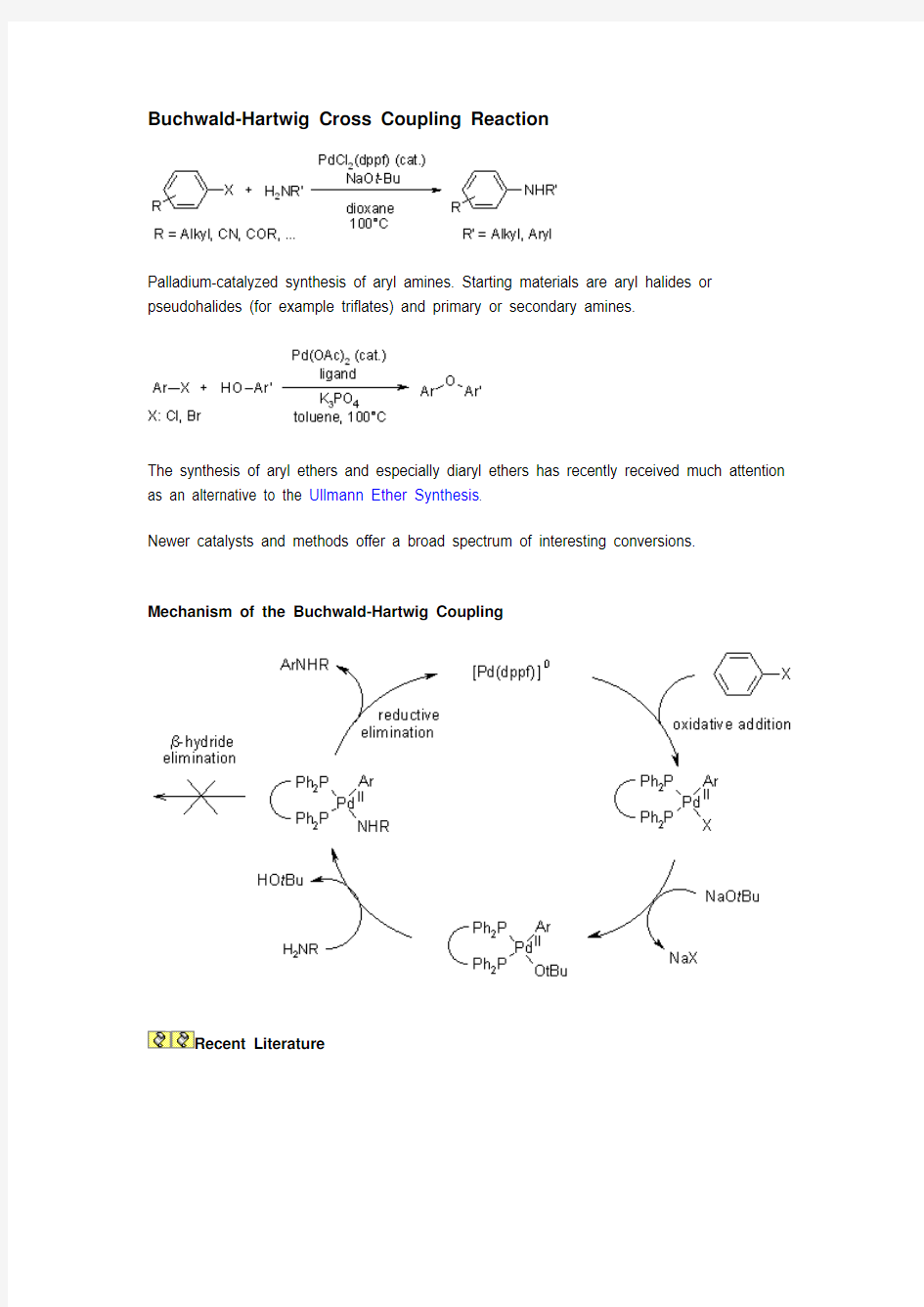

Buchwald-Hartwig Cross Coupling Reaction
Palladium-catalyzed synthesis of aryl amines. Starting materials are aryl halides or pseudohalides (for example triflates) and primary or secondary amines.
The synthesis of aryl ethers and especially diaryl ethers has recently received much attention as an alternative to the Ullmann Ether Synthesis .
Newer catalysts and methods offer a broad spectrum of interesting conversions.
