2006Arabidopsis PASTICCINO2作为去磷酸化酶调节周期素激酶A
- 格式:pdf
- 大小:395.59 KB
- 文档页数:13


某理工大学《细胞生物学》课程试卷(含答案)__________学年第___学期考试类型:(闭卷)考试考试时间:90 分钟年级专业_____________学号_____________ 姓名_____________1、判断题(95分,每题5分)1. 在普通光学显微镜不能看清核被膜的细微结构。
()答案:正确解析:相差显微镜下可以看出核被膜的界限,只有在电子显微镜下才能看清核被膜的细微结构2. 因为DNA的两条链是互补的,所以给定基因的mRNA能以任一链为模板合成。
()答案:错误解析:转录起始子的位置决定了转录进行的方向和以哪条DNA链作为模板链。
按相反方向进行转录会产生序列完全不同的(很可能是无意义的)mRNA链。
3. 普通光镜样品的制备,在包埋、切片后可直接进行观察,一般不需要组织染色。
()答案:错误解析:普通光镜主要用于对染色后的细胞切片进行观察。
4. 一棵树的干重大部分来自于根部吸收的矿物质。
()答案:错误解析:树的大部分干重为光合作用合成的有机化合物。
5. 分泌功能旺盛的细胞,其糙面内质网的数量越多。
()[浙江师范大学2010研]答案:正确解析:糙面内质网是内质网与核糖体共同形成的复合机能结构,其主要功能是合成分泌性的蛋白质和多种膜蛋白,因此在分泌细胞和分泌抗体的浆细胞中,糙面内质网非常发达。
6. 同一个生物中的所有细胞都具有相同数量的染色体(卵细胞与精细胞除外)。
()答案:正确解析:染色体的个数随生物而异,但是在同一个生物的所有细胞中它是恒定的。
7. IP3是PKC系统中的第二信使,它直接激活内质网上的钙泵,动员Ca2+的释放。
()答案:错误解析:IP3不能激活钙泵,只能激活内质网膜中的钙离子通道。
8. 溶酶体及过氧化物酶体是分解废物的场所。
()答案:错误解析:过氧化物酶体与溶酶体含有一些酶,胞质溶胶中产生的一些物质或被细胞摄入的物质由这些酶分解。
不过,这些物质中有许多被降解并可利用,并不全是废物。
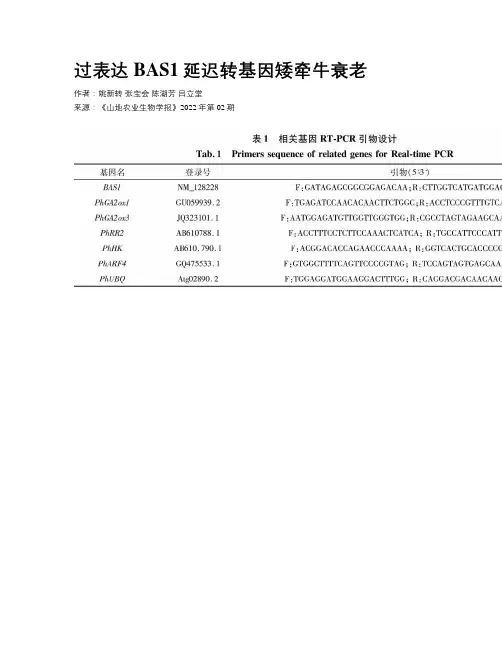
过表达BAS1延迟转基因矮牵牛衰老作者:姚新转张宝会陈湖芳吕立堂来源:《山地农业生物学报》2022年第02期摘要:油菜素类固醇(BRs)是必不可少的植物激素,在植物的生长、繁殖以及逆境响应中发挥重要作用。
为了验证该基因的功能,本文构建了植物遗传表达载体,利用农杆菌介导遗传转化法遗传转化矮牵牛,获得29 株转基因植株;转基因植物表现为节间缩短,分枝增多,生长后期表现出叶片延迟衰老;叶绿素含量、可溶性总糖含量、酶活明显高于野生型, MDA 含量明顯低于野生型;BAS1基因在矮牵牛中的超表达可以提高矮牵牛植株中衰老相关基因的表达,从而延缓了转基因矮牵牛的衰老。
因此,BAS1可用作调控植物花衰老和延长花寿命的潜在候选基因。
关键词:BAS1基因;矮牵牛;抗氧化酶活性;相关基因表达;衰老中图分类号:Q812文献标识码:A文章编号:1008-0457(2022)02-0038-006国际DOI编码:10.15958/ki.sdnyswxb.2022.02.006BAS1基因编码具有羟化酶活性的细胞色素P450单加氧酶,该酶催化油菜素固醇(BR)C-26的羟基羟化作用,导致BR生理活性的降低或丧失[1]。
作为成年植物,BR突变体基本上表现出缺少红光受体phyB突变体的相反表型。
BR突变体是深绿色,生长缓慢,叶片萎缩且茎短小。
另外,BAS1突变体表现出延迟衰老[2]。
过表达BAS1的转基因植物显著降低BR含量,从而导致叶片萎缩,叶片深绿和茎短[3-4]。
BAS1过表达诱导的叶片衰老与烟草植物中细胞分裂素过表达导致的表型非常相似。
异戊烯基转移酶(IPT)催化细胞分裂素合成的限速步骤。
Gan等[5]研究表明,通过精确控制IPT表达来引发特定的发育反应。
SAG12启动子仅在衰老开始时才激活IPT表达,这种激活导致衰老过程的抑制[5-6]。
通过IPT表达抑制叶片衰老导致衰老特异性启动子的减弱[7],从而防止了细胞分裂素的过量产生,干扰了发育的其他方面[8]。

作者单位:中南大学湘雅二医院心内科,湖南长沙410000E -m a i l :w a n g c h k e k e @163.c o m综 述普罗布考抗动脉粥样硬化作用机制及研究进展王 畅 综述,李向平 审校【文章编号】1005-2194(2007)09-0700-03 【中图分类号】R 5 【文献标志码】A 【关键词】 普罗布考;动脉粥样硬化K e y w o r d s P r o b u c o l ;A t h e r o s c l e r o s i s 普罗布考(p r o b u c o l )又名丙丁酚,化学名为4,4′-[(1-甲基亚乙基)双(硫基)]双[2,6-双(1,1-二甲基乙基苯酚],分子式为C 13H 48O 2S 2。
早期大量的药理及临床实验发现普罗布考具有调脂、抗氧化和抗动脉粥样硬化(A S )作用,但由于其可降低高密度脂蛋白胆固醇(H D L -C ),使其在临床上的应用受到影响。
晚近的研究证明,普罗布考虽然降低血H D L -C 浓度,但并不降低其转运胆固醇酯的作用,甚至增加其转运能力。
有关普罗布考抗A S 作用及机制的研究一直是人们关注的热点,本文就此方面的研究进展进行综述。
1 调脂作用早在20世纪70年代,人们就发现普罗布考具有调脂作用,其可有效降低总胆固醇(T C )和低密度脂蛋白胆固醇(L D L -C )水平,但同时也降低H D L -C 水平。
1.1 降低T C 和L D L -C 研究表明,普罗布考可使T C 和L D L -C 降低25%以上。
而且是纯合子型家族性高胆固醇血症患者少数有效降脂的药物之一,并可消退皮肤及肌腱黄色瘤。
由于普罗布考可通过非受体的机制刺激L D L -C 的清除,而不依赖L D L 受体途径,故在治疗家族性纯合子型高胆固醇血症中具有特殊地位[1]。
目前认为,普罗布考降胆固醇的作用机制如下:(1)竞争性抑制胆固醇合成系统中的限速酶-甲基羟戊二酰辅酶A (H M C -C o A ),抑制胆固醇的生物合成。

性范围与低剂量(<100mg )阿司匹林相当,而胃肠道出血的范围则优于标准剂量(100~325mg )的阿司匹林。
低剂量阿司匹林与氯吡格雷合用在预防中风中的安全性已被确证。
在C URE 和CRE DO 中都未发现这一疗法会使有急性冠脉症状的患者发生额外的出血性中风。
噻氯匹定和氯吡格雷都与TTP 的发生率上升有关。
7 结语对那些可缓解的危险因素的识别和积极治疗是中风二级预防的关键因素。
抗血小板治疗被推荐用于中风的二级预防。
中风患者处于在其他血管床上发生局部缺血性疾病的危险之中,抗血小板治疗在这一领域中提供了额外的保护。
阿司匹林作为应用范围最广的抗血小板药物,价廉而且通常容易耐受。
然而,对那些高危患者和对阿司匹林治疗无效的患者,需要改用其他的抗血小板药物如氯吡格雷或采用抗血小板药物合并治疗的方法。
对阿司匹林与氯吡格雷合用在中风的二级预防的有效性和安全性目前仍在评价中。
中风二级预防的方法包括生活方式的改变、消除危险因素和药物治疗,且应当在住院之前就开始,并且在门诊患者这一层面继续进行。
与阿尔茨海默病密切相关的α2,β2和γ2分泌酶的研究进展马 波综述 张建军审校(中国医学科学院/中国协和医科大学药物研究所,北京 100050)摘要:阿尔茨海默病(AD )是一种进行性的脑内神经元退变性疾病。
β淀粉样蛋白(A β)学说认为,Aβ凝集和聚积是AD 病理发生、发展的起始因素,而其他的病理改变如脑内神经纤维缠结、神经元的功能紊乱和丢失等,均被认为是由于A β的解离与凝聚、清除与产生的失衡所引发的。
目前认为,β2和γ2分泌酶抑制剂是最合理和有效的AD 治疗药物。
此外,α2分泌酶激动剂也可减少A β的产生。
近年来,人们对于分泌酶的认识取得了新的进展,本文重点对这方面进行综述。
关键词:阿尔茨海默病;β淀粉样蛋白学说;分泌酶;早老素中图分类号:R974.1+6 文献标识码:A 文章编号:100120971(2005)0120022205 收稿日期:2004209203〗1 Aβ级联假说阿尔茨海默病(Alzheimer ′s disease ,AD )是一种进行性的脑内神经元退变性疾病,包括家族性AD 和散发性AD 。

果糖-1,6-二磷酸酶果糖-1,6-二磷酸酶(Fructose-1,6-bisphosphatase,简称为 FBPase)是一种参与葡萄糖代谢的酶,存在于几乎所有真核生物、许多原核生物以及一些病毒中。
它是Gluconeogenesis(糖异生)途径中的一个重要酶,负责将2分子果糖-1,6-二磷酸(FBP)水解为2分子磷酸果糖(F6P),从而使糖异生途径前进。
该酶是糖异生途径和糖原合成途径中的限速酶之一,在空腹时,肝脏中糖异生途径占主导地位,FBPase的活性是肝脏维持正常生理状态所必需的。
结构与功能FBPase 主要由 3 个结构域构成:N 端结构域、中间结构域和 C 端结构域,这些结构域在进化过程中具有高度的保守性。
N 端结构域含有磷酸化酶催化所需的脯氨酸残基,是酶催化和调节的关键区域。
中间结构域含有两个反向同构的催化中心,这些催化中心使 FBPase 能够高效地水解两个 FBP 分子,从而生成两个 F6P。
C 端结构域则对物质基础进行分类和整合,为 FBPase 酶学和生物学特性的整合提供基础。
FBPase 对许多环境因素,如 pH 值,离子强度和温度等,均表现出高度的敏感性。
稳定剂和抑制剂都能够显著地影响酶的活性。
对于细胞与机体内的 FBPase 来说,该酶的调节显得尤为重要。
急性调节通常由磷酸化和解磷酸化等化学修饰实现,而长期调节则主要由基因表达调控实现。
FBPase 的磷酸化和解磷酸化磷酸化和解磷酸化修饰对于 FBPase 的调节非常重要。
当细胞需要进入糖异生途径时,活性化的酶被磷酸化,并透过蛋白水解酶4的参与,磷酸化 FBPase 的活性在糖异生途径的起始阶段被激发,以产生更多的葡萄糖。
与此相反,当细胞需要停止进行糖异生途径时,F6P 是其调节的关键物质,彼时解磷酸化的 FBPase 更方便形成稳定的 F6P/FBP 催化活性平衡,从而使糖异生途径的前进效率下降。
改变 FBPase 的活性和构象调整代表了一种非常快速和可靠的方式,调整葡萄糖代谢产物的浓度,从而使细胞适应外界环境的需求。

蛋白磷酸酶2调节亚基5a
蛋白磷酸酶2调节亚基5a (PP2A-B55a) 是一种重要的细胞信号调节蛋白,它在细胞周期、细胞分化和细胞凋亡等过程中发挥关键作用。
在细胞内,PP2A-B55a与PP2A催化亚基和PP2A结构亚基组合形成复合物,调控多种蛋白底物的磷酸化状态,从而影响细胞的生理功能。
PP2A-B55a通过调节多个信号通路的活性,参与了许多重要的生物学过程。
例如,PP2A-B55a可以调节细胞周期的进程,控制细胞的有丝分裂和有丝分裂。
它在细胞周期的G2期末期和M期初期发挥重要作用,通过去磷酸化和激活多个关键蛋白,如CDK1和CDC25C,促进细胞进入有丝分裂。
PP2A-B55a还参与了细胞分化和细胞凋亡等过程。
在细胞分化中,PP2A-B55a可以调节多个转录因子的磷酸化状态,影响基因的表达,从而促进细胞分化和特化。
在细胞凋亡中,PP2A-B55a可以调节多个凋亡信号通路的活性,控制细胞的生存和死亡决策。
值得注意的是,PP2A-B55a的功能受到多种调控机制的影响。
例如,某些研究表明,PP2A-B55a的磷酸化状态与其活性和稳定性密切相关。
此外,PP2A-B55a的表达水平也受到多种信号通路的调控,如Wnt信号通路和MAPK信号通路等。
