MnO2Poly(3,4-ethylenedioxythiophene) Coaxial Nanowires
- 格式:pdf
- 大小:142.79 KB
- 文档页数:2


收稿日期2020-11-16基金项目江西省自然科学基金面上项目(编号:20192BAB206039);南昌大学研究生创新专项资金项目(编号:CX2019118);“大学生创新创业训练计划”项目(编号:S202010403017)。
作者简介张亚宣(1994—),男,硕士研究生。
通信作者欧阳二明(1976—),男,副教授,博士,硕士研究生导师。
总第537期2021年第3期金属矿山METAL MINECo-g-C 3N 4/La-TiO 2复合材料光催化降解废水中乙基黄药张亚宣1姚振龙2汪遵盛1汪存会1欧阳二明3(1.南昌大学建筑工程学院,江西南昌330031;2.南昌大学际銮书院,江西南昌330031;3.南昌大学资源环境与化工学院,江西南昌330031)摘要为了降解选矿废水中残留的浮选药剂乙基黄药,采用溶胶-凝胶法制备了一种复合型光催化材料Co-g-C 3N 4/La-TiO 2,通过SEM、XRD、UV-Vis、光致发光光谱和XPS 等对样品的形貌、晶型结构、光化学性质和表面化学性质进行表征,研究了催化剂用量、模拟废水pH、乙基黄药初始浓度和时间对光催化效果的影响,并利用活性自由基捕捉试验确定光催化过程中起作用的活性基团。
结果表明:Co-g-C 3N 4/La-TiO 2中La 和Co 以La 3+和Co 2+形式存在,其禁带宽度减小,吸收带边红移,光催化效率较高。
在催化剂投加量为1g/L,乙基黄药浓度为60mg/L,pH 为10的条件下,光催化60min 后乙基黄药的降解率达到了99.1%。
空穴(h +)、羟基自由基(·OH)、超氧自由基(·O 2-)都在光催化氧化降解乙基黄药的过程中起到了重要作用,对光催化的贡献程度为:·OH>h +>·O 2-。
关键词乙基黄药二氧化钛石墨相氮化碳金属掺杂光催化氧化中图分类号X703文献标志码A文章编号1001-1250(2021)-03-206-08DOI 10.19614/ki.jsks.202103029Photocatalytic Degradation of Ethyl Xanthate in Wastewater by Co -g -C 3N 4/La -TiO 2Composite MaterialZHANG Yaxuan 1YAO Zhenlong 2WANG Zunsheng 1WANG Cunhui 1OUYANG Erming 1(1.School of Architectural Engineering ,Nanchang University ,Nanchang 330031,China ;2.Jiluan Academy ,NanchangUniversity ,Nanchang 330031,China ;3.School of Resources Environmental and Chemical Engineering ,NanchangUniversity ,Nanchang 330031,China )AbstractIn order to degrade the flotation agent ethyl xanthate remaining in the beneficiation wastewater ,a compos⁃ite photocatalytic material Co -g -C 3N 4/La -TiO 2was prepared by the sol -gel method.The morphology ,crystal structure ,photo⁃chemical properties and surface chemical properties of the samples were characterized by SEM ,XRD ,UV -Vis ,PL and XPS spectrum.The effects of catalyst dosage ,simulated wastewater pH ,initial concentration of ethyl xanthate and time on the photocatalytic effect were studied.Free radical capture experiments have also been carried out to determine the active groups that play a role in the photocatalysis process.The results showed that La and Co in Co -g -C 3N 4/La -TiO 2exist in the form of La 3+and Co 2+.The band gap decreases and the absorption band edge was red -shifted ,thus the photocatalytic efficien⁃cy is high.Under the conditions of catalyst dosage of 1g/L ,ethyl xanthate concentration of 60mg/L ,and pH of 10,the degradation rate of ethyl xanthate reached 99.1%after 60minutes of photocatalysis reaction.Holes (h +),hydroxyl radicals (·OH )and superoxide radicals (·O 2-)all have a great contribution to the degradation of ethyl xanthate during the processof photocatalytic oxidative ,and the degree of contribution is ranked as ·OH >h +>·O 2-.Keywords ethyl xanthate ,titanium dioxide ,graphite phase carbon nitride ,metal doping ,photocatalytic oxidationSeries No.537March 2021黄药作为最常用的捕收剂,大量存在于硫化矿的浮选废水中[1]。

