
Materials Chemistry and Physics 128 (2011) 303–310
Contents lists available at ScienceDirect
Materials Chemistry and
Physics
j o u r n a l h o m e p a g e :w w w.e l s e v i e r.c o m /l o c a t e /m a t c h e m p h y
s
Study of Ni-catalyst for electroless Ni–P deposition on glass ?ber
Libo Li ?,Bo Liu
School of Chemical and Environmental Engineering,Harbin University of Science and Technology,No.4Lin Yuan Road,Harbin 150040,People’s Republic of China
a r t i c l e i n f o Article history:
Received 1August 2010
Received in revised form 26January 2011Accepted 14March 2011
Keywords:
Glass ?ber surfaces
Electroless nickel–phosphorus alloy coatings
Electron microscopy Adhesion
a b s t r a c t
The glass ?ber surface is metalized with electroless nickel–phosphorus deposition.The roughening and activation processes are optimized by the orthogonal experiments.A new nickel-catalyst method is devel-oped to activate the glass ?ber surface.When the activation is completed,a layer of continuous and dense ?lm is formed on the substrate.The activated ?lm contains a great deal of nickel oxide particles which can become the active sites after they are deoxidized in the electroless bath.In the activated ?lm on the glass ?ber,the content of Ni element is 41.01wt.%,the content of O element is 45.64wt.%and the content of P element is 13.35wt.%.Scanning electron microscopy (SEM)shows that the Ni–P coatings obtained under the optimum pretreatment conditions are uniform,continuous and adhered to the glass ?ber surface.Energy dispersive X-ray spectrometry (EDS)points out that the content of the nickel and the phosphorus in the deposits is 87.41wt.%and 12.59wt.%,respectively.X-ray photoelectron spectroscopy (XPS)analysis reveals that the O signal on electroless Ni-coated glass surface corresponds to oxygen in the glass substrate.X-ray diffraction pattern (XRD)indicates the Ni–P coatings are amorphous.
Crown Copyright ? 2011 Published by Elsevier B.V. All rights reserved.
1.Introduction
Glass ?ber is frequently used as the ?ller of macromolecule materials [1].Fiber reinforced polymer composites are an impor-tant class of materials and widely used in electronics industry due to high strength-to-weight ratios and high rigidities.Unfor-tunately,they lack electromagnetic shielding capability;however,electroless plating (EN)can be used to remedy this shortcom-ing [2].Electroless nickel–phosphorus coatings are preferred in many industries such as the oil,chemical,plastic,mechanical,and electronic industries because of their excellent corrosion,wear resistance,hardness,lubricity,uniformity of deposit regardless of geometries,solderability and nonmagnetic properties [3–7].Espe-cially in the electronics industry,a variety of applications including the production of ohmic contacts,shielded materials,chip-level interconnects and printed circuit boards have been described [8,9].The metallization technique of electroless nickel plating is a suit-able method to provide the required surface conductivity for these nonmetallic parts [10,11].The adhesive strength plays an important role in the coatings quality,so the pretreatment is a key process in electroless nickel plating.Palladium salt is used as the traditional catalysts for electroless plating,but it needs the high costs of pro-duction [8].In our study,a nickel salt activation process is used to catalytically activate the glass ?ber,thus,to introduce metallic Ni sites onto the non-metal surface for electroless nickel plating.The
?Corresponding author.Tel.:+86045186848183;fax:+86045186392708.E-mail address:llbo2002@https://www.doczj.com/doc/c115729707.html, (L.Li).
metallic Ni sites work effectively in electroless deposition of nickel without any surface-active impurity.
2.Experimental
The diameter of the glass ?ber in this work was about 12?m.Electroless nickel–phosphorus alloy deposition on the non-metal substrate involved a multi-step procedure.Firstly,surface cleaning and roughening were commonly performed to improve the adhesion.Secondly,chemical treatments (“accelerations”)of the surface-bound activated solution were required to prepare the catalyst for the metal deposition.The Ni catalysts,which were anchored to the surface site,would then be used for electroless Ni–P plating.Finally,the plating process was performed in a 1L glass reaction vessel with a heater and a stirring device.
Speci?cally,the metallization was performed following a procedure in four steps:
?Cleaning in the acetone for 5min,and washing with the deionised water.
?Immersion in a solution consisted of H 2O 2,HF and HNO 3at room temperature for 30s,and washing with the deionised water.The concentration of the roughening solutions was varied according to the needs of the study.The roughening process of electroless Ni–P plating was optimized by the orthogonal experiment method.The three compositions of the roughening solution mentioned above,which were represented by A,B and C,respectively,were taken to be three investigation fac-tors.The levels of the factors for the orthogonal experiment of the roughening process were listed in Table 1.
?Immersion in the activation solution including NiSO 4·6H 2O,NaH 2PO 2·2H 2O and C 2H 6O with ultrasonic at room temperature;then heat treatment in the vacuum oven.The immersion time,the heat treatment time and temperature were varied according to the needs of the study.The activation process of electroless Ni–P plating was carried out by the orthogonal experiment method.The compositions of the three activation solutions and the parameters of the conditions mentioned above,which were represented by A,B,C,D,E and F,respectively,were taken to be six investigation factors.Table 2was the levels of the factors for the orthogonal experiment of the activation process.
0254-0584/$–see front matter Crown Copyright ? 2011 Published by Elsevier B.V. All rights reserved.doi:10.1016/j.matchemphys.2011.03.026
304L.Li,B.Liu /Materials Chemistry and Physics 128 (2011) 303–310
Table 1
Levels of factors for orthogonal experiment of roughening process (L 9(33)).No.A
H 2O 2(ml L ?1)B
HF (ml L ?1)C
HNO 3(ml L ?1)1100200502150400753
200
600
100
Fig.1.The glass ?ber after degreasing.
?The electroless nickel plating was performed by immersing the glass ?ber in an acidic plating solution with pH of 4.4at 90?C for 20min.The composition of the electroless plating solution was given in Table 3.Nickel sulphate was the source for metal ions,sodium hypophosphite was the reductant agent,and sodium cit-rate and lactic acid were used as complexing agent and sodium acetate acted as buffering agent to control pH of the bath during plating process.Ammonia was used to adjust pH of the bath.The chemicals used in this experiment were all of analytical reagent grade.After electroless plating,the samples were rinsed in the deionised water.To obtain reliable results,5specimens were prepared and coated for each exper-imental condition,so ?nal results are average values of 5measurements.
