抗Xa因子活性测定评价依诺肝素在心导管室中应用的安全性和有效性
- 格式:pdf
- 大小:181.96 KB
- 文档页数:4

抗Xa因子在高风险患者群体中抗凝给药的监测作用
李雪;杨卫;李永旺;戴冬雪;马蓓洁;张静波;蔡会欣
【期刊名称】《标记免疫分析与临床》
【年(卷),期】2024(31)2
【摘要】静脉血栓栓塞症(VTE)是全世界重点关注的公共健康问题,由于其较高的发病率与致死率,国内外多项VTE防治指南提出了预防策略,但仍有VTE事件的发生。
低分子肝素(LMWH)是临床上目前抗凝的首要选择,监测其抗凝疗效,可以降低血栓发生的风险。
研究表明抗Xa活性水平可以很好地评估LMWH的抗凝效果,本文将对特殊人群在预防性抗凝治疗中抗Xa因子指标的作用进行综述,评估抗Xa因子监测抗凝用药的安全性和有效性。
【总页数】6页(P387-392)
【作者】李雪;杨卫;李永旺;戴冬雪;马蓓洁;张静波;蔡会欣
【作者单位】承德医学院研究生学院;保定市第一中心医院
【正文语种】中文
【中图分类】R364.1
【相关文献】
1.抗Xa因子在日间CRRT低分子肝素抗凝监测中的临床应用
2.抗Xa活性检测在抗凝药物监测中的应用
3.抗Xa活性在普通肝素抗凝监测中应用的研究进展
4.抗Xa因子活性检测在血液透析患者低分子肝素抗凝中的应用进展
5.新生儿体外膜肺氧合患者肝素抗凝抗Xa因子活性及活化部分凝血活酶时间监测评价
因版权原因,仅展示原文概要,查看原文内容请购买。

抗Xa试剂说明书一、产品概述抗Xa试剂是一种用于测定和监测肝素和类似物抗凝血活性的试剂。
本试剂基于活化部分凝血活酶时间(APTT)测定原理,通过与抗Xa抗体的结合,测定样品中抗凝血因子Xa的活性,从而评估肝素和类似物的抗凝血效果。
二、试剂成分•抗Xa抗体:本试剂中包含经过专业筛选和纯化的抗Xa抗体。
•缓冲液:提供了适宜的试剂pH值和离子浓度。
三、试剂储存条件本试剂应储存在温度为2-8摄氏度的冰箱中,避免阳光直射和脱水。
未开封的试剂在正确储存条件下可以保质期为12个月。
四、试剂使用方法1.取出所需的试剂,并恢复至常温。
2.将试剂与样品按照指定比例混合,轻轻摇动以混匀。
3.在混合溶液中加入适量的凝血酶,启动凝血反应。
4.使用常规凝血时间测定仪器记录凝血时间。
5.将测得的凝血时间与标准曲线比对,从而计算出样品中抗凝血因子Xa的活性浓度。
五、注意事项•请勿将试剂接触皮肤和黏膜,如不慎接触,应立即用大量清水冲洗。
•请避免试剂受到高温和冷冻的影响,以免影响试剂的稳定性和准确性。
•请在使用前确认试剂是否过期,过期试剂严禁使用。
•请按照指定比例混合试剂与样品,以保证测定结果的准确性。
•每次测定均应配制新鲜的标准曲线,且应配制多个浓度水平的曲线点。
六、结果解读本试剂可测定样品中抗凝血因子Xa的活性浓度,通过与标准曲线比对,可以得到相应的浓度值。
较高的活性浓度表示样品中抗凝血因子Xa的活性较高,说明样品中的抗凝血效果较好;较低的活性浓度表示样品中抗凝血因子Xa的活性较低,说明样品中的抗凝血效果较差。
七、性能指标•灵敏度:本试剂的灵敏度为0.1 IU/mL。
•精密度:同批样品重复性CV值小于5%。
•准确度:与国际标准物质的相关性大于0.95。
•交叉反应性:本试剂对其他相关物质的交叉反应极低,具有良好的特异性。
八、故障排除常见故障及解决方法:故障现象:测定结果异常偏高或偏低。
解决方法:请检查试剂和样品是否按照说明书要求混合,在保证试剂和样品质量的前提下重新测定。

初探抗凝血因子Xa活性检测在血液透析抗凝治疗中的应用的
开题报告
1. 研究背景
血液透析是一种常见的肾脏替代治疗,可以有效地清除体内代谢废物和毒素,帮助肾功能丧失的患者维持生命。
在血液透析过程中,需要使用抗凝剂来防止血液在透析器内凝固,从而保证透析效果。
目前常用的抗凝剂有肝素和低分子肝素,但这些药物使用后可能会出现出血等不良反应,因此需要对抗凝剂的使用进行严密监测。
