2007 WS2 sensitized mesoporous TiO2 for efficient photocatalytic hydrogen production from water
- 格式:pdf
- 大小:160.74 KB
- 文档页数:5

高能晶面TiO2的可控合成及其光催化性能张秀芳;王庆娟;王聪;李蒋【摘要】利用水热法合成了形貌规则并具有高表面能{101}和{001}晶面的锐钛矿T iO2单晶,通过X射线衍射和扫描电镜等对样品的形貌和结构进行了表征,系统考察了氢氟酸浓度对所得样品中{001}晶面比例的影响以及后处理对样品性能的影响.光催化降解罗丹明B反应表明,当氢氟酸为58 mmol/L、合成晶体{001}晶面比例为48%时光催化效果最强.{001}晶面不是唯一影响光催化效率的因素,{001}晶面与{101}晶面的协同作用也同样重要.【期刊名称】《大连工业大学学报》【年(卷),期】2018(037)005【总页数】5页(P357-361)【关键词】二氧化钛;高表面能晶面;光催化;污染物【作者】张秀芳;王庆娟;王聪;李蒋【作者单位】大连工业大学轻工与化学工程学院 ,辽宁大连 116034;大连工业大学轻工与化学工程学院 ,辽宁大连 116034;大连工业大学轻工与化学工程学院 ,辽宁大连 116034;大连工业大学轻工与化学工程学院 ,辽宁大连 116034【正文语种】中文【中图分类】X131.20 引言半导体的出现及发展引起了催化化学、材料研发等多方面的研究,为能源的有效利用和环境治理提供了一个新的研究方向。
自1972年Fluishima 和Honda[1]发现二氧化钛(TiO2)电极可以在光照下将水分解成氢气和氧气以来,掀起了科学家对TiO2的研究热潮。
而且,由于TiO2制备成本低廉,无毒性,化学性质稳定,且在紫外光范围内具有很强的光催化活性,在污水治理、空气净化、光分解水制氢等领域有着广泛的应用前景[2-4]。
TiO2有板钛矿、锐钛矿和金红石三种晶型,其中锐钛矿TiO2在光催化[5-7]和太阳能电池[8-10]等方面表现出优异的性能。
其中,TiO2的光催化性能不仅与尺寸、晶相、形貌等有关,还与高能晶面的暴露有关[11-12]。

广 东 化 工 2008年第1期· 10 · 第35卷总第177期石蜡熔化蓄热的实验研究谢望平,朱冬生,汪南,剧霏(华南理工大学传热强化与过程节能教育部重点实验室,广东广州 510640)[摘 要]石蜡是一种常见的相变材料,文章对石蜡的熔化蓄热进行了单管实验研究,并进行了差示扫描量热(DSC)测试,以所得的石蜡的相变温度为基础,研究石蜡在不同温度下的熔化情况,利用Agilent 34970A系统采集实验数据并处理,对其温度场的分布进行了分析比较。
实验结果表明,石蜡的熔化是遵循一定规律的,开始阶段通过热传导熔化,等到出现一定液相时出现自然对流,并且加热温度越高,溶解越快,自然对流出现越早。
石蜡导热系数比较低,因此应用范围受到了限制,文章为进一步改进石蜡的导热性能、使其得到更为广泛的应用提供了实验基础。
[关键词]石蜡;蓄热;相变材料;导热系数Experimental Research on Melting Heat Storage of ParaffinXie Wangping, Zhu Dongsheng, Wang Nan, Ju Fei(The Key Lab of Enhancement Heat Transfer and Energy Conversion, the Education Ministry,South China University of Technology, Guangzhou 510640, China)Abstract: Paraffin is a common kind of phase change materials(PCMs). The experiment on melting heat storage of paraffin was conducted in a tube. The phase change temperature was measured by DSC, and the melting of the paraffin was studied at different temperatures. The data was input to the computer by Agilent 34970A. The melting curve and temperature field were analyzed. The results showed that at the initial stage conduction occupied the main status in the heat transfer, but as time passed, natural convection appeared and made important effect, and the temperature was higher, the convection appeared earlier. The application of the paraffin was limited because of the low conductivity. This paper could help to improve the thermal properties and extend the application of paraffin.Keywords: paraffin;heat storage;phase change materials(PCMs);thermal conductivity蓄热技术在许多工业和建筑采暖等能量利用系统中广泛应用,它是提高能源利用效率和保护环境的重要技术,可用于解决热能供给与需求在时间和强度上不匹配的矛盾[1],有效降低能量供应和需求时间性的差异造成的能量利用的浪费,因而蓄热技术在太阳能利用、电缆的“移峰填谷”、废热和余热的回收利用以及工业与民用建筑采暖与空调的节能等领域具有广泛的应用前景[2-4]。



Morphology-photovoltaic property correlation in perovskite solar cells: One-step versus two-step deposition of CH3NH3PbI3Jeong-Hyeok Im, Hui-Seon Kim, and Nam-Gyu ParkCitation: APL Materials 2, 081510 (2014); doi: 10.1063/1.4891275View online: /10.1063/1.4891275View Table of Contents: /content/aip/journal/aplmater/2/8?ver=pdfcovPublished by the AIP PublishingArticles you may be interested inDouble functions of porous TiO2 electrodes on CH3NH3PbI3 perovskite solar cells: Enhancement of perovskite crystal transformation and prohibition of short circuitingAPL Mat. 2, 081511 (2014); 10.1063/1.4891597Mechanical properties of hybrid organic-inorganic CH3NH3BX3 (B = Sn, Pb; X = Br, I) perovskites for solar cell absorbersAPL Mat. 2, 081801 (2014); 10.1063/1.4885256CdS quantum dots grown by in situ chemical bath deposition for quantum dot-sensitized solar cellsJ. Appl. Phys. 110, 044313 (2011); 10.1063/1.3624944High efficiency mesoporous titanium oxide PbS quantum dot solar cells at low temperatureAppl. Phys. Lett. 97, 043106 (2010); 10.1063/1.3459146Quantum-dot-sensitized solar cells: Assembly of CdS-quantum-dots coupling techniques of self-assembled monolayer and chemical bath depositionAppl. Phys. Lett. 90, 143517 (2007); 10.1063/1.2721373APL MATERIALS2,081510(2014)Morphology-photovoltaic property correlation in perovskite solar cells:One-step versus two-step depositionof CH3NH3PbI3Jeong-Hyeok Im,Hui-Seon Kim,and Nam-Gyu Park aSchool of Chemical Engineering and Department of Energy Science,SungkyunkwanUniversity,Suwon440-746,South Korea(Received21April2014;accepted14July2014;published online28July2014)Perovskite CH3NH3PbI3light absorber is deposited on the mesoporous TiO2layervia one-step and two-step coating methods and their photovoltaic performances arecompared.One-step coating using a solution containing CH3NH3I and PbI2showsaverage power conversion efficiency(PCE)of7.5%,while higher average PCE of13.9%is obtained from two-step coating method,mainly due to higher voltage andfill factor.The coverage,pore-filling,and morphology of the deposited perovskiteare found to be critical in photovoltaic performance of the mesoporous TiO2basedperovskite solar cells.©2014Author(s).All article content,except where otherwisenoted,is licensed under a Creative Commons Attribution3.0Unported License.[/10.1063/1.4891275]Perovskite solar cell is emerging photovoltaic technology because of low cost and high efficiency. Since the reports on the all-solid-state perovskite solar cells with power conversion efficiencies (PCEs)of∼10%in2012,1,2rapid progress has been made for the past one and half years.As a consequence,PCEs as high as over16%have been achieved.3,4CH3NH3PbI3and CH3NH3PbI3-x Cl x are currently the front-and-center materials for high efficiency perovskite solar cell.Since perovskite wasfirst used as a sensitizer in dye-sensitized type solar cell in the early stage,5,6perovskite has been tried to be deposited on the surface of TiO2.Spin-coating of the solutions containing CH3NH3I and PbI2for CH3NH3PbI3or CH3NH3I and PbCl2for CH3NH3PbI3−x Cl x led to the scattered nanodots1 or extremely thin layer.2This method requires infiltration of hole transporting material(HTM),such as2,2 ,7,7 -tetrakis(N,N-di-p-methoxyphenylamine)-9,9-spirobifluorene(spiro-MeOTAD),into the pores of the metal oxidefilms.Photovoltaic performance relies significantly on the extent of pore-filling with HTM.This issue was addressed byfilling the pores with perovskite instead of HTM,7 which resulted in a PCE of12%.Building up the perovskite thin layer in the mesoporous metal oxide matrix(nanocomposite structure)eventually led to construction of heterojunction structure without the metal oxide layer.4Recent progress in perovskite solar cell and its basic understanding can be found in the latest literatures.8–12For pore-filling with CH3NH3PbI3perovskite,sequential deposition technique via two-step dipping was found to one of effective ways to achieve reproducibly high efficiency perovskite solar cell.3Average PCE of12%with small standard deviation of±0.5was obtained using two-step dipping method.A slight modification of dipping condition led to a PCE of15%.It was mentioned that uncontrolled precipitation of CH3NH3PbI3perovskite in a single step deposition produced large morphological variation and thereby inconsistent photovoltaic performance.However,no compara-tive study between the one-step and two-step deposition has been carried out.Here we have studied morphology and photovoltaic performance depending on deposition procedure of CH3NH3PbI3. We performed two-step sequential spin-coating procedure for CH3NH3PbI3deposition which was slightly different from two-step dipping method.3Both one-step and two-step coating methods a Author to whom correspondence should be addressed.Electronic mail:npark@.Tel.:+82-31-290-7241.Fax: +82-31-290-7272.2,081510-12166-532X/2014/2(8)/081510/8©Author(s)2014resulted in reproducible photovoltaic performance,but significant difference in especially photo-voltage andfill factor.Electron life time was dependent on coating procedure.Such difference in photovoltaic performance was found to correlate to morphology of the deposited CH3NH3PbI3.CH3NH3I was synthesized according to method reported in Ref.6.Methylamine(27.86ml, 40%in methanol,TCI)and hydroiodic acid(30ml,57wt.%in water,Aldrich)were mixed at0◦C and stirred for2h.The precipitate was recovered by evaporation at50◦C for1h.The product was washed with diethyl ether three times and thenfinally dried at60◦C in vacuum oven for24h.Anatase TiO2nanoparticles with diameter of∼40nm were synthesized by two-step hydrother-mal method.The seed particles with diameter of∼20nm were synthesized by acetic acid catalyzed hydrolysis of titanium isopropoxide(97%,Aldrich)and autoclaving at230◦C for12h.The seed particles were washed with ethanol and collected using centrifuge.Hydrothermal treatment was performed again with the seed particles to grow the particle size.TiO2paste was prepared by mixing the TiO2particles(∼40nm)with terpineol(99.5%,Aldrich),ethyl cellulose(EC)(46cp,Aldrich), and lauric acid(LA)(96%,Fluka)at nominal ratio of TiO2:TP:EC:LA=1.25:6:0.6:0.1.The paste was further treated with three-roll-mill for40min.FTO(Fluorine-doped Tin Oxide)glass substrate(Pilkington,TEC-8,8 /sq)with dimension of 2.5cm×2.5cm was cleaned in an ultrasonic bath containing ethanol for20min,which was treated in UVO(Ultraviolet Ozone)cleaner for20min.TiO2blocking layer(BL)was spin-coated on a FTO substrate at2000rpm for20s using0.15M titanium diisopropoxide bis(acetylacetonate)(75wt.% in isopropanol,Aldrich)in1-butanol(99.8%,Aldrich)solution,which was heated at125◦C for 5min.After cooling down to room temperature,the TiO2paste was spin-coated on the BL layer at 2000rpm for10s,where the pristine paste was diluted in ethanol(0.1g/ml).After drying at100◦C for5min,thefilm was annealed at550◦C for30min.The mesoporous TiO2film was immersed in 0.02M aqueous TiCl4(>98%,Aldrich)solution at90◦C for10min.After washing with de-ionized (DI)water and drying,thefilm was heated at500◦C for30min.To make the perovskite precursor solution,the synthesized CH3NH3I(0.395g)was mixed with PbI2(1.157g,99%Aldrich)in2ml N,N-dimethylacetamide(DMA,>99%Sigma)at60◦C for12h under stirring.Twenty microliters perovskite precursor solution was spin-coated on the mesoporous TiO2layer at3000rpm for20s.Thefilm was dried consecutively at40◦C for3min and100◦C for5min.Twenty microliters of spiro-MeOTAD solution was spin-coated on the CH3NH3PbI3 perovskite layer at4000rpm for30s.A spiro-MeOTAD solution was prepared by dissolving 72.3mg of spiro-MeOTAD in1ml of chlorobenzene,to which28.8μl of4-tert-butyl pyridine(TBP) and17.5μl of lithium bis(trifluoromethanesulfonyl)imide(Li-TFSI)solution(520mg Li-TSFI in 1ml acetonitrile(Sigma-Aldrich,99.8%))were added.Finally,an80-nm-thick gold electrode was thermally deposited on the spiro-MeOTAD coatedfilm.The one substrate containsfive cells and the photoactive layer of each cell was ca.0.2cm2(Figure S1of the supplementary material).13 In1ml N,N-dimethylformamide(DMF,99.8%Sigma-Aldrich),462mg PbI2was dissolved at 70◦C to make1M PbI2solution.Twenty microliters PbI2solution was spin-coated on the meso-porous TiO2layer at3000rpm for20s,which was dried at40◦C for3min and100◦C for5min consecutively.One hundred microliters of0.063M CH3NH3I solution in2-propanol(Aldrich) (10mg/ml)was loaded on the PbI2-coated substrate for20s,which was spun at4000rpm for 20s and then dried at100◦C for5min.It took4s to reach4000rpm,the duration for acceleration. The HTM and Au layer were formed by the same way in the one-step coating procedure.Photocurrent and voltage were measured from a solar simulator equipped with450W Xenon lamp(Newport6279NS)and a Keithley2400source meter.Light intensity was adjusted with the NREL-calibrated Si solar cell having KG-2filter for approximating one sun light intensity (100mW cm−2).While measuring current and voltage,the cell was covered with a black mask having an aperture.Incident photon-to-electron conversion efficiency(IPCE)was measured using a specially designed IPCE system(PV measurement,Inc.).A75W Xenon lamp was used as a light source for generating monochromatic beam.Calibration was accomplished using a silicon photodiode,which was calibrated using the NIST-calibrated photodiode G425as a standard.IPCE data were collected at DC mode.Afield-emission scanning electron microscope(FE-SEM,Jeol JSM6700F)was used to investigate surface and cross sectional morphology of the perovskite solar cells.FIG.1.One-step and two-step spin-coating procedures for CH3NH3PbI3formation.PbI2was mixed with CH3NH3I in N,N-dimethylacetamide(DMA),which was spin-coated and heated for one-step coating.For two-step coating,a PbI2-dissolved N,N-dimethylformamide(DMF)solution wasfirst spin-coated on the substrate,dried and then a CH3NH3I-dissolved isopropyl alcohol(IPA)solution was spin-coated on the PbI2coated substrate.For transient photovoltage measurement,535nm and680nm of wavelength lasers were used as probe and bias light source,respectively.The probe light was incident over the bias light generating steady-state charge where the incident charge was rapidly decreased showingfirst order exponential decay.Both light intensities were varied by a neutral densityfilter.The transient photovoltage signal was amplified using a low-noise preamplifier,Stanford Research System SR560and monitored by an oscilloscope,TDS3054B.Impedance spectra were measured in dark with an Autolab302B with varying a bias potential from0V to1.0V where the potential step is0.1V.AC20mV perturbation was applied with a frequency from1MHz to1Hz.The resulted impedance spectra werefit using Z-View software.The Nyquist plots and the bestfit results(Figure S2of the supplementary material)13 based on an equivalent circuit were described in the supplementary material.In Figure1one-step and two-step coating procedures are schematically illustrated.For one-step coating of CH3NH3PbI3,the DMA solution containing equimolar CH3NH3I and PbI2is spin-coated on the mesoporous TiO2layer.PbI2is formedfirst for two-step coating procedure,which was followed by spin-coating the CH3NH3I solution.In two-step procedure,compared to two-step dipping method,3two-step spin-coating procedure is well defined method because of quantitatively managed process.The amount of CH3NH3I and spin-coating condition should be carefully adjusted in terms of the amount of deposited PbI2.For coating with20μl of1M PbI2solution,100μl of0.063M CH3NH3I is found to be sufficient to convert PbI2into CH3NH3PbI3as confirmed by no presence of PbI2peak in X-ray diffraction spectrum(data are not shown).Detailed method for two-step coating is described in the experimental part.As can be seen in SEM images in Figure2,morphology of the deposited CH3NH3PbI3is remarkably different.One-step coating produces shapeless CH3NH3PbI3(Figure2(b)),whereas cube-like crystals are formed by two-step coating method(Figure2(c)).Besides morphological difference,TiO2layer is not completely covered by the perovskite using one-step spin-coating method compared to full coverage with perovskite by two-step coating procedure.Insufficient coverage in one-step coating is probably related to wettability,associated with high ionic strength (1.25M of CH3NH3+and Pb2+and3.75M of I−)of coating solution,14and/or competition betweenpositive ions of CH3NH3+and Pb2+.Contrary to one-step method,close packing with cube-likeFIG.2.Surface SEM images(left:low magnification,right:high magnification)of(a)mesoporous TiO2coating on the compact blocking layer deposited FTO substrate,(b)one-step deposition of CH3NH3PbI3on the mesoporous TiO2layer, and(c)two-step deposition of CH3NH3PbI3on the mesoporous TiO2layer.crystal with dimension of about100–150nm is induced by two-step method,which indicates that spin-coating of20μl of1M solution of PbI2in DMF covers fully the TiO2film.PbI2is layered structure and well known to undergo intercalation reaction,15in which Lewis base molecules such as pyridine and methylamine were found to be intercalated into interlayer of PbI2.It was mentioned that charge transfer was not obvious during intercalation reaction and the dipole moment of Lewis base molecule or hydrogen bonding by the N–H