PP2A-B55a作为一种重要的蛋白磷酸酶调节亚基,在细胞周期、细
胞分化和细胞凋亡等生物学过程中发挥着重要作用。
对于进一步理解细胞信号调控的机制和疾病的发生与发展具有重要意义。
因此,深入研究PP2A-B55a的功能和调控机制,对于揭示细胞生物学的奥秘和疾病治疗具有重要意义。

拓扑异构酶i和ii名词解释拓扑异构酶I和II名词解释导论在人类体内,存在着一种重要的酶类物质,被称为拓扑异构酶,它在维持DNA的结构和功能中起着至关重要的作用。
本文将对拓扑异构酶I和II进行详细解释,并分析它们在细胞中的功能和影响。
一、拓扑异构酶I的定义和特点1.1 定义拓扑异构酶I(Topoisomerase I)是一种能够介导DNA断裂和连接的酶,它能够调节DNA的拓扑结构,维持DNA的超螺旋状态。
该酶通过在DNA链上切割,松弛或整合 DNA 的连结,帮助细胞进行染色体复制、转录和重组。
1.2 功能拓扑异构酶I具有以下几个主要功能:(1)解旋:在DNA复制和转录过程中,DNA链的双螺旋结构需要解开,以使DNA聚合酶获得访问基因序列的机会。
拓扑异构酶I能够切割一个DNA链未配对部分的DNA,减小其超螺旋的紧张程度,从而实现DNA的解旋。
(2)断链:拓扑异构酶I能够切割DNA链中的磷酸二酯键,从而在DNA链上产生一个短暂的断裂。
这对于染色体重组和机械性拓扑学变化等过程至关重要。
(3)连接:拓扑异构酶I不仅能够断裂DNA链,还能够在适当的时间和位置上重新连接它们,以确保DNA链的完整性。
1.3 影响拓扑异构酶I的功能异常或缺陷可能导致多种疾病的发生和发展。
在肿瘤细胞中,拓扑异构酶I的活性增强可能导致DNA拓扑结构的不稳定,从而促进染色体异常和癌症的发生。
一些抗肿瘤药物,如喜树碱,通过抑制拓扑异构酶I的活性,阻碍了肿瘤细胞的DNA复制和修复,进而抑制了肿瘤细胞的生长和扩散。
二、拓扑异构酶II的定义和特点2.1 定义拓扑异构酶II(Topoisomerase II)是一种双链DNA分子的切割和连接酶,它在DNA复制和细胞分裂中发挥着关键作用。
拓扑异构酶II 可以解开DNA双链,对染色体进行结构改变,并帮助维持染色体的拓扑构型。
2.2 功能拓扑异构酶II的功能主要包括:(1)DNA切割:拓扑异构酶II能够切割DNA的两个链,并且在需要的时候重新连接这些链。

以磷酸二酯酶为主要靶点的阿魏酸抗阿尔兹海默病作用机制研究越来越多证据表明,磷酸二酯酶(Phosphodiesterase,PDE)在学习和记忆过程中发挥了很大作用,其相应的抑制剂能通过调控细胞内环磷酸腺苷(cyclic adenosine monophosphate,c AMP)含量来改善中枢神经系统退行性疾病所致的学习记忆障碍。
因此寻找特异性PDE抑制剂是目前国内外各大药理实验室的研究热点。
我们近期的实验结果显示,阿魏酸(ferulic acid,FA)能调节PDE的表达及活性,从而影响生物体的各种代谢。
但是FA是如何对PDE进行调控以及面对PDE 这样一个庞大的超家族,FA是针对其中某一种亚型发挥特异性调节作用还是发挥非特异性PDE调节作用并不明确。
本项目通过现代分子生物学手段明确FA对PDE亚型表达有抑制作用,并对其下游信号通路具有调节作用,并在整体动物学实验上验证FA抗阿尔茨海默病(Alzheimer’s disease,AD)样炎症作用与其对PDE的表达抑制作用密切相关。
这些工作将不仅为AD的特异性靶向治疗提供安全有效的天然药物,同时也为FA 的药理作用及机制深入研究提供理论支撑。
本课题展开了相关的研究工作,包括以下四个部分。
第一,运用分子对接的方法模拟FA与PD4B2的相互作用。
分子对接结果表明,FA可与包括Tyr233、His234、Met347、Asn395、Phe414、Gln443、和Phe446在内的氨基酸残基发生强烈的相互作用,数据还表明,FA可进入PDE4B2的结合腔,并与氨基酸残基Phe446和Phe414形成π<sup>π</sup>相互作用。
在FA与氨基酸残基Gln443和His234之间可发现有氢键。
FA的构象位于疏水腔区域,表明FA与PDE4B2之间存在静电和疏水性相互作用。
分子对接结果从理论上支持了FA对PDE4B酶活性及表达的潜在影响,表明FA可被用作基本结构来设计PDE4抑制剂,应进行进一步的研究来证实该相互作用。

2006中国科学院研究生院硕士研究生入学考试试题科目名称:生物化学与分子生物学一、是非题(20题,每题1.5分,共30分)1.蛋白质变性的实质是非共价键断裂,天然构象解体,但共价键未发生断裂。
2.有些抗生素是多肽或其衍生物。
3.“自杀性底物”是指在亲和标记法中使用的共价修饰剂。
4.糖原磷酸化酶只催化1→4糖苷键的磷酸解。
5.到目前为止发现的信号转导蛋白G蛋白都是由三种亚基构成的。
6.红外光谱可用以研究蛋白质溶液构象。
7.丙氨酸具有光学活性。
8.线粒体内膜固有蛋白大多是由线粒体DNA编码的。
9.转录因子ⅢA(TFⅢA)能与5S RNA以及5S RNA基因调控区结合,因而5S RNA能对自身的合成进行负反馈调节。
10.酶催化的反应速度总是随着底物浓度升高而加快。
11.线粒体呼吸链复合物II的三维结构已被我国科学家解析。
这个复合物催化琥珀酸脱氢辅酶Q还原的同时产生跨膜质子移位。
12.去垢剂是一类既有亲水部分又有疏水部分的分子。
13.质量性状是由单个基因支配,而数量性状是由多个基因决定的。
14.生物体的遗传性状完全是由基因序列决定的。
15.脑细胞有CD4表面受体,所以HIV病毒也能感染脑细胞。
16.线粒体基因采用的密码子与细胞的密码子有不同。
17.原核生物的mRNA的翻译起始区有一个SD序列,它与23S RNA的3’端结合而起始翻译。
18.葡萄糖—6—磷酸脂酶是高尔基体的标志酶。
19.孟德尔遗传定律和摩尔根遗传定律分别反映了位于不同染色体和相同染色体上的基因的遗传规律。
20.限制性内切酶是识别同时切割某特定DNA序列的酶。
二、选择题(30题,每题1.5分,共45分)1.肌原纤维中的粗丝和细丝____________。
A、分别由肌动蛋白和肌球蛋白组成B、分别由肌球蛋白和肌动蛋白组成C、皆由肌动蛋白组成D、皆由肌球蛋白组成2.参与蛋白质组成的氨基酸是L—型的,其多肽链折叠成的螺旋结构___________。

某大学生物工程学院《普通生物化学》课程试卷(含答案)__________学年第___学期考试类型:(闭卷)考试考试时间:90 分钟年级专业_____________学号_____________ 姓名_____________1、判断题(140分,每题5分)1. 在生物体内,肽链是在合成结束后才开始折叠的。
()答案:错误解析:2. 胆固醇虽然不是生物膜的主要成分,但却具有调节生物膜流动性的作用。
()答案:正确解析:3. DNA连接酶能将两条游离的DNA单链连接起来。
()[中国科技大学2001、研]答案:错误解析:E.coli的DNA连接酶要求断开的两条链由互补链将它们聚在一起,形成双螺旋结构,而不能将两条游离的DNA单链连接起来。
4. 蛋白质分子的亚基与结构域是同义词。
()[西南农业大学研]答案:错误解析:5. 辅酶A中含有泛酸,其主要作用是转移一碳基团。
()答案:错误解析:辅酶A中含有泛酸,主要功能是酰基转移,传递一碳基团的辅酶是四氢叶酸。
6. DNA变性是指互补碱基之间的氢键断裂。
()[中山大学2018研]答案:正确解析:7. 生物膜上的脂质主要是磷脂。
()答案:正确解析:8. 核酸是由许多核苷酸组成的生物大分子,是机体必需的营养素。
()[中山大学2018研]答案:错误解析:核酸在体内可以自身合成,不是机体必需的营养素。
9. 琥珀酸脱氢酶是三羧酸循环中唯一掺入线粒体内膜的酶。
()答案:正确解析:琥珀酸脱氢酶是TCA循环中唯一一个整合于膜上的多亚基酶,在真核生物中结合于线粒体内膜,在原核生物中整合于细胞膜上,其是连接氧化磷酸化与电子传递的枢纽之一,可为真核细胞线粒体和多种原核细胞需氧和产能的呼吸链提供电子。
10. 真核生物的所有mRNA都含有poly(A)结构。
()答案:错误解析:11. DNA和RNA聚合酶都具有校对活性,以减少复制和转录的错误。
()[中山大学2018研]答案:错误解析:RNA聚合酶没有校对活性。
谈蛋白磷酸酶2A 的结构、功能和活性调节本文从网络收集而来,上传到平台为了帮到更多的人,如果您需要使用本文档,请点击下载按钮下载本文档(有偿下载),另外祝您生活愉快,工作顺利,万事如意!蛋白质的可逆性磷酸化是许多细胞生物学过程的极其重要的调节机制。
概括起来说, 这种翻译后修饰可以改变信号通路中许多关键蛋白质的性质,如酶活性、细胞内定位等, 从而影响细胞的增殖、分化和凋亡。
过去对蛋白质可逆性磷酸化的研究主要集中在蛋白激酶(protein kinase , PK)的调控方面。
现在认为蛋白磷酸酶(protein phosphatase , PP)和PK一样受到严密调控, 同样起重要的作用。
根据氨基酸顺序的一致性和三维结构的相似性, PP 分为三个家族:磷酸化酪氨酸残基蛋白磷酸酶(phosphotyrosine residues phosphatases , PTP)、磷酸化丝/苏氨酸残基蛋白磷酸酶(phosphoserine andphosphothreonine residues phosphatases , PPP)和Mg2 +依赖的磷酸化丝/苏氨酸残基蛋白磷酸酶(Mg2 +-dependentphosphoserine and phosphothreonine residuesphosphatases , PPM)。
其中PTP 家族还存在一类能使磷酸化酪氨酸和丝/苏氨酸残基脱磷酸的蛋白磷酸酶。
PPM 家族包括蛋白磷酸酶2C 和位于线粒体的丙酮酸脱氢酶。
最近还发现一类磷酸化组氨酸残基的蛋白磷酸酶。
根据酶对底物选择的特异性、对不同抑制剂的敏感性及对不同二价阳离子的依赖性, 真核生物中PPP 家族可分为PP1 、PP2A 、PP2B 、PP2C 四类。
PP1主要作用于磷酸化酶激酶的β 亚单位;PP2 作用于磷酸化酶激酶的α亚单位;PP2B 需要Ca2+激活;PP2C 需要Mg2+作为辅助因子。
后来又发现PP3 、PP4 、PP5 、PP6 、PP7 等新成员, 对酶抑制剂敏感性不同。
某工业大学生物工程学院《细胞生物学》课程试卷(含答案)__________学年第___学期考试类型:(闭卷)考试考试时间:90 分钟年级专业_____________学号_____________ 姓名_____________1、判断题(50分,每题5分)1. 所有的动物细胞都有一种相类似的控制程序性细胞死亡的机制,即通过一个自杀性蛋白酶家族的介导。
自杀性死亡途径受信号控制,若细胞外的信号促性程序性细胞死亡,则属于死亡的负控制;若细胞外信号抑制细胞的程序性死亡,则是死亡的正控制。
()答案:错误解析:若细胞外的信号促进程序性细胞死亡,则属于死亡的正控制;若细胞外信号抑制细胞的程序性死亡,则是死亡的负控制。
2. 各种正常细胞的体积大小不同,但它们细胞核的大小通常差距不大。
()答案:正确解析:3. 分泌功能旺盛的细胞,其糙面内质网的数量越多。
()[浙江师范大学2010研]答案:正确解析:糙面内质网是内质网与核糖体共同形成的复合机能结构,其主要功能是化学合成分泌性的蛋白质和多种膜蛋白,因此在分泌细胞和分泌抗体的浆细胞中,糙面内质网非常发达。
4. 中心粒的复制是在细胞周期S期全部完成的。
()答案:错误解析:中心粒在G1期末复制,在S期由一对中心体连接在一起,到G2期一对中心体开始分离并逐渐向细胞两极移动。
5. 胞质溶胶含有膜结合细胞器,如溶酶体。