最近一项研究表明,抗凝血因子Xa活性检测可以作为血液透析抗凝治疗中的一种新的监测方法。
2. 研究目的
本研究旨在探讨抗凝血因子Xa活性检测在血液透析抗凝治疗中的应用,比较其与传统监测方法的差异和优劣,为血液透析患者的抗凝治疗提供一种更为准确和安全的监测方法。
3. 研究方法
采用前瞻性、随机对照的研究方法,将80名需要进行血液透析的患者随机分为两组,一组使用传统的肝素监测方法,另一组使用抗凝血因子Xa活性检测方法。
对两组患者的抗凝剂使用情况、出血事件等数据进行统计和分析,比较两种监测方法之间的差异。
4. 预期结果
预期结果是抗凝血因子Xa活性检测方法能够更准确地监测血
液透析患者的抗凝剂使用情况,降低出血等不良反应的发生率。
同时,这种新的监测方法能够为临床医生提供更及时、更准确的参考信息,帮助调整抗凝治疗方案,提高治疗效果和安全性。
5. 结论
本研究的结论将为血液透析患者的抗凝治疗提供一种更为安全和有效的监测方法,为临床医生提供更准确的参考信息,对于改善透析效果和患者生活质量具有积极意义。

依诺肝素应用的临床科室依诺肝素是一种用来预防和治疗血栓的药物,适用于多种临床场合。
在医院的临床科室中,依诺肝素有着广泛的应用,下面将就依诺肝素在不同临床科室中的应用进行介绍。
一、内科科室在内科科室中,依诺肝素通常用于治疗各种原因引起的血栓性疾病,如心脏病、肺栓塞、中风等。
内科患者常常存在多种潜在的血栓形成风险因素,依诺肝素可以有效预防和治疗这些情况,减少并发症的发生。
二、外科科室在外科科室中,依诺肝素常常用于手术前后的抗凝治疗。
手术患者术后易发生血栓形成,特别是大型手术或高危手术患者,依诺肝素可以有效减少血栓风险,降低手术并发症的发生率。
三、妇产科科室在妇产科科室中,依诺肝素常用于妊娠期和产后的血栓预防。
孕妇和产妇血液黏稠度增高,易发生静脉血栓栓塞症,依诺肝素可以有效预防此类并发症的发生,保障母婴健康。
四、急诊科室在急诊科室中,依诺肝素常用于抢救危重患者。
对于急需抗凝的患者,依诺肝素可以迅速起效,有效减轻血栓带来的危害,帮助患者渡过生命危机。
五、重症监护室在重症监护室中,依诺肝素常用于重症患者的预防和治疗。
危重病患者易发生全身性炎症反应综合征和DIC等疾病,依诺肝素可以调节凝血机制,防止出现严重的凝血障碍。
六、心血管科室在心血管科室中,依诺肝素常用于冠心病、心肌梗死、心房颤动等患者的治疗。
这些患者存在较高的血栓形成风险,依诺肝素可以维持血液通畅,保护心脏功能。
七、其他科室除了上述几个主要科室,依诺肝素还可以在其他临床科室中应用,如神经内科、肾内科、呼吸内科等。
根据患者的具体病情和需要,医生会综合考虑使用依诺肝素的适宜性和剂量。
综上所述,依诺肝素作为一种有效的抗血栓药物,在临床科室中有着广泛的应用。
医护人员在使用依诺肝素时应遵循相关的临床指南和用药原则,确保患者获得最佳的治疗效果,减少药物引起的不良反应和并发症。
希望本文对依诺肝素在临床科室中的应用有所启发和帮助。

华法令抗凝观察指标-概述说明以及解释1.引言1.1 概述华法令抗凝观察指标是指用于观察华法令抗凝药物治疗效果的一系列指标。
华法令是一种抗凝药物,被广泛应用于心血管疾病和血栓症的治疗中。
准确观察并评估华法令的抗凝效果对于患者的治疗非常重要。
随着华法令的应用范围不断扩大,抗凝观察指标的研究和应用也越来越受到关注。
这些指标包括但不限于:凝血酶原时间(PT)、国际标准化比值(INR)、活化部分凝血活酶时间(APTT)、凝血酶时间(TT)和抗凝血酶(anti-Xa)活性等。
PT是一种常用的血液检测指标,用于评估华法令对凝血酶原的抑制效果。
通过测量患者的PT值,并与正常参考范围进行比较,可以判断患者的抗凝治疗是否达到预期效果。
INR是一种标准化的PT值,它可以解决不同实验室测试方法之间的差异性,使血液检测结果更加准确可靠。
INR值是判断患者抗凝治疗是否达标的重要参考指标之一。