Surface morphology was examined by using a Philips FEI Sirion Scanning Elec-tron Microscopy (SEM),and the accelerating voltage of the SEM is 20kV.The coating composition was determined from quantitative energy dispersive X-ray spectrom-etry (EDS)attached to the SEM.Crystallographic structure was studied by X-ray diffraction (XRD)using an automatic Japanese Rigaku RTP 300PC powder diffrac-tometer with monochromatic Cu K ?radiation.X-ray photoelectron spectroscopy (XPS,PHI-5700,ESCA System)with an Al K ?X-ray source (1486.6eV)and at 45?take-off angle was used to analyze the surface electronic states;the pass energy used to record the wide scan XPS spectra was 187.85eV and the pass energy was 29.35eV for high resolution scans.All XPS peaks were referenced to the C1s signal at a binding energy of 285eV.
The adhesion between Ni–P coatings and the glass ?ber was tested by Ther-mal Shock Method (TSM):the plated ?bers were placed in an oven at 250?C for 1h and then quenched in a mixture of ice and water at 0?C to observe whether it was peeled off or not.The thermal shock experiments were repeated until the coatings were peeled off.The cycle times of the thermal shock exper-iments were recorded to indicate the adhesion strength.The more the
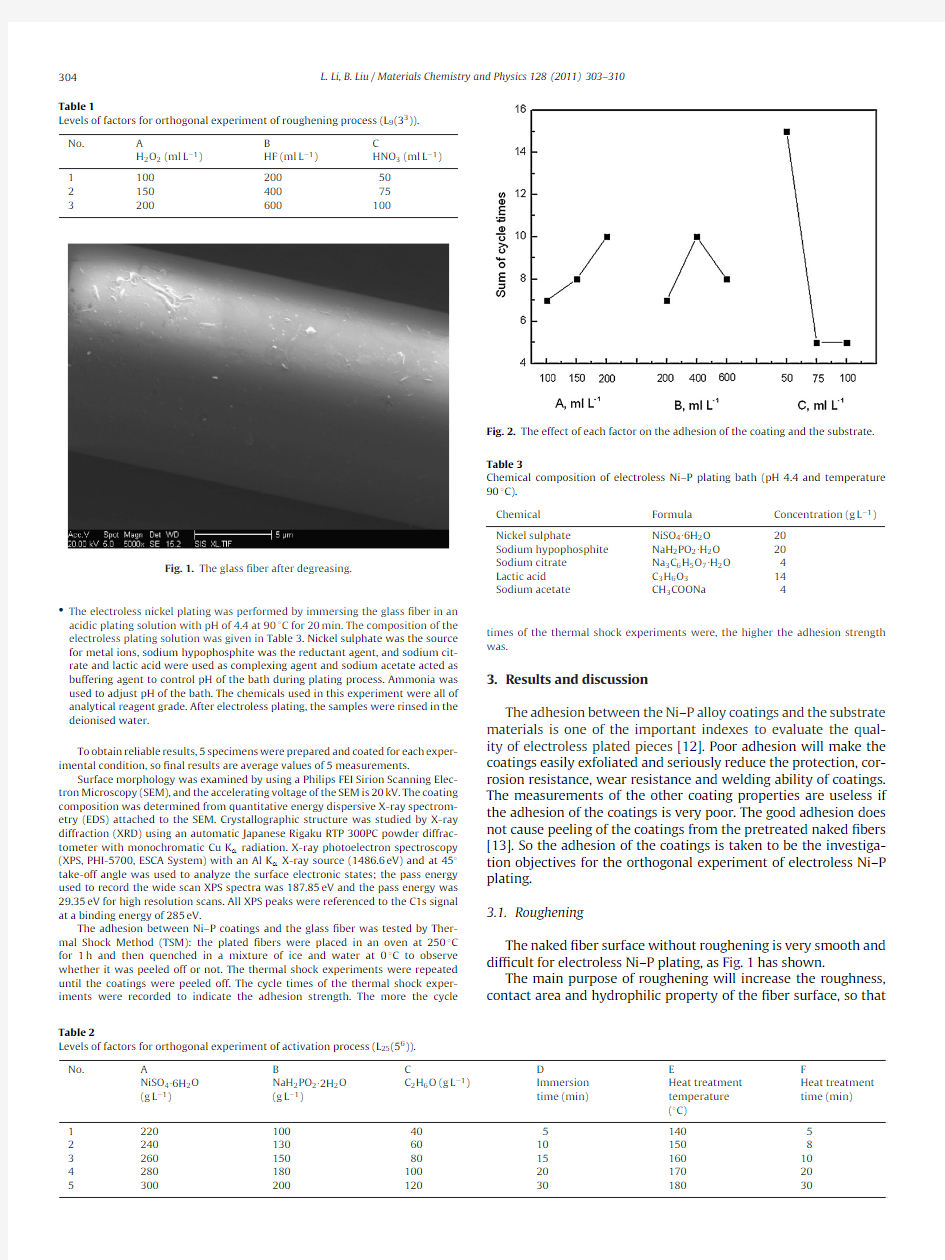
cycle Fig.2.The effect of each factor on the adhesion of the coating and the substrate.Table 3
Chemical composition of electroless Ni–P plating bath (pH 4.4and temperature 90?C).Chemical
Formula Concentration (g L ?1)Nickel sulphate
NiSO 4·6H 2O 20Sodium hypophosphite NaH 2PO 2·H 2O 20Sodium citrate Na 3C 6H 5O 7·H 2O 4Lactic acid
C 3H 6O 3
14Sodium acetate
CH 3COONa
4
times of the thermal shock experiments were,the higher the adhesion strength was.
3.Results and discussion
The adhesion between the Ni–P alloy coatings and the substrate materials is one of the important indexes to evaluate the qual-ity of electroless plated pieces [12].Poor adhesion will make the coatings easily exfoliated and seriously reduce the protection,cor-rosion resistance,wear resistance and welding ability of coatings.The measurements of the other coating properties are useless if the adhesion of the coatings is very poor.The good adhesion does not cause peeling of the coatings from the pretreated naked ?bers [13].So the adhesion of the coatings is taken to be the investiga-tion objectives for the orthogonal experiment of electroless Ni–P plating.
3.1.Roughening
The naked ?ber surface without roughening is very smooth and dif?cult for electroless Ni–P plating,as Fig.1has shown.
The main purpose of roughening will increase the roughness,contact area and hydrophilic property of the ?ber surface,so that
Table 2
Levels of factors for orthogonal experiment of activation process (L 25(56)).No.
