418ANNALES DE CARDIOLOGIE ET D ANGEIOLOGIE11446ANNALES DE CHIMIE-SCIENCE DES MATERIAUX8856ANNALES DE CHIRURGIE PLASTIQUE ESTHETIQUE8360ANNALES DE DERMATOLOGIE ET DE VENEREOLOGIE8580ANNALES DE L INSTITUT FOURIER5407ANNALES DE L INSTITUT HENRI POINCARE-ANALYSE NON LINEAIRE6882ANNALES DE L INSTITUT HENRI POINCARE-PROBABILITES ET STATISTIQUES 9130ANNALES DE LA SOCIETE ENTOMOLOGIQUE DE FRANCE6589ANNALES DE LIMNOLOGIE-INTERNATIONAL JOURNAL OF LIMNOLOGY 6342ANNALES DE PALEONTOLOGIE10468ANNALES DE PATHOLOGIE7534ANNALES FRANCAISES D ANESTHESIE ET DE REANIMATION4267ANNALES GEOPHYSICAE5230ANNALES HENRI POINCARE11096ANNALES MEDICO-PSYCHOLOGIQUES9709ANNALES POLONICI MATHEMATICI5731ANNALES SCIENTIFIQUES DE L ECOLE NORMALE SUPERIEURE8057ANNALES SOCIETATIS GEOLOGORUM POLONIAE6853ANNALES ZOOLOGICI6613ANNALES ZOOLOGICI FENNICI7820ANNALI DELL ISTITUTO SUPERIORE DI SANITA7192ANNALI DELLA SCUOLA NORMALE SUPERIORE DI PISA-CLASSE DI SCIENZE 7175ANNALI DI MATEMATICA PURA ED APPLICATA8290ANNALI ITALIANI DI CHIRURGIA5761ANNALS ACADEMY OF MEDICINE SINGAPORE2115ANNALS OF ALLERGY ASTHMA & IMMUNOLOGY3281ANNALS OF ANATOMY-ANATOMISCHER ANZEIGER9809ANNALS OF ANIMAL SCIENCE3551ANNALS OF APPLIED BIOLOGY5006ANNALS OF APPLIED PROBABILITY4238ANNALS OF APPLIED STATISTICS1231ANNALS OF BEHAVIORAL MEDICINE1547ANNALS OF BIOMEDICAL ENGINEERING1487ANNALS OF BOTANY9017ANNALS OF CARNEGIE MUSEUM。
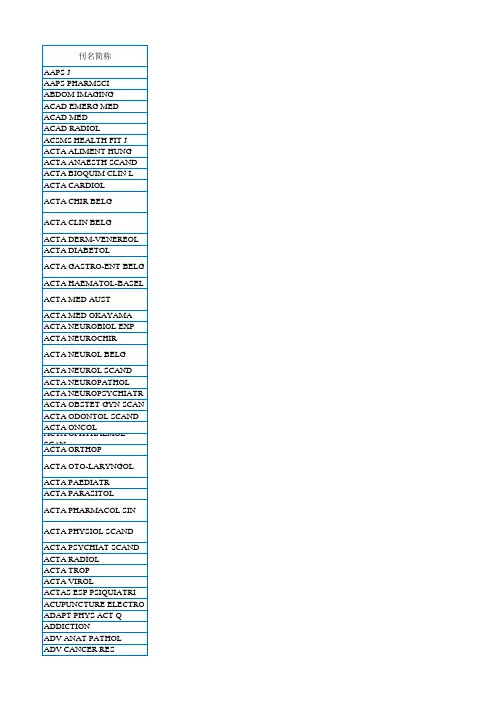
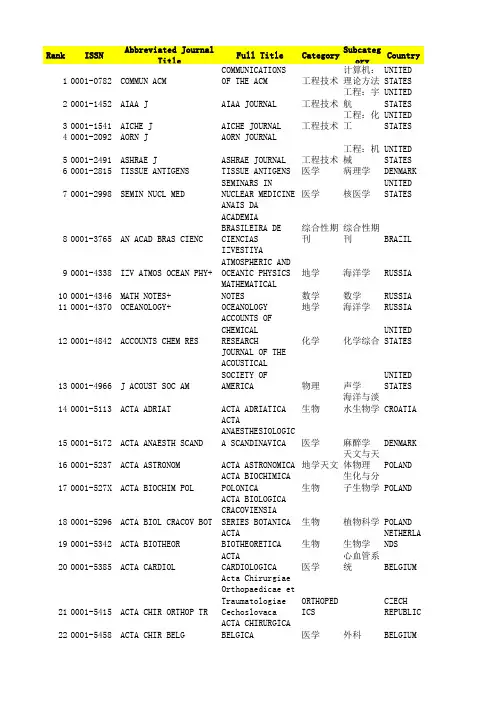
Rank ISSN Abbreviated Journal Title Full Title Category SubcategoryCountryUNITED STAT 10001-0782COMMUN ACM COMMUNICATIONS OF T工程技术计算机:理论20001-1452AIAA J AIAA JOURNAL工程技术工程:宇航UNITED STAT 30001-1541AICHE J AICHE JOURNAL工程技术工程:化工UNITED STAT 40001-2092AORN J AORN JOURNAL50001-2491ASHRAE J ASHRAE JOURNAL工程技术工程:机械UNITED STAT 60001-2815TISSUE ANTIGENS TISSUE ANTIGENS医学病理学DENMARK 70001-2998SEMIN NUCL MED SEMINARS IN NUCLEAR医学核医学UNITED STAT 80001-3765AN ACAD BRAS CIENC ANAIS DA ACADEMIA B综合性期刊综合性期刊BRAZIL90001-4338IZV ATMOS OCEAN PHY+IZVESTIYA ATMOSPHER地学海洋学RUSSIA 100001-4346MATH NOTES+MATHEMATICAL NOTES数学数学RUSSIA 110001-4370OCEANOLOGY+OCEANOLOGY地学海洋学RUSSIA 120001-4842ACCOUNTS CHEM RES ACCOUNTS OF CHEMICA化学化学综合UNITED STAT 130001-4966J ACOUST SOC AM JOURNAL OF THE ACOU物理声学UNITED STATCROATIA 140001-5113ACTA ADRIAT ACTA ADRIATICA生物海洋与淡水生150001-5172ACTA ANAESTH SCAND ACTA ANAESTHESIOLOG医学麻醉学DENMARKPOLAND 160001-5237ACTA ASTRONOM ACTA ASTRONOMICA地学天文天文与天体物POLAND 170001-527X ACTA BIOCHIM POL ACTA BIOCHIMICA POL生物生化与分子生180001-5296ACTA BIOL CRACOV BOT ACTA BIOLOGICA CRAC生物植物科学POLAND 190001-5342ACTA BIOTHEOR ACTA BIOTHEORETICA生物生物学NETHERLANDS 200001-5385ACTA CARDIOL ACTA CARDIOLOGICA医学心血管系统BELGIUM 210001-5415ACTA CHIR ORTHOP TR Acta Chirurgiae Ort ORTHOPEDICS CZECH REPUB 220001-5458ACTA CHIR BELG ACTA CHIRURGICA BEL医学外科BELGIUM 230001-5555ACTA DERM-VENEREOL ACTA DERMATO-VENERE医学皮肤病学NORWAY 240001-5644ACTA GASTRO-ENT BELG ACTA GASTRO-ENTEROL医学胃肠肝病学BELGIUM 250001-5652HUM HERED HUMAN HEREDITY生物遗传学SWITZERLAND 260001-5709ACTA GEOL POL ACTA GEOLOGICA POLO地学地质学POLANDPEOPLES R C 270001-5733CHINESE J GEOPHYS-CH CHINESE JOURNAL OF 地学地球化学与地280001-5792ACTA HAEMATOL-BASEL ACTA HAEMATOLOGICA医学血液学SWITZERLANDGERMANY 290001-5903ACTA INFORM ACTA INFORMATICA工程技术计算机:信息300001-5962ACTA MATH-DJURSHOLM ACTA MATHEMATICA数学数学SWEDEN 310001-5970ACTA MECH ACTA MECHANICA物理力学AUSTRIA 320001-6268ACTA NEUROCHIR ACTA NEUROCHIRURGIC医学临床神经学AUSTRIA 330001-6314ACTA NEUROL SCAND ACTA NEUROLOGICA SC医学临床神经学DENMARK 340001-6322ACTA NEUROPATHOL ACTA NEUROPATHOLOGI医学病理学GERMANY 350001-6349ACTA OBSTET GYN SCAN ACTA OBSTETRICIA ET医学妇产科学DENMARKNORWAY 360001-6357ACTA ODONTOL SCAND ACTA ODONTOLOGICA S医学牙科与口腔外370001-6454ACTA ORNITHOL ACTA ORNITHOLOGICA生物鸟类学POLAND 380001-6462ACTA ORTHOP BELG Acta Orthopaedica B医学整形外科BELGIUM 390001-6489ACTA OTO-LARYNGOL ACTA OTO-LARYNGOLOG医学耳鼻喉科学NORWAY 400001-6497B-ENT B-ENT医学耳鼻喉科学BELGIUM 410001-6837ACTA POL PHARM ACTA POLONIAE PHARM医学药学POLAND 420001-690X ACTA PSYCHIAT SCAND ACTA PSYCHIATRICA S医学精神病学ENGLAND 430001-6977ACTA SOC BOT POL ACTA SOCIETATIS BOT生物植物科学POLAND 440001-7051ACTA THERIOL ACTA THERIOLOGICA生物动物学POLAND 450001-706X ACTA TROP ACTA TROPICA医学寄生虫学NETHERLANDS 460001-7213ACTA VET BRNO ACTA VETERINARIA BR农林科学兽医学CZECH REPUB 470001-723X ACTA VIROL ACTA VIROLOGICA医学病毒学SLOVAKIA 480001-7272ACTA ZOOL-STOCKHOLM ACTA ZOOLOGICA生物动物学ENGLANDUNITED STAT 490001-7701GEN RELAT GRAVIT GENERAL RELATIVITY 物理天文与天体物GERMANY 500001-7868AKTUEL UROL AKTUELLE UROLOGIE医学泌尿学与肾脏510001-8244BEHAV GENET BEHAVIOR GENETICS生物行为科学UNITED STATENGLAND 520001-8678ADV APPL PROBAB ADVANCES IN APPLIED数学统计学与概率530001-8686ADV COLLOID INTERFAC ADVANCES IN COLLOID化学物理化学NETHERLANDS 540001-8708ADV MATH ADVANCES IN MATHEMA数学数学UNITED STATENGLAND 550001-8732ADV PHYS ADVANCES IN PHYSICS物理物理:凝聚态560001-9054AEQUATIONES MATH Aequationes Mathema MATHEMATICS MATHEMATICS SWITZERLAND 570001-9240AERONAUT J AERONAUTICAL JOURNA工程技术工程:宇航ENGLAND 580001-9704AFINIDAD AFINIDAD化学化学综合SPAIN 590002-0443J NURS ADMIN JOURNAL OF NURSING 医学护理UNITED STAT 600002-0729AGE AGEING AGE AND AGEING医学老年医学ENGLAND 610002-1121GER J AGR ECON German Journal of A AGRICULTURAL ECONOMI GERMANYUNITED STAT 620002-1482AGR HIST AGRICULTURAL HISTOR农林科学科学史与科学630002-1857AGROCHIMICA AGROCHIMICA农林科学土壤科学ITALY 640002-1962AGRON J AGRONOMY JOURNAL农林科学农艺学UNITED STAT 650002-5100ALDRICHIM ACTA ALDRICHIMICA ACTA化学有机化学UNITED STAT 660002-5232ALGEBR LOG+Algebra and Logic数学数学RUSSIA 670002-5240ALGEBR UNIV ALGEBRA UNIVERSALIS数学数学SWITZERLAND 680002-5852ALLG FORST JAGDZTG ALLGEMEINE FORST UN农林科学林学GERMANY 690002-6980AMBIX Ambix HISTORY & PHILOSOPHY ENGLAND 700002-7014AMEGHINIANA AMEGHINIANA地学古生物学ARGENTINA 710002-7685AM BIOL TEACH AMERICAN BIOLOGY TE BIOLOGY EDUCATION, UNITED STATUNITED STAT 720002-7812AM CERAM SOC BULL AMERICAN CERAMIC SO工程技术材料科学:硅UNITED STAT 730002-7820J AM CERAM SOC JOURNAL OF THE AMER工程技术材料科学:硅740002-7863J AM CHEM SOC JOURNAL OF THE AMER化学化学综合UNITED STATUNITED STAT 750002-8177J AM DENT ASSOC JOURNAL OF THE AMER医学牙科与口腔外760002-8320T AM ENTOMOL SOC TRANSACTIONS OF THE生物昆虫学UNITED STAT 770002-838X AM FAM PHYSICIAN AMERICAN FAMILY PHY医学医学:内科UNITED STAT 780002-8444AM FERN J AMERICAN FERN JOURN生物植物科学UNITED STAT 790002-8487T AM FISH SOC TRANSACTIONS OF THE农林科学渔业UNITED STAT 800002-8614J AM GERIATR SOC JOURNAL OF THE AMER医学老年医学UNITED STAT 810002-8703AM HEART J AMERICAN HEART JOUR医学心血管系统UNITED STAT 820002-8711J AM HELICOPTER SOC JOURNAL OF THE AMER工程技术工程:宇航UNITED STATUNITED STAT 830002-9092AM J AGR ECON AMERICAN JOURNAL OF管理科学农业经济与政840002-9122AM J BOT AMERICAN JOURNAL OF生物植物科学UNITED STAT 850002-9149AM J CARDIOL AMERICAN JOURNAL OF医学心血管系统UNITED STAT 860002-9165AM J CLIN NUTR AMERICAN JOURNAL OF医学营养学UNITED STAT 870002-9173AM J CLIN PATHOL AMERICAN JOURNAL OF医学病理学UNITED STATUNITED STAT 880002-9254AM J ENOL VITICULT AMERICAN JOURNAL OF农林科学生物工程与应UNITED STAT 890002-9262AM J EPIDEMIOL AMERICAN JOURNAL OF医学公共卫生、环900002-9270AM J GASTROENTEROL AMERICAN JOURNAL OF医学胃肠肝病学UNITED STAT 910002-9297AM J HUM GENET AMERICAN JOURNAL OF生物遗传学UNITED STAT 920002-9327AM J MATH AMERICAN JOURNAL OF数学数学UNITED STAT 930002-9343AM J MED AMERICAN JOURNAL OF医学医学:内科UNITED STAT 940002-936X AM J NURS AMERICAN JOURNAL OF医学护理UNITED STAT 950002-9378AM J OBSTET GYNECOL AMERICAN JOURNAL OF医学妇产科学UNITED STAT 960002-9394AM J OPHTHALMOL AMERICAN JOURNAL OF医学眼科学UNITED STAT 970002-9440AM J PATHOL AMERICAN JOURNAL OF医学病理学UNITED STAT 980002-9459AM J PHARM EDUC AMERICAN JOURNAL OF医学学科教育UNITED STAT 990002-9483AM J PHYS ANTHROPOL AMERICAN JOURNAL OF生物进化生物学UNITED STAT 1000002-9505AM J PHYS AMERICAN JOURNAL OF物理物理:综合UNITED STAT 1010002-953X AM J PSYCHIAT AMERICAN JOURNAL OF医学精神病学UNITED STATUNITED STAT 1020002-9599AM J SCI AMERICAN JOURNAL OF地学地球科学综合1030002-9610AM J SURG AMERICAN JOURNAL OF医学外科UNITED STAT 1040002-9629AM J MED SCI AMERICAN JOURNAL OF医学医学:内科UNITED STATUNITED STAT 1050002-9637AM J TROP MED HYG AMERICAN JOURNAL OF医学公共卫生、环1060002-9645AM J VET RES AMERICAN JOURNAL OF农林科学兽医学UNITED STAT1070002-9726J AM LEATHER CHEM AS JOURNAL OF THE AMER工程技术材料科学:纺UNITED STAT 1080002-9890AM MATH MON AMERICAN MATHEMATIC MATHEMATICS UNITED STAT 1090002-9939P AM MATH SOC PROCEEDINGS OF THE 数学数学UNITED STAT 1100002-9947T AM MATH SOC TRANSACTIONS OF THE数学数学UNITED STATUNITED STAT 1113月7日B AM METEOROL SOC BULLETIN OF THE AME地学气象与大气科1123月31日AM MIDL NAT AMERICAN MIDLAND NA环境科学生态学UNITED STAT 1130003-004X AM MINERAL AMERICAN MINERALOGI地学地球化学与地UNITED STAT 1140003-0082AM MUS NOVIT AMERICAN MUSEUM NOV环境科学动物学UNITED STAT 1150003-0090 B AM MUS NAT HIST BULLETIN OF THE AME环境科学生态学UNITED STAT 1160003-0147AM NAT AMERICAN NATURALIST环境科学进化生物学UNITED STAT 1170003-021X J AM OIL CHEM SOC JOURNAL OF THE AMER工程技术食品科技UNITED STAT 1180003-0996AM SCI AMERICAN SCIENTIST综合性期刊综合性期刊UNITED STAT 1190003-1062J AM SOC HORTIC SCI JOURNAL OF THE AMER农林科学园艺UNITED STAT 1200003-1305AM STAT AMERICAN STATISTICI数学统计学与概率UNITED STAT 1210003-1348AM SURGEON AMERICAN SURGEON医学外科UNITED STAT 1220003-1488JAVMA-J AM VET MED A JAVMA-JOURNAL OF TH农林科学兽医学UNITED STAT 1230003-2409ANAESTHESIA ANAESTHESIA医学麻醉学ENGLAND 1240003-2417ANAESTHESIST ANAESTHESIST医学麻醉学GERMANY 1250003-2654ANALYST ANALYST化学分析化学ENGLAND 1260003-2670ANAL CHIM ACTA ANALYTICA CHIMICA A化学分析化学NETHERLANDS 1270003-2697ANAL BIOCHEM ANALYTICAL BIOCHEMI生物分析化学UNITED STAT 1280003-2700ANAL CHEM ANALYTICAL CHEMISTR化学分析化学UNITED STAT 1290003-2719ANAL LETT ANALYTICAL LETTERS化学分析化学UNITED STAT 1300003-2778J ANAT SOC INDIA Journal of the Anat ANATOMY & MORPHOLOGY INDIA 1310003-2999ANESTH ANALG ANESTHESIA AND ANAL医学麻醉学UNITED STAT 1320003-3022ANESTHESIOLOGY ANESTHESIOLOGY医学麻醉学UNITED STAT 1330003-3197ANGIOLOGY ANGIOLOGY医学外周血管病UNITED STATUNITED STAT 1340003-3219ANGLE ORTHOD ANGLE ORTHODONTIST医学牙科与口腔外1350003-3472ANIM BEHAV ANIMAL BEHAVIOUR生物动物学ENGLANDENGLAND 1360003-3790ANN SCI ANNALS OF SCIENCE综合性期刊科学史与科学1370003-3804ANN PHYS-BERLIN ANNALEN DER PHYSIK物理物理:综合GERMANY 1380003-3847ANN BOT FENN ANNALES BOTANICI FE生物植物科学FINLANDFRANCE 1390003-3898ANN BIOL CLIN-PARIS ANNALES DE BIOLOGIE医学医学:研究与1400003-4088ANN LIMNOL-INT J LIM ANNALES DE LIMNOLOG地学湖沼学FRANCEFRANCE 1410003-4266ANN ENDOCRINOL-PARIS ANNALES D ENDOCRINO医学内分泌学与代1420003-4347ANN TELECOMMUN Annals of Telecommu工程技术电信学FRANCE 1430003-4487ANN MED-PSYCHOL ANNALES MEDICO-PSYC医学精神病学FRANCE 1440003-4541ANN ZOOL ANNALES ZOOLOGICI生物动物学POLAND 1450003-455X ANN ZOOL FENN ANNALES ZOOLOGICI F生物动物学FINLAND 1460003-4746ANN APPL BIOL ANNALS OF APPLIED B生物农业综合UNITED STAT 1470003-4800ANN HUM GENET ANNALS OF HUMAN GEN生物遗传学ENGLAND 1480003-4819ANN INTERN MED ANNALS OF INTERNAL 医学医学:内科UNITED STAT 1490003-486X ANN MATH ANNALS OF MATHEMATI数学数学UNITED STAT 1500003-4878ANN OCCUP HYG ANNALS OF OCCUPATIO医学毒理学ENGLAND 1510003-4894ANN OTO RHINOL LARYN ANNALS OF OTOLOGY R医学耳鼻喉科学UNITED STAT 1520003-4916ANN PHYS-NEW YORK ANNALS OF PHYSICS物理物理:综合UNITED STAT 1530003-4932ANN SURG ANNALS OF SURGERY医学外科UNITED STAT 1540003-4967ANN RHEUM DIS ANNALS OF THE RHEUM医学风湿病学ENGLAND 1550003-4975ANN THORAC SURG ANNALS OF THORACIC 医学呼吸系统UNITED STAT 1560003-5599ANTI-CORROS METHOD M ANTI-CORROSION METH工程技术冶金工程ENGLAND 1570003-6072ANTON LEEUW INT J G ANTONIE VAN LEEUWEN生物微生物学NETHERLANDS 1580003-6811APPL ANAL APPLICABLE ANALYSIS数学应用数学ENGLAND 1590003-682X APPL ACOUST APPLIED ACOUSTICS物理声学ENGLANDRUSSIA 1600003-6838APPL BIOCHEM MICRO+APPLIED BIOCHEMISTR生物生物工程与应1610003-6862APPL ENTOMOL ZOOL APPLIED ENTOMOLOGY 生物昆虫学JAPAN 1620003-6870APPL ERGON APPLIED ERGONOMICS工程技术工程:工业ENGLAND 1630003-6900APPL MECH REV Applied Mechanics R物理力学UNITED STAT 1640003-6951APPL PHYS LETT APPLIED PHYSICS LET物理物理:应用UNITED STAT 1650003-7028APPL SPECTROSC APPLIED SPECTROSCOP工程技术光谱学UNITED STAT 1660003-7214J WATER SUPPLY RES T JOURNAL OF WATER SU环境科学工程:土木ENGLANDENGLAND 1670003-813X ARCHAEOMETRY ARCHAEOMETRY地学地球科学综合1680003-889X ARCH MATH ARCHIV DER MATHEMAT数学数学SWITZERLAND 1690003-925X J FOOD SAF FOOD QUAL Journal of Food Safety and Food Quality-ArchivGERMANY 1700003-9438ARCH TIERZUCHT ARCHIV FUR TIERZUCH农林科学奶制品与动物1710003-9438ARCH ANIM BREED Archives Animal BreedingGERMANY 1720003-9519ARCH HIST EXACT SCI ARCHIVE FOR HISTORY管理科学科学史与科学1730003-9527ARCH RATION MECH AN ARCHIVE FOR RATIONA物理力学GERMANY 1740003-9829ARCH ITAL BIOL ARCHIVES ITALIENNES医学神经科学ITALY 1750003-9861ARCH BIOCHEM BIOPHYS ARCHIVES OF BIOCHEM生物生化与分子生UNITED STAT 1760003-9888ARCH DIS CHILD ARCHIVES OF DISEASE医学小儿科ENGLANDENGLAND 1770003-9969ARCH ORAL BIOL ARCHIVES OF ORAL BI医学牙科与口腔外1780003-9985ARCH PATHOL LAB MED ARCHIVES OF PATHOLO医学病理学UNITED STAT 1790003-9993ARCH PHYS MED REHAB ARCHIVES OF PHYSICA医学康复医学UNITED STAT 1800004-0614ARCH ESP UROL ARCHIVOS ESPANOLES UROLOGY & NEPHROLOGY SPAIN 1810004-0622ARCH LATINOAM NUTR ARCHIVOS LATINOAMER医学营养学VENEZUELA 1820004-069X ARCH IMMUNOL THER EX ARCHIVUM IMMUNOLOGI医学免疫学POLAND 1830004-0843ARCTIC ARCTIC地学环境科学CANADA 1840004-1254ARH HIG RADA TOKSIKO Arhiv za Higijenu R PUBLIC, ENV TOXICOLOGY C ROATIA 1850004-2080ARK MAT ARKIV FOR MATEMATIK数学数学SWEDEN 1860004-2730ARQ BRAS ENDOCRINOL Arquivos Brasileiro医学内分泌学与代BRAZIL 1870004-2749ARQ BRAS OFTALMOL ARQUIVOS BRASILEIRO OPHTHALMOLOGY BRAZIL 1880004-282X ARQ NEURO-PSIQUIAT ARQUIVOS DE NEURO-P医学精神病学BRAZILNETHERLANDS 1890004-3702ARTIF INTELL ARTIFICIAL INTELLIG工程技术计算机:人工UNITED STAT 1900004-5411J ACM JOURNAL OF THE ACM工程技术计算机:理论1910004-5632ANN CLIN BIOCHEM ANNALS OF CLINICAL BIOCHEMISTRYUNITED STAT 1920004-6256ASTRON J ASTRONOMICAL JOURNA地学天文天文与天体物1930004-6264PUBL ASTRON SOC JPN PUBLICATIONS OF THE地学天文天文与天体物JAPANUNITED STAT 1940004-6280PUBL ASTRON SOC PAC PUBLICATIONS OF THE地学天文天文与天体物GERMANY 1950004-6337ASTRON NACHR ASTRONOMISCHE NACHR地学天文天文与天体物UNITED STAT 1960004-637X ASTROPHYS J ASTROPHYSICAL JOURN地学天文天文与天体物NETHERLANDS 1970004-640X ASTROPHYS SPACE SCI ASTROPHYSICS AND SP地学天文天文与天体物1980004-8038AUK AUK生物鸟类学UNITED STAT 1990004-8380AUSTRALAS J DERMATOL AUSTRALASIAN JOURNA医学皮肤病学AUSTRALIA 2000004-8666AUST NZ J OBSTET GYN AUSTRALIAN & NEW ZE医学妇产科学AUSTRALIA 2010004-8674AUST NZ J PSYCHIAT AUSTRALIAN AND NEW 医学精神病学ENGLAND 2020004-9158AUST FORESTRY AUSTRALIAN FORESTRY FORESTRY AUSTRALIA 2030004-9425AUST J CHEM AUSTRALIAN JOURNAL 化学化学综合AUSTRALIA 2040004-959X AUST J ZOOL AUSTRALIAN JOURNAL 生物动物学AUSTRALIA 2050004-9727 B AUST MATH SOC BULLETIN OF THE AUS数学数学AUSTRALIA 2060005-0423AUST VET J AUSTRALIAN VETERINA农林科学兽医学ENGLANDUNITED STAT 2070005-1098AUTOMATICA AUTOMATICA工程技术工程:电子与2080005-1144AUTOMATIKA Automatika AUTOMATION ENGINEERING CROATIA 2090005-1179AUTOMAT