APTT是一种测量血浆凝血酶原时间的指标,它的变化可以反映华法令对内源性凝血通路的影响。
通过监测APTT值的变化,可以了解华法令的抗凝作用是否正常进行。
TT是一种血浆凝血酶时间测定的指标,它主要用于观察华法令对外源性凝血通路的影响。
通过检测TT值的改变,可以判断华法令对血浆外源性凝血酶的抑制效果。
抗凝血酶(anti-Xa)活性是一种直接测定华法令抗凝效果的指标,它可以准确反映华法令对Xa因子的抑制效果。
通过测量患者的anti-Xa活性,可以更加精确地评估华法令的抗凝效果。
因此,对于华法令抗凝治疗来说,准确观察和评估抗凝观察指标是必不可少的。
这些指标的监测和分析将有助于医生调整患者的华法令剂量,以达到最佳的抗凝效果,提高治疗的安全性和有效性。
此外,随着医疗技术的不断进步,可能会有更多更准确的抗凝观察指标被应用于临床实践中,为华法令抗凝治疗提供更好的指导。
1.2文章结构1.2 文章结构本文主要分为三个部分,即引言、正文和结论。
引言部分主要包括概述、文章结构和目的三个小节。

微量生色底物法测定肝素钠肌醇烟酸酯乳膏中抗Xa因子效价目的建立肝素钠肌醇烟酸酯乳膏中抗Xa因子效价的测定方法。
方法对肝素钠肌醇烟酸酯乳膏进行破乳处理后,采用微量生色底物法、生物检定统计法,测定乳膏中抗Xa因子的效价。
结果建立了肝素钠肌醇烟酸酯乳膏中肝素钠抗Xa因子的测定方法,抗Xa因子在0.0250~0.1020 IU/mL范围内与吸光度(A)值线性关系良好(r=0.996);低、中、高浓度的平均回收率为99.5%,RSD为1.92%(n=9)。
抗Xa因子和抗Ⅱa因子的效价比值在0.9~1.1范围内。
结论该方法结果准确,重复性好,可用于肝素钠肌醇烟酸酯乳膏中抗Xa因子效价的测定,有效控制该制剂的质量。
[Abstract] Objective To establish a method for determination of anti-FXa potency in heparin sodium and inositol nicotinate cream. Methods The emulsion was broken. The anti-FXa potency was determined by micro-chromogenic substrate method and biological test statistics method. Results The determination method was established for anti-FXa potency in heparin sodium and inositol nicotinate cream. The linear range of anti-FXa was during 0.0250-0.1020 IU/mL (r=0.996). The average recovery in three levels was 99.5% (RSD=1.92%,n=9). The ratio of potency was during 0.9-1.1 for anti-FXa and anti-FⅡa. Conclusion The method is accurate and reproduceable. It can be used for the determination of anti-FXa potency in order to control the quality of heparin sodium and inositol nicotinate cream.[Key words] Micro-chromogenic substrate method;Heparin sodium and inositol nicotinate cream;Anti-FXa;Potency凍伤是指机体长时间暴露于寒冷环境,导致全身或局部体温下降而产生的损伤[1-2]。
