PEDOT as a Conductive Polymer-Principles and Applications(1)
- 格式:pdf
- 大小:779.39 KB
- 文档页数:15


PEDOT及与磁性复合材料的制备和吸波性能研究的开题报告题目:PEDOT及与磁性复合材料的制备和吸波性能研究一、简介随着信息技术的飞速发展,电磁波辐射对人体的危害越来越受到重视。
吸波材料在电磁波防护领域发挥着重要作用,因此研究吸波材料已成为当前研究的热点和难点之一。
PEDOT(聚3,4-乙烯基噻吩)是一种导电聚合物,在吸波材料的研究中具有重要潜力。
另外,将PEDOT与磁性复合材料进行混合,制备出PEDOT磁性复合材料,可以进一步提高吸波性能。
二、研究内容1. PEDOT单一材料的制备:选择适当的聚合催化剂、溶剂和配合物,制备出高导电性的PEDOT材料。
2. 磁性纳米材料的制备:选择肥料或硝晶石为原料,合成出不同尺寸和形状的磁性纳米颗粒。
3. PEDOT磁性复合材料的制备:将PEDOT和磁性纳米颗粒进行混合,并通过化学还原法、电化学沉积法等方法制备PEDOT磁性复合材料。
4. 吸波性能研究:利用微波吸收仪测试吸波材料的吸波性能,分析PEDOT磁性复合材料的吸波性能特点。
三、研究意义1. 提高吸波材料的性能:PEDOT磁性复合材料可以利用PEDOT的导电性和磁性纳米材料的磁性特性,实现对不同频率、不同强度电磁波的吸收,从而提高吸波材料的性能。
2. 拓展PEDOT的应用领域:PEDOT在导电、催化等领域已有广泛应用。
将PEDOT应用于吸波材料,可以拓展其应用领域,实现多功能化。
3. 推动磁性纳米材料的应用:磁性纳米材料在医学、环境治理等领域有很广泛的应用,将其应用于吸波材料的研究,可以推动其在电磁波防护中的应用。
四、研究方法1. PEDOT单一材料的制备方法:化学氧化聚合法、电化学聚合法等方法。
2. 磁性纳米材料制备方法:共沉淀法、热分解法、控制结晶法等方法。
3. PEDOT磁性复合材料制备方法:化学还原法、电化学沉积法等方法。
4. 吸波性能测试方法:微波吸收仪测试法。
五、研究进度1. 已经完成了PEDOT单一材料的制备及其物性测试;2. 正在进行磁性纳米材料的制备及其物性测试;3. 将在接下来的研究中进行PEDOT磁性复合材料的制备及其性能测试。

乙二醇掺杂使pedotpss电导率提高的机理研究乙二醇(ethylene glycol, EG)是一种常见的有机化合物,具有良好的溶解性和稳定性。
聚(3,4-乙砜二(噻吩-2,5-二甲氧基)苯磺酸盐)(PEDOT:PSS)是一种常用的导电高分子材料,具有良好的导电性和可溶性。
研究发现,将乙二醇掺杂PEDOT:PSS可以显著提高其电导率。
本文将探讨乙二醇掺杂PEDOT:PSS电导率提高的机理。
乙二醇在PEDOT:PSS中的掺杂可以改善PEDOT和PSS之间的相互作用,从而提高PEDOT:PSS的导电性。
乙二醇分子具有两个羟基(OH)官能团,在PEDOT:PSS中与PEDOT和PSS发生相互作用。
乙二醇的氧原子与PEDOT的酸性硫原子形成氢键,增强PEDOT:PSS中PEDOT的导电性。
乙二醇的羟基官能团还可以通过氢键与PSS中的硫酸根离子结合,减少PEDOT:PSS中的离子交联,从而减小PEDOT粒子之间的距离,提高电子的传导性。
此外,乙二醇还可以改善PEDOT:PSS薄膜的结构和形貌,进一步提高导电性。
PEDOT:PSS薄膜通常呈现出颗粒状、颗粒团状或网络状结构,这些结构会导致电子在PEDOT:PSS薄膜中的传导受阻。
乙二醇的掺杂能够改变PEDOT:PSS颗粒的排列和形貌,使其呈现出更加均匀和致密的结构,减少界面和颗粒之间的间隙,提高电子的传导性。
乙二醇的掺杂还可以减少PEDOT:PSS薄膜中的电荷传输势垒,增加电子的迁移率。
PEDOT:PSS薄膜中的电荷传输势垒是电子从PEDOT向PSS转移所需克服的能量势垒。
乙二醇的掺杂可以改变PEDOT和PSS之间的相互作用,降低电荷传输势垒,使电子更容易从PEDOT向PSS转移,提高电导率。
此外,乙二醇的掺杂还可以改善PEDOT:PSS薄膜的稳定性,延长其使用寿命。
PEDOT:PSS薄膜在潮湿环境中容易发生降解和失去导电性。
乙二醇的掺杂可以与PSS形成更强的氢键,增强PEDOT:PSS薄膜的稳定性,减缓其降解速度。

聚⼄炔导电性介绍第18卷 第5期⼤学化学2003年10⽉知识介绍聚⼄炔导电性介绍包咏(沈阳⼴播电视⼤学 沈阳110003)摘要 ⽩川英树等⼈因发现导电聚合物⽽荣获2000年诺贝尔化学奖。本⽂⽤“孤⼦理论”对聚⼄炔的导电机制作了简介。通常来讲⾼聚物是绝缘材料,20世纪70年代⽩川英树(H.Shirakawa)等⼈⾸次合成聚⼄炔薄膜[1],后⼜通过掺杂发现⾼聚物也具有导电性。导电⾼聚物既具有⾦属的⾼导电率,⼜具有聚合物的可塑性,质量⼜轻,是⼀类具有⼴阔应⽤前景的新材料。⾼聚物导电性的发现拓宽了⼈类对导体材料的认识及应⽤领域。⽩川英树、麦克迪尔⽶德(A.G.Mac Diarmid)和⿊格(A.J.Heeger)3⼈因发现⾼聚物的⾦属导电性⽽荣获了2000年诺贝尔化学奖。
对于导电⾼聚物的导电机理,苏武沛等⼈[2,3]运⽤孤⼦理论较好地解释了聚⼄炔掺杂的导电机理。值得⼀提的是,苏武沛是施⾥弗(J.R.Schrieffer)的研究⽣,⽽施⾥弗是与巴丁、库伯共同荣获1972年Nobel物理学奖的理论物理学家。⽩川英树是有机化学家,麦克迪尔⽶德是熟悉物理的化学家,⿊格是熟悉化学的实验物理学家,他们之间密切合作,使化学与物理、实验与理论结合起来,解决了材料制备、物理和化学性能测试、实验数据和理论机制分析,开创了化学和物理相结合的活跃的新边缘学科。
1 孤⼦概念⾼聚物导电可以⽤孤⼦理论来解释。现以最简单的导电聚合物———聚⼄炔为例来描述孤⼦形成的物理图景及其导电机制。先了解⼀下孤⼦的⼀般形态和性质。孤⼦的概念来源于“孤波”。1834年秋,英国科学家罗素(S.Rusell)在运河岸边看到由两匹马拉着⼀条迅速⾏驶的船。当船突然停⽌时,在船头激起⼀个沿着河⾯滚动的波包,其⼤⼩、形状和速度变化都很慢,罗素追随此波包2~3km,直到运河转了⼏个弯以后,波包才逐渐消失。罗素把这种孤⽴的波包称为“孤波”。在罗素逝世100周年(1982年)时,⼈们在罗素发现孤波的运河边树⽴⼀座罗素纪念像,以纪念他这⼀不寻常的发现。

pedot化学式Pedot是一种具有广泛应用前景的有机半导体材料,它有着良好的导电性、稳定性和氧化还原性能。
Pedot化学式为(C8H5O2) n,其中n为大于等于10的整数,它的化学结构如下:[C=C-C(=O)-O-]n要了解Pedot化学式的含义,我们需要对其化学结构进行解析。
首先,Pedot由苯环和咪唑环组成,其分子结构中含有苯环和咪唑环上的氮、氧、碳等多种原子。
其次,Pedot具有链状结构,其中每个咪唑环都通过共轭键与相邻的苯环相连。
这种共轭结构使得Pedot具有很好的导电性。
Pedot的制备方法有多种,其中最常见的方法是聚合反应。
聚合反应是利用咪唑单元的亲电性官能团与二元碘化物等化合物发生偶联反应,形成共轭芳香烃的聚合物。
在聚合反应中,反应条件和催化剂的选择对生成的Pedot结构和性能会有显著影响。
常用的聚合条件包括电化学聚合、化学聚合、母体聚合等。
此外,单体物种的选择也是影响Pedot性质的重要因素,例如通过引入一些有机基团或杂原子(如硫、氮、氧等),可以提高Pedot的导电率和稳定性。
Pedot材料具有广泛的应用前景,最为突出的是其在有机电子学领域中的应用。
Pedot可用作有机场效应晶体管材料,可以制备出灵活、高性能的柔性电子设备。
此外,Pedot还可用作光电材料,应用于太阳能电池和电致变色显示器等领域。
除此之外,Pedot还可以作为锂离子电池的电极材料,充分发挥其优异的电导性和稳定性。
总之,Pedot化学式的掌握对于理解其在有机电子学领域中的应用具有重要意义。
Pedot具有良好的导电性、稳定性和氧化还原性能,其制备方法和单体材料的选择也对其性质具有显著影响。
在未来的应用中,Pedot将会有着广泛的应用前景,为有机电子学领域的发展注入新的活力。
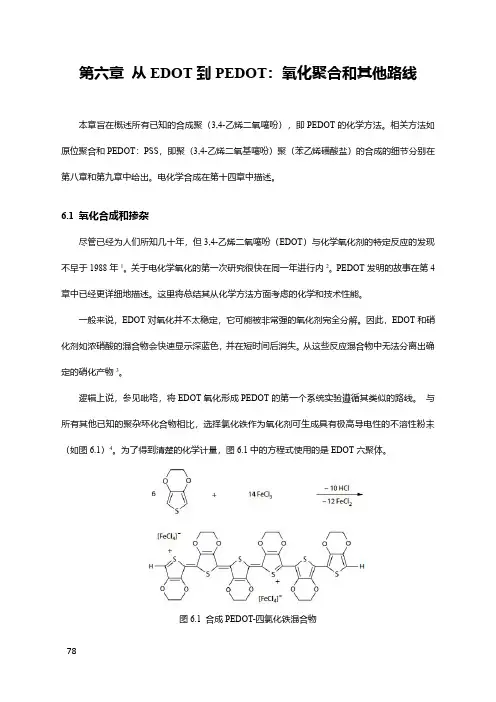
第六章从EDOT到PEDOT:氧化聚合和其他路线本章旨在概述所有已知的合成聚(3,4-乙烯二氧噻吩),即PEDOT的化学方法。
相关方法如原位聚合和PEDOT:PSS,即聚(3,4-乙烯二氧基噻吩)聚(苯乙烯磺酸盐)的合成的细节分别在第八章和第九章中给出。
电化学合成在第十四章中描述。
6.1氧化合成和掺杂尽管已经为人们所知几十年,但3,4-乙烯二氧噻吩(EDOT)与化学氧化剂的特定反应的发现不早于1988年1。
关于电化学氧化的第一次研究很快在同一年进行内2。
PEDOT发明的故事在第4章中已经更详细地描述。
这里将总结其从化学方法方面考虑的化学和技术性能。
一般来说,EDOT对氧化并不太稳定,它可能被非常强的氧化剂完全分解。
因此,EDOT和硝化剂如浓硝酸的混合物会快速显示深蓝色,并在短时间后消失。
从这些反应混合物中无法分离出确定的硝化产物3。
逻辑上说,参见吡咯,将EDOT氧化形成PEDOT的第一个系统实验遵循其类似的路线。
与所有其他已知的聚杂环化合物相比,选择氯化铁作为氧化剂可生成具有极高导电性的不溶性粉末(如图6.1)4。
为了得到清楚的化学计量,图6.1中的方程式使用的是EDOT六聚体。
图6.1合成PEDOT-四氯化铁混合物在沸腾的乙腈(沸点82℃)中所合成的PEDOT-四氯化铁的电导率,是相同条件下合成的聚吡咯×FeCl4–的3000倍:15S/cm对5×10-3S/cm,通过压片计算。
令人意外的是,通过延长EDOT和FeCl3在沸腾的苄腈中的反应(6h,188°C),可以进一步提高PEDOT-四氯化铁的电导率。
这个有趣的效应,作为这一方向的最初提示之一,清楚地表明了PEDOT强烈的温度稳定性。
其它几种金属离子也已经在不同程度上成功地应用于氧化EDOT。
高氧化态的锰,特别是二氧化锰,在商业上用于特殊应用中。
PEDOT能够被沉积在MnO2层上,以进一步用作于恒电流电镀铜的导电基底5-7。


导电聚合物的研究论文导读:导电聚合物大多都有一个较长的π共轭主链,因此又称为共轭聚合物。
聚吡咯具有质量轻,电导率高,易于制备与掺杂,空气稳定性好,合成方便,电化学可逆性强等优点,但价格及工艺流程比较昂贵,因此还没有大规模推广。
聚苯胺以其良好的热稳定性,化学稳定性,电化学可逆性,优良的电磁微波吸收性能,潜在的溶液和熔融加工性能,原料易得,合成方法简便,还有独特的掺杂现象等特性,成为现在研究进展最快的导电高分子材料之一;聚噻吩作为高分子材料的一种,具有极其小的尺寸、丰富潜在的功能,导电能力,可以在酸性体系中聚合,生成粉末状不溶物或者液态的聚合物但是由于导电高分子聚吡咯聚噻吩聚苯胺链的强刚性和链间的强的相互作用使得它们的溶解性极差,相应的可加工性也差,限制了它们在技术上的广泛应用。