REM CONTR+AUTOMATION AND REMO工程技术仪器仪表RUSSIA 2100005-2086AVIAN DIS AVIAN DISEASES农林科学兽医学UNITED STATNETHERLANDS 2110005-2728BBA-BIOENERGETICS BIOCHIMICA ET BIOPH生物生化与分子生2120005-2736BBA-BIOMEMBRANES BIOCHIMICA ET BIOPH生物生化与分子生NETHERLANDS 2130005-6650BAUINGENIEUR-GERMANY Bauingenieur工程技术工程:土木GERMANY 2140005-7959BEHAVIOUR BEHAVIOUR生物动物学NETHERLANDS2150005-9366BERL MUNCH TIERARZTL BERLINER UND MUNCHE农林科学兽医学GERMANYGERMANY 2160005-9900BETON- STAHLBETONBAU Beton- und Stahlbet工程技术材料科学:表UNITED STAT 2170006-291X BIOCHEM BIOPH RES CO BIOCHEMICAL AND BIO生物生化与分子生UNITED STAT 2180006-2928BIOCHEM GENET BIOCHEMICAL GENETIC生物生化与分子生2190006-2952BIOCHEM PHARMACOL BIOCHEMICAL PHARMAC医学药学UNITED STATUNITED STAT 2200006-2960BIOCHEMISTRY-US BIOCHEMISTRY生物生化与分子生2210006-2979BIOCHEMISTRY-MOSCOW+BIOCHEMISTRY-MOSCOW生物生化与分子生RUSSIA 2220006-3088BIOLOGIA BIOLOGIA生物生物学SLOVAKIA 2230006-3134BIOL PLANTARUM BIOLOGIA PLANTARUM生物植物科学NETHERLANDS 2240006-3185BIOL BULL-US BIOLOGICAL BULLETIN生物海洋与淡水生UNITED STAT 2250006-3207BIOL CONSERV BIOLOGICAL CONSERVA环境科学环境科学ENGLAND 2260006-3223BIOL PSYCHIAT BIOLOGICAL PSYCHIAT医学精神病学UNITED STAT 2270006-324X P BIOL SOC WASH PROCEEDINGS OF THE 生物生物学UNITED STAT 2280006-3363BIOL REPROD BIOLOGY OF REPRODUC生物生殖生物学UNITED STAT 2290006-341X BIOMETRICS BIOMETRICS生物生物学UNITED STAT 2300006-3444BIOMETRIKA BIOMETRIKA生物生物学ENGLAND 2310006-3495BIOPHYS J BIOPHYSICAL JOURNAL生物生物物理UNITED STATUNITED STAT 2320006-3525BIOPOLYMERS BIOPOLYMERS生物生化与分子生NETHERLANDS 2330006-355X BIORHEOLOGY BIORHEOLOGY医学工程:生物医2340006-3568BIOSCIENCE BIOSCIENCE生物生物学UNITED STATUNITED STAT 2350006-3592BIOTECHNOL BIOENG BIOTECHNOLOGY AND B工程技术生物工程与应2360006-3606BIOTROPICA BIOTROPICA环境科学生态学UNITED STAT 2370006-3657BIRD STUDY BIRD STUDY生物鸟类学ENGLANDNETHERLANDS 2380006-3835BIT BIT NUMERICAL MATHE数学计算机:软件2390006-4971BLOOD BLOOD医学血液学UNITED STAT 2400006-5196BLUMEA BLUMEA生物植物科学NETHERLANDS 2410006-579X BOIS FOR TROP BOIS ET FORETS DES FORESTRY FRANCEITALY 2420006-6729 B GEOFIS TEOR APPL Bollettino di Geofi地学地球化学与地GERMANY 2430006-8055BOT MAR BOTANICA MARINA生物海洋与淡水生2440006-8101BOT REV BOTANICAL REVIEW生物植物科学UNITED STAT 2450006-8241BOTHALIA BOTHALIA生物植物科学SOUTH AFRICNETHERLANDS 2460006-8314BOUND-LAY METEOROL BOUNDARY-LAYER METE地学气象与大气科2470006-8950BRAIN BRAIN医学临床神经学ENGLAND 2480006-8977BRAIN BEHAV EVOLUT BRAIN BEHAVIOR AND 医学行为科学SWITZERLAND 2490006-8993BRAIN RES BRAIN RESEARCH医学神经科学NETHERLANDS 2500006-9248BRATISL MED J Bratislava Medical 医学医学:内科SLOVAKIA 2510007-0610BRIT DENT J BRITISH DENTAL JOUR医学牙科与口腔外ENGLAND 2520007-070X BRIT FOOD J British Food Journa工程技术食品科技ENGLANDENGLAND 2530007-0882BRIT J PHILOS SCI BRITISH JOURNAL FOR管理科学科学史与科学2540007-0912BRIT J ANAESTH BRITISH JOURNAL OF 医学麻醉学ENGLAND 2550007-0920BRIT J CANCER BRITISH JOURNAL OF 医学肿瘤学ENGLAND 2560007-0963BRIT J DERMATOL BRITISH JOURNAL OF 医学皮肤病学ENGLAND 2570007-1048BRIT J HAEMATOL BRITISH JOURNAL OF 医学血液学ENGLANDENGLAND 2580007-1102BRIT J MATH STAT PSY BRITISH JOURNAL OF 医学数学跨学科应2590007-1145BRIT J NUTR BRITISH JOURNAL OF 医学营养学ENGLAND 2600007-1161BRIT J OPHTHALMOL BRITISH JOURNAL OF 医学眼科学ENGLAND 2610007-1188BRIT J PHARMACOL BRITISH JOURNAL OF 医学药学ENGLAND 2620007-1250BRIT J PSYCHIAT BRITISH JOURNAL OF 医学精神病学ENGLAND 2630007-1285BRIT J RADIOL BRITISH JOURNAL OF 医学核医学ENGLAND 2640007-1323BRIT J SURG BRITISH JOURNAL OF 医学外科ENGLAND 2650007-1420BRIT MED BULL BRITISH MEDICAL BUL医学医学:内科ENGLAND 2660007-1668BRIT POULTRY SCI BRITISH POULTRY SCI农林科学奶制品与动物ENGLAND 2670007-196X BRITTONIA BRITTONIA生物植物科学UNITED STAT 2680007-215X BRODOGRADNJA Brodogradnja ENGINEERING, MARINE C ROATIA2690007-2745BRYOLOGIST BRYOLOGIST生物植物科学UNITED STAT 2700007-4497 B SCI MATH BULLETIN DES SCIENC数学应用数学FRANCE 2710007-4551 B CANCER BULLETIN DU CANCER医学肿瘤学FRANCE 2720007-4853 B ENTOMOL RES BULLETIN OF ENTOMOL生物昆虫学ENGLAND 2730007-4861 B ENVIRON CONTAM TOX BULLETIN OF ENVIRON环境科学毒理学UNITED STATRUSSIA 2740007-4888 B EXP BIOL MED+BULLETIN OF EXPERIM医学医学:研究与2750007-4977 B MAR SCI BULLETIN OF MARINE 地学海洋学UNITED STAT 2760007-5027LAB MED LABORATORY MEDICINEUNITED STAT 2770007-5140 B HIST MED BULLETIN OF THE HIS医学科学史与科学2780007-5922MITT KLOSTERNEUBURG MITTEILUNGEN KLOSTE FOOD SCIENC HORTICULTUR Austria 2790007-8506CIRP ANN-MANUF TECHN CIRP ANNALS-MANUFAC工程技术工程:工业SWITZERLAND 2800007-9235CA-CANCER J CLIN CA-A CANCER JOURNAL医学肿瘤学UNITED STATFRANCE 2810007-9723CAH BIOL MAR CAHIERS DE BIOLOGIE生物海洋与淡水生2820008-0624CALCOLO CALCOLO数学数学ITALY 2830008-0845CALIF AGR CALIFORNIA AGRICULT农林科学农业综合UNITED STAT 2840008-1078CALIF FISH GAME CALIFORNIA FISH AND农林科学动物学UNITED STAT 2850008-347X CAN ENTOMOL CANADIAN ENTOMOLOGI生物昆虫学CANADA 2860008-350X CAN FAM PHYSICIAN CANADIAN FAMILY PHY医学医学:内科CANADACANADA 2870008-3674CAN GEOTECH J CANADIAN GEOTECHNIC地学地球科学综合CANADA 2880008-3976CAN J AGR ECON CANADIAN JOURNAL OF管理科学农业经济与政2890008-3984CAN J ANIM SCI CANADIAN JOURNAL OF农林科学奶制品与动物CANADA 2900008-4034CAN J CHEM ENG CANADIAN JOURNAL OF工程技术工程:化工CANADA 2910008-4042CAN J CHEM CANADIAN JOURNAL OF化学化学综合CANADA 2920008-4077CAN J EARTH SCI CANADIAN JOURNAL OF地学地球科学综合CANADA 2930008-414X CAN J MATH CANADIAN JOURNAL OF数学数学CANADA 2940008-4166CAN J MICROBIOL CANADIAN JOURNAL OF生物免疫学CANADA 2950008-4174CAN J OCCUP THER Canadian Journal of REHABILITATION CANADA 2960008-4182CAN J OPHTHALMOL CANADIAN JOURNAL OF医学眼科学CANADA 2970008-4204CAN J PHYS CANADIAN JOURNAL OF物理物理:综合CANADA 2980008-4212CAN J PHYSIOL PHARM CANADIAN JOURNAL OF医学生理学CANADA 2990008-4220CAN J PLANT SCI CANADIAN JOURNAL OF生物农艺学CANADA 3000008-4271CAN J SOIL SCI CANADIAN JOURNAL OF农林科学土壤科学CANADA 3010008-428X CAN J SURG CANADIAN JOURNAL OF医学外科CANADA 3020008-4301CAN J ZOOL CANADIAN JOURNAL OF生物动物学CANADA 3030008-4395CAN MATH BULL CANADIAN MATHEMATIC数学数学CANADA 3040008-4433CAN METALL QUART CANADIAN METALLURGI工程技术冶金工程CANADA 3050008-4476CAN MINERAL CANADIAN MINERALOGI地学矿物学CANADA 3060008-5286CAN VET J CANADIAN VETERINARY农林科学兽医学CANADA 3070008-543X CANCER-AM CANCER SOC CANCER医学肿瘤学UNITED STAT 3080008-5472CANCER RES CANCER RESEARCH医学肿瘤学UNITED STATNETHERLANDS 3090008-6215CARBOHYD RES CARBOHYDRATE RESEAR化学生化与分子生UNITED STAT 3100008-6223CARBON CARBON工程技术材料科学:综3110008-6312CARDIOLOGY CARDIOLOGY医学心血管系统SWITZERLAND 3120008-6363CARDIOVASC RES CARDIOVASCULAR RESE医学心血管系统ENGLANDUNITED STAT 3130008-6452CARIBB J SCI CARIBBEAN JOURNAL O综合性期刊生物多样性保SWITZERLAND 3140008-6568CARIES RES CARIES RESEARCH医学牙科与口腔外3150008-7114CARYOLOGIA CARYOLOGIA生物遗传学ITALY 3160008-7475CASTANEA CASTANEA生物植物科学UNITED STAT 3170008-8749CELL IMMUNOL CELLULAR IMMUNOLOGY生物免疫学UNITED STATENGLAND 3180008-8846CEMENT CONCRETE RES CEMENT AND CONCRETE工程技术材料科学:综3190008-8994CENTAURUS CENTAURUS HISTORY & PHILOSOPHY ENGLAND 3200009-0352CEREAL CHEM CEREAL CHEMISTRY工程技术食品科技UNITED STAT 3210009-2347CHEM ENG NEWS CHEMICAL & ENGINEER工程技术工程:化工UNITED STAT 3220009-2363CHEM PHARM BULL CHEMICAL & PHARMACE医学化学综合JAPAN3230009-2460CHEM ENG-NEW YORK CHEMICAL ENGINEERIN工程技术工程:化工UNITED STAT 3240009-2509CHEM ENG SCI CHEMICAL ENGINEERIN工程技术工程:化工UNITED STATNETHERLANDS 3250009-2541CHEM GEOL CHEMICAL GEOLOGY地学地球化学与地NETHERLANDS 3260009-2614CHEM PHYS LETT CHEMICAL PHYSICS LE化学物理:原子、3270009-2665CHEM REV CHEMICAL REVIEWS化学化学综合UNITED STAT 3280009-2673 B CHEM SOC JPN BULLETIN OF THE CHE化学化学综合JAPAN 3290009-2770CHEM LISTY CHEMICKE LISTY化学化学综合CZECH REPUB 3300009-2797CHEM-BIOL INTERACT CHEMICO-BIOLOGICAL 生物毒理学NETHERLANDSGERMANY 3310009-2819CHEM ERDE-GEOCHEM CHEMIE DER ERDE-GEO地学地球化学与地3320009-2851CHEM UNSERER ZEIT CHEMIE IN UNSERER Z化学化学综合GERMANY 3330009-286X CHEM-ING-TECH CHEMIE INGENIEUR TE工程技术工程:化工GERMANY 3340009-3068CHEM IND-LONDON CHEMISTRY & INDUSTR化学应用化学ENGLANDNETHERLANDS 3350009-3084CHEM PHYS LIPIDS CHEMISTRY AND PHYSI生物生化与分子生3360009-3092CHEM TECH FUELS OIL+CHEMISTRY AND TECHN工程技术工程:化工RUSSIA 3370009-3122CHEM HETEROCYCL COM+Chemistry of Hetero化学有机化学LATVIA 3380009-3130CHEM NAT COMPD+CHEMISTRY OF NATURA化学有机化学UZBEKISTAN 3390009-3157CHEMOTHERAPY CHEMOTHERAPY医学药学SWITZERLAND 3400009-4293CHIMIA CHIMIA化学化学综合SWITZERLAND 3410009-4536J CHIN CHEM SOC-TAIP JOURNAL OF THE CHIN化学化学综合TAIWAN 3420009-4722CHIRURG CHIRURG医学外科GERMANY 3430009-5893CHROMATOGRAPHIA CHROMATOGRAPHIA化学分析化学GERMANY 3440009-5915CHROMOSOMA CHROMOSOMA生物生化与分子生GERMANY 3450009-7322CIRCULATION CIRCULATION医学外周血管病UNITED STAT 3460009-7330CIRC RES CIRCULATION RESEARC医学外周血管病UNITED STAT 3470009-739X CIR ESPAN CIRUGIA ESPANOLA SURGERY SPAIN 3480009-7411CIR CIR Cirugia y Cirujanos医学外科MEXICO 3490009-8558CLAY MINER CLAY MINERALS地学矿物学ENGLANDUNITED STAT 3500009-8604CLAY CLAY MINER CLAYS AND CLAY MINE地学地球科学综合NETHERLANDS 3510009-8981CLIN CHIM ACTA CLINICA CHIMICA ACT医学医学实验技术3520009-9104CLIN EXP IMMUNOL CLINICAL AND EXPERI医学免疫学ENGLAND 3530009-9120CLIN BIOCHEM CLINICAL BIOCHEMIST医学医学实验技术CANADAUNITED STAT 3540009-9147CLIN CHEM CLINICAL CHEMISTRY医学医学实验技术3550009-9163CLIN GENET CLINICAL GENETICS医学遗传学DENMARK 3560009-9201CLIN OBSTET GYNECOL CLINICAL OBSTETRICS医学妇产科学UNITED STAT 3570009-921X CLIN ORTHOP RELAT R CLINICAL ORTHOPAEDI医学外科UNITED STAT 3580009-9228CLIN PEDIATR CLINICAL PEDIATRICS医学小儿科UNITED STAT 3590009-9236CLIN PHARMACOL THER CLINICAL PHARMACOLO医学药学UNITED STAT 3600009-9260CLIN RADIOL CLINICAL RADIOLOGY医学核医学ENGLAND 3610010-0285COGNITIVE PSYCHOL COGNITIVE PSYCHOLOG医学心理学UNITED STAT 3620010-065X COLEOPTS BULL COLEOPTERISTS BULLE生物昆虫学UNITED STAT 3630010-0757COLLECT MATH Collectanea Mathema数学数学SPAIN 3640010-1354COLLOQ MATH-WARSAW COLLOQUIUM MATHEMAT MATHEMATICS POLAND 3650010-2180COMBUST FLAME COMBUSTION AND FLAM工程技术工程:化工UNITED STAT 3660010-2202COMBUST SCI TECHNOL COMBUSTION SCIENCE 工程技术工程:化工UNITED STAT 3670010-2571COMMENT MATH HELV COMMENTARII MATHEMA数学数学SWITZERLANDGERMANY 3680010-3616COMMUN MATH PHYS COMMUNICATIONS IN M物理物理:数学物3690010-3624COMMUN SOIL SCI PLAN COMMUNICATIONS IN S农林科学分析化学UNITED STAT 3700010-3640COMMUN PUR APPL MATH COMMUNICATIONS ON P数学数学UNITED STAT 3710010-437X COMPOS MATH COMPOSITIO MATHEMAT数学数学NETHERLANDS 3720010-440X COMPR PSYCHIAT COMPREHENSIVE PSYCH医学精神病学UNITED STATENGLAND 3730010-4485COMPUT AIDED DESIGN COMPUTER-AIDED DESI工程技术计算机:软件ENGLAND 3740010-4620COMPUT J COMPUTER JOURNAL工程技术计算机:软件NETHERLANDS 3750010-4655COMPUT PHYS COMMUN COMPUTER PHYSICS CO物理计算机:跨学UNITED STAT 3760010-4825COMPUT BIOL MED COMPUTERS IN BIOLOG工程技术工程:生物医AUSTRIA 3770010-485X COMPUTING COMPUTING工程技术计算机:理论RUSSIA 3780010-5082COMBUST EXPLO SHOCK+COMBUSTION EXPLOSIO工程技术材料科学:综3790010-5422CONDOR CONDOR生物鸟类学UNITED STAT 3800010-7514CONTEMP PHYS CONTEMPORARY PHYSIC物理物理:综合ENGLAND 3810010-7824CONTRACEPTION CONTRACEPTION医学妇产科学UNITED STATGERMANY 3820010-7999CONTRIB MINERAL PETR CONTRIBUTIONS TO MI地学地球化学与地NETHERLANDS 3830010-8545COORDIN CHEM REV COORDINATION CHEMIS化学无机化学与核3840010-9312CORROSION-US CORROSION MATERIALS S METALLURGY UNITED STAT 3850010-938X CORROS SCI CORROSION SCIENCE工程技术材料科学:综ENGLAND 3860010-9452CORTEX CORTEX医学行为科学ITALY 3870010-9525COSMIC RES+COSMIC RESEARCH地学天文工程:宇航RUSSIA 3880011-1643CROAT CHEM ACTA CROATICA CHEMICA AC化学化学综合CROATIA 3890011-183X CROP SCI CROP SCIENCE农林科学农艺学UNITED STATNETHERLANDS 3900011-216X CRUSTACEANA CRUSTACEANA生物海洋与淡水生3910011-2240CRYOBIOLOGY CRYOBIOLOGY生物生理学UNITED STAT 3920011-2275CRYOGENICS CRYOGENICS物理热力学ENGLAND 3930011-3840CURR PROB SURG CURRENT PROBLEMS IN医学外科UNITED STAT 3940011-3891CURR SCI INDIA CURRENT SCIENCE综合性期刊综合性期刊INDIA 3950011-4162CUTIS CUTIS医学皮肤病学UNITED STAT 3960011-4545CYTOLOGIA CYTOLOGIA CELL BIOLOG GENETICS & Japan 3970011-4642CZECH MATH J CZECHOSLOVAK MATHEM数学数学CZECH REPUB 3980011-5029DM-DIS MON DM DISEASE-A-MONTH医学医学:内科UNITED STAT 3990011-748X DEFENCE SCI J DEFENCE SCIENCE JOU综合性期刊综合性期刊INDIA 4000011-9059INT J DERMATOL INTERNATIONAL JOURN医学皮肤病学UNITED STAT 4010011-9164DESALINATION DESALINATION工程技术工程:化工NETHERLANDS 4020012-0413DEUT LEBENSM-RUNDSCH DEUTSCHE LEBENSMITT工程技术食品科技GERMANY 4030012-0472DEUT MED WOCHENSCHR DEUTSCHE MEDIZINISC医学医学:内科GERMANY 4040012-1592DEV GROWTH DIFFER DEVELOPMENT GROWTH 生物发育生物学JAPAN 4050012-1606DEV BIOL DEVELOPMENTAL BIOLO生物发育生物学UNITED STAT 4060012-1622DEV MED CHILD NEUROL DEVELOPMENTAL MEDIC医学临床神经学ENGLAND 4070012-1630DEV PSYCHOBIOL DEVELOPMENTAL PSYCH生物发育生物学UNITED STATUNITED STAT 4080012-1797DIABETES DIABETES医学内分泌学与代GERMANY 4090012-186X DIABETOLOGIA DIABETOLOGIA医学内分泌学与代4100012-2661DIFF EQUAT+DIFFERENTIAL EQUATI数学数学RUSSIA 4110012-2823DIGESTION DIGESTION医学胃肠肝病学SWITZERLAND 4120012-365X DISCRETE MATH DISCRETE MATHEMATIC数学数学NETHERLANDS 4130012-3692CHEST CHEST医学呼吸系统UNITED STAT 4140012-3706DIS COLON RECTUM DISEASES OF THE COL医学外科UNITED STAT 4150012-3862DISS MATH Dissertationes Math数学数学POLAND 4160012-4486DOC OPHTHALMOL DOCUMENTA OPHTHALMO医学眼科学NETHERLANDS 4170012-5008DOKL CHEM DOKLADY CHEMISTRY化学化学综合UNITED STAT 4180012-5016DOKL PHYS CHEM DOKLADY PHYSICAL CH化学物理化学RUSSIA 4190012-6667DRUGS DRUGS医学毒理学NEW ZEALAND 4200012-6772DRVNA IND Drvna Industrija MATERIALS SCIENCE, P CROATIA 4210012-7094DUKE MATH J DUKE MATHEMATICAL J数学数学UNITED STAT 4220012-7361DYNA-BILBAO Dyna工程技术工程:综合SPAINNETHERLANDS 4230012-821X EARTH PLANET SC LETT EARTH AND PLANETARY地学地球化学与地NETHERLANDS 4240012-8252EARTH-SCI REV EARTH-SCIENCE REVIE地学地球科学综合4250012-9593ANN SCI ECOLE NORM S ANNALES SCIENTIFIQU数学数学FRANCE 4260012-9615ECOL MONOGR ECOLOGICAL MONOGRAP环境科学生态学UNITED STAT 4270012-9658ECOLOGY ECOLOGY环境科学生态学UNITED STATENGLAND 4280012-9682ECONOMETRICA ECONOMETRICA社会科学数学跨学科应4290013-0001ECON BOT ECONOMIC BOTANY生物植物科学UNITED STAT 4300013-0915P EDINBURGH MATH SOC PROCEEDINGS OF THE 数学数学ENGLAND4310013-1644EDUC PSYCHOL MEAS EDUCATIONAL AND PSY医学数学跨学科应UNITED STATUNITED STAT 4320013-4651J ELECTROCHEM SOC JOURNAL OF THE ELEC工程技术材料科学:膜4330013-4686ELECTROCHIM ACTA ELECTROCHIMICA ACTA工程技术电化学ENGLANDENGLAND 4340013-5194ELECTRON LETT ELECTRONICS LETTERS工程技术工程:电子与4350013-5585BIOMED ENG-BIOMED TE Biomedical Engineer ENGINEERING MEDICAL INF GERMANY 4360013-7006ENCEPHALE ENCEPHALE-REVUE DE 医学精神病学FRANCEUNITED STAT 4370013-7227ENDOCRINOLOGY ENDOCRINOLOGY医学内分泌学与代4380013-726X ENDOSCOPY ENDOSCOPY医学外科GERMANY 4390013-791X ENG ECON ENGINEERING ECONOMI ENGINEERING OPERATIONS UNITED STAT 4400013-7944ENG FRACT MECH ENGINEERING FRACTUR物理力学ENGLANDNETHERLANDS 4410013-7952ENG GEOL ENGINEERING GEOLOGY地学地球科学综合4420013-8029ENG J AISC ENGINEERING JOURNAL工程技术工程:土木UNITED STAT 4430013-8703ENTOMOL EXP APPL ENTOMOLOGIA EXPERIM生物昆虫学ENGLAND 4440013-872X ENTOMOL NEWS ENTOMOLOGICAL NEWS生物昆虫学UNITED STAT 4450013-8746ANN ENTOMOL SOC AM ANNALS OF THE ENTOM生物昆虫学UNITED STAT 4460013-8797P ENTOMOL SOC WASH PROCEEDINGS OF THE 生物昆虫学UNITED STAT 4470013-9157ENVIRONMENT ENVIRONMENT环境科学环境科学UNITED STATUNITED STAT 4480013-9351ENVIRON RES