A
NiSO 4·6H 2O (g L ?1)B
NaH 2PO 2·2H 2O (g L ?1)C
C 2H 6O (g L ?1)
D
Immersion time (min)E
Heat treatment temperature (?C)F
Heat treatment time (min)122010040514052240130601015083260150801516010428018010020170205300
200
120
30
180
30
L.Li,B.Liu/Materials Chemistry and Physics128 (2011) 303–310
305
Fig.3.SEM photograph of the glass?ber and the glass surface after roughening.(a) The glass?ber surface after the roughening;(b)the glass surface after the roughen-ing.
improve the adhesion between the?ber surface and the Ni–P coat-ing.
The results of the orthogonal experiment(L9(33))for roughen-ing process of electroless Ni–P plating on the glass?ber surface are listed in Table4.
By comparing K in column2–4of Table4,it can be seen that the level of each factor with the largest K are A3,B2,and C1,respec-tively,and they may be combined into level group of A3B2C1to get the coating with higher adhesion.This level group is accordance with that one of No.8experiment in Table4and its result shows that the coating is not peeling after the7th cycle of the thermal shock experiments.Therefore,the level group of A3B2C1,that is, H2O2of200ml L?1,HF of400ml L?1and HNO3of50ml L?1,can be considered as the optimum roughening conditions for electroless Ni–P plating on the glass?ber surface.A factor with larger range value(R)means that the factor has more effect on the adhesion. It can be note that from Table4that the R value of factor C is10 which is larger than that of factors A and B,indicating the former have more effect on the adhesion that the latter.Fig.2is the effect of each factor on the adhesion of the coating and the substrate. The adhesion mainly increases with increase of H2O2concentra-tion(factor A)and decreases with increase of HNO3concentration (factor C).
Fig.3shows the SEM images of the glass?bers(a)and the glass (b)after the roughening obtained from the optimum conditions. From Fig.3,it can be seen that the roughening treatment creates some cracks on the?ber surface to increase the roughness and
area.Fig.4.SEM micrograph of electroless Ni–P plating on the glass?ber surface and the glass surface.(a)The activated?lm on the glass?ber surface;(b)the activated?lm on the glass surface.A layer of continuous and dense?lm is formed.
3.2.Activation
Electroless plating with the catalytic metals is an effective way for the necessary surface treatments[14–17],and the coated lay-ers can serve as the medium for the adhesion and the transferring loads.Palladium generally has been the catalyst of choice[18–21]; however,since this metal is also an excellent hydrogenation and reduction catalyst for a variety of the other chemical reaction, its price has begun to increase because of a supply de?ciency. Accordingly,we have made effort to discover a new,
reasonably Fig.5.EDS spectrum of the activated glass?ber surface.
306L.Li,B.Liu/Materials Chemistry and Physics128 (2011) 303–310
Table4
Results of orthogonal experiment(L9(33))for roughening process of electroless Ni–P plating.
No.A
H2O2(ml L?1)B
HF(ml L?1)
C
HNO3(ml L?1)
Cycle times of TSM
11(100)1(200)1(50)3 21(100)2(400)2(75)2 31(100)3(600)3(100)2 42(150)1(200)2(75)2 52(150)2(400)3(100)1 62(150)3(600)1(50)5 73(200)1(200)3(100)2 83(200)2(400)1(50)7 92(200)3(600)2(75)1 K17715
K28105
K31085
R3310
K1,K2,K3—sum of cycle times of level1,level2and level3,respectively.
R—range:the difference between the largest K value and the smallest K value of each factor.
price catalyst Ni to replace Pd as the catalyst for electroless nickel plating.
The activation conditions are optimized by the orthogonal experiment,and the results are listed in Table5.
The level with larger K value is better than that with smaller K value for getting good coating adhesion.After K values in col-umn2–6of Table5are compared,the level of each factor with the largest K are A5,B5,C2,D1,E2and F5.However,this level group is not included in Table5.The test with the level group of A5B5C2D1E2F5 is set in order to validate the results.The test result has shown that the coatings are perfect and not peeling after the8th cycle of the thermal shock experiments,meaning the higher adhesion obtained by the level group of A5B5C2D1E2F5.Therefore,the opti-mum conditions of the activation process are as follow:immersion in activation solution consisted of300g L?1NiSO4·6H2O,200g L?1 NaH2PO2·2H2O and60ml L?1C2H6O at25?C for5min,then heat treatment in the oven at150?C for30min.The glass?ber is too small to discern the activated surface,even if the scanning electron microscopy is used.Therefore,the activation process is also brought into effect on the glass to observe the images and investigate the surface electronic states.Figs.4and5are the SEM images and EDS spectrum of the glass and glass?ber surface after the activation under the optimum conditions,respectively.A layer of continuous and dense?lm is formed,containing a great deal of the particles.
Table5
Results of orthogonal experiment(L25(56))for activation process of electroless Ni–P plating.
No.A
NiSO4·6H2O
(g L?1)B
NaH2PO2·2H2O
(g L?1)
C
C2H6O(g L?1)
D
Immersion
time(min)
E
Heat treatment
temperature
(?C)
F
Heat treatment
time(min)
Cycle times of TSM
11(220)1(100)1(40)1(5)1(140)1(5)–21(220)2(130)2(60)2(10)2(150)2(8)3 31(220)3(150)3(80)3(15)3(160)3(10)1 41(220)4(180)4(100)4(20)4(170)4(20)1 51(220)5(200)5(120)5(30)5(180)5(30)7 62(240)1(100)2(60)3(15)4(170)5(30)–72(240)2(130)3(80)4(20)5(180)1(5)1 82(240)3(150)4(100)5(30)1(140)2(8)–92(240)4(180)5(120)1(5)2(150)3(10)1 102(240)5(200)1(40)2(10)3(160)4(20)1 113(260)1(100)3(80)5(30)2(150)4(20)–123(260)2(130)4(100)1(5)3(160)5(30)1 133(260)3(150)5(120)2(10)4(170)1(5)1 143(260)4(180)1(40)3(15)5(180)2(8)1 153(260)5(200)2(60)4(20)1(140)3(10)7 164(280)1(100)4(100)2(10)5(180)3(10)1 174(280)2(130)5(120)3(15)1(140)4(20)–184(280)3(150)1(40)4(20)2(150)5(30)–194(280)4(180)2(60)5(30)3(160)1(5)1 204(280)5(200)3(80)1(5)4(170)2(8)1 215(300)1(100)5(120)4(20)3(160)2(8)–225(300)2(130)1(40)5(30)4(170)3(10)–235(300)3(150)2(60)1(5)5(180)4(20)7 245(300)4(180)3(80)2(10)1(140)5(30)2 255(300)5(200)4(100)3(15)2(150)1(5)7 K1121210910
K235188115
K310959410
K43610939
K51622981017
R1321162145
K1,K2,K3,K4,K5—sum of cycle times of level1,level2,level3,level4and level5,respectively.