目录第一章引言 (1)1.1 四氧化三锰的构造、性质 (1)1.1.1 四氧化三锰的简介 (1)1.1.2 四氧化三锰的性质 (1)1.1.3 四氧化三锰的构造 (2) (3)1.2.1 工艺的根本概况 (3)1.2.2 工艺的方法 (4) (5)1.4 行业现状 (6)1.5 四氧化三锰的应用 (6)1.6 国内四氧化三锰的研究方向 (7)1.6.1 提高产品质量, 提升产品价值 (7)1.6.2加强市场调研, 开拓国际市场 (7)第二章实验局部 (9) (9)2.1.1 反响原理 (9)2.1.2 工艺流程 (10)2.2 试剂、仪器 (10)内容 (11)2.3.1 Mn3O4的制备思路 (11)2.3.2 实验步骤 (11)Mn3O4产品的检测 (12)第三章结果与讨论 (13) (13)第四章结论 (19)参考文献 (20)致谢 (21)第一章引言1.1 四氧化三锰的构造、性质1.1.1 四氧化三锰的简介四氧化三锰,化学式:Mn3O4,英文名:manganous manganic oxide。
大多数情况下,它是棕红色粉末,通常认为是一种锰的混合氧化物,即MnO·Mn2O3,由于Mn3O4中的Mn2+的核外电子排布式为3d5,Mn3+的核外电子排布式为3d',两种离子均有未成对电子,两种电子构造均具有磁性,因而Mn3O4具有磁性。
四氧化三锰的外观1.1.2 四氧化三锰的性质天然条件下,Mn304常以黑锰矿的形式存在,天然黑锰矿为浅红色或褐色,在锰的氧化为重最稳定.相对密度为 4.8578/cm3,熔点为1563℃,相对分子质量为228. 82,锰的理论含量72 .3%,硬度为5,不溶于水,能够在硝酸,盐酸和硫酸中溶解。
其它任何锰的氧化物在空气中灼烧都可得到棕红色的粉末,如硫酸锰在970℃的加热条件下、二氧化锰在空气中加热到940℃、都能够生成Mn3O4;二氧化锰与二氧化碳作用也生成Mn3O4。

纳米二氧化锰的可控制备及其电化学储能机理研究吴彻平;彭家惠【摘要】通过添加十二烷基溴化氨(CTAB)利用液相沉淀法制备了不同形貌的纳米二氧化锰,用扫描电镜、X射线衍射、孔结构以及循环伏安法等分析研究了CTAB添加量对二氧化锰形貌和电化学性能的影响.结果表明,随着CTAB添加量的增加,二氧化锰形貌从长棒状到均匀球状发生规律变化,此外随着颗粒尺寸变小,二氧化锰比容量从162F/g提高到了213F/g.%A series of nanosized manganese dioxides have been synthesized via a co-precipitation process with additive of CTAB. SEM result indicated that with additive of CTAB,MnO2 nanosphere with about 20nm in diameter and a narrow particle size distribution has been obtained, comparable with nanorode morphology with a wide particle size distribution synthesized without CTAB. The adding amount of CTAB has been studied by SEM,XRD, N2 adsorption and desorption and cyclic voltammetric tests. The results found that as the decrease in particle sizeof MnO2 ,the specific capacitance of MnO2 increased from 162 to 213F/g.【期刊名称】《功能材料》【年(卷),期】2011(042)002【总页数】3页(P359-361)【关键词】二氧化锰;十二烷基溴化氨;共沉淀法;形貌【作者】吴彻平;彭家惠【作者单位】重庆大学材料科学与工程学院,重庆,400045;重庆大学材料科学与工程学院,重庆,400045【正文语种】中文【中图分类】TB332随着低炭经济的到来,新型绿色能源的综合高效开发利用已得到研究人员的广泛关注。
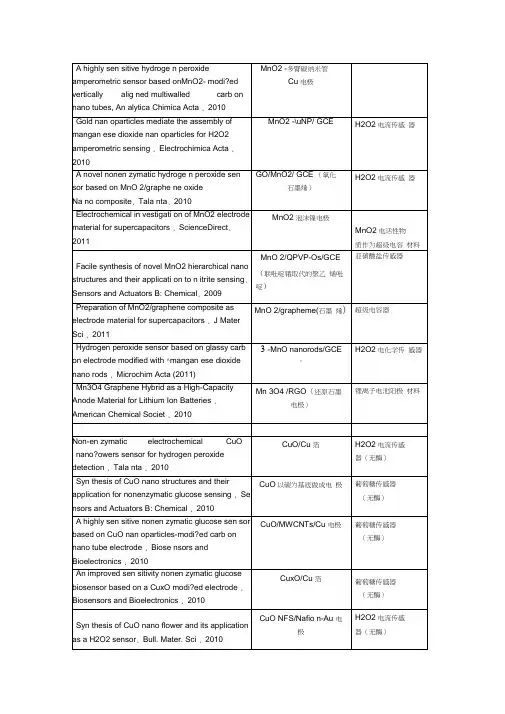
f-MWCNTs /GCESWCNH/GCE (单臂碳纳米管) graphe ne /GCEGR -CS/GCE,(石墨烯-壳聚糖)chitosan-graphene-GCE (壳聚糖-石墨烯) nano-Cu/PPy/GCE (聚吡咯) CPB/chitosan/GCE 溴化十六烷基吡啶 Chitosa n-CTAB /GCE亚硝酸盐MnO2/QPVP-Os/GCE (联吡啶锇取代的 聚乙烯吡啶) nano-Au/Ch/GCE (氯化胆碱)CR-GO/GCE (还原型氧化石墨烯) Nano-AI2O3 /GCEDAB /GCE (双十二烷基溴化铵) 对乙酰氨基酚 f-MWCNTs /GCE MWNT or SWNT/EPPGMWCNTs:graphite/GCE 多臂碳纳米管和石墨混合 Carbon nano tubes paste electrodes 碳纳米管糊电极 graphene /GCE (石墨烯)PAMAMPAMAM/Fe3O4 /GCE PAMAM/CoTe /GCE纳米AI2O3,对有机磷分子有较好的氧化还原活性。