ENVIRONMENTAL RESEA环境科学公共卫生、环4490013-936X ENVIRON SCI TECHNOL ENVIRONMENTAL SCIEN环境科学工程:环境UNITED STAT 4500013-9580EPILEPSIA EPILEPSIA医学临床神经学UNITED STAT 4510013-9998ERDE ERDE GEOGRAPHY, GEOSCIENCES GERMANY 4520014-0015ERDKUNDE Erdkunde地学自然地理GERMANY 4530014-0139ERGONOMICS ERGONOMICS工程技术工程:工业ENGLAND 4540014-0309ERWERBS-OBSTBAU Erwerbs-Obstbau农林科学园艺GERMANY 4550014-2336EUPHYTICA EUPHYTICA农林科学农艺学NETHERLANDS 4560014-2565REV CLIN ESP REVISTA CLINICA ESP医学医学:内科SPAIN 4570014-2972EUR J CLIN INVEST EUROPEAN JOURNAL OF医学医学:内科GERMANY 4580014-2980EUR J IMMUNOL EUROPEAN JOURNAL OF医学免疫学UNITED STAT 4590014-2999EUR J PHARMACOL EUROPEAN JOURNAL OF医学药学NETHERLANDS 4600014-3022EUR NEUROL EUROPEAN NEUROLOGY医学临床神经学SWITZERLAND 4610014-3057EUR POLYM J EUROPEAN POLYMER JO化学高分子科学ENGLAND 4620014-3065POTATO RES POTATO RESEARCH AGRONOMY NETHERLANDS 4630014-312X EUR SURG RES EUROPEAN SURGICAL R医学外科SWITZERLAND 4640014-3820EVOLUTION EVOLUTION环境科学进化生物学UNITED STAT 4650014-4797EXP AGR EXPERIMENTAL AGRICU农林科学农艺学ENGLAND 4660014-4800EXP MOL PATHOL EXPERIMENTAL AND MO医学病理学UNITED STAT 4670014-4819EXP BRAIN RES EXPERIMENTAL BRAIN NEUROSCIENCES GERMANY 4680014-4827EXP CELL RES EXPERIMENTAL CELL R医学细胞生物学UNITED STAT 4690014-4835EXP EYE RES EXPERIMENTAL EYE RE医学眼科学UNITED STAT 4700014-4851EXP MECH EXPERIMENTAL MECHAN物理材料科学:表ENGLAND 4710014-4886EXP NEUROL EXPERIMENTAL NEUROL医学神经科学UNITED STAT 4720014-4894EXP PARASITOL EXPERIMENTAL PARASI医学寄生虫学UNITED STATNETHERLANDS 4730014-5793FEBS LETT FEBS LETTERS生物生化与分子生4740014-8237FARMACIA FARMACIA医学药学ROMANIA 4750015-0193FERROELECTRICS FERROELECTRICS工程技术材料科学:综ENGLAND 4760015-0282FERTIL STERIL FERTILITY AND STERI医学妇产科学UNITED STATRUSSIA 4770015-0541FIBRE CHEM+FIBRE CHEMISTRY工程技术材料科学:纺4780015-1882FILTR SEPARAT FILTRATION & SEPARA工程技术工程:化工ENGLANDUNITED STAT 4790015-2684FIRE TECHNOL FIRE TECHNOLOGY工程技术材料科学:综4800015-363X FLEISCHWIRTSCHAFT FLEISCHWIRTSCHAFT工程技术食品科技GERMANY 4810015-4040FLA ENTOMOL FLORIDA ENTOMOLOGIS生物昆虫学UNITED STAT 4820015-4628FLUID DYNAM+Fluid Dynamics MECHANICSPHYSICS, FL UNITED STAT 4830015-4725FLUORIDE FLUORIDE PUBLIC, ENV TOXICOLOGY N EW ZEALAND 4840015-5497FOLIA BIOL-KRAKOW FOLIA BIOLOGICA-KRA医学生物学POLAND。
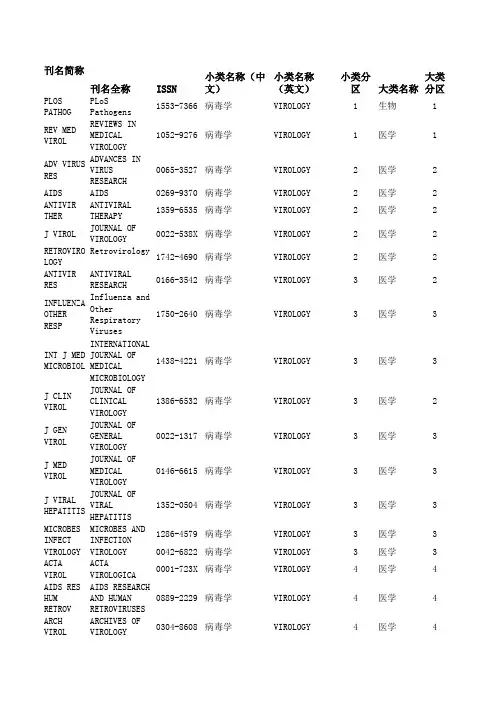
小类名称(英文小类分区大类名称大类分区刊名简称刊名全称ISSN小类名称(中文)PLOS PATHOG P LoS Pathogens1553-7366病毒学VIROLOGY1生物1 REV MED VIRO REVIEWS IN MEDIC1052-9276病毒学VIROLOGY1医学1 ADV VIRUS RE ADVANCES IN VIRU0065-3527病毒学VIROLOGY2医学2 AIDS AIDS0269-9370病毒学VIROLOGY2医学2 ANTIVIR THER ANTIVIRAL THERAP1359-6535病毒学VIROLOGY2医学2J VIROL JOURNAL OF VIROL0022-538X病毒学VIROLOGY2医学2 RETROVIROLOG Retrovirology 1742-4690病毒学VIROLOGY2医学2 ANTIVIR RES A NTIVIRAL RESEAR0166-3542病毒学VIROLOGY3医学2 INFLUENZA OT Influenza and Ot1750-2640病毒学VIROLOGY3医学3INT J MED MI INTERNATIONAL JO1438-4221病毒学VIROLOGY3医学3J CLIN VIROL JOURNAL OF CLINI1386-6532病毒学VIROLOGY3医学2J GEN VIROL J OURNAL OF GENER0022-1317病毒学VIROLOGY3医学3J MED VIROL J OURNAL OF MEDIC0146-6615病毒学VIROLOGY3医学3J VIRAL HEPA JOURNAL OF VIRAL1352-0504病毒学VIROLOGY3医学3 MICROBES INF MICROBES AND INF1286-4579病毒学VIROLOGY3医学3 VIROLOGY VIROLOGY0042-6822病毒学VIROLOGY3医学3 ACTA VIROLACTA VIROLOGICA0001-723X病毒学VIROLOGY4医学4 AIDS RES HUM AIDS RESEARCH AN0889-2229病毒学VIROLOGY4医学4 ARCH VIROLARCHIVES OF VIRO0304-8608病毒学VIROLOGY4医学4 CURR HIV RES CURRENT HIV RESE1570-162X病毒学VIROLOGY4医学3 FOOD ENVIRON Food and Environ1867-0334病毒学VIROLOGY4医学4 FUTURE VIROL Future Virology1746-0794病毒学VIROLOGY4医学4 INDIAN J VIR Indian Journal o0970-2822病毒学VIROLOGY4医学4 INTERVIROLOG INTERVIROLOGY0300-5526病毒学VIROLOGY4医学4J NEUROVIROL JOURNAL OF NEURO1355-0284病毒学VIROLOGY4医学3J VIROL METH JOURNAL OF VIROL0166-0934病毒学VIROLOGY4医学3S AFR J HIV SOUTHERN AFRICAN1608-9693病毒学VIROLOGY4医学4 VIRAL IMMUNO VIRAL IMMUNOLOGY0882-8245病毒学VIROLOGY4医学4 VIROL J Virology Journal1743-422X病毒学VIROLOGY4医学3 VIROLOGIE VIROLOGIE1267-8694病毒学VIROLOGY4医学4 VIRUS GENES V IRUS GENES0920-8569病毒学VIROLOGY4医学4 VIRUS RES VIRUS RESEARCH0168-1702病毒学VIROLOGY4医学3 VIRUSES-BASE Viruses-Basel1999-4915病毒学VIROLOGY4医学4 ACTA NEUROPA ACTA NEUROPATHOL0001-6322病理学PATHOLOGY1医学1AM J PATHOL A MERICAN JOURNAL0002-9440病理学PATHOLOGY1医学2 ANNU REV PAT Annual Review of1553-4006病理学PATHOLOGY1医学1J PATHOL JOURNAL OF PATHO0022-3417病理学PATHOLOGY1医学1AM J SURG PA AMERICAN JOURNAL0147-5185病理学PATHOLOGY2医学2 BRAIN PATHOL BRAIN PATHOLOGY1015-6305病理学PATHOLOGY2医学2 DIS MODEL ME Disease Models &1754-8403病理学PATHOLOGY2医学2 EXPERT REV M EXPERT REVIEW OF1473-7159病理学PATHOLOGY2医学2 HISTOPATHOLO HISTOPATHOLOGY0309-0167病理学PATHOLOGY2医学2J MOL DIAGN J OURNAL OF MOLEC1525-1578病理学PATHOLOGY2医学2J NEUROPATH JOURNAL OF NEURO0022-3069病理学PATHOLOGY2医学2 LAB INVESTLABORATORY INVES0023-6837病理学PATHOLOGY2医学2 MODERN PATHO MODERN PATHOLOGY0893-3952病理学PATHOLOGY2医学2 NEUROPATH AP NEUROPATHOLOGY A0305-1846病理学PATHOLOGY2医学2 SEMIN IMMUNO Seminars in Immu1863-2297病理学PATHOLOGY2医学2 ADV ANAT PAT ADVANCES IN ANAT1072-4109病理学PATHOLOGY3医学3 ALZ DIS ASSO ALZHEIMER DISEAS0893-0341病理学PATHOLOGY3医学3ANAL CELL PA Analytical Cellu2210-7177病理学PATHOLOGY3医学2 ARCH PATHOL ARCHIVES OF PATH0003-9985病理学PATHOLOGY3医学3 CANCER CYTOP CANCER CYTOPATHO1934-662X病理学PATHOLOGY3医学3 CYTOM PART B CYTOMETRY PART B1552-4949病理学PATHOLOGY3医学3 DIS MARKERS D ISEASE MARKERS0278-0240病理学PATHOLOGY3医学4 EXP MOL PATH EXPERIMENTAL AND0014-4800病理学PATHOLOGY3医学3 HISTOL HISTO HISTOLOGY AND HI0213-3911病理学PATHOLOGY3生物3 HUM PATHOLHUMAN PATHOLOGY0046-8177病理学PATHOLOGY3医学3 INT J EXP PA INTERNATIONAL JO0959-9673病理学PATHOLOGY3医学4 INT J IMMUNO INTERNATIONAL JO0394-6320病理学PATHOLOGY3医学3 J CLIN PATHO JOURNAL OF CLINI0021-9746病理学PATHOLOGY3医学3 PATHOBIOLOGY PATHOBIOLOGY1015-2008病理学PATHOLOGY3医学3 PATHOLOGY PATHOLOGY0031-3025病理学PATHOLOGY3医学3 TISSUE ANTIG TISSUE ANTIGENS0001-2815病理学PATHOLOGY3医学3 TOXICOL PATH TOXICOLOGIC PATH0192-6233病理学PATHOLOGY3医学3 VIRCHOWS ARC VIRCHOWS ARCHIV-0945-6317病理学PATHOLOGY3医学3 ACTA CYTOLACTA CYTOLOGICA0001-5547病理学PATHOLOGY4生物4 AM J FOREN M AMERICAN JOURNAL0195-7910病理学PATHOLOGY4医学4 ANN DIAGN PA Annals of Diagno1092-9134病理学PATHOLOGY4医学4 ANN PATHOLANNALES DE PATHO0242-6498病理学PATHOLOGY4医学4 APMIS APMIS0903-4641病理学PATHOLOGY4医学4 APPL IMMUNOH APPLIED IMMUNOHI1062-3345病理学PATHOLOGY4医学4 BRAIN TUMOR Brain Tumor Path1433-7398病理学PATHOLOGY4医学4 CARDIOVASC P CARDIOVASCULAR P1054-8807病理学PATHOLOGY4医学4 CLIN NEUROPA CLINICAL NEUROPA0722-5091病理学PATHOLOGY4医学4 CYTOPATHOLOG CYTOPATHOLOGY0956-5507病理学PATHOLOGY4生物4 DIAGN CYTOPA DIAGNOSTIC CYTOP8755-1039病理学PATHOLOGY4医学4 DIAGN MOL PA DIAGNOSTIC MOLEC1052-9551病理学PATHOLOGY4医学4 DIAGN PATHOL Diagnostic Patho1746-1596病理学PATHOLOGY4医学4 ENDOCR PATHO ENDOCRINE PATHOL1046-3976病理学PATHOLOGY4医学4 EXP TOXICOL EXPERIMENTAL AND0940-2993病理学PATHOLOGY4医学4 FETAL PEDIAT Fetal and Pediat1551-3815病理学PATHOLOGY4医学4 FOLIA NEUROP FOLIA NEUROPATHO1641-4640病理学PATHOLOGY4医学4 FORENSIC SCI Forensic Science1547-769X病理学PATHOLOGY4医学4 INDIAN J PAT Indian Journal o0377-4929病理学PATHOLOGY4医学4 INT J GYNECO INTERNATIONAL JO0277-1691病理学PATHOLOGY4医学4 INT J SURG P INTERNATIONAL JO1066-8969病理学PATHOLOGY4医学4 J COMP PATHO JOURNAL OF COMPA0021-9975病理学PATHOLOGY4生物4 J CUTAN PATH JOURNAL OF CUTAN0303-6987病理学PATHOLOGY4医学4 J ORAL PATHO JOURNAL OF ORAL 0904-2512病理学PATHOLOGY4医学4 J TOXICOL PA Journal of Toxic0914-9198病理学PATHOLOGY4医学4 KOREAN J PAT Korean Journal o1738-1843病理学PATHOLOGY4医学4 LEPROSY REV L EPROSY REVIEW0305-7518病理学PATHOLOGY4医学4 MED MOL MORP Medical Molecula1860-1480病理学PATHOLOGY4医学4 MED NUCL Medecine Nucleai0928-1258病理学PATHOLOGY4医学4 NEUROPATHOLO NEUROPATHOLOGY0919-6544病理学PATHOLOGY4医学4 PATHOL BIOL P ATHOLOGIE BIOLO0369-8114病理学PATHOLOGY4医学4 PATHOL INTPATHOLOGY INTERN1320-5463病理学PATHOLOGY4医学4 PATHOL ONCOL PATHOLOGY & ONCO1219-4956病理学PATHOLOGY4医学4 PATHOL RES P PATHOLOGY RESEAR0344-0338病理学PATHOLOGY4医学4 PATHOLOGE PATHOLOGE0172-8113病理学PATHOLOGY4医学4POL J PATHOL POLISH JOURNAL O1233-9687病理学PATHOLOGY4医学4 SCI JUSTICE S CIENCE & JUSTIC1355-0306病理学PATHOLOGY4医学4 SEMIN DIAGN SEMINARS IN DIAG0740-2570病理学PATHOLOGY4医学4 ULTRASTRUCT ULTRASTRUCTURAL 0191-3123病理学PATHOLOGY4医学4 VET PATHOLVETERINARY PATHO0300-9858病理学PATHOLOGY4农林科学3 POLYM TESTPOLYMER TESTING0142-9418材料科学:表征与测MATERIALS SCIENC1工程技术3MATERIALS SCIENC1工程技术1 PROG CRYST G PROGRESS IN CRYS0960-8974材料科学:表征与测MATERIALS SCIENC2物理3 EXP MECH EXPERIMENTAL MEC0014-4851材料科学:表征与测MATER CHARAC MATERIALS CHARAC1044-5803材料科学:表征与测MATERIALS SCIENC2工程技术3MATERIALS SCIENC2工程技术3 NANOSC MICRO Nanoscale and Mi1556-7265材料科学:表征与测MATERIALS SCIENC2工程技术3 NDT&E INT NDT & E INTERNAT0963-8695材料科学:表征与测ENG FAIL ANA ENGINEERING FAIL1350-6307材料科学:表征与测MATERIALS SCIENC3工程技术4MATERIALS SCIENC3工程技术4 J NONDESTRUC JOURNAL OF NONDE0195-9298材料科学:表征与测J SANDW STRU JOURNAL OF SANDW1099-6362材料科学:表征与测MATERIALS SCIENC3工程技术4MATERIALS SCIENC3工程技术4 J STRAIN ANA JOURNAL OF STRAI0309-3247材料科学:表征与测MATERIALS SCIENC3工程技术4 MECH ADV MAT MECHANICS OF ADV1537-6494材料科学:表征与测MECH TIME-DE MECHANICS OF TIM1385-2000材料科学:表征与测MATERIALS SCIENC3工程技术4MATERIALS SCIENC3工程技术4 RES NONDESTR RESEARCH IN NOND0934-9847材料科学:表征与测MATERIALS SCIENC3工程技术4 STRAIN STRAIN0039-2103材料科学:表征与测MATERIALS SCIENC4工程技术4 ADV STEEL CO Advanced Steel C1816-112X材料科学:表征与测MATERIALS SCIENC4工程技术4 ARCH MECH ARCHIVES OF MECH0373-2029材料科学:表征与测MATERIALS SCIENC4工程技术4 BETON- STAHL Beton- und Stahl0005-9900材料科学:表征与测MATERIALS SCIENC4工程技术4 COMPUT CONCR Computers and Co1598-8198材料科学:表征与测MATERIALS SCIENC4工程技术4 EXP TECHNIQU EXPERIMENTAL TEC0732-8818材料科学:表征与测MATERIALS SCIENC4工程技术4 INSIGHT INSIGHT1354-2575材料科学:表征与测MATERIALS SCIENC4工程技术4 J TEST EVAL J OURNAL OF TESTI0090-3973材料科学:表征与测MATERIALS SCIENC4工程技术4 MATER EVALMATERIALS EVALUA0025-5327材料科学:表征与测MATERIALS SCIENC4工程技术4 MATER PERFOR MATERIALS PERFOR0094-1492材料科学:表征与测MATERIALS SCIENC4工程技术4 MATER TESTMaterials Testin0025-5300材料科学:表征与测MATERIALS SCIENC4工程技术4 NONDESTRUCT Nondestructive T1058-9759材料科学:表征与测MATERIALS SCIENC4工程技术4 PART PART SY PARTICLE & PARTI0934-0866材料科学:表征与测MATERIALS SCIENC4工程技术4 POLYM POLYM POLYMERS & POLYM0967-3911材料科学:表征与测MATERIALS SCIENC4工程技术4 POWDER DIFFR POWDER DIFFRACTI0885-7156材料科学:表征与测MATERIALS SCIENC4工程技术4 QIRT J QIRT Journal1768-6733材料科学:表征与测MATERIALS SCIENC4工程技术4 RUSS J NONDE RUSSIAN JOURNAL 1061-8309材料科学:表征与测MATERIALS SCIENC4工程技术4 STRENGTH MAT STRENGTH OF MATE0039-2316材料科学:表征与测DYES PIGMENT DYES AND PIGMENT0143-7208材料科学:纺织MATERIALS SCIENC1工程技术2 CELLULOSE CELLULOSE0969-0239材料科学:纺织MATERIALS SCIENC2工程技术2 COLOR TECHNO COLORATION TECHN1472-3581材料科学:纺织MATERIALS SCIENC2工程技术4 TEXT RES JTEXTILE RESEARCH0040-5175材料科学:纺织MATERIALS SCIENC2工程技术4 FIBER POLYM F IBERS AND POLYM1229-9197材料科学:纺织MATERIALS SCIENC3工程技术4 IND TEXTILA I ndustria Textil1222-5347材料科学:纺织MATERIALS SCIENC3工程技术4 J AM LEATHER JOURNAL OF THE A0002-9726材料科学:纺织MATERIALS SCIENC3工程技术4 J ENG FIBER Journal of Engin1558-9250材料科学:纺织MATERIALS SCIENC3工程技术4 J IND TEXTJournal of Indus1528-0837材料科学:纺织MATERIALS SCIENC3工程技术4 WOOD FIBER S WOOD AND FIBER S0735-6161材料科学:纺织MATERIALS SCIENC3工程技术4 AATCC REV AATCC REVIEW1532-8813材料科学:纺织MATERIALS SCIENC4工程技术4 FIBRE CHEM+F IBRE CHEMISTRY0015-0541材料科学:纺织MATERIALS SCIENC4工程技术4 FIBRES TEXT FIBRES & TEXTILE1230-3666材料科学:纺织MATERIALS SCIENC4工程技术4 INT J CLOTH International Jo0955-6222材料科学:纺织MATERIALS SCIENC4工程技术4 J NAT FIBERS Journal of Natur1544-0478材料科学:纺织MATERIALS SCIENC4工程技术4J TEXT I JOURNAL OF THE T0040-5000材料科学:纺织MATERIALS SCIENC4工程技术4 J VINYL ADDI JOURNAL OF VINYL1083-5601材料科学:纺织MATERIALS SCIENC4工程技术4 SEN-I GAKKAI SEN-I GAKKAISHI0037-9875材料科学:纺织MATERIALS SCIENC4工程技术4 TEKST KONFEK Tekstil ve Konfe1300-3356材料科学:纺织MATERIALS SCIENC4工程技术4 TEKSTIL TEKSTIL0492-5882材料科学:纺织MATERIALS SCIENC4工程技术4 COMPOS SCI T COMPOSITES SCIEN0266-3538材料科学:复合MATERIALS SCIENC1工程技术2 COMPOS PART COMPOSITES PART 1359-835X材料科学:复合MATERIALS SCIENC2工程技术2 COMPOS PART COMPOSITES PART 1359-8368材料科学:复合MATERIALS SCIENC2工程技术3 COMPOS STRUC COMPOSITE STRUCT0263-8223材料科学:复合MATERIALS SCIENC2工程技术3 APPL COMPOS APPLIED COMPOSIT0929-189X材料科学:复合MATERIALS SCIENC3工程技术4 CEMENT CONCR CEMENT & CONCRET0958-9465材料科学:复合MATERIALS SCIENC3工程技术3 J COMPOS CON JOURNAL OF COMPO1090-0268材料科学:复合MATERIALS SCIENC3工程技术4 J COMPOS MAT JOURNAL OF COMPO0021-9983材料科学:复合MATERIALS SCIENC3工程技术4 J SANDW STRU JOURNAL OF SANDW1099-6362材料科学:复合MATERIALS SCIENC3工程技术4 MECH ADV MAT MECHANICS OF ADV1537-6494材料科学:复合MATERIALS SCIENC3工程技术4 POLYM COMPOS POLYMER COMPOSIT0272-8397材料科学:复合MATERIALS SCIENC3工程技术4 ADV COMPOS L ADVANCED COMPOSI0963-6935材料科学:复合MATERIALS SCIENC4工程技术4 ADV COMPOS M ADVANCED COMPOSI0924-3046材料科学:复合MATERIALS SCIENC4工程技术4 BETON- STAHL Beton- und Stahl0005-9900材料科学:复合MATERIALS SCIENC4工程技术4 CEM WAPNO BE Cement Wapno Bet1425-8129材料科学:复合MATERIALS SCIENC4工程技术4 COMPOS INTER COMPOSITE INTERF0927-6440材料科学:复合MATERIALS SCIENC4工程技术4 J REINF PLAS JOURNAL OF REINF0731-6844材料科学:复合MATERIALS SCIENC4工程技术4 J THERMOPLAS JOURNAL OF THERM0892-7057材料科学:复合MATERIALS SCIENC4工程技术4 MECH COMPOS MECHANICS OF COM0191-5665材料科学:复合MATERIALS SCIENC4工程技术4 PLAST RUBBER PLASTICS RUBBER 1465-8011材料科学:复合MATERIALS SCIENC4工程技术4 POLYM POLYM POLYMERS & POLYM0967-3911材料科学:复合MATERIALS SCIENC4工程技术4 PROG RUBBER Progress in Rubb1477-7606材料科学:复合MATERIALS SCIENC4工程技术4 SCI ENG COMP SCIENCE AND ENGI0334-181X材料科学:复合MATERIALS SCIENC4工程技术4 STEEL COMPOS STEEL AND COMPOS1229-9367材料科学:复合MATERIALS SCIENC4工程技术4 J EUR CERAM JOURNAL OF THE E0955-2219材料科学:硅酸盐MATERIALS SCIENC1工程技术2 CERAM INT CERAMICS INTERNA0272-8842材料科学:硅酸盐MATERIALS SCIENC2工程技术3 INT J APPL C International Jo1546-542X材料科学:硅酸盐MATERIALS SCIENC2工程技术3 J AM CERAM S JOURNAL OF THE A0002-7820材料科学:硅酸盐MATERIALS SCIENC2工程技术2 ADV APPL CER Advances in Appl1743-6753材料科学:硅酸盐MATERIALS SCIENC3工程技术4 J CERAM SOC JOURNAL OF THE C1882-0743材料科学:硅酸盐MATERIALS SCIENC3工程技术4 J ELECTROCER JOURNAL OF ELECT1385-3449材料科学:硅酸盐MATERIALS SCIENC3工程技术4 J NON-CRYST JOURNAL OF NON-C0022-3093材料科学:硅酸盐MATERIALS SCIENC3工程技术3 J SOL-GEL SC JOURNAL OF SOL-G0928-0707材料科学:硅酸盐MATERIALS SCIENC3工程技术3 AM CERAM SOC AMERICAN CERAMIC0002-7812材料科学:硅酸盐MATERIALS SCIENC4工程技术4 BOL SOC ESP BOLETIN DE LA SO0366-3175材料科学:硅酸盐MATERIALS SCIENC4工程技术4 CERAM-SILIKA CERAMICS-SILIKAT0862-5468材料科学:硅酸盐MATERIALS SCIENC4工程技术4 CFI-CERAM FO CFI-CERAMIC FORU0173-9913材料科学:硅酸盐MATERIALS SCIENC4工程技术4 GLASS CERAM+GLASS AND CERAMI0361-7610材料科学:硅酸盐MATERIALS SCIENC4工程技术4 GLASS PHYS C GLASS PHYSICS AN1087-6596材料科学:硅酸盐MATERIALS SCIENC4工程技术4 GLASS TECHNO GLASS TECHNOLOGY1753-3546材料科学:硅酸盐MATERIALS SCIENC4工程技术4 IND CERAM INDUSTRIAL CERAM1121-7588材料科学:硅酸盐MATERIALS SCIENC4工程技术4 J CERAM PROC JOURNAL OF CERAM1229-9162材料科学:硅酸盐MATERIALS SCIENC4工程技术4 J INORG MATE JOURNAL OF INORG1000-324X材料科学:硅酸盐MATERIALS SCIENC4工程技术4 MATER WORLD M ATERIALS