()答案:错误解析:胞质溶胶是指除各种细胞器之外的细胞质部分,叶绿体的可流动部分,并且是膜结合细胞器线粒体外的流动部分,神经细胞属于膜结合细胞器。
6. 细胞外配体与受体酪氨酸激酶结合,并通过单次穿膜的α螺旋的构象变化激活了细胞内催化结构域的活性。
()[中山大学2009研]答案:错误解析:受体酪氨酸激酶与配体结合后活化的制度不是构象顺式的变化,而是其发生自身磷酸化而获得蛋白激酶的活性。
7. 秋水仙碱可同微丝的(+)端结合,并阻止新的单体加入。
()答案:错误解析:秋水仙碱只能作用于微管。
某理工大学《细胞生物学》课程试卷(含答案)__________学年第___学期考试类型:(闭卷)考试考试时间:90 分钟年级专业_____________学号_____________ 姓名_____________1、判断题(95分,每题5分)1. 细菌中,编码核糖体RNA、转运RNA和mRNA的基因是被不同的RNA聚合酶转录的。
()答案:错误解析:细菌只有一种RNA聚合酶,转录所有的基因。
相反,真核细胞有三种不同的聚合酶,每一种用于三类基因中的一类。
2. 细胞内的生化过程总是能完全在试管内实现。
()答案:错误解析:在细胞内与试管内的生化过程的根本区别是:细胞表现为有严格程序的、自动控制的代谢体系。
在试管内完全再现细胞内的生物过程,有待进一步发展。
3. 磷脂极性头部是带正电荷的,因此它可以直接与带负电荷的氨基酸残基直接相互作用。
()答案:错误解析:磷脂极性头部是带负电荷的,它可以直接与带正电荷的氨基酸残基直接相互作用,与带负电荷的氨基酸残基作用时,需通过Ca2+、Mg2+等阳离子为中介。
4. 胆固醇使得脂质在双层中堆积更紧密,但它并没有降低膜的流动性。
()[复旦大学2019研]答案:正确解析:胆固醇使得脂质在双层中堆积更为紧密,但它并没有降低膜脂的流动性,在大部分动物细胞膜中高浓度的胆固醇还可以防止碳氢链聚合以及结晶态的形成。
5. 程序性细胞死亡是由一类特殊的胞内蛋白酶介导的,其中的一个成员能降解核纤层蛋白。
()答案:正确解析:程序性细胞死亡是由特殊功能的一些蛋白酶所执行的一个主动过程。
6. 一些真核细胞不仅在细胞核内存在遗传物质,也可有核外DNA,如质粒。
()答案:正确解析:酵母细胞中存在质粒分子,真核细胞的叶绿体、线粒体中都含有少量的DNA。
7. 细胞凋亡与细胞坏死的一个共同特征是细胞膨胀,体积显著增大,趋于解体。
()解析:细胞坏死过程中,细胞膨胀,细胞体积增大,最终胞膜破裂,细胞体内容物散逸。
钙蛋白酶小亚基1在细胞凋亡、细胞周期及肿瘤中作用机制的研究进展曹伟;许贤林;何小舟【期刊名称】《中国医药生物技术》【年(卷),期】2015(010)004【总页数】3页(P323-325)【作者】曹伟;许贤林;何小舟【作者单位】213003 常州,苏州大学附属第三医院肿瘤生物诊疗中心;213003 常州,苏州大学附属第三医院肿瘤生物诊疗中心;213003 常州,苏州大学附属第三医院肿瘤生物诊疗中心【正文语种】中文钙蛋白酶小亚基1在细胞凋亡、细胞周期及肿瘤中作用机制的研究进展曹伟,许贤林,何小舟DOI:10.3969/cmba.j.issn.1673-713X.2015.04.010作者单位:213003 常州,苏州大学附属第三医院肿瘤生物诊疗中心通信作者:何小舟,Email:**********************收稿日期:2015-06-08钙蛋白酶(calpain)是一种存在于动物细胞中的钙离子依赖性的中性半胱氨酸蛋白酶。
钙蛋白酶小亚基 1 (calpain-s1,capn4)作为 calpain 的调节小亚基,对细胞周期、细胞凋亡及肿瘤的发生、发展等具有一定的影响。
本文就近年来有关capn4 在细胞周期、细胞凋亡及肿瘤中的作用机制作一综述。
1 Calpain 简介Calpain 是由 Guroff[1]在 1964 年于大鼠的脑组织中首次发现并研究。
Calpain1 和calpain2 存在于哺乳动物中,由于其激活所需的 Ca2+浓度不同,分为μ-calpain 和m-calpain。
calpain1 和 calpain2 在结构上是由一个 80 kD的大催化亚基和一个 28 kD 的小调节亚基组成[2]。
Calpain参与细胞的增殖、凋亡、迁徙及转移等能力。
钙蛋白酶的水解活性是其主要表现,研究表明,calpain 可以通过水解踝蛋白(talin)的Q433-Q434 位点和 talin-R 端的 K2493-K2494位点来影响整合素的激活和黏着斑的形成[3-5]。
Arabidopsis PASTICCINO2Is an Antiphosphatase Involvedin Regulation of Cyclin-Dependent Kinase A WMarco Da Costa,a,1Lieˆn Bach,a Isabelle Landrieu,b Yannick Bellec,a Olivier Catrice,c Spencer Brown,cLieven De Veylder,d Guy Lippens,b Dirk Inze´,d and Jean-Denis Faure a,2a Laboratoire de Biologie Cellulaire,Institut National de la Recherche Agronomique,F-78026Versailles Cedex,Franceb Institut Pasteur de Lille,Centre National de la Recherche Scientifique,Unite´Mixte de Recherche8525,Universite´de Lille,F-59019Lille Cedex,Francec Dynamique de la Compartimentation Cellulaire,Institut des Sciences du Ve´ge´tal,Unite´Propre de Recherche2355,Centre National de la Recherche Scientifique,F-91198Gif-sur-Yvette,Franced Department of Plant Systems Biology,Flanders Interuniversity Institute for Biotechnology,Ghent University,B-9052Ghent,BelgiumPASTICCINO2(PAS2),a member of the protein Tyr phosphatase-like family,is conserved among all eukaryotes and is characterized by a mutated catalytic site.The cellular functions of the Tyr phosphatase-like proteins are still unknown,even if they are essential in yeast and mammals.Here,we demonstrate that PAS2interacts with a cyclin-dependent kinase(CDK)that is phosphorylated on Tyr and not with its unphosphorylated isoform.Phosphorylation of the conserved regulatory Tyr-15is involved in the binding of CDK to PAS2.Loss of the PAS2function dephosphorylated Arabidopsis thaliana CDKA;1and upregulated its kinase activity.In accordance with its role as a negative regulator of the cell cycle,overexpression of PAS2 slowed down cell division in suspension cell cultures at the G2-to-M transition and early mitosis and inhibited Arabidopsis seedling growth.The latter was accompanied by altered leaf development and accelerated cotyledon senescence.PAS2was localized in the cytoplasm of dividing cells but moved into the nucleus upon cell differentiation,suggesting that the balance between cell division and differentiation is regulated through the interaction between CDKA;1and the antiphosphatase PAS2.INTRODUCTIONIn multicellular organisms,cell division and cell differentiation have to be coordinated during development.This statement is especially true for plants that carry on continuous organogenesis in the meristems,where cells have to maintain their proliferative potential as well as initiate differentiation to produce new organs. Cell cycle regulators are required for the control of cell cycle transitions but seem also to be involved in coordinating transi-tions between cell proliferation and cell differentiation(Gutierrez, 2005).In eukaryotes,cell division is regulated by phosphorylation events performed by cyclin-dependent kinases(CDKs)(Inze´, 2005).The cell cycle machinery is conserved among eukaryotes, and,in particular,several CDKs have been characterized in plants(Vandepoele et al.,2002).CDKA has been identified as the bonafide CDK because of the presence of the conserved PSTAIRE motif and by its ability to complement the yeast Schizosaccharomyces pombe cdc2mutant(Colasanti et al., 1991;Ferreira et