其氮原子上一对孤对电子易于从溶液中结合一个氢质子•。
人们充分利用生物高分子壳聚糖的生物相容性、低 不断开发它的应用领域。
在分析化学上,己用于分离富Kazunori 等用壳聚糖修饰电极测定 北京大学叶宪曾研究组曾用壳聚糖修饰玻Au (lll ),Ag (l ),Pt (ll )和 Pd (ll )。
尿酸和乙酰氨基酚对葡萄糖检测的干扰。
多巴胺花状 ZnO/GCE多巴胺传感器同时检测多巴胺和对乙酰氨基酚 同时检测尿酸、多巴胺和抗坏血酸 抗坏血酸存在下检测多巴胺 抗坏血酸存在下检测多巴胺 同时检测抗坏血酸、多巴胺、尿酸 同时测定多巴胺和尿酸 同时检测多巴胺和抗坏血酸 同时检测多巴胺和抗坏血酸亚硝酸盐传感器 亚硝酸盐传感器亚硝酸盐传感器 对亚硝酸盐的检测检测水样中的亚硝酸盐同时检测多巴胺和对乙酰氨基酚 测定对乙酰基氨基酚 对乙酰氨基酚(扑热息痛) 对乙酰氨基酚对乙酰氨基酚的检测,不受多巴 胺和抗坏血酸的干扰测定牛奶中的双酚 A 测定水中的双酚A壳聚糖分子链上有许多游离的氨基, 而使壳聚糖成为带正电荷的聚电解质 毒性、生物可降解性以及可食用性, 集痕量Ni (ll ) , Cu (ll ) , Cd (ll )。

物 理 化 学 学 报Acta Phys. -Chim. Sin. 2022, 38 (5), 2006059 (1 of 8)Received: June 23, 2020; Revised: July 28, 2020; Accepted: August 12, 2020; Published online: August 17, 2020. *Correspondingauthors.Emails:******************.cn(J.D.);******************.cn(Q.L.).The project was supported by the National Natural Science Foundation of China (21975281, 21773293, 21603264), the National Key Research and Development Program of China (2016YFA0203301), the Jiangsu Planned Projects for Postdoctoral Research Funds, China (2019K048), and the Suzhou Science and Technology Plan Project, China (SYG201926).国家自然科学基金(21975281, 21773293, 21603264), 国家重点研发计划(2016YFA0203301), 江苏省博士后基金(2019K048), 苏州市科技计划项目(SYG201926)资助© Editorial office of Acta Physico-Chimica Sinica[Article] doi: 10.3866/PKU.WHXB202006059 A Polypyrrole-Coated MnO 2/Carbon Nanotube Film Cathode for Rechargeable Aqueous Zn-Ion BatteriesXiaofan Shen 1,2, Xiaona Wang 1, Nengsheng Yu 1,2, Wei Yang 1, Yurong Zhou 1,2, Yanhong Shi 1, Yulian Wang 1,3, Lizhong Dong 1,3, Jiangtao Di 1,3,*, Qingwen Li 1,3,*1 Key Laboratory of Multifunctional Nanomaterials and Smart Systems, Advanced Materials Division, Suzhou Institute ofNano-Tech and Nano-Bionics, Chinese Academy of Sciences, Suzhou 215123, Jiangsu Province, China.2 Nano Science and Technology Institute, University of Science and Technology of China, Suzhou 215123, Jiangsu Province, China.3 School of Nano-Tech and Nano-Bionics, University of Science and Technology of China, Hefei 230026, China.Abstract: Rechargeable aqueous Zinc-ion batteries (ZIBs) have emerged as potential energy storage devices due to their high energy density, low cost, and safety. To date, numerous cathodes based on manganese dioxide, vanadium dioxide, and polyanionic compounds have been