聚苯胺可看作是苯二胺与醌二亚胺的共聚物,y值用于表征聚苯胺的氧化还原程度,(1-y)值代表了聚苯胺的氧化状态。
关键词:导电聚合物,聚噻吩,聚苯胺,聚吡咯,对比1、引言1977白川英树等人发现了碘掺杂的聚乙炔具有很高的导电性,比一般的有机高分子材料高约13个数量级。
这一惊人发现,彻底改变了人们以往的观念-—有机高分子是绝缘体。
导电聚合物大多都有一个较长的π共轭主链,因此又称为共轭聚合物。
论文大全。
共轭分子中,σ键是定域键,构成分子骨架;而垂直于分子平面的p轨道组合成离域π键,所有π电子在整个分子骨架内运动。
离域π键的形成,增大了π电子活动范围,使体系能级降低、能级间隔变小,增加物质的导电性能。
交替的单键、双键共轭结构是导电高分子材料的共同特征,若进行掺杂可使其电导率增加若干数量级,接近于金属电导率,这为导电高分子进入市场提供了强劲的力量。
2.三种导电高分子的对比本文导电高分子材料研究主要是聚噻吩,聚苯胺,聚吡咯这三种聚合物,其中只有聚苯胺初步形成了工业化规模,由此可见他们之间存在一定程度的差异,接下来将从以下四个方面对三种物质的性质进行对比:2.1优缺点比较:聚吡咯具有质量轻,电导率高,易于制备与掺杂,空气稳定性好,合成方便,电化学可逆性强等优点,但价格及工艺流程比较昂贵,因此还没有大规模推广;聚苯胺以其良好的热稳定性,化学稳定性,电化学可逆性,优良的电磁微波吸收性能,潜在的溶液和熔融加工性能,原料易得,合成方法简便,还有独特的掺杂现象等特性,成为现在研究进展最快的导电高分子材料之一;聚噻吩作为高分子材料的一种,具有极其小的尺寸、丰富潜在的功能, 导电能力,可以在酸性体系中聚合,生成粉末状不溶物或者液态的聚合物但是由于导电高分子聚吡咯聚噻吩聚苯胺链的强刚性和链间的强的相互作用使得它们的溶解性极差,相应的可加工性也差,限制了它们在技术上的广泛应用。

10.8电致变色行为10.8.1引言一些材料根据所施加的电化学电位的变化而改变颜色,这种现象称为电致变色。
电致变色材料早就为大家所知[241,242]。
所有电致变色材料的共同点是电化学触发氧化还原过程,从而改变了材料的光吸收。
玻璃工业已经利用电致变色效应开发了电致变色的多层玻璃(“智能窗”)。
然而,因为严苛的寿命要求,以及其复杂而高成本的生产过程,导致其高价格难以为市场所接受,所以,直到现在,这些窗户没有成功应用与推广。
Sage Electrochromics公司(一家位于美国的公司)和已经退出市场的Velux/USA一起联盟商业化了一个电致变色屋顶窗。
主要的玻璃制造商,例如,Pilkington/FLABEG(德国)和Saint Gobain (法国)尝试将建筑和汽车玻璃中的电致变色窗进行商业化,但都没有成功。
Schott-Donnelly(美国),Saint-Gobain(法国)和Central Glass(日本)证明电致变色窗在汽车天窗玻璃上的应用是可行的,并且已经应用在豪华车的天窗。
所有商业电致变色玻璃基于三氧化钨作为电致变色材料,其必须在高真空下利用溅射工艺喷涂在玻璃表面上。
由于缺乏长期稳定性和液体电解质的低接受性,利用有机紫精化学开发的窗户系统的所有努力已经证明是失败的。
但是其开发了液体和凝胶型汽车后视镜系统(Gentex,美国)和相机的电致变色显示器(尼康,日本)应用技术[243]。
目前,电致变色显示器(特别是基于PEDOT的)在印刷电子领域中受到广泛关注。
其主要目的是利用印刷技术开发纸状显示器。
典型的电致变色材料是联吡啶鎓盐(紫罗碱)[244,245]。
类似于紫罗碱的染料通常由不同的化学物质组成,其电解质在电极处被氧化或还原以形成自由基阳离子和在扩散后电荷重组的阴离子(图10.38和图10.39)。
在这种系统中,需要小但明显的电流来维持着色状态。
与作为电致变色239240剂和液体电解质的电致变色的紫罗碱可以与PEDOT 组合作为透明电极材料[246]。

Published:May 12,2011COMMUNICATION /JACSInterface-Directed Assembly of One-Dimensional Ordered Architecture from Quantum Dots Guest and Polymer HostShengyang Yang,Cai-Feng Wang,and Su Chen*State Key Laboratory of Materials-Oriented Chemical Engineering,and College of Chemistry and Chemical Engineering,Nanjing University of Technology,Nanjing 210009,P.R.ChinabSupporting Information ABSTRACT:Assembly of inorganic semiconductor nano-crystals into polymer host is of great scienti fic and techno-logical interest for bottom-up fabrication of functional devices.Herein,an interface-directed synthetic pathway to polymer-encapsulated CdTe quantum dots (QDs)has been developed.The resulting nanohybrids have a highly uniform fibrous architecture with tunable diameters (ranging from several tens of nanometers to microscale)and enhanced optical performance.This interfacial assembly strategy o ffers a versatile route to incorporate QDs into a polymer host,forming uniform one-dimensional nanomaterials po-tentially useful in optoelectronic applications.Similar to the way that atoms bond to form molecules and complexes,inorganic nanoparticles (NPs)can be combined to form larger ensembles with multidimensional ordered hier-archical architecture,evoking new collective functions.To this end,the development of the controlled self-assembly method for well-de fined structures of these ensembles is signi ficant for creating new and high-performance tunable materials and hence has aroused appealing scienti fic and industrial interest.1Particu-larly,much e ffort has been devoted to the construction of one-dimensional (1D)structures of NPs,owing in part to their application as pivotal building blocks in fabricating a new generation of optoelectronic devices.2In this context,directed host Àguest assembly of NPs into polymer matrices is an e ffective “bottom-up ”route to form 1D ordered functional materials with advantageous optical,electrical,magnetic,and mechanical properties.3Some typical routes have been developed for the generation of these 1D hybrids so far,involving template-assisted,4seeding,5and electro-static approaches.6However,the challenge still remains to precisely manipulate assembly of aqueous NPs and water-insoluble polymers into uniform 1D nanocomposites with a high aspect ratio because of phase separation and aggregation.7Moreover,facile synthetic strate-gies are highly needed to fabricate homogeneous 1D composites in which each component still preserves favorable properties to produce optimal and ideal multifunctional materials.A liquid Àliquid interface o ffers an ideal platform to e fficiently organize NPs into ordered nanostructures driven by a minimiza-tion of interfacial energy.8While much of this research has been directed toward NP hybrids with diverse morphologies based on small organic ligand-directed assembly,9some success has also been achieved in polymer-based NPs nanocomposites.10Russelland co-workers developed