R—range:the difference between the largest K value and the smallest K value of each factor.
(–)—the cycle times of thermal shock experiments are0,that is,the coating is peeling after electroless plating.
L.Li,B.Liu /Materials Chemistry and Physics 128 (2011) 303–310
307
Fig.6.XPS wide scan spectrum of the glass surface after the activation under the optimum conditions.Ni and P photoelectron signals are observed in the
spectrum.
Fig.7.XPS high-resolution spectrum of nickel on the glass after the activation.The peak at 854.3and 857.4eV is assigned to characteristic of Ni 2±in the NiO and Ni 3±in the Ni 2O 3,
respectively.
Fig.8.XPS high-resolution spectrum of the O1s on the glass after the heat treatment during activation process.The peak at 532.0eV can be assigned as O 2?
species.
Fig.9.SEM photograph of the activated glass surface after removed from the NaH 2PO 2solution.The spherical granulas wrapped in the ?lm are smaller,thinner and
discontinuous.
Fig.10.XPS wide scan spectrum of the activated glass surface deoxidized by NaH 2PO 2solution.After deoxidized by NaH 2PO 2,the active ?lms are too thin to completely cover the glass,so the peaks of Si and O are detected.
On the activated surface,the content of Ni element is 41.01wt.%,the content of O element is 45.64wt.%and the content of P element is 13.35wt.%.
Fig.6shows the XPS wide scan spectrum of the glass surface after the activation under the optimum conditions.Ni and P pho-toelectron signals are observed in the spectrum.
308L.Li,B.Liu /Materials Chemistry and Physics 128 (2011) 303–310
Fig.11.XPS high-resolution spectrum of nickel on the activated glass surface after removed from the NaH 2PO 2solution.The peaks at 853.4eV in Ni2p 3/2level are ascribed to Ni metal.
Fig.12.XPS high-resolution spectrum of the O1s on the activated glass surface after removed from the NaH 2PO 2solution.The O1s peak at 532.4eV corresponds to oxygen in the glass substrate.
Fig.13.SEM images of the glass and the glass ?bers after electroless plating.(a)The plated glass surface (5000×);(b)the plated glass observed at 45?angle (1000×);(c)the plated glass ?ber surface (5000×);(d)the plated glass ?ber surface (1000×);(e)the cross-section of the glass ?ber (1000×).The deposit layer composed of the granulas is uniform,smooth and adhered to the substrate.
L.Li,B.Liu/Materials Chemistry and Physics128 (2011) 303–310
309
Fig.14.EDS spectrum of the plated glass?ber surface.
Fig.7shows XPS high-resolution spectrum of nickel on the glass after the activation.The double peaks of Ni2p3/2at about854.3and 857.4eV indicate that nickel exists in oxidation states of±2(in the NiO)and±3(in the Ni2O3)[22],respectively.It also found that O exists in the?2oxidation state.In Fig.8,the peak at532.0eV can be assigned as O2?species[23].
From the above results,it can been found the active?lms com-prise a great deal of the nickel oxide particles which maybe become the active sites after they are deoxidized in the electroless plating bath.
In order to investigate the formation process of the active sites in the electroless plating bath,the activated glass?ber and glass are put into a simple aqueous hypophosphite solution for10min.Fig.9 shows SEM photographs of the glass surface after removed from the NaH2PO2solution.The nickel oxide particles in the active?lms have deoxidized into the spherical granulas like the electroless Ni coating.But the spherical granulas wrapped in the?lm are smaller, thinner and discontinuous.
Fig.10shows XPS wide scan spectrum of the activated glass surface after removed from the NaH2PO2solution.After deoxidized by NaH2PO2,the active?lms are so thin that it cannot completely cover the glass as has shown in Fig.9,and therefore the peaks of Si and O are detected.
Fig.11is the high-resolution Ni2p spectrum for the activated glass surface after removed from the NaH2PO2solution.The peaks at853.4eV in Ni2p3/2level are ascribed to Ni metal[24,25].Fig.12 shows O1s spectrum of the activated glass surface after removed from the NaH2PO2solution.The O1s peak at532.4eV corresponds to oxygen in the glass substrate[26].The above SEM,EDS and XPS results show that the nickel oxide particles in the active?lms formed in process of activation heat treatment can be deoxidated into the Ni metal(the active sites)by the NaH2PO2in the electroless plating bath.
3.3.Electroless Ni–P plating
When the pretreatments under the optimum roughening and activation conditions are?nished,electroless Ni–P plating on the glass?bers are carried out by putting the sample in the electroless bath.Fig.13shows the SEM images of the glass and the glass?bers after electroless plating.The micrographs of these alloys reveal that the deposit layer composed of the granulas is uniform,smooth and adhered to the substrate.Fig.14is the EDS spectrum of the plated glass?ber surface.The nickel and phosphorus content in the deposits is87.41wt.%and12.59wt.%,respectively.
Fig.15is XPS wide scan spectrum of electroless Ni–P alloy on the glass.It is found that Ni–P alloy dominates on the glass surface during the electroless nickel-plating process,and the peaks inten-sity of Si and O in the glass substrate is greatly lower than one
of
Fig.15.XPS wide scan spectrum of electroless Ni–P alloy on the
glass.
Fig.16.XPS high-resolution spectrum of nickel for electroless Ni-coated glass sur-face.The narrower and sharper Ni2p3/2peak at853.4eV and Ni2p1/2at870.7eV are characteristic of Ni(0).
Fig.10,indicating the higher coverage of the coatings.In XPS high-resolution spectrum of nickel for electroless Ni plating on the glass (Fig.16),the narrower and sharper Ni2p3/2peak at853.4eV and Ni2p1/2at870.7eV are characteristic of Ni(0)[24].Fig.17is
XPS
Fig.17.XPS high-resolution spectrum of the O1s for electroless Ni-coated glass.The O1s peak at532.5eV corresponds to oxygen in the glass.
310L.Li,B.Liu/Materials Chemistry and Physics
128 (2011) 303–310
Fig.18.XRD patters of as-plated deposits.The peak of electroless Ni–P alloy is broad in the X-ray diffraction pattern,indicating that the coating is amorphous.
high-resolution spectrum of the O1s of the Ni-coated glass surface after electroless plating.The O1s peak at532.5eV attributes to oxy-gen in the glass[26],which explains that oxygen signal in the EDS spectrum of the plated glass?ber comes from substrate.
