Enhanced alpha-ketoglutaric acid production in Yarrowia lipolytica WSH-Z06
- 格式:pdf
- 大小:469.12 KB
- 文档页数:6


![与多糖结合的治疗性肽的无菌复合物[发明专利]](https://uimg.taocdn.com/b91ba59aa26925c52dc5bf89.webp)
专利名称:与多糖结合的治疗性肽的无菌复合物专利类型:发明专利
发明人:B·库伦,D·斯尔科克,P·范罗伊文,W·哈维申请号:CN99802742.1
申请日:19991206
公开号:CN1290180A
公开日:
20010404
专利内容由知识产权出版社提供
摘要:本发明提供含有治疗性肽和多糖的复合物的无菌组合物,多糖选自纤维素衍生物、几丁质、脱乙酰几丁质、半乳甘露聚糖及其混合物,其中该组合物用电离辐射灭菌。
多糖的存在令人惊奇地稳定了治疗性肽,使其能抵抗在电离辐射下、特别是γ辐射下的分解。
本发明还包括无菌组合物的制备方法和无菌治疗性肽的制备方法。
申请人:庄臣及庄臣医药有限公司
地址:英国爱丁堡
国籍:GB
代理机构:中国专利代理(香港)有限公司
更多信息请下载全文后查看。
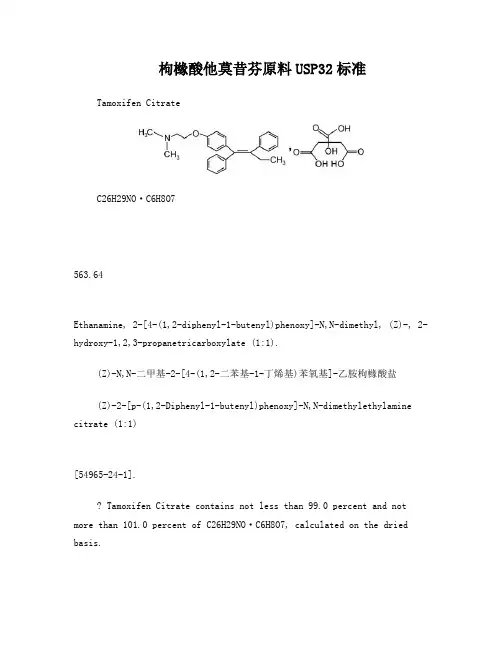
枸橼酸他莫昔芬原料USP32标准Tamoxifen CitrateC26H29NO·C6H8O7563.64Ethanamine, 2-[4-(1,2-diphenyl-1-butenyl)phenoxy]-N,N-dimethyl, (Z)-, 2-hydroxy-1,2,3-propanetricarboxylate (1:1).(Z)-N,N-二甲基-2-[4-(1,2-二苯基-1-丁烯基)苯氧基]-乙胺枸橼酸盐(Z)-2-[p-(1,2-Diphenyl-1-butenyl)phenoxy]-N,N-dimethylethylamine citrate (1:1)[54965-24-1].Tamoxifen Citrate contains not less than 99.0 percent and not more than 101.0 percent of C26H29NO·C6H8O7, calculated on the dried basis.枸橼酸他莫昔芬按干燥品计算,含C26H29NO·C6H8O7不得少于99.0%,不得多于101.0%。
Packaging and storage— Preserve in well-closed, light-resistant containers.包装和贮存—在密闭、耐光的容器中保存。
Description: White, fine, crystalline powder. Soluble in methanol; very slightly soluble in water, in acetone, in chloroform, and in alcohol.性状:本品为白色结晶性粉末。
本品在甲醇中溶解,在水、丙酮、三氯甲烷和乙醇中极微溶解。
Melting Point: Melts at about 142°, with decomposition.熔点:本品的熔点大约为142℃,熔融时同时分解。

生物科学词汇AABO血型ABC blood groupA带A-banda螺旋alpha helixa酮戊二酸alpha-ketoglutaric acid阿米巴痢疾;变形虫性痢疾amoebic dysentery阿士匹灵; 阿司匹林aspirin癌cancer;carcinoma癌细胞cancerous cell爱迪生氏病;阿狄森氏病Addison’s disease安非他明;苯异丙胺amphetamine安全期safe period安全期避孕法rhythm method胺amine氨ammonia氨芐青霉素;氨芐青霉素ampicillin氨化;氨化作用ammonification氨化细菌ammonifying bacteria氨基amino group氨基化合物amide氨基甲酸脂carbamate氨基酸amino acid氨基酸顺序;氨基酸序列amino acid sequence氨基转移; 氨基转移作用transamination安达卢西亚Andalusian fowl安瓿;针药管ampoule按蚊属Anopheles暗带dark band暗反应dark reactions凹穴玻片cavity slide螯;钳爪chela螯角; 钳爪chelicera螯虾crayfish螯足cheliped奥陶纪Ordovician periodB巴氏体; 巴尔氏小体; 性染色质小体Barr body巴斯德消毒法pasteurization白氨酸;亮氨酸leucine白粉霉病;白粉霉病powdery mildew白化;白化现象,白化症albinism白化体,白化病患者albino白喉diphtheria白菌属Albugo白蛉sandfly白内障cataract白色体leucoplast白纤维软骨white fibrocartilage白血病leukaemia白血球;白血细胞white blood cell;white blood corpuscle;leucocyte 白蚁termite白质white matter白垩纪Cretaceous period斑macula斑叶variegated leaf斑疹伤寒typhus;typhus fever板根;板状根buttress root板鳃亚纲Elasmobranchii半保留复制semiconservative replication半变态hemimetamorphosis半变态的hemimetabolous半变态发育hemimetabolous development半翅目Hemiptera半关节面demifacet半合基因hemizygous gene半合子hemizygote半花图half-flower diagram半固体尿液semisolid urine半胱氨酸cysteine半规管semicircular canal半寄生物;兼性寄生物hemiparasite半乳糖galactose半衰期half-life半索动物;半索类hemichordate半透性的; 半渗透性的semi-permeable半透性膜semi-permeable membrane半纤维素hemicellulose半月瓣semilunar valve伴细胞; 伴胞companion cell瓣;瓣膜,壳瓣,裂片valve瓣鳃动物lamellibranch瓣鳃纲Lamellibranchia棒眼bar eye包埋技术embedding technique包皮prepuce包皮腺preputial gland苞鳞bract scale苞片bract胞壁质murein胞间连丝plasmodesmata胞间隙intercellular space胞间液intercellular fluid胞嘧啶cytosine胞内的;细胞内的intracellular胞内消化;细胞内消化intracellular digestion胞囊形成encystment胞体; 细胞体cell body胞外的;细胞外的extracellular胞外消化;细胞外消化extracellular digestion胞外液;细胞外液extracellular fluid胞芽gemma胞芽杯gemma cup胞衣;胎盘胎膜afterbirth胞质分裂; 细胞质分裂cytokinesis胞质环流cyclosis胞质桥; 胞质间桥cytoplasmic bridge孢间连丝; 孢间连体disjunctor孢囊柄sporangiophore孢子spore孢子虫sporozoon孢子囊spore sac;sporangium;sorus孢子囊,被囊幼虫; 胞蚴sporocyst孢子生殖sporogony孢子体sporogonium;sporophyte孢子小体;子孢子sporozoite孢子形成sporulation孢子叶sporophyll薄壁组织parenchyma宝血草sea-lavender爱护; 储存conservation爱护色protective colouration保水力;保水量water retention capacity;water-retaining capacity 保卫细胞guard cell保幼激素juvenile hormone (JH)饱和脂肪酸saturated fatty acid曝气池aeration tank鲍曼氏囊, 肾小球囊Bowman's capsule杯形托,胞芽杯,杯形器,壳斗cupule杯状细胞goblet cell背,背甲,背板tergum背膘backfat背膘厚度backfat thickness背大动脉dorsal aorta背的; 背面的; 背侧的dorsal背腹的dorsi-ventral背腹肌dorsoventral muscle背根dorsal root背根节dorsal root ganglion背甲,背片tergite背孔dorsal pore背裂dorsal fissure背须dorsal cirrus背叶notopodium被动免疫passive immunity被动免疫法passive immunization被囊动物tunicate被囊动物亚门Tunicata被子植物angiosperm被子植物纲Angiospermae苯胺染料aniline dye苯丙氨酸;苯基丙氨酸phenylalanine苯丙酮酸phenylpyruvic acid苯丙酮酸尿症phenylketonuria苯醌quinine苯酸; 苯甲酸benzoic acid本底辐射; 本底放射background radiation 本地种indigenous species本立德溶液Benedict's solution本立德试剂Benedict's reagent本立德试验Benedict's test本能instinct本能行为instinctive behaviour本生灯Bunsen burner本体感受器proprioceptor;proprioreceptor 贲门cardium贲门括约肌cardiac sphincter鼻隔nasal septum鼻腔nasal cavity鼻腺nasal gland比较胚胎学comparative embryology比较器comparator比目鱼flatfish比重计hydrometer必需氨基酸essential amino acid臂动脉brachial artery臂静脉brachial vein壁虎gecko壁压wall pressure避孕contraception避孕屏障contraceptive barrier避孕套condom避孕丸contraceptive pill蓖麻籽castor bean边材; 液材sapwood边缘区域fringe area边缘胎座式marginal placentation鞭毛flagellum鞭毛虫flagellate鞭毛虫纲Flagellata鞭毛纲Mastigophora扁虫flatworm扁桃体tonsil扁形动物门Platyheliminthes变态metamorphosis变温动物poikilotherm变温性poikilothermy变形虫amoeba变形运动amoeboid movement变性蛋白; 变性蛋白质denatured protein变性作用denaturation变异variation便秘constipation标本specimen标本瓶specimen bottle标志重捕法mark-and-recapture method标准差; 标准偏差; 标准误差standard deviation 标准曲线standard curve标准溶液standard solution表胚层periblast表皮epidermis表皮细胞epidermal cell表面玻璃watch glass表面积; 表面面积surface area表面循环superficial circulation表面张力surface tension表膜pellicle表土topsoil表现型phenotype表现型比;表现型比值phenotypic ratio鳔swim bladder濒危物种endangered species冰醋酸;无水醋酸glacial acetic acid兵蟹soldier crab滨草,沙地芦苇marram grass滨螺periwinkle丙氨酸alanine丙二醇propylene glycol丙糖triose丙酮acetone丙酮酸pyruvic acid丙酮酸盐; 丙酮酸脂pyruvate柄,花柄,pedicel柄,叶柄petiole饼图;圆瓣图pie chart病rust病毒virus病毒感染viral infection病毒粒子virion病毒性肝炎virus hepatitis病毒学virology病菌;病原菌,胚;胚芽;胚原基germ病原体pathogen波尔效应Bohr effect波浪作用wave action玻璃液;玻璃状液vitreous humour玻片; 切片slide钵口幼虫scyphistoma钵水母纲Scyphozoa勃起肌;立肌erector勃起组织erectile tissue补偿点compensation point补呼气量expiratory reserve volume补吸气量inspiratory reserve volume哺乳动物;哺乳类mammal哺乳纲Mammalia捕虫盘beating tray捕食的predatory捕食者predator捕食者猎物相亘作用predator-prey interaction 不饱和脂肪酸unsaturated fatty acid不定根adventitious root不动关节fixed joint;immovable joint不分离现象non-disjunction不兼容性;不亲和性incompatibility不可降解的;非降解性的nondegradable不可再生资源;非再生资源non-renewable resources不连续变异discontinuous variation不连续生长discontinuous growth不裂的;不开裂的indehiscent不随意的involuntary不随意肌involuntary muscle不透的impermeable不透性层impervious layer不完全变态;不全变态incomplete metamorphosis不完全显性incomplete dominance不完全菌类;半知菌类;半知菌亚门;不完全菌纲Fungi Imperfecti 不应期; 不反应期; 休复期refractory period不育性sterility步带ambulacrum布伦纳氏腺; 十二指肠腺Brunner's gland部分显性partial dominanceC残余物,残基residue操纵子operon糙皮病;癞皮病pellagra槽,齿槽socket草本层herbaceous layer草本植物herb草醯醋酸oxaloacetic acid草履虫paramecium草原生境grassland habitat草质茎herbaceous stem侧根lateral root侧茅lateral bud侧膜胎座式parietal placentation侧片pleurite侧神经节pleural ganglion侧生分生组织lateral meristem侧丝paraphysis侧线lateral line侧足parapodium测定; 检定assay测交test cross测微计micrometer层stratum差示空气温度计; 相异空气温度计differential air thermometer差异生殖differential reproduction差异透性; 分别透性differential permeability差异透性膜; 分别透性膜differentially permeable membrane插条,插条法cutting颤搐twitch颤蚓属Tubifex产孢子组织sporogenous tissue产乳量; 产奶量milk yield长匐茎runner长骨long bone长日照植物long-day plant长牙tusk常染色体; 体染色体autosome常染色质euchromatin肠enteron;intestine肠表皮;肠皮层gastrodermis肠系膜mesentery肠系膜动脉mesenteric artery肠系膜后动脉posterior mesenteric artery肠系膜前动脉anterior mesenteric artery肠腺intestinal gland肠液intestinal juice;succus entericus超极化hyperpolarization超滤;超滤作用ultrafiltration超速离心机ultracentrifuge超微结沟ultrastructure潮虫wood-louse潮间带;潮间带;滨海带;海岸带;潮汐带intertidal zone;tidal zone;littoral zone 潮气; 呼吸气tidal air潮气量tidal volume潮汐节律tidal rhythm潮汐作用tidal action潮下带; 亚海岸带sublittoral zone沉淀;降水量precipitation沉淀素precipitin沉积岩sedimentary rock沈降池; 沈淀池sedimentation tank成层现象stratification成虫imago成带;成带现象zonation成带型zonation pattern成骨细胞osteoblast成花素;成花激素florigen成年期adulthood成软骨细胞chondroblast成熟maturation成熟,成熟度maturity成纤维细胞fibroblast都市生态系;都市生态系统urban ecosystem 池, 潴池cisterna池黾;水马pond skater持水量;持水力water-holding capacity迟发过敏性delayed hypersensitivity尺water measurer尺骨ulna齿槽tooth socket齿根tooth root齿冠tooth crown齿颈tooth neck齿舌radula齿式dental formula齿突;齿状突odontoid process齿型tooth type齿虚位; 牙虚位diastema齿龈gum耻骨pubis耻骨联合pubic symphysis豉豆虫;豉虫whirligig beetle赤道板equatorial plate赤霉酸;赤霉酸gibberellic acid;gibberellin 赤藓红;藻红;真曙红erythrosine翅,翼瓣wing虫hookworm虫媒传粉insect pollination虫媒花insert-pollinated flower重瓣胃omasum重吸取; 重吸取作用reabsorption重演说recapitulation theory重组recombination重组DNA技术recombinant DNA technology 重组质粒recombinant plasmid重组子recon抽样; 取样sampling臭氧ozone臭氧层ozone layer出汗perspiration出鳃动脉efferent branchial artery出鳃静脉efferent branchial vein出生率birth rate;natality rate出水管excurrent siphon;exhalant siphon出土的epigeal出土式萌发epigeal germination出血haemorrhage出芽, 出芽生殖; 芽殖, 芽接budding初级精母细胞primary spermatocyte初级淋巴组织primary lymphoid tissue初级卵母细胞primary oocyte初级生产力primary productivity初级消费者primary consumer初乳colostrums初生分生组织primary meristem初生根primary root初生木质部primary xylem初生韧皮部primary phloem初生生长primary growth初生体重birth weight初生细胞壁primary cell wall初始质壁分离incipient plasmolysis除草剂;除莠剂herbicide;weed-killer除虫菊pyrethrum储备溶液stock solution储唾囊; 储涎囊salivary receptacle储蓄泡reservoir触角antenna触觉感受器touch receptor触觉纤毛tactile cilium触毛; 触须, 蔓足cirrus触手; 触须tentacle传出神经;输出神经efferent nerve传出神经元;输出神经元efferent neurone传导,输导conduction传导组织conductive tissue传粉;传粉作用pollination传染病infectious disease传染媒介vector of infection传入神经;输入神经afferent nerve传入神经元;输入神经元afferent neurone垂唇hypostome垂管manubrium垂体;脑下垂体pituitary body;pituitary gland;hypophysis 垂体后叶;脑下垂体后叶posterior pituitary垂体前叶;脑下垂体前叶anterior pituitary垂直成层结构vertical stratification锤骨hammer;malleus春化作用vernalization纯合的homozygous纯合子;同型接合子homozygote纯酒精;无水酒精absolute alcohol纯培养; 纯种培养; 纯培养物pure culture纯群聚;同型同种群聚gregation纯系pure line纯系; 无性系; 无性繁育系; 克隆clone纯系化; 无性系化; 无性繁育系化cloning纯系生长; 无性系生长; 无性繁育系生长clonal growth纯系选择理论; 无性系选择理论; 无性繁育系选择理论clonal selection theory 纯种purebred唇,下唇,下唇瓣labium唇基clypeus唇须;下唇须labial palp唇足纲Chilopoda慈菇arrowhead雌苞;雌器苞perichaetium雌苞叶perichaetial leaves雌二醇;雌甾二醇;雌固二醇oestradiol雌激素;雌甾激素oestrogen雌配子female gamete雌蕊pistil雌蕊花pistillate flower雌蕊群gynoecium雌蕊先熟; 雌性先熟protogyny雌三醇;雌甾三醇;雌固三醇oestriol雌酮;雌甾酮;雌固酮oestrone雌雄同体hermaphrodite雌雄同株; 雌雄同体monoecious雌雄同表达象hermaphroditism雌雄异株; 雌雄异体dioecious雌原核female pronucleus次级精母细胞secondary spermatocyte次级淋巴组织secondary lymphoid tissue次级卵母细胞secondary oocyte次级消费者secondary consumer次氯酸钠sodium hypochlorite次生分生组织secondary meristem次生根secondary root次生加厚secondary thickening次生木质部secondary xylem次生韧皮部secondary phloem次生生长secondary growth次生细胞壁secondary cell wall刺激stimulus刺激收缩偶联机制excitation-contraction coupling mechanism刺激物;刺激剂irritant刺丝囊nematocyst刺吸式piercing and sucking type刺细胞nematoblast; cnidoblast刺血针lancet从辐触手adradial tentacle粗糙食物; 基糠roughage粗蛋白质crude protein粗调剂器; 粗调焦轮coarse adjustment knob粗纤维crude fibre粗线期pachytene促黑激素; 促黑素细胞激素melanocyte stimulating hormone (MSH)促黄体激素;促黄体生成激素luteinizing hormone (LH)促甲状腺素thyrotropin;thyrotropic hormone;thyrotrophin促甲状腺激素thyroid stimulating hormone;thyrotrophic