ASTM D 1505-03用密度梯度法测定塑料密度的试验方法
- 格式:pdf
- 大小:67.50 KB
- 文档页数:7

ASTM D792-00用位移法测定塑料密度和比重的标准试验方法 1. 范围1.1这些测试方法描述的是对固定塑料包括片状、棒状、管状和模状等的相对密度和比重的测定方法。
1.2 描述了两种测试方法。
1.2.1测试方法A—在水中测试固体塑料。
1.2.2测试方法B—其它液体中测试固体塑料。
注1— 作为选择,D 1505测试方法可以用于多数测试形式,也用于薄膜和片状材料。
1.3 在SI 单位中规定的值可认为是标准值。
1.4此标准没有支持所有安全规定的所有标准,假如有的话,与它的使用相联系。
建立安全和健康实际相关的准则是使用者的责任。
注2—这里没有与ISO 标准相似或相等的标准。
2.参考文献2.1ASTM标准D 618 Practice for Conditioning Plastics and Electrical Insulating Materials for TestingD 891 Test Methods for Specific Gravity, Apparent, of Liquid Industrial ChemicalsD 1505 Test Method for Density of Plastics by the Density- Gradient TechniqueD 1622 Test Method for Apparent Density of Rigid Cellular PlasticsD 1898 Practice for Sampling of PlasticsE 1 Specification for ASTM ThermometersE 12 Terminology Relating to Density and Specific Gravity of Solids, Liquids, and GasesE 380 Practice for Use of the International System of Units (SI) (the Modernized Metric System)E 691 Practice for Conducting an Interlaboratory Study to Determine the Precision of a Test Method3.术语3.1 概要— 在这些测试方法中的单位,符号,缩写与E 380惯例相联系。

实验 密度梯度管法测定聚合物的密度和结晶度密度梯度法是测定聚合物密度的方法之一。
聚合物的密度是聚合物的重要参数。
聚合物结晶过程中密度变化的测定,可研究结晶度和结晶速率;拉伸、退火可以改变取向度和结晶度,也可通过密度来进行研究;对许多结晶性聚合物其结晶度的大小对聚合物的性能、加工条件选择及应用都有很大影响。
聚合物的结晶度的测定方法虽有X 射线衍射法、红外吸收光谱法、核磁共振法、差热分析、反相色谱等等,但都要使用复杂的仪器设备。
而用密度梯度管法从测得的密度换算到结晶度,既简单易行又较为准确。
而且它能同时测定一定范围内多个不同密度的样品,尤其对很小的样品或是密度改变极小的一组样品,需要高灵敏的测定方法来观察其密度改变,此法既方便又灵敏。
一、实验目的:1.掌握用密度梯度法测定聚合物密度、结晶度的基本原理和方法。
2.利用文献上某些结晶性聚合物PE 和PP 晶区和非晶区的密度数据,计算结晶度。
二、基本原理:由于高分子结构的不均一性,大分子内摩擦的阻碍等原因,聚合物的结晶总是不完善的,而是晶相与非晶相共存的两相结构,结晶度f w 即表征聚合物样品中晶区部分重量占全部重量的百分数:在结晶聚合物中(如PP 、PE 等),晶相结构排列规则,堆砌紧密,因而密度大;而非晶结构排列无序,堆砌松散,密度小。
所以,晶区与非晶区以不同比例两相共存的聚合物,结晶度的差别反映了密度的差别。
测定聚合物样品的密度,便可求出聚合物的结晶度。
密度梯度法测定结晶度的原理就是在此基础上,利用聚合物比容的线性加和关 系,即聚合物的比容是晶区部分比容与无定形部分比容之和。
聚合物的比容V 和结晶度w f 有如下关系:()1c w a w V V f V f =+- --------------------------------- (2) 式中c V 为样品中结晶区比容,可以从X 光衍射分析所得的晶胞参数计算求得;a V 为样品中无定形区的比容,可以用膨胀计测定不同温度时该聚合物熔体的比容,然后外推得到该温度时非晶区的比容a V 的数值。

塑料密度和相对密度的测试方法1范围1.1这些试验方法讲述了片状,棒条状,管状或铸模件固体塑料相对密度和密度的测定方法。
1.2讲述了两种试验方法:1.2.1试验方法A---在水中测试,1.2.2试验方法B---在其他液体中测试。
1.3 SI为标准单位。
1.4该标准并不旨在讨论所有的安全问题,如有,仅与其使用相关。
该标准的使用者责任制定相关适用的安全和健康规范,并在使用前确定规范的适用性。
2参考文件3术语3.1总则---该标准中使用的单位,符号和缩写与规范E380—致。
3.2定义:3.2.1相对密度---在23E的温度下材料不渗透部分单位体积质量与相同温度下同体积同密度无气蒸馏水的质量之比。
表达形式为:相对密度23/23 C (或sp gr 23/23 C)。
3.2.2密度---在23E的温度下,材料无渗透部分每立方米的千克质量。
表达式为:D,千克/立方米注4---E380中定义的SI标准单位是千克/立方米。
克/立方厘米X 1000转换为千克/立方米。
注5---相对密度23/23 C可以通过下式转换成密度23 T,千克/立方米。
D23C ,千克/立方米二相对密度23/23 °CX 997.64试验方法概述4.1测定固体塑料样品在空气中的质量。
然后将其浸入液体中,测出表观质量,然后计算相对密度。
5意义和使用5.1相对密度或密度6抽样6.1测定相对密度的抽样单位应该要能够代表产品的数量,所要求的数据按照D1898进行。
6.1.1如果已知或怀疑样品中含有两层或多层相对密度不同的材料,或者将成品部分或横切部分作为样品测试,或者将样品分层测试相对密度。
整体部分的相对密度不能将各层的相对密度相加获取,除非将各层的相对百分比考虑在内。
7调节7.1调节---在试验前,按照D618的规定将试验样品在23± 2C的温度和50± 5%勺相对湿度下至少放置40小时。
以防出现不一致,温度可上下浮动「C,相对湿度浮动土2%7.2试验条件---在23土2C,50± 5%@对湿度的标准实验室环境下进行试验,除非在试验方法或本标准中有其他规定。

Designation:D 1505–03Standard Test Method forDensity of Plastics by the Density-Gradient Technique 1This standard is issued under the fixed designation D 1505;the number immediately following the designation indicates the year of original adoption or,in the case of revision,the year of last revision.A number in parentheses indicates the year of last reapproval.A superscript epsilon (e )indicates an editorial change since the last revision or reapproval.This standard has been approved for use by agencies of the Department of Defense.1.Scope*1.1This test method covers the determination of the density of solid plastics.1.2This test method is based on observing the level to which a test specimen sinks in a liquid column exhibiting a density gradient,in comparison with standards of known density.N OTE 1—The comparable ISO document is ISO 1183–2.There has not been any data generated to date comparing the results of the ISO method with this method.1.3The values stated in SI units are to be regarded as the standard.1.4This standard does not purport to address all of the safety concerns,if any,associated with its use.It is the responsibility of the user of this standard to establish appro-priate safety and health practices and determine the applica-bility of regulatory limitations prior to use.2.Referenced Documents 2.1ASTM Standards:2D 941Test Method for Density and Relative Density (Spe-cific Gravity)of Liquids by Lipkin Bicapillary Pycnometer D 2839Practice for Use of a Melt Index Strand for Deter-mining Density of PolyethyleneD 4703Practice for Compression Molding Thermoplastic Materials into Test Specimens,Plaques,or SheetsE 691Practice for Conducting an Interlaboratory Study to Determine the Precision of a Test Method 2.2ISO Standard:ISO 1183-2Methods for Determining the Density and Rela-tive Density of Noncellular Plastics 33.Terminology 3.1Definition:3.1.1density of plastics —the weight per unit volume of material at 23°C,expressed as follows:D 23C ,g/cm 3(1)N OTE 2—Density is to be distinguished from specific gravity,which is the ratio of the weight of a given volume of the material to that of an equal volume of water at a stated temperature.4.Significance and Use4.1The density of a solid is a conveniently measurable property which is frequently useful as a means of following physical changes in a sample,as an indication of uniformity among samples,and a means of identification.4.2This test method is designed to yield results accurate to better than 0.05%.N OTE 3—Where accuracy of 0.05%or better is desired,the gradient tube shall be constructed so that vertical distances of 1mm shall represent density differences no greater than 0.0001g/cm.3The sensitivity of the column is then 0.0001g/cm 3·mm.Where less accuracy is needed,the gradient tube shall be constructed to any required sensitivity.5.Apparatus5.1Density-Gradient Tube —A suitable graduate with ground-glass stopper.45.2Constant-Temperature Bath —A means of controlling the temperature of the liquid in the tube at 2360.1°C.A thermostatted water jacket around the tube is a satisfactory and convenient method of achieving this.5.3Glass Floats —A number of calibrated glass floats cov-ering the density range to be studied and approximately evenly distributed throughout this range.5.4Pycnometer ,for use in determining the densities of the standard floats.5.5Liquids ,suitable for the preparation of a density gradi-ent (Table 1).N OTE 4—It is very important that none of the liquids used in the tube1This test method is under the jurisdiction of ASTM Committee D20on Plastic and is the direct responsibility of Subcommittee D20.70on Analytical Methods (Section D20.70.01).Current edition approved November 1,2003.Published January 2004.Originally approved in st previous edition approved in 1998as D 1505-98.2For referenced ASTM standards,visit the ASTM website,,or contact ASTM Customer Service at service@.For Annual Book of ASTM Standards volume information,refer to the standard’s Document Summary page on the ASTM website.3Available from American National Standards Institute (ANSI),25W.43rd St.,4th Floor,New York,NY 10036.4Tubes similar to those described in Refs (6)and (12)may also be used.1*A Summary of Changes section appears at the end of this standard.Copyright ©ASTM International,100Barr Harbor Drive,PO Box C700,West Conshohocken,PA 19428-2959,United States.exert a solvent or chemical effect upon the test specimens during the time of specimen immersion.5.6Hydrometers —A set of suitable hydrometers covering the range of densities to be measured.These hydrometers should have 0.001density graduations.5.7Analytical Balance ,with a sensitivity of 0.001g.5.8Siphon or Pipet Arrangement ,for filling the gradient tube.This piece of equipment should be constructed so that the rate of flow of liquid may be regulated to 1065mL/min.6.Test Specimen6.1The test specimen shall consist of a piece of the material under test.The piece may be cut to any shape convenient for easy identification,but should have dimensions that permit the most accurate position measurement of the center of volume of the suspended specimen (Note 5).Care should be taken in cutting specimens to avoid change in density resulting from compressive stress.N OTE 5—The equilibrium positions of film specimens in the thickness range from 0.025to 0.051mm (0.001to 0.002in.)may be affected by interfacial tension.If this affect is suspected,films not less than 0.127mm (0.005in.)in thickness should be tested.6.2The specimen shall be free of foreign matter and voids and shall have no cavities or surface characteristics that will cause entrapment of bubbles.7.Preparation of Density-Gradient Columns7.1Preparation of Standard Glass Floats 5—Prepare glass floats by any convenient method such that they are fully annealed,approximately spherical,have a maximum diameter less than one fourth the inside diameter of the column,and do not interfere with the test specimens.Prepare a solution (400to 600mL)of the liquids to be used in the gradient tube such that the density of the solution is approximately equal to the desired lowest density.When the floats are at room temperature,drop them gently into the solution.Save the floats that sink very slowly,and discard those that sink very fast,or save them for another tube.If necessary to obtain a suitable range of floats,grind selected floats to the desired density by rubbing the head part of the float on a glass plate on which is spread a thin slurry of 400or 500-mesh silicon carbide (Carborundum)or otherappropriate abrasive.Progress may be followed by dropping the float in the test solution at intervals and noting its change in rate of sinking.7.2Calibration of Standard Glass Floats (see Appendix X1):7.2.1Place a tall cylinder in the constant-temperature bath maintained at 2360.1°C.Then fill the cylinder about two thirds full with a solution of two suitable liquids selected from Table 1,the density of which can be varied over the desired range by the addition of either liquid to the mixture.After the cylinder and solution have attained temperature equilibrium,place the float in the solution,and if it sinks,add the denser liquid by suitable means with good stirring until the float reverses direction of movement.If the float rises,add the less dense liquid by suitable means with good stirring until the float reverses direction of movement.7.2.2When reversal of movement has been observed,re-duce the amount of the liquid