bond was necessary requirement for intercalation.Thus, reaction of PbI2with CH3NH3I may be regarded as pseudo-intercalation because I−in CH3NH3I salt acts as an electron donor.Reaction of PbI2with I−forms presumably(PbI3)−via I2-I−interaction, which is followed by reaction with CH3NH3+to form CH3NH3PbI3.It was reported that a vacuum deposited PbI2was converted to CH3NH3PbI3when it was dipped in CH3NH3I solution,where full conversion required more than1h.16However,solution processed PbI2film reduces significantly reaction time for conversion.According to single crystal X-ray diffraction analysis,need-like crystals collected after cooling1M solution of PbI2in DMF revealed that one DMF molecule was coordinated to Pb via oxygen bridge.17Thus,substitution of CH3NH3I for DMF could also explain the two-step formation of CH3NH3PbI317and the faster reaction than the vacuum deposited PbI2beginning with surface reaction.Investigation from cross-sectional SEM images also confirms imperfect pore-filling of per-ovskite by one-step coating,which leads to contact between FTO and HTM as can be seen in Figure3. On the other hand,pores are completelyfilled with perovskite by using two-step coating.As can be seen in schematic illustrations based on SEM images,one-step coating leads to perovskite island but two-step one results in void-free perovskite layer.Mesoporous TiO2layer thickness is about 100nm and perovskite overlayer is around200nm.Photovoltaic parameters are plotted in Figure4,where the data obtained from40cells arestatistically analyzed.All the parameters of two-step deposited perovskite are superior to those ofFIG.3.Cross sectional SEM images of(a)one-step deposition of CH3NH3PbI3and(b)two-step deposition of CH3NH3PbI3. One-step deposition leads to imperfect pore-filling as shown in the high magnification SEM image.Two-step deposition showsthat pores of TiO2layer are fullyfilled with CH3NH3PbI3as confirmed by void-free interface.FIG.4.Short-circuit current density(Jsc),open-circuit voltage(V oc),fill factor(FF),and power conversion efficiency(PCE) for the perovskite solar cells based on one-step and two-step deposition procedure.The data were statistically analyzed from40cells.FIG.5.Normalized IPCE for the perovskite solar cells based on one-step(black line)and two-step(red line)process.one-step deposited one.Average short-circuit current density(J sc),open-circuit voltage(V oc),fill factor(FF),and power conversion efficiency(PCE)of19.15mA/cm2,0.828V,0.470,and7.5%are observed from the one-step deposited perovskite solar cells,while higher values of21.47mA/cm2, 1.024V,0.634,and13.9%are obtained from the two-step deposited ones.Standard deviation for PCE is as small as±0.6and±0.4for the one-step and two-step deposited devices,respectively, which indicates that the data are reproducible.Morphology-property relation can explain difference in photovoltaic performance.Higher J sc for the two-step deposition is due to better pore-filling of perovskite compared to its island structure for the one-step deposition.As shown in Figure3,the absence of perovskite at FTO interface loses absorption of short wavelength light,asfirmed by IPCE measurement in Figure5,which is responsible for lower J sc for one-step deposition.Recombination kinetics of devices based on one-step and two-step procedure are investigated using a transient photovoltage measurement and impedance spectra.The electron life time is obtained from a transient photovoltage signal byfitting it withfirst order exponential decay.In the transient photovoltage measurement the electron life time is strongly dependent on the light intensity where strong light intensity induces high electron and hole density and thus,a fast recombination is resulted. Contrariwise,longer electron life time is attributed to the lower density of electron and hole induced by weak light intensity.18It is reported that CH3NH3PbI3perovskite solar cell also shows the power law dependence of electron life time on the light intensity or open circuit voltage,19,20as shown in Figure6(a).The electron life time of two-step deposited perovskite is about one order of magnitude longer than that of one-step implying that the recombination kinetics highly depends on the perovskite structure determined by deposition method.This suggests that the voids generated in one-step coating allow HTM to infiltrate into perovskite layer,which increases a potential recombination site and leads ten times faster recombination than in two-step deposited perovskite.The recombination resistance is obtained from impedance spectra where thefirst arc in high frequency region is related to the transport in sprio-MeOTAD21and the second arc is related to the recombination.22,23The two arcs arefitted using a simplified equivalent circuit(resistance-parallel resistance,capacitance-parallel resistance,and capacitance in series)and the resulted recombination resistance(R r)is plotted as a function of an applied bias voltage in Figure6(b).R r shows little change in the low applied voltage (V app<0.5V)region but it starts to decrease rapidly as the Fermi level in photoanode increases by applying high bias voltage(V app>0.5V).1R r for one-step and two-step deposited perovskite are similar as expected in the region of low applied voltage(V app<0.5V),but R r for one-step shows lower value than that for two-step as the applied bias voltage increases more than0.5V describing that the recombination kinetics for one-step is faster than that of two-step perovskite. This result is also accordance with the tendency of electron life time.Likewise,the two-stepperovskite shows enhanced recombination kinetics due to its well established layer with free-voidparison of(a)electron life time for one-step(black)and two-step(red)deposited perovskite with varying open circuit voltage and(b)recombination resistance for one-step(black)and two-step(red)deposited perovskite by applying bias voltage.enabling to prevent the HTM infiltration and thus decrease the recombination probability.The lowered recombination rate for two-step deposited perovskite layer has a significant impact on the open-circuit voltage24leading200mV higher V oc than that for one-step deposited perovskite.It was reported that photovoltaic performance was found to be strongly dependent on degree of perovskite pore-filling,where decrease in perovskite pore-filling fraction led to deterioration of J sc,V oc,and fill factor.25In addition,incomplete perovskite pore-filling resulted in fast charge recombination of the injected electron in TiO2with spiro-MeOTAD.25Thus,we propose here that removal of the exposed TiO2allowing unwanted contact with spiro-MeOTAD is important to improve photovoltaic performance of the mesoporous TiO2based perovskite solar cell.Photovoltaic property-morphology relation was systematically evaluated from the diverse depo-sition methodologies of perovskite CH3NH3PbI3.Reproducible photovoltaic parameters extracted from statistical analysis were found to have strong correlation with the morphology of the deposited perovskite along with degree of the perovskite coverage.Recombination kinetics was significantly affected by the resulting morphology of the perovskite.The exposed TiO2by one-step coating was responsible for fast recombination and short electron life time.On the other hand,the complete pore-filling with perovskite by two-step method resulted in a significant improvement of photo-voltaic performance.It is concluded that photovoltaic performance is strongly dependent on degree of perovskite coverage on the mesoporous TiO2layer and morphology of the deposited perovskite in the mesoporous TiO2based perovskite solar cells.This work was supported by the National Research Foundation of Korea(NRF)grants funded by the Ministry of Science,ICT&Future Planning(MSIP)of Korea under Contract Nos.NRF-2010-0014992,NRF-2012M1A2A2671721,NRF-2012M3A7B4049986(Nano Material Technol-ogy Development Program),and NRF-2012M3A6A7054861(Global Frontier R&D Program on Center for Multiscale Energy System).H.S.K.is grateful to NRF for funding the global Ph.D.grant. 1H.-S.Kim,C.-R.Lee,J.-H.Im,K.-B.Lee,T.Moehl,A.Marchioro,S.-J.Moon,R.Humphry-Baker,J.-H.Yum,J.E. Moser,M.Gr¨a tzel,and N.-G.Park,Sci.Rep.2,591(2012).2M.M.Lee,J.Teuscher,T.Miyasaka,T.N.Murakami,and H.J.Snaith,Science338,643(2012).3J.Burschka,N.Pellet,S.-J.Moon,R.Humphry-Baker,P.Gao,M.K.Nazeeruddin,and M.Gr¨a tzel,Nature(London)499, 316(2013).4M.Liu,M.B.Johnston,and H.J.Snaith,Nature(London)501,395(2013).5A.Kojima,K.Teshima,Y.Shirai,and T.Miyasaka,J.Am.Chem.Soc.131,6050(2009).6J.-H.Im,C.-R.Lee,J.-W.Lee,S.-W.Park,and N.-G.Park,Nanoscale3,4088(2011).7J.H.Heo,S.H.Im,J.H.Noh,T.N.Mandal,C.-S.Lim,J.A.Chang,Y.H.Lee,H.-j.Kim,A.Sarkar,M.K.Nazeeruddin, M.Gr¨a tzel,and S.I.Seok,Nat.Photon.7,486(2013).8N.-G.Park,J.Phys.Chem.Lett.4,2423(2013).9H.J.Snaith,J.Phys.Chem.Lett.4,3623(2013).10H.-S.Kim,S.H.Im,and N.-G.Park,J.Phys.Chem.C118,5615(2014).11S.Kazim,M.K.Nazeeruddin,M.Gr¨a tzel,and S.Ahmad,Angew.Chem.Inter.Ed.53,2812(2014).12P.P.Boix,K.Nonomura,N.Mathews,and S.G.Mhaisalkar,Mater.Today17,16(2014).13See supplementary material at /10.1063/1.4891275for the real device configuration and for the Nyquist plots and theirfits based on an equivalent circuit.14R.Steitz,W.Jaeger,and R.V.Klitzing,Langmuir17,4471(2001).15C.C.Coleman,H.Goldwhite,and W.Tikkanen,Chem.Mater.10,2794(1998).16K.Liang,D.B.Mitzi,and M.T.Prikas,Chem.Mater.10,403(1998).17A.Wakamiya,M.Endo,T.Sasamori,N.Tokitoh,Y.Ogomi,S.Hayase,and Y.Murata,Chem.Lett.43,711–713(2014). 18K.Zhu,S.-R Jang,and A.J.Frank,J.Phys.Chem.Lett.2,1070(2011).19D.Bi,S.-J.Moon,L.Haggman,G.Boschloo,L.Yang,E.M.J.Johansson,M.K.Nazeeruddin,M.Gr¨a tzel,and A.Hagfeldt, RCS Adv.3,18762(2013).20Y.Zhao,A.M.Nardes,and K.Zhu,J.Phys.Chem.Lett.5,490(2014).21F.Fabregat-Santiago,J.Bisquert,L.Cevey,P.Chen,M.Wang,S.M.Zakeeruddin,and M.Gr¨a tzel,J.Am.Chem.Soc. 131,558(2009).22H.-S.Kim,I.Mora-Sero,V.Gonzalez-Pedro,F.Fabregat-Santiago,E.J.Juarez-Perez,N.-G.Park,and J.Bisquert,Nat. Commun.4,2242(2013).23H.-S.Kim,J.-W.Lee,N.Yantara,P.P.Boix,S.A.Kulkarni,S.Mhaisalkar,M.Gr¨a tzel,and N.-G.Park,Nano Lett.13, 2412(2013).24A.Zaban,M.Greenshtein,and J.Bisquert,Chem.Phys.Chem.4,859(2003).25T.Leijtens,uber,G.E.Eperon,S.D.Stranks,and H.J.Snaith,J.Phys.Chem.Lett.5,1096(2014).。

工作场所有害因素职业接触限值中华人民共和国卫生部2007-04-12 发布2007-11-01实施第1部分:化学有害因素Occupational exposure limits for hazardous agents in the workplacePart 1:Chemical hazardous agents前言此次修订将GBZ 2-2002 《工作场所有害因素职业接触限值》分为GBZ 2.1 《工作场所有害因素职业接触限值第1部分:化学有害因素》和GBZ 2.2 《工作场所有害因素职业接触限值第2部分:物理因素》。
自本部分实施之日起,GBZ2-2002中相应的内容作废。
本部分与GBZ 2-2002相比主要修改如下:a)进一步明确了职业卫生标准所采用的概念及其定义,并增加了以下内容:——超限倍数及其应用;——总粉尘、呼吸性粉尘和空气动力学直径的定义;——化学物质的致癌性参考分类、标识及其应用;——致敏性物质的标识及其应用;——经皮标识的应用。
b)对某些标准值进行了调整:——修订了乙腈、乙酸甲酯的接触限值;——增订了百草枯、毒死蜱、氯乙酸、钡及其可溶性化合物、萤石混合性粉尘呼尘的接触限值。
c)删除了GBZ2-2002中47种粉尘的PC-STEL值和164种化学物质的带*号的PC-STEL值。
d)增加参考致癌性标识59项,致敏性标识9项,经皮标识10项。
本部分的附录A为规范性附录。
本部分由全国职业卫生标准委员会提出。
本部分由中华人民共和国卫生部批准。
本部分主要起草单位:中国疾病预防控制中心职业卫生与中毒控制所、复旦大学公共卫生学院、华中科技大学同济公共卫生学院、北京大学公共卫生学院等。
本部分主要起草人:苏志、李涛、梁友信、杨磊、王生、张敏、吕伯钦、吴维皑、徐伯洪、刘占元、郑玉新、闫慧芳、陈卫红、谷京宇、杜燮祎、周志俊、夏昭林、何丽华、赵一鸣、黄汉林、缪剑影、刘晓延、张幸、雷玲、朱菊一。
本部分所代替标准的历次版本发布情况为:——GBZ 2-2002。

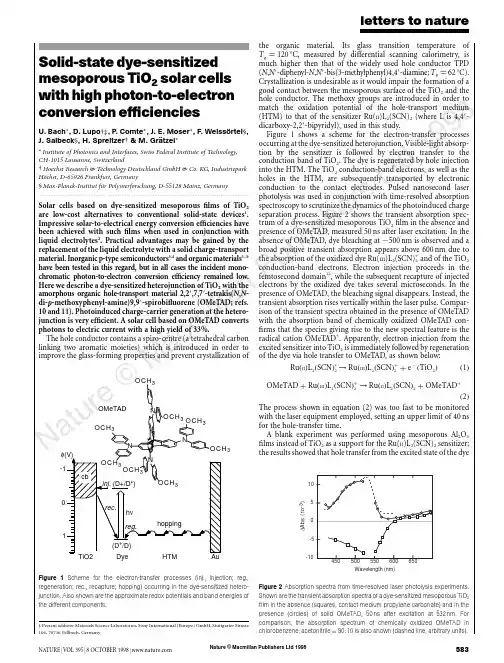
Nature © Macmillan Publishers Ltd 19988Solid-state dye-sensitized mesoporous TiO 2solar cells with high photon-to-electron conversion efficienciesU.Bach *,D.Lupo †‡,P .Comte *,J.E.Moser *,F .Weissortel §,J.Salbeck §,H.Spreitzer †&M.Gratzel **Institute of Photonics and Interfaces,Swiss Federal Institute of Technology,CH-1015Lausanne,Switzerland†Hoechst Research &Technology Deutschland GmbH &Co.KG,Industriepark Ho¨chst,D-65926Frankfurt,Germany §Max-Planck-Institut fu¨r Polymerforschung,D-55128Mainz,Germany .........................................................................................................................Solar cells based on dye-sensitized mesoporous films of TiO 2are low-cost alternatives to conventional solid-state devices 1.Impressive solar-to-electrical energy conversion efficiencies have been achieved with such films when used in conjunction with liquid electrolytes 2.Practical advantages may be gained by the replacement of the liquid electrolyte with a solid charge-transport material.Inorganic p-type semiconductors 3,4and organic materials 5–9have been tested in this regard,but in all cases the incident mono-chromatic photon-to-electron conversion efficiency remained low.Here we describe a dye-sensitized heterojunction of TiO 2with the amorphous organic hole-transport material 2,2Ј,7,7Ј-tetrakis(N ,N -di-p -methoxyphenyl-amine)9,9Ј-spirobifluorene (OMeTAD;refs.10and 11).Photoinduced charge-carrier generation at the hetero-junction is very efficient.A solar cell based on OMeTAD converts photons to electric current with a high yield of 33%.The hole conductor contains a spiro-centre (a tetrahedral carbon linking two aromatic moieties)which is introduced in order to improve the glass-forming properties and prevent crystallization ofthe organic material.Its glass transition temperature of T g ¼120ЊC,measured by differential scanning calorimetry,is much higher then that of the widely used hole conductor TPD (N ,N Ј-diphenyl-N ,N Ј-bis(3-methylphenyl)4,4Ј-diamine;T g ¼62ЊC).Crystallization is undesirable as it would impair the formation of a good contact between the mesoporous surface of the TiO 2and the hole conductor.The methoxy groups are introduced in order to match the oxidation potential of the hole-transport medium (HTM)to that of the sensitizer Ru(II )L 2(SCN)2(where L is 4,4Ј-dicarboxy-2,2Ј-bipyridyl),used in this study.Figure 1shows a scheme for the electron-transfer processes occurring at the dye-sensitized heterojunction.Visible-light absorp-tion by the sensitizer is followed by electron transfer to the conduction band of TiO 2.The dye is regenerated by hole injection into the HTM.The TiO 2conduction-band electrons,as well as the holes in the HTM,are subsequently transported by electronic conduction to the contact electrodes.Pulsed nanosecond laser photolysis was used in conjunction with time-resolved absorption spectroscopy to scrutinize the dynamics of the photoinduced charge separation process.Figure 2shows the transient absorption spec-trum of a dye-sensitized mesoporous TiO 2film in the absence and presence of OMeTAD,measured 50ns after laser excitation.In the absence of OMeTAD,dye bleaching at ϳ500nm is observed and a broad positive transient absorption appears above 600nm due to the absorption of the oxidized dye Ru(III )L 2(SCN)+2and of the TiO 2conduction-band electrons.Electron injection proceeds in the femtosecond domain 12,while the subsequent recapture of injected electrons by the oxidized dye takes several microseconds.In the presence of OMeTAD,the bleaching signal disappears.Instead,the transient absorption rises vertically within the laser par-ison of the transient spectra obtained in the presence of OMeTAD with the absorption band of chemically oxidized OMeTAD con-firms that the species giving rise to the new spectral feature is the radical cation OMeTAD +.Apparently,electron injection from the excited sensitizer into TiO 2is immediately followed by regeneration of the dye via hole transfer to OMeTAD,as shown below:Ru ðII ÞL 2ðSCN Þء2→Ru ðIII ÞL 2ðSCN Þþ2þe ϪðTiO 2Þð1ÞOMeTAD þRu ðIII ÞL 2ðSCN Þþ2→Ru ðII ÞL 2ðSCN Þ2þOMeTADþð2ÞThe process shown in equation (2)was too fast to be monitored with the laser equipment employed,setting an upper limit of 40ns for the hole-transfer time.A blank experiment was performed using mesoporous Al 2O 3films instead of TiO 2as a support for the Ru(II )L 2(SCN)2sensitizer;the results showed that hole transfer from the excited state of the dyeletters to natureNATURE |VOL 395|8OCTOBER 1998|5833OCH 3φ(V)0OMeTAD Figure 1Scheme for the electron-transfer processes (inj.,injection;reg.,regeneration;rec.,recapture;hopping)occurring in the dye-sensitized hetero-junction.Also shown are the approximate redox potentials and band energies of the different components.