hormone促进作用;加速作用acceleration;facilitation促卵泡激素follicle stimulating hormone (FSH)促乳素; 促乳激素prolactin促肾上腺皮质激素adrenocorticotrophic hormone促生长素; 生长激素somatotrophin;somatotropin促生长激素; 生长激素somatotrophic hormone;somatotropic hormone促胃液素gastrin促性腺素;促性腺激素gonadotrophin;gonadotropin;gonadotropic hormone 醋酸acetic acid醋酸地衣红染剂aceto-orcein stain醋酸杆菌属Acetobacter醋酸酒精acetic alcohol醋酸铅试纸lead acetate paper催产素oxytocin催汗神经元sudomotor neurone催化剂catalyst催泪反射lachrymal reflexDDNA 聚合物DNA polymer大孢子megaspore大孢子囊megasporangium大孢子叶megasporophyll大肠large intestine大穿刺丝囊;大穿刺囊penetrant大动脉aorta大动脉弓aortic arch大颚,上颚,下颔骨;下颚骨mandible大分子macromolecule大核macronucleus大量营养素;要紧营养表macronutrient大裂球;大分裂球macromere大陆漂移continental drift大麻,大麻纤维hemp大麦芽bark大脑cerebrum大脑半球cerebral hemisphere大脑皮层; 大脑皮质cerebral cortex大薸water lettuce大试管boiling tube大田作物;农作物field crop大蚊幼虫cranefly larva大纤丝;大型纤维macrofibril大型生物区系;大型生物相macrobiota;macrofauna 大型消费者macroconsumer大型植物区系;大型植物相macroflora大阴唇labia majora达尔文主义Darwinism呆小病cretinism代era;generation代谢; 代谢作用; 新陈代谢metabolism代谢废物metabolic waste代谢活动metabolic activity代谢率metabolic rate代谢途径metabolic pathway代谢物metabolite带girdle带间的interzonal单倍世代haploid generation单倍数haploid number单倍体monoploid;haploid单侧刺激unilateral stimulus单纯系抗体; 单克隆抗体monoclonal antibody单果simple fruit单核白血球; 单核白血细胞monocyte单基因遗传monohybrid inheritance单基因杂交monohybrid cross单基因杂交比monohybrid ratio单基因杂种monohybrid单链DNA single-stranded DNA单亲遗传uniparental inheritance单球菌monococcus单染色体monosome单糖monosaccharide单体monomer单体性monosomy单体雄蕊的monadelphous单突触反射弧monosynaptic reflex arc单细胞蛋白质single cell protein (SCP)单细胞的unicellular单性果实parthenocarpous fruit单性结实parthenocarpy单性生殖;孤雌生殖parthenogenesis单循环single circulation单眼ocellus;simple eye单叶simple leaf单种裁培; 单株培养monoculture单子叶植物monocot;monocotyledonous plant单子叶植物纲Monocotyledoneae丹宁; 鞣酸,鞣料; 鞣质tannin担孢子basidiospore担孢子梗,叶座sterigma担轮幼虫trochophore担子basidium担子菌baisdiomycete担子菌纲Basidiomycetes担子菌门Basidiomycota担子菌亚门Basidiomycotina胆固醇; 胆甾醇cholesterol胆管bile duct胆红素bilirubin胆碱choline胆碱能的; 胆碱功能的,胆碱功能药物cholinergic胆碱能神经元; 胆碱功能神经元cholinergic neurone 胆绿素biliverdin胆囊gall bladder胆色素bile pigments胆石gallstone胆小管bile canaliculi;bile ductule胆盐bile salts胆汁bile胆总管common bile duct淡水生境freshwater habitat蛋白;蛋清,清蛋白;白蛋白,胚乳albumen 蛋白间质proteose蛋白酶类protease蛋白水解; 蛋白水解作用proteolysis蛋白细胞,胚乳细胞albuminous cell蛋白质; 蛋白; 朊protein蛋白质合成protein synthesis弹尾目Collembola氮肥nitrogen fertilizer氮循环nitrogen cycle导管vessel导管分子vessel element导热性thermal conductivity倒位inversion倒位纯合子inversion homozygote倒位杂合子inversion heterozygote德国麻疹German measles等长生长isometric growth等长收缩isometric contraction等翅目Isoptera等电点isoelectric point等量样本;等分部份aliquot等摩尔的equimolar等渗的,等张的isostonie等渗溶液isotonic solution等位基因allele;allelomorph等显性; 共显性, 等显势codominance等张收缩isotonic contration等足动物isopod镫骨stirrup;stapes低倍物镜;低倍接物镜low power objective低聚糖;寡糖oligosaccharide低渗的hypotonic低渗溶液hypotonic solution低温的hypothermic;hypothermia滴定; 滴定法titration滴定管burette滴管dropper滴瓶dropping bottle滴试板spot plate滴液漏斗dropping funnel滴液移液管dropping pipette绦虫cestode;tapeworm绦虫纲Cestoda绦虫属Taenia绦虫幼虫; 绦虫蚴proscolex底土subsoil第二寄主secondary host第二性征secondary sexual character第二子代; 子二代second filial (F2) generation第三纪Tertiary period第四纪Quaternary period第一寄主primary host第一子代;子一代first filial (F1) generation递质; 传递介质; 传导物质transmitter地理分布geographic distribution地理隔离geographic isolation地理障碍geographic barrier地钱属Marchantia地热能geothermal energy地蜈蚣earwig地下区域subterranean zone地衣lichen地衣红orcein地质年代表geologic time scale地中海贫血; 地中海贫血症thalassaemia点滴试验; 亮点法spot test点突变point mutation点样方point quadrat;pin quadrat碘iodine碘液iodine solution碘液试验iodine test电感骨器electroreceptor电解质electrolyte电离辐射ionizing radiation电泳electrophoresis电子传递链electron transport chain电子传递体系electron transport system电子供体electron donor电子受体electron acceptor电子显微技术;电子显微镜术electron microscopy 电子显微镜electron microscope电子显微照片electron micrograph电子载体electron carrier淀粉starch淀粉碘化物试纸starch iodide paper淀粉核pyrenoid淀粉酵素amylase淀粉粒starch grain碟状幼体ephyra顶,头顶vertex顶端分生组织apical meristem顶端优势; 顶芽优势apical dominance顶盖tectum顶极群落climax community顶级食肉动物; 顶极食肉动物top carnivore 顶体acrosome顶细胞apical cell顶芽apical bud;terminal bud顶叶parietal lobe定点诱变site-directed mutagenesis定积土sedentary soil定位mapping定向orientation定向反应directional response定向选择directional selection冬眠hibernation动脉artery动脉导管ductus arteriosus动脉干truncus arteriosus动脉压; 动脉血压arterial pressure动脉系; 动脉系统arterial system动脉血栓形成arterial thrombosis动脉硬化; 动脉硬化症arteriosclerosis动脉锥; 动脉圆锥conus arteriosus动情期oestrus动情期同步化oestrus synchronization动情前期pro-oestrus动情周期oestrous cycle动丝;动纤丝kinetodesma动态;运动kinesis动态平稳dynamic equilibrium动物极animal pole动物区系;动物相fauna动物式营养holozoic nutrition动物育种;家畜繁育animal breeding动作电势;动作电位action potential冻干切片术freeze-drying microtomy冻蚀法;冰冻蚀刻法freeze-etching technique 冻原tundra豆科Leguminosae豆科植物leguminous plant豆娘假设虫damselfly nymph痘病毒poxvirus窦; 腔antrum;sinus窦房结sinoatrial node窦状隙sinusoid独立分配independent assortment独立分配定律law of independent assortment独立分配原理principle of independent assortment 毒力;毒性;致病力virulence毒蛇venomous snake毒神经的neurotoxic毒素toxin毒雾smog毒性toxicity毒性菌株virulent strain毒性噬菌体;烈性噬菌体virulent phage毒液venom杜匀氏漏斗; 生物分离漏斗Tullgren funnel端脑telencephalon短日照植物short-day plant短生的;短命的ephermeral短枝dwarf shoot断裂;断裂生殖fragmentation断乳体重weaning weight堆肥compost对氨基苯酸;对氨基苯甲酸para-aminbenzoic acid 对比性状contrasting character对称symmetry对数期;对数生长期log phase;logarithmic phase 对数曲线logarithmic curve对虾prawn对比control对比实验control experiment盾鳞placoid scale多倍体polyploidy多倍性plordy多核的coenocytic;multinucleate多核糖体polysome;polyribosome多核体; 多核细胞coenocyte多基因遗传polygenic inheritance多极神经元multipolar neurone多孔动物门Porifera多毛动物;多毛类polychaete多毛纲Polychaeta多年生perennation多年生植物perennial plant多糖polysaccharide多细胞的multicellular多线染色体polytene chromosome多形核白血球;多形核白血细胞polymorphonuclear leucocyte多形核颗粒白血球;多形合颗粒白血细胞polymorphonuclear granulocyte 多形状现象;多态性polymorphism多样性diversity多原型polyarch多趾polydactyly多足动物; 多足类myriapod多足纲MyriapodaE额frons额叶frontal lobe锇酸osmic acid恶性肿瘤malignant tumour阻遏基因repressor gene阻遏物repressor萼片sepal颚;颌jaw儿童期childhood耳垂ear lobe耳膜eardrum耳砂otoconium耳石otolith耳蜗cochlea耳蜗管cochlear duct二倍数diploid number二倍体diploid二叉分枝式dichotomous branchng二叉式检索表dichotomous key二次消费者second order consumer二叠纪Permian period二分裂; 二分体分裂binary fission二级结构secondary structure二价的,二价染色体bivalent二尖瓣bicuspid valve二磷酸果糖fructose bisphosphate二磷酸核酮糖ribulose bisphosphate (RuBP)二硫键disulphide bond二氯酚靛酚dichlorophenol indophenol (DCPIP)二年生的biennial二年生植物biennial plant二歧聚伞花序; 二歧聚伞花序dichasial cyme 二羧酸dicarboxylic acid二头肌biceps二氧化碳carbon dioxide二氧化硫sulphur dioxide二支的; 二岔的biramousF F纤毛F-pilusF因子 F factorFAA固定剂FAA fixative发酵;发酵作用fermentation发酵器fermenter发生率incidence发生器电势;发生器电位generator potential 发育development法类皮欧氏管;输卵管Fallopian tube番红safranine繁育propagation繁育体propagule反刍regurgitation反刍动物ruminant反口面aboral surface反馈feedback反馈机制feedback mechanism反馈操纵feedback control反馈抑制feedback inhibition反密码子anticodon反射动作reflex action反射弧reflex arc反射中枢reflex centre反射作用;分射reflex反突变back mutation反硝化菌; 脱氮菌denitrifier反硝化细菌; 脱氮细菌denitrifying bacteria 反硝化作用; 脱氮作用denitrification反应reaction;response反应器effector反应时刻reaction time反应物reactant反足细胞antipodal cell返巢本能homing instinct泛酸pantothenic acid方格网;格网grid纺锤状原始细胞fusiform initial防腐剂preservative防腐剂; 消毒剂; 抗菌剂antiseptic防备反应defense reaction房室瓣atrioventricular valve房室结atrioventricular node纺锤丝spindle fibre纺锤体spindle纺织腺spinning-gland放大镜hand lens放牧牲畜grazing animal放射虫纲Radiata放射肌radial muscle放射性的radioactive放射性示踪物radioactive tracer放射性同位素radioisotope放射性同位素定年法; 放射性同位素年份测定radioisotope dating 放线菌actinomycete放线菌目Actinomycetales放线菌素actinomycin飞沬传染droplet infection非必需氨基酸non-essential amino acid非等位基因non-allelic gene非叠密码non-overlapping code非还原糖non-reducing sugar非极性的non-polar非竞争性抑制noncompetitive inhibition非生物的,无生命的abiotic非特异性免疫nonspecific immunity非细胞的acellular非原质体apoplast非整倍体aneuploid非整倍性aneuploidy非洲睡病;非洲昏睡病African sleeping disease非姊妹染色单体non-sister chromatid菲令氏溶液Fehling’s solution菲令氏试验Fehling’s test肥大细胞mast cell腓骨fibula肺;肺脏lung肺尘埃沉病pneumonoconiosis肺动脉pulmonary artery肺活量vital capacity肺静脉pulmonary vein肺量计; 呼吸量计spirometer肺螺亚纲Pulmonata肺泡,齿槽;牙槽alveolus肺气肿emphysema肺循环pulmonary circulation肺炎pneumonia肺炎球菌pneumonococcus肺炎球菌属Pneumococcus废料循环waste recycling废水effluent废物;废料waste product废物处理waste treatment分贝decibel (dB)分布类型distribution pattern分布区重叠的; 同域的sympatric分布曲线distribution curve分化; 分化作用differentiation分节; 分节现象segmentation分节的metameric分节现象metamerism分解; 分解作用decomposition分解,辨论率; 清晰度resolution分解代谢catabolism分解纤维素的celluloytic分解者decomposer分离segregation分类classification分类; 分类学taxonomy分类单元; 分类群taxon分类阶梯; 分类阶层系统taxonomic hierarchy 分离定律law of segregation分离原理principle of segregation分裂,裂殖fission分流; 分路途径shunt pathway分流血管shunt vessel分泌; 分泌作用,分泌物secretion分泌过多hypersecretion分泌过少hyposecretion分泌器官secretory organ分泌素; 肠促胰液素secretin分娩birth;labour;parturition分生孢子conidium分生孢子梗; 分生孢子柄conidiophore分生组织meristem分压partial pressure分子式molecular formula酚phenol粉管受精的siphonogamous粉砂silt粪便dung;faece粪肥manure丰度;多度abundance丰度指数;多度指数index of abundance 风化;风化作用weathering风媒传粉wind pollination风媒花wind-pollinated flower风疹rubella蜂窝胃,网状结构reticulum凤尾蕨属Pteris缝; 骨缝suture跗骨tarsal跗骨,跗节,睑板tarsus辐管; 辐水管radial canal辐卵裂radial cleavage辐射; 放射radiation辐射对称radial symmetry辐射对称花actinomorphic flower孵化率hatchability浮肋floating rib浮浪幼体planula浮萍,青萍duckweed浮游动物zooplankton浮游生物plankton浮游生物网plankton net浮游幼虫planktonic larva浮游植物phytoplankton蜉蝣目Ephemeroptera蜉蝣假设虫mayfly nymph佛尔克曼氏管V olkmann’s canal氟化物fluoride辅基prosthetic group辅酵素coenzyme辅因子cofactor辅阻遏物corepressor腐败; 腐解decay腐败细菌putrefying bacteria腐败作用putrefaction腐坏spoilage腐生; 腐生现象saprophytism腐生物; 腐生生物saprobiont腐生营养saprophytic nutrition腐生植物saprophyte腐生植物的,腐生的saprophytic腐食性生物; 腐生物saprotroph腐殖酸humic acid腐殖土humic soil腐殖质humus腐殖质池humus tank腐质消费者; 碎屑消费者detritus consumer脯氨酸proline父本male parent复层上皮stratified epithelium复等位基因; 多等位基因multiple alleles复分裂multiple fission复果multiple fruit复合肥料compound fertilizer复合物complex复极化; 再极化repolarization复糖complex sugar复眼compound eye复叶compound leaf复制duplication;replication复制平册培养; 复制平皮培养; 复制平皿培养法; 复制平板培养法replica plating 复制物,印模replica复壮; 回春rejuvenation腹;腹部abdomen;venter腹大动脉;腹侧大动脉ventral aorta腹的;腹面的;腹侧的ventral腹根ventral root腹甲plastron;sternite腹裂ventral fissure腹面沟ventral groove腹膜,围脏膜peritoneum腹鳍pelvic fin腹腔abdominal cavity腹腔动脉coeliac artery腹泻diarrhea腹须ventral cirrus腹支ventral ramus腹足pleopod腹足动物;腹足类gastropod腹足纲Gastropoda副产品by-product副淀粉paramylum副萼epicalyx副交感神经parasympathetic nerve副交感神经系统parasympathetic nervous system 副细胞subsidiary cell负反馈negative feedback负染色法negative staining technique负性加速期negative acceleration phase富养化作用eutrophication附;附力,粘连adhesion附睪epididymis附加体;游离体episome附力;黏附力adhesive force附器hapteron附生植物epiphyte附腺;副腺accessory glands附肢, 附器appendage附肢骨骼appendicular skeleton覆膜tectorial membrane覆瓦状的imbricateG 伽玛射线gamma ray盖; 被盖tegmentum盖,鳃盖,蒴盖operculum盖玻片cover glass;cover slip盖度; 覆盖度degree of coverage钙化; 钙化作用calcification钙化醇calciferol钙质壳calcareous shell洪涝,洪涝度aridity干化; 干燥作用desiccation干扰interference干扰素interferon干细胞stem cell干重dry weight肝;肝脏liver肝动脉hepatic artery肝静脉hepatic vein肝盲囊hepatic caecum肝门静脉hepatic portal vein肝素heparin肝细胞hepatocyte肝吸虫;肝蛭liver