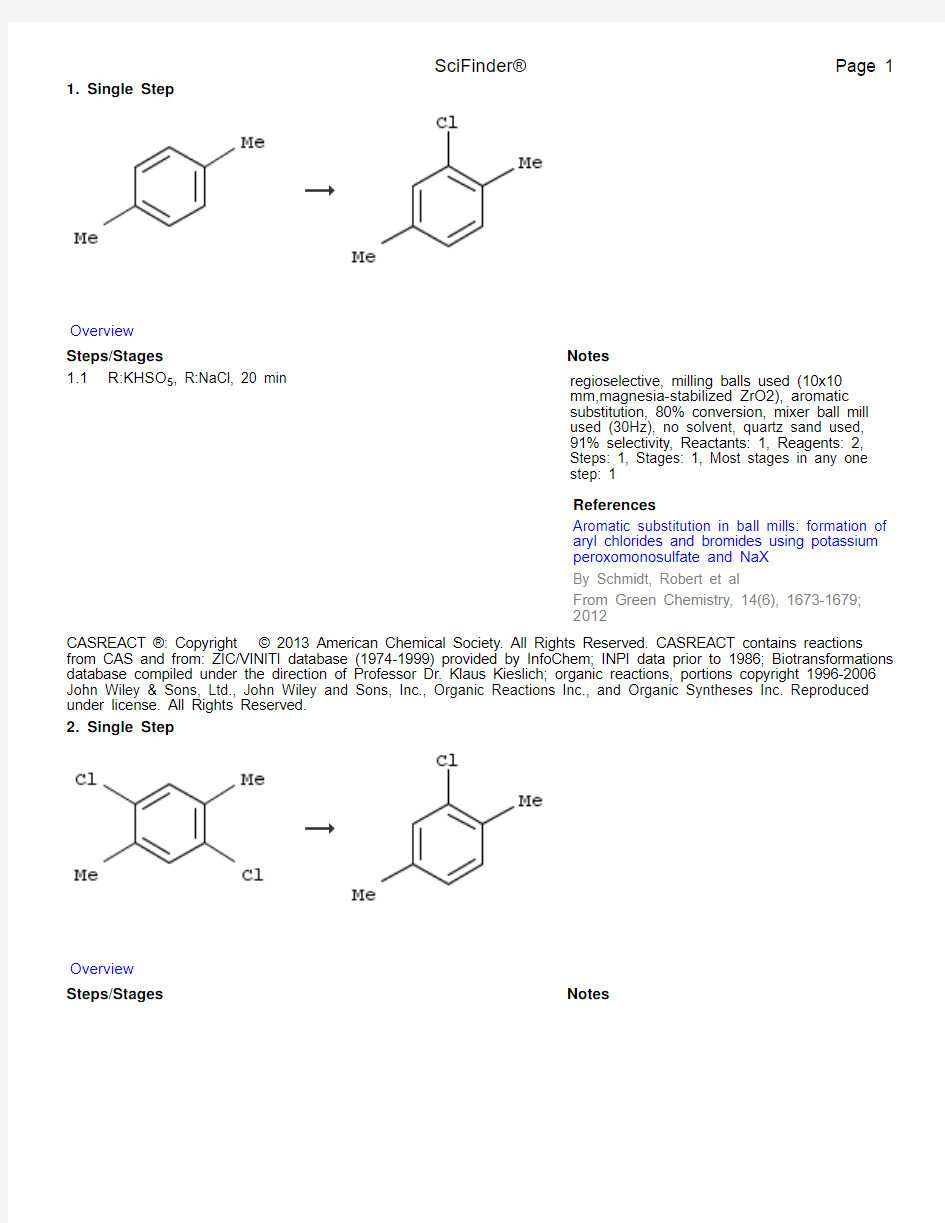
1. Single Step
Overview
Steps/Stages Notes
1.1R:KHSO5, R:NaCl, 20 min regioselective, milling balls used (10x10
mm,magnesia-stabilized ZrO2), aromatic
substitution, 80% conversion, mixer ball mill
used (30Hz), no solvent, quartz sand used,
91% selectivity, Reactants: 1, Reagents: 2,
Steps: 1, Stages: 1, Most stages in any one
step: 1
References
Aromatic substitution in ball mills: formation of
aryl chlorides and bromides using potassium
peroxomonosulfate and NaX
By Schmidt, Robert et al
From Green Chemistry, 14(6), 1673-1679;
2012
CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.