ultrathin membranes and capsules of quantum dots (QDs)stabilized by cross-linked polymers at the toluene/water interface.10a,11Brinker ’s group reported the fab-rication of free-standing,patternable NP/polymer monolayer arrays via interfacial NP assembly in a polymeric photoresist.12Herein,a simple host Àguest assembly route is developed to facilely create homogeneous 1D CdTe/polymer hybrids without any indication of phase separation at the aqueous/organic inter-face for the first time.The CdTe nanocrystal is a semiconductor that has been used extensively for making thin film for solar cells.13Some elegant studies have been made in synthesizing pure inorganic 1D CdTe nanowires via assembly from corre-sponding individual CdTe nanocrystals.14In this work,CdTe QDs are covalently grafted with poly(N -vinylcarbazole-co -glycidylmethacrylate)(PVK-co -PGMA)to form uniform fibrous fluorescent composites at the water/chloroform interface via the reaction between epoxy groups of PVK-co -PGMA and carboxyl groups on the surface of CdTe QDs (Scheme 1).15These 1D composite fibers can be allowed to grow further in the radial direction by “side-to-side ”assembly.Additionally,this type of interfacial QD Àpolymer assembly can observably improve the fluorescence lifetime of semiconductor QDs incorporated in theScheme 1.Schematic Representation of the Synthesis of PVK-co -PGMA/CdTe QDs Composite Nano fibersReceived:February 8,2011polymeric matrix.It can be expected that this example of both linear axial organization and radial assembly methodology can be applied to fabricate spatial multiscale organic Àinorganic com-posites with desired properties of NPs and polymers.Figure 1a shows a typical scanning electron microscope (SEM)image of PVK-co -PGMA/CdTe QDs composite nano fi-bers obtained at the water/chloroform interface after dialysis.The as-prepared fibers have uniform diameters of about 250nm and typical lengths in the range of several tens to several hundreds of micrometers (Figures 1a and S4Supporting In-formation [SI]).Interestingly,PVK-co -PGMA/CdTe composite fibers can randomly assemble into nestlike ring-shaped patterns (Figures 1b and S5[SI]).Given the interaction among epoxy groups,the formation of nestlike microstructures could be attributed to incidental “head-to-tail ”assembly of composite fibers.Moreover,in order to establish the relationship between the role of epoxy groups and the formation of composite nano fibers,control experiments were performed,in which pure PGMA or PVK was used to couple CdTe QDs.The PGMA/CdTe composites could be obtained with fibrous patterns (Figure S6[SI]),but no fibrous composites were achieved at the biphase interface with the use of PVK under the same conditions.The microstructures and fluorescence properties of PVK-co -PGMA/CdTe composite fibers were further character-ized using laser confocal fluorescence microscopy (LCFM).Confocal fluorescence micrographs of composite fibers show that the di fferently sized QDs have no obvious in fluence on the morphology of composites (Figure 1c Àe).Clearly,uniform and strong fluorescence emission is seen throughout all the samples,and the size-dependent fluorescence trait of CdTe QDs in PVK-co -PGMA matrix remains well.In order to verify the existence and distribution of CdTe QDs in the fibers,transmission electron microscopy (TEM)was employed to examine the assembled structures.Figure 2a shows a TEM image of PVK-co -PGMA/CdTe QDs composite nano fi-bers,indicating each composite fiber shown in Figure 1a was assembled from tens of fine nano fibers.An individual fine nano fiber with the diameter of about 30nm is displayed in Figure 2b,from which we can see that CdTe QDs have been well anchored into the fiber with polymeric protection layer,revealing this graft-form process at the interface e ffectively avoidednon-uniform aggregation in view of well-dispersed CdTe QDs within the composite fiber,consistent with the LCFM observa-tion.Unlike previous works where the nanoparticles were ad-sorbed onto the polymer fibers,16CdTe QDs were expelled from the surface of fibers (∼2.5nm)in our system (Figure 2c),albeit the high percentage of QDs in the polymer host (23wt %)was achieved (Figure S7[SI]).This peculiarity undoubtedly confers CdTe QDs with improved stability.The clear di ffuse rings in the selected area electron di ffraction (SAED)pattern further indicate excellent monodispersion and finely preserved crystalline struc-ture of QDs in the nano fibers (Figure 2d).The SAED data correspond to the cubic zinc blende structure of CdTe QDs.A possible mechanism for the assembly of 1D nanostructure was proposed,as illustrated