‡Present address:Materials Science Laboratories,Sony International (Europe)GmbH,Stuttgarter Strasse 106,70736Fellbach,Germany.Wavelength (nm)1050-5-10∆A b s . (10-3)Figure 2Absorption spectra from time-resolved laser photolysis experiments.Shown are the transient absorption spectra of a dye-sensitized mesoporous TiO 2film in the absence (squares,contact medium propylene carbonate)and in the presence (circles)of solid OMeTAD,50ns after excitation at 532nm.Forcomparison,the absorption spectrum of chemically oxidized OMeTAD in chlorobenzene :acetonitrile ¼90:10is also shown (dashed line,arbitrary units).Nature © Macmillan Publishers Ltd 19988to the OMeTAD does not contribute significantly to the photo-induced charge-separation phenomena observed.The photovoltaic performance of the dye-sensitized heterojunc-tion was studied by means of sandwich-type cells,shown schema-tically in Fig.3a.The working electrode consisted of conducting glass (F-doped SnO 2,sheet resistance 10Q per square)onto which a compact TiO 2layer was deposited by spray pyrolysis 13.This avoids direct contact between the HTM layer and the SnO 2which would short-circuit the cell.A 4.2-m-thick mesoporous film of TiO 2was deposited by screen printing onto the compact layer 14,and deriva-tized with Ru(II )L 2(SCN)2by adsorption from acetonitrile.The HTM was introduced into the mesopores by spin-coating a solution of OMeTAD in chlorobenzene onto the TiO 2film,and subsequent evaporation of the solvent.A semi-transparent gold back contact was evaporated on top of the hole conductor under vacuum.Figure 3b shows the photocurrent action spectrum of a typical cell under short-circuit conditions.The given values are not corrected for reflection and absorption losses of the conducting glass,which are estimated to be at least 15%in the visible region of the spectrum.The spectrum closely matches the absorption spectrum of the dye,confirming that the observed photocurrent arises from electron injection by the sensitizer.The maximum value of the incident photo-to-electron conversion efficiency (IPCE)is 33%,which is more than two orders of magnitude larger than the previously reported value for a similar dye-sensitized solid heterojunction 9and only a factor of ϳ2lower than with liquid electrolytes 2.The coating solution used for the device in Fig.3b contained 0.33mM N(PhBr)3SbCl 6and 15mM Li[(CF 3SO 2)2N]in addition to 0.17M OMeTAD.In the absence of these additives,the maximum IPCE was only 5%.N(PhBr)3SbCl 6acts as a dopant,introducing free charge carriers in the HTM by oxidation,as confirmed by spectro-electrochemical measurements.Partial oxidation of OMeTAD by N(PhBr)3SbCl 6is a convenient way to control the dopant level 15.On adding N(PhBr)3SbCl 6to a solution of OMeTAD in chlorobenzene,the radical cation OMeTAD +is instantly formed.The spectral features of OMeTAD +remained unchanged during solvent eva-poration and glass formation,except for a small hypochromic shift.No subsequent absorption changes were detectable over several weeks,confirming the temporal stability of OMeTAD +in the HTM.The second additive,Li[(CF 3SO 2)2N],is a source of Li +ions,which are known to be potential-determining for TiO 2(ref.16).Along with the protons from the carboxylic acid groups of Ru(II )L 2(SCN)2,they confer a positive charge on the surface of the oxide.As the sensitizer is negatively charged a local electrostatic field is produced,assisting electron injection into the TiO 2while retard-ing recapture of the electron by the oxidized dye.The lithium salt may also compensate for space-charge effects.Under light illumina-tion of the heterojunction,a net positive space charge is expected to be formed in the HTM,inducing a local field that impairs current flow.The lithium salt could screen this field,thereby eliminating the space-charge control of the photocurrent.Improvement of the photovoltaic performance of dye-sensitized heterojunctions by immersion in LiClO 4solutions was also reported by Murakoshi et al.5.Figure 4shows current-density/voltage curves employing the device structure shown in Fig.3a.Curves I and II were obtained with hole conductor containing both the N(PhBr)3SbCl 6dopant and the Li[(CF 3SO 3)2N]salt,whereas these additives were absent for curve III.Curve I was measured in the dark,whereas II and III were obtained under light illumination.The device that contains the hole conductor without additives performs poorly,the conversion yield being only 0.04%at a white-light illumination of 9.4mW cm −2.Addition of the dopant and Li +salt increases the overall conversion efficiency to 0.74%.Under full sunlight (100mW cm −2,air mass 1.5),the short-circuit photocurrent density reached 3.18mA cm −2,a value which is unprecedented for solar cells based on organic solids.Further improvement of the photovoltaic performance is expected,as many parameters of the cell assembly have not yet been opti-mized.Preliminary stability tests performed over 80h using the visible output of a 400W Xe lamp showed that the photocurrent was stable within Ϯ20%,while the open-circuit voltage and the fill factor (see Methods)increased.The total charge passed through the cell during illumination was 300C cm −2;corresponding to turnover numbers of about 8,400and 60,000for the OMeTAD and the dye,respectively.This shows that the hole conductor can sustain photo-voltaic operation without significance degradation.From the present findings,the concept of dye-sensitized hetero-junctions emerges as a very interesting and viable option for futureletters to nature584NATURE |VOL 395|8OCTOBER 1998|Wavelength (nm)abI P C E (%)Figure 3Structure and spectral response of the photovoltaic devices.a ,Structure 1,conducting F-doped SnO 2-coated glass;2,compact TiO 2layer;3,dye-sensitized heterojunction;4,gold electrode.b ,Photocurrent action spectrum for a dye-sensitized heterojunction,the structure of which is shown above.The IPCE value corresponds to the number of electrons generated by monochromatic light in the external circuit,divided by the number of incident photons.The 4.2-m-thick mesoporous TiO 2film was sensitized with Ru(II )L 2(SCN)2,spin-coated with a solution of 0.17M OMeTAD,0.33mM N(PhBr)3SbCl 6and 15mM Li[(CF 3SO 2)2N in chlorobenzene with 5%acetonitrile added.0Voltage (V)C u r r e n t d e n s i t y (m A c m –2)Figure 4Current-density/voltage characteristics.Shown are characteristics of the same device as in Fig.3,obtained in the dark (I)and under white-light illumination at 9.4mW cm −2(II).The spectral distribution corresponded to global air mass 1.5corrected for spectral mismatch.The short-circuit current density was 0.32mA cm −2,the open-circuit voltage 342mV and the fill factor 62%corresponding to an overall conversion efficiency of 0.74%.For comparison,the photocurrent-density/voltage characteristic of a cell containing no N(PhBr)3SbCl 6or Li[(CF 3SO 2)2N is also shown (III).Nature © Macmillan Publishers Ltd 19988low-cost solid-state solar cells.Photodiodes based on interpenetrat-ing polymer networks of poly(phenylenevinylene)derivatives 17,18present a related approach.The main difference to our system is that at least one component of the polymer network needs to function simultaneously as an efficient light absorber and a good charge-transport material.The dye-sensitized heterojunction cell offers a greater flexibility,as the light absorber and charge-transport material can be selected independently to obtain optimum solar-energy harvesting and high photovoltaic output.Ⅺ.........................................................................................................................MethodsCompounds.OMeTAD was pure according to 1H-NMR and HPLC analysis.The synthesis will be reported elsewhere.Ru(II )L 2(SCN)2was prepared as previously described 2.Transient absorption spectroscopy.This was carried out with a Nd-YAG laser as excitation light source,producing a 6-ns pulse at 532nm of typically 1mJ cm −2,with a repetition frequency of 30Hz.The probe light was provided by a Xe lamp,which was spectrally narrowed by cut-off and interference filters before passing the device.A monochromator combined with a photomultiplier was used as detection system.A T ektronix 524TDS oscilloscope was used to record and store the data.For the laser experiments,dye-sensitized mesoporous semiconductor films were deposited on ordinary glass.Photocurrent-voltage characteristics.These were measured with a Keithley 2400Source Meter and a 400W Xe lamp.A Schott KG3filter was used in order to approach the spectral distribution of the lamp to air mass 1.5G.The light intensity was regulated to the desired energy output by using a silicon solar cell,calibrated at the ISE-Fraunhofer Institut in Freiburg Germany.Efficiencies were corrected for the spectral mismatch.The fill factor (FF)is defined as FF ¼V opt I opt =I sc V oc ,where V opt and I opt are respectively current and voltage for maximum power output,and I sc and U oc are the short-circuit current and open-circuit voltage,respectively.Received 8May;accepted 13July 1998.1.O’Regan,B.&Gra¨tzel,M.A low-cost,high-efficiency solar cell based on dye-sensitized colloidal TiO 2films.Nature 353,737–739(1991).2.Nazeeruddin,M.K.et al .Conversion of light to electricity by cis-X 2bis(2,2Ј-bipyridyl-4,4Ј-dicarbox-ylate)ruthenium(II)charge-transfer sensitizers (X ¼Cl −,Br −,I −,CN −and SCN −)on nanocrystalline TiO 2electrodes.J.Am.Chem.Soc.115,6382–6390(1993).3.O’Regan,B.&Schwarz,rge enhancement in photocurrent efficiency caused by UVillumination of the dye-sensitized heterojunction TiO 2/RuLL ЈNCS/CuSCN:initiation and potential mechanism.Chem.Mater.10,1501–1509(1998).4.T ennakone,K.,Kumara,G.R.R.A.,Kumarasinghe,A.R.,Wijayantha,K.G.U.&Sirimanne,P .M.Dye-sensitized nano-porous solid-state photovoltaic cell.Semicond.Sci.Technol.10,1689–1693(1995).5.Murakoshi,K.,Kogure,R.&Yanagida,S.Solid state dye-sensitized TiO 2solar cell with polypyrrole ashole transport layer.Chem.Lett.5,471–472(1997).6.Bach,U.et al .Ultrafast hole injection from dye molecules into an organic hole conductor for dyesensitized solid state solar cells.Abstract Book,Bayreuth Polymer &Materials Research Symposium ,P28(Bayreuth,1997).7.Weisso¨rtel, F.Amorphous niedermolekulare Ladungstransportmaterialien fu ¨r nanokristalline Solarzellen.Thesis,Univ.Regensburg (1996).8.Gra¨tzel,M.in Future Generation Photovoltaic Technologies Vol.404(ed.McConnell,R.)119–126(Am.Inst.Phys.,Denver,1997).9.Hagen,J.et al .Novel hybrid solar cells consisting of inorganic nanoparticles and an organic holetransport material.Synth.Met.89,215–220(1997).10.Salbeck,J.,Weisso¨rtel,F.&Bauer,J.Spiro linked compounds for use as active materials in organic light emitting diodes.Macromol.Symp.125,121–132(1997).11.Salbeck,J.,Yu,N.,Bauer,J.,Weisso¨rtel,F.&Bestgen,H.Low molecular organic glasses for blue electroluminescence.Synth.Met.91,209–215(1997).12.Tachibana,Y.,Moser,J.E.,Gra¨tzel,M.,Klug,D.R.&Durrant,J.R.Subpicosecond interfacial charge separation in dye-sensitized nanocrystalline titanium dioxide films.J.Phys.Chem.100,20056–20062(1996).13.Kavan,L.&Gra¨tzel,M.Highly efficient semiconducting TiO 2photoelectrodes prepared by aerosol pyrolysis.Electrochim.Acta 40,643–652(1995).14.Barbe´,C.J.et al .Nanocrystalline titanium oxide electrodes for photovoltaic applications.J.Am.Ceram.Soc.80,3157–3171(1997).15.Abkowitz,M.&Pai,parison of the drift mobility measured under transient and steady-state conditions in a prototypical hopping system.Phil.Mag.B 53,192–216(1986).16.Enright,B.,Redmond,G.&Fitzmaurice,D.Spectroscopic determination of flat-band potentials forpolycrystalline TiO 2electrodes in mixed-solvent systems.J.Phys.Chem.97,1426–1430(1994).17.Halls,J.J.M.et al .Efficient photodiodes from interpenetrating polymer networks.Nature 376,498–500(1995).18.Yu,G.,Gao,J.,Hummelen,J.C.,Wudl,F.&Heeger,A.J.Polymer photovoltaic cells:enhancedefficiencies via a network of internal donor acceptor heterojunctions.Science 270,1789–1791(1995).Acknowledgement.This work was supported by the Swiss National Science Foundation and the European Joule III programme (OFES).Correspondence and requests for materials should be addressed to M.G.(e-mail:michael.graetzel@epfl.ch).letters to natureNATURE |VOL 395|8OCTOBER 1998|585Accumulation of persistent organochlorine compounds in mountains of western CanadaJules M.Blais *†,David W.Schindler *,Derek C.G.Muir †‡,Lynda E.Kimpe §,David B.Donald k &Bruno Rosenberg ¶*Department of Biological Sciences,University of Alberta,Edmonton,Alberta,Canada T6G 2E9‡Department of Fisheries and Oceans,Freshwater Institute,501University Crescent,Winnipeg,Manitoba,Canada R3T 2N6§Public Health Sciences,University of Alberta,Edmonton,Alberta,Canada T6G 2G3k Environment Canada,Room 300Park Plaza,2365Albert Street,Regina,Saskatchewan,Canada S4P 4K1¶Freshwater Institute,Winnipeg,Manitoba,Canada R3T 2N6.........................................................................................................................Persistent,semi-volatile organochlorine compounds,including toxic industrial pollutants and agricultural pesticides,are found everywhere on Earth,including in pristine polar and near-polar locations 1–4.Higher than expected occurrences of these com-pounds in remote regions are the result of long-range transport in the atmosphere,precipitation and ‘cold condensation’—the progressive volatilization in relatively warm locations and sub-sequent condensation in cooler environments 3,4which leads to enhanced concentrations at high latitudes.The upper reaches of high mountains are similar to high-latitude regions in that they too are characterized by relatively low average temperatures,but the accumulation of organochlorine compounds as a function of altitude has not yet been documented.Here we report organo-chlorine deposition in snow from mountain ranges in western Canada that show a 10-to 100-fold increase between 770and 3,100m altitude.In the case of less-volatile compounds,the observed increase by a factor of 10is simply due to a 10-fold increase in snowfall over the altitude range of the sampling sites.In the case of the more-volatile organochlorines,cold-condensa-tion effects further enhance the concentration of these com-pounds with increasing altitude.These findings demonstrate that temperate-zone mountain regions,which tend to receiveT able 1Correlation between organochlorine concentrations in snow and site elevationsCompound Correlation coefficientVapour pressure(Pa).............................................................................................................................................................................␣-HCH0.85*0.1Heptachlorepoxide 0.75*0.1␥-HCH 0.73*0.03Dieldrin0.42*0.016Endosulphan-I 0.76*0.008c-Chlordane 0.42*0.003t-Chlordane 0.340.003p p ЈDDT 0.000.0001.............................................................................................................................................................................PCBs.............................................................................................................................................................................S Dichloro-0.54*0.2(0.008–0.60)S Trichloro-0.53*0.04(0.003–0.022)S Tetrachloro-0.000.006(0.003–0.10)S Pentachloro-0.000.001(0.0003–0.009)S Hexachloro-0.110.0002(7ϫ10Ϫ4Ϫ0:012)S Heptachloro-0.173ϫ10Ϫ4(2:7ϫ10Ϫ5Ϫ0:0015).............................................................................................................................................................................Correlation coefficients (r )are shown for organochlorine concentrations (ng l −1)in snow and site elevation (m.a.s.l.)for the equation conc :¼a e b Elev :,where a and b are fitted constants.Asterisks show significance at P р0:05,for 19degrees of freedom.Sub-cooled liquid vapour pressures are included for pesticides at 20ЊC (ref.21)and PCBs at 25ЊC (ref.22).Published vapour pressures vary considerably,so these values represent mean reported values for all PCBs in that class.Ranges of published vapour pressures for each PCB category are shown in brackets.Only compounds with mean sample concentrations that were ten times higher than blanks were considered.†Present addresses:Department of Biology,University of Ottawa,30Marie Curie Street,PO Box 450Stn.A,Ottawa,Ontario,Canada K1N 6N5(J.M.B.);Environment Canada,867Lakeshore Road,Burlington,Ontario,Canada L7R 4A6(D.C.G.M).。