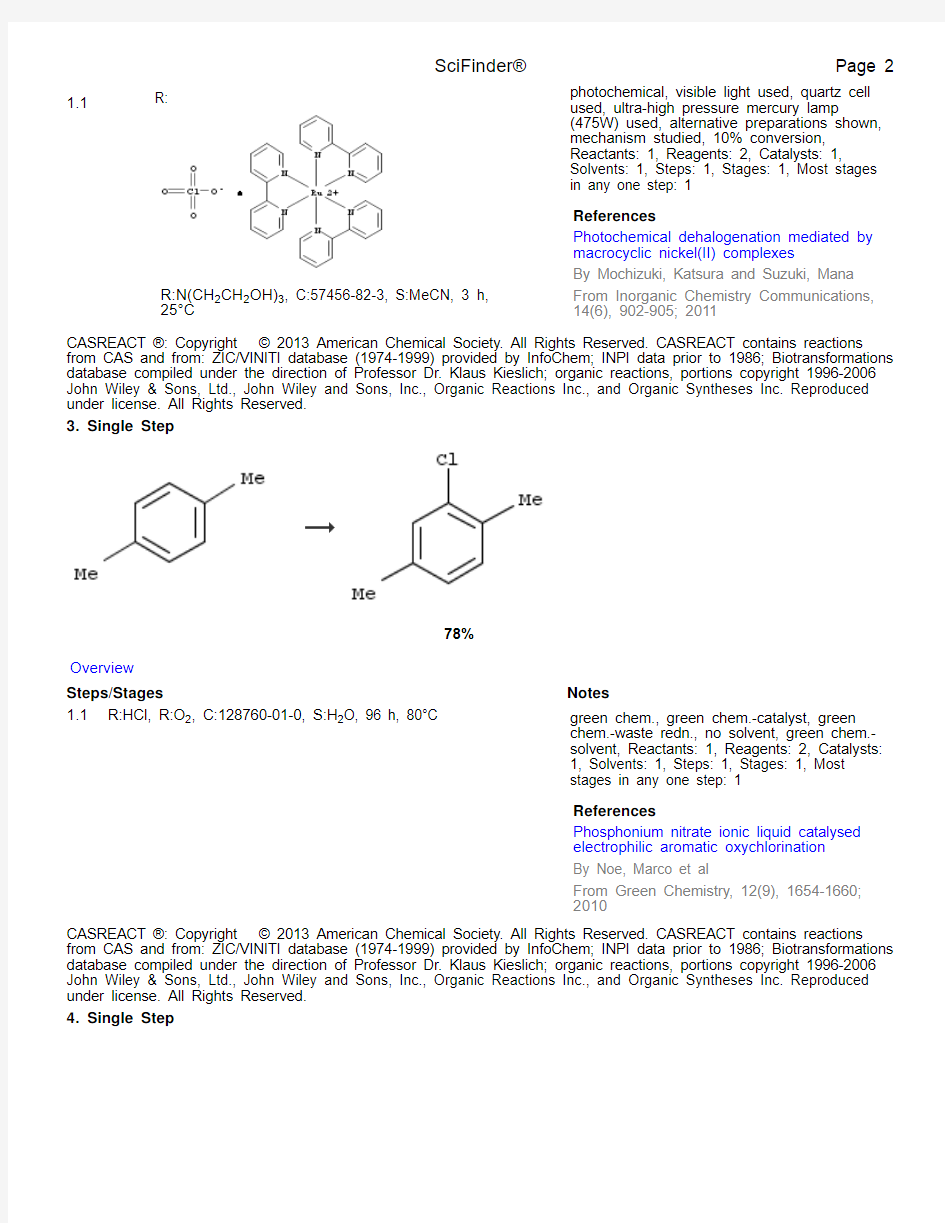
2. Single Step
Overview
Steps/Stages Notes
1.1
R:
R:N(CH2CH2OH)3, C:57456-82-3, S:MeCN, 3 h,
25°C photochemical, visible light used, quartz cell used, ultra-high pressure mercury lamp (475W) used, alternative preparations shown, mechanism studied, 10% conversion, Reactants: 1, Reagents: 2, Catalysts: 1, Solvents: 1, Steps: 1, Stages: 1, Most stages in any one step: 1
References
Photochemical dehalogenation mediated by macrocyclic nickel(II) complexes
By Mochizuki, Katsura and Suzuki, Mana From Inorganic Chemistry Communications, 14(6), 902-905; 2011
CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.