WORLD0967-8638材料科学:硅酸盐MATERIALS SCIENC4工程技术4 PHYS CHEM GL Physics and Chem1753-3562材料科学:硅酸盐MATERIALS SCIENC4工程技术4 POWDER METAL POWDER METALLURG1068-1302材料科学:硅酸盐MATERIALS SCIENC4工程技术4SCI SINTERSCIENCE OF SINTE0350-820X材料科学:硅酸盐MATERIALS SCIENC4工程技术4 T INDIAN CER Transactions of 0371-750X材料科学:硅酸盐MATERIALS SCIENC4工程技术4 J ELECTROCHE JOURNAL OF THE E0013-4651材料科学:膜MATERIALS SCIENC1工程技术2 CHEM VAPOR D CHEMICAL VAPOR D0948-1907材料科学:膜MATERIALS SCIENC2工程技术3 SURF COAT TE SURFACE & COATIN0257-8972材料科学:膜MATERIALS SCIENC2工程技术2 THIN SOLID F THIN SOLID FILMS0040-6090材料科学:膜MATERIALS SCIENC2工程技术3 APPL SURF SC APPLIED SURFACE 0169-4332材料科学:膜MATERIALS SCIENC3工程技术3 J THERM SPRA JOURNAL OF THERM1059-9630材料科学:膜MATERIALS SCIENC3工程技术3 J VAC SCI TE JOURNAL OF VACUU0734-2101材料科学:膜MATERIALS SCIENC3工程技术3 PROG ORG COA PROGRESS IN ORGA0300-9440材料科学:膜MATERIALS SCIENC3工程技术3 CORROS REVCORROSION REVIEW0334-6005材料科学:膜MATERIALS SCIENC4工程技术4 INT J SURF S International Jo1749-785X材料科学:膜MATERIALS SCIENC4工程技术4 J COAT TECHN Journal of Coati1547-0091材料科学:膜MATERIALS SCIENC4工程技术4 J PLAST FILM JOURNAL OF PLAST8756-0879材料科学:膜MATERIALS SCIENC4工程技术4 JCT COATINGS JCT COATINGSTECH1547-0083材料科学:膜MATERIALS SCIENC4工程技术4 KORROZ FIGY K orrozios Figyel0133-2546材料科学:膜MATERIALS SCIENC4工程技术4 PIGM RESIN T Pigment & Resin 0369-9420材料科学:膜MATERIALS SCIENC4工程技术4 SURF COAT IN SURFACE COATINGS1754-0925材料科学:膜MATERIALS SCIENC4工程技术4 SURF ENG SURFACE ENGINEER0267-0844材料科学:膜MATERIALS SCIENC4工程技术4 T I MET FINI TRANSACTIONS OF 0020-2967材料科学:膜MATERIALS SCIENC4工程技术3MATERIALS SCIENC1工程技术1 BIOMATERIALS BIOMATERIALS0142-9612材料科学:生物材料MATERIALS SCIENC2工程技术1 ACTA BIOMATE Acta Biomaterial1742-7061材料科学:生物材料MATERIALS SCIENC2生物2 EUR CELLS MA EUROPEAN CELLS &1473-2262材料科学:生物材料MATERIALS SCIENC2工程技术2 J MECH BEHAV Journal of the M1751-6161材料科学:生物材料MATERIALS SCIENC2生物3 MACROMOL BIO MACROMOLECULAR B1616-5187材料科学:生物材料MATERIALS SCIENC3生物3 BIOINTERPHAS Biointerphases 1559-4106材料科学:生物材料MATERIALS SCIENC3生物3 COLLOID SURF COLLOIDS AND SUR0927-7765材料科学:生物材料MATERIALS SCIENC3工程技术2 DENT MATERDENTAL MATERIALS0109-5641材料科学:生物材料MATERIALS SCIENC3工程技术2 J BIOACT COM JOURNAL OF BIOAC0883-9115材料科学:生物材料MATERIALS SCIENC3工程技术2 J BIOMAT SCI JOURNAL OF BIOMA0920-5063材料科学:生物材料MATERIALS SCIENC3工程技术2 J BIOMED MAT JOURNAL OF BIOME1549-3296材料科学:生物材料J BIOMED MAT JOURNAL OF BIOME1552-4973材料科学:生物材料MATERIALS SCIENC3工程技术2MATERIALS SCIENC4工程技术4 ARTIF CELL B ARTIFICIAL CELLS1073-1199材料科学:生物材料MATERIALS SCIENC4工程技术3 BIOFABRICATI Biofabrication1758-5082材料科学:生物材料BIOINSPIR BI Bioinspiration &1748-3182材料科学:生物材料MATERIALS SCIENC4工程技术3MATERIALS SCIENC4工程技术3 BIOMED MATER Biomedical Mater1748-6041材料科学:生物材料MATERIALS SCIENC4工程技术4 BIO-MED MATE BIO-MEDICAL MATE0959-2989材料科学:生物材料MATERIALS SCIENC4工程技术4 CELL POLYMCELLULAR POLYMER0262-4893材料科学:生物材料MATERIALS SCIENC4医学4 DENT MATER J DENTAL MATERIALS0287-4547材料科学:生物材料MATERIALS SCIENC4生物4 J APPL BIOMA Journal of Appli1722-6899材料科学:生物材料MATERIALS SCIENC4工程技术3 J BIOBASED M Journal of Bioba1556-6560材料科学:生物材料MATERIALS SCIENC4工程技术2 J BIOMATER A JOURNAL OF BIOMA0885-3282材料科学:生物材料MATERIALS SCIENC4工程技术4 J BIONIC ENG Journal of Bioni1672-6529材料科学:生物材料MATERIALS SCIENC4工程技术2 J MATER SCI-JOURNAL OF MATER0957-4530材料科学:生物材料MATERIALS SCIENC4工程技术2 MAT SCI ENG Materials Scienc0928-4931材料科学:生物材料MATERIALS SCIENC2工程技术3 BIORESOURCES BioResources1930-2126材料科学:纸与木材MATERIALS SCIENC2工程技术2 CELLULOSE CELLULOSE0969-0239材料科学:纸与木材MATERIALS SCIENC2工程技术3 HOLZFORSCHUN HOLZFORSCHUNG0018-3830材料科学:纸与木材MATERIALS SCIENC2工程技术3 WOOD SCI TEC WOOD SCIENCE AND0043-7719材料科学:纸与木材EUR J WOOD W European Journal0018-3768材料科学:纸与木材MATERIALS SCIENC3工程技术4MATERIALS SCIENC3工程技术3 J WOOD CHEM JOURNAL OF WOOD 0277-3813材料科学:纸与木材MATERIALS SCIENC3工程技术4 NORD PULP PA NORDIC PULP & PA0283-2631材料科学:纸与木材MATERIALS SCIENC3工程技术4 TAPPI J TAPPI JOURNAL0734-1415材料科学:纸与木材MATERIALS SCIENC3工程技术4 WOOD FIBER S WOOD AND FIBER S0735-6161材料科学:纸与木材MATERIALS SCIENC4工程技术4 APPITA J APPITA JOURNAL1038-6807材料科学:纸与木材CELL CHEM TE CELLULOSE CHEMIS0576-9787材料科学:纸与木材MATERIALS SCIENC4工程技术4MATERIALS SCIENC4工程技术4 DREWNO Drewno1644-3985材料科学:纸与木材MATERIALS SCIENC4工程技术4 DRVNA IND Drvna Industrija0012-6772材料科学:纸与木材MATERIALS SCIENC4工程技术4 FOREST PROD FOREST PRODUCTS 0015-7473材料科学:纸与木材MATERIALS SCIENC4工程技术4 J PULP PAP S JOURNAL OF PULP 0826-6220材料科学:纸与木材MATERIALS SCIENC4工程技术4 MADERAS-CIEN Maderas-Ciencia 0717-3644材料科学:纸与木材MATERIALS SCIENC4工程技术4 MOKUZAI GAKK MOKUZAI GAKKAISH0021-4795材料科学:纸与木材MATERIALS SCIENC4工程技术4 PAP PUU-PAP PAPERI JA PUU-PA0031-1243材料科学:纸与木材MATERIALS SCIENC4工程技术4 PULP PAP-CAN PULP & PAPER-CAN0316-4004材料科学:纸与木材MATERIALS SCIENC4工程技术4 WOCHENBL PAP WOCHENBLATT FUR 0043-7131材料科学:纸与木材MATERIALS SCIENC4工程技术4 WOOD RES-SLO WOOD RESEARCH1336-4561材料科学:纸与木材ACS NANO ACS Nano 1936-0851材料科学:综合MATERIALS SCIENC1工程技术1 ADV FUNCT MA ADVANCED FUNCTIO1616-301X材料科学:综合MATERIALS SCIENC1工程技术1 ADV MATER ADVANCED MATERIA0935-9648材料科学:综合MATERIALS SCIENC1工程技术1 ANNU REV MAT ANNUAL REVIEW OF1531-7331材料科学:综合MATERIALS SCIENC1工程技术1 MAT SCI ENG MATERIALS SCIENC0927-796X材料科学:综合MATERIALS SCIENC1工程技术1 MATER TODAY M aterials Today1369-7021材料科学:综合MATERIALS SCIENC1工程技术1 NANO LETT NANO LETTERS1530-6984材料科学:综合MATERIALS SCIENC1工程技术1 NANO TODAYNano Today1748-0132材料科学:综合MATERIALS SCIENC1工程技术1 NAT MATER NATURE MATERIALS1476-1122材料科学:综合MATERIALS SCIENC1工程技术1 NAT NANOTECH Nature Nanotechn1748-3387材料科学:综合MATERIALS SCIENC1工程技术1 PROG MATER S PROGRESS IN MATE0079-6425材料科学:综合MATERIALS SCIENC1工程技术1 ACTA MATERACTA MATERIALIA1359-6454材料科学:综合MATERIALS SCIENC2工程技术1 CARBON CARBON0008-6223材料科学:综合MATERIALS SCIENC2工程技术1 CHEM MATERCHEMISTRY OF MAT0897-4756材料科学:综合MATERIALS SCIENC2工程技术1 CRIT REV SOL CRITICAL REVIEWS1040-8436材料科学:综合MATERIALS SCIENC2物理1 CRYST GROWTH CRYSTAL GROWTH &1528-7483材料科学:综合MATERIALS SCIENC2化学2 CURR OPIN SO CURRENT OPINION 1359-0286材料科学:综合MATERIALS SCIENC2工程技术1 EXP MECH EXPERIMENTAL MEC0014-4851材料科学:综合MATERIALS SCIENC2物理3 INT J PLASTI INTERNATIONAL JO0749-6419材料科学:综合MATERIALS SCIENC2工程技术1 INT MATER RE INTERNATIONAL MA0950-6608材料科学:综合MATERIALS SCIENC2工程技术1 J MATER CHEM JOURNAL OF MATER0959-9428材料科学:综合MATERIALS SCIENC2工程技术1 J MECH PHYS JOURNAL OF THE M0022-5096材料科学:综合MATERIALS SCIENC2物理2 J PHYS CHEM Journal of Physi1932-7447材料科学:综合MATERIALS SCIENC2化学2 LANGMUIR LANGMUIR0743-7463材料科学:综合MATERIALS SCIENC2化学2 MICROSC MICR MICROSCOPY AND M1431-9276材料科学:综合MATERIALS SCIENC2工程技术2 MRS BULL MRS BULLETIN0883-7694材料科学:综合MATERIALS SCIENC2工程技术1 NANO RES Nano Research1998-0124材料科学:综合MATERIALS SCIENC2化学2 NANOSCALE Nanoscale2040-3364材料科学:综合MATERIALS SCIENC2工程技术1 NANOTECHNOLO NANOTECHNOLOGY0957-4484材料科学:综合MATERIALS SCIENC2工程技术1 ORG ELECTRON ORGANIC ELECTRON1566-1199材料科学:综合MATERIALS SCIENC2工程技术1 PLASMONICS Plasmonics 1557-1955材料科学:综合MATERIALS SCIENC2工程技术1 SMALL SMALL1613-6810材料科学:综合MATERIALS SCIENC2工程技术1 SOFT MATTER S oft Matter1744-683X材料科学:综合MATERIALS SCIENC2工程技术1 SOL ENERG MA SOLAR ENERGY MAT0927-0248材料科学:综合MATERIALS SCIENC2工程技术1 THIN SOLID F THIN SOLID FILMS0040-6090材料科学:综合MATERIALS SCIENC2工程技术3 ACS APPL MAT ACS Applied Mate1944-8244材料科学:综合MATERIALS SCIENC3工程技术2APPL PHYS A-APPLIED PHYSICS 0947-8396材料科学:综合MATERIALS SCIENC3工程技术3 CEMENT CONCR CEMENT AND CONCR0008-8846材料科学:综合MATERIALS SCIENC3工程技术2 CMC-COMPUT M CMC-Computers Ma1546-2218材料科学:综合MATERIALS SCIENC3工程技术3 COMP MATER S COMPUTATIONAL MA0927-0256材料科学:综合MATERIALS SCIENC3工程技术3 CORROS SCICORROSION SCIENC0010-938X材料科学:综合MATERIALS SCIENC3工程技术2 CURR APPL PH CURRENT APPLIED 1567-1739材料科学:综合MATERIALS SCIENC3物理3 CURR NANOSCI Current Nanoscie1573-4137材料科学:综合MATERIALS SCIENC3工程技术2 DIAM RELAT M DIAMOND AND RELA0925-9635材料科学:综合MATERIALS SCIENC3工程技术2 DIG J NANOMA Digest Journal o1842-3582材料科学:综合MATERIALS SCIENC3工程技术2 ELECTROCHEM ELECTROCHEMICAL 1099-0062材料科学:综合MATERIALS SCIENC3工程技术2 EUR PHYS J E EUROPEAN PHYSICA1292-8941材料科学:综合MATERIALS SCIENC3工程技术2 FUNCT MATER Functional Mater1793-6047材料科学:综合MATERIALS SCIENC3工程技术2 GOLD BULL GOLD BULLETIN0017-1557材料科学:综合MATERIALS SCIENC3工程技术2 IEEE T NANOT IEEE TRANSACTION1536-125X材料科学:综合MATERIALS SCIENC3工程技术2 INT J ADHES INTERNATIONAL JO0143-7496材料科学:综合MATERIALS SCIENC3工程技术3 INT J DAMAGE INTERNATIONAL JO1056-7895材料科学:综合MATERIALS SCIENC3工程技术3 INT J FATIGU INTERNATIONAL JO0142-1123材料科学:综合MATERIALS SCIENC3工程技术3 INTERMETALLI INTERMETALLICS0966-9795材料科学:综合MATERIALS SCIENC3工程技术2 J ALLOY COMP JOURNAL OF ALLOY0925-8388材料科学:综合MATERIALS SCIENC3工程技术2 J MATER RES J OURNAL OF MATER0884-2914材料科学:综合MATERIALS SCIENC3工程技术3 J MATER SCI J OURNAL OF MATER0022-2461材料科学:综合MATERIALS SCIENC3工程技术3 J MICROMECH JOURNAL OF MICRO0960-1317材料科学:综合MATERIALS SCIENC3工程技术2 J NANOPART R JOURNAL OF NANOP1388-0764材料科学:综合MATERIALS SCIENC3工程技术2 J NANOSCI NA JOURNAL OF NANOS1533-4880材料科学:综合MATERIALS SCIENC3工程技术3 J NON-CRYST JOURNAL OF NON-C0022-3093材料科学:综合MATERIALS SCIENC3工程技术3 J NUCL MATER JOURNAL OF NUCLE0022-3115材料科学:综合MATERIALS SCIENC3工程技术3 MACROMOL MAT MACROMOLECULAR M1438-7492材料科学:综合MATERIALS SCIENC3工程技术3 MAT SCI ENG MATERIALS SCIENC0921-5093材料科学:综合MATERIALS SCIENC3工程技术2 MATER CHEM P MATERIALS CHEMIS0254-0584材料科学:综合MATERIALS SCIENC3工程技术2 MATER LETTMATERIALS LETTER0167-577X材料科学:综合MATERIALS SCIENC3工程技术2 MATER RES BU MATERIALS RESEAR0025-5408材料科学:综合MATERIALS SCIENC3工程技术2 MATER SCI EN Materials Scienc0921-5107材料科学:综合MATERIALS SCIENC3工程技术3 MECH ADV MAT MECHANICS OF ADV1537-6494材料科学:综合MATERIALS SCIENC3工程技术4 MECH MATERMECHANICS OF MAT0167-6636材料科学:综合MATERIALS SCIENC3工程技术2 METALL MATER METALLURGICAL AN1073-5623材料科学:综合MATERIALS SCIENC3工程技术3 MICROPOR MES MICROPOROUS AND 1387-1811材料科学:综合MATERIALS SCIENC3化学3 NANOSCALE RE Nanoscale Resear1931-7573材料科学:综合MATERIALS SCIENC3工程技术2 OPT MATER OPTICAL MATERIAL0925-3467材料科学:综合MATERIALS SCIENC3工程技术3 PHOTONIC NAN Photonics and Na1569-4410材料科学:综合MATERIALS SCIENC3工程技术2 PHYS CHEM MI PHYSICS AND CHEM0342-1791材料科学:综合MATERIALS SCIENC3地学3 PHYS STATUS Physica Status S1862-6254材料科学:综合MATERIALS SCIENC3物理2 SCI ADV MATE Science of Advan1947-2935材料科学:综合MATERIALS SCIENC3工程技术2 SCI TECHNOL SCIENCE AND TECH1468-6996材料科学:综合MATERIALS SCIENC3工程技术2 SCRIPTA MATE SCRIPTA MATERIAL1359-6462材料科学:综合MATERIALS SCIENC3工程技术2 SMART MATER SMART MATERIALS 0964-1726材料科学:综合MATERIALS SCIENC3工程技术3 SOFT MATERSOFT MATERIALS1539-445X材料科学:综合MATERIALS SCIENC3工程技术2 SYNTHETIC ME SYNTHETIC METALS0379-6779材料科学:综合MATERIALS SCIENC3工程技术2 WEAR WEAR0043-1648材料科学:综合MATERIALS SCIENC3工程技术3 ACI MATER J A CI MATERIALS JO0889-325X材料科学:综合MATERIALS SCIENC4工程技术4 ACI STRUCT J ACI STRUCTURAL J0889-3241材料科学:综合MATERIALS SCIENC4工程技术4 ACTA MECH SO ACTA MECHANICA S0894-9166材料科学:综合MATERIALS SCIENC4物理4ADV MATER PR ADVANCED MATERIA0882-7958材料科学:综合MATERIALS SCIENC4工程技术4 ANN CHIM-SCI ANNALES DE CHIMI0151-9107材料科学:综合MATERIALS SCIENC4工程技术4 ARCH CIV MEC Archives of Civi1644-9665材料科学:综合MATERIALS SCIENC4工程技术4 ATOMIZATION ATOMIZATION AND 1044-5110材料科学:综合MATERIALS SCIENC4工程技术4 B MATER SCI B ULLETIN OF MATE0250-4707材料科学:综合MATERIALS SCIENC4工程技术4 CHALCOGENIDE Chalcogenide Let1584-8663材料科学:综合MATERIALS SCIENC4工程技术4 CIRCUIT WORL CIRCUIT WORLD0305-6120材料科学:综合MATERIALS SCIENC4工程技术4 COMBUST EXPL COMBUSTION EXPLO0010-5082材料科学:综合MATERIALS SCIENC4工程技术4 CONSTR BUILD CONSTRUCTION AND0950-0618材料科学:综合MATERIALS SCIENC4工程技术3 CORROS ENG S CORROSION ENGINE1478-422X材料科学:综合MATERIALS SCIENC4工程技术4 CORROSION CORROSION0010-9312材料科学:综合MATERIALS SCIENC4工程技术4 ELECTRON MAT Electronic Mater1738-8090材料科学:综合MATERIALS SCIENC4工程技术3 FATIGUE FRAC FATIGUE & FRACTU8756-758X材料科学:综合MATERIALS SCIENC4工程技术4 FERROELECTRI FERROELECTRICS0015-0193材料科学:综合MATERIALS SCIENC4工程技术4 FIBRE CHEM+F IBRE CHEMISTRY0015-0541材料科学:综合MATERIALS SCIENC4工程技术4 FIRE MATERFIRE AND MATERIA0308-0501材料科学:综合MATERIALS SCIENC4工程技术4 FIRE SAFETY FIRE SAFETY JOUR0379-7112材料科学:综合MATERIALS SCIENC4工程技术4 FIRE TECHNOL FIRE TECHNOLOGY0015-2684材料科学:综合MATERIALS SCIENC4工程技术4 FULLER NANOT FULLERENES NANOT1536-383X材料科学:综合MATERIALS SCIENC4工程技术4 GEOSYNTH INT GEOSYNTHETICS IN1072-6349材料科学:综合MATERIALS SCIENC4地学4 GRANUL MATTE GRANULAR MATTER1434-5021材料科学:综合MATERIALS SCIENC4物理4 HIGH TEMP MA HIGH TEMPERATURE0334-6455材料科学:综合MATERIALS SCIENC4工程技术4 HIGH TEMP MA HIGH TEMPERATURE1093-3611材料科学:综合MATERIALS SCIENC4工程技术4 IEEE T ADV P IEEE TRANSACTION1521-3323材料科学:综合MATERIALS SCIENC4工程技术3 IEEE T COMPO IEEE TRANSACTION1521-3331材料科学:综合MATERIALS SCIENC4工程技术4 INDIAN J ENG INDIAN JOURNAL O0971-4588材料科学:综合MATERIALS SCIENC4工程技术4 INFORM MIDEM INFORMACIJE MIDE0352-9045材料科学:综合MATERIALS SCIENC4工程技术4 INORG MATER+INORGANIC MATERI0020-1685材料科学:综合MATERIALS SCIENC4工程技术4 INT J FRACTU INTERNATIONAL JO0376-9429材料科学:综合MATERIALS SCIENC4物理4 INT J MATER INTERNATIONAL JO0268-1900材料科学:综合MATERIALS SCIENC4工程技术4 INT J MIN ME International Jo1674-4799材料科学:综合MATERIALS SCIENC4工程技术4 INT J NANOTE International Jo1475-7435材料科学:综合MATERIALS SCIENC4工程技术3 INT J NUMER INTERNATIONAL JO0363-9061材料科学:综合MATERIALS SCIENC4工程技术3 INT J REFRAC INTERNATIONAL JO0263-4368材料科学:综合MATERIALS SCIENC4工程技术3 INT J SURF S International Jo1749-785X材料科学:综合MATERIALS SCIENC4工程技术4 J ADHES SCI JOURNAL OF ADHES0169-4243材料科学:综合MATERIALS SCIENC4工程技术4 J ADHESIONJOURNAL OF ADHES0021-8464材料科学:综合MATERIALS SCIENC4工程技术4 J ADV CONCR Journal of Advan1346-8014材料科学:综合MATERIALS SCIENC4工程技术4 J ADV MATER-JOURNAL OF ADVAN1070-9789材料科学:综合MATERIALS SCIENC4工程技术4 J COMPUT THE Journal of Compu1546-1955材料科学:综合MATERIALS SCIENC4工程技术4 J CULT HERIT JOURNAL OF CULTU1296-2074材料科学:综合MATERIALS SCIENC4综合性期刊3 J ELASTICITY JOURNAL OF ELAST0374-3535材料科学:综合MATERIALS SCIENC4工程技术3 J ELASTOM PL JOURNAL OF ELAST0095-2443材料科学:综合MATERIALS SCIENC4工程技术4 J ELECTRON M JOURNAL OF ELECT0361-5235材料科学:综合MATERIALS SCIENC4工程技术3 J ENERG MATE Journal of Energ0737-0652材料科学:综合MATERIALS SCIENC4工程技术4 J ENG MATER-JOURNAL OF ENGIN0094-4289材料科学:综合MATERIALS SCIENC4工程技术4 J EXP NANOSC Journal of Exper1745-8080材料科学:综合MATERIALS SCIENC4工程技术4 J FIRE PROT Journal of Fire 1042-3915材料科学:综合MATERIALS SCIENC4工程技术4 J FIRE SCIJOURNAL OF FIRE 0734-9041材料科学:综合MATERIALS SCIENC4工程技术4 J FRICT WEAR Journal of Frict1068-3666材料科学:综合MATERIALS SCIENC4工程技术4 J INTEL MAT JOURNAL OF INTEL1045-389X材料科学:综合MATERIALS SCIENC4工程技术3J MAGN MAGN JOURNAL OF MAGNE0304-8853材料科学:综合MATERIALS SCIENC4物理4 J MATER CIVI JOURNAL OF MATER0899-1561材料科学:综合MATERIALS SCIENC4工程技术4 J MATER ENG JOURNAL OF MATER1059-9495材料科学:综合MATERIALS SCIENC4工程技术4 J MATER PROC JOURNAL OF MATER0924-0136材料科学:综合MATERIALS SCIENC4工程技术3 J MATER SCI JOURNAL OF MATER1005-0302材料科学:综合MATERIALS SCIENC4工程技术4 J MATER SCI-JOURNAL OF MATER0957-4522材料科学:综合MATERIALS SCIENC4工程技术4 J MECH MATER Journal of