in Figure S8[SI].The hydrophilic epoxy groups of the PVK-co -PGMA chain in the oil phase orient toward the biphase interface and then react with carboxyl groups on the surface of CdTe QDs in the aqueous phase to a fford premier PVK-co -PGMA/CdTe QDs composites.Such nanocomposites will reverse repeatedly,resulting from iterative reaccumulation of epoxy groups at the interface and the reaction between the active pieces (i.e.,epoxy or carboxyl groups)in the composites with intact CdTe QDs or PVK-co -PGMA,forming well-de fined nano-fibers.The control experiments showing that the diameter of composite fibers increases with the increase in the concentration of PVK-co -PGMA are in agreement with the proposed mechan-ism (Figure S9[SI]).In addition,it is expected that the pure polymeric layer on the surface of the fibers (red rectangular zone in Figure 2c)will allow further assembly of fine fibers into thick fibers,and these fibers also could randomly evolve into rings,forming nestlike microstructures when the “head ”and “tail ”of fibers accidentally meet (Figure 1b).To further examine the assembly behavior of composite fibers,the sample of PVK-co -PGMA/CdTe QDs composite nano fibers were kept at the water/chloroform interface for an additional month in a close spawn bottle at room temperature (Figure S10[SI]).With longer time for assembly,thicker composite fibers with tens of micrometers in diameter were obtained (Figure 3a).These micro-fibers have a propensityto form twisted morphology (Figure 3a,b),Figure 1.(a,b)SEM images of PVK-co -PGMA/CdTe QDs composite nano fibers.(c Àe)Fluorescence confocal microscopy images of PVK-co -PGMA/CdTe QDs composite nano fibers in the presence of di fferently sized QDs:(c)2.5nm,(d)3.3nm,and (e)3.6nm.The excitation wavelengths are 488(c),514(d),and 543nm (e),respectively.Figure 2.(a,b)TEM images of PVK-co -PGMA/CdTe QDs composite nano fibers,revealing composite nano fiber assemblies.(c)HRTEM image and (d)SAED pattern of corresponding PVK-co -PGMA/CdTe QDs composite nano fibers.while their re fined nanostructures still reveal relatively parallel character and con firm the micro fibers are assembled from countless corresponding nano fibers (Figure 3c).The corresponding LCFM image of an individual micro fiber is shown in Figure 3d (λex =488nm),indicating strong and homogeneous green fluorescence.Another indication is the fluorescent performance of PVK-co -PGMA/CdTe QDs composite micro fibers (Figure 4a).The fluorescent spectrum of composite fibers takes on emission of both PVK-co -PGMA and CdTe QDs,which suggests that this interfacial assembly route is e ffective in integrating the properties of organic polymer and inorganic nanoparticles.It is worth noting that there is a blue-shift (from 550to 525nm)and broadening of the emission peak for CdTe QDs upon their incorporation into polymeric hosts,which might be ascribed to the smaller QD size and less homogeneous QD size distribution resulting from the photooxidation of QD surfaces.17Since the emission spectra of PVK-co -PGMA spectrally overlap with the CdTe QD absorption (Figure S11[SI]),energy transfer from the copolymer to the CdTe QDs should exist.18However,the photoluminescence of PVK-co -PGMA does not vanish greatly in the tested sample in comparison with that of polymer alone,revealing inferior energy transfer between the polymer host and the QDs.Although e fficient energy transfer could lead to hybrid materials that bring together the properties of all ingredients,18it is a great hurdle to combine and keep the intrinsic features of all constituents.19In addition,by changing the polymeric compo-nent and tailoring the element and size of QDs,it should be possible to expect the integration of organic and inorganic materials with optimum coupling in this route for optoelectronic applications.Finally,to assess the stability of CdTe QDs in the composite micro fibers,time-resolved photoluminescence was performed using time-correlated single-photon counting (TCSPC)parative TCSPC studies for hybrid PVK-co -PGMA/CdTe QDs fibers and isolated CdTe QDs in the solid state are presented in Figure 4b.We can see that the presence of PVK-co -PGMA remarkably prolongs the fluorescence lifetime (τ)of CdTe QDs.Decay traces for the samples were well fittedwith biexponential function Y (t )based on nonlinear least-squares,using the following expression.20Y ðt Þ¼R 1exp ðÀt =τ1ÞþR 2exp ðÀt =τ2Þð1Þwhere R 1,R 2are fractional contributions of time-resolved decaylifetimes τ1,τ2and the average lifetime τhcould be concluded from the eq 2:τ¼R 1τ21þR 2τ22R 1τ1þR 2τ2ð2ÞFor PVK-co -PGMA/CdTe QDs system,τh is 10.03ns,which is