Fig.18shows the structure of the as-plated deposits.The peak of electroless Ni–P alloy is broad in the X-ray diffraction pattern, indicating that the coating is amorphous[21].
It gives clear evidence that more reduced nickel metal is deposited on the isolated Ni catalytic centers(as has shown in Fig.9) in electroless plating bath.With the reaction time increasing,Ni2±can be reduced continuously and then deposited on the tops of deposited Ni layer and/or?lled in the voids and gaps between the existing nickels fractious due to the self-catalytic nature of nickel particles(as Fig.13has shown).
4.Conclusion
A uniform nickel–phosphorus alloy coating is successfully deposited on the glass?ber substrate by electroless plating after the proper roughening and nickel activation.The optimum rough-ening conditions,that is,H2O2of200ml L?1,HF of400ml L?1and HNO3of50ml L?1,can be obtained by the orthogonal experiment. The roughening treatment creates some cracks on the?ber sur-face to increase the roughness and area,therefore,the adhesion between the substrate and the coating is higher under the optimum roughening conditions.The optimum conditions of the activa-tion process are achieved after the orthogonal experiment,that is to say,immersion in activation solution consisted of300g L?1NiSO4·6H2O,200g L?1NaH2PO2·2H2O and60ml L?1C2H6O at25?C for5min,then heat treatment in the oven at150?C for30min.A layer of continuous and dense?lm is formed,containing a great deal of nickel oxide particles after the nickel activation.The nickel oxide particles could become the active sites after they are deoxi-dized in the electroless plating bath.The coating on the glass?ber is uniform,smooth and adhered to the substrate by the optimum pretreatment technique.The nickel and the phosphorus content in the deposits is87.41wt.%and12.59wt.%,respectively.The result of XRD determination shows that the as-deposited Ni–P?lm is amorphous.
Acknowledgement
This work is supported?nancially by Educational Commission of Heilongjiang Province of China(No.11521041)and Natural Science Foundation of Heilongjiang Province of China(No.B201007). References
[1]C.K.Lee,Surf.Coat.Technol.202(2008)4868.
[2]Y.Huang,K.Shi,Z.Liao,Y.Wang,L.Wang,F.Zhu,Mater.Lett.61(2007)1742.
[3]S.C.Domenech,E.Lima Jr.,V.Drago,J.C.De Lima,N.G.Borges Jr.,A.O.V.Avila,V.
Soldi,Appl.Surf.Sci.220(2003)238.
[4]A.Abdel Aal,A.Shaaban,Z.Abdel Hamid,Appl.Surf.Sci.254(2008)1966.
[5]M.Palaniappa,G.Veera Babu,K.Balasubramanian,Mater.Sci.Eng.A471(2007)
165.
[6]M.Benounis,N.Jaffrezic-Renault,Sens.Actuators B100(2004)1.
[7]J.H.Moon,K.H.Kim,H.W.Choi,S.W.Lee,S.J.Park,Ultramicroscopy108(2008)
1307.
[8]W.J.Dressick,L.M.Kondracki,M.S.Chen,S.L.Brandow,E.Matijevi′c,J.M.Calvert,
Colloids Surf.A:Physicochem.Eng.Aspects108(1996)101.
[9]C.K.Lee,Mater.Chem.Phys.114(2009)125.
[10]A.Shibata,Y.Imamura,M.Sone,C.Ishiyama,Y.Higo,Thin Solid Films517
(2009)1935.
[11]S.Abderrahmane,A.Himour,R.Kherrat,E.Chailleux,N.Jaffrezic-Renault,G.
Stremsdoerfer,Sens.Actuators B75(2001)1.
[12]J.Kurdi,H.Ardelean,P.Marcus,P.Jonnard,F.Are?-Khonsari,Appl.Surf.Sci.189
(2002)119.
[13]B.Jiang,L.Xiao,S.Hu,Opt.Mater.31(2009)1532.
[14]M.Charbonnier,M.Romand,Y.Goepfert,D.Léonard,M.Bouadi,Surf.Coat.
Technol.200(2006)5478.
[15]Q.Li,S.Fan,W.Han,C.Sun,W.Liang,Jpn.J.Appl.Phys.36(1997)L501.
[16]X.Chen,J.Xia,J.Feng,W.Li,S.Xie,Compos.Sci.Technol.(2000)301.
[17]L.M.Ang,T.S.A.Hor,G.Q.Xu,C.H.Tung,S.P.Zhan,J.L.S.Wang,Carbon38(2000)
363.
[18]C.R.Xia,X.X.Guo,F.-P.Li,D.-K.Peng,Colloids Surf.A:Physicochem.Eng.Aspects
179(2001)229.
[19]M.Charbonnier,M.Romand,Int.J.Adhes.Adhes.23(2003)277.
[20]S.J.Park,Y.S.Jang,K.Y.Rhee,J.Colloid Interface Sci.245(2002)383.
[21]X.C.Wang,W.B.Cai,W.J.Wang,H.T.Liu,Z.Z.Yu,Surf.Coat.Technol.168(2003)
300.
[22]S.J.Park,Y.S.Jang,J.Colloid Interface Sci.263(2003)170.
[23]X.H.Yu,H.Y.Wang,Z.R.Yang,P.Yin,X.Q.Xin,Appl.Surf.Sci.158(2000)335.
[24]H.Li,H.X.Li,W.L.Dai,W.J.Wang,Z.G.Fang,Appl.Surf.Sci.152(1999)25.
[25]H.X.Li,W.J.Wang,H.Li,J.F.Deng,J.Catal.194(2000)211.
[26]M.Schneider,V.A.Gasparov,W.Richter,M.Deckwerth,C.Riissel,J.Non-Cryst.
Solids215(1997)201.