additions to that equivalent to 0.0001-g/cm 3density.When an addition equivalent to 0.0001-g/cm 3density causes a reversal of movement,or when the float remains completely stationary for at least 15min,the float and liquid are in satisfactory balance.The cylinder must be covered whenever it is being observed for balance,and the liquid surface must be below the surface of the liquid in the constant-temperature bath.After vigorous stirring,the liquid may continue to move for a considerable length of time;make sure that the observed movement of the float is not due to liquid motion by waiting at least 15min after stirring has stopped before observing the float.7.2.3When balance has been obtained,fill a freshly cleaned and dried pycnometer with the solution and place it in the 2360.1°C bath for sufficient time to allow temperature equilib-rium of the glass.Determine the density of the solution by normal methods (Test Method D 941)and make “in vacuo”corrections for all weighings.Record this as the density of the float.Repeat the procedure for each float.7.3Gradient Tube Preparation (see appendix for details):7.3.1Method A —Stepwise addition.7.3.2Method B —Continuous filling (liquid entering gradi-ent tube becomes progressively less dense).7.3.3Method C —Continuous filling (liquid entering gradi-ent tube becomes progressively more dense).8.Conditioning8.1Test specimens whose change in density on conditioning may be greater than the accuracy required of the density determination shall be conditioned before testing in accordance with the method listed in the applicable ASTM material specification.9.Procedure9.1Wet three representative test specimens with the less dense of the two liquids used in the tube and gently place them in the tube.Allow the tube and specimens to reach equilibrium,which will require 10min or more.Thin films of 1to 2mils in thickness require approximately 11⁄2h to settle,and rechecking after several hours is advisable (Note 4).9.2Read the height of each float and each specimen by a line through the individual center of volume and averaging the5Glass floats may be purchased from American Density Materials,3826Springhill Rd.Staunton,V A 24401,Ph:(540)887-1217.TABLE 1Liquid Systems for Density-Gradient TubesSystemDensity Range,g/cm 3Methanol-benzyl alcohol 0.80to 0.92Isopropanol-water0.79to 1.00Isopropanol-diethylene glycol 0.79to 1.11Ethanol-carbon tetrachloride 0.79to 1.59Toluene-carbon tetrachloride 0.87to 1.59Water-sodium bromide 1.00to 1.41Water-calcium nitrate1.00to 1.60Carbon tetrachloride-trimethylene dibromide 1.60to 1.99Trimethylene dibromide-ethylene bromide 1.99to2.18Ethylene bromide-bromoform2.18to2.89three values.When a cathetometer is used,measure the height of the floats and specimens from an arbitrary level using a line through their center of volume.If equilibrium is not obtained,the specimen may be imbibing the liquid.9.3Old samples can be removed without destroying the gradient by slowly withdrawing a wire screen basket attached to a long wire (Note 6).This can be conveniently done by means of a clock motor.Withdraw the basket from the bottom of the tube and,after cleaning,return it to the bottom of the tube.It is essential that this procedure be performed at a slow enough rate (approximately 30min/300-mm length of column)so that the density gradient is not disturbed.N OTE 6—Whenever it is observed that air bubbles are collecting on samples in the column,a vacuum applied to the column will correct this.10.Calculation10.1The densities of the samples may be determined graphically or by calculation from the levels to which the samples settle by either of the following methods:10.1.1Graphical Calculation —Plot float position versus float density on a chart large enough to be read accurately to 61mm and the desired precision of density.Plot the positions of the unknown specimens on the chart and read their corre-sponding densities.10.1.2Numerical Calculation —Calculate the density by interpolation as follows:Density at x 5a 1[~x 2y !~b 2a !/~z 2y !#(2)where:a andb =densities of the two standard floats,y and z =distances of the two standards,a and b ,respec-tively,bracketing the unknown measured from an arbitrary level,andx =distance of unknown above the same arbitrarylevel.11.Report11.1Report the following information:11.1.1Density reported as D 23C ,in grams per cubic centimetre,as the average for three representative test speci-mens,11.1.2Number of specimens tested if different than three,11.1.3Sensitivity of density gradient in grams per cubic centimetre per millimetre,11.1.4Complete identification of the material tested,and 11.1.5Date of the test.12.Precision and Bias 612.1Specimens Molded in One Laboratory and Tested in Several Laboratories —An interlaboratory test was run in 1981in which randomized density plaques were supplied to 22laboratories.Four polyethylene samples of nominal densities of 0.92to 0.96g/cm 3were molded in one laboratory.The data were analyzed using Practice E 691,and the results are given in Table 2.12.2Specimens Molded and Tested in Several Laboratories :12.2.1Samples Prepared Using Practice D 4703in Each Laboratory —Table 3is based on a round robin 9conducted in 1994in accordance with Practice E 691,involving seven materials tested by 7to 11laboratories.For each material,all of the samples were prepared by each laboratory,molded in accordance with Procedure C of Annex A1of Practice D 4703,and tested using this test method.The data are for comparison with the data of the same samples tested by Practice D 2839.Each test result is an individual determination.Each laboratory obtained six test results for each material.12.2.2Samples Prepared Using Practice D 2839in Each Laboratory —Table 4is based on a round robin 9conducted in 1994in accordance with Practice E 691,involving seven materials tested by 10to 15laboratories.For each material,all of the samples were prepared by each laboratory in accordance with Practice D 2839.Each test result is an individual deter-mination.Each laboratory obtained six test results for each material.12.3Concept of r and R —Warning—The following expla-nations of r and R (12.3-12.3.3)are only intended to present a meaningful way of considering the approximate precision of this test method.The data in Table 1should not be rigorously applied to acceptance or rejection of material,as those data are specific to the round robin and may not be representative of other lots,conditions,materials,or ers of this test method should apply the principles outlined in Practice E 691to generate data specific to their laboratory and materi-als,or between specific laboratories.The principles of 12.3-12.3.3would then be valid for each data.If S r and S R have been calculated from a large enough body of data,and for test results that were averages from testing one specimen:12.3.1Repeatability Limit,r (Comparing two test results for the same material,obtained by the same operator using the6Supporting data are available from ASTM Headquarters.Request RR:D20-1123.TABLE 2Precision Data Summary—Polyethylene DensityMaterial Average Density,g/cm 3S r A S R B r C R D 10.91960.000290.001060.000820.004520.93190.000120.000800.000340.002330.95270.000330.001160.000930.003340.96230.000620.001140.001800.0033AS r =within-laboratory standard deviation for the indicated material.It is obtained by pooling the within-laboratory standard deviations of the test results from all of the participating laboratories.BS R =between-laboratories reproducibility,expressed as standard deviation,for the indicated material.Cr =within-laboratory repeatability limit =2.8S r .DR =between-laboratories reproducibility limit =2.8S R.same equipment on the same day)—The two test results should be judged not equivalent if they differ by more than the r value for that material.12.3.2Reproducibility Limit,R (Comparing two test results for the same material,obtained by different operators using different equipment in different laboratories)—The two test results should be judged not equivalent if they differ by more than the R value for that material.12.3.3Any judgment in accordance with 12.2.1or 12.2.2would have an approximate 95%(0.95)probability of being correct.12.3.4Bias —There are no recognized standards by which to estimate the bias of this test method.13.Keywords13.1density;film;gradient;plaque;polyolefins;polyeth-ylene;polypropylene;preparationAPPENDIXES(Nonmandatory Information)X1.FLOAT CALIBRATION—ALTERNATIVE TEST METHODX1.1This test method of float calibration has been found by one laboratory to save time and give the same accuracy as the standard test method.Its reliability has not been demon-strated by round-robin data.X1.1.1Prepare a homogeneous solution whose density is fairly close to that of the float in question.X1.1.2Fill a graduate about 3⁄4full with the solution,drop in the float,stopper,and place in a thermostatted water bath near 23°C.Fill a tared two-arm pycnometer (Test Method D 941,or equivalent)with the solution.Place the pycnometer in the bath.X1.1.3Vary the bath temperature until the solution density is very near to that of the float.