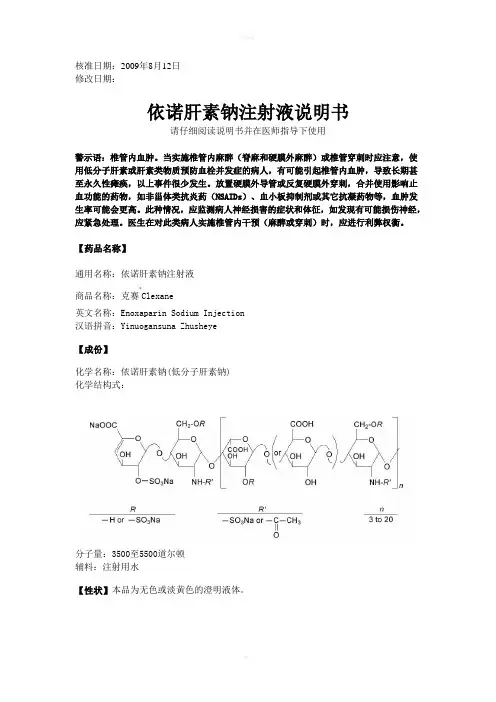
核准日期:2009年8月12日修改日期:依诺肝素钠注射液说明书请仔细阅读说明书并在医师指导下使用警示语:椎管内血肿。
当实施椎管内麻醉(脊麻和硬膜外麻醉)或椎管穿刺时应注意,使用低分子肝素或肝素类物质预防血栓并发症的病人,有可能引起椎管内血肿,导致长期甚至永久性瘫痪,以上事件很少发生。
放置硬膜外导管或反复硬膜外穿刺,合并使用影响止血功能的药物,如非甾体类抗炎药(NSAIDs)、血小板抑制剂或其它抗凝药物等,血肿发生率可能会更高。
此种情况,应监测病人神经损害的症状和体征,如发现有可能损伤神经,应紧急处理。
医生在对此类病人实施椎管内干预(麻醉或穿刺)时,应进行利弊权衡。
【药品名称】通用名称:依诺肝素钠注射液商品名称:克赛®Clexane英文名称:Enoxaparin Sodium Injection 汉语拼音:Yinuogansuna Zhusheye【成份】化学名称:依诺肝素钠(低分子肝素钠) 化学结构式:分子量:3500至5500道尔顿辅料:注射用水【性状】本品为无色或淡黄色的澄明液体。
【适应症】2000 Axa IU 和4000 Axa IU注射液:•预防静脉血栓栓塞性疾病 (预防静脉内血栓形成) ,特别是与骨科或普外手术有关的血栓形成。
6000 Axa IU, 8000 Axa IU 和10000 Axa IU注射液:• 治疗已形成的深静脉栓塞,伴或不伴有肺栓塞,临床症状不严重,不包括需要外科手术或溶栓剂治疗的肺栓塞。
• 治疗不稳定性心绞痛及非Q波心肌梗死,与阿司匹林合用。
• 用于血液透析体外循环中,防止血栓形成。
• 治疗急性ST段抬高型心肌梗死,与溶栓剂联用或同时与经皮冠状动脉介入治疗(PCI)联用。
【规格】(1)0.2ml :2000 AxaIU (2)0.4ml: 4000 AxaIU (3)0.6ml: 6000 AxaIU (4)0.8ml :8000 AxaIU (5)1.0ml :10000 AxaIU【用法用量】预防静脉血栓栓塞性疾病,治疗深静脉栓塞,治疗不稳定性心绞痛及非Q波心肌梗死时应采用深部皮下注射给予依诺肝素;血液透析体外循环时为血管内途径给药;对于ST段抬高型急性心肌梗死,初始的治疗为静脉注射,随后改为皮下注射治疗。

抗xa参考范围-回复什么是抗xa参考范围?抗xa参考范围是指在药物治疗期间,通过检测患者血液中的抗xa活性来确定是否达到理想的治疗效果。
抗xa活性是指抗凝药物华法林或肝素对于血液中凝血因子xa的抑制能力。
如何确定抗xa参考范围?确定抗xa参考范围需要进行以下步骤:1. 收集参考样本:从大量的各年龄段、性别、种族的正常健康个体中收集血液样本。
这些样本将用于建立参考范围。
2. 测定抗xa活性:使用标准化的测定方法,如凝血酶时间(PT)法或抗凝血酶时间(aPTT)法来测定参考样本中的抗xa活性。
这些测定方法可以反映出凝血时间的延长程度,从而评估抗xa活性。
3. 数据分析:将收集到的抗xa活性数据进行统计分析,包括计算平均值、标准差和百分位数等。
这些分析结果将帮助确定正常参考范围。
4. 确定参考范围:根据统计分析的结果,可以确定一个正常的抗xa参考范围。
通常,参考范围被定义为正常个体中抗xa活性的95置信区间,即在这个范围内的个体被认为处于正常范围内。
5. 验证参考范围:为了验证参考范围的准确性和适用性,通常会对大量的患者样本进行测定,并与参考范围进行比较。
如果患者样本的抗xa活性在参考范围内,则说明该参考范围是准确可信的。
为什么抗xa参考范围重要?抗xa参考范围的确定对于药物治疗的安全和有效性至关重要。
如果抗xa 活性低于参考范围,表示患者可能存在凝血风险,需要适当调整药物剂量,以避免出血等并发症。
相反,如果抗xa活性超出参考范围,说明患者可能没有得到足够的抗凝效果,需要增加药物剂量。