al.,1991).Of the plant-specific CDKs with the PPTALRE and PPTTLRE motifs,CDKB can not complement the S.pombe cdc2mutant but is nonetheless involved in the G2-to-M transition(Porceddu et al.,2001;Boudolf et al.,2004).Similarly to CDKs,a large family of cyclins has been described in several plant species(Inze´,2005).Correct cell cycle progression re-quires a tight control of protein and activity levels of CDK/cyclin complexes(De Veylder et al.,2003;Dewitte and Murray,2003). For instance,transcriptional regulation of the expression of cyclins is important for the G1-to-S transition,but posttransla-tional modifications that affect CDK complex stability and activity are essential for many cell cycle steps(Inze´,2005).Irreversible inactivation of the CDK/cyclin complex results from ubiquitin-mediated degradation of the cyclins(Genschik et al.,1998; Koepp et al.,1999).One of the most important posttranslational modifications of CDKs involves protein phosphorylation.CDKs are initially activated by cyclin association and by phosphoryla-tion on a Thr residue in the conserved T-loop(Connell-Crowley et al.,1993;Solomon,1993).CDK/cyclin complexes are revers-ibly inactivated by phosphorylation of Thr-14and Tyr-15of CDK (Morgan,1995).Phosphorylation is mediated by the WEE1/ MYK1/MYT protein kinase,while dephosphorylation is caused by the dual specificity protein Tyr phosphatase(dsPTP)CDC25.1Current address:Cell Proliferation Group,Medical Research CouncilClinical Sciences Centre,Imperial College Faculty of Medicine,Hammersmith Hospital Campus,Du Cane Road,London,W120NN,UK.2To whom correspondence should be addressed.E-mail jean-denis.faure@versailles.inra.fr;fax33-130-83-3099.The author responsible for distribution of materials integral to thefindings presented in this article in accordance with the policy describedin the Instructions for Authors()is:Jean-Denis Faure(jean-denis.faure@versailles.inra.fr).W Online version contains Web-only data.Article,publication date,and citation information can be found at/cgi/doi/10.1105/tpc.105.040485.The Plant Cell,Vol.18,1426–1437,June2006,ª2006American Society of Plant BiologistsDephosphorylation of CDK by CDC25at the G2-to-M transition is a rate-limiting step for CDK activation and,thus,for cell cycle progression(Donzelli and Draetta,2003).The plant WEE1regulatory kinase was identified in Arabidop-sis thaliana and maize(Zea mays)(Sun et al.,1999;Sorrell et al., 2002).Until recently,no gene with significant primary sequence similarity to CDC25could be detected in the Arabidopsis ge-nome(Arabidopsis Genome Initiative,2000),although the CDC25-like activity was found to be involved in the G2-to-M transition of Nicotiana plumbaginifolia cells(Zhang et al.,1996, 2005).Accordingly,an Arabidopsis CDC25isoform with a tertiary structure similar to that of the human CDC25and able to activate in vitro the CDK/cyclin complex has only recently been cloned (Landrieu et al.,2004a,2004b).In animals,tumorigenesis is often preceded by the deregula-tion of cell cycle components that triggers ectopic cell prolifer-ation.Surprisingly,plants seem to be strikingly resistant to unrestricted growth similar to mammalian hyperplasia or cancer when cell cycle genes are deregulated(Cockcroft et al.,2000; Gutierrez,2005).Nonetheless,direct genetic screens for ectopic cell proliferation and tumor-like development have identified several mutants in Arabidopsis(Faure et al.,1998;Bouton et al., 2002;Frank et al.,2002),among which the pasticcino(pas) mutants(pas1,pas2,and pas3)have been characterized by ectopic cell proliferation in the apical part,which is a cytokinin-enhanced process(Faure et al.,1998).These pas mutants have impaired embryo and seedling development associated with modified cytokinin and auxin sensitivity(Harrar et al.,2003).The three PAS genes have been identified as members of a con-served gene family in eukaryotes(Vittorioso et al.,1998;Bellec et al.,2002;Baud et al.,2004).PAS2encodes the Arabidopsis member of a new PTP family,the PTP-LIKE(PTPL)family, originally described in mice and humans and characterized by mutations in the active site that conferred phosphatase inactivity (Uwanogho et al.,1999;Bellec et al.,2002;Pele´et al.,2005).The cellular function of this protein family is still unknown but seems to be critical because the absence of the PAS2homolog in yeast is lethal and in mammals leads to severe defects in myofibril differentiation(Bellec et al.,2002;Pele´et al.,2005).The discov-ery of a putative inactive PTP involved in plant cell proliferation raised the possibility that the plant cell cycle might be regulated by an antiphosphatase.Here,we show that PAS2is indeed a component of the CDKA;1/cyclin complex by its interaction with phosphorylated CDKA;1.PAS2protects the phosphorylated CDKA;1residues from phosphatase activities and thus regulates CDKA;1phos-phorylation status and kinase activity;however,its absence causes CDKA;1dephosphorylation and increases cell compe-tence to divide.A mechanism