(If the float was initially on the bottom of the graduate,lower the bath temperature until the float rises;if the float floated initially,raise the bath tempera-ture until the float sinks to the bottom.)X1.1.4Change the bath temperature in the appropriate direction in increments corresponding to solution density increments of about 0.0001g/cm 3until the float reverses direction of movement as a result of the last change.This must be done slowly (at least 15-min intervals between incremental changes on the temperature controller).Read the volume of liquid in the pycnometer.X1.1.5Change the bath temperature in increments in the opposite direction,as above,until a change in the float position again occurs.Read the volume of liquid in the pycnometer.N OTE X1.1—The float should rise off the bottom of its own volition.As a precaution against surface tension effects when the float is floating,the float should be pushed about halfway down in the liquid column and then observed as to whether it rises or falls.For this purpose,a length of Nichrome wire,with a small loop on the lower end and an inch or so of length extending above the liquid surface,is kept within the graduate throughout the course of the run.To push a floating float down,the cylinder is unstoppered and the upper wire end grasped with tweezers for the manipulations.The cylinder is then quickly restoppered.X1.1.6Remove the pycnometer from the bath,dry the outside,and set aside until the temperature reaches ambient temperature.Weigh and calculate the “in vacuo”mass of solution to ing the average of the two observed solution volumes,calculate the density of the solution to 0.0001g/cm 3.This solution density is also the float density.X1.1.7The pycnometer used should be calibrated for vol-ume from the 23°C calibration,although the reading is taken at a different temperature.The alternative test method is based on a number of unsupported assumptions but generally gives the same results as that described in 7.2within the accuracyTABLE 3Precision Data—Density,g/cm 3Material Number ofLaboratoriesDensity,g/cm 3S r A S R B r C R DB 70.91390.000290.000880.000810.00245F 80.91770.000180.000790.000510.00221G 80.92200.000280.000710.000780.00197A 110.93560.000360.001050.001000.00294E 110.95280.000460.001180.001290.00331C 100.96190.001000.001000.001030.00281D90.96330.000360.001370.001010.00384AS r =within-laboratory standard deviation for the indicated material.It is obtained by pooling the within-laboratory standard deviations of the test results from all of the participating laboratories.BS R =between-laboratories reproducibility,expressed as standard deviation,for the indicated material.Cr =within-laboratory repeatability limit =2.8S r .DR =between-laboratories reproducibility limit =2.8S R .TABLE 4Density,g/cm 3,Samples Prepared in Accordance WithPractice D 2839MaterialNumber ofLaboratoriesDensity,g/cm 3S r A S R B r C R D B 100.91390.000260.000780.000720.00219F 120.91790.000200.000780.000550.00220G 130.92220.000300.000730.000850.00206A 150.93570.000410.000800.001150.00225E 140.95300.000390.000920.001090.00258C 110.96150.000300.000730.000850.00206D100.96260.000530.001090.001480.00305AS r =within-laboratory standard deviation for the indicated material.It is obtained by pooling the within-laboratory standard deviations of the test results from all of the participating laboratories.BS R =between-laboratories reproducibility,expressed as standard deviation,for the indicated material.Cr =within-laboratory repeatability limit =2.8S r .DR =between-laboratories reproducibility limit =2.8S R.required.In case of disagreement,the method described in7.2shall be the referee method.X2.GRADIENT TUBE PREPARATIONX2.1Method A—Stepwise Addition:X2.1.1Using the two liquids that will give the desireddensity range,and sensitivity(S)in grams per cubic centimetreper millimetre,prepare four or more solutions such that eachdiffers from the next heavier by80S g/cm3.The number ofsolutions will depend upon the desired density range of thecolumn and shall be determined as follows:Numbers of solutions to prepare density2gradient(X2.1)column~Note X2.1!5~11D22D1!/80S(X2.1)where:D2=upper limit of density range desired,D1=lower limit of density range desired,andS=sensitivity,in grams per cubic centimetre per milli-metre.N OTE X2.1—Correct the value of(1+D2−D1)/80S to the nearestwhole number.To prepare these solutions,proceed as follows:Using the hydrometers,mix the two liquids in the proportions necessary to obtain the desired solutions.Remove the dissolved air from the solutions by gentle heating or an applied vacuum.Then check the density of the solutions at2360.1°C by means of the hydrometers and,if necessary,add the appropriate air-free liquid until the desired density is obtained.N OTE X2.2—Where aqueous mixtures are used,0.5%aqueous sodium acetate should be used to prepare the mixture.This reduces the formation of bubbles from dissolution.N OTE X2.3—In order to obtain a linear gradient in the tube,it is very important that the solutions be homogeneous and at the same temperature when their densities are determined.It is also important that the density difference between the solutions consecutively introduced into the tube be equal.X2.1.2By means of a siphon or pipet,fill the gradient tube with an equal volume of each liquid starting with the heaviest, taking appropriate measures to prevent air from being dis-solved in the liquid.After the addition of the heaviest liquid, very carefully and slowly pour an equal volume of the second heaviest liquid down the side of the column by holding the siphon or pipet against the side of the tube at a slight angle. Avoid excess agitation and turbulence.In this manner,the “building”of the tube shall be completed.N OTE X2.4—Density gradients may also be prepared by reversing the procedure described in X2.1.1and X2.1.2.When this procedure is used, the lightest solution is placed in the tube and the next lightest solution is very carefully and slowly“placed”in the bottom of the tube by means of a pipet or siphon which just touches the bottom of the tube.In this manner the“building”of the tube shall be completed.X2.1.3If the tube is not already in a constant-temperature bath,transfer the tube,with as little agitation as possible,to the constant-temperature bath maintained at2360.1°C.The bath level should approximately equal that of the solution in the tube,and provision should be made for vibrationless mounting of the tube.X2.1.4For every254mm of length of tube,dip a minimum offive clean calibratedfloats,spanning the effective range of the column,into the less dense solvent used in the preparation of the gradient tube and add them to the tube.By means of a stirrer(for example,a small coiled wire or other appropriate stirring device)mix the different layers of the tube gently by stirring horizontally until the least dense and most densefloats span the required range of the gradient tube.If,at this time,it is observed that thefloats are“bunched”together and not spread out evenly in the tube,discard the solution and repeat the procedure.Then cap the tube and keep it in the constant-temperature bath for a minimum of24h.X2.1.5At the end of this time,plot the density offloats versus the height offloats to observe whether or not a fairly smooth and nearly linear curve is obtained.Some small irregularities may be seen,but they should be slight.Whenever an irregular curve is obtained,the solution in the tube shall be discarded and a new gradient prepared.N OTE X2.5—Gradient systems may remain stable for several months. X2.2Method B—Continuous Filling with Liquid Entering Gradient Tube Becoming Progressively Less Dense:X2.2.1Assemble the apparatus as shown in Fig.X2.1,using beakers of the same diameter.Then select an appropriate amount of two suitable liquids which previously have been carefully deaerated by gentle heating or an applied vacuum. Typical liquid systems for density-gradient tubes are listed in Table1.The volume of the more dense liquid used in the mixer (Beaker B shown in Fig.X2.1)must be equal to at least one half of the total volume desired in the gradient tube.An FIG.X2.1Apparatus for Gradient TubePreparationestimate of the volume of the less dense liquid required in Beaker A to establishflow from A to B can be obtained from the following inequality:V A.d B V B/d A(X2.2) where:V A=starting liquid volume in Beaker A,V B=starting liquid volume in Beaker B,d A=density of the starting liquid in Beaker A,andd B=density of the starting liquid in Beaker B.A small excess(not exceeding5%)over the amount indicated by the preceding equality will induce the required flow from A toB and yield a very nearly linear gradient column.X2.2.2Place an appropriate volume of the denser liquid into Beaker B of suitable size.Prime the siphon between Beaker B and the gradient tube with liquid from Beaker B and then close the stopcock.The delivery end of this siphon should be equipped with a capillary tip forflow control.N OTE X2.6—Techniques acceptable for transfer of liquid into the gradient tube are siphon/gravity,vacuum-filling,use of a peristatic pump, or any other technique useful to transfer liquids in a controlled manner.It is important to control theflow in order to maintain a desirable gradient. X2.2.3Place an appropriate volume of the less dense liquid into Beaker A.Prime the siphon between Beakers A and B with the liquid from Beaker A and close the stopcock.Start the highspeed,propeller-type stirrer in Beaker B and adjust the speed of stirring such that the surface of the liquid does not fluctuate greatly.X2.2.4Start the delivery of the liquid to the gradient tube by opening the necessary siphon-tube stopcocks simultaneously. Adjust theflow of liquid into the gradient tube at a very slow rate,permitting the liquid toflow down the side of the tube.Fill the tube to the desired level.N OTE X2.7—Preparation of a suitable gradient tube may require1to 11⁄2h or longer,depending upon the volume required in the gradient tube. X2.3Method C—Continuous Filling with Liquid Entering Gradient Tube Becoming Progressively More Dense:X2.3.1This method is essentially the same as Method B with the following exceptions:X2.3.2The lighter of the two liquids is placed in Beaker B. X2.3.3The liquid introduced into the gradient column is introduced at the bottom of the column.Thefirst liquid introduced is the lighter end of the gradient and is constantly pushed up in the tube as the liquid being introduced becomes progressively heavier.X2.3.4The liquid from Beaker A must be introduced into Beaker B by directflow from the bottom of Beaker A to the bottom of Beaker B,rather than being siphoned over as it is in Method B.Filling the tube by this method may be done more rapidly than by Methods A or B.The stopcock between Containers A and B should be of equal or larger bore than the outlet stopcock.A schematic drawing of the apparatus for Method C is shown in Fig.X2.2.REFERENCES (1)Linderstrøm-Lang,K.,“Dilatometric Ultra-Micro-Estimation of Pep-tidase Activity,”Nature,NATRA,V ol139,1937,p.713.(2)Linderstrøm-Lang,K.,and Lanz,H.,“Enzymic Histochemistry XXIXDilatometric Micro-Determination of Peptidase Activity,”Comptesrendus des gravaus de laboratorie Carlsberg,Serie Chimique,V ol21,1938,p.315.(3)Linderstrøm-Lang,K.,Jacobsen,O.,and Johansen,G.,“Measurementof the Deuterium Content in Mixtures of H2O and D2O,”ibid.,V ol23,1938,p.17.(4)Jacobsen,C.F.,and Linderstrøm-Lang,K.,“Method for Rapid Deter-mination of Specific Gravity,”Acta Physiologica Scandinavica,AP-SCA,V ol1,1940,p.149.(5)Boyer,R.F.,Spencer,R.S.,and Wiley,R.M.,“Use of Density-Gradient Tube in the Study of High Polymers,”Journal of Polymer Science,JPSCA,V ol1,1946,p.249.(6)Anfinsen,C.,“Preparation and Measurement of Isotopic Tracers:ASymposium Prepared for the Isotope Research Group,”Edwards,J.W.,Publishers,Ann Arbor,MI,1946,p.61.(7)Tessler,S.,Woodberry,N.T.,and Mark,H.,“Application of theDensity-Gradient Tube in Fiber Research,”Journal of Polymer Sci-ence,JPSCA,V ol1,1946,p.437.(8)Low,B.W.,and Richards,F.M.,“The Use of the Gradient Tube forthe Determination of Crystal Densities,”Journal of the American Chemical Society,JACSA,V ol74,1952,p.1660.(9)Sperati,C.A.,Franta,W.A.,and Starkweather,H.W.,Jr.,“TheMolecular Structure of Polyethylene V,the Effect of Chain Branching and Molecular Weight on Physical Properties,”Journal of the Ameri-can Chemical Society,JACSA,V ol75,1953,p.6127.(10)Tung,L.H.,and Taylor,W.C.,“An Improved Method of PreparingDensity Gradient Tubes,”Journal of Polymer Science,JPSCA,V ol 21,1956,p.144.(11)Mills,J.M.,“A Rapid Method of Construction Linear DensityGradient Columns,”Journal of Polymer Science,V ol19,1956,p.585.(12)Wiley,R.E.,“Setting Up a Density Gradient Laboratory,”PlasticsTechnology,PLTEA,V ol8,No.3,1962,p.31.FIG.X2.2Apparatus for Gradient TubePreparation。