Mechanism of the Buchwald-Hartwig Coupling
Recent Literature
[(CyPF-t Bu)PdCl2]: An Air-Stable, One-Component, Highly Efficient Catalyst for Amination of Heteroaryl and Aryl Halides
Q. Sheng, J. F. Hartwig, Org. Lett., 2008, 10, 4109-4112.
A Multiligand Based Pd Catalyst for C-N Cross-Coupling Reactions
B. P. Fors, S. L. Buchwald, J. Am. Chem. Soc., 2010, 132, 15914-15917.
Palladium-Catalyzed Coupling of Ammonia with Aryl Chlorides, Bromides, Iodides, and Sulfonates: A General Method for the Preparation of Primary Arylamines
G. D. Vo, J. F. Hartwig, J. Am. Chem. Soc., 2009, 131, 11049-11061.
(IPr)Pd(acac)Cl: An Easily Synthesized, Efficient, and Versatile Precatalyst for C-N and C-C Bond Formation
N. Marion, E. C. Ecarnot, O. Navarro, D. Amoroso, A. Bell, S. P. Nolan, J. Org. Chem., 2006, 71, 3816-3821.
Synthesis of Bulky and Electron-Rich MOP-type Ligands and Their Applications in Palladium-Catalyzed C-N Bond Formation
X. Xie, T. Y. Zhang, Z. Zhang, J. Org. Chem., 2006, 71, 6522-6529.
Triazole-Based Monophosphine Ligands for Palladium-Catalyzed Cross-Coupling Reactions of Aryl Chlorides
Q. Dai, W. Gao, D. Liu, L. M. Kapes, X. Zhang, J. Org. Chem., 2006, 71, 3928-3934.
Bulky Alkylphosphines with Neopentyl Substituents as Ligands in the Amination of Aryl Bromides and Chlorides
L. L. Hill, L. R. Moore, R. Huang, R. Craciun, A. J. Vincent, D. A. Dixon, J. Chou, C. J. Woltermann, K. H. Shaughnessy, J. Org. Chem., 2006, 71, 4951-4955.
Water-Mediated Catalyst Preactivation: An Efficient Protocol for C-N Cross-Coupling Reactions
B. P. Fors, P. Krattiger, E. Strieter, S. L. Buchwald, Org. Lett., 2008, 10, 3505-3508.
A Highly Active Catalyst for Pd-Catalyzed Amination Reactions: Cross-Coupling Reactions
Using Aryl Mesylates and the Highly Selective Monoarylation of Primary Amines Using Aryl Chlorides
B. P. Fors, D. A. Watson, M. R. Biscoe, S. L. Buchwald, J. Am. Chem. Soc., 2008, 130, 13552-13554.
Palladium-Catalyzed Monoarylation of Aryl Amine with Aryl Tosylates
X. Xie, G. Ni, F. Ma, L. Ding, S. Xu, Z. Zhang, Synlett, 2011, 955-958.
Cross-Coupling and Dehalogenation Reactions Catalyzed by (N-Heterocyclic
carbene)Pd(allyl)Cl Complexes
O. Navarro, H. Kaur, P. Mahjoor, S. P. Nolan, J. Org. Chem., 2004, 69, 3173-3180.
Expedited Palladium-Catalyzed Amination of Aryl Nonaflates through the Use of Microwave-Irradiation and Soluble Organic Amine Bases
R. E. Tundel, K. W. Anderson, S. L. Buchwald, J. Org. Chem., 2006, 71, 430-433.
Palladium- and Nickel-Catalyzed Aminations of Aryl Imidazolylsulfonates and Sulfamates L. Ackermann, R. Sandmann, W. Song, Org. Lett., 2011, 13, 1784-1786.
An Air and Thermally Stable One-Component Catalyst for the Amination of Aryl Chlorides D. Zim, S. L. Buchwald, Org. Lett., 2003, 5, 2413-2415.
(t-Bu)2PNP(i-BuNCH2CH2)3N: New Efficient Ligand for Palladium-Catalyzed C-N Couplings of Aryl and Heteroaryl Bromides and Chlorides and for Vinyl Bromides at Room Temperature Ch. V. Reddy, J. V. Kingston, J. G. Verkade, J. Org. Chem., 2008, 73, 3047-3062.
Use of Polymer-Supported Dialkylphosphinobiphenyl Ligands for Palladium-Catalyzed Amination and Suzuki-Reaktions
C. A. Parrish, S. L. Buchwald, J. Org. Chem., 2001, 66, 3820-3827.
(t-Bu)2PNP(i-BuNCH2CH2)3N: New Efficient Ligand for Palladium-Catalyzed C-N Couplings of Aryl and Heteroaryl Bromides and Chlorides and for Vinyl Bromides at Room Temperature Ch. V. Reddy, J. V. Kingston, J. G. Verkade, J. Org. Chem., 2008, 73, 3047-3062.
A Pd(0)-catalyzed C-N bond-forming reaction enables the synthesis of brominated indoles in the presence of P t Bu3 as phosphine ligand. The bulky ligand serves to prevent inhibition of the catalyst by facilitating reversible oxidative addition into the product C-Br bond.
S. G. Newman, M. Lautens, J. Am. Chem. Soc., 2010, 132, 11416-11417.
A new reactivity pattern for vinyl bromides: cine-substitution via palladium catalysed C-N coupling/Michael addition reactions
M. C. Willis, J. Chauhan, W. G. Whittinham, Org. Biomol. Chem., 2005, 3, 3094-3095.
Significantly Improved Method for the Pd-Catalyzed Coupling of Phenols with Aryl Halides: Understanding Ligand Effects
C. H. Burgos, T. E. Barder, X. Huang, S. L. Buchwald, Angew. Chem. Int. Ed., 2006, 45, 4321-4326.