先丰客户发表文章Publications Featuring XFNANO Graphene, Carbon Nanotubes and Others.此统计数据日期截至2014年02月22日,由于文章较多,此处仅统计先丰客户英文文章且直接引用先丰公司英文名称”Nanjing XFNANO Materials Tech Co.,Ltd”,截至到现在已经有超过500篇文章(包括英文/中文/专利)署名先丰纳米,我司现整理出242篇高质量英文文章,总影响因子超过1000,平均影响因子3.993。
其中2014年前两个月客户已经发表高质量英文文章50篇;2013年客户发表200多篇高质量的英文文章,其中有JACS,AM,AFM,CC,JPCC,JMC 等等,值得骄傲的是这些材料都是实验的主体材料,在国际上宣传了”XFNANO”,为先丰带来了声誉和很多国际客户,这也说明了国外杂志对我司的认可,也为后来客户发表文章直接引用我司提供了很多方便和印证。
先丰纳米公司从09年发展至今,一直专注于提供高质量的石墨烯产品。
我司现摘录部分英文文章如下,一是为宣传我司;二是也是为广大客户更信任我司产品,启迪客户科研思路,用好我司提供的材料;三是推动我司继续前进,履行“先进纳米材料制造商以及技术服务商”的宗旨。
另外我司代理的产品,国内客户发表文章的也有上百篇,由于署名不是我司,较难查找,我司以后会摘录几篇影响因子较高的客户文章,同时也欢迎客户反馈文章发表信息。
反馈一篇我司此列表中未摘录的英文文章包括会议论文奖励一百元,作者亲自反馈的除奖金外,购买我司产品一律享受VIP待遇。
以下为影响因子和文章概述,经我司计算,客户以我司名义发表SCI文章影响因子平均高达:3.993影响因子(Impact Factor)概述:大于等于10:期刊:Advanced Materials 2013 影响因子:14.829文章Vertically Oriented Graphene Bridging Active-Layer/Current-Collector Interface for Ultrahigh Rate Supercapacitors期刊:Advanced Functional Materials 2012 影响因子:10.179文章: Layered H2Ti6O13-Nanowires: A New Promising Pseudocapacitive Material in Non-Aqueous Electrolyte南京先丰纳米材料科技有限公司Nanjing XFNANO Materials Tech Co.,Ltd 地址:南京市鼓楼区南京大学国家大学科技园Add:Nanjing Jiangsu Province China大于等于6:期刊:Nanoscale 2014 影响因子:6.233文章:Vertical junction photodetectors based on reduced graphene oxide/silicon Schottky diodes.期刊:Biomaterials 影响因子:7.604文章:Inhibitory effect of silver nanomaterials on transmissible virus-induced host cell infections.期刊:Biosensors and Bioelectronics 2014 影响因子: 5.437文章A general strategy to prepare homogeneous and reagentless GO/lucigenin&enzyme biosensors for detection of small biomolecules期刊:Biosensors and Bioelectronics 2014 影响因子: 5.437文章Simultaneous electrochemical detection of cervical cancer markers using reduced graphene oxide-tetraethylene pentamine as electrode materials and distinguishable redox probes as labels期刊:Biosensors and Bioelectronics 2014 影响因子: 5.437文章Electrochemical determination of cefotaxime based on a three-dimensional molecularly imprinted film sensor期刊:Biosensors and Bioelectronics 2014 影响因子: 5.437文章Femtomole level photoelectrochemical aptasensing for mercury ions using quercetin–copper(II) complex as the DNA intercalator期刊:analytical chemistry 2014 影响因子: 5.695文章 A Homogeneous Signal-On Strategy for the Detection of rpoB Genes of Mycobacterium tuberculosis Based on Electrochemiluminescent Graphene Oxide and Ferrocene Quenching期刊:Biosensors and Bioelectronics 2014影响因子: 5.437文章Investigation of the effect of phytohormone on the expression of microRNA-159a in Arabidopsis thaliana seedlings based on mimic enzyme catalysis systematic electrochemical biosensor期刊:Biosensors and Bioelectronics 2014 影响因子: 5.437文章Target-induced electronic switch for ultrasensitive detection of Pb2+ based on three dimensionally ordered macroporous Au–Pd bimetallic electrode期刊:Biosensors and Bioelectronics 2014 影响因子: 5.437文章Electrochemical immunoassay for procalcitonin antigen detection based on signal amplification strategy of multiple nanocomposites期刊:Carbon 2014 影响因子: 5.868文章Enhanced nonlinear optical and optical limiting properties of graphene/ZnO hybrid organic glasses期刊:Carbon 2014 影响因子: 5.868文章Reductive dechlorination of hexachloroethane by sulfide in aqueous solutions mediated by graphene oxide and carbon nanotubes文章Facile and novel electrochemical preparation of a graphene–transition metal oxide nanocomposite for ultrasensitive electrochemical sensing of acetaminophen and phenacetin期刊:Biomaterials 2014 影响因子:7.604文章Graphene oxide doped conducting polymer nanocomposite film for electrode-tissue interface 期刊:Nanoscale 2014 影响因子:6.233南京先丰纳米材料科技有限公司Nanjing XFNANO Materials Tech Co.,Ltd 地址:南京市鼓楼区南京大学国家大学科技园Add:Nanjing Jiangsu Province China文章Fabrication and application of flexible graphene silk composite film electrodes decorated with spiky Pt nanospheres期刊:Journal of Materials Chemistry A 2014 影响因子: 6.101文章Binder-free phenyl sulfonated graphene/sulfur electrodes with excellent cyclability for lithium sulfur batteries期刊:Journal of Materials Chemistry A 2014 影响因子: 6.101文章A 3D hierarchical porous α-Ni(OH)2/graphite nanosheet composite as an electrode material for supercapacitors期刊:Chemical Communications 2012 影响因子:6.169文章: Graphene electrochemical supercapacitors: the influence of oxygen functional groups期刊:Advanced Functional Materials 2013 影响因子:9.765文章Highly Electron Transparent Graphene for Field Emission Triode Gates期刊:Biomaterials 2013 影响因子:7.604文章Nanodiamonds-mediated doxorubicin nuclear delivery to inhibit lung metastasis of breast cancer期刊:Nanoscale 2013 影响因子:6.233期刊:Nanoscale 2013 影响因子: 6.233文章Using ruthenium polypyridyl functionalized ZnO mesocrystals and gold nanoparticle dotted graphene composite for biological recognition and electrochemiluminescence biosensing期刊:Nanoscale 2013 影响因子: 6.233文章One-pot, water-based and high-yield synthesis of tetrahedral palladium nanocrystal decorated graphene期刊:Journal of Materials Chemistry A 2013影响因子:6.101文章Graphene-wrapped silver/porous silicon composite with enhanced electrochemical performance for lithium-ion batteries期刊:Biomaterials 2013 影响因子:7.604文章:Protein-assisted fabrication of nano-reduced graphene oxide for combined in vivo photoacoustic imaging and photothermal therapy大于等于5:期刊:Biosensors and Bioelectronics 2014 影响因子:5.437文章:Mild and Novel Electrochemical Preparation of β-Cyclodextrin/Graphene Nanocomposite Film for Super-Sensitive Sensing of Quercetin期刊:Anal. Chem. 2014 影响因子:5.695文章:In Situ Growth of Porous Platinum Nanoparticles on Graphene Oxide for Colorimetric Detection of Cancer Cells期刊:Journal of Materials Chemistry A 2013 影响因子:5.968文章: Highly loaded CoO/graphene nanocomposites as lithium-ion anodes with superior reversible capacity期刊:Journal of Materials Chemistry 2012 影响因子:5.968文章: Graphene/porous cobalt nanocomposite and its noticeable electrochemical hydrogen storage ability at room temperature南京先丰纳米材料科技有限公司Nanjing XFNANO Materials Tech Co.,Ltd 地址:南京市鼓楼区南京大学国家大学科技园Add:Nanjing Jiangsu Province China期刊:Journal of Materials Chemistry 2012 影响因子:5.968文章: Graphene/polyaniline nanorod arrays: synthesis and excellent electromagnetic absorption properties期刊:Journal of Materials Chemistry 2012 影响因子:5.968文章: A novel Fe3O4–graphene–Au multifunctional nanocomposite: green synthesis and catalytic application期刊:Journal of Materials Chemistry 2013 影响因子:5.968文章: Enhanced photovoltaic performance of dye-sensitized solar cells based on TiO2 nanosheets/graphene composite films期刊:Journal of Materials Chemistry A 2013 影响因子:5.968文章: Stabilization of NaZn(BH4)3via nanoconfinement in SBA-15 towards enhanced hydrogen release期刊:Applied Catalysis B: Environmental 2012 影响因子:5.625文章: Enhanced photocatalytic activity of hierarchical macro/mesoporous TiO2–graphene composites for photodegradation of acetone in air期刊:Biosensors and Bioelectronics 2012 影响因子:5.602文章: Acetylcholinesterase biosensor based on chitosan/prussian blue/multiwall carbon nanotubes/hollow gold nanospheres nanocomposite film by one-step electrodeposition期刊:Biosensors and Bioelectronics 2012 影响因子:5.602文章: Label-free colorimetric sensor for ultrasensitive detection of heparin based on color quenching of gold nanorods by graphene oxide期刊:Biosensors and Bioelectronics 2012 影响因子:5.602文章: Direct electron transfer glucose biosensor based on glucose oxidase self-assembled on electrochemically reduced carboxyl graphene期刊:Biosensors and Bioelectronics 2012 影响因子:5.602文章: DNA electrochemical biosensor based on thionine-graphene nanocomposite期刊:Carbon 2012 影响因子:5.378文章: Synthesis of electrochemiluminescent graphene oxide functionalized with a ruthenium(II) complex and its use in the detection of tripropylamine期刊:Carbon 2013 影响因子: 5.868文章Preparation and tribological properties of TiAl matrix composites reinforced by multilayer graphene期刊:Biosensors and Bioelectronics 2013 影响因子: 5.437文章Simple Approach for Ultrasensitive Electrochemical Immunoassay of Clostridium difficile toxin B Detection期刊:Biosensors and Bioelectronics 2013 影响因子: 5.437文章Target-induced Electronic Switch for Ultrasensitive Detection of Pb2+ Based on Three Dimensionally Ordered Macroporous Au-Pd Bimetallic Electrode期刊:Biosensors and Bioelectronics 2014 影响因子: 5.437文章Direct electron transfer of glucose oxidase and biosensing for glucose based on PDDA-capped gold nanoparticle modified graphene/multi-walled carbon nanotubes electrode南京先丰纳米材料科技有限公司Nanjing XFNANO Materials Tech Co.,Ltd 地址:南京市鼓楼区南京大学国家大学科技园Add:Nanjing Jiangsu Province China期刊:Analytical chemistry 2013 影响因子: 5.695文章Graphene Oxide–Peptide Nanocomplex as a Versatile Fluorescence Probe of Protein Kinase Activity Based on Phosphorylation Protection against Carboxypeptidase Digestion期刊:Lab on a Chip 2013 影响因子: 5.697文章On-chip selective capture of cancer cells and ultrasensitive fluorescence detection of survivin mRNA in a single living cell期刊:Environmental Science & Technology 2013 影响因子:5.257文章Graphene and g-C3N4 Nanosheets Cowrapped Elemental α-Sulfur As a Novel Metal-Free Heterojunction Photocatalyst for Bacterial Inactivation under Visible-Light期刊:Biosensors and Bioelectronics 2014 影响因子:5.437文章A highly sensitive and wide-ranged electrochemical zinc(II) aptasensor fabricated on core–shell SiO2-Pt@meso-SiO2期刊:Analytical chemistry 2013 影响因子:5.695文章Electrochemiluminescent Quenching of Quantum Dots for Ultrasensitive Immunoassay through Oxygen Reduction Catalyzed by Nitrogen-Doped Graphene-Supported Hemin期刊:Biosensors and Bioelectronics 2013 影响因子:5.437文章A novel ionic liquid stabilized molecularly imprinted optosensing material based on quantum dots and graphene oxide for specific recognition of vitamin E期刊:APPLIED MATERIALS & INTERFACES 2013 影响因子:5.008文章Dye-Sensitization-Induced Visible-Light Reduction of Graphene Oxide for the Enhanced TiO2 Photocatalytic Performance期刊:Biosensors and Bioelectronics 2014 影响因子:5.437文章Graphene oxide as nanogold carrier for ultrasensitive electrochemical immunoassay of Shewanella oneidensis with silver enhancement strategy期刊:ACS APPLIED MATERIALS & INTERFACES 2013 影响因子:5.008文章Graphene-Wrapped CoS Nanoparticles for High-Capacity Lithium-Ion Storage期刊:Biosensors and Bioelectronics 2013 影响因子:5.437文章Combination of cascade chemical reactions with graphene–DNA interaction to develop new strategy for biosensor fabrication期刊:Biosensors and Bioelectronics 2013 影响因子:5.437文章 A graphene oxide-based FRET sensor for rapid and sensitive detection of matrix metalloproteinase 2 in human serum sample期刊:Biosensors and Bioelectronics 2014 影响因子:5.437文章Ultrasensitive photoelectrochemical immunoassay of indole-3-acetic acid based on the MPA modified CdS/RGO nanocomposites decorated ITO electrode期刊:Environ. Sci. Technol 2013 影响因子:5.228文章:Graphene Oxide-Facilitated Reduction of Nitrobenzene in Sulfide-Containing Aqueous Solutions期刊:Journal of Materials Chemistry A 2013 影响因子:5.968文章:Nitrogen-doped mesoporous carbons originated from ionic liquids as electrode materials for supercapacitors南京先丰纳米材料科技有限公司Nanjing XFNANO Materials Tech Co.,Ltd 地址:南京市鼓楼区南京大学国家大学科技园Add:Nanjing Jiangsu Province China期刊:Nanoscale 2013 影响因子:5.914文章:Label-free Electrochemical Impedance Genosensor Based on 1-Aminopyrene/Graphene Hybrids 期刊:Chemistry - A European Journal 2013 影响因子:5.925文章:Three-Dimensional Hierarchical Architectures Constructed by Graphene/MoS2 Nanoflake Arrays and Their Rapid Charging/Discharging Properties as Lithium-Ion Battery Anodes期刊:Chemistry - A European Journal 2013 影响因子:5.925文章:Structural Engineering for High Energy and Voltage Output Supercapacitors期刊:Chemistry - A European Journal 2013 影响因子:5.925文章: Label-Free Detection of MicroRNA: Two-Step Signal Enhancement with a Hairpin-Probe-Based Graphene Fluorescence Switch and Isothermal Amplification大于等于4:期刊:Analytica Chimica Acta 2014 影响因子:4.378文章:In situ synthesis of ceria nanoparticles in the ordered mesoporous carbon as a novel electrochemical sensor for the determination of hydrazine.期刊:Journal of Power Sources 2014 影响因子:4.675文章:Three-dimensional macroporous anodes based on stainless steel fiber felt for high-performance microbial fuel cells.期刊:Journal of Power Sources 2014 影响因子:4.675文章:Sulfonated poly(ether ether ketone)/mesoporous silica hybrid membrane for high performance vanadium redox flow battery期刊:Journal of Power Sources 2012 影响因子:4.951文章: Carbon felt supported carbon nanotubes catalysts composite electrode for vanadium redox flow battery application期刊:Journal of Power Sources 2012 影响因子:4.951文章: A new method for fabrication of graphene/polyaniline nanocomplex modified microbial fuel cell anodes期刊:J. Phys. Chem. C 2012 影响因子:4.805文章: Alignment of Single-Walled Carbon Nanotubes with Ferroelectric Liquid Crystal期刊:Analytica Chimica Acta 2012 影响因子:4.555文章: Highly sensitive luminol electrochemiluminescence immunosensor based on ZnO nanoparticles and glucose oxidase decorated graphene for cancer biomarker detection期刊:Journal of Chromatography A 2012 影响因子:4.531文章: Simultaneous determination of 10 β2-agonists in swine urine using liquid chromatography–tandem mass spectrometry and multi-walled carbon nanotubes as a reversed dispersive solid phase extraction sorbent期刊:ACS Applied Materials & Interfaces 2012 影响因子:4.525文章: “Turn-on”Fluorescence Detection of Lead Ions Based on Accelerated Leaching of Gold Nanoparticles on the Surface of Graphene期刊:Chemistry-An Asian Journal 2012 影响因子:4.5南京先丰纳米材料科技有限公司Nanjing XFNANO Materials Tech Co.,Ltd 地址:南京市鼓楼区南京大学国家大学科技园Add:Nanjing Jiangsu Province China文章: Dispersion of Reduced Graphene Oxide in Multiple Solvents with an Imidazolium-Modified Hexa-peri-hexabenzocoronene期刊:Analyst 2012 影响因子:4.23文章: Glucose sensor based on an electrochemical reduced graphene oxide-poly(L-lysine) composite film modified GC electrode期刊:Analyst 2012 影响因子:4.23文章: A functional graphene oxide-ionic liquid composites/gold nanoparticles sensing platform for ultrasensitive electrochemical detection of Hg2+期刊:Analyst 2012 影响因子:4.23文章: Ultrasensitive colorimetric detection of heparin based on self-assembly of gold nanoparticles on graphene oxide期刊:PLOS ONE 2012 影响因子:4.092文章: Obstruction of Photoinduced Electron Transfer from Excited Porphyrin to Graphene Oxide: A Fluorescence Turn-On Sensing Platform for Iron (III) Ions期刊:Pharmaceutical Research 2012 影响因子:4.093文章: PEGylated Multi-Walled Carbon Nanotubes for Encapsulation and Sustained Release of Oxaliplatin期刊:Electrochemistry Communications 2014 影响因子: 4.425文章A Novel Electrochemical Immunosensor for Golgi Protein 73 Assay期刊:Journal of Power Sources 2014 影响因子: 4.675文章Mesoporous Li3V2(PO4)3@CMK-3 nanocomposite cathode material for lithium ion batteries期刊:Analytica Chimica Acta 2014 影响因子: 4.387文章Sensitive and selective electrochemical determination of quinoxaline-2-carboxylic acid based on bilayer of novel poly(pyrrole) functional composite using one-step electro-polymerization and molecularly imprinted poly(o-phenylenediamine)期刊:Journal of Membrane Science 2014 影响因子:4.093文章Poly(vinyl alcohol)–graphene oxide nanohybrid “pore-filling” membrane for pervaporation of toluene/n-heptane mixtures期刊:Journal of Power Sources 2014 影响因子: 4.675文章Non-aqueous hybrid supercapacitors fabricated with mesoporous TiO2 microspheres and activated carbon electrodes with superior performance期刊:Journal of Power Sources 2014 影响因子: 4.675文章Preparation of three-dimensional hybrid nanostructure-encapsulated sulfur cathode for high-rate lithium sulfur batteries期刊:Bioresource Technology 2013 影响因子: 4.75文章Selective production of chemicals from biomass pyrolysis over metal chlorides supported on zeolite期刊:Journal of Chromatography A 2013 影响因子: 4.612文章Simultaneous determination of six resorcylic acid lactones in feed using liquid chromatography–tandem mass spectrometry and multi-walled carbon nanotubes as a dispersive solid phase extraction sorbent南京先丰纳米材料科技有限公司Nanjing XFNANO Materials Tech Co.,Ltd 地址:南京市鼓楼区南京大学国家大学科技园Add:Nanjing Jiangsu Province China期刊:Journal of Power Sources 2013 影响因子: 4.675文章Reduced graphene oxide film as a shuttle-inhibiting interlayer in a lithium–sulfur battery期刊:The Journal of Physical Chemistry C 2013 影响因子: 4.814文章Electromagnetic Wave Absorption Properties of Reduced Graphene Oxide Modified by Maghemite Colloidal Nanoparticle Clusters期刊:Journal of Power Sources 2013 影响因子: 4.675文章Improving the performance of lithium–sulfur batteries by graphene coating期刊:Journal of Chromatography A 2013 影响因子:4.612文章Application of graphene as the stationary phase for open-tubular capillary electrochromatography 期刊:Journal of Power Sources 2013 影响因子:4.675文章Design, hydrothermal synthesis and electrochemical properties of porous birnessite-type manganese dioxide nanosheets on graphene as a hybrid material for supercapacitors期刊:Appl. Mater. Interfaces 2013 影响因子:4.525文章:One-Pot Environmentally Friendly Approach toward Highly Catalytically Active Bimetal-Nanoparticle-Graphene Hybrids期刊:Electrochemistry Communications 2013 影响因子:4.859文章:Fabrication of streptavidin functionalized silver nanoparticle decorated graphene and its application in disposable electrochemical sensor for immunoglobulin E期刊:ACS Appl. Mater. Interfaces 2013 影响因子:4.525文章:Facile Fabrication and Enhanced Photocatalytic Performance of Ag/AgCl/rGO Heterostructure Photocatalyst期刊:Analyst 2013 影响因子:4.23文章:One-pot green synthesis of graphene oxide/gold nanocomposites as SERS substrates for malachite green detection大于等于3:期刊:Sensors and Actuators B: Chemical影响因子:3.535文章:Facile preparation of highly water-stable and flexible PEDOT:PSS organic/inorganic composite materials and their application in electrochemical sensors.期刊:Electrochimica Acta影响因子:3.777文章:Inhibitory effect of silver nanomaterials on transmissible virus-induced host cell infections期刊:Microchimica Acta 2014 影响因子:3.434文章:Graphene oxide functionalized magnetic nanoparticles as adsorbents for removal of phthalate esters.期刊:Nanotechnology 2013 影响因子:3.979文章: Facile and straightforward synthesis of superparamagnetic reduced graphene oxide–Fe3O4 hybrid composite by a solvothermal reaction期刊:Sensors and Actuators B: Chemical 2012 影响因子:3.898文章: Sensitive DNA biosensor improved by 1,10-phenanthroline cobalt complex as indicator based on the electrode modified by gold nanoparticles and graphene期刊:Electrochimica Acta 2013 影响因子:3.832南京先丰纳米材料科技有限公司Nanjing XFNANO Materials Tech Co.,Ltd 地址:南京市鼓楼区南京大学国家大学科技园Add:Nanjing Jiangsu Province China文章: Electrochemical biosensor based on reduced graphene oxide modified electrode with Prussian blue and poly(toluidine blue O) coating期刊:Electrochimica Acta 2012 影响因子:3.832文章: High sensitive determination of theophylline based on gold nanoparticles/l-cysteine/Graphene/Nafion modified electrode期刊:Electrochimica Acta 2013 影响因子:3.832文章: Graphene oxide/nickel oxide modified glassy carbon electrode for supercapacitor and nonenzymatic glucose sensor期刊:Electrochimica Acta 2012 影响因子:3.832文章: Graphene oxide nanoribbon and polyhedral oligomeric silsesquioxane assembled composite frameworks for pre-concentrating and electrochemical sensing of 1-hydroxypyrene期刊:Bioelectrochemistry 2012 影响因子:3.759文章: Nonenzymatic amperometric determination of glucose by CuO nanocubes–graphene nanocomposite modified electrode期刊:Chemical Engineering Journal 2012 影响因子:3.461文章: Self-assembly of graphene oxide and polyelectrolyte complex nanohybrid membranes for nanofiltration and pervaporation期刊:Fuel 2012 影响因子:3.248文章: Experimental study on bio-oil upgrading over catalyst in supercritical ethanol期刊:RSC Advances 2013 2011年创刊预计影响因子:大于3.0文章: Sandwich nanocomposites of polyaniline embedded between graphene layers and multi-walled carbon nanotubes for cycle-stable electrode materials of organic supercapacitors期刊:RSC Advances 2012 2011年新刊,预计影响因子:大于3.0文章: Electrochemically-driven and dynamic enhancement of drug metabolism via cytochrome P450 microsomes on colloidal gold/graphene nanocomposites期刊:Electrochimica Acta 2014 影响因子: 3.777文章(4-Ferrocenylethyne) Phenylamine Functionalized Graphene Oxide Modified Electrode for Sensitive Nitrite Sensing期刊:Sensors and Actuators B: Chemical 2014 影响因子: 3.535文章Simultaneous determination of dihydroxybenzene isomers based on graphene-graphene oxide nanocomposite modified glassy carbon electrode期刊:Sensors and Actuators B: Chemical 2014 影响因子: 3.535文章Sensitive electrochemiluminescence sensor based on ordered mesoporous carbon composite film for dopamine期刊:Talanta 2014 影响因子: 3.498文章Square wave anodic stripping voltammetric determination of Cd2+ and Pb2+ at bismuth-film electrode modified with electroreduced graphene oxide-supported thiolated thionine期刊:Sensors and Actuators B: Chemical 2014 影响因子: 3.535文章A multiple-promoted silver enhancement strategy in electrochemical detection of target virus期刊:Nanotechnology 2014 影响因子: 3.842南京先丰纳米材料科技有限公司Nanjing XFNANO Materials Tech Co.,Ltd 地址:南京市鼓楼区南京大学国家大学科技园Add:Nanjing Jiangsu Province China文章Ag–graphene hybrid conductive ink for writing electronics期刊:Analyst 2014 影响因子: 3.969文章Capillary electrophoresis-based immobilized enzyme reactor using graphene oxide as support via layer by layer electrostatic assembly期刊:Microchimica Acta 2014 影响因子: 3.434文章Fluorescent aptasensor for the determination of Salmonella typhimurium based on a graphene oxide platform期刊:Talanta 2014 影响因子:3.498文章Tannic acid functionalized N-doped graphene modified glassy carbon electrode for the determination of bisphenol A in food package期刊:Composites Science and Technology 2013 影响因子: 3.328文章Fabrication of graphene/polylactide nanocomposites with improved properties期刊:Microporous and Mesoporous Materials 2014 影响因子: 3.365文章Synthesis, characterization and CO2 capture of mesoporous SBA-15 adsorbents functionalized with melamine-based and acrylate-based amine dendrimers期刊:Analyst 2013 影响因子: 3.969文章Graphene based electrochemical biosensor for label-free measuring the activity and inhibition of protein tyrosine kinase期刊:Electrochimica Acta 2013 影响因子: 3.777文章Preparation and charaterization of Pt/functionalized graphene and its electrocatalysis for methanol oxidation期刊:Plos One 2013 影响因子: 3.73文章Synergistic Removal of Pb(II), Cd(II) and Humic Acid by Fe3O4@Mesoporous Silica-Graphene Oxide Composites期刊:Electrochimica Acta 2013 影响因子: 3.777文章Electrocatalytic oxidation and detection of N-acetylcysteine based on magnetite/reduced graphene oxide composite-modified glassy carbon electrode期刊:Catalysis Science & Technology 2013 影响因子: 3.753文章The role of reducing agent in perylene tetracarboxylic acid coating on graphene sheets enhances Pd nanoparticles-electrocalytic ethanol oxidation期刊:Acta Materialia 2013 影响因子: 3.941文章Nanoconfinement significantly improves the thermodynamics and kinetics of co-infiltrated 2LiBH4–LiAlH4 composites: Stable reversibility of hydrogen absorption/resorption期刊:Microchimica Acta 2013 影响因子:3.434文章Highly sensitive and selective voltammetric detection of mercury(II) using an ITO electrode modified with 5-methyl-2-thiouracil, graphene oxide and gold nanoparticles期刊:Composites Science and Technology 2013 影响因子:3.328文章Porous graphene sandwich/poly(vinylidene fluoride) composites with high dielectric properties 期刊:Electrochimica Acta 2013 影响因子: 3.777文章Cu2O/NiOx/graphene oxide modified glassy carbon electrode for the enhanced electrochemical oxidation of reduced glutathione and nonenzyme glucose sensor南京先丰纳米材料科技有限公司Nanjing XFNANO Materials Tech Co.,Ltd 地址:南京市鼓楼区南京大学国家大学科技园Add:Nanjing Jiangsu Province China期刊:Electrochimica Acta 2013 影响因子: 3.777文章Direct electrodeposion of reduced graphene oxide and dendritic copper nanoclusters on glassy carbon electrode for electrochemical detection of nitrite期刊:Analyst 2013 影响因子: 3.969文章Realization of on-tissue protein identification by highly efficient in situ digestion with graphene-immobilized trypsin for MALDI imaging analysis期刊:Food Chemistry 2014 影响因子:3.334文章Electrochemical determination of toxic ractopamine at an ordered mesoporous carbon modified electrode期刊:Talanta 2013 影响因子:3.498文章Simultaneous Determination of Dopamine and Uric Acid Using Layer-by-Layer Graphene and Chitosan Assembled Multilayer Films期刊:Electrochimica Acta 2013 影响因子:3.777文章Electrochemically Cathodic Exfoliation of Graphene Sheets in Room Temperature Ionic Liquids N-Butyl, methylpyrrolidinium Bis(trifluoromethylsulfonyl)imide and Their Electrochemical Properties 期刊:Journal of Applied Physics 2013 影响因子:2.21文章An experimental investigation on fluidic behaviors in a two-dimensional nanoenvironment期刊:Journal of Molecular Catalysis A: Chemical 2013 影响因子:3.187文章Enhancing the photocatalytic activity of lead molybdate by modifying with fullerene期刊:Physical Chemistry Chemical Physics 2013 影响因子:3.829文章Improving the antifouling property of polysulfone ultrafiltration membrane by incorporation of isocyanate-treated graphene oxide期刊:Sensors and Actuators B: Chemical 2013 影响因子:3.535文章Electrochemical modification of graphene oxide bearing different types of oxygen functional species for the electro-catalytic oxidation of reduced glutathione期刊:Sensors and Actuators B: Chemical 2013 影响因子:3.535文章A novel graphene oxide-based fluorescence assay for RNA endonuclease activity of mammalian Argonaute2 protein期刊:Sensors and Actuators B: Chemical 2013 影响因子:3.535文章Enhanced room temperature sensing of Co3O4-intercalated reduced graphene oxide based gas sensors期刊:Talanta 2013 影响因子:3.498文章Graphene matrix for signal enhancement in ambient plasma assisted laser desorption ionization mass spectrometry期刊:Sensors and Actuators B: Chemical 2013影响因子:3.535文章Electrodeposition of electroreduced graphene oxide-Au nanoparticles composite film at glassy carbon electrode for anodic stripping voltammetric analysis of trace arsenic(III)期刊:Physical Chemistry Chemical Physics 2013 影响因子:3.829文章Enhanced reverse saturable absorption in graphene/Ag2S organic glasses期刊:Electrochimica Acta 2013 影响因子:3.777南京先丰纳米材料科技有限公司Nanjing XFNANO Materials Tech Co.,Ltd 地址:南京市鼓楼区南京大学国家大学科技园Add:Nanjing Jiangsu Province China文章Electrochemical immunoassay platform for high sensitivity detection of indole-3-acetic acid期刊:Analyst 2013 影响因子:3.969文章Aptamer-linked biosensor for thrombin based on AuNPs/Thionine-graphene nanocomposite期刊:Journal of Colloid and Interface Science 2013 影响因子:3.172文章Green Synthesis and Photo-catalytic Performances for ZnO-Reduced Graphene Oxide Nanocomposites期刊:Electrochimica Acta 2013 影响因子:3.832文章: Insight into effects of graphene in Li4Ti5O12/carbon composite with high rate capability as anode materials for lithium ion batteries期刊:Dalton Transactions 2013 影响因子:3.838文章:Remarkable improvements in the stability and thermal conductivity of graphite/ethylene glycol nanofluids caused by a graphene oxide percolation structure期刊:Talanta 2013 影响因子:3.794文章:Selective and sensitive determination of uric acid in the presence of ascorbic acid and dopamine by PDDA functionalized graphene/graphite composite electrode期刊:ELECTROPHORESIS 2013 影响因子:3.303文章:Graphene oxide and reduced graphene oxide as novel stationary phases via electrostatic assembly for open-tubular capillary electrochromatography期刊:Sensors and Actuators B 2013 影响因子:3.898文章:A reduced graphene oxide based biosensor for high-sensitive detection of phenols in water samples期刊:Sensors and Actuators B: Chemical 2013 影响因子:3.898文章:Amperometric biosensor for NADH and ethanol based on electroreduced graphene oxide–polythionine nanocomposite film南京先丰纳米材料科技有限公司Nanjing XFNANO Materials Tech Co.,Ltd 地址:南京市鼓楼区南京大学国家大学科技园Add:Nanjing Jiangsu Province China。
Photoelectronic Responses in Solution-Processed Perovskite CH3NH3PbI3Solar CellsStudied by Photoluminescenceand Photoabsorption Spectroscopy Yasuhiro Yamada,Toru Nakamura,Masaru Endo,Atsushi Wakamiya,and Yoshihiko KanemitsuAbstract—Photoelectronic responses of organic–inorganic hy-brid perovskite CH3NH3PbI3on mesoporous TiO2electrodes are investigated.On the basis of near-band-edge optical absorption and photoluminescence spectra,the bandgap energy and exciton binding energy as a function of temperature are obtained.The ex-citon binding energy is much smaller than thermal energy at room temperature,which means that most excitons are thermally disso-ciated,and optical processes are determined by the photoexcited electrons and holes.We determined the temperature dependence of exciton binding energy,which changes fromß30meV at13K to 6meV at300K.In addition,the bandgap energy and the exciton binding energy show abrupt changes at150K due to structural phase transition.Our fundamental optical studies provide essen-tial information for improving the device performance of solar cells based on halide perovskite semiconductors.Index Terms—Photoluminescence,semiconductor devices,solar energy.I.I NTRODUCTIONL EAD halide perovskites,i.e.,CH3NH3PbX3[X=Cl,Br, and I],have recently emerged as a promising candidate material for efficient light-energy conversion devices.Although CH3NH3PbX3itself is a3-D semiconductor,organic–inorganic hybrid lead halide perovskite semiconductors have previously been examined extensively with regard to the unique optical properties of natural lower dimensional layered perovskite-type compounds[1],[2].In2009,Kojima et al.