塑料密度快速测定法与3h(小时)密度梯度法的对比实验摘要:密度是塑料的一个特征物理量,不同的塑料具有不同的密度范围。
对于HDPE,LLDPE等,ASTM,ISO,GB均有相应测定标准,这些标准检测周期比较长,测试相对比较麻烦。
作者进行密度快速测定法、与3h密度梯度法在HDPE和LLDPE的对比实验的研究。
根据实验结果分析得出:快速密度测定法与3h密度梯度法存在规定差值,即:0.0020-0.0030之间,在精确度要求不高的情况下,快速密度测定法可以代替3h密度梯度法以更快的速度、更少的二次污染来测定塑料密度。
关键词:塑料密度快速测定法一、仪器与试剂密度梯度计:英国RAY-RAN公司型号RR/DGA1恒温水浴:温度波动不大于士0.1℃。
密度计:密度范围适合配管密度的要求,精确到士0.001 g/cm3。
密度梯度管:刻有精确分度的玻璃管,直径不小于40mm,长不小于250mm。
标准玻璃浮标:经过精确校正,密度范围适合配管要求熔融指数仪:挤出样条异丙醇二、试样制备3h密度梯度法:用熔融指数样条作为密度样条,对于LLDPE待样条凝固后经400ml蒸馏水煮沸30min,对于HDPE经200ml沸水煮沸30min,待样条自然冷却至室温,用单面刀片截取样条中间3~5mm作为密度样条。
快速法:用熔融指数样条作为密度样条,直接冷却至室温后,用单面刀片截取样条中间3~5mm作为密度样条。
三、密度梯度柱配制用两个尺寸相同的玻璃容器,按GB/T 1033-2010中规定【1】,配制成相应的重液和轻液【2】,脱除气饱,起动电磁搅拌器,均匀搅拌,使混合液缓缓沿着梯度管壁流入管中,直至所需液位。
根据所需密度范围,选用5个以上的标准玻璃浮子,沿壁轻轻放入梯度柱中,使这一组标准玻璃浮子均匀分布于梯度柱的有效范围内。
将自己制好的密度梯度柱放在温度23±0.1℃下静置不少于2h待浮子位置稳定后,测量每个浮子的几何中心高度,精确到1mm。

塑料熔体(质量、体积)流动速率及熔体密度的测定摘要介绍塑料熔体(质量、体积)流动速率、熔体密度的测定方法及熔体流动速率比、表观粘度的计算。
关键词熔体流动速率熔体密度熔体流动速率比表观粘度熔体流动速率,原称熔融指数,其定义为:在规定条件下,一定时间内挤出的热塑性物料的量,也即熔体每10min通过标准口模毛细管的质量,用MFR表示,单位为g/10min。
熔体流动速率可表征热塑性塑料在熔融状态下的粘流特性,对保证热塑性塑料及其制品的质量,对调整生产工艺,都有重要的指导意义。
近年来,熔体流动速率从“质量”的概念上,又引伸到“体积”的概念上,即增加了熔体体积流动速率。
其定义为:熔体每10min通过标准口模毛细管的体积,用MVR表示,单位为cm3/10min[1]。
从体积的角度出发,对表征热塑性塑料在熔融状态下的粘流特性,对调整生产工艺,又提供了一个科学的指导参数。
对于原先的熔体流动速率,则明确地称其为熔体质量流动速率,仍记为MFR。
熔体质量流动速率与熔体体积流动速率已在最近的ISO标准中明确提出,我国的标准也将作相应修订,而在进出口业务中,熔体体积流动速率的测定也将很快得到应用。
1 熔体质量流动速率(MFR)的测定方法熔体质量流动速率的测定,按方法分为切割(手工或自动定时)测定与自动(半自动)测定。
1.1 切割测定根据定义,当熔体在负荷的作用下通过口模毛细管挤出,由操作人员使用切割刀具将流经口模出口的一段熔料割取,并记录该段熔料自口模流出的时间,经称重并换算至流出时间为10min时的质量,即为熔体质量流动速率值MFR。
配置有自动定时切割装置的设备,可根据需要设置切割间隔时间。
任何型号的熔体流动速率测定仪都可进行手工切割测定。
1.2 自动(半自动)测定自动(半自动)测定不需对流出熔料进行切割。
它的原理是:在测定仪上预先设定熔料的流出体积,再由测定仪上的计时器自动记录流出该体积的熔料所需的时间。
这样,只要知道熔料的密度(注意:是该材料在特定试验温度下的熔体密度),即可按(1)式计算出熔体质量流动速率:式中:L───测定仪预先设定的活塞移动有效距离,cm;ρ──熔体密度,g/cm3;t───活塞移动有效距离所需的时间,s。

ASTM化工标准目录(2004)(上)1 新制定标准涂料、塑料、橡胶及石油产品等D6943-03 工业防护涂料浸没试验规程D6990-03 船舶涂料系统污垢热阻和物理特性的评价规程D6989-03 溶剂基和水基油墨树脂溶液的制备规程D6976-03 橡胶避孕阴道隔膜规格E2313-03 一甘醇酯中醛的分光光度试验方法D6977-04 医用氯丁橡胶检验手套规格D6891-03 序列JVA火花点火发动机中评价汽车发动机油的试验方法D6969-03 分析用煅烧石油焦炭样品的制备规程D6970-03 分析用煅烧石油焦炭样品的收集规程D6973-03 在高压恒容叶片泵中标明石油水力流体耐磨性的试验方法D6974-03 液体燃料中能生活的细菌和霉菌的计数规程过滤和培养方法D6984-03 在序列ⅢF火花点火发动机中评价汽车发动机油的试验方法D 6986-03 航空燃料中游离水分、颗料和其它污染的试验方法目视检测法D6968-03 用气相色谱仪和原子发射检测装置同时测定天然气和气体燃料中硫化合物和微量烃的试验方法D6901-03 美术用的有色铅笔规格D6957-03 漆刷填充材料里卷曲物的测量规程D6979-03 聚氨酯原料的试验方法:多元醇碱度的测定方法,以氮百分率表示F2263-03 评价三通(T)的试验方法D6988-03 塑料薄膜试样厚度的测定指南D6866-04 用放射性碳和同位素比质量计分析自然分布区物质的生物基含量试验方法F2330-04 评价多层壁聚烯烃塑料管道对热氯化水的氧化稳定性试验方法2新修订标准第06卷涂料及有关涂层等D2065-03 在表面活性剂促进水分吸收条件下测定复合木材产品边棱性能的试验方法D2697-03 透明或着色涂料中不挥发分体积的测试方法D4827-03 胶乳中未反应单体的毛细管柱气相色谱测定方法D4834-03 涂料中铅含量的直接抽气原子吸收光谱测定方法D2066-03 糊状印刷油墨分散体的相对着色力测试方法D 5324-03 水型建筑涂料的检验指南D2803-03 金属有机涂层耐丝状锈蚀的试验指南D 5401-03 木制品用透明防水涂料的评价试验方法D 1640-03 室温下有机涂料干燥、老化或膜形成的试验方法D 5146-03 溶剂型建筑涂料的测试指南D1439-03 羧甲基纤维素钠的试验方法D1726-03 液体环氧树脂的可水解氯化物含量的试验方法D 5400-03 羟丙基纤维素的试验方法D5201-03 a 涂料及涂层配方物理常数的计算规程D2199-03 增速剂从乙烯基织物迁移至喷漆的测定方法D 5098-03 丙烯树脂乳液绘画漆规格D5722—03 用聚焦阳光和浸泡冷冻融化方法进行成批涂布压花硬质板的室外耐候性加速试验实施规程D6279—03 高光泽涂层耐擦伤性的试验方法D1078—03 挥发性有机液体馏程的试验方法D1613—03 用于涂料、清漆、喷漆及有关产品的挥发性溶剂和化学中间体中酸度的试验方法D1718—03 乙酸异丁酯(95%级)规格D2086—03 乙烯基乙酸酯和乙醛中酸度的试验方法D3130—03 乙酸正丙酯(96%级)规格D3728—03 乙酸2-乙氧基乙酯(99%级)规格D4835—03 丙二醇单甲醚乙酸酯规格第08卷塑料工艺D785—03 塑料及电绝缘材料洛氏硬度的测定方法D3895—03 用扫描差示热量法测定烯烃氧化诱导时间的试验方法D4662—03 聚氨酯原料的试验方法:多元醇的酸、碱值的测定D5491—03 用作模塑料及挤压料的回收聚乙烯薄膜原料的分类D5988—03 测定土壤中塑料材料或堆肥后残留塑料材料的有氧生物降解试验方法D1922—03 a 塑料薄膜和薄板抗撕裂扩展性的试验方法摆锤法D3262—03 玻璃纤维增强热固性树脂排污管规格D3517—03 玻璃纤维增强热固性树脂耐压管规格D3839—02ε1玻璃纤维增强热固性树脂管地下安装指南D2657—03 聚烯烃管及配件的热融接合规程D3035—03 基于控制外径聚乙烯(PE)塑料管(DrPR)规格F2019—03 用拉到原地玻璃增强塑料装置、原地固化热塑性树脂管进行现有管线及导管的修理规程F2513—03b 热塑性气体耐压管、软管及配件规格D1505—03 塑料密度测定方法密度梯度法D2343—03 增强塑料用玻璃纤维单纱,纱线和粗纱抗拉特性的试验方法D1622—03 硬泡沫塑料表观密度的测定方法D 3647—03 根据成分对增强塑料拉挤成型的型材分类的规程D 410l—03 b 聚丙烯注射及挤压材料规格D 3296—03 (FEP)氟化乙丙烯碳氟化合物管材规格D4000—03a 给定塑性材料的分类体系D4067—03 采用ASTM方法的增强和填充聚苯硫(PPS)注射模塑和挤压材料的分类体系D2291—03 玻璃树脂复合材料用环形试样的制备规程D4476—03 测定用纤维增强拉挤塑料棒抗弯性能的试验方法D4059—03 聚氨酯原材料的试验方法:异氰酸酯比重的测定方法D4878—03 聚氨酯原材料的试验方法:多元醇粘度的测定方法D5117—03 坚实的玻璃纤维增强挤拔料的染料渗透性试验方法D 5336—03 聚酞酰胺(PPA)注射模塑料规格D 5857—03 b 用ISO标准草案和方法的聚丙烯注射和挤压料规格D6289—03 测量模制热固性塑料由模型尺寸收缩的试验方法D 6778—03 聚甲醛(POM)模塑料和挤压料的分类D4661—03 聚氨酯原材料的试验方法:异氰酸酯中总氯量的测定方法D 5204—03 聚酰胺-酰亚胺模塑料及挤压料的分类体系D 3982—03 接触模压的玻璃纤维增强热固性树脂导管及通风橱罩的规格D 6864—03a 固体有色塑料壁板制品的颜色和外观稳定性规格F1807—03 SDK 9交联聚乙烯(PEX)管用的由铜卷曲环金属插入配件规格D256—03 塑料摆式抗冲击性的试验方法D638-03 塑料抗张性能的试验方法D3418-03 用扫描差示热量计测定聚合物转变温度的试验方法D4272-03 用锥体坠落器测定塑料薄膜耐总能量冲击的试验方法D2578-04 聚乙烯和聚丙烯薄膜润湿张力的试验方法D3307-04 全氟烷氧基(PFA) 碳氟化合物模塑料及挤压料规格D2241-04 聚氯乙烯额定压力管(SDR系列)规格D2513-04 热塑性气体耐压管、软管及配件规格F905-03 聚乙烯鞍型熔融接头质量检验规程F1335-04 高温设备用额定压力复合材料管和配件规格F3035-03a 依据控制外径的Dr-Br聚乙烯管规格D788-04 聚甲基丙烯酸甲酯模塑料及挤压料的分类体系D3014-04 硬质热固性泡沫塑料在垂直状态时火焰高度、燃烧时间和重量损失的试验方法D 3159-04 改性ETFE—含氟聚合物模塑料及挤压料规格D3368-04 (FEP)氟化乙丙烯炭氟化合物树脂薄板及薄膜规格D4000-04 指定塑性材料的分类体系D4894-04 聚四氟乙烯(PTFE)粒状模塑料及柱状挤压料规格D 5857-04 用ISO草案及方法的丙烯塑料注型料及挤压料规格D5946-04 采用水触角测量法对电晕处理过聚合物薄膜的试验方法D6339-04 间同(立构)聚苯乙烯模塑料及挤压料规格D2665-04 聚氯乙烯塑料排水、排污和通风管及配件规格F1803-04 依据控制内径的聚氯乙烯封闭异型重力管及配件规格D2103-03 聚乙烯薄膜及薄板规格D2457-03 塑料薄膜及固体塑料镜面光泽的试验方法D2732-03 测定塑料薄膜自由线性热收缩性能的试验方法D 4101-03 a 聚丙烯注塑料及挤压料规格D 3748-03 评价高密度硬质多孔塑料规程D1004-03 塑料薄膜及薄板抗撕裂(Graves撕裂)的试验方法D4549-03 聚苯乙烯及橡胶改性聚苯乙烯模塑料及挤压料(PS)规格D6338-03 高度交联热塑性硫化产品(HCT—PVS)分类体系D 5740-03 依照分类D4000书写材料标准的指南D 3679-03ε1硬质聚氯乙烯档板规格F794-03 受控内径的聚氯乙烯(PVC)异型动力污水管和配件规格F1281-03 交联聚乙烯/铝佼联聚乙烯(Pex-Al-pex)压力管规格F1282-03 聚乙烯—铝—聚乙烯(PE-A1-PE)复合压力管规格F949-03 光滑内壁聚氯乙烯(PVC)波纹污水管和配件规格D2665-02ε1聚氯乙烯(PVC)塑料排水管、废水管及通风管以及配件规格第09卷橡胶工业及其它D3568-03 橡胶评价乙烯-丙烯—二烯烃三元共聚物(EPDM)(包括用油混炼)的试验方法D3848-03 橡胶评价掺和炭黑的丙烯腈-丁二烯共聚物(NBR)的试验方法D5900-03 工业参比物质(IRM)的物理和化学特性规格D1417-03 a合成橡胶胶乳的试验方法D3566-03 在氯存在下用氧燃烧法测定橡胶溴含量的操作规程D2000-03 汽车用橡胶制品的分类体系D1510-03 炭黑的试验方法碘吸附值D1799-03 a 炭黑操作规程成包装运货物的采样D3765-03a 炭黑的试验方法十六烷基三甲基溴化铵(CTAB)表面积D6915-03a 炭黑操作规程标准参比炭黑的评价D429-03 橡胶性能的试验方法与刚性基底的粘合性D6746-03 生橡胶及未硫化化合物的湿抗拉强度的试验方法D4821-03a 炭黑试验方法精确度和偏差的确认指南D 5817-03a 颗粒炭黑试验用总样的缩分、混合和干燥的操作规程F1364-03 使用校准设备验证干涉激光成象无损轮胎检验系统检验能力的规程D3767-03 橡胶尺寸的测量规程D1566-04 有关橡胶的术语D1646-04 橡胶试验方法用门尼粘度计测定粘度、应力松弛及预硫化性能的试验方法D2000-03a 汽车用橡胶制品分类体系D3493-04 炭黑试验方法压制样的油吸收值试验方法D1765-04 橡胶制品用炭黑分类体系D5230-04 炭黑试验方法自动单粒硬度D6602-03b 采样和检验炭黑的易排放物和、或其它环境颗粒物的操作规程D3194-04 天然橡胶塑性保持值(PRI)的试验方法D4483-03 橡胶及炭黑制造工业评价试验方法标准精确度的规程第15卷胶粘剂等D 5363-03 厌氧的单一成分胶粘剂规格D6005-03 测定地毯胶粘剂抗滑动性的试验方法D6471-03 汽车和轻型货车用最小预稀释到50体积乙二醇基发动机冷却剂溶液规格D6472-03 汽车和轻型货车用提浓回收乙二醇基发动机冷却剂规格E1226-00ε1可燃粉剂的压力和压力提高率的试验方法E2019-03ε1空气中尘雾最小点火能量的试验方法D6586-03 在水系中GAC上,用快速小型柱子试验预告污染物吸附的规程D6446-03 测定粒状和丸状活性炭快速硫化氢突破容量的试验方法D4995-04 电子级和脱脂级1,1,2-三氯1,2,2-三氟乙烷溶剂规格D 5248-04 再生1,1,2—三氯1,2,2—三氟乙烷规格D 5396-04 再生全氯乙烯规格D 5646-04 再生三氯乙烯规格D 905-03 在压缩负荷下粘合体剪切强度的试验方法D950-03 粘合体冲击强度的试验方法D 3167-03 a 胶粘剂抗浮辊撕裂性能的试验方法E180-03 工业和特殊化学品分析和检验用ASTM方法准确度的确定规程第05卷石油产品等D 341-03 液体石油产品粘度温度关系曲线图D 665-03 加抑制剂的矿物油水存在下防锈特性的试验方法D 910-03a 航空汽油规格D1319-03 液体石油产品中烃类型的试验方法荧光指示剂吸附法D1655-03a航空涡轮机燃料规格D1835-03a 液化石油气规格D1840-03 航空涡轮机燃料中萘烃的试验方法紫外线分光光度法D 2274-03 a 馏分燃料油氧化稳定性的试验方法加速法D2532-03 航空涡轮机润滑剂粘度及承受低温后粘度变化的试验方法D2887-03 石油馏分沸程分布的试验方法气相色谱法D2892-03a 原油的蒸馏试验方法15块理论塔板蒸馏塔法D2896-03 石油产品碱值的试验方法高氯酸电位滴定法D 3228-03 润滑油和燃料油中总氮的试验方法改进的克揶达法D 3427-03 石油空气释放性的试验方法D4171-03 燃料系统用的防冻剂规格D4294—03 石油和石油产品中硫的试验方法能量分散x射线荧光光度法D4422—03 石油焦炭分析中灰分的试验方法D4485—03a 发动机油的性能规格D4625—03 柴油燃料在43℃(110‘F)贮存稳定性的试验方法D4693—03 加脂润滑的车轮轴承低温扭矩的试验方法D4814—03a 汽车火花点火发动机燃料规格D4857—03 非弦外操舟机用二冲程汽油发动机润滑油最大限度减小环粘接和活塞沉渍物能力的试验方法D 5191—03 石油产品蒸气压的试验方法微量法D5275—03 含流体聚合物的燃料注入器剪叨稳定性试验(FISST)方法D5001—03 航空涡轮机燃料润滑性测定方法用球在圆柱体润滑性评价器(BOCLE)法D 5006—03 航空燃料中燃料系统醚型防冻剂的测定方法D 5704—03 a 人工传动装置和最终驱动轴用润滑油热及氧化稳定性的评价试验方法D5705—03 残留燃料油上方的气相中硫化氢测定方法D 5800—03 a 润滑油蒸发损失的试验方法阿科(Noack)法D 5862—03 二冲程循环涡轮增压6V92TA2柴油发动机中评价发动机油的试验方法D 6121—03a 评价准双曲面凿轮驱动轴用润滑剂在低速和高扭矩条件下承载能力的试验方法D6352—03 在174 ℃~700 ℃沸腾范围内用气相色谱法测定石油馏分的沸程分布的试验方法D 6377—03 测定原油蒸气压的试验方法:VPCRx(膨胀法)D6378—03 测定石油产品、烃和烃—氧化混合物的蒸气压(VPx)的试验方法(三级膨胀法)D6424—03 天然吸气的火花点火航空发动机辛烷值的实施规程D 6447—03 航空涡轮燃料氢过氧化值的试验方法伏安分析法D6557—03 评价汽车发动机油防锈性能的试验方法D 6593—03 a 在汽油为燃料的火花点火内燃发动机内,低温、轻负载的运行条件下,评价汽车发动机油抑制沉渍物形成的试验方法D6594—03 评价柴油发动机油在135℃腐蚀性的试验方法D6709—03 在序列Ⅷ火花点火发动机(CLR油试验发动机)中,评价汽车发动机油的试验方法D6751—03 a 中间馏分燃料用的生物柴油燃料掺油料B100规格D6812—03 汽轮增压和增加负荷的火花点火航空发动机的地面辛烷值实施规程D 6837—03 在序列VIB火花点火发动机中测量汽车发动机油对客车和轻型货车节约燃料效果的试验方法D 6897—03 a 液化石油气(LPG)蒸气压的试验方法(膨胀法)D2700—03b 火花点火发动机燃料的马达辛烷值的试验方法D1977—03 用氢氟酸/硫酸分解和原子光谱分析法测定FCC平衡催化剂中镍和钒的试验方法D3663—03 催化剂和催化剂载体表面积的试验方法D 3908—03 容量真空法测定有载体的铂催化剂氢化学吸附性能的试验方法D4164—03 成型催化剂及催化剂载体机械轻击的堆积密度试验方法D41S0—03 成型催化剂及催化剂载体的振动堆积密度试验方法D4222—03 用静态容量测定法测定催化剂和催化剂载体氮吸附和脱附等温线的试验方法D4512—03 细催化剂和催化剂载体颗粒及粉体的振动表观堆积密度试验方法D4567—03 连续流动法用氮吸附单点测定催化剂及催化剂载体比表面的试验方法D4781—03 细催化剂颗粒和催化剂载体颗粒机械轻击堆积密度的试验方法D4824—03 用氨化学吸附法测定催化剂酸度的试验方法D6175—03 挤出催化剂和催化剂载体颗粒径向压碎强度的试验方法D3907—03 用微活性试验法进行流化催化裂解(FCC)催化剂的试验方法D5154—03 测定(FCC)催化剂活性和选择性的微活性试验方法3新撤消标准涂料、塑料等D2571—95 木制家具用喷漆的试验指南D1347—72(1995) 甲基纤维素试验方法D1358—86(1995) 分光光度法测定脱水蓖麻油及其衍生物二烯值的试验方法D1466—86(1995) 涂料、清漆及有关物料中常用的液体油和脂肪酸的采样方法D1467—89(1995) 防护涂料中脂肪酸的试验方法D1615—60(1995) 醇酸树脂中甘油、乙二醇和季戊四醇的试验方法D1950—86(1995) 热聚合干性油中丙酮允许量的试验方法D1951—86(1995) 干性油和脂肪酸中灰分的试验方法D1952—86(1995) 定量测定干性油裂解的试验方法D1954—86(1995) 生亚麻子油中油脚的试验方法D1955—95(1995) 干性油凝胶时间的试验方法D1958—86(1995) 奥气油中氯仿不溶物的试验方法D1960—86(1995) 干性油加热损失的试验方法D1964—85(1995) 桐油质量的试验方法D1967—86(1995) 干性油加热后色度测定的试验方法D1983—90(1995) 甲基酯气液色谱法分析脂肪酸组分的试验方法D2078—86(1995) 脂肪族季铵氯化物碘值的试验方法D 3447—0l 