3. Single Step
78%
Overview
Steps/Stages Notes
1.1R:HCl, R:O2, C:128760-01-0, S:H2O, 96 h, 80°C green chem., green chem.-catalyst, green
chem.-waste redn., no solvent, green chem.-
solvent, Reactants: 1, Reagents: 2, Catalysts:
1, Solvents: 1, Steps: 1, Stages: 1, Most
stages in any one step: 1
References
Phosphonium nitrate ionic liquid catalysed
electrophilic aromatic oxychlorination
By Noe, Marco et al
From Green Chemistry, 12(9), 1654-1660;
2010
CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.
4. Single Step
34%12%
Overview
Steps/Stages Notes
1.1R:CuCl2, R:N-Chlorosuccinimide, 4 min, 102°C, 3.5 bar microwave irradiation, regioselective,
Reactants: 1, Reagents: 2, Steps: 1, Stages:
1, Most stages in any one step: 1
References
Microwave assisted solid additive effects in
simple dry chlorination reactions with N-
chlorosuccinimide
By Bucos, Madalina et al
From Tetrahedron, 66(11), 2061-2065; 2010 CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.
5. Single Step
3%48%
Overview
Steps/Stages Notes
1.1R:KBr, R:N-Chlorosuccinimide, 4 min, 102°C, 3.5 bar microwave irradiation, regioselective,
Reactants: 1, Reagents: 2, Steps: 1, Stages:
1, Most stages in any one step: 1
References
Microwave assisted solid additive effects in
simple dry chlorination reactions with N-
chlorosuccinimide
By Bucos, Madalina et al
From Tetrahedron, 66(11), 2061-2065; 2010 CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.
6. Single Step
72%14%
Overview
Steps/Stages Notes
1.1R:Isocyanuric chloride, C:657414-80-7, S:657414-80-7, 48 h, 56-
58°C
green chem., optimized on time, ionic liquid
used, optimization study, Reactants: 1,
Reagents: 1, Catalysts: 1, Solvents: 1, Steps:
1, Stages: 1, Most stages in any one step: 1
References
Chlorination of Aromatics with
Trichloroisocyanuric Acid (TCICA) in
Bronsted-Acidic Imidazolium Ionic Liquid
[BMIM(SO3H)][OTf]: an Economical, Green
Protocol for the Synthesis of Chloroarenes
By Hubbard, Abigail et al
From Australian Journal of Chemistry, 60(12),
923-927; 2007
CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.
7. Single Step
Overview
Steps/Stages Notes
1.1R:N-Chlorosuccinimide, C:Montmorillonite, S:ClCH2CH2Cl, 16-18
h, 80°C regioselective, Reactants: 1, Reagents: 1, Catalysts: 1, Solvents: 1, Steps: 1, Stages: 1, Most stages in any one step: 1
References
Regiospecific chlorination of xylenes using K-10 montmorillonite clay
By Thirumamagal, B. T. S. et al
From Synthetic Communications, 38(16), 2820-2825; 2008
Experimental Procedure
General/Typical Procedure: Typical Procedure for Chlorination of Aromatic Compound using NCS and
K-10 Montmorrillonite Clay in 1,2-Dichloroethane To a solution of aromatic compound (100mmol) in a
dry 1,2-dichloroethane (50mL), NCS (100mmol) and K-10 montmorrillonite clay (4g) were added and
heated at 80°C for 16-18 h. The crystallized succinimide along with the clay was filtered off and
separated by washing with dichloromethane. The solvent was removed by distillation from the filtrate.