Mecha1559-3959材料科学:综合MATERIALS SCIENC4工程技术4 J MICRO-NANO Journal of Micro1932-5150材料科学:综合MATERIALS SCIENC4工程技术4 J NANO RES-S Journal of Nano 1662-5250材料科学:综合MATERIALS SCIENC4工程技术4 J NANOMATER J ournal of Nanom1687-4110材料科学:综合MATERIALS SCIENC4工程技术3 J NEW MAT EL JOURNAL OF NEW M1480-2422材料科学:综合MATERIALS SCIENC4工程技术4 J OPTOELECTR JOURNAL OF OPTOE1454-4164材料科学:综合MATERIALS SCIENC4工程技术4 J OVONIC RES Journal of Ovoni1584-9953材料科学:综合MATERIALS SCIENC4工程技术4 J PHASE EQUI JOURNAL OF PHASE1547-7037材料科学:综合MATERIALS SCIENC4工程技术4 J PHYS CHEM Journal of Physi1948-7185材料科学:综合MATERIALS SCIENC4化学4 J POROUS MAT JOURNAL OF POROU1380-2224材料科学:综合MATERIALS SCIENC4工程技术4 J SOC INF DI Journal of the S1071-0922材料科学:综合MATERIALS SCIENC4工程技术4 J SUPERHARD Journal of Super1063-4576材料科学:综合MATERIALS SCIENC4工程技术4 J WUHAN UNIV JOURNAL OF WUHAN1000-2413材料科学:综合MATERIALS SCIENC4工程技术4 JOM-US JOM-JOURNAL OF T1047-4838材料科学:综合MATERIALS SCIENC4工程技术3 KONA POWDER KONA Powder and 0288-4534材料科学:综合MATERIALS SCIENC4工程技术4 KOREAN J MET Korean Journal o1738-8228材料科学:综合MATERIALS SCIENC4工程技术4 KOVOVE MATER KOVOVE MATERIALY0023-432X材料科学:综合MATERIALS SCIENC4工程技术4 LASER ENG LASERS IN ENGINE0898-1507材料科学:综合MATERIALS SCIENC4工程技术4 MACH SCI TEC MACHINING SCIENC1091-0344材料科学:综合MATERIALS SCIENC4工程技术4 MAG CONCRETE MAGAZINE OF CONC0024-9831材料科学:综合MATERIALS SCIENC4工程技术4 MAT SCI SEMI MATERIALS SCIENC1369-8001材料科学:综合MATERIALS SCIENC4工程技术4 MATER CONSTR MATERIALES DE CO0465-2746材料科学:综合MATERIALS SCIENC4工程技术4 MATER CORROS MATERIALS AND CO0947-5117材料科学:综合MATERIALS SCIENC4工程技术4 MATER DESIGN MATERIALS & DESI0261-3069材料科学:综合MATERIALS SCIENC4工程技术3 MATER HIGH T MATERIALS AT HIG0960-3409材料科学:综合MATERIALS SCIENC4工程技术4 MATER MANUF MATERIALS AND MA1042-6914材料科学:综合MATERIALS SCIENC4工程技术4 MATER RES IN MATERIALS RESEAR1432-8917材料科学:综合MATERIALS SCIENC4工程技术4 MATER RES-IB Materials Resear1516-1439材料科学:综合MATERIALS SCIENC4工程技术4 MATER SCI TE MATERIALS SCIENC0267-0836材料科学:综合MATERIALS SCIENC4工程技术4 MATER SCI+MATERIALS SCIENC1068-820X材料科学:综合MATERIALS SCIENC4工程技术4 MATER SCI-ME Materials Scienc1392-1320材料科学:综合MATERIALS SCIENC4工程技术4 MATER SCI-PO MATERIALS SCIENC0137-1339材料科学:综合MATERIALS SCIENC4工程技术4 MATER STRUCT MATERIALS AND ST1359-5997材料科学:综合MATERIALS SCIENC4工程技术4 MATER TECHNO MATERIALS TECHNO1066-7857材料科学:综合MATERIALS SCIENC4工程技术4 MATER TEHNOL Materiali in Teh1580-2949材料科学:综合MATERIALS SCIENC4工程技术4 MATER TRANS M ATERIALS TRANSA1345-9678材料科学:综合MATERIALS SCIENC4工程技术4 MATER WORLD M ATERIALS WORLD0967-8638材料科学:综合MATERIALS SCIENC4工程技术4 MATERIA-BRAZ Materia-Rio de J1517-7076材料科学:综合MATERIALS SCIENC4工程技术4 MATERIALWISS MATERIALWISSENSC0933-5137材料科学:综合MATERIALS SCIENC4工程技术4 MATH MECH SO MATHEMATICS AND 1081-2865材料科学:综合MATERIALS SCIENC4工程技术4 MET MATER IN METALS AND MATER1598-9623材料科学:综合MATERIALS SCIENC4工程技术3 METALL MATER METALLURGICAL AN1073-5615材料科学:综合MATERIALS SCIENC4工程技术4 METALLOFIZ N METALLOFIZIKA I 1024-1809材料科学:综合MATERIALS SCIENC4工程技术4 MICRO NANO L Micro & Nano Let1750-0443材料科学:综合MATERIALS SCIENC4工程技术4 MICROELECTRO MICROELECTRONICS1356-5362材料科学:综合MATERIALS SCIENC4工程技术4。
阈值期刊数期刊数3.4585225序号1234567891011121314151617181920212223242526272829303132ISSN刊名简称1476-1122NAT MATER1748-3387NAT NANOTECHNOL1区2区阈值阈值1.931 1.1123区期刊数0360-1285PROG ENERG COMBUST1530-6984NANO LETT1523-9829ANNU REV BIOMED ENG1087-0156NAT BIOTECHNOL1301-8361ENERGY EDUC SCI TECH0935-9648ADV MATER0927-796X MAT SCI ENG R1748-0132NANO TODAY0142-9612BIOMATERIALS0079-6425PROG MATER SCI0958-1669CURR OPIN BIOTECH0167-7799TRENDS BIOTECHNOL1616-301X ADV FUNCT MATER1531-7331ANNU REV MATER RES1936-0851ACS NANO1369-7021MATER TODAY0734-9750BIOTECHNOL ADV1549-9634NANOMED-NANOTECHNOL0079-6816PROG SURF SCI1473-0197LAB CHIP0360-0300ACM COMPUT SURV1558-3724POLYM REV1947-5438ANNU REV CHEM BIOMOL1613-6810SMALL0950-6608INT MATER REV0897-4756CHEM MATER0960-8974PROG CRYST GROWTH CH1062-7995PROG PHOTOVOLTAICS0018-9219P IEEE0021-9517J CATAL377分区11111111111111111111111111111111ACPROGRESSCIENCETREBIOTECHNOLOGYADVANCEMATERIALSPROGRESSSCIENCEMATERIAENGINEERING R-REPORTSNanPROGRESSCOMBUSTION SCIENCENANOANNUALBIOMEDICAL ENGINEERINGADVANCEANNUALMATERIALS RESEARCH刊NATURENature NNATURE BIEnergy EducTechnologyLAB OINTERMATERIALS REVIEWSNanoNanotechnology Biology andPROPHOTOVOLTAICSPROCEEDIEEEJOURNALBIOTECADVANCESCURRENBIOTECHNOLOGYMaterBIOMPROGRESGROWTH ANDCHARACTERIZATION OFMATERIALSACM COMPPolymCHEMISTRYAnnual Revand Biomolecular EngineeringS33 34 35 36 373839 40 41 42 43 44 45 46 4748 49 50 51 52 5354 55 56 57 58 59 60 61 62 63 641096-7176METAB ENG0883-7694MRS BULL1884-4049NPG ASIA MATER0956-5663BIOSENS BIOELECTRON0959-9428J MATER CHEM0079-6727PROG QUANT ELECTRON0749-6419INT J PLASTICITY0162-8828IEEE T PATTERN ANAL1553-877X IEEE COMMUN SURV TUT1053-5888IEEE SIGNAL PROC MAG1364-0321RENEW SUST ENERG REV0738-8551CRIT REV BIOTECHNOL0024-9297MACROMOLECULES0008-6223CARBON1613-4125MOL NUTR FOOD RES2040-3364NANOSCALE0065-2156ADV APPL MECH1744-683X SOFT MATTER1742-7061ACTA BIOMATER1754-6834BIOTECHNOL BIOFUELS0276-7783MIS QUART1388-2481ELECTROCHEM COMMUN0960-8524BIORESOURCE TECHNOL1936-4954SIAM J IMAGING SCI0920-5691INT J COMPUT VISION0378-7753J POWER SOURCES1040-8398CRIT REV FOOD SCI0278-0046IEEE T IND ELECTRON1022-1336MACROMOL RAPID COMM1089-778X IEEE T EVOLUT COMPUT1359-0286CURR OPIN SOLID ST M0927-0248SOL ENERG MAT SOL C11111111111111111111111111111111NPG ABIOSEBIOELECTRONICSSoActa BINTERNATIOF PLASTICITYBiotechnolNaIEEE CoSurveys and TutorialsIEEE SIGNAMAGAZINECAPROGRESELECTRONICSMRSJOURNALCHEMISTRYMETABOLICMIS QSIAM JouSciencesIEEE TRANPATTERN ANALYSIS ANDMACHINE INTELLIGENCEMACRORENESUSTAINABLE ENERGYCRITICALBIOTECHNOLOGYSOLAR ENEAND SOLAR CELLSJOURNASOURCESCRITICALFOOD SCIENCE ANDCURRENSOLID STATE & MATERIALSBIORTECHNOLOGYADVANCEMECHANICSELECTROCOMMUNICATIONSMOLECULAFOOD RESEARCHINTERNATIOF COMPUTER VISIONIEEE TRANEVOLUTIONARYIEEE TRANINDUSTRIAL ELECTRONICSMACROMOCOMMUNICATIONS65 6667 68 69 70 71 7273 74 75 7677 7879 8081 82 83 84 85 86 87 8889 90 91 92 93 949596 970360-3199INT J HYDROGEN ENERG1359-6454ACTA MATER0733-8716IEEE J SEL AREA COMM1475-2859MICROB CELL FACT0129-0657INT J NEURAL SYST0304-3894J HAZARD MATER1361-8415MED IMAGE ANAL1566-1199ORG ELECTRON0924-2244TRENDS FOOD SCI TECH0730-0301ACM T GRAPHIC0737-0024HUM-COMPUT INTERACT1941-1413ANNU REV FOOD SCI T0885-8993IEEE T POWER ELECTR0961-9534BIOMASS BIOENERG0306-2619APPL ENERG1944-8244ACS APPL MATER INTER0957-4484NANOTECHNOLOGY0013-4686ELECTROCHIM ACTA1613-4982MICROFLUID NANOFLUID0006-3592BIOTECHNOL BIOENG1557-1955PLASMONICS1077-260X IEEE J SEL TOP QUANT1063-6706IEEE T FUZZY SYST0376-7388J MEMBRANE SCI0925-4005SENSOR ACTUAT B-CHEM1741-2560J NEURAL ENG0308-8146FOOD CHEM0570-4928APPL SPECTROSC REV0740-0020FOOD MICROBIOL1387-2176BIOMED MICRODEVICES0360-5442ENERGY0016-2361FUEL1565-1339INT J NONLIN SCI NUM111111111122221111111122121122222MicrobialInternationalSystemsINTERNATIOF HYDROGEN ENERGYJOURNAL OMATERIALSMEDICAL IMHUMANINTERACTIONIEEE JOSELECTED TOPICS INQUANTUM ELECTRONICSIEEE TRANFUZZY SYSTEMSACM TRANGRAPHICSJOURNALSCIENCEBIOTECHBIOENGINEERINGIEEE TRANPOWER ELECTRONICSBIOMASSELECTROTRENDS IN& TECHNOLOGYIEEE JOSELECTED AREAS INORGANICACTA MSENSORS AB-CHEMICALAPPLIED SREVIEWSAnnual RScience and TechnologyNANOTEAPPLIEACS ApplInterfacesINTERNATIOF NONLINEAR SCIENCESAND NUMERICALFOOD MIBIOMMICRODEVICESFOOD CPlaJournal of NMicrofluidicsEN98 99 100101 102 103 104 105106 107108 109 110 111 112 113 114 115116 117 118 119120 121 122 123 124125 126127 1280018-9200IEEE J SOLID-ST CIRC1570-1646CURR PROTEOMICS1617-7959BIOMECH MODEL MECHAN0175-7598APPL MICROBIOL BIOT0010-2180COMBUST FLAME1431-9276MICROSC MICROANAL1468-6996SCI TECHNOL ADV MAT0268-005X FOOD HYDROCOLLOID0168-1605INT J FOOD MICROBIOL1178-2013INT J NANOMED1751-6161J MECH BEHAV BIOMED0010-938X CORROS SCI0109-5641DENT MATER1385-8947CHEM ENG J1548-7660J STAT SOFTW1615-6846FUEL CELLS1388-0764J NANOPART RES0278-3649INT J ROBOT RES1364-8152ENVIRON MODELL SOFTW0020-0255INFORM SCIENCES1436-2228MAR BIOTECHNOL0143-7208DYES PIGMENTS0163-6804IEEE COMMUN MAG1452-3981INT J ELECTROCHEM SC1392-3730J CIV ENG MANAG1057-7149IEEE T IMAGE PROCESS0266-3538COMPOS SCI TECHNOL0168-1656J BIOTECHNOL1540-7489P COMBUST INST1083-4419IEEE T SYST MAN CY B1556-603X IEEE COMPUT INTELL M2222222222222222222222222222222SCIETECHNOLOGY OFFOOD HYDIEEE JOURSTATE CIRCUITSINTERNATIOF FOOD MICROBIOLOGYAPPLIED MAND BIOTECHNOLOGYInternatioNanomedicineCHEMICALJOURNALCurrenBiomechanicMechanobiologyJournal of Cand ManagementIEEE TRANIMAGE PROCESSINGDENTALCOMPOSITETECHNOLOGYIEEE COMMAGAZINEFuJOUNANOPARTICLE RESEARCHMARINE BIOCORROSMICROMICROANALYSISJournal ofBehavior of BiomedicalCOMBUSTIJOUBIOTECHNOLOGYIEEE CIntelligence MagazineJournal of SINFORMATINTERNATIOF ROBOTICS RESEARCHENVIRMODELLING & SOFTWAREIEEE TRANSYSTEMS MAN ANDCYBERNETICS PART B-CYBERNETICSInternatioElectrochemical SciencePROCEEDCOMBUSTION INSTITUTEDYES AN129130131 132133 134 135 136137138 139 140 141142 143144 145 146 147 148 149 150 151 152 153 154155 156157158 159 1601359-6462SCRIPTA MATER0896-8446J SUPERCRIT FLUID0017-1557GOLD BULL1093-9687COMPUT-AIDED CIV INF1932-4529IEEE IND ELECTRON M1383-5866SEP PURIF TECHNOL1045-9227IEEE T NEURAL NETWOR1549-3296J BIOMED MATER RES A0378-3820FUEL PROCESS TECHNOL0969-0239CELLULOSE0741-3106IEEE ELECTR DEVICE L1532-4435J MACH LEARN RES1066-8888VLDB J0018-8646IBM J RES DEV0004-5411J ACM0018-9448IEEE T INFORM THEORY1931-7573NANOSCALE RES LETT1472-6750BMC BIOTECHNOL0883-9115J BIOACT COMPAT POL1947-2935SCI ADV MATER1758-5082BIOFABRICATION0098-5589IEEE T SOFTWARE ENG1466-8564INNOV FOOD SCI EMERG1743-0003J NEUROENG REHABIL0724-6145ADV BIOCHEM ENG BIOT0963-9969FOOD RES INT0376-0421PROG AEROSP SCI1556-4959J FIELD ROBOT0004-3702ARTIF INTELL0956-7135FOOD CONTROL0066-4200ANNU REV INFORM SCI1541-1672IEEE INTELL SYST22222222222222222222222222222222JOURNALLEARNING RESEARCHVLDBSCRIPTAIBM JORESEARCH ANDCELGOLDCOMPUTEAND INFRASTRUCTUREENGINEERINGJOURNAL OMATERIALS RESEARCHJOURNALJOURNALAND COMPATIBLEJOUSUPERCRITICAL FLUIDSIEEE TRANNEURAL NETWORKSJournal ofARTIFICIALIEEE IndusMagazineSEPARPURIFICATIONBMC BIOTFOODPROGRESSSCIENCESBiofIEEE TRANSOFTWARE ENGINEERINGFOODINTERNATIONALNanoscaleIEEE ELECLETTERSIEEE TRANINFORMATION THEORYFUEL PTECHNOLOGYANNUALINFORMATION SCIENCEAND TECHNOLOGYIEEE INSYSTEMSScience of AADVABIOCHEMICALENGINEERING /InnovativeEmerging TechnologiesJournal of Nand Rehabilitation161 162 163164 165166167168 169 170171 172173174 175 176 177178 179 180 181 182 183 184 185 186187 188189 190 1910736-6205BIOTECHNIQUES0960-1481RENEW ENERG1083-4435IEEE-ASME T MECH1524-9050IEEE T INTELL TRANSP1369-703X BIOCHEM ENG J1069-2509INTEGR COMPUT-AID E1552-3098IEEE T ROBOT0005-1098AUTOMATICA1942-0862MABS-AUSTIN1541-4337COMPR REV FOOD SCI F0196-2892IEEE T GEOSCI REMOTE1089-7801IEEE INTERNET COMPUT0885-8969IEEE T ENERGY CONVER0278-6915FOOD CHEM TOXICOL0887-0624ENERG FUEL1570-8268J WEB SEMANT1053-587X IEEE T SIGNAL PROCES0031-3203PATTERN RECOGN0272-1732IEEE MICRO1359-835X COMPOS PART A-APPL S1381-5148REACT FUNCT POLYM0733-8724J LIGHTWAVE TECHNOL8755-2930EARTHQ SPECTRA0268-3962J INF TECHNOL1536-1233IEEE T MOBILE COMPUT0360-1315COMPUT EDUC0924-2716ISPRS J PHOTOGRAMM1540-7977IEEE POWER ENERGY M0013-4651J ELECTROCHEM SOC1751-7575ENTERP INF SYST-UK0008-8846CEMENT CONCRETE RES2222222222222222222222222222222Journal ofIEEE TRANSIGNAL PROCESSINGBIOCENGINEERING JOURNALPATTERNFOOD ANTOXICOLOGYIEEE TransaAUTIEEECOMPUTINGIEEE TRANINTELLIGENTTRANSPORTATIONRENEWAIEEE-ASMEON MECHATRONICSBIOTEIEEEEARTHQUINTEGRATEAIDED ENGINEERINGIEEE TRANGEOSCIENCE AND REMOTESENSINGCOMPREHEIN FOOD SCIENCE ANDFOOD SAFETYJOURNAL OTECHNOLOGYCEMENT ARESEARCHIEEE TRANMOBILE COMPUTINGCOMPUTEREnterprisSystemsREACTIVEPOLYMERSENERGCOMPOSAPPLIED SCIENCE ANDMANUFACTURINGIEEE TRANENERGY CONVERSIONJOURNAL OTECHNOLOGYJOURNELECTROCHEMICALISPRS JPHOTOGRAMMETRY ANDREMOTE SENSINGIEEE PoMagazine192 193 194 195 196 197 198 199200 201 202 203 204 205206 207 208 209 210 211 212 213 214 215 216217 218219 2200928-4931MAT SCI ENG C-MATER0378-7206INFORM MANAGE-AMSTER1134-3060ARCH COMPUT METHOD E0090-6964ANN BIOMED ENG0733-5210J CEREAL SCI0191-2615TRANSPORT RES B-METH1748-0221J INSTRUM1568-4946APPL SOFT COMPUT0885-8950IEEE T POWER SYST0021-9614J CHEM THERMODYN0958-6946INT DAIRY J0018-9383IEEE T ELECTRON DEV0955-2219J EUR CERAM SOC1066-033X IEEE CONTR SYST MAG0824-7935COMPUT INTELL-US0921-9668PLANT FOOD HUM NUTR1943-0655IEEE PHOTONICS J1536-1268IEEE PERVAS COMPUT0957-4174EXPERT SYST APPL1322-7130AUST J GRAPE WINE R1943-0604IEEE T AUTON MENT DE8756-7938BIOTECHNOL PROGR0023-6438LWT-FOOD SCI TECHNOL1367-5435J IND MICROBIOL BIOT0309-1740MEAT SCI0260-8774J FOOD ENG0009-2509CHEM ENG SCI0924-1868USER MODEL USER-ADAP1063-6560EVOL COMPUT22222222222222222222222222222ARCCOMPUTATIONALANNALS OENGINEERINGJOURNASCIENCETRANSRESEARCH PART B-JOURNALTHERMODYNAMICSIEEE TraAutonomous MentalBIOTECPROGRESSIEEE TRANPOWER SYSTEMSLEBENWISSENSCHAFT UND-TECHNOLOGIE-FOODEXPERT SAPPLICATIONSIEEE TRANELECTRON DEVICESJOURNEUROPEAN CERAMICIEEE PhoAPPLIED SOINFORMANAGEMENTJournal ofMateriaEngineering C-Materials forJOURNAL OMICROBIOLOGY &BIOTECHNOLOGYCHEMICALSCIENCEINTERNAJOURNALPLANT FOONUTRITIONIEEE CONTMAGAZINECOMPINTELLIGENCEJOURNENGINEERINGAUSTRALIAGRAPE AND WINEMEATIEEE PCOMPUTINGUSER MOUSER-ADAPTEDEVOLCOMPUTATION221 222 223 224 225 226227 228 229 230 231 232 233 234 235 236 237 238 239 240 241 242 243 244 245 246 2471934-8630BIOINTERPHASES1536-1276IEEE T WIREL COMMUN1536-1284IEEE WIREL COMMUN1932-4553IEEE J-STSP0001-0782COMMUN ACM0038-092X SOL ENERGY1569-4410PHOTONIC NANOSTRUCT0950-3293FOOD QUAL PREFER0963-8687J STRATEGIC INF SYST0957-4530J MATER SCI-MATER M1748-6041BIOMED MATER0018-9286IEEE T AUTOMAT CONTR0254-0584MATER CHEM PHYS0925-8388J ALLOY COMPD0167-739X FUTURE GENER COMP SY0219-1377KNOWL INF SYST0304-3991ULTRAMICROSCOPY0889-1575J FOOD COMPOS ANAL1532-2882J AM SOC INF SCI TEC1077-2626IEEE T VIS COMPUT GR1552-4973J BIOMED MATER RES B0045-7825COMPUT METHOD APPL M0167-9236DECIS SUPPORT SYST0011-9164DESALINATION0166-5162INT J COAL GEOL1064-5462ARTIF LIFE0002-7820J AM CERAM SOC222222222222222222222222222JOURNALSCIENCE-MATERIALS INMEDICINEBiomedBioinIEEE TRANAUTOMATIC CONTROLFOOD QPREFERENCEIEEE WCOMMUNICATIONSIEEE JourTopics in Signal ProcessingSOLAMATERIALAND PHYSICSULTRAMIEEE TRANWIRELESSCOMMUNICACMKNOWINFORMATION SYSTEMSARTIFINTERNATIOF COAL GEOLOGYJOURNAMERICAN