卤化有机溶剂纯度的试验方法D4757—98 蒸气脱脂溶剂的标记规程D 5320—96(2000) 稳定的三氯乙烯和四氯乙烯中1,1,1—三氯乙烷及二氯甲烷含量的试验方法D 5223—03 发动机冷却剂级丙二醇规格D 5999—96再悬浮中胶粘剂无干扰的试验方法D2069—9L(1998) 海船用燃料规格D 3520—88(1998) 热处理液淬火时间的试验方法磁性淬火计法D5119—02 车用发动机油在CRC—L—38火花点火发动机中的评价方法D 5302—0l a 车用发动机油在火花点火内燃机中,用汽油作燃料、低温、轻负荷条件下,抑制沉渍物生成和磨损的评价试验方法D 5480—95(1999) 用气相色谱法测定发动机油挥发度的试验方法D 5533—98 车用发动机油在程序ⅢE火花点火发动机中的评价试验方法D 5844—98 车用发动机油抑制生锈的评价试验方法(程序ⅡD)D1848—88(1998) 室外用乳胶漆漆膜破裂特性记录的分类D284—88(1999) 氧化汞颜料的化学分析方法D604—81(1996)ε1硅藻土颜料的规格D 656—87(1999) 纯甲苯胺红调色剂规格D 719—91(1999) 硅藻土颜料的分析方法D 970—86(1999) 对位红和甲苯胺红颜料的试验方法D 3360—96 液体比重计法测定普通白色体质颜料粒度分布的试验方法2004年ASTM化工标准目录(中)1 新制定标准涂料、塑料、橡胶及石油产品等D7054-04 分布于北美涂料涂敷器用的延伸标杆警告标记规格D6865-04 丙烯腈苯乙烯丙烯酸酯(Asa)和丙烯腈乙丙二烯单体苯乙烯(Aes)塑料以及复合物的模塑料和挤压料分类体系D7026-04 通过炭同位素分析法确定物料生物基含量结果用的采样及报告指南D6954-04 在氧化和生物降解联合作用环境里降解的塑料曝露和试验指南D 7029-04 在180.0 ftiF(82.2 ffic)下测定不饱和聚酯及乙烯基酯反应性的试验方法F2389-04 压力分级聚丙烯(PP)管系统规格D7050-04 天然橡胶规程按照预示工艺性能的采样及捆包分类D7057-04 气相色谱法(外部标准)分析异丙苯的试验方法D7038-04 评价汽车齿轮润滑剂耐湿度腐蚀的试验方法D 7039-04 用单色波长分散X—射线荧光分光法测定汽油和柴油机燃料中硫含量的试验方法D 7044-04 可生物降解的耐火水力流体规格D 6985-04 燃料油中间馏分的规格海军用F 2331-04 测定用于热塑性螺纹管及配件材料的螺纹密封胶化学相容性的试验方法2新修订标准第06卷涂料及有关涂层等D2369-04 涂料挥发物含量的测试方法D3960-04 色漆和有关涂料中挥发性有机化合物(VOC)含量的测试方法D1725-04 树脂溶液粘度的测试方法D1353-03 色漆、清漆、喷漆及有关产品用挥发性溶剂中不挥发物质的测试方法D1720-03 硝酸纤维溶液中活性溶剂稀释比的试验方法D3329-03 气相色谱法测定甲基异丁基酮纯度的试验方法D1308-02ε1家用化学品对透明和着色有机面漆影响的试验方法D6132-04 用超声波仪表非破坏性测量应用有机涂料干膜厚度的试验方法D1641-04 清漆户外曝露试验实施规程D3258-04 用着色法的白色或近白色涂料涂膜孔隙率的试验方法D 3793-04 乳胶漆膜低温凝聚试验方法D 5068-04 评定用试漆刷具的制备规程D6488-04 关于印刷问题术语D6583-04 用矿物油吸附法的漆膜孔隙度试验方法D6901-04 美术彩色铅笔规格D4139-04 颜料挥发物和非挥发物含量测定指南D6687-04 印刷油墨载体及其成分的试验指南D1133-04 烃类溶剂的贝壳杉脂一丁醇值的试验方法D2380-04 甲醛溶液的甲醇含量试验方法D2379—04 甲醛溶液的酸度试验方法D2916—04 异佛尔酮规格D3548—04 丙烯酸乙酯规格D 3620—04 冰乙酸规格D4416—04 丙烯酸规格D5399—04 气相色谱法的烃类溶剂沸点分布试验方法第08卷塑料工艺D4877—04 聚氨基甲酸乙酯原料的试验方法:异氰酸酯中APHA色度的测定D6110—04 塑料切口试样耐却贝冲击的试验方法F1056—04 用于熔融套接聚乙烯管、管子及配件的熔融套接工具规格F1673—04 腐蚀性废料排泄系统用聚偏氟乙烯(PVDF)规格F1807—04 Sdr9交联聚乙烯(PEX)管用的卷曲铜环金属内插件规格F1947—04 往现有排水管和通气管中安装折叠聚氯乙烯PVC管的操作规程F2023—04 评估交联聚乙烯(PEX)管道和系统对热氯化水抗氧化性能的试验方法D1238—04 测量热塑性塑料熔体流动速率挤压塑性仪法D1248—04 电线和电缆用聚乙烯塑料的挤压料规格D4895—04 用分散法生产的聚四氟乙烯(PTFE)规格D 5260—04 聚氯乙烯(PVC)均聚物和共聚物以及氯化聚氯乙烯(CPVC)化合物耐化学药剂性能的分类D5420—04 使用落锤冲击(Gardner冲击)法的扁平硬质塑料试样的冲击试验方法D 5675—04 含氟聚合物微粉规格D3679—04 硬质聚氯乙烯档板规格D4477—04 硬质未增塑聚氯乙烯拱腹规格D5813—04 就地固化的热固性树脂排污管规格D2774—04 热塑性压力管系统的地下安装规程F1974—04 聚乙烯/铝/聚乙烯和交链聚乙烯/铝/交链聚乙烯复合压力管用金属内插配件规格D746—04 塑料及弹性体脆化温度的试验方法用冲击法D 3262—04 玻璃纤维增强的热固性树脂排污管规格D3517—04 玻璃纤维增强的热固性树脂压力管规格D3754—04 玻璃纤维增强的热固性树脂耐压排污管及工业管规格D256—04 塑料抗摆锤冲击性的试验方法D789—04 聚酰胺(PA)相对粘度的测定方法D2115—04 聚氯乙烯组成的炉热稳定性规程D3012—04 用炉内的样本旋转器测定聚丙烯热氧化稳定性的试验方法D4101—04 聚丙烯注射及挤压料规格D4441—04 聚四氟乙烯水分散体规格D4889—04 聚氨基甲酸乙酯原料的试验方法:原料或改性异氰酸酯粘度的测定D4976—04 聚乙烯塑料模塑及挤压料规格D2665—04 a 聚氯乙烯(PVC)塑料排水、排污及通风管及配件规格F876—04 交联聚乙烯(PEX)管子规格F2098—04 固定Sdr 9交联聚乙烯管在金属内插配件上用的不锈钢夹子规格D1785—04 聚氯乙烯塑料管(40、80及120号表)的规格D2241—04 a 聚氯乙烯耐不同级别压力管、Sdr系列规格D2513—04a 热塑性塑料气体压力管、软管及配件规格D2837—04 获得热塑性管材流体静力学设计基础或热塑性管制晶压力设计基础的试验方法D3034—04 Psm型聚氯乙烯(PVC)污水管及配件规格F493—04 氯化聚氯乙烯(CPVC)塑料管及配件用液态粘固剂规格第09卷橡胶工业及其它D454-04 橡胶的试验方法用加热与空气加压的变质作用D 572-04 橡胶的试验方法用加热和加氧的变质作用D926-04 橡胶特性的试验方法用平行板法的塑性及复原性D2702-04 橡胶化学品用的规程红外线吸收特性的测定D3765-04 炭黑的试验方法CTAB(十六烷基三甲溴化铵)表面积D4821-04 炭黑导则试验方法精密度及偏差的确认D 750-04 用人工气候装置测定橡胶变质的试验方法第15卷胶粘剂等D896-04 粘合体耐化学试剂的试验方法D907-04胶粘剂的术语D1337—04 用稠度和粘结强度确定胶粘剂贮存寿命的试验方法D5656-04 拉伸载荷法测定胶粘剂剪切下应力—应变性能用的厚粘合金属搭接受剪节点的试验方法D6004-04 测定地毯胶粘剂的粘合剪切强度的试验方法D6105-04 为了粘接应用放电表面处理活化塑料的操作规程D853-04 工业芳烃中硫化氢和二氧化硫含量(定性)测定方法D2360-04 气相色谱法测定单环芳烃中痕量杂质的试验方法D 3792-04 邻二甲苯气相色谱分析方法D4734-04 精制苯-545规格—甲基苯乙烯(AMS)的毛细管气相色谱分析方法αD6144-04D 5159-04 颗粒活性炭的粉化磨损指南D 3878-04 复合材料术语D6484/D6484 M-04 聚合物基块复合层在制品空心抗压强度的试验方法D3943-04 新氧化铝基催化剂中总钼量的试验方法D4481-04 新氧化铝基催化剂中总镍量的试验方法D4782-04 用湿化学方法对分子筛催化剂内钯的试验方法E1064-04 有机溶液中水的试验方法库仑-费休滴定法E1615-04 用锌铁合金法的微量铁定量方法D2809-04 带有发动机冷却剂铝泵的空气腐蚀及磨耗的试验方法D4725-04 发动机冷却剂术语D5752-04 用于重载发动机的预装冷却剂中附加冷却添加剂(SCAs)的规格D847-04 苯、甲苯、二甲苯、溶剂石脑油和类似工业芳烃酸度的试验方法—甲基苯乙烯)规格αD6367-04 AMS(D2659-04 室外潮湿环境中结构层压木制品用胶粘剂规格D2867-04 活性炭中水分的试验方法第05卷石油产品等D86-04 常压下石油产品蒸馏方法D97-04 石油产品倾点的测试方法D664-04 石油产品的酸值试验方法电位滴定法D975-04 燃料柴油规格D2983-04 测定润滑油低温粘度的试验方法布鲁克菲耳德粘度计法D4683-04 在高温和高剪切速率下测定粘度的试验方法锥形轴承模拟器法D5188-04 燃料的汽液比温度试验方法真空室法D6304-04 测定石油产品、润滑油和添加剂中水分含量的试验方法卡尔费休滴定法D86-04 b 常压下石油产品蒸馏方法D445-04 透明和不透明液体运动粘度的试验方法及动力粘度的计算法D 611-04 石油产品及烃类溶剂苯胺点和混合苯胺点测试方法D975-04 a 燃料柴油规格D4055-04 戊烷不溶物的膜过滤测试方法D4485-04 发动机润滑油性能规格D4806-04 汽车点火发动机燃料用的与汽油混合的变性燃料酒精的规格D5293-04 发动机油-5℃~-30℃表观粘度的试验方法冷启动模拟法D 5372-04 烷基导热液的评价指南D5481-04 高温高剪切速率下表观粘度的测试方法多孔毛细管粘度计法D5579-04 手动传动润滑剂热稳定性试验方法循环耐久性试验法D5704-04 主传动和主驱动轴用润滑油热及氧化稳定性的评价试验方法D 5769-04 成品汽油中苯、甲苯和总芳烃含量的色谱/质谱测定方法D6201-04 P2进样阀沉积物形成的无铅火花点火发动机燃料功率计评价试验方法D6304-04a 测定石油产品、润滑油及添加剂中水分含量的库仑—费休滴定法D6379-04 测定航空燃料和石油馏分中芳烃类的试验方法带有折射率检测的高效液相色谱仪D6593-04 低温和轻负荷条件下运行的以汽油为燃料的火花点火内燃发动机内抑制沉积物形成的汽车机油评价的试验方法D6594-04 135℃柴油机油腐蚀性的评价试验方法D6792-04 石油产品和润滑油试验室中质量系统的指南D6837-04 在序列VIB火花点火发动机中测定汽车机油对客车和轻负荷货车节能影响的试验方法D6838-04 Cummins M11高烟灰试验方法D6973-04 高压恒容量叶片泵中标明石油水力流体耐磨性的试验方法D6974-04 在液体燃料中能生活的细菌和霉菌的计数规程过滤和培养方法D130-04 石油产品对铜腐蚀的试验方法铜条法D938-04 石油蜡包括凡士林冻凝点的试验方法D943-04 加抑制剂矿物油氧化特性的试验方法D 1265-04 液化石油(LP)气取样规程手动法D2158-04 液化石油(LP)气中残留物的试验方法D2887-04 石油馏分沸程分布的试验方法气相色谱法D2893-04 极压润滑油氧化特性的试验方法D3227-04 汽油、煤油、航空气轮机燃料及馏分燃料中硫醇态硫含量的试验方法电位差法。

塑料密度的测定(梯度柱法)1 适用范围本方法适用于非泡沫成品状态下的塑料,无论机械加工还是其他方法成型但不能是粉料。
测定密度的范围在0.9~1.0g/cm3。
2 引用标准GB/T1033.2-2010 《非泡沫塑料密度的测定第2部分:密度梯度柱法》3 方法概述将样品在异丙醇中处理后,放入相对密度由上到下均匀地增加的梯度柱中。
在规定的恒温(23±0.5℃)下,由已知密度的浮子高度和样品静止的高度差,即可求出被测样品的密度。
4 仪器和试剂4.1 密度梯度柱:配有精准分度,装有按要求配制的水和异丙醇混合液,密度由下到上逐渐递减,内部放有标准玻璃浮子。
4.2 恒温水浴:温度波动不大于±0.1℃。
4.3 玻璃浮子。
浮子间隔:0.0020g/cm3,精度:0.0002g/cm3。
4.4 恒温水浴。
4.5自动打捞装置:升降速度:0.9~7.5cm/min。
4.6自动配液装置:配液瓶(2L)4个、硅塞4个、聚四氟乙烯管2米、聚乙烯管2米、流量调节阀2个、安全移液器1个、重锤导向筒1个、磁力搅拌器1个。
4.7 异丙醇AR。
4.8 超纯水。
5 操作步骤5.1 检查水浴运转情况,梯度柱液温度,浮子分布情况是否正常。
5.2 在测定MFR时,选出无气泡、无杂质、垂直自然晾凉约20min的样条,切下约2-3mm 试样3段。
5.3 将试样浸入盛有200mL沸腾的蒸馏水的烧杯中盖上盖煮沸30min进行退火,然后将该烧杯置于试验室环境温度下冷却1h。
5.4 用镊子将试样放入轻液浸润后,轻轻放入梯度柱内,待小试样不再下沉时,约30min,记下试样几何中心高度。
5.5 测量试样上、下浮子的高度,输入软件中。
5.6 试样密度计算:在所绘制的浮标密度(ρ)—浮标高度(H)的工作曲线上输入试样的几何中心高度,读取试样所对应的密度,取三位有效数字。
6 测定结果测定结果根据下式计算:ρt =ρb+(ρa-ρb)*x/y (1)式中:ρt :所求试样的密度,单位:g/cm3。
实验1 密度梯度管法测定高聚物的密度和结晶度高聚物的密度是高聚物的重要物理参数之一,它对于指导高聚物的合成、成型工艺以及探索结构与性能之间的关系等方面都是不可缺少的数据。
而对于结晶高聚物来说,结晶度反映了物质内部结构规则程度,影响着其许多物理、化学性能和应用性能,密度和结晶度之间有着密切的关系。
因此,测定高聚物的密度和结晶度,对研究其结构状态进而控制材料的性能有着很大的实用意义。
测定高聚物结晶度的方法很多,有X-射线衍射法、红外吸收光谱法、核磁共振法、差热分析法、反相色谱法、化学方法(水解法、甲酰化法、氘交换法)、密度法等等。
其中前几种方法都需要使用复杂的仪器设备,而密度法是从较容易测定的高聚物密度换算成结晶度,既简单易行,又较为准确。
凡是能测定出高聚物试样密度的方法都属于密度法。
本实验采用密度法中的一种方法 ── 密度梯度管法测定高聚物的结晶度。
一、实验目的1. 了解用密度梯度管法测定高聚物的密度和结晶度的基本原理和方法。
2. 学会用连续灌注法制备密度梯度管的技术及密度梯度管的标定方法。
3. 用密度梯度管测定结晶高聚物试样的密度,并计算其结晶度。
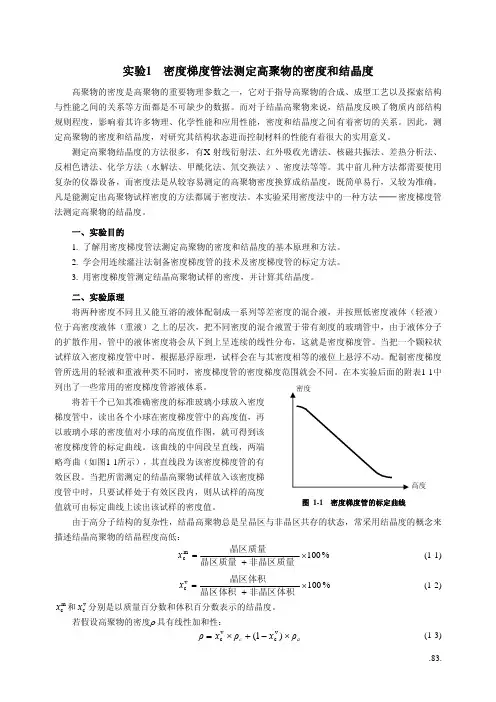
二、实验原理将两种密度不同且又能互溶的液体配制成一系列等差密度的混合液,并按照低密度液体(轻液)位于高密度液体(重液)之上的层次,把不同密度的混合液置于带有刻度的玻璃管中,由于液体分子的扩散作用,管中的液体密度将会从下到上呈连续的线性分布,这就是密度梯度管。
当把一个颗粒状试样放入密度梯度管中时,根据悬浮原理,试样会在与其密度相等的液位上悬浮不动。
配制密度梯度管所选用的轻液和重液种类不同时,密度梯度管的密度梯度范围就会不同。
在本实验后面的附表1-1中列出了一些常用的密度梯度管溶液体系。
高度图 1-1 密度梯度管的标定曲线将若干个已知其准确密度的标准玻璃小球放入密度梯度管中,读出各个小球在密度梯度管中的高度值,再以玻璃小球的密度值对小球的高度值作图,就可得到该密度梯度管的标定曲线。

ASTM D类最新标准目录(二).docD985-97(2002) 纸浆、纸和纸板亮度的试验方法(方向反射系数457nm)D991-89(2005) 橡胶特性.导电橡胶及抗静电橡胶制品的体电阻系数测试方法D994-98(2003) 混凝土用预制伸缩缝填充料(沥青型)D995-95b(2002) 热搅拌、热铺沥青铺面混合料用搅拌设备D996-04 包装及分配环境的术语D999-01 船运集装箱的振动试验方法D1000-04 电气设备用压合敏感胶粘剂涂覆带的试验方法D1002-05 用拉力负载测定金属之间胶粘剂抗剪切强度特性的试验方法D1003-00 透明塑料混浊度和透光系数的测试方法D1004-03 塑料薄膜和薄板的抗撕裂强度的测试方法D1005-95(2001) 用千分尺测量有机涂层干膜厚度的试验方法D1006-01 木材表面涂料的室外暴露试验D1007-05 仲丁醇D1013-93(1998) 树脂和塑料中总氮量的测试方法D1014-02 钢材表面涂料室外暴露试验方法D1015-05 高纯度烃冻结点的测试方法D1016-05e1 冻结点测定烃纯度的试验方法D1018-00(2005) 石油馏分中氢含量的测试方法D1025-00(2004) 聚合级丁二烯中不挥发性残余物的试验方法D1030-95(1999) 纸和纸板纤维分析的试验方法D1036-99(2005) 木电杆的静态试验方法D1037-99 木质纤维板和刨花板的性能评定方法D1038-83(2005) 薄板和胶合板相关术语的定义D1039-94(2004) 电绝缘用玻璃粘合云母的试验方法D1042-01a 在加速使用状态下塑料线性尺寸变化的标准试验方法D1043-02 用扭转试验法测定温度对塑料强度变化的试验方法D1044-05 透明塑料表面耐磨蚀性的试验方法D1045-95(2001) 塑料用增塑剂的取样与试验方法D1047-95(2001) 电线及电缆用聚氯乙烯套管D1048-05 橡胶绝缘毡层D1049-98(2002)e1 橡胶绝缘封盖物D1050-05e1 橡胶绝缘衬里套管D1051-02 橡胶绝缘管D1052-05 用罗斯挠曲装置测定橡胶切口扩展的试验方法D1053-92a(2001)e1 橡胶特性试验.挠性聚合物和涂覆制品的低温劲度测试方法D1054-02e1 用回跳摆锤法测定橡胶弹性的试验方法D1055-04 韧性多孔材料.泡沫胶乳D1056-00 韧性多孔材料-海绵状或膨胀橡胶D1059-01 基于短长度样品的纱线支数试验方法D1060-96(2005) 为测定净毛纤维百分率从成包原毛中心取样D1061-95(2004)e1 石棉卷标准规范D1062-02 金属间粘结力抗裂强度的标准试验方法D1065-96(2001) 松脂制品(包括松香、松油和相关产品)中不可皂化物的测试方法D1066-97(2001) 蒸汽的抽样方法D1067-02 水的酸性和碱性的测试方法D1068-05e1 水中铁的测试方法D1070-03 气体燃料相关密度的试验方法D1071-83(2003) 气体燃料试样容积的测量方法D1072-06 燃料气中总硫量的测试方法D1073-06 沥青铺面混合料用细集料D1074-02 沥青混合料抗压强度的测试方法D1075-96(2005) 水对压实的沥青混合料粘结力影响的试验方法D1076-06 橡胶.浓缩的、氨储存的、乳状的和离心处理的天然胶乳D1078-05 挥发性有机液体馏程的试验方法D1079-05a 铺屋面材料、防水材料和沥青材料的相关术语D1082-00(2005) 云母耗散因数和介电常数的测量方法D1084-97(2005) 胶粘剂粘度的测试方法D1091-00(2005) 润滑油和添加剂中磷含量的测试方法D1092-05 润滑脂表观粘度的试验方法D1093-04 液态烃和它们的蒸馏残余物酸度的测试方法D1094-00(2005) 航空燃料水反应性的试验方法D1101-97a(2006) 室外用层压结构木制品的胶合接头完整性的试验方法D1102-84(2001) 木材中灰分的测试方法D1105-96(2001) 无提取物木材的制备D1106-96(2001) 木材中酸不溶木素的测试方法D1107-96(2001) 木材的醇苯可溶性试验方法D1108-96(2001) 木材中二氯甲烷溶解物的测试方法D1109-84(2001) 木材在百分之一的烧碱中可溶性的试验方法D1110-84(2001) 木材水溶解度的测试方法D1113-90a(2001) 洗净羊毛中植物性物质和其它碱性不溶杂质的测试方法D1117-01 无纺织物试验方法D1118-95(2004)e2 石棉纤维和石棉织物磁参数的标准测试方法D1119-05 发动机冷却剂与防锈剂的灰分含量的测试方法D1120-94(2004) 发动机冷却剂沸点的测试方法D1121-98(2003) 发动机抗冻剂、防锈剂和冷却剂储备碱度的测试方法D1122-97a(2002) 用液体比重计测量发动机冷却剂及其浓缩物比重的试验方法D1123-99(2003) 用KarlFischer试剂法测试发动机冷却剂浓缩物的试验方法D1125-95(2005) 水的电导性和电阻率的测试方法D1126-02 水硬度的测试方法D1129-06 与水相关的术语D1133-04 烃类溶剂的贝壳杉脂丁醇值的测试方法D1135-86(2004) 蓝色颜料化学分析的标准试验方法D1139-00(2004) 单层或多层沥青表面处理用集料D1140-00 土壤中小于200号(75微米)筛孔的材料总量的测试方法D1141-98(2003) 海水代用品D1142-95(2006) 露点温度法测定气体燃料中蒸汽含量的试验方法D1143-81(1994)e1 静态轴向压力荷载下桩柱的试验方法D1144-99(2005) 胶粘剂粘结强度提高的测定方法D1146-00 有效粘结层粘结点的试验方法D1148-95(2001) 橡胶变质.受热及紫外线使浅颜色表面退色的试验方法D1149-99 橡胶变质试验.在小室中橡胶表面臭氧龟裂D1151-00 潮气和温度对胶粘剂粘结能力影响的试验方法D1152-06 甲醇(甲基醇)D1153-06 甲基异丁基甲酮D1155-03 玻璃球圆度的测试方法D1157-91(2004) 轻质烃总抑制剂含量(TBC)的测试方法D1159-01 电化学滴定法测量石油馏分及商用脂族烯烃的溴值试验方法D1160-06 石油产品减压蒸馏方法D1165-03 生活中用硬木和软木的命名法D1166-84(2001) 木材及有关材料中甲氧基团的测试方法D1168-99(2003) 电绝缘用石蜡的测试D1169-02e1 电绝缘液体电阻率(电阻系数)的测试方法D1171-99 橡胶变质试验.室外或小室内橡胶表面臭氧龟裂(三角形试样)D1172-95(2001) 肥皂和洗涤剂水溶液pH值的测试方法D1173-53(2001) 表面活性剂起泡性试验方法D1176-98(2002) 试验用发动机冷却剂或防锈剂水溶液的取样和制备方法D1177-05 发动机冷却剂溶液冻结点的测试方法D1179-04 水中氟化物离子的测试方法D1183-03 胶粘剂耐周期性实验室老化条件的标准试验方法D1184-98(2004) 胶粘剂粘结的层压部件抗挠强度的试验方法D1185-98a(2003) 在材料搬运和船运中托盘和有关设备的试验方法D1186-01 铁基非磁性涂层干膜厚度的无损测量方法D1187-97(2002)e1 金属保护涂层用沥清乳液D1188-96(2002)e1 用涂石蜡样品测定压实的沥青混合料的体积比重和密度的试验方法D1193-06 试剂水(联邦试验方法No.7916)D1195-93(2004) 用于公路路面和机场跑道设计和评定的土壤与韧性路面成份的往复静态平板荷载的测试方法D1196-93(2004) 用于公路和机场路面设计和评定的土壤与韧性路面成份的非往复静立平板荷载试验方法D1198-93(1998) 胺基树脂溶剂容限的测试方法D1199-86(2003) 碳酸钙颜料技术规范D1200-94(2005) 福特粘度杯测定粘度的试验方法D1201-99(2004) 热固聚酯模制化合物D1203-94(2003) 用活性碳法测定塑料的挥发损失的试验方法D1204-02 高温下非硬性热塑塑料薄板或薄膜线性尺寸变化的测试方法D1208-96(2002) 某些颜料通性的试验方法D1209-05 透明液体色度的试验方法(铂钴标度)D1210-05 颜料载体体系分散细度的测试方法D1212-91(2001) 木材用透明硝基清漆漆膜耐温度变化的试验方法D1214-04 有机涂层湿膜厚度的测试方法D1217-93(2003)e1 用宾汉比重法测定液体密度和相对密度(比重)的试验方法D1218-02 液态烃的折射率和折射分散度的测试方法D1223-93(1998) 75度下纸和纸板镜面光泽的试验方法D1224-92(2001) 纸中锌和镉的试验方法D1227-95(2000) 屋面保护涂层用乳化沥青D1229-03 橡胶特性试验方法.