[3]first reported the development of a sensitized solar cell using perovskite CH3NH3PbI3as a light-harvesting sensitizer with mesoporous TiO2electrodes and liquid electrolyte.The further breakthrough was the realization of an efficient all-solid-state sensitized so-lar cell with power conversion efficiency above10%based on perovskite CH3NH3PbI3,with the use of the solid-state hole-transporting material Spiro-OMeTAD[4],[5].With ongoing re-search,the power conversion efficiency of perovskite-based de-vices has improved rapidly and is currently at over19%[3]–[10],Manuscript received July10,2014;accepted October8,2014.This work was supported by The Sumitomo Electric Industries Group CSR foundation, JST-PRESTO,and JST-CREST.The authors are with the Institute for Chemical Research,Kyoto Uni-versity,Uji,Kyoto611-0011,Japan(e-mail:yamada.yasuhiro.6c@kyoto-u. ac.jp;nakamura.tooru.25z@;endo@hydrogen.kuicr.kyoto-u.ac.jp; wakamiya@scl.kyoto-u.ac.jp;kanemitu@scl.kyoto-u.ac.jp).Color versions of one or more of thefigures in this paper are available online at .Digital Object Identifier10.1109/JPHOTOV.2014.2364115which is much higher than that of conventional dye-sensitized solar cells.More recently,the planar heterojunction-type per-ovskite solar cell has also been developed[11],[12].In order to further contribute to the development of increas-ingly efficient perovskite solar cells,it is necessary to identify the key mechanism that determines the advanced photovoltaic properties of perovskite semiconductors.To date,it has been demonstrated that long carrier diffusion length contributes to the high efficiency of these solar cells[13],[14].The estimation of diffusion length was conducted using time-resolved photolu-minescence(PL)spectroscopy in perovskite thinfilms,assum-ing the1-D exciton diffusion model.However,it has not been clarified whether the exciton or free-carrier mechanism is an appropriate interpretation of the optical properties.To answer this question,we need to evaluate the exciton binding energy of CH3NH3PbI3.Although several research works have been made to evaluate the exciton binding energy of CH3NH3PbI3 bulk crystals and thinfilms,the reported literature values are scattered over a wide range[15]–[18],and it is still under discus-sion.Therefore,in order to promote an improved understanding of the intrinsic characteristics of perovskite solar cells,it is nec-essary to reveal fundamental optical properties of perovskite semiconductors and evaluate the exciton binding energy.In our previous report,we obtained the room-temperature bandgap energy of CH3NH3PbI3on mesoporous TiO2elec-trodes based on their PL,PL excitation,and transient absorption [19].The combination of these different optical spectroscopy techniques provides detailed information on optical properties [20],[21].It was found that the PL and PLE spectra have peaks at1.60and1.64eV,respectively,and we observed a negative TA peak at1.61eV due to photobleaching,which usually appears at the bandgap energy of direct bandgap semiconductors.We, therefore,determined that the direct bandgap energy is1.61eV at room temperature.In this paper,we discuss the optical absorption spectra mea-sured through total optical transmittance(TT)and diffuse re-flectance(DR)analyses in a wide temperature range.The fabri-cation process of the perovskite thinfilms and perovskite-based solar cells used in this study is outlined.The optical absorption spectra of these samples are then obtained and analyzed,taking excitonic effects into consideration,and the temperature depen-dence of the bandgap energy,the exciton binding energy,and the broadening parameter are determined.Temperature-dependent PL spectra are also measured.2156-3381©2014IEEE.Personal use is permitted,but republication/redistribution requires IEEE permission.See /publications standards/publications/rights/index.html for more information.Fig.1.Schematic illustration of the perovskite solar cells and samples.Optical measurements were performed on the sample without hole transporting material and gold electrode.The CH3NH3PbI3layer was formed on the mesoporous TiO2electrode.II.S AMPLES AND E XPERIMENTAL S ETUP Perovskite thinfilms were fabricated on mesoporous TiO2 electrodes using the two-step method[8].The porous TiO2 layer was deposited on the glass substrate through spin coat-ing using a TiO2paste.The porous TiO2films were infiltrated with PbI2(99.999%,Sigma-Aldrich)through the spin-coating of a PbI2solution in N,N-dimethylformamide in an argon-filled glove box.After maintaining a temperature of70°C for30min to allow the material to dry,thefilms were dipped in a solution of CH3NH3I in2-propanol and then rinsed with2-propanol. We then conducted optical measurements on these samples. Perovskite-based photovoltaic cells were also fabricated by at-taching hole-transporting material and gold electrodes to the samples(see[19]and[22]for this fabrication method).The structures of the samples used in the optical and photovoltaic measurements are illustrated schematically in Fig.1.Before and during the experiment,the samples were maintained in an argon atmosphere to avoid degradation due to air exposure.In addition,the samples were left for more than three days after fabrication to allow the intrinsic material properties to stabilize. To evaluate the near-band-edge optical properties,we mea-sured TT,which is the angle-integrated intensity of forward scattered light,using an integrating sphere because of the strong light-scattering due to the mesoporous TiO2.In addition,DR spectroscopy was used to obtain temperature-dependent optical absorption spectra.The acquired DR spectrum was converted to the Kubelka–Munk function F(R),which is approximately pro-portional to the absorption coefficient,according to the relation F(R)=(1−R)2/(2R),where R is the diffuse reflectivity.PL measurements were performed using an Si CCD camera with a monochromator.III.R ESULTS AND D ISCUSSIONWe confirmed that the solar cell with the produced CH3NH3PbI3works well,particularly with regard to power-conversion efficiency.Fig.2shows the solar-cell performance of our perovskite-based solar cell.We estimated the power conversion efficiency under AM 1.5conditions.It shows high open-circuit voltage(V OC=0.98V),short-circuit cur-rent(J SC=19.1mA/cm2),andfill-factor(F F=0.68).The power-conversion efficiency was12.7%.To evaluate the optical properties of CH3NH3PbI3,we con-ducted optical absorption measurements,as shown in Fig.3. Although DR measurement of perovskite solar cells hasbeen Fig.2.(a)Current-density–voltage curves under illumination(solid curve) and in the dark(broken curve)and(b)external quantum efficiency spectrum of our perovskite solarcell.Fig.3.(a)Optical absorption spectra obtained by TT(circles)and DR(tri-angle)measurements.The solid curve is thefit given by(1)and(2).The bro-ken curve represents the Gaussian broadening function G(E),which is used in (2).We obtained w=75meV as a bestfit parameter,which corresponds to the width of the Gaussian broadening function.conducted previously[5],[6],[19],we also carried out TT mea-surement to achieve a more accurate optical absorption spec-trum.For comparison,the DR spectrum is normalized and offset, and it was found that the TT and DR spectra were in good agree-ment with each other.However,the TT spectra had less signal-to-noise ratio compared with the DR measurements.Therefore, the TT spectra were used for analysis of the perovskite solar cell properties at room temperature.As we have stated previously,CH3NH3PbI3is a direct-gap semiconductor[19].Its TT spectrum has no excitonic peaks, suggesting the exciton binding energy is much smaller than the thermal energy at room temperature(ß25meV).However,even in this case,it is known that the near-band-edge optical absorp-tion is modified due to excitonic effects[23]–[25].According to [23]and[24],the near-band-edge optical absorption spectrum for a dipole-allowed direct-gap semiconductor,taking excitonic effects into consideration,is expressed asα∝A∞n=11n3δ(E−E n)/E2+(E−E g)1/2τeτE sinhτ(1)Y AMADA et al.:PHOTOELECTRONIC RESPONSES IN SOLUTION-PROCESSED PEROVSKITE CH3NH3PBI3SOLAR CELLS3whereτ=π|E b/(E−E g)|.E,E g,and E b are the photon en-ergy,bandgap energy,and exciton binding energy,respectively.A is a proportionality coefficient that depends on temperature, while E n is the exciton resonance energy and typically followsE n=E g−E b/n2,where n(=1,2,3,...)is the quantum number.The actual absorption spectrum is modified by intrinsicor extrinsic effects such as thermal broadening and inhomoge-neous broadening.To take these broadening effects into account,the optical absorption is convolved by the Gaussian broadeningfunction G(E),i.e.,αr=∞α(E)G(V−E)dV.(2) Wefit the TT spectra using(1)and(2).Thefit result is shown in Fig.3as a solid curve,which conforms to the experimen-tal data well,and the broadening function is given as a broken curve in the samefigure.The obtained bestfit parameters were E g=1.614eV and E b=6meV,while the broadening Gaus-sian function width(the broadening parameter)is w=75meV. The estimated bandgap energy shows good agreement with our previous report.Note that the broadening parameter is much higher than the thermal energy at room temperature.We are of the view that this originates from the Urbach tail[19].Based on the exciton binding energy result of6meV,we canconclude that the majority of the excitons in CH3NH3PbI3arethermally dissociated at room temperature.However,this exci-ton binding energy is much smaller than that given by previousreports,which had results in the range of37–50meV[15]–[17].Since these values were estimated at low temperatures of ap-proximately10K,we propose that the exciton binding energyvaries with temperature.It has been reported that CH3NH3PbI3undergoes phase transition at150and330K[19],[26],andlarge modification of optical properties is anticipated near thephase transition temperature[19].To examine this suggestion,we conducted PL and DR mea-surements in a wide temperature range from13to300K,asshown in Fig.4.For the PL measurements,the excitation pho-ton energy was1.91eV.At room temperature,the PL spectrumshows a broad peak at1.60eV.According to the estimatedbandgap energy of1.614eV,there is only a small Stokes shift,and therefore,we hold that the PL origin is the band-to-bandradiative recombination of electrons at the conduction band andthe holes at the valence band.We confirmed that the PL spectrum shape is independent ofexcitation intensity and excitation photon energy under our ex-perimental conditions.This means that the PL processes are in-dependent of photoexcited carrier density.The low-temperaturePL spectra are rather complicated:Three PL components wereobserved.With a decrease in temperature,a new PL peak ap-pears at approximately1.65eV and two-step optical absorptionis observed.This is related to the structural phase transition inthe region of this temperature[26].We believe that the twophases coexist even below the phase transition temperature as,below50K,a new PL peak appears at1.60eV.Regarding optical absorption spectra,a broad peak is ob-served near the band-edge in the optical absorption spectrum atlow temperatures(below50K).It is our view that thisbroad Fig.4.PL and DR spectra at various temperatures.The solid curves represent thefit given by(1)and(2).F(R)=(1−R)2/(2R),where R is the diffusereflectivity.Fig.5.Temperature dependence of the broadening parameter(w),exciton binding energy(E b),and bandgap energy(E g).Thefitting errors for E g were below10meV.peak corresponds to the n=1excitons.The DR spectra werefit using(1)and(2),and at low temperatures(below150K)where two optical absorption edges coexist,wefit the high-energy absorption edge.Thefit results are plotted as solid curves in Fig.4.We obtained the bandgap energy,exciton binding en-ergy,and broadening parameter as best-fit parameters,which are indicated in Fig.5.The bandgap energy and broadening pa-rameter show abrupt changes at150K,which is the structural phase transition temperature.It can be seen that the broadening parameter gradually increases with temperatures above150K, and the exciton binding energy is also temperature-dependent. At13K,the estimated exciton binding energy is30meV,which is consistent with the previously reported value[15]–[17].It is clear that the exciton binding energy decreases with tem-perature,and a gradual change appears at the phase transition temperature.We hold that the temperature-dependent exciton4IEEE JOURNAL OF PHOTOVOLTAICSbinding energy can be explained by dielectric permittivity.The dielectric constant of CH3NH3PbI3should vary with temper-ature because of the phase transition temperature at150and 330K,and the exciton binding energy is inversely proportional to the square of the dielectric permittivity.In fact,it has been reported that the dielectric permittivity measured at90GHz increases with temperature above150K[27].This trend is con-sistent with the observed temperature dependence of the exciton binding energy.In addition,it was pointed out that excitons are almost entirely screened at room temperature,yielding free car-riers,due to optical phonons and collective rotational motion of the organic cations[28].Ourfindings suggest that excitons in CH3NH3PbI3thinfilms are not stable at room temperature because of the smaller exciton binding energy(6meV)than the thermal energy(25meV),and photoexcited electrons and holes behave as free carriers.This result is consistent with our recent study on time-resolved PL and transient absorption,which provides clear evidence that the radiative two-carrier recombination of free electrons and holes determines the optical processes in CH3NH3PbI3thinfilms[29]. Comparison with the previous reports should be mentioned. D’Innocenzo et al.recently reported the exciton binding energy of50meV that was estimated from the temperature depen-dence of exciton linewidth in optical absorption spectra above the phase transition temperature[18].To estimate the exci-ton binding energy,they assumed the temperature-independent inhomogeneous linewidth.However,CH3NH3PbI3thinfilms show an increase of the bandgap energy with temperature above the phase transition temperature,and this trend is contrary to typical semiconductors,suggesting that material parameters in CH3NH3PbI3might change with temperature above the phase transition.On the other hand,we evaluated the exciton binding energy using whole spectrum shape near the bandgap energy without such an assumption.Based on these discussions,we be-lieve that our estimation of exciton binding energy well account for the near-band-edge optical properties of CH3NH3PbI3.It is significant to employ various spectroscopic techniques to reveal the near-band-edge optical and excitonic properties of semiconductors.Through such research works,we can clarify the detailed mechanism for photovoltaic processes in emerging solar-cell materials,i.e.,lead halide perovskites CH3NH3PbX3 [X=Cl,Br,and I].IV.C ONCLUSIONIn conclusion,we have studied the near-band-edge optical spectra of organic–inorganic hybrid perovskite CH3NH3PbI3 on mesoporous TiO2electrodes by means of TT,DR,and PL measurements.At room temperature,no exciton peaks were observed in the optical absorption spectra.These spectra were then analyzed,taking excitonic effects into account,and an exciton binding energy of6meV was obtained.This result is much smaller than that given in previous reports estimated at low temperatures.This indicates that the exciton binding energy is temperature dependent.We also examined the temperature-dependent DR and PL spectra.At room temperature,almost no Stokes shift was observed.With a decrease in temperature, the exciton binding energy decreases,and the PL peak and DR onset energy suddenly show a blue shift at approximately150K, which is a structural phase transition temperature.We believe that ourfindings provide the requisite information to develop efficient perovskite solar cells.R EFERENCES[1] D.B.Mitzi,S.Wang,C.A.Feild,C.A.Chess,and A.M.Guloy,“Conduct-ing layered organic-inorganic halides containing<110>-oriented per-ovskite sheets,”Science,vol.267,pp.1473–1476,Mar.1995.[2]T.Ogawa and Y.Kanemitsu,Optical Properties of Low-Dimensional Ma-terials.Singapore:World Scientific,1995,ch.6.[3] A.Kojima,K.Teshima,Y.Shirai,and T.Miyasaka,“Organometal halideperovskites as visible-light sensitizers for photovoltaic cells,”J.Amer.Chem.Soc.,vol.131,pp.6050–6051,Apr.2009.[4]J.-H.Im,C.-R.Lee,J.-W.Lee,S.-W.Park,and N.-G.Park,“6.5%ef-ficient perovskite quantum-dot-sensitized solar cell,”Nanoscale,vol.3, pp.4088–4093,Sep.2011.[5]H.-S.Kim,C.-R.Lee,J.-H.Im,K.-B.Lee,T.Moehl,A.Marchioro,S.-J.Moon,R.Humphry-Baker,J.-H.Yum,J.E.Moser,M.Gr¨a tzel,and N.-G.Park,“Lead iodide perovskite sensitized all-solid-state submicron thinfilm mesoscopic solar cell with efficiency exceeding9%,”Sci.Rep., vol.2,pp.591-1–591-7,Aug.2012.[6]M.M.Lee,J.Teuscher,T.Miyasaka,T.N.Murakami,and H.J.Snaith,“Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites,”Science,vol.338,pp.643–647,Nov.2012.[7]J.H.Heo,S.H.Im,J.H.Noh,T.N.Mandal,C.-S.Lim,J.A.Chang,Y.H.Lee,H.-J.Kim,A.Sarkar,M.K.Nazeeruddin,M.Gr¨a tzel,and S.I.Seok,“Efficient inorganic–organic hybrid heterojunction solar cells containing perovskite compound and polymeric hole conductors,”Nat.Photon.,vol.7,pp.486–491,May2013.[8]J.Burschka,N.Pellet,S.-J.Moon,R.Humphry-Baker,P.Gao,M.K.Nazeeruddin,and M.Gr¨a tzel,“Sequential deposition as a route to high-performance perovskite-sensitized solar cells,”Nature,vol.499, pp.316–319,Jul.2013.[9]P.Docampo,J.M.Ball,M.Darwich,G.E.Eperon,and H.J.Snaith,“Effi-cient organometal trihalide perovskite planar-heterojunction solar cells on flexible polymer substrates,”mun.,vol.4,pp.2761-1–2761-6, Nov.2014.[10]H.Zhou,Q.Chen,G.Li,S.Luo,T.Song,H.Duan,Z.Hong,J.You,Y.Liu,and Y.Yang,“Interface engineering of highly efficient perovskite solar cells,”Science,vol.345,pp.542–546,Aug.2014.[11]M.Liu,M.B.Johnston,and H.J.Snaith,“Efficient planar hetero-junction perovskite solar cells by vapour deposition,”Nature,vol.501, pp.395–398,Sep.2013.[12]G.E.Eperon,V.M.Burlakov,P.Docampo,A.Goriely,and H.J.Snaith,“Morphological control for high performance,solution-processed pla-nar heterojunction perovskite solar cells,”Adv.Func.Mater.,vol.24, pp.151–157.Jan.2014.