reported. Among them, MnO 2 cathodes are particularly desirable candidates for commercialization owing to their tunnel structure and affordability. In particular, the parasitic reaction of Mn-based cathodes in alkaline batteries can be suppressed in mild aqueous electrolytes, resulting in enthusiasm for the development of rechargeable Zn||MnO 2 batteries. Even though various MnO 2 phases have been reported as hosts for Zn 2+/H + insertion, MnO 2 crystal structures undergo significant,irreversible transformations during cycling, which is a major challenge in Zn||MnO 2 batteries. In addition, the tunnel structure can collapse under the insertion of the hydrated cation resulting in Mn 2+ dissolution into the electrolyte and significant loss in capacity over long cycling periods. The MnO 2 cathode also has low intrinsic electronic conductivity due to the large charge transfer resistance, which limits the diffusivity of divalent ions. Despite the achievements made in the field of ZIBs so far, designing active materials and ZIBs systems to meet commercial requirements is a significant challenge. In this study, we report the preparation of polypyrrole-wrapped MnO 2/carbon nanotubes (PPy@MnO 2/CNT) as composite cathodes for aqueous ZIBs. A combination of design strategies was used to increase structural stability and improve electronic conductivity, including increased electrode/electrolyte interaction by using nano-sized structures, shortened diffusion pathways through multistage composites, and enhanced electrical conductivity with conductive composites. The three-dimensional (3D) structured PPy/CNT network can facilitate mass and charge transport during the charge and discharge processes. The structure of MnO 2 wrapped by polypyrrole effectively prevents the dissolution of MnO 2. Thus, the assembled Zn||MnO 2 batteries, using PPy@MnO 2/CNT composite cathodes, exhibit a high capacity of 210 mAh·g −1 at 1 A·g −1, and achieve 85.7% capacity retention after 1000 charge/discharge cycles. Moreover, a high specific capacity of 100 mAh·g −1 could be maintained at 2 A·g −1, exhibiting excellent kinetic performance. The assembled quasi-solid Zn//MnO 2 battery, benefiting from the xanthan gum electrolyte and flexible CNT film, possesses intrinsic safety, bending resistance, and high potential in wearable applications.Key Words: Zinc-ion battery; PPy@MnO 2; Carbonnanotube; Flexible battery; Longlife聚吡咯@二氧化锰/碳纳米管薄膜电极的制备及在高性能锌离子电池中的应用沈晓帆1,2,王晓娜1,俞能晟1,2,杨薇1,周雨融1,2,石艳红1,王玉莲1,3,董立忠1,3,邸江涛1,3,*,李清文1,3,*1中国科学院苏州纳米技术与纳米仿生研究所,先进材料部,中国科学院多功能材料与轻巧系统重点实验室,江苏苏州 2151232中国科学技术大学纳米科学技术学院,江苏苏州 2151233中国科学技术大学纳米技术与纳米仿生学院,合肥 230026摘要:中性/弱酸性水系锌锰电池因其能量密度高、价格低廉、环境友好等优势受到广泛关注。