低温时的压缩变形率D1230-94(2001) 服装纺织品的易燃性的测试方法D1234-85(2001) 含脂羊毛的手扯长度的取样和试验方法D1238-04c 用挤压塑性计测定热塑性塑料流率的测试方法D1239-98 用化学制剂测量塑料薄膜的抗萃取性测试方法D1240-02 包括松香,高脂油和相关产品中心存储的松香酸含量的测试方法D1241-00 土壤集料次底层、底层和表层用料D1243-95(2000)e1 氯乙烯聚合物的稀溶液粘度的试验方法D1244-98(2005) 纱线结构的名称D1245-84(2003) 用化学显微镜作水沉积物的检验D1246-05 水中的溴化物离子的测试方法D1248-05 聚乙烯塑料模制和挤压材料D1249-92(1999) 辛基正酞酸酯增塑剂D1250-04 石油测量表D1252-06 水化学氧需要量(重铬酸盐氧需要量)的测试方法D1253-03 水中残余氯的测试方法D1257-90(2001) 高比重甘油D1258-05 高比重甘油的试验方法D1259-06 树脂溶液的不挥发物含量的测试方法D1263-94(2005)e1 汽车轮轴承润滑脂泄漏倾向的试验方法D1264-03e1 润滑脂水洗出性能的试验方法D1265-05 液化石油(LP)气取样(手工法)D1266-98(2003)e1 石油产品中硫的试验方法(燃灯法)D1267-02 液化石油(LP)气气压的测试方法(液化石油气法)D1272-56(2002) 五氯苯酚D1274-95(2000) 五氯苯酚的化学分析方法D1275-06 电绝缘油中腐蚀性硫的测试方法D1278-91a(2002)e1 天然橡胶的试验方法.化学分析D1280-00 浸槽式金属除垢剂的全浸腐蚀试验的试验方法D1282-05 用气流阻力表示羊毛毛条,生条和洗净羊毛的平均纤维直经的测试方法D1283-05 羊毛碱溶性的测试方法D1287-91(2002) 发动机冷却剂和防锈剂的pH值的试验方法D1290-95(2002) 用离心机测定水乳化抛光剂中沉积物的试验方法D1291-01 氯的需求量或水的需求量或两者兼有的评估D1292-05 水的气味的测试方法D1293-99(2005) 水的pH值测试方法D1294-05 在1英寸(25.4毫米)长度内羊毛纤维拉伸强度和断裂强度试验方法D1296-01 挥发性溶剂和烯释剂气味的试验方法D1298-99(2005) 用石油密度计法测定原油和液体石油产品密度,相对密度(比重)或API比重的试验方法D1301-91(2003) 白铅颜料化学分析的标准试验方法D1304-99(2005) 与电绝缘试验相关的胶粘剂D1305-99(2004) 电绝缘纸和纸板.硫酸盐(牛皮纸)层型D1306-88(1996)e1 醇酸树脂和含有其它二元酸的酯类的酞酸含量测试方法(重量法)D1308-02e1 家用化学品对透明和着色有机面漆影响的试验方法D1309-93(2004) 贮藏期间交通标志用色漆的沉淀特性的测试方法D1310-01 用泰格开口杯装置测定液体闪点和燃点的测试方法D1312-93(1998) 涂层用合成酚醛树脂或溶液中表观游离酚的测试方法D1316-06 用美国油墨研究会研磨测量计测定油墨研磨细度的测试方法D1318-00(2005) 测定残留燃料油中钠试验方法(火焰光度测定法)D1319-03e1 用萤光指示剂吸附法测定液态石油产品中烃类物质的试验方法D1321-04 石油蜡针入度测试方法D1322-97(2002)e1 航空涡轮机燃料烟点的试验方法D1325-94(2000) 氨化砷酸铜D1326-94(2000) 氨化砷酸铜的化学分析法D1327-04 铺屋面和防水用浸沥青物质的机织粗麻布D1329-02 评定橡胶特性的试验方法.低温下的回缩(TR试验)D1330-04 薄橡胶衬垫D1331-89(2001) 表面活性剂溶液的表面张力与界面张力的试验方法D1334-05 原毛毛含量的测试方法.商业尺度D1335-05 毛绒地毯绒毛束粘合试验方法D1336-97(2003) 机织纱线扭曲度试验方法D1337-04 用稠度和粘结强度测定胶粘剂贮藏寿命的试验方法D1338-99(2005) 用稠度和粘结强度测定液态或糊状胶粘剂使用寿命的试验方法D1342-92(2002) 巴西棕榈蜡中石蜡型烃含量的测试方法D1343-95(2006) 用落球法测定纤维素衍生物粘度的测试方法D1348-94(2003) 纤维素中湿度的测试方法D1349-99(2005) 橡胶.试验温度D1351-02 电线和电缆用聚乙烯绝缘材料D1352-02 电线和电缆用耐臭氧的丁基橡胶绝缘材料D1353-03 色漆,清漆,喷漆及相关产品用挥发性溶剂中不挥发物质的试验方法D1356-05 与大气取样和分析相关的术语D1357-95(2005) 制定外围大气的取样计划D1360-98(2004) 涂料阻燃性试验方法(小室法)D1363-06 丙铜和甲醇的高锰酸盐时间的测试方法D1364-02 挥发性溶剂中水的测试方法(费歇尔试剂滴定法)D1366-86(2003) 颜料粒度特性报告D1367-96(2001)e1 石墨润滑质量的测试方法D1369-84(2006) 沥青表面处理用材料的数量D1370-00 沥青材料间接触相容性的试验方法(奥林萨斯试验)D1384-05e1 玻璃器皿中发动机冷却剂腐蚀试验的测试方法D1385-01 水中肼含量的测试方法D1386-98(2004) 合成和天然蜡的酸值(经验)的测试方法D1387-89(2002) 合成和天然蜡皂化值(经验)的测试方法D1388-96(2002) 织物硬挺性测试方法D1389-97a(2004) 薄固体电绝缘材料的校验电压试验的试验方法D1392-92(1998) 红花油D1394-76(2003) 钛白颜料化学分析的标准试验方法D1396-92(1998) 聚乙烯缩丁醛化学分析的标准试验方法D1397-93(1998) 醇酸树脂和树脂溶液中不皂化物的测试方法D1398-93(1998) 醇酸树脂和醇酸树脂溶液的脂肪酸含量测试方法D1399-95(2000) 磷酸三甲苯酯不可皂化物含量的测试方法D1400-00 非铁金属基表面非传导涂层干膜厚度无损测量的测量方法D1401-02 石油和合成液体水溶解性的试验方法D1403-02 用1/4和1/2标度锥形设备测定润滑脂锥入度的试验方法D1404/D1404M-99(2003) 评定润滑脂中有害粒子的测试方法D1405-01(2006) 平定航空燃料净燃烧热的测试方法D1411-04 分级集料筑路搅拌物中作为掺合物的溶水氯化物的测试方法D1412-04 在96%-97%相对湿度和30℃时煤的平衡湿气的测试方法D1413-05b 用实验室土块培养法测试木材防腐剂D1414-94(2003) O型橡胶圈的试验方法D1415-05 橡胶特性的测试方法.国际硬度D1417-03a 合成橡胶胶乳的测试方法.D1418-06 橡胶和橡胶胶乳.命名D1422-99 退捻加捻法测定单纱捻数的试验方法D1423-02 直接计算法测定纱线捻数的试验方法D1424-96(2004) 埃尔曼多夫落锤仪测定机织物抗撕裂的试验方法D1426-03 用电容测试设备测定纱线条干不匀度的测试方法D1429-03 水中氨态氮的测试方法D1430-03 水和盐水的比重的测试方法D1434-82(2003) 聚氯三氟乙烯(PCTFE)塑料D1435-05 测定塑料薄膜和薄片透气性能的测试方法D1436-97(2002) 塑料的室外风化D1439-03 试验用乳胶地板基质抛光剂的使用D1440-96(2002) 羧甲基纤维素钠盐的测试方法D1441-06 棉纤维长度和长度分布的试验方法(列阵法)D1442-06 试验用棉纤维取样D1445-05 棉纤维成熟度的试验方法(烧碱膨胀与偏振光法)D1447-00 用纤维照相法测定棉纤维的长度和长度均匀度的测试方法D1448-05 棉纤维的马克隆尼读数的试验方法D1452-80(2000) 用螺旋钻作土壤勘探和取样D1455-87(2002) 乳化地板抛光剂60度镜面光泽度的试验方法D1456-86(2005) 橡胶特性的测试方法.特定应力下的延伸D1458-01 电绝缘用完全硫化硅橡胶涂层的玻璃布与玻璃带的试验方法D1459-93(2003) 电绝缘用硅清漆涂层的玻璃布和玻璃带D1460-86(2005) 橡胶性能的测试方法.液体浸没期间的长度变化D1461-85(2006) 沥青铺砌混合料中水份或挥发性馏份含量的测试方法D1462-92(1998) 精制豆油技术规范D1464-90(2002) 棉花染色差异性的试验方法D1465-04 石油蜡的熔点和粘着点的测试方法D1468-93(2001) 磷酸三甲苯酯中挥发物质的试验方法D1474-98(2002) 有机涂层压印硬度的试验方法D1475-98(2003) 色漆,清漆,喷漆及相关产品密度的试验方法D1476-02 喷漆溶剂的庚烷混溶性试验方法D1478-02 滚珠轴承润滑脂低温转矩的试验方法D1480-02e1 用宾汉比重计测定粘性材料密度和相对密度(比重)的试验方法D1481-02 用利普金双毛细管比重计测定粘性材料密度和相对密度(比重)的试验方法D1483-95(2002) 用加纳尔--科尔曼法测定颜料的吸油量的试验方法D1485-86(2002) 从天然原料中获取橡胶的方法.取样和样品制备D1488-00 胶粘剂中淀粉物质的测试方法D1489-97(2004) 含水胶粘剂不挥发物含量的测试方法D1490-01 脲甲醛树脂溶液中不挥发物含量的测试方法D1492-02 库仑计滴定法测定芳烃溴指数的测定方法D1494-97(2001)e1 增强塑料板的散射光透射系数的测试方法D1498-00 水的氧化还原潜能D1499-05 操纵塑料暴露用曝光和曝水装置(碳弧型)D1500-04a 石油产品ASTM颜色的试验方法(ASTM比色度)D1505-03 用密度梯度法测定塑料密度的试验方法D1506-99 碳黑的测试方法.灰分含量D1508-02e2 粒状碳黑的测试方法.精粒含量D1509-95(2000) 碳黑的测试方法.加热损耗D1510-06a 炭黑碘吸收值的测试方法D1511-00(2006) 碳黑的测试方法.片状尺寸分布D1512-05 碳黑的测试方法.pH值D1513-05 片状碳黑的测试方法.顷注密度D1514-04 碳黑测试方法.筛渣D1516-05 皮革宽度的测试方法D1517-04e1 与皮革相关的术语定义D1518-85(2003) 纺织材料的热传导的试验方法D1519-95(2004)e1 橡胶化学制品的试验方法.溶化范围D1523-00 工作温度为90℃的电线和电缆用合成橡胶绝缘材料D1524-94(2004) 用过的石油制电绝缘油的现场目测检查的方法D1525-06 塑料维卡(Vicat)软化温度的测试方法D1527-99(2005) 丙烯腈-丁二稀-苯乙稀塑料管.40号表和80号表D1531-01e1 用液体位移法测定介电常数与耗散系数的试验方法D1533-00(2005) 绝缘液体中水的试验方法(卡耳费瑟反应法)D1534-95(2002)e1 用比色指示剂滴定法测定电绝缘液近似酸度的试验方法D1535-06 用孟塞尔制规定颜色的测试方法D1537-60(1998) 蒸馏的大豆脂肪酸D1538-60(1998) 蒸馏的亚麻籽脂肪酸D1539-60(1998) 脱水蓖麻籽脂酸D1541-97 干性油及其衍生物总碘值的试验方法D1544-04 透明液体颜色的测试方法(加德纳色度)D1545-98 用起泡时间法测定透明液体粘度的试验方法D1546-96(2003) 透明地板密封剂的性能试验D1550-94(2005) ASTM丁二烯测量表D1552-03 石油产品中硫含量的试验方法(高温法)D1554-01(2005) 与木基纤维和刨花板材料相关的名词术语定义D1555-04a 工业芳烃体积和重量的计算方法D1555M-04a 工业香烃类计量的体积和重量计算的标准测试方法D1556-00 用砂锥法现场测定土壤密度和单位重量的测试方法D1557-02e1 用修正作用力56000ft-Ibf/ft(2700 KN-m/m)测量土壤实验室压实性能的测试方法D1558-99(2004)e1 细粒土水含量渗透阻力关系的试验方法D1560-05e1 用赫维门(Hveem)装置测定沥青混合物抗变形和粘结性的试验方法D1561-92(2005)e1 用搅拌压实机法制作沥青混合物试样D1562-05 纤维素丙酸盐模制和挤压物D1566-06 与橡胶相关的术语D1568-05 烷基苯磺酸盐的取样与化学分析的标准试验方法D1569-05 烷基洗涤剂的试验方法D1570-95(2003) 脂肪烷基硫酸盐的取样与化学分析的测试方法D1571-95(2004)e1 石棉布的标准规范D1573-95(2004)e1 石棉织物热老化的标准测试方法D1574-04 Standard Test Method for Extractable Matter in Wool andOther Animal Fibers D1575-90(2001) 洗净羊毛及生条中羊毛纤维长度的测试方法D1576-90(2001) 用炉烘干法测定羊毛内水分的试验方法D1577-01 纺织纤维线密度的测试方法D1578-93(2006)e1 绞纱形式下纱线的断裂强度的试验方法D1579-01 苯酚,间苯二酚和三聚氰胺胶粘剂中填料含量的测试方法D1582-98(2004) 液相苯酚,间苯二酚和三聚氰胺胶粘剂中不挥发物含量的测试方法D1583-01 干粘膜中氢离子浓度的测试方法D1585-96(2003) 中心仓库中松香,妥尔油和相关产品的脂肪酸含量测试方法D1586-99 渗透试验和土壤开管取样的试验方法D1587-00 泥土薄壁管抽样D1593-99 非硬性氯乙烯塑料薄板D1596-97(2003) 包装材料减震性能的试验方法D1598-02 恒定内压下塑料管的破裂时间的测试方法D1599-99(2005) 塑料管,管道和配件的短时破裂水压的测试方法D1600-99 与塑料相关的缩略名词术语D1601-99(2004) 乙烯聚合物稀溶液粘度的测试方法D1603-06 烯烃塑料中碳黑测试方法D1607-91(2005) 大气中二氧化氮含量的测试方法(格里斯-沙耳茨曼反应)D1608-98(2003) 气态燃烧产物中氧化氮的测试方法(苯酚二磺酸法)D1610-01 试验用皮革和皮革制品的修整D1611-00(2005) 皮革与金属接触时产生腐蚀的试验方法D1612-05 甲醇(木精)中丙酮含量的测试方法D1613-06 色漆,清漆,喷漆和有关产品用挥发性溶剂和化学介质中酸度的试验方法D1614-03 丙酮中碱度的试验方法D1617-90(2001) 溶剂和稀释剂酯化值的试验方法D1618-99(2004) 碳黑的可萃取性测验方法.甲苯脱色D1619-03 碳黑的测试方法.硫含量D1621-04a 硬质泡沫塑料的压缩特性的试验方法D1622-03 硬质泡沫塑料的表观密度的测试方法D1623-03 硬质泡沫塑料张力和张力粘合性能的试验方法D1624-71(2000) 酸性铬酸铜D1625-71(2000) 铬酸盐砷酸铜D1627-94(2000) 酸性铜铬酸盐的化学分析D1628-94(2000) 铬酸盐砷酸铜的化学分析的测试方法D1630-06 橡胶特性的试验.耐磨性(NBS磨损机)D1631-99(2004) 用碘试剂法测定苯酚和有关原料中水分的试验方法D1632-96 试验室中水泥土压缩试样和挠曲试样的制作和养护D1633-00 模制水泥土圆筒的抗压强度的试验方法D1634-00(2006) 用横梁弯曲断裂部分测定水泥土抗压强度的试验方法(改良立方体法) D1635-00(2006) 用简支梁三点负荷法测定水泥土抗挠强度的试验方法D1636-99(2004)e1 烯丙基模制化合物D1639-90(1996)e1 有机涂料酸值试验方法D1640-03 室温下有机涂料干燥,固化及成膜试验方法D1641-04 外用清漆耐用性的试验方法D1644-01(2006) 清漆中不挥发物含量试验方法D1646-04 橡胶的测试方法.粘度和硫化特性(穆尼粘度计)D1647-89(1996)e1 清漆干膜耐水性和耐碱性试验方法D1648-86(2003) 碱式硅铬酸盐颜料D1649-01 铬酸锶颜料D1652-04 环氧树脂中环氧含量的试验方法D1653-03 有机涂层薄膜水蒸气渗透性测试方法D1654-05 腐蚀环境中涂漆或加涂层样件评估的标准试验方法D1655-06a 航空涡轮机燃料D1657-02 用压力液体比重计测定轻烃类物质的密度或相对密度的标准试验方法D1662-92(2002) 切削润滑油中活性硫的测试方法D1665-98(2003) 柏油制品的恩氏比粘度的测试方法D1666-87(2004) 木材和木基材料的传导机械试验D1667-05 软质泡沫材料.氯乙烯聚合物及共聚物(闭孔泡沫)D1668-97a(2006) 铺屋面和防水用玻璃织物(机织和经处理)D1669-03 沥青材料在加速风化与室外风化用试片的制备D1670-04 沥青材料在加速风化与室外风化中端点损坏的测试方法D1673-94(2004) 电绝缘用充气泡沫塑料的介电常数与耗散系数的测试方法D1675-03 聚四氟乙烯管的试验方法D1676-03 带绝缘薄膜的磁性导线的测试方法D1677-02 电绝缘用未处理云母纸的取样和试验D1679-02 工作温度为75℃的电线和电缆用合成橡胶耐热防潮绝缘材料D1681-05 按阳离子滴定程序对洗涤剂中合成阴离子活性成分的测试方法D1683-04 机织物缝纫接缝瑕疵的试验方法D1684-96(2002) 颜色分级用棉分级室的照明D1685-05 用分光光度计测定苯中微量噻吩的试验方法D1686-96(2004) 溶融状态中固体芳烃与相关材料的颜色的测试方法(铂钴标度) D1687-02 水中铬含量的测试方法D1688-02 水中铜含量的测试方法D1691-02 水中锌含量的测试方法D1693-05 乙烯塑料的环境应力破裂的试验方法D1694-95(2000) 玻璃纤维(玻璃纤维增强热固树脂)管用60度螺纹(短管)D1695-96(2001) 纤维素和纤维素衍生物的术语D1696-95(2006) 纤维素在氢氧化钠中溶解度的试验方法D1698-03 用过的老化绝缘油中沉淀物与可溶性淤渣的试验方法D1708-06 用微型抗拉试样对塑料抗拉特性的测试方法D1709-04 用钢锥自由下落法对塑料薄膜的抗冲击强度的测试方法D1710-02 聚四氟乙烯(PTFE)基本型材,棒材和厚壁管材D1711-02 电绝缘术语D1712-03 塑料抗硫化物污染D1718-03 乙酸异丁酯(95%)D1719-05 异丁醇D1720-03 &%。

聚丙烯腈碳纤维性能表征规范聚丙烯腈碳纤维的性能主要有力学性能、热物理性能和电学性能。
对于碳纤维材料来说,拉伸力学性能,包括拉伸强度、拉伸模量以及断裂伸长率是其主要力学性能指标。
由于纤维材料本身的特点,很难对其压缩力学性能进行有效的表征,因此基本不考虑纤维本身的压缩性能。
碳纤维的热物理性能包括热容、导热系数、线膨胀系数等,也是材料应用的重要指标。
电性能主要为体积电阻率以及电磁屏蔽方面的性能。
对于碳纤维的拉伸力学性能测试,各国都已经基本形成了相应的测试标准系列,这些标准系列同时包括了在力学性能测试时需要的线密度、体密度、上浆量等相关的测试。
对于热物理性能,相关的测试标准较少。
5.5.1 碳纤维性能测试标准序号标准号标准名称1 JIS R7601-1986 碳纤维试验方法2 JIS R7602-1995 碳纤维织物试验方法3 JIS R7603-1999 碳纤维-密度的试验方法4 JIS R7604-1999 碳纤维-上浆剂附着率的试验方法5 JIS R7605-1999 碳纤维-线密度的试验方法6 JIS R7606-2000 碳纤维单纤维拉伸性能试验方法7 JIS R7607-2000 碳纤维单纤维直径及断面面积试验方法8 JIS R7608-2007 碳纤维-树脂浸渍丝拉伸性能测试方法9 JIS R7609-2007 碳纤维体积电阻率测试方法10 JIS R7601-2006 碳纤维试验方法(修正1)日本东丽公司作为世界聚丙烯腈基碳纤维生产能力和水平最高的企业,也有自己的碳纤维力学性能测试内部规范,测试规范号和名称为TY-030B-01《碳纤维拉伸强度、拉伸弹性模量和断裂延伸率测试方法》。
序号标准号中文标准名称1 ASTM D4018-2011 连续碳纤维和石墨纤维束性能的测试方法2 ASTM D1505-2010 采用密度梯度法测试塑料密度的测试方法3 ASTM D1577-2007 纺织纤维线密度的测试方法4 ASTM D7633-2013 炭黑碳含量的测试方法5 ASTM D482-2007 石油产品灰分的测试方法6 ASTM D2257-1998 纺织品中可萃取物的测试方法7 ASTMD4102-1982 碳纤维的耐热氧化性的试验方法序号标准号中文标准名称1 ISO10618-2004 碳纤维.树脂浸渍纱线拉伸特性测定2 ISO10548-2003 碳纤维.尺寸的测定3 ISO10119-1992 碳纤维.密度测定4 ISO10120-1991 碳纤维.线性密度的测定5 ISO10548-2003 碳纤维.胶料含量的测定6 ISO11567-1996 碳纤维.长丝直经和横截面积的测定7 ISO11566-1996 碳纤维.单丝样品抗拉性能的测定8 ISO 碳纤维长丝纱.试验方法和通用规范序号标准号标准名称1 GB/T3362-1982 碳纤维复丝拉伸性能试验方法2 GB/T3362-2005 碳纤维复丝拉伸性能试验方法3 GB/T3364-2008 碳纤维直径和根数试验方法4 GB/T3366-1996 碳纤维增强塑料纤维体积含量试验方法5 GB/T3855-2005 碳纤维增强塑料树脂含量试验方法6 GB/T26749-2011 碳纤维浸胶纱拉伸性能的测定7 GB/T30019-2013 碳纤维密度的测定8 GB/T29762-2013 碳纤维纤维直径和横截面积的测定9 GB/T29761-2013 碳纤维浸润剂含量的测定10 GB/T23442-2009 聚丙烯腈基碳纤维原丝结构和形态的测定11 GB/T26752-2011 聚丙烯腈基碳纤维12 QJ3074-1998 碳纤维及其复合材料电阻率测试方法13 GB18530-2001 车间空气中碳纤维粉尘职业接触限值碳纤维单丝力学性能测试单丝力学性能测试可以较为简单快速的得到碳纤维拉伸力学性能,需要样品量少,通常十厘米左右的纤维样品就可以完成对碳纤维的力学性能表征,因此在早期应用较为普遍。
塑料粒子密度检测方法
嘿,你知道塑料粒子的密度咋检测不?其实超简单!先准备一个天平,还有测量体积的工具。
把一定量的塑料粒子放在天平上称出质量,这就好比你去菜市场买菜称重量一样。
然后用排水法之类的方法测量塑料粒子的体积,就像你测量一个不规则形状的小玩具的体积那样。
检测的时候可得小心点,别弄洒了塑料粒子,不然就像撒了一地的豆子,难捡得很呢!这过程安全得很,只要你操作得当,不会有啥危险。
稳定性也不错,只要你认真按照步骤来,结果一般都挺靠谱。
那这检测方法啥时候用呢?比如说在塑料生产厂里,得知道塑料粒子的密度是不是合格呀!这就好比厨师做菜得知道食材的好坏一样。
优势可多了,能快速准确地了解塑料粒子的质量,让你心里有底。
我给你说个实际案例哈。
有个塑料厂,以前不知道塑料粒子密度检测方法,生产出来的产品质量参差不齐。
后来用了这个方法,哇塞,一下子就找出问题所在了,产品质量大大提高。
这效果,杠杠的!