approximately 2.7times that of isolated CdTe QDs (3.73ns).Photooxidation of CdTe QDs during the assembly process can increase the surface states of QDs,causing a delayed emission upon the carrier recombination.21Also,the polymer host in this system could prevent the aggregation of QDs,avoid self-quench-ing,and delay the fluorescence decay process.22The increased fluorescence lifetime could be also ascribed to energy transfer from PVK-co -PGMA to CdTe QDs.18c The result suggests that this host Àguest assembly at the interface could find signi ficant use in the fabrication of QDs/polymer hybrid optoelectronic devices.In summary,we have described the first example of liquid/liquid interfacial assembly of 1D ordered architecture with the incorporation of the QDs guest into the polymer host.The resulting nanohybrids show a highly uniform fibrous architecture with tunable diameter ranging from nanoscale to microscale.The procedure not only realizes the coexistence of favorable properties of both components but also enables the fluorescence lifetime of QDs to be enhanced.This interesting development might find potential application for optoelectronic and sensor devices due to high uniformity of the 1D structure.Further e fforts paid on optimal regulation of QDs and polymer composition into 1D hybrid nanostructure could hold promise for the integration of desirable properties of organic and inorganic compositions for versatile dimension-dependent applications.In addition,this facile approach can be easily applied to various semiconductor QDs and even metal NPs to develop highly functional 1D nanocomposites.’ASSOCIATED CONTENTbSupporting Information.Experimental details,FT-IR,GPC,UV Àvis,PL,SEM,TGA analysis,and complete ref 9c.This material is available free ofcharge via the Internet at .Figure 3.(a,b)SEM and (c)FESEM images of PVK-co -PGMA/CdTe QDs composite micro fibers.(d)Fluorescence confocal microscopy images of PVK-co -PGMA/CdTe QDs composite micro fibers inthe presence of green-emitting QDs (2.5nm).Figure 4.(a)Fluorescence spectra of PVK-co -PGMA,CdTe QD aqueous solution,and PVK-co -PGMA/CdTe QDs composite micro-fibers.(b)Time-resolved fluorescence decay curves of CdTe QDs (2.5nm diameter)powders (black curve)and the corresponding PVK-co -PGMA/CdTe QDs composite micro fibers (green curve)mea-sured at an emission peak maxima of 550nm.The samples were excited at 410nm.Biexponential decay function was used for satisfactory fitting in two cases (χ2<1.1).’AUTHOR INFORMATIONCorresponding Authorchensu@’ACKNOWLEDGMENTThis work was supported by the National Natural Science Foundation of China(21076103and21006046),National Natural Science Foundation of China-NSAF(10976012),the Natural Science Foundations for Jiangsu Higher Education Institutions of China(07KJA53009,09KJB530005and10KJB5 30006),and the Priority Academic Program Development of Jiangsu Higher Education Institutions(PAPD).’REFERENCES(1)(a)Kashiwagi,T.;Du,F.;Douglas,J.F.;Winey,K.I.;Harris, R.H.;Shields,J.R.Nat.Mater.2005,4,928.(b)Shenhar,R.;Norsten, T.B.;Rotello,V.M.Adv.Mater.2005,17,657.(c)Akcora,P.;Liu,H.; Kumar,S.K.;Moll,J.;Li,Y.;Benicewicz,B.C.;Schadler,L.S.;Acehan, D.;Panagiotopoulos,A.Z.;Pryamitsyn,V.;Ganesan,V.;Ilavsky,J.; Thiyagarajan,P.;Colby,R.H.;Douglas,J.F.Nat.Mater.2009,8,354.(d)Dayal,S.;Kopidakis,N.;Olson,D.C.;Ginley,D.S.;Rumbles,G. J.Am.Chem.Soc.2009,131,17726.(e)Lin,Y.;B€o ker,A.;He,J.;Sill,K.; Xiang,H.;Abetz,C.;Li,X.;Wang,J.;Emrick,T.;Long,S.;Wang,Q.; Balazs,A.;Russell,T.P.Nature2005,434,55.(f)Park,S.;Lim,J.ÀH.; Chung,S.W.;Mirkin,C.A.Science2004,303,348.(g)Mai,Y.; Eisenberg,A.J.Am.Chem.Soc.2010,132,10078.(h)Mallavajula, R.K.;Archer,L.A.Angew.Chem.,Int.Ed.2011,50,578.(i)Kim,J.;Piao, Y.;Hyeon,T.Chem.Soc.Rev.2009,38,372.(2)(a)Xia,Y.;Yang,P.;Sun,Y.;Wu,Y.;Mayers,B.;Gates,B.;Yin, Y.;Kim,F.;Yan,H.Adv.Mater.2003,15,353.(b)Lu,X.;Wang,C.;Wei, Y.Small2009,5,2349.(c)Nie,Z.;Fava,D.;Kumacheva,E.;Zou,S.; Walker,G.C.;Rubinstein,M.Nat.Mater.2007,6,609.(3)(a)Huynh,W.U.;Dittmer,J.J.;Alivisatos,A.P.Science2002, 295,2425.(b)Balazs,A.C.;Emrick,T.;Russell,T.P.Science2006, 314,1107.(c)Ramanathan,T.;Abdala,A.A.;Stankovich,S.;Dikin, D.A.;Herrera-Alonso,M.;Piner,R.D.;Adamson,D.H.;Schniepp, H.C.;Chen,X.;Ruoff,R.S.;Nguyen,S.T.;Aksay,I.A.;Prud’homme, R.K.;Brinson,L.C.Nat.Nanotechnol.2008,3,327.(d)Tomczak,N.; Janczewski,D.;Han,M.;Vancso,G.J.Prog.Polym.Sci.2009,34,393.(e)Zhao,Y.;Thorkelsson,K.;Mastroianni,A.J.;Schilling,T.;Luther, J.M.;Rancatore,B.J.;Matsunaga,K.;Jinnai,H.;Wu,Y.;Poulsen,D.; Frechet,J.M.J.;Alivisatos,A.P.;Xu,T.Nat.Mater.2009,8,979.(f) Colfen,H.;Mann,S.Angew.Chem.,Int.Ed.2003,42,2350.(g)Sone,E.D.;Stupp,S.I.J.Am.Chem.Soc.2004,126,12756.