is proposed based on the com-petitive action of an antiphosphatase and a dsPTP on CDKA;1to control cell competence for division.RESULTSPAS2Interacts with CDKA;1in VivoThe sequence similarity between PAS2and members of the PTPL family suggested that PAS2could be involved in protein phosphorylation.The fact that PTPL members are characterized by a mutated catalytic site of PTP prompted us to consider PAS2 as a competitor of an active PTP for a phosphorylated substrate. Arabidopsis CDKA;1appeared to be a potential target of PAS2 for several reasons.First,this main regulator of the cell cycle is one of the few plant proteins in which a regulatory Tyr phospho-rylation site is conserved.Then,CDKA dephosphorylation by a CDC25-like activity is required for cell cycle progression of N. plumbaginifolia(Zhang et al.,2005).In particular,a CDC25-like activity has been found to be necessary for cytokinin regulation of the G2-to-M transition of the cell cycle.Finally,the pas2 mutants show cell dedifferentiation and proliferation,which are specifically enhanced by cytokinins,suggesting that both PAS2 and cytokinins could regulate CDKA;1phosphorylation by tar-geting CDC25activity.We tested whether PAS2and CDKA;1interact in vivo by purifying PAS2-interacting proteins by pull-down assays(Figure 1A).Total proteins from Arabidopsis cell cultures(lane1)were loaded on a column with PAS2(lane2)or on an empty control column(lane3).The analysis of PAS2binding proteins by protein gel blots showed that CDKA;1was specifically retained on the PAS2column(Figure1B).The reverse experiment was per-formed by affinity purification of the active CDKA;1complexes from Arabidopsis cell cultures with p10CKS1At columns(Figure 1C,bottom).Protein gel blot analysis with anti-PAS2serum indicated that PAS2copurified with CDKA;1(Figure1C,top).Figure1.Interaction of PAS2with CDKA;1in Vivo.(A)and(B)Purification of CDKA;1by recombinant PAS2(PAS2:His)from total protein extracts of Arabidopsis cells.The pull-down assay was performed with a PAS2:His column.Proteins were detected by Coo-massie staining(A)and by protein gel blots(B)from total protein extract of Arabidopsis cells(1),protein extract after purification on PAS2:His column(2),and protein extract after purification on empty column(3). Protein gel blot analysis was performed with anti-PSTAIRE(PSTAIRE) and antiphosphotyrosine(PTYR).(C)Copurification of PAS2with the CDK complex.Detection of PAS2 (anti-PAS2;PAS2)and CDKA;1(PSTAIRE)after CDK purification by p10CKS1At beads from protein extracts of Arabidopsis cells.(D)Association of PAS2with the CDK complex in Arabidopsis seedlings. Detection of PAS2and CDKA;1(PSTAIRE)after CDK purification by p10CKS1At beads from protein extracts of wild-type plants or pas2-1 mutant plants.Antiphosphatase Regulates Cell Division1427A similar experiment was performed with wild-type Arabidopsis seedlings,and,as expected,PAS2was also associated with CDKA;1(Figure1D).As anticipated,a low amount of PAS2was found in the complex with CDKA;1in a weak pas2allele,which was characterized by a strongly reduced steady state level of the PAS2mRNA(Bellec et al.,2002).Altogether,these data dem-onstrate that PAS2interacts with the CDKA;1protein complex in cell cultures as well as in seedlings.PAS2Interacts Only with Phosphorylated CDK and Inhibits CDKA;1DephosphorylationThe PTP motif in the PAS2sequence suggested that protein phosphorylation was involved in the PAS2function.The recom-binant PAS2had no phosphatase activity(Bellec et al.,2002),but the protein could have still retained its ability to bind a phos-phorylated substrate.We tested this hypothesis using an in vitro binding assay with the recombinant PAS2:His and CDKA;1:mal-tose binding protein(MBP).The recombinant CDKA;1:MBP was purified from Escherichia coli and phosphorylated by Src Tyr kinase in vitro.Phosphorylation was monitored by radioactive incorporation of g32P-ATP(data not shown)and by protein gel blots with an antiphosphotyrosine antibody(Figure2A).We verified that Tyr-15,which is an important regulatory residue of CDKA;1and a substrate of CDC25,was phosphorylated by Src Tyr kinase.Both the unphosphorylated and phosphorylated recombinant CDKA;1:MBP proteins were loaded onto a PAS2 column,and bound proteins were eluted and analyzed by protein gel blots(Figure2B).Only the phosphorylated CDKA;1:MBP was retained on the PAS2column,demonstrating that the PAS2-CDKA;1interaction requires CDKA;1phosphorylation on Tyr residues.Accordingly,a Tyr phosphorylated protein of a size similar to that of CDKA;1was also affinity purified by PAS2from Arabidopsis cell culture extract(Figure1B).We then investigated the role of Tyr-15phosphorylation in PAS2binding to CDK using the specific kinase WEE1.Because the phosphorylation specificity of the Arabidopsis WEE1had never clearly been addressed,we used the well-characterized human WEE1kinase that phosphorylated specifically Tyr-15of CDK2(Morgan,1997).Human CDK2and Arabidopsis CDKA;1 share69%identity at the protein level and a complete identity over17residues around Tyr-15.The glutathione S-transferaseFigure2.Interaction of PAS2with Phosphorylated CDKA;1.