此外,抗xa参考范围还可以帮助临床医生评估患者的凝血状态,并在需要时调整药物治疗。
根据抗xa参考范围的指导,医生可以个体化地调整药物剂量,以达到最佳的治疗效果。
总结:抗xa参考范围的确定是一个复杂的过程,需要收集样本、测定抗xa活性、进行数据分析和验证参考范围的有效性。
抗xa参考范围的确定对于药物治疗的安全和有效至关重要,可以帮助医生个体化地调整药物剂量,以达到最佳的治疗效果。

使用依诺肝素48 h行冠脉造影的安全性和有效性邢云利;贾三庆;王雷【期刊名称】《中华老年多器官疾病杂志》【年(卷),期】2007(6)1【摘要】目的低分子量肝素(LMWH)可以有效取代普通肝素(UH)应用于急性冠脉综合征(ACS)的治疗.然而,这些患者行冠脉造影(CAG)的最佳抗凝策略尚不明了.国外有关LMWH在冠脉造影中的应用研究表明,皮下应用低分子肝素至少48h(≥4次)冠脉造影,术中不追加抗凝剂是安全有效的,但此方案不一定适合我国.我科室曾沿用此法人选176例ACS患者,结果显示CAG前肝素抗-X a因子活性(0.808±0.265)IU/ml,93.2%的患者抗-X a活性>0.5 IU/ml.本研究扩大样本量,试图进一步评价LMWH在心导管室应用的安全性和有效性,探索适合国人的心导管检查抗凝策略.方法与结果人选278例ACS患者.所有患者按照1 mg(100 IU)/kg 每隔12 h(7 am~7 pm)皮下注射依诺肝素(克赛),在接受至少48 h(≥4次)的LMWH后进行导管检查.末次注射(7 am)距离冠脉造影不超过8 h(3 pm之前).穿刺前(≈末次注射的3~5 h内)取血测定抗-X a活性.如病情需要,可行经皮冠脉介入术(PCI),术中追加普通肝素5000 IU.结果显示冠脉造影前肝素抗-X a活性为(0.745±0.304)IU/ml,79.7%的患者抗-X a活性>0.5 IU/ml,5.4%抗-X a活性>1.2 IU/ml.1例在造影过程中,另3例在PCI中出现血栓,无严重出血事件.结论皮下注射依诺肝素至少48h行冠脉造影抗凝强度偏低,可能需要寻找新的抗凝方案.【总页数】3页(P27-29)【作者】邢云利;贾三庆;王雷【作者单位】100050,北京市,首都医科大学附属北京友谊医院心内科;100050,北京市,首都医科大学附属北京友谊医院心内科;100050,北京市,首都医科大学附属北京友谊医院心内科【正文语种】中文【中图分类】R9【相关文献】1.依诺肝素联合替罗非班治疗急性心肌梗死的有效性与安全性分析 [J], 李江;朱佳佳;康铁朵;宁尚秋;杨鲲;盛瑾;陈立颖;刘文娴2.老年冠心病合并慢性肾功能不全患者介入治疗围术期应用不同剂量依诺肝素抗凝的有效性和安全性 [J], 王磊;曹佳;谷沫丽;夏云峰;刘润梅;翟红霞;李良;殷亚昕;张津津;陈海威;李娜;赵志坤3.冠脉介入治疗术中应用依诺肝素(0.5mg/kg)的安全性和有效性 [J], 黄兆辉;周忠江;杨秦南;侯玉清4.在替罗非班和阿司匹林治疗的非ST段抬高性急性冠脉综合征患者:依诺肝素与普通肝素的安全性及有效性的随机对照试验 [J], Blazing M.A.;De LemosJ.A.;White H.D.;姜馨5.合并门静脉血栓的乙型肝炎肝硬化患者,使用不同剂量依诺肝素抗凝治疗的安全性和有效性 [J], 王中峰;Cui SB因版权原因,仅展示原文概要,查看原文内容请购买。

低分子肝素钠治疗 PICC导管血栓的有效性及安全性评价摘要:[目的]探讨低分子肝素在PICC导管相关血栓预防中的有效性及安全性评价。
[方法]将32例多发性骨髓瘤、淋巴瘤 PICC 置管患者为研究对照随机分成对照组和低分子肝素钠组,各16例,PICC 导管常规用0.9%生理盐水 20ml 进行封管,导管发生血栓后使用低分子肝素钠进行治疗,观察患者临床症状和血栓发生情况。
[结果]两组患者基本信息无显著性差异;与对照组对比,低分子肝素钠组堵管以及血栓发生率显著降低(P<0.05)。