[13]S.D.Stranks,G.E.Eperon,G.Grancini,C.Menelaou,M.J.P.Alcocer,T.Leijtens,L.M.Herz,A.Petrozza,and H.J.Snaith,“Electron-hole diffusion lengths exceeding1micrometer in an organometal trihalide perovskite absorber,”Science,vol.342,pp.341–344,Oct.2013. [14]G.Xing,N.Mathews,S.Sun,S.S.Lim,m,M.Gr¨a tzel,S.Mhaisalkar,and T.C.Sum,“Long-range balanced electron-and hole-transport lengths in organic-inorganic CH3NH3PbI3,”Science,vol.342, pp.344–347,Oct.2013.[15]T.Ishihara,“Optical properties of PbI-based perovskite structures,”J.Lumin.,vol.60–61,pp.269–274,Apr.1994.[16]M.Hirasawa,T.Ishihara,T.Goto,K.Uchida,and N.Miura,“Mag-netoabsorption of the lowest exciton in perovskite-type compound (CH3NH3)PbI3,”Physica B,vol.201,pp.427–431,Jul.1994.[17]K.Tanaka,T.Takahashi,T.Ban,T.Kondo,K.Uchida,and N.Miura,“Bandgap and exciton binding energies in lead-iodide-based natural quantum-well crystals,”Solid State Commun.,vol.127,pp.619–623, Sep.2003.[18]V.D’Innocenzo,G.Grancini,M.J.P.Alcocer,A.R.S.Kandada,S.D.Stranks,M.M.Lee,nzani,H.J.Snaith,and A.Petrozza,“Excitons versus free charges in organo-lead tri-halide perovskites,”mun., vol.5,pp3586-1–3586-6,Aug.2014.[19]Y.Yamada,T.Nakamura,M.Endo,A.Wakamiya,and Y.Kanemitsu,“Near-band-edge optical responses of solution-processed organic–inorganic hybrid perovskite CH3NH3PbI3on mesoporous TiO2elec-trodes,”Appl.Phys.Exp.,vol.7,pp.032302-1–032302-4,Mar.2014.Y AMADA et al.:PHOTOELECTRONIC RESPONSES IN SOLUTION-PROCESSED PEROVSKITE CH3NH3PBI3SOLAR CELLS5[20]Y.Yamada and Y.Kanemitsu,“Determination of electron and hole life-times of rutile and anatase TiO2single crystals,”Appl.Phys.Lett., vol.101,pp.133907-1–133907-4,Sep.2012.[21]L.Q.Phuong,M.Okano,Y.Yamada,A.Nagaoka,K.Yoshino,andY.Kanemitsu,“Photocarrier localization and recombination dynamics in Cu2ZnSnS4single crystals,”Appl.Phys.Lett.,vol.103,pp.191902-1–191902-4,Nov.2013.[22] A.Wakamiya,M.Endo,T.Sasamori,N.Tokitoh,Y.Ogomi,S.Hayase,andY.Murata,“Reproducible fabrication of efficient perovskite-based solar cells:X-ray crystallographic studies on the formation of CH3NH3PbI3 layers,”Chem.Lett.,vol.43,pp.711–713,Feb.2014.[23]P.Y.Yu and M.Cardona,Fundamentals of Semiconductors:Physics andMaterial Properties,3rd ed.Berlin,Germany:Springer-Verlag,2005, ch.6.[24]R.J.Elliott,“Intensity of optical absorption by excitons,”Phys.Rev.,vol.108,pp.1384–1389,Dec.1957.[25]M.D.Sturge,“Optical absorption of gallium arsenide between0.6and2.75eV,”Phys.Rev.,vol.127,pp.768–773,Aug.1962.[26]N.Onoda-Yamamuro,T.Matsuo,and H.Suga,“Calorimetric and IR spec-troscopic studies of phase transitions in methylammonium trihalogeno-plumbates(II),”J.Phys.Chem.Solids,vol.51,pp.1383–1395,Jul.1990.[27] A.Poglitsch and D.Weber,“Dynamic disorder in methylammoni-umtrihalogenoplumbates(II)observed by millimeterwave spectroscopy,”J.Chem.Phys.,vol.87,pp.6373–6378,Aug.1987.[28]J.Even,L.Pedesseau,and C.Katan,“Analysis of multivalley andmultibandgap absorption and enhancement of free carriers related to exciton screening in hybrid perovskites,”J.Phys.Chem.C,vol.118, pp.11566–11572,May2014.[29]Y.Yamada,T.Nakamura,M.Endo,A.Wakamiya,and Y.Kanemitsu,“Photocarrier recombination dynamics in perovskite CH3NH3PbI3for solar cell applications,”J.Amer.Chem.Soc.,vol.136,pp11610–11613, Aug.2014.Authors’photographs and biographies not available at the time of publication.。

第40卷第2期2021年3月Vol.40No.2Mar.2021大连工业大学学报JournalofDalianPolytechnicUniversityDOI:10.19670/ki.dlgydxxb.2021.0210二氧化钛表面超强酸化光氧复合降解罗丹明B温宇,杨大伟(大连工业大学轻工与化学工程学院,辽宁大连116034)摘要:采用共结晶方法制备了锌锆共掺杂的介孔二氧化钛,前驱体用硫酸处理使其具有超强酸性。
将制备的介孔二氧化钛用于降解废水模拟物罗丹明B,测试其光催化与氧催化降解能力。
通过紫外-可见分光光度计、X射线衍射、电镜扫描等对催化剂进行表征,实验结果表明,在强酸修饰二氧化钛前驱体的影响下,掺杂锌锆的介孔二氧化钛具有光催化与氧催化活性。
锌锆共掺杂介孔二氧化钛的光催化与氧催化效率分别达到了72%与25%o硫酸处理后在TiO2与掺杂原子表明形成酸性中心,在无光条件下氧化降解废水效率为30%,提高了降解效率。
关键词:二氧化钛;光催化;酸催化;罗丹明B中图分类号:X703.5文献标志码:A文章编号:1674-1404(2021)02-0136-04Composite degradation of rhodamine B using TiO2withphotocatalytic oxygen and super acidWEN Yu,YANG Dawei(SchoolofLightndustryandChemicalEngineering,DalianPolytechnicUniversity,Dalian116034,China) Abstract:The mesoporous titania doped with zinc oxide,zirconium dioxide,zinc and zirconium were prepared by the co-crystallization method and the precursor of mesoporous titania was pretreated with sulfuric acid to endowed it super acidic.The mesoporous titania was used for degradation of rhodamine B in simulated wastewater and its photocatalytic activity and oxygen catalytic ability was analyzed by UV-visible spectrophotometer,X ray diffraction,scanning electron microscopy.The results showed that the T1O2doped metal oxides and super acid exhibited excellent photocatalytic and oxygen catalytic ability.The degradation rate of rhodamine B photocatalyzed and oxygen catalyzed by the prepared catalysts were72%and25%,respectively.After treatment with sulfuric acid,the acidic centers were formed between the doped atoms and the surface of titanium dioxide,which improved the oxygen degrading efficiency of wastewater to30%.Keywords:TiO2;photocatalytic;acidic catalysis;rhodamine B0引言工业生产中生成的有机废水对环境造成严重污染,国家对废水排放标准执行越来越严格,如何降低或消除有机废水中大分子有机物成为研究的重点。
【关键字】论文BRIEF COMMUNICATIONPreparation and photoelectric properties of mesoporous ZnO filmsMing Ming Wu •Yue Shen •Feng Gu •Yi An Xie •Jian Cheng Zhang •Lin Jun WangReceived:24June 2009/Accepted:21October 2009/Published online:6November 2009ÓSpringer Science+Business Media,LLC 2009Abstract Mesoporous ZnO films doped with Ti 4?(M-ZnOhave been prepared by doping process and sol–gel method.The films have mesoporous structures and consist of nano-crystalline phase,as evidenced from small angle X-ray diffraction and high resolution transmission electron microscopy.The wide angle X-ray diffraction of M-ZnO films confirms that M-ZnO has hexagonal wurtzite structure and ternary ZnTiO3phases.Ultraviolet–visible transmittance spectra,absorbance spectra and energy gaps of the films were measured.The Eg of M-ZnO is intensity of M-ZnO centered at 380nm increases obviously with the excitation power,which is due to the doping process and enhanced emiss ion efficiency.M-ZnO thin films display a positive photovoltaic effect compared to mesoporous TiO 2(M-TiO 2films.Keywords Photoelectric propertiesÁMesoporous ÁZnO ÁTiO 21IntroductionIt has been recently shown that semiconducting mesoporous metal oxides,e.g.,SnO 2[1,2]or TiO 2[3],with large specific surface areas and uniform pore widths show interesting properties which are superior to non porous samples of the same metal oxides.Zinc oxide (ZnOis attracting tremendous research interest due to its vast spectrum properties and applications.ZnO is an n-type direct band-gap semiconductorwith E g =3.37eV and an exciton-binding energy of 60meV.It has been applied for light-emitting diodes [4–6],lasers [7],photovoltaic solar cells [8],UV-photodetectors [9]and sensors [10].Particularly,it has attracted great attention in Dye-sensitized solar cells (DSSC.To date,the highest solar-to-electric conversion effi-ciency of over 11%has been achieved with films that consist of mesoporous TiO 2nanocrystallites sensitized by ruth e-nium-based dyes [11].Besides the optical properties similar to TiO 2,ZnO has other advantages such as higher light absorbance below 400nm than TiO 2[12],improved elec-tronic transfer rate and hindered dark current generation [13,14].Nevertheless,ZnOnano structure electrodes seem to have insufficient internal surface areas,which limits their energy conversion efficiency at a relatively low level,for example,1.5–2.4%for ZnO nanocrystalline films [15–17],0.5–1.5%for ZnO nanowire films [18–20],2.7–3.5%for uniform ZnO aggregate films [21,22]and 5.4%for poly-disperse ZnO aggregates [8].In spite of a great deal of effort to successfully synthesize mesoporous ZnO powders successfully [23,24],however,many barriers still exist due to the intrinsic properties of zinc versus silicon.To the best of our knowledge,there were few reports about ordered mesoporous ZnO thin film prepared by wet chemical method.The main hurdles in the synthesis of well-ordered mesoporous ZnO are the high reactivity of Zn ion precursors toward hydro lysis [25]and difficulty for Zn to form the three-dimensional network structure of Zn-O as compared to Si and Ti [26].In this work,we report a highly reproducible synthetic method to produce thermally stable M-ZnO films through doping process and sol–gel method.Photoelectric proper-ties of M-ZnO films were studied and compared with M-TiO 2films,which can get the highest solar-to-electric conversion efficiency.ÁY.Shen (&ÁF.Gu Á Á ÁSchool of Materials Science and Engineering,Shanghai University,Shanghai 200072,Chinae-mail:yueshen@;J Sol-Gel Sci Technol (201053:470–474DOI 10.1007/s10971-009-2099-72ExperimentalThe Pluronic P123triblock co polymer(EO20PO70EO20 with a molar weightof5800was kindly donated by BASF. All other chemicals were of analytical grade and used as received.M-ZnOfilms were prepared by doping process and sol–gel method via the following procedure:1.6ml concentrated HCl was slowly added to0.17ml tetrabutyl titanate(TBOT, [98%purityand2.085g zinc acetate dihydrate(Zn(Ac2, [99%purityat room temperature under vigorous stirring. Separately,0.75g P123wasfirst dissolved in8.3ml 1-butanol([99%purity,then added to the HCl/TBOT/ Zn(Ac2solution.At last,2ml acetylacetone(AcAcwas added.This solution was subsequently aged with stirring at room temperature for6h.The molar ratio of P123/1-buta-nol/Zn(Ac2/TBOT//AcAcwas0.013:9:0.95:0.05:2:2.M-ZnOfilms were prepared by spin coating the freshsolution onto Indium tin oxides(ITOsubstrate at900rpm for10s and3,300rpmfor20s.The as-synthesizedfilms were aged at40°C for1days and then annealed at120°C for5h at vacuum.The thickfilms were prepared by repeating the above stepsfor5times.Thefilms were sub-sequently calcined at a rate of1K min-1to350°C for5h. ITO glasses had been eroded to form plan electrodes before the spin coating process,and cleaned successively in de-ionized water,acetone and ethanol,for10min each.For ease of comparison,we prepared mesoporous TiO2(M-TiO2thin films using the same process.Themolar ratio of P123/ 1-butanol/TBOT/HCl/AcAc was0.013:9:1:2:2.The thick-ness of thinfilms is about100nm.Thefilms were characterized by(X-ray diffractometer, RigakuD/MAX-2550,Tokyowith Cu K a radiation (k=1.54056A˚,operated at40kV and200mA.The small angle scanning range was from0.5°to3°with a scanning rate of0.25°min-1.Transmission electron microscopic(TEMimages of M-ZnO thinfilms were obtained using Japan JEM-2010F microscope operating at an acceleration voltage of200kV.A JASCO V570spec-trophotometer was used to measure the optical spectra of the thinfilms.PL spectra were measured at room temper-ature with a spectrometer(Horiba Jobin Yvon HR800 using the excitation source of the325nm line of a He-Cd laser.Current-voltage measurements were carried out by semiconductor characterization system(Keithley4200, Americawith a tungsten lamp(250W.All measurements were performed at room temperature in air.3Results and discussionsSAXRD and HRTEM are two typical ways to investigate the order properties of mesoporous materials.The SAXRD patterns of M-ZnO and M-TiO2thinfilms are shown in Fig.1,and illustrate characteristic peaks at2h=0.62°and 0.75°,respectively,suggesting that the M-ZnO and M-TiO2thinfilms exhibited mesoporous structure.The diameter/d value,determined as distance between meso walls,is calculated from the2h values of the characteristic peaks by the Bragg equation. Further structural characterization of M-ZnO was per-formed using HRTEM and is shown in presents a honeycomb-like porous structure and the pore size is conforming to the results of SAXRD.In image(b, there are obviou s lattice fringes,which indicate thefilms have nano-crystalline phase structure.Figure3shows the wide angle X-ray diffraction patterns of M-ZnO thickfilms(on ITO substratesand ITO sub-strates,respectively.It can be seen that M-ZnO thickfilms exhibit hexagonal wurtzite structure and ternary ZnTiO3 phases,together.Yet no peaks corresponding to titanium and/or titanium oxide were detected.The crystal latticeconstants of M-ZnO calculated from the wide-angle X-ray diffraction are a=3.243A˚and c=5.190A˚,which are close to the card JCPDS No.36-1451,a=3.250A˚and c=5.207A˚.The differences result from the introduction of Ti ion in ZnO,because the Ti4?radius(0.68A˚is smaller than that of Zn2?(0.74A˚.The slight change of lattice parameters of M-ZnOconfirms that the Ti io ns have been incorporated into the ZnO lattice.Ultraviolet-visible(UV/vistransmittance spectra of M-ZnO and M-TiO2thinfilms were measured in Fig.4. Compared to the M-TiO2thinfilm,the fundamental transmittance edge of the M-ZnO thinfilm shows a blue shift fro m350to300nm.The inset graph is the absorbance spectra of M-ZnO and M-TiO2thinfilms.It illustrates that the absorption rate of M-ZnO is greater than that of M-TiO2in the visible range,suggesting the highzinc Fig.1SAXRD patterns of(aM-ZnO and(bM-TiO2content M-ZnO composite material can increase the light-harvesting capability as photoelectrode film.The plot of (a h m 1/2versus h m of M-ZnO and M-TiO 2films is shown in Fig.5,where a is the absorption coefficient,h m is the photon energy.Following the well-known Tauc function:(a h m 1/2µ(h m -Egand extrapolating the linear portion to (a h m 1/2=0,the optical-gap energy (Egcan be deter-mined.It could be found that the Eg of M-ZnO and M-TiO 2were 3.25and 3.37eV,respectively.Figure 6shows the room-temperature PL spectra of M-ZnO thick films as a function of the excitation power density.The five excitation power intensities are 2,20,50,100,and 200mW,respectively.The spot radius is 1l m.Dominant emission peaks of M-ZnO centered at 380nm,corresponding to 3.26eV,are ascribed to direct electron-hole recombination which should be equal to the M-ZnO band gap.It is worth noting that there is asignificantFig.2TEM images of a M-ZnO (50,0009and b M-ZnO (200,0009Fig.3The wide angle X-ray diffraction of M-ZnO thick films and ITOsubstratesFig.4Transmittance spectra of (a M-ZnO and (b M-TiO 2(inset:UV/vis absorbance spectra of (a M-ZnO and (b M-TiO 2Fig.5Energy gap (Egof (a M-ZnO and (b M-TiO 2increase of PL intensity of M-ZnO thick films at 380nm as compared to the visible bands emission with excitation powers increasing from 2to 200mW.This result is con-sistent to literature [27]and can be expected to be caused by the doping process due to enhanced emission efficiency from free exciton emission [27].For M-ZnO films,Ti atoms occupy Zn atom sites in the lattice of ZnO.When incident UV light excite the carriers in the films,the photocarriers may escape more easily from Ti ions than from Zn ions,which leads to the quick diffusion of excitons and increased exciton concentration in the M-ZnO films.Current-voltage properties of M-ZnO and M-TiO 2thin films were tested in dark and under irradiation for 5s with a tungsten lamp (250W,height to the film was 15cm.As shown in Fig.7b,photoconductivity of the M-TiO 2thin film was 6.023910-10S and dark conductivity was 1.070910-9S at bias voltage of 1V,photoconductivitydecreased about 1.8times under irradiation compared with that in thedark.However,under the same irradiation condition,it was interesting to find that the M-ZnO thin film exhibits a positive p hotovoltaic effect.Photoconduc-tivity of the M-ZnO thin film reached 9.718910-7S while dark conductivity was 3.256910-7S,photocon-ductivity increased about 3times as shown in TiO 2was widely used in DSSC,it has a low electron transfer rate and high combination rate of the pair of excited electrons [8,9],which induced a negative pho-tovoltaic effect itself.While ZnO has very high electron mobility,which is about 155cm 2V -1s -1[28],ZnO materials can improve the electronic transfer rate and hinder the dark current generation [13,14].Furthermore,it contains some intrinsic defects,which can act as capture centers of photoelectrons andthereby