中等分辨率制备分离的 快速色谱技术 W. Clark Still,* Michael K a h n , and Abhijit Mitra Departm(7nt o/ Chemistry, Columbia Uniuersity,1Veu York, Neu; York 10027 ReceiLied January 26, 1978 我们希望找到一种简单的吸附色谱技术用于有机化合物的常规净化。这种技术是适于传统的有机物大规模制备分离,该技术需使用长柱色谱法。尽管这种技术得到的效果非常好,但是其需要消耗大量的时间,并且由于频带拖尾经常出现低复原率。当分离的样本剂量大于1或者2g时,这些问题显得更加突出。近年来,几种制备系统已经进行了改进,能将分离时间减少到1-3h,并允许各成分的分辨率ΔR f≥(使用薄层色谱分析进行分析)。在这些方法中,在我们的实验室中,媒介压力色谱法1和短柱色谱法2是最成功的。最近,我们发现一种可以将分离速度大幅度提升的技术,可用于反应产物的常规提纯,我们将这种技术称为急骤色谱法。虽然这种技术的分辨率只是中等(ΔR f≥),而且构建这个系统花费非常低,并且能在10-15min内分离重量在的样本。4 急骤色谱法是以空气压力驱动的混合介质压力以及短柱色谱法为基础,专门针对快速分离,介质压力以及短柱色谱已经进行了优化。优化实验是在一组标准条件5下进行的,优化实验使用苯甲醇作为样本,放在一个20mm*5in.的硅胶柱60内,使用Tracor 970紫外检测器监测圆柱的输出。分辨率通过持续时间(r)和峰宽(w,w/2)的比率进行测定的(Figure 1),结果如图2-4所示,图2-4分别放映分辨率随着硅胶颗粒大小、洗脱液流速和样本大小的变化。
南京航空航天大学金城学院 毕业设计(论文)外文文献翻译 系部经济系 专业国际经济与贸易 学生姓名陈雅琼学号2011051115 指导教师邓晶职称副教授 2015年5月
Economic policy,tourism trade and productive diversification (Excerpt) Iza Lejárraga,Peter Walkenhorst The broad lesson that can be inferred from the analysis is that promoting tourism linkages with the productive capabilities of a host country is a multi-faceted approach influenced by a variety of country conditions.Among these,fixed or semi-fixed factors of production,such as land,labor,or capital,seem to have a relatively minor influence.Within the domain of natural endowments,only agricultural capital emerged as significant.This is a result that corresponds to expectations,given that foods and beverages are the primary source of demand in the tourism economy.Hence,investments in agricultural technology may foment linkages with the tourism market.It is also worth mentioning that for significant backward linkages to emerge with local agriculture,a larger scale of tourism may be important. According to the regression results,a strong tourism–agriculture nexus will not necessarily develop at a small scale of tourism demand. It appears that variables related to the entrepreneurial capital of the host economy are of notable explanatory significance.The human development index(HDI), which is used to measure a country's general level of development,is significantly and positively associated with tourism linkages.One plausible explanation for this is that international tourists,who often originate in high-income countries,may feel more comfortable and thus be inclined to consume more in a host country that has a life-style to which they can relate easily.Moreover,it is important to remember that the HDI also captures the relative achievements of countries in the level of health and education of the population.Therefore,a higher HDI reflects a healthier and more educated workforce,and thus,the quality of local entrepreneurship.Related to this point,it is important to underscore that the level of participation of women in the host economy also has a significantly positive effect on linkages.In sum, enhancing local entrepreneurial capital may expand the linkages between tourism and other sectors of the host country.
快速外文文献翻译 在科研过程中阅读翻译外文文献是一个非常重要的环节,许多领域高水平的文献都是外文文献,借鉴一些外文文献翻译的经验是非常必要的。由于特殊原因我翻译外文文献的机会比较多,慢慢地就发现了外文文献翻译过程中的三大利器:Google“翻译”频道、金山词霸(完整版本)和CNKI“翻译助手"。 具体操作过程如下: 1.先打开金山词霸自动取词功能,然后阅读文献; 2.遇到无法理解的长句时,可以交给Google处理,处理后的结果猛一看,不堪入目,可是经过大脑的再处理后句子的意思基本就明了了; 3.如果通过Google仍然无法理解,感觉就是不同,那肯定是对其中某个“常用单词”理解有误,因为某些单词看似很简单,但是在文献中有特殊的意思,这时就可以通过CNKI的“翻译助手”来查询相关单词的意思,由于CNKI的单词意思都是来源与大量的文献,所以它的吻合率很高。 另外,在翻译过程中最好以“段落”或者“长句”作为翻译的基本单位,这样才不会造成“只见树木,不见森林”的误导。 注: 1、Google翻译:https://www.doczj.com/doc/c115729707.html,/language_tools google,众所周知,谷歌里面的英文文献和资料还算是比较详实的。我利用它是这样的。一方面可以用它查询英文论文,当然这方面的帖子很多,大家可以搜索,在此不赘述。回到我自己说的翻译上来。下面给大家举个例子来说明如何用吧比如说“电磁感应透明效应”这个词汇你不知道他怎么翻译, 首先你可以在CNKI里查中文的,根据它们的关键词中英文对照来做,一般比较准确。 在此主要是说在google里怎么知道这个翻译意思。大家应该都有词典吧,按中国人的办法,把一个一个词分着查出来,敲到google里,你的这种翻译一般不太准,当然你需要验证是否准确了,这下看着吧,把你的那支离破碎的翻译在google里搜索,你能看到许多相关的文献或资料,大家都不是笨蛋,看看,也就能找到最精确的翻译了,纯西式的!我就是这么用的。 2、CNKI翻译:https://www.doczj.com/doc/c115729707.html, CNKI翻译助手,这个网站不需要介绍太多,可能有些人也知道的。主要说说它的有点,你进去看看就能发现:搜索的肯定是专业词汇,而且它翻译结果下面有文章与之对应(因为它是CNKI检索提供的,它的翻译是从文献里抽出来的),很实用的一个网站。估计别的写文章的人不是傻子吧,它们的东西我们可以直接拿来用,当然省事了。网址告诉大家,有兴趣的进去看看,你们就会发现其乐无穷!还是很值得用的。https://www.doczj.com/doc/c115729707.html, 3、网路版金山词霸(不到1M):https://www.doczj.com/doc/c115729707.html,/6946901637944806 翻译时的速度: 这里我谈的是电子版和打印版的翻译速度,按个人翻译速度看,打印版的快些,因为看电子版本一是费眼睛,二是如果我们用电脑,可能还经常时不时玩点游戏,或者整点别的,导致最终SPPEED变慢,再之电脑上一些词典(金山词霸等)在专业翻译方面也不是特别好,所以翻译效果不佳。在此本人建议大家购买清华大
外文文献及翻译 题目:利用固定化过氧化氢酶 回收纺织品漂染的废水 专业食品科学与工程 学生姓名梁金龙 班级B食品072 学号0710308119 指导教师郑清