[结论]低分子肝素钠能够减少血栓的发生和出血事件的情况,为临床提供科学可靠的依据,证明低分子肝素钠在治疗血液性相关恶性肿瘤携带PICC 导管血栓具有一定效果和安全性。
关键词:PICC 导管;血栓;低分子肝素钠;普通肝素钠;有效性;安全性中心静脉导管(CVCs)是许多癌症患者和血液透析、肠道外营养、检测中心静脉压、血浆置换或介入治疗中不可缺少的组成部分,主要用于超过25%的住院非重症监护病房患者以及需要输液、输血、抗生素和化疗药物的门诊病人[1]。
虽然应用置留中心静脉导管明显地提高了需要长期静脉给药的患者治疗的安全性和生活质量,但导管相关性静脉血栓的形成是其不可避免的并发症,引起了临床的极大关注[2]。
据报道,在成人中CVC相关血栓形成的风险在2%到42%之间,而在儿童中高达12%到44%[3]。
因此,我科通过对低分子肝素能治疗成人中心静脉导管相关血栓效果的观察,研究低分子肝素钠治疗预防的有效性及安全性,为临床降低血栓发生提供数据依据。
1 方法1.1研究对象与分组:收集32名在2016-2019年在医院多发性骨髓瘤、淋巴瘤 PICC 置管患者为研究对象,采用随机数字表法将其分为对照组、低分子肝素钠组各16例,作为研究低分子肝素钠在血液系统相关血栓治疗中的有效性及安全性评价。
所有患者在取得患者的知情同意后,采用问卷调查的方式收集患者的病例资料和基本信息。
中华心血管病杂志2003年2月第31卷第2期clIin 0l! , : : 抗Xa因子活性测定评价依诺肝素在 中应用的安全性和有效性 王雷 贾三庆 严松彪 侯晓霞 张宇晨赵敏 王敏 心导 临床研究. 管室
【摘要】 目的低分子量肝素可以有效的取代普通肝素应用于急性冠状动脉综合征(ACS)的治 疗。然而,ACS患者在行心导管检查时最佳的抗凝策略尚不明了。本研究旨在用抗xa因子活性检测 评价低分子量肝素在心导管室中应用的安全性和有效性,探索适合国人的心导管检查及经皮冠状动 脉介入治疗(PCI)的抗凝策略。方法 共入选ACS患者176例。在每12 h(7:00—19:00)皮下注射依诺 肝素l mg/kg至少48 h后,不追加普通肝素或低分子量肝素于心导管室行冠状动脉造影,不进行凝血 系统监测。60例(34.1%)患者继之行PCI。结果 在心导管检查前的肝素抗)【a因子活性是(0.81± 0.27)IU/ml,93.2%的患者抗)【a因子活性>0.50 IU/ml,且抗xa因子活性与从注射到开始导管检查 的时间无关(P=0.097)。PCI组术后30 d无死亡、急性冠状动脉再闭塞或急诊血管重建事件。3例 (5.0%)PCI患者术中出现血栓和(或)栓塞事件。单纯冠状动脉造影组有l例因三支血管病变在术后 17 d等待冠状动脉旁路移植术时发生急性心肌梗死而行急诊PCI;另l例患者在冠状动脉造影后21 d 死于十二指肠穿孔。176例入选患者无一例出现严重出血事件;PCI组有3例(5.0%)患者出现轻度穿 刺部位出血,单纯冠状动脉造影组为5例(4.3%)。结论本研究初步表明皮下注射依诺肝素48 h后 行冠状动脉造影及PCI对ACS患者是安全有效的,但尚需进一步的大样本随机对照研究进行评价。 【关键词】 冠状动脉疾病; 血管成形术,经腔,经皮冠状动脉; 因子Xa; 依诺肝素
Evaluation of the朝fety and eMcacy of enoxaparin admini. ̄qration in the cardiac catheter laboratory by measurement of factor Xa activity WANG Lei,JIA San-qing,yAⅣSong-biao,et a1.Heart&Blood Vessel Center of Friendship Hospital,Capital Uni ̄rsity ofMedical Sciences,Beij ̄100050,China