stop the recombina-tion of photoelectrons and photo-holes.This may improve the energy conversion efficiency of M-ZnO in DSSC.4ConclusionIn conclusion,M-ZnO films doped with Ti 4?were pre-pared by sol–gel and spin coating method.Eg of M-ZnO is 3.25eV,which is smaller than that of bulk ZnO.M-ZnO films exhibit hexagonal wurtzite structure and ternary ZnTiO 3phases.The PL intensi ty of M-ZnO centered at 380nm is increased obviously with the excitation power,which is expected to be caused by enhanced emission efficiency from free excitonemission.Current-voltage properties of M-ZnO films display a positive photovoltaic effect and indicate the promising applications in DSSC.Acknowledgments The work was supported by Innovation Pro-gram of Shanghai Municipal Education Commission (08YZ08,AM and other Research Foundation of Shanghai City Committee of Sci-ence and Technology(0852*******,0752nm016,07JC14058and Shanghai Leading Academic Disciplines(S30107.We thank Dr.Qiang Li,Bo Lu and Jian Huang for their assistance in the measurement at Shanghai University.References1.Wagner T,Kohl CD,Fro¨ba M,Tiemann M (2006Sensors 6:3182.Hyodo T,Abe S,Shimizu Y,Egashira M (2003Sens Actuators B 93:5903.Choi H,Stathatos E,Dionysiou DD (2006Appl Catal B 63:604.Keem K,Jeong DY,Kim S,Lee MS,Yeo IS,Chung U,Moon JT (2006Nano Lett 6:14545.Konenkamp R,Word RC,Godinez M (2005Nano Lett 5:20056.Spanhel L (2008J Sol-Gel Sci Technol 39:77.Kim YJ,Shang HM,Cao GZ (2006J Sol-Gel Sci Technol 38:798.Zhang QF,Chou TP,Russo B,Jenekhe SA,Cao GZ (2008Angew Chem 120:24369.Monroy E,Omnes F,Calle F (2003Semicond Sci Technol 18:3310.Yan CL,Xue DF (2007J Alloys Compounds431:241Fig.6PL spectra of M-ZnO thick films with various excitation power densities at roomtemperatureFig.7Current-voltage characteristics of a M-ZnO and b M-TiO 2(filled square in dark,(filled triangle under irradiation11.Gra¨tzel M(2005Inorg Chem44:684112.Sakthivel S,Neppolian B,Shankar MV,Arabindoo B,Palanich-amy M,Murugesan V(2003Sol Energy Mater Sol Cells77:6513.Mane RS,Lee WJ,Pathan HM(2005J Phys Chem B109:2425414.Wang ZS,Huang CH,Huang YY(2001Chem Mater13:67815.Otsuka A,Funabiki K,Sugiyama N,Yoshida T(2006Chem Lett35:66616.Zeng LY,Dai SY,Xu WW,Wang KJ(2006Plasma Sci Technol8:17217.Lee WJ,Suzuki A,Imaeda K,Okada H,Wakahara A,Yoshida A(2004Jpn J Appl Phys43:152w M,Greene LE,Johnson JC,Saykally R,Yang PD(2005Nat Mater44:5519.Baxter JB,Aydil ES(2005Appl Phys Lett86:05311420.Pasquier AD,Chen HH,Lu YC(2006Appl Phys Lett89:321.Zhang QF,Chou TP,Russo B,Jenekhe SA,Cao GZ(2008AdvFunct Mater18:165422.Chou TP,Zhang QF,Fryxell GE,Cao GZ(2007Adv Mater19:258823.Wagner T,Waitz T,Roggenbuck J,Fro¨ba M,Kohl CD,TiemannM(2007Thin Solid Films515:836024.Polarz S,Orlov AV,Schu¨th F,Lu AH(2007Chem Eur J13:59225.Soler-Illia GJ,Sanchez C,Lebeau B,Patarin J(2002Chem Rev102:409326.Yang PD,Zhao DY,Margolese DI(1999Chem Mater11:281327.Zhang Y,Zhang ZY,Lin BX,Fu ZX,Xu J(2005J Phys Chem B109:1920028.Kaidashev EM,Lorenz M,von Wenckstern H,Rahm A,Sem-melhack H-C,Han K-H,Benndorf G,Bundesmann C,Hochmuth H,Grundmann M(2003Appl Phys Lett82:3901此文档是由网络收集并进行重新排版整理.word可编辑版本!。
Yiying Wu (吴屹影)The Ohio State University Phone: (614) 247-7810 Department of Chemistry Fax: (614)-292-1685 100 W 18th Avenue E-mail:***********************.edu Columbus, OH 43210EDUCATION:Dec. 2002 Ph.D. in Chemistry, University of California at Berkeley.Advisor: Prof. Peidong Yang.June. 1998 B.S. in chemical physics, University of Science and Technology of China.EMPLOYMENT:2005-present Assistant Professor, Chemistry Department, The Ohio StateUniversity. Interest: nanostructured functional materials.2003-2005 Postdoctoral Researcher, University of California at Santa Barbara, Department of Chemistry and Biochemistry, Advisor: Prof. Galen D.Stucky.HONORS:2010 NSF-CAREER Award2008 Cottrell Scholar Award, Research Corporation2001 MRS Graduate Student Silver Award, Boston.2001-2002Cal@Silicon Valley Fellowship, University of California at Berkeley. PROFESSIONAL MEMBERSHIPS:2001 American Chemical Society2001 Materials Research SocietyPUBLICATION LIST:u=undergraduate, g=graduate student, p=postdoc, s=senior personnel, *=corresponding author45. Y. Li g, G. Natu g, Y. Wu*. “LiFePO4/Graphene Composite as the CathodeMaterial for High-Power Lithium Ion Batteries” submitted to Nano Letters(2010).44. P. Hasin g, M. A. Alpuche-Aviles p, Y. Wu*.“Electrocatalytic activity ofgraphene multilayers towards I-/I3-: effect of preparation conditions andpolyelectrolyte modification” submitted to J. Physical Chemistry C (2010).43. G. Natu g, Y. Wu*. “Photoelectrochemical Study of the Ilmenite Polymorph ofCdSnO3 and its Photoanodic Application in Dye-Sensitized Solar Cells” J.Physical Chemistry C, accepted (2010).42. Y. Li g, P. Hasin g, Y. Wu*. “Ni x Co3-x O4 Nanowire Arrays for ElectrocatalyticOxygen Evolution”, Advanced Materials, accepted (2010).41. J. Baxter s, G. Chen s, D. Daniielson s, M. S. Dresselhaus s*, A. G. Fedorov s*, T. S.Fisher s, C. W. Jones s, E. Maginn s, U. Kortshagen s, A. Manthiram s, A. Nozik s, D.Rolison s, T. Sands s, L. Shi s*, D. Sholl s, Y. Wu s. “Nanoscale Design to Enablethe Revolution in Renewable Energy”, Energy & Environmental Science. 2(6), 559 (2009)40. Y. Li g, Y. Wu*. “Coassembly of Graphene Oxide and Nanowires for Large-Area Nanowire Alignment”, J. Am. Chem. Soc.131(16) 5851-5857 (2009).39. M. A. Alpuche-Aviles p, Y. Wu*. “Photoelectrochemical Study of the Bandstructure of Zn2SnO4Prepared by the Hydrothermal method”, J. Am. Chem.Soc.131(9) 3216-3224 (2009).38. P. Hasin g, M. A. Alpuche-Aviles p, Y. Li g, Y. Wu*. “Mesoporous Nb-dopedTiO2 as Pt Support for Counter Electrode in Dye-Sensitized Solar Cells”, J.Phys. Chem. C. 113(17) 7456-7460 (2009).37. Y. Li g, Y. Wu*. “Formation of Na0.44MnO2 nanowires via stress-inducedsplitting of birnessite nanosheets”,Nano Research, 2(1): 54-60 (2009). 36. Y. Li g, B. Tan p, Y. Wu*. "Mesoporous Co3O4 Nanowire Arrays for LithiumIon Batteries with High Capacity and Rate Capacity", Nano Letters, 8:265-270 (2008).35. Y. Li g, B. Tan p, Y. Wu*. "Ammonia-Evaporation-Induced Synthetic Methodfor Metal (Cu, Zn, Cd, Ni) Hydroxide/Oxide Nanostructures", Chem.Mater.20: 567-576 (2008).34. B. Tan p, E. Toman u, Y. Li g, Y. Wu*, "Zinc Stannate (Zn2SnO4) Dye-SensitizedSolar Cells", J. Am. Chem. Soc. 129(14), 4162 (2007).33. Y. Li g, B. Tan p, Y. Wu*, "Freestanding mesoporous quasi-single-crystallineCo3O4 nanowire arrays", J. Am. Chem. Soc. 128(44), 14258-14259 (2006)(highlighted by Nature Nanotech. (Oct. 2006)).32. B. Tan p, Y. Wu*, “Dye-Sensitized Solar Cells Based on Anatase TiO2Nanoparticle/Nanowire Composites”, J. Phys. Chem. B110: 15932-15938(2006).(Postdoc work)31. A. Thomas, M. Schierhorn, Y. Wu, G. Stucky, “Assembly of SphericalMicelles in 2D Physical Confinements and Their Replication intoMesoporous Silica Nanorods”, J. Mater. Chem. 17: 4558-4562 (2007). 30. M. Moskovits, D.H. Jeong, T. Livneh, Y.Y. Wu, G.D. Stucky, "Engineeringnanostructures for single-molecule surface-enhanced Raman spectroscopy", Isreal Journal. of Chemistry, 46: 283-291 (2006).29. Y. Zhang , J. Christofferson, A. Shakouri, D. Li, A. Majumdar, Y. Wu, R. Fan,P. Yang, “Characterization of heat transfer along Si Nanowire”, IEEETransactions on Nanotechnology, 5, 67 (2006).28.J. F. Wang, C.-K. Tsung, R. C. Hayward, Y. Wu, G. D. Stuck. “Single-crystal mesoporous silica ribbons”, Angew. Chem. Int. Ed.44: 332-336 (2005).27.Y. Wu, G. S. Cheng, K. Katsov, S. W. Sides, J. F. Wang, J. Tang, G. H.Fredrickson, M. Moskovits, G. D. Stucky, “Composite mesostructures bynano-confinement”, Nature Materials3, 816-822 (2004). (Highlighted byScience306, 943 (2004)).26.Y. Wu, T. Livneh, Y. X. Zhang, G. S. Cheng, J. F. Wang, J. Tang, M.Moskovits, G. D. Stucky, “Templat ed synthesis of highly orderedmesostructured nanowires and nanowire array”, Nano Letters 4, 2337(2004) (cover story).25.J. F. Wang, C.-K. Tsung, W. B. Hong, Y. Wu, J. Tang, G. D. Stucky,"Synthesis of mesoporous silica nanofibers with controlled porearchitectures", Chem. Mater. 16, 5169 (2004).24.J. Tang, Y. Wu, E. W. McFarland, G. D. Stucky, “Synthesis andphotocatalytic properties of highly crystalline and ordered mesoporousTiO2 thin films”, Chem. Comm. (14), 1670-1671 (2004).(Graduate work)23.A. R. Abramson, W. C. Kim, S. T. Huxtable, H. Q. Yan, Y. Wu, A. Majumdar,C.-K. Tien, P.D. Yang, "Fabrication and characterization of ananowire/polymer-based nanocomposite for a prototype thermoelectricdevice", Journal of Microelectromechanical Systems, 13(3), 505 (2004).).22.D. Y. Li, Y. Wu, R. Fan, P. D. Yang, A. Majumdar, “Thermal conductivity ofSi/SiGe longitudinal heterostructure nanowires” Appl. Phys. Lett. 83(15),3186 (2003).21.D. Y. Li, Y. Wu, P. Kim, L. Shi, N. Mingo, Y. Liu, P. D. Yang, A. Majumdar,“Thermal conductivity of individual silicon nanowires” Appl Phys. Lett.83(14), 2934 (2003).20.R. Fan, Y. Wu, D. Y. Li, M. Yue, A. Majundar, P. D. Yang, “Fabrication ofSilica Nanotube Arrays from Vertical Silicon Nanowire Templates”, J. Am.Chem. Soc.125(18), 5254-5255 (2003).19.Y. N. Xia, P. D. Yang, Y. Sun, Y. Wu, B. Mayers, B. Gates, Y. D. Yin, F. Kim,H. Yan, “One-dimensional Nanostructures: Synthesis, Characterization, andApplications”, Adv. Mater. 15(5), 353-389 (2003).18.Y. Wu, R. Fan, P. D. Yang, "Block-by-block growth of single-crystallineSi/SiGe superlattice nanowires", Nano letters, 2, 83 (2002).17.Y. Wu, H. Yan, M. Huang, B. Messer, J. Song, P. D. Yang, “Inoragnicsemiconductor nanowires: rational growth, assemblies and novel properties”, Chemistry, Euro. J., 8, 1260 (2002).16.Y. Wu, H. Yan, P. D. Yang, "Semiconductor nanowire array: potentialsubstrates for photocatalysis and photovoltaics", Topics in Catalysis, 19(2), 197 (2002).15.B. Gates, B. Mayers, Y. Wu, Y. Sun, B. Cattle, P. D. Yang, Y. N. Xia,“Synthesis and characterization of crystalline Ag2Se nanowires through atemplate-engaged reaction at room temperature”, Adv. Func. Mater. 12(10), 679-686 (2002).14.P. D. Yang, Y. Wu, R. Fan, “Inorganic semiconductor nanowires”,International Journal of Nanoscience,1(1), 1-39 (2002).13.B. Zheng, Y. Wu, P. D. Yang, J. Liu, “Synthesis of ultra-long and highly-oriented silicon oxide nanowires from alloy liquid”, Adv. Mater. 14, 122(2002).12.Y. Wu, P. D. Yang, “Direct observation of vapor-liquid-solid nanowiregrowth”, J. Am. Chem. Soc. 123, 3165 (2001).11.Y. Wu, B. Messer, P. D. Yang, "Superconducting MgB2 nanowires", Adv.Mater.13, 1487 (2001).10.Y. Wu, P. D. Yang, “Melting and welding semiconductor nanowires innanotubes”, Adv. Mater. 13, 520 (2001).9.M. Huang, S. Mao, H. Feick, H. Yan, Y. Wu, H. Kind, E. Weber, R. Russo, P.D. Yang, "Room-temperature ultraviolet nanowire nanolasers", Science,292, 1897 (2001).8.M. Huang, Y. Wu, H. Feick, N. Tran, E. Weber, P. D. Yang, “Catalytic growthof zinc oxide nanowi res through vapor transport”, Adv. Mater. 13(2), 113(2001).7.J. Song, Y. Wu, B. Messer, H. Kind, P. D. Yang, "Metal nanowire formationusing Mo3Se3- as reducing and sacrificing templates", J. Am. Chem. Soc.123, 10397 (2001).6. B. Gates, Y. Wu, Y. Yin, P. D. Yang, Y. D. Xia, “Single-crystalline nanowiresof Ag2Se can be synthesized by templating against nanowires of trigonalSe”, J. Am. Chem. Soc. 123, 11500 (2001).5.J. Song, B. Messer, Y. Wu, H. Kind P. D. Yang, "MMo3Se3 (M=Li+, Na+, Rb+,Cs+, NMe4+) nanowire formation via cation exchange in organic solution",J. Am. Chem. Soc. 123, 9714 (2001).4.Y. Li, J. Wang, Z. Deng, Y. Wu, X. Sun, S. Fan, D. Yu, P. D. Yang, “Bismuthnanotubes: a rational low-temperature synthetic route”, J. Am. Chem. Soc.123, 9904 (2001).3.Y. Wu, P. D. Yang, “Germanium/carbon core-sheath nanostructures”, Appl.Phys. Lett. 77, 43 (2000).2.Y. Wu, P. D. Yang, “Germanium nanowire growth via simple vapor transport”,Chem. Mater. 12, 605 (2000).1. B. Messer, J. H. Song, M. Huang, Y. Wu, F. Ki m, P. Yang, “Surfactantinduced mesoscopic assemblies of inorganic molecular chains”, Adv.Mater. 12, 1526 (2000).GRANTS and AW ARDS:9/07-8/10 Department of Energy, “Designing nanoparticle/nanowire composites and "nanotree" arrays as electrodes for efficient dye-sensitized solarcells”, $750,000 total9/06-9/08 Petroleum Research Fund PRF-43833, “Functional nanocrystal-nanowire composite materials: synthesis and electron transportproperties”, $35,000 total.7/08- Research Co rporation (Cottrell Scholar Award), “Searching for New Electrode Materials and Nanostructured Architectures for EfficientDye-Sensitized Solar Cells”; $100,000 total.2/10-2/15 NSF-CAREER, “Black Cobalt Oxide Nanowire Arrays: Synthesis, Properties, and Ene rgy Applications”; $575,000 totalINVITED PRESENTATIONS:Conferences/Workshops/Symposia16. FACCS conference, Lousville, KY, October 19, 2009.15. IMR Materials Week, Ohio State Unversity, August 31 – September 3, 2009.14. North American Solid State Conference, Ohio State University, June 17-20,2009.13. Central Regional Meeting of the American Chemical Society, Cleveland, May20-23, 2009.12. Materials Research Society meeting, San Francisco, April 13-17, 2009.11. Indo-US Workshop on nanoscale materials and interfaces, Purdue University,10-12 March 2009.10. Nanoparticles in Energy Applications Workshop, Argonne National Lab, Feb.23, 2009.9. ASME Heat transfer, Fluids, Energy Sustainability and Nanotechnology,Jacksonville, FL, Aug. 12, 20088. Central Regional Meeting of American Chemical Society, Columbus, OH,June 13, 20087. 35th Annual Spring Symposium, Michigan Chapter of the American VacuumSociety, Toledo, Oh, May 28, 20086. Wright Center PVIC Semi Annual meeting, Columbus, OH, April 17, 2008 5. 235th ACS National Meeting, New Orleans, LA, April 9, 20084. SPIE Optics East, Boston, MA, Sept. 9-12, 20073. Advanced Materials Workshop, Dalian, China, June 23-24, 20072. 223rd ACS national meeting, Chicago, IL, March 25-28, 20071. 41st ACS Midwest Regional Meeting, Quincy, IL, Oct. 25-27, 2006 Universities/Colleges13. IUPUI, Department of Mechanical Engineering, February 4, 2010.12. UC Davis, Department of Chemistry, January 5, 2010.11. University of Michigan, Department of Chemistry, October 30, 2009.10. Penn State University, Department of Chemistry &MRSEC, October 5, 2009.9. Purdue University, Department of Chemistry, September 16, 2009.8. The Ohio State University, ENCOMM, February 13, 20097. The Ohio State University, Department of Chemistry, January 14, 2009 (4th-year review).6. Indiana University, Department of Chemistry, April 22, 2008.5. Miami University, Department of Chemistry and Biochemistry, February 7,2008.4. Ohio University, Condensed matter and surface science seminar, May 17,2007.3. OSU, Department of Materials Science and Engineering, April 6, 2007.2. OSU, Department of Biomedical Engineering, February 2007.1. Kent State University, Department of Chemistry, September 22, 2005. PATENTS:4. “Graphene Compo sites as the Cathode Material of High-Power Lithium IonBatteries”, U.S. provisional patent, OSU1159-288A (2010).3. “Fluidic Nanotubes and Devices”, Patent No. US 7,355,216 B2 (April 8, 2008) 2. “Sacrificial Template Method of Fabricating a Nanotube”, Pa tent No.: US7,211,143 B2 (May 1, 2007).1. “Methods of fabricating nanostructures and nanowires and devices fabricatedtherefrom”, Patent N0.: WO02080280 (2002).LAB PERSONNEL:Present:Postdoctoral Associates:Dr. Dan Wang (May 2009-present)Graduate Students:Yanguang Li (5th year, Ph.D candidate); Panitat Hasin (3nd year); Gayatri Natu (3nd year); Ishika Sinha (3nd year); Tushar Kabre (2st year);。
矿产资源开发利用方案编写内容要求及审查大纲
矿产资源开发利用方案编写内容要求及《矿产资源开发利用方案》审查大纲一、概述
㈠矿区位置、隶属关系和企业性质。
如为改扩建矿山, 应说明矿山现状、
特点及存在的主要问题。
㈡编制依据
(1简述项目前期工作进展情况及与有关方面对项目的意向性协议情况。
(2 列出开发利用方案编制所依据的主要基础性资料的名称。
如经储量管理部门认定的矿区地质勘探报告、选矿试验报告、加工利用试验报告、工程地质初评资料、矿区水文资料和供水资料等。
对改、扩建矿山应有生产实际资料, 如矿山总平面现状图、矿床开拓系统图、采场现状图和主要采选设备清单等。
二、矿产品需求现状和预测
㈠该矿产在国内需求情况和市场供应情况
1、矿产品现状及加工利用趋向。
2、国内近、远期的需求量及主要销向预测。
㈡产品价格分析
1、国内矿产品价格现状。
2、矿产品价格稳定性及变化趋势。
三、矿产资源概况
㈠矿区总体概况
1、矿区总体规划情况。
2、矿区矿产资源概况。
3、该设计与矿区总体开发的关系。
㈡该设计项目的资源概况
1、矿床地质及构造特征。
2、矿床开采技术条件及水文地质条件。