利用固定化过氧化氢酶回收纺织品漂染的废水 Silgia A. Costa1, Tzanko Tzanov1, Filipa Carneiro1, Georg M. Gübitz2 &Artur Cavaco-Paulo1,? 1纺织工程系, 米尼奥大学, 4810吉马尔, 葡萄牙 2环境生物技术系, 格拉茨技术大学, 8010格拉茨, 奥地利 ?作者联系方式(Fax: +351 253 510293; E-mail: artur@det.uminho.pt) 关键词:过氧化氢酶的固定化,酶稳定,过氧化氢,纺织印染 摘要 过氧化氢酶固定在氧化铝载体上并用戊二醛交联,在再循环塔反应器和CSTR反应器中研究贮存稳定性,温度和pH值对酶的活性。固定化酶的在的活性维持在44%,pH值11(30?C),对照组是活性为90%,pH值7(80?C),过氧化氢酶固定化的半衰期时间被提高到2小时(pH12,60?C)。用过氧化氢漂白织物后,洗涤过程中的残留水被固定化酶处理,可以用于再次印染时,记录实验数据。 1 序言 由于新的法规的出台,从生态经济的角度来看(Dickinson1984年),对于纺织行业中存在的成本和剩余水域的污染问题,必须给予更多的关注。过氧化氢是一种漂白剂,广泛应用于工业纺织工艺(Spiro & Griffith1997年)。在去除H2O2时,会消耗大量的水和能源(Weck 1991, St?hr & Petry 1995),以避免对氧气敏感的染料(Jensen 2000)产生后续问题。过氧化氢酶可用于降低过氧化氢的含量(Vasudevan & Thakur 1994, Emerson et al. 1996),从而减少水分消耗或方便回收印染洗涤液。过氧化氢酶的使用主要问题出在漂白时温度和清洗液碱度过高。以前,我们试图通过筛选新的嗜热嗜碱的微生物(Paar et al. 2001)或使用不同的酶稳定剂(Costa et al. 2001)来解决此问题。但是染料与蛋白质之间的潜在相互作用(Tzanov et al. 2001a, b)表明,可溶性过氧化氢酶的使用是不恰当的。固定化过氧化氢酶的使用还有一种选择(Costa et al. 2001, Amar et al. 2000)。在这项研究中,我们对氧化铝进行共价固定并使用戊二醛作为交联剂,这种方法在商业中得到验证。本项研究的目的就是探讨过氧化氢酶的固定化动力学,及其稳定性和工艺条件,这将使我们能够应用此系统,以处理可能被用于清洗染色的反复使用的酒。 2 材料和方法 2.1 酶 Terminox(EC1.11.1.6),50L以上,由AQUITEX- Maia提供,葡萄牙产。 2.2 过氧化氢酶的固定化 取Al2O3颗粒或薄片(Aldrich),直径分别为3和7毫米,在45摄氏度下,先经浓度4%的γ-氨丙基三乙氧基硅烷(Sigma)烷基化,再放入丙酮中浸泡24小时。用蒸馏水洗涤硅烷化载体后,放入浓度为2%戊二醛水溶液中室温下浸泡2小时(Aldrich),重复清洗一次并在60?C下干燥1小时。取五克的烷基化载体,室温24?C下浸泡在25毫升粗酶制剂中(Costa et al. 2001)。得出,每克Al2O3
Advantages of Managed Code Microsoft intermediate language shares with Java byte code the idea that it is a low-level language witha simple syntax , which can be very quickly translated intonative machine code. Having this well-defined universal syntax for code has significant advantages. Platform independence First, it means that the same file containing byte code instructions can be placed on any platform; atruntime the final stage of compilation can then be easily accomplished so that the code will run on thatparticular platform. In other words, by compiling to IL we obtain platform independence for .NET, inmuch the same way as compiling to Java byte code gives Java platform independence. Performance improvement IL is actually a bit more ambitious than Java bytecode. IL is always Just-In-Time compiled (known as JIT), whereas Java byte code was ofteninterpreted. One of the disadvantages of Java was that, on execution, the process of translating from Javabyte code to native executable resulted in a loss of performance. Instead of compiling the entire application in one go (which could lead to a slow start-up time), the JITcompiler simply compiles each portion of code as it is called (just-in-time). When code has been compiled.once, the resultant native executable is stored until the application exits, so that it does not need to berecompiled the next time that portion of code is run. Microsoft argues that this process is more efficientthan compiling the entire application code at the start, because of the likelihood that large portions of anyapplication code will not actually be executed in any given run. Using the JIT compiler, such code willnever be compiled.
New technique of the computer network Abstract The 21 century is an ages of the information economy, being the computer network technique of representative techniques this ages, will be at very fast speed develop soon in continuously creatively, and will go deep into the people's work, life and study. Therefore, control this technique and then seem to be more to deliver the importance. Now I mainly introduce the new technique of a few networks in actuality live of application. keywords Internet Network System Digital Certificates Grid Storage 1. Foreword Internet turns 36, still a work in progress Thirty-six years after computer scientists at UCLA linked two bulky computers using a 15-foot gray cable, testing a new way for exchanging data over networks, what would ultimately become the Internet remains a work in progress. University researchers are experimenting with ways to increase its capacity and speed. Programmers are trying to imbue Web pages with intelligence. And work is underway to re-engineer the network to reduce Spam (junk mail) and security troubles. All the while threats loom: Critics warn that commercial, legal and political pressures could hinder the types of innovations that made the Internet what it is today. Stephen Crocker and Vinton Cerf were among the graduate students who joined UCLA professor Len Klein rock in an engineering lab on Sept. 2, 1969, as bits of meaningless test data flowed silently between the two computers. By January, three other "nodes" joined the fledgling network.