fluke肝炎hepatitis肝总管common hepatic duct甘氨酸glycine甘油glycerine;glycerol甘油二酯diglyceride甘油三脂triglyceride甘油一酯monoglyceride甘油酯glyceride坩埚crucible坩埚钳crucible tongs杆菌bacillus杆状体rhabdite感化性; 感药性chemonasty感受sensation感受棍rhopalium感受毛细胞sensory hair cell感受囊sensory capsule感受器官sense organ感受区sensory area感受神经末鞘sensory nerve ending感受神经纤维sensory nerve fibre感受神经元sensory neurone感受细胞sensory cell感热性thermonasty感受器,受体receptor感受器电势; 感受器电位receptor potential 感性nasty感性反应nastic response感性运动nastic movement感旋光性photonasty感夜性nyctinasty感应性irritability感震性seismonasty刚毛,剌毛,须,鬃bristle; chaeta;eta刚毛囊; 毛囊chaetal sac纲class纲翅目Dictyoptera肛门anus杠杆系统lever system高柏氏腺; 尿道球腺Cowper's gland高倍物镜;高倍接物镜high power objective 高尔基器Golgi apparatus高尔基体Golgi body高能化合物energy-rich compound高渗的hypertonic高渗溶液hypertonic solution高渗性hypertonicity高血糖素;胰高血糖素glucagons高血压hypertension高压灭菌器; 消毒蒸锅autoclave高原适应;高空适应altitude acclimatization 哥蒂氏器;螺旋器organ of Corti格拉夫氏卵泡;囊状卵泡Graafian follicle 格兰氏染剂Gram stain格兰氏阳性细菌Gram-positive bacteria格兰氏阴性细菌Gram-negative bacteria 隔; 中隔; 隔膜septum隔离机制isolating mechanism隔膜; 隔板,横膈膜,子宫帽,光栏diaphragm 膈神经phrenic nerve铬酸chromic acid个体发生ontogeny个体生态学autecology根端压片; 根尖压片root tip squash根冠root cap根茎rhizome根瘤root nodule根瘤菌nodule bacteria;root nodule bacteria 根瘤菌属Rhizobium根毛root hair根毛层piliferous layer根霉属; 根霉属Rhizopus根培养基root medium根托rhizophore根压root pressure根状菌丝rhizoidal hypha根足; 根状伪足rhizopodium根足虫rhizopod根足虫纲Rhizopoda更新世Pleistocene epoch亘换interchange工业黑化现象industrial melanism弓形动脉arcuate artery供体donor巩膜sclera;sclerotic coat汞; 水银mercury汞中毒mercury poisoning共体; 共肉coenosarc共生; 共生现象symbiosis共生生物symbiont共生者; 共栖者commensal共质体symplasma;symplast沟sulcus佝偻病rickets菇类; 蕈类mushroom古动物学palaeozoology古生代Palaeozoic era古生物学palaeontology古新世Palaeocene epoch古植物学palaeophytology谷氨醯胺;谷氨酰胺glutamine谷氨酸glutamic acid谷氨酸钠; 味精monosodium glutamate (MSG) 骨板bone lamella骨干diaphysis骨骼skeleton骨骼肌skeletal muscle骨胳系统skeletal system骨化;骨化作用ossification骨迷路; 骨性迷路bony labyrinth骨膜periosteum骨盆,肾盂pelvis骨盆腔pelvic cavity骨髓bone marrow骨细胞osteocyte骨针,骨片spicule骨组织bone tissue股骨,腿节femur股动脉femoral artery股骨; 大腿骨thigh bone股静脉femoral vein鼓膜tympanum;tympanic membrane鼓藻desmid钴胺素cobalamin固醇; 甾醇sterol固氮;固氮作用nitrogen fixation固氮菌azobacter固氮菌属Azotobacter固氮细菌nitrogen fixing bacteria固的sedentary固定,固定法,定影;定像fixation固定剂fixative固化; 硬化;成熟(干酪),食品加工curing固器holdfast固碳作用carbon fixation固有层lamina propia刮勺spatula寡毛动物;寡毛类oligochaete寡毛纲Oligochaeta关节joint关节囊joint capsule;articular capsule关节软骨articular cartilage关节突zygapophysis关节炎arthritis管胞tracheid管虫tube worm管分泌tubular secretion管核tube nuclei管瓶;管形瓶;小药瓶vial管细胞solenocyte管足tube feet冠心病; 冠状心脏疾病coronary heart disease 冠状动脉coronary artery冠状动脉血栓形成coronary thrombosis冠状静脉coronary vein冠状循环; 冠脉循环coronary circulation灌溉irrigation灌木shrub灌木层shrub layer光导形状发生photomorphogenesis光反应light reactions光感受器photoreceptor光合室photosynthetic chamber光合丝photosynthetic filament光合自养生物;光能自养生物photoautotroph 光合作用photosynthesis光化毒雾photochemical smog光化反应photochemical reactions光呼吸;光呼吸作用photorespiration光激活;光活化photoactivation光解作用photolysis光磷酸化;光磷酸化作用photophosphorylation 光敏素;光敏色素phytochrome光谱,谱spectrum光系统I photosystem I光系统II photosystem II。

AA-bandA带abdomen腹;腹部abdominal cavity腹腔abductor外展肌;展肌abiogenesis自然发生说;无生源说abiotic(1)非生物的,(2)无生命的ABC blood groupABO血型abomasum皱胃aboral surface反口面abortion流产abscisic acid脱落酸abscission脱离;脱落abscission layer离层absolute alcohol纯酒精;无水酒精absolute refractory period 绝对不应期;绝对休复期absorbance吸光度absorbancy吸光度absorption吸收;吸收作用absorption spectrum 吸收光谱abundance丰度;多度abyssal region深海区acceleration促进作用;加速作用acceptor受体;接受体acceptor site受体部位accessory glands附腺;副腺acclimation(1)驯化;风土驯化,(2)适应;acclimatization(1)驯化;风土驯化,(2)适应;accommodation调节;视觉调节;调整作用acellular非细胞的Acetabularia伞藻属acetaldehyde乙醛acetic acid醋酸acetic alcohol醋酸酒精aceto-orcein stain醋酸地衣红染剂Acetobacter醋酸杆菌属acetone丙酮acetylcholine (ACh)乙醯胆碱;乙?胆碱acetylcholinesterase乙醯胆碱脂?;乙?胆碱脂?acetylcoenzyme A乙醯辅?A;乙?辅?Aachene瘦果acicula针;刺acid hydrolysis酸解;加酸水解acid rain酸雨acid-base balance酸碱平衡acid-tolerant耐酸的acidophil(1)嗜酸的,(2)嗜酸生物,(3)acidophilic嗜酸的acinour gland泡状腺acinus腺泡acoelomate无体腔动物acquired active immunity获得性自动免疫;后天性自动免疫acquired immune deficiencysyndrome (AIDS)acquired immunity获得性免疫;后天性免疫acquired passive immunity获得性被动免疫;后天性被动免疫Acrania无头类acrosome顶体actin肌动蛋白;肌纤蛋白actinomorphic flower辐射对称花Actinomycetales放线菌目actinomycete放线菌actinomycin放线菌素action potential动作电势;动作电位action spectrum作用光谱activated macrophage活化巨噬细胞activated sludge method 活性污泥法;活性污泥处理法activated T-cell活化T细胞activation活化;激活activation energy活化能activator激活物active immunity自动免疫active site活性部位active transport主动运输actomyosin肌动球蛋白acuity锐度;敏度;分辨能力adaptation适应adaptive behaviour 适应行为adaptive convergence 适应趋同adaptive immunity适应性免疫adaptive migration适应迁移adaptive radiation适应辐射Addison¢s disease爱迪生氏病;阿狄森氏病additive effect加性效应;累加效应additive gene action基因加性作用adductor(1)内收肌;收肌,(2)闭壳肌adenine腺嘌呤adenohypophysis腺垂体;垂体腺性部adenosine腺?adenosine diphosphate (ADP) 腺?二磷酸:二磷酸腺?adenosine monophosphate (AMP) 腺?二磷酸:一磷酸腺?adenosine triphosphate (ATP) 腺?二磷酸:三磷酸腺?adenovirus腺病毒adhesion(1)附 ;附 力,(2)粘连adhesive force附 力;黏附力adipose tissue脂肪组织adolescence青年期adradial tentacle从辐触手adrenal cortex肾上腺皮质adrenal gland肾上腺adrenal medulla肾上腺髓质adrenaline肾上腺素adrenergic(1)肾上腺素能的;肾上腺素功能的,(2)肾上腺素功能药物adrenergic neurone肾上腺素能神经元;肾上腺素功能神经元adrenocorticotrophic hormone促肾上腺皮质激素(ACTH)adrenocorticotropic hormone (ACTH)adsorption吸附;吸附作用adulthood成年期adventitious root不定根aeration tank曝气池aerenchyma通气组织aerial root气生根Aerobacter气杆菌属aerobe需氧生物;需气菌aerobic respiration需氧呼吸aerosol(1)气溶胶;气溶胶体,(2) 雾剂;喷雾剂aerotropism向氧性;向气性aestivation(1)夏蛰;夏眠,(2)花被卷叠式afferent arteriole输入小动脉afferent branchial artery入鳃动脉afferent branchial vein入鳃静脉afferent nerve传入神经;输入神经afferent neurone传入神经元;输入神经元affinity(1)亲和性;亲合性,(2)亲和力;亲合力afforestation造林;植林African sleeping disease非洲睡病;非洲昏睡病afterbirth胞衣;胎盘胎膜afterimage后像;残留影像agar琼脂;琼胶;洋菜胶agar culture琼脂培养agar medium琼脂培养基agar plate琼脂平面agar slant琼脂斜面agaric伞菌;蕈Agaricus伞菌属age distribution 年龄分布age structure年龄结构agglutination凝集;凝集作用agglutinin凝集素agglutinogen凝集原;凝集素原aggregate fruit聚合果aggregation(1)聚集;聚合,(2)群聚;群栖agranulocyte无颗粒白血球;无颗粒白血细胞air chamber气室air pollutant空气污染物air pollution空气污染air pore气孔air sac(1)气囊,(2)肺泡air space气室alanine丙氨酸alary muscle翼状肌albinism(1)白化;白化现象,(2)白化症albino(1)白化体,(2)白化病患者Albugo白 菌属albumen(1)蛋白;蛋清,(2)清蛋白;白蛋白,(3)胚乳albumin清蛋白;白蛋白albuminous cell(1)蛋白细胞,(2)胚乳细胞alcohol酒精;醇alcoholic fermentation 酒精发酵alderfly larva鱼蛉幼虫aldosterone醛甾酮,醛固酮aleurone layer糊粉层alga藻;藻类algal bloom藻花;藻类过量繁殖现象alimentary canal消化道;消化管aliquot等量样本;等分部份alkalaemia碱血症alkaline medium碱性介质;碱性介体alkaloid生物碱all-or-none response 全或无反应all-or-nothing law 全或无定律allantochorion尿囊绒膜allantoic acid尿囊酸allantoin尿囊素allantois尿囊allele等位基因allelomorph等位基因allergen过敏素;致敏素;变应素allergy过敏反应;变态反应allopolyploid异源多倍体allopolyploidy异源多倍性Alocasia海芋属alpha helixa螺旋alpha-ketoglutaric acida酮戊二酸alternation of generations 世代交替altitude acclimatization 高原适应;高空适应alveolus(1)肺泡,(2)齿槽;牙槽ambulacrum步带ametabolous无变态的ametabolous development无变态发育amide醯胺;?胺amine胺amino acid氨基酸amino acid sequence 氨基酸顺序;氨基酸序列amino group氨基amitosis无丝分裂ammonia氨ammonification氨化;氨化作用ammonifying bacteria 氨化细菌ammoniotelic排氨的ammonium molybdate钼酸铵amniocentesis羊膜穿刺术amnion羊膜amniotic cavity羊膜腔amniotic fluid羊水amniotic membrane羊膜amniotic sac羊膜囊amoeba变形虫amoebic dysentery阿米巴痢疾;变形虫性痢疾amoeboid movement变形运动amphetamine安非他明;苯异丙胺Amphibia两栖纲amphibian两栖动物;两栖类amphoteric两性的ampicillin氨芐青霉素;氨芐青霉素ampoule安瓿;针药管ampulla壶腹amylase淀粉?amylopectin支链淀粉amylose直链淀粉anabolism组成代谢;合成代谢anaemia贫血anaerobe厌氧生物;厌氧菌anaerobic respiration 缺氧呼吸anal cercus尾须anal style尾针analgesic镇痛剂;止痛剂analogous organs同功器官anaphase后期anatomy(1)解剖,(2)解剖学Andalusian fowl安达卢西亚androecium雄蕊群androgen雄激素;雄性激素androsterone雄酮;雄甾酮;雄固酮aneuploid非整倍体aneuploidy非整倍性angiosperm被子植物Angiospermae被子植物纲angstrom (A)*埃aniline dye苯胺染料aniline hydrochloride 氢氯化苯胺;盐酸苯胺aniline sulphate硫酸苯胺anilinium chloride氯化苯胺animal breeding动物育种;家畜繁育animal husbandry 畜牧;畜牧业animal pole动物极anion阴离子anisogamete异型配子anisogamy异配生殖annelid环节动物:环节类Annelida环节动物门annual plant一年生植物anual ring年轮annular thickening 环状加厚annulus环带Anopheles按蚊属anoxia缺氧;缺氧症antagonism拮抗作用;对抗作用antagonistic muscles 拮抗肌antenna触角antennule小触角anterior choroid plexus前脉络丛anterior mesenteric artery 肠系膜前动脉anterior pituitary垂体前叶;脑下垂体前叶anterior sucker前吸盘anterior vena cava前腔静脉;前腔大静脉anther花药antheridium精子器;藏精器;精器antherozoid游动精子anthocyanidin花色素:;花青素anthocyanin花色素?;花青素?Anthozoa珊瑚虫纲anthropoid类人猿Anthropoidea人猿亚目antibacterial action 抗细菌作用antibiotic抗生素;抗菌素antibiotic disc抗生片;抗菌素片antibody抗体anticancer agent抗癌剂anticoagulant抗凝血剂anticodon反密码子antidiabetic agent抗糖尿病剂antidiuretic hormone (ADH) 抗利尿激素antigen抗原antigen-antibody complex 抗原抗体复合物antigen-antibody reaction 抗原抗体反应antigenic stimulus抗原刺激antinistamine抗组胺药 ; 抗组织胺药antimalarial抗疟药antipodal cell反足细胞antipyretic退热剂 ; 解热剂antiseptic防腐剂 ; 消毒剂 ; 抗菌剂antiserum抗血清antitoxin抗毒素antiviral抗病毒的antrum窦 ; 腔anus肛门anvil砧骨aorta大动脉aortic arch大动脉弓aphid蚜虫apical bud顶芽apical cell顶细胞apical dominance 顶端优势 ; 顶芽优势apical meristem顶端分生组织Apis蜜蜂属apocarpous离心皮的apoenzyme脱辅基? ; ?元 ; ?蛋白apoplast非原质体appendage(1) 附肢 , (2) 附器appendicitis阑尾炎appendicular skeleton 附肢骨骼appendix阑尾; 蚓突aquaculture(1) 水产养殖, (2) 溶液培养aquarium水族箱aquatic habitat水生生境aquatic plant水生植物aqueous humour水状液arachnid蜘蛛类动物Arachnida蛛形纲arachnoid membrane蛛网膜arboreal树栖的Archaeopteryx始祖鸟属archegonium颈卵器; 藏卵器 ; 卵器archenteron原肠arcuate artery弓形动脉areolar connective tissue 网状结缔组织arginine精氨酸aridity(1) 干旱, (2) 干旱度arolium中垫arrector立肌 ; 毛肌arrector pili毛肌 ; 立毛肌arrowhead慈菇arterial pressure动脉压 ; 动脉血压arterial system动脉系 ; 动脉系统arterial thrombosis 动脉血栓形成arteriole小动脉arteriosclerosis动脉硬化 ; 动脉硬化症artery动脉arthritis关节炎arthropod节肢动物 ; 节肢类Arthropoda节肢动物门articular capsule关节囊articular cartilage关节软骨artificial insemination人工授精artificial parthenogenesis 人工单性生殖 ; 人工孤雌生殖artificial selection人工选择asbestos石棉asbestosis石棉沉着病Ascaris蛔虫属ascocarp子囊果ascomycete子囊菌Ascomycota子囊菌门Ascomycotina 子囊菌亚门ascorbate抗坏血酸盐ascorbic acid 抗坏血酸ascospore子囊孢子ascus子囊asepsis无菌; 无菌法aseptate无隔膜的aseptic technique无菌技术asexual reproduction无性生殖asparagine天冬醯胺; 天冬?胺; 天门冬醯胺;天门冬?胺aspartase天冬氨酸?; 天门冬氨酸?aspartic acid天冬氨酸; 天门冬氨酸Aspergillus曲霉属; 曲霉属aspirator吸气瓶aspirin阿士匹灵 ; 阿司匹林assay测定 ; 检定assimilation同化 ; 同化作用association area联合区association centre 联合中枢association neurone 联合神经元aster星体aster fibre星丝Asteroidea海星亚纲asthma气喘; 哮喘astigmatism散光athlete¢s foot脚癣atlas寰惟atrial systole(1) 心房收缩 ; (2) 心房收缩期atrioventricular node房室结atrioventricular valve 房室瓣atrium心房attached ear lobe连生耳垂attenuated organism减毒生物attenuated vaccine减毒疫苗attenuation减毒 ; 减毒作用auditory canal听道auditory nerve听神经auditory ossicles听小骨Aurelia海月水母属aureomycin金霉素; 金霉素auricle(1) 心耳, (2) 叶耳autecology个体生态学autoclave高压灭菌器; 消毒蒸锅autogamy自体受精; 自核交配autoimmune disease自身免疫性疾病; 自体免疫性疾病autoimmunity自身免疫性; 自体免疫性autolysis自体溶解; 自溶autonomic nervous system 自主神经系统autopolyploid同源多倍体autopolyploidy同源多倍性autosome常染色体 ; 体染色体autotomy自切 ; 自割autotroph自养生物 ; 自营生物autotrophic nutrition自养营养 ; 自营营养auxin生长素Aves鸟纲axial filament轴丝axial skeleton中轴骨胳axil腋axile placentation 中轴胎座式axillary artery腋动脉axillary bud腋芽axillary vein腋静脉axis枢椎axon轴突azobacter固氮菌Azoic era无生代Azotobacter 固氮菌属azygous vein 奇静脉Bbacillus杆菌back mutation 反突变backbone脊柱backcross回交backfat背膘backfat thickness背膘厚度background radiation 本底辐射 ; 本底放射backswimmer仰泳bacterial toxin细菌毒素bactericidal杀菌的bactericide杀菌剂bacteriochlorophyll 细菌叶绿素; 菌绿素。