The liquid chlorinated product was purified by passing through a silica-gel column using hexanes/ethyl
acetate (98:2) as the eluent. 2-Chloro-1,4-xylene (6): liquid; yield (8.6 g, 62%). 1H NMR (CDCl3): δ 6.8-
6.9 (m, 3H, Ar), 2.35 (s, 3H, Me), 2.31 (s, 3H, Me); 13C NMR 13
7.7, 135.5, 131.0, 130.8, 12
8.3, 126.9,
20.7, 11.9; MS: m/z (%) 140.51 (100.0), 142.34 (32.0).
CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.
8. Single Step
Overview
Steps/Stages Notes
1.1C:AlCl3, 1 h, 25°C Chlorination, Reactants: 2, Catalysts: 1, Steps:
1, Stages: 1, Most stages in any one step: 1
References
CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.
9. Single Step
Overview
Steps/Stages Notes
1.1
R:
R:HCl, S:H2O, 48 h, 80°C
regioselective, Reactants: 1, Reagents: 2,
Solvents: 1, Steps: 1, Stages: 1, Most stages
in any one step: 1
References
[Hmim][NO3]-an efficient solvent and
promoter in the oxidative aromatic
chlorination
By Chiappe, Cinzia et al
From Green Chemistry, 8(8), 742-745; 2006 CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.
10. 3 Steps
[Step 2.1]
Overview
Steps/Stages Notes
1.1R:H2SO4, R:HNO3, S:H2O, 0°C; 2 h, 60°C
2.1R:NaOH, S:EtOH, 70 h; 2 h, reflux
3.1R:N2H4, S:EtOH, 2 h, 80°C Reactants: 2, Reagents: 4, Solvents: 2, Steps: 3, Stages: 3, Most stages in any one step: 1
References
Preparation and spectroscopic study of 13-substituted 2,11-
dithiahexahydro[3.3]paracyclophanes
By Lin, Shaw-Tao et al
From Journal of Chemical Research, (11), 708-711; 2005
CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.
11. 2 Steps
Overview
Steps/Stages Notes
1.1R:NaOH, S:EtOH, 70 h; 2 h, reflux
2.1R:N2H4, S:EtOH, 2 h, 80°C
Reactants: 2, Reagents: 2, Solvents: 1, Steps:
2, Stages: 2, Most stages in any one step: 1
References
Preparation and spectroscopic study of 13-
substituted 2,11-
dithiahexahydro[3.3]paracyclophanes
By Lin, Shaw-Tao et al
From Journal of Chemical Research, (11),
708-711; 2005
CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.
12. Single Step
60%
Overview
Steps/Stages Notes
1.1R:N2H4, S:EtOH, 2 h, 80°C Reactants: 1, Reagents: 1, Solvents: 1, Steps:
1, Stages: 1, Most stages in any one step: 1
References
Preparation and spectroscopic study of 13-
substituted 2,11-
dithiahexahydro[3.3]paracyclophanes
By Lin, Shaw-Tao et al
From Journal of Chemical Research, (11),
708-711; 2005
CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.
13. Single Step
89%
Overview
Steps/Stages Notes
1.1R:Cl2, C:AlCl3, 5 h, 70-75°C optimization study, optimized on catalyst,
temperature, Reactants: 1, Reagents: 1,
Catalysts: 1, Steps: 1, Stages: 1, Most stages
in any one step: 1
References
Preparation of 3(2)-chloro-para-methylbenzyl
chloride
By Mao, Limin et al
From Faming Zhuanli Shenqing Gongkai
Shuomingshu, 1817832, 16 Aug 2006 CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.
14. Single Step
68%
Overview
Steps/Stages Notes
1.1R:NaCl, R:PhI(OAc)2, 0.5 h, rt Grindstone chem., no solvent, regioselective,
solid state, Reactants: 1, Reagents: 2, Steps:
1, Stages: 1, Most stages in any one step: 1
References
Grindstone chemistry:
(diacetoxyiodo)benzene-mediated oxidative
nuclear halogenation of arenes using NaCl,
NaBr or I2
By Karade, N. N. et al
From Journal of Chemical Research, (6), 366-
368; 2006
CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.