Inventory management Inventory Control On the so-called "inventory control", many people will interpret it as a "storage management", which is actually a big distortion. The traditional narrow view, mainly for warehouse inventory control of materials for inventory, data processing, storage, distribution, etc., through the implementation of anti-corrosion, temperature and humidity control means, to make the custody of the physical inventory to maintain optimum purposes. This is just a form of inventory control, or can be defined as the physical inventory control. How, then, from a broad perspective to understand inventory control? Inventory control should be related to the company's financial and operational objectives, in particular operating cash flow by optimizing the entire demand and supply chain management processes (DSCM), a reasonable set of ERP control strategy, and supported by appropriate information processing tools, tools to achieved in ensuring the timely delivery of the premise, as far as possible to reduce inventory levels, reducing inventory and obsolescence, the risk of devaluation. In this sense, the physical inventory control to achieve financial goals is just a means to control the entire inventory or just a necessary part; from the perspective of organizational functions, physical inventory control, warehouse management is mainly the responsibility of The broad inventory control is the demand and supply chain management, and the whole company's responsibility. Why until now many people's understanding of inventory control, limited physical inventory control? The following two reasons can not be ignored: First, our enterprises do not attach importance to inventory control. Especially those who benefit relatively good business, as long as there is money on the few people to consider the problem of inventory turnover. Inventory control is simply interpreted as warehouse management, unless the time to spend money, it may have been to see the inventory problem, and see the results are often very simple procurement to buy more, or did not do warehouse departments . Second, ERP misleading. Invoicing software is simple audacity to call it ERP, companies on their so-called ERP can reduce the number of inventory, inventory control, seems to rely on their small software can get. Even as SAP, BAAN ERP world, the field of
毕业设计说明书 英文文献及中文翻译 学院:专 2011年6月 电子与计算机科学技术软件工程
https://www.doczj.com/doc/c115729707.html, Overview https://www.doczj.com/doc/c115729707.html, is a unified Web development model that includes the services necessary for you to build enterprise-class Web applications with a minimum of https://www.doczj.com/doc/c115729707.html, is part of https://www.doczj.com/doc/c115729707.html, Framework,and when coding https://www.doczj.com/doc/c115729707.html, applications you have access to classes in https://www.doczj.com/doc/c115729707.html, Framework.You can code your applications in any language compatible with the common language runtime(CLR), including Microsoft Visual Basic and C#.These languages enable you to develop https://www.doczj.com/doc/c115729707.html, applications that benefit from the common language runtime,type safety, inheritance,and so on. If you want to try https://www.doczj.com/doc/c115729707.html,,you can install Visual Web Developer Express using the Microsoft Web Platform Installer,which is a free tool that makes it simple to download,install,and service components of the Microsoft Web Platform.These components include Visual Web Developer Express,Internet Information Services (IIS),SQL Server Express,and https://www.doczj.com/doc/c115729707.html, Framework.All of these are tools that you use to create https://www.doczj.com/doc/c115729707.html, Web applications.You can also use the Microsoft Web Platform Installer to install open-source https://www.doczj.com/doc/c115729707.html, and PHP Web applications. Visual Web Developer Visual Web Developer is a full-featured development environment for creating https://www.doczj.com/doc/c115729707.html, Web applications.Visual Web Developer provides an ideal environment in which to build Web sites and then publish them to a hosting https://www.doczj.com/doc/c115729707.html,ing the development tools in Visual Web Developer,you can develop https://www.doczj.com/doc/c115729707.html, Web pages on your own computer.Visual Web Developer includes a local Web server that provides all the features you need to test and debug https://www.doczj.com/doc/c115729707.html, Web pages,without requiring Internet Information Services(IIS)to be installed. Visual Web Developer provides an ideal environment in which to build Web sites and then publish them to a hosting https://www.doczj.com/doc/c115729707.html,ing the development tools in Visual Web Developer,you can develop https://www.doczj.com/doc/c115729707.html, Web pages on your own computer.
英文翻译 英语原文: . Introducing Classes The only remaining feature we need to understand before solving our bookstore problem is how to write a data structure to represent our transaction data. In C++ we define our own data structure by defining a class. The class mechanism is one of the most important features in C++. In fact, a primary focus of the design of C++ is to make it possible to define class types that behave as naturally as the built-in types themselves. The library types that we've seen already, such as istream and ostream, are all defined as classesthat is,they are not strictly speaking part of the language. Complete understanding of the class mechanism requires mastering a lot of information. Fortunately, it is possible to use a class that someone else has written without knowing how to define a class ourselves. In this section, we'll describe a simple class that we canuse in solving our bookstore problem. We'll implement this class in the subsequent chapters as we learn more about types,expressions, statements, and functionsall of which are used in defining classes. To use a class we need to know three things: What is its name? Where is it defined? What operations does it support? For our bookstore problem, we'll assume that the class is named Sales_item and that it is defined in a header named Sales_item.h. The Sales_item Class The purpose of the Sales_item class is to store an ISBN and keep track of the number of copies sold, the revenue, and average sales price for that book. How these data are stored or computed is not our concern. To use a class, we need not know anything about how it is implemented. Instead, what we need to know is what operations the class provides. As we've seen, when we use library facilities such as IO, we must include the associated headers. Similarly, for our own classes, we must make the definitions associated with the class available to the compiler. We do so in much the same way. Typically, we put the class definition into a file. Any program that wants to use our class must include that file. Conventionally, class types are stored in a file with a name that, like the name of a program source file, has two parts: a file name and a file suffix. Usually the file name is the same as the class defined in the header. The suffix usually is .h, but some programmers use .H, .hpp, or .hxx. Compilers usually aren't picky about header file names, but IDEs sometimes are. We'll assume that our class is defined in a file named Sales_item.h. Operations on Sales_item Objects
英文翻译 A comprehensive overview of substations Along with the economic development and the modern industry developments of quick rising, the design of the power supply system become more and more completely and system. Because the quickly increase electricity of factories, it also increases seriously to the dependable index of the economic condition, power supply in quantity. Therefore they need the higher and more perfect request to the power supply. Whether Design reasonable, not only affect directly the base investment and circulate the expenses with have the metal depletion in colour metal, but also will reflect the dependable in power supply and the safe in many facts. In a word, it is close with the economic performance and the safety of the people. The substation is an importance part of the electric power system, it is consisted of the electric appliances equipments and the Transmission and the Distribution. It obtains the electric power from the electric power system, through its function of transformation and assign, transport and safety. Then transport the power to every place with safe, dependable, and economical. As an important part of power’s transport and control, the transformer substation must change the mode of the traditional design and control, then can adapt to the modern electric power system, the development of modern industry and the of trend of the society life. Electric power industry is one of the foundations of national industry and national economic development to industry, it is a coal, oil, natural gas, hydropower, nuclear power, wind power and other energy conversion into electrical energy of the secondary energy industry, it for the other departments of the national economy fast and stable development of the provision of adequate power, and its level of development is a reflection of the country's economic development an important indicator of the level. As the power in the industry and the importance of the national economy, electricity transmission and distribution of electric energy used in these areas is an indispensable component.。Therefore, power transmission and distribution is critical. Substation is to enable superior power plant power plants or power after adjustments to the lower load of books is an important part of power transmission. Operation of its functions, the capacity of a direct impact on the size of the lower load power, thereby affecting the industrial production and power consumption.Substation system if a link failure, the system will protect the part of action. May result in power outages and so on, to the production and living a great disadvantage. Therefore, the substation in the electric power system for the protection of electricity reliability,