DOI :10.13193/j.issn.1673-7717.2021.02.001肠道菌群驱动巨噬细胞糖代谢重编程与动脉粥样硬化“脾虚痰瘀”贾连群1,隋国媛1,宋囡1,2,吕美君2,3,杜莹1,2,陈丽娟2,3,战凯璇2,曹慧敏2,曹媛2,杨关林1,3(1.中医脏象理论及应用教育部重点实验室,辽宁沈阳110847;2.辽宁中医药大学中医药创新工程技术中心,辽宁沈阳110847;3.心脑合病中西医结合防治技术国家地方联合工程实验室,辽宁沈阳110847)摘要:脾主运化,为气血生化之源。
脾失健运,痰瘀互结,瘀滞日久,脉道不利,最终引发动脉粥样硬化(atherosclero-sis ,AS )发生。
中医认为维持肠道菌群稳态是脾主运化的重要生理功能。
肠道菌群作为人体内具有生物屏障、营养、免疫等多种生物学作用的重要“器官”,其稳态失衡,将影响人体正常健康状态,其机制可能为肠道菌群失调诱导脂多糖(lipopolysaccharide ,LPS )释放驱动巨噬细胞糖代谢重编程引发炎症反应继而导致AS 发生。
关键词:脾失健运;糖代谢重编程;肠道菌群;巨噬细胞中图分类号:R259.431.2;R378.2文献标志码:A文章编号:1673-7717(2021)02-0001-03Gut Microbiota Drives Macrophage Glycobolism Reprogramming andAtherosclerosis "Spleen Deficiency and Blood Stasis -Phlegm"JIA Lianqun 1,SUI Guoyuan 1,SONG Nan 1,2,LYU Meijun 2,3,DU Ying 1,2,CHEN Lijuan 2,3,ZHAN Kaixuan 2,CAO Huimin 2,CAO Yuan 2,YANG Guanlin 1,3(1.Key Laboratory of Ministry of Education for Traditional Chinese Medicine Viscera -State Theory and Applications ,Ministry of Education of China ,Shenyang 110847,Liaoning ,China ;2.Chinese Medicine Innovation Engineering Technology Center ,Shenyang 110847,Liaoning ,China ;3.National and Local Joint Engineering Laboratory for Integrated Chinese and Western Medicine Prevention and Treatment Technology onAngina Pectoris Complicated with Atherosclerotic Thrombotic Cerebral Infarction ,Shenyang 110847,Liaoning ,China )Abstract :Spleen governing transportation and transformation is the source of Qi and blood.Dysfunction of spleen in trans-portation ,blood stasis -phlegm ,long -term stagnation and vessels obstruction eventually lead to atherosclerosis (AS ).Tradi-tional Chinese medicine thinks that maintaining gut microbiota is an important physiological function of spleen governing transpor-tation and transformation.Gut microbiota is an important "organ"with biological barriers ,nutrition ,immunity and other biologi-cal functions in the human body ,and its steady -state imbalance will affect the normal health of the human body ,and its mecha-nism is that lipopolysaccharide (LPS )which is induced by gut microbiota imbalance drives macrophage glycobolism reprogram-ming ,and triggers an inflammatory response which in turn leads to AS.Keywords :dysfunction of spleen in transportation ;glycobolism reprogramming ;gut microbiota ;macrophage 基金项目:国家自然科学基金(81774022,81803860,81974548);沈阳市高层次人才创新计划(RC170248)作者简介:贾连群(1975-),女,辽宁营口人,教授,博士研究生导师,博士,研究方向:中西医结合防治动脉粥样硬化。

生物科学词汇AABO血型ABC blood groupA带A-banda螺旋alpha helixa酮戊二酸alpha-ketoglutaric acid阿米巴痢疾;变形虫性痢疾amoebic dysentery阿士匹灵; 阿司匹林aspirin癌cancer;carcinoma癌细胞cancerous cell爱迪生氏病;阿狄森氏病Addison’s disease安非他明;苯异丙胺amphetamine安全期safe period安全期避孕法rhythm method胺amine氨ammonia氨芐青霉素;氨芐青霉素ampicillin氨化;氨化作用ammonification氨化细菌ammonifying bacteria氨基amino group氨基化合物amide氨基甲酸脂carbamate氨基酸amino acid氨基酸顺序;氨基酸序列amino acid sequence氨基转移; 氨基转移作用transamination安达卢西亚Andalusian fowl安瓿;针药管ampoule按蚊属Anopheles暗带dark band暗反应dark reactions凹穴玻片cavity slide螯;钳爪chela螯角; 钳爪chelicera螯虾crayfish螯足cheliped奥陶纪Ordovician periodB巴氏体; 巴尔氏小体; 性染色质小体Barr body巴斯德消毒法pasteurization白氨酸;亮氨酸leucine白粉霉病;白粉霉病powdery mildew白化;白化现象,白化症albinism白化体,白化病患者albino白喉diphtheria白菌属Albugo白蛉sandfly白内障cataract白色体leucoplast白纤维软骨white fibrocartilage白血病leukaemia白血球;白血细胞white blood cell;white blood corpuscle;leucocyte 白蚁termite白质white matter白垩纪Cretaceous period斑macula斑叶variegated leaf斑疹伤寒typhus;typhus fever板根;板状根buttress root板鳃亚纲Elasmobranchii半保留复制semiconservative replication半变态hemimetamorphosis半变态的hemimetabolous半变态发育hemimetabolous development半翅目Hemiptera半关节面demifacet半合基因hemizygous gene半合子hemizygote半花图half-flower diagram半固体尿液semisolid urine半胱氨酸cysteine半规管semicircular canal半寄生物;兼性寄生物hemiparasite半乳糖galactose半衰期half-life半索动物;半索类hemichordate半透性的; 半渗透性的semi-permeable半透性膜semi-permeable membrane半纤维素hemicellulose半月瓣semilunar valve伴细胞; 伴胞companion cell瓣;瓣膜,壳瓣,裂片valve瓣鳃动物lamellibranch瓣鳃纲Lamellibranchia棒眼bar eye包埋技术embedding technique包皮prepuce包皮腺preputial gland苞鳞bract scale苞片bract胞壁质murein胞间连丝plasmodesmata胞间隙intercellular space胞间液intercellular fluid胞嘧啶cytosine胞内的;细胞内的intracellular胞内消化;细胞内消化intracellular digestion胞囊形成encystment胞体; 细胞体cell body胞外的;细胞外的extracellular胞外消化;细胞外消化extracellular digestion胞外液;细胞外液extracellular fluid胞芽gemma胞芽杯gemma cup胞衣;胎盘胎膜afterbirth胞质分裂; 细胞质分裂cytokinesis胞质环流cyclosis胞质桥; 胞质间桥cytoplasmic bridge孢间连丝; 孢间连体disjunctor孢囊柄sporangiophore孢子spore孢子虫sporozoon孢子囊spore sac;sporangium;sorus孢子囊,被囊幼虫; 胞蚴sporocyst孢子生殖sporogony孢子体sporogonium;sporophyte孢子小体;子孢子sporozoite孢子形成sporulation孢子叶sporophyll薄壁组织parenchyma宝血草sea-lavender保护; 保存conservation保护色protective colouration保水力;保水量water retention capacity;water-retaining capacity 保卫细胞guard cell保幼激素juvenile hormone (JH)饱和脂肪酸saturated fatty acid曝气池aeration tank鲍曼氏囊, 肾小球囊Bowman's capsule杯形托,胞芽杯,杯形器,壳斗cupule杯状细胞goblet cell背,背甲,背板tergum背膘backfat背膘厚度backfat thickness背大动脉dorsal aorta背的; 背面的; 背侧的dorsal背腹的dorsi-ventral背腹肌dorsoventral muscle背根dorsal root背根节dorsal root ganglion背甲,背片tergite背孔dorsal pore背裂dorsal fissure背须dorsal cirrus背叶notopodium被动免疫passive immunity被动免疫法passive immunization被囊动物tunicate被囊动物亚门Tunicata被子植物angiosperm被子植物纲Angiospermae苯胺染料aniline dye苯丙氨酸;苯基丙氨酸phenylalanine苯丙酮酸phenylpyruvic acid苯丙酮酸尿症phenylketonuria苯醌quinine苯酸; 苯甲酸benzoic acid本底辐射; 本底放射background radiation 本地种indigenous species本立德溶液Benedict's solution本立德试剂Benedict's reagent本立德试验Benedict's test本能instinct本能行为instinctive behaviour本生灯Bunsen burner本体感受器proprioceptor;proprioreceptor 贲门cardium贲门括约肌cardiac sphincter鼻隔nasal septum鼻腔nasal cavity鼻腺nasal gland比较胚胎学comparative embryology比较器comparator比目鱼flatfish比重计hydrometer必需氨基酸essential amino acid臂动脉brachial artery臂静脉brachial vein壁虎gecko壁压wall pressure避孕contraception避孕屏障contraceptive barrier避孕套condom避孕丸contraceptive pill蓖麻籽castor bean边材; 液材sapwood边缘区域fringe area边缘胎座式marginal placentation鞭毛flagellum鞭毛虫flagellate鞭毛虫纲Flagellata鞭毛纲Mastigophora扁虫flatworm扁桃体tonsil扁形动物门Platyheliminthes变态metamorphosis变温动物poikilotherm变温性poikilothermy变形虫amoeba变形运动amoeboid movement变性蛋白; 变性蛋白质denatured protein变性作用denaturation变异variation便秘constipation标本specimen标本瓶specimen bottle标志重捕法mark-and-recapture method标准差; 标准偏差; 标准误差standard deviation 标准曲线standard curve标准溶液standard solution表胚层periblast表皮epidermis表皮细胞epidermal cell表面玻璃watch glass表面积; 表面面积surface area表面循环superficial circulation表面张力surface tension表膜pellicle表土topsoil表现型phenotype表现型比;表现型比值phenotypic ratio鳔swim bladder濒危物种endangered species冰醋酸;无水醋酸glacial acetic acid兵蟹soldier crab滨草,沙地芦苇marram grass滨螺periwinkle丙氨酸alanine丙二醇propylene glycol丙糖triose丙酮acetone丙酮酸pyruvic acid丙酮酸盐; 丙酮酸脂pyruvate柄,花柄,pedicel柄,叶柄petiole饼图;圆瓣图pie chart病病毒virus病毒感染viral infection病毒粒子virion病毒性肝炎virus hepatitis病毒学virology病菌;病原菌,胚;胚芽;胚原基germ病原体pathogen波尔效应Bohr effect波浪作用wave action玻璃液;玻璃状液vitreous humour玻片; 切片slide钵口幼虫scyphistoma钵水母纲Scyphozoa勃起肌;立肌erector勃起组织erectile tissue补偿点compensation point补呼气量expiratory reserve volume补吸气量inspiratory reserve volume哺乳动物;哺乳类mammal哺乳纲Mammalia捕虫盘beating tray捕食的predatory捕食者predator捕食者猎物相亘作用predator-prey interaction 不饱和脂肪酸unsaturated fatty acid不定根adventitious root不动关节fixed joint;immovable joint不分离现象non-disjunction不兼容性;不亲和性incompatibility不可降解的;非降解性的nondegradable不可再生资源;非再生资源non-renewable resources不连续变异discontinuous variation不连续生长discontinuous growth不裂的;不开裂的indehiscent不随意的involuntary不随意肌involuntary muscle不透的impermeable不透性层impervious layer不完全变态;不全变态incomplete metamorphosis不完全显性incomplete dominance不完全菌类;半知菌类;半知菌亚门;不完全菌纲Fungi Imperfecti 不应期; 不反应期; 休复期refractory period不育性sterility步带ambulacrum布伦纳氏腺; 十二指肠腺Brunner's gland部分显性partial dominanceC残余物,残基residue操纵子operon糙皮病;癞皮病pellagra槽,齿槽socket草本层herbaceous layer草本植物herb草醯醋酸oxaloacetic acid草履虫paramecium草原生境grassland habitat草质茎herbaceous stem侧根lateral root侧茅lateral bud侧膜胎座式parietal placentation侧片pleurite侧神经节pleural ganglion侧生分生组织lateral meristem侧丝paraphysis侧线lateral line侧足parapodium测定; 检定assay测交test cross测微计micrometer层stratum差示空气温度计; 相异空气温度计differential air thermometer差异生殖differential reproduction差异透性; 分别透性differential permeability差异透性膜; 分别透性膜differentially permeable membrane插条,插条法cutting颤搐twitch颤蚓属Tubifex产孢子组织sporogenous tissue产乳量; 产奶量milk yield长匐茎runner长骨long bone长日照植物long-day plant长牙tusk常染色体; 体染色体autosome常染色质euchromatin肠enteron;intestine肠表皮;肠皮层gastrodermis肠系膜mesentery肠系膜动脉mesenteric artery肠系膜后动脉posterior mesenteric artery肠系膜前动脉anterior mesenteric artery肠腺intestinal gland肠液intestinal juice;succus entericus超极化hyperpolarization超滤;超滤作用ultrafiltration超速离心机ultracentrifuge超微结沟ultrastructure潮虫wood-louse潮间带;潮间带;滨海带;海岸带;潮汐带intertidal zone;tidal zone;littoral zone 潮气; 呼吸气tidal air潮气量tidal volume潮汐节律tidal rhythm潮汐作用tidal action潮下带; 亚海岸带sublittoral zone沉淀;降水量precipitation沉淀素precipitin沉积岩sedimentary rock沈降池; 沈淀池sedimentation tank成层现象stratification成虫imago成带;成带现象zonation成带型zonation pattern成骨细胞osteoblast成花素;成花激素florigen成年期adulthood成软骨细胞chondroblast成熟maturation成熟,成熟度maturity成纤维细胞fibroblast城市生态系;城市生态系统urban ecosystem 池, 潴池cisterna池黾;水马pond skater持水量;持水力water-holding capacity迟发过敏性delayed hypersensitivity尺water measurer尺骨ulna齿槽tooth socket齿根tooth root齿冠tooth crown齿颈tooth neck齿舌radula齿式dental formula齿突;齿状突odontoid process齿型tooth type齿虚位; 牙虚位diastema齿龈gum耻骨pubis耻骨联合pubic symphysis豉豆虫;豉虫whirligig beetle赤道板equatorial plate赤霉酸;赤霉酸gibberellic acid;gibberellin 赤藓红;藻红;真曙红erythrosine翅,翼瓣wing虫hookworm虫媒传粉insect pollination虫媒花insert-pollinated flower重瓣胃omasum重吸收; 重吸收作用reabsorption重演说recapitulation theory重组recombination重组DNA技术recombinant DNA technology 重组质粒recombinant plasmid重组子recon抽样; 取样sampling臭氧ozone臭氧层ozone layer出汗perspiration出鳃动脉efferent branchial artery出鳃静脉efferent branchial vein出生率birth rate;natality rate出水管excurrent siphon;exhalant siphon出土的epigeal出土式萌发epigeal germination出血haemorrhage出芽, 出芽生殖; 芽殖, 芽接budding初级精母细胞primary spermatocyte初级淋巴组织primary lymphoid tissue初级卵母细胞primary oocyte初级生产力primary productivity初级消费者primary consumer初乳colostrums初生分生组织primary meristem初生根primary root初生木质部primary xylem初生韧皮部primary phloem初生生长primary growth初生体重birth weight初生细胞壁primary cell wall初始质壁分离incipient plasmolysis除草剂;除莠剂herbicide;weed-killer除虫菊pyrethrum储备溶液stock solution储唾囊; 储涎囊salivary receptacle储蓄泡reservoir触角antenna触觉感受器touch receptor触觉纤毛tactile cilium触毛; 触须, 蔓足cirrus触手; 触须tentacle传出神经;输出神经efferent nerve传出神经元;输出神经元efferent neurone传导,输导conduction传导组织conductive tissue传粉;传粉作用pollination传染病infectious disease传染媒介vector of infection传入神经;输入神经afferent nerve传入神经元;输入神经元afferent neurone垂唇hypostome垂管manubrium垂体;脑下垂体pituitary body;pituitary gland;hypophysis 垂体后叶;脑下垂体后叶posterior pituitary垂体前叶;脑下垂体前叶anterior pituitary垂直成层结构vertical stratification锤骨hammer;malleus春化作用vernalization纯合的homozygous纯合子;同型接合子homozygote纯酒精;无水酒精absolute alcohol纯培养; 纯种培养; 纯培养物pure culture纯群聚;同型同种群聚gregation纯系pure line纯系; 无性系; 无性繁殖系; 克隆clone纯系化; 无性系化; 无性繁殖系化cloning纯系生长; 