多晶型MnO_(2)-Ru复合催化剂的制备及其电催化水解析氧性能的研究李佳;袁仲纯;姚梦琴;刘飞;马俊【期刊名称】《人工晶体学报》【年(卷),期】2024(53)2【摘要】在二氧化锰(MnO_(2))中引入杂原子是调整电化学水氧化催化活性位点的有效方法。
在电催化析氧反应(OER)中,虽然已经研究出了许多MnO_(2)的改性方法,但很少有研究以MnO_(2)为主体,讨论MnO_(2)晶型对催化活性的影响。
基于此,本文制备了4种晶型的MnO_(2)(α-MnO_(2)、β-MnO_(2)、γ-MnO_(2)和δ-MnO_(2)),并系统地研究了Ru加入MnO_(2)制备得到的催化剂(x-MnO_(2)-Ru)的OER性能。
线性扫描伏安法和计时电位法测试结果表明,β-MnO_(2)在Ru加入后得到的催化剂(β-MnO_(2)-Ru)电化学性能最佳,在电流密度为10 mA·cm^(-2)时拥有300 mV的较低过电位,而且运行24 h后仍保持较好的催化活性。
结合表征发现,β-MnO_(2)-Ru具有较多的Mn^(3+)和缺陷氧空位,从而具有优异的电催化性能。
【总页数】8页(P336-343)【作者】李佳;袁仲纯;姚梦琴;刘飞;马俊【作者单位】贵州大学化学与化工学院【正文语种】中文【中图分类】TQ032.4【相关文献】1.炭载Ru-Fe催化剂对直接甲酸燃料电池中氧还原的电催化性能研究2.过渡金属掺杂Ru-Se簇合物电催化剂氧还原性能的对比研究3.ZIF-8/C60复合物衍生电催化剂的制备及其水氧化性能4.核壳结构Ru@PtRu纳米花电催化剂的制备及碱性氢析出反应性能研究因版权原因,仅展示原文概要,查看原文内容请购买。

不同溶剂中导电聚合物P EDO T 的化学氧化聚合及光谱研究3汪斌华1,2,邓永红3,戈 钧3,周 啸3,王晓工3,杨邦朝1(1.电子科技大学微电子与固体电子学院,四川成都610041;2.厦门信达电子有限公司企业博士后工作站,福建厦门361021;3.清华大学化工系,北京100084)摘 要: 利用3,42乙撑二氧噻吩单体和对甲苯磺酸铁,分别以异丙醇、四氢呋喃和乙氰为溶剂,用化学氧化法合成了导电聚合物聚(3,42乙撑二氧噻吩),3种溶剂中的反应速率有较大差异,乙氰中反应速率最快,四氢呋喃中反应速率最慢。
三者的聚合产物都具有较高的电导率,但四氢呋喃中合成产物的电导率略高于另外两个产物。
红外光谱和拉曼光谱显示,3种溶剂中合成的聚(3,42乙撑二氧噻吩)在化学结构、分子共轭长度、掺杂情况等方面基本相同。
关键词: 聚(3,42乙撑二氧噻吩);化学氧化合成;反应速率;红外光谱;拉曼光谱中图分类号: O631.5文献标识码:A 文章编号:100129731(2005)10216102031 引 言导电聚合物PEDO T (poly (3,42et hylenedioxy 2t hiop hene ))(聚(3,42乙撑二氧噻吩))具有较高的导电性、良好的化学稳定性和热稳定性等优点,因而可以在诸多领域(如固体电解质电容器阴极材料、塑料抗静电涂层、传感器材料以及可充电电池等)得到应用。
化学氧化法合成导电聚合物PEDO T 的要素包括单体EDO T 、氧化剂、掺杂剂以及一定的溶剂,典型的氧化(掺杂)剂是Fe (Ⅲ)盐(如FeCl 3、Fe (O Ts )3等),化学氧化法合成PEDO T 的化学方程式如图1所示[1]。
实验中我们发现,在该反应体系中,(单体+氧化剂)的浓度对反应速率的影响很大,当单体浓度较低时,混合溶液可以在室温下稳定存在数小时,当单体浓度稍高时,聚合反应速度迅速增加。
这意味着混合溶液在浓度稍高时存在着短的“使用寿命”,这在某些应用场合,比如固体铝电容器的制备过程,是将电容器芯子浸渍到上述混合溶液中并使单体和氧化剂在芯子中原位聚合形成导电阴极,这时,希望使用浓度更高的浸渍液以便形成更多的导电聚合物。

第35卷 第12期 无 机 材 料 学 报Vol. 35No. 122020年12月Journal of Inorganic Materials Dec., 2020收稿日期: 2020-03-02; 收到修改稿日期: 2020-05-07基金项目: 国家自然科学基金(61775131, 61376009); 上海高校特聘教授(东方学者)岗位计划(2013-70)National Natural Science Foundation of China (61775131, 61376009); The Program for Professor of Special Ap-pointment (Eastern Scholar) at Shanghai Institutions of Higher Learning (2013-70)作者简介: 王金敏(1975–), 男, 教授.E-mail:*******************.cn文章编号: 1000-324X(2020)12-1307-08 DOI: 10.15541/jim20200105纳米二氧化锰的制备及其应用研究进展王金敏, 于红玉, 马董云(上海第二工业大学 工学部, 环境与材料工程学院, 上海 201209)摘 要: 二氧化锰作为一种重要的过渡金属氧化物, 因其储量丰富、晶型多样、性能优异而备受关注。
将二氧化锰纳米化后, 其颗粒尺寸变小、比表面积变大、材料性能优化、应用领域得以拓宽。