(A)In vitro phosphorylation of CDKA;1:MBP.CDKA;1:MBP was incubated(þ)or not(ÿ)with Src kinase and analyzed by protein gel blotting with antiphosphotyrosine(PTYR),antiphosphotyrosine15(P-Y15),and anti-PSTAIRE antibodies.(B)Tyr phosphorylation of CDKA;1is required for the interaction with PAS2.Incubation of CDKA;1:MBP phosphorylated or not by Src kinase with PAS2:His column.Interaction between PAS2and CDKA;1:MBP or CDKA;1:MBP Y-P was monitored by protein gel blots with the anti-PSTAIRE antibody.(1)CDKA;1:MBP,(2)elution of CDKA;1:MBP from PAS2:His column,and(3)elution of CDKA;1:MBP Y-P from PAS2:His column.(C)In vitro phosphorylation of GST:CDK2by WEE1.GST:CDK2was coproduced in E.coli with(þ)or without(ÿ)the WEE1kinase and analyzed by protein gel blotting with antiphosphotyrosine15(P-Y15)and anti-PSTAIRE antibodies.(D)WEE1phosphorylation of CDK2is required for the interaction with PAS2.The GST:CDK2fusion protein phosphorylated or not by WEE1kinase was incubated with PAS2:His column,and the interaction was monitored by protein gel blots with the anti-PSTAIRE antibody.(E)The interaction of PAS2with phosphorylated CDK2is competed off with pTyr-15peptide.The experiment was similar to(D)except that the conserved peptide EKVEKIGEGTYGVVYK phosphorylated on Thr(pT14)or phosphorylated on Tyr(pT15)was included or not(ÿ)in the binding experiment,and the interaction was monitored by protein gel blots with the anti-PSTAIRE antibody.1428The Plant Cell(GST):CDK2fusion protein was produced in E.coli in the ab-sence or in the presence of WEE1as previously described (Welburn and Endicott,2004).The fusion proteins were purified, and Tyr-15was found to be phosphorylated only in the presence of WEE1(Figure2C).Both fusion proteins were then incubated with a PAS2:His column and an empty column.Only the WEE1-treated GST:CDK2fusion protein interacted with PAS2, demonstrating that phosphorylation of the Tyr-15residue was necessary for PAS2binding.Finally,to confirm the involvement of pTyr-15,we performed competition experiments with the conserved peptide EKVEKIGEGTYGVVYK overlapping Thr-14–Tyr-15.Peptides phosphorylated on Tyr-15but also on Thr-14 were used to displace WEE1-phosphorylated GST:CDK2bind-ing to PAS2.As shown in Figure2E,both pTyr-15and pThr-14 peptides competed off GST:CDK2binding to PAS2even if phosphorylation of Tyr-15seemed more efficient in the compe-tition experiment.Altogether,our results showed that PAS2 binds only phosphorylated CDK and that the conserved Tyr-15 and Thr-14are involved in the interaction.We checked whether PAS2binding could protect CDKA;1 from dephosphorylation.According to this hypothesis,loss of PAS2should improve the access of phosphatases to CDKA;1 and,in turn,decrease CDKA;1Tyr phosphorylation.The CDKA;1 complex was purified from the wild type and the pas2-1mutant, and the CDKA;1phosphorylation status and kinase activity were pared with the wild type,the CDKA;1Tyr phos-phorylation level in the pas2-1mutant had decreased severely, and,accordingly,the associated CDKA;1kinase activity had increased(Figures3A and3B).In conclusion,PAS2maintains CDKA;1in an inactive state by protecting CDKA;1from dephos-phorylation by phosphatases.PAS2Overexpression Alters Cell Division in Tobacco Cells Loss-of-function of PAS2leads to ectopic cell proliferation.This feature is consistent with its role as a CDKA;1inhibitor.By contrast,an increase in the PAS2protein in the cell should alter cell division and,eventually,impair development.To assess whether PAS2is involved in the regulation of cell division,we overexpressed PAS2in tobacco(Nicotiana tabacum)Bright Yellow-2(BY-2)suspension cells.Because the PAS2effect could be rapidly counterselected after several rounds of subcul-tures,we expressed PAS2under the control of an inducible Cre/ Lox system(Joube`s et al.,2004).Briefly,in this system,the PAS2 gene is cloned under the control of the constitutive cauliflowermosaic virus35S promoter,but a greenfluorescent protein(GFP) spacer with a transcription termination site prevents its expres-sion.The GFP spacer isflanked with the Lox recombination sites and can be excised in the presence of the CRE recombinase.A ligand-dependent CRE-GR construct expressed under a heat shock promoter was used to tightly control the recombinase activity.Thus,GFP excision and PAS2expression are induced by the expression of the construct pHSP-CRE:GR after heat shock(HS)and dexamethasone(Dex)treatment(Figures4A and 4B).The HS/Dex treatment did not affect the BY-2cell cultures (Figure4C;Joube`s et al.,2004).Without HS/Dex treatment,90to 95%of the BY-2cells harboring the35S-lox:GFP:lox:PAS2 construct(PAS2loxGFPlox)were GFP positive,and,correspond-ingly,only a low basal level of PAS2expression was detected in the culture(Figures4A and4B).By contrast,upon HS/Dex treatment,>80%of the cells became GFP negative after3d and accumulated PAS2transcripts as early as7h after treatment (Figures4A and4B).The effect of the PAS2expression could be observed24h after induction by a decrease in cell culture growth (Figure4C).To check whether the effect of PAS2on culture growth was related to cell division,we analyzed the cell cycle progression in synchronized PAS2loxGFPlox cells.BY-2cells were first treated with HS/Dex and24h later blocked at the G1-to-S transition by aphidicolin treatment.Aphidicolin reversibly arrests cells in early S phase by inhibiting DNA polymerase a and d(Sala et al.,1983;Nagata et al.,1992).Cells were released fromthe Figure3.Inhibition of CDKA;1Phosphorylation and Activity by PAS2.