无性系生长; 无性繁殖系生长clonal growth纯系选择理论; 无性系选择理论; 无性繁殖系选择理论clonal selection theory 纯种purebred唇,下唇,下唇瓣labium唇基clypeus唇须;下唇须labial palp唇足纲Chilopoda慈菇arrowhead雌苞;雌器苞perichaetium雌苞叶perichaetial leaves雌二醇;雌甾二醇;雌固二醇oestradiol雌激素;雌甾激素oestrogen雌配子female gamete雌蕊pistil雌蕊花pistillate flower雌蕊群gynoecium雌蕊先熟; 雌性先熟protogyny雌三醇;雌甾三醇;雌固三醇oestriol雌酮;雌甾酮;雌固酮oestrone雌雄同体hermaphrodite雌雄同株; 雌雄同体monoecious雌雄同体现象hermaphroditism雌雄异株; 雌雄异体dioecious雌原核female pronucleus次级精母细胞secondary spermatocyte次级淋巴组织secondary lymphoid tissue次级卵母细胞secondary oocyte次级消费者secondary consumer次氯酸钠sodium hypochlorite次生分生组织secondary meristem次生根secondary root次生加厚secondary thickening次生木质部secondary xylem次生韧皮部secondary phloem次生生长secondary growth次生细胞壁secondary cell wall刺激stimulus刺激收缩偶联机制excitation-contraction coupling mechanism刺激物;刺激剂irritant刺丝囊nematocyst刺吸式piercing and sucking type刺细胞nematoblast; cnidoblast刺血针lancet从辐触手adradial tentacle粗糙食物; 基糠roughage粗蛋白质crude protein粗调节器; 粗调焦轮coarse adjustment knob粗纤维crude fibre粗线期pachytene促黑激素; 促黑素细胞激素melanocyte stimulating hormone (MSH)促黄体激素;促黄体生成激素luteinizing hormone (LH)促甲状腺素thyrotropin;thyrotropic hormone;thyrotrophin促甲状腺激素thyroid stimulating hormone;thyrotrophic hormone促进作用;加速作用acceleration;facilitation促卵泡激素follicle stimulating hormone (FSH)促乳素; 促乳激素prolactin促肾上腺皮质激素adrenocorticotrophic hormone促生长素; 生长激素somatotrophin;somatotropin促生长激素; 生长激素somatotrophic hormone;somatotropic hormone促胃液素gastrin促性腺素;促性腺激素gonadotrophin;gonadotropin;gonadotropic hormone 醋酸acetic acid醋酸地衣红染剂aceto-orcein stain醋酸杆菌属Acetobacter醋酸酒精acetic alcohol醋酸铅试纸lead acetate paper催产素oxytocin催汗神经元sudomotor neurone催化剂catalyst催泪反射lachrymal reflexDDNA 聚合物DNA polymer大孢子megaspore大孢子囊megasporangium大孢子叶megasporophyll大肠large intestine大穿刺丝囊;大穿刺囊penetrant大动脉aorta大动脉弓aortic arch大颚,上颚,下颔骨;下颚骨mandible大分子macromolecule大核macronucleus大量营养素;主要营养表macronutrient大裂球;大分裂球macromere大陆漂移continental drift大麻,大麻纤维hemp大麦芽bark大脑cerebrum大脑半球cerebral hemisphere大脑皮层; 大脑皮质cerebral cortex大薸water lettuce大试管boiling tube大田作物;农作物field crop大蚊幼虫cranefly larva大纤丝;大型纤维macrofibril大型生物区系;大型生物相macrobiota;macrofauna 大型消费者macroconsumer大型植物区系;大型植物相macroflora大阴唇labia majora达尔文主义Darwinism呆小病cretinism代era;generation代谢; 代谢作用; 新陈代谢metabolism代谢废物metabolic waste代谢活动metabolic activity代谢率metabolic rate代谢途径metabolic pathway代谢物metabolite带girdle带间的interzonal单倍世代haploid generation单倍数haploid number单倍体monoploid;haploid单侧刺激unilateral stimulus单纯系抗体; 单克隆抗体monoclonal antibody单果simple fruit单核白血球; 单核白血细胞monocyte单基因遗传monohybrid inheritance单基因杂交monohybrid cross单基因杂交比monohybrid ratio单基因杂种monohybrid单链DNA single-stranded DNA单亲遗传uniparental inheritance单球菌monococcus单染色体monosome单糖monosaccharide单体monomer单体性monosomy单体雄蕊的monadelphous单突触反射弧monosynaptic reflex arc单细胞蛋白质single cell protein (SCP)单细胞的unicellular单性果实parthenocarpous fruit单性结实parthenocarpy单性生殖;孤雌生殖parthenogenesis单循环single circulation单眼ocellus;simple eye单叶simple leaf单种裁培; 单株培养monoculture单子叶植物monocot;monocotyledonous plant单子叶植物纲Monocotyledoneae丹宁; 鞣酸,鞣料; 鞣质tannin担孢子basidiospore担孢子梗,叶座sterigma担轮幼虫trochophore担子basidium担子菌baisdiomycete担子菌纲Basidiomycetes担子菌门Basidiomycota担子菌亚门Basidiomycotina胆固醇; 胆甾醇cholesterol胆管bile duct胆红素bilirubin胆碱choline胆碱能的; 胆碱功能的,胆碱功能药物cholinergic胆碱能神经元; 胆碱功能神经元cholinergic neurone 胆绿素biliverdin胆囊gall bladder胆色素bile pigments胆石gallstone胆小管bile canaliculi;bile ductule胆盐bile salts胆汁bile胆总管common bile duct淡水生境freshwater habitat蛋白;蛋清,清蛋白;白蛋白,胚乳albumen 蛋白间质proteose蛋白酶类protease蛋白水解; 蛋白水解作用proteolysis蛋白细胞,胚乳细胞albuminous cell蛋白质; 蛋白; 朊protein蛋白质合成protein synthesis弹尾目Collembola氮肥nitrogen fertilizer氮循环nitrogen cycle导管vessel导管分子vessel element导热性thermal conductivity倒位inversion倒位纯合子inversion homozygote倒位杂合子inversion heterozygote德国麻疹German measles等长生长isometric growth等长收缩isometric contraction等翅目Isoptera等电点isoelectric point等量样本;等分部份aliquot等摩尔的equimolar等渗的,等张的isostonie等渗溶液isotonic solution等位基因allele;allelomorph等显性; 共显性, 等显势codominance等张收缩isotonic contration等足动物isopod镫骨stirrup;stapes低倍物镜;低倍接物镜low power objective低聚糖;寡糖oligosaccharide低渗的hypotonic低渗溶液hypotonic solution低温的hypothermic;hypothermia滴定; 滴定法titration滴定管burette滴管dropper滴瓶dropping bottle滴试板spot plate滴液漏斗dropping funnel滴液移液管dropping pipette绦虫cestode;tapeworm绦虫纲Cestoda绦虫属Taenia绦虫幼虫; 绦虫蚴proscolex底土subsoil第二寄主secondary host第二性征secondary sexual character第二子代; 子二代second filial (F2) generation第三纪Tertiary period第四纪Quaternary period第一寄主primary host第一子代;子一代first filial (F1) generation递质; 传递介质; 传导物质transmitter地理分布geographic distribution地理隔离geographic isolation地理障碍geographic barrier地钱属Marchantia地热能geothermal energy地蜈蚣earwig地下区域subterranean zone地衣lichen地衣红orcein地质年代表geologic time scale地中海贫血; 地中海贫血症thalassaemia点滴试验; 亮点法spot test点突变point mutation点样方point quadrat;pin quadrat碘iodine碘液iodine solution碘液试验iodine test电感骨器electroreceptor电解质electrolyte电离辐射ionizing radiation电泳electrophoresis电子传递链electron transport chain电子传递体系electron transport system电子供体electron donor电子受体electron acceptor电子显微技术;电子显微镜术electron microscopy 电子显微镜electron microscope电子显微照片electron micrograph电子载体electron carrier淀粉starch淀粉碘化物试纸starch iodide paper淀粉核pyrenoid淀粉酵素amylase淀粉粒starch grain碟状幼体ephyra顶,头顶vertex顶端分生组织apical meristem顶端优势; 顶芽优势apical dominance顶盖tectum顶极群落climax community顶级食肉动物; 顶极食肉动物top carnivore 顶体acrosome顶细胞apical cell顶芽apical bud;terminal bud顶叶parietal lobe定点诱变site-directed mutagenesis定积土sedentary soil定位mapping定向orientation定向反应directional response定向选择directional selection冬眠hibernation动脉artery动脉导管ductus arteriosus动脉干truncus arteriosus动脉压; 动脉血压arterial pressure动脉系; 动脉系统arterial system动脉血栓形成arterial thrombosis动脉硬化; 动脉硬化症arteriosclerosis动脉锥; 动脉圆锥conus arteriosus动情期oestrus动情期同步化oestrus synchronization动情前期pro-oestrus动情周期oestrous cycle动丝;动纤丝kinetodesma动态;运动kinesis动态平衡dynamic equilibrium动物极animal pole动物区系;动物相fauna动物式营养holozoic nutrition动物育种;家畜繁育animal breeding动作电势;动作电位action potential冻干切片术freeze-drying microtomy冻蚀法;冰冻蚀刻法freeze-etching technique 冻原tundra豆科Leguminosae豆科植物leguminous plant豆娘若虫damselfly nymph痘病毒poxvirus窦; 腔antrum;sinus窦房结sinoatrial node窦状隙sinusoid独立分配independent assortment独立分配定律law of independent assortment独立分配原理principle of independent assortment 毒力;毒性;致病力virulence毒蛇venomous snake毒神经的neurotoxic毒素toxin毒雾smog毒性toxicity毒性菌株virulent strain毒性噬菌体;烈性噬菌体virulent phage毒液venom杜匀氏漏斗; 生物分离漏斗Tullgren funnel端脑telencephalon短日照植物short-day plant短生的;短命的ephermeral短枝dwarf shoot断裂;断裂生殖fragmentation断乳体重weaning weight堆肥compost对氨基苯酸;对氨基苯甲酸para-aminbenzoic acid 对比性状contrasting character对称symmetry对数期;对数生长期log phase;logarithmic phase 对数曲线logarithmic curve对虾prawn对照control对照实验control experiment盾鳞placoid scale多倍体polyploidy多倍性plordy多核的coenocytic;multinucleate多核糖体polysome;polyribosome多核体; 多核细胞coenocyte多基因遗传polygenic inheritance多极神经元multipolar neurone多孔动物门Porifera多毛动物;多毛类polychaete多毛纲Polychaeta多年生perennation多年生植物perennial plant多糖polysaccharide多细胞的multicellular多线染色体polytene chromosome多形核白血球;多形核白血细胞polymorphonuclear leucocyte多形核颗粒白血球;多形合颗粒白血细胞polymorphonuclear granulocyte 多形态现象;多态性polymorphism多样性diversity多原型polyarch多趾polydactyly多足动物; 多足类myriapod多足纲MyriapodaE额frons额叶frontal lobe锇酸osmic acid恶性肿瘤malignant tumour阻遏基因repressor gene阻遏物repressor萼片sepal颚;颌jaw儿童期childhood耳垂ear lobe耳膜eardrum耳砂otoconium耳石otolith耳蜗cochlea耳蜗管cochlear duct二倍数diploid number二倍体diploid二叉分枝式dichotomous branchng二叉式检索表dichotomous key二次消费者second order consumer二叠纪Permian period二分裂; 二分体分裂binary fission二级结构secondary structure二价的,二价染色体bivalent二尖瓣bicuspid valve二磷酸果糖fructose bisphosphate二磷酸核酮糖ribulose bisphosphate (RuBP)二硫键disulphide bond二氯酚靛酚dichlorophenol indophenol (DCPIP)二年生的biennial二年生植物biennial plant二歧聚伞花序; 二歧聚伞花序dichasial cyme 二羧酸dicarboxylic acid二头肌biceps二氧化碳carbon dioxide二氧化硫sulphur dioxide二支的; 二岔的biramousF F纤毛F-pilusF因子 F factorFAA固定剂FAA fixative发酵;发酵作用fermentation发酵器fermenter发生率incidence发生器电势;发生器电位generator potential 发育development法类皮欧氏管;输卵管Fallopian tube番红safranine繁殖propagation繁殖体propagule反刍regurgitation反刍动物ruminant反口面aboral surface反馈feedback反馈机制feedback mechanism反馈控制feedback control反馈抑制feedback inhibition反密码子anticodon反射动作reflex action反射弧reflex arc反射中枢reflex centre反射作用;分射reflex反突变back mutation反硝化菌; 脱氮菌denitrifier反硝化细菌; 脱氮细菌denitrifying bacteria 反硝化作用; 脱氮作用denitrification反应reaction;response反应器effector反应时间reaction time反应物reactant反足细胞antipodal cell返巢本能homing instinct泛酸pantothenic acid方格网;格网grid纺锤状原始细胞fusiform initial防腐剂preservative防腐剂; 消毒剂; 抗菌剂antiseptic防御反应defense reaction房室瓣atrioventricular valve房室结atrioventricular node纺锤丝spindle fibre纺锤体spindle纺织腺spinning-gland放大镜hand lens放牧牲畜grazing animal放射虫纲Radiata放射肌radial muscle放射性的radioactive放射性示踪物radioactive tracer放射性同位素radioisotope放射性同位素定年法; 放射性同位素年份测定radioisotope dating 放线菌actinomycete放线菌目Actinomycetales放线菌素actinomycin飞沬传染droplet infection非必需氨基酸non-essential amino acid非等位基因non-allelic gene非叠密码non-overlapping code非还原糖non-reducing sugar非极性的non-polar非竞争性抑制noncompetitive inhibition非生物的,无生命的abiotic非特异性免疫nonspecific immunity非细胞的acellular非原质体apoplast非整倍体aneuploid非整倍性aneuploidy非洲睡病;非洲昏睡病African sleeping disease非姊妹染色单体non-sister chromatid菲令氏溶液Fehling’s solution菲令氏试验Fehling’s test肥大细胞mast cell腓骨fibula肺;肺脏lung肺尘埃沉病pneumonoconiosis肺动脉pulmonary artery肺活量vital capacity肺静脉pulmonary vein肺量计; 呼吸量计spirometer肺螺亚纲Pulmonata肺泡,齿槽;牙槽alveolus肺气肿emphysema肺循环pulmonary circulation肺炎pneumonia肺炎球菌pneumonococcus肺炎球菌属Pneumococcus废料循环waste recycling废水effluent废物;废料waste product废物处理waste treatment分贝decibel (dB)分布类型distribution pattern分布区重叠的; 同域的sympatric分布曲线distribution curve分化; 分化作用differentiation分节; 分节现象segmentation分节的metameric分节现象metamerism分解; 分解作用decomposition分解,分辨率; 清晰度resolution分解代谢catabolism分解纤维素的celluloytic分解者decomposer分离segregation分类classification分类; 分类学taxonomy分类单元; 分类群taxon分类阶梯; 分类阶层系统taxonomic hierarchy 分离定律law of segregation分离原理principle of segregation分裂,裂殖fission分流; 分路途径shunt pathway分流血管shunt vessel分泌; 分泌作用,分泌物secretion分泌过多hypersecretion分泌过少hyposecretion分泌器官secretory organ分泌素; 肠促胰液素secretin分娩birth;labour;parturition分生孢子conidium分生孢子梗; 分生孢子柄conidiophore分生组织meristem分压partial pressure分子式molecular formula酚phenol粉管受精的siphonogamous粉砂silt粪便dung;faece粪肥manure丰度;多度abundance丰度指数;多度指数index of abundance 风化;风化作用weathering风媒传粉wind pollination风媒花wind-pollinated flower风疹rubella蜂窝胃,网状结构reticulum凤尾蕨属Pteris缝; 骨缝suture跗骨tarsal跗骨,跗节,睑板tarsus辐管; 辐水管radial canal辐卵裂radial cleavage辐射; 放射radiation辐射对称radial symmetry辐射对称花actinomorphic flower孵化率hatchability浮肋floating rib浮浪幼体planula浮萍,青萍duckweed浮游动物zooplankton浮游生物plankton浮游生物网plankton net浮游幼虫planktonic larva浮游植物phytoplankton蜉蝣目Ephemeroptera蜉蝣若虫mayfly nymph佛尔克曼氏管V olkmann’s canal氟化物fluoride辅基prosthetic group辅酵素coenzyme辅因子cofactor辅阻遏物corepressor腐败; 腐解decay腐败细菌putrefying bacteria腐败作用putrefaction腐坏spoilage腐生; 腐生现象saprophytism腐生物; 腐生生物saprobiont腐生营养saprophytic nutrition腐生植物saprophyte腐生植物的,腐生的saprophytic腐食性生物; 腐生物saprotroph腐殖酸humic acid腐殖土humic soil腐殖质humus腐殖质池humus tank腐质消费者; 碎屑消费者detritus consumer脯氨酸proline父本male parent复层上皮stratified epithelium复等位基因; 多等位基因multiple alleles复分裂multiple fission复果multiple fruit复合肥料compound fertilizer复合物complex复极化; 