15. Single Step
82%16%
Overview
Steps/Stages Notes
1.1R:HCl, 55-65°C chemoselective, electrochem. (6000 A/m2),
Reactants: 1, Reagents: 1, Steps: 1, Stages:
1, Most stages in any one step: 1
References
Some features of electrochemical chlorination
of alkyl aromatic hydrocarbons on a side
chain
By Yuzbekov, Yu. A. and Maksimov, Kh. A.
From Sakartvelos Mecnierebata Akademiis
Macne, Kimiis Seria, 28(3-4), 246-252; 2002 CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.
16. Single Step
54%35%
Overview
Steps/Stages Notes
1.1R:HCl, 55-65°C chemoselective, electrochem. (2000 A/m2),
Reactants: 1, Reagents: 1, Steps: 1, Stages:
1, Most stages in any one step: 1
References
Some features of electrochemical chlorination
of alkyl aromatic hydrocarbons on a side
chain
By Yuzbekov, Yu. A. and Maksimov, Kh. A.
From Sakartvelos Mecnierebata Akademiis
Macne, Kimiis Seria, 28(3-4), 246-252; 2002 CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.
17. Single Step
100%
Overview
Steps/Stages Notes
1.1R:HCl, R:H2O2, C:F3CCH2OH, S:H2O, 15 h, 25°C Reactants: 1, Reagents: 2, Catalysts: 1,
Solvents: 1, Steps: 1, Stages: 1, Most stages
in any one step: 1
References
Electrophilic Aromatic Chlorination and
Haloperoxidation of Chloride Catalyzed by
Polyfluorinated Alcohols: A New Manifestation
of Template Catalysis
By Ben-Daniel, Revital et al
From Journal of the American Chemical
Society, 125(40), 12116-12117; 2003 Experimental Procedure
Product: 2-chloro-p-xylene (100) Yield: 98%
CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.
18. Single Step
86%
Overview
Steps/Stages Notes
1.1R:AcCl, C:Ce(NH4)2(NO3)6, S:MeCN, 7-8 h, rt
1.2S:Et2O, rt
1.3R:NaHCO3, R:NaCl, S:H2O, rt
regioselective, Reactants: 1, Reagents: 3,
Catalysts: 1, Solvents: 3, Steps: 1, Stages: 3,
Most stages in any one step: 3
References
A novel and efficient ceric ammonium nitrate
catalyzed oxidative nuclear chlorination of
activated aromatic compounds by acetyl
chloride
By Roy, Subhas Chandra et al
From Synlett, (2), 221-222; 2003 CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.
19. Single Step
Overview
Steps/Stages Notes
1.1R:Selectfluor, R:NaCl, S:MeCN52% conversion, Reactants: 1, Reagents: 2,
Solvents: 1, Steps: 1, Stages: 1, Most stages
in any one step: 1
References
Novel process for generating useful
electrophiles from common anions using
Selectfluor fluorination agent
By Syvret, Robert G. et al
From Journal of Organic Chemistry, 67(13),
4487-4493; 2002
Experimental Procedure
General/Typical Procedure: General Procedure. In a typical experiment, Selectfluor fluorination agent
(7.1 g, 20.0 mmol), NaSCN (1.6 g, 19.7 mmol), and 200 mL of ACN were combined in a 500 mL round
bottom flask containing a magnetic stir bar. Compound 2a (2.2 g, 20.3 mmol) was added, and the
contents of the flask were stirred under nitrogen and sampled periodically to monitor the reaction
progress. After the mixture was stirred for 20 h at room temperature, analysis by GC and GC-MS
indicated that 67% conversion of the 2a had been achieved, with a product distribution of 1 and 62%
ortho- and para-methoxyphenylthiocyanate 2e, respectively. At this point, the solvent was removed in
a vacuum, and the resulting residue was treated with 200 mL of deionized water. This aqueous mixture
was extracted with 2 100 mL portions of methylene chloride; the methylene chloride extracts were
combined, dried (MgSO4), and then evaporated to a crude product residue. This residue was purified
by chromatography on silica gel using a mixture of 5% ethyl acetate and 95% hexane. A product
fraction was collected, dried over MgSO4, and then analyzed by NMR, GCMS, and GC-IR and shown
to be a mixture of ortho and para isomers of 2e, but predominately the para isomer. chloro-p-xylene
10c, 100% mol.
CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.
20. Single Step
100%
Overview
Steps/Stages Notes
1.1R:Selectfluor, R:NaCl, S:DMF
1.2S:H2O
alternative prepn. gave lower yields,
Reactants: 1, Reagents: 2, Solvents: 2, Steps:
1, Stages: 2, Most stages in any one step: 2
References
Process for generating electrophiles from
anions by reaction with electrophilic
fluorinating agent
By Syvret, Robert George et al
From Eur. Pat. Appl., 1138657, 04 Oct 2001 Experimental Procedure
Example 14: Chlorination of p-xylene 15.0 g (42 mmol) of F-TEDA-BF4 fluorination agent, 1.17g (20
mmol) of NaCl, and 200 mL of acetonitrile were added to a 500-mL round bottom flask containing a
magnetic stir bar and stirring was commenced. To this was added 2.13 g (20 mmol) of p-xylene and
the contents of the flask are stirred under nitrogen. After 93 hours of stirring at room temperature,
analysis by GC and GC-MS indicated that complete conversion of the p-xylene starting material had
been achieved. At this point, the solvent was removed under vacuum on a Rotavap. To the resulting
residue was added 100 mL of de-ionized water. This aqueous mixture was extracted with 3 x 100 mL
portions of chloroform. The chloroform extracts were combined, dried with MgSO4, and evaporated to
a product residue which weighed 2.02 g. A portion of this residue was dissolved in acetonitrile and
analyzed by GC-MS and shown to contain primarily 2-chloro-1,4-dimeth-ylbenzene as well as some
dichlorinated and fluorinated products. The product residue was purified by chromatography on silica
gel using hexane as the eluent. Two product fractions were collected, evaporated to residues, and
then analyzed separately by NMR spectroscopy and GC-MS. The analysis were consistent with 2-
chloro-1,4-dimethylbenzene as the primary product in each fraction. 2-chloro-1,4-dimethylbenzene
NMR (CDCl3): (1H) 2.31 ppm (s, 3H), 2.36 ppm (s, 3H), 6.94 -7.18 ppm (m, 3H); 13C{1H}: 19.41 ppm
(s, 1C), 20.62 ppm (s; 1C), 127.32 ppm (s, 1C), 129.62 ppm (s, 1C), 130.66 ppm (s, 1C), 132.79 ppm
(s, 1C), 134.25 ppm (s, 1C), 136.93(s, 1C); MS (El): m/e (relative intensity): 140 (M+, 42), 125(10),
105(100).
CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.
21. Single Step
Overview
Steps/Stages Notes
1.1R:t-BuOCl, C:SiO2, S:CCl478% conversion, mono:dichloro = 67:33,
Reactants: 1, Reagents: 1, Catalysts: 1,
Solvents: 1, Steps: 1, Stages: 1, Most stages
in any one step: 1
References
Selective mono-chlorination of aromatic
compounds
By Smith, Keith et al
From Green Chemistry, 1(2), 83-90; 1999 CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.
22. Single Step
94%
Overview
Steps/Stages Notes
1.1 -Reactants: 1, Steps: 1, Stages: 1, Most stages
in any one step: 1
References
Hydrogen peroxide salts as reagents in the
oxidative halogenation of aromatic
compounds
By Rudakova, N. I. et al
From Zhurnal Obshchei Khimii, 65(2), 315-17;
1995
CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.
23. Single Step
Overview
Steps/Stages Notes
1.1 -Reactants: 1, Steps: 1, Stages: 1, Most stages
in any one step: 1
References
Mechanisms of free-radical reactions. Nature
of the chain-growth stage in free-radical
halogenations of alkylaromatic hydrocarbons
with trichloromethanesulfonyl chloride and
bromotrichloromethane
By Dneprovskii, A. S. et al
From Zhurnal Organicheskoi Khimii, 26(4),
819-23; 1990
CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.