(A)Decreased Tyr phosphorylation in pas2-1mutant by CDKA;1.CDK complexes were purified on p10CKS1At from wild-type and pas2-1mutant plants.Phosphorylated CDKA;1was revealed by anti-PTYR and anti-PSTAIRE protein gel blots.(B)Increase of the CDK-associated kinase activity in pas2-1mutant plants.The Arabidopsis CDK complexes were purified on p10CKS1At,and the kinase activity was quantified by monitoring the histone H1phos-phorylation.Data were normalized according to the CDKA;1protein level as quantified by the protein gel blots with anti-PSTAIRE antibody.The kinase activity represents the result of three independent experiments. Error bars represent SE.Antiphosphatase Regulates Cell Division1429Figure 4.Inducible PAS2Overexpression in BY-2Cells.(A)Extensive GFP activity in BY-2cells transformed with PAS2loxGFPlox (left).Three days after HS and Dex induction,no GFP fluorescence could be detected (right).For each condition,BY-2cells were analyzed in light transmission and for GFP fluorescence.Bars ¼30m m.(B)Increase in PAS2expression after HS/Dex treatment.Time course of PAS2expression was measured by PCR on reverse transcription of RNA from PAS2loxGFPlox BY-2cells induced (þ)or not (ÿ).(C)Growth inhibition of induced (pink)versus uninduced (blue)PAS2loxGFPlox cell cultures.HS/Dex treatment did not affect growth of the control GFP cell culture (blue dashed line)compared with the untreated condition (pink dashed line).1430The Plant Cellaphidicolin block,and cell cycle progression was monitored by measuring the mitotic index.The aphidicolin block associated with HS and Dex did not modify cell cycle progression because control35S-GFP transgenic BY-2cells entered mitosis at the same time whether they had been treated with HS/Dex or not (Figure4D).Untreated PAS2loxGFPlox showed,like the control cell lines,a peak of mitosis at12h and then at28h after aphidicolin release.By contrast,HS/Dex-treated PAS2loxGFPlox cells reached mitosis13and30h after aphidicolin release,corresponding to a 1-and2-h delay compared with untreated cells,respectively (Figure4E).This delay in mitosis was not caused by a stress response from the combination of HS and aphidicolin treatment because a similar delay was observed in the second mitosis.The lag in mitosis entry of PAS2loxGFPlox-treated cells was on average 1.6h60.9h forfive independent experiments,while control cells treated or not always entered into mitosis simultaneously.The fact that entry into mitosis had already been delayed during the first cycle after aphidicolin block suggested that PAS2acts in S or G2phases or at the transition between G2and mitosis.To discriminate between these hypotheses,the cell cycle phases were measured byflow cytometry for thefirst25h of the synchronization experiment(Figure4F).Treated PAS2loxGFPlox cells were arrested earlier in S phase than untreated cells after aphidicolin block,but time of entry into G2phase was similar for both cell lines(Figure4G,top and middle).Thus,the delay observed in S phase could not explain the difference in mitosis timing between treated and untreated cell cultures.While both types of cells entered the G2phase synchronously,HS/Dex-treated cells had a shorter G2phase because cells exited G21h earlier than control cells(Figure4G,top).The same pattern of early entry in G2of HS/Dex-treated PAS2loxGFPlox cells was observed in two independent experiments.The delay in mitosis of the HS/Dex cell population must then originate from an inhibition of the cell progression through mitosis.Thus,we checked whether PAS2expression had an effect on specific mitotic phases by scoring the frequency of the different mitotic figures.Treated cells had an unbalanced prophase:metaphase ratio(Figure4H),suggesting that the PAS2overexpression inhibited early mitosis.While the cell cycle study on BY-2 cultures demonstrated that PAS2has a direct negative effect on cell division,this effect is probably not the only cause of growth inhibition.The reduction of PAS2loxGFPlox cell culture growth rate after induction could also result from an inhibition of cell expansion.The measurements of PAS2loxGFPlox cell length 48h after HS/Dex induction showed that PAS2induction led to a shift of the cell length distribution toward smaller cells(Figure4I). In summary,the