再极化repolarization复糖complex sugar复眼compound eye复叶compound leaf复制duplication;replication复制平册培养; 复制平皮培养; 复制平皿培养法; 复制平板培养法replica plating 复制物,印模replica复壮; 回春rejuvenation腹;腹部abdomen;venter腹大动脉;腹侧大动脉ventral aorta腹的;腹面的;腹侧的ventral腹根ventral root腹甲plastron;sternite腹裂ventral fissure腹面沟ventral groove腹膜,围脏膜peritoneum腹鳍pelvic fin腹腔abdominal cavity腹腔动脉coeliac artery腹泻diarrhea腹须ventral cirrus腹支ventral ramus腹足pleopod腹足动物;腹足类gastropod腹足纲Gastropoda副产品by-product副淀粉paramylum副萼epicalyx副交感神经parasympathetic nerve副交感神经系统parasympathetic nervous system 副细胞subsidiary cell负反馈negative feedback负染色法negative staining technique负性加速期negative acceleration phase富养化作用eutrophication附;附力,粘连adhesion附睪epididymis附加体;游离体episome附力;黏附力adhesive force附器hapteron附生植物epiphyte附腺;副腺accessory glands附肢, 附器appendage附肢骨骼appendicular skeleton覆膜tectorial membrane覆瓦状的imbricateG 伽玛射线gamma ray盖; 被盖tegmentum盖,鳃盖,蒴盖operculum盖玻片cover glass;cover slip盖度; 覆盖度degree of coverage钙化; 钙化作用calcification钙化醇calciferol钙质壳calcareous shell干旱,干旱度aridity干化; 干燥作用desiccation干扰interference干扰素interferon干细胞stem cell干重dry weight肝;肝脏liver肝动脉hepatic artery肝静脉hepatic vein肝盲囊hepatic caecum肝门静脉hepatic portal vein肝素heparin肝细胞hepatocyte肝吸虫;肝蛭liver fluke肝炎hepatitis肝总管common hepatic duct甘氨酸glycine甘油glycerine;glycerol甘油二酯diglyceride甘油三脂triglyceride甘油一酯monoglyceride甘油酯glyceride坩埚crucible坩埚钳crucible tongs杆菌bacillus杆状体rhabdite感化性; 感药性chemonasty感觉sensation感觉棍rhopalium感觉毛细胞sensory hair cell感觉囊sensory capsule感觉器官sense organ感觉区sensory area感觉神经末鞘sensory nerve ending感觉神经纤维sensory nerve fibre感觉神经元sensory neurone感觉细胞sensory cell感热性thermonasty感受器,受体receptor感受器电势; 感受器电位receptor potential 感性nasty感性反应nastic response感性运动nastic movement感旋光性photonasty感夜性nyctinasty感应性irritability感震性seismonasty刚毛,剌毛,须,鬃bristle; chaeta;eta刚毛囊; 毛囊chaetal sac纲class纲翅目Dictyoptera肛门anus杠杆系统lever system高柏氏腺; 尿道球腺Cowper's gland高倍物镜;高倍接物镜high power objective 高尔基器Golgi apparatus高尔基体Golgi body高能化合物energy-rich compound高渗的hypertonic高渗溶液hypertonic solution高渗性hypertonicity高血糖素;胰高血糖素glucagons高血压hypertension高压灭菌器; 消毒蒸锅autoclave高原适应;高空适应altitude acclimatization 哥蒂氏器;螺旋器organ of Corti格拉夫氏卵泡;囊状卵泡Graafian follicle 格兰氏染剂Gram stain格兰氏阳性细菌Gram-positive bacteria格兰氏阴性细菌Gram-negative bacteria 隔; 中隔; 隔膜septum隔离机制isolating mechanism隔膜; 隔板,横膈膜,子宫帽,光栏diaphragm 膈神经phrenic nerve铬酸chromic acid个体发生ontogeny个体生态学autecology根端压片; 根尖压片root tip squash根冠root cap根茎rhizome根瘤root nodule根瘤菌nodule bacteria;root nodule bacteria 根瘤菌属Rhizobium根毛root hair根毛层piliferous layer根霉属; 根霉属Rhizopus根培养基root medium根托rhizophore根压root pressure根状菌丝rhizoidal hypha根足; 根状伪足rhizopodium根足虫rhizopod根足虫纲Rhizopoda更新世Pleistocene epoch亘换interchange工业黑化现象industrial melanism弓形动脉arcuate artery供体donor巩膜sclera;sclerotic coat汞; 水银mercury汞中毒mercury poisoning共体; 共肉coenosarc共生; 共生现象symbiosis共生生物symbiont共生者; 共栖者commensal共质体symplasma;symplast沟sulcus佝偻病rickets菇类; 蕈类mushroom古动物学palaeozoology古生代Palaeozoic era古生物学palaeontology古新世Palaeocene epoch古植物学palaeophytology谷氨醯胺;谷氨酰胺glutamine谷氨酸glutamic acid谷氨酸钠; 味精monosodium glutamate (MSG) 骨板bone lamella骨干diaphysis骨骼skeleton骨骼肌skeletal muscle骨胳系统skeletal system骨化;骨化作用ossification骨迷路; 骨性迷路bony labyrinth骨膜periosteum骨盆,肾盂pelvis骨盆腔pelvic cavity骨髓bone marrow骨细胞osteocyte骨针,骨片spicule骨组织bone tissue股骨,腿节femur股动脉femoral artery股骨; 大腿骨thigh bone股静脉femoral vein鼓膜tympanum;tympanic membrane鼓藻desmid钴胺素cobalamin固醇; 甾醇sterol固氮;固氮作用nitrogen fixation固氮菌azobacter固氮菌属Azotobacter固氮细菌nitrogen fixing bacteria固的sedentary固定,固定法,定影;定像fixation固定剂fixative固化; 硬化;成熟(干酪),食品加工curing固器holdfast固碳作用carbon fixation固有层lamina propia刮勺spatula寡毛动物;寡毛类oligochaete寡毛纲Oligochaeta关节joint关节囊joint capsule;articular capsule关节软骨articular cartilage关节突zygapophysis关节炎arthritis管胞tracheid管虫tube worm管分泌tubular secretion管核tube nuclei管瓶;管形瓶;小药瓶vial管细胞solenocyte管足tube feet冠心病; 冠状心脏疾病coronary heart disease 冠状动脉coronary artery冠状动脉血栓形成coronary thrombosis冠状静脉coronary vein冠状循环; 冠脉循环coronary circulation灌溉irrigation灌木shrub灌木层shrub layer光导形态发生photomorphogenesis光反应light reactions光感受器photoreceptor光合室photosynthetic chamber光合丝photosynthetic filament光合自养生物;光能自养生物photoautotroph 光合作用photosynthesis光化毒雾photochemical smog光化反应photochemical reactions光呼吸;光呼吸作用photorespiration光激活;光活化photoactivation光解作用photolysis光磷酸化;光磷酸化作用photophosphorylation 光敏素;光敏色素phytochrome光谱,谱spectrum光系统I photosystem I光系统II photosystem II。

DNA双加氧酶TET在中枢神经系统的研究进展刘洁;米亚静【摘要】DNA双加氧酶TET家族是新发现的一类表观遗传修饰蛋白,能够将DNA的5-甲基胞嘧啶氧化为5-羟甲基胞嘧啶,进而调控基因的表达。
多项研究显示,TET1-3在中枢神经系统表达丰富,其潜在的生物功能也被广泛关注。
本文从TET蛋白结构功能概述、TET蛋白在中枢神经系统的表达及功能以及针对TET 家族的基因敲除小鼠实验三方面作一综述。
%DNA dioxygenase TET family is a recently discovered proteins involved epigenetic modification, which can oxidize the 5-methylcytosine of DNA into 5-hydroxymethylcytosine and thereby regulate gene expression. Accumulative studies have shown that TET1-3 was abundantly expressed in the central nervous system, and its poten-tial functions were beginning to be studied. This paper gives an overview of the structure and functions of TET, its po-tential roles in the central nervous system, and also the TET gene knockout experiments.【期刊名称】《海南医学》【年(卷),期】2015(000)014【总页数】3页(P2107-2108,2109)【关键词】TET;表观遗传修饰;中枢神经系统;基因敲除【作者】刘洁;米亚静【作者单位】西安医学院基础医学研究所,陕西西安 710021;西安医学院基础医学研究所,陕西西安 710021【正文语种】中文【中图分类】R338.2表观遗传学修饰(Epigenetic modification)是指DNA碱基序列没有改变的前提下,基因表达水平发生改变。
Enhanced a -ketoglutaric acid production in Yarrowia lipolytica WSH-Z06by an improved integrated fed-batch strategyZongzhong Yu a ,Guocheng Du b ,c ,Jingwen Zhou a ,b ,⇑,Jian Chen a ,b ,⇑aState Key Laboratory of Food Science and Technology,Jiangnan University,1800Lihu Road,Wuxi,Jiangsu 214122,ChinabThe Key Laboratory of Carbohydrate Chemistry and Biotechnology,Ministry of Education,Jiangnan University,1800Lihu Road,Wuxi,Jiangsu 214122,China cSchool of Biotechnology and Key Laboratory of Industrial Biotechnology,Ministry of Education,Jiangnan University,1800Lihu Road,Wuxi,Jiangsu 214122,Chinaa r t i c l e i n f o Article history:Received 24December 2011Received in revised form 5March 2012Accepted 6March 2012Available online 14March 2012Keywords:a -Ketoglutaric acid pH control strategy Fed-batchYarrowia lipolytica Overproductiona b s t r a c tThis study aimed at enhancing a -ketoglutaric acid (a -KG)production by Yarrowia lipolytica WSH-Z06.Batch culture experiments demonstrated that CaCO 3and a relatively low pH (3.0)in the a -KG production phase contributed to a -KG ing a two-stage pH control strategy,in which pH was buffered by CaCO 3in the growth phase and then maintained at 3.0in the a -KG production phase,the yield of a -KG reached 53.4g L À1.In the later phase of batch fermentation,the glycerol was exhausted but synthesis of a -KG did not cease.Therefore,glycerol was fed with an integrated fed-batch mode,and a -KG production increased to 66.2g L À1with a productivity of 0.35g L À1h Àpared to optimal batch culture,a -KG production and productivity were enhanced by 23.9%and 16.7%,respectively.The two-stage pH control strategy,constant feeding approach and lower pH in later phase would be useful for a -KG industrial production.Ó2012Elsevier Ltd.All rights reserved.1.Introductiona -Ketoglutaric acid (a -KG),is an important multifunctional or-ganic acid generated in the tricarboxylic acid (TCA)cycle.It has a broad range of industrial applications in the food,pharmaceutical,fine chemistry and animal feed industries (Finogenova et al.,2005;Otto et al.,2011).As a precursor of glutamic acid,a -KG has a spar-ing effect on endogenous glutamine pools,and is closely linked with the synthesis of proline,arginine and polyamines (Matzi et al.,2007).Additionally,a -KG plays a pivotal role in the interme-diary metabolism to modulate nitrogen balance (De Bandt et al.,1998).In clinical nutrition and healthcare,a -KG can improve gut morphology and function,counteract trauma-induced dysimmuni-ty and play an important role in metabolic adaptation to injury (Cynober,1999).The L -arginine and a -KG mixture is a popular nutrition enhancer in functional beverages to improve blood flow to muscle,reduce catabolism and increase protein synthesis during resistance training,resulting in improved training adaptations (Campbell et al.,2006).Barrett and Yousaf (2008)proposed that a -KG could be further exploited as a poly-(triol-a -ketoglutarate)with new potential applications,e.g.tissue engineering and drug delivery.Currently,a -KG is produced via multi-step chemical synthesis.However,it is hard to overcome shortcomings that include low yield,low purity,residual cyanides and other toxic waste (Otto et al.,2011).Biotechnological processes,as attractive alternative methods of a -KG production,have been studied for several dec-ades (Finogenova et al.,2005).Many bacteria and yeasts have been screened for a -KG overproduction.Among them,Yarrowia lipolyti-ca has been the most intensively studied non-conventional yeast due to its several advantages:e.g.wide substrate spectrum,intense secretory ability,higher product yield,waste minimization and an existing efficient system for genetic engineering transformation (Holz et al.,2010;Madzak et al.,2004;Mirbagheri et al.,2011).Moreover,Y.lipolytica is considered by the Food and Drug Admin-istration (USA)as nonpathogenic and many processes based on this organism are classified as Generally Recognized as Safe (GRAS)(Barth and Gaillardin,1997).In recent years,there have been many significant studies concerning the overproduction of a -KG (Otto et al.,2011).Chernyavskaya et al.(2000)identified the principal conditions affecting a -KG oversynthesis by the mutant Y.lipolytica N1from ethanol,and a a -KG concentration of 49g L À1was achieved;however,the concentration of ethanol in the broth should be maintained at <2.5g L À1.Liu et al.