【Abstract】 Objective To evaluate the safety and eficacy of subcutaneous low—molecular-weight heparin (LMWH)used in cardiac catheterization,and tried to seek an optimal anticoagulation strategy.Methods A total of 176 patien ̄with acute coronary syndrome(ACS)were treated for at least 48 hours with subcutaneous enoxaparin(1 mg/kg every 12 hours,cycled at 7:0o and 19:0o).Without additional bolus of udWH or unfractionated heparin and without coagulation monitoring,all 176 patients underwent coronary angiographies (CAG)within 8 hours after udWH injection,foHowed by immediate percutaneous coronary intervention(PCI)in 60 patients(34.1%).Results Anti一)【a activity atthetime of catheterizationwas(0.81±0.27)IU/III1.93.2% of the patients had anti—Xa activity>0.50 IU/m1.It was not correlated to the UⅥWH injection-to—catheterization time(r=一0.147,P=0.097).Therewere noin—hospital abrupt closures or urgentrevascularizations afterPCI. In the CAG group,one patient with 3 vessels disease was striken by recurrent acute myocardial infarction while waiting for coronary artery bypass grafting(17 days after CAG)and primary PC1 was performed successfully. Another patient died of non-cardiac cause before coronary artery bypass grafting.Thrombosis and/or embolism occurred in 3 patients(5.0%)after PCI.Minor hemorrhage was observed in 3 patients(5.0%)of PCI group, and 5 patients(4.3%)in CAG group.No major hemorrhage occurred.Conclusion PC1 within 8 hours ofthe last dose after≥48 hours enoxaparin subcutaneous injection seems to be safe and effective. 【Key words】 Coronary disease; Angloplasty,transluminal,percutaneous coronary; Factor Xa; Enoxaparin
有关经皮冠状动脉介入治疗(PCI)的ESSENCE 和TIMI.1lB研究[1,2]提示,对急性冠状动脉综合征