(4)Chan,C.S.;De Stasio,G.;Welch,S.A.;Girasole,M.;Frazer,B.H.;Nesterova,M.V.;Fakra,S.;Banfield,J.F.Science2004,303,1656.(5)Tran,H.D.;Li,D.;Kaner,R.B.Adv.Mater.2009,21,1487.(6)Yuan,J.;M€u ller,A.H.E.Polymer2010,51,4015.(7)(a)Greenham,N.C.;Peng,X.;Alivisatos,A.P.Phys.Rev.B 1996,54,17628.(b)Lopes,W.A.;Jaeger,H.M.Nature2001,414,735.(c)Gupta,S.;Zhang,Q.;Emrick,T.;Balazs,A.Z.;Russell,T.P.Nat. Mater.2006,5,229.(8)(a)Wang,X.;Zhuang,J.;Peng,Q.;Li,Y.Nature2005,437,121.(b)Huang,J.;Kaner,R.B.J.Am.Chem.Soc.2004,126,851.(c)Binder, W.H.Angew.Chem.,Int.Ed.2005,44,5172.(d)Capito,R.M.;Azevedo, H.S.;Velichko,Y.S.;Mata,A.;Stupp,S.I.Science2008,319,1812.(e)Yin,Y.;Skaff,H.;Emrick,T.;Dinsmore,A.D.;Russell,T.P.Science 2003,299,226.(f)Arumugam,P.;Patra,D.;Samanta,B.;Agasti,S.S.; Subramani,C.;Rotello,V.M.J.Am.Chem.Soc.2008,130,10046.(g)Hou,L.;Wang,C.F.;Chen,L.;Chen,S.J.Mater.Chem.2010, 20,3863.(9)(a)Duan,H.;Wang,D.;Kurth,D.G.;Mohwald,H.Angew. Chem.,Int.Ed.2004,43,5639.(b)B€o ker,A.;He,J.;Emrick,T.;Russell,T.P.Soft Matter2007,3,1231.(c)Russell,J.T.;et al.Angew.Chem.,Int. Ed.2005,44,2420.(10)(a)Lin,Y.;Skaff,H.;B€o ker,A.;Dinsmore,A.D.;Emrick,T.; Russell,T.P.J.Am.Chem.Soc.2003,125,12690.(b)B€o ker,A.;Lin,Y.; Chiapperini,K.;Horowitz,R.;Thompson,M.;Carreon,V.;Xu,T.; Abetz,C.;Skaff,H.;Dinsmore,A.D.;Emrick,T.;Russell,T.P.Nat. Mater.2004,3,302.(11)Skaff,H.;Lin,Y.;Tangirala,R.;Breitenkamp,K.;B€o ker,A.; Russell,T.P.;Emrick,T.Adv.Mater.2005,17,2082.(12)Pang,J.;Xiong,S.;Jaeckel,F.;Sun,Z.;Dunphy,D.;Brinker,C.J.J.Am.Chem.Soc.2008,130,3284.(13)Fulop,G.;Doty,M.;Meyers,P.;Betz,J.;Liu,C.H.Appl.Phys. Lett.1982,40,327.(14)(a)Tang,Z.;Kotov,N.A.;Giersig,M.Science2002,297,237.(b)Zhang,H.;Wang,D.;Yang,B.;M€o hwald,H.J.Am.Chem.Soc.2006, 128,10171.(c)Yang,P.;Ando,M.;Murase,N.Adv.Mater.2009, 21,4016.(d)Srivastava,S.;Santos,A.;Critchley,K.;Kim,K.-S.; Podsiadlo,P.;Sun,K.;Lee,J.;Xu,C.;Lilly,G.D.;Glotzer,S.C.;Kotov, N.A.Science2010,327,1355.(15)Reis,A.V.;Fajardo,A.R.;Schuquel,I.T.A.;Guilherme,M.R.; Vidotti,G.J.;Rubira,A.F.;Muniz,.Chem.2009,74,3750.(16)(a)Djalali,R.;Chen,Y.;Matsui,H.J.Am.Chem.Soc.2002, 124,13660.(b)George,J.;Thomas,K.G.J.Am.Chem.Soc.2010, 132,2502.(17)(a)Yang,S.;Li,Q.;Chen,L.;Chen,S.J.Mater.Chem.2008, 18,5599.(b)Wang,Y.;Herron,N.J.Phys.Chem.1991,95,525.(c)Zhang,Y.;He,J.;Wang,P.N.;Chen,J.Y.;Lu,Z.J.;Lu,D.R.;Guo,J.; Wang,C.C.;Yang,W.L.J.Am.Chem.Soc.2006,128,13396.(d)Carrillo-Carri o n,C.;C a rdenas,S.;Simonet,B.M.;Valc a rcel,M. mun.2009,5214.(18)(a)Tessler,N.;Medvedev,V.;Kazes,M.;Kan,S.;Banin,U. Science2002,295,1506.(b)Zhang,Q.;Atay,T.;Tischler,J.R.;Bradley, M.S.;Bulovi c,V.;Nurmikko,A.V.Nat.Nanotechnol.2007,2,555.(c)Lutich,A.A.;Jiang,G.X.;Susha,A.S.;Rogach,A.L.;Stefani,F.D.; Feldmann,J.Nano Lett.2009,9,2636.(19)Li,M.;Zhang,J.;Zhang,H.;Liu,Y.;Wang,C.;Xu,X.;Tang,Y.; Yang,B.Adv.Funct.Mater.2007,17,3650.(20)Schr€o der,G.F.;Alexiev,U.;Grubm€u ller,H.Biophys.J.2005, 89,3757.(21)(a)Zhong,H.Z.;Zhou,Y.;Ye,M.F.;He,Y.J.;Ye,J.P.;He,C.; Yang,C.H.;Li,Y.F.Chem.Mater.2008,20,6434.(b)Sun,H.;Zhang, H.;Zhang,J.;Ning,Y.;Yao,T.;Bao,X.;Wang,C.;Li,M.;Yang,B. J.Phys.Chem.C2008,112,2317.(22)Kagan,C.R.;Murray,C.B.;Bawendi,M.G.Phys.Rev.B1996, 54,8633.。

第l 4卷第3期 2002年5月 化 学进展
PROGRESS I CHEMISTRY VO】.14 NO.3
May,2002
导电聚合物 李永舫 (中善科学院化学研究所有机固体旨家重点实验室 北京100080)
摘 要 导电聚合物是2O世纪7o年代发展起来蜘一A斫究领域,因其诱^曲应用前景受到广泛重翘。 本文介绍了导电聚台物的发现和发展历史,综述了导电聚台物的结构和掺杂特征、制吾方詹、号导和电亿学
特^生及其本征态共轭聚合物的光电特性 对导电聚合物'-3前的研究热点卸应周前景进行了讨论。 关键词 导电聚台物 共轭聚台物 掺杂 电导 皂化学^生质 光电子器件 中图分类号:O 631文献标识码:A文章编号:1005 281X L 2002)03—0207—06
Conducting Polymers Li Yong ng (Key Laboratory of Organic Solids,Institute()f Chemistry,Chinese Academy of Sciences.Beijing l 00080,China)
Abstract The research field of conducting polymers has been developed since l 970s,and has attracted much attent Lon due tO their promising applica Lions.In this paper,the discovery and deve]opments。f conducting polymers were introduced The struclures,doping characteristics,preparation methods.conductivity and electrochemical properties of conducting polymers,as we]l as the photoelecttonic properties of conjugated polymers.were reviewed.In addition,possible applications and present hot topics of conducting polymers were discussed. Key words conducting polymers;conjugated polymers;doping;electric conductivity;elcctroe— hemical propert ies;polymer ph0t。e1ectr。n Lc devices
不同溶剂中导电聚合物P EDO T 的化学氧化聚合及光谱研究3汪斌华1,2,邓永红3,戈 钧3,周 啸3,王晓工3,杨邦朝1(1.电子科技大学微电子与固体电子学院,四川成都610041;2.厦门信达电子有限公司企业博士后工作站,福建厦门361021;3.清华大学化工系,北京100084)摘 要: 利用3,42乙撑二氧噻吩单体和对甲苯磺酸铁,分别以异丙醇、四氢呋喃和乙氰为溶剂,用化学氧化法合成了导电聚合物聚(3,42乙撑二氧噻吩),3种溶剂中的反应速率有较大差异,乙氰中反应速率最快,四氢呋喃中反应速率最慢。
三者的聚合产物都具有较高的电导率,但四氢呋喃中合成产物的电导率略高于另外两个产物。
红外光谱和拉曼光谱显示,3种溶剂中合成的聚(3,42乙撑二氧噻吩)在化学结构、分子共轭长度、掺杂情况等方面基本相同。