(2007)found that lit-tle a -KG was detected as a byproduct in the pyruvic acid fermen-tation broth of Torulopsis glabrata .However,redistributing the metabolic flux to a -KG by manipulating cofactor levels resulted in pyruvic acid concentration decreasing from 69to 21.8g L À1,while a -KG concentration increasing to 43.7g L À1.Other studies0960-8524/$-see front matter Ó2012Elsevier Ltd.All rights reserved./10.1016/j.biortech.2012.03.021⇑Corresponding authors.Address:School of Biotechnology,Jiangnan University,1800Lihu Road,Wuxi,Jiangsu 214122,China.Tel.:+8651085329031;fax:+8651085918309.E-mail address:zhoujw1982@ (J.Zhou).have focused on changing related enzyme activities to influence the amounts and ratios of the organic acids produced(Holz et al., 2010;Zhang et al.,2009).Little literature is available on the optimization of a-KG produc-tion by batch and fed-batch fermentation in Y.lipolytica using glyc-erol as the sole carbon source.Recently,glycerol as a waste derived from hydrophobic substrates(e.g.oils,fats,fatty acids and n-alkanes)and biodiesel production has been considered as a potential substrate for a-KG production by Y.lipolytica(Hama et al.,2011; Mirbagheri et al.,2011).Glycerol was used as the sole carbon source throughout the present study to achieve the goal of a-KG industrial production.In this work,relatively low pH as well as CaCO3were key factors leading to a-KG accumulation.By a two-stage pH control strategy,a-KG production increased to53.4g LÀ1.Finally,a novel integrated fed-batch culture led to a significant enhancement in a-KG production by a further23.9%,reaching66.2g LÀ1.2.Methods2.1.MicroorganismsThe thiamine-auxotrophic Y.lipolytica WSH-Z06,screened in a previous study(Zhou et al.,2010),was used throughout.2.2.MediumMedium composition for slant and seed cultures was(all in g LÀ1):20glucose,10peptone,0.5MgSO4Á7H2O and1KH2PO4 (Zhou et al.,2009).The medium initial pH was adjusted to5.5with 4mol LÀ1NaOH.In the slant medium,20g LÀ1agar was added.The fermentation medium had the following composition(in g LÀ1):100glycerol,3(NH4)2SO4,3KH2PO4,1.2MgSO4Á7H2O,0.5 NaCl,0.1K2HPO4,and0.4l g LÀ1thiamine-HCl.The medium initial pH was adjusted to4.5with4mol LÀ1NaOH.The thiamine-HCl wasfilter-sterilized before addition to the medium.CaCO3used as pH buffer was dry-heat sterilized at160°C for30min before being added into the medium.Other components were autoclaved for15min at115°C.2.3.Culture conditionsThe seed culture was inoculated with healthy yeast,grown on an agar slant and incubated in a500-mLflask containing50mL of seed culture medium for24h on a reciprocal shaker at200rpm.The batch and fed-batch fermentations were performed in500-mLflasks containing50mL of fermentation medium,or in a7-L jar fermentor(BioFlo415-7L;New Brunswick Scientific,Enfield,CT, USA)containing4L of fermentation medium.The inoculation size was10%(v/v).Theflask cultures were grown for168h,the rotation rate controlled at200rpm,and the medium buffered by20g LÀ1 CaCO3.In fermentor cultures,the agitation speed and aeration rate were controlled at600rpm and2.5vvm(volume air per volume broth per minute),respectively.pH was controlled with sterile 4mol LÀ1NaOH solution and CaCO3according to the different de-signed strategies.All cultivations were carried out at28°C.2.4.Analytical methodSamples were taken for off-line analysis every12h after starting the fermentations.The optical density of the culture broth was mea-sured using a spectrophotometer(Biospe-1601;Shimadzu Co, Kyoto,Japan)at570nm after an appropriate dilution,and converted to dry cell weight(DCW)according to a predetermined calibration equation,OD570:DCW(g LÀ1)=1:0.223.For measurement of glyc-erol,pyruvic acid and a-KG,fermentation broth was centrifuged at10,000g for10min.The supernatant was diluted50times andfil-tered through a membrane(pore size=0.22l m).a-KG,glycerol and pyruvic acid in supernatant were simultaneously determined by HPLC(Agilent1100series,Santa Clara,CA,USA)with a Aminex HPX-87H column(300mmÂ7.8mm;Bio-Rad Laboratories Inc., Hercules,CA,USA).The mobile phase was5mmol LÀ1sulfuric acid in distilled,de-ionized waterfiltered to0.22l m.The mobile phase flow rate was0.6mL minÀ1.The column temperature was main-tained at35°C,and the injection volume was10l L.The a-KG and pyruvic acid were detected by UV(wavelength at210nm)detector. Glycerol was detected by a differential refraction index detector.All values are the means of three independent extraction processes. 2.5.Statistical analysisStudent’s t-test was employed to investigate statistical differ-ences,and samples with P<0.05were considered significant.3.Results and discussion3.1.Influence of initial glycerol concentration on a-KG production in shakerflasksThe effect of various initial glycerol concentrations of50,80, 100,150,200,250and300g LÀ1on cell growth and a-KG produc-tion inflasks are shown in Fig.1.When glycerol concentration was within50–200g LÀ1,thefinal biomass was very similar(about 11g LÀ1DCW)for each batch.However,cells grew a little faster in the presence of50or80g LÀ1glycerol,with the maximal bio-mass occurring24h earlier with these low glycerol concentrations, indicating that maintaining a low glycerol concentration was favorable for cell growth in prophase.Using100g LÀ1of glycerol was found to produce the maximum a-KG concentration of36.7g LÀ1at168h,with thefinal biomass of 11.3g LÀ1DCW.With increasing initial glycerol concentration,the a-KG production declined.When initial glycerol concentration was 250g LÀ1,both cell growth and a-KG production were significantly inhibited:final biomass and a-KG production were very low at8.1 and19.4g LÀ1,respectively.The results showed that a-KG produc-tion was more efficient at glycerol concentration<100g LÀ1.How-ever,lower initial glycerol concentrations resulted in lower total amounts of a-KG,indicating that100g LÀ1initial glycerol concen-tration was most suitable for a-KG production in batchculture.598Z.Yu et al./Bioresource Technology114(2012)597–6023.2.Improving pH control strategy of a-KG production in batch fermentationThree different pH control strategies were respectively applied in batch fermentations of a-KG production in a7-L fermentor (Fig.2).In strategy A,pH was maintained at4.5using4mol LÀ1 NaOH for the whole process(Fig.2A)(Chernyavskaya et al., 2000).The Y.lipolytica grew well and reached afinal biomass of 9.6g LÀ1DCW.In the broth,there was23.8g LÀ1residual glycerol until168h.The maximum a-KG concentration was22.0g LÀ1at 156h,while pyruvic acid was up to36.9g LÀ1.Lower a-KG produc-tion and more pyruvic acid were obtained in7-L fermentor com-pared to500-mLflasks.In order to determine the reasons, culture conditions betweenflasks and fermentor were compared. The pH control strategy likely played a key role in a-KG accumula-tion,and consequently strategy B was tested.The pH was buffered with20g LÀ1CaCO3added into the medium just after inoculation to simulate conditions in theflasks(Fig.2B).Using strategy B,40.3 and31.8g LÀ1of a-KG and pyruvic acid accumulated,respectively, when glycerol was exhausted at180h.However,the production rate of a-KG differed for the different pHs(Fig.2B).pH variation in the process of a-KG production was investigated using strategy B in an attempt to determine the opti-mal pH(Table1).During0–84h,pH declined from6.4to3.5,and biomass reached its maximum of12.8g LÀ1DCW,with only11.2 and16.1g LÀ1of a-KG and pyruvic acid produced,respectively. The accumulation rates of a-KG and pyruvic acid were0.13and 0.19g LÀ1hÀ1,respectively.Within84–120h(i.e.elapsed36h), pH continued to decline from3.5to2.7,and further16.5and 8.1g LÀ1of a-KG and pyruvic acid accumulated,respectively,with corresponding rates of0.46and0.23g LÀ1hÀ1.Thus accumulation rates of a-KG and pyruvic acid increased by250%and20%,respec-tively.Within120–180h,pH varied between2.7and2.4,and the accumulation rate of a-KG decreased to0.21g LÀ1hÀ1.The staged analysis of the strategy B results indicated that a relatively low pH (3.0)could significantly facilitate the a-KG synthesis.Consequently,an improved two-stage pH control strategy(strat-egy C)was proposed:pH was buffered with CaCO3in the growth phase and then maintained at3.0with4mol LÀ1NaOH during the a-KG production ing strategy C,pH naturally declined to 3.0owing to the organic acids accumulation,and a-KG production phase started spontaneously.As expected,a-KG accumulated at a higher rate when maintained at pH3.0(Fig.2C).Final yield of a-KG reached to53.4g LÀ1,and pyruvic acid was low with 21.3g LÀ1.Besides,the lower pH would have many advantages in industrial production:it would reduce the need for neutralizing agents such as NaOH,thereby reduce costs in downstream process-ing and alleviate environmental pollution.Furthermore,low pH could protect Y.lipolytica from other living contaminants,which is an advantage for industrial fermentation.3.3.The effect of pH control strategy on a-KG production in batch fermentationThe results of the a-KG-producing fermentations by three dif-ferent pH control strategies are shown in Table2.Strategy A(Fig.2A)resulted infinal yields of pyruvic acid(36.9g LÀ1)beingmuch greater than of a-KG(22.0g LÀ1),suggesting that excessive pyruvic acid could be potentially redistributed into the citric acid cycle to produce a-KG.Ca2+was reported to enhance the activity of pyruvate carboxylase in mitochondria(Walajtys-Rhode et al., 1992),and CO2À3could be used as substrate of pyruvate carboxyl-ase.Therefore,both Ca2+and CO2À3could facilitate the pyruvate car-boxylation process.So more carbonflux could be channeled into the citric acid cycle.Liu et al.(2007)improved the rate of a-KG/ pyruvic acid using CaCO3as a pH regulating agent in T.glabrata.Strategy B(Fig.2B)resulted in more pyruvic acid being redistrib-uted into the citric acid cycle,probably due to the presence of CaCO3.As a result,a-KG production and productivity were im-proved by83.2%and84.0%,respectively.Using strategy C(Fig.2C),the100g LÀ1of glycerol was ex-hausted after132h of culture.At the same time,the concentration of pyruvic acid reached its peak of41.8g LÀ1,and then slowly de-creased to21.3g LÀ1at180h.However,the Y.lipolytica still could synthesize a-KG efficiently for a long time.Rymowicz et al.(2010) prolongated the active citric acid biosynthesis(107g LÀ1)byZ.Yu et al./Bioresource Technology114(2012)597–602599Y.lipolytica up to300h using cell recycle with a productivity of 1.42g LÀ1hÀ1.Afther132h,the concentration of a-KG continued to increase,and reached a maximum of54.0g LÀ1at168h,suggest-ing that excessive pyruvic acid accumulation may be transported into cells and used for a-KG synthesis under glycerol deficiency (Huang et al.,2006),a likely reasonable route to reduce this byprod-uct.However,when the concentration of pyruvic acid decreased to 21.3g LÀ1,this was insufficient to meet the requirement of succes-sive a-KG accumulation,and so a-KG accumulation began to decrease.3.4.Fed-batch fermentation by pulse feeding inflasksIn the later phase of batch fermentation(Fig.2C),the cells still had latent capacity to product a-KG,but this was limited by glyc-erol deficiency.Glycerol was fed to investigate whether the results would change.The a-KG production by fed-batch fermentations in flasks using20,50,80and100g LÀ1of initial glycerol is shown in Fig.3.There was5mL of glycerol solution(500g LÀ1)added to eachflask at72h.For initial glycerol of20g LÀ1,the biomass was only 5.8g LÀ1DCW at48h,and then ceased increasing (Fig.3A).After glycerol was fed at72h,cells continued to grow and reached a biomass of8.5g LÀ1DCW at144h.Although glyc-erol(total of70g LÀ1)was nearly exhausted at168h,only 23.9g LÀ1a-KG and13.6g LÀ1pyruvic acid were detected in the broth.When the initial glycerol was50g LÀ1,i.e.a total of100g LÀ1,the biomass reached9.3g LÀ1DCW at72h(Fig.3B),and thefinal yields of a-KG and