induction of PAS2in BY-2cells reduced the growth of cell culture,which was caused by a combined inhibi-tion of cell cycle progression and cell expansion.PAS2Overexpression Alters Plant GrowthThe effect of PAS2overexpression in Arabidopsis was analyzed with transgenic plants overexpressing PAS2or PAS2:GFP fu-sions.All the constructs were functional because they could complement the pas2-1mutant(see Supplemental Figure1on-line).High GFP-producing plants were retained for further anal-ysis because PAS2accumulation could be monitored easily in the whole seedlings throughout development(Figures5A and 5B).PAS2expression relative to EF1a was quantified by RT-PCR in35S-PAS2:GFP seedlings compared with the wild type (Columbia-0)(Figure5C).The transgenic plants accumulated 30-fold more PAS2transcripts than untransformed wild type. Several independent transgenic lines with high GFP staining had obvious developmental defects with slowed growth and stunted phenotype(Figure5A).Cotyledons were smaller in35S-PAS2: GFP seedlings compared with those in the wild type and seemed to have an accelerated senescence.The development offirst leaves was often altered:either thefirst leaves were absent or had a delayed growth with primordia-like shapes or reduced leaf blades(Figure5D).Epidermal cells of young cotyledons of 35S-PAS2:GFP seedlings were similar to those of the wild type,suggesting that the smaller size of the cotyledons was caused by a reduced number of cells(Figure5E).PAS2Subcellular DistributionThe intracellular distribution of PAS2protein was investigated in the root cells of transgenic Arabidopsis expressing the construct 35S-PAS2:GFP(Figures5F and5G).PAS2:GFP was excluded from the nucleus in the root meristem(Figure5F)and in the elongation zone as well(data not shown).On the contrary,most of the cells in the differentiated zone displayed cytosolic but also nuclear distribution of the PAS2:GFP protein fusion(Figure5G). The presence of PAS2in the nucleus could also be seen in root hairs(see Supplemental Figure2online).Figure4.(continued).(D)and(E)Delay in cell cycle progression induced by PAS2.HS/Dex treatments did not modify cell cycle progression.Mitotic index was measured, after release from aphidicolin block,in35S-GFP and PAS2loxGFPlox cell cultures that were induced(pink line)or not(blue line).While HS/Dex treatment did not affect growth of the control GFP cell culture(D),it delayed PAS2loxGFPlox cell mitosis(E).(F)Cytometric analysis of the cell cycle of synchronized cells uninduced(top)and induced(bottom)from the experiment presented in(E).(G)Time course progression through the cell cycle of uninduced(blue)and induced(pink)PAS2loxGFPlox cells in G2(top panel),S(middle panel),and G1 phases(bottom panel),respectively.(H)Distribution of prophase,metaphase,and anaphase plus telophase during mitosis of uninduced(blue)and induced(pink)PAS2loxGFPlox cells.Mitotic figures were sampled infive time points during thefirst peak of mitosis of synchronized cells described in(E).Approximately200mitoticfigures were counted for each time point.Error bars represent SE.(I)Cell length distribution of uninduced(blue)and induced(pink)PAS2loxGFPlox cells.Cell length was measured48h after induction on439uninduced and575induced cells.Antiphosphatase Regulates Cell Division1431Figure 5.PAS2:GFP Expression in Arabidopsis .(A)Growth inhibition by stable expression of 35S-PAS2:GFP in 10-d-old seedlings.(B)GFP activity in seedling meristem and primary root of transgenic seedlings compared with control (Columbia-0).(C)Expression level of PAS2in 35S-PAS2:GFP seedlings compared with the wild type.Real time RT-PCR amplification of PAS2transcript was normalized against EF1a .Error bars represent SE .(D)Altered development of young leaves in 10-d-old transgenic 35S-PAS2:GFP seedlings with fused (left),finger-shaped (right),or missing leaf primordia (inset).(E)Cotyledon epidermis cells of 35S-PAS2:GFP plants compared with the wild type.(F)and (G)Subcellular distribution of PAS2:GFP changed according to cell type.In root meristem with actively dividing cells,PAS2:GFP fusion is mainly present in the cytosol (F).In differentiated cells in the root hair initiation zone,PAS2:GFP shows cytosolic and nuclear localization (G).For each panel,the GFP signal,the DRAQ5nuclear staining,and merged image are shown in the left,middle,and right panels,respectively.(H)to (L)Subcellular distribution of PAS2:GFP in BY-2cells.Most of the interphasic cells showed PAS2:GFP accumulation outside the nucleus (H)but could also be detected strongly accumulated inside the nucleus in a minority of cells (I).PAS2:GFP fusion was found to embrace chromosomes during the different phases of mitosis as shown for metaphase (J),anaphase (K),and telophase (L).For each panel,the GFP signal,the DRAQ5nuclear staining,and merged image are shown in the top,middle,and bottom panels,respectively.Bars ¼1mm ([A],[B],and [D]),25m m (E),5m m ([F]and [G]),and 30m M ([H]to [L]).1432The Plant Cell。