关键词: 聚(3,42乙撑二氧噻吩);化学氧化合成;反应速率;红外光谱;拉曼光谱中图分类号: O631.5文献标识码:A 文章编号:100129731(2005)10216102031 引 言导电聚合物PEDO T (poly (3,42et hylenedioxy 2t hiop hene ))(聚(3,42乙撑二氧噻吩))具有较高的导电性、良好的化学稳定性和热稳定性等优点,因而可以在诸多领域(如固体电解质电容器阴极材料、塑料抗静电涂层、传感器材料以及可充电电池等)得到应用。
化学氧化法合成导电聚合物PEDO T 的要素包括单体EDO T 、氧化剂、掺杂剂以及一定的溶剂,典型的氧化(掺杂)剂是Fe (Ⅲ)盐(如FeCl 3、Fe (O Ts )3等),化学氧化法合成PEDO T 的化学方程式如图1所示[1]。
实验中我们发现,在该反应体系中,(单体+氧化剂)的浓度对反应速率的影响很大,当单体浓度较低时,混合溶液可以在室温下稳定存在数小时,当单体浓度稍高时,聚合反应速度迅速增加。
这意味着混合溶液在浓度稍高时存在着短的“使用寿命”,这在某些应用场合,比如固体铝电容器的制备过程,是将电容器芯子浸渍到上述混合溶液中并使单体和氧化剂在芯子中原位聚合形成导电阴极,这时,希望使用浓度更高的浸渍液以便形成更多的导电聚合物。
Researchers at Linköping University in Sweden have created analog1 and digital electronics circuits inside living plants. The group at the Laboratory of Organic Electronics (LOE), under the leadership of Professor Magnus Berggren, have usedthe vascular2 system of living roses to build key components3 of electronic circuits. The article featured in the journal Science Advances demonstrates wires, digital logic4, and even displays elements - fabricated inside the plants - that could develop new applications for organic electronics and new tools in plant science.Plants are complex organisms that rely on the transport of ionic signals and hormones5 to perform necessary functions. However, plants operate on a much slower time scale making interacting with and studying plants difficult. Augmenting6 plants with electronic functionality would make it possible to combine electric signals with the plant's own chemical processes. Controlling and interfacing7 with chemical pathways in plants could pave the way to photosynthesis-based fuel cells, sensors8 and growth regulators, and devices that modulate9 the internal functions of plants."Previously10, we had no good tools for measuring the concentration of various molecules11 in living plants. Now we'll be able to influence the concentration of the various substances in the plant that regulate growth and development. Here, I see great possibilities for learning more," says Ove Nilsson, professor of plant reproduction biology and director of the Umeå Plant Science Center, as well as a co-author of the article.The idea of putting electronics directly into trees for the paper industry originated in the 1990s while the LOE team at Linköping University was researching printed electronics on paper. Early efforts to introduce electronics in plants were attempted by Assistant Professor Daniel Simon, leader of the LOE's bioelectronics team, and Professor Xavier Crispin, leader of the LOE's solid-state device team, but a lack of funding from skeptical12 investors13 halted these projects.Thanks to independent research money from the Knut and Alice Wallenberg Foundation in 2012, Professor Berggren was able to assemble a team of researchers to reboot the project. The team tried many attempts of introducing conductive polymers through rose stems. Only one polymer, called PEDOT-S, synthesized by Dr. Roger Gabrielsson, successfully assembled itself inside the xylem channels as conducting wires, while still allowing the transport of water and nutrients14. Dr. Eleni Stavrinidou used the material to create long (10 cm) wires in the xylem channels of the rose. By combining the wires with the electrolyte that surrounds these channels she was able to create an electrochemical transistor15, a transistor that converts ionic signals to electronic output. Using the xylem transistors16 she also demonstrated digital logic gate function.词汇解析:1 analogn.类似物,模拟参考例句:The analog signal contains high-frequency video information,which helps make up the picture.模拟信号包括有助于构成图像的高频视频信息。
定义:导电聚合物又称导电高分子,是指通过掺杂等手段,能使得电导率在半导体和导体范围内的聚合物。
通常指本征导电聚合物,这一类聚合物主链上含有交替的单键和双键,从而形成了大的共轭π体系。
π电子的流动产生了导电的可能性。
简介:没有经过掺杂处理的导电聚合物电导率很低,属于绝缘体。
其原因在于导电聚合物的能隙很宽(一维半导体的不稳定性),室温下反键轨道(空带)基本没有电子。
但经过氧化掺杂(使主链失去电子)或还原掺杂(使主链得到电子),在原来的能隙中产生新的极化子、双极化子或孤子能级,其电导率能上升到10~10000 S/cm2,达到半导体或导体的电导率范围。
导电聚合物(聚乙炔)由日本科学家白川英树最先发现,美国科学家Heeger 和MacDiarmid 也是这一研究领域的先驱。
这三位科学家由于在导电聚合物研究中的突出贡献,共同获得了2000年的诺贝尔化学奖。
种类:自1970年代第一种导电聚合物——聚乙炔发现以来,一系列新型的导电高聚物相继问世。
常见的导电聚合物有:聚乙炔、聚噻吩、聚吡咯、聚苯胺、聚苯撑、聚苯撑乙烯和聚双炔等。
聚乙炔是最先报道具有高电导率的、结构最简单的共轭高聚物。
1987年,德国BASF 公司的科学家改进了白川英树的聚合方法,得到的聚乙炔经碘掺杂并拉伸取向后电导率高达2×10^5西/厘米,此数值大约相当于铜电导率(6×105西/厘米)的1/3。
在相同质量的情况下,它显示出比铜高2~3倍的电导率。
由于聚乙炔具有特殊的光学、电学和磁学性质以及可逆的电化学性质,它在二次电池和光电化学电池方面显示诱人的应用前景,但最致命的弱点是它在空气中不稳定。
聚噻吩和聚吡咯具有将聚乙炔的氢用硫或NH取代的结构,尽管它们的电导率没有聚乙炔高,但其稳定性好,能够用于制备电子器件。
被称为“苯胺黑”的聚苯胺粉末早在1910年已经合成出来,然而直到从酸性的水溶液介质中通过苯胺单体的氧化聚合而制备的聚苯胺才具有较高的电导率。
Principles and Applications ofan Intrinsically Conductive Polymer
PEDOT
© 2011 by Taylor and Francis Group, LLCCRC Press is an imprint of theTaylor & Francis Group, an informa business
Boca Raton London New York