所以说,塑料粒子密度检测方法真的很有用,能帮你把好质量关,让你的生产更顺利。
实验 密度梯度管法测定聚合物的密度和结晶度密度梯度法是测定聚合物密度的方法之一。
聚合物的密度是聚合物的重要参数。
聚合物结晶过程中密度变化的测定,可研究结晶度和结晶速率;拉伸、退火可以改变取向度和结晶度,也可通过密度来进行研究;对许多结晶性聚合物其结晶度的大小对聚合物的性能、加工条件选择及应用都有很大影响。
聚合物的结晶度的测定方法虽有X 射线衍射法、红外吸收光谱法、核磁共振法、差热分析、反相色谱等等,但都要使用复杂的仪器设备。
而用密度梯度管法从测得的密度换算到结晶度,既简单易行又较为准确。
而且它能同时测定一定范围内多个不同密度的样品,尤其对很小的样品或是密度改变极小的一组样品,需要高灵敏的测定方法来观察其密度改变,此法既方便又灵敏。
一、实验目的:1.掌握用密度梯度法测定聚合物密度、结晶度的基本原理和方法。
2.利用文献上某些结晶性聚合物PE 和PP 晶区和非晶区的密度数据,计算结晶度。
二、基本原理:由于高分子结构的不均一性,大分子内摩擦的阻碍等原因,聚合物的结晶总是不完善的,而是晶相与非晶相共存的两相结构,结晶度f w 即表征聚合物样品中晶区部分重量占全部重量的百分数:在结晶聚合物中(如PP 、PE 等),晶相结构排列规则,堆砌紧密,因而密度大;而非晶结构排列无序,堆砌松散,密度小。
所以,晶区与非晶区以不同比例两相共存的聚合物,结晶度的差别反映了密度的差别。
测定聚合物样品的密度,便可求出聚合物的结晶度。
密度梯度法测定结晶度的原理就是在此基础上,利用聚合物比容的线性加和关 系,即聚合物的比容是晶区部分比容与无定形部分比容之和。
聚合物的比容V 和结晶度w f 有如下关系:()1c w a w V V f V f =+- --------------------------------- (2) 式中c V 为样品中结晶区比容,可以从X 光衍射分析所得的晶胞参数计算求得;a V 为样品中无定形区的比容,可以用膨胀计测定不同温度时该聚合物熔体的比容,然后外推得到该温度时非晶区的比容a V 的数值。
ICS 11.040.40 备案号:YYYY/T ××××—××××/ASTM F 1925:1999中 华 人 民 共 和 国 医 药 行 业 标 准外科植入物用纯 L—聚乳酸树脂的标准规 范Standard Specification for Virgin Poly(L—Lactic Acid)Resin for Surgical Implants(ASTM F 1925:1999,IDT)(征求意见稿)200×-××-××发布200×-××-××实施 发布国家食品药品监督管理局YY/T ××××—××××/ASTM F 1925:1999目次前言 ................................................................................. II 1 范围 ................................................................................ 1 2 规范性引用文件 ...................................................................... 1 3 术语 ................................................................................ 1 4 L—聚乳酸的要求 ..................................................................... 2 5 制样 ................................................................................ 3 6 试验方法 ............................................................................ 3 7 包装和标签 .......................................................................... 3 8 生物相容性 .......................................................................... 3 9 关键词 .............................................................................. 3 附录 A................................................................................. 5IYY/T ××××—××××/ASTM F 1925:1999前言本标准等同采用ASTM F 1925:1999《外科植入物用纯L—聚乳酸树脂的标准规范》。
实验六 密度梯度管法测定聚合物的密度和结晶度一、实验目的1.掌握密度梯度管法测定聚合物密度和结晶度的基本原理;2.掌握连续注入法制各密度梯度管的技术及密度梯度的标定;3.用密度梯度管法测定聚合物的密度并计算聚合物的结晶度。
二、实验原理结晶度是表征聚合物性质的一个重要指标,它是反映聚合物内部结构规则程度的物理量,对聚合物的力学性能、热性能、光学性质、溶解性和耐腐蚀性都有着非常显著的影响。
聚合物结晶度的测定方法很多,如X 射线衍射法、红外吸收光谱法、核磁共振法、差热分析和反相色谱等。
与以上各种实验手段相比较,用密度梯度管法测定聚合物密度和结晶度设备简单,操作便利,又有非常好的实验精确度。
不仅如此,密度梯度管法还可以同时对一定范围内不同密度的一组样品进行测定,是确定聚合物密度和结晶度的一种行之有效的实验方法。
需要指出的是,尽管结晶度的概念已沿用了很久,但是由于聚合物的晶区与非晶区的界限不明确,在一个样品中,实际上同时存在着不同程度的有序状态,这样就使得准确确定结晶部分的含量十分困难,又由于各种测定结晶度的方法涉及不同的有序状态,测定结果常常有较大出入,有时数据的差别超过测量误差,因此,在指出某种聚合物的结晶度时,应说明测量的方法,也只有这样才能正确理解和比较结晶度。
对结晶性聚合物而言,当其处于结晶温度时,即处于玻璃化转变温度以上、结晶融化温度以下时,便开始结晶。
由于高分子结构的复杂性,大分子内摩擦阻碍等原因,使得聚合物的结晶与小分子晶体相比较会有更多的缺陷,所以结晶总是不完善的,成为一种晶区和非晶区共存的体系。
结晶度f w 即表征聚合物样品中晶区部分重量占全部重量的百分数:%100f w ×+=晶区重量非晶区重量晶区重量 在实际结晶聚合物中,晶区部分和非晶区部分的界限并不是想象的那么明显,每个高分子可以同时贯穿几个晶区和非晶区,而且晶区和非晶区两相间的交替部分有着半有序的过渡状态。
即使是晶区部分,往往又有很多缺陷,这些缺陷同样表现为无序态的性质,因此实际测定的结晶度并不是想象中的那样具有非常明确的物理意义。
塑料密度和相对密度的测试方法1范围1.1 这些试验方法叙述了片状,棒条状,管状或铸模件固体塑料相对密度和密度的测定方法。
1.2 叙述了两种试验方法:1.2.1 试验方法 A--- 在水中测试,1.2.2 试验方法 B---在其余液体中测试。
1.3SI 为标准单位。
1.4 该标准其实不旨在议论全部的安全问题,若有,仅与其使用有关。
该标准的使用者责任拟订有关合用的安全和健康规范,并在使用前确立规范的合用性。
2参照文件3术语3.1 总则 ---该标准中使用的单位,符号和缩写与规范E380 一致。
3.2 定义:3.2.1 相对密度 --- 在 23℃的温度下资料不浸透部分单位体积质量与相同温度下同体积同密度无气蒸馏水的质量之比。
表达形式为:相对密度 23/23℃(或spgr23/23 ℃)。
3.2.2 密度 --- 在 23℃的温度下,资料无浸透部分每立方米的千克质量。
表达式为:D23,千克 / 立方米注 4---E380 中定义的 SI 标准单位是千克 / 立方米。
克 / 立方厘米× 1000 变换为千克 / 立方米。
注 5--- 相对密度 23/23 ℃能够经过下式变换成密度 23℃,千克 / 立方米。
D23℃ , 千克 / 立方米 =相对密度 23/23 ℃× 997.64试验方法概括4.1 测定固体塑料样品在空气中的质量。
而后将其浸入液体中,测出表观质量,而后计算相对密度。
5意义和使用5.1 相对密度或密度6抽样6.1 测定相对密度的抽样单位应当要能够代表产品的数目,所要求的数据依据D1898进行。
6.1.1 假如已知或思疑样品中含有两层或多层相对密度不一样的资料,或许将成品部分或横切部分作为样品测试,或许将样品分层测试相对密度。
整体部分的相对密度不可以将各层的相对密度相加获得,除非将各层的相对百分比考虑在内。
7调理7.1 调理 --- 在试验前,依据 D618的规定将试验样品在 23±2℃的温度和 50±5% 的相对湿度下起码搁置 40 小时。
Designation:D 1505–03Standard Test Method forDensity of Plastics by the Density-Gradient Technique 1This standard is issued under the fixed designation D 1505;the number immediately following the designation indicates the year of original adoption or,in the case of revision,the year of last revision.A number in parentheses indicates the year of last reapproval.A superscript epsilon (e )indicates an editorial change since the last revision or reapproval.This standard has been approved for use by agencies of the Department of Defense.1.Scope*1.1This test method covers the determination of the density of solid plastics.1.2This test method is based on observing the level to which a test specimen sinks in a liquid column exhibiting a density gradient,in comparison with standards of known density.N OTE 1—The comparable ISO document is ISO 1183–2.There has not been any data generated to date comparing the results of the ISO method with this method.1.3The values stated in SI units are to be regarded as the standard.1.4This standard does not purport to address all of the safety concerns,if any,associated with its use.It is the responsibility of the user of this standard to establish appro-priate safety and health practices and determine the applica-bility of regulatory limitations prior to use.2.Referenced Documents 2.1ASTM Standards:2D 941Test Method for Density and Relative Density (Spe-cific Gravity)of Liquids by Lipkin Bicapillary Pycnometer D 2839Practice for Use of a Melt Index Strand for Deter-mining Density of PolyethyleneD 4703Practice for Compression Molding Thermoplastic Materials into Test Specimens,Plaques,or SheetsE 691Practice for Conducting an Interlaboratory Study to Determine the Precision of a Test Method 2.2ISO Standard:ISO 1183-2Methods for Determining the Density and Rela-tive Density of Noncellular Plastics 33.Terminology 3.1Definition:3.1.1density of plastics —the weight per unit volume of material at 23°C,expressed as follows:D 23C ,g/cm 3(1)N OTE 2—Density is to be distinguished from specific gravity,which is the ratio of the weight of a given volume of the material to that of an equal volume of water at a stated temperature.4.Significance and Use4.1The density of a solid is a conveniently measurable property which is frequently useful as a means of following physical changes in a sample,as an indication of uniformity among samples,and a means of identification.4.2This test method is designed to yield results accurate to better than 0.05%.N OTE 3—Where accuracy of 0.05%or better is desired,the gradient tube shall be constructed so that vertical distances of 1mm shall represent density differences no greater than 0.0001g/cm.3The sensitivity of the column is then 0.0001g/cm 3·mm.Where less accuracy is needed,the gradient tube shall be constructed to any required sensitivity.5.Apparatus5.1Density-Gradient Tube —A suitable graduate with ground-glass stopper.45.2Constant-Temperature Bath —A means of controlling the temperature of the liquid in the tube at 2360.1°C.A thermostatted water jacket around the tube is a satisfactory and convenient method of achieving this.5.3Glass Floats —A number of calibrated glass floats cov-ering the density range to be studied and approximately evenly distributed throughout this range.5.4Pycnometer ,for use in determining the densities of the standard floats.5.5Liquids ,suitable for the preparation of a density gradi-ent (Table 1).N OTE 4—It is very important that none of the liquids used in the tube1This test method is under the jurisdiction of ASTM Committee D20on Plastic and is the direct responsibility of Subcommittee D20.70on Analytical Methods (Section D20.70.01).Current edition approved November 1,2003.Published January 2004.Originally approved in st previous edition approved in 1998as D 1505-98.2For referenced ASTM standards,visit the ASTM website,,or contact ASTM Customer Service at service@.For Annual Book of ASTM Standards volume information,refer to the standard’s Document Summary page on the ASTM website.3Available from American National Standards Institute (ANSI),25W.43rd St.,4th Floor,New York,NY 10036.4Tubes similar to those described in Refs (6)and (12)may also be used.1*A Summary of Changes section appears at the end of this standard.Copyright ©ASTM International,100Barr Harbor Drive,PO Box C700,West Conshohocken,PA 19428-2959,United States.exert a solvent or chemical effect upon the test specimens during the time of specimen immersion.5.6Hydrometers —A set of suitable hydrometers covering the range of densities to be measured.These hydrometers should have 0.001density graduations.5.7Analytical Balance ,with a sensitivity of 0.001g.5.8Siphon or Pipet Arrangement ,for filling the gradient tube.This piece of equipment should be constructed so that the rate of flow of liquid may be regulated to 1065mL/min.6.Test Specimen6.1The test specimen shall consist of a piece of the material under test.The piece may be cut to any shape convenient for easy identification,but should have dimensions that permit the most accurate position measurement of the center of volume of the suspended specimen (Note 5).Care should be taken in cutting specimens to avoid change in density resulting from compressive stress.N OTE 5—The equilibrium positions of film specimens in the thickness range from 0.025to 0.051mm (0.001to 0.002in.)may be affected by interfacial tension.If this affect is suspected,films not less than 0.127mm (0.005in.)in thickness should be tested.6.2The specimen shall be free of foreign matter and voids and shall have no cavities or surface characteristics that will cause entrapment of bubbles.7.Preparation of Density-Gradient Columns7.1Preparation of Standard Glass Floats 5—Prepare glass floats by any convenient method such that they are fully annealed,approximately spherical,have a maximum diameter less than one fourth the inside diameter of the column,and do not interfere with the test specimens.Prepare a solution (400to 600mL)of the liquids to be used in the gradient tube such that the density of the solution is approximately equal to the desired lowest density.When the floats are at room temperature,drop them gently into the solution.Save the floats that sink very slowly,and discard those that sink very fast,or save them for another tube.If necessary to obtain a suitable range of floats,grind selected floats to the desired density by rubbing the head part of the float on a glass plate on which is spread a thin slurry of 400or 500-mesh silicon carbide (Carborundum)or otherappropriate abrasive.Progress may be followed by dropping the float in the test solution at intervals and noting its change in rate of sinking.7.2Calibration of Standard Glass Floats (see Appendix X1):7.2.1Place a tall cylinder in the constant-temperature bath maintained at 2360.1°C.Then fill the cylinder about two thirds full with a solution of two suitable liquids selected from Table 1,the density of which can be varied over the desired range by the addition of either liquid to the mixture.After the cylinder and solution have attained temperature equilibrium,place the float in the solution,and if it sinks,add the denser liquid by suitable means with good stirring until the float reverses direction of movement.If the float rises,add the less dense liquid by suitable means with good stirring until the float reverses direction of movement.7.2.2When reversal of movement has been observed,re-duce the amount of the liquid additions to that equivalent to 0.0001-g/cm 3density.When an addition equivalent to 0.0001-g/cm 3density causes a reversal of movement,or when the float remains completely stationary for at least 15min,the float and liquid are in satisfactory balance.The cylinder must be covered whenever it is being observed for balance,and the liquid surface must be below the surface of the liquid in the constant-temperature bath.After vigorous stirring,the liquid may continue to move for a considerable length of time;make sure that the observed movement of the float is not due to liquid motion by waiting at least 15min after stirring has stopped before observing the float.7.2.3When balance has been obtained,fill a freshly cleaned and dried pycnometer with the solution and place it in the 2360.1°C bath for sufficient time to allow temperature equilib-rium of the glass.Determine the density of the solution by normal methods (Test Method D 941)and make “in vacuo”corrections for all weighings.Record this as the density of the float.Repeat the procedure for each float.7.3Gradient Tube Preparation (see appendix for details):7.3.1Method A —Stepwise addition.7.3.2Method B —Continuous filling (liquid entering gradi-ent tube becomes progressively less dense).7.3.3Method C —Continuous filling (liquid entering gradi-ent tube becomes progressively more dense).8.Conditioning8.1Test specimens whose change in density on conditioning may be greater than the accuracy required of the density determination shall be conditioned before testing in accordance with the method listed in the applicable ASTM material specification.9.Procedure9.1Wet three representative test specimens with the less dense of the two liquids used in the tube and gently place them in the tube.Allow the tube and specimens to reach equilibrium,which will require 10min or more.Thin films of 1to 2mils in thickness require approximately 11⁄2h to settle,and rechecking after several hours is advisable (Note 4).9.2Read the height of each float and each specimen by a line through the individual center of volume and averaging the5Glass floats may be purchased from American Density Materials,3826Springhill Rd.Staunton,V A 24401,Ph:(540)887-1217.TABLE 1Liquid Systems for Density-Gradient TubesSystemDensity Range,g/cm 3Methanol-benzyl alcohol 0.80to 0.92Isopropanol-water0.79to 1.00Isopropanol-diethylene glycol 0.79to 1.11Ethanol-carbon tetrachloride 0.79to 1.59Toluene-carbon tetrachloride 0.87to 1.59Water-sodium bromide 1.00to 1.41Water-calcium nitrate1.00to 1.60Carbon tetrachloride-trimethylene dibromide 1.60to 1.99Trimethylene dibromide-ethylene bromide 1.99to2.18Ethylene bromide-bromoform2.18to2.89three values.When a cathetometer is used,measure the height of thefloats and specimens from an arbitrary level using a line through their center of volume.If equilibrium is not obtained, the specimen may be imbibing the liquid.9.3Old samples can be removed without destroying the gradient by slowly withdrawing a wire screen basket attached to a long wire(Note6).This can be conveniently done by means of a clock motor.Withdraw the basket from the bottom of the tube and,after cleaning,return it to the bottom of the tube.It is essential that this procedure be performed at a slow enough rate(approximately30min/300-mm length of column) so that the density gradient is not disturbed.N OTE6—Whenever it is observed that air bubbles are collecting on samples in the column,a vacuum applied to the column will correct this.10.Calculation10.1The densities of the samples may be determined graphically or by calculation from the levels to which the samples settle by either of the following methods:10.1.1Graphical Calculation—Plotfloat position versus float density on a chart large enough to be read accurately to 61mm and the desired precision of density.Plot the positions of the unknown specimens on the chart and read their corre-sponding densities.10.1.2Numerical Calculation—Calculate the density by interpolation as follows:Density at x5a1[~x2y!~b2a!/~z2y!