SOCIETY FORINFORMATION SCIENCEIEEE TRANVISUALIZATION ANDCOMPUTER GRAPHICSDESAFUTURECOMPUTER SYSTEMSJOURNALINFORMATION SYSTEMSJOURNAL OCOMPOUNDSPhotonics anFundamentals andJOURNAMERICAN CERAMICJOURNCOMPOSITION ANDDECISIOSYSTEMSJOURNAL OMATERIALS RESEARCHPART B-APPLIEDCOMPUTEAPPLIED MECHANICS ANDENGINEERING248 249250 251252 253254255 256257 258259 260261262 263 264 265 266267 268269 270 271 272 273 2740018-9197IEEE J QUANTUM ELECT1552-6283INT J SEMANT WEB INF1063-6692IEEE ACM T NETWORK0167-577X MATER LETT0360-1323BUILD ENVIRON0899-7667NEURAL COMPUT0885-3282J BIOMATER APPL0960-1317J MICROMECH MICROENG1083-4427IEEE T SYST MAN CY A0169-7439CHEMOMETR INTELL LAB0017-9310INT J HEAT MASS TRAN0890-8044IEEE NETWORK0263-8223COMPOS STRUCT0001-1541AICHE J0378-3812FLUID PHASE EQUILIBR0742-1222J MANAGE INFORM SYST1070-9932IEEE ROBOT AUTOM MAG1051-8215IEEE T CIRC SYST VID0933-2790J CRYPTOL0966-9795INTERMETALLICS0196-8904ENERG CONVERS MANAGE1551-3203IEEE T IND INFORM1432-8488J SOLID STATE ELECTR0098-1354COMPUT CHEM ENG0018-9294IEEE T BIO-MED ENG1057-7157J MICROELECTROMECH S1094-6977IEEE T SYST MAN CY C222222222222222222222222222IEEE-ACM TON NETWORKINGMATERIAJOUMICROMECHANICS ANDJOURNAL OINTERJOUBIOMATERIALSENERGY COMANAGEMENTIEEE RAUTOMATION MAGAZINECHEMOMINTELLIGENT LABORATORYSYSTEMSINTERNATIOF HEAT AND MASSFLUID PHANEURAL CInternatioSemantic Web and InformationBUILDENVIRONMENTIEEE JOQUANTUM ELECTRONICSIEEE TraIndustrial InformaticsIEEE TRANBIOMEDICAL ENGINEERINGIEEE TRANSYSTEMS MAN ANDCYBERNETICS PART A-SYSTEMS AND HUMANSAICHEIEEE NCOMPOSITCOMPUTERENGINEERINGIEEE TRANCIRCUITS AND SYSTEMSFOR VIDEO TECHNOLOGYJOURNAL OELECTROCHEMISTRYJOURNAL OINFORMATION SYSTEMSJOUMICROELECTROMECHANICAL SYSTEMSIEEE TRANSYSTEMS MAN ANDCYBERNETICS PART C-APPLICATIONS AND RE275 276 277 278 279 280281 282283284 285 286 287288 289290 291 292 293 294 295 296297 298 2990888-5885IND ENG CHEM RES1822-427X BALT J ROAD BRIDGE E0025-5408MATER RES BULL1944-0049FOOD ADDIT CONTAM A0142-0615INT J ELEC POWER1069-4730J ENG EDUC0921-5093MAT SCI ENG A-STRUCT1536-9323J ASSOC INF SYST0304-386X HYDROMETALLURGY0893-6080NEURAL NETWORKS0268-2575J CHEM TECHNOL BIOT1292-8941EUR PHYS J E0890-6955INT J MACH TOOL MANU0378-7788ENERG BUILDINGS1550-4859ACM T SENSOR NETWORK0363-907X INT J ENERG RES0920-5063J BIOMAT SCI-POLYM E0964-1726SMART MATER STRUCT0029-5981INT J NUMER METH ENG1793-6047FUNCT MATER LETT1041-1135IEEE PHOTONIC TECH L0739-5175IEEE ENG MED BIOL0167-4730STRUCT SAF0018-9480IEEE T MICROW THEORY0143-7496INT J ADHES ADHES2222222222222222222222222NEURALJOURNALTECHNOLOGY ANDBIOTECHNOLOGYINDUENGINEERING CHEMISTRYEUROPEJOURNAL EJournal of thInformation SystemsMATERIALBULLETINFOOD ADCONTAMINANTSJOURNAL OEDUCATIONINTERNATIOF MACHINE TOOLS &MANUFACTUREJOUBIOMATERIALS SCIENCE-Baltic JourBridge EngineeringINTERNATIOF ELECTRICAL POWER &ENERGY SYSTEMSINTERNATIOF ENERGY RESEARCHINTERNATIOF ADHESION ANDINTERNATIFOR NUMERICAL METHODSIN ENGINEERINGFunctionalIEEE TRANMICROWAVE THEORY ANDTECHNIQUESACM TransaNetworksHYDROMENERGY AMATERIALSENGINEERING A-STRUCTURAL MATERIALSPROPERTIES MICROSTSMART MSTRUCTURESSTRUCTUIEEE PTECHNOLOGY LETTERSIEEE ENGMEDICINE AND BIOLOGYMAGAZINE300 301 302303304305 306 307308 309310311312 313314 315 316 317 318 319 320 321 322 323 324325 326327 328 3291359-4311APPL THERM ENG1536-125X IEEE T NANOTECHNOL0018-926X IEEE T ANTENN PROPAG0167-6636MECH MATER0891-2017COMPUT LINGUIST1551-319X J DISP TECHNOL1099-0062ELECTROCHEM SOLID ST1226-086X J IND ENG CHEM1615-7591BIOPROC BIOSYST ENG0923-9820BIODEGRADATION0888-613X INT J APPROX REASON0378-3839COAST ENG1751-8741IET NANOBIOTECHNOL0257-8972SURF COAT TECH1041-4347IEEE T KNOWL DATA EN0032-5910POWDER TECHNOL1384-5810DATA MIN KNOWL DISC1559-1131ACM T WEB0958-9465CEMENT CONCRETE COMP0305-0548COMPUT OPER RES1438-7492MACROMOL MATER ENG1756-4646J FUNCT FOODS1475-9217STRUCT HEALTH MONIT0965-8564TRANSPORT RES A-POL0379-6779SYNTHETIC MET0362-028X J FOOD PROTECT0888-3270MECH SYST SIGNAL PR0959-1524J PROCESS CONTR1290-0729INT J THERM SCI0929-5607ADSORPTION222222222333333332233333333333IEEE TRANANTENNAS ANDMECHANICSCOMPLINGUISTICSJournal of DiBIODEGMACROMATERIALS ANDJournal of FBIOPROBIOSYSTEMS ENGINEERINGSTRUCTUMONITORING-ANINTERNATIONAL JOURNALCEMENTCOMPOSITESCOASTALIET NanoDATA MKNOWLEDGE DISCOVERYJOURNAL OAND ENGINEERINGIEEE TRANNANOTECHNOLOGYELECTROCSOLID STATE LETTERSAPPLIEENGINEERINGTRANSRESEARCH PART A-POLICYMECHANIAND SIGNAL PROCESSINGINTERNATIOF APPROXIMATEPOWDERSURFACETECHNOLOGYIEEE TRANKNOWLEDGE AND DATAENGINEERINGADSORPTIOTHE INTERNATIONALADSORPTION SOCIETYJOURNPROTECTIONCOMOPERATIONS RESEARCHSYNTHEACM TransaJOURNALCONTROLINTERNATIOF THERMAL SCIENCES330 331332 333 334335 336337 338 339 340 341 342 343 344 345 346 347348 349350351 352 353 354 355 356 3570160-564X ARTIF ORGANS1077-3142COMPUT VIS IMAGE UND0925-9635DIAM RELAT MATER1366-5545TRANSPORT RES E-LOG1520-9210IEEE T MULTIMEDIA0040-6090THIN SOLID FILMS0195-6574ENERG J1932-4545IEEE T BIOMED CIRC S0018-9162COMPUTER0098-3500ACM T MATH SOFTWARE0169-4332APPL SURF SCI0300-9440PROG ORG COAT0734-2071ACM T COMPUT SYST0021-9568J CHEM ENG DATA0018-9316IEEE T BROADCAST1556-6013IEEE T INF FOREN SEC0261-3069MATER DESIGN0925-3467OPT MATER0948-1907CHEM VAPOR DEPOS1545-5963IEEE ACM T COMPUT BI1876-4517FOOD SECUR1531-1309IEEE MICROW WIREL CO0924-4247SENSOR ACTUAT A-PHYS0272-4324PLASMA CHEM PLASMA P0016-0032J FRANKLIN I0255-2701CHEM ENG PROCESS0098-9886INT J CIRC THEOR APP0968-090X TRANSPORT RES C-EMER3333333333333333333333333333COMACM TRANMATHEMATICAL SOFTWAREARTIFICAPPLIESCIENCEDIAMONDMATERIALSTRANSRESEARCH PART E-LOGISTICS ANDTHIN SPROGRESCOATINGSIEEE TraInformation Forensics andCOMPUTEIMAGE UNDERSTANDINGIEEE TRANMULTIMEDIAJOURNAL OINSTITUTE-ENGINEERINGAND APPLIEDIEEE TRANBROADCASTINGCHEMICALAND PROCESSINGPLASMA CPLASMA PROCESSINGOPTICALCHEMIDEPOSITIONSENSORS AA-PHYSICALJOURNALAND ENGINEERING DATAIEEE TraBiomedical Circuits andACM TRANCOMPUTER SYSTEMSENERGINTERNATIOF CIRCUIT THEORY ANDAPPLICATIONSTRANSRESEARCH PART C-EMERGING TECHNOLOGIESMATERIAIEEE MICWIRELESS COMPONENTSLETTERSIEEE-ACMComputational Biology andBioinformaticsFood358359 360361362 363 364 365366 367368 369370 371 372373 374375376377378379380381382 383384385386387 388389390 3913920950-7051KNOWL-BASED SYST1752-1416IET RENEW POWER GEN0022-2461J MATER SCI1042-7147POLYM ADVAN TECHNOL1424-8220SENSORS-BASEL0379-5721FOOD NUTR BULL0149-1423AAPG BULL0022-3115J NUCL MATER1382-3256EMPIR SOFTW ENG1566-2535INFORM FUSION0142-9418POLYM TEST0273-2289APPL BIOCHEM BIOTECH1559-128X APPL OPTICS1748-3182BIOINSPIR BIOMIM0043-1648WEAR1080-5370GPS SOLUT1359-8368COMPOS PART B-ENG1557-1858FOOD BIOPHYS0142-727X INT J HEAT FLUID FL0097-5397SIAM J COMPUT1056-7895INT J DAMAGE MECH1350-4533MED ENG PHYS1089-7771IEEE T INF TECHNOL B0885-6125MACH LEARN1367-5788ANNU REV CONTROL1071-5819INT J HUM-COMPUT ST1573-4137CURR NANOSCI0003-021X J AM OIL CHEM SOC1598-5032MACROMOL RES0141-5492BIOTECHNOL LETT0022-2720J MICROSC-OXFORD0723-4864EXP FLUIDS0026-1394METROLOGIA0740-7459IEEE SOFTWARE1063-6536IEEE T CONTR SYST T33333333333333333333333333333333333JOURNALSCIENCECOMPOSENGINEERINGFoodFOOD ANBULLETININTERNATIOF HEAT AND FLUID FLOWWJOURNALMATERIALSEMPIRICAENGINEERINGAPPLIESEIET ReneGenerationPOLYMERSTECHNOLOGIESKNOWLESYSTEMSSIAM JOCOMPUTINGMACHINAAPGAPPLIED BAND BIOTECHNOLOGYInformPOLYMIEEE TRANINFORMATIONTECHNOLOGY INMETEXPERIMEINTERNATIOF HUMAN-COMPUTERCurrentJOURNAL OOXFORDMEDICAL EPHYSICSGPS SINTERNATIOF DAMAGE MECHANICSBioinspiratioIEEE SIEEE TRANCONTROL SYSTEMSTECHNOLOGYANNUALCONTROLBIOTECHNOJOURNAMERICAN OIL CHEMISTSMACRORESEARCH393 394395 396397398399 400 401 402 403 404405 406407408 409410 411412 413 414 415 416 4170965-0393MODEL SIMUL MATER SC1996-1944MATERIALS1438-7697EUR J LIPID SCI TECH1862-832X MACROMOL REACT ENG1842-3582DIG J NANOMATER BIOS0045-7949COMPUT STRUCT1549-8328IEEE T CIRCUITS-I0022-1147J FOOD SCI1059-9630J THERM SPRAY TECHN1530-4388IEEE T DEVICE MAT RE1063-8016J DATABASE MANAGE1570-8705AD HOC NETW0947-8396APPL PHYS A-MATER1049-331X ACM T SOFTW ENG METH0272-1716IEEE COMPUT GRAPH0007-8506CIRP ANN-MANUF TECHN1558-7916IEEE T AUDIO SPEECH1049-8923INT J ROBUST NONLIN0142-1123INT J FATIGUE0265-2048J MICROENCAPSUL0003-7028APPL SPECTROSC0021-891X J APPL ELECTROCHEM0340-1200BIOL CYBERN0885-3010IEEE T ULTRASON FERR0168-7433J AUTOM REASONING3333333333333333333333333EUROPEALIPID SCIENCE ANDMacromolEngineeringINTERNATIOF ROBUST ANDNONLINEAR CONTROLINTERNATIOF FATIGUEIEEE TRANCIRCUITS AND SYSTEMS I-FUNDAMENTAL THEORYAND APPLICATJOUMICROENCAPSULATIONCIRPMANUFACTURINGTECHNOLOGYJOURNALSPRAY TECHNOLOGYIEEE TRANDEVICE AND MATERIALSRELIABILITYACM TRANSOFTWARE ENGINEERINGAND METHODOLOGYCOMPSTRUCTURESMDigesNanomaterials andMODESIMULATION IN MATERIALSSCIENCE ANDAPPLIED SIEEE TRANULTRASONICSFERROELECTRICS ANDFREQUENCY CONTROLJOURNSCIENCEAPPLIEDMATERIALS SCIENCE &JOURNALMANAGEMENTAd HoBIOLOGICAIEEE TransSpeech and LanguageJOURNAELECTROCHEMISTRYIEEE COMPUAND APPLICATIONSJOURNAL OREASONING418 419 420 421 422 423 424425 426 427428429430 431432433 434 435 436437438 439 440 441442 443444 4451022-1344MACROMOL THEOR SIMUL0263-4368INT J REFRACT MET H0018-9545IEEE T VEH TECHNOL1876-1070J TAIWAN INST CHEM E0272-8842CERAM INT1943-068X IEEE T COMP INTEL AI0169-023X DATA KNOWL ENG0968-4328MICRON0266-8254LETT APPL MICROBIOL1076-9757J ARTIF INTELL RES1387-2532AUTON AGENT MULTI-AG1095-4244WIND ENERGY0967-0661CONTROL ENG PRACT1556-7265NANOSC MICROSC THERM0301-679X TRIBOL INT1023-8883TRIBOL LETT0306-4379INFORM SYST1073-5623METALL MATER TRANS A0924-0136J MATER PROCESS TECH1040-7782NUMER HEAT TR A-APPL1527-3342IEEE MICROW MAG0737-3937DRY TECHNOL0167-7055COMPUT GRAPH FORUM0929-5593AUTON ROBOT0262-8856IMAGE VISION COMPUT0140-7007INT J REFRIG0921-5107MATER SCI ENG B-ADV0043-7719WOOD SCI TECHNOL3333333333333333333333333333AUTONOMAND MULTI-AGENTWINDMACROTHEORY AND SIMULATIONSCONTROLPRACTICELETTERSMICROBIOLOGYIEEE TRANVEHICULAR TECHNOLOGYJournal of thof Chemical EngineersDATA & KENGINEERINGNanoscaleThermophysical EngineeringMETALLUMATERIALS TRANSACTIONSA-PHYSICAL METALLURGYAND MATERIALINTERNATIOF REFRACTORY METALS &HARD MATERIALSIEEE TraComputational Intelligence andCERAMICSINFORMATMaterialsEngineering B-AdvancedFunctional Solid-StateINTERNATIOF REFRIGERATION-REVUEINTERNATIONALE DU FROIDNUMERTRANSFER PART A-IEEE MMAGAZINEIMAGECOMPUTINGTRIBOLOJOURNALINTELLIGENCE RESEARCHTRIINTERNATIONALMWOOD STECHNOLOGYJOURNALPROCESSINGAUTONOMDRYING TCOMPUTFORUM。
刊名简称刊名全称CHEM REV=L CHEMICAL REVIEWS 化学评论 美国ACCOUNTS CHEM RES ac ACCOUNTS OF CHEMICAL RESEARCH 化学研究述评 美国PROG POLYM SCI pr PROGRESS IN POLYMER SCIENCECHEM SOC REV ch CHEMICAL SOCIETY REVIEWS 化学会评论 英国ALDRICHIM ACTA al ALDRICHIMICA ACTAANNU REV PHYS CHEM an ANNUAL REVIEW OF PHYSICAL CHEMISTRYSURF SCI REP su SURFACE SCIENCE REPORTSSURF SCI REP su SURFACE SCIENCE REPORTSANGEW CHEM INT EDIT an ANGEWANDTE CHEMIE-INTERNATIONAL EDITION 德国应用化学COORDIN CHEM REV co COORDINATION CHEMISTRY REVIEWSNAT PROD REP na NATURAL PRODUCT REPORTSNAT PROD REP na NATURAL PRODUCT REPORTSNAT PROD REP na NATURAL PRODUCT REPORTSADV CATAL ad ADVANCES IN CATALYSISJ AM CHEM SOC jo JOURNAL OF THE AMERICAN CHEMICAL SOCIETY 美国化学会志CATAL REV ca CATALYSIS REVIEWS-SCIENCE AND ENGINEERINGINT REV PHYS CHEM in INTERNATIONAL REVIEWS IN PHYSICAL CHEMISTRYJ PHOTOCH PHOTOBIO C jo JOURNAL OF PHOTOCHEMISTRY AND PHOTOBIOLOGY C-PHOTOCHEM ADV POLYM SCI ad ADVANCES IN POLYMER SCIENCEANAL CHEM an ANALYTICAL CHEMISTRYTOP CURR CHEM to TOPICS IN CURRENT CHEMISTRYTRAC-TREND ANAL CHEM tr TRAC-TRENDS IN ANALYTICAL CHEMISTRYCHEM-EUR J ch CHEMISTRY-A EUROPEAN JOURNAL 化学 德国ADV SYNTH CATAL ad ADVANCED SYNTHESIS & CATALYSISADV SYNTH CATAL ad ADVANCED SYNTHESIS & CATALYSISCHEM COMMUN ch CHEMICAL COMMUNICATIONS 化学通讯 英国ADV ORGANOMET CHEM ad ADVANCES IN ORGANOMETALLIC CHEMISTRYADV ORGANOMET CHEM ad ADVANCES IN ORGANOMETALLIC CHEMISTRY 有机金属化学进展 ORG LETT or ORGANIC LETTERSCURR OPIN COLLOID IN cu CURRENT OPINION IN COLLOID & INTERFACE SCIENCE FARADAY DISCUSS fa FARADAY DISCUSSIONSGREEN CHEM gr GREEN CHEMISTRYSTRUCT BOND st STRUCTURE AND BONDINGSTRUCT BOND st STRUCTURE AND BONDINGADV INORG CHEM ad ADVANCES IN INORGANIC CHEMISTRYCRYST GROWTH DES cr CRYSTAL GROWTH & DESIGNCRYST GROWTH DES cr CRYSTAL GROWTH & DESIGNCRYST GROWTH DES cr CRYSTAL GROWTH & DESIGNCHEM-ASIAN J ch Chemistry-An Asian JournalJ COMPUT CHEM jo JOURNAL OF COMPUTATIONAL CHEMISTRYJ PHYS CHEM B jo JOURNAL OF PHYSICAL CHEMISTRY BJ CHEM THEORY COMPUT jo Journal of Chemical Theory and ComputationADV COLLOID INTERFAC ad ADVANCES IN COLLOID AND INTERFACE SCIENCEINORG CHEM in INORGANIC CHEMISTRYLANGMUIR la LANGMUIR 兰格缪尔 美国BIOMACROMOLECULES bi BIOMACROMOLECULESBIOMACROMOLECULES bi BIOMACROMOLECULESBIOMACROMOLECULES bi BIOMACROMOLECULESJ ORG CHEM jo JOURNAL OF ORGANIC CHEMISTRY 有机化学杂志 美国ORGANOMETALLICS or ORGANOMETALLICSORGANOMETALLICS or ORGANOMETALLICSJ CHROMATOGR A jo JOURNAL OF CHROMATOGRAPHY AJ CHROMATOGR A jo JOURNAL OF CHROMATOGRAPHY AJ ANAL ATOM SPECTROM jo JOURNAL OF ANALYTICAL ATOMIC SPECTROMETRYJ ANAL ATOM SPECTROM jo JOURNAL OF ANALYTICAL ATOMIC SPECTROMETRYJ POLYM SCI POL CHEM jo JOURNAL OF POLYMER SCIENCE PART A-POLYMER CHEMISTRY CRYSTENGCOMM cr CRYSTENGCOMMCRYSTENGCOMM cr CRYSTENGCOMMCHEMPHYSCHEM ch CHEMPHYSCHEMCHEMPHYSCHEM ch CHEMPHYSCHEMANALYST an ANALYSTCURR ORG CHEM cu CURRENT ORGANIC CHEMISTRYPHYS CHEM CHEM PHYS ph PHYSICAL CHEMISTRY CHEMICAL PHYSICSPHYS CHEM CHEM PHYS ph PHYSICAL CHEMISTRY CHEMICAL PHYSICSJ BIOL INORG CHEM jo JOURNAL OF BIOLOGICAL INORGANIC CHEMISTRYJ BIOL INORG CHEM jo JOURNAL OF BIOLOGICAL INORGANIC CHEMISTRYJ PHYS CHEM C jo Journal of Physical Chemistry CJ PHYS CHEM C jo Journal of Physical Chemistry CJ PHYS CHEM C jo Journal of Physical Chemistry CJ AM SOC MASS SPECTR jo JOURNAL OF THE AMERICAN SOCIETY FOR MASS SPECTROMETRY J AM SOC MASS SPECTR jo JOURNAL OF THE AMERICAN SOCIETY FOR MASS SPECTROMETRY J AM SOC MASS SPECTR jo JOURNAL OF THE AMERICAN SOCIETY FOR MASS SPECTROMETRY J CHEM INF MODEL jo Journal of Chemical Information and ModelingJ CHEM INF MODEL jo Journal of Chemical Information and ModelingJ CHEM INF MODEL jo Journal of Chemical Information and Modeling DALTON T da DALTON TRANSACTIONSCHEM REC ch CHEMICAL RECORDORG BIOMOL CHEM or ORGANIC & BIOMOLECULAR CHEMISTRYTALANTA ta TALANTAJ COMB CHEM jo JOURNAL OF COMBINATORIAL CHEMISTRYJ COMB CHEM jo JOURNAL OF COMBINATORIAL CHEMISTRYJ COMB CHEM jo JOURNAL OF COMBINATORIAL CHEMISTRYANAL CHIM ACTA an ANALYTICA CHIMICA ACTAPOLYMER po POLYMERAPPL CATAL A-GEN ap APPLIED CATALYSIS A-GENERALAPPL CATAL A-GEN ap APPLIED CATALYSIS A-GENERALJ MASS SPECTROM jo JOURNAL OF MASS SPECTROMETRYJ MASS SPECTROM jo JOURNAL OF MASS SPECTROMETRYJ MASS SPECTROM jo JOURNAL OF MASS SPECTROMETRYJ PHYS CHEM REF DATA jo JOURNAL OF PHYSICAL AND CHEMICAL REFERENCE DATAJ PHYS CHEM REF DATA jo JOURNAL OF PHYSICAL AND CHEMICAL REFERENCE DATAJ PHYS CHEM REF DATA jo JOURNAL OF PHYSICAL AND CHEMICAL REFERENCE DATAJ PHYS CHEM A jo JOURNAL OF PHYSICAL CHEMISTRY AJ PHYS CHEM A jo JOURNAL OF PHYSICAL CHEMISTRY AANAL BIOANAL CHEM an ANALYTICAL AND BIOANALYTICAL CHEMISTRYANAL BIOANAL CHEM an ANALYTICAL AND BIOANALYTICAL CHEMISTRYMAR CHEM ma MARINE CHEMISTRYMAR CHEM ma MARINE CHEMISTRYEUR J ORG CHEM eu EUROPEAN JOURNAL OF ORGANIC CHEMISTRYTETRAHEDRON te TETRAHEDRONCURR ORG SYNTH cu CURRENT ORGANIC SYNTHESISRAPID COMMUN MASS SP ra RAPID COMMUNICATIONS IN MASS SPECTROMETRYRAPID COMMUN MASS SP ra RAPID COMMUNICATIONS IN MASS SPECTROMETRY ELECTROANAL el ELECTROANALYSISELECTROANAL el ELECTROANALYSISSYNLETT sy SYNLETTNEW J CHEM ne NEW JOURNAL OF CHEMISTRYCRIT REV ANAL CHEM cr CRITICAL REVIEWS IN ANALYTICAL CHEMISTRYCOMMENT INORG CHEM co COMMENTS ON INORGANIC CHEMISTRYMATCH-COMMUN MATH CO ma MATCH-COMMUNICATIONS IN MATHEMATICAL AND IN COMPUTER C MATCH-COMMUN MATH CO ma MATCH-COMMUNICATIONS IN MATHEMATICAL AND IN COMPUTER C MATCH-COMMUN MATH CO ma MATCH-COMMUNICATIONS IN MATHEMATICAL AND IN COMPUTER C J MOL CATAL A-CHEM jo JOURNAL OF MOLECULAR CATALYSIS A-CHEMICALEUR J INORG CHEM eu EUROPEAN JOURNAL OF INORGANIC CHEMISTRYCATAL TODAY ca CATALYSIS TODAYCATAL TODAY ca CATALYSIS TODAYCATAL TODAY ca CATALYSIS TODAYJ SEP SCI jo JOURNAL OF SEPARATION SCIENCETETRAHEDRON-ASYMMETR te TETRAHEDRON-ASYMMETRYTETRAHEDRON-ASYMMETR te TETRAHEDRON-ASYMMETRYTETRAHEDRON-ASYMMETR te TETRAHEDRON-ASYMMETRY 四面体 英国TETRAHEDRON LETT te TETRAHEDRON LETTERS 四面体通讯 英国MICROPOR MESOPOR MAT mi MICROPOROUS AND MESOPOROUS MATERIALSMICROPOR MESOPOR MAT mi MICROPOROUS AND MESOPOROUS MATERIALSMICROPOR MESOPOR MAT mi MICROPOROUS AND MESOPOROUS MATERIALSMICROPOR MESOPOR MAT mi MICROPOROUS AND MESOPOROUS MATERIALSADV PHYS ORG CHEM ad ADVANCES IN PHYSICAL ORGANIC CHEMISTRYADV PHYS ORG CHEM ad ADVANCES IN PHYSICAL ORGANIC CHEMISTRYJ ELECTROANAL CHEM jo JOURNAL OF ELECTROANALYTICAL CHEMISTRYEUR J MED CHEM eu EUROPEAN JOURNAL OF MEDICINAL CHEMISTRYTHEOR CHEM ACC th THEORETICAL CHEMISTRY ACCOUNTSPROG SOLID STATE CH pr PROGRESS IN SOLID STATE CHEMISTRYULTRASON SONOCHEM ul ULTRASONICS SONOCHEMISTRYULTRASON SONOCHEM ul ULTRASONICS SONOCHEMISTRYCATAL COMMUN ca CATALYSIS COMMUNICATIONSSYNTHESIS-STUTTGART sy SYNTHESIS-STUTTGARTJ COLLOID INTERF SCI jo JOURNAL OF COLLOID AND INTERFACE SCIENCETOP CATAL to TOPICS IN CATALYSISTOP CATAL to TOPICS IN CATALYSISCHEM PHYS LETT ch CHEMICAL PHYSICS LETTERSCHEM PHYS LETT ch CHEMICAL PHYSICS LETTERSADV CHROMATOGR ad ADVANCES IN CHROMATOGRAPHYAUST J CHEM au AUSTRALIAN JOURNAL OF CHEMISTRYCOMB CHEM HIGH T SCR co COMBINATORIAL CHEMISTRY & HIGH THROUGHPUT SCREENING COMB CHEM HIGH T SCR co COMBINATORIAL CHEMISTRY & HIGH THROUGHPUT SCREENING COMB CHEM HIGH T SCR co COMBINATORIAL CHEMISTRY & HIGH THROUGHPUT SCREENINGJ FLUORESC jo JOURNAL OF FLUORESCENCEJ FLUORESC jo JOURNAL OF FLUORESCENCEPOLYM DEGRAD STABIL po POLYMER DEGRADATION AND STABILITYEUR POLYM J eu EUROPEAN POLYMER JOURNALPURE APPL CHEM pu PURE AND APPLIED CHEMISTRY 理论化学与应用化学 美国J PHOTOCH PHOTOBIO A jo JOURNAL OF PHOTOCHEMISTRY AND PHOTOBIOLOGY A-CHEMISTRY J ORGANOMET CHEM jo JOURNAL OF ORGANOMETALLIC CHEMISTRYJ ORGANOMET CHEM jo JOURNAL OF ORGANOMETALLIC CHEMISTRYMACROMOL CHEM PHYS ma MACROMOLECULAR CHEMISTRY AND PHYSICSCARBOHYD POLYM ca CARBOHYDRATE POLYMERSCARBOHYD POLYM ca CARBOHYDRATE POLYMERSCARBOHYD POLYM ca CARBOHYDRATE POLYMERSJ SOLID STATE CHEM jo JOURNAL OF SOLID STATE CHEMISTRYJ SOLID STATE CHEM jo JOURNAL OF SOLID STATE CHEMISTRYADV HETEROCYCL CHEM ad ADVANCES IN HETEROCYCLIC CHEMISTRYMICROCHEM J mi MICROCHEMICAL JOURNALCHEM PHYS ch CHEMICAL PHYSICSCHEM PHYS ch CHEMICAL PHYSICSORG PROCESS RES DEV or ORGANIC PROCESS RESEARCH & DEVELOPMENTORG PROCESS RES DEV or ORGANIC PROCESS RESEARCH & DEVELOPMENTCATAL LETT ca CATALYSIS LETTERSINORG CHEM COMMUN in INORGANIC CHEMISTRY COMMUNICATIONSVIB SPECTROSC vi VIBRATIONAL SPECTROSCOPYVIB SPECTROSC vi VIBRATIONAL SPECTROSCOPYVIB SPECTROSC vi VIBRATIONAL SPECTROSCOPYSURF SCI su SURFACE SCIENCESURF SCI su SURFACE SCIENCEJ ANAL APPL PYROL jo JOURNAL OF ANALYTICAL AND APPLIED PYROLYSISJ ANAL APPL PYROL jo JOURNAL OF ANALYTICAL AND APPLIED PYROLYSISUSP KHIM+us USPEKHI KHIMIIPOLYHEDRON po POLYHEDRONPOLYHEDRON po POLYHEDRONCARBOHYD RES ca CARBOHYDRATE RESEARCHCARBOHYD RES ca CARBOHYDRATE RESEARCHCARBOHYD RES ca CARBOHYDRATE RESEARCHSUPRAMOL CHEM su SUPRAMOLECULAR CHEMISTRYINORG CHIM ACTA in INORGANICA CHIMICA ACTAMINI-REV ORG CHEM mi MINI-REVIEWS IN ORGANIC CHEMISTRYSOLID STATE SCI so SOLID STATE SCIENCESSOLID STATE SCI so SOLID STATE SCIENCESSOLID STATE SCI so SOLID STATE SCIENCESCOLLOID SURFACE A co COLLOIDS AND SURFACES A-PHYSICOCHEMICAL AND ENGINEERIN MICROCHIM ACTA mi MICROCHIMICA ACTAADV QUANTUM CHEM ad ADVANCES IN QUANTUM CHEMISTRYJ MOL MODEL jo JOURNAL OF MOLECULAR MODELINGJ MOL MODEL jo JOURNAL OF MOLECULAR MODELINGJ MOL MODEL jo JOURNAL OF MOLECULAR MODELINGJ MOL MODEL jo JOURNAL OF MOLECULAR MODELINGPOLYM INT po POLYMER INTERNATIONALCURR ANAL CHEM cu Current Analytical ChemistryANAL SCI an ANALYTICAL SCIENCESCHEM LETT ch CHEMISTRY LETTERSSEP PURIF REV se SEPARATION AND PURIFICATION REVIEWSSEP PURIF REV se SEPARATION AND PURIFICATION REVIEWSSEP PURIF REV se SEPARATION AND PURIFICATION REVIEWSTHERMOCHIM ACTA th THERMOCHIMICA ACTATHERMOCHIM ACTA th THERMOCHIMICA ACTAJ FLUORINE CHEM jo JOURNAL OF FLUORINE CHEMISTRYJ FLUORINE CHEM jo JOURNAL OF FLUORINE CHEMISTRYCOLLOID POLYM SCI co COLLOID AND POLYMER SCIENCECOLLOID POLYM SCI co COLLOID AND POLYMER SCIENCEJ PHYS ORG CHEM jo JOURNAL OF PHYSICAL ORGANIC CHEMISTRYJ PHYS ORG CHEM jo JOURNAL OF PHYSICAL ORGANIC CHEMISTRYB CHEM SOC JPN bu BULLETIN OF THE CHEMICAL SOCIETY OF JAPAN 日本化学会通J MOL STRUCT jo JOURNAL OF MOLECULAR STRUCTUREJ THERM ANAL CALORIM jo JOURNAL OF THERMAL ANALYSIS AND CALORIMETRYJ THERM ANAL CALORIM jo JOURNAL OF THERMAL ANALYSIS AND CALORIMETRYMAGN RESON CHEM ma MAGNETIC RESONANCE IN CHEMISTRYMAGN RESON CHEM ma MAGNETIC RESONANCE IN CHEMISTRYMAGN RESON CHEM ma MAGNETIC RESONANCE IN CHEMISTRYHELV CHIM ACTA he HELVETICA CHIMICA ACTACALPHAD ca CALPHAD-COMPUTER COUPLING OF PHASE DIAGRAMS AND THERMO CALPHAD ca CALPHAD-COMPUTER COUPLING OF PHASE DIAGRAMS AND THERMO J INORG ORGANOMET P jo JOURNAL OF INORGANIC AND ORGANOMETALLIC POLYMERSJ IRAN CHEM SOC jo Journal of the Iranian Chemical SocietyJ CHEMOMETR jo JOURNAL OF CHEMOMETRICSJ CHEMOMETR jo JOURNAL OF CHEMOMETRICSJ CHEMOMETR jo JOURNAL OF CHEMOMETRICSJ CHEMOMETR jo JOURNAL OF CHEMOMETRICSJ CHEMOMETR jo JOURNAL OF CHEMOMETRICSJ CHEMOMETR jo JOURNAL OF CHEMOMETRICSPOLYM J po POLYMER JOURNALCR CHIM co COMPTES RENDUS CHIMIEJ BRAZIL CHEM SOC jo JOURNAL OF THE BRAZILIAN CHEMICAL SOCIETYINT J QUANTUM CHEM in INTERNATIONAL JOURNAL OF QUANTUM CHEMISTRYINT J QUANTUM CHEM in INTERNATIONAL JOURNAL OF QUANTUM CHEMISTRYINT J QUANTUM CHEM in INTERNATIONAL JOURNAL OF QUANTUM CHEMISTRYSTRUCT CHEM st STRUCTURAL CHEMISTRYSTRUCT CHEM st STRUCTURAL CHEMISTRYSTRUCT CHEM st STRUCTURAL CHEMISTRYSURF INTERFACE ANAL su SURFACE AND INTERFACE ANALYSISAPPL ORGANOMET CHEM ap APPLIED ORGANOMETALLIC CHEMISTRYAPPL ORGANOMET CHEM ap APPLIED ORGANOMETALLIC CHEMISTRYSOLVENT EXTR ION EXC so SOLVENT EXTRACTION AND ION EXCHANGEANAL LETT an ANALYTICAL LETTERSCHROMATOGRAPHIA ch CHROMATOGRAPHIACHROMATOGRAPHIA ch CHROMATOGRAPHIAZ ANORG ALLG CHEM ze ZEITSCHRIFT FUR ANORGANISCHE UND ALLGEMEINE CHEMIE CAN J CHEM ca CANADIAN JOURNAL OF CHEMISTRY-REVUE CANADIENNE DE CHIM CATAL SURV ASIA ca CATALYSIS SURVEYS FROM ASIAMOL SIMULAT mo MOLECULAR SIMULATIONMOL SIMULAT mo MOLECULAR SIMULATIONJ INCL PHENOM MACRO jo JOURNAL OF INCLUSION PHENOMENA AND MACROCYCLIC CHEMIST J INCL PHENOM MACRO jo JOURNAL OF INCLUSION PHENOMENA AND MACROCYCLIC CHEMIST J APPL POLYM SCI jo JOURNAL OF APPLIED POLYMER SCIENCEINT J CHEM KINET in INTERNATIONAL JOURNAL OF CHEMICAL KINETICSJ MATH CHEM jo JOURNAL OF MATHEMATICAL CHEMISTRYJ MATH CHEM jo JOURNAL OF MATHEMATICAL CHEMISTRYARKIVOC ar ARKIVOCJ SOLUTION CHEM jo JOURNAL OF SOLUTION CHEMISTRYB KOR CHEM SOC bu BULLETIN OF THE KOREAN CHEMICAL SOCIETYRADIOCHIM ACTA ra RADIOCHIMICA ACTARADIOCHIM ACTA ra RADIOCHIMICA ACTAJ PORPHYR PHTHALOCYA jo JOURNAL OF PORPHYRINS AND PHTHALOCYANINESMONATSH CHEM mo MONATSHEFTE FUR CHEMIEJ MOL STRUC-THEOCHEM jo JOURNAL OF MOLECULAR STRUCTURE-THEOCHEMJ MOL LIQ jo JOURNAL OF MOLECULAR LIQUIDSJ MOL LIQ jo JOURNAL OF MOLECULAR LIQUIDSJ COAT TECHNOL RES jo Journal of Coatings Technology and ResearchJ PHOTOPOLYM SCI TEC jo JOURNAL OF PHOTOPOLYMER SCIENCE AND TECHNOLOGY HETEROCYCLES he HETEROCYCLESPOLYM BULL po POLYMER BULLETINMOLECULES mo MOLECULESBIOINORG CHEM APPL bi BIOINORGANIC CHEMISTRY AND APPLICATIONSBIOINORG CHEM APPL bi BIOINORGANIC CHEMISTRY AND APPLICATIONSBIOINORG CHEM APPL bi BIOINORGANIC CHEMISTRY AND APPLICATIONS HETEROATOM CHEM he HETEROATOM CHEMISTRYSYNTHETIC COMMUN sy SYNTHETIC COMMUNICATIONSLETT ORG CHEM le LETTERS IN ORGANIC CHEMISTRYJ CHEM SCI jo JOURNAL OF CHEMICAL SCIENCESJ CHROMATOGR SCI jo JOURNAL OF CHROMATOGRAPHIC SCIENCEJ CHROMATOGR SCI jo JOURNAL OF CHROMATOGRAPHIC SCIENCEJ CLUST SCI jo JOURNAL OF CLUSTER SCIENCEJ LIQ CHROMATOGR R T jo JOURNAL OF LIQUID CHROMATOGRAPHY & RELATED TECHNOLOGIE J LIQ CHROMATOGR R T jo JOURNAL OF LIQUID CHROMATOGRAPHY & RELATED TECHNOLOGIECHIMIA ch CHIMIAJPC-J PLANAR CHROMAT jp JPC-JOURNAL OF PLANAR CHROMATOGRAPHY-MODERN TLC TRANSIT METAL CHEM tr TRANSITION METAL CHEMISTRYACTA CHIM SLOV ac ACTA CHIMICA SLOVENICARADIAT PHYS CHEM ra RADIATION PHYSICS AND CHEMISTRYRADIAT PHYS CHEM ra RADIATION PHYSICS AND CHEMISTRYRADIAT PHYS CHEM ra RADIATION PHYSICS AND CHEMISTRYJ CARBOHYD CHEM jo JOURNAL OF CARBOHYDRATE CHEMISTRYJ CARBOHYD CHEM jo JOURNAL OF CARBOHYDRATE CHEMISTRYJ COORD CHEM jo JOURNAL OF COORDINATION CHEMISTRYJ THEOR COMPUT CHEM jo JOURNAL OF THEORETICAL & COMPUTATIONAL CHEMISTRY COLLECT CZECH CHEM C co COLLECTION OF CZECHOSLOVAK CHEMICAL COMMUNICATIONS QUIM NOVA qu QUIMICA NOVAE-POLYMERS e-E-POLYMERSJ POLYM RES jo JOURNAL OF POLYMER RESEARCHJ HETEROCYCLIC CHEM jo JOURNAL OF HETEROCYCLIC CHEMISTRYJ DISPER SCI TECHNOL jo JOURNAL OF DISPERSION SCIENCE AND TECHNOLOGYACTA CHROMATOGR ac ACTA CHROMATOGRAPHICAZ NATURFORSCH B ze ZEITSCHRIFT FUR NATURFORSCHUNG SECTION B-A JOURNAL OF Z NATURFORSCH B ze ZEITSCHRIFT FUR NATURFORSCHUNG SECTION B-A JOURNAL OF INT J MOL SCI in INTERNATIONAL JOURNAL OF MOLECULAR SCIENCESCHINESE J CHEM ch CHINESE JOURNAL OF CHEMISTRYACTA CHIM SINICA ac ACTA CHIMICA SINICAJ MACROMOL SCI A jo JOURNAL OF MACROMOLECULAR SCIENCE-PURE AND APPLIED CHE NAT PROD RES na NATURAL PRODUCT RESEARCHNAT PROD RES na NATURAL PRODUCT RESEARCHHIGH PERFORM POLYM hi HIGH PERFORMANCE POLYMERSPHYS CHEM LIQ ph PHYSICS AND CHEMISTRY OF LIQUIDSPHYS CHEM LIQ ph PHYSICS AND CHEMISTRY OF LIQUIDSORG PREP PROCED INT or ORGANIC PREPARATIONS AND PROCEDURES INTERNATIONAL CROAT CHEM ACTA cr CROATICA CHEMICA ACTACHINESE J ORG CHEM ch CHINESE JOURNAL OF ORGANIC CHEMISTRYPOLYCYCL AROMAT COMP po POLYCYCLIC AROMATIC COMPOUNDSDES MONOMERS POLYM de DESIGNED MONOMERS AND POLYMERSCHINESE J STRUC CHEM ch CHINESE JOURNAL OF STRUCTURAL CHEMISTRYCHINESE J STRUC CHEM ch CHINESE JOURNAL OF STRUCTURAL CHEMISTRYJ ADV OXID TECHNOL jo JOURNAL OF ADVANCED OXIDATION TECHNOLOGIESCENT EUR J CHEM ce CENTRAL EUROPEAN JOURNAL OF CHEMISTRYMENDELEEV COMMUN me MENDELEEV COMMUNICATIONSJ SYN ORG CHEM JPN jo JOURNAL OF SYNTHETIC ORGANIC CHEMISTRY JAPANIRAN POLYM J ir IRANIAN POLYMER JOURNALTURK J CHEM tu TURKISH JOURNAL OF CHEMISTRYTURK J CHEM tu TURKISH JOURNAL OF CHEMISTRYISR J CHEM is ISRAEL JOURNAL OF CHEMISTRYCHEM J CHINESE U ch CHEMICAL JOURNAL OF CHINESE UNIVERSITIES-CHINESEJ CHIN CHEM SOC-TAIP jo JOURNAL OF THE CHINESE CHEMICAL SOCIETYBEILSTEIN J ORG CHEM be Beilstein Journal of Organic ChemistryCHINESE J POLYM SCI ch CHINESE JOURNAL OF POLYMER SCIENCECOLLOID J+co COLLOID JOURNALINDIAN J CHEM A in INDIAN JOURNAL OF CHEMISTRY SECTION A-INORGANIC BIO-IN PHOSPHORUS SULFUR ph PHOSPHORUS SULFUR AND SILICON AND THE RELATED ELEMENTS KINET CATAL+ki KINETICS AND CATALYSISREV ANAL CHEM re REVIEWS IN ANALYTICAL CHEMISTRYACTA PHYS-CHIM SIN ac ACTA PHYSICO-CHIMICA SINICAJ CHEM CRYSTALLOGR jo JOURNAL OF CHEMICAL CRYSTALLOGRAPHYJ CHEM CRYSTALLOGR jo JOURNAL OF CHEMICAL CRYSTALLOGRAPHYANN CHIM-ROME an ANNALI DI CHIMICAANN CHIM-ROME an ANNALI DI CHIMICAINORG REACT MECH in INORGANIC REACTION MECHANISMSINT J POLYM ANAL CH in INTERNATIONAL JOURNAL OF POLYMER ANALYSIS AND CHARACTE SCI CHINA SER B sc SCIENCE IN CHINA SERIES B-CHEMISTRYRES CHEM INTERMEDIAT re RESEARCH ON CHEMICAL INTERMEDIATESJ ANAL CHEM+jo JOURNAL OF ANALYTICAL CHEMISTRYREACT KINET CATAL L re REACTION KINETICS AND CATALYSIS LETTERSCHEM LISTY ch CHEMICKE LISTYCHINESE J INORG CHEM ch CHINESE JOURNAL OF INORGANIC CHEMISTRYJ RADIOANAL NUCL CH jo JOURNAL OF RADIOANALYTICAL AND NUCLEAR CHEMISTRYJ RADIOANAL NUCL CH jo JOURNAL OF RADIOANALYTICAL AND NUCLEAR CHEMISTRYJ RADIOANAL NUCL CH jo JOURNAL OF RADIOANALYTICAL AND NUCLEAR CHEMISTRY CHEM ANAL-WARSAW ch CHEMIA ANALITYCZNAJ CHIL CHEM SOC jo JOURNAL OF THE CHILEAN CHEMICAL SOCIETYPOLYM SCI SER A+po POLYMER SCIENCE SERIES AACTA POLYM SIN ac ACTA POLYMERICA SINICAJ SERB CHEM SOC jo JOURNAL OF THE SERBIAN CHEMICAL SOCIETYIONICS io IONICSIONICS io IONICSIONICS io IONICSRUSS J ORG CHEM+ru RUSSIAN JOURNAL OF ORGANIC CHEMISTRYPROG CHEM pr PROGRESS IN CHEMISTRYHIGH ENERG CHEM+hi HIGH ENERGY CHEMISTRYJ CHEM EDUC jo JOURNAL OF CHEMICAL EDUCATIONJ CHEM EDUC jo JOURNAL OF CHEMICAL EDUCATIONRUSS CHEM B+ru RUSSIAN CHEMICAL BULLETINCHINESE J ANAL CHEM ch CHINESE JOURNAL OF ANALYTICAL CHEMISTRYPOL J CHEM po POLISH JOURNAL OF CHEMISTRYRUSS J COORD CHEM+ru RUSSIAN JOURNAL OF COORDINATION CHEMISTRYCHEM PAP ch CHEMICAL PAPERS-CHEMICKE ZVESTICAN J ANAL SCI SPECT ca CANADIAN JOURNAL OF