Principles and Applications ofan Intrinsically Conductive Polymer
PEDOT
Andreas ElschnerStephan KirchmeyerWilfried LövenichUdo MerkerKnud Reuter
© 2011 by Taylor and Francis Group, LLCCRC PressTaylor & Francis Group6000 Broken Sound Parkway NW, Suite 300Boca Raton, FL 33487-2742
© 2011 by Taylor and Francis Group, LLCCRC Press is an imprint of Taylor & Francis Group, an Informa business
No claim to original U.S. Government worksPrinted in the United States of America on acid-free paper10 9 8 7 6 5 4 3 2 1
International Standard Book Number: 978-1-4200-6911-2 (Hardback)This book contains information obtained from authentic and highly regarded sources. Reasonable efforts have been made to publish reliable data and information, but the author and publisher cannot assume responsibility for the validity of all materials or the consequences of their use. The authors and publishers have attempted to trace the copyright holders of all material reproduced in this publication and apologize to copyright holders if permission to publish in this form has not been obtained. If any copyright material has not been acknowledged please write and let us know so we may rectify in any future reprint.
Except as permitted under U.S. Copyright Law, no part of this book may be reprinted, reproduced, transmit-ted, or utilized in any form by any electronic, mechanical, or other means, now known or hereafter invented, including photocopying, microfilming, and recording, or in any information storage or retrieval system, without written permission from the publishers.
For permission to photocopy or use material electronically from this work, please access www.copyright.com (http://www.copyright.com/) or contact the Copyright Clearance Center, Inc. (CCC), 222 Rosewood Drive, Danvers, MA 01923, 978-750-8400. CCC is a not-for-profit organization that provides licenses and registration for a variety of users. For organizations that have been granted a photocopy license by the CCC, a separate system of payment has been arranged.
Trademark Notice: Product or corporate names may be trademarks or registered trademarks, and are used only for identification and explanation without intent to infringe.
Library of Congress Cataloging-in-Publication DataPEDOT : principles and applications of an intrinsically conductive polymer / Andreas Elschner … [et al.].p. cm.Includes bibliographical references and index.Summary: “A handbook and a guide, this resource provides the full chemical, physical, and technical information about the most forwardly developed electrically conductive polymer, Poly (3,4-ethylenedioxythiophene), otherwise known as PEDOT. Discussing basic knowledge and exploring technical applications, the book is based on information generated by universities and academic research, as well as by industrial scientists, giving the full picture of the experimental and the practical. While there is information available in handbooks on polythiophene chemistry and physics, under which PEDOT falls, until now there has been no book focusing exclusively on this important conducting polymer”-- Provided by publisher.ISBN 978-1-4200-6911-2 (hardback)1. Conducting polymers. 2. Polythiophenes. 3. Polyethylene. I. Elschner, Andreas. II. Title.
QD382.C66P43 2011547’.594--dc22 2010035012
© 2011 by Taylor and Francis Group, LLCv
ContentsForeword .................................................................................................................xiPreface ...................................................................................................................xiiiAcknowledgments .............................................................................................xviiAuthors .................................................................................................................xixAbbreviations ......................................................................................................xxi
1. The Discovery and Development of Conducting Polymers ..................11.1 The Scope of This Historical Overview .............................................11.2 Introduction ...........................................................................................21.3 An Early Example: Polyaniline ...........................................................41.4 The First Electrically Conductive Poly(Heterocycle): Polypyrrole .............................................................................................91.5 The Fundamental Breakthrough: Doped Polyacetylene ...............10References .......................................................................................................15