pyruvic acid were increased to41.9and18.8g LÀ1at 168h,respectively.This results illuminated that cell growth was ex-tremely important preparation for a-KG synthesis;only when high biomass was obtained in the early phase could high a-KG produc-tion be achieved.When initial glycerol was80g LÀ1(Fig.3C), although18.9g LÀ1glycerol remained in the broth,56.6and 21.2g LÀ1of a-KG and pyruvic acid were achieved within the same culture time,respectively.When initial glycerol was increased to 100g LÀ1,only3.8g LÀ1more a-KG was obtained,with25.3g LÀ1 glycerol remained in the broth.These results indicated that sufficient glycerol,up to20g LÀ1,in the broth would aid a-KG production.After glycerol was fed at72h,a-KG and pyruvic acid contents de-creased;however,they both exhibited a desirable trend in returning to a higher level after120h of culture(Fig.3).It was concluded that cells could not adapt to sudden changes in the extracellular environ-ment,and that a-KG and pyruvic acid werefirstly utilized by tem-porarily active protective mechanisms.With the expression of proteins involved in energy maintenance,transport and RNA trans-lation(van Duuren et al.,2011),a new adaptive mode may form,and thereby,a-KG production resume.In the new mode,it seemed that pyruvic acid was rapidly channeled into the TCA cycle to produce a-KG that pyruvic acid was kept at a low level.3.5.Fed-batch fermentation by constant feeding in a7-L fermentorTo avoid potential delays or inhibition resulting from the sud-den increases of glycerol,a constant feeding approach was applied in fed-batch culture(Kim et al.,2009;Zhu et al.,2010).Of150g LÀ1 total glycerol,124.3g LÀ1was used(Fig.3D).Thus,80g LÀ1initial glycerol was chosen,and50g LÀ1was bining the pH con-trol of strategy C resulted in an improved fed-batch culture mode (Fig.4)(Tang et al.,2009).The delay was then negligible and both a-KG and pyruvic acid accumulated during the feeding time (Fig.4).Consequently,66.2and26.1g LÀ1of a-KG and pyruvic acid were obtained at192h,respectively.The total130g LÀ1of glycerol was exhausted and the maximal biomass was11.4g LÀ1DCW.The biomass reached9.0g LÀ1DCW12h earlier(at60h)in comparison with the batch mode,i.e.a shorter growth phase,with the a-KG production phase beginning12h earlier.This indicated that the higher nutrient concentrations in the initial medium increased the specific growth rate.In the fed-batch culture process with con-stant feeding,glycerol was generally maintained at30–50g LÀ1 during the feeding course.a-KG production was significantly en-hanced,and a-KG productivity attained0.35g LÀ1hÀ1–a16.7%in-crease compared with the optimal batch culture(Fig.2C).Thefinal yield of pyruvic acid was very similar to that of the batch mode.For the purpose of lower pyruvic acid and higher a-KG production,ge-netic modification could redistribute the carbonflux from the pyruvic acid node to a-KG and also block further metabolism of a-KG(Zhang et al.,2009).Fed-batch culture is a preferred operational mode,using feeding substrate into a batch culture in an appropriate way,and has advantages of achieving a higher cell density by overcoming sub-strate inhibition and increasing production of the desired product (Li et al.,2010).From an economic point of view,the integrated fed-batch mode has huge advantages.In fed-batch mode(Fig.4), 12.8g LÀ1more a-KG was obtained compared to batch culture (Fig.2C)by feeding30g LÀ1glycerol–a cheap raw material.Addi-tionally,much energy consumption and labor would be saved when an equal amount of a-KG is produced by fed-batch mode compared to batch culture(Akerberg and Zacchi,2000).These advantages could significantly facilitate low-cost industrial a-KG production.Table1pH variation in the process of a-KG production by strategy B.pH Culture time(h)a-KG(g LÀ1)PA a(g LÀ1)Accumulation rate of a-KG(g LÀ1hÀ1)Accumulation rate of PA(g LÀ1hÀ1)6.4–3.50–8411.216.10.130.193.5–2.784–12016.58.10.460.232.7–2.4120–18012.67.60.210.13a PA:pyruvic acid.Table2Comparison of three pH control strategies.pH control strategy DCW(g LÀ1)a-KG(g LÀ1)PA a(g LÀ1)r a-KG/PA a(g gÀ1)r a-KG/glycerol a(g gÀ1)a-KG productivity(g LÀ1hÀ1)A9.622.036.90.590.290.13B12.840.331.8 1.270.420.24C11.553.421.3 2.510.530.30a PA:pyruvic acid;r a-KG/PA :the ratio offinal a-KG production tofinal PA production;r a-KG/glycerol:ratio offinal a-KG production to exhausted glycerol.600Z.Yu et al./Bioresource Technology114(2012)597–6024.ConclusionsIn the present study,pH was identified as a key factor in a -KG production by Y.lipolytica WSH-Z06.The production of a -KG was enhanced by applying a two-stage pH control strategy,with final production of 53.4g L À1.In addition,a novel integrated fed-batchmode of combining pH-shift and substrate-feeding strategies was used,and the maximum a -KG production was improved to 66.2g L À1,with a yield of glycerol to a -KG reaching 0.51g g À1.The lower pH of the fermentation process could significantly de-crease the risk of living contaminants and the usage of neutralizing agents.AcknowledgementsThis work was supported by grants from the Major State Basic Research Development Program of China (973Program,No.2012CB720806),the Key Program of National Natural Science Foundation of China (No.31130043),the National Natural Science Foundation of China (No.31171638),the Priority Academic Pro-gram Development of Jiangsu Higher Education Institutions and the 111Project (No.111-2-06).ReferencesAkerberg, C.,Zacchi,G.,2000.An economic evaluation of the fermentativeproduction of lactic acid from wheat flour.Bioresour.Technol.75,119–126.Barrett, D.G.,Yousaf,M.N.,2008.Poly (triol a -ketoglutarate)as biodegradable,chemoselective,and mechanically tunable elastomers.Macromolecules 41,6347–6352.Barth,G.,Gaillardin,C.,1997.Physiology and genetics of the dimorphic fungusYarrowia lipolytica .FEMS Microbiol.Rev.19,219–237.Campbell,B.,Roberts,M.,Kerksick,C.,Wilborn,C.,Marcello,B.,Taylor,L.,Nassar,E.,Leutholtz,B.,Bowden,R.,Rasmussen,C.,Greenwood,M.,Kreider,R.,2006.Pharmacokinetics,safety,and effects on exercise performance of L -arginine a -ketoglutarate in trained adult men.Nutrition 22,872–881.Chernyavskaya,O.G.,Shishkanova,N.V.,Il’chenko, A.P.,Finogenova,T.V.,2000.Synthesis of alpha-ketoglutaric acid by Yarrowia lipolytica yeast grown on ethanol.Appl.Microbiol.Biotechnol.53,152–158.Cynober,L.A.,1999.The use of alpha-ketoglutarate salts in clinical nutrition andmetabolic care.Curr.Opin.Clin.Nutr.2,33–37.Z.Yu et al./Bioresource Technology 114(2012)597–602601De Bandt,J.P.,Coudray-Lucas, C.,Lioret,N.,Lim,S.K.,Saizy,R.,Giboudeau,J.,Cynober,L.,1998.A randomized controlled trial of the influence of the mode ofenteral ornithine a-ketoglutarate administration in burn patients.J.Nutr.128, 563–569.Finogenova,T.V.,Morgunov,I.G.,Kamzolova,S.V.,Chernyavskaya,O.G.,2005.Organic acid production by the yeast Yarrowia lipolytica:a review of prospects.Appl.Biochem.Microbiol.41,418–425.Hama,S.,Tamalampudi,S.,Yoshida,A.,Tamadani,N.,Kuratani,N.,Noda,H.,Fukuda,H.,Kondo,A.,2011.Process engineering and optimization of glycerol separationin a packed-bed reactor for enzymatic biodiesel production.Bioresour.Technol.102,10419–10424.Holz,M.,Otto,C.,Kretzschmar,A.,Yovkova,V.,Aurich,A.,Pötter,M.,Marx,A.,Barth,G.,2010.Overexpression of alpha-ketoglutarate dehydrogenase in Yarrowialipolytica and its effect on production of organic acids.Appl.Microbiol.Biotechnol.89,1519–1526.Huang,H.J.,Liu,L.M.,Li,Y.,Du,G.C.,Chen,J.,2006.Redirecting carbonflux inTorulopsis glabrata from pyruvate to a-ketoglutaric acid by changing metabolic co-factors.Biotechnol.Lett.28,95–98.Kim,Y.B.,Yun,H.S.,Kim,E.K.,2009.Enhanced sophorolipid production by feeding-rate-controlled fed-batch culture.Bioresour.Technol.100,6028–6032.Li,Y.,Jiang,H.,Du,X.,Huang,X.,Zhang,X.,Xu,Y.,2010.Enhancement of phenazine-1-carboxylic acid production using batch and fed-batch culture of gacAinactivated Pseudomonas sp.M18G.Bioresour.Technol.101,3649–3656.Liu,L.,Li,Y.,Zhu,Y.,Du,G.,Chen,J.,2007.Redistribution of carbonflux in Torulopsisglabrata by altering vitamin and calcium level.Metab.Eng.9,21–29.Madzak,C.,Gaillardin,C.,Beckerich,J.M.,2004.Heterologous protein expressionand secretion in the non-conventional yeast Yarrowia lipolytica:a review.J.Biotechnol.109,63–81.Matzi,V.,Lindenmann,J.,Muench,A.,Greilberger,J.,Juan,H.,Wintersteiger,R.,Maier,A.,Smolle-Juettner,F.M.,2007.The impact of preoperative micronutrientsupplementation in lung surgery.A prospective randomized trial of oralsupplementation of combined alpha-ketoglutaric acid and5-hydroxymethylfurfural.Eur.J.Cardio-thorac.32,776–782.Mirbagheri,M.,Nahvi,I.,Emtiazi,G.,Darvishi,F.,2011.Enhanced Production of Citric Acid in Yarrowia lipolytica by Triton X-100.Appl.Biochem.Biotechnol.165,1068–1074.Otto, C.,Yovkova,V.,Barth,G.,2011.Overproduction and secretion of alpha-ketoglutaric acid by microorganisms.Appl.Microbiol.Biotechnol.92,689–695. Rymowicz,W.,Fatykhova,A.R.,Kamzolova,S.V.,Rywinska,A.,Morgunov,I.G.,2010.Citric acid production from glycerol-containing waste of biodiesel industry by Yarrowia lipolytica in batch,repeated batch,and cell recycle regimes.Appl.Microbiol.Biotechnol.87,971–979.Tang,Y.J.,Zhang,W.,Zhong,J.J.,2009.Performance analyses of a pH-shift and DOT-shift integrated fed-batch fermentation process for the production of ganoderic acid and Ganoderma polysaccharides by medicinal mushroom Ganoderma lucidum.Bioresour.Technol.100,1852–1859.van Duuren,J.B.J.H.,Wijte,D.,Karge,B.,Santos,V.A.P.M.d.,Yang,Y.,Mars,A.E., Eggink,G.,2011.pH-stat fed-batch process to enhance the production of Cis, Cis-muconate from benzoate by Pseudomonas putida KT2440-JD1.Biotechnol.Progr.1002,792–801.Walajtys-Rhode,E.,Zapatero,J.,Moehren,G.,Hoek,J.,1992.The role of the matrix calcium level in the enhancement of mitochondrial pyruvate carboxylation by glucagon pretreatment.J.Biol.Chem.267,370–379.Zhang, D.,Liang,N.,Shi,Z.,Liu,L.,Chen,J.,Du,G.,2009.Enhancement of a-ketoglutarate production in Torulopsis glabrata:Redistribution of carbonflux from pyruvate to a-ketoglutarate.Biotechnol.Bioproc.E.14,134–139. Zhou,J.W.,Dong,Z.Y.,Liu,L.M.,Du,G.C.,Chen,J.,2009.A reusable method for construction of non-marker large fragment deletion yeast auxotroph strains:a practice in Torulopsis glabrata.J.Microbiol.Methods76,70–74.Zhou,J.W.,Zhou,H.Y.,Du,G.C.,Liu,L.M.,Chen,J.,2010.Screening of a thiamine-auxotrophic yeast for alpha-ketoglutaric acid overproduction.Lett.Appl.Microbiol.51,264–271.Zhu,Y.,Li,J.,Tan,M.,Liu,L.,Jiang,L.,Sun,J.,Lee,P.,Du,G.,Chen,J.,2010.Optimization and scale-up of propionic acid production by propionic acid-tolerant Propionibacterium acidipropionici with glycerol as the carbon source.Bioresour.Technol.101,8902–8906.602Z.Yu et al./Bioresource Technology114(2012)597–602。