ANALYTICAL SCIENCES AND SPECTROSCO CAN J ANAL SCI SPECT ca CANADIAN JOURNAL OF ANALYTICAL SCIENCES AND SPECTROSCO STUD CONSERV st STUDIES IN CONSERVATIONSTUD CONSERV st STUDIES IN CONSERVATIONSTUD CONSERV st STUDIES IN CONSERVATIONHETEROCYCL COMMUN he HETEROCYCLIC COMMUNICATIONSREV INORG CHEM re REVIEWS IN INORGANIC CHEMISTRYJ STRUCT CHEM+jo JOURNAL OF STRUCTURAL CHEMISTRYJ STRUCT CHEM+jo JOURNAL OF STRUCTURAL CHEMISTRYLC GC EUR lc LC GC EUROPEPOLYM-KOREA po POLYMER-KOREADOKL PHYS CHEM do DOKLADY PHYSICAL CHEMISTRYCHEM WORLD-UK ch CHEMISTRY WORLDINDIAN J CHEM B in INDIAN JOURNAL OF CHEMISTRY SECTION B-ORGANIC CHEMISTR RUSS J GEN CHEM+ru RUSSIAN JOURNAL OF GENERAL CHEMISTRYCHEM NAT COMPD+ch CHEMISTRY OF NATURAL COMPOUNDSJ RARE EARTH jo JOURNAL OF RARE EARTHSS AFR J CHEM-S-AFR T so SOUTH AFRICAN JOURNAL OF CHEMISTRY-SUID-AFRIKAANSE TYD CHIM OGGI ch CHIMICA OGGI-CHEMISTRY TODAYCHIM OGGI ch CHIMICA OGGI-CHEMISTRY TODAYRUSS J PHYS CHEM A+ru RUSSIAN JOURNAL OF PHYSICAL Chemistry AJ MED PLANTS RES jo Journal of Medicinal Plants ResearchRUSS J INORG CHEM+ru RUSSIAN JOURNAL OF INORGANIC CHEMISTRYCHEM RES CHINESE U ch CHEMICAL RESEARCH IN CHINESE UNIVERSITIESCHINESE CHEM LETT ch CHINESE CHEMICAL LETTERSDOKL CHEM do DOKLADY CHEMISTRYMAIN GROUP MET CHEM ma MAIN GROUP METAL CHEMISTRYMAIN GROUP MET CHEM ma MAIN GROUP METAL CHEMISTRYCHEM UNSERER ZEIT ch CHEMIE IN UNSERER ZEITPROG REACT KINET MEC pr PROGRESS IN REACTION KINETICS AND MECHANISMJ INDIAN CHEM SOC jo JOURNAL OF THE INDIAN CHEMICAL SOCIETYLC GC N AM lc LC GC NORTH AMERICABUNSEKI KAGAKU bu BUNSEKI KAGAKUREV CHIM-BUCHAREST re REVISTA DE CHIMIEREV CHIM-BUCHAREST re REVISTA DE CHIMIEPOLYM SCI SER B+po POLYMER SCIENCE SERIES BINDIAN J HETEROCY CH in INDIAN JOURNAL OF HETEROCYCLIC CHEMISTRYCHEM IND-LONDON ch CHEMISTRY & INDUSTRYOXID COMMUN ox OXIDATION COMMUNICATIONSREV ROUM CHIM re REVUE ROUMAINE DE CHIMIERUSS J APPL CHEM+ru RUSSIAN JOURNAL OF APPLIED CHEMISTRYASIAN J CHEM as ASIAN JOURNAL OF CHEMISTRYB CHEM SOC ETHIOPIA bu BULLETIN OF THE CHEMICAL SOCIETY OF ETHIOPIA AFINIDAD af AFINIDADJ AUTOM METHOD MANAG jo JOURNAL OF AUTOMATED METHODS & MANAGEMENT IN CHEMISTRY J AUTOM METHOD MANAG jo JOURNAL OF AUTOMATED METHODS & MANAGEMENT IN CHEMISTRY KOBUNSHI RONBUNSHU ko KOBUNSHI RONBUNSHUJ CHEM SOC PAKISTAN jo JOURNAL OF THE CHEMICAL SOCIETY OF PAKISTANJ CHEM RES-S jo JOURNAL OF CHEMICAL RESEARCH-SACTUAL CHIMIQUE ac ACTUALITE CHIMIQUERUSS J PHYS CHEM B+ru Russian Journal of Physical Chemistry BCHEM PHYS CARBON ch CHEMISTRY AND PHYSICS OF CARBONCHEM PHYS CARBON ch CHEMISTRY AND PHYSICS OF CARBONCHEM PHYS CARBON ch CHEMISTRY AND PHYSICS OF CARBONJ APPL CRYSTALLOGR jo JOURNAL OF APPLIED CRYSTALLOGRAPHYACTA CRYSTALLOGR B ac ACTA CRYSTALLOGRAPHICA SECTION B-STRUCTURAL SCIENCE ACTA CRYSTALLOGR A ac ACTA CRYSTALLOGRAPHICA SECTION AJ CRYST GROWTH jo JOURNAL OF CRYSTAL GROWTHLIQ CRYST li LIQUID CRYSTALSCRYST RES TECHNOL cr CRYSTAL RESEARCH AND TECHNOLOGYACTA CRYSTALLOGR C ac ACTA CRYSTALLOGRAPHICA SECTION C-CRYSTAL STRUCTURE COM MOL CRYST LIQ CRYST mo MOLECULAR CRYSTALS AND LIQUID CRYSTALSACTA CRYSTALLOGR E ac ACTA CRYSTALLOGRAPHICA SECTION E-STRUCTURE REPORTS ONL CRYSTALLOGR REP+cr CRYSTALLOGRAPHY REPORTSZ KRIST-NEW CRYST ST ze ZEITSCHRIFT FUR KRISTALLOGRAPHIE-NEW CRYSTAL STRUCTURE小类名称(英文)小类分区大类名称大类分区2008年影响因ISSN小类名称(中文0009-2665化学综合CHEMISTRY, MULTIDISCIPLINA1化学123.592 0001-4842化学综合CHEMISTRY, MULTIDISCIPLINA1化学112.176 0079-6700高分子科学POLYMER SCIENCE1化学116.819 0306-0012化学综合CHEMISTRY, MULTIDISCIPLINA1化学117.419 0002-5100有机化学CHEMISTRY, ORGANIC1化学116.733 0066-426X物理化学CHEMISTRY, PHYSICAL1化学114.688 0167-5729物理化学CHEMISTRY, PHYSICAL1化学112.808 0167-5729物理:凝聚态物PHYSICS, CONDENSED MATTER1化学112.808 1433-7851化学综合CHEMISTRY, MULTIDISCIPLINA1化学110.879CHEMISTRY, INORGANIC & NUC1化学110.566 0010-8545无机化学与核化0265-0568医药化学CHEMISTRY, MEDICINAL1化学17.45 0265-0568有机化学CHEMISTRY, ORGANIC1化学17.45 0265-0568生化与分子生物BIOCHEMISTRY & MOLECULAR B2化学17.45 0360-0564物理化学CHEMISTRY, PHYSICAL1化学1 4.812 0002-7863化学综合CHEMISTRY, MULTIDISCIPLINA2化学18.091 0161-4940物理化学CHEMISTRY, PHYSICAL1化学1 5.625 0144-235X物理化学CHEMISTRY, PHYSICAL2化学1 6.892 1389-5567物理化学CHEMISTRY, PHYSICAL2化学1 5.36 0065-3195高分子科学POLYMER SCIENCE1化学2 6.802 0003-2700分析化学CHEMISTRY, ANALYTICAL1化学2 5.712 0340-1022化学综合CHEMISTRY, MULTIDISCIPLINA2化学2 5.27 0165-9936分析化学CHEMISTRY, ANALYTICAL1化学2 5.485 0947-6539化学综合CHEMISTRY, MULTIDISCIPLINA2化学2 5.454 1615-4150应用化学CHEMISTRY, APPLIED1化学2 5.619 1615-4150有机化学CHEMISTRY, ORGANIC1化学2 5.619 1359-7345化学综合CHEMISTRY, MULTIDISCIPLINA2化学2 5.34 0065-3055无机化学与核化CHEMISTRY, INORGANIC & NUC1化学2 3.571 0065-3055有机化学CHEMISTRY, ORGANIC2化学2 3.571 1523-7060有机化学CHEMISTRY, ORGANIC2化学2 5.128 1359-0294物理化学CHEMISTRY, PHYSICAL2化学2 5.493 1364-5498物理化学CHEMISTRY, PHYSICAL2化学2 4.604 1463-9262化学综合CHEMISTRY, MULTIDISCIPLINA2化学2 4.542 0081-5993物理化学CHEMISTRY, PHYSICAL2化学2 6.511 0081-5993无机化学与核化CHEMISTRY, INORGANIC & NUC2化学2 6.511CHEMISTRY, INORGANIC & NUC2化学2 4.214 0898-8838无机化学与核化1528-7483晶体学CRYSTALLOGRAPHY1化学2 4.215MATERIALS SCIENCE, MULTIDI2化学2 4.215 1528-7483材料科学:综合1528-7483化学综合CHEMISTRY, MULTIDISCIPLINA2化学2 4.215 1861-4728化学综合CHEMISTRY, MULTIDISCIPLINA2化学2 4.197 0192-8651化学综合CHEMISTRY, MULTIDISCIPLINA3化学2 3.39 1520-6106物理化学CHEMISTRY, PHYSICAL2化学2 4.189 1549-9618化学综合CHEMISTRY, MULTIDISCIPLINA3化学2 4.274 0001-8686物理化学CHEMISTRY, PHYSICAL3化学2 5.333CHEMISTRY, INORGANIC & NUC2化学2 4.147 0020-1669无机化学与核化0743-7463物理化学CHEMISTRY, PHYSICAL3化学2 4.097 1525-7797高分子科学POLYMER SCIENCE2化学2 4.1461525-7797有机化学CHEMISTRY, ORGANIC2化学2 4.146BIOCHEMISTRY & MOLECULAR B3化学2 4.146 1525-7797生化与分子生物0022-3263有机化学CHEMISTRY, ORGANIC2化学2 3.952CHEMISTRY, INORGANIC & NUC2化学2 3.815 0276-7333无机化学与核化0276-7333有机化学CHEMISTRY, ORGANIC2化学2 3.815 0021-9673分析化学CHEMISTRY, ANALYTICAL2化学2 3.756 0021-9673生化研究方法BIOCHEMICAL RESEARCH METHO2化学2 3.756 0267-9477分析化学CHEMISTRY, ANALYTICAL2化学2 4.028 0267-9477光谱学SPECTROSCOPY2化学2 4.028 0887-624X高分子科学POLYMER SCIENCE2化学2 3.821 1466-8033晶体学CRYSTALLOGRAPHY2化学2 3.535 1466-8033化学综合CHEMISTRY, MULTIDISCIPLINA3化学2 3.535PHYSICS, ATOMIC, MOLECULAR1化学2 3.636 1439-4235物理:原子、分1439-4235物理化学CHEMISTRY, PHYSICAL3化学2 3.636 0003-2654分析化学CHEMISTRY, ANALYTICAL2化学2 3.761 1385-2728有机化学CHEMISTRY, ORGANIC2化学2 3.184PHYSICS, ATOMIC, MOLECULAR2化学2 4.064 1463-9076物理:原子、分1463-9076物理化学CHEMISTRY, PHYSICAL3化学2 4.064CHEMISTRY, INORGANIC & NUC2化学2 3.6 0949-8257无机化学与核化BIOCHEMISTRY & MOLECULAR B3化学2 3.6 0949-8257生化与分子生物MATERIALS SCIENCE, MULTIDI2化学2 3.396 1932-7447材料科学:综合1932-7447物理化学CHEMISTRY, PHYSICAL3化学2 3.396 1932-7447纳米科技NANOSCIENCE & NANOTECHNOLO3化学2 3.396 1044-0305分析化学CHEMISTRY, ANALYTICAL2化学2 3.181 1044-0305光谱学SPECTROSCOPY2化学2 3.181 1044-0305物理化学CHEMISTRY, PHYSICAL3化学2 3.181 1549-9596计算机:信息系COMPUTER SCIENCE, INFORMAT1化学2 3.643COMPUTER SCIENCE, INTERDIS2化学2 3.643 1549-9596计算机:跨学科1549-9596化学综合CHEMISTRY, MULTIDISCIPLINA3化学2 3.643CHEMISTRY, INORGANIC & NUC2化学2 3.58 1477-9226无机化学与核化1527-8999化学综合CHEMISTRY, MULTIDISCIPLINA3化学3 3.477 1477-0520有机化学CHEMISTRY, ORGANIC2化学3 3.55 0039-9140分析化学CHEMISTRY, ANALYTICAL2化学3 3.206 1520-4766应用化学CHEMISTRY, APPLIED1化学3 3.011 1520-4766化学综合CHEMISTRY, MULTIDISCIPLINA3化学3 3.011 1520-4766医药化学CHEMISTRY, MEDICINAL3化学3 3.011 0003-2670分析化学CHEMISTRY, ANALYTICAL2化学3 3.146 0032-3861高分子科学POLYMER SCIENCE2化学3 3.331 0926-860X环境科学ENVIRONMENTAL SCIENCES2化学3 3.19 0926-860X物理化学CHEMISTRY, PHYSICAL3化学3 3.19 1076-5174光谱学SPECTROSCOPY2化学3 2.94 1076-5174生物物理BIOPHYSICS3化学3 2.94 1076-5174有机化学CHEMISTRY, ORGANIC3化学3 2.94 0047-2689物理化学CHEMISTRY, PHYSICAL3化学3 2.424 0047-2689化学综合CHEMISTRY, MULTIDISCIPLINA3化学3 2.424 0047-2689物理:综合PHYSICS, MULTIDISCIPLINARY3化学3 2.424PHYSICS, ATOMIC, MOLECULAR2化学3 2.871 1089-5639物理:原子、分1089-5639物理化学CHEMISTRY, PHYSICAL3化学3 2.871 1618-2642分析化学CHEMISTRY, ANALYTICAL2化学3 3.328 1618-2642生化研究方法BIOCHEMICAL RESEARCH METHO3化学3 3.328 0304-4203海洋学OCEANOGRAPHY2化学3 2.977 0304-4203化学综合CHEMISTRY, MULTIDISCIPLINA3化学3 2.977 1434-193X有机化学CHEMISTRY, ORGANIC3化学3 3.016 0040-4020有机化学CHEMISTRY, ORGANIC3化学3 2.897 1570-1794有机化学CHEMISTRY, ORGANIC3化学3 2.61 0951-4198分析化学CHEMISTRY, ANALYTICAL2化学3 2.772 0951-4198光谱学SPECTROSCOPY3化学3 2.772 1040-0397分析化学CHEMISTRY, ANALYTICAL2化学3 2.901 1040-0397电化学ELECTROCHEMISTRY3化学3 2.901 0936-5214有机化学CHEMISTRY, ORGANIC3化学3 2.659 1144-0546化学综合CHEMISTRY, MULTIDISCIPLINA3化学3 2.942 1040-8347分析化学CHEMISTRY, ANALYTICAL3化学3 3.5CHEMISTRY, INORGANIC & NUC3化学32 0260-3594无机化学与核化MATHEMATICS, INTERDISCIPLI1化学3 3.5 0340-6253数学跨学科应用COMPUTER SCIENCE, INTERDIS2化学3 3.5 0340-6253计算机:跨学科0340-6253化学综合CHEMISTRY, MULTIDISCIPLINA3化学3 3.5 1381-1169物理化学CHEMISTRY, PHYSICAL3化学3 2.814CHEMISTRY, INORGANIC & NUC3化学3 2.694 1434-1948无机化学与核化0920-5861工程:化工ENGINEERING, CHEMICAL1化学3 3.004 0920-5861应用化学CHEMISTRY, APPLIED2化学3 3.004 0920-5861物理化学CHEMISTRY, PHYSICAL3化学3 3.004 1615-9306分析化学CHEMISTRY, ANALYTICAL3化学3 2.746 0957-4166物理化学CHEMISTRY, PHYSICAL3化学3 2.796 0957-4166无机化学与核化CHEMISTRY, INORGANIC & NUC3化学3 2.796 0957-4166有机化学CHEMISTRY, ORGANIC3化学3 2.796 0040-4039有机化学CHEMISTRY, ORGANIC3化学3 2.538MATERIALS SCIENCE, MULTIDI2化学3 2.555 1387-1811材料科学:综合1387-1811应用化学CHEMISTRY, APPLIED2化学3 2.555 1387-1811物理化学CHEMISTRY, PHYSICAL3化学3 2.555 1387-1811纳米科技NANOSCIENCE & NANOTECHNOLO3化学3 2.555 0065-3160物理化学CHEMISTRY, PHYSICAL3化学3 1.833 0065-3160有机化学CHEMISTRY, ORGANIC3化学3 1.833 1572-6657分析化学CHEMISTRY, ANALYTICAL3化学3 2.484 0223-5234医药化学CHEMISTRY, MEDICINAL3化学3 2.882 1432-881X物理化学CHEMISTRY, PHYSICAL3化学3 2.37 0079-6786无机化学与核化CHEMISTRY, INORGANIC & NUC3化学3 2.938 1350-4177声学ACOUSTICS2化学3 2.796 1350-4177化学综合CHEMISTRY, MULTIDISCIPLINA3化学3 2.796 1566-7367物理化学CHEMISTRY, PHYSICAL3化学3 2.791 0039-7881有机化学CHEMISTRY, ORGANIC3化学3 2.47 0021-9797物理化学CHEMISTRY, PHYSICAL3化学3 2.443 1022-5528应用化学CHEMISTRY, APPLIED2化学3 2.212 1022-5528物理化学CHEMISTRY, PHYSICAL3化学3 2.212 0009-2614物理化学CHEMISTRY, PHYSICAL3化学3 2.169。
Journal of Integrative Neuroscience
Topical Review
Models of Dendritic and Astrocytic Networks for fMRI
Roman R. Poznanski and Jorge J. Riera CRIAMS, Claremont Graduate University, Claremont, CA 91711-3988 USA and NICHe, Tohoku University, Aobaku Sendai 980-8579 Japan
Received: 19/12/2005
Abstract In order to elucidate the relationships between hierarchical structures within the neocortical neuropil and the information carried by an ensemble of neurons encompassing a single voxel, it is essential to predict through volume conductor modeling LFPs representing average extracellular potentials, which are expressed in terms of interstitial potentials of individual cells in networks of gap-junctionally connected astrocytes and synaptically connected neurons. These relationships have been provided and can be then used to investigate how the underlying neuronal population activity can be inferred from the measurement of the BOLD signal through electrovascular coupling mechanisms across the blood-brain barrier. The importance of both synaptic and extrasynaptic transmission as the basis of electrophysiological indices triggering vascular responses between dendritic and astrocytic networks, and sequential configurations of firing patterns in composite neural networks is emphasized. The purpose of this review is to show how fMRI data may be used to draw conclusions about the information transmitted by individual neurons in populations generating the BOLD signal.
Keywords: sequential configurations; astrocytes; gap-junctions; sub-assemblies; dynamic connectivity; multi-hierarchy; neocortex; functional neuroimaging; nested networks; volume conductor modeling.
1. Introduction The largest subdivision of the cerebral cortex is the neocortex, also called the isocortex. It operates by activating distinct cortical modules or columns (Mountcastle, 1957). These modules are local neural circuits of approximately 104 neurons linked together by a complex intramodular circuitry (Abeles, 1982, 1991). The modular
view of neocortical organization has been explored most clearly in the posterior regions, including visual, somatosensory and auditory cortices. However, its generality to both the posterior and anterior regions is implied (Eccles, 1981; Szentagothai, 1975, 1978). That each cortical module has reciprocal connections (e.g., associative, commissural, and projection fibers) between 10 to 30 other modules (see Mountcastle, 1979) precludes an experimental evaluation of extrinsic connectivity of the neocortex with electrophysiological techniques, such as with multi-unit microelectrodes (e.g., Buzsaki, 2004). Functional neuroimaging devices, on the other hand, have a spatial resolution that is large compared with the size of neurons. Such a spatial resolution involves a high degree of interconnectedness at the systems level (Felleman and van Essen, 1991), requiring large-scale theories of extrinsic connectivity of the neocortex which have yet to be developed (see e.g., Friston et al., 1995; Stevens, 1994). An understanding of the functional organization of the neocortical module as a precisely connected but nested distributed system requires that the intrinsic organization of the neocortex be functionally sub-divided into brain regions composed of large numbers of modular elements linked together in echeloned, parallel and serial arrangements that may be compared with the idealized notion of “brain cell assemblies” first proposed by Hebb (1949). In view of the nested columnar organization of the neocortex, such neocortical assemblies (macro-scale) should be comprised of “sub-assemblies” as has been recently observed within functional columns in the rat visual cortex (Yoshimura et al., 2005). These neocortical sub-assemblies (meso-scale) can than be further sub-divided into modules or local neural circuits consisting of approximately 104 neurons. Although a large number of
theoretical studies have hypothesized how cognitive events emerge through the modeling of neocortical assemblies as neuronal masses (Braitenberg, 1978; Palm, 1982; Wickelgren, 1992; Eichenbaum, 1993; Miller, 1996; Eichenbaum and Davis, 1998) little effort has been given to theoretical elucidation of the functionality of neocortical sub-assemblies in the context of an integrative theory of cognition (Poznanski, 2002b). In particular, there are no theoretical studies showing directly how one hierarchical level of neuronal organization affects another level, with each lower level manifesting an emergent behavior at the higher level.