24. Single Step
Overview
Steps/Stages Notes
1.1 -Reactants: 1, Steps: 1, Stages: 1, Most stages
in any one step: 1
References
Mechanisms of free-radical reactions. Nature
of the chain-growth stage in free-radical
halogenations of alkylaromatic hydrocarbons
with trichloromethanesulfonyl chloride and
bromotrichloromethane
By Dneprovskii, A. S. et al
From Zhurnal Organicheskoi Khimii, 26(4),
819-23; 1990
CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.
25. Single Step
16%
33%
Overview
Steps/Stages Notes
1.1R:HCl, S:Et2O Reactants: 2, Reagents: 1, Solvents: 1, Steps:
1, Stages: 1, Most stages in any one step: 1
References
Reactivity of 3,6-dimethoxy-3,6-
dimethylcyclohexa-1,4-diene: nuclear versus
benzylic nucleophilic substitution
By Alonso, Francisco et al
From Tetrahedron, 46(6), 2069-80; 1990 CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.
26. Single Step
84%
Overview
Steps/Stages Notes
1.1R:SO2Cl2zeolite ZF520, Reactants: 1, Reagents: 1,
Steps: 1, Stages: 1, Most stages in any one
step: 1
References
Versatility of zeolites as catalysts for ring or
side-chain aromatic chlorinations by sulfuryl
chloride
By Delaude, Lionel and Laszlo, Pierre
From Journal of Organic Chemistry, 55(18),
5260-9; 1990
CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.
27. Single Step
66%
Overview
Steps/Stages Notes
1.1R:PhCH2NMe3 ICl4, S:AcOH Reactants: 1, Reagents: 1, Solvents: 1, Steps:
1, Stages: 1, Most stages in any one step: 1
References
Halogenation using quaternary ammonium
polyhalides. XIX. Aromatic chlorination of
arenes with benzyltrimethylammonium
tetrachloroiodate
By Kajigaeshi, Shoji et al
From Bulletin of the Chemical Society of
Japan, 62(6), 2096-8; 1989
CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.
28. Single Step
76%
Overview
Steps/Stages Notes
1.1R:Ca(OCl)2, C:AcOH, S:H2O, S:Me2CO Reactants: 1, Reagents: 1, Catalysts: 1,
Solvents: 2, Steps: 1, Stages: 1, Most stages
in any one step: 1
References
Ring chlorination of benzenoid compounds
using calcium hypochlorite [Ca(OCl)2]
By Nwaukwa, Stephen O. and Keehn, Philip
M.
From Synthetic Communications, 19(5-6),
799-804; 1989
CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.
29. Single Step
Overview
Steps/Stages Notes
1.1R:Cl2Reactants: 1, Reagents: 1, Steps: 1, Stages:
1, Most stages in any one step: 1
References
Dichloro-di-p-xylylene
By Polievka, Milan et al
From Czech., 247592, 15 Jan 1987 CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.
30. Single Step
9%17%
Overview
Steps/Stages Notes
1.1R:NOCl, R:F3CCO2H, S:F3CCO2H, S:CH2Cl2Reactants: 1, Reagents: 2, Solvents: 2, Steps:
1, Stages: 1, Most stages in any one step: 1
References
Oxidative chlorination of aromatic compounds
in the presence of nitrogen-containing
oxidizing agents
By Makhon'kov, D. I. et al
From Zhurnal Organicheskoi Khimii, 24(2),
241-8; 1988
CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.
31. Single Step
9%
48%8%
31%
33%
Overview
Steps/Stages Notes
1.1C:SnCl4, S:p-C6H4Me2Reactants: 2, Catalysts: 1, Solvents: 1, Steps:
1, Stages: 1, Most stages in any one step: 1
References
Lewis acid-catalyzed sulfenylations of
methylbenzenes by p-chlorobenzenesulfenyl
chloride
By Grant, Douglas W. et al
From Journal of Chemical Research,
Synopses, (12), 392-3; 1987
CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.
32. Single Step
Overview
Steps/Stages Notes
1.1R:SO2Cl2Reactants: 1, Reagents: 1, Steps: 1, Stages:
1, Most stages in any one step: 1
References
Electrophilic chlorination by sulfuryl chloride in
the presence of silica gel
By Hojo, Masaru and Masuda, Ryoichi
From Synthetic Communications, 5(3), 169-
71; 1975
CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.