本文在引言部分从介绍二氧化锰的应用着手, 指出纳米化和晶型多变对二氧化锰的结构和性能有着重要的影响。
正文部分主要从纳米二氧化锰的制备方法和纳米二氧化锰的应用两个方面对近年来的研究进展进行了总结和评述。
(1)介绍了水热法、溶胶-凝胶法、化学沉淀法、固相合成法等纳米二氧化锰的制备方法, 对各种制备方法的优点与缺点以及所制备纳米二氧化锰的形貌与性能进行了总结。

MnO2/Poly(3,4-ethylenedioxythiophene)CoaxialNanowiresbyOne-Step
CoelectrodepositionforElectrochemicalEnergyStorage
RanLiuandSangBokLee*DepartmentofChemistryandBiochemistry,UniVersityofMaryland,CollegePark,Maryland20742ReceivedDecember19,2007;E-mail:slee@umd.edu
One-dimensional(1D)nanostructuredmaterialshavebeenintensivelyinvestigatedasbuildingcomponentsinelectrochemicalenergystorage1andsolarenergyconversion2devicesbecausethey
provideshortdiffusionpathlengthstoionsandexcitons,leadingtohighcharge/dischargerates1andhighsolarenergyconversion
efficiency.2Morerecently,coaxialnanowireshaveattracted
greaterattentioninthisfieldduetotheiraddedsynergicproperties(e.g.,highconductivity)3aorfunctionalities(e.g.,core/shellp-n
junction)3b,carisingfromthecombinationofdifferentmaterials.3Variousmaterialssuchassemiconductor/semiconductor,metal/metaloxide,andmetaloxide/metaloxide,havebeenemployedascore/shellincoaxialnanowires.3However,therehavebeen
fewstudiesonthecoaxialnanowireswithtransitionmetaloxideandconductivepolymer,althoughbothofthemareimportantelectroactivematerialsusedinelectrochemicalenergystorage.4Thecombinationofthesetwomaterialsat1Dnanostructuresmayexhibitexcellentelectrical,electrochemical,andmechanicalpropertiesforelectrochemicalenergystorage.Todate,onlyafewreportshavebeenpublishedonthesynthesisofmetaloxide/conductivepolymerwithcore/shellstructures.5Inallofthese
reports,astepwisesyntheticapproachwasadopted:metaloxidenanoparticles,5ananostrands,5bornanotubes5cwerefirstsynthesized
andsubsequentlycoatedchemicallybyconductivepolymersasshells.Inthispaper,weintroduceasimpleone-stepmethodtosynthesizeMnO2/poly(3,4-ethylenedioxythiophene)(PEDOT)co-
axialnanowiresbycoelectrodepositioninaporousaluminatemplate.6MnO2isoneofthemostpopularelectrochemicalenergy
storagematerialsbecauseofitshighenergydensity,lowcost,environmentalfriendliness,andnaturalabundance,7butithaspoor
conductivity.4cPEDOThasmeritsofexcellentconductivity,high
stability,andmechanicalflexibility,8butitprovideslowelectro-
chemicalenergydensity.Electrodepositionisusedherebecauseitisasimpleyetversatilemethodincontrollingstructuresandtheircompositionbytuningappliedpotentialsandelectrolyteingredi-ents.9Inthisreport,MnO2/PEDOTcoaxialnanowiresarefoundto
bepromisingelectrochemicalenergystoragematerials.ThecoreMnO2provideshighenergystoragecapacity,whilethehighly
conductive,porous,andflexiblePEDOTshellfacilitatestheelectrontransportandiondiffusionintothecoreMnO2andprotectsitfrom
structurallysignificantcollapsingandbreaking.Thesecombinedpropertiesenablethecoaxialnanowirestohaveveryhighspecificcapacitancesathighcurrentdensities.Scheme1illustratesthegrowthofMnO2/PEDOTcoaxial
nanowires.Underaconstantpotential(typically0.75VvsAg/AgCl),Mn2+(10mMmanganeseacetate)isconvertedtoitshigher
oxidizationstate,whichcanreadilyundergohydrolysistoyieldMnO2.7Simultaneously,EDOTmonomer(80mM)iselectropo-
lymerizedintoPEDOTintheporesofthetemplate.10Very
interestingly,thiscoelectrodepositiongivesrisetoformationofcoaxialnanowires.
Figure1ashowstheSEMimageoffree-standingcoaxialnanowiresgrownat0.75Vafterremovaloftemplate.Figure1bshowstheTEMimageofasinglecoaxialnanowire.AlthoughthecoreMnO2andshellPEDOTcanbeeasilydistinguishedbytheir
morphologiesinTEMimages,energydispersiveX-rayspectro-scopic(EDS)elementalmapsofSandMn(Figure1candd)fromthedesignatedareainFigure1bclearlyconfirmsthecoaxialnanostructure.TheelectrondiffractionpatternrevealsthatthecoreMnO2isamorphous(seeSupportinginformation).
WecaneasilycontrolthestructuresofcoaxialnanowiressuchasPEDOTshellthicknessandnanowirelengthbyvaryingtheappliedpotential.Thisshouldprovideuswiththeabilitytotuneelectrochemicalpropertiesofthecoaxialnanowires.SincetheonsetgrowthpotentialofMnO2(0.5V)islowerthanthatofPEDOT
(0.75V),MnO2nanowirescanbeselectivelygrownbelow0.6V,
whilePEDOTnanowiresaregrownatthepotentialsabove0.85VduetothehighergrowthrateofPEDOTgiventhattheconcentrationofEDOTmonomeris8timesthatofMn2+.Betweenthesetwo
extremepotentials,coaxialnanowireswithvariousPEDOTshellthicknesses(25-100nm)canbeobtained,asshowninFigure1e.AftertheMnO2coresinthesecoaxialnanowiresareselectively
removedbywetetching,PEDOTnanotubeswithdifferentwallthicknessesareobtainedandclearlyobservedbyTEM(Supportinginformation).Interestingly,theinnersurfacemorphologyofPEDOTnanotubesappearssomewhatroughandspiky.ItsuggeststhePEDOTmayhavegrownintotheMnO2corelayer.Thiscanbe
supportedfurtherbyanEDSline-scanprofileonasinglecoaxialnanowire(Supportinginformation).SuchPEDOTpenetrationsmayplayimportantrolesinfurtherimprovingthecoreconductivity.Thegrowthmechanismofcoaxialnanowires,althoughnotcompletelyunderstood,isbrieflysuggestedasfollows.Wehavepreviouslyprovedthatthesputteredring-shapeAuelectrodesatthebottomoftheporescandirectthegrowthofPEDOTnanotubesatlowoverpotential.11Thismayexplainthepreferentialformation