#(2) where:a and b=densities of the two standardfloats,y and z=distances of the two standards,a and b,respec-tively,bracketing the unknown measured froman arbitrary level,andx=distance of unknown above the same arbitrary level.11.Report11.1Report the following information:11.1.1Density reported as D23C,in grams per cubic centimetre,as the average for three representative test speci-mens,11.1.2Number of specimens tested if different than three, 11.1.3Sensitivity of density gradient in grams per cubic centimetre per millimetre,11.1.4Complete identification of the material tested,and 11.1.5Date of the test.12.Precision and Bias612.1Specimens Molded in One Laboratory and Tested in Several Laboratories—An interlaboratory test was run in1981 in which randomized density plaques were supplied to22 laboratories.Four polyethylene samples of nominal densities of0.92to0.96g/cm3were molded in one laboratory.The data were analyzed using Practice E691,and the results are given in Table2.12.2Specimens Molded and Tested in Several Laboratories: 12.2.1Samples Prepared Using Practice D4703in Each Laboratory—Table3is based on a round robin9conducted in 1994in accordance with Practice E691,involving seven materials tested by7to11laboratories.For each material,all of the samples were prepared by each laboratory,molded in accordance with Procedure C of Annex A1of Practice D4703, and tested using this test method.The data are for comparison with the data of the same samples tested by Practice D2839. Each test result is an individual determination.Each laboratory obtained six test results for each material.12.2.2Samples Prepared Using Practice D2839in Each Laboratory—Table4is based on a round robin9conducted in 1994in accordance with Practice E691,involving seven materials tested by10to15laboratories.For each material,all of the samples were prepared by each laboratory in accordance with Practice D2839.Each test result is an individual deter-mination.Each laboratory obtained six test results for each material.12.3Concept of r and R—Warning—The following expla-nations of r and R(12.3-12.3.3)are only intended to present a meaningful way of considering the approximate precision of this test method.The data in Table1should not be rigorously applied to acceptance or rejection of material,as those data are specific to the round robin and may not be representative of other lots,conditions,materials,or ers of this test method should apply the principles outlined in Practice E691to generate data specific to their laboratory and materi-als,or between specific laboratories.The principles of12.3-12.3.3would then be valid for each data.If S r and S R have been calculated from a large enough body of data,and for test results that were averages from testing one specimen:12.3.1Repeatability Limit,r(Comparing two test results for the same material,obtained by the same operator using the 6Supporting data are available from ASTM Headquarters.Request RR:D20-1123.TABLE2Precision Data Summary—Polyethylene DensityMaterial Average Density,g/cm3S r A S R B r C R D10.91960.000290.001060.000820.004520.93190.000120.000800.000340.002330.95270.000330.001160.000930.003340.96230.000620.001140.001800.0033A Sr=within-laboratory standard deviation for the indicated material.It is obtained by pooling the within-laboratory standard deviations of the test results from all of the participating laboratories.B SR =between-laboratories reproducibility,expressed as standard deviation,for the indicated material.C r=within-laboratory repeatability limit=2.8Sr .D R=between-laboratories reproducibility limit=2.8SR.same equipment on the same day)—The two test results should be judged not equivalent if they differ by more than the r value for that material.12.3.2Reproducibility Limit,R (Comparing two test results for the same material,obtained by different operators using different equipment in different laboratories)—The two test results should be judged not equivalent if they differ by more than the R value for that material.12.3.3Any judgment in accordance with 12.2.1or 12.2.2would have an approximate 95%(0.95)probability of being correct.12.3.4Bias —There are no recognized standards by which to estimate the bias of this test method.13.Keywords13.1density;film;gradient;plaque;polyolefins;polyeth-ylene;polypropylene;preparationAPPENDIXES(Nonmandatory Information)X1.FLOAT CALIBRATION—ALTERNATIVE TEST METHODX1.1This test method of float calibration has been found by one laboratory to save time and give the same accuracy as the standard test method.Its reliability has not been demon-strated by round-robin data.X1.1.1Prepare a homogeneous solution whose density is fairly close to that of the float in question.X1.1.2Fill a graduate about 3⁄4full with the solution,drop in the float,stopper,and place in a thermostatted water bath near 23°C.Fill a tared two-arm pycnometer (Test Method D 941,or equivalent)with the solution.Place the pycnometer in the bath.X1.1.3Vary the bath temperature until the solution density is very near to that of the float.(If the float was initially on the bottom of the graduate,lower the bath temperature until the float rises;if the float floated initially,raise the bath tempera-ture until the float sinks to the bottom.)X1.1.4Change the bath temperature in the appropriate direction in increments corresponding to solution density increments of about 0.0001g/cm 3until the float reverses direction of movement as a result of the last change.This must be done slowly (at least 15-min intervals between incremental changes on the temperature controller).Read the volume of liquid in the pycnometer.X1.1.5Change the bath temperature in increments in the opposite direction,as above,until a change in the float position again occurs.Read the volume of liquid in the pycnometer.N OTE X1.1—The float should rise off the bottom of its own volition.As a precaution against surface tension effects when the float is floating,the float should be pushed about halfway down in the liquid column and then observed as to whether it rises or falls.For this purpose,a length of Nichrome wire,with a small loop on the lower end and an inch or so of length extending above the liquid surface,is kept within the graduate throughout the course of the run.To push a floating float down,the cylinder is unstoppered and the upper wire end grasped with tweezers for the manipulations.The cylinder is then quickly restoppered.X1.1.6Remove the pycnometer from the bath,dry the outside,and set aside until the temperature reaches ambient temperature.Weigh and calculate the “in vacuo”mass of solution to ing the average of the two observed solution volumes,calculate the density of the solution to 0.0001g/cm 3.This solution density is also the float density.X1.1.7The pycnometer used should be calibrated for vol-ume from the 23°C calibration,although the reading is taken at a different temperature.The alternative test method is based on a number of unsupported assumptions but generally gives the same results as that described in 7.2within the accuracyTABLE 3Precision Data—Density,g/cm 3Material Number ofLaboratoriesDensity,g/cm 3S r A S R B r C R DB 70.91390.000290.000880.000810.00245F 80.91770.000180.000790.000510.00221G 80.92200.000280.000710.000780.00197A 110.93560.000360.001050.001000.00294E 110.95280.000460.001180.001290.00331C 100.96190.001000.001000.001030.00281D90.96330.000360.001370.001010.00384AS r =within-laboratory standard deviation for the indicated material.It is obtained by pooling the within-laboratory standard deviations of the test results from all of the participating laboratories.BS R =between-laboratories reproducibility,expressed as standard deviation,for the indicated material.Cr =within-laboratory repeatability limit =2.8S r .DR =between-laboratories reproducibility limit =2.8S R .TABLE 4Density,g/cm 3,Samples Prepared in Accordance WithPractice D 2839MaterialNumber ofLaboratoriesDensity,g/cm 3S r A S R B r C R D B 100.91390.000260.000780.000720.00219F 120.91790.000200.000780.000550.00220G 130.92220.000300.000730.000850.00206A 150.93570.000410.000800.001150.00225E 140.95300.000390.000920.001090.00258C 110.96150.000300.000730.000850.00206D100.96260.000530.001090.001480.00305AS r =within-laboratory standard deviation for the indicated material.It is obtained by pooling the within-laboratory standard deviations of the test results from all of the participating laboratories.BS R =between-laboratories reproducibility,expressed as standard deviation,for the indicated material.Cr =within-laboratory repeatability limit =2.8S r .DR =between-laboratories reproducibility limit =2.8S R.required.In case of disagreement,the method described in7.2shall be the referee method.X2.GRADIENT TUBE PREPARATIONX2.1Method A—Stepwise Addition:X2.1.1Using the two liquids that will give the desireddensity range,and sensitivity(S)in grams per cubic centimetreper millimetre,prepare four or more solutions such that eachdiffers from the next heavier by80S g/cm3.The number ofsolutions will depend upon the desired density range of thecolumn and shall be determined as follows:Numbers of solutions to prepare density2gradient(X2.1)column~Note X2.1!5~11D22D1!/80S(X2.1)where:D2=upper limit of density range desired,D1=lower limit of density range desired,andS=sensitivity,in grams per cubic centimetre per milli-metre.N OTE X2.1—Correct the value of(1+D2−D1)/80S to the nearestwhole number.To prepare these solutions,proceed as follows:Using the hydrometers,mix the two liquids in the proportions necessary to obtain the desired solutions.Remove the dissolved air from the solutions by gentle heating or an applied vacuum.Then check the density of the solutions at2360.1°C by means of the hydrometers and,if necessary,add the appropriate air-free liquid until the desired density is obtained.N OTE X2.2—Where aqueous mixtures are used,0.5%aqueous sodium acetate should be used to prepare the mixture.This reduces the formation of bubbles from dissolution.N OTE X2.3—In order to obtain a linear gradient in the tube,it is very important that the solutions be homogeneous and at the same temperature when their densities are determined.It is also important that the density difference between the solutions consecutively introduced into the tube be equal.X2.1.2By means of a siphon or pipet,fill the gradient tube with an equal volume of each liquid starting with the heaviest, taking appropriate measures to prevent air from being dis-solved in the liquid.After the addition of the heaviest liquid, very carefully and slowly pour an equal volume of the second heaviest liquid down the side of the column by holding the siphon or pipet against the side of the tube at a slight angle. Avoid excess agitation and turbulence.In this manner,the “building”of the tube shall be completed.N OTE X2.4—Density gradients may also be prepared by reversing the procedure described in X2.1.1and X2.1.2.When this procedure is used, the lightest solution is placed in the tube and the next lightest solution is very carefully and slowly“placed”in the bottom of the tube by means of a pipet or siphon which just touches the bottom of the tube.In this manner the“building”of the tube shall be completed.X2.1.3If the tube is not already in a constant-temperature bath,transfer the tube,with as little agitation as possible,to the constant-temperature bath maintained at2360.1°C.The bath level should approximately equal that of the solution in the tube,and provision should be made for vibrationless mounting of the tube.X2.1.4For every254mm of length of tube,dip a minimum offive clean calibratedfloats,spanning the effective range of the column,into the less dense solvent used in the preparation of the gradient tube and add them to the tube.By means of a stirrer(for example,a small coiled wire or other appropriate stirring device)mix the different layers of the tube gently by stirring horizontally until the least dense and most densefloats span the required range of the gradient tube.If,at this time,it is observed that thefloats are“bunched”together and not spread out evenly in the tube,discard the solution and repeat the procedure.Then cap the tube and keep it in the constant-temperature bath for a minimum of24h.X2.1.5At the end of this time,plot the density offloats versus the height offloats to observe whether or not a fairly smooth and nearly linear curve is obtained.Some small irregularities may be seen,but they should be slight.Whenever an irregular curve is obtained,the solution in the tube shall be discarded and a new gradient prepared.N OTE X2.5—Gradient systems may remain stable for several months. X2.2Method B—Continuous Filling with Liquid Entering Gradient Tube Becoming Progressively Less Dense:X2.2.1Assemble the apparatus as shown in Fig.X2.1,using beakers of the same diameter.Then select an appropriate amount of two suitable liquids which previously have been carefully deaerated by gentle heating or an applied vacuum. Typical liquid systems for density-gradient tubes are listed in Table1.The volume of the more dense liquid used in the mixer (Beaker B shown in Fig.X2.1)must be equal to at least one half of the total volume desired in the gradient tube.An FIG.X2.1Apparatus for Gradient TubePreparationestimate of the volume of the less dense liquid required in Beaker A to establishflow from A to B can be obtained from the following inequality:V A.d B V B/d A(X2.2) where:V A=starting liquid volume in Beaker A,V B=starting liquid volume in Beaker B,d A=density of the starting liquid in Beaker A,andd B=density of the starting liquid in Beaker B.A small excess(not exceeding5%)over the amount indicated by the preceding equality will induce the required flow from A toB and yield a very nearly linear gradient column.X2.2.2Place an appropriate volume of the denser liquid into Beaker B of suitable size.Prime the siphon between Beaker B and the gradient tube with liquid from Beaker B and then close the stopcock.The delivery end of this siphon should be equipped with a capillary tip forflow control.N OTE X2.6—Techniques acceptable for transfer of liquid into the gradient tube are siphon/gravity,vacuum-filling,use of a peristatic pump, or any other technique useful to transfer liquids in a controlled manner.It is important to control theflow in order to maintain a desirable gradient. X2.2.3Place an appropriate volume of the less dense liquid into Beaker A.Prime the siphon between Beakers A and B with the liquid from Beaker A and close the stopcock.Start the highspeed,propeller-type stirrer in Beaker B and adjust the speed of stirring such that the surface of the liquid does not fluctuate greatly.X2.2.4Start the delivery of the liquid to the gradient tube by opening the necessary siphon-tube stopcocks simultaneously. Adjust theflow of liquid into the gradient tube at a very slow rate,permitting the liquid toflow down the side of the tube.Fill the tube to the desired level.N OTE X2.7—Preparation of a suitable gradient tube may require1to 11⁄2h or longer,depending upon the volume required in the gradient tube. X2.3Method C—Continuous Filling with Liquid Entering Gradient Tube Becoming Progressively More Dense:X2.3.1This method is essentially the same as Method B with the following exceptions:X2.3.2The lighter of the two liquids is placed in Beaker B. X2.3.3The liquid introduced into the gradient column is introduced at the bottom of the column.Thefirst liquid introduced is the lighter end of the gradient and is constantly pushed up in the tube as the liquid being introduced becomes progressively heavier.X2.3.4The liquid from Beaker A must be introduced into Beaker B by directflow from the bottom of Beaker A to the bottom of Beaker B,rather than being siphoned over as it is in Method B.Filling the tube by this method may be done more rapidly than by Methods A or B.The stopcock between Containers A and B should be of equal or larger bore than the outlet stopcock.A schematic drawing of the apparatus for Method C is shown in Fig.X2.2.REFERENCES (1)Linderstrøm-Lang,K.,“Dilatometric Ultra-Micro-Estimation of Pep-tidase Activity,”Nature,NATRA,V ol139,1937,p.713.(2)Linderstrøm-Lang,K.,and Lanz,H.,“Enzymic Histochemistry XXIXDilatometric Micro-Determination of Peptidase Activity,”Comptesrendus des gravaus de laboratorie Carlsberg,Serie Chimique,V ol21,1938,p.315.(3)Linderstrøm-Lang,K.,Jacobsen,O.,and Johansen,G.,“Measurementof the Deuterium Content in Mixtures of H2O and D2O,”ibid.,V ol23,1938,p.17.(4)Jacobsen,C.F.,and Linderstrøm-Lang,K.,“Method for Rapid Deter-mination of Specific Gravity,”Acta Physiologica Scandinavica,AP-SCA,V ol1,1940,p.149.(5)Boyer,R.F.,Spencer,R.S.,and Wiley,R.M.,“Use of Density-Gradient Tube in the Study of High Polymers,”Journal of Polymer Science,JPSCA,V ol1,1946,p.249.(6)Anfinsen,C.,“Preparation and Measurement of Isotopic Tracers:ASymposium Prepared for the Isotope Research Group,”Edwards,J.W.,Publishers,Ann Arbor,MI,1946,p.61.(7)Tessler,S.,Woodberry,N.T.,and Mark,H.,“Application of theDensity-Gradient Tube in Fiber Research,”Journal of Polymer Sci-ence,JPSCA,V ol1,1946,p.437.(8)Low,B.W.,and Richards,F.M.,“The Use of the Gradient Tube forthe Determination of Crystal Densities,”Journal of the American Chemical Society,JACSA,V ol74,1952,p.1660.(9)Sperati,C.A.,Franta,W.A.,and Starkweather,H.W.,Jr.,“TheMolecular Structure of Polyethylene V,the Effect of Chain Branching and Molecular Weight on Physical Properties,”Journal of the Ameri-can Chemical Society,JACSA,V ol75,1953,p.6127.(10)Tung,L.H.,and Taylor,W.C.,“An Improved Method of PreparingDensity Gradient Tubes,”Journal of Polymer Science,JPSCA,V ol 21,1956,p.144.(11)Mills,J.M.,“A Rapid Method of Construction Linear DensityGradient Columns,”Journal of Polymer Science,V ol19,1956,p.585.(12)Wiley,R.E.,“Setting Up a Density Gradient Laboratory,”PlasticsTechnology,PLTEA,V ol8,No.3,1962,p.31.FIG.X2.2Apparatus for Gradient TubePreparation。