作者单位:100050北京,首都医科大学附属北京友谊医院心血管 疾病诊治研究中心
(ACS)患者,在行导管检查前和术中应从低分子量 肝素(LMWH)治疗转向普通肝素(UH)治疗。但这种 方法在临床应用时有可能增加出血的风险。有研究 表明,由于没有内源性的血小板激活,LMWH可以改
维普资讯 http://www.cqvip.com 中华心血管病杂志2003年2月第3l卷第2期 Chin J Cardiol,Februar ̄2003,v01.3l N0.2 善ACS患者的临床预后;同时,因其半衰期长,使用 简单,生物利用度较好,不需进行常规凝血系统监 测。对Xa因子的拮抗较强,总体费用较低,已显示出 较UH更为有效的潜在优势。C0llet等 进行的前 瞻性研究证实,不稳定性心绞痛患者于心导管术前 应用LMWH是安全有效的,但国内尚缺乏此方面的 系统研究。本研究旨在评价LMWH应用于ACS患 者心导管诊治中的安全性和有效性,并探索一条较 好的心导管检查及治疗前抗凝策略。 资料与方法 1.研究对象:入选标准:(1)不稳定性心绞痛患 者;(2)非Q波心肌梗死患者;(3)急性心肌梗死患 者。根据入院前24 h内出现的典型胸痛、伴随的心 电图改变和(或)心肌酶升高而确诊。排除标准:(1) 年龄>80岁;(2)血肌酐>176.8 mmol/L(2.0 ms/on); (3)有使用抗凝剂的禁忌证,如活动性溃疡、脏器活 动性出血、感染性心内膜炎、不能控制的高血压[收 缩压(SBP)>200 mln rig,或舒张压(DBP)>120 mln Hg,1 mln Hg=0.133 kPa]、3个月内新发中风;(4)其 他有较高出血风险的患者。 根据上述标准,我们入选了176例ACS患者。 所有患者在无禁忌证的情况下均口服阿司匹林、B. 受体阻滞剂、硝酸酯类、血管紧张素转换酶抑制剂 (ACEI)及他汀等药物。按照1 ms/ks体重皮下注射 依诺肝素(商品名克赛,安万特医药有限公司生产), 每隔12 h(7:00.19:0o)注射1次,至少48 h(≥5次)。 在接受至少48 h的LMWH皮下注射后进行心导管 检查。全部176例患者行冠状动脉造影(CAG)检 查,其中60例(34.1%)继之行PCI。在心导管检查 前不间断皮下注射依诺肝素,患者末次注射(7:oo) 8 h内(15:00之前)行导管检查。在穿刺前(末次注 射的2—8 h内)取血测定抗Xa因子活性。术中不 追加UH和(或)LMWH,不监测凝血活性。在末次注 射10 h(16:0o)内拔除鞘管。术后不再使用LMWH。 2.临床随访:住院期间的检查主要包括体格检 查、心电图、肌酸激酶(CK)和肌钙蛋白T(TnT)水平。 术后30 d的随访通过电话访问或门诊进行。主要 终点事件包括死亡(包括心原性和非心原性)和再发 心肌梗死。后者定义为复发胸痛,并出现≥1项下 述变化:(1)CK大于正常高限的2倍,并超过最近一 次CK值的0.5倍,伴TnT阳性;(2)新出现左束支传 导阻滞或病理性Q波。急诊血运重建是指为缓解 复发缺血进行的急诊PCI或冠状动脉旁路移植术 (CABG)。次要终点事件包括血栓和(或)栓塞事件 及出血事件。血栓和(或)栓塞事件是指冠状动脉造 影时肉眼可见血栓。如果发生出血致死、颅内或眼 内出血或血红蛋白下降≥5 g/dl称为严重出血事 件;轻度出血事件包括鼻衄、瘀斑、血肿、肉眼血尿或 临床未发现出血但血红蛋白下降>4 g/dl。 3.抗Xa因子活性生物学测定:导管穿刺前取血 2.7 rnl枸橼酸钠抗凝,在30 min内以3 000 r/min离 心15 min分离血浆,一80℃保存,采用DADE BEHRING公司Berichrom(r)试剂盒、德国BE全自动 血凝仪成批测定。 4.统计分析:计量资料用均数4-标准差(牙4-s) 表示。采用线性相关分析连续性变